Objective:
Improved understanding of the effect of HIV infection on Kaposi sarcoma (KS) presentation and outcomes will guide development of more effective KS staging and therapeutic approaches. We enrolled a prospective cohort of epidemic (HIV-positive; HIV+KS) and endemic (HIV-negative; HIV−KS) KS patients in Uganda to identify factors associated with survival and response.
Methods:
Adults with newly diagnosed KS presenting for care at the Uganda Cancer Institute (UCI) in Kampala, Uganda, between October 2012 and December 2019 were evaluated. Participants received chemotherapy per standard guidelines and were followed over 1 year to assess overall survival (OS) and treatment response.
Results:
Two hundred participants were enrolled; 166 (83%) had HIV+KS, and 176 (88%) were poor-risk tumor (T1) stage. One-year OS was 64% (95% confidence interval [CI] 57–71%), with the hazard of death nearly threefold higher for HIV+KS (hazard ratio [HR] = 2.93; P = 0.023). Among HIV+KS, abnormal chest X-ray (HR = 2.81; P = 0.007), lower CD4+ T-cell count (HR = 0.68 per 100 cells/μl; P = 0.027), higher HIV viral load (HR = 2.22 per log10 copies/ml; P = 0.026), and higher plasma Kaposi sarcoma-associated herpesvirus (KSHV) copy number (HR = 1.79 per log10 copies/ml; P = 0.028) were associated with increased mortality. Among HIV−KS, factors associated with mortality included Karnofsky score <70 (HR = 9.17; P = 0.045), abnormal chest X-ray (HR = 8.41; P = 0.025), and higher plasma KSHV copy number (HR = 6.21 per log10 copies/ml; P < 0.001).
Conclusions:
Although survival rates were better for HIV−KS than HIV+KS, the high mortality rate seen in both groups underscores the urgent need to identify new staging and therapeutic approaches. Factors associated with mortality, including high plasma KSHV, may serve as important targets of therapy.
Keywords: endemic Kaposi sarcoma, HHV-8, HIV, Kaposi sarcoma-associated herpesvirus, Kaposi sarcoma
Introduction
Kaposi sarcoma (KS) is among the most common HIV-associated malignancies worldwide and a leading cause of cancer in several African countries [1,2]. In sub-Saharan Africa (SSA), KS presents as epidemic (HIV-associated; HIV+KS) and endemic (HIV-negative; HIV−KS) forms of the disease. Prior to the HIV pandemic, endemic KS represented 4–10% of recognized adult cancers in Eastern and Southern Africa [3–6]. The incidence of KS rose dramatically with widespread HIV infection, which increases the risk of KS several thousand-fold among those co-infected with Kaposi sarcoma-associated herpesvirus (KSHV), the causative viral agent of all forms of KS [7]. Despite progress in controlling the HIV pandemic, both epidemic and endemic KS continue to cause significant morbidity and mortality in many regions of SSA [8].
Although endemic KS has been recognized for decades, there have been few systematic studies of endemic KS, particularly in adults. Although the AIDS Clinical Trials Group (ACTG) staging criteria is widely accepted for HIV+KS despite its limited prognostic value in SSA [9–12], there is no universally adopted staging system for endemic KS. Further, treatment recommendations for HIV-negative KS are largely derived from those used for HIV-associated KS and have not been comprehensively evaluated.
Comparing epidemic and endemic KS provides a unique opportunity to better define KS pathogenesis. Heterogeneity in clinical presentation and outcomes between HIV+KS and HIV−KS may suggest differences in underlying disease processes, likely mediated by HIV infection. Identifying these differences could yield insights into KS biology and enhance current staging systems and treatment approaches. In this study, we sought to comprehensively describe clinical presentation and outcomes in a prospective cohort of epidemic and endemic KS patients in Uganda and to identify factors associated with overall survival and response.
Methods
Study participants and setting
We enrolled adults with KS who presented for care at the Uganda Cancer Institute (UCI) in Kampala, Uganda, between October 2012 and December 2019. Eligibility requirements were: age ≥18 years; histologic confirmation of KS; naïve to antiretroviral therapy (ART); and no prior chemotherapy or radiation treatment for KS.
Study procedures
Participants completed an enrollment visit and up to 10 follow-up visits over ∼1 year. At enrollment, participants completed a standardized medical history and physical examination. Peripheral blood and an oral swab were collected for KSHV testing. HIV serology was performed, and CD4+ T-cell and plasma HIV RNA testing was completed for HIV-seropositive participants. UCI medical charts were reviewed to record routine blood count data and chest X-ray findings. At follow-up visits, participants completed an interim medical history and detailed physical exam to assess treatment response.
Participants received chemotherapy at the UCI per standard guidelines independent of the study. First-line therapy consisted of combination bleomycin and vincristine (BV) or paclitaxel; both regimens were given every 3 weeks for six cycles. HIV-seropositive participants not already in HIV care were referred to local HIV clinics for management and initiation of ART according to the Ugandan Ministry of Health guidelines.
Laboratory procedures
Plasma and oral swab samples were evaluated for KSHV DNA by quantitative, real-time PCR at the UCI-Fred Hutch Cancer Centre Laboratory in Kampala, Uganda as described previously [13,14]. Samples with >150 copies/ml of KSHV DNA were considered positive [15]. CD4+ T-cell counts were measured by flow cytometry, and HIV-1 RNA levels were measured using real-time RT-PCR.
