Abstract
The epidermal growth factor receptor (EGFR) is expressed robustly in the placenta, and critical processes of pregnancy such as placental growth and trophoblast fusion are dependent on EGFR function. However, the role that aberrant EGFR signaling might play in the etiology and/or maintenance of preeclampsia (PE) remains largely unexplored. Recently, we have shown that overexpression of EGFR in cultured uterine artery endothelial cells (UAEC), which express little endogenous EGFR, remaps responsiveness away from vascular endothelial growth factor receptor (VEGFR) signaling and towards EGFR, suggesting that endothelial EGFR expression may be kept low to preserve VEGFR control of angiogenesis. Here we will consider evidence for the possibility that the endothelial dysfunction observed in PE might in some cases result from elevation of endothelial EGFR. During pregnancy, trophoblasts are known to synthesize large amounts of EGFR protein, and the placenta regularly releases syncytiotrophoblast (STB)-derived exosomes and microparticles into the maternal circulation. Although there are no reports of elevated EGFR gene expression in preeclamptic endothelial cells, the ongoing shedding of placental vesicles into the vascular system raises the possibility that EGFR-rich vesicles might fuse with endothelium, thereby contributing to the symptoms of PE by interrupting angiogenesis and blocking pregnancy-adapted vasodilatory function.
Keywords: EGFR, exosome, endothelium, preeclampsia, pregnancy
Introduction
Preeclampsia (PE) is a hypertensive disease of pregnancy that is both a leading cause of maternal morbidity and mortality worldwide, and confers an increased risk for developing cardiovascular diseases later in life (reviewed by Lee & Tubby 2015). To date, nothing can definitively prevent PE, and the only ‘curative’ treatment is still delivery of the baby and placenta. Attempts to map PE-specific patterns of placental gene expression have identified at least three major subclasses of PE, each with its own unique expression profile for known PE markers (Leavey et al. 2015). Significant progress has been made, however, in understanding the mechanisms of normal vascular adaptation to pregnancy, which comprises early angiogenic events and enhancement of pro-vasodilatory signaling, facilitated by the augmentation of connexin 43 (Cx43) gap junction-mediated intercellular Ca2+ signaling (Yi et al. 2010). We have previously pointed out that PE is associated with alterations in both growth factors and cytokines in a manner reminiscent of ‘wounding’ responses, and these same factors bring about loss of pregnancy-adapted Cx43 function and vasodilation (reviewed in Bird et al. 2013). While growth factors and cytokines are indeed essential for the establishment of pregnancy and the continuing development of the fetus, they require precise regulation to avoid the pathologies of pregnancy that result from deficient or excessive endocrine and paracrine signaling.
Some growth factors, such as vascular endothelial growth factor (VEGF) and placental growth factor (PlGF), have been studied extensively in the context of PE, but the role that altered epidermal growth factor (EGF) receptor signaling might play in promoting PE remains comparatively unexamined. Although accumulating data in rodent models suggest that aberrant EGFR signaling is likely a factor in the development and/or maintenance of general hypertensive diseases (Florian & Watts 1999; Sambhi et al. 1992; Kagiyama et al. 2002, 2003; Kim et al. 2006; Ying & Sanders 2005; Kelly et al. 2017), there is little published research on EGFR signaling in the context of hypertensive disease during pregnancy, probably because EGFR is not considered a major regulator of normal angiogenesis or vasodilatory function in either uterine or umbilical endothelium. Nonetheless, transcriptome meta-analysis has suggested that altered gene expression of EGFR and/or its ligands may be a factor in the impaired placental function associated with PE (Moslehi et al. 2013), but there has been no direct evidence to support changes in endothelial cell EGFR expression being associated with endothelial dysfunction in PE.
Interestingly, EGFR is generally expressed at low levels in ‘normal’ endothelial cells. In the vascular endothelium, the VEGF receptor (VEGFR) system is considered the key regulator of angiogenesis (reviewed in Ferrara & Davis-Smyth 1997), while the fibroblast growth factor receptor (FGFR) system is the key mediator of mitogenesis (Lindner et al. 1990). Recently, we have shown that artificial increases in EGFR expression in cultured uterine artery endothelial cells (UAEC) leads to a shift in responsiveness away from VEGFR and towards EGFR, suggesting that endothelial EGFR expression may normally be low to avoid interference with VEGFR control of angiogenesis (Clemente et al. 2020). Indeed, the purpose of this review is to now explore the evidence that endothelial dysfunction observed in PE might indeed result from elevation of endothelial EGFR through exosomal transfer from the PE placenta.
During a healthy pregnancy, trophoblasts are known to synthesize large amounts of EGFR protein (Uhlén et al. 2015), and the placenta releases syncytiotrophoblast (STB)-derived exosomes and microparticles into the maternal circulation (reviewed in Tannetta et al. 2017). The plasma concentration of placenta-derived exosomes has also been shown to rise markedly in each subsequent trimester (Salomon et al. 2014), and this is even greater in the third trimester in a subset of PE cases (Pillay et al. 2016). Although there are no reports of elevated EGFR gene transcript expression in preeclamptic endothelial cells, the ongoing shedding of placental vesicles into the vascular system raises the possibility that EGFR-rich vesicles might be taken up by the endothelium, promoting the symptoms of PE by interrupting angiogenesis and blocking pregnancy-adapted vasodilatory function.
Before proposing a new model for placental EGFR-mediated endothelial dysfunction, this review will (1) provide a brief overview of EGFR signaling in general, (2) review ways in which EGFR is critical for placental function and embryonic and fetal development, (3) cite the limited and contradictory data on altered EGFR expression and activity in PE, and (4) examine preliminary evidence suggesting that improperly regulated EGFR signaling can have deleterious effects on pregnancy-adapted vasodilation.
It should be noted that the term “EGFR signaling” should only be interpreted to mean “any signaling event that involves EGFR,” and not “signaling performed exclusively by EGFR.” Effector molecules that are activated by EGFR in one cellular environment may also be mimicked by another receptor (e.g. VEGFR2, FGFR1) in a different setting.
The epidermal growth factor receptor
EGFR is a 170 kDa transmembrane protein expressed in a wide variety of cell types. It is one of four members of the ErbB family of receptor tyrosine kinases comprising EGFR (ErbB1), HER2 (ErbB2), the kinase-impaired ErbB3, and the EGFR-like ErbB4. In healthy cells, EGFR sits dormant in the plasma membrane as a monomer (Yamashita et al. 2015) or inactive symmetric dimer (Jura et al. 2009) until it is activated by binding with one of seven known ligands, including EGF, heparin-binding EGF-like growth factor (HBEGF), and transforming growth factor alpha (TGFα) (Wilson et al. 2009). Binding of ligand to EGFR induces a conformational change in its transmembrane domain that releases EGFR from its intrinsically disordered basal inactive state (Shan et al. 2012), so allowing the formation of active asymmetric dimers (Zhang et al. 2006; reviewed in Purba et al. 2017). This asymmetric dimerization stimulates EGFR’s tyrosine kinase activity, which results in the autophosphorylation of several tyrosines in the receptor’s C-terminal tail, and subsequently receptor internalization. These phosphotyrosine residues serve as docking sites for a diverse array of effector molecules that initiate signaling cascades along major pathways, including PI3K/Akt, MAPK, JAK-STAT, and PLCγ (reviewed in Wee & Wang 2017; Jones & Rappoport 2014). In some cellular environments, EGFR can also phosphorylate tyrosine residues of other receptors, and be transactivated by G-protein coupled receptor (GPCR) agonists (Daub et al. 1996).
Under normal conditions, EGFR plays key roles in cell proliferation, tissue homeostasis, organismal growth and development, wound healing, immune function, and embryogenesis (reviewed in Wee & Wang 2017). However, precisely because of its critical importance in so many biological processes, the consequences of aberrant EGFR signaling can be deadly. Deficient EGFR signaling is associated with skin disorders, inflammatory bowel disease, and improper cartilage formation leading to osteoarthritis (Brooke et al. 2014, Jia et al. 2016), while excessive EGFR signaling due to overexpression or activating mutation drives the progression of many cancers, including aggressive cancers of the breast, ovary, prostate, lung, colon, and brain (reviewed in Normanno et al. 2006).
EGFR in healthy pregnancy
Expression and localization of EGFR and its ligands in a healthy pregnancy
The critical importance of EGFR in pregnancy is immediately suggested by its high level of expression in the placenta, which far exceeds the levels observed in all other organs (Uhlen et al. 2015). Placental expression of EGFR varies in localization over the course of pregnancy, as does expression of EGFR ligands. Immunohistochemical staining of human placental tissues from weeks 4 and 5 of the first trimester showed that almost all expression of EGF and EGFR was localized in cytotrophoblasts (CTBs). During weeks 6 to 12, however, both EGF and EGFR were localized primarily in STBs (Ladines-Llave et al. 1991), suggesting that the role of placental EGFR changes during different stages of fetal development. Other EGFR ligands, including amphiregulin (AREG) (Lysiak et al. 1995) and betacellulin (BTC) (Tanimura et al. 2004), are also expressed in the placenta in a compartmentalized and time-dependent manner. TGFα is moderately expressed and HBEGF is robustly expressed in 1st trimester decidua and in CTBs and extravillous trophoblasts (EVTB) during all three trimesters (Lysiak et al. 1993; Birdsall et al. 1996; Leach et al. 1999). TGFα is also expressed in the STB during all three trimesters (Lysiak et al. 1993), but HBEGF expression is poor in the STB during the first trimester (Yoo et al. 1997), when EGFR in the STB appears to be activated primarily by other ligands in an autocrine manner (Ladines-Llave et al. 1991; Lysiak et al. 1993; Lysiak et al. 1995; Tanimura et al. 2004). At term, EGFR protein is abundant on the microvillus and basolateral surfaces of STBs (Rao et al. 1985), suggesting activation by maternal and fetal ligands, respectively. It is also detected in intracellular organelles, including the endoplasmic reticulum, Golgi apparatus, and lysosomes, consistent with ongoing synthesis and degradation (Ramani et al. 1986). The complex spatiotemporal distribution of EGFR and its ligands strongly suggests the necessity for precise regulation of the receptor over the course of pregnancy.
