Abstract
2-Difluoromethoxyestratriene derivatives were designed to improve potency and in vivo stability of the drug candidate 2-methoxyestradiol (2ME2). Compound evaluation in vitro against the proliferation of MCF-7 and MDA MB-231 breast cancer cells, as inhibitors of tubulin polymerisation and also steroid sulfatase (STS) both in cell lysates and in whole cells, showed promising activities. In antiproliferative assays 2-difluoromethoxyestradiol was less potent than 2ME2, but its sulfamates were often more potent than the corresponding non-fluorinated analogues. The fluorinated bis-sulfamate is a promising anti-proliferative agent in MCF-7 cells (GI50 0.28 μM) vs the known 2-methoxyestradiol-3,17-O,O-bissulfamate (STX140, GI50 0.52 μM), confirming the utility of our approach. Compounds were also evaluated in the NCI 60-cell line panel and the fluorinated bis-sulfamate displayed very good overall activities with a sub-micromolar average GI50. It was a very potent STS inhibitor in whole JEG-3 cells (IC50 3.7 nM) similar to STX140 (4.2 nM) and additionally interferes with tubulin assembly in vitro and colchicine binding to tubulin. An X-ray study of 2-difluoromethoxy-3-benzyloxyestra-1,3,5(10)-trien-17-one examined conformational aspects of the fluorinated substituent. The known related derivative 2-difluoromethyl-3-sulfamoyloxyestrone was evaluated for STS inhibition in whole JEG-3 cells and showed an excellent IC50 of 55 pM.
Keywords: difluoromethoxy isosteres, microtubule disruptors, tubulin assembly, colchicine binding, steroid sulfatase inhibition
Graphical Abstract

Difluoromethoxyestratriene-based steroid sulfatase (STS) inhibitors have been designed. Six difluoromethoxy-substituted steroid dervatives were synthesised and evaluated in vitro against the proliferation of MCF-7 and MDA MB-231 breast cancer cells. Bis-sulfamate 10 was identified as a promising anti-proliferative agent (GI50 0.28 μM in MCF-7 cells), a potent STS inhibitor (IC50 3.7 nM in JEG-3 cells) and an inhibitor of tubulin assembly in vitro and colchicine binding to tubulin.
Introduction
2-Methoxyestradiol 1 (2ME2) is a natural, endogenous and non-estrogenic metabolite of estradiol and has been developed as an anti-cancer drug candidate as it prevents the formation of new blood vessels that tumours need for growth.[1] Several studies showed that 2ME2 is also a microtubule inhibitor[2] and is effective inter alia against prostate cancer in rodents.[3] However, by the end of 2015 all clinical development of 2ME2 had been suspended or discontinued.[4] The main reasons were its poor bioavailability (ca 1%) and its extensive conjugative metabolism.[5] Therefore, chemical modification of 2ME2 has been a productive area of research for some years.[6] We have pioneered the use of sulfamoylated derivatives in oncology.[7] In previous studies, we described the design of sulfamoylated derivatives based around 2ME2 1, (Figure 1) as anticancer agents.[8–15] These agents were developed as part of a programme addressing the design of inhibitors of the emerging drug target steroid sulfatase (STS).[7a,16] In particular, an aryl sulfamate pharmacophore motif 2 imbued potent irreversible inhibition against STS with novel red blood cell delivery properties in vivo. Our non-steroidal clinical drug Irosustat 3 (Figure 1) and steroidal drugs bearing this motif have entered 20 clinical trials up to phase II, primarily in oncology, with clinical activity demonstrated in humans for hormone replacement therapy and hormone-dependent diseases, such as breast and endometrial cancers and endometriosis.[7b] Unmet monotherapy potential also exists in ovarian, bladder and colorectal cancers. A phase I trial in men with prostate cancer has also taken place with a phase II trial envisaged. The sulfamoylated estratrienes also showed potent STS inhibitory activity.[11] These compounds exhibit potent anti-proliferative activity against a range of human cancer cell lines and also inhibit angiogenesis. One of these of great promise is 2-methoxyestradiol-3,17-O,O-bissulfamate, STX140 4 (Figure 1), which is highly active against hormone-independent tumours. In addition to good oral bioavailability[17,18] and excellent in vivo activities,[7a,13] it also inhibited the growth of taxane-resistant cancer cells[19–21] and HUVEC proliferation (a commonly used marker for anti-angiogenic activity)[22,23] and could have significant promise as a therapy for triple-negative breast cancer and castrate-resistant prostate cancer. Moreover, unlike Irosustat 3, STX140 4 is more than just an STS inhibitor. Because of its cytotoxic activity it has the potential to target ovarian cancer (itself a viable STS monotherapy target - vide infra, but also responsive to cytotoxic chemotherapy) in a dual fashion.
Figure 1.
Structures of 2ME2 1, the aryl sulfamate pharmacophore 2, Irosustat 3, and STX140 4.
Another considerable facet of interest relates to the relatively unexplored immunological and immuno-oncology applications of this class of compound and of STS inhibitors more generally. STS, present in macrophages within lymphoid tissues, has a crucial role in regulating part of the immune response, and STS inhibitors are known to have activities in the immune system alone,[24] but this has not yet been thoroughly explored. Moreover, 2ME2 itself was shown to suppress dramatically the development of mouse experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS).[25] It inhibits in vitro lymphocyte activation, cytokine production and proliferation. Treatment of lymphocytes specifically reduced the nuclear translocation and transcriptional activity of nuclear factor of activated T-cells (NFAT), whereas NF-κB and activator protein 1 (AP-1) activation were not adversely affected. 2ME2 may attenuate EAE through disruption of the NFAT pathway and subsequent lymphocyte activation. A molecular rationale for the use of 2ME2 as an immunomodulatory agent for the treatment of autoimmune disorders such as MS in humans has been proposed.[24,25]
As already mentioned, 2ME2 itself, although already studied in several US oncology clinical trials (Entremed), is a very poor pharmaceutical drug with poor bioavailability and is also very heavily metabolised at both hydroxyl groups. Therefore, patient doses were as high as 6 g of drug per day. The problem of metabolism is significantly reduced in STX140 4 as, like Irosustat 3, it is transported in vivo in red blood cells in a novel fashion, not metabolised and has a bioavailability of ca 85%.[26] The two sulfamate groups of STX140 efficiently block conjugative metabolism and add further attractive biological and pharmaceutical properties, but there is still the small (but finite) interfering prospect of in vivo demethylation at the 2-methoxy substituent, that may regenerate some undesirable in vivo estrogenicity.[27] To date this reaction has only been demonstrated for 2ME2, but it seems likely that this could also apply to compounds of the STX140 class, hence the relevance of this current study. Recently, we demonstrated excellent activities for steroidal sulfamates related to STX140 in an in vitro EAE model of MS.[28] The results seem to imply that 2ME2 and related steroid sulfamates can act by blocking calcium entry to lymphocytes, an essential part of the T cell activation process, and that this might be through blockade of the Orai/STIM calcium entry system.[28] Thus, the intrinsic anti-inflammatory activity of 2ME2[29,30] and STX140 provides significant potential for the compounds as immunomodulatory agents alone or in immuno-oncology. Since STX140 itself is a potent inhibitor of STS, the combination of its wider properties with the known activity of STS in the immune system may generate significant and novel synergies to exploit. Dose-dependent inhibitory effects of Irosustat 3 on LPS-induced NO and PGE2 production in macrophages have been demonstrated.[31]
A common strategy to improve metabolic stability is the use of fluorinated isosteres.[32] The main reason fluorinated groups such as OCF3 or OCF2H are explored as a replacement for OCH3 is to avert a biotransformation pathway involving O-demethylation and to decrease the overall rate of oxidative metabolism.[33] 2ME2 is itself non-estrogenic, but it is known to undergo O-demethylation as a reverse reaction, leading to the significantly estrogenic 2-hydroxyestradiol.[34,35] Fluorinated isosteres are an intuitive approach, since the rate-limiting step in this metabolic reaction is the abstraction of a hydrogen atom from the OCH3 group, a process that cannot occur when hydrogen is replaced by fluorine. Unfortunately, most synthetic preparations of OCF3 groups occur under relatively harsh reaction conditions[36] and are usually unsuitable for the transformation of hydroxyl groups in the presence of more sensitive functional groups. Only a few milder transformations are described to convert phenols into their corresponding OCF3 derivatives.[37] However, the scope of these usually two-step reaction procedures remains limited to the transformation of more electron-deficient phenols. The main problem of using electron-rich phenols is the second step in these procedures, because usually an electrophilic or a radical fluorine species is generated. These react preferably at an electron-rich ring carbon and not, as intended, at the introduced difluorinated carbon. One-step reactions using CF3 transfer reagents are also described.[38] However, many of the reagents used in these reactions are unstable, and the reactions often suffer from a variety of problems, including the ones mentioned above for two-step procedures. Therefore, the use of OCF2H as a metabolically-stable isostere in bioactive compounds has gained significant attention in recent years.[32] In comparison with OCF3, the OCF2H group is described as being of similar stability but of slightly decreased lipophilicity.[32] Initial attempts to transform the steroidal aromatic 2-hydroxyl group under mild reaction conditions into OCF3 were made but failed.[37] The introduction of OCF2H, on the other hand, seemed much more promising in terms of delivering a reliable reaction procedure, and we wanted to evaluate the potential benefits of increased metabolic stability and anti-proliferative and other activities compared to the methoxy derivatives.
As mentioned, a large variety of mono- and bis-sulfamoylated steroidal derivatives were previously synthesised and evaluated as potential anti-cancer agents.[8–15] Various alkyl groups were introduced in the 2-position (see compounds of type 5 and 6, Figure 2),[9] but only the 2-ethyl-substituted bis-sulfamate derivative[11] delivered potency similar to that of 4. For this reason, only 2-ethyl and 2-methoxy substituted derivatives (compounds of type 7 and 8, Figure 2) were later considered when various hydrogen bond acceptors were installed at the 17β-position.[13–15] In the present work, we explored the OCF2H group as a replacement for the OCH3 substituent in the 2-position of the steroidal A-ring and determined initially if introduction of this metabolically more stable and more lipophilic group could lead to more potent anti-cancer agents (Figure 2). Thus, difluoromethoxy derivatives 9–12 with all possible combinations of hydroxy and sulfamoyloxy groups in both 3- and 17β-position were the initial priority targets towards a tightly focused SAR (Figure 2). STX140 is a multi-targeted drug and we also report here the effects of this new substitution on other activities of interest.
Figure 2.
Design of difluoromethoxy derivatives 9–12.
Results and Discussion
Chemistry
All candidate 2-difluoromethoxy estratriene compounds were synthesised starting from 2-hydroxy-3-benzyloxyestrone 13.[39] In order to difluoromethylate the 2-hydroxy group, we used diethyl (bromodifluoromethyl)phosphonate and potassium hydroxide in a mixture of acetonitrile and water at room temperature.[40] Compound 14 was achieved in moderate yields as reaction mixtures usually showed conversions of about 60–70%. Treatment of 14 with hydrogen in the presense of palladium on charcoal gave 2-difluoromethoxyestrone 15 in good yield. Compound 15 was then reacted with sulfamoyl chloride in N,N-dimethylacetamide (DMA)[41] to furnish 2-difluoromethoxy-3-sulfamoyloxyestrone 16 in good yield. Diol 9, the direct analogue of 2-ME2, was achieved by treatment of 14 with sodium borohydride in tetrahydrofuran and iso-propanol and subsequent removal of the benzyl protecting group using hydrogen in the presence of palladium on charcoal (Scheme 1).
Scheme 1.
Synthesis of difluoromethoxy derivatives 9, 15 and 16. Reagents and conditions: a) (EtO)2P(O)CF2Br, KOH, MeCN, H2O, 25 °C; b) H2, Pd/C, 25 °C; c) NaBH4, THF, i-PrOH, 0 °C to 25 °C; d) H2NSO2Cl, DMA, 25 °C.
Attempts were made to synthesise sulfamates 10 and 12 in the same manner as described for the 2-methoxy derivatives.[8,11] Unfortunately, treatment of 16 with sodium borohydride did not lead to the corresponding 3-sulfamoyloxy-17β-hydroxy derivative 12 but instead gave exclusively diol 9, as the sulfamoyl group was cleaved even under these mild reaction conditions (Scheme 2). It seems that the introduction of an electron-withdrawing 2-OCF2H group leads to a significantly more hydrolysis-labile 3-sulfamoyloxy group than for the corresponding 2-OCH3 derivatives, where this reaction works well.[8] Another reaction that did not occur in the same manner for these novel OCF2H derivatives was the bis-sulfamoylation of diol 9, as this only delivered a mixture of 10 and 12 in a 2:1 ratio unlike the facile reaction for the parent steroid 1.[11] Annoyingly, these two compounds proved inseparable by column chromatography. An attempt to achieve complete conversion into 10 by repeating the sulfamoylation reaction using the above described mixture did not result in any additional conversion and virtually the same product ratio as before was observed by 1H NMR spectroscopy. Therefore, different synthetic strategies were required to achieve sulfamates 10 and 12.
Scheme 2.
Attempted synthesis of difluoromethoxy derivatives 10 and 12. Reagents and conditions: a) NaBH4, THF, i-PrOH, 0 °C to 25 °C; b) H2NSO2Cl, DMA, 25 °C.
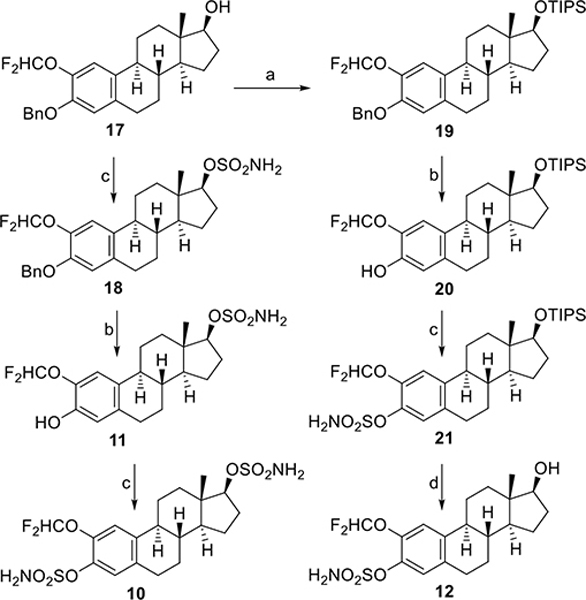
As the sulfamoylation was more easily achievable in the 3-position than in the 17β-position, we chose a strategy that included two separate mono-sulfamoylation steps with the 17β-hydroxy group being converted first (Scheme 3). Therefore, 17 was treated with sulfamoyl chloride in DMA to give 18. Subsequent hydrogenation then achieved phenol 11. Finally, sulfamoylation of 11 furnished the target bis-sulfamate 10 in good overall yield (Scheme 3). Compound 17 also served as the starting material for the synthesis of the 3-sulfamoyloxy-17β-hydroxy derivative 12. Treatment of 17 with TIPSCl and imidazole in N,N-dimethylformamide (DMF) led to 19 in low yield (18%), and 60% of the starting material 17 was recovered. Subsequent hydrogenation of 19 gave phenol 20. Sulfamoylation of 20 then led to 21. The silyl protecting group in the 17β-position was then removed by treatment with HF/pyridine in dichloromethane to give 12 in good yield (Scheme 3).
Scheme 3.
Synthesis of difluoromethoxy derivatives 10–12. Reagents and conditions: a) TIPSCl, imidazole, DMF, 25 °C; b) H2, Pd/C, THF, i-PrOH, 25 °C; c) H2NSO2Cl, DMA, 25 °C; d) HF/pyridine, CH2Cl2, −40 °C to 0 °C.
The lability of the sulfamate observed during the reduction of 16 led us to carry out an additional experiment. We studied the relative effects of the 2-OCF2H group on the aromatic ring by conducting hydrolysis experiments in wet DMSO-d6 using both 4 (STX140) and 10, as these two compounds all had features in common apart from the substituent at the 2-position. At the start of the experiment, the amount of water present in our batch of wet DMSO-d6 worked out as a water/substrate ratio of ~60:1. It appears that difluoromethoxy-substituted sulfamate 10 is far more sensitive to hydrolysis than its corresponding methoxy derivative 4 (Table 1). This experiment was conducted over 30 days. At the end, 92% of 10 had hydrolysed into 11. In contrast, 4 hydrolysed three times more slowly, and 31% conversion into 22 was observed after 30 days at room temperature. We worked out T1/2 parameters for both 4 and 10. For 10, T1/2 = ~10 days, as could be estimated from Table 1. For STX140, T1/2 was much longer and could only be roughly estimated as T1/2 = ~60–80 days. Both experiments showed only hydrolysis of the aromatic sulfamoyl group. The aliphatic sulfamoyl group in the 17β-position was stable under the experimental conditions, as expected. This may be purely a consequence of a lower pKa of the corresponding phenol for OCF2H in comparison with OCH3 derivatives (calculated pKa’s: ~8.52 for OCF2H vs ~9.22 for OCH3). It has been well established[42] that decreasing the pKa of the leaving phenol of an aromatic sulfamate ester leads to greater instability, presumably by means of an E1cB elimination process,[42] and it is not unexpected that introduction of an electron-withdrawing group onto the aryl ring would facilitate this. A very good example of this is provided by our earlier study of 2-difluoromethylestrone sulfamate 23.[43] Compound 23 was subjected to the same hydrolysis procedure to give 24.[43] The reaction occurred slightly faster than for the conversion of 10 into 11. The calculated pKa’s of the leaving phenols 11 and 24 are very similar (~8.52 for OCF2H vs ~8.49 for CF2H). However, conformational factors of the OCF2H group, and possibly also of the CF2H group, cannot be completely ruled out.
Table 1.
Hydrolysis of compounds 4, 10 and 23 in wet DMSO-d6 at 25 °C.[a]
|
| |||||
|---|---|---|---|---|---|
|
| |||||
| Cpd | R2 | R3, R4 | 0 days | 15 days | 30 days |
|
| |||||
| 4 | OCH3 | H, OSO2NH2 | 100 | 81 | 69 |
| 22 | OCH3 | H, OSO2NH2 | 0 | 19 | 31 |
|
| |||||
| 10 | OCF2H | H, OSO2NH2 | 100 | 32 | 8 |
| 11 | OCF2H | H, OSO2NH2 | 0 | 68 | 92 |
|
| |||||
| 23 | CF2H | =O | 100 | 24 | 5 |
| 24 | CF2H | =O | 0 | 76 | 95 |
Results are % values of the corresponding compound in the reaction mixture, as determined by 1H NMR spectroscopy.
Therefore, we tried to establish whether any kind of hydrogen bonding between the hydrogen of the 2-difluoromethoxy group and the oxygen in the neighbouring 3-position on the aromatic ring is likely to exist. Such interaction, if found, could be responsible, in addition to the expected inductive effects above, for the much faster cleavage of a leaving group such as sulfamate by neighbouring group participation through the increased acidity of the OCF2H proton.[32] Hydrolysis was identified as a potential problem for all 2-difluoromethoxy-3-sulfamoyloxy derivatives. Therefore, we selected benzyl- protected derivative 14 for crystallisation to explore the conformation of the new substituent. Thin long needles of 14 crystallised from methanol and were analysed by single crystal X-ray diffraction (Figure 3). However, the hydrogen of the difluoromethoxy group in the solved structure does not point towards the oxygen in the 3-position. In fact, the OCF2H group is not in plane with the aromatic ring system, as is observed for the methoxy group in the X-ray crystal structure of 1 (2ME2)[44] and indeed for STX140 itself[44] and also for the related compound 8a (STX641).[15] This can be explained in anisoles by anomeric interactions of the C–F σ* orbitals with the oxygen lone pair orbitals that weaken the π conjugation when more hydrogen atoms of OCH3 are subsequently replaced with fluorine atoms.[32] Therefore, aromatic OCF3 groups are usually found in an orthogonal orientation.[32] For aromatic OCF2H groups a distinct orientational preference has not been identified and either one or two of the C–F bonds are found in an anomeric orientation.[32] The OCF2H group in the X-ray structure of 14 is placed orthogonal to the aromatic ring as one would expect for an OCF3 group (Figure 4) and both C–F σ* orbitals show anomeric interactions with the oxygen lone pair orbitals (endo-endo conformation). The hydrogen atom of OCF2H is not close enough for any direct interaction with the oxygen atom in the 3-position.
Figure 3.
A) Single crystal X-ray structure of 14. B) Part of crystal lattice packing diagram of 14 to illustrate the orthogonal orientation of the OCF2H group within the crystal layers.
However, rotation of the O–CF2H bond would allow access to two different endo-exo conformations (Scheme 4B), one of which (endo-exo 1) may still lead to an increased rate of hydrolysis via a mechanism that involves a water molecule in order to bridge the distance between the hydrogen of the OCF2H group and the oxygen in the 3-position. Obviously, in solution direct hydrogen bonding may still present a possibility and cannot be completely ruled out (Scheme 4A). The obvious limitations of the use of 14 as wider a model are clear, but due to the significantly increased lability of the sulfamoyloxy group, we were so far unable to crystallise the corresponding sulfamate derivative to explore this more directly, although this has indeed been achieved for the more stable STX140.[44]
Scheme 4.
Potential mechanisms of accerated hydrolysis of 2-difluoromethoxy-3-sulfamoyloxyestratriene derivatives to their corresponding phenols and sulfamic acid by neighbouring group H-bonding. A) Direct. B) Water assisted.
Biology
All six compounds were tested in vitro against the proliferation of MCF-7 and MDA MB-231 breast cancer cell lines (Table 2). Data obtained for both cell lines were in good agreement. All three difluoromethoxy sulfamates were 5–10 fold more potent than their corresponding phenols with bis-sulfamate 10 displaying overall the best anti-proliferative activity with GI50’s of 0.28 μM (MCF-7) and 0.74 μM (MDA MB-231), respectively, very similar to that of 4 (STX140). Of all OCF2H substituted phenols that were evaluated, only diol 9 shows good activity (GI50’s 2.6 μM and 3.03 μM). Both, 11 and 15, with a 17β-sulfamate or 17-keto motif respectively, proved either weaker or essentially inactive. Upon first look, it seems surprising that the antiproliferative activities against MCF-7- and MDA-MB-231-cells are very similar (Table 2). For compounds with expected STS inhibitory activity like 4, 10, 12 and 16, better antiproliferative activities against MCF-7 would be expected as these cells, in contrast to MDA-MB-231 cells, are estrogen-receptor positive (ER+). Therefore, it should be noted that during the experiments, the MCF-7 cells were not grown in estrogen-deprived conditions but in full media. This would then contain estradiol (E2) from the fetal bovine serum (FBS), cutting out the need for removal of sulfate. To test the effects of STS inhibition on proliferation, estrogen-deprived conditions plus addition of estradiol sulfate (E2S) or estrone sulfate (E1S) would have been required to demonstrate that E2S stimulates MCF-7 growth, which would be inhibited by the addition of compound. Overall, the results observed for the MCF-7 cell assays are, however, very good and could have been even more impressive with use of an estrogen-deprived media setting.
Table 2.
Anti-proliferative activity of difluoromethoxy-substituted estratriene derivatives against MCF-7 and MDA MB-231 human breast cancer cells in vitro.[a,b]

| |||||
|---|---|---|---|---|---|
|
| |||||
| Cpd | R1 | R2 | R3, R4 | MCF-7 | MDA MB-231 |
|
| |||||
| 1 | H | OCH3 | H, OH | 0.6 | 1.7 |
| 4 | SO2NH2 | OCH3 | H, OSO2NH2 | 0.52 | 0.29 |
| 9 | H | OCF2H | H, OH | 2.6 | 3.03 |
| 10 | SO2NH2 | OCF2H | H, OSO2NH2 | 0.28 | 0.74 |
| 11 | H | OCF2H | H, OSO2NH2 | >5 | >10 |
| 12 | SO2NH2 | OCF2H | H, OH | 1.1 | 1.11 |
| 15 | H | OCF2H | =O | >5 | >10 |
| 16 | SO2NH2 | OCF2H | =O | 0.58 | 0.99 |
Results are GI50 values in μM and are the mean of three determinations for MDA MB-231 and two determinations for MCF-7.
Compounds 10–12 were selected for further anti-cancer evaluation in the NCI 60-cell line assay. Data from six cell lines are presented along with the mean activity across the whole panel (MGM values) in Table 3. Overall, all three compounds exhibited sub-micromolar anti-proliferative activities. Surprisingly, this includes phenol 11, and the NCI results for 11 are not in good agreement with our own data, but the reasons for this are unclear. We then established the microtubule disruptor activity of the new compounds alongside the established potent microtubule disruptor combretastatin A-4 (CA-4) and the steroids 1 (2ME2) and 4 (STX140) (Table 4). The bis-sulfamate derivative 10 inhibited tubulin assembly reasonably well with an IC50 of 3.9 μM and is about 3-fold less active than CA-4. The concentration in these tubulin-based assays far exceeds the anti-proliferative dose. It should also be noted that the nominal compound concentration recorded is that of agent added to the culture medium, and is not the concentration within cells.
Table 3.
Anti-proliferative activity of difluoromethoxy-substituted estratriene derivatives against various cancer cell lines from the NCI-60 cell line panel.[a]
| Cpd | Lung HOP-62 | Colon HCT-116 | CNS SF-539 | Melan-oma UACC-62 | Ovarian OVCAR-3 | Renal SN12C | MGM |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 10 | 1.98 | 0.474 | 0.266 | 0.564 | 0.527 | 0.567 | 0.661 |
| 11 | 0.648 | 0.375 | 0.427 | 0.460 | 0.383 | 1.22 | 0.813 |
| 12 | 0.634 | 0.406 | 0.313 | 0.498 | 0.386 | 0.692 | 0.646 |
Results are GI50 values in μM and the mean of three determinations. The MGM represents the mean concentration that caused 50% growth inhibition in all 60 cell lines.
Table 4.
Activity of difluoromethoxy-substituted estratriene derivatives as inhibitors of tubulin polymerisation and [3H]colchicine binding (5 μM inhibitor) to tubulin.[a]
| Tubulin assembly | Colchicine binding | |
|---|---|---|
| Compound | IC50 (μM) | (% Inhibition) |
|
| ||
| CA-4 | 1.2 ± 0.04 | 98 ± 1 |
| 1 | 8.3 ± 0.8 | 36 ± 3 |
| 4 | 2.3 ± 0.1 | 33 ± 3 |
| 9 | 12.4 ± 0.1 | N.D. |
| 10 | 3.9 ± 0.5 | 19 ± 0.5 |
| 11 | >20 | N.D. |
| 12 | 6.6 ± 0.7 | 22 ± 1 |
| 15 | >20 | N.D. |
| 16 | 4.3 ± 0.2 | 32 ± 4 |
Values are the mean ±SD of at least two determinations. N.D. not determined.
The inhibition of colchicine binding to tubulin for these fluorinated compounds was also determined, with 16 being the best, showing 32% inhibition at 5 μM whereas 10 showed only 19% inhibition. It thus appears reasonable to suggest that the interaction of these novel compounds can at least partially be ascribed to their ability to disrupt the normal dynamic polymerisation of tubulin by interaction at the colchicine binding site. It is interesting that introduction of OCF2H in 10 vs OCH3 in 4, in compounds with equivalent sulfamate substitution decreased colchicine binding, but in the related 16 vs 15, sulfamoylation significantly increased inhibition of tubulin assembly over the phenol. Likewise, in 10 vs 11 the additional phenolic sulfamoylation considerably improved this activity. We know from recent work where two of our non-steroidal sulfamate derivatives have been co-crystallised with the α/β-tubulin dimer and where the binding modes are visible in atomic detail, that a sulfamate group is able to engage in interactions with residues in the colchicine site that are not reached by colchicine itself.[45,46] This would be expected for steroidal derivatives as well, but the activity of 4 vs 10 shows that this is likely not the only consideration. As mentioned earlier, X-ray analysis of 14 has shown OCF2H placed in an orthogonal position relative to the aromatic ring whereas the OCH3 is usually found in a co-planar orientation. This is also observed in tubulin co-crystal structures of two non-steroidal heterocyclic sulfamates.[45,46] In both cases the OCH3 neighbouring the sulfamoyloxy group is almost co-planar with the aromatic ring. Changing from OCH3 to OCF2H may therefore not only add some additional bulk to the substituent but may also force it towards a less favourable position in the binding site of the αβ-tubulin heterodimer. This could possibly account for the slightly reduced overall activity of these new difluoromethoxy derivatives in tubulin assays when compared to the parent methoxy analogues.
