Abstract
Objective:
To evaluate gut microbiota (GMB) alterations and metabolite profile perturbations associated with bone mineral density (BMD) in the context of HIV infection.
Design:
Cross-sectional studies of 58 women with chronic HIV infection receiving antiretroviral therapy and 33 women without HIV infection.
Methods:
We examined associations of GMB and metabolites with BMD among 91 women. BMD was measured by dual-energy X-ray absorptiometry, and T-scores of lumbar spine or total hip <−1 defined low BMD. GMB was measured by 16S rRNA V4 region sequencing on fecal samples and plasma metabolites were measured by liquid chromatography-tandem mass spectrometry. Associations of GMB with plasma metabolites were assessed in a larger sample (418 women; 280 HIV+ and 138 HIV−).
Results:
Relative abundances of five predominant bacterial genera (Dorea, Megasphaera, unclassified Lachnospiraceae, Ruminococcus, and Mitsuokella) were higher in women with low BMD compared to those with normal BMD (all linear discriminant analysis scores >2.0). A distinct plasma metabolite profile was identified in women with low BMD, featuring lower levels of several metabolites belonging to amino acids, carnitines, caffeine, fatty acids, pyridines, and retinoids, compared to those with normal BMD. BMD-associated bacterial genera, especially Megasphaera, were inversely associated with several BMD-related metabolites (e.g., 4-pyridoxic acid, C4 carnitine, creatinine, and dimethylglycine). The inverse association of Megasphaera with dimethylglycine was more pronounced in women with HIV infection compared to those without HIV infection (P for interaction=0.016).
Conclusions:
Among women with and at risk of HIV infection, we identified altered GMB and plasma metabolite profiles associated with low BMD.
Keywords: bone mineral density, HIV infection, gut microbiota, metabolomics
Introduction
Human immunodeficiency virus (HIV) infection has been associated with low bone mineral density (BMD) and increased fracture risk [1, 2]. HIV infection alone may cause bone loss, which can be accelerated with antiretroviral therapy (ART) initiation [3–6]. In the Women’s Interagency HIV Study (WIHS), we have shown that HIV infection was associated with decreased BMD [7]. However, no significant difference was observed in the rate of BMD decline by HIV status in another cohort [8]. Thus, the relationship between HIV infection, ART and bone health has not been fully understood.
Emerging evidence suggests a close relationship between the gut microbiota and bone metabolism, and alterations in gut microbiota have been associated with bone health. For example, in studies of participants without HIV infection, several genera of Clostridiales were higher in individuals with low BMD or osteoporosis compared to those with normal BMD [9–11]. Although mechanisms underlying the associations between gut microbiota and bone metabolism remain largely unknown, microbiota-related metabolites (e.g., short-chain fatty acids) have been suggested as potential mediators linking gut microbiota to bone health and disease. In recent metabolomic studies, many metabolites such as lipids (particularly sphingolipids) and amino acids were associated with BMD [12–14]. However, host metabolomic data have not been well-integrated with gut microbiome data in previous bone studies, and data are sparse among people living with HIV.
We previously found alterations in gut microbiota and related plasma metabolite profiles among women with HIV in the WIHS [15]. We conducted analyses utilizing data on gut microbiome, plasma metabolites, and dual-energy X-ray absorptiometry (DXA) scans in a subset of WIHS participants to identify potential gut microbiome and metabolite signatures for BMD among women living with and without HIV infection.
Methods
Study Population
The WIHS is a prospective cohort study of women living with HIV infection and sociodemographically similar women without HIV infection. Detailed information on study design and methods has been previously described [16]. WIHS participants underwent semiannual core study visits with the completion of a structured in-person interviewer, a physical examination, and collection of biological specimens. From 2015 to 2018, 516 women enrolled in the Bronx, Brooklyn, and Chicago sites of the WIHS provided fecal samples. We excluded 27 participants who reported taking antibiotics in the prior six months. Among the 489 women remaining, a subset of 91 women (58 HIV+; 33 HIV−) who underwent DXA scanning during 2012–2016 was included in the primary analyses. A total of 418 women who had both gut microbiome and metabolome data (but not all with DXA data) were included in secondary association analyses of gut microbiota and plasma metabolites. The studies were reviewed and approved by each site’s Institutional Review Board. All individuals provided written informed consent.
BMD Assessment
BMD at the lumbar spine and total hip were measured by DXA (General Electric/Lunar Prodigy; Madison, WI) [17]. All BMD measurements were performed by trained technicians using a standardized protocol. Established instrument calibration and quality control procedures were performed every day. The T-scores for BMD of the lumbar spine and total hip were computed by comparing with the BMD of healthy young people of the same sex and race and used to categorize BMD based on World Health Organization criteria [18] as normal (T-scores of −1 and above), osteopenia (T-scores of −2.5 to −1), and osteoporosis (T-scores of −2.5 and below). The low-BMD group was defined as the T-scores for either lumbar spine or total hip were less than −1.0, and the normal-BMD group was defined as the T-scores for the lumbar spine and total hip were greater than or equal to −1.0.
Microbiome Measurement
Fecal samples were collected using a home-based self-collection kit, and genomic DNA extraction and 16S rRNA gene V4 hypervariable region PCR amplification were conducted according to previously described procedures [15, 19]. The size integrity of the amplicons with Illumina indices were validated using a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA), and the high-throughput amplicon sequencing was performed on a MiSeq platform (Illumina, San Diego, CA. RRID: SCR_016379) using 2×300 paired-end fragment reads at the Genomics Core and Sequencing Core at the Albert Einstein College of Medicine. The bioinformatics analysis was performed using the QIIME2 (version 2020.2.0) [20]. Detailed methods are described in the Supplementary Methods. Finally, the genus level was profiled for the downstream taxonomic analyses.
Plasma Metabolomic Profiling
Fasting plasma samples were collected and stored at −80°C at the core visit. Untargeted metabolomic profiling was performed at the Broad Institute Metabolomics Platform (Cambridge, MA). Briefly, metabolites were detected in plasma and quantified using a Nexera X2 Ultra-High Performance Liquid Chromatography (Shimadzu Corp., Marlborough, MA) coupled to an Exactive Plus mass spectrometer (Thermo Fisher Scientific, Waltham, MA). Raw mass data were processed using TraceFinder software (Thermo Fisher Scientific, Waltham, Massachusetts) and Progenesis QI (Nonlinear Dynamics, Newcastle upon Tyne, United Kingdom). Detailed methods of plasma metabolomic profiling have been described previously [21]. A total of 504 metabolites were detected with known identification. Metabolites with coefficients of variation > 15% or missing rates > 20% were removed. Eventually, 467 metabolites were included in the current analysis. Missing values (under detectable levels) were imputed with half of the minimum value for a given metabolite. A rank-based inverse normal transformation was performed for the metabolite data before analyses [22, 23].
