Abstract
The consequences of adenovirus infections are generally mild. However, despite the perception that adenoviruses are harmless, infections can cause severe disease in certain individuals, including newborns, the immunocompromised, and those with pre-existing conditions including respiratory or cardiac disease. In addition, adenovirus outbreaks remain relatively common events and the recent emergence of more pathogenic genomic variants of various genotypes has been well documented. Coupled with evidence of zoonotic transmission, inter-species recombination and the lack of approved adenovirus antivirals or widely available vaccines, adenoviruses remain a threat to public health. At the same time, the detailed understanding of adenovirus biology garnered over nearly 7 decades of study has made this group of viruses a molecular workhorse for vaccine and gene therapy applications.
Keywords: Adenovirus, pathogenesis, outbreaks, persistence, adenovirus vectors, therapeutics
Human adenoviruses and their medical importance
The human adenoviruses (HAdVs) have a predictable pattern of unpredictable emergence. Tackling the emergence and re-emergence of HAdVs has been an area of recent interest, alongside improved understanding of HAdV epidemiology and zoonoses. Additionally, rapidly growing interest in harnessing HAdV as a tool for vaccine and therapeutic applications requires continued detailed study of the interplay between infection and innate and adaptive immune responses. This has also recently pushed understudied human and non-human adenoviruses into the research spotlight. This review provides a broad overview of the current state of HAdV research and highlights several research areas of increasing medical importance.
Characteristics of HAdVs
Although adenoviruses (AdVs) are highly species specific, collectively they infect all vertebrates, including humans, other mammals, birds, reptiles, and fish. HAdVs are non-enveloped, with a doubled stranded DNA (dsDNA) genome contained inside an ~90 nm icosahedral capsid, comprised of the three main structural proteins (hexon, penton, fiber) and four minor structural proteins (IIIa, VI, VIII, and IX) [1–4]. Over 110 HAdV types are classified into seven different species (A-G), originally based on their neutralization phenotypes (serotype) and currently based on their unique genomic characteristics (genotype) [5] (http://hadvwg.gmu.edu). Tissue tropism differs across HAdV species, and this impacts the clinical manifestation of infection (Table 1).
Table 1.
Characteristics of the 7 human adenovirus species.
| Species | Common HAdV Types Associated with Disease | Major Site of Infection | Primary Entry Receptor | Associated with Outbreak? | Refs |
|---|---|---|---|---|---|
| A | 12,31 | Respiratory tract, urinary tract, GI tract | CAR | yes | [6,45,51] |
| B | 3,7,14, 21, 34,55 | Respiratory tract, conjunctiva, urinary tract | CD46, DSG2 | yes | [6,9,10,23,47] |
| C | 1,2,5,6 | Respiratory tract, urinary tract | CAR | yes | [45,51,136] |
| D | 8,19,37,53,54,56 | Conjunctiva | CD46, Sialic acid | yes | [16,19,21,46,87,88,128,137] |
| E | 4 | Respiratory tract, conjunctiva | CAR | yes | [20,23,49,90] |
| F | 40,41 | GI tract | CAR | yes | [6,45,52,112] |
| G | GI tract | CAR, Polysialic acid | no | [7,50] |
HAdV prevalence and transmission
HAdV infections are highly prevalent, with nearly all individuals infected with at least one type by 6 years of age [6]. Infections in immunocompetent individuals typically resolve within 7–10 days without treatment or hospitalization [7]. In contrast, immunocompromised individuals and children are at risk for more severe disease [8]. Immunocompromised populations at risk for HAdV infection include recipients of hematopoietic stem cell and solid organ transplants, or individuals with primary immunodeficiencies, such as severe combined immunodeficiency syndrome, HIV infection, or undergoing immunosuppressive therapies [7]. Clinical manifestations of HAdV infection, such as pneumonia and hepatitis (Box 1), can prove fatal in immunocompromised patients and occasionally even in the immunocompetent, as observed historically in military recruits [9–11].
Box 1. HAdV-F41 and AAV-2: dynamic duo or innocent bystanders?
A worldwide outbreak of severe acute hepatitis of unknown etiology in seemingly healthy children was declared in April 2022. As of July 2022, there were over 1000 probable cases, including 22 deaths. Many suspected HAdV as a likely culprit, as HAdV-F41 was the most frequently detected pathogen in clinical samples [110,111]. Additionally, serum viral loads were higher in children with liver failure compared to children who spontaneously improved [110,111]. Although rare, HAdV infection can cause hepatitis, typically in pediatric, immunocompromised individuals [11]. However, HAdV-F41 is not associated with hepatitis and does not display liver tropism [112]. HAdV-F41 typically causes gastroenteritis, particularly in young children [7]. Previous hypotheses suggested that genomic variations in HAdV-F41 could explain this atypical presentation. However, preliminary WGS data suggested there was no evidence of common single nucleotide polymorphisms in HAdV-F41 samples or evidence of recombination with other HAdVs [113]. Additionally, immunohistochemistry and electron microscopy failed to detect HAdV in liver biopsy samples from children with acute hepatitis of unknown cause [110,111]. Preliminary investigations suggested evidence of high levels of adeno-associated virus 2 (AAV-2) DNA and RNA in blood samples and liver cells compared to controls with HAdV infection or hepatitis of another etiology, shedding light on another potential culprit [113,114].
In 1965, the AAV family was discovered as “contaminants” in HAdV preparations [115]. These small viruses, previously thought to be non-pathogenic and often used as vectors for gene therapy, require a “helper” virus to replicate, such as a papillomavirus, herpesvirus, or AdV (the latter two identified in children with acute hepatitis) [113–115]. AAV can also inhibit HAdV replication in co-infected cells [115]. Five HAdV genes, E1A, E1B55K, E2A, E4orf6, and VA RNA, constitute the minimal core required for successful AAV replication [115]. These genes activate AAV promotors, facilitate export and splicing of AAV mRNA, and promote viral DNA replication and protein synthesis [115]. Importantly, preliminary evidence suggested no new mutations in E1A, E2A and E4A proteins in detected HAdV-F41 samples [113].
It is unknown if HAdV-F41 co-infection is of specific importance to this clinical manifestation, or simply an innocent bystander as a common cause of pediatric infection. Given the frequent detection of human herpesvirus 6B, another virus that “helps” AAV, in these cases of acute hepatitis, it remains possible that several distinct types of coinfections have contributed to the current outbreak. However, further research is necessary to elucidate the potential role of HAdV-F41.
HAdV infections in children
Over 80% of HAdV infections are diagnosed in children under 5 years of age due to the lack of pre-existing immunity [7]. Most HAdV infections, especially with HAdV species A and D, are mild or asymptomatic [6]. HAdV accounts for 5–10% of all childhood respiratory tract infections, which are commonly caused by HAdV types 1–7 [12–14]. HAdV-B3 and -B7 are two of the most common genotypes associated with acute respiratory disease (ARD) and hospitalization in pediatric cases [12–14]. More severe disease and enhanced cytotoxicity in cell culture was recently associated with HAdV-B7 infection compared to HAdV-B3 [15]. HAdV-B7 also displayed more robust replication in cell culture and induced higher levels of inflammatory cytokines combined with more severe airway inflammation in infected mice compared to HAdV-B3 [15]. This provides potential insight into the enhanced pathogenicity of HAdV-B7 in humans [15].
HAdV transmission, incubation, and viral shedding
HAdV infections typically spread via exposure to respiratory droplets, the fecal-oral route, and contact with contaminated food, water, or surfaces [6]. Direct inoculation into the eye is also possible, demonstrated by an outbreak in an ophthalmology clinic of HAdV associated-epidemic keratoconjunctivitis due to a contaminated bottle of multi-use eye drops [16]. The incubation period for HAdV-associated disease manifestations ranges from 2 to 14 days [7].
Post-recovery, prolonged asymptomatic viral shedding can occur from the gastrointestinal and respiratory tract, typically lasting longer in immunocompromised individuals [8]. A recent study of HAdV-B55 positive respiratory infections in immunocompetent patients detected viral shedding in respiratory samples up to 52 days after fever onset [17]. Viral shedding in hospitalized children with severe HAdV pneumonia lasted 96.9 days and 51.4 days in HAdV-B7 and -B3 positive infections, respectively [18]. HAdV shedding is significantly prolonged compared to shedding of other respiratory viruses in healthy patients, such as influenza virus (up to 18 days), respiratory syncytial virus (mean duration 4 days) and rhinovirus (mean duration 11 days) [17]. These data have practical implications for nosocomial infection control strategies and surveillance policies for HAdV outbreaks.
Outbreaks and emerging HAdVs
HAdV outbreaks have no seasonality and often occur in closed populations, such as hospitals, daycare and long-term care facilities, college dormitories, and military barracks [14]. Due to the typical self-limiting and mild nature of HAdV infections in immunocompetent individuals, HAdV outbreaks often remain undetected and under-reported. Nevertheless, novel and re-emerging HAdVs have caused outbreaks associated with significant morbidity and mortality in infants, children, immunocompromised individuals, and immunocompetent individuals with no apparent pre-existing risk factors [9,10,16,19–23]. Several noteworthy outbreaks have been specifically associated with recombinant and genomic variant HAdVs, described below (Figure 1).
Figure 1.
