Abstract
Background:
Causes of death and their trends among Veterans with HIV (VWH) are different than those in the general population with HIV, but this has not been fully described. The objective was to understand the trends in, and risk factors for, all-cause and cause-specific mortality across eras of combination antiretroviral therapy (cART) among VWH.
Setting:
The HIV Atlanta VA Cohort Study (HAVACS) includes all VWH who ever sought care at the Atlanta VA Medical Center.
Methods:
Age-adjusted all-cause and cause-specific mortality rates were calculated annually and compared between pre- (1982–1996), early- (1997–2006), and late-cART (2007–2016) eras. Trends were assessed using Kaplan-Meier curves, cumulative incidence functions, and join-point regression models. Risk factors were identified by Cox proportional hazards models.
Results:
Of the 4,674 VWH in HAVACS, 1,752 died; of whom, 1,399 (79.9%), 301 (17.2%), and 52 (3.0%) were diagnosed with HIV in the pre-, early-, and late-cART eras, respectively. Significant increases were observed in rates of all-cause, AIDS-related, and non-AIDS-related mortality in the pre-cART era, followed by declines in the early- and late-cART eras. All-cause, AIDS-related, and non-AIDS-related mortality rates plummeted by 65%, 81%, and 45%, respectively, from the pre- to late-cART eras. However, VWH continue to die at higher rates due to AIDS-related infections, non-AIDS-related malignancies, respiratory disease, cardiovascular disease, and renal failure than those in the general population with HIV.
Conclusions:
In older populations with HIV, it is important that providers not only monitor for and treat diseases associated with aging, but also intervene and address lifestyle risk factors.
Keywords: HIV, AIDS, mortality, antiretroviral therapy, Veterans, United States
Introduction
Of the more than 9 million U.S. Veterans enrolled in the Department of Veterans Affairs (VA), it is estimated that 3.7% have HIV infection, compared to only 0.5% of U.S. adults.1–3 Moreover, cohort studies in Europe and the U.S. estimate that the all-cause mortality rate among people with HIV (PWH) ranges from 6 to 13 per 1,000 person-years (PY), compared to 40 per 1,000 PY in U.S. Veterans with HIV (VWH).4 The burden of HIV and its associated mortality is clearly higher among Veterans, but this has not been fully described in the scientific literature.
Compared to the general population, Veterans are more likely to be male, older, and African-American, and have higher rates of substance abuse, mental illness, unemployment, and homelessness.4–6 These individual-level barriers are associated with decreased retention in care and adherence to combination antiretroviral treatment (cART). Despite this, the proportions of VWH diagnosed with HIV, retained in care, prescribed cART, and virally suppressed are significantly greater than those in the general population.6–7 For example, it is estimated that only 4.9% of Veterans have undiagnosed HIV compared to 18.1% of the general population.6 This is a testament to the VA as an integrated health care system that reduces institutional-level barriers by providing care with little to no out-of-pocket costs and curtailing interruptions in care due to life changes.5–6,8 Other institutional-level policies unique to Veterans during and after their military service include required HIV screening every two years in the Department of Defense, enacted in 1986, and routine HIV testing requiring only verbal consent in the VA, enacted in 2009, respectively.5–6,9 However, a critical component of the armamentarium against HIV was the development and evolution of ART, particularly the advent of cART in 1996, and the decades of treatment advocacy that have helped PWH adhere to ART.10
In the general population, it is estimated that the all-cause mortality rate for PWH decreased from 233 per 1,000 PY in the pre-cART era to 6 per 1,000 PY in the post-cART era.11–12 In the post-cART era, PWH on ART who have maintained a CD4 count ≥500 cells/mL have been shown to have an all-cause mortality rate approaching that of the general population without HIV.12 With respect to cause-specific mortality, it is estimated that the rates due to AIDS and causes unrelated to AIDS (non-AIDS) in the post-cART era are 5.1 per 1,000 PY and 0.9 per 1,000 PY, respectively.13–14 In VWH specifically, it is estimated that the all-cause mortality rate decreased from 25 deaths per 100 patients in the pre-cART era to 3 deaths per 100 patients in the post-cART era, or about 40 per 1,000 PY.4,15 No data have been published on cause-specific mortality among VWH.
The objective of the current work is to fill in this knowledge gap for VWH – to understand the trends in, and risk factors for, all-cause and cause-specific mortality. Improved knowledge may better enable VA providers to focus diagnostic and therapeutic efforts, and improve overall care in this distinct, understudied population. Moreover, as the general population with HIV continues to age, the results from this work may provide further insight into the psychosocial challenges and social inequities that older populations with HIV continue to experience.
Methods
Study population
The HIV Atlanta VA Cohort Study (HAVACS) was initiated in 1982 and is an ongoing, open cohort of all VWH who ever sought care at the Atlanta VA Medical Center (AVAMC). A complete profile of the study has been previously described.15 All HAVACS patients with a documented HIV diagnosis between January 1, 1982 and December 31, 2016 were included in the current analysis; the 15th of the month was imputed for missing dates and those without at least a month and year were excluded. Dates and causes of death were collected and cross-referenced from a compendium of available sources, including vital statistic agencies and hospitals or through physician report and active follow-up. The Coding Causes of Death in HIV Project protocol was adapted to classify causes of death.16 If diagnostic codes were available, causes of death were classified by a clinician and a computer algorithm; if they were not available, two clinicians independently classified each death.17 Deaths were classified as AIDS-related if there was a AIDS defining condition prior to death and/or a CD4 count <100 cells/mL within a year (18 months if off ART) of death, and a diagnosis compatible with AIDS as a cause of death.18 AIDS-related causes were categorized as infection or malignancy; non-AIDS-related causes were categorized as cardiovascular disease, infection, liver-related (including hepatocellular carcinoma), malignancy, renal failure, respiratory disease (including pulmonary infection), sudden death, or violence (including suicide and drug-related). Deaths for which the cause was unknown were included in analyses of all-cause mortality. The HAVACS cohort was approved by Emory University’s Institutional Review Board and the AVAMC Research and Development Committee.
Descriptive analyses
Descriptive analyses were performed to characterize patients who had died during the study period by socio-demographics (ages at HIV and AIDS diagnosis, sex, race/ethnicity, and transmission category) and clinical factors (first [≤6 months after HIV diagnosis], nadir, and last [≤6 months before death] CD4 counts; last HIV viral load [≤6 months before death], ART status, times from HIV diagnosis to ART initiation and AIDS diagnosis, AIDS status, CDC stage, and hepatitis B [HBV, HBsAg test] and C [HCV, HCV antibody test] serostatus). Fisher’s exact tests or chi-square tests for categorical data, and one-way ANOVA for continuous data with Bonferroni correction were performed, as appropriate, to identify factors associated with death among those who had been diagnosed with HIV in the pre-cART (1982–1996) and post-cART (early [1997–2006], late [2007–2016]) eras; post-hoc tests included Tukey’s tests and partitioning the likelihood ratio tests, as appropriate.
