Abstract
Background:
Lumbar puncture is recommended for individuals with syphilis who have neurological symptoms, however symptoms have poor sensitivity for predicting symptomatic neurosyphilis. Neurofilament light chain (NfL) is a marker for neuroaxonal injury; cerebrospinal fluid concentrations are higher in symptomatic neurosyphilis than in uncomplicated syphilis or asymptomatic neurosyphilis.
Methods:
Serum NfL was quantified in 20 individuals with uncomplicated syphilis, 10 with asymptomatic neurosyphilis and 10 with symptomatic neurosyphilis using an ultrasensitive single molecule array assay; it was repeated a median of 12.5 months after neurosyphilis therapy. Serum NfL concentration was age-adjusted using a published formula.
Results:
Age-adjusted serum NfL concentration was significantly higher in symptomatic neurosyphilis compared to each of the other two groups. It was above the highest value in uncomplicated syphilis in one of 10 participants with asymptomatic neurosyphilis and 3 of 10 with symptomatic neurosyphilis. Serum NfL concentration increased in one participant with asymptomatic neurosyphilis with possible treatment failure.
Conclusions:
If confirmed in a larger study, serum NfL may be a useful adjunct for identifying central nervous system infection by T. pallidum.
Keywords: neurosyphilis, neurological symptoms, neurofilament light chain
Short summary
Age-adjusted serum neurofilament light (NfL) concentration was elevated in one of 10 individuals with asymptomatic neurosyphilis, and in three of 10 with symptomatic neurosyphilis, suggesting neuroaxonal injury in these persons.
Introduction
The indications for lumbar puncture (LP) in patients with syphilis are controversial. The US Centers for Disease Control and Prevention (CDC) recommend cerebrospinal fluid (CSF) analysis for individuals with syphilis who have “clinical evidence of neurologic involvement” (1). The guidelines ignore the possibility of neurological injury in those with CSF abnormalities but no symptoms, even though there are no data in the current treatment era to suggest that asymptomatic neurosyphilis is benign in all patients.
Several studies show that neurological symptoms may not be adequate markers of risk for central nervous system (CNS) T. pallidum infection. (2–4). Neurofilament light chain (NfL) is a marker for neuroaxonal injury; previous work shows that CSF concentrations are higher in patients with symptomatic neurosyphilis than in those with uncomplicated syphilis or asymptomatic neurosyphilis (5, 6). Recent methodological advances have made it possible to measure this marker in blood (7), suggesting that blood NfL could be a more objective marker of CNS T. pallidum infection than neurological symptoms.
Materials and Methods
Study Participants
Participants were enrolled in a study of CSF abnormalities in syphilis (8). Participants underwent study visits at entry at which a standardized history and neurological examination, LP and blood draw were performed. The study clinician categorized each participant as having uncomplicated syphilis, asymptomatic neurosyphilis, or symptomatic neurosyphilis based on clinical and CSF findings. Individuals with abnormal CSF at entry underwent follow-up visits at 12, 24 and 52 weeks at which the same standardized history and neurological examination, and blood draw were performed. CSF examination was repeated in individuals treated for neurosyphilis at week 12, and at weeks 24 and 52, if the previous CSF profile was abnormal. If the CSF profile remained abnormal at the end of study participation at ~52 weeks, participants were referred for clinical follow-up. The study was approved by the University of Washington Institutional Review Board, and written consent was obtained from all participants.
Samples Chosen for Analysis
Samples from 40 individuals were randomly chosen for this analysis from a list of participants divided into three groups: 1) uncomplicated syphilis (no neurological symptoms attributable to syphilis, CSF white blood cells (WBC) < 6/uL and nonreactive CSF- Venereal Disease Research Laboratory [VDRL]); 2) asymptomatic neurosyphilis (no neurological symptoms attributable to syphilis, CSF WBCs > 20/uL or reactive CSF-VDRL); 3) symptomatic neurosyphilis (neurological symptoms attributable to syphilis, CSF WBCs > 20/uL or reactive CSF-VDRL). For each group, half of the participants were persons with HIV (PWH); all were taking antiretroviral therapy, and PWH and participants without HIV were matched by age within each group. The researchers who chose the participants’ samples were blinded to other characteristics, including neurosyphilis treatment response. For individuals with asymptomatic and symptomatic neurosyphilis, serum samples collected at the end of study participation were also assayed.