Definitions
Among baseline clinical characteristics, body mass index (BMI) was categorized as underweight if BMI <18.5 kg/m2[16]. The Karnofsky score, a measure of performance status, was categorized as a score ≥70 and <70 to distinguish those who can and cannot care for themselves without assistance [17].
KS stage was defined according to the AIDS Clinical Trials Group (ACTG) classification [9,18]. Tumor stage was defined as T1 (poor risk) if there was tumor-associated edema or ulceration, or oral involvement beyond the hard palate. Immune status was defined as poor risk (I1) if CD4+ T-cell count was less than 200 cells/μl. Systemic symptoms were defined as poor risk (S1) if the patient had a history of opportunistic infections, presence of “B” symptoms, such as weight loss of 10% or more, unexplained fevers and night sweats, or Karnofsky performance status <70. Treatment response was evaluated as the best response achieved over the follow-up period as defined by the ACTG KS response criteria [9].
Statistical methods
Baseline demographic and clinical characteristics were summarized. Statistical significance of differences between HIV+KS and HIV−KS participants was assessed with the chi-squared test for categorical variables, or the Wilcoxon rank-sum test for continuous variables. To estimate overall survival (OS), we constructed Kaplan-Meier survival functions from Visit 1 until death, loss to follow-up, or to 1-year survival. Cox proportional hazards regression was applied to estimate mortality hazard ratios (HRs) with 95% confidence intervals (CIs). Response to therapy was dichotomized into good response [complete clinical response (CR)/partial response (PR)] or poor response [stable disease (SD)/progressive disease (PD)], and logistic regression was used to estimate odds ratios (ORs) of response associated with participant characteristics. Variables with P <0.1 in univariable models were selected for multivariable models. All tests were two-sided. Primary analysis excluded participants missing information for variables included in the model. In a sensitivity analysis, multiple imputation with chained equations was used to impute missing data (Supplemental Methods, Supplemental Digital Content). We used Stata version 17 (StataCorp, College Station, Texas, USA) and R version 4 (R Foundation for Statistical Computing, Vienna, Austria) to conduct analysis.
Ethical considerations
Approval for the study was obtained from the Makerere University School of Medicine Research and Ethics Committee, Fred Hutchinson Cancer Research Center IRB, and the Uganda National Council for Science and Technology. All participants provided documentation of informed consent.
Results
Characteristics of study participants
Among 246 persons screened prior to December 31, 2019, 200 participants were enrolled in the study. The main reason for ineligibility was lack of KS diagnosis on pathology review (N = 10); eight declined study participation, seven had received prior KS chemotherapy, six were already receiving ART, two declined KS chemotherapy, and one was withdrawn from the study due to severe illness and inability to complete study procedures. Eleven eligible participants died and one was lost to follow up prior to completing the Visit 1.
Participants with HIV+KS comprised 83% (166) of the study cohort (Table 1). The majority (N = 156; 78%) were men, which did not differ significantly by HIV status. The median age was 32 years (range, 18–75 years) among HIV+KS and 46 years (range, 18–90) among HIV−KS (P < 0.001). Twenty-six (14.1%) participants were considered underweight with a BMI <18.5 kg/m2, which was similar among epidemic and endemic KS groups (P = 0.44).
Table 1.
Participant characteristics by HIV serostatus.
| Overall | HIV+KS | HIV−KS | P | |
| N = 200 | N = 166 | N = 34 | ||
| Male, N (%) | 156 (78) | 127 (76.5) | 29 (85.3) | 0.26 |
| Age, median (range) | 34 (18, 90) | 32 (18, 75) | 46 (19, 90) | <0.001 |