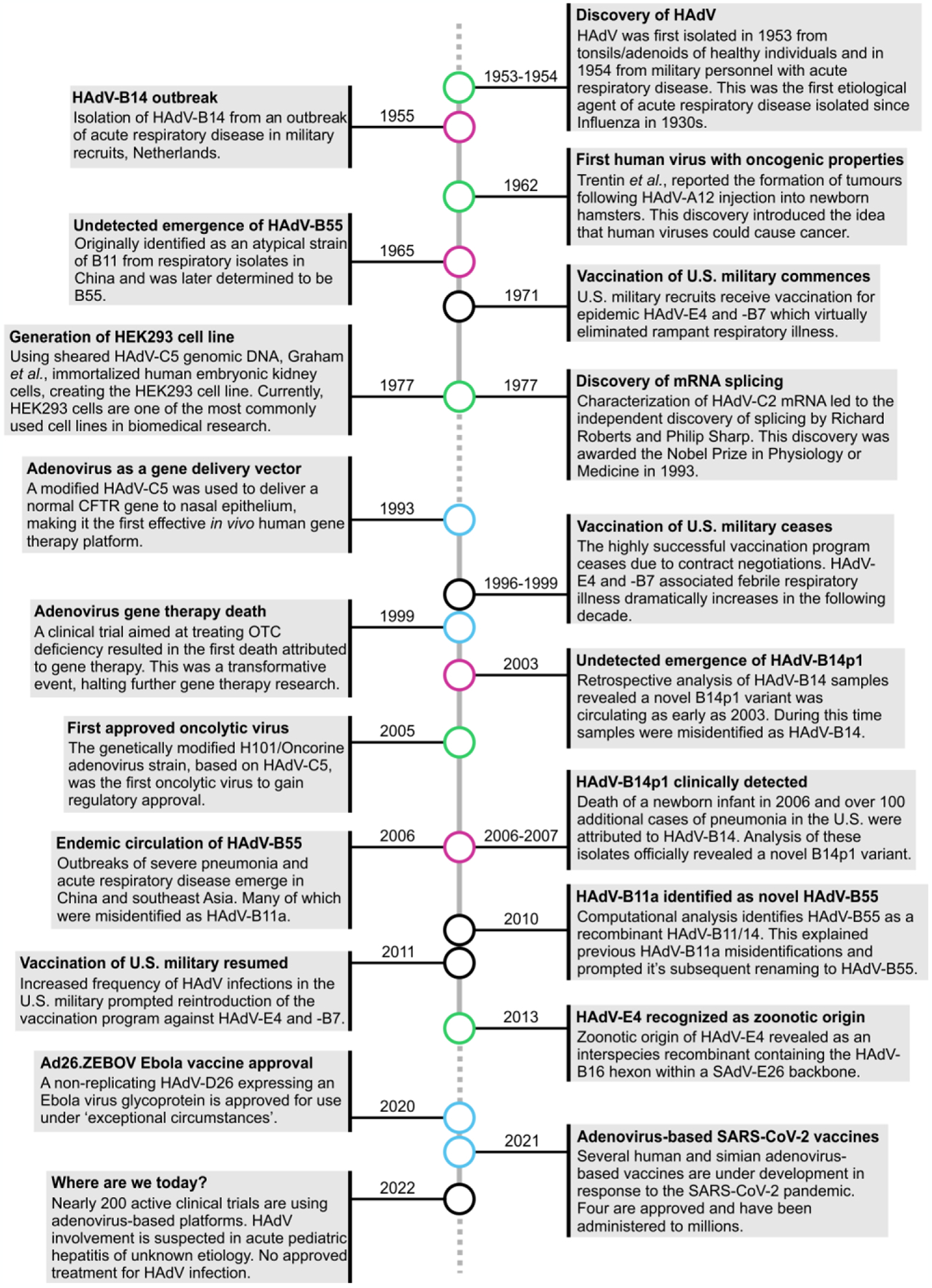
Timeline of key human adenovirus historic events, emergences, and technologies over 69 years.
Human adenoviruses (HAdV) have been associated with advancements in bio-medical research technologies (blue circles), and many milestone discoveries (green circles) since its identification in 1953–1954 [138,139]. Additionally, this timeline follows the emergence and re-emergence of HAdV-B55 and HAdV-B14/HAdV-B14p1 (pink circles) [7,28,29,32]. In 1962, HAdV gained its distinction as the first human virus to cause cancer, albeit in a rodent model [140]. HAdV has also severed as a tool to study molecular biology. HAdV was instrumental in the discovery of splicing and generation of the popular human embryonic kidney (HEK) 293 cell line [141–143]. The U.S. military experienced severe HAdV outbreaks in their recruits, and contracted Wyeth Laboratories Inc. to produce and supply them with the only approved HAdV vaccine (against HAdV-B7 and HAdV-E4, which was latter discovered to have a zoonotic origin) [6,34]. The vaccination program lasted from 1971 to 1996 when contract renegotiations failed, and the program ceased [6]. Without vaccine protection, HAdV outbreaks in the military drastically increased over the next decade until the program was reinstated in 2011 [6,7]. HAdVs are used as vectors in gene therapy, initially to deliver the cystic fibrosis transmembrane conductance regulator (CFTR) gene to humans in vivo, and later being associated with a patient death in a clinical trial on ornithine transcarbamylase (OTC) deficiency [108,144]. HAdV was also the first approved oncolytic virus [145]. HAdV has been successfully used as a vaccine platform for Ebola virus (Ad26.ZEBOV) and the SARS-CoV-2 pandemic, with many ongoing HAdV vector clinical trials [109].
Genetic diversity in emerging HAdVs and its effects on pathogenesis
Genetic diversity in HAdV can arise from spontaneous mutations during viral genome replication, despite the proof-reading abilities and high fidelity of the HAdV DNA-dependent DNA polymerase. Homologous recombination (see Glossary) also introduces genetic diversity and functions as a major driver of HAdV evolution and the emergence of novel pathogenic strains [24]. Recombination between different HAdVs most frequently occurs during co-infection of the same cell with different HAdV types of the same species [25]. Typically, recombinant and genomic variant HAdVs are only identified if they attract attention as a result of their apparent increased virulence (i.e., severe or unusual disease and fatal outcome) [20,26].
HAdV-B14p1 is a clinically important genomic variant that is now circulating worldwide [27]. HAdV-B14p1 has been associated with numerous outbreaks of pneumonia and ARD in relatively healthy individuals, resulting in higher fatality rates compared to the HAdV-B14 prototype strain [7,14,28]. HAdV-B55 is an intertypic recombinant of HAdV-B11 and HAdV-B14 and has also been associated with outbreaks of pneumonia and ARD in both immunocompetent and immunocompromised individuals [14,28]. The HAdV-B55 genome has the backbone of HAdV-B14 and a HAdV-B11-like hexon protein, thus creating a “trojan horse-like” virus, in which neutralizing antibodies are produced against an HAdV-B11 epitope, but the pathogenic profile is that of HAdV-B14 [29]. Evidently, HAdV-B55 was initially mistaken as a genomic variant of HAdV-B11 [30–32].
The re-emergence of HAdV-B55 and shift from sporadic outbreaks to endemic status in Southeast Asia has garnered attention concerning the possibility of global transmission events [32]. To examine HAdV-B55 prevalence from a global perspective, a study analyzed whole genome sequencing (WGS) of 72 HAdV-B55 clinical isolates from 6 countries and identified amino acid residue differences between HAdV-B55 strains in proteins essential for replication and virus maturation [32]. This highlighted the potential for random mutations to affect HAdV transmission, and/or pathogenesis.
Gain-of-function and loss-of-function mutations in emerging HAdVs
Little is known about the impact of recombination and random mutations on HAdV transmission and pathogenesis. Recently, gain-of-function mutations suspected to enhance replication in human cells were identified in HAdV-E4 isolated from a pediatric population presenting with flu-like symptoms [33]. Sequencing of HAdV-E4 isolates revealed a critical replication motif termed nuclear factor I (NF-I) [33]. This motif is highly conserved within the inverted terminal repeats of many HAdV strains and is required for efficient viral replication in human cells, but is absent in the prototype HAdV-E4 strain [33–35]. HAdV-E4 infections have typically been identified in military populations and more recently, they have been reported globally in civilian populations [20,36]. This motif may confer a replicative advantage over the prototype HAdV-E4, enabling HAdV-E4 to cross into the immune-naïve civilian population. Moreover, this may explain the observed wider distribution of HAdV-E4.
Loss-of-function mutations could also have an impact on pathogenesis. For example, in vitro studies of a HAdV-C5 E1B-19K gene deletion mutant was associated with enhanced proinflammatory responses by activated macrophages compared to wild-type HAdV-C5 infection [37]. This suggests that E1B-19K may play a role in limiting local innate inflammation during infection [37]. Conversely, altered E1B-19K function may play a role in the increased host inflammatory response seen in emerging, pathogenic HAdV strains. Further research and characterization of novel and re-emerging viruses is necessary to understand how gain- or loss-of-function mutations impact pathogenesis and clinical presentation.
Emerging AdVs and their zoonotic potential
HAdV-E4 was the first identified HAdV with a zoonotic origin (Figure 1) [34]. The HAdV-E4 genome contains a partial HAdV-B16 hexon recombined into the chimpanzee simian adenovirus (SAdV)-E26 backbone [34]. This significant human pathogen has caused outbreaks of febrile respiratory illness and pneumonia in military and civilian populations. Its high prevalence in military recruits led to the development of the only HAdV vaccine, a live virus, oral vaccine for military personnel against HAdV-E4 (and HAdV-B7) (Figure 1) [7].
HAdV-E4 is not the only AdV with zoonotic links. Humans in Côte d’Ivoire, Cameroon, and Nigeria exhibit neutralizing antibodies against chimpanzee SAdV-C [38]. HAdV has also been detected in fecal samples from pigs, dogs, sheep, and goats in Côte d’Ivoire, as well as in captive and wild non-human primates [39,40]. More recently, a HAdV-C1 related virus isolated from a cat was reported to replicate in human cells [41]. Analysis of two SAdVs isolated from chimpanzees and baboons revealed that HAdV-B76 was derived from a recombination event between human, chimpanzee, and baboon AdVs, suggesting back-and-forth interspecies transmission [42]. Overall, a growing emphasis has been placed on exploring the potential of non-human AdVs to cross species barriers and understanding their interactions with the human immune system.
HAdV genotyping and its limitations in studying pathogenicity
Advancements in molecular methods for genotyping HAdV strains have been essential for epidemiological investigations in addition to the surveillance of emerging HAdVs [14]. Typically, PCR amplification and sequencing of the hexon gene, with emphasis on the type-specific hypervariable regions, is sufficient for HAdV typing [43]. However, this could lead to misidentification of recombinant viruses, highlighting the necessity of sequencing two or more targets for molecular typing, such as the penton base, hexon, and fiber genes [24,32]. Additionally, detection of novel animal viruses in humans is unlikely with standard clinical diagnostics and would require next generation sequencing. Recent advancements in WGS allow for high-throughput sequencing directly from clinical samples with low viral loads [44]. The use of WGS and phylogenetic analysis can provide critical information to establish epidemiological links among cases of infection with any given HAdV type and aid in the development of nosocomial infection control policies [44,45].