Temporal trends
All-cause and cause-specific mortality rates and 95% confidence intervals (CI) were calculated annually and for each cART era, and age-adjusted to the 2000 U.S. standard population. Incidence density ratios (IDR) and 95% CIs were calculated to compare all-cause and cause-specific mortality rates between cART eras. Person-time was computed from date of HIV diagnosis to date of death. Regardless of whether a patient survived from one era to another, all person-time was contributed to the cART era in which they were diagnosed with HIV. Patients were censored at the end of the study period or the date of their last visit if they had not been seen after December 31, 2014. Join-point regression models were fitted to estimate annual percent changes (APC) and 95% CIs during periods of time when statistically significant changes in mortality rates were observed. Because all-cause and cause-specific mortality rates were age-adjusted, results were compared with those found in the general population with HIV, which naturally has a different age distribution.
Inferential analyses
Survival probabilities with respect to all-cause mortality for each cART era were estimated and compared using the Kaplan-Meier approach and log-rank tests, respectively. Stacked cumulative incidence functions with respect to cause-specific mortality for each cART era were estimated to account for competing risks. Adjusted hazard ratios (aHR) were estimated using multivariable Cox proportional hazards models to identify risk factors associated with all-cause and cause-specific mortality; factors of interest included age at HIV diagnosis, race/ethnicity, ever using injection drugs (IDU), ART status, ever receiving an AIDS diagnosis, and last CD4 count and HIV viral load. Hazard ratios adjusted for all subsets of the covariates of interest were compared to the fully adjusted model using the 10% change-in-estimate criterion. Stratified Cox models or Cox models with time-dependent covariates were performed when the proportional hazards assumption was violated. All analyses were performed in SAS 9.4 (SAS Institute, Cary, NC) or Joinpoint Trend Analysis 4.7 (NCI, Bethesda, MD), and probability values <0.05 were considered statistically significant.
Results
Sample characteristics
Of the 4,674 patients in the analytic sample (22–79 years of age at enrollment), 1,752 died; of whom, 1,399 (79.9%), 301 (17.2%), and 52 (3.0%) were diagnosed with HIV in the pre-, early-, and late-cART eras, respectively. Of the patients who died, 46 (2.6%) did not have adequate date of death data. Of the 2,922 patients who were censored, 1,013 (34.7%) were lost-to-follow-up (LTFU) and 1,909 (65.3%) survived past the study period. In the pre-, early-, and late-cART eras, patients contributed 35,687 PY (mean [standard deviation, SD] = 14.2 [9.8] PY), 15,569 PY (mean [SD] = 12.0 [5.1] PY), and 3,817 PY (mean [SD] = 4.9 [2.8] PY), respectively. Socio-demographic and clinical characteristics of those who died were summarized in Table 1. After Bonferroni correction (α = 0.05/15 = 0.003), patients who were diagnosed with HIV in each era and subsequently died were significantly different by all socio-demographic and clinical characteristics assessed (except for last CD4 count, last HIV viral load, and ART status) than those who also died but were diagnosed with HIV in another era. Of note, ART initiation trended upwards in the late-cART era, nearing 90%.
Table 1.
Characteristics of Veterans who died by cART era in which they were diagnosed with HIV.
| Characteristics | HAVACS N=1,752 |
Pre-cART era1 N = 1,399 |
Early-cART era1 N = 301 |
Late-cART era1 N = 52 |
P-value3 |
|---|---|---|---|---|---|
|
| |||||
| n (%)2 | n (%)2 | n (%)2 | n (%)2 | ||
|
| |||||
| Sociodemographics | |||||
| Age at HIV diagnosis (years),4 mean (SD) | 39.7 (10.9) | 37.4 (9.7)ac | 47.3 (10.6)ab | 55.8 (10.2)bc | <0.001 |
| Sex | 0.003 | ||||
| Female | 24 (1.4%) | 13 (0.9)ac | 8 (2.7)a | 3 (5.8)c | |
| Male | 1,728 (98.6) | 1,386 (99.1)ac | 293 (97.3)a | 49 (94.2)c | |
| Race/ethnicity5 | <0.001 | ||||
| African-American | 1,121 (64.7) | 839 (60.8)ac | 243 (81.0)a | 39 (75.0)c | |
| Hispanic | 21 (1.2) | 19 (1.4)ac | 1 (0.3)a | 1 (1.9)c | |
| White | 590 (34.1) | 522 (37.8)ac | 56 (18.7)a | 12 (23.1)c | |
| Transmission category5 | <0.001 | ||||
| Heterosexual | 54 (4.3) | 30 (2.8)ac | 18 (10.2)ab | 6 (27.3)bc | |
| IDU | 426 (33.6) | 354 (33.1)ac | 69 (39.0)ab | 3 (13.6)bc | |
| MSM | 715 (56.3) | 617 (57.7)ac | 86 (48.6)ab | 12 (54.6)bc | |
| MSM/IDU | 74 (5.8) | 69 (6.5)ac | 4 (2.3)ab | 1 (4.6)bc | |
| Clinical | |||||
| First CD45,6 (cells/mL) | <0.001 | ||||
| < 200 | 303 (77.9) | 151 (84.8)ac | 130 (75.1)ab | 22 (57.9)bc | |
| ≥ 200 | 86 (22.1) | 27 (15.2)ac | 43 (24.9)ab | 16 (42.1)bc | |
| Nadir CD45 (cells/mL) | <0.001 | ||||
| < 50 | 1,267 (86.8) | 1,038 (92.7)ac | 210 (72.2)ab | 19 (39.6)bc | |
| ≥ 50 | 192 (13.2) | 82 (7.3)ac | 81 (27.8)ab | 29 (60.4)bc | |
| Last CD45,6 (cells/mL) | 0.067 | ||||
| < 200 | 542 (67.8) | 398 (70.0) | 123 (63.7) | 21 (55.3) | |
| ≥ 200 | 258 (32.3) | 171 (30.1) | 70 (36.3) | 17 (44.7) | |
| Last HIV viral load5,6 (copies/mL) | 0.558 | ||||
| < 400 | 155 (34.6) | 92 (33.5) | 49 (35.0) | 14 (42.4) | |
| ≥ 400 | 293 (65.4) | 183 (66.6) | 91 (65.0) | 19 (57.6) | |
| ART initiated (any) | 1,168 (66.7) | 923 (66.0) | 215 (71.4) | 30 (57.7) | 0.072 |
| HIV diagnosis to ART initiation (months),4,5 mean (SD) | 33.0 (42.5) | 38.5 (44.9)ac | 12.6 (21.9)a | 9.1 (15.8)c | <0.001 |
| HIV diagnosis to AIDS diagnosis (years),4,5 mean (SD) | 3.7 (4.4) | 4.2 (4.5)ac | 1.6 (3.0)a | 0.7 (1.6)c | <0.001 |
| AIDS status5 | 0.006 | ||||
| No | 196 (11.2) | 143 (10.2)c | 42 (14.0) | 11 (22.9)c | |
| Yes | 1,552 (88.8) | 1,256 (89.8)c | 259 (86.1) | 37 (77.1)c | |
| CDC stage5 | <0.001 | ||||
| A | 435 (24.9) | 316 (22.6)ac | 95 (31.6)a | 24 (48.0)c | |
| B | 285 (16.3) | 218 (15.6)ac | 58 (19.3)a | 9 (18.0)c | |
| C | 1,028 (58.8) | 863 (61.8)ac | 148 (49.2)a | 17 (34.0)c | |
| Hepatitis B serostatus5,7 | <0.001 | ||||
| Negative | 278 (45.0) | 135 (38.1)ac | 118 (53.2)a | 25 (59.5)c | |
| Positive | 340 (55.0) | 219 (61.9)ac | 104 (46.9)a | 17 (40.5)c | |
| Hepatitis C serostatus5,7 | <0.001 | ||||
| Negative | 1,375 (78.6) | 1,138 (81.4)a | 196 (65.6)a | 41 (78.9) | |
| Positive | 374 (21.4) | 260 (18.6)a | 103 (34.5)a | 11 (21.2) | |
AIDS, acquired immune deficiency syndrome; ANOVA, analysis of variance; ART, antiretroviral therapy; cART, combination antiretroviral therapy; CDC, Centers for Disease Control and Prevention; HAVACS, HIV Atlanta Veterans Affairs Cohort Study; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug use; mL, milliliter; MSM, men who have sex with men; SD, standard deviation.