HIV Infection Related Assessments
HIV infection was ascertained at baseline and each follow-up visit for those who tested seronegative using enzyme-linked immunosorbent assay (ELISA) and when positive, confirmed by western blot. CD4+ T-cell counts were measured by flow cytometry in AIDS Clinical Trials Group-certified laboratories. HIV-1 viral load was quantified using the isothermal nucleic acid sequence-based amplification method. The lower limit of quantification for HIV-1 viral load was 20 copies per milliliter. Antiretroviral therapy exposure and virologic suppression status were assessed using a standard method [24].
Statistical Analysis
A total of 91 women who had both BMD data and gut microbiome data were included in the main analysis. Characteristics of participants were compared by normal versus low BMD status, using Fisher’s exact test for categorical variables and Mann-Whitney U test for continuous variables. The α-diversity indices (Chao 1 index, Shannon index, and Simpson’s index) at genus level were compared by BMD and HIV serostatus using the Mann-Whitney U test. β-diversity Bray-Curtis distance metrics were calculated to estimate the dissimilarity of microbial community compositions in different groups. Permutational multivariate ANOVA (PerMANOVA) and principal coordinates analysis (PCoA) were used for the microbial β-diversity analyses. Cumulative sum scaling normalization was applied to the genus-level abundance of taxonomic units for subsequent analyses [25]. Linear discriminant analysis (LDA) effect size (LefSe) was used to identify bacterial genera associated with low BMD, with an LDA score of 2.0 as cutoff [26]. Multivariable linear regression models were further used to examine the associations between identified gut bacterial genera and low BMD, after adjustment for age, race (Black, Hispanic, or other), annual income (≥$12,000 or <$12,000), education (less than high school or high school and above), body mass index (BMI), a potential confounder of marijuana use (yes or no)[15, 27, 28], menopausal status (yes, no, or N/A due to hysterectomy; defined by self-reported menopause at 2 consecutive visits for women aged ≥45 years), nucleoside reverse transcriptase inhibitor (NRTI) use (yes or no), non-nucleoside reverse transcriptase inhibitor (NNRTI) use (yes or no), protease inhibitors (PI) use (yes or no), HIV serostatus (positive or negative), and HIV viral load (detectable or undetectable). Sensitivity analyses were conducted by further adjustment for calcium and/or vitamin D supplement use and physical activity levels, and excluding pre-menopausal women.
From the 91 women, a subset of women (n=76) who also had metabolomic data was included in the association analysis of plasma metabolites with BMD status. Partial least-squares discriminant analysis (PLS-DA) was used to identify plasma metabolites associated with low BMD with variable importance in projection (VIP) score of 2.0 as a cutoff [29]. We further used multivariable linear regression models to assess the associations between identified metabolites and low BMD, after adjustment for age, race, annual income, education, BMI, marijuana use, menopausal status, NRTI use, NNRTI use, PI use, HIV serostatus, and HIV viral load. Benjamini-Hochberg false discovery rate (FDR) method was used to correct multiple testing [30]. Sensitivity analyses were conducted by further adjustment for calcium and/or vitamin D supplement use and physical activity levels, and excluding pre-menopausal women. For the secondary correlation analyses of gut microbiota and plasma metabolites, we included 418 women who had both gut microbiome and metabolome data. Spearman correlation analysis was conducted to estimate the correlation coefficients among identified plasma metabolites and BMD-related gut bacterial genera. A two-sided P < 0.05 was considered statistically significant. All analyses were performed using R version 3.6.3 (https://www.r-project.org/) with the phyloseq [31] and vegan [32] packages.
Results
Participant Characteristics
A total of 91 women with a mean age of 53.8 years were included in the primary analyses. Table 1 shows the characteristics of study participants by BMD status including 31 with low BMD and 60 with BMD in the normal range. Comparisons of demographic, socioeconomic, and behavioral variables between the normal-BMD and low-BMD groups were generally similar, although not unexpectedly the women with low BMD tended to be older (P=0.053) and had lower BMI (P=0.034) compared to women with the normal-BMD. As expected, women with low BMD had a lower spine and hip BMD and T-scores (all P<0.001). The HIV status did not differ by BMD status. Among the women with HIV, all were receiving ART and the majority (81.0%) had CD4 counts 500 cells/mm3 or greater, as well as undetectable HIV RNA viral load (82.8%). There were no significant differences in CD4 T-cell count and viral suppression status between HIV+ BMD groups.
Table 1.
Characteristics of study participants
| Women with normal BMD (n=60) | Women with low BMD (n=31) | P-value | |
|---|---|---|---|
| Age, years | 53.2 ± 4.6 | 55.0 ± 4.5 | 0.053 |
| BMI, kg/m 2 | 31.2 ± 6.4 | 28.6 ± 8.6 | 0.034 |
| Race | |||
| Black | 44 (73.3) | 20 (64.5) | 0.13 |
| Hispanic | 12 (20.0) | 11 (35.5) | |
| Other | 4 (6.7) | 0 (0.0) | |
| Annual income, ≥ $12,000 | 37 (61.7) | 22 (71.0) | 0.66 |
| Education, less than high school | 43 (71.7) | 27 (87.1) | 0.12 |
| Smoking | |||
| Never | 8 (13.3) | 1 (3.2) | 0.25 |
| Current | 30 (50.0) | 20 (64.5) | |
| Former | 22 (36.7) | 10 (32.3) | |
| Drinking | |||
| Abstainer | 33 (55.0) | 18 (58.1) | 0.59 |
| <7 drinks/week | 21 (35.0) | 9 (29.0) | |
| 7–12 drinks/week | 2 (3.3) | 3 (9.7) | |
| >12 drinks/week | 4 (6.7) | 1 (3.2) | |
| Physical activity | 0.42 | ||
| Physically inactive | 7 (11.7) | 3 (9.7) | |
| Moderately active | 32 (53.3) | 21 (67.7) | |
| Very active | 21 (35.0) | 7 (22.6) | |
| Calcium and/or vitamin D supplement use, yes | 6 (10.0) | 8 (25.8) | 0.07 |
| Marijuana use, yes | 10 (16.7) | 5 (16.1) | 1.00 |
| Menopause | |||
| Yes | 36 (60.0) | 25 (80.6) | 0.15 |
| No | 17 (28.3) | 4 (12.9) | |
| N/A due to hysterectomy | 7 (11.7) | 2 (6.5) | |
| L1L4 BMD, g/cm 2 | 1.3 ± 0.1 | 1.0 ± 0.2 | <0.001 |
| L1L4 T-score | 1.0 ± 1.2 | −1.7 ± 1.3 | <0.001 |
| Hip BMD, g/cm 2 | 1.1 ± 0.1 | 0.9 ± 0.1 | <0.001 |
| Hip T-score | 0.7 ± 1.0 | −1.2 ± 0.9 | <0.001 |
| HIV+ serostatus | 38 (63.3) | 20 (64.5) | 1.00 |
| HIV-specific factors (HIV+ only) | |||
| CD4 T-cell count ≥500 cells/mm3 | 30 (78.9) | 17 (85.0) | 0.73 |
| Undetectable viral load, ≤20 copies/ml | 31 (81.6) | 17 (85.0) | 0.99 |
| HIV viral load, copies/ml* | 46.0 (35.0, 5026.0) | 91.0 (80.0, 480.0) | <0.001 |
| NRTI use, yes | 36 (94.7) | 19 (95.0) | 1.00 |
| NNRTI use, yes | 10 (26.3) | 8 (40.0) | 0.37 |
| PI use, yes | 15 (39.5) | 6 (30.0) | 0.57 |
Data are presented as mean ± standard error or median (interquartile range) for continuous variables or n (%) for categorical variables.
Median (interquartile range) of the HIV viral load was calculated among the women with detectable viral load only.
HIV-specific characteristics (high CD4 T-cell count, viral load, and undetectable viral load) were analyzed only in the HIV+ women.
P values for comparisons between normal and low BMD groups were estimated from the Mann-Whitney U test for continuous variables and Fisher’s exact test for categorical variables.
The low-BMD group was defined as the T-scores for either lumbar spine or total hip were less than −1.0, and the normal-BMD group was defined as the T-scores for the lumbar spine and total hip were greater than or equal to −1.0.
BMD, bone mineral density; BMI, body mass index; HIV, human immunodeficiency virus; NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; and PI, protease inhibitors.
Gut Microbiome and BMD Status
First, we examined the relationship between three different measures of bacterial community α-diversity (i.e., Chao 1, Shannon, and Simpson) and BMD status, and did not find associations irrespective of HIV serostatus (all P > 0.05, Supplementary Figure 1A). We also used PCoA to visualize the Bray-Curtis dissimilarity among the samples. As shown in Supplementary Figure 1B, the first and second principal coordinates explained 27% and 17% of the total variance, and no clear separation was observed by BMD status or HIV serostatus. No significant associations of β-diversity with BMD or HIV serostatus were observed in the PerMANOVA analysis (both R2 < 0.1, P > 0.05).
Among the 81 identified gut microbial genera (relative abundance ≥ 0.01% and prevalence ≥ 10%), the LEfSe analysis showed that six genera were associated with BMD status (all LDA scores > 2.0) (Figure 1A). Five of them (Dorea, Megasphaera, unclassified Lachnospiraceae, Ruminococcus, and Mitsuokella) were more abundant, and one other (unclassified Mollicutes RF39) was less abundant in the low-BMD group compared to the normal-BMD group. As the prevalence (17.6%) and relative abundance (0.9%) of unclassified Mollicutes RF39 were relatively low, and it can only be classified at the order level, we focused on the other five low BMD-associated genera in the subsequent analyses. All of the five genera belong to the Clostridiales order, within the most abundant phylum Firmicutes (Figure 1B). After multivariable adjustment and further adjustment for calcium and/or vitamin D supplement intake and physical activity levels, the associations between the five bacterial genera and low BMD did not change (Supplementary Tables 1 and 2). We also got similar results after excluding the pre-menopausal women (Supplementary table 3). The associations of these bacterial genera with BMD were generally consistent between women with and without HIV infection, except for Mitsuokella. The positive association between Mitsuokella and low BMD was only observed in women with HIV infection but not in those without HIV infection (P for interaction = 0.03) (Supplementary Table 1).
Figure 1.
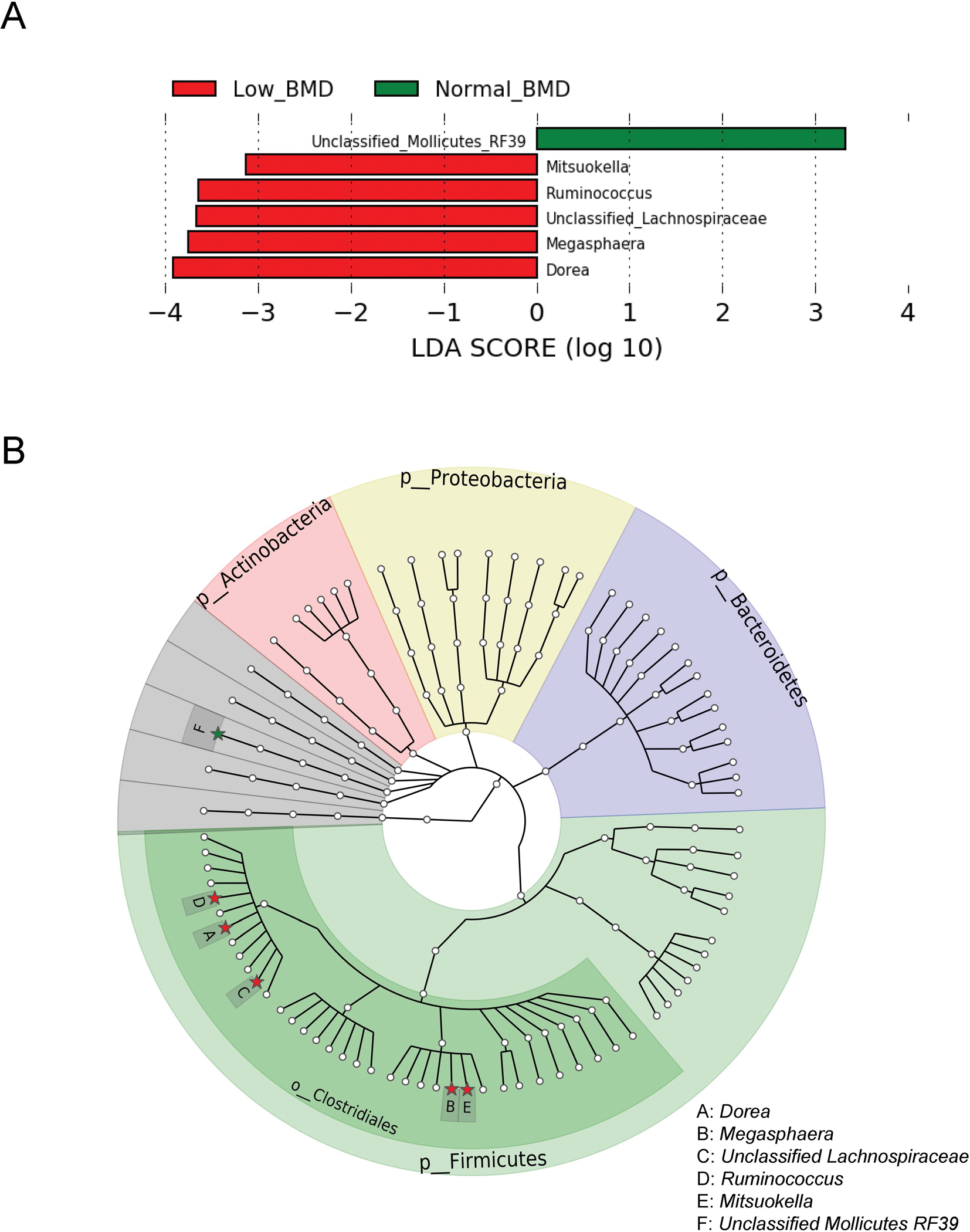
Microbial genera associated with BMD status.