Laboratory Methods
Serum RPR and treponemal test reactivity, CSF WBC enumeration and CSF-VDRL reactivity were determined in a Clinical Laboratory Improvement Amendments (CLIA)-approved hospital laboratory. Plasma HIV RNA concentration, and peripheral blood CD4+ T lymphocyte concentration collected within 90 days of the LP were obtained from medical records. Serum NfL concentration was measured using the Simoa (single molecule array) NF-Light assay on an HD-X instrument according to instructions from the manufacturer (Quanterix, Billerica, MA). Serum was frozen at −20C and thawed just before analysis. All samples were analyzed in one round of experiments using one batch of reagents by board-certified laboratory technicians who were blinded to clinical data. Calibrators were run in duplicates. Samples were run in singlets with a 4-fold dilution. For a quality control (QC) sample with a concentration of 24.8 pg/mL, the intra-assay coefficient of variation (CV) was 5.5%. For a QC sample with a concentration of 73.7 pg/mL, the intra-assay CV was 7.1%.
Statistical Methods
Serum NfL concentrations were normalized for age using a published formula that adjusts the value to a reference age of 18 years based on values from 118 healthy donors aged 24–66 years (9). Continuous variables were expressed as median (interquartile range, IQR). Relationships between continuous variables were determined by Mann-Whitney U, Kruskal-Wallis, or Spearman rank sum tests. Pearson chi square test was used to compare proportions.
Results
Of the 10 individuals with symptomatic neurosyphilis, 2 had vision loss alone, 3 had hearing loss alone, 1 had meningitis symptoms alone, 2 had vision and hearing loss plus meningitis symptoms, 1 had vision loss and meningitis symptoms, and 1 had hearing loss plus meningitis symptoms. Characteristics of all participants are shown in Table 1. Age did not differ by group, but more participants with uncomplicated syphilis and symptomatic neurosyphilis had early syphilis compared to those with asymptomatic neurosyphilis (p=0.001), and serum RPR titer was higher in the those with asymptomatic or symptomatic neurosyphilis compared to those with uncomplicated syphilis (p=0.018).
Table 1.
Participant characteristics
| Uncomplicated Syphilis N=20 |
Asymptomatic NS N=10 |
Symptomatic NS N=10 |
|
|---|---|---|---|
|
| |||
| Male | 20 | 10 | 10 |
| Age, years | 38 (34–48) | 46 (29–48) | 46 (36–56) |
| PWH | 10 (50.0%) | 5 (50.0%) | 5 (50.0%) |
| CD4/uL | 534 (305–606) n=9 | 180 (144–470) n=4 | 574 (411–717) n=4 |
| Plasma HIV RNA c/mL | 40 (30–67) n=9 | 5600 (39–224,565) n=4 | 35 (23–40) n=4 |
| Early syphilis | 12 (70.6%) n=17* | 2 (20.0%) | 10 (100%) |
| Serum RPR titer | 32 (4–128) | 128 (32–2048) | 256 (64–2048) |
| CSF WBC/uL | 5 (2–5) | 19 (5–48) | 29 (24–45) |
| CSF-VDRL reactive | 0 (0.0%) | 8 (80.0%) | 5 (50.0%) |
| Age-adjusted serum NfL pg/mL | 6.52 (5.15–10.53) | 7.04 (5.00–11.70) | 11.91 (7.91–19.00) |
Values are n (%) or median (interquartile range).