Pre-cART era (1984–1996), early-cART era (1997–2006), late-cART era (2007–2016).
Percentages may not add to 100% due to rounding up to the nearest tenth.
Fisher’s exact and chi-square tests for categorical data, as appropriate; one-way ANOVA for continuous data.
Kolmogorov-Smirnov tests were performed to confirm continuous variables were normally distributed.
Missing: age at AIDS diagnosis (n=327), race/ethnicity (n=20), transmission category (n=483), first CD4 (n=1,363), nadir CD4 (n=293), last CD4 (n=952), last HIV viral load (n=1,304), HIV diagnosis to ART initiation (n=584), HIV diagnosis to AIDS diagnosis (n=346), AIDS status (n=4), CDC stage (n=4), hepatitis B (n=1,134), hepatitis C (n=3).
First CD4: ≤6 months after HIV diagnosis; last CD4 and HIV viral load: ≤6 months before death.
Hepatitis B and C serostatus assessed by HBsAg and HCV antibody tests, respectively.
Pre- vs. early-cART era, statistically significantly different by Tukey’s post-hoc test or partitioning the likelihood ratio test, as appropriate.
Early- vs. late-cART era, statistically significantly different by Tukey’s post-hoc test or partitioning the likelihood ratio test, as appropriate.
Pre- vs. late-cART era, statistically significantly different by Tukey’s post-hoc test or partitioning the likelihood ratio test, as appropriate.
Temporal trends
Annual age-adjusted rates for all-cause, AIDS- and non-AIDS-related, and infection- and cancer-related mortality are depicted in Figures 1A, 1B, and 1C, respectively; statistically significant trends are denoted by joinpoints. In the pre-cART era, a notable shift occurred in 1986 when the rates of all-cause, AIDS-related, and infection-related mortality, which had all been significantly decreasing since 1984 (APC: −49.3, −52.6, and −52.8, respectively), suddenly pivoted and continued to increase until 1995 (APC: 5.9, 2.8, and 2.8, respectively). In parallel, significantly increasing rates of non-AIDS-related and cancer-related mortality were observed (APC: 3.1 and 0.8, respectively). Beginning in 1995, however, just at the precipice of the post-cART era, the all-cause mortality rate decreased significantly (APC=−29.9), a trend that continued through to the end of the post-cART era (APC=−1.1). Similarly, in the post-cART era, a gradual yet significant decrease occurred in the rates of AIDS-, non-AIDS-, and infection-related mortality (APC: −0.4, −0.7, and −0.7, respectively); no significant change in the cancer-related mortality rate was observed since the advent of cART.
Figure 1. Joinpoint regression analysis of age-adjusted mortality rates in Veterans with HIV during the pre-, early-, and late-cART eras (n=4,674).
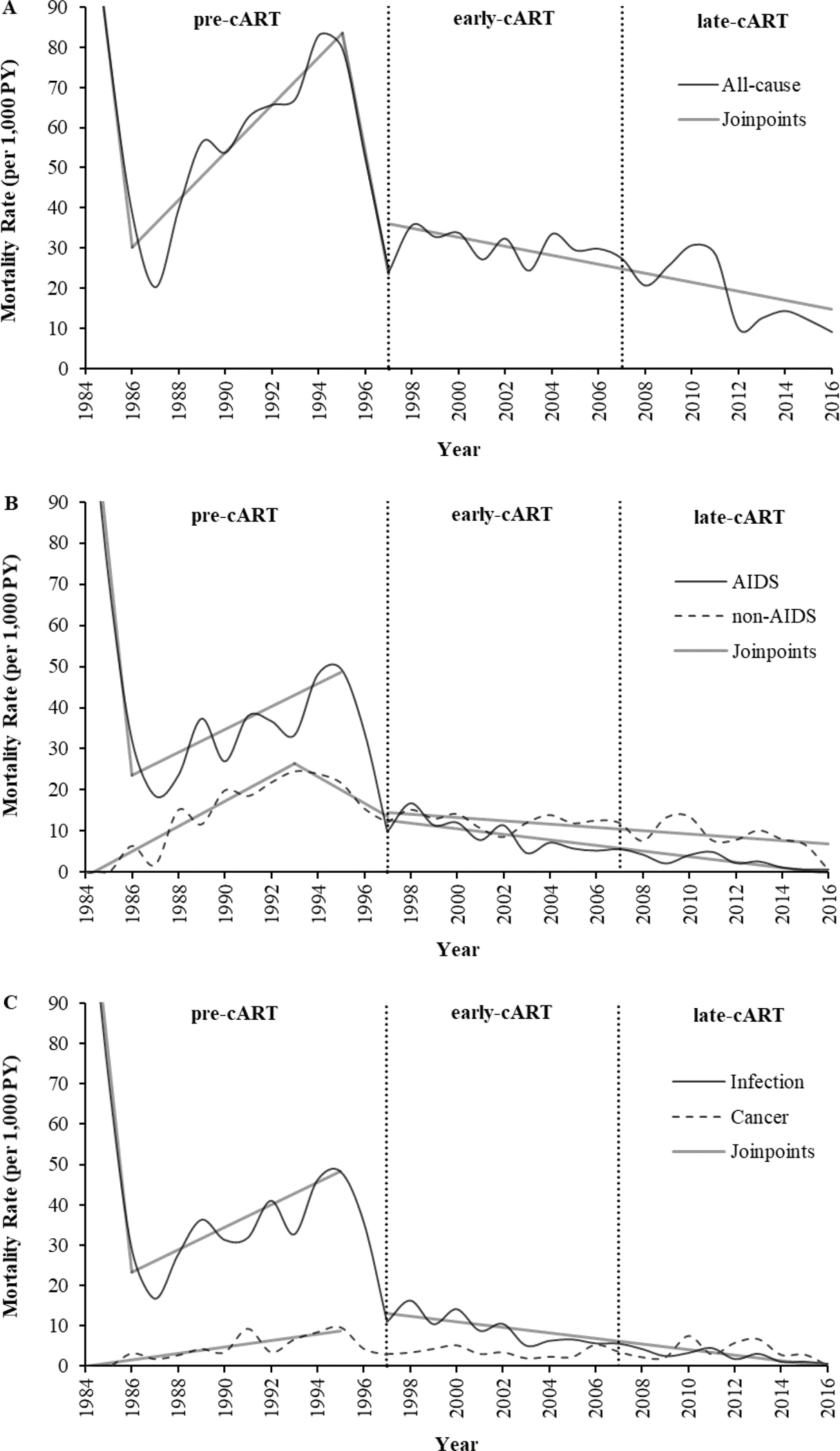
A, all-cause mortality rates (n=1,752); APC: 1984–1986 (−49.3), 1986–1995 (5.9), 1995–1997 (−29.9), 1997–2016 (−1.1). B, AIDS- (n=724) and non-AIDS-related (n=687) mortality rates; APC (AIDS): 1984–1986 (−52.6), 1986–1995 (2.8), 1997–2016 (−0.4); APC (non-AIDS): 1984–1993 (3.1), 1993–1997 (−3.2), 1997–2016 (−0.7). C, infection (including AIDS- and non-AIDS-related infections; n=727) and cancer (including AIDS- and non-AIDS-related cancers; n=221) mortality rates; APC (infection): 1984–1986 (−52.8), 1986–1995 (2.8), 1997–2016 (−0.7); APC (cancer): 1984–1995 (0.8). Age-adjusted mortality rates depicted have been smoothed. AIDS, acquired immune deficiency syndrome; APC, annual percent change; cART, combination antiretroviral therapy; early-cART era (1997–2006); HIV, human immunodeficiency virus; late-cART era (2007–2016); pre-cART era (1984–1996); PY, person-year.