One additional signaling molecule worth mentioning, although not formally recognized as an EGFR ligand, is EGF-like domain 7. EGFL7 is a secreted angiogenic factor whose overexpression has been positively correlated with the phospho-activation of EGFR. Conversely, knockdown of the EGFL7 gene was shown to inhibit activation of EGFR signaling cascades, including the PI3K/Akt and MEK/ERK pathways (Luo et al. 2014; Wang et al. 2017; Liu et al. 2018). Generally speaking, adult tissues express little to no EGFL7 except in those tissues that are heavily vascularized such as lung, heart, kidney, ovary, and uterus. EGFL7 mRNA is abundantly expressed in both human umbilical vein endothelial cells (HUVEC) and bovine aortic endothelial cells (BAEC), whereas a variety of nonendothelial cell lines have been found to express little to none (Fitch et al. 2004). Under normal conditions, EGFL7 promotes VEGF-induced sprouting angiogenesis and supports endothelial barrier function via tyrosine phosphorylation of VE-cadherin, while knockdown of EGFL7 inhibits these processes (Usuba et al. 2019). Primary human trophoblasts have been shown to respond to hypoxia by increasing EGFL7 transcription, presumably in an attempt to improve cellular oxygenation. When cultured in 1% O2 for 24 hours, the cells exhibited a HIF1α-independent 2.2-fold upregulation of EGFL7 mRNA (P < 0.0001) (Whitehead et al. 2018).
EGFR in fertility and fetal development
Egfr knockout studies in mice have revealed the vital function of EGFR in pregnancy, as evidenced by the fact that embryos of whole body Egfr knockout mice die in gestation (Sibilia & Wagner 1995; Threadgill et al. 1995). To determine the importance of EGFR and other members of the ERBB family of receptors in fertility, Large et al. (2014) created a mouse model in which Egfr expression was ablated exclusively in the uterus, leaving expression at normal levels in all other tissues. Of the seven females tested, four gave birth to only a few pups in a six-month period, and three produced no pups at all. This appeared to be related to incomplete decidualization caused by the absence of expression of critical EGFR effectors such as Bmp2 (Lee et al. 2007) and Wnt4 (Franco et al. 2011), which mediate decidual cell proliferation, survival, and differentiation. In comparison, knockout of uterine Erbb2 (Her2) or Erbb3, which are also expressed in the endometrium, produced only a small reduction in average litter size, suggesting that EGFR is the only member of the ERBB receptor family that is necessary and sufficient for proper endometrial function. Large et al. further demonstrated the essential role of EGFR in decidualization using a primary human endometrial stromal cell (HESC) culture model. Decidualization markers such as IGFBP1 and prolactin are known to be induced in HESC culture when these cells are treated with a combination of estrogen, progestin and cAMP (Giudice et al. 1992; Huang et al. 1987). However, expression of these markers was significantly reduced when HESC was transfected with EGFR siRNA, and the cells exhibited an aberrant morphology (Large et al. 2014).
EGFR is also involved in preimplantation development of the embryo. Administration of EGF has been shown to enhance the in vitro rate of blastocyst formation of several species, including mice (Dadi et al. 2007), pigs (Abeydeera et al. 1998; Wei et al. 2001), cows (Lonergan et al. 1996; Watson et al. 2000), and sheep (Guler et al. 2000). In cultures of fertilized sheep oocytes, for example, EGF more than doubled the number of blastocysts formed (1.4+/−0.1 vs. 0.6+/−0.2 per ewe) for the duration of the culture period (Grazul-Bilska et al. 2003). The most important EGFR ligand in the earliest days of pregnancy, however, may be HBEGF. The functions of HBEGF are numerous, including a central role in the establishment of pregnancy, development of human embryos to the blastocyst stage and beyond, and mediation of trophoblast motility (reviewed in Jessmon et al. 2009). During implantation, endometrial HBEGF serves as both a pro-survival growth factor and, in its transmembrane “proHBEGF” form, an attachment factor for blastocysts. Plasma membrane-tethered proHBEGF binds to either EGFR or heparin sulfate proteoglycan on the surface of an adjacent blastocyst, facilitating both the adherence of the blastocyst and the initiation of juxtacrine signaling (Raab et al. 1996).
EGFR promotes trophoblast proliferation
EGFR ligands have been shown to promote trophoblast proliferation in vitro. In cultures of first trimester human trophoblasts, EGF (Maruo et al, 1992), TGFα (Lysiak et al. 1993), and AREG (Lysiak et al. 1995) were each shown to increase the rate of proliferation. Filla et al. (1993) additionally showed that EGF and TGFα stimulated growth in cultures derived from second trimester trophoblasts. The unique expression profiles of EGFR and HER2 in first trimester CTB further suggest that EGFR mediates proliferation, as evidenced by the presence of EGFR and absence of HER2 in proliferative villous CTB. Inversely, HER2 appears to mediate invasion and differentiation, since HER2 was expressed in invasive, extravillous CTB, but EGFR was undetected in these non-proliferative cells (Jokhi et al. 1994). However, in cultured CTB derived from term placentas, EGFR activation by EGF induced differentiation into STBs (Morrish et al. 1987), suggesting that the role of EGFR changes over the duration of pregnancy.
EGFR signaling protects trophoblasts from apoptosis
Apoptosis, or programmed cell death, is a necessary occurrence in the differentiation of tissues. The hypoxic environment and elevated levels of inflammatory cytokines observed in PE placentas, however, can stimulate excessive apoptosis, resulting in placental dysfunction and retarded growth. One of the most important functions of EGFR during pregnancy is protection against apoptosis. Indeed, EGFR activation can protect against apoptosis induced by hypoxia (Perkins et al. 2002; Armant et al. 2006; Humphrey et al. 2008), reactive oxygen species (Moll et al. 2007), and inflammatory cytokines (Garcia-Lloret et al. 1996) in a number of cell models. Interestingly, supraphysiological concentrations of EGF (>50 ng/ml) provided reduced protection against cytokine-induced trophoblast death, suggesting that excessive stimulation of EGFR activity can also harm trophoblast viability (Garcia-Lloret et al. 1996), perhaps due to compensatory downregulation of the receptor itself, or associated signaling kinases.
EGFR signaling and angiogenesis
While under normal circumstances VEGF is the key regulator of angiogenesis in the vascular endothelium, treatment of HUVEC with HBEGF or EGF can induce VEGF-independent migration, capillary tube formation, and angiogenesis (Mehta & Besner 2007). HBEGF also strongly stimulated expression of eNOS mRNA, increased eNOS protein synthesis, and boosted the release of nitric oxide (NO). Pretreatment of HUVEC with the PI3K kinase inhibitor wortmannin or the EGFR kinase inhibitor AG1478 blocked these effects, showing EGFR-mediated eNOS activity to be regulated via the PI3K pathway (Mehta et al. 2008). EGFR expression in endothelial cells tends to be modest, as stated earlier, but these data suggest that EGFR could assume direct control of angiogenesis and enhance eNOS function in an environment where it outcompeted VEGFR1/2 for access to the relevant effector molecules.
Soluble EGFR in the placenta
In addition to its robust expression of mRNA encoding the full-length 170 kDa EGFR, the placenta also expresses a number of naturally occurring alternative EGFR transcripts that encode truncated variants of the full-length receptor termed “soluble EGFR” (sEGFR). These include the 1.8-kb mRNA transcript that encodes a secreted 60 kDa isoform (p60 EGFR) and the 3.0-kb transcript that encodes a plasma membrane-tethered 110 kDa isoform (p110 EGFR) (Reiter & Maihle 2003). Both sEGFR variants lack all of the typical domains of the full-length receptor except the extracellular ligand-binding domain, rendering them devoid of any kinase activity. These alternative transcripts are poorly expressed in most human tissues, but the highest expression level occurs in placenta (Reiter & Maihle 2003).
Since the ligand-binding domain of sEGFR isoforms is identical to that of the full-length receptor, sEGFR isoforms are capable of forming complexes with EGFR ligands, but does not induce sEGFR homodimerization (Gunther et al. 1990; Flickinger et al. 1992). sEGFR is generally thought to be a negative regulator of EGFR signaling, but it is unclear whether inhibition of EGFR kinase activity is achieved by “mopping up” available ligand (Flickinger et al. 1992) or by dimerizing with the full-length EGFR (Basu et al. 1989). In hindsight, it seems likely that these two distinct actions—ligand binding and dimerization with full-length EGFR—are distinct steps in a single mechanism.
Dysregulation of EGFR during pregnancy
EGFR in preeclampsia
Relatively few studies have addressed the subject of EGFR signaling in the context of PE. A transcriptome meta-analysis found that the EGFR gene and transcription regulators of EGF-dependent pathways were significantly upregulated in preeclamptic placentas, leading the authors to postulate that EGFR was among the 50 or so genes “most relevant to preeclampsia” (Moslehi et al. 2013). Surprisingly, the upregulation of EGFR and its transcriptional regulators was associated with a deficiency of EGFR signaling rather than an increase (Moslehi et al. 2013), which is supported by observations of stunted syncytial differentiation in PE (reviewed in Huppertz 2011). When cultured trophoblasts were exposed to a hypoxic environment for the first 24 hours, they exhibited significantly more apoptosis than cells experiencing hypoxia at 48 to 72 hours, suggesting that less differentiated trophoblasts are more vulnerable to the negative effects of hypoxia than are more differentiated trophoblasts (Levy et al. 2000). This is also consistent with the characterization of PE as a condition of reduced EGF/EGFR signaling (Moslehi et al. 2013), since EGF is known to promote trophoblastic differentiation (Morrish et al. 1987). In contrast, Hastie et al. (2019) found evidence that EGFR mRNA expression and phosphorylation of EGFR and ERK1/2 were significantly increased in severe early-onset preeclamptic placentas compared with control placentas, resulting in the secondary upregulation of sFlt1. The authors speculated that the increased EGFR pathway activity in preeclamptic placenta might be a response to hypoxia. EGFR mRNA and protein expression were found to be elevated in primary cytotrophoblasts cultured in 1% oxygen for 24 h, just as in severe early-onset PE. Polymorphisms of EGF (Chenthuran et al. 2014) and HBEGF (Harendra et al. 2012) have also been associated with PE, further suggesting that aberrant EGFR signaling can have negative consequences during pregnancy.
Too little or too much EGFR activity can be harmful during pregnancy
Since, as previously noted, the complete absence of EGFR results in embryonic lethality, it is appropriate to investigate the activity level of EGFR required for healthy placental development. To study the physiological consequences of attenuated EGFR signaling, Dackor et al. (2009a) created strains of mice that were homozygous for a hypomorphic allele of Egfr (termed 'Egfrwa2'). The spongiotrophoblast layer of the placentas of mice expressing Egfrwa2 was significantly thinner or absent, depending on the strain. Embryo growth was retarded in the third week of development, leading the authors to suggest that Egfrwa2 homozygous mice could model intrauterine growth restriction (IUGR) in humans. Additionally, to study the effects of excessive EGFR signaling, Dackor et al. (2009b) created strains of mice that were homozygous or heterozygous for the hypermorphic EgfrDsk5 allele. EgfrDsk5 heterozygous females exhibited enlarged placentas with a thickened spongiotrophoblast layer, which was associated with low fertility and pregnancy loss in some strains, independent of embryo genotype. EgfrDsk5 homozygotes showed even greater placental enlargement, and this too was often associated with embryo death prior to day 15.5. Together, these experiments support the idea of a “Goldilocks zone” for maternal and fetal EGFR signaling, in which EGFR plays an essential role in pregnancy, but either too little or too much activity can have deleterious consequences. As previously mentioned, PE has already been associated with reduced EGFR signaling. Is it possible that excessive EGFR activity could also, at least in some cases, exacerbate the symptoms of PE?
EGFR ligand expression in PE
The current evidence for expression levels of EGFR and its ligands in PE is limited and contradictory. Kosovic et al. (2017) compared EGF and EGFR expression separately in decidual cells (DC), villous trophoblasts (VTB), and extravillous trophoblasts (EVTB) obtained from either normal (38 wks) or preeclamptic placentas (38 wks). There was no significant difference between the normal and PE groups in maternal and gestational age, neonatal sex, or birth weight. No between-group differences were found in the expression levels of EGF or EGFR in DC, VTB, or EVTB. Similarly, no difference was observed in urine EGF concentrations at 10-18 weeks between mothers with PE and healthy matched controls (Lindqvist et al. 1999). Alternatively, immunohistochemical labeling of six EGFR ligands (EGF, HBEGF, TGFα, AREG, EREG, and BTC) in the trophoblasts of placentas from women with PE (mean 32.6 wks), preterm labor (32.9 wks), small for gestational age fetuses (32.9 wks), and normal term placentas (39 wks) found that EGF, HBEGF, and TGFα were significantly reduced in the basal plate of PE placentas compared to all other groups (Armant et al. 2015). One study of serum EGF concentration in PE pregnancy (32 wks, median = 93.13 pg/ml) found EGF levels to be more than double those observed in normotensive pregnancy (38.5 wks, median = 38.82 pg/ml) (Kupsamy et al. 2019). Yet another study reported that when maternal serum levels of EGF, HBEGF, and TGFα were measured in 20 pregnant women of similar maternal and gestational age (~34 wks) with or without PE that eventually delivered at term, the median serum concentration of EGF in the PE group (147.5 pg/ml) was less than half that of the median level in the normotensive group (324.3 pg/ml). Interestingly, however, levels of HBEGF and TGFα were not reduced (Armant et al. 2015), and other evidence suggests HBEGF may actually be significantly elevated (Charkiewicz et al. 2018). This is physiologically significant if true, because HBEGF appears to be the most commonly utilized ligand in known ligand-dependent EGFR transactivation mechanisms.
To summarize, these confusing data on EGFR ligand expression in PE illustrate why the expression of EGFR ligands in PE and their potential role in the etiology and maintenance of the disease is far from settled. It is likely that each subtype of PE expresses unique tissue-specific levels of EGF ligands, but the impact of these differences on PE pathology remains unclear.
Soluble EGFR in PE
One novel way that the availability of EGFR ligands could be increased in some types of PE is by reduced expression of sEGFR. Measurement of serum concentrations of sEGFR in 172 normotensive or preeclamptic pregnant women showed levels to be significantly lower in preterm PE (33.0 ± 2.68 wks, P < 0.001) and term PE (38.8 ± 1.14 wks, P < 0.01) than matched controls. Conversely, sFlt1 was significantly higher in preterm preeclampsia (P < 0.001) and term preeclampsia (P < 0.01) than matched controls, and the sFlt1:sEGFR ratio correlated strongly (R = 0.711) with the severity of preterm preeclampsia (Cui et al. 2018a). Although serum sEGFR level alone could not differentiate between mild and severe forms of preterm PE, alterations in the levels of sEGFR and three other proteins (VEGF ↑, sEndoglin ↑, PlGF ↓) were each shown to be independent predictive markers for PE, (Cui et al. 2018b). Citing known effects of EGFR on vascular tone and sodium retention in arterial hypertension (Beltowski & Lowicka 2009), Cui et al. speculated that reduced expression of serum sEGFR in PE might increase the availability of EGFR ligands in the vasculature, thereby boosting EGFR signaling and exacerbating hypertension in PE. Ilekis et al. (1995) found an inverse relationship in the placenta between expression of the p60 sEGFR transcript and that of the full-length EGFR transcript, opening the possibility that a high concentration of the full-length EGFR protein may necessitate a low concentration of sEGFR and vice versa. This means that higher expression of EGFR may typically be accompanied by greater availability of ligands, further potentiating EGFR activity.
EGFL7 in PE
Initial measurements of changes in EGFL7 expression in PE pregnancies have been contradictory. Whitehead et al. (2018) observed no significant difference in EGFL7 mRNA expression in early or late onset PE compared to gestational age-matched normotensive controls. However, elevations in EGFL7 protein levels of PE patients were reported by Massimiani et al. (2019), who were unable to detect circulating EGFL7 in nonpregnant women but found that it was detectable during pregnancy. In PE patients, the mean circulating level of EGFL7 protein at weeks 35-40 was significantly higher (44 vs. 25 μg/mL). Similarly, villous explant cultures from PE placentas at weeks 35-37 released three times as much EGFL7 into the culture media in 24 hrs (mean = 257.99 μg/mL) than did explant samples from controls at weeks 37-39 (mean = 86.76 μg/mL). Notably, the variance was high between samples, with many PE-derived samples exhibiting no difference from controls and others showing stark differences, suggesting that elevated EGFL7 may only be a feature of particular PE subtypes. Thus, while EGFL7 may play a role in the response to hypoxia, it is not likely to be a fundamental initiating event. In the absence of EGFR elevation, we doubt changes in EGFL7 would be a principal determinant of endothelial dysfunction. However, in endothelial cells with elevated EGFR expression, EGFL7 might further drive excessive EGFR activity.
EGFR transactivation
One last important EGFR activation mechanism that may yet be found relevant in PE is “transactivation” by agonists of G-protein coupled receptors (GPCRs) via induction of matrix metalloproteinase (MMP) cleavage of membrane-tethered EGFR ligand (e.g. proHBEGF, proEGF, etc.). GPCR-mediated EGFR transactivation can also occur independently of MMP activity or ligand through interaction with a specific Src family kinase (SFK), such as in the case of the muscarinic M2 acetylcholine receptor in COS-7 cells via a mechanism that involves direct association of EGFR with Fyn, but is independent of Src and Yes (Stirnweiss et al. 2006). Cytokines such as TNFα, IL-8, IL-1α, IL-1β, and IFNγ are known to be significantly elevated in PE (reviewed in LaMarca et al. 2007; Lau et al. 2013, Bird et al. 2013), and all of these have been reported to transactivate EGFR (Argast et al. 2004; Chen et al. 2004; Itoh et al. 2005; Lee et al. 2015; Tanida et al. 2004). IL-8 transactivation of EGFR, for example, has been observed in microvascular endothelium (Schraufstatter et al. 2003), and it has been shown to be capable of inducing phosphorylations in Src, ERK1/2, FAK, Jnk, p38-MAPK, STAT1 and STAT3 (Kyriakakis et al. 2011). Interestingly, in addition to transporting EGFR, placental exosomes may also be a source of these transactivating cytokines. When BeWo cells, a placental choriocarcinoma cell line commonly used as an in vitro model for the placenta, were treated with exosomes isolated from the serum of early onset PE or late onset PE pregnancies, expression of the pro-inflammatory interleukin IL-8 increased, and expression of the anti-inflammatory interleukin IL-10 decreased (Maduray et al. 2020).
A new model for endothelial dysfunction in preeclampsia
Pregnancy-adapted programming of the endothelium
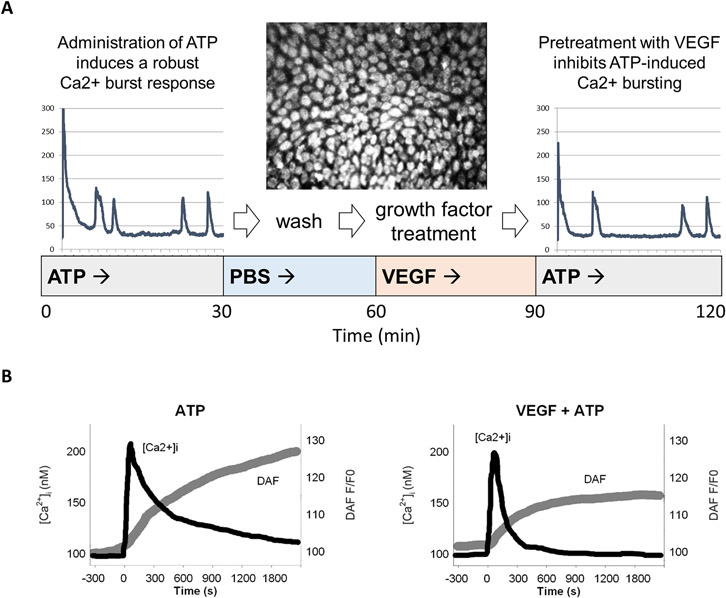
Pregnancy requires significant enhancements of vascular function to meet the needs of the mother and developing fetus, including increased cardiac output, blood volume, and capacity for vasodilation. These adaptations are dependent on numerous changes in cell signaling that we have termed 'pregnancy-adapted programming.' One particular adaptation we have studied extensively is the augmented capacity for Cx43 gap junctional communication. Using cultured uterine artery endothelial cells obtained from pregnant ewes (P-UAEC), which retain their pregnancy-enhanced capacity for pro-vasodilatory signaling (Bird et al. 2000; Gifford et al. 2003), we have shown Cx43 gap junctional enhancement to be necessary and sufficient for optimizing the intercellular Ca2+ signaling that drives sustained pregnancy-enhanced NO production (Yi et al. 2010). A consequence of this fact is that any signaling event that diminishes Cx43 gap junctional communication could potentially inhibit pregnancy-adapted vasodilation. Supporting this assertion, we have established that treatment of P-UAEC monolayers with growth factors and cytokines reduces the agonist-induced Ca2+ bursting otherwise required for pregnancy-adapted vasodilation [Figure 1] (Yi et al. 2011; Boeldt et al. 2015; Ampey et al. 2019).