| ACTG stage, N (%) | ||||
| T1 | 176 (88) | 144 (86.7) | 32 (94.1) | 0.23 |
| I1 | 93 (56.4) | |||
| S1 | 147 (73.5) | 124 (74.7) | 23 (67.6) | 0.40 |
| Karnofsky <70, N (%) | 58 (29) | 47 (28.3) | 11 (32.4) | 0.64 |
| B-symptoms, N (%) | 138 (69) | 118 (71.1) | 20 (58.8) | 0.16 |
| Opportunistic infections, N (%) | 29 (14.5) | 27 (16.3) | 2 (5.9) | 0.12 |
| BMI (kg/m2), median (IRQ)a | 26 (14.1) | 21.5 (19.8, 23.3) | 21.7 (19.7, 23.9) | 0.44 |
| Hemoglobin, median (IQR)a | 10.3 (8.9, 12.5) | 10 (8.7, 11.9) | 12.8 (11.1, 14.9) | <0.001 |
| Platelets, median (IQR)a | 232 (155, 297) | 223 (138, 297) | 245.5 (199, 305) | 0.08 |
| Abnormal chest X-ray finding, N (%)a,b | 65 (38.7) | 61 (43.9) | 4 (13.7) | 0.002 |
| Months since first KS lesion, median (IQR)a | 7 (3, 17) | 6 (3, 12) | 22 (8, 48) | <0.001 |
| Distinct sites with KS lesions | 5 (2, 7) | 5 (3, 7) | 2 (1, 3) | <0.001 |
| Any edema, N (%) | 165 (82.5) | 135 (81.3) | 30 (88.2) | 0.33 |
| Any macular lesions, N (%) | 195 (97.5) | 162 (97.6) | 33 (97.1) | 0.86 |
| Any nodular lesions, N (%) | 134 (67) | 107 (64.5) | 27 (79.4) | 0.09 |
| Any fungating lesions, N (%) | 93 (46.5) | 70 (42.2) | 23 (67.7) | 0.007 |
| Months since HIV diagnosis, median (IQR)a | 2 (1, 5) | |||
| HIV viral load, log10(copies/ml) median (IQR)c | 5.3 (4.9, 5.7) | |||
| CD4+ count, median (IQR) | 167 (49, 338) | |||
| KSHV detected in plasma, N (%)a | 188 (95.4) | 158 (96.3) | 30 (90.9) | 0.17 |
| Plasma KSHV titer, log10(copies/ml), median (IQR)d | 4 (3.6, 4.5) | 4.1 (3.7, 4.5) | 3.8 (3.1, 4) | <0.001 |
| KSHV detected in oral swaba, N (%) | 115 (57.8) | 101 (61.2) | 14 (41.2) | 0.03 |
| Oral KSHV titer log10(copies/ml), median (IQR)e | 3.6 (2.9, 4.5) | 3.6 (2.9, 4.5) | 3.3 (2.6, 5.1) | 0.80 |
ACTG, AIDS Clinical Trials Group; IQR, interquartile range; KHSV, Kaposi sarcoma-associated herpesvirus; KS, Kaposi sarcoma.
Percentages, medians, ranges, and P-values exclude participants missing data (Table 1, Supplemental Digital Content).
Infiltrate or effusion, unilateral or bilateral.
Excludes one participant for whom viral load was not assessed, and two participants with undetectable viral load.
Among participants with detectable plasma KSHV.
Among participants with detectable oral KSHV.
By ACTG staging criteria, 176 (88%) were T1 stage and 147 (74%) were poor-risk systemic illness (S1) stage. For the key criteria contributing to S status, 58 (29%) had Karnofsky score <70, 138 (69%) reported B-symptoms, and 29 (15%) had a history of opportunistic infection. The proportion of participants with T1 and S1 stage did not differ by HIV status. Among those with HIV-associated KS, the median CD4+ T-cell count was 167 cells/μl (interquartile range [IQR]: 49–338 cells/μl) and the median HIV-1 RNA level was 5.3 log10 copies/ml (IQR: 4.9–5.7 log10 copies/ml).
Kaposi sarcoma clinical presentation
The median duration since the participant noticed their first KS lesion was 7 months (IQR: 3–17 months), but HIV−KS participants reported a longer time since their first lesion (median 22 months; IQR: 8–48 months) compared to HIV+KS participants (median 6 months; IQR: 3–12 months) (P < 0.001).
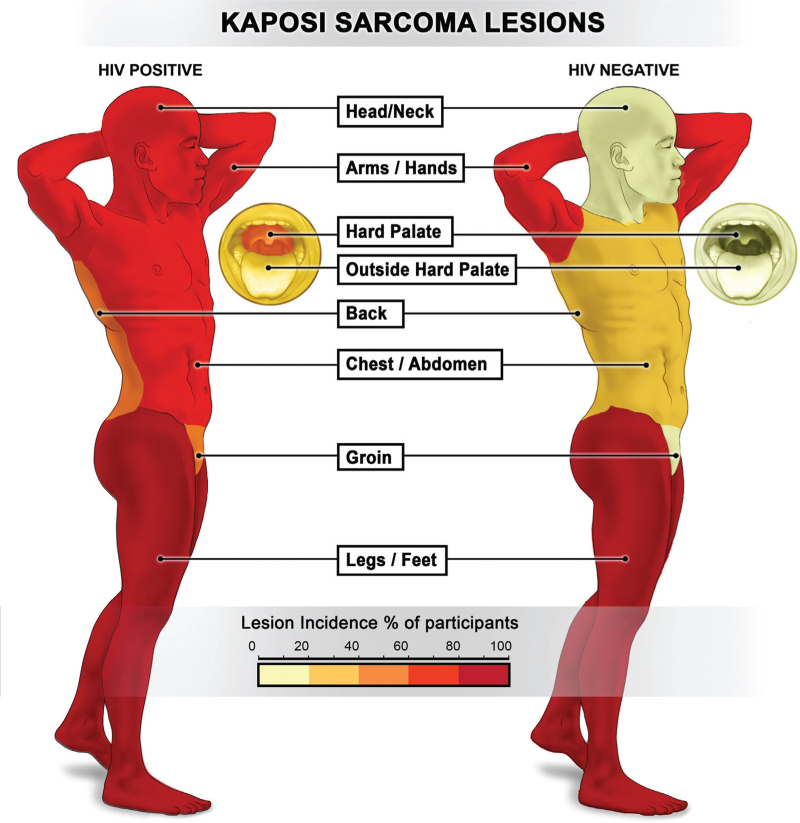
Lesions involved the following anatomic locations at KS presentation (Fig. 1): 108 (54%) head or neck; 95 (48%) hard palate; 54 (27%) oral cavity outside the hard palate; 140 (70%) upper extremities; 123 (62%) chest or abdomen; 101 (51%) back; 88 (44%) groin; and 187 (94%) lower extremities. The most commonly involved sites for both HIV+KS and HIV−KS were lower extremities (94 vs. 91%, P = 0.55) and upper extremities (71 vs. 65%, P = 0.46), but HIV+KS participants were more likely to have lesions at other anatomic sites compared to HIV−KS participants: head or neck (62 vs. 15%, P < 0.001), hard palate (56 vs. 6%, P < 0.001), oral cavity outside hard palate (31 vs. 6%, P = 0.002), chest or abdomen (68 vs. 32%, P < 0.001), back (56 vs. 24%, P = 0.001), and groin (50 vs. 15%, P < 0.001).
Fig. 1.
KS lesion distribution by HIV status. KS, Kaposi sarcoma.
HIV+KS participants generally had KS lesions disseminated over more anatomic sites (median 5 sites; IQR: 3–7 sites) compared to HIV−KS participants (median 2 sites; IQR: 1–3 sites) (P < 0.001). HIV+KS participants presented with more lesions (median 82 lesions, IQR: 39–179 lesions) than HIV−KS participants (median 62 lesions, IQR: 35–101 lesions), although the difference was not statistically significant (P = 0.23).
For lesion morphotype, 195 (98%) participants had macular lesions, 134 (67%) had nodular lesions, 93 (47%) had fungating lesions; 150 (75%) had more than one type of lesion. Fungating lesions were more common among HIV−KS than HIV+KS participants (68 v. 42%, P = 0.007); macular and nodular lesions did not differ significantly by HIV status (P = 0.86 and 0.09, respectively).
Edema was observed in 165 (83%) participants, with no significant difference based on HIV status (P = 0.33). HIV+KS and HIV−KS participants had a similar frequency of edema involving lower extremities (74 vs. 85%, P = 0.15) and upper extremities (10 vs. 15%, P = 0.45), but HIV+KS participants were more likely to have edema involving head or neck (22 vs. 6%, P = 0.03) and groin (34 vs. 9%, P = 0.01).
Laboratory and imaging studies
Baseline hemoglobin values were slightly lower in HIV+KS (median 10 g/dl; IQR: 8.7–11.9 g/dl) compared to HIV−KS (median 12.8 g/dl; IQR, 11.1–14.9 g/dl) (P < 0.001). Median platelet levels were 232 × 109/l (IQR: 155–297 × 109/l), with no difference by HIV status (P = 0.08). Of 168 participants with chest X-ray results (139 HIV+KS, 29 HIV−KS), abnormal chest X-rays were identified in 65 (36%) participants, with abnormal findings more commonly observed in HIV+KS than HIV−KS (44% vs. 14%, P = 0.002).
KSHV was detected in plasma at baseline in 188 (95%) participants. The frequency of detecting KSHV in plasma was similar in HIV+KS (96%) and HIV−KS (91%) (P = 0.17); however, KSHV copy number was higher in plasma among epidemic KS (median 4.1 log10 copies/ml; IQR: 3.7–4.5 log10 copies/ml) compared to endemic KS (median 3.8 log10 copies/ml; IQR: 3.1–4.0 log10 copies/ml) (P < 0.001). KSHV was detected in oral swabs from 115 (58%) participants, more commonly among HIV+KS (101; 61%) compared to HIV−KS (14; 41%) (P = 0.03). Of the 115 oral samples in which KSHV DNA was detected, the median KSHV copy number was 3.6 log10 copies/ml (IQR: 2.9–4.5 log10 copies/ml), which did not differ significantly by HIV status (P = 0.80).
Treatment received
Following study enrollment, 176 (88%) participants received chemotherapy (Table 1, Supplemental Digital Content). Among these, the majority (140; 80%) received bleomycin-vincristine (BV) combination therapy as the first-line regimen while 34 (20%) participants received paclitaxel. Those with HIV−KS were more likely to receive paclitaxel compared to HIV+KS (32 vs. 14%; P = 0.006).
All HIV+KS participants were naive to antiretroviral therapy (ART) on enrollment, and 121 (73%) initiated ART during the observation period. The most common ART regimen was efavirenz (EFV)-based (69; 57%), generally given with tenofovir (TDF) and emtricitabine (FTC). Other ART regimens included: dolutegravir (DTG) with TDF/3TC (15; 12%); nevirapine (NVP)-based (11; 9%); or a boosted protease inhibitor-based regimen [lopinavir (LPV) or atazanavir (ATV)] in 11 (9%) participants. Among those who started ART, the median time to initiation was 25 days (IQR: 2–67 days) following Visit 1.
Overall survival
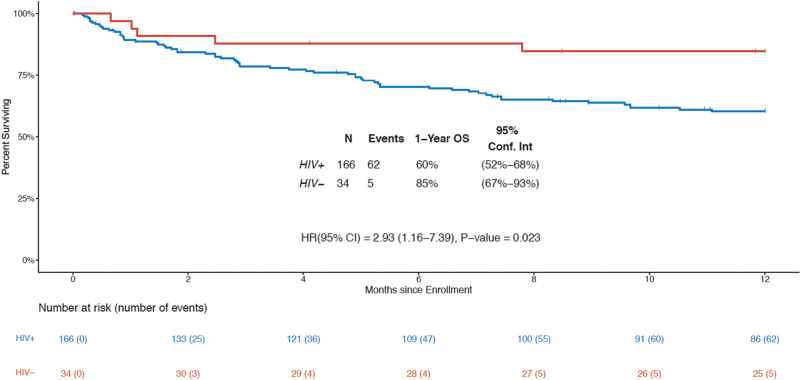
At 1 year following enrollment, 111 participants were alive, 67 had died, and 22 were censored prior to 1 year of follow up (median 132 days of follow up). The 1-year OS was 64% (95% CI 57–71). For HIV+KS, 1-year OS was 60% (95% CI 52–68), and for HIV−KS was 85% (95% CI 67–93) (Fig. 2). The hazard of death was nearly three times higher for epidemic KS compared to endemic KS (HR = 2.93, 95% CI 1.16–7.39; P = 0.023).
Fig. 2.
Overall survival by HIV status.
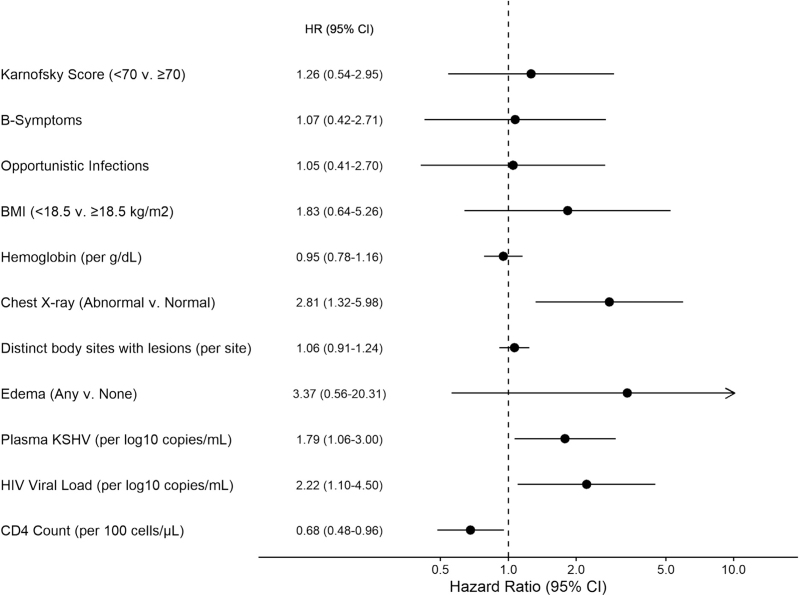
Among HIV+KS, multiple baseline factors were associated with increased hazard of death in univariable analysis (Figure 1, Supplemental Digital Content). In multivariable analysis (Fig. 3), abnormal chest X-ray (HR = 2.81, [95% CI 1.32–4.18]; P = 0.007), lower baseline CD4+ T-cell count (HR = 0.68 per 100 cells/μl increase, [95% CI 0.48–0.96]; P = 0.027), higher baseline HIV viral load (HR =2.22 per log10 copies/ml increase, [95% CI 1.10–4.50]; P = 0.026), and higher plasma KSHV copy number (HR = 1.79 per log10 copies/ml increase, [95% CI 1.06–3.00]; P = 0.028) were associated with increased hazard of mortality.
Fig. 3.
Factors associated with Mortality: HIV+KS multivariable. Arrow indicates 95% CI upper limit >10. All variables included in the multivariable model are displayed. CI, confidence interval; KS, Kaposi sarcoma.
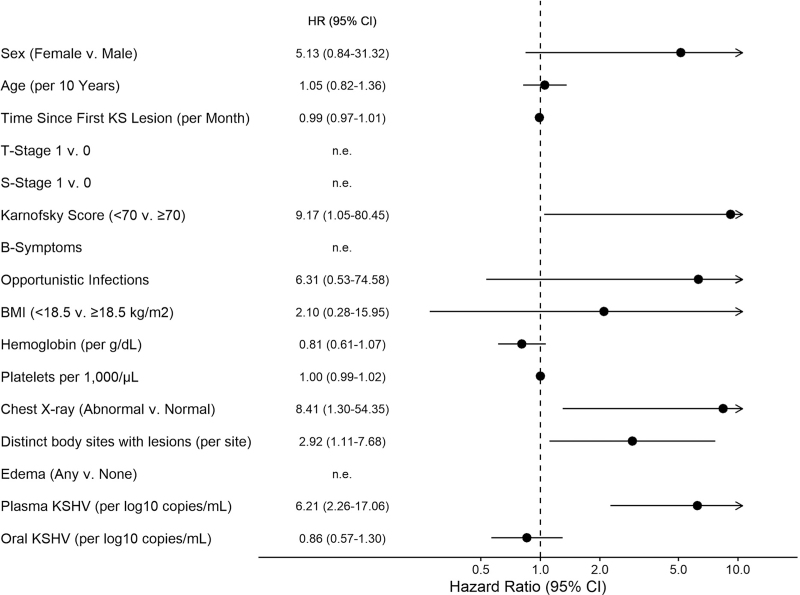
Among HIV−KS, factors associated with increased risk of death in univariable analysis included Karnofsky score <70 (HR = 9.17, [95% CI 1.05–80.45]; P = 0.045), anatomically disseminated disease (HR per each anatomic site with lesions = 2.92 [95% CI 1.11–7.68]; P = 0.029), abnormal chest X-ray (HR = 8.41, [95% CI 1.30–54.35]; P = 0.025), and higher plasma KSHV copy number (HR = 6.21 per log10 copies/ml increase, [95% CI 2.26–17.06]; P < 0.001) (Fig. 4). No multivariable model was identified for HIV-KS survival because of the small number of deaths (5 in total).
Fig. 4.
Factors associated with mortality: HIV−KS univariable. Arrow indicates 95% CI upper limit >10. CI, confidence interval.
Results of survival analysis with imputed data were not substantially different from the primary complete-case analysis (Supplemental Materials, Supplemental Digital Content).
Treatment response
Among the 176 participants initiating chemotherapy, the best response achieved was clinical complete response in 19 (11%), partial response in 84 (48%), stable disease in 23 (13%), and progressive disease in 25 (13%) participants. Response was not evaluable in 26 (15%) participants (Table 2, Supplemental Digital Content). Participants with HIV+KS were less likely to have a good response (CR/PR) than HIV−KS (OR = 0.32 [95% CI 0.10–1.00]; P = 0.05). For HIV+KS, higher CD4+ cell count (OR =1.34 per 100 cells/μl increase [95% CI 1.10–1.65]; P = 0.004), less disseminated disease (OR =0.85 per additional anatomic site with lesions [95% CI 0.72–1.00]; P = 0.048), and higher plasma KSHV titer (OR = 0.44 per log10 copies/ml increase, [95% CI 0.24–0.82]; P = 0.01) were significantly associated with complete or partial response. Higher baseline hemoglobin (OR = 1.21 per Hbg g/dl increase, [95% CI 1.00–1.47]) was associated with good response with a P-value = 0.054 (Figure 2, Supplemental Digital Content). Among HIV−KS participants, low platelets (OR = 0.99 per 1000/μl [95% CI 0.98, 1.00]; P = 0.027) and higher oral KSHV titer (OR = 0.41 per log10 copies/mL [95% CI 0.21, 0.80]; P = 0.008) were significantly associated with good response in the univariate models (Figure 3, Supplemental Digital Content).
Discussion
This is the first prospective study to our knowledge to rigorously characterize both epidemic and endemic KS in SSA, revealing several important observations about the effect of HIV infection on KS presentation and outcomes. Our cohort demonstrated that HIV−KS is generally less disseminated than HIV+KS, with endemic KS having lesions at fewer anatomic sites, rare oral manifestations, and less common chest x-ray abnormalities, which likely represent KS pulmonary involvement. Adults with endemic KS also presented at an older age and reported longer periods of time from noticing their first KS lesion before seeking care, suggesting a more indolent clinical course than epidemic KS. Although our cohort included a large proportion of advanced stage HIV+KS patients, the observation that endemic KS is generally less fulminant than epidemic KS is consistent with previous reports [3,4]. Together, these findings support the hypothesis that HIV infection accelerates KS disease processes, perhaps through impaired host immune function [19–21], inflammation [22], activation of KSHV replication [23], or increased expression of markers of immune exhaustion in chronic HIV infection [24–26]. Translational studies utilizing biologic samples from the cohort are ongoing to evaluate these potential mechanisms and gain insights into the biology of KS pathogenesis.
Survival outcomes among adults with epidemic KS were worse than those with endemic KS, with a nearly threefold increased risk of death at one year following initiation of treatment. The poor 1-year OS of 60% among epidemic KS participants in our cohort is similar to previously reported survival outcomes among adults with HIV+KS from Uganda [11], but somewhat worse than the ∼80% 1-year OS achieved in a recent multisite clinical trial of BV vs. paclitaxel in SSA [27]. To our knowledge, this is the first prospective study to report survival outcomes in adults with endemic KS, and although survival rates were better for HIV−KS than HIV+KS, the high mortality rate seen in both groups underscores the need to identify new therapeutic approaches.
A striking finding of our study was the high rate of plasma KSHV viremia and oral KSHV shedding observed in both HIV+KS and HIV−KS participants, which is consistent with our group's prior studies in Uganda [28]. Higher plasma KSHV copy number and more common oral KSHV shedding was seen among adults with epidemic KS, perhaps reflecting poorer immune control of the virus and more disseminated KS disease, including oral involvement, at presentation. Importantly, plasma KSHV copy number was associated with survival among both epidemic and endemic KS. High pretreatment plasma KSHV quantity has been associated with poor outcomes in other cohorts of HIV+KS in SSA [29,30], but this is the first study to evaluate the association between plasma KSHV and survival in adults with endemic KS. Interestingly, higher baseline KSHV viremia was associated with a greater hazard for death for endemic KS compared to epidemic KS [HR 6.21 (2.26–17.06) vs. 1.79 (1.06–3.00)]. Together, these finding suggests that ongoing KSHV replication is a key feature of disease in all forms of KS, and measurement of baseline KSHV viral load may have role in augmenting current KS staging systems.