Despite the modernization of HAdV genotyping, there are still limitations. The shift in diagnostics away from viral isolation in cell culture towards direct analysis precludes banking, characterizing, and distributing viral isolates to research laboratories. This will undoubtedly limit understanding of the pathogenicity of emerging variants or zoonotic events. However, this significant challenge may be ameliorated by the ability to synthesize entire viral genomes de novo using WGS data [46].
The HAdV replication cycle: entry, replication, and egress
Viral entry begins with the HAdV capsid binding to primary receptors via the fiber knob domain. These receptors include coxsackie-adenovirus receptor (CAR), CD46, desmoglein-2 (DSG2), or sialic acid (Table 1) [47–52]. Next, the arginine-glycine-aspartic acid (RGD) motif in the HAdV penton base interacts with cellular integrins to stimulate endocytosis [53]. To avoid endosomal degradation, fiber proteins are removed by disruptions from primary receptor interactions, exposing protein VI that mediates endosomal escape into the cytoplasm [54,55]. After escape, the capsid is trafficked to the nucleus [56]. Following capsid disassembly, the condensed viral genome enters the nucleus through the nuclear pore [49,57].
HAdV gene expression occurs in a temporal manner. The early phase of gene expression synthesizes proteins required for viral genome replication, while the late phase synthesizes proteins involved in virion assembly [58]. Accumulation of early gene products triggers viral genome replication and the switch to late phase gene expression [58]. After late phase gene expression, virion assembly occurs in the nucleus, followed by lytic viral egress [59].
Little is known about viral egress for HAdV species beyond species C. For HAdV-C, viral egress is mediated by the adenovirus death protein (ADP), which ruptures membranes at late stages of infections [59]. Recently, a nonlytic cell-to-cell transmission route for HAdV-C was described [60]. Lytic and non-lytic egress could have opposite effects on virus-induced inflammation and pathogenesis, and therefore potential implications for disease management.
The immune response to HAdV infection
Activation of innate immune responses to HAdV infection
HAdV entry into the cell triggers a rapid response from various innate immune signaling pathways (Figure 2A), causing the production and release of pro-inflammatory cytokines and chemokines that serve as warning signals to neighbouring cells to activate antiviral defences [8,61]. Several extracellular proteins also target HAdV to facilitate uptake by phagocytes or impede viral entry (Box 2) (Figure 2A). Upon escape from endosomes and entry into the cytosol, the cyclic guanine adenine synthase (cGAS) signaling pathway is involved in detecting HAdV and is activated by the presence of dsDNA in the cytosol [62]. It is generally assumed that damage of the protein-wrapped viral DNA in the cytosol exposes DNA to activate cGAS, although the entirety of this activation mechanism remains unknown [63]. Activation of cGAS leads to stimulator of interferon genes (STING) activation and interferon regulatory factor 3 (IRF3)-dependent production of type I interferons (IFN), including IFN-α and IFN-β [62]. HAdV genomic DNA can also be sensed in the nuclei of infected cells by hnRNPA2B1, a heterogeneous nuclear riboprotein [64]. Upon activation, hnRNPA2B1 dimerizes and activates TANK-binding kinase 1 (TBK1) leading to IRF3 phosphorylation and activation of the type I IFN response [64].
Figure 2.
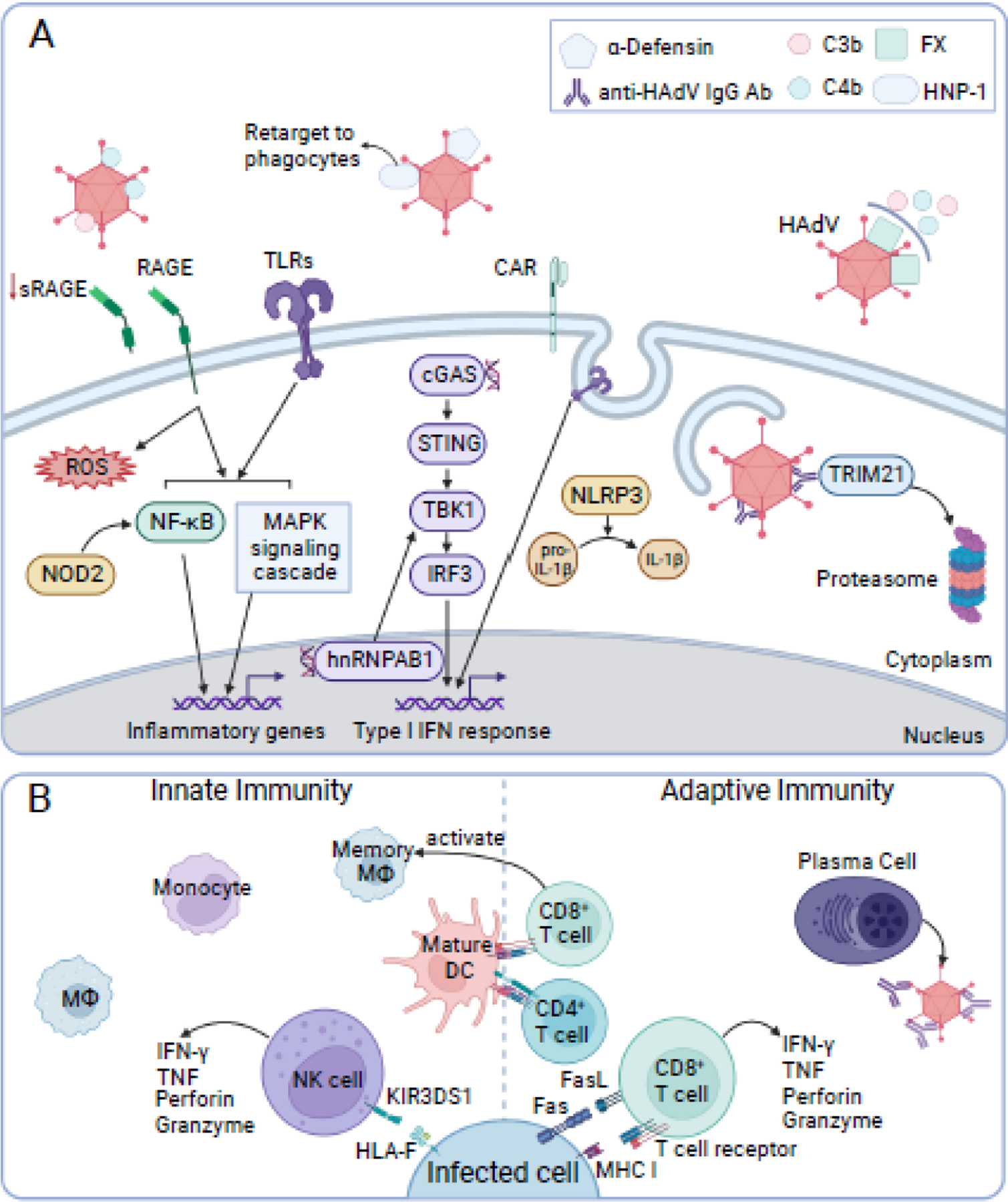
The cellular immune response, extracellular proteins, and molecular mechanisms involved during human adenovirus infection.
This non-comprehensive overview of anti-HAdV immune responses emphasizes recent findings. (A) Extracellular proteins α-defensin and complement protein C4b can bind human adenovirus (HAdV) and impede entry. Binding of HNP-1 re-targets the virion to phagocytes, while binding of complement protein C3b leads to virion opsonization. FX binding protects HAdV from complement. RAGE activation produces reactive oxygen species (ROS). RAGE and TLR activation during infection ultimately results in NF-κB and MAPK signaling and transcription of inflammatory genes. A NOD receptor, NOD2, can also activate NF-κB. Signaling from endosomal TLRs, sensing of cytosolic double-stranded DNA (dsDNA) via cGAS/STING pathway or nuclear viral dsDNA via hnRNPAB1 stimulates transcription of type I IFN genes. Activation of NLRP3, a NOD-like receptor, helps regulate secretion of IL-1β. TRIM21 binds to cytosolic antibody-HAdV complexes, targeting the virion for proteasomal degradation. (B) Macrophages (MΦ), monocytes, dendritic cells (DCs), and natural killer (NK) cells are recruited and activated following HAdV infection. NK cell receptor KIR3DS1 recognizes HLA-F on infected cell surfaces. NK cell activation leads to degranulation (perforin and granzyme) and production of IFN-γ and TNF. Mature DCs present viral peptides to activate CD8+ and CD4+ T cells. CD8+ T cells produce IFN-γ and TNF, release perforin and granzyme, induce apoptosis via Fas-Fas ligand (FasL) interactions, and lyse cells presenting viral peptides via MHC I. In alveolar mucosal tissues, CD8+ T cells can induce alveolar memory MΦ, which provide protection against re-infection. Plasma cells produce high-affinity, HAdV-specific antibodies against HAdV structural proteins to inhibit receptor binding, neutralize/aggregate virions, and activate complement. Created with BioRender.com
Box 2. The role of extracellular proteins in HAdV infection.
Several extracellular factors, discussed below, can “tag” the HAdV virion prior to viral entry. These factors provide the HAdV-immunocomplex access to a different set of receptors and mechanisms to enter cells, such as macrophages and dendritic cells, compared to the virus alone. These HAdV-immunocomplexes, such as HAdV-α-defensin or HAdV-IgG, enhance entry into dendritic cells and cause a more potent release of cytokines than the HAdV alone [116–118].
α-defensin
Previously, human α-defensins were shown to stabilize the viral capsid by bridging the penton-fiber base to prevent fiber shedding, protein VI exposure, and endosome escape [119]. Recent work revealed that α-defensin neutrophil protein 1 (HNP-1) binds the capsids of HAdV-C5, -D26, and -B35 to re-target it towards TLR-4 on primary human phagocytes, thereby inducing NLRP3 inflammasome formation and release of pro-inflammatory cytokine IL-1β [118]. This enhanced uptake of defensin-bound HAdV by immune cells contrasts with the reduced uptake of HAdV by epithelial cells and may suggest that defensins are redirecting HAdV towards innate immune cells to “kick-start” the innate immune response [118,119].
Complement
Both IgM and IgG activate the classic complement pathway during HAdV-infection. The classical pathway involves the interaction between the complement C1 complex and antibody bound HAdV, which triggers a cleavage cascade, producing C3b and C4b, both of which can covalently attach to the surface of the virion [67,120]. C3b activates proinflammatory signaling pathways and the proteasome-mediated degradation of C3b-opsonized HAdV [121]. C4b blocks capsid disassembly and prevents entry of the viral genome into the nucleus, in a mechanism analogous to human α-defensin-mediated HAdV-C5 neutralization [120]. Recent in vivo evidence suggested this C4b mechanism operates synergistically with TRIM21, in which viruses that manage to escape inactivation by C4 can then be targeted by cytosolic TRIM21 for proteasomal degradation [106,120].
Vitamin K-dependent coagulation factor X
Vitamin K-dependent coagulation Factor X (FX) can protect HAdV-C5 from neutralization by complement proteins in mouse and human serum and contributes to the characteristic hepatocyte liver tropism of HAdV-C5 [122]. The FX γ-carboxyl glutamic acid (GLA) domains bind to hypervariable regions on the HAdV-C5 hexon [123]. FX serves to bridge the attachment between HAdV-C5 to heparan sulfate proteoglycan (HSPG) on the hepatocyte cell surface [123]. However, this relationship is not conserved across HAdV species, as many genotypes displayed minimal affinity for FX [123]. The impact of the interaction with FX and HAdV neutralization in human serum continues to be explored for HAdV vector candidates [122].
Other innate receptors activated upon HAdV infection include the nucleotide-binding and oligomerization domain (NOD), NOD-like (NLR), and Toll-like (TLR) families of receptors, whose activation leads to release of early innate and inflammatory response factors such as IL-6, IL-12p40, IL-1β, tumour necrosis factor (TNF), IFN-α and IFN-β [65–67]. Initial innate immune responses in the airway mucosal epithelia are particularly important, as these tissues are specifically targeted by HAdV and present the first line of defense against infection. As one example, the receptor for advanced glycation end products (RAGE) is constitutively expressed in lung tissue and immune cells and is involved in the inflammatory response to HAdV infection [68]. RAGE signaling promotes reactive oxygen species (ROS) formation, as well as NF-κB and MAPK pathway activation. As a result, proinflammatory genes such as IL-18, vascular cell adhesion protein 1 (VCAM-1), and TNF are expressed, initiating and maintaining inflammation, unless RAGE activation is blocked by its soluble form sRAGE [69]. Patients with HAdV infections exhibiting decreased levels of sRAGE, were more likely to develop pneumonia, likely as a result of excessive inflammation and severe tissue injury [68].
Cellular innate response to HAdV infection
Innate effector cells, particularly natural killer (NK) cells, are recruited and activated in response to HAdV infection (Figure 2B). NK cells are innate immune cells that express a variety of activating and inhibitory receptors that recognize the altered expression of proteins on the surface of target cells. The balance between activating and inhibitory signals control their cytolytic function, which involves the release of perforin and granzyme-containing granules, INF-γ, and TNF [70].
Interestingly, upregulation of human leukocyte antigen (HLA)-F, the ligand for activation of the NK cell receptor KIR3DS1, allows for enhanced recognition and killing of HAdV-infected cells in a 3D organoid model [71]. Additionally, HAdV-infected pediatric hematopoietic stem cell transplant (HSCT) recipients who received KIR3DS1+/HLA-Bw4+ donor cells were protected against severe HAdV infection [71]. This KIR3DS1/HLA-F axis may be a promising target for the treatment of HAdV infections in children and immunocompromised individuals [71].
Innate immunity memory, or trained immunity, is an emerging concept based upon recent findings that following primary immunological exposure, innate NK cells, monocytes, and macrophages can be programmed to carry out an immune memory response to enhance host defence [72,73]. Acute respiratory adenovirus infections with HAdV-C5 have been found to induce memory alveolar macrophages (AMs) in mucosal tissues. These exhibit higher major histocompatibility complex (MHC) II expression, elevated chemokine production, increased glycolytic metabolism, and a “defense-ready” gene signature [74]. These long-lasting memory AMs were activated by effector CD8+ T cells in an IFN-γ- and contact-dependent manner [74]. The generation of broadly protective memory macrophages in addition to antigen-specific T and B cell immunity produces a key immunological memory response against HAdV reinfection and illustrates this new paradigm of immunological memory [74].
Adaptive immune response to HAdV infection
Innate immunity supports the activation and proliferation of antigen-specific adaptive immune responses against HAdV (Figure 2B) [8]. HAdV-specific CD4+ and CD8+ T cells, activated by mature dendritic cells (DCs) and the release of proinflammatory cytokines, show cross-reactivity with different HAdV species, indicating that T cells recognize conserved amino acid epitopes from HAdV structural proteins [8]. An immunodominant T cell targets is the HAdV hexon protein, which contains antigenic components common to all adenovirus species [75]. CD8+ T cells produce proinflammatory cytokines, such as IFN-γ and TNF, as well as kill infected cells presenting viral peptides bound to MHC I via the release of perforin and granzyme-containing granules, or by inducing apoptosis through Fas-Fas ligand (FasL) interactions [76–79]. However, excessive inflammatory responses are linked to severe outcomes following AdV infection in mouse models and clinical cases, including deleterious pulmonary inflammation [76,80].
B cells produce high-affinity, type-specific antibodies against the HAdV capsid proteins. Anti-HAdV antibodies, particularly of the IgG class, can target HAdV for opsonization, activate the classical complement pathway, neutralize viral particles, and induce inflammasome activation in cells internalizing opsonized virus [51]. Despite extracellular anti-hexon antibody recognition of HAdV, neutralization does not occur until after viral-mediated entry and endosomal escape. After endosomal escape, the cytosolic antibody receptor TRIM21 binds the antibody-HAdV complex and catalyzes the formation of ubiquitin chains, targeting the virion for proteasomal degradation [81,82]. Overall, studies of the adaptive immune response to HAdV have practical implications for improving current HAdV vectors (Box 3) used for gene therapy and vaccines in order to protect these vectors from immune responses, thereby increasing their efficacy.
Box 3. HAdV vectors for gene delivery.
There is longstanding interest in using HAdV as a vectors for gene therapy and vaccines [108]. The dsDNA viral genome, tolerance to genetic modifications, and ability to induce robust immunogenic responses makes HAdVs particularly appealing for these applications. Due to the seroprevalence of antibodies for common HAdV species, many researchers have transitioned to using rare HAdVs (e.g., HAdV-B11, D26 and B35) or even animal AdVs (e.g., pigs, dogs, cattle, and non-human primates) to prevent antibody-mediated neutralization of the vector [109,124–127]. A high-profile example is the modified chimpanzee AdV vector, ChAdOx, used for the Oxford-AstraZeneca vaccine for SARS-CoV-2, with more than 1 billion doses administered (Figure 1) [128]. A current challenge of using AdVs with low seroprevalence is that they are historically understudied compared to more common HAdVs. This has led to a recent reinvigoration of investigation related to basic HAdV biology, such as primary receptor engagement, interactions with factors in the innate immune system, and induction of adaptive immunity [106,120,129]. With hundreds of clinical trials using AdV vectors, this area highlights an exciting future for AdV research.
Severe acute inflammatory responses to HAdV infection
Typically, HAdV infections are effectively cleared by the immune response, although severe acute inflammatory responses can occur [8]. However, severe inflammatory responses have also been associated with some HAdV infections in immunocompetent hosts and disseminated infections in immunocompromised hosts [13,15,83]. For example, epidemic keratoconjunctivitis (EKC) caused by HAdV results in severe inflammation of the conjunctiva and cornea, which can also persist for months to years [84]. High levels of inflammatory cytokines and chemokines, including IL-1, IL-6, IL-8 IL-10, IFN-γ, and TNF are associated with stronger inflammatory responses and increased disease severity and may have diagnostic and prognostic implications for HAdV infections [15,61,80]. Further investigation to understand the immune mechanisms causing severe acute inflammatory responses to HAdV infection, such as observed with EKC, will aid in prevention and management of severe disease [8,68,69,76,80].
Ocular manifestations of HAdV infections
HAdV is the most frequent cause of viral conjunctivitis and is often caused by HAdV species D (e.g., HAdV‐D8, D19, D37, D53, D54, D56, and more recently, D82 and D85); however, HAdV-B, C, and E have also been implicated as causal agents [84–87]. Ocular manifestations of HAdV infection include simple follicular conjunctivitis, pharyngoconjunctival fever, and EKC [84]. EKC is a severe and highly contagious eye disease with clinical presentations typically including bilateral follicular conjunctivitis (i.e., red, irritated eyes) and preauricular lymphadenopathy (i.e., swelling of preauricular lymph nodes) [84]. Other clinical signs unique to EKC are conjunctival membrane formation, and corneal inflammation (keratitis) that can persist or re-occur for months to years after infection and lead to impaired vision [84,88].
HAdV-D8 is the most commonly detected HAdV type among cases of EKC and has been associated with sporadic outbreaks in health care settings, such as hospitals and ophthalmology clinics [16,21,22,87,89]. However, these outbreaks of HAdV-D8 are often under reported as detection of the causal agent of EKC is not required for clinical diagnosis and treatment [21]. Outbreaks of HAdV conjunctivitis can also occur in community settings such as swimming pools, as observed in an outbreak which saw 55 individuals contract pharyngoconjunctival fever from swimming in HAdV-E4 contaminated water [90].
While there is currently no specific therapy to treat EKC, recent studies of the viral entry of EKC-associated HAdV-D have revealed potential targets for the development of viral entry inhibitor therapies [91].
HAdV persistence
While HAdV infections are typically acute and lytic, some infections can enter a persistent, subclinical state in tissues that is characterized by long-term, low levels of viral replication and virion production (Box 4) [92]. Persistent infection can be problematic in immunocompromised individuals, in whom reactivation of persistent infection can lead to viremia and disseminated adenoviral disease [93–95]. HAdV persistence has been well-documented in tonsillar and adenoid tissues, and there is supporting evidence of HAdV persistence in the gastrointestinal tract and other tissues [96–98].
Box 4. Molecular mechanisms of HAdV persistence.
The molecular details of why and how HAdVs establish persistent infections are not entirely understood. Changes in epigenetic signatures and chromatin structure likely lead to the establishment and maintenance of HAdV persistence. Histone deacetylase inhibitors, which target the epigenome, can trigger reactivation of persistent HAdV [130]. Interestingly, the added insult of a bacterial co-infection in patients with persistent HAdV may influence resurgence of acute viral infection. Bacterial components like lipopolysaccharide (LPS) can stimulate inflammatory responses that induce histone acetylation and euchromatinization (i.e., opening of chromatin), which may positively influence reactivation of persistent HAdV [130].
Besides changes in host chromatin structure, many viral components have been linked to HAdV persistence. The adenovirus death protein (ADP), a late viral protein unique to HAdV-C and responsible for cytolysis of the infected cell, is expressed at very low levels in persistent infections [59]. Persistent cells also show reduced E1A expression, which is not surprising, as E1A is responsible for driving the early phase of viral replication [58,131]. However, the viral-associated RNAs (VA-RNA I and II) are highly expressed during persistence [132]. VA RNAs are commonly associated with protecting HAdV from the innate immune sensor PKR and in repurposing the RISC complex to interfere with cellular gene expression [133,134], but recent association of VA-RNAs with the persistence phenotype points to a previously underappreciated function of these viral RNAs.
During acute lytic infections, cellular homeostasis is dramatically disrupted. Conversely, the dormant nature of persistent infections exhibits a more balanced cellular environment [135]. HAdV persistence can be established via pre-exposure of cells to IFNs, which are classic antiviral cytokines. IFN exposure reduces transcription of E1A, leading to a subsequent deficiency of E1A function early in infection, which helps steer infection towards persistence. [131,135]. More recently, it was shown that HAdV E3–19K glycoprotein activates the IRE1α nuclease to initiate mRNA splicing of X-box binding protein-1 (XBP1), followed by XBP1 binding to the E1A promoter, maintaining transcription of E1A [135]. This pathway contributes to lytic infection in the absence of IFN, and persistent infection in the presence of IFN [135]. Clearly, the effects of IFN on E1A play a multi-faceted role in directing the phenotypic outcome of infection, likely in a context-dependent manner. Overall, these important findings highlight the molecular intricacies of how the fate of a HAdV infection is decided, and that much remains to be learned in this area.
The gastrointestinal tract appears to be an important reservoir for persistent infections. Intriguingly, persistent HAdV is found in lymphocytes in the gut lamina propria, not gut epithelial cells [97]. Once persistent infections have reactivated, HAdV is strictly found within gut epithelial cells. This change in cell preference suggests that lymphoid cells represent poor host environments for acute HAdV replication and are a “last resort” for viral persistence [97]. Indeed, HAdV-infected lymphocytes in the gastrointestinal tract are likely the primary source for HAdV reactivation in pediatric HSCT recipients [93]. Lymphocytes are presumably important reservoirs of persistent HAdV infections in all tissues.
Persistent infection may influence host responses and clinical outcomes even in the absence of reactivation. For instance, in a recent study using a mouse model of adenovirus pathogenesis, persistent infection with a mouse adenovirus exacerbated graft-versus-host disease (GVHD)-like inflammation following allogeneic bone marrow transplantation [99]. HAdV persistence has also been linked to other conditions such as chronic lung disease (e.g., asthma and chronic obstructive lung disease) and chronic heart disease [98,100].
Treatment of HAdV infections
Clinically, HAdV infection poses a significant risk for immunosuppressed transplant recipients. In these cases, the most effective strategy for controlling HAdV infections is to reduce immunosuppressive treatment, restoring the natural ability to combat infection [101]. Unfortunately, there are no clinically approved treatments for HAdV (see Clinician’s corner). Broad-spectrum antivirals such as ganciclovir, ribavirin, and cidofovir have all been reported to have varying degrees of efficacy at controlling HAdV infections, but cidofovir is the most commonly used, despite concern for significant nephrotoxicity [102]. Supplementing cidofovir treatment with intravenous immunoglobulin (IVIG) from pooled doners may exhibit some efficacy, but it is not considered standard therapy [103].
Clinician’s corner.
The human adenoviruses (HAdVs) are common causes of human disease. Sporadic HAdV outbreaks can occur, but endemic HAdV infections are identified year-round across the globe. HAdVs can cause many different types of illness, including upper and lower respiratory tract infection, gastrointestinal tract infection, keratoconjunctivitis, myocarditis, and hemorrhagic cystitis. HAdV infections are typically mild, although severe disease can occasionally occur in immunocompetent individuals. Severe and sometimes fatal infections are more common in immunocompromised individuals.
HAdVs may not be specifically identified in infected individuals, because the mild infections that they often cause are typically not brought to clinical attention. HAdV infection may be suspected based on epidemiologic or clinical features in some cases, but there can be substantial overlap in symptoms caused by HAdV infection and infections caused by other viruses. Most HAdVs grow well in culture, but viral cultures are no longer routinely performed by clinical microbiology laboratories. Instead, a specific diagnosis can be made using molecular assays to detect HAdV in samples such as respiratory specimens, stool, and blood. Specific diagnosis of HAdV infection is most important in immunocompromised individuals. For instance, measurement of the HAdV genome copy number in blood can help to make a diagnosis and assist in gauging response to therapy.
Supportive care is the mainstay of treatment for HAdV infections. The nucleoside analog cidofovir is used topically in cases of keratoconjunctivitis. Although no prospective, randomized, controlled trials exist that formally demonstrate clinical benefit of intravenous cidofovir therapy for HAdV infections, it is frequently used to treat HAdV infections in immunocompromised patients. However, its use can be complicated by clinically significant nephrotoxicity. There is increasing evidence that adoptive immunotherapy using infusion of HAdV-specific T cells can be successfully used to treat severe HAdV infections in immunocompromised individuals.
Oral live virus vaccine against HAdV-E4 and HAdV-B7 is used in the United States military, but no HAdV-specific vaccine is available to the general population.
HAdV-based vectors have been used in human gene therapy trials. HAdVs have also been used as vaccine platforms, most recently as part of vaccine efforts against SARS-CoV-2.
Routine monitoring for HAdV infection is often used in individuals at high risk for severe disease, particularly HSCT patients. Pre-emptive monitoring for HAdV viremia is performed using quantitative PCR-based detection of viral DNA in blood samples [101]. Continued monitoring is used to gauge the success of any intervention. If HAdV levels rise during treatment, a new strategy may be needed to control the infection [101]. As immunosuppression is a key aspect of transplant success, it is important to determine the shortest time frame needed to control infection before resuming immunosuppression [101].
Many clinically approved compounds for the treatment of other pathologies have been investigated for anti-HAdV activity. These compounds interrupt the HAdV replicative cycle at different points to exert their antiviral activity. While there are promising in vitro and in vivo data supporting the efficacy of these drugs, none have been approved for treatment of HAdV infection in humans [102]. If any of these repurposed compounds exhibit strong enough anti-HAdV activity to be considered for clinical trials, their implementation could be streamlined since tolerated dosage and safety data already exist [102].
There is growing interest in, and experience with, the use of HAdV-specific CD8+ T cells as therapy. Donor-derived peripheral blood mononuclear cells are cultured in the presence of viral protein, peptide, lysate, or viral antigen-presenting cells, followed by in vitro or in vivo expansion to generate virus-specific T cells [104]. These mono- or multi-virus specific T cells are then administered to patients to provide exogenous cellular immunity to combat HAdV infection. Clinical trials using this T cell therapy report good efficacy, low toxicity, and protection against a broad range of HAdV types, making them increasingly attractive as an off-the-shelf therapeutic, especially for emerging HAdV outbreaks [104,105].
Another potential therapeutic strategy is the use of engineered, antiviral monoclonal antibodies. Of the 4 classes of human IgG, anti-hexon IgG3 demonstrated enhanced TRIM21 activity and complement C1/C4-mediated neutralization in an IgG3 hinge-dependent manner [106]. Overall, IgG3 delivered the most potent neutralization of HAdV-C5, providing novel insights that can be exploited to guide antibody engineering [106].
Concluding Remarks
As the frequency and breadth of viral disease outbreaks seemingly increases, understanding and combating disease emergence is important. The self-limited nature of HAdV infections, accompanied by recombination events, evolutionary gain- or loss-of-function mutations, and gaps in knowledge about zoonotic potential complicates the detection of emerging HAdVs. Current surveillance efforts are inconsistent, often region-specific and may rely on voluntary reporting or provide insufficient HAdV genotyping data [14]. Recent evidence supporting a collaborative “local-to-global” approach to surveillance underscores the importance of casting a wide net for HAdV detection and monitoring [107].
There is still much to learn about the many different facets of HAdV pathogenesis (see Outstanding questions). There is a need for improved understanding of how individual genetic variations and co-infections can affect transmission, replication, and host response, along with continued research into prevention and treatment of HAdV infection. Moreover, this is an exciting era for HAdV virology, as the output of decades of seminal research has allowed these viruses to be harnessed for their therapeutic potential as our partners for gene therapy and vaccine development [108,109]. Indeed, while HAdV may be an innocuous pathogen to many, to some it may become a formidable predator.
Outstanding questions.
Outside of the well-studied HAdV-C5, do other HAdV species display lytic or non-lytic egress?
What level of variability in HAdV infection exists at a cellular and organism level, such as genetic modifications, and how does this affect infection severity, clinical manifestations, and outcomes?
What features are responsible for a sudden change or increase in pathogenicity of circulating HAdV? How do some genomic variants of HAdVs, such as HAdV-B14p1, induce more severe disease manifestation, mortality, and cellular inflammation compared to their protype strain?
Where are the hotspots for detecting adenovirus before outbreaks occur? Should testing be performed more frequently in hospitals or in animals? How important is genotyping of these adenoviruses? How quick and inexpensive can it be?
What role does the relationship between viral structural protein sequences and function play in enhanced immuno-pathologies?
Do HAdV infections that cause more severe clinical manifestation induce different cytokine/chemokine profiles from immune cells such as dendritic cells, T cells, B cells, and NK cells?
Can HAdV persistence be treated or avoided? Is there a possibility to reactivate HAdV prior to transplant to prevent immunosuppressive reactivation and severe disease?
Are there potential antiviral combinations that could be used in extreme cases to safely treat HAdV? If clinically approved compounds are used, could this be an attainable goal? What is the balance between host disruption and crippling viral replication?
Can co-infections with HAdV and other viruses affect the severity and clinical manifestation of HAdV infection?
Highlights.
Genomic variants and recombinant viruses such as human adenovirus (HAdV) -B14p1 and HAdV-B55 have caused global outbreaks and severe disease in both immunocompetent and immunocompromised individuals.
While cidofovir is commonly used to treat HAdV infections, there are no clinically approved treatments. There is potential to develop alternative therapeutics, such as HAdV-specific CD8+ T cells.
Detailed studies of the immune response to HAdV infections, such as antibody neutralization and the interactions between natural killer cell receptor KIR3DS1 and HLA-F on infected cells, revealed potential therapeutic targets and applications.
HAdV vectors are widely popular for vaccines and gene therapy. This prompted an interest in understudied HAdVs, such as HAdV-B11, -D26 and -B35, and has evolved the understanding of entry and immune system responses for these viruses.
Acknowledgements
This work was supported by a grant from the Canadian Institute of Health Research (MOP-148689) to J.S.M. J.B.W is supported by a National Institute of Health grant (R21 AI163720) K.M.M. holds a Natural Sciences and Engineering Research Council CGS-D award. M.J.D currently holds an Ontario Graduate Scholarship. A.M.E. holds a RGE Murray Scholarship. We apologize to the many colleagues whose excellent research was not cited directly in this review.
Glossary
- Graft-versus-host disease
A systemic condition that occurs when host tissue is seen as foreign and attacked by healthy, transplanted (graft) stem cells introduced into the recipient’s body.
- Homologous recombination
The genetic recombination of nucleotide sequences from similar or identical DNA strands.
- Hypervariable regions
Regions that exist in the HAdV hexon protein. These differ in length and residue composition across different HAdV genotypes but are relatively conserved among the same genotypes.
- Inflammasome
Stimuli-induced, cytosolic, multiprotein sensor and receptor complexes that can activate an inflammatory response.
- Lymphocytes
White blood cells that provide an immune response derived from the lymphoid lineage. These include B cells, T cells, and natural killer cells.
- MAPK
A signaling cascade pathway that relays signals from an array of stimuli to activate a number of cellular responses, including inflammation.
- Nephrotoxicity
A rapid deterioration in kidney function in response to the toxic effect of drugs or chemicals.
- Neutralizing antibodies
Antibodies that bind the pathogen (i.e., virus) target in a way that prevents the virus from infecting the cell or exerting its biological effect.
- NF-κB
A transcription factor in the NF-κB signaling pathway. Translocates to the nucleus to bind DNA of various target genes, including those involved in inflammation.
- NOD-like receptors (NLR)
Recognizes cytoplasmic pathogen-associated molecular patterns in response to viral and intracellular pathogen infections. One member is NLRP3, which initiates the formation of the inflammasome.
- Nucleotide-binding and oligomerization domain (NOD)
A family of proteins that act as pattern recognition receptors. For example, NOD2 is an intracellular sensor required for innate immune responses to HAdV infection.
- Opsonization
The tagging of foreign pathogens with host-produced proteins and lipids, such as antibodies or complement proteins, targeting the pathogen for destruction by phagocytosis.
- Reactive oxygen species (ROS)
A highly reactive, unstable, oxygen-containing molecule. Build up can cause cell death and damage to genomic material and proteins.
- Seroprevalence
The prevalence in the populations of individuals with antibodies against a specific infectious agent (i.e., virus)
- Toll-like receptors (TLRs)
A large family of transmembrane pattern recognition receptors found on plasma membranes and endosomal compartments that activate a variety of pathways in the innate immune system, leading to the production of inflammatory cytokines and the type I interferon response.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no conflict of interest.
References
- 1.Dai X et al. (2017) Atomic Structures of Minor Proteins VI and VII in Human Adenovirus. J. Virol 91, e00850–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu X et al. (2017) Cryo-EM structure of human adenovirus D26 reveals the conservation of structural organization among human adenoviruses. Sci. Adv 3, e1602670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rafie K et al. (2021) The structure of enteric human adenovirus 41-A leading cause of diarrhea in children. Sci. Adv 7, eabe0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pérez-Illana M et al. (2021) Cryo-EM structure of enteric adenovirus HAdV-F41 highlights structural variations among human adenoviruses. Sci. Adv 7, eabd9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hearing P (2021) Adenoviridae: The Viruses and Their Replication. In Fields Virology,Volume 2: DNA viruses (7th edn) (Howley PM et al. , eds), pp. 98–128, Wolters Kluwer:Philadelphia, PA, USA [Google Scholar]
- 6.Gray GC and Erdman DD Adenovirus Vaccines. , Plotkin’s Vaccines. (2018), 121–133.e8 [Google Scholar]
- 7.Lynch JP 3rd and Kajon AE (2021) Adenovirus: Epidemiology, Global Spread of Novel Types, and Approach to Treatment. Semin. Respir. Crit. Care Med 42, 800–821 [DOI] [PubMed] [Google Scholar]
- 8.Lion T (2014) Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev 27, 441–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Killerby ME et al. (2019) Respiratory Illness Associated With Emergent Human Adenovirus Genome Type 7d, New Jersey, 2016–2017. Open forum Infect. Dis 6, ofz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudding BA et al. (1972) Fatal pneumonia associated with adenovirus type 7 in three military trainees. N. Engl. J. Med 286, 1289–1292 [DOI] [PubMed] [Google Scholar]
- 11.Schaberg KB et al. (2017) Adenovirus Hepatitis: Clinicopathologic Analysis of 12 Consecutive Cases From a Single Institution. Am. J. Surg. Pathol 41, 810–819 [DOI] [PubMed] [Google Scholar]
- 12.Lee J et al. (2010) Comprehensive serotyping and epidemiology of human adenovirus isolated from the respiratory tract of Korean children over 17 consecutive years (1991–2007). J. Med. Virol 82, 624–631 [DOI] [PubMed] [Google Scholar]
- 13.Yao L et al. (2019) Human adenovirus among hospitalized children with respiratory tract infections in Beijing, China, 2017–2018. Virol. J 16, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajon AE et al. (2019) Emergence and re-emergence of respiratory adenoviruses in the United States. Curr. Opin. Virol 34, 63–69 [DOI] [PubMed] [Google Scholar]
- 15.Fu Y et al. (2019) Human adenovirus type 7 infection causes a more severe disease than type 3. BMC Infect. Dis 19, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller MP et al. (2018) Adenovirus-related epidemic keratoconjunctivitis outbreak at a hospital-affiliated ophthalmology clinic. Am. J. Infect. Control 46, 581–583 [DOI] [PubMed] [Google Scholar]
- 17.Huh K et al. (2019) Prolonged shedding of type 55 human adenovirus in immunocompetent adults with adenoviral respiratory infections. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol 38, 793–800 [DOI] [PubMed] [Google Scholar]
- 18.Zeng S-Z et al. (2021) Persistent viral shedding of human adenovirus type 7 in children with severe pneumonia. J. Med. Virol 93, 4846–4855 [DOI] [PubMed] [Google Scholar]
- 19.Hysmith ND et al. (2018) Use of real-time semiquantitative PCR data in management of a neonatal intensive care unit adenovirus outbreak. Infect. Control Hosp. Epidemiol 39, 1074–1079 [DOI] [PubMed] [Google Scholar]
- 20.Kajon AE et al. (2018) Adenovirus Type 4 Respiratory Infections among Civilian Adults, Northeastern United States, 2011–2015. Emerg. Infect. Dis 24, 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miro E et al. (2021) Whole-genome analysis to describe a human adenovirus D8 conjunctivitis outbreak in a tertiary hospital. J. Med. Virol 93, 4840–4845 [DOI] [PubMed] [Google Scholar]
- 22.Lamson Bs DM et al. (2018) Molecular typing and whole genome next generation sequencing of human adenovirus 8 strains recovered from four 2012 outbreaks of keratoconjunctivitis in New York State. J. Med. Virol 90, 1471–1477 [DOI] [PubMed] [Google Scholar]
- 23.Kujawski SA et al. (2021) Outbreaks of Adenovirus-associated Respiratory Illness on 5 College Campuses in the United States, 2018–2019. Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. Am 72, 1992–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhingra A et al. (2019) Molecular Evolution of Human Adenovirus (HAdV) Species C. Sci. Rep 9, 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ismail AM et al. (2018) Genomic analysis of a large set of currently-and historically-important human adenovirus pathogens. Emerg. Microbes Infect 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heo JY et al. (2018) Molecular Epidemiology of Human Adenovirus-Associated Febrile Respiratory Illness in Soldiers, South Korea. Emerg. Infect. Dis 24, 1221–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Q et al. (2017) Comparative genomic analysis of two emergent human adenovirus type 14 respiratory pathogen isolates in China reveals similar yet divergent genomes. Emerg. Microbes Infect 6, e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajon AE et al. (2010) Molecular Epidemiology and Brief History of Emerging Adenovirus 14—Associated Respiratory Disease in the United States. J. Infect. Dis 202, 93–103 [DOI] [PubMed] [Google Scholar]
- 29.Walsh MP et al. (2010) Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J. Clin. Microbiol 48, 991–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hierholzer JC et al. (1974) Occurrence of respiratory illness due to an atypical strain of adenovirus type 11 during a large outbreak in Spanish military recruits. Am. J. Epidemiol 99, 434–442 [DOI] [PubMed] [Google Scholar]
- 31.Li QG et al. (1991) Genetic relationship between thirteen genome types of adenovirus 11, 34, and 35 with different tropisms. Intervirology 32, 338–350 [DOI] [PubMed] [Google Scholar]
- 32.Hang J et al. (2020) Human Adenovirus Type 55 Distribution, Regional Persistence, and Genetic Variability. Emerg. Infect. Dis 26, 1497–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J et al. (2019) A Survey of Recent Adenoviral Respiratory Pathogens in Hong Kong Reveals Emergent and Recombinant Human Adenovirus Type 4 (HAdV-E4) Circulating in Civilian Populations. Viruses 11, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dehghan S et al. (2013) Computational analysis of four human adenovirus type 4 genomes reveals molecular evolution through two interspecies recombination events. Virology 443, 197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatfield L and Hearing P (1991) Redundant elements in the adenovirus type 5 inverted terminal repeat promote bidirectional transcription in vitro and are important for virus growth in vivo. Virology 184, 265–276 [DOI] [PubMed] [Google Scholar]
- 36.Wang B et al. (2018) Seroepidemiological investigation of HAdV-4 infection among healthy adults in China and in Sierra Leone, West Africa. Emerg. Microbes Infect 7, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radke JR et al. (2014) Adenovirus E1B 19-kilodalton protein modulates innate immunity through apoptotic mimicry. J. Virol 88, 2658–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang Z et al. (2006) Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis 12, 1596–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pauly M et al. (2015) Adenovirus in Rural Côte D’Ivoire: High Diversity and Cross-Species Detection. Ecohealth 12, 441–452 [DOI] [PubMed] [Google Scholar]
- 40.Wevers D et al. (2011) Novel adenoviruses in wild primates: a high level of genetic diversity and evidence of zoonotic transmissions. J. Virol 85, 10774–10784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ongrádi J et al. (2019) Adenovirus Isolated From a Cat Is Related to Human Adenovirus 1. Front. Microbiol 10, 1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dehghan S et al. (2019) A Zoonotic Adenoviral Human Pathogen Emerged through Genomic Recombination among Human and Nonhuman Simian Hosts. J. Virol 93, e00564–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarantis H et al. (2004) Comprehensive detection and serotyping of human adenoviruses by PCR and sequencing. J. Clin. Microbiol 42, 3963–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houldcroft CJ et al. (2018) Use of Whole-Genome Sequencing of Adenovirus in Immunocompromised Pediatric Patients to Identify Nosocomial Transmission and Mixed-Genotype Infection. J. Infect. Dis 218, 1261–1271 [DOI] [PubMed] [Google Scholar]
- 45.Myers CE et al. (2021) Using Whole Genome Sequences to Investigate Adenovirus Outbreaks in a Hematopoietic Stem Cell Transplant Unit. Front. Microbiol 12, 667790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noyce RS et al. (2018) Construction of an infectious horsepox virus vaccine from chemically synthesized DNA fragments. PLoS One 13, e0188453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vassal-Stermann E et al. (2019) CryoEM structure of adenovirus type 3 fibre with desmoglein 2 shows an unusual mode of receptor engagement. Nat. Commun 10, 1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David PB et al. (2021) Human species D adenovirus hexon capsid protein mediates cell entry through a direct interaction with CD46. Proc. Natl. Acad. Sci 118, e2020732118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greber UF and Flatt JW (2019) Adenovirus Entry: From Infection to Immunity. Annu. Rev. Virol 6, 177–197 [DOI] [PubMed] [Google Scholar]
- 50.Lenman A et al. (2018) Polysialic acid is a cellular receptor for human adenovirus 52. Proc. Natl. Acad. Sci 115, E4264–E4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freimuth P et al. (1999) Coxsackievirus and adenovirus receptor amino-terminal immunoglobulin V-related domain binds adenovirus type 2 and fiber knob from adenovirus type 12. J. Virol 73, 1392–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rajan A et al. (2018) Enteric Species F Human Adenoviruses use Laminin-Binding Integrins as Co-Receptors for Infection of Ht-29 Cells. Sci. Rep 8, 10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veesler D et al. (2014) Single-particle EM reveals plasticity of interactions between the adenovirus penton base and integrin αVβ3. Proc. Natl. Acad. Sci. U. S. A 111, 8815–8819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernando-Pérez M et al. (2020) Dynamic competition for hexon binding between core protein VII and lytic protein VI promotes adenovirus maturation and entry. Proc. Natl. Acad. Sci. U. S. A 117, 13699–13707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maier O et al. (2012) Spatiotemporal dynamics of adenovirus membrane rupture and endosomal escape. J. Virol 86, 10821–10828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou J et al. (2018) Role of kinesins in directed adenovirus transport and cytoplasmic exploration. PLOS Pathog. 14, e1007055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bauer M et al. (2019) The E3 Ubiquitin Ligase Mind Bomb 1 Controls Adenovirus Genome Release at the Nuclear Pore Complex. Cell Rep. 29, 3785–3795.e8 [DOI] [PubMed] [Google Scholar]
- 58.Crisostomo L et al. (2019) Temporal dynamics of adenovirus 5 gene expression in normal human cells. PLoS One 14, e0211192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murali VK et al. (2014) Adenovirus death protein (ADP) is required for lytic infection of human lymphocytes. J. Virol 88, 903–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Georgi F et al. (2020) The FDA-Approved Drug Nelfinavir Inhibits Lytic Cell-Free but Not Cell-Associated Nonlytic Transmission of Human Adenovirus. Antimicrob. Agents Chemother 64, e01002–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Biserni GB et al. (2021) Potential Diagnostic and Prognostic Biomarkers for Adenovirus Respiratory Infection in Children and Young Adults. Viruses 13, 1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lam E et al. (2014) Adenovirus detection by the cGAS/STING/TBK1 DNA sensing cascade. J. Virol 88, 974–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hopfner K-P and Hornung V (2020) Molecular mechanisms and cellular functions of cGAS–STING signalling. Nat. Rev. Mol. Cell Biol 21, 501–521 [DOI] [PubMed] [Google Scholar]
- 64.Wang L et al. (2019) Nuclear hnRNPA2B1 initiates and amplifies the innate immune response to DNA viruses. Science 365, eaav0758. [DOI] [PubMed] [Google Scholar]
- 65.Muruve DA et al. (2008) The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452, 103–107 [DOI] [PubMed] [Google Scholar]
- 66.Smith JS et al. (2011) The role of endosomal escape and mitogen-activated protein kinases in adenoviral activation of the innate immune response. PLoS One 6, e26755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Doronin K et al. (2012) Coagulation factor X activates innate immunity to human species C adenovirus. Science 338, 795–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu W et al. (2022) Soluble Receptor for Advanced Glycation End Product Is Involved in the Inflammatory Response of Human Adenovirus-Infected Patients. Front. Microbiol 13, 923215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hudson BI and Lippman ME (2018) Targeting RAGE Signaling in Inflammatory Disease. Annu. Rev. Med 69, 349–364 [DOI] [PubMed] [Google Scholar]
- 70.Hammer Q et al. (2018) Natural killer cell specificity for viral infections. Nat. Immunol 19, 800–808 [DOI] [PubMed] [Google Scholar]
- 71.Jung JM et al. (2021) KIR3DS1 directs NK cell-mediated protection against human adenovirus infections. Sci. Immunol 6, eabe2942. [DOI] [PubMed] [Google Scholar]
- 72.Romee R et al. (2016) Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med 8, 357ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quintin J et al. (2012) Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yao Y et al. (2018) Induction of Autonomous Memory Alveolar Macrophages Requires T Cell Help and Is Critical to Trained Immunity. Cell 175, 1634–1650.e17 [DOI] [PubMed] [Google Scholar]
- 75.Serangeli C et al. (2010) Ex vivo detection of adenovirus specific CD4+ T-cell responses to HLA-DR-epitopes of the Hexon protein show a contracted specificity of T(HELPER) cells following stem cell transplantation. Virology 397, 277–284 [DOI] [PubMed] [Google Scholar]
- 76.Molloy CT et al. (2017) Contributions of CD8 T cells to the pathogenesis of mouse adenovirus type 1 respiratory infection. Virology 507, 64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chávez-Galán L et al. (2009) Cell Death Mechanisms Induced by Cytotoxic Lymphocytes. Cell. Mol. Immunol 6, 15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wohlleber D et al. (2012) TNF-induced target cell killing by CTL activated through cross-presentation. Cell Rep. 2, 478–487 [DOI] [PubMed] [Google Scholar]
- 79.Sánchez-Céspedes J et al. (2021) T-cells immune response controls the high incidence of adenovirus infection in adult allogenic hematopoietic transplantation recipients. Haematologica 106, 275–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng R et al. (2021) Changes of Host Immunity Mediated by IFN-γ(+) CD8(+) T Cells in Children with Adenovirus Pneumonia in Different Severity of Illness. Viruses 13, 2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bottermann M et al. (2018) TRIM21 mediates antibody inhibition of adenovirus-based gene delivery and vaccination. Proc. Natl. Acad. Sci 115, 10440–10445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McEwan WA et al. (2013) Intracellular antibody-bound pathogens stimulate immune signaling via the Fc receptor TRIM21. Nat. Immunol 14, 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campbell SJ et al. (2017) Disseminated adenovirus infection causing severe ARDS. BMJ Case Rep. 2017, bcr2016217524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rajaiya J et al. (2021) Adenovirus and the Cornea: More Than Meets the Eye. Viruses 13, 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaneko H et al. (2020) Five Cases of Epidemic Keratoconjunctivitis Due to Human Adenovirus Type 85 in Fukushima, Japan. Jpn. J. Infect. Dis 73, 316–319 [DOI] [PubMed] [Google Scholar]
- 86.Lee CS et al. (2018) Determinants of Outcomes of Adenoviral Keratoconjunctivitis. Ophthalmology 125, 1344–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gu J et al. (2021) Adenovirus diseases: a systematic review and meta-analysis of 228 case reports. Infection 49, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Butt AL and Chodosh J (2006) Adenoviral keratoconjunctivitis in a tertiary care eye clinic. Cornea 25, 199–202 [DOI] [PubMed] [Google Scholar]
- 89.Killerby ME et al. (2017) Notes from the Field: Epidemic Keratoconjunctivitis Outbreak Associated with Human Adenovirus Type 8 - U.S. Virgin Islands, June-November 2016. MMWR. Morb. Mortal. Wkly. Rep 66, 811–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li J et al. (2018) A swimming pool-associated outbreak of pharyngoconjunctival fever caused by human adenovirus type 4 in Beijing, China. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis 75, 89–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chandra N et al. (2019) Sialic Acid-Containing Glycans as Cellular Receptors for Ocular Human Adenoviruses: Implications for Tropism and Treatment. Viruses 11, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y et al. (2010) Modeling adenovirus latency in human lymphocyte cell lines. J. Virol 84, 8799–8810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hum RM et al. (2018) Molecular monitoring of adenovirus reactivation in faeces after haematopoietic stem-cell transplantation to predict systemic infection: a retrospective cohort study. Lancet. Haematol 5, e422–e429 [DOI] [PubMed] [Google Scholar]
- 94.Kosulin K et al. (2019) Diagnostic Parameters of Adenoviremia in Pediatric Stem Cell Transplant Recipients. Front. Microbiol 10, 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ali S et al. (2019) The yield of monitoring adenovirus in pediatric hematopoietic stem cell transplant patients. Pediatr. Hematol. Oncol 36, 161–172 [DOI] [PubMed] [Google Scholar]
- 96.Proenca-Modena JL et al. (2019) Human adenovirus replication and persistence in hypertrophic adenoids and palatine tonsils in children. J. Med. Virol 91, 1250–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kosulin K et al. (2016) Persistence and reactivation of human adenoviruses in the gastrointestinal tract. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis 22, 381.e1–381.e8 [DOI] [PubMed] [Google Scholar]
- 98.Kühl U et al. (2005) Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation 112, 1965–1970 [DOI] [PubMed] [Google Scholar]
- 99.Chang CJ et al. (2022) Mouse Adenovirus Type 1 Persistence Exacerbates Inflammation Induced by Allogeneic Bone Marrow Transplantation. J. Virol 96, e0170621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marin J et al. (2000) Persistence of viruses in upper respiratory tract of children with asthma. J. Infect 41, 69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Al-Heeti OM et al. (2022) Adenovirus Infection and Transplantation. Transplantation 106, 920–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dodge MJ et al. (2021) Emerging antiviral therapeutics for human adenovirus infection: Recent developments and novel strategies. Antiviral Res. 188, 105034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haq A et al. (2022) Treatment of Viral Hepatitis Due to Adenovirus in a Liver Transplantation Recipient: The Clinical Use of Cidofovir and Intravenous Immunoglobulin. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc 28, 505–507 [DOI] [PubMed] [Google Scholar]
- 104.Dailey Garnes NJM et al. (2019) Adenovirus infection and disease in recipients of hematopoietic cell transplantation. Curr. Opin. Infect. Dis 32, 591–600 [DOI] [PubMed] [Google Scholar]
- 105.Tzannou I et al. (2017) Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol 35, 3547–3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Foss S et al. (2022) Potent TRIM21 and complement-dependent intracellular antiviral immunity requires the IgG3 hinge. Sci. Immunol 7, eabj1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.O’Brien E and Xagoraraki I (2019) A water-focused one-health approach for early detection and prevention of viral outbreaks. One Heal. (Amsterdam, Netherlands) 7, 100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crystal RG (2014) Adenovirus: the first effective in vivo gene delivery vector. Hum. Gene Ther 25, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sakurai F et al. (2022) Adenovirus vector-based vaccine for infectious diseases. Drug Metab. Pharmacokinet 42, 100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gutierrez Sanchez LH et al. (2022) A Case Series of Children with Acute Hepatitis and Human Adenovirus Infection. N. Engl. J. Med 387, 620–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kelgeri C et al. (2022) Clinical Spectrum of Children with Acute Hepatitis of Unknown Cause. N. Engl. J. Med 387, 611–619 [DOI] [PubMed] [Google Scholar]
- 112.do Nascimento LG et al. (2022) Human enteric adenovirus F40/41 as a major cause of acute gastroenteritis in children in Brazil, 2018 to 2020. Sci. Rep 12, 11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morfopoulou S et al. (2022) GENOMIC INVESTIGATIONS OF ACUTE HEPATITIS OF UNKNOWN AETIOLOGY IN CHILDREN. medRxiv DOI: 10.1101/2022.07.28.22277963 [DOI] [Google Scholar]
- 114.Ho A et al. (2022) Adeno-associated virus 2 infection in children with non-A-E hepatitis. medRxiv DOI: 10.1101/2022.07.19.22277425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meier AF et al. (2020) The Interplay between Adeno-Associated Virus and Its Helper Viruses. Viruses 12, 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zaiss AK et al. (2009) Antiviral antibodies target adenovirus to phagolysosomes and amplify the innate immune response. J. Immunol 182, 7058–7068 [DOI] [PubMed] [Google Scholar]
- 117.Eichholz K et al. (2016) Immune-Complexed Adenovirus Induce AIM2-Mediated Pyroptosis in Human Dendritic Cells. PLoS Pathog. 12, e1005871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karsten E et al. (2022) Adenovirus-α-Defensin Complexes Induce NLRP3-Associated Maturation of Human Phagocytes via Toll-Like Receptor 4 Engagement. J. Virol 96, e01850–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith JG and Nemerow GR (2008) Mechanism of adenovirus neutralization by Human alpha-defensins. Cell Host Microbe 3, 11–19 [DOI] [PubMed] [Google Scholar]
- 120.Bottermann M et al. (2019) Complement C4 Prevents Viral Infection through Capsid Inactivation. Cell Host Microbe 25, 617–629.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tam JCH et al. (2014) Intracellular sensing of complement C3 activates cell autonomous immunity. Science 345, 1256070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Harmon AW et al. (2018) Hexons from adenovirus serotypes 5 and 48 differentially protect adenovirus vectors from neutralization by mouse and human serum. PLoS One 13, e0192353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Waddington SN et al. (2008) Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132, 397–409 [DOI] [PubMed] [Google Scholar]
- 124.Baker AT et al. (2019) Diversity within the adenovirus fiber knob hypervariable loops influences primary receptor interactions. Nat. Commun 10, 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tian X et al. (2021) Seroprevalence of Neutralizing Antibodies against Six Human Adenovirus Types Indicates the Low Level of Herd Immunity in Young Children from Guangzhou, China. Virol. Sin 36, 373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao H et al. (2018) Seroprevalence of Neutralizing Antibodies against Human Adenovirus Type-5 and Chimpanzee Adenovirus Type-68 in Cancer Patients. Front. Immunol 9, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yi H et al. (2022) Seroprevalence of neutralizing antibodies against adenovirus type 26 and 35 in healthy populations from Guangdong and Shandong provinces, China. Virol. Sin S1995–820X, 00112–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Voysey M et al. (2021) Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet (London, England) 397, 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.T. BA, et al. (2022) Human adenovirus type 26 uses sialic acid–bearing glycans as a primary cell entry receptor. Sci. Adv 5, eaax3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang L et al. (2020) Histone Deacetylase Inhibitors Promote Latent Adenovirus Reactivation from Tonsillectomy Specimens. J. Virol 94, e00100–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zheng Y et al. (2016) E2F/Rb Family Proteins Mediate Interferon Induced Repression of Adenovirus Immediate Early Transcription to Promote Persistent Viral Infection. PLoS Pathog. 12, e1005415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Furuse Y et al. (2013) Persistently adenovirus-infected lymphoid cells express microRNAs derived from the viral VAI and especially VAII RNA. Virology 447, 140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hood IV et al. (2019) Crystal structure of an adenovirus virus-associated RNA. Nat. Commun 10, 2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kamel W et al. (2014) Small RNA Sequence Analysis of Adenovirus VA RNA-Derived MiRNAs Reveals an Unexpected Serotype-Specific Difference in Structure and Abundance. PLoS One 9, e105746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prasad V et al. (2020) The UPR sensor IRE1α and the adenovirus E3–19K glycoprotein sustain persistent and lytic infections. Nat. Commun 11, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chehadeh W et al. (2018) Adenovirus types associated with severe respiratory diseases: A retrospective 4-year study in Kuwait. J. Med. Virol 90, 1033–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hemsath JR et al. (2022) Ex Vivo and In Vivo CD46 Receptor Utilization by Species D Human Adenovirus Serotype 26 (HAdV26). J. Virol 96, e0082621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.ROWE WP et al. (1953) Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. Soc. Exp. Biol. Med. (New York, N.Y.) 84, 570–573 [DOI] [PubMed] [Google Scholar]
- 139.Hllleman MR and Werner JH (1954) Recovery of New Agent from Patients with Acute Respiratory Illness. Proc. Soc. Exp. Biol. Med 85, 183–188 [DOI] [PubMed] [Google Scholar]
- 140.Trentin JJ et al. (1962) The quest for human cancer viruses. Science 137, 835–841 [DOI] [PubMed] [Google Scholar]
- 141.Berget SM et al. (1977) Spliced segments at the 5’ terminus of adenovirus 2 late mRNA. Proc. Natl. Acad. Sci. U. S. A 74, 3171–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chow LT et al. (1977) An amazing sequence arrangement at the 5’ ends of adenovirus 2 messenger RNA. Cell 12, 1–8 [DOI] [PubMed] [Google Scholar]
- 143.Graham FL et al. (1977) Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol 36, 59–74 [DOI] [PubMed] [Google Scholar]
- 144.Raper SE et al. (2003) Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab 80, 148–158 [DOI] [PubMed] [Google Scholar]
- 145.Zhang R et al. (2021) A Recombinant Human Adenovirus Type 5 (H101) Combined With Chemotherapy for Advanced Gastric Carcinoma: A Retrospective Cohort Study. Front. Oncol 11, 752504. [DOI] [PMC free article] [PubMed] [Google Scholar]