Figure 1. VEGF pretreatment of an endothelial cell monolayer inhibits the ATP-induced Ca2+ burst response.
Pooled passage 4 P-UAEC grown in 35-mm glass-bottom microwell dishes to 95-100% confluence were then loaded with Fura-2 AM, a free Ca2+ dye. Cells were then incubated with 100 μM ATP and the data recorded for 80-100 cells simultaneously for 30 min. After the initial ATP treatment, the dish was washed and allowed to sit for 30 min. Then, VEGF (or another growth factor or cytokine) was added and recorded for 30 min before a subsequent treatment of ATP was added for an additional 30 min. Ca2+ bursts were then counted for the “before” and “after” VEGF treatments. Finally, before and after counts of cell burst numbers were compared (Boeldt et al. 2015). (A) The tracing from a single cell stimulated with AP before (left) or after (right) treatment with VEGF is shown. Note the narrowing of the initial peak and the fewer subsequent peaks of Ca2+ following VEGF treatment, especially in the period 5 to 15 minutes. These findings are consistent with (B), where intact human umbilical vein tissue is loaded with Fura-2 and DAF-2 (to measure free Ca2+ and NO, respectively) and treated with ATP before and after VEGF treatment (Yi et al. 2010). This data shows overall endothelium response, i.e. the mean level of Ca2+ elevated above the baseline and NO production. Before VEGF exposure, Ca2+ and NO responses are robust. Following VEGF treatment, the mean level of free Ca2+ falls more quickly, and NO production is suppressed.
Recently, we have shown more specifically that Ca2+ bursting is attenuated in P-UAEC via Src- and ERK-mediated phosphorylations of Cx43 in gap junctional plaques (Boeldt et al. 2015, Ampey et al. 2019). Since these same phosphorylation events occur in wound healing, it is worthwhile to review briefly the local environment of an acute wound site. When tissue is wounded, the immediate elevation of growth factors and cytokines results in inhibitory phosphorylations of Cx43 (reviewed in Barrientos et al. 2008; Widgerow 2012). The inhibition of Cx43 function reduces cell-to-cell connectivity, which allows the removal of damaged tissue and the proliferation and migration of new cells into the wounded area. As this process completes, the local concentrations of growth factors and cytokines return to basal levels and Cx43 function returns to normal (Coutinho et al. 2003). In a chronically wounded state, however, the wound site may continue to exhibit elevated concentrations of cytokines, including TNFα, IL-1, and IL-6, and growth factors such as VEGF, bFGF and EGF (reviewed in Barrientos et al. 2008), resulting in sustained inhibition of Cx43 gap junctional assembly and channel function (Laird 2005; Moreno 2005). Indeed, the parallels between wound healing and the suppression of vascular adaptation to pregnancy have led us to ask if the poor vasodilation observed in PE is in fact due to the same signaling pathways observed in chronic wounding (reviewed in Bird et al. 2013). Could some subclasses of PE essentially be the body mistakenly reacting to pregnancy as though it were a non-healing wound?
EGFR-mediated endothelial dysfunction in cultured uterine artery cells
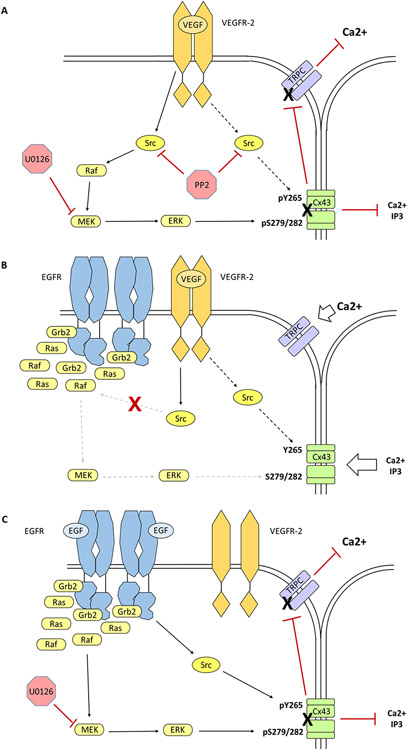
We have recently confirmed that one such pregnancy-associated and wounding site growth factor, VEGF (acting through VEGFR2), induces inhibitory phosphorylations of Cx43 in P-UAEC via Src and ERK (Boeldt et al. 2015). EGF has also been shown to inhibit gap junctional communication (GJC) in several cell types (Madhukar et al. 1989; Lau et al. 1992; Oh et al. 1993); however, EGF is not able to inhibit Ca2+ bursting in P-UAEC as VEGF does, suggesting that the low endogenous receptor number (~7000 EGFR/cell) is insufficient to couple to the signaling pathways that mediate inhibitory Cx43 phosphorylations (Clemente et al. 2020). To determine if the EGF/EGFR system could substitute for the VEGF/VEGFR2 system, we overexpressed EGFR in P-UAEC via adenoviral transduction. Although these P-UAEC now exhibited constitutive EGFR autophosphorylation, this high basal activity was not sufficient to suppress Ca2+ bursting. However, adding EGF treatment to these modified cells induced phosphorylations of ERK1/2 and Cx43, and inhibited Ca2+ bursting to the same degree as VEGF. Interestingly, VEGF treatment no longer inhibited Ca2+ bursting in P-UAEC with added EGFR, as if EGFR had 'hijacked' the molecular machinery formerly utilized by VEGFR2 [Figure 2]. We tentatively proposed that overexpression of EGFR resulted in receptor localization in membrane regions where it was normally absent and so could now recruit pools of key effector molecules (e.g. adapter proteins, intermediate kinases, etc.) away from VEGFR2, and allowing EGFR to mediate Cx43 phosphorylation instead. If this is indeed the case, it follows that the low endogenous EGFR expression in P-UAEC in vivo may be necessary for VEGFR-2 to retain control of ERK-mediated regulation of GJC, a committing step to initiating angiogenesis (Clemente et al. 2020).
Figure 2. Overexpression of EGFR in P-UAEC suppresses the ability of VEGFR2 to activate Cx43-associated ERK1/2.
(A) VEGF administration (10 ng/mL) triggers the formation of activated VEGFR-2 homodimers, which initiates Src-mediated phosphorylation of Cx43 Y265 and Src-dependent ERK-mediated phosphorylation of Cx43 S279/282, which results in gap junctional gate closure and loss of periodic TRP channel-mediated Ca2+ entry needed for pregnancy-adapted vasodilation. While the exact nature of communication between Cx43 opening and TRPC channel activation remains unclear, pharmacological inhibition of either Src (PP2, 10 μM) or MEK (U0126, 10 μM) protects the sustained Ca2+ burst response from VEGF-induced downregulation. (B) Overexpression of EGFR appears to have significant effects on the local environment at the plasma membrane, as evidenced by the fact that VEGF was not able to inhibit ATP-stimulated Ca2+ bursting in the presence of concentrated EGFR. We proposed that the overexpression of EGFR in P-UAEC localizes the receptor in membrane regions where it is normally absent, thereby conferring access to effector molecules that it would otherwise be denied. The constitutively autophosphorylated C-terminal tail of EGFR serves as a continuous source of active phosphotyrosine docking sites, thereby acting as a sink for effector molecules such as SOS, Grb2, the Ras-GTPase, Raf-1, Shc, Cbl and PLCγ. This effectively reduces their availability for other receptors such as VEGFR2. Of note, EGFR does not prevent VEGF-activated Src phosphorylation of Cx43 Y265, which is known to initiate gap junction disassembly but is apparently not associated with acute gate closure of the gap junction. (C) Normally, treatment of P-UAEC with EGF has no significant effect on gap junctional communication or Ca2+ bursting, presumably due to the low expression level of EGFR. However, when P-UAEC that overexpress EGFR are treated with EGF (10 ng/mL), Src- and ERK-mediated phosphorylations of Cx43 occur and Ca2+ bursting is inhibited. Unlike VEGFR2-mediated Ca2+ burst inhibition, the activation of MAPK pathway by EGFR does not require Src. Only pretreatment with the MEK inhibitor U0126 protects against EGFR-induced gap junctional closure and impaired Ca2+ bursting.
The observation that EGFR could induce PE-like endothelial cell dysfunction in our experimental model led us to ask if this mechanism might actually be active in the uterine arteries of women with PE. For our in vitro model of EGFR-mediated dysfunction to occur in vivo, the uterine arteries would need to overexpress EGFR; however, excess EGFR (and perhaps its ligands) might not need to be synthesized by the arterial endothelium. It could instead come from the richest source of EGFR in the mother's body - the placenta - through the shedding of EGFR rich exosomes and other extracellular vesicles.
Could placenta-derived EGFR suppress pregnancy-adapted vasodilation in PE?
Exosomes are extracellular vesicles that originate in the endocytic pathway, characterized by a cup-shaped appearance and a diameter of 40 to 160 nm (reviewed in Kalluri & LeBleu 2020). They are secreted by many tissue types and possess common identifying markers (e.g. CD63). Exosomes of placental origin are uniquely identified by the presence of placental alkaline phosphatase (PLAP), an enzyme isoform produced exclusively by the placenta (Sabapatha et al. 2006). Placental (PLAP+) and non-placental exosomes can be detected in maternal plasma at six weeks of pregnancy and increase in concentration throughout the first trimester (Salomon et al. 2014; Sarker et al. 2014). In healthy pregnant women, plasma concentration of exosomes in the first trimester (N = 20, 6-12 weeks) was found to be 50X higher than measured in non-pregnant women (N = 9), and the level of PLAP+ exosomes increased markedly in each subsequent trimester (Salomon et al. 2014). In preeclamptic pregnancies, Pillay et al. (2016) found that the plasma concentration of PLAP+ exosomes increased in the third trimester only in early onset PE and decreased in plasma from late onset PE, suggesting that the shedding of PLAP+ exosomes may be dependent on PE type. Furthermore, extracellular vesicles (EVs) from healthy placentas and diseased placentas differ in content. When peripheral blood mononuclear cells were exposed to microparticles from the plasma membranes of hypoxic trophoblasts, they experienced a faster and more robust inflammatory response than when the cells were exposed to microparticles from normal trophoblasts (Lee et al. 2012). Similarly, microparticles from the peripheral venous blood of healthy pregnant women protected against apoptosis and promoted migration and tube formation in early-stage trophoblasts. In contrast, these processes were inhibited when the cells were incubated with microparticles from women with gestational hypertension or PE (Shomer et al. 2013). Another study found that the content, quantity, and bioactivity of CTB-derived exosomes obtained from conditioned media correlated inversely with oxygen tension. Exosomes from the most hypoxic environment (1% O2) stimulated the greatest invasion and proliferation of EVTB, suggesting that exosome-induced EVTB migration is a normal adaptive response to placental hypoxia. The authors concluded that CTBs regulate EVTB phenotype by altering the protein content and secretion rate of exosomes in response to oxygen tension (Salomon et al. 2013). In this context, Whigham et al. (2019) proposed using the content of placenta-derived EVs in the maternal circulation as a tool for diagnosing PE and other diseases of pregnancy, and designing appropriate therapies.
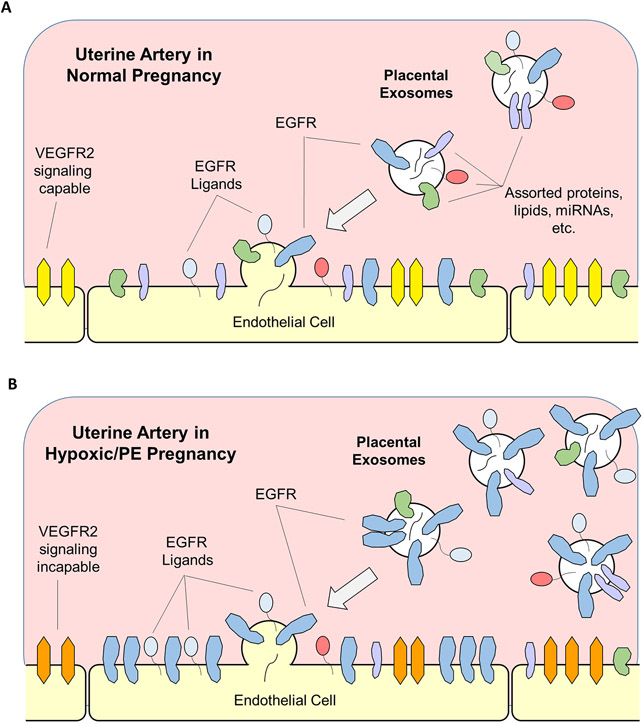
Placental trophoblasts are known to express increasing amounts of EGFR mRNA and protein over the course of pregnancy (Chen et al. 1988; Magid et al. 1985), and we have identified shed PLAP+/EGFR+ extracellular vesicles in first trimester plasma samples using nanoscale high resolution flow cytometry (Clemente et al. 2020). These observations led us to hypothesize that some of these placenta-derived EGFR+ exosomes and/or microparticles could be taken up by the arterial endothelium, thereby transferring EGFR from the placenta to the uterine and systemic vasculature. While exosomal transfer of RNAs, proteins, etc. appears to be a normal part of pregnancy, excessive transfer of EGFR, its ligands, and associated signaling molecules in endothelial cells could negatively impact maternal vasodilation and perhaps drive some subtypes of PE [Figure 3].
Figure 3. Putative mechanism for EGFR-mediated endothelial cell dysfunction in PE.
(A) Exosomes and microparticles containing a variety of proteins, micro RNAs, etc. are released from the placenta into the uterine and systemic vasculature, some of which may fuse with arterial endothelium. This exosomal fusion is likely to be benign or beneficial. As delivery of exogenous EGFR (and potentially ligands, EGFL7, etc.) increases with time, there may be a limit placed on the ability of VEGFR (yellow) to control endothelial function and particularly late term angiogenesis. (B) In PE pregnancy, where exosomal delivery may further increase, excessive delivery of EGFR and related signaling molecules to the endothelial plasma membrane now overwhelms the VEGFR2 (orange) signaling system completely, rendering the receptor incapable of endothelial cell control. The hypoxia and/or inflammation associated with PE is likely to alter not only EGFR concentration, but signaling adaptors, kinase subtypes, and miRNA co-delivered in the same PE placenta-derived vesicles, reflecting the placenta response to physiologic distress.
Although this mechanism has yet to be demonstrated in PE, cellular pathology due to transfer of EGFR+ exosomes has been observed in cancer. Gastric cancer cells have been shown to secrete EGFR+ exosomes that fuse with the plasma membranes of liver stromal cells. The exogenous EGFR facilitates cancer proliferation by providing an otherwise absent binding site for the c-MET receptor on metastatic cancer cells (Zhang et al. 2017). Similarly, exosomes secreted by cultured hypoxic prostate cancer cells have been reported to induce prostate stromal cells to exhibit a cancer-associated fibroblast phenotype and become more invasive (Ramteke et al. 2015). More recently, transfer of exosomal wild-type EGFR to cancer cells that express activating EGFR mutations was found to induce resistance to the tyrosine kinase inhibitor osimertinib (Wu et al. 2021). It should also be noted that although we are the first to specifically propose EGFR itself from placental vesicles as a driver of endothelial cell dysfunction in PE, negative effects of placental vesicles on vasodilation were first reported over two decades ago. When STB microvillous membrane vesicles were perfused through preconstricted subcutaneous arteries obtained from fat biopsies, the arteries displayed a reduced relaxation response to acetylcholine treatment (Cockell et al. 1997). Additionally, overnight incubation with microparticles from preeclamptic serum caused abolishment of the bradykinin-mediated relaxation otherwise observed in the presence of healthy pregnant microparticles (van Wijk et al. 2002).
When considering the plausibility of placental EGFR-mediated endothelial dysfunction as a factor in PE, it is sensible to ask why no one has reported observing this mechanism before. However, two key features of the mechanism explain why it might have eluded detection. Firstly, since the putative influx of EGFR would originate in the placenta, transcriptome analysis of endothelial cells from PE patients would not detect it. Secondly, cultured endothelial cells (e.g. HUVEC) from PE patients would quickly lose any exogenous EGFR due to cell division and protein turnover with passage in ‘normal’ serum conditions. Therefore, only the direct quantification of PLAP and EGFR proteins in freshly isolated endothelium from PE patients could reveal the existence of such a mechanism. Leavey et al. (2015) used the term “canonical” to name the early onset subtype of PE characterized by upregulation of known PE markers such as sFlt1 and sENG, which are not significantly elevated in the other subtypes. The authors described this well-defined cluster as a disorder in which the placenta appears as “an injured tissue in a hypoxic environment, secreting increased amounts of placental products into the maternal compartment.” If placental hypoxia does indeed stimulate greater EGFR expression and activity that results in increased sFlt1 levels (Hastie et al. 2019), then our proposed mechanism of EGFR-mediated endothelial dysfunction is most likely to be detected in cases of early onset PE that exhibit elevated sFlt1.
Conclusions
The complex spaciotemporal regulation of expression of EGFR and its ligands in reproductive tissues over the course of pregnancy suggests distinct developmental roles for the receptor. EGFR is critical for both fertility and fetal development. In the placenta, it promotes trophoblast proliferation and protects trophoblasts from apoptosis. Insufficient EGFR activity is associated with pathologies of pregnancy such as IUGR and PE, and excessive EGFR activity is known to produce aberrant placental development in at least one experimental model, but the effects of EGFR overexpression in the endothelium remain unclear. The findings outlined above suggest that it could indeed impair normal angiogenesis, allow EGF to impair otherwise pregnancy-adapted vasodilation of the maternal vasculature, and provide novel transactivation pathways for other endocrine factors.
Based on the steadily increasing rate of placental (PLAP+) exosome and microparticle secretion into the maternal vasculature with each successive trimester, and our recent observation of PLAP+/EGFR+ extracellular vesicles (EVs) in maternal plasma, it appears likely that some level of EGFR transfer from the placenta to the vascular endothelium is a normal part of pregnancy. Under ordinary circumstances, this is likely benign, or even beneficial, perhaps increasing placental EGFR transfer with time to increasingly limit VEGFR2 function and slow angiogenesis in the second half of pregnancy. EVs from healthy placentas have even been shown to induce positive adaptive responses when administered to endothelial cells in hypoxic conditions, appearing to attempt restoration of healthy cellular oxygenation. Given exosomal effects appear to be a double-edged sword, we should also acknowledge there may also be dependence on other cargo in the exosomes (e.g. signaling adaptors, kinases, miRNA) that are altered in the hypoxic and/or PE placenta. It is also important to note this proposed mechanism is not unique to pregnancy. Our recent findings that EGFR overexpression in cultured P-UAEC remaps signaling pathways away from VEGFR2 and artificially suppresses the Cx43-dependent Ca2+ signaling required for sustained vasodilation are similar to a mechanism reported in vivo in cancer cells, whereby EVs secreted by tumors promote proliferation and metastasis by incorporating into healthy cells and hijacking normal processes using newly delivered EGFR. We speculate that increased vesicle shedding from the placenta over time in response to hypoxia above that of normal pregnancy may mediate symptoms of hypertensive PE by transferring too much EGFR protein (and possibly EGFR ligands and signaling complexes) to the endothelium. This mechanism is also consistent with the observation that the only truly effective treatment for PE to date is removal of the placenta.
Acknowledgments
We would like to thank Paul Bertics (University of Wisconsin - Madison) and Terry Morgan (Oregon Health & Science University) for helpful discussions in formulating these ideas.
Funding
Early studies leading to this review were performed under NIH awards R25 GM083252 and T32HD041921 and Unity Point Meriter Foundation Grant 2020-701. Preparation of this manuscript was performed under T32 HD101384.
Footnotes
Declaration of Competing Interests
There are no conflicts of interest to disclose. Dr Ian Bird is a Senior Editor on the Journal of Endocrinology & Journal of Molecular Endocrinology join editorial board. Dr Ian Bird was not involved in the review or editorial process for this paper, on which he is listed as an author.
References
- Abeydeera LR, Wang WH, Cantley TC, Rieke A, Prather RS & Day BN 1998. Presence of epidermal growth factor during in vitro maturation of pig oocytes and embryo culture can modulate blastocyst development after in vitro fertilization. Mol Reprod Dev 51 395–401. [DOI] [PubMed] [Google Scholar]
- Ampey AC, Boeldt DS, Clemente L, Grummer MA, Yi F, Magness RR & Bird IM 2019. TNF-alpha inhibits pregnancy-adapted Ca. Mol Cell Endocrinol 488 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argast GM, Campbell JS, Brooling JT & Fausto N 2004. Epidermal growth factor receptor transactivation mediates tumor necrosis factor-induced hepatocyte replication. J Biol Chem 279 34530–34536. [DOI] [PubMed] [Google Scholar]
- Armant DR, Kilburn BA, Petkova A, Edwin SS, Duniec-Dmuchowski ZM, Edwards HJ, Romero R & Leach RE 2006. Human trophoblast survival at low oxygen concentrations requires metalloproteinase-mediated shedding of heparin-binding EGF-like growth factor. Development 133 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armant DR, Fritz R, Kilburn BA, Kim YM, Nien JK, Maihle NJ, Romero R & Leach RE 2015. Reduced expression of the epidermal growth factor signaling system in preeclampsia. Placenta 36 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos S, Stojadinovic O, Golinko MS, Brem H & Tomic-Canic M 2008. Growth factors and cytokines in wound healing. Wound Repair Regen 16 585–601. [DOI] [PubMed] [Google Scholar]
- Basu A, Raghunath M, Bishayee S & Das M 1989. Inhibition of tyrosine kinase activity of the epidermal growth factor (EGF) receptor by a truncated receptor form that binds to EGF: role for interreceptor interaction in kinase regulation. Mol Cell Biol 9 671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bełtowski J & Lowicka E 2009. EGF receptor as a drug target in arterial hypertension. Mini Rev Med Chem 9 526–538. [DOI] [PubMed] [Google Scholar]
- Bird IM, Boeldt DS, Krupp J, Grummer MA, Yi FX & Magness RR 2013. Pregnancy, programming and preeclampsia: gap junctions at the nexus of pregnancy-induced adaptation of endothelial function and endothelial adaptive failure in PE. Curr Vasc Pharmacol 11 712–729. [DOI] [PubMed] [Google Scholar]
- Bird IM, Sullivan JA, Di T, Cale JM, Zhang L, Zheng J & Magness RR 2000. Pregnancy-dependent changes in cell signaling underlie changes in differential control of vasodilator production in uterine artery endothelial cells. Endocrinology 141 1107–1117. [DOI] [PubMed] [Google Scholar]
- Birdsall MA, Hopkisson JF, Grant KE, Barlow DH & Mardon HJ 1996. Expression of heparin-binding epidermal growth factor messenger RNA in the human endometrium. Mol Hum Reprod 2 31–34. [DOI] [PubMed] [Google Scholar]
- Boeldt DS, Grummer MA, Yi F, Magness RR & Bird IM 2015. Phosphorylation of Ser-279/282 and Tyr-265 positions on Cx43 as possible mediators of VEGF-165 inhibition of pregnancy-adapted Ca2+ burst function in ovine uterine artery endothelial cells. Mol Cell Endocrinol 412 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke MA, O'Toole EA & Kelsell DP 2014. Exoming into rare skin disease: EGFR deficiency. J Invest Dermatol 134 2486–2488. [DOI] [PubMed] [Google Scholar]
- Charkiewicz K, Jasinska E, Goscik J, Koc-Zorawska E, Zorawski M, Kuc P, Raba G, Kluz T, Kalinka J, Sakowicz A, et al. 2018. Angiogenic factor screening in women with mild preeclampsia - New and significant proteins in plasma. Cytokine 106 125–130. [DOI] [PubMed] [Google Scholar]
- Chen CF, Kurachi H, Fujita Y, Terakawa N, Miyake A & Tanizawa O 1988. Changes in epidermal growth factor receptor and its messenger ribonucleic acid levels in human placenta and isolated trophoblast cells during pregnancy. J Clin Endocrinol Metab 67 1171–1177. [DOI] [PubMed] [Google Scholar]
- Chen WN, Woodbury RL, Kathmann LE, Opresko LK, Zangar RC, Wiley HS & Thrall BD 2004. Induced autocrine signaling through the epidermal growth factor receptor contributes to the response of mammary epithelial cells to tumor necrosis factor alpha. J Biol Chem 279 18488–18496. [DOI] [PubMed] [Google Scholar]
- Chenthuran T, Galhenagey GH, Jayasekara RW & Dissanayake VH 2014. Polymorphism in the epidermal growth factor gene is associated with pre-eclampsia and low birthweight. J Obstet Gynaecol Res 40 1235–1242. [DOI] [PubMed] [Google Scholar]
- Clemente L, Boeldt DS, Grummer MA, Morita M, Morgan TK, Wiepz GJ, Bertics PJ & Bird IM 2020. Adenoviral transduction of EGFR into pregnancy-adapted uterine artery endothelial cells remaps growth factor induction of endothelial dysfunction. Mol Cell Endocrinol 499 110590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockell AP, Learmont JG, Smárason AK, Redman CW, Sargent IL & Poston L 1997. Human placental syncytiotrophoblast microvillous membranes impair maternal vascular endothelial function. Br J Obstet Gynaecol 104 235–240. [DOI] [PubMed] [Google Scholar]
- Coutinho P, Qiu C, Frank S, Tamber K & Becker D 2003. Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell Biol Int 27 525–541. [DOI] [PubMed] [Google Scholar]
- Cui L, Shu C, Liu Z, Tong W, Cui M, Wei C, Tang JJ, Liu X, Hai H, Jiang J, et al. 2018a. Serum protein marker panel for predicting preeclampsia. Pregnancy Hypertens 14 279–285. [DOI] [PubMed] [Google Scholar]
- Cui L, Shu C, Liu Z, Tong W, Cui M, Wei C, Tang JJ, Liu X, Hu J, Jiang J, et al. 2018b. The expression of serum sEGFR, sFlt-1, sEndoglin and PLGF in preeclampsia. Pregnancy Hypertens 13 127–132. [DOI] [PubMed] [Google Scholar]
- Dackor J, Caron KM & Threadgill DW 2009a. Placental and embryonic growth restriction in mice with reduced function epidermal growth factor receptor alleles. Genetics 183 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackor J, Li M & Threadgill DW 2009b. Placental overgrowth and fertility defects in mice with a hypermorphic allele of epidermal growth factor receptor. Mamm Genome 20 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadi TD, Li MW & Lloyd KC 2007. EGF and TGF-alpha supplementation enhances development of cloned mouse embryos. Cloning Stem Cells 9 315–326. [DOI] [PubMed] [Google Scholar]
- Daub H, Weiss FU, Wallasch C & Ullrich A 1996. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 379 557–560. [DOI] [PubMed] [Google Scholar]
- Ferrara N & Davis-Smyth T 1997. The biology of vascular endothelial growth factor. Endocr Rev 18 4–25. [DOI] [PubMed] [Google Scholar]
- Filla MS, Zhang CX & Kaul KL 1993. A potential transforming growth factor alpha/epidermal growth factor receptor autocrine circuit in placental cytotrophoblasts. Cell Growth Differ 4 387–393. [PubMed] [Google Scholar]
- Fitch MJ, Campagnolo L, Kuhnert F & Stuhlmann H 2004. Egfl7, a novel epidermal growth factor-domain gene expressed in endothelial cells. Dev Dyn 230 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flickinger TW, Maihle NJ & Kung HJ 1992. An alternatively processed mRNA from the avian c-ErbB gene encodes a soluble, truncated form of the receptor that can block ligand-dependent transformation. Mol Cell Biol 12 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian J & Watts S 1999. Epidermal growth factor: a potent vasoconstrictor in experimental hypertension. American Journal of Physiology-Heart and Circulatory Physiology 276 H976–H983. [DOI] [PubMed] [Google Scholar]
- Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK, et al. 2011. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J 25 1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lloret MI, Yui J, Winkler-Lowen B & Guilbert LJ 1996. Epidermal growth factor inhibits cytokine-induced apoptosis of primary human trophoblasts. J Cell Physiol 167 324–332. [DOI] [PubMed] [Google Scholar]
- Gifford SM, Cale JM, Tsoi S, Magness RR & Bird IM 2003. Pregnancy-specific changes in uterine artery endothelial cell signaling in vivo are both programmed and retained in primary culture. Endocrinology 144 3639–3650. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Dsupin BA & Irwin JC 1992. Steroid and peptide regulation of insulin-like growth factor-binding proteins secreted by human endometrial stromal cells is dependent on stromal differentiation. J Clin Endocrinol Metab 75 1235–1241. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Choi JT, Bilski JJ, Weigl RM, Kirsch JD, Kraft KC, Reynolds LP & Redmer DA 2003. Effects of epidermal growth factor on early embryonic development after in vitro fertilization of oocytes collected from ewes treated with follicle stimulating hormone. Theriogenology 59 1449–1457. [DOI] [PubMed] [Google Scholar]
- Guler A, Poulin N, Mermillod P, Terqui M & Cognié Y 2000. Effect of growth factors, EGF and IGF-I, and estradiol on in vitro maturation of sheep oocytes. Theriogenology 54 209–218. [DOI] [PubMed] [Google Scholar]
- Günther N, Betzel C & Weber W 1990. The secreted form of the epidermal growth factor receptor. Characterization and crystallization of the receptor-ligand complex. J Biol Chem 265 22082–22085. [PubMed] [Google Scholar]
- Harendra GG, Jayasekara RW & Dissanayake VH 2012. Haplotypes of heparin-binding epidermal-growth-factor-like growth factor gene are associated with pre-eclampsia. J Obstet Gynaecol Res 38 239–246. [DOI] [PubMed] [Google Scholar]
- Hastie R, Brownfoot FC, Pritchard N, Hannan NJ, Cannon P, Nguyen V, Palmer K, Beard S, Tong S & Kaitu'u-Lino TJ 2019. EGFR (Epidermal Growth Factor Receptor) Signaling and the Mitochondria Regulate sFlt-1 (Soluble FMS-Like Tyrosine Kinase-1) Secretion. Hypertension 73 659–670. [DOI] [PubMed] [Google Scholar]
- Huang JR, Tseng L, Bischof P & Jänne OA 1987. Regulation of prolactin production by progestin, estrogen, and relaxin in human endometrial stromal cells. Endocrinology 121 2011–2017. [DOI] [PubMed] [Google Scholar]
- Humphrey RG, Sonnenberg-Hirche C, Smith SD, Hu C, Barton A, Sadovsky Y & Nelson DM 2008. Epidermal growth factor abrogates hypoxia-induced apoptosis in cultured human trophoblasts through phosphorylation of BAD Serine 112. Endocrinology 149 2131–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B 2011. Trophoblast differentiation, fetal growth restriction and preeclampsia. Pregnancy Hypertens 1 79–86. [DOI] [PubMed] [Google Scholar]
- Ilekis JV, Stark BC & Scoccia B 1995. Possible role of variant RNA transcripts in the regulation of epidermal growth factor receptor expression in human placenta. Mol Reprod Dev 41 149–156. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Joh T, Tanida S, Sasaki M, Kataoka H, Itoh K, Oshima T, Ogasawara N, Togawa S, Wada T, et al. 2005. IL-8 promotes cell proliferation and migration through metalloproteinase-cleavage proHB-EGF in human colon carcinoma cells. Cytokine 29 275–282. [DOI] [PubMed] [Google Scholar]
- Jessmon P, Leach RE & Armant DR 2009. Diverse functions of HBEGF during pregnancy. Mol Reprod Dev 76 1116–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Ma X, Tong W, Doyran B, Sun Z, Wang L, Zhang X, Zhou Y, Badar F, Chandra A, et al. 2016. EGFR signaling is critical for maintaining the superficial layer of articular cartilage and preventing osteoarthritis initiation. Proc Natl Acad Sci U S A 113 14360–14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokhi PP, King A & Loke YW 1994. Reciprocal expression of epidermal growth factor receptor (EGF-R) and c-erbB2 by non-invasive and invasive human trophoblast populations. Cytokine 6 433–442. [DOI] [PubMed] [Google Scholar]
- Jones S & Rappoport JZ 2014. Interdependent epidermal growth factor receptor signalling and trafficking. Int J Biochem Cell Biol 51 23–28. [DOI] [PubMed] [Google Scholar]
- Jura N, Endres NF, Engel K, Deindl S, Das R, Lamers MH, Wemmer DE, Zhang X & Kuriyan J 2009. Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment. Cell 137 1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagiyama S, Eguchi S, Frank G, Inagami T, Zhang Y & Phillips M 2002. Angiotensin II-induced cardiac hypertrophy and hypertension are attenuated by epidermal growth factor receptor antisense. Circulation 106 909–912. [DOI] [PubMed] [Google Scholar]
- Kagiyama S, Qian K, Kagiyama T & Phillips M 2003. Antisense to epidermal growth factor receptor prevents the development of left ventricular hypertrophy. Hypertension 41 824–829. [DOI] [PubMed] [Google Scholar]
- Kalluri R & LeBleu VS 2020. The biology, function, and biomedical applications of exosomes. Science 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly NJ, Radder JE, Baust JJ, Burton CL, Lai YC, Potoka KC, Agostini BA, Wood JP, Bachman TN, Vanderpool RR, et al. 2017. Mouse Genome-Wide Association Study of Preclinical Group II Pulmonary Hypertension Identifies Epidermal Growth Factor Receptor. Am J Respir Cell Mol Biol 56 488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee C, Park H, Kim H, So H, Lee K, Lee H, Roh H, Choi W, Park T, et al. 2006. Epidermal growth factor induces vasoconstriction through the phosphatidylinositol 3-kinase-mediated mitogen-activated protein kinase pathway in hypertensive rats. Journal of Pharmacological Sciences 101 135–143. [DOI] [PubMed] [Google Scholar]
- Kosovic I, Prusac IK, Berkovic A, Marusic J, Mimica M & Tomas SZ 2017. Expression of EGF, EGFR, and proliferation in placentas from pregnancies complicated with preeclampsia. Hypertens Pregnancy 36 16–20. [DOI] [PubMed] [Google Scholar]
- Kupsamy K, Moodley J & Naicker T 2019. Hepatocyte growth factor and epidermal growth factor in HIV infected women with preeclampsia. Eur J Obstet Gynecol Reprod Biol 240 9–14. [DOI] [PubMed] [Google Scholar]
- Kyriakakis E, Cavallari M, Pfaff D, Fabbro D, Mestan J, Philippova M, De Libero G, Erne P & Resink TJ 2011. IL-8-mediated angiogenic responses of endothelial cells to lipid antigen activation of iNKT cells depend on EGFR transactivation. J Leukoc Biol 90 929–939. [DOI] [PubMed] [Google Scholar]
- Ladines-Llave CA, Maruo T, Manalo AS & Mochizuki M 1991. Cytologic localization of epidermal growth factor and its receptor in developing human placenta varies over the course of pregnancy. Am J Obstet Gynecol 165 1377–1382. [DOI] [PubMed] [Google Scholar]
- Laird DW 2005. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim Biophys Acta 1711 172–182. [DOI] [PubMed] [Google Scholar]
- LaMarca BD, Ryan MJ, Gilbert JS, Murphy SR & Granger JP 2007. Inflammatory cytokines in the pathophysiology of hypertension during preeclampsia. Curr Hypertens Rep 9 480–485. [DOI] [PubMed] [Google Scholar]
- Large MJ, Wetendorf M, Lanz RB, Hartig SM, Creighton CJ, Mancini MA, Kovanci E, Lee KF, Threadgill DW, Lydon JP, et al. 2014. The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet 10 e1004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau AF, Kanemitsu MY, Kurata WE, Danesh S & Boynton AL 1992. Epidermal growth factor disrupts gap-junctional communication and induces phosphorylation of connexin43 on serine. Mol Biol Cell 3 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SY, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V & Chamley LW 2013. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol 70 412–427. [DOI] [PubMed] [Google Scholar]
- Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R & Armant DR 1999. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab 84 3355–3363. [DOI] [PubMed] [Google Scholar]
- Leavey K, Bainbridge SA & Cox BJ 2015. Large scale aggregate microarray analysis reveals three distinct molecular subclasses of human preeclampsia. PLoS One 10 e0116508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G & Tubby J 2015. Preeclampsia and the risk of cardiovascular disease later in life--A review of the evidence. Midwifery 31 1127–1134. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP & DeMayo FJ 2007. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol 27 5468–5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Romero R, Lee YJ, Park IS, Park CW & Yoon BH 2012. Systemic inflammatory stimulation by microparticles derived from hypoxic trophoblast as a model for inflammatory response in preeclampsia. Am J Obstet Gynecol 207 337.e331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner V, Majack RA & Reidy MA 1990. Basic fibroblast growth factor stimulates endothelial regrowth and proliferation in denuded arteries. J Clin Invest 85 2004–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist P, Grennert L & Marsál K 1999. Epidermal growth factor in maternal urine--a predictor of intrauterine growth restriction? Early Hum Dev 56 143–150. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhang J, Gao H, Yuan T, Kang J, Jin L, Gui S & Zhang Y 2018. Role of EGFL7/EGFR-signaling pathway in migration and invasion of growth hormone-producing pituitary adenomas. Sci China Life Sci 61 893–901. [DOI] [PubMed] [Google Scholar]
- Lonergan P, Carolan C, Van Langendonckt A, Donnay I, Khatir H & Mermillod P 1996. Role of epidermal growth factor in bovine oocyte maturation and preimplantation embryo development in vitro. Biol Reprod 54 1420–1429. [DOI] [PubMed] [Google Scholar]
- Luo BH, Xiong F, Wang JP, Li JH, Zhong M, Liu QL, Luo GQ, Yang XJ, Xiao N, Xie B, et al. 2014. Epidermal growth factor-like domain-containing protein 7 (EGFL7) enhances EGF receptor-AKT signaling, epithelial-mesenchymal transition, and metastasis of gastric cancer cells. PLoS One 9 e99922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysiak JJ, Han VK & Lala PK 1993. Localization of transforming growth factor alpha in the human placenta and decidua: role in trophoblast growth. Biol Reprod 49 885–894. [DOI] [PubMed] [Google Scholar]
- Lysiak JJ, Johnson GR & Lala PK 1995. Localization of amphiregulin in the human placenta and decidua throughout gestation: role in trophoblast growth. Placenta 16 359–366. [DOI] [PubMed] [Google Scholar]
- Madhukar BV, Oh SY, Chang CC, Wade M & Trosko JE 1989. Altered regulation of intercellular communication by epidermal growth factor, transforming growth factor-beta and peptide hormones in normal human keratinocytes. Carcinogenesis 10 13–20. [DOI] [PubMed] [Google Scholar]
- Maduray K, Moodley J & Mackraj I 2020. The impact of circulating exosomes derived from early and late onset preeclamptic pregnancies on inflammatory cytokine secretion by BeWo cells. Eur J Obstet Gynecol Reprod Biol 247 156–162. [DOI] [PubMed] [Google Scholar]
- Magid M, Nanney LB, Stoscheck CM & King LE 1985. Epidermal growth factor binding and receptor distribution in term human placenta. Placenta 6 519–526. [DOI] [PubMed] [Google Scholar]
- Maruo T, Matsuo H, Murata K & Mochizuki M 1992. Gestational age-dependent dual action of epidermal growth factor on human placenta early in gestation. J Clin Endocrinol Metab 75 1362–1367. [DOI] [PubMed] [Google Scholar]
- Massimiani M, Lacko LA, Burke Swanson CS, Salvi S, Argueta LB, Moresi S, Ferrazzani S, Gelber SE, Baergen RN, Toschi N, et al. 2019. Increased circulating levels of Epidermal Growth Factor-like Domain 7 in pregnant women affected by preeclampsia. Transl Res 207 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta VB & Besner GE 2007. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors 25 253–263. [DOI] [PubMed] [Google Scholar]
- Mehta VB, Zhou Y, Radulescu A & Besner GE 2008. HB-EGF stimulates eNOS expression and nitric oxide production and promotes eNOS dependent angiogenesis. Growth Factors 26 301–315. [DOI] [PubMed] [Google Scholar]
- Moll SJ, Jones CJ, Crocker IP, Baker PN & Heazell AE 2007. Epidermal growth factor rescues trophoblast apoptosis induced by reactive oxygen species. Apoptosis 12 1611–1622. [DOI] [PubMed] [Google Scholar]
- Moreno AP 2005. Connexin phosphorylation as a regulatory event linked to channel gating. Biochim Biophys Acta 1711 164–171. [DOI] [PubMed] [Google Scholar]