Among HIV+KS participants, lower CD4+ cell count was associated with worse OS, but other ACTG staging criteria – tumor stage and systemic symptoms – were not associated with OS for either epidemic or endemic KS. This lack of association could be related to the fact that the majority of cohort participants had advanced stage disease and the study may not have had power to discern differences in T and S stage. Prior work, however, has demonstrated the limited prognostic value of ACTG staging criteria in SSA, particularly in the era of widely available ART [11]. These findings further highlight the need to identify new staging criteria, ideally based on mechanisms of disease pathogenesis, to better predict clinical outcomes.
Our study has several important limitations. As a non-trial prospective cohort study, there was some variation in the type of chemotherapy participants received. Endemic KS patients were more likely to receive paclitaxel therapy than epidemic KS, but we did not observe that this impacted overall survival in sub-analysis. This is consistent with the findings of AMC 067 trial, which found that paclitaxel had superior progression-free survival compared to combination bleomycin-vincristine, but that the regimens were equivalent in terms of overall survival [27]. In addition, not all HIV+KS patients received ART during the study. While ART is an essential component of optimal epidemic KS care, some participants died prior to initiating ART and others faced challenges engaging in HIV care despite study team efforts to facilitate HIV clinic enrollment. Unfortunately, failure to initiate timely ART reflects real-world challenges even in the era of widely-available ART, and it likely contributes to the poorer survival outcomes observed in our cohort compared to KS clinical trial cohorts [27]. Successful implementation of HIV “test and treat” programs will be an important strategy for both preventing KS and improving KS outcomes. Further, we recruited participants from a tertiary cancer care center, so it is likely that our cohort represents more advanced epidemic KS and endemic KS than seen in the general population. However, the UCI is among the few facilities able to provide chemotherapy in the country, and we believe our advanced KS cohort reflects those seeking chemotherapy for KS in Uganda. We were also not able to fully evaluate for visceral disease in our cohort and relied on chest X-ray alone to assess for possible pulmonary involvement, but we believe this reflects standard of care in most centers in SSA and helps to identify predictor variables that will be most realistically incorporated into future staging classifications. Finally, our cohort includes fewer HIV−KS compared to HIV+KS, which may obscure some important differences between the groups that we did not have power to detect. However, our cohort distribution reflects the KS patient population at the UCI, with ∼10% endemic KS, and represents one of the largest prospective cohorts of epidemic and endemic KS reported to date.
In summary, our study demonstrated that HIV−KS is generally less disseminated and has better outcomes than HIV+KS, but high mortality rates were observed in both groups. Factors associated with mortality, including high plasma KSHV, may serve as important biomarkers and targets of therapy. Additional clinical and translational studies are needed to better define KS pathogenesis and factors associated with survival to inform improved staging and therapeutic approaches for both epidemic and endemic KS patients in SSA and worldwide.
Acknowledgements
We are grateful to the study participants and the study team, whose commitment made this study possible.
Author contributions: W.P.: study design, data acquisition, data review, statistical analysis, manuscript preparation; S.A.: data review, statistical analysis, manuscript preparation; P.M.: data acquisition, manuscript preparation; J.K.: data acquisition, manuscript preparation; S.S.: data acquisition, manuscript preparation; D.M.: data acquisition, manuscript preparation; J.N.: data acquisition, manuscript preparation; C.N.: data acquisition, manuscript preparation; L.O.: data acquisition, data review, manuscript preparation; E.N.: data review, statistical analysis, manuscript preparation; S.K.: data acquisition, manuscript preparation; K.B.: data review, statistical analysis, manuscript preparation; M.R.: data review, statistical analysis, manuscript preparation; C.C.: study design, data review, manuscript preparation; J.O.: study design, data review, manuscript preparation; EHW: study design, data review, manuscript preparation.
Research support: K23 CA 150931 (PI W.P.), R01 CA217138 (PI E.H.W., W.P.), R01 CA239287 (PI W.P., E.H.W.), U54 CA190146 (PI, C.C., J.O., W.P.), P30 AI027757 (PI, Celum).
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71:209–249. [DOI] [PubMed] [Google Scholar]
- 2.Wabinga HR, Parkin DM, Wabwire-Mangen F, Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br J Cancer 2000; 82:1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ziegler JL. Endemic Kaposi's sarcoma in Africa and local volcanic soils. Lancet 1993; 342:1348–1351. [DOI] [PubMed] [Google Scholar]
- 4.Hutt MS. Kaposi's sarcoma. Br Med Bull 1984; 40:355–358. [DOI] [PubMed] [Google Scholar]
- 5.McHardy J, Williams EH, Geser A, de-The G, Beth E, Giraldo G. Endemic Kaposi's sarcoma: incidence and risk factors in the West Nile District of Uganda. Int J Cancer 1984; 33:203–212. [DOI] [PubMed] [Google Scholar]
- 6.Cook-Mozaffari P, Newton R, Beral V, Burkitt DP. The geographical distribution of Kaposi's sarcoma and of lymphomas in Africa before the AIDS epidemic. Br J Cancer 1998; 78:1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beral V. The epidemiology of cancer in AIDS patients. AIDS 1991; 5: (Suppl 2): S99–S103. [DOI] [PubMed] [Google Scholar]
- 8.Bender Ignacio RA, Ghadrshenas M, Low D, Orem J, Casper C, Phipps W. HIV status and associated clinical characteristics among adult patients with cancer at the Uganda Cancer Institute. J Global Oncol 2017; 4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krown SE, Metroka C, Wernz JC. Kaposi's sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 1989; 7:1201–1207. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen HQ, Magaret AS, Van Rompaey SE, Kitahata MM, Wald A, Casper C. Persistent Kaposi sarcoma in the era of HAART: characterizing the predictors of clinical response. AIDS 2008; 22:937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okuku F, Krantz EM, Kafeero J, Kamya MR, Orem J, Casper C, et al. Evaluation of a predictive staging model for HIV-associated Kaposi sarcoma in Uganda. J Acquir Immune Defic Syndr 2017; 74:548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman EE, Semeere A, McMahon DE, Byakwaga H, Laker-Oketta M, Regan S, et al. Beyond T staging in the “Treat-All” era: severity and heterogeneity of Kaposi sarcoma in East Africa. J Acquir Immune Defic Syndr 2021; 87:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pauk J, Huang ML, Brodie SJ, Wald A, Koelle DM, Schacker T, et al. Mucosal shedding of human herpesvirus 8 in men. N Engl J Med 2000; 343:1369–1377. [DOI] [PubMed] [Google Scholar]
- 14.Casper C, Krantz E, Selke S, Kuntz SR, Wang J, Huang ML, et al. Frequent and asymptomatic oropharyngeal shedding of human herpesvirus 8 among immunocompetent men. J Infect Dis 2007; 195:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magaret AS, Wald A, Huang M-L, Selke S, Corey L. Optimizing PCR positivity criterion for detection of herpes simplex virus DNA on skin and mucosa. J Clin Microbiol 2007; 45:1618–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. WHO. Obesity: preventing and managing the global epidemic. Report on a WHO consultation on obesity, Geneva, 3–5 June 1997. Technical report series number 894.2000; Geneva: World Health Organization. [PubMed] [Google Scholar]
- 17.Karnofsky D, Burchenal JH. MacLeod C. The clinical evaluation of chemotherapeutic agents in cancer. Evaluation of chemotherapeutic agents. New York: Columbia University Press; 1949. 199–205. [Google Scholar]
- 18.Krown S, Testa M, Huang J. AIDS-related Kaposi's sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 1997; 15:3085–3092. [DOI] [PubMed] [Google Scholar]
- 19.Wijnveen AC, Persson H, Bjorck S, Blohme I. Disseminated Kaposi's sarcoma--full regression after withdrawal of immunosuppressive therapy: report of a case. Transplant Proc 1987; 19:3735–3736. [PubMed] [Google Scholar]
- 20.Nagy S, Gyulai R, Kemeny L, Szenohradszky P, Dobozy A. Iatrogenic Kaposi's sarcoma: HHV8 positivity persists but the tumors regress almost completely without immunosuppressive therapy. Transplantation 2000; 69:2230–2231. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson LP, Jenkins FJ, Springer G, Munoz A, Shah KV, Phair J, et al. Interaction of human immunodeficiency virus type 1 and human herpesvirus type 8 infections on the incidence of Kaposi's sarcoma. J Infect Dis 2000; 181:1940–1949. [DOI] [PubMed] [Google Scholar]
- 22.Mercader M, Taddeo B, Panella JR, Chandran B, Nickoloff BJ, Foreman KE. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am J Pathol 2000; 156:1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrington W, Jr, Sieczkowski L, Sosa C, Chan-a-Sue S, Cai JP, Cabral L, et al. Activation of HHV-8 by HIV-1 tat [letter]. Lancet 1997; 349:774–775. [DOI] [PubMed] [Google Scholar]
- 24.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006; 443:350–354. [DOI] [PubMed] [Google Scholar]
- 25.Epple HJ, Loddenkemper C, Kunkel D, Troger H, Maul J, Moos V, et al. Mucosal but not peripheral FOXP3+ regulatory T cells are highly increased in untreated HIV infection and normalize after suppressive HAART. Blood 2006; 108:3072–3078. [DOI] [PubMed] [Google Scholar]
- 26.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 2008; 205:2763–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krown SE, Moser CB, MacPhail P, Matining RM, Godfrey C, Caruso SR, et al. Treatment of advanced AIDS-associated Kaposi sarcoma in resource-limited settings: a three-arm, open-label, randomised, noninferiority trial. Lancet 2020; 395:1195–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston C, Orem J, Okuku F, Kalinaki M, Saracino M, Mbidde E, et al. Impact of HIV infection and Kaposi sarcoma on human herpesvirus-8 mucosal replication and dissemination in Uganda. PLoS One 2009; 4:e4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El Amari E, Toutous-Trellu L, Gayet-Ageron A. Predicting the evolution of Kapsoi sarcoma in the highly active antiretroviral therapy era. AIDS 2008; 22:1019–1028. [DOI] [PubMed] [Google Scholar]
- 30.Borok M, Fiorillo S, Gudza I, Putnam B, Ndemera B, White IE, et al. Evaluation of plasma human herpesvirus 8 DNA as a marker of clinical outcomes during antiretroviral therapy for AIDS-related Kaposi sarcoma in Zimbabwe. Clin Infect Dis 2010; 51:342–349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.