Age-adjusted mortality rates
Causes of death were determined for 1,487 patients; 265 (15.1%) deaths occurred during the study period for which the cause of death is unknown. The distribution of death classifications is summarized in Table 2, along with the cause-specific mortality rates by cART era in which patients were diagnosed with HIV. Overall, the proportions of AIDS- and non-AIDS-related deaths were approximately equal (51.3% and 48.7%, respectively). AIDS-related deaths were predominantly due to infection (87.2%), while non-AIDS-related deaths had no one predominant cause but were more likely to be due to malignancy (18.6%), respiratory disease (18.0%), liver disease (14.1%), infection (14.0%), or sudden death (10.2%). The rates of all-cause and cause-specific mortality were statistically significantly lower during the early-cART era than during the pre-cART era; except for non-AIDS-related cardiovascular disease, malignancies, and sudden death. Most notably, cause-specific mortality rates that decreased ≥75% in the early-cART era include respiratory disease (IDR=0.19, 95% CI [0.09–0.58]), AIDS-related malignancies (IDR=0.22, 95% CI [0.11–0.46]), violence (IDR=0.23, 95% CI [0.09–0.58]), and non-AIDS-related infections (IDR=0.24, 95% CI [0.12, 0.48]). None of the cause-specific mortality rates were significantly lower during the late-cART era than during the early-cART era; only the all-cause mortality rate experienced a statistically significant 30% reduction in the late-cART era (IDR=0.70, 95% CI [0.53, 0.95]).
Table 2.
Cause-specific mortality rates per 1,000 person-years in Veterans by cART era in which they were diagnosed with HIV (n=4,674).
| Causes of death | n (%)1 | MR (95% CI) per 1,000 PY | Early- vs. pre-cART eras | Late- vs. early-cART eras | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Pre-cART era2 | Early-cART era2 | Late-cART era2 | IDR (95% CI) | P-value | IDR (95% CI) | P-value | ||
|
| ||||||||
| All-cause3 | 1,752 (100.0) | 39.10 (37.05, 41.15) | 19.33 (17.15, 21.52) | 13.62 (9.92, 17.33) | 0.49 (0.44, 0.56) | <0.001 | 0.70 (0.53, 0.95) | 0.020 |
| AIDS-related4 | 724 (51.3) | 17.64 (16.26, 19.01) | 5.14 (4.01, 6.26) | 3.41 (1.55, 5.26) | 0.29 (0.23, 0.37) | <0.001 | 0.66 (0.37, 1.19) | 0.169 |
| Infection | 631 (44.7) | 15.32 (14.03, 16.60) | 4.62 (3.56, 5.69) | 2.88 (1.18, 4.59) | 0.30 (0.24, 0.39) | <0.001 | 0.62 (0.33, 1.18) | 0.144 |
| Malignancy | 93 (6.6) | 2.32 (1.82, 2.82) | 0.51 (0.16, 0.87) | 0.52 (0.00, 1.25) | 0.22 (0.11, 0.46) | <0.001 | 1.02 (0.22, 4.80) | 0.980 |
| Non-AIDS-related4 | 687 (48.7) | 14.37 (13.12, 15.61) | 9.19 (7.68, 10.69) | 7.86 (5.05, 10.67) | 0.64 (0.53, 0.77) | <0.001 | 0.86 (0.58, 1.27) | 0.438 |
| Cardiovascular disease | 67 (4.8) | 1.12 (0.77, 1.46) | 1.41 (0.82, 2.00) | 1.31 (0.16, 2.46) | 1.26 (0.75, 2.13) | 0.378 | 0.93 (0.35, 2.45) | 0.879 |
| Infection5 | 96 (6.8) | 2.40 (1.90, 2.91) | 0.58 (0.20, 0.96) | 0.26 (0.00, 0.78) | 0.24 (0.12, 0.48) | <0.001 | 0.45 (0.06, 3.58) | 0.453 |
| Liver-related6 | 97 (6.9) | 2.21 (1.72, 2.70) | 1.09 (0.57, 1.61) | 0.26 (0.00, 0.78) | 0.49 (0.29, 0.84) | 0.008 | 0.24 (0.03, 1.80) | 0.165 |
| Malignancy6 | 128 (9.1) | 2.10 (1.62, 2.57) | 2.57 (1.77, 3.37) | 3.41 (1.55, 5.26) | 1.23 (0.84, 1.80) | 0.299 | 1.33 (0.71, 2.48) | 0.377 |
| Renal failure | 49 (3.5) | 0.95 (0.63, 1.27) | 0.96 (0.48, 1.45) | 0.26 (0.00, 0.78) | 1.01 (0.55, 1.86) | 0.965 | 0.27 (0.04, 2.06) | 0.207 |
| Respiratory disease5 | 124 (8.8) | 3.10 (2.53, 3.68) | 0.58 (0.20, 0.96) | 1.05 (0.02, 2.08) | 0.19 (0.09, 0.37) | <0.001 | 1.81 (0.56, 5.89) | 0.322 |
| Sudden death | 70 (5.0) | 1.09 (0.75, 1.43) | 1.67 (1.03, 2.31) | 1.31 (0.16, 2.46) | 1.53 (0.93, 2.52) | 0.092 | 0.78 (0.30, 2.04) | 0.619 |
| Violence6 | 56 (4.0) | 1.40 (1.01, 1.78) | 0.32 (0.04, 0.60) | 0.26 (0.00, 0.78) | 0.23 (0.09, 0.58) | 0.002 | 0.82 (0.10, 6.98) | 0.853 |
AIDS, acquired immune deficiency syndrome; cART, combination antiretroviral therapy; CI, confidence interval; HCC, hepatocellular carcinoma; HIV, human immunodeficiency virus; IDR, incidence density ratio; MR, mortality rate; PY, person-years.
Percentages may not add to 100% due to rounding up to the nearest tenth.
Pre-cART, 1984–1996 (n=1,366); early-cART, 1997–2006 (n=291); late-cART, 2007–2016 (n=49).