- Morrish DW, Bhardwaj D, Dabbagh LK, Marusyk H & Siy O 1987. Epidermal growth factor induces differentiation and secretion of human chorionic gonadotropin and placental lactogen in normal human placenta. J Clin Endocrinol Metab 65 1282–1290. [DOI] [PubMed] [Google Scholar]
- Moslehi R, Mills JL, Signore C, Kumar A, Ambroggio X & Dzutsev A 2013. Integrative transcriptome analysis reveals dysregulation of canonical cancer molecular pathways in placenta leading to preeclampsia. Sci Rep 3 2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello M, Carotenuto A, De Feo G, Caponigro F & Salomon D 2006. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 366 2–16. [DOI] [PubMed] [Google Scholar]
- Oh SY, Schmidt SA & Murray AW 1993. Epidermal growth factor inhibits gap junctional communication and stimulates serine-phosphorylation of connexin43 in WB cells by a protein kinase C-independent mechanism. Cell Adhes Commun 1 143–149. [DOI] [PubMed] [Google Scholar]
- Perkins J, St John J & Ahmed A 2002. Modulation of trophoblast cell death by oxygen and EGF. Mol Med 8 847–856. [PMC free article] [PubMed] [Google Scholar]
- Pillay P, Maharaj N, Moodley J & Mackraj I 2016. Placental exosomes and pre-eclampsia: Maternal circulating levels in normal pregnancies and, early and late onset preeclamptic pregnancies. Placenta 46 18–25. [DOI] [PubMed] [Google Scholar]
- Purba ER, Saita EI & Maruyama IN 2017. Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: The "Rotation Model". Cells 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab G, Kover K, Paria BC, Dey SK, Ezzell RM & Klagsbrun M 1996. Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin-binding EGF-like growth factor. Development 122 637–645. [DOI] [PubMed] [Google Scholar]
- Ramani N, Chegini N, Rao CV, Woost PG & Schultz GS 1986. The presence of epidermal growth factor binding sites in the intracellular organelles of term human placenta. J Cell Sci 84 19–40. [DOI] [PubMed] [Google Scholar]
- Ramteke A, Ting H, Agarwal C, Mateen S, Somasagara R, Hussain A, Graner M, Frederick B, Agarwal R & Deep G 2015. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol Carcinog 54 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao CV, Ramani N, Chegini N, Stadig BK, Carman FR, Woost PG, Schultz GS & Cook CL 1985. Topography of human placental receptors for epidermal growth factor. J Biol Chem 260 1705–1710. [PubMed] [Google Scholar]
- Reiter J & Maihle NJ 2003. Characterization and expression of novel 60-kDa and 110-kDa EGFR isoforms in human placenta. Ann N Y Acad Sci 995 39–47. [DOI] [PubMed] [Google Scholar]
- Sabapatha A, Gercel-Taylor C & Taylor DD 2006. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am J Reprod Immunol 56 345–355. [DOI] [PubMed] [Google Scholar]
- Salomon C, Kobayashi M, Ashman K, Sobrevia L, Mitchell MD & Rice GE 2013. Hypoxia-induced changes in the bioactivity of cytotrophoblast-derived exosomes. PLoS One 8 e79636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon C, Torres MJ, Kobayashi M, Scholz-Romero K, Sobrevia L, Dobierzewska A, Illanes SE, Mitchell MD & Rice GE 2014. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLoS One 9 e98667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambhi M, Swaminathan N, Wang H & Rong H 1992. Increased EGF binding and EGFR messenger-RNA expression in rat aorta with chronic administration of pressor angiotension-II. Biochemical Medicine and Metabolic Biology 48 8–18. [DOI] [PubMed] [Google Scholar]
- Sarker S, Scholz-Romero K, Perez A, Illanes SE, Mitchell MD, Rice GE & Salomon C 2014. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med 12 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstatter IU, Trieu K, Zhao M, Rose DM, Terkeltaub RA & Burger M 2003. IL-8-mediated cell migration in endothelial cells depends on cathepsin B activity and transactivation of the epidermal growth factor receptor. J Immunol 171 6714–6722. [DOI] [PubMed] [Google Scholar]
- Shan Y, Eastwood MP, Zhang X, Kim ET, Arkhipov A, Dror RO, Jumper J, Kuriyan J & Shaw DE 2012. Oncogenic mutations counteract intrinsic disorder in the EGFR kinase and promote receptor dimerization. Cell 149 860–870. [DOI] [PubMed] [Google Scholar]
- Shomer E, Katzenell S, Zipori Y, Sammour RN, Isermann B, Brenner B & Aharon A 2013. Microvesicles of women with gestational hypertension and preeclampsia affect human trophoblast fate and endothelial function. Hypertension 62 893–898. [DOI] [PubMed] [Google Scholar]
- Sibilia M & Wagner EF 1995. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science 269 234–238. [DOI] [PubMed] [Google Scholar]
- Stirnweiss J, Valkova C, Ziesché E, Drube S & Liebmann C 2006. Muscarinic M2 receptors mediate transactivation of EGF receptor through Fyn kinase and without matrix metalloproteases. Cell Signal 18 1338–1349. [DOI] [PubMed] [Google Scholar]
- Tanida S, Joh T, Itoh K, Kataoka H, Sasaki M, Ohara H, Nakazawa T, Nomura T, Kinugasa Y, Ohmoto H, et al. 2004. The mechanism of cleavage of EGFR ligands induced by inflammatory cytokines in gastric cancer cells. Gastroenterology 127 559–569. [DOI] [PubMed] [Google Scholar]
- Tanimura K, Nakago S, Murakoshi H, Takekida S, Moriyama T, Matsuo H, Hashimoto K & Maruo T 2004. Changes in the expression and cytological localization of betacellulin and its receptors (ErbB-1 and ErbB-4) in the trophoblasts in human placenta over the course of pregnancy. Eur J Endocrinol 151 93–101. [DOI] [PubMed] [Google Scholar]
- Tannetta D, Collett G, Vatish M, Redman C & Sargent I 2017. Syncytiotrophoblast extracellular vesicles - Circulating biopsies reflecting placental health. Placenta 52 134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K & Harris RC 1995. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269 230–234. [DOI] [PubMed] [Google Scholar]
- Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, et al. 2015. Proteomics. Tissue-based map of the human proteome. Science 347 1260419. [DOI] [PubMed] [Google Scholar]
- Usuba R, Pauty J, Soncin F & Matsunaga YT 2019. EGFL7 regulates sprouting angiogenesis and endothelial integrity in a human blood vessel model. Biomaterials 197 305–316. [DOI] [PubMed] [Google Scholar]
- Vanwijk MJ, Svedas E, Boer K, Nieuwland R, Vanbavel E & Kublickiene KR 2002. Isolated microparticles, but not whole plasma, from women with preeclampsia impair endothelium-dependent relaxation in isolated myometrial arteries from healthy pregnant women. Am J Obstet Gynecol 187 1686–1693. [DOI] [PubMed] [Google Scholar]
- Wang FY, Kang CS, Wang-Gou SY, Huang CH, Feng CY & Li XJ 2017. EGFL7 is an intercellular EGFR signal messenger that plays an oncogenic role in glioma. Cancer Lett 384 9–18. [DOI] [PubMed] [Google Scholar]
- Watson AJ, De Sousa P, Caveney A, Barcroft LC, Natale D, Urquhart J & Westhusin ME 2000. Impact of bovine oocyte maturation media on oocyte transcript levels, blastocyst development, cell number, and apoptosis. Biol Reprod 62 355–364. [DOI] [PubMed] [Google Scholar]
- Wee P & Wang Z 2017. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers (Basel) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Park KW, Day BN & Prather RS 2001. Effect of epidermal growth factor on preimplantation development and its receptor expression in porcine embryos. Mol Reprod Dev 60 457–462. [DOI] [PubMed] [Google Scholar]
- Whigham CA, MacDonald TM, Walker SP, Hannan NJ, Tong S & Kaitu'u-Lino TJ 2019. The untapped potential of placenta-enriched molecules for diagnostic and therapeutic development. Placenta 84 28–31. [DOI] [PubMed] [Google Scholar]
- Whitehead CL, Kaitu'u-Lino TJ, Binder NK, Beard S, De Alwis N, Brownfoot F, Tong S & Hannan NJ 2018. EGFL7 gene expression is regulated by hypoxia in trophoblast and altered in the plasma of patients with early preeclampsia. Pregnancy Hypertens 14 115–120. [DOI] [PubMed] [Google Scholar]
- Widgerow AD 2012. Cellular resolution of inflammation--catabasis. Wound Repair Regen 20 2–7. [DOI] [PubMed] [Google Scholar]
- Wilson KJ, Gilmore JL, Foley J, Lemmon MA & Riese DJ 2009. Functional selectivity of EGF family peptide growth factors: implications for cancer. Pharmacol Ther 122 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Luo M, To KKW, Zhang J, Su C, Zhang H, An S, Wang F, Chen D & Fu L 2021. Intercellular transfer of exosomal wild type EGFR triggers osimertinib resistance in non-small cell lung cancer. Mol Cancer 20 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Yano Y, Kawano K & Matsuzaki K 2015. Oligomerization-function relationship of EGFR on living cells detected by the coiled-coil labeling and FRET microscopy. Biochim Biophys Acta 1848 1359–1366. [DOI] [PubMed] [Google Scholar]
- Yi FX, Boeldt DS, Magness RR & Bird IM 2011. [Ca2+]i signaling vs. eNOS expression as determinants of NO output in uterine artery endothelium: relative roles in pregnancy adaptation and reversal by VEGF165. Am J Physiol Heart Circ Physiol 300 H1182–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi FX, Boeldt DS, Gifford SM, Sullivan JA, Grummer MA, Magness RR & Bird IM 2010. Pregnancy enhances sustained Ca2+ bursts and endothelial nitric oxide synthase activation in ovine uterine artery endothelial cells through increased connexin 43 function. Biol Reprod 82 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying WZ & Sanders PW 2005. Enhanced expression of EGF receptor in a model of salt-sensitive hypertension. Am J Physiol Renal Physiol 289 F314–321. [DOI] [PubMed] [Google Scholar]
- Yoo HJ, Barlow DH & Mardon HJ 1997. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Dev Genet 21 102–108. [DOI] [PubMed] [Google Scholar]
- Zhang H, Deng T, Liu R, Bai M, Zhou L, Wang X, Li S, Yang H, Li J, Ning T, et al. 2017. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun 8 15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Gureasko J, Shen K, Cole PA & Kuriyan J 2006. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 125 1137–1149. [DOI] [PubMed] [Google Scholar]