Abstract
Antibodies reactive with C polysaccharide (PS) were found in healthy adults, pneumococcal patients, and vaccinees. These antibodies were not directed to the phosphocholine determinant of the C PS, as they appear to be in mice, since the human antibodies were inhibitable only with C PS. We found another population of phosphocholine-specific antibodies inhibitable only by phosphocholine and related compounds.
The pneumococcal C polysaccharide (C PS) is a cell wall surface PS common to all pneumococci. The chemical composition and initial structural studies on C PS were first reported by Liu and Gotschlich (11). The complete structure of C PS was shown to contain two phosphocholine (PC) moieties per repeat unit (5, 8); however, more recent information shows that the C PSs from some strains have only one PC per repeat (9). The C PS is covalently attached to the cell wall peptidoglycan and through the peptidoglycan to the type-specific capsular PS (15). The purified type-specific PSs therefore contain contaminating C PS, meaning that the licensed 23-valent pneumococcal PS vaccines also contain C PS (15).
Human antibodies to the pneumococcal C PS are not opsonic and not protective (12, 17). Most published studies relating to the specificity of C PS antibodies state that the PC moiety is the immunologically dominant epitope of C PS, based almost entirely on mouse data (1, 14). There are several reports dealing with human antibodies selected for their reactivity to PC (3, 7, 14), but we are not aware of reports examining the epitope specificity of antibodies selected initially for reactivity to purified pneumococcal C PS.
Since the C PS is present in all pneumococcal vaccines, it is important to understand the specificity of human anti-C PS antibodies. It has been reported that the pneumococcal C PS induces anti-PC antibodies and that these antibodies contribute to protection against pneumococcal disease, based upon studies in mice. The present study was therefore undertaken to determine whether human anti-C PS antibodies are PC specific. We examined the epitope specificity of human antibodies to purified C PS in healthy adults and in individuals following vaccination or pneumococcal disease, and we found that C PS antibodies are C PS specific and not inhibitable by PC and that adults also have PC antibodies, largely non-cross-reactive with C PS.
For antibody measurements by enzyme-linked immunosorbent assay (ELISA), C PS, obtained from State Serum Institute of Denmark, was admixed at 3.0 μg/ml with methylated human serum albumin at 3.0 and 1.0 μg/ml and used to coated Immulon-1 plates (Dynatech, Chantilly, Va.), which were then incubated overnight. PC conjugated to bovine serum albumin (PC-BSA) was used to coat Immulon-4 plates at 5 μg/ml of protein. The remainder of the ELISA procedure was as described previously (4). Cross-reactivity and specificity of the C PS and PC antibodies were measured using competitive inhibition, in which a serum dilution from the upper linear region of a dilution curve was mixed with decreasing twofold concentrations of the inhibitors and then added to the antigen-coated ELISA plates.
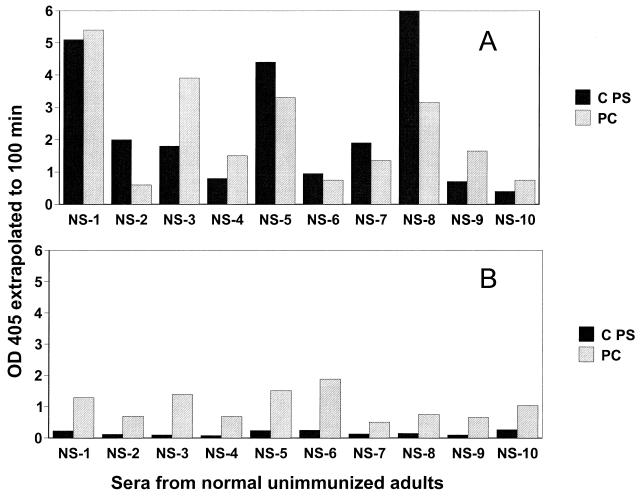
Sera from approximately 50 healthy nonvaccinated adults all contained measurable antibodies to both C PS and PC (using PC-BSA) as measured by ELISA. The relative levels of immunoglobulin G (IgG) and IgM antibody to C PS and to PC in sera from 10 representative healthy adults are shown in Fig. 1. Most of the anti-C PS antibodies were IgG, while similar levels of IgG and IgM antibodies were reactive with PC.
FIG. 1.
Concentrations of antibody to C PS and PC in sera from healthy adults not immunized with the pneumococcal PS vaccine. IgG antibodies (A) and IgM antibodies (B) were measured by ELISA using purified C PS and PC-BSA, all at a serum dilution of 1:800. OD, optical density; NS, normal serum.
Acute- and convalescent-phase sera from six adults with culture-confirmed invasive pneumococcal disease were examined by ELISA, and little or no increase in either anti-C PS or anti-PC antibodies (IgM or IgG) was found in the convalescent-phase sera (data not shown). The antibody levels in acute-phase sera were not different from those of healthy adults.
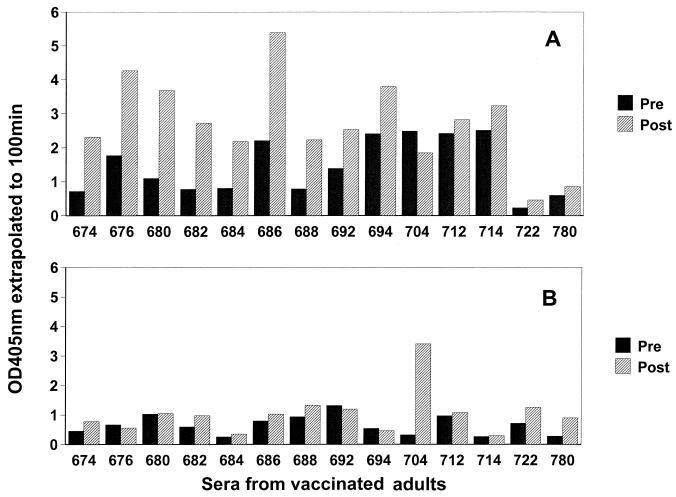
Pre- and postimmunization sera from 24 adults immunized with a 23-valent pneumococcal PS vaccine were examined for increases in IgG and IgM antibodies to C PS and PC. Forty-two percent (10 of 24) of the vaccinees responded with at least a twofold increase in levels of IgG antibody to the C PS, while only 8% (2 of 24) responded with IgM antibodies. In contrast, only one individual (no. 704) responded with a ≥2-fold increase in anti-PC antibodies. The IgG and IgM anti-C PS responses of 14 vaccinees with increased C PS or PC antibodies are shown in Fig. 2. Two vaccinees, no. 704 and 780, had a ≥2-fold increase only in IgM anti-C PS antibodies, with vaccinee 704 having a >10-fold increase.
FIG. 2.
Antibody response to C PS following immunization of adults with a pneumococcal PS vaccine. IgG (A) and IgM (B) antibodies were measured by ELISA before and after immunization. OD, optical density.
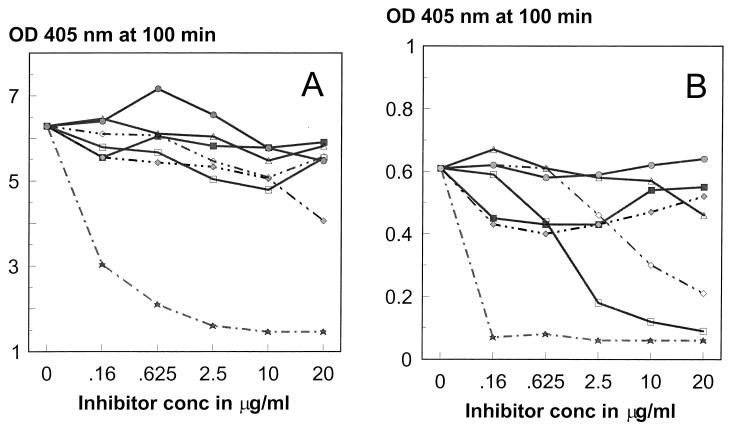
Interestingly, the IgG and IgM antibodies present in the serum of vaccinee 704 had different specificities (Fig. 3). The IgG antibodies were strongly inhibited by C PS, and to a lesser degree by p-nitrophenyl PC, at 20 μg/ml. By contrast, the IgM antibodies were strongly inhibited by C PS, but also by phosphatidylcholine and acetylcholine, while no inhibition was seen with PC. Thus, the IgG antibodies were specific for C PS, but not for a PC epitope, while the IgM antibodies were apparently choline specific.
FIG. 3.
Specificity of anti-C PS antibodies in postvaccination serum (from subject 704). IgG (A) and IgM (B) antibodies were examined for inhibition by C PS (stars), PC (filled squares), p-nitrophenyl PC (solid diamonds), phosphatidylcholine (open squares), acetylcholine (open diamonds), butyrylcholine (open triangles), and tricholine (circles). Note that the y axis maximum values are 8 and 1 for panels A and B, respectively. OD, optical density.
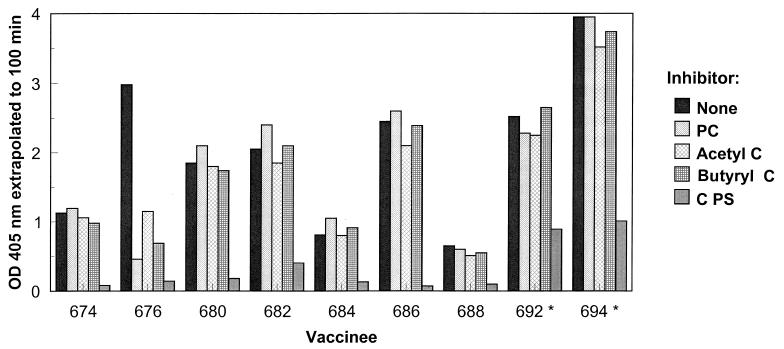
The specificities of IgG antibodies to C PS in postimmunization sera from nine vaccinees are shown in Fig. 4. One individual, no. 676, responded with an increase in IgG antibody to C PS (Fig. 2), but not to PC (data not shown). Antibodies from this individual were maximally inhibited by C PS but were also strongly inhibited by choline. The anti-C PS antibodies in the other eight individuals were inhibited only by C PS.
FIG. 4.
Specificity of IgG antibodies to C PS following immunization of adults with the pneumococcal PS vaccine. The competitive inhibitors at a concentration of 5 μg/ml were PC, acetylcholine (acetyl C), butyrylcholine (butyryl C), and C PS. Purified C PS was attached to the plate. The asterisks indicate that the inhibitor concentration was 10 μg/ml. OD, optical density.
The specificities of IgG antibodies to C PS and to PC in sera of adults postimmunization and in convalescence from pneumococcal bacteremia were compared to those in healthy adults, and one such comparison is shown in Table 1. The antibodies reactive with C PS in each of the three individuals were strongly inhibited only by C PS and much less so by p-nitrophenyl PC. Antibodies reactive with PC in the same individuals were not inhibited by C PS and were maximally inhibited by p-nitrophenyl PC. Thus, anti-PC antibodies are distinctly different from those reactive with the C PS, and the major reactive epitope of anti-C PS antibodies in human sera is not as previously supposed, the PC moiety. By contrast, a mouse monoclonal antibody against PC reacted strongly with both C PS and PC-BSA and was fully inhibited by C PS and PC (data not shown).
TABLE 1.
Specificities of human IgG antibodies reactive with either C PS or PC-BSA attached to the ELISA plate
| ELISA antigen | Individuala | % Inhibition byb:
|
||||||
|---|---|---|---|---|---|---|---|---|
| C PS | PC | P-nitro PC | PT choline | Acetylcholine | Butyrylcholine | Tricholine | ||
| C PS | Vaccinee | 76.8 | 8.1 | 19.7 | 23.8 | 19.2 | 12.9 | 15.9 |
| Healthy adult | 99.3 | 0 | 4.0 | 17.0 | 18.0 | 14.6 | 4.8 | |
| Patient | 99.1 | 17.7 | 23.0 | 19.6 | 23.0 | 12.2 | 1.1 | |
| PC | Vaccinee | 11.5 | 35.3 | 76.1 | 0 | 12.4 | 23.4 | 23.4 |
| Healthy adult | 9.6 | 54.4 | 73.3 | 3.2 | 21.1 | 22.0 | 26.6 | |
| Patient | 12.7 | 42.6 | 79.3 | 6.1 | 47.3 | 44.9 | 53.4 | |
Representative adults included a vaccinee (no. 704), a healthy adult, and a pneumococcal disease patient.
Abbreviations: P-nitro, p-nitrophenyl; PT, phosphatidyl.
To conclude, this is the first study with humans to show two distinct populations of antibodies, one specific for C PS and the other specific for PC. We have examined the specificity of human antibodies selected initially for reactivity with pneumococcal C PS. In a number of earlier studies antibodies were selected for reactivity with PC and then were shown to be PC inhibitable, leading to the assumption that these antibodies were reactive with pneumococcal C PS (2).
The specifications for the purified pneumococcal PSs used in formulation of the 23-valent vaccine manufactured by Merck & Company and by Wyeth-Lederle do not include a specification for allowable C PS. Unpublished studies in our laboratory using 31P nuclear magnetic resonance and an inhibition ELISA indicate that these PSs contain between 5 and 10% (by weight) C PS. This C PS is both free and covalently bound to the capsular PS, probably through peptidoglycan fragments (15). The presence of the C PS interferes with estimation of concentrations of antibody to the type-specific capsular PS (10). Furthermore, opsonization of pneumococci by human sera is mediated primarily by antibodies to the capsular PSs, with no correlation between levels of antibody to C PS and opsonic titers (17).
The literature commonly states that the PC moiety on the C PS is the immunologically dominant epitope. This statement appears to be based primarily on mouse data showing that anti-PC monoclonal antibodies are readily obtained following immunization with C PS (1). While we found that a mouse monoclonal antibody reacts strongly with C PS and PC-BSA and that PC strongly inhibited binding of the mouse antibody to either antigen, we found that human IgG antibodies to C PS were not inhibitable by up to 25 μg of PC yet were strongly inhibited with <1 μg of C PS per ml.
Briles et al. showed that children rapidly develop anti-PC antibodies through natural exposure and that these antibodies reach adult levels by about 3 years of age (2). They found that adsorption with either C PS or cells from the pneumococcal capsule-minus mutant R36A inhibited binding of these sera by more than 85%. By contrast, we found very little reactivity of anti-PC antibodies with C PS.
Stein and Sigal (16) found that human IgM anti-PC antibodies had fine specificity patterns distinct from those of their murine counterparts. The human anti-PC antibodies had a much higher relative affinity for glycerol PC and choline than the murine anti-PC antibodies. In related studies, Brown et al. (3) found that human IgG anti-PC antibodies were inhibited more strongly by p-nitrophenyl PC than by PC, while the IgM antibodies were inhibited equally by these two compounds. We did not examine the specificity of IgM anti-PC antibodies, but we also found that the IgG antibodies were inhibited more by p-nitrophenyl PC than by PC. Like Brown et al. (3), we found that anti-PC antibodies did not increase following immunization with the pneumococcal PS vaccine.
We found that most adults have elevated C PS antibodies and that postimmunization, 42% have twofold rises in C PS antibodies, almost entirely IgG. Pedersen et al. (13) vaccinated adults and older children having risk factors for pneumococcal disease, such as splenectomy, with pneumococcal vaccine. They observed <2-fold increases in anti-C PS antibody in both adults and children postimmunization.
Koskela measured concentrations of antibody to the C PS in acute- and convalescent-phase sera of children with culture confirmed pneumococcal otitis media (10). All of the children had both IgG and IgM anti-C PS antibodies in their acute-phase sera, and half of them had small antibody rises, less than twofold, in convalescence. These children were then vaccinated with the PS vaccine, and no child had a ≥2-fold increase in antibody. Similarly, we found high levels of IgG anti-C PS antibody in the acute-phase sera of six adult patients with pneumococcal bacteremia, with no increase in the levels in their convalescent-phase sera.
In summary, most adults have detectable levels of antibody to both C PS and to PC, but these are two distinct populations of antibody, often of differing in immunoglobulin class. Exposure of humans to pneumococcal vaccines or pneumococcal infections does not induce anti-PC antibody, and the C PS-reactive antibodies are likely induced by exposure to the ubiquitous pneumococcus. Importantly, this study demonstrates that immune specificities, both vaccine induced and naturally acquired, shown in one animal species may differ markedly from those observed in humans.
REFERENCES
- 1.Briles D E, Forman C, Horowitz J C, Volanakis J E, Benjamin W H, Jr, McDaniel L S, Eldridge J, Brooks J. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect Immun. 1989;57:1457–1464. doi: 10.1128/iai.57.5.1457-1464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briles D E, Scott G B, Gray B, Crain M J, Blaese M, Nahm M, Scott V, Haber P. Naturally occurring antibodies to phosphocholine as a potential index of antibody responsiveness to polysaccharides. J Infect Dis. 1987;155:1307–1314. doi: 10.1093/infdis/155.6.1307. [DOI] [PubMed] [Google Scholar]
- 3.Brown M, Schiffman G, Rittenberg M B. Subpopulations of antibodies to phosphocholine in human serum. J Immunol. 1984;132:1323–1328. [PubMed] [Google Scholar]
- 4.Concepcion N, Frasch C E. Evaluation of previously assigned antibody concentrations in pneumococcal polysaccharide reference serum 89SF by the method of cross-standardization. Clin Diagn Lab Immunol. 1998;5:199–204. doi: 10.1128/cdli.5.2.199-204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer W, Behr T, Peter-Katalinic J, Egge H. Teichoic acid and lipoteichoic acid of Streptococcus pneumoniae possess identical chain structures. A reinvestigation of teichoic acid (C polysaccharide) Eur J Biochem. 1993;215:851–857. doi: 10.1111/j.1432-1033.1993.tb18102.x. [DOI] [PubMed] [Google Scholar]
- 6.Goebel W F, Adams M H. The immunological properties of the heterofile antigen and somatic polysaccharide of pneumococcus. J Exp Med. 1943;77:435–449. doi: 10.1084/jem.77.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray B M, Dillon H C, Jr, Briles D E. Epidemiological studies of Streptococcus pneumoniae in infants: development of antibody to phosphocholine. J Clin Microbiol. 1983;18:1102–1107. doi: 10.1128/jcm.18.5.1102-1107.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jennings H J, Lugowski C, Young N M. Structure of the complex polysaccharide C-substance from Streptococcus pneumoniae type 1. Biochemistry. 1980;19:4712–4719. doi: 10.1021/bi00561a026. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson C, Jansson P-E, Sorensen U B S. The pneumococcal common antigen C-polysaccharide occurs in different forms: mono- or disubstituted with phosphorylcholine. Eur J Biochem. 1999;265:1091–1097. doi: 10.1046/j.1432-1327.1999.00835.x. [DOI] [PubMed] [Google Scholar]
- 10.Koskela M. Serum antibodies to pneumococcal C polysaccharide in children: response to acute pneumococcal otitis media or to vaccination. Pediatr Infect Dis J. 1997;6:519–526. doi: 10.1097/00006454-198706000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Liu T Y, Gotschlich E C. The chemical composition of pneumococcal C-polysaccharide. J Biol Chem. 1963;238:1928–1934. [PubMed] [Google Scholar]
- 12.Musher D M, Watson D A, Baughn R E. Does naturally acquired IgG antibody to cell wall polysaccharide protect human subjects against pneumococcal infection? J Infect Dis. 1990;161:736–740. doi: 10.1093/infdis/161.4.736. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen F K, Henrichsen J, Sorensen U S, Nielsen L L. Anti-C-carbohydrate antibodies after pneumococcal vaccination. Acta Pathol Microbiol Immunol Scand Sect C. 1982;90:353–355. doi: 10.1111/j.1699-0463.1982.tb01462.x. [DOI] [PubMed] [Google Scholar]
- 14.Scott M G, Briles D E, Shackelford P G, Smith D S, Nahm M H. Human antibodies to phosphocholine. IgG anti-PC antibodies express restricted numbers of V and C regions. J Immunol. 1987;138:3325–3331. [PubMed] [Google Scholar]
- 15.Sorensen U B S, Henrichsen J, Chen H-C, Szu S C. Covalent linkage between the capsular polysaccharide of Streptococcus pneumoniae revealed by immunochemical methods. Microb Pathog. 1990;8:325–334. doi: 10.1016/0882-4010(90)90091-4. [DOI] [PubMed] [Google Scholar]
- 16.Stein L D, Sigal N H. Heterogenicity of the human phosphocholine-specific B cell repertoire. J Immunol. 1984;132:1329–1335. [PubMed] [Google Scholar]
- 17.Vioarsson G, Jónsdóttir I, Jónsson S, Valdimarsson H. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J Infect Dis. 1994;170:592–599. doi: 10.1093/infdis/170.3.592. [DOI] [PubMed] [Google Scholar]