Includes deaths for which the cause is unknown (n=265) or other (n=76).
Includes only those sub-categories enumerated below.
Pulmonary infections included in respiratory, not infection.
HCC included in liver, not malignancy.
Includes suicide and drug-related causes of death.
Survival and incidence
Kaplan-Meier curves of all-cause mortality in the pre-, early-, and late-cART eras are depicted in Figure 2a; they are significantly different (χ2[2] = 262, p<0.001). The 10-year cumulative survival probability increased dramatically from 63.6% in the pre-cART era to 81.8% and 89.8% in the early- and late-cART eras, respectively. Figures 2b, 2c, and 2d present the stacked cumulative incidences for AIDS-related, non-AIDS-related, and undetermined mortality in the pre-, early-, and late-cART eras, respectively. In the pre-cART era, despite a gradual waning, the mortality rate due to AIDS significantly outpaced that of non-AIDS-related and undetermined mortality; such that, cumulatively, 48.9% of patients died due to an AIDS-related condition, followed by non-AIDS-related (34.0%) and undetermined (17.0%) mortality. In contrast, the cumulative incidences in the early-cART era were all substantially diminished, even more so in the late-cART era, but also gradually waned. In the early-cART era, the mortality rate due to non-AIDS-related conditions markedly outpaced that of AIDS-related and undetermined mortality; such that, cumulatively 9.5% of patients died due to a non-AIDS-related condition, followed by AIDS-related (5.7%) and undetermined (4.3%) mortality. Similarly, in the late-cART era, the mortality rate due to non-AIDS-related conditions markedly outpaced that of AIDS-related and undetermined mortality; such that, cumulatively 3.4% of patients died due to a non-AIDS-related condition, followed by AIDS-related (1.4%) and undetermined (0.9%) mortality.
Figure 2. Estimates of survival probability and cumulative incidence of mortality in Veterans by cART era in which they were diagnosed with HIV (n=4,674).
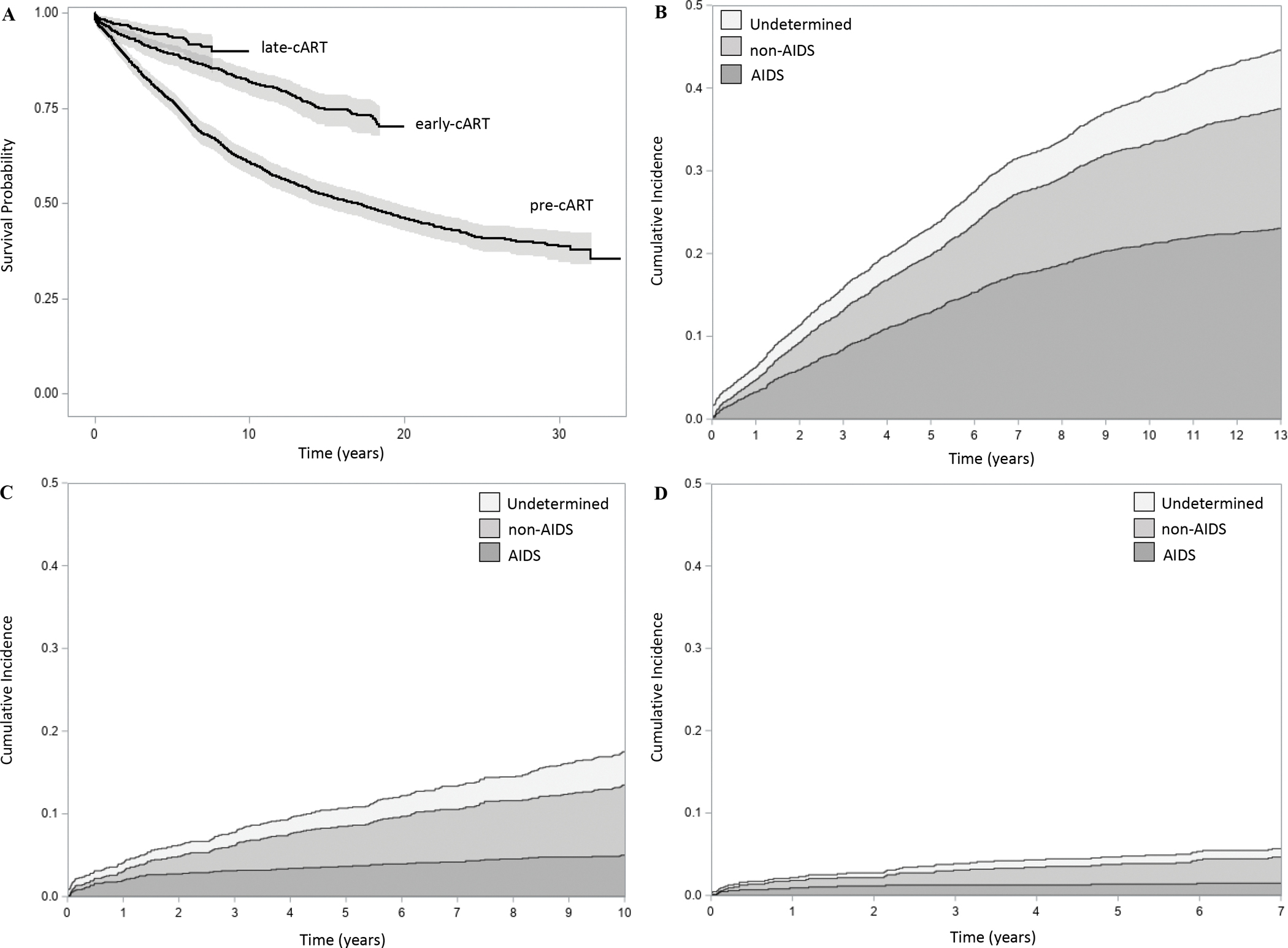
A, KM survival curves (equal-precision confidence bands) of all-cause mortality in the pre-, early-, and late-cART eras (log-rank test, p<0.001). B, stacked CIF plots of undetermined, non-AIDS- and AIDS-related mortality in the pre-cART era (n=2,552). C, stacked CIF plots of undetermined, non-AIDS- and AIDS-related mortality in the early-cART era (n=1,320). D, stacked CIF plots of undetermined, non-AIDS- and AIDS-related mortality in the late-cART era (n=802). AIDS, acquired immune deficiency syndrome; cART, combination antiretroviral therapy; CIF, cumulative incidence function; early-cART era (1997–2006); HIV, human immunodeficiency virus; KM, Kaplan-Meier; late-cART era (2007–2016); pre-cART era (1984–1996).