All new 2-difluoromethoxy sulfamates were also tested for steroid sulfatase (STS) inhibition, an emerging strategy for endocrine therapy[7] as an adjunct to the well-established aromatase inhibition.[7,47] All three 2-difluoromethoxy-3-sulfamoyloxy compounds displayed excellent activities in the JEG-3 human choriocarcinoma lysate assay with IC50’s in the nanomolar region (44–75 nM; Table 5). As expected, phenol 11 with its aliphatic 17β-sulfamoyloxy substituent was inactive. The best activity was observed for bis-sulfamate 10 in the whole cell assay with an IC50 of 3.7 nM (Table 5). The previously synthesised compounds estrone sulfamate 25[41] and 2-difluoromethyl estrone sulfamate 23[43] that in comparision to present compounds is missing the bridging oxygen atom to the CHF2 moiety, were also evaluated for comparison and showed a similar level of activity in the JEG-3 lysate assay, with 23 only about 3-times more potent than 16. 23 had, however, not previously been evaluated in a whole cell system, only in placental microsomes, and compounds 23 and 25 proved significantly more potent than all the 2-difluoromethoxy derivatives or STX140 with IC50’s of 0.055 nM and 0.118 nM, respectively (Table 5). In direct comparison, 2-difluoromethyl estrone sulfamate 23 proved about 100-times more potent than the corresponding 2-difluoromethoxy estrone sulfamate 16.
Table 5.
Steroid sulfatase inhibition of difluoromethoxy-substituted estratriene sulfamate derivatives in JEG-3 human choriocarcinoma assays in vitro.[a]

| |||||
|---|---|---|---|---|---|
|
| |||||
| Cpd | R1 | R2 | R3, R4 | Cell lysate | Whole cells |
|
| |||||
| 4 | SO2NH2 | OCH3 | H, OSO2NH2 | 99 | 4.2 |
| 10 | SO2NH2 | OCF2H | H, OSO2NH2 | 44 | 3.7 |
| 11 | H | OCF2H | H, OSO2NH2 | >5000 | N.D. |
| 12 | SO2NH2 | OCF2H | H, OH | 75 | 18.6 |
| 16 | SO2NH2 | OCF2H | =O | 56 | 5.6 |
| 23 | SO2NH2 | CF2H | =O | 19 | 0.055 |
| 25 | SO2NH2 | H | =O | 79 | 0.118 |
Results are IC50 values in nM and are the mean of three determinations. N.D. not determined.
Conclusions
Steroid sulfamate derivatives have been further explored as potential anti-cancer agents. Novel 2-difluoromethoxy-substituted derivatives showed excellent in vitro activities against the proliferation of MCF-7 and MDA MB-231 breast cancer cells and were also evaluated in NCI-60 cell line assays. The most cytotoxic of the new agents, bis-sulfamate 10, displayed GI50’s of 0.28 μM and 0.74 μM in MCF-7 and MDA MB-231 breast cancer cells, respectively. 10 inhibited tubulin assembly and [3H]colchicine binding to tubulin. However, activities were slightly reduced compared to STX140. An X-ray analysis of benzyl protected compound 14 showed that the OCF2H moiety is almost orthogonal to the aromatic ring, as compared to the OCH3 group, which generally has a co-planar orientation. The reduced activities of 9 and 10 compared to 1 (2ME2) and 4 (STX140) in tubulin assays might therefore indicate a less favourable orientation of OCF2H in the binding site compared to OCH3. Additionally, 10 showed excellent STS inhibition in a JEG-3 human choriocarcinoma cell lysate and whole cell assays, with IC50’s of 44 nM and 3.7 nM, respectively, showing significant improvement in comparison with STX140 (99 nM and 4.2 nM, respectively), although removal of the bridging oxygen to the CF2H in 23 produced exceptional activity particularly in whole cells, with IC50 values of 19 nM and 0.055 nM respectively. Replacement of OCH3 with the metabolically more stable OCF2H led to an increased hydrolysis rate of the aromatic sulfamate ester and at a rate qualitatively similar to just a simple CF2H substitution. This may be solely a result of a lower pKa of the corresponding phenol for OCF2H substituted derivatives but other factors may contribute. Importantly, this should not be of undue concern in vivo where STX140 has been shown to be sequestered in red blood cells with the sulfamate protected from metabolism. It remains to be seen from future in vivo work if the 2-position substitution here reduces any likely back-demethylation reaction, potentially with positive overall consequences for activity and further development.
Experimental Section
Biology.
In vitro studies, cell lines:
MCF-7 (estrogen-receptor-positive) breast cancer cells were generously provided by the NCI cancer drug screen. Cells were maintained in a 5% CO2 humidified atmosphere at 37 °C in RPMI 1640, supplemented with 17% fetal bovine serum, l-glutamine (2 mM), and 12 μg/mL gentamicin sulfate. MDA MB-231 (metastatic pleural effusion of breast adenocarcinoma) breast cancer cells were obtained from ATCC Global Bioresource Center. Cells were maintained in a 5% CO2 humidified atmosphere at 37 °C in RPMI-1640 medium, supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (0.1 mg/mL). To ascertain IC50 values, 8000 cells in their appropriate growth medium were added to each well of a 96-well microtitre plate (Falcon; BD Biosciences, Cowley, UK). Plates were incubated for 24 h at 37 °C in a 5% CO2 humidified atmosphere before addition of compounds at a final concentration of 10−10–10−2 M.
Antiproliferative assays:
MCF-7 cells were seeded into 96-well microtitre plates (5000 cells per well) and were grown for 24 h without compound. They were then treated with compound at 10−9–10−4 M or with vehicle control. At 96 h post-treatment, IC50 values were determined by measuring cell protein with sulforhodamine B.[48] MDA MB-231 cells were seed into 96-well microtitre plates (8000 cells per well), allowed to adhere over 24 h, and then treated with compounds or vehicle control. At 48 h post treatment, a BrdU incorporation assay (Roche, Welwyn Garden City, UK) was performed as per the manufacturer’s instructions. Incorporation of BrdU results were expressed as a percentage of mean control values resulting in the calculation of the 50% growth inhibition (GI50). All experiments were performed in triplicate.
Tubulin Assays.
Bovine brain tubulin, prepared as described previously,[49] was used in the studies presented here. Assembly IC50’s were determined as described in detail elsewhere.[50] Briefly, 1.0 mg/mL (10 μM) tubulin was preincubated without GTP with varying compound concentrations for 15 min at 30 °C. Reaction mixtures were placed on ice, and GTP (final concentration, 0.4 mM) was added. The reaction mixtures were transfered to cuvettes held at 0 °C in a recording spectrophotometer. Baselines were established at 0 °C, and increase in turbidity was followed for 20 min following a rapid (< 30 s) jump to 30 °C. Compound concentrations required to reduce the turbidity increase by 50% were determined. The method for measuring inhibition of the binding of [3H]colchicine to tubulin was described in detail previously.[51] Reaction mixtures contained 0.1 mg/mL (1.0 μM) tubulin, 5.0 μM [3H]colchicine, and potential inhibitor at 5.0 μM. Compounds were compared to CA-4, a particularly potent inhibitor of the binding of colchicine to tubulin.[52] Reaction mixtures were incubated for 10 min at 37 °C, a time point at which the binding of colchicine in control reaction mixtures is generally 40–60% complete.
In vitro cell-free sulfatase assay:
A compound’s ability to block STS activity was measured using the lysate of JEG-3, a human placenta choriocarcinoma cell line which has high STS activity. To ascertain STS inhibition, enzyme activity was measured in the absence and presence of the inhibitor (10−11–10−5 M) using [3H]estrone sulfate (E1S; 4 × 105 dpm, Perkin Elmer) adjusted to 20 μM with unlabelled E1S substrate. After incubation of the substrate and inhibitor with JEG-3 lysate (125 μg of protein/mL) for 1 h, the product formed, estrone (E1), was separated from the mixture by extraction with toluene. [4–14C]E1 (American Radiolabelled Chemicals) was also used throughout the assay to monitor procedural losses. An organic phase aliquot was added to scintillation fluid, and the 3H and 14C content measured by scintillation spectrometry. The mass of E1S hydrolyzed was calculated from the 3H counts detected (corrected for the volume of medium and organic solvent used and for recovery of 14C counts) and the specific activity of the substrate.
In vitro whole cell sulfatase assay:
Intact monolayers of JEG-3 cells were incubated for 20 h at 37 °C with [3H]E1S (5 pmol, 7 × 105 dpm, 60 Ci/mmol) in serum-free Eagle’s Minimal Essential Medium (1.0 mL) with or without inhibitors (10−12–10−5 M). After incubation, medium (0.5 mL) was removed and product E1 separated from E1S by solvent partition using toluene. [4-14C]E1 (7 × 103 dpm, 52 mCi/mmol) was used to correct for procedural losses. The mass of E1S hydrolyzed was calculated as outlined above.
Chemistry.
All chemicals were either purchased from Sigma Aldrich (now Merck: Gillingham, UK), Alfa Aesar (Heysham, UK) or Fluorochem (Hadfield, UK). Organic solvents of HPLC grade (PE, EtOAc, CH2Cl2, MeCN, MeOH) or ACS reagent grade (Et2O, i-PrOH) were supplied by Merck and used as supplied. The petroleum ether (PE) was of fractions 40–60 °C. N,N-Dimethylacetamide (DMA), N,N-dimethylformamide (DMF) and tetrahydrofuran (THF) were purchased from Merck and stored under a positive pressure of N2 after use. Sulfamoyl chloride was prepared by an adaptation of the method of Appel and Berger[53] and was stored in the refrigerator under positive pressure of N2 as a solution in toluene as described by Woo et al.[54] An appropriate volume of this solution was freshly concentrated in vacuo immediately before use. Compound 13 was prepared according to a literature procedures.[39] Reactions were carried out at room temperature unless stated otherwise. Flash column chromatography was performed on silica gel (MatrexC60). 1H NMR spectra were recorded with a Varian Mercury VX 400 NMR spectrometer at 400 MHz. Chemical shifts are reported in parts per million (ppm) relative to the residual solvent peak as internal standard. High resolution time-of-flight mass spectra were performed on an Agilent single quadrupole with CTC-PAL autosampler or a Bruker Daltonics microTOF mass spectrometer using electrospray ionisation (ESI). Melting points were determined using a Stanford Research Systems Optimelt MPA100 melting point apparatus (Stanford Research Systems, Sunnyvale, CA, USA) and are uncorrected. All compounds were ≥ 95% pure by 1H NMR spectroscopy.
Crystallographic methods:
Low temperature single crystal X-ray diffraction data of 14 were collected using a (Rigaku) Oxford Diffraction SuperNova diffractometer. Raw frame data were reduced using CrysAlisPro, and the structures were solved using ‘Superflip’[55] before refinement with CRYSTALS[56] as per the SI (CIF). Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC 2169044 and is accessible via www.ccdc.cam.ac.uk/data_request/cif.
2-Difluoromethoxy-3-benzyloxyestra-1,3,5(10)-trien-17-one 14.
Compound 13[39] (1.135 g, 3.0 mmol) was placed in a sealed tube with a magnetic stirring bar and dissolved in MeCN (15 mL) and then treated with potassium hydroxide (3.37 g, 60.3 mmol, in 15 mL H2O) at 0 °C. Diethyl bromodifluoromethyl phosphonate (1.61 g, 6.03 mmol) was added. After 5 mins the reaction was allowed to warm to RT and stirred vigurously overnight. The solution was then diluted with Et2O (400 mL) and washed with water (400 mL). The aqueous layer was extracted with Et2O (400 mL). The combined organic layers were filtered through solid NaCl and concentrated in vacuo. Purification by flash column chromatography (PE→PE/EtOAc 9:1) afforded 14 as a white solid (581 mg, 45%). A small sample was recrystallised for X-ray analysis; mp: 133–135 °C (MeOH). 1H NMR (400 MHz, CDCl3): δ = 0.91 (3H, s), 1.36–1.68 (6H, m), 1.92–2.28 (5H, m), 2.30–2.40 (1H, m), 2.45–2.57 (1H, m), 2.82–2.89 (2H, m), 5.09 (2H, s), 6.55 (1H, t, J = 75.9 Hz), 6.74 (1H, s), 7.10 (1H, s), 7.29–7.46 ppm (5H, m); 19F NMR (376 MHz, CDCl3): δ = −81.15 ppm (2F, d, J = 75.9 Hz); 13C NMR (100 MHz, CDCl3): δ = 14.0, 21.7, 26.0, 26.5, 29.4, 31.6, 36.0, 38.1, 43.9, 48.1, 50.4, 71.0, 115.0, 116.6 (t, J = 259.7 Hz), 120.3, 127.3, 128.2, 128.7, 133.2, 135.1, 136.7, 138.4 (t, J = 3.2 Hz), 148.3, 220.8 ppm; HRMS (ES+): m/z found 449.1899; C26H28F2NaO3+ (M++Na) requires 449.1899. Single Crystal Data for 14: C26H28F2O3, Mr = 426.50. 150 K – triclinic, P1, a = 6.6907(3) Å, b = 7.9038(3) Å, c = 10.4268(5) Å, α = 94.683(4)°, β = 94.963(4)°, γ = 102.395(4)°, V = 533.67(4) Å3, Data/restraints/parameters – 4099/3/281, Flack = 0.063(205) for 1597 Friedel pairs, Rint = 0.021, Final R1 = 0.0548, wR2 = 0.1542 (I>2σ(I)).
2-Difluoromethoxy-3-hydroxyestra-1,3,5(10)-trien-17-one 15.
Compound 14 (513 mg, 1.2 mmol) was dissolved in MeOH (18 mL) and THF (68 mL), degassed and treated with hydrogen in the presence of Pd/C (10%, 85 mg) at RT for 3 h. The mixture was filtered through celite and rinsed with EtOAc. The filtrate was concentrated in vacuo to afford 15 as a white solid (402 mg, >99%); 1H NMR (400 MHz, CDCl3): δ = 0.91 (3H, s),1.42–1.44 (1H, m), 1.45–1.59 (4H, m), 1.59–1.66 (1H, m), 1.93–2.09 (3H, m), 2.09–2.18 (1H, m), 2.18–2.27 (1H, m), 2.27–2.36 (1H, m), 2.47–2.55 (1H, m), 2.81–2.88 (2H, m), 6.48 (1H, t, J = 74.0 Hz), 6.74 (1H, s), 7.01 ppm (1H, s); 19F NMR (376 MHz, CDCl3): δ = −79.86 ppm (2F, d, J = 74.0 Hz); 13C NMR (100 MHz, CDCl3): δ = 14.0, 21.7, 26.1, 26.5, 29.1, 31.6, 36.0, 38.1, 44.0, 48.1, 50.4, 116.7 (t, J = 261.2 Hz), 116.8, 117.7, 132.6, 135.6, 136.2 (t, J = 2.8 Hz), 145.5, 221.2 ppm; HRMS (ES−): m/z found 335.1461; C19H21F2O3− (M-H)− requires 335.1464.
2-Difluoromethoxy-3-sulfamoyloxyestra-1,3,5(10)-trien-17-one 16.
Sulfamoyl chloride (11 mL, 0.63 M in toluene) was concentrated in vacuum and cooled to 0 °C until it solidified. DMA (2.5 mL) was added, and the mixture was cooled to 0 °C. Compound 15 (293 mg, 0.87 mmol) was added, and the solution was stirred for 10 min at 0 °C and then at RT for 18 h. The reaction mixture was diluted with EtOAc (250 mL) and washed with 4:1 water/brine (3 × 300 mL). The organic layer was filtered through solid NaCl and concentrated in vacuo. The resulting pale yellow residue was washed with EtOAc (2 mL) and Et2O (2 × 2 mL). The procedure was repeated with the resulting filtrate to yield more material. Compound 16 was obtained as a white amorphous powder (157 mg, 34%); 1H NMR (400 MHz, acetone-d6): δ = 0.92 (3H, s), 1.40–1.75 (7H, m), 1.85–1.90 (1H, m), 2.05–2.13 (2H, m), 2.29–2.49 (3H, m), 2.88–2.93 (2H, m), 6.86 (1H, t, J = 74.5 Hz), 7.23 (2H, s), 7.21 ppm (2H, s); 19F NMR (376 MHz, acetone-d6): δ = −81.84 ppm (2F, d, J = 74.5 Hz); 13C NMR (100 MHz, acetone-d6): δ = 14.1, 22.1, 26.5, 26.9, 29.5, 32.5, 36.1, 38.6, 45.0, 48.3, 51.1, 117.8 (t, J = 258.6 Hz), 119.5, 124.8, 136.1, 140.3, 140.6, 142.2 (t, J = 3.3 Hz), 219.3 ppm; HRMS (ES−): m/z found 414.1188; C19H22F2NO5S− (M-H)− requires 414.1192.
2-Difluoromethoxy-3-benzyloxy-17β-hydroxyestra-1,3,5(10)-triene 17.
Compound 14 (575 mg, 1.35 mmol) was dissolved in THF/isopropanol (1:1, 40 mL) and cooled to 0 °C. Sodium borohydride (306 mg, 8.09 mmol) was then added portionwise as a solid. After 0.5 h the reaction was allowed to warm to RT and stirred for 2.5 h. Ammonium chloride (sat. 80 mL) was added dropwise. The solution was diluted with Et2O (400 mL) and washed with water (400 mL). The aqueous layer was extracted with Et2O (300 mL), and the organic layers filtered through NaCl and evaporated in vacuo to yield a yellow foam (560 mg). Purification by flash column chromatography (PE→PE/EtOAc 5:1) afforded 17 as a beige solid (283 mg, 57% yield) still containing EtOAc (2.2 wt% by 1H NMR); 1H NMR (400 MHz, CDCl3): δ = 0.79 (3H, s), 1.14–1.23 (1H, m), 1.25–1.55 (6H, m), 1.65–1.75 (1H, m), 1.85–1.92 (1H, m), 1.93–1.99 (1H, m), 2.07–2.22 (2H, m), 2.22–2.30 (1H, m), 2.75–2.88 (2H, m), 3.74 (1H, t, J = 8.6 Hz), 5.09 (2H, s), 6.55 (1H, t, J = 75.6 Hz), 6.73 (1H, s), 7.10 (1H, s), 7.30–7.45 ppm (5H, m); 19F NMR (376 MHz, CDCl3): δ = −81.04 ppm (2F, d, J = 75.8 Hz); 13C NMR (100 MHz, CDCl3): δ = 11.2, 23.2, 26.3, 27.3, 29.6, 30.7, 36.7, 38.6, 43.4, 44.0, 50.1, 71.1, 81.9, 115.1, 116.7 (t, J = 258.7 Hz), 120.3, 127.3, 128.1, 128.7, 133.9, 135.3, 136.8, 138.4 (t, J = 3.1 Hz), 148.1 ppm; HRMS (ES+): m/z found 451.2056; C26H30F2NaO3+ (M+Na)+ requires 451.2055.
2-Difluoromethoxy-3,17β-bis-hydroxyestra-1,3,5(10)-triene 9.
Compound 17 (250 mg, 0.58 mmol) was dissolved in methanol (8 mL) and THF (32 mL), degassed and treated with hydrogen in the presence of Pd/C (10%, 40 mg) at RT for 3 h. The mixture was filtered through celite and rinsed with EtOAc. The filtrate was concentrated in vacuo to afford 9 as a white solid (195 mg, >99%) still containing EtOAc (2.3 wt% by 1H NMR); 1H NMR (400 MHz, CDCl3): δ = 0.78 (3H, s), 1.13–1.22 (1H, m), 1.26–1.55 (6H, m), 1.64–1.74 (1H, m), 1.83–1.90 (1H, m), 1.92–1.98 (1H, m), 2.06–2.18 (2H, m), 2.19–2.26 (1H, m), 2.75–2.82 (2H, m), 3.74 (1H, t, J = 8.5 Hz), 6.48 (1H, t, J = 74.5 Hz), 6.71 (1H, s), 7.01 ppm (1H, s); 19F NMR (376 MHz, CDCl3): δ = −79.67 ppm (2F, d, J = 74.5 Hz); 13C NMR (100 MHz, CDCl3): δ = 11.2, 23.2, 26.5, 27.2, 29.2, 30.7, 36.7, 38.6, 43.4, 44.0, 50.1, 82.0, 116.7, 116.8 (t, J = 261.3 Hz), 117.6, 133.3, 135.8, 136.2 (t, J = 2.5 Hz), 145.3 ppm; HRMS (ES−): m/z found 337.1618; C19H23F2O3− (M-H)− requires 337.1621.
2-Difluoromethoxy-3-benzyloxy-17β-sulfamoyloxyestra-1,3,5(10)-triene 18.
Method as for 16 using compound 17 (240 mg, 0.56 mmol) and sulfamoyl chloride (0.51 M in toluene, 5.5 mL, 2.8 mmol) in DMA (2 mL) at RT for 16 h. The resulting mixture was then diluted with EtOAc (100 mL) and washed with water containing 20% brine (4 × 100 mL), dried and concentrated in vacuo. Purification by flash column chromatography (CH2Cl2→CH2Cl2/EtOAc 9:1) afforded 18 as a white solid (145 mg, 51%) still containing EtOAc (1.9 wt% by 1H NMR); 1H NMR (400 MHz, DMSO-d6): δ = 0.77 (3H, s), 1.16–1.44 (6H, m), 1.62–1.75 (2H, m), 1.77–1.86 (1H, m), 1.89–1.97 (1H, m), 2.11–2.29 (3H, m), 2.73–2.82 (2H, m), 4.34 (1H, t, J = 8.3 Hz), 5.11 (2H, s), 6.93 (1H, s), 6.99 (1H, t, J = 75.5 Hz), 7.05 (1H, s), 7.37 (2H, s, br, NH2), 7.30–7.48 ppm (5H, m); 19F NMR (376 MHz, DMSO-d6): δ = −80.68 ppm (1F, d, J = 75.4 Hz), −80.69 ppm (1F, d, J = 75.4 Hz); HRMS (ES+): m/z found 530.1785; C26H31F2NNaO5S− (M+Na)+ requires 530.1783.
2-Difluoromethoxy-3-hydroxy-17β-sulfamoyloxyestra-1,3,5(10)- triene 11.
Method as for 15 using compound 18 (135 mg, 0.266 mmol), Pd/C (10%, 20 mg) and hydrogen in THF (16 mL) and MeOH (4 mL) at RT for 3 h. The reaction mixture was then filtered through celite and washed with MeOH (20 mL). The filtrate was concentrated in vacuo to afford 11 as a white amorphous solid (110 mg, 99% yield); 1H NMR (400 MHz, DMSO-d6): δ = 0.76 (3H, s), 1.13–1.42 (6H, m), 1.59–1.74 (2H, m), 1.74–1.83 (1H, m), 1.85–1.97 (1H, m), 2.05–2.27 (3H, m), 2.63–2.75 (2H, m), 4.33 (1H, t, J = 8.3 Hz), 6.63 (1H, s), 6.92 (1H, t, J = 75.3 Hz), 6.94 (1H, s), 7.39 ppm (1H, s, br); 19F NMR (376 MHz, DMSO-d6): δ = −80.69 ppm (1F, d, J = 75.5 Hz), −80.70 ppm (1F, d, J = 75.5 Hz); 13C NMR (100 MHz, DMSO-d6): δ = 11.6, 22.5, 25.6, 26.6, 27.5, 28.4, 35.8, 37.9, 42.6, 43.0, 48.6, 87.4, 116.8 (t, J = 257.2 Hz), 116.9, 118.5, 130.8, 134.3, 136.5 (t, J = 3.1 Hz), 146.4 ppm; HRMS (ES−): m/z found 416.1349; C19H24F2NO5S− (M-H)− requires 416.1349.
2-Difluoromethoxy-3–17β-bis-sulfamoyloxyestra-1,3,5(10)-triene 10.
Method as for 16 using compound 11 (80 mg, 0.19 mmol) and sulfamoyl chloride (0.51 M in toluene, 2.0 mL, 1.0 mmol) in DMA (0.7 mL) at RT for 16 h. The reaction mixture was then diluted with EtOAc (100 mL), washed with water with 20% brine (4 × 100 mL), dried and concentrated in vacuo to afford 10 as a white amorphous powder (75 mg, 80%); 1H NMR (400 MHz, DMSO-d6): δ = 0.77 (3H, s), 1.18–1.45 (6H, m), 1.61–1.76 (2H, m), 1.79–1.87 (1H, m), 1.88–1.97 (1H, m), 2.10–2.32 (3H, m), 2.74–2.86 (2H, m), 4.34 (1H, t, J = 8.4 Hz), 7.04 (1H, t, J = 74.1 Hz), 7.15 (1H, s), 7.17 (1H, s), 7.38 (2H, s), 8.09 ppm (2H, s); 19F NMR (376 MHz, DMSO-d6): δ = −80.98 ppm (1F, d, J = 74.2 Hz), −80.99 ppm (1F, d, J = 74.2 Hz); HRMS (ES−): m/z found 495.1072; C19H25F2N2O7S2− (M-H)− requires 495.1077.
2-Difluoromethoxy-3-benzyloxy-17β-triisopropylsilyloxyestra-1,3,5(10)-triene 19.
Compound 17 (200 mg, 0.47 mmol) and imidazole (96 mg, 1.41 mmol) were dissolved in anhydrous DMF (3 mL). TIPS-Cl (136 mg, 0.71 mmol) was added, and the reaction mixture was stirred at RT for 18 h. An additional portion of TIPS-Cl (100 mg, 0.52 mmol) was added, and the reaction mixture was stirred for an additional 1 h. Water (100 mL) was added, and the aqueous layer was extracted with EtOAc (2 × 100 mL), and the combined organic layers were washed with water (100 mL) and water with 20% brine (100 mL), dried through NaCl and concentrated in vacuo to afford 400 mg of a colourless oil. Purification by flash column chromatography (PE→PE/EtOAc 19:1→7:1→3:1) afforded 19 as a colourless glass (50 mg, 18%; recovery of 17: 120 mg, 60%); 1H NMR (400 MHz, CDCl3): δ = 0.78 (3H, s), 1.05–1.08 (21H, m), 1.20–1.59 (7H, m), 1.60–1.70 (1H, m), 1.83–1.91 (1H, m), 1.95–2.06 (2H, m), 2.09–2.19 (1H, m), 2.19–2.28 (1H, m), 2.73–2.89 (2H, m), 3.81 (1H, t, J = 8.3 Hz), 5.09 (2H, s), 6.54 (1H, t, J = 75.8 Hz), 6.72 (1H, s), 7.10 (1H, s), 7.29–7.45 ppm (5H, m); 19F NMR (376 MHz, CDCl3): δ = −81.03 ppm (2F, d, J = 75.8 Hz; HRMS (ES+): m/z found 607.3386; C35H50F2NaO3Si+ (M+Na)+ requires 607.3389.
2-Difluoromethoxy-3-hydroxy-17β-triisopropylsilyloxyestra-1,3,5(10)-triene 20.
Method as for 15 using compound 19 (46 mg, 0.08 mmol), Pd/C (10%, 15 mg), MeOH (2 mL) and THF (8 mL) at RT for 4 h. The mixture was filtered through celite and rinsed with EtOAc (2 × 5 mL). The filtrate was concentrated in vacuo to afford 20 as a colourless glass (38 mg, 98%); 1H NMR (400 MHz, CDCl3): δ = 0.78 (3H, s), 1.05–1.09 (21H, m), 1.10–1.17 (1H, m), 1.21–1.60 (6H, m), 1.60–1.71 (1H, m), 1.82–1.90 (1H, m), 1.94–2.02 (2H, m), 2.10–2.24 (2H, m), 2.75–2.82 (2H, m), 3.81 (1H, t, J = 8.2 Hz), 6.48 (1H, t, J = 74.5 Hz), 6.72 (1H, s), 7.01 ppm (1H, s); 19F NMR (376 MHz, CDCl3): δ = −79.77 ppm (2F, d, J = 74.5 Hz); 13C NMR (100 MHz, CDCl3): δ = 11.6, 12.6, 18.3, 18.3, 23.4, 26.7, 27.3, 29.3, 31.6, 37.5, 38.8, 44.1, 44.3, 49.7, 82.1, 116.7, 116.8 (t, J = 260.6 Hz), 117.6, 133.5, 135.8, 136.3 (t, J = 2.4 Hz), 145.3 ppm; HRMS (ES−): m/z found 493.2951; C28H43F2O3Si− (M-H)− requires 493.2955.
2-Difluoromethoxy-3-sulfamoyloxy-17β-triisopropylsilyloxyestra-1,3,5(10)-triene 21.
Method as for 16 using compound 20 (37 mg, 0.076 mmol) and sulfamoyl chloride (0.51 M in toluene, 0.8 mL, 0.41 mmol) in DMA (1.5 mL) at RT for 4 h. The reaction mixture was then diluted with EtOAc (100 mL), washed with water (4 × 50 mL), filtered through solid NaCl and concentrated in vacuo to give 19 as a pale beige glass (42 mg, 98%); 1H NMR (400 MHz, acetone-d6): δ = 0.84 (3H, s), 1.10 (21H, s), 1.19–1.62 (7H, m), 1.66–1.77 (1H, m), 1.88–1.96 (1H, m), 1.98–2.03 (1H, m), 2.04–2.13 (1H, m), 2.20–2.37 (2H, m), 2.83–2.87 (2H, m), 3.91 (1H, t, J = 8.3 Hz), 6.84 (1H, t, J = 74.5 Hz), 7.20 (1H, s), 7.22 (1H, s), 7.27 ppm (2H, s, br), 19F NMR (376 MHz, acetone-d6): δ = −81.75 ppm (2F, d, J = 74.7 Hz); 13C NMR (100 MHz, acetone-d6): δ = 11.9, 13.2, 18.5, 18.6, 23.9, 27.1, 27.6, 29.6, 32.3, 38.2, 39.3, 44.9, 45.0, 50.2, 82.8, 117.8 (t, J = 258.6 Hz), 119.5, 124.8, 136.1, 140.1, 141.0, 142.2 ppm (t, J = 3.3 Hz); HRMS (ES+): m/z found 596.2643; C28H45F2NaNO5SSi+ (M+Na)+ requires 596.2648.
2-Difluoromethoxy-3-sulfamoyloxy-17β-hydroxyestra-1,3,5(10)-triene 12.
Compound 21 (40 mg, 0.070 mmol) was dissolved in CH2Cl2 (2 mL) and cooled to −40 °C. Hydrogen fluoride pyridine complex (0.10 mL, ~70% HF, ~3.8 mmol) was added dropwise via syringe, and the reaction mixture was stirred for 5 min allowing the reaction mixture to warm to 0 °C. The solution was then diluted with CH2Cl2 (40 mL) and washed with water (40 mL). The organic layer was filtered through solid NaCl and concentrated in vacuo. Purification by column chromatography (CH2Cl2/EtOAc 9:1→4:1→3:2) afforded 12 as a white amorphous solid (18 mg, 61%). 1H NMR (400 MHz, CDCl3): δ = 0.78 (3H, s), 1.13–1.76 (8H, m), 1.86–2.01 (2H, m), 2.08–2.30 (3H, m), 2.76–2.94 (2H, m), 3.74 (1H, t, J = 8.5 Hz), 5.05 (2H, s, br), 6.48 (1H, t, J = 74.0 Hz), 7.15 (1H, s), 7.17 (1H, s); 19F NMR (376 MHz, CDCl3): δ = −79.77 ppm (2F, d, J = 74.0 Hz); HRMS (ES+): m/z found 440.1314; C19H25F2NaNO5S+ (M+Na)+ requires 440.1314.
Supplementary Material
Acknowledgements
This research was supported in part by the Developmental Therapeutics Program in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute, which includes federal funds under Contract No. HHSN261200800001E and in part by a summer studentship grant from the Lister Institute of Preventive Medicine and by bursaries from Merton and Somerville Colleges, Oxford (to H.A. and W.G.).
Footnotes
Conflict of Interest
The authors declare no conflcit of interest.
Disclaimer: The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organisations imply endorsement by the U.S. Government.
References
- [1].a) Pribluda VS, Gubish ER, Lavallee TM, Treston A, Swartz GM, Green SJ, Cancer Metast. Rev. 2000, 19, 173–179; [DOI] [PubMed] [Google Scholar]; b) Brueggemeier RW, Bhat AS, Lovely CJ, Coughenour HD, Joomprabutra S, Weitzel DH, Vandre DD, Yusuf F, Burak WE Jr., J. Steroid Biochem. Mol. Biol. 2001, 78, 145–156; [DOI] [PubMed] [Google Scholar]; c) Fotsis T, Zhang Y, Pepper MS, Adlercreutz H, Montesane R, Nawroth PP, Schweigerer L, Nature 1994, 368, 237–239. [DOI] [PubMed] [Google Scholar]
- [2].Lakhani NJ, Sarkar MA, Venitz J, Figg WD, Pharmacotherapy, 2003, 23, 165–172. [DOI] [PubMed] [Google Scholar]
- [3].Sato F, Fukuhara H, Basilion JP, Neoplasia 2005, 7, 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Fallah J, Rini BI, Curr. Oncol. Rep. 2019, 21:6; [DOI] [PubMed] [Google Scholar]; b) Harrison MR, Hahn NM, Pili R, Oh WK, Hammers H, Sweeney C, Kim KM, Perlman S, Arnott J, Sidor C, Wilding G, Liu G, Invest. New Drugs 2011, 29,1465–1474; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Bruce JY, Eickhoff J, Pili R, Logan T, Carducci M, Arnott J, Treston A, Wilding G, Liu G, Invest. New Drugs 2012, 30, 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Perez-Sepulveda A, Espana-Perrot PP, Norwitz ER, Illanes SE, Redrop. Sci. 2013, 20, 1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) Edsall AB, Mohanakrishan AK, Yang D, Fanwick PE, Hamel E, Hanson AD, Agoston GE, Cushman M, J. Med. Chem. 2004, 47, 5126–5139; [DOI] [PubMed] [Google Scholar]; b) Pert DJ, Ridley DD, Aust. J. Chem. 1987, 40, 303–309; [Google Scholar]; c) Woo LWL, Leblond B, Purohit A, Potter BVL, Bioorg. Med. Chem. 2012, 20, 2506–2519; [DOI] [PubMed] [Google Scholar]; d) Bulman Page PC, Hussain F, Maggs JL, Morgan P, Park BK, Tetrahedron 1990, 46, 2059–2068; [Google Scholar]; e) Maltais R, Poirier D, Steroids, 2011, 76, 929–948. [DOI] [PubMed] [Google Scholar]
- [7].a) Thomas MP, Potter BVL, J. Med. Chem. 2015, 58, 7634–7658; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Potter J BVL. Mol. Endocrinol. 2018, 61, T233–252. [DOI] [PubMed] [Google Scholar]
- [8].Leese MP, Hejaz HAM, Mahon MF, Newman SP, Purohit A, Reed MJ, Potter BVL, J. Med. Chem. 2005, 48, 5243–5256. [DOI] [PubMed] [Google Scholar]
- [9].Bubert C, Leese MP, Mahon MF, Ferrandis E, Regis-Lydi S, Kasprzyk PG, Newman SP, Ho YT, Purohit A, Reed MJ, Potter BVL, J. Med. Chem. 2007, 50, 4431–4443. [DOI] [PubMed] [Google Scholar]
- [10].Jourdan F, Bubert C, Leese MP, Smith A, Ferrandis E, Regis-Lydi S, Newman SP, Purohit A, Reed MJ, Potter BVL, Org. Biomol. Chem. 2008, 6, 4108–4119. [DOI] [PubMed] [Google Scholar]
- [11].Leese MP, Leblond B, Smith A, Newman SP, Di Fiore A, De Simone G, Supuran CT, Purohit A, Reed MJ, Potter BVL, J. Med. Chem. 2006, 49, 7683–7696. [DOI] [PubMed] [Google Scholar]
- [12].Leese MP, Newman SP, Purohit A, Reed MJ, Potter BVL, Bioorg. Med. Chem. Lett. 2004, 14, 3135–3138. [DOI] [PubMed] [Google Scholar]
- [13].Jourdan F, Leese MP, Dohle W, Ferrandis E, Newman SP, Chander S, Purohit A, Potter BVL, J. Med. Chem. 2011, 54, 4863–4879. [DOI] [PubMed] [Google Scholar]
- [14].Jourdan F, Leese MP, Dohle W, Hamel E, Ferrandis E, Newman SP, Purohit A, Reed MJ, Potter BVL, J. Med. Chem. 2010, 53, 2942–2951. [DOI] [PubMed] [Google Scholar]
- [15].Leese MP, Jourdan FL, Gaukroger K, Mahon MF, Newman SP, Foster PA, Stengel C, Regis-Lydi S, Ferrandis E, Di Fiore A, De Simone G, Supuran CT, Purohit A, Reed MJ, Potter BVL, J. Med. Chem. 2008, 52, 1295–1308. [DOI] [PubMed] [Google Scholar]
- [16].Thomas MP, Potter BVL, Steroid Biochem J. Mol. Biol. 2015, 153, 160–169. [DOI] [PubMed] [Google Scholar]
- [17].a) Elger W, Schwarz S, Hedden AM, Reddersen G, Schneider B, Steroid Biochem J. Mol. Biol. 1995, 55, 395–403; [DOI] [PubMed] [Google Scholar]; b) Schwarz S, Thieme I, Richter M, Undeutsch B, Henkel H, Elger W, Steroids 1996, 61, 710–717. [DOI] [PubMed] [Google Scholar]
- [18].Ireson CR, Chander SK, Purohit A, Perera S, Newman SP, Parish D, Leese MP, Smith AC, Potter BVL, Reed MJ, Br. J. Cancer 2004, 90, 932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Suzuki RN, Newman SP, Purohit A, Leese MP, Potter BVL, Reed MJ, Steroid Biochem J. Mol. Biol. 2003, 84, 269–278. [DOI] [PubMed] [Google Scholar]
- [20].Newman SP, Foster PA, Stengel C, Day JM, Ho YT, Judde JG, Lassalle M, Prevost G, Leese MP, Potter BVL, Reed MJ, Purohit A, Clin. Cancer Res. 2008, 14, 597–606. [DOI] [PubMed] [Google Scholar]
- [21].Day JM, Foster PA, Tutill HJ, Newman SP, Ho YT, Leese MP, Potter BVL, Reed MJ, Purohit A, Br. J. Cancer 2009, 100, 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Newman SP, Leese MP, Purohit A, James DRC, Rennie CE, Potter BVL, Reed MJ, Int. J. Cancer 2004, 109, 533–540. [DOI] [PubMed] [Google Scholar]
- [23].Chander SK, Foster PA, Leese MP, Newman SP, Potter BVL, Purohit A, Reed MJ, Br. J. Cancer 2007, 96, 1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Reed MJ, Purohit A, Woo LWL, Newman SP, Potter BVL, Endocrine Rev. 2005, 26, 171–202. [DOI] [PubMed] [Google Scholar]
- [25].Duncan GS, Brenner D, Tusche MW, Brüstle A, Knobbe CB, Elia AJ, Mock T, Bray MR, Krammer PH, Mak TW, Proc. Natl. Acad. Sci. USA 2012, 109, 21034–21039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].a) Foster PA, Stengel C, Ali T, Leese MP, Potter BVL, Reed MJ, Purohit A, Newman SP, Anticancer Res. 2008, 28, 1483–1491; [PubMed] [Google Scholar]; b) Andring JT, Dohle W, Potter BVL, McKenna R, J. Med. Chem. 2019, 62, 2202–2212; [DOI] [PubMed] [Google Scholar]; c) Stengel C, Newman SP, Leese MP, Thomas MP, Potter BVL, Reed MJ, Purohit A, Foster PA, Anticancer Res. 2015, 35, 5249–5262. [PMC free article] [PubMed] [Google Scholar]
- [27].Eriksson AL, Wilhelmson AS, Fagman JB, Ryberg H, Koskela A, Tuukkanen J, Tivesten Å, Ohlsson C, Endocrinology 2016, 157, 4200–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Löhndorf A, Hosang L, Dohle W, Ordoardi F, Waschkowski S-A, Rosche A, Bauche A, Winzer R, Tolosa E, Windhorst S, Marry S, Flügel A, Potter BVL, Diercks B-P, Guse AH, BBA Mol. Cell Res. 2021, 1868:118988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stubelius A, Andréasson E, Karlsson A, Ohlsson C, Tivesten Å, Islander U, Carlsten H, Clin. Immunol. 2011, 140, 37–46. [DOI] [PubMed] [Google Scholar]
- [30].Stubelius A, Erlandsson MC, Islander U, Carlsten H, Clin. Immunol. 2014, 153, 40–48. [DOI] [PubMed] [Google Scholar]
- [31].Jang H-L, El-Gamal MI, Choi H-E, Choi H-Y, Lee K-T, Oh C-H, Bioorg. Med. Chem. Lett. 2014, 24, 571–575. [DOI] [PubMed] [Google Scholar]
- [32].a) Zafrani Y, Sod-Moriah G, Yeffet D, Berliner A, Amir D, Marciano D, Elias S, Katalan S, Ashkenazi N, Madmon M, Gershonov E, J. Med. Chem. 2019, 62, 5628–5637; [DOI] [PubMed] [Google Scholar]; b) Zafrani Y, Yeffet D, Sod-Moriah G, Berliner A, Amir D, Marciano D, Gershonov E, Saphier S, J. Med. Chem. 2017, 60, 797–804; [DOI] [PubMed] [Google Scholar]; c) Xing L, Blakemore DC, Narayanan A, Unwalla R, Lovering F, Denny RA, Zhou H, Bunnage ME, Chem. Med. Chem. 2015, 10, 715–726; [DOI] [PubMed] [Google Scholar]; d) Müller K, Faeh C, Diederich F, Science 2007, 317, 1881–1886; [DOI] [PubMed] [Google Scholar]; e) Müller K, Chimia, 2014, 68, 356–362. [DOI] [PubMed] [Google Scholar]
- [33].Guengerich FP, Methods in Enzymology 2017, 596, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sutherland TE, Anderson RL, Hughes RA, Altmann E, Schuliga M, Ziogas J, Stewart AG, Drug Disc. Today 2007, 12, 577–584. [DOI] [PubMed] [Google Scholar]
- [35].LaVallee TM, Zhan XH, Herbstritt CJ, Kough EC, Green SJ, Pribluda VS, Cancer Res. 2002, 62, 3691–3697. [PubMed] [Google Scholar]
- [36].a) Leroux FR, Manteau B, Vors J-P, Pazenok S, Beilstein J Org. Chem. 2008, 4:13; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Feiring AE, J. Org. Chem. 1979, 44, 2907–2910; [Google Scholar]; c) Mathey F, Bensoam J, Tetrahedron Lett. 1973, 14, 2253–2256; [Google Scholar]; d) Sheppard WA, J. Org. Chem. 1964, 29, 1–11. [Google Scholar]
- [37].a) Kanie K, Tanaka Y, Suzuki K, Kuroboshi M, Hiyama T, Bull. Chem. Soc. Jpn. 2000, 73, 471–484; [Google Scholar]; b) Kuroboshi M, Kanie K, Hiyama T, Adv. Synth. Catal. 2001, 343, 235–250; [Google Scholar]; c) Kuroboshi M, Suzuki K, Hiyama T, Tetrahedron Lett. 1992, 33, 4173–4176; [Google Scholar]; d) Shimizu M, Hiyama T, Angew. Chem., Int. Ed. 2005, 44, 214–231. [DOI] [PubMed] [Google Scholar]
- [38].a) Stanek K, Koller R, Togni A, J. Org. Chem. 2008, 73, 7678–7685; [DOI] [PubMed] [Google Scholar]; b) Liang A, Han S, Liu Z, Wang L, Li J, Zou D, Wu Y, Wu Y, Chem. Eur. J. 2016, 22, 5102–5106; [DOI] [PubMed] [Google Scholar]; c) Umemoto T, Zhang B, Zhu T, Zhou X, Zhang P, Hu S, Li Y, J. Org. Chem. 2017, 82, 7708–7719; [DOI] [PubMed] [Google Scholar]; d) Umemoto T, Adachi K, Ishihara S, J. Org. Chem. 2007, 72, 6905–6917; [DOI] [PubMed] [Google Scholar]; e) Umemoto T, Ishihara S, Tetrahedron Lett. 1990, 31, 3579–3582; [Google Scholar]; f) Umemoto T, Ishihara S, J. Am. Chem. Soc. 1993, 115, 2156–2164; [Google Scholar]; g) Umemoto T, Adachi K, J. Org. Chem. 1994, 59, 5692–5699. [Google Scholar]
- [39].Nambara T, Honma S, Akiyama S, Chem. Pharm. Bull. 1970, 18, 474–480. [Google Scholar]
- [40].Zafrani Y, Sod-Moriah G, Segall Y, Tetrahedron 2009, 65, 5278–5283. [Google Scholar]
- [41].Okada M, Iwashita S, Koizumi N, Tetrahedron Lett. 2000, 41, 7047–7051. [Google Scholar]
- [42].a) Spillane WJ, Malaubier J-B, Tetrahedron Lett. 2010, 51, 2059–2062; [Google Scholar]; b) Spillane WJ, MacGrath P, Brack C, O’Byrne AB, J. Org. Chem. 2001, 66, 6313–6316; [DOI] [PubMed] [Google Scholar]; c) McCaw CJA, Spillane WJ, J. Phys. Org. Chem. 2006, 19, 512–519; [Google Scholar]; d) Spillane WJ, Thea S, Cevasco G, Hynes MJ, McCaw CJA, Maguire NP, Org. Biomol. Chem. 2011, 9, 523–530; [DOI] [PubMed] [Google Scholar]; e) Spillane WJ, O’Byrne A, McCaw CJA, Eur. J. Org. Chem. 2008, 4200–4205. [Google Scholar]
- [43].Reed JE, Woo LWL, Robinson JJ, Leblond B, Leese MP, Purohit A, Reed MJ, Potter BVL, Biochem. Biophys. Res. Commun. 2004, 317, 169–175. [DOI] [PubMed] [Google Scholar]
- [44].a) Caira MR, Bourne SA, Samsodien H, Pharm. Drug Del. Pharm. Tech. 2015, 104, 3418–3425; [DOI] [PubMed] [Google Scholar]; b) Cairo MR, Bourne SA, Samsodien H, Smith VJ, Beilstein J Org. Chem. 2015, 11, 2616–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dohle W, Jourdan FL, Menchon G, Prota AE, Foster PA, Mannion P, Hamel E, Thomas MP, Kasprzyk PG, Ferrandis E, Steinmetz MO, Leese MP, Potter BVL, J. Med. Chem. 2018, 61, 1031–1044 [DOI] [PubMed] [Google Scholar]
- [46].Dohle W, Prota AE, Menchon G, Hamel E, Steinmetz MO, Potter BVL, ACS Omega 2019, 4, 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Foster PA, Molecules 2021, 26, 2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Monks A, Scudiero D, Shoemaker R, Paull K, Visitica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd M, Natl J. Cancer Inst. 1991, 83, 757–766. [DOI] [PubMed] [Google Scholar]
- [49].Hamel E, Lin CM, Biochemistry 1984, 23, 4173–4184. [DOI] [PubMed] [Google Scholar]
- [50].Hamel E, Cell Biochem. Biophys. 2003, 38, 1–22. [DOI] [PubMed] [Google Scholar]
- [51].Verdier-Pinard P, Lai JY, Yoo HD, Yu J, Marquez B, Nagle DG, Nambu M, White JD, Falck JR, Gerwick WH, Day BW, Hamel E, Mol. Pharmacol. 1998, 53, 62–76. [DOI] [PubMed] [Google Scholar]
- [52].Lin CM, Ho HH, Pettit GR, Hamel E, Biochemistry 1989, 28, 6984–6991. [DOI] [PubMed] [Google Scholar]
- [53].Appel R, Berger G, Chem. Ber. 1958, 91, 1339–1341. [Google Scholar]
- [54].Woo LWL, Lightowler M, Purohit A, Reed MJ, Potter BVL, Steroid Biochem J. Mol. Biol. 1996, 57, 79–88. [DOI] [PubMed] [Google Scholar]
- [55].Palatinus L, Chapuis G, J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar]
- [56].a) Parois P, Cooper RI, Thompson AL, Chem. Cent. J. 2015, 9:30; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Cooper RI, Thompson AL, Watkin DJ, J. Appl. Cryst. 2010, 43, 1100–1107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.