Three participants had treated syphilis and were not included in the denominator.
CSF, cerebrospinal fluid; NfL, neurofilament light chain; NS, neurosyphilis; PWH, person with HIV; RPR, rapid plasma reagin; WBC, white blood cells
There was no significant difference in adjusted serum NfL concentrations in PWH compared to those without HIV, and there was no significant relationship between adjusted serum NfL concentration and plasma HIV RNA, peripheral blood CD4+ T cell concentration, serum RPR titer, reactive CSF-VDRL or CSF-VDRL titer. Serum NfL concentration was higher with higher CSF WBCs (r=0.43, p=0.005).
Adjusted serum NfL concentration did not differ between those with uncomplicated syphilis and those with asymptomatic neurosyphilis (Table 1, Figure 1), but adjusted serum NfL concentration was significantly higher in those with symptomatic neurosyphilis compared to the other two groups (Table 1; p=0.006 compared to uncomplicated syphilis, and p=0.035 compared to asymptomatic neurosyphilis).
Figure 1.
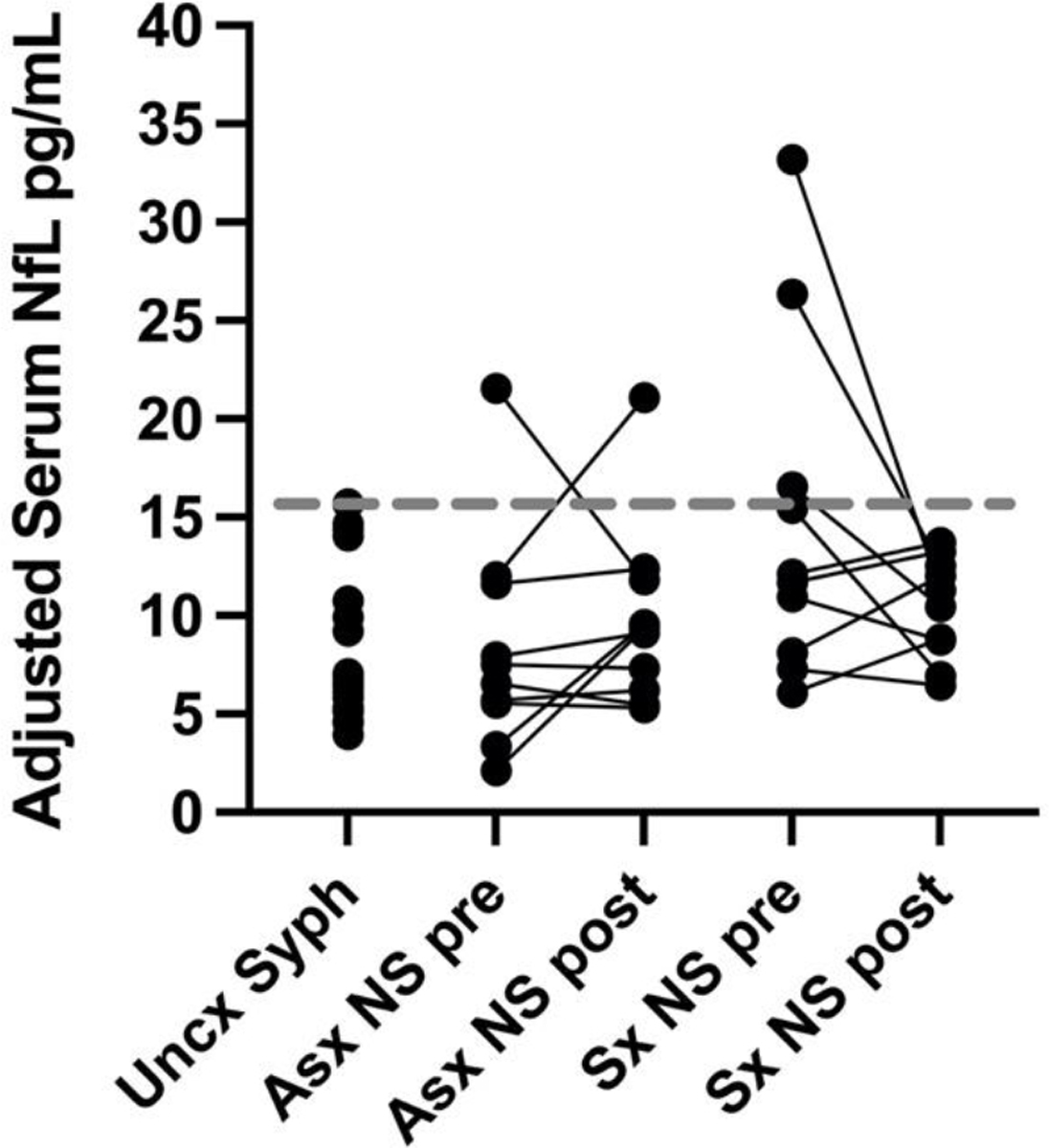
Age-adjusted serum neurofilament light chain (NfL) concentrations in uncomplicated syphilis (Uncx Syph); and in asymptomatic neurosyphilis (Asx NS) and symptomatic neurosyphilis (Sx NS) pre-therapy (pre) and post-therapy (post). The gray dotted line represents the highest adjusted serum NfL concentration in individuals with uncomplicated syphilis. One of 10 individuals with asymptomatic neurosyphilis and three of 10 with symptomatic neurosyphilis had adjusted serum NfL concentrations above the highest value in individuals with uncomplicated syphilis. Additionally, one individual with asymptomatic neurosyphilis had an almost doubling of their serum concentration 14 months after appropriate treatment in the setting of possible treatment failure.