Risk factors
Adjusted hazard ratios of risk factors for all-cause and cause-specific mortality are summarized in Table 3. Older age at HIV diagnosis (≥40 years) was associated with two-fold increased risks for all-cause mortality (aHR=1.90, 95% CI [1.60–2.26]) and deaths due to AIDS-related infections (aHR=2.04, 95% CI [1.55–2.68]), liver disease (aHR=2.88, 95% CI [1.54–5.36]), and non-AIDS-related malignancies (aHR=2.64, 95% CI [1.37–5.10]), and a five-fold increased risk for death due to cardiovascular disease (aHR=4.57, 95% CI [1.77–11.79]). Non-White patients had a 30% increased risk of all-cause mortality (aHR=1.30, 95% CI [1.14–1.48]), driven predominantly by increased risks of death due to renal failure (aHR=5.36, 95% CI [1.06–27.15]), sudden death (aHR=2.58, 95% CI [1.12–5.94]), and cardiovascular disease (aHR=2.45, 95% CI [1.01–5.94]). IDU patients had a two- to three-fold increased risk of death due to liver disease and violence (aHR [95% CI]: 2.62 [1.38–4.98] and 2.40 [1.09–5.29], respectively). Initiating ART reduced the risks of all-cause and AIDS-related mortality by 32–44% (aHR [95% CI]: 0.68 [0.57–0.81] and 0.56 [0.43–0.72], respectively), driven predominantly by a 48% reduced risk of death due to AIDS-related infections (aHR=0.52, 95% CI [0.40, 0.67]). Similarly, patients with a low last CD4 count (<200 cells/mL) had twice the risk of all-cause mortality (aHR=1.72, 95% CI [1.35–2.20]) and a four-fold increased risk of AIDS-related mortality (aHR=4.16, 95% CI [2.26–7.67]), driven predominantly by a five-fold increased risk of death due to an AIDS-related infection (aHR=4.72, 95% CI [2.36–9.46]). As expected, patients with AIDS had a three-fold increased risk of death due to an AIDS-related condition (aHR=2.66, 95% CI [1.02–6.99]). After adjustment, last HIV viral load was not a statistically significant risk factor for all-cause or cause-specific mortality.
Table 3.
Adjusted hazard ratios of risk factors for cause-specific mortality in Veterans with HIV (n=4,674).
| Causes of death | aHR (95% CI) |
||||||
|---|---|---|---|---|---|---|---|
| Age1,2 ≥40 (vs. <40 years) | Non-White3,4 (vs. White) | IDU3,5 (vs. non-IDU) | ART initiated6 (vs. uninitiated) | AIDS3,7 (vs. no AIDS) | CD42,3,8 <200 (vs. ≥200 cells/mL) | HIV viral load2,3,9 ≥400 (vs. <400 copies/mL) | |
|
| |||||||
| All-cause10 | 1.90 (1.60, 2.26) | 1.30 (1.14, 1.48) | 1.12 (0.97, 1.29) | 0.68 (0.57, 0.81) | 0.71 (0.50, 1.00) | 1.72 (1.35, 2.20) | 1.15 (0.92, 1.45) |
| AIDS-related | 1.83 (1.41, 2.37) | 1.07 (0.89, 1.29) | 0.93 (0.75, 1.15) | 0.56 (0.43, 0.72) | 2.66 (1.02, 6.99) | 4.16 (2.26, 7.67) | 1.44 (0.91, 2.27) |
| Infection | 2.04 (1.55, 2.68) | 1.12 (0.87, 1.45) | 1.09 (0.82, 1.44) | 0.52 (0.40, 0.67) | 2.00 (0.73, 5.48) | 4.72 (2.36, 9.46) | 1.56 (0.92, 2.64) |
| Malignancy | 0.96 (0.47, 1.98) | 0.90 (0.49, 1.62) | 0.49 (0.23, 1.04) | 1.02 (0.51, 2.02) | 1.10 (1.01, 1.20) | 3.18 (0.75, 13.51) | 1.10 (0.32, 3.77) |
| Non-AIDS-related | 1.94 (1.55, 2.41) | 1.46 (1.17, 1.81) | 1.21 (0.97, 1.50) | 0.86 (0.65, 1.13) | 0.47 (0.31, 0.72) | 1.17 (0.82, 1.68) | 1.16 (0.84, 1.59) |
| Cardiovascular disease | 4.57 (1.77, 11.79) | 2.45 (1.01, 5.94) | 0.87 (0.38, 1.98) | 1.45 (0.49, 4.32) | 0.50 (0.16, 1.64) | 1.19 (0.48, 2.99) | 0.87 (0.32, 2.34) |
| Infection11 | 1.55 (0.79, 3.02) | 1.94 (1.06, 3.57) | 1.01 (0.53, 1.92) | 1.07 (0.56, 2.05) | 1.46 (0.30, 7.10) | 1.03 (0.42, 2.51) | 1.23 (0.46, 3.34) |
| Liver-related12 | 2.88 (1.54, 5.36) | 1.07 (0.58, 1.95) | 2.62 (1.38, 4.98) | 0.72 (0.39, 1.34) | 0.24 (0.09, 0.65) | 1.62 (0.67, 3.97) | 0.94 (0.44, 2.04) |
| Malignancy12 | 2.64 (1.37, 5.10) | 0.94 (0.49, 1.77) | 0.49 (0.23, 1.02) | 0.94 (0.45, 1.96) | 0.50 (0.18, 1.38) | 1.81 (0.73, 4.48) | 0.79 (0.34, 1.86) |
| Renal failure | 2.05 (0.75, 5.59) | 5.36 (1.06, 27.15) | 1.76 (0.55, 5.65) | 0.31 (0.08, 1.22) | 0.58 (0.04, 8.62) | 1.97 (0.20, 19.65) | 1.87 (0.24, 14.72) |
| Respiratory disease11 | 1.62 (0.89, 2.93) | 1.50 (0.83, 2.72) | 0.77 (0.41, 1.43) | 1.44 (0.72, 2.87) | 0.26 (0.06, 1.17) | 2.21 (0.65, 7.54) | 0.91 (0.31, 2.70) |
| Sudden death | 1.96 (0.95, 4.07) | 2.58 (1.12, 5.94) | 1.70 (0.84, 3.44) | 0.63 (0.29, 1.37) | 0.51 (0.20, 1.29) | 0.69 (0.30, 1.59) | 2.06 (1.00, 4.26) |
| Violence13 | 0.61 (0.19, 1.98) | 0.97 (0.38, 2.45) | 2.40 (1.09, 5.29) | 0.41 (0.18, 0.97) | 0.92 (0.27, 3.14) | 0.39 (0.14, 1.09) | 2.09 (0.78, 5.54) |
aHR, adjusted hazard ratio; AIDS, acquired immune deficiency syndrome; ART, antiretroviral therapy; cART, combination antiretroviral therapy; CI, confidence interval; early-cART era (1997–2006); HCC, hepatocellular carcinoma; HIV, human immunodeficiency virus; IDU, injection drug use; late-cART era (2007–2016); pre-cART era (1984–1996).
Models adjusted for sex, race/ethnicity, transmission category, ART initiation, AIDS status, last CD4, last HIV viral load, and cART era.
Age at HIV diagnosis; last CD4 and HIV viral load: ≤6 months before censorship.
Missing: race/ethnicity (n=70), transmission category (n=1,280), AIDS status (n=651), last CD4 (n=3,626), last HIV viral load (n=3,310).
Models adjusted for cART era only.
Models adjusted for sex, race/ethnicity, and cART era.
Models adjusted for sex, race/ethnicity, transmission category, AIDS status, last CD4, last HIV viral load, and cART era.
Models adjusted for sex, race/ethnicity, transmission category, ART initiation, last CD4, last HIV viral load, and cART era.
Models adjusted for age at HIV diagnosis, race/ethnicity, transmission category, ART initiation, AIDS status, last HIV viral load, and cART era.
Models adjusted for age at HIV diagnosis, race/ethnicity, transmission category, ART initiation, AIDS status, last CD4, and cART era.
Includes deaths for which the cause is unknown (n=265) or other (n=76).
Pulmonary infections included in respiratory, not infection.
HCC included in liver, not malignancy.
Includes suicide and drug-related causes of death.
Discussion
The work presented represents the first analysis of cause-specific mortality among VWH and the first to examine mortality over a prolonged period of time. Compared to the general population with HIV, HAVACS patients were more likely to be older, male, MSM, IDU, and diagnosed with AIDS.14,19–21 It is not surprising then that the causes attributed to their deaths were also different.
Age-adjusted all-cause mortality
Early in the epidemic within the pre-cART era, the all-cause mortality rate among HAVACS patients was substantially lower than in the general population with HIV (39 vs. 233 per 1,000 PY).11 Continuing this trend, in the early-cART era, the all-cause mortality rate among HAVACS patients receiving care at AVAMC plummeted by 51%, even more than the rate nationally among all VWH enrolled in the VA (19 vs. 35 per 1,000 PY)22; this rate continues to decline (14 per 1,000 PY in the late-cART era) and is now approaching the general population with HIV (6–13 per 1,000 PY).4 In regard to survival in the post-cART era, the probability of survival at 65 years of age is considerably higher among HAVACS patients than the general population with HIV (59% vs. 48%, respectively)23 but substantially lower than the general population without HIV (59% vs. 84%, respectively).24–25 With respect to risk factors, HAVACS patients who were non-White and had a last CD4 count <200 cells/mL were at a 30% and 72% increased risk for all-cause mortality, respectively; whereas those who initiated ART had a 32% reduced risk for all-cause mortality, aligning with findings from other studies in the general population with HIV.4,19–20,26–27 Additional risk factors identified in other studies that were either not assessed or were not statistically significant among HAVACS patients include older age, male sex, IDU, AIDS, HCV, and a detectable HIV viral load.20,26–28
Age-adjusted AIDS-related mortality
The cause-specific mortality rate due to AIDS plummeted by 71% from the pre- to the early-cART era (17.6 vs. 5.1 per 1,000 PY, respectively) – driven largely by significant reductions in the rates of infections and malignancies – and this rate continues to decline (3.4 per 1,000 PY in the late-cART era). In the late-cART era, the mortality rate due to infections is considerably higher in HAVACS patients than in the general population with HIV (2.9 vs. 2.4 per 1,000 PY, respectively), but the mortality rate due to malignancies is substantially lower (0.5 vs. 1.5 per 1,000 PY, respectively); regardless, the overall mortality rate due to AIDS is considerably lower in HAVACS patients (3.4 vs. 5.1 per 1,000 PY, respectively) and its declining trend is corroborated by previous studies.14,32–33 With respect to risk factors, HAVACS patients who were diagnosed with AIDS and had a last CD4 count <200 cells/mL had almost a three- and four-fold increased risk for AIDS-related mortality, respectively; whereas those who initiated ART had a 44% reduced risk for AIDS-related mortality, substantiating findings from other studies in the general population with HIV.14,20–21,32 Additional risk factors identified in other studies that were either not assessed or were not statistically significant among HAVACS patients include younger age, late ART initiation, IDU, and a detectable HIV viral load.13–14,20–21,32
Age-adjusted non-AIDS-related mortality
The cause-specific mortality due to non-AIDS diminished by 36% from the pre- to the early-cART era (14.4 vs. 9.2 per 1,000 PY, respectively) – driven predominantly by significant reductions in the rates of respiratory disease, violence, and infections – and this rate continues to decline (7.9 per 1,000 PY in the late-cART era) but is notably higher than the mortality rate due to AIDS (3.4 per 1,000 PY in the late-cART era). In the late-cART era, the mortality rates due to malignancies (3.4 vs. 1.2 per 1,000 PY, respectively), cardiovascular disease (1.3 vs. 0.8 per 1,000 PY, respectively), respiratory disease (1.1 vs. 0.2 per 1,000 PY, respectively), and renal failure (0.3 vs. 0.2 per 1,000 PY, respectively) are all considerably higher in HAVACS patients than in the general population with HIV, but the mortality rates due infections (0.3 vs. 0.9 per 1,000 PY, respectively), violence (0.3 vs. 0.8 per 1,000 PY, respectively), and liver disease (0.3 vs. 0.7 per 1,000 PY, respectively) are all lower.14 Regardless, the overall mortality rate due to non-AIDS in HAVACS patients is 50% higher than the corresponding rate in the general population with HIV (7.9 vs. 5.3 per 1,000 PY, respectively), but its slow declining trend is corroborated by previous studies. 14,32–33 With respect to risk factors, HAVACS patients who were non-White had a 46% increased risk non-AIDS-related mortality while those who were diagnosed with AIDS had a 53% reduced risk for non-AIDS-related mortality, substantiating findings from other studies in the general population with HIV.14,21,32 Additional risk factors identified in other studies that were either not assessed or were not statistically significant among HAVACS patients include older age, early ART initiation, IDU, a CD4 count <200 cells/mL, and a detectable HIV viral load.13–14,20–21,32,34
Strengths and limitations
Strengths of this study include its complete enrollment of all patients with HIV ever seen at the AVAMC; long 35-year study duration; access to national patient-level VA data; generalizability to all VWH; and use of a standardized protocol to classify the cause of death. Limitations of this study include that the dates of HIV diagnosis were left-censored because data on the last negative HIV test were unavailable; considerable LTFU (34.7%) that may, if differential between groups, introduce selection bias; immortal time bias as Veterans must survive longer to be eligible for VA benefits; sparse data in the beginning of the epidemic, resulting in imprecise annual mortality rates; exclusion of patients with dates of death missing month or year (2.6%) from all analyses and of patients who died with an unknown cause of death (15.1%) from all analyses of cause-specific mortality; no available data on the causal chain of mortality (immediate, underlying, and comorbid); other risk factors of interest were unmeasured; and ART regimens were not assessed to evaluate the evolution of treatment guidelines over time.
Conclusions
Despite the advent of potent cART, HAVACS patients continue to die at considerably higher rates due to AIDS-related infections and non-AIDS-related malignancies, respiratory disease, cardiovascular disease, and renal failure than those in the general population with HIV; many of which can be attributed to social and lifestyle risk factors. For example, among PWH, the most common non-AIDS-related malignancy and respiratory disease are lung cancer and chronic obstructive pulmonary disease, both likely due to smoking; many cardiovascular-related deaths are attributed to endocarditis, likely due to IDU; and the leading risk factor of chronic kidney disease is diabetes – all of which are modifiable risk factors amenable to intervention.14,20,32,35–36 After all, PWH have been shown to be just as likely to achieve conventional risk factor treatment goals as people without HIV.37 As VWH live longer, VA providers must not only recognize the shift in HIV disease management towards chronic comorbidities, but also the opportunity to intervene and address lifestyle risk factors, including risk reduction programs that address smoking, alcohol and drug misuse, and treatment advocacy programs to mitigate ART nonadherence.14,32
Acknowledgements
The completion of this work could not have been possible without the participation of all the patients in HAVACS, the ID clinic staff at AVAMC, and David Kendrick and Rincy Varughese, who were instrumental for data collection and retrieval, respectively – thank you.
KJV received support from NICHD 1U19HD089881. VCM received support from Emory CFAR P30 AI050409.
Footnotes
VCM has received investigator-initiated research grants (to the institution) and consultation fees (both unrelated to the current work) from Eli Lilly, Bayer, Gilead Sciences and ViiV. The remaining authors have no conflicts of interest.
Data from this manuscript were presented at the virtual IDWeek conference, September 29 – October 3, 2021, and at the International Workshop on HIV and Hepatitis Observational Databases in Athens, Greece, March 23 – 25, 2022.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- 1.Owens DK, Sundaram V, Lazzeroni LC, et al. Prevalence of HIV infection among inpatients and outpatients in Department of Veterans Affairs health care systems: Implications for screening programs for HIV. Am J Public Health. 2007;97:2173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HIV Surveillance Report: Diagnoses of HIV Infection in the Unites States and Dependent Areas. Center for Disease Control and Prevention, June 24, 2021. Available at: https://www.cdc.gov/hiv/statistics/overview/index.html.
- 3.2019 Population Estimates by Age, Sex, Race, and Hispanic Origin. U.S. Census Bureau, June 25, 2020. Available at: https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html.
- 4.HIV-CAUSAL Collaboration. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS. 2010;24:123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guest JL, Weintrob AC, Rimland D, et al. A comparison of HAART outcomes between the US military HIV Natural History Study (NHS) and HIV Atlanta Veterans Affairs Cohort Study (HAVACS). PLoS One. 2013;8:e62273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangal JP, Rimland D, Marconi VC. The continuum of HIV care in a veterans’ affairs clinic. AIDS Res Hum Retroviruses. 2014;30:409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnett PG, Chow A, Joyce VR, et al. Determinants of the cost of health services used by veterans with HIV. Med Care. 2011;49:848–56. [DOI] [PubMed] [Google Scholar]
- 9.Armed Forces Health Surveillance Branch. Update: Routine screening for antibodies to human immunodeficiency virus, civilian applicants for US military service and US armed forces, active and reserve components, January 2014 – June 2019. MSMR. 2019;26:2–9. [PubMed] [Google Scholar]
- 10.Tseng A, Seet J, Phillips EJ. The evolution of three decades of antiretroviral therapy: Challenges, triumphs, and the promise of the future. Br J Clin Pharmacol. 2015;79:182–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mocroft A, Vella S, Benfield TL, et al. Changing patterns of mortality across Europe in patients with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725–30. [DOI] [PubMed] [Google Scholar]
- 12.Bhaskaran K, Hamouda O, Sannes M, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300:51–9. [DOI] [PubMed] [Google Scholar]
- 13.Wada N, Jacobson LP, Cohen M, et al. Cause-specific mortality among HIV-infected individuals, by CD4+ cell count at HAART initiation, compared with HIV-uninfected individuals. AIDS. 2014;28:257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996 – 2006: Collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guest JL, Moanna A, Wirtz SS, et al. Cohort profile: The HIV Atlanta Veterans Affairs Cohort Study (HAVACS). Int J Epidemiol. 2017;46:790–1g. [DOI] [PubMed] [Google Scholar]
- 16.Kowalska JD, Friis-Moller N, Kirk O, et al. The Coding Causes of Death in HIV (CoDe) Project: Initial results and evaluation of methodology. Epidemiology. 2011;22:516–23. [DOI] [PubMed] [Google Scholar]
- 17.Lewden C, May T, Rosenthal E, et al. Changes in causes of death among adults infected with HIV between 2000 and 2005: The “Mortalite 2000 and 2005” surveys (ANRS EN19 and Mortavic). J Acquir Immune Defic Syndr. 2008;48:590–8. [DOI] [PubMed] [Google Scholar]
- 18.Ingle SM, May MT, Gill MJ, et al. Impact of risk factors for specific causes of death in the first and subsequent years of antiretroviral therapy among HIV-infected patients. Clin Infect Dis. 2014;59:287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: A collaborative analysis of cohort studies. Lancet. 2017;4:E349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trickey A, May MT, Vehreschild J, et al. Cause-specific mortality in HIV-positive patients who survived ten years after starting antiretroviral therapy. PLoS One. 2016;11:e0160460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pettit AC, Giganti MJ, Ingle SM, et al. Increased non-AIDS mortality among persons with AIDS-defining events after antiretroviral therapy initiation. J Int AIDS Soc. 2018;21:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006;44:S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obel N, Omland LH, Kronborg G, et al. Impact of non-HIV and HIV risk factors on survival in HIV-infected patients on HAART: A population-based nationwide cohort study. PLoS One. 2011;6:e22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Population Prospects. Department of Economic and Social Affairs, United Nations Population Division, 2019. Available at: https://population.un.org/wpp/. [Google Scholar]
- 25.National Surveys on Drug Use and Health. Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services, March, 2012. Available at: https://www.samhsa.gov/data/sites/default/files/report_1969/Spotlight-1969.html. [Google Scholar]
- 26.Akgun K, Gordon K, Pisani M, et al. Risk factors for hospitalization and medical intensive care unit (MICU) admission among HIV-infected veterans. J Acquir Immune Defic Syndr. 2013;62:52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heltemes BR. Mortality and risk stratification of HIV infected individuals. J Insur Med. 2015;45:142–52. [DOI] [PubMed] [Google Scholar]
- 28.Justice AC, Modur S, Tate JP, et al. Predictive accuracy of the Veterans Aging Cohort Study (VACS) Index for mortality with HIV infection: A North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62:149–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKinnell JA, Willig JH, Westfall AO, et al. Antiretroviral prescribing patterns in treatment-naïve patients in the United States. AIDS Patient Care STDs. 2010;24:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monforte AA, Sabin CA, Phillips A, et al. The changing incidence of AIDS events in patients receiving highly active antiretroviral therapy. Arch Intern Med. 2005;165:416–23. [DOI] [PubMed] [Google Scholar]
- 31.Justice AC, Freiberg MS, Tracy R, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54:984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowell A, Sheroni Sv, Kyriakides TC, et al. Trends in hospital deaths among HIV-infected patients during the antiretroviral therapy era, 1995–2011. J Hosp Med. 2015;10:608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kowalska JD, Reekie J, Mocroft A, et al. Long-term exposure to combination antiretroviral therapy and risk of death from specific causes: No evidence for any previously unidentified increased risk due to antiretroviral therapy. AIDS. 2012;26:315–23. [DOI] [PubMed] [Google Scholar]
- 34.Park LS, Tate JP, Sigel K, et al. Viral suppression is associated with lower AIDS-defining and non-AIDS-defining cancer incidence in HIV-infected veterans. Ann Intern Med. 2018;169:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gingo MR, Nouraie M, Kessinger CJ. Decreased lung function and all-cause mortality in HIV-infected individuals. Ann Am Thorac Soc. 2018;15:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winston J, Deray G, Hawkins T, et al. Kidney disease in patients with HIV infection and AIDS. Clin Infect Dis. 2008;47:1449–57. [DOI] [PubMed] [Google Scholar]
- 37.Adeyemi O, Vibhakar S, Max B. Are we meeting the American Diabetes Association goals for HIV-infected patients with diabetes mellitus? Clin Infect Dis. 2009;49:799–802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.