A. Taxonomic linear discriminative analysis (LDA) effect size by BMD status.
B. Phylogenetic tree of taxonomic features associated with BMD status. Genera associated with BMD status were highlighted as solid stars and noted by capital letters.
Green bars and stars indicate the genus was enriched in the normal-BMD group, whereas red ones indicate the genus was enriched in the low-BMD group. BMD, bone mineral density.
We found no associations of these bacterial genera with HIV serostatus, CD4 T-cell count, and HIV viral load (Supplementary Table 4).
Plasma Metabolites and BMD Status
The PLS-DA revealed that the metabolite profile of women with low BMD was different from those with normal BMD (Figure 2A). A total of 27 metabolites were main contributors by BMD status (VIP scores > 2.0) as selected by PLS-DA in unadjusted analyses. After multivariable adjustment, 17 of the selected metabolites were still associated with BMD status (all FDR-adjusted P < 0.05, Supplementary Tables 5). The association results were similar after further adjustment for calcium and/or vitamin D supplement intake and physical activity levels (Supplementary Table 6), as well as excluding the pre-menopausal women (Supplementary table 7). Of the 17 identified metabolites, metabolites within the same class (e.g., 1,7-dimethyluric acid and 5-acetylamino-6-amino-3-methyluracil produced from caffeine) showed moderate-to-high correlations with each other (Supplementary Figure 2). Plasma serine was higher, while levels of the other 16 metabolites were lower in women with low BMD compared with those with normal BMD (Figure 2B). We did not find effect modification by HIV serostatus on the associations between these metabolites and low BMD (All P for interaction ≥ 0.09, Supplementary Table 5). We also examined the associations of these metabolites with HIV-specific characteristics and found that 5 metabolites (creatinine, N1-methyl-2-pyridone-5-carboxamide, dimethylglycine, 4-pyridoxic acid, and retinol) were higher, while 2 metabolites (homoarginine and serine) were lower in women with HIV infection compared to those without infection (Supplementary Table 8). In addition, three metabolites (caffeine, retinol, and dimethylglycine) were associated with CD4 T-cell count. Caffeine and retinol were enriched, while dimethylglycine was depleted in women with higher CD4 T-cell counts. However, no metabolites were associated with viral load.
Figure 2.
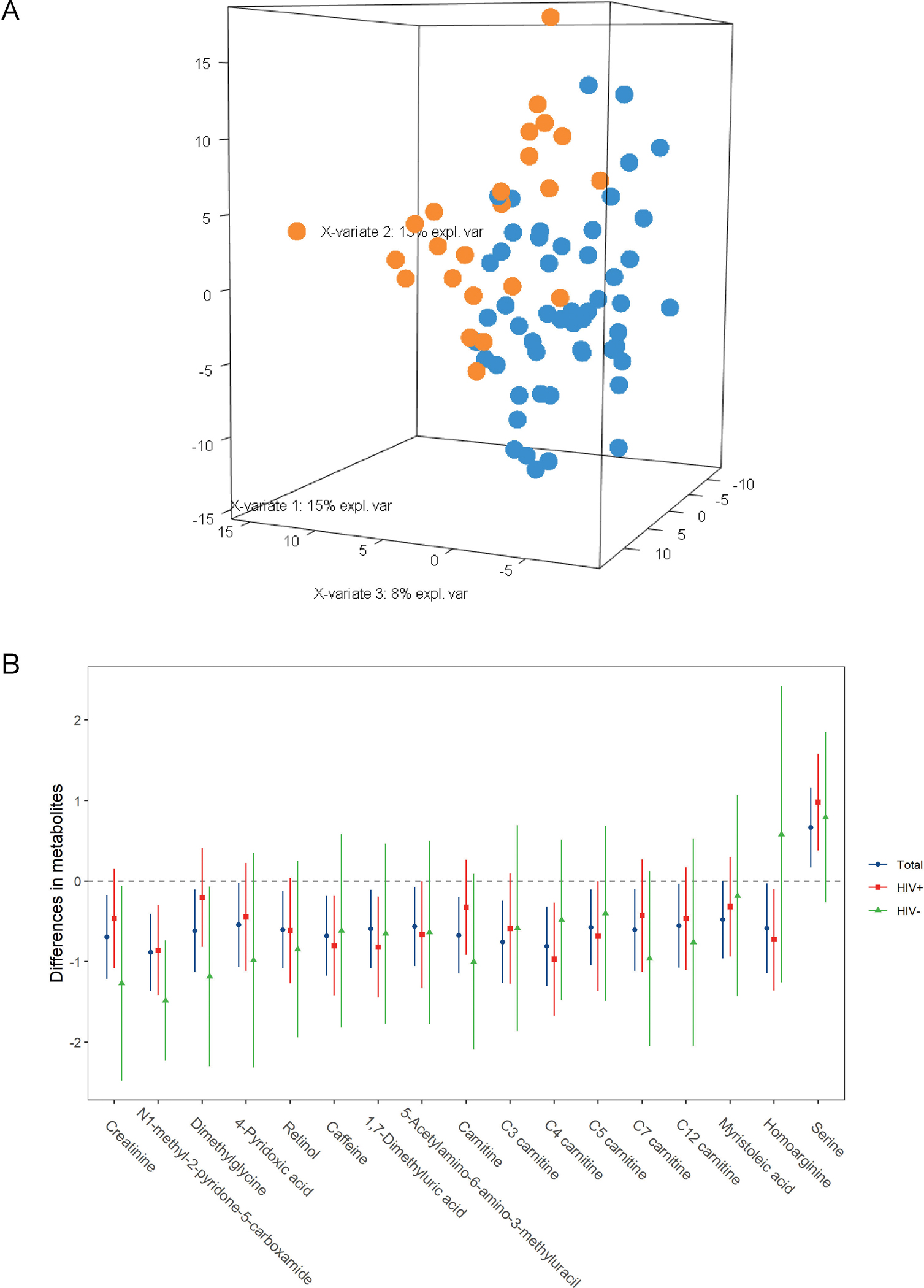
Plasma metabolites associated with BMD status.
A. PLS-DA plot on BMD status
B. Error bar plot of metabolites associated with low BMD among total, HIV+, and HIV− groups. The number of participants was 76, 50, and 26 in the total, HIV+, and HIV− groups, respectively. These metabolites were selected by PLS-DA (VIP scores ≥2) in total samples. Data are differences (95% confident intervals) in metabolites between women with low and normal BMD, after adjustment for age, race, education, annual income, smoking, alcohol drinking, marijuana use, body mass index, menopausal status, three types of antiviral therapies and HIV viral load (HIV+ and total samples only), and HIV serostatus (total samples only).
BMD, bone mineral density.
Gut Microbiome and Plasma Metabolites
We further examined correlations of the BMD status-associated gut bacterial genera and host plasma metabolites among 418 women who had both gut microbiome and plasma metabolomics data. As shown in Figure 3, four of the five BMD-related bacterial genera were associated with at least one of the selected 17 metabolites in all samples (r= −0.21 to −0.14, all FDR-adjusted P < 0.05). The four genera (Dorea, Megasphaera, unclassified Lachonospiraceae, and Ruminococcus) were all inversely correlated with plasma creatinine (all FDR-adjusted P < 0.05). Megasphaera was inversely correlated with plasma 4-pyridoxic acid, C4 carnitine, C5 carnitine, and dimethylglycine (all FDR-adjusted P < 0.05). Dorea was also inversely associated with plasma 4-pyridoxic acid and C4 carnitine (both FDR-adjusted P < 0.05). The correlations between bacterial genera and plasma metabolites identified from the total population were generally consistent in women with and without HIV infection, although most of them were not significant in women without HIV infection, which might be due to relatively small sample size in this group (n=138). In addition, the inverse correlation between Megasphaera and dimethylglycine was more pronounced in women with HIV infection compared to those without HIV infection (P for interaction = 0.016).
Figure 3.
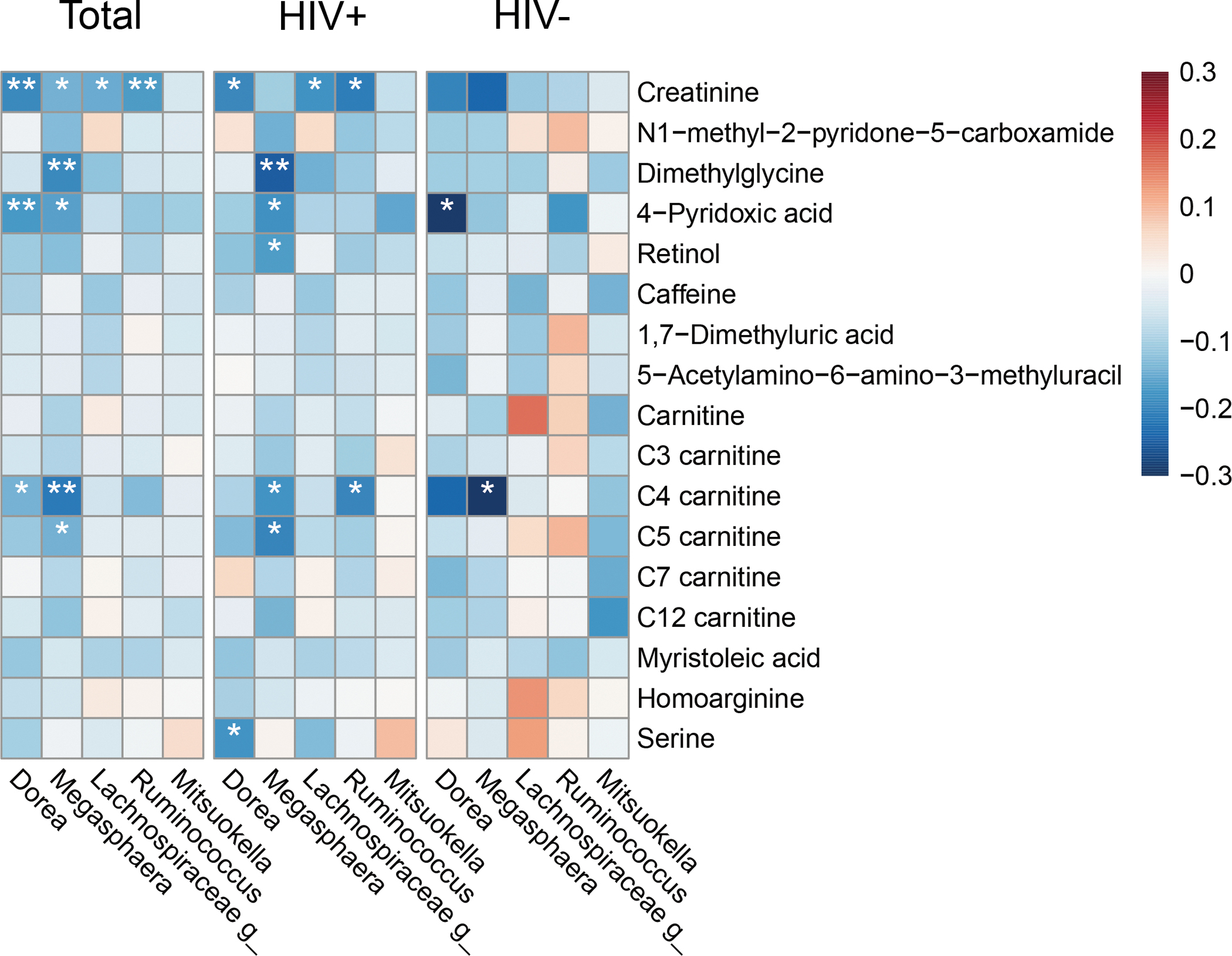
Spearman correlation heatmap of BMD-associated gut bacterial genera and BMD-associated plasma metabolites.
The number of participants was 418, 280, and 138 in the total, HIV+, and HIV− groups, respectively. HIV, human immunodeficiency virus.
*False discovery rate-adjusted P < 0.05; **False discovery rate-adjusted P < 0.01.
Discussion
To the best of our knowledge, this is the first study to examine the associations of gut microbiota and plasma metabolites with BMD status in the context of HIV infection. Previous studies in populations without HIV have investigated the relationship between gut microbial composition and BMD but results are controversial [9–11, 33]. While one study reported that post-menopausal women with osteoporosis tended to have the lowest α-diversity compared with those with osteopenia and normal BMD [33], similar to our findings, other studies that included post-menopausal women or both sexes did not observe a significant difference in α-diversity among different BMD groups [9–11]. Future studies with larger sample sizes are warranted to investigate the relationship between gut bacterial composition and BMD status.
At the genus level, we observed that the relative abundances of five genera were higher in women with low BMD compared to those with normal BMD. These five genera belong to the order Clostridiales. Two genera (Megasphaera and Mitsuokella) belong to the family Veillonellaceae and three genera (Dorea, Ruminococcus, and unclassified Lachnospiraceae) belong to the family Lachnospiraceae. These results were partially consistent with studies that reported several genera from the Clostridiales order that were higher in low-BMD or osteoporosis groups compared to those with normal-BMD [9–11]. More specifically, in agreement with a study in post-menopausal women [33], we found that Megasphaera was enriched in women with low BMD. This study also observed that Megasphaera was positively associated with markers of bone formation (N-terminal propeptide of type I procollagen) and resorption (C-telopeptide of type I collagen) [33], which indicated that this bacterial genus might reflect high bone metabolic turnover. We also found that Megasphaera was inversely associated with several metabolites that were associated with lower levels in the low-BMD group compared to the normal-BMD group. Taken together, these data suggest that gut Megasphaera might be associated with an unfavorable metabolite profile for bone health, though further population and experimental studies are needed to validate our findings and investigate the potential mechanisms.
Similar to Megasphaera, another genus Mitsuokella, in family Veillonellaceae, was enriched in the low-BMD group. This is partially consistent with the aforementioned study in post-menopausal women, which reported that family Veilonellaceae was enriched in the osteopenia group compared to women with normal BMD[33]. Interestingly, the association of Mitsuokella with low BMD was stronger in women with HIV compared to women without HIV, suggesting potential effect modification, which needs further investigation. The other three genera enriched in the low-BMD group all belong to family Lachnospiraceae. In support of our findings, a study using a two-sample Mendelian randomization approach found and successfully replicated an inverse causal association of Clostridiales and Lachnospiraceae with estimated heel BMD in the Caucasians [34]. In contrast, another study in the Chinese elderly reported that the abundance of Clostridiales and Lachnospiraceae was positively associated with BMD and T-scores [10]. More studies are needed to clarify the relationship between gut Lachnospiraceae and bone health and underlying mechanisms.
In agreement with previous metabolomic studies in populations without HIV[12, 35], we also observed distinct plasma metabolite profiles between women with low BMD and those with normal BMD. Interestingly, most of the metabolites were lower in the low-BMD than normal-BMD groups. Congruent with our findings, the Hordaland Health Study also found that lower dimethylglycine levels were associated with higher odds of low BMD [36]. Of note, HIV infection tended to modify this association, which was stronger among women without HIV compared to women with HIV (P-interaction = 0.088). Dimethylglycine belongs to the choline oxidation pathway and is demethylated for the formation of sarcosine and glycine in mitochondria [37]. It has been proposed that dimethylglycine might modulate bone metabolism by increasing the activity of peroxisome proliferator-activated receptor alpha [36, 38]. Notably, the genus Megasphaera was inversely associated with plasma dimethylglycine. This is concordant with a previous study showing that Megashpaera elsdenii carried electron-transferring flavoprotein [39], a substrate of the reaction converting dimethylglycine to sarcosine (glycine betaine degradation I pathway) [40]. Intriguingly, the association between Megasphaera and dimethylglycine was also modified by HIV infection status (P-interaction = 0.016). Together, these suggest a complex interplay of HIV infection, Megasphaera, and dimethylglycine on BMD, though further functional studies are warranted to unravel the underlying mechanisms. Consistent with published studies [41, 42], we also found that plasma creatinine level was lower in women with low BMD compared to those with normal BMD. In adults with normal kidney function, circulating creatinine is considered a surrogate marker of skeletal muscle mass [43], which has been positively associated with BMD [44]. Moreover, lower levels of creatinine were correlated with four gut bacterial genera enriched in the low-BMD group. It has been shown that creatinine could be eliminated from the host by intestinal microbiota [45]. These data suggest that these unfavorable bacteria might reduce host creatinine levels, although their impact on bone health is unclear. Nevertheless, since diet influences serum creatinine levels [46] and gut microbiota [47], we cannot rule out potential confounding by dietary factors. In addition, previous studies in populations without HIV infection also found that caffeine-associated metabolites and homoarginine were associated with higher BMD [48, 49].
Our study has several limitations. The small sample size limits the power to identify associations of gut bacteria and plasma metabolites with BMD status, which may be modified by HIV serostatus. Due to the observational nature and cross-sectional study design, we are unable to demonstrate causal relationships among gut bacteria, plasma metabolites, and BMD. Future prospective studies with larger sample sizes are needed to better understand the relationships of gut microbiota and plasma metabolites with bone health in the context of HIV infection. Furthermore, dietary intake and immune activation were not measured in this study. In addition, the 16S sequencing method limits our investigation of the microbial species and functional profiles related to BMD. Finally, this study only focused on women, and findings need to be validated in men and other HIV-infected populations.
In summary, this study found that alterations of several gut microbial genera and related plasma metabolites were associated with BMD status in women with HIV infection or a group of women at risk of HIV infection. In addition, there was a potential effect modification of HIV infection on the interrelationships among the genus Megasphaera, plasma dimethylglycine, and BMD. Future studies with larger sample sizes and metagenomics data are warranted to shed light on the specific microbes and their functional roles along with metabolite profiles in bone metabolism in the context of HIV infection.
Supplementary Material
Acknowledgements
Q.Q., Z.M., M.T.Y, and A.S. designed the study. M.T.Y., A.S., Z.W., A.C., K.W., R.R., D.G., R.C.K., R.D.B., and Q.Q. contributed to data collection. Z.M. and Z.W. performed statistical analyses. Z.M. drafted the manuscript. Q.Q., M.T.Y., and A.S. critically revised the manuscript. All authors edited and reviewed the manuscript. Q.Q. is the guarantor of this work and has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final version of the manuscript.
This study was supported by the National Heart, Lung, and Blood Institute (NHLBI) K01HL129892 and R01HL140976, and Feldstein Medical Foundation Research Grant to Q.Q., R.C.K. was supported by NHLBI 5R01HL126543, R01 HL132794 and the National Institute on Mental Health (NIMH) 5R01MD011389–03, and J.R.K. was supported by NHLBI R01 HL132794 and K24 HL135413. Other funding sources include NHLBI R01HL083760, and R01HL095140, the National Institute of Allergy and Infectious Diseases (NIAID) U01 AI035004, and the Einstein Cancer Research Center (P30CA013330), the Einstein Liver Research Center (P30DK041296), the Einstein-Rockefeller-CUNY Center for AIDS Research funded by the NIAID (P30AI124414), and the Stable Isotope and Metabolomics Core Facility of the Einstein-Mount Sinai Diabetes Research Center (ES-DRC) of the Albert Einstein College of Medicine funded by National Cancer Institute (P60DK020541). M.T.Y. was supported by R01 AI-095089.
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos and Anjali Sharma), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; ConnieWofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR000454 (Atlanta CTSA), and P30-AI-050410 (UNC CFAR).
Footnotes
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
- 1.Moore AL, Vashisht A, Sabin CA, Mocroft A, Madge S, Phillips AN, et al. Reduced bone mineral density in HIV-positive individuals. AIDS (London, England) 2001; 15(13):1731–1733. [DOI] [PubMed] [Google Scholar]
- 2.Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Lo Y, Klein RS. Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. AIDS (London, England) 2007; 21(5):617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tebas P, Powderly WG, Claxton S, Marin D, Tantisiriwat W, Teitelbaum SL, et al. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS (London, England) 2000; 14(4):F63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruera D, Luna N, David DO, Bergoglio LM, Zamudio J. Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS (London, England) 2003; 17(13):1917–1923. [DOI] [PubMed] [Google Scholar]
- 5.Teichmann J, Stephan E, Lange U, Discher T, Friese G, Lohmeyer J, et al. Osteopenia in HIV-infected women prior to highly active antiretroviral therapy. The Journal of infection 2003; 46(4):221–227. [DOI] [PubMed] [Google Scholar]
- 6.Amiel C, Ostertag A, Slama L, Baudoin C, N’Guyen T, Lajeunie E, et al. BMD is reduced in HIV-infected men irrespective of treatment. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2004; 19(3):402–409. [DOI] [PubMed] [Google Scholar]
- 7.Sharma A, Ma Y, Scherzer R, Wheeler AL, Cohen M, Gustafson DR, et al. Brief Report: Association of Adipokines With Bone Mineral Density in HIV-Infected and HIV-Uninfected Women. Journal of acquired immune deficiency syndromes (1999) 2016; 73(4):433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tinago W, Cotter AG, Sabin CA, Macken A, Kavanagh E, Brady JJ, et al. Predictors of longitudinal change in bone mineral density in a cohort of HIV-positive and negative patients. AIDS (London, England) 2017; 31(5):643–652. [DOI] [PubMed] [Google Scholar]
- 9.Das M, Cronin O, Keohane DM, Cormac EM, Nugent H, Nugent M, et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology (Oxford, England) 2019; 58(12):2295–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Huang Q, Yang R, Dai Y, Zeng Y, Tao L, et al. Gut microbiota composition and bone mineral loss-epidemiologic evidence from individuals in Wuhan, China. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2019; 30(5):1003–1013. [DOI] [PubMed] [Google Scholar]
- 11.Palacios-González B, Ramírez-Salazar EG, Rivera-Paredez B, Quiterio M, Flores YN, Macias-Kauffer L, et al. A Multi-Omic Analysis for Low Bone Mineral Density in Postmenopausal Women Suggests a RELATIONSHIP between Diet, Metabolites, and Microbiota. Microorganisms 2020; 8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Q, Shen H, Su KJ, Zhang JG, Tian Q, Zhao LJ, et al. Metabolomic profiles associated with bone mineral density in US Caucasian women. Nutrition & metabolism 2018; 15:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi H, Bao J, An G, Ouyang G, Zhang P, Wang C, et al. Association between the metabolome and bone mineral density in pre- and post-menopausal Chinese women using GC-MS. Molecular bioSystems 2016; 12(7):2265–2275. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Yan D, Zhao A, Hou X, Zheng X, Chen P, et al. Discovery of potential biomarkers for osteoporosis using LC-MS/MS metabolomic methods. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2019; 30(7):1491–1499. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Usyk M, Sollecito CC, Qiu Y, Williams-Nguyen J, Hua S, et al. Altered Gut Microbiota and Host Metabolite Profiles in Women With Human Immunodeficiency Virus. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2020; 71(9):2345–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12(9):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma A, Ma Y, Tien PC, Scherzer R, Anastos K, Cohen MH, et al. HIV Infection Is Associated With Abnormal Bone Microarchitecture: Measurement of Trabecular Bone Score in the Women’s Interagency HIV Study. Journal of acquired immune deficiency syndromes (1999) 2018; 78(4):441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organization technical report series 1994; 843:1–129. [PubMed] [Google Scholar]
- 19.Moon JY, Zolnik CP, Wang Z, Qiu Y, Usyk M, Wang T, et al. Gut microbiota and plasma metabolites associated with diabetes in women with, or at high risk for, HIV infection. EBioMedicine 2018; 37:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature biotechnology 2019; 37(8):852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi Q, Hua S, Clish CB, Scott JM, Hanna DB, Wang T, et al. Plasma Tryptophan-Kynurenine Metabolites Are Altered in Human Immunodeficiency Virus Infection and Associated With Progression of Carotid Artery Atherosclerosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2018; 67(2):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mei Z, Chen GC, Wang Z, Usyk M, Yu B, Baeza YV, et al. Dietary factors, gut microbiota, and serum trimethylamine-N-oxide associated with cardiovascular disease in the Hispanic Community Health Study/Study of Latinos. The American journal of clinical nutrition 2021; 113(6):1503–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J, Loos RJ, Powell JE, Medland SE, Speliotes EK, Chasman DI, et al. FTO genotype is associated with phenotypic variability of body mass index. Nature 2012; 490(7419):267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanna DB, Post WS, Deal JA, Hodis HN, Jacobson LP, Mack WJ, et al. HIV Infection Is Associated With Progression of Subclinical Carotid Atherosclerosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2015; 61(4):640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paulson JN, Stine OC, Bravo HC, Pop M. Differential abundance analysis for microbial marker-gene surveys. Nature methods 2013; 10(12):1200–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome biology 2011; 12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fulcher JA, Hussain SK, Cook R, Li F, Tobin NH, Ragsdale A, et al. Effects of Substance Use and Sex Practices on the Intestinal Microbiome During HIV-1 Infection. The Journal of infectious diseases 2018; 218(10):1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sophocleous A, Robertson R, Ferreira NB, McKenzie J, Fraser WD, Ralston SH. Heavy Cannabis Use Is Associated With Low Bone Mineral Density and an Increased Risk of Fractures. The American journal of medicine 2017; 130(2):214–221. [DOI] [PubMed] [Google Scholar]
- 29.Westerhuis JA, Hoefsloot HC, Smit S, Vis DJ, Smilde AK, van Velzen EJ, et al. Assessment of PLSDA cross validation. Metabolomics 2008; 4(1):81–89. [Google Scholar]
- 30.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 1995; 57(1):289–300. [Google Scholar]
- 31.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS one 2013; 8(4):e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oksanen J, Kindt R, Legendre P, O’Hara B, Stevens MHH, Oksanen MJ, et al. The vegan package. Community ecology package 2007; 10(631–637):719. [Google Scholar]
- 33.He J, Xu S, Zhang B, Xiao C, Chen Z, Si F, et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging 2020; 12(9):8583–8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni JJ, Yang XL, Zhang H, Xu Q, Wei XT, Feng GJ, et al. Assessing causal relationship from gut microbiota to heel bone mineral density. Bone 2021; 143:115652. [DOI] [PubMed] [Google Scholar]
- 35.Mei Z, Dong X, Qian Y, Hong D, Xie Z, Yao G, et al. Association between the metabolome and bone mineral density in a Chinese population. EBioMedicine 2020; 62:103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Øyen J, Svingen GF, Gjesdal CG, Tell GS, Ueland PM, Lysne V, et al. Plasma dimethylglycine, nicotine exposure and risk of low bone mineral density and hip fracture: the Hordaland Health Study. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2015; 26(5):1573–1583. [DOI] [PubMed] [Google Scholar]
- 37.Lever M, George PM, Dellow WJ, Scott RS, Chambers ST. Homocysteine, glycine betaine, and N,N-dimethylglycine in patients attending a lipid clinic. Metabolism: clinical and experimental 2005; 54(1):1–14. [DOI] [PubMed] [Google Scholar]
- 38.Stunes AK, Westbroek I, Gustafsson BI, Fossmark R, Waarsing JH, Eriksen EF, et al. The peroxisome proliferator-activated receptor (PPAR) alpha agonist fenofibrate maintains bone mass, while the PPAR gamma agonist pioglitazone exaggerates bone loss, in ovariectomized rats. BMC endocrine disorders 2011; 11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato K, Nishina Y, Shiga K. Purification of electron-transferring flavoprotein from Megasphaera elsdenii and binding of additional FAD with an unusual absorption spectrum. Journal of biochemistry 2003; 134(5):719–729. [DOI] [PubMed] [Google Scholar]
- 40.Smith LT, Pocard JA, Bernard T, Le Rudulier D. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. Journal of bacteriology 1988; 170(7):3142–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moayyeri A, Cheung CL, Tan KC, Morris JA, Cerani A, Mohney RP, et al. Metabolomic Pathways to Osteoporosis in Middle-Aged Women: A Genome-Metabolome-Wide Mendelian Randomization Study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2018; 33(4):643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Xu H, Li GH, Long MT, Cheung CL, Vasan RS, et al. Metabolomics Insights into Osteoporosis Through Association With Bone Mineral Density. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huh JH, Choi SI, Lim JS, Chung CH, Shin JY, Lee MY. Lower Serum Creatinine Is Associated with Low Bone Mineral Density in Subjects without Overt Nephropathy. PloS one 2015; 10(7):e0133062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu K, Briffa K, Smith A, Mountain J, Briggs AM, Lye S, et al. Gender differences in the relationships between lean body mass, fat mass and peak bone mass in young adults. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2014; 25(5):1563–1570. [DOI] [PubMed] [Google Scholar]
- 45.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiological reviews 2000; 80(3):1107–1213. [DOI] [PubMed] [Google Scholar]
- 46.Pimenta E, Jensen M, Jung D, Schaumann F, Boxnick S, Truebel H. Effect of Diet on Serum Creatinine in Healthy Subjects During a Phase I Study. Journal of clinical medicine research 2016; 8(11):836–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nature reviews Gastroenterology & hepatology 2019; 16(1):35–56. [DOI] [PubMed] [Google Scholar]
- 48.Chau YP, Au PCM, Li GHY, Sing CW, Cheng VKF, Tan KCB, et al. Serum Metabolome of Coffee Consumption and its Association With Bone Mineral Density: The Hong Kong Osteoporosis Study. The Journal of clinical endocrinology and metabolism 2020; 105(3). [DOI] [PubMed] [Google Scholar]
- 49.Pilz S, Meinitzer A, Tomaschitz A, Kienreich K, Dobnig H, Schwarz M, et al. Associations of homoarginine with bone metabolism and density, muscle strength and mortality: cross-sectional and prospective data from 506 female nursing home patients. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2013; 24(1):377–381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