One of 10 individuals with asymptomatic neurosyphilis and three of 10 individuals with symptomatic neurosyphilis had pretreatment adjusted serum NfL concentrations above the highest concentration seen in individuals with uncomplicated syphilis. Adjusted serum NfL concentrations, determined a median of 12.5 (IQR, 12–13) months after neurosyphilis treatment in those with asymptomatic and symptomatic neurosyphilis, remained below this concentration with the exception of one individual with asymptomatic neurosyphilis (Table 2). This individual was a person without HIV who had late syphilis and was treated with intramuscular procaine penicillin G and oral probenecid per CDC guidelines (1) for 10 days. While serum RPR and CSF-VDRL titers declined over the course of 14-month follow-up, CSF WBCs increased from 5/uL to 10/uL between the third and the final visit, suggesting treatment failure.
Table 2.
Characteristics of participant with increase in adjusted serum NfL concentration after treatment for asymptomatic neurosyphilis.
| Time relative to NS treatment | Adjusted serum NfL pg/mL | CSF WBCs/uL | CSF-VDRL titer | Serum RPR titer |
|---|---|---|---|---|
|
| ||||
| 2 weeks before | 11.99 | 50 | 8 | 64 |
| 3 months after | -- | 20 | 2 | 32 |
| 6 months after | -- | 5 | 2 | 32 |
| 14 months after | 21.09 | 10 | 2 | 16 |
CSF, cerebrospinal fluid; NfL, neurofilament light chain; NS, neurosyphilis; RPR, rapid plasma reagin; WBC, white blood cells.
Discussion
NfL has been studied as a biomarker for neurological injury in many conditions (7). While initial studies focused on CSF concentrations, subsequent work showed that, using the Simoa platform, NfL could be measured in serum and plasma, and that these concentrations correlated with CSF concentrations (7). NfL concentrations increase with age, and this observation has been a barrier to identifying “elevated” concentrations in individual patients. However, age-correction is now possible (9). We found that age-adjusted serum NfL concentrations were significantly higher in individuals with symptomatic neurosyphilis compared to those with asymptomatic neurosyphilis or uncomplicated syphilis. Additionally in our study, one of 10 individuals with asymptomatic neurosyphilis and three of 10 with symptomatic neurosyphilis had adjusted serum NfL concentrations above the highest value in individuals with uncomplicated syphilis. Moreover, one individual with asymptomatic neurosyphilis had an almost doubling of their serum concentration 14 months after appropriate treatment in the setting of possible treatment failure. These findings suggest that individuals with asymptomatic and symptomatic neurosyphilis, based on our current definitions, can have neurological injury as a consequence of T. pallidum infection of the CNS, and they add additional support to the contention that neurological symptoms are an imperfect screen for CNS infection. Our results also suggest that increases in serum NfL concentration after neurosyphilis treatment may signal treatment failure.
Our study has limitations that should be considered in interpreting our findings. We assayed a convenience sample that was small. Neurosyphilis categorization was made by the study clinician, which may have introduced bias. We required that individuals with neurosyphilis have CSF WBCs > 20/uL or a reactive CSF-VDRL, which may limit generalizability of our findings. We used a published formula to adjust the serum NfL values to a reference age of 18 years; this same procedure would need to be followed by others to compare to our findings.
From a practical standpoint, our findings suggest an objective method for assessing CNS injury in patients with syphilis, which could be used as an adjunct in identifying individuals with syphilis who should undergo LP, and to assess treatment success. While assessment of serum NfL would not have affected the decision to undergo LP in our patients with symptoms, we required evidence of CSF inflammation in our definition of symptomatic neurosyphilis. Patients with ocular and otosyphilis may not have concomitant meningitis (1), and we did not assess the usefulness of assessment of serum NfL to detect neuroaxonal injury in such individuals. This assessment could be particularly useful in suspected otosyphilis, where the diagnosis is particularly challenging (10). We also demonstrate elevated serum NfL in an individual with asymptomatic neurosyphilis, suggesting that the test could also be useful in determining whether asymptomatic individuals at high risk for syphilitic meningitis, for example, because of high serum RPR titer (11, 12), should undergo LP. A larger and more comprehensive study is required to confirm our findings.
Acknowledgments
This work was supported by grants from the US National Institutes of Health (NS34235 and NS082120) to CMM. HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018–02532), the European Research Council (#681712 and #101053962), Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809–2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21–831376-C, #ADSF-21–831381-C and #ADSF-21–831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022–0270), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme – Neurodegenerative Disease Research (JPND2021–00694), and the UK Dementia Research Institute at UCL (UKDRI-1003).
Footnotes
Conflicts of interest: HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Passage Bio, Pinteon Therapeutics, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. The other authors report no conflicts of interest related to this work.
References
- 1.Workowski KA, Bachmann LH, Chan PA, et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis AP, Stern J, Tantalo L, et al. How Well Do Neurologic Symptoms Identify Individuals With Neurosyphilis? Clin Infect Dis. 2018;66(3):363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiva F, Short CE, Goldmeier D, Winston A. Predictive value of neurological symptoms in persons with suspected neurosyphilis. Sex Transm Infect. 2022;98(3):228–9. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Ayala P, Quinonez-Flores A, Gonzalez-Hernandez LA, et al. Clinical features associated with neurosyphilis in people living with HIV and late latent syphilis. Int J STD AIDS. 2022;33(4):330–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W, Chang H, Wu W, et al. Increased CSF Soluble TREM2 Concentration in Patients With Neurosyphilis. Front Neurol. 2020;11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu DM, Cai SN, Li R, Wu Y, Liu SA, Lun WH. Elevation of Cerebrospinal Fluid Light and Heavy Neurofilament Levels in Symptomatic Neurosyphilis. Sex Transm Dis. 2020;47(9):634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalil M, Teunissen CE, Otto M, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14(10):577–89. [DOI] [PubMed] [Google Scholar]
- 8.Dunaway SB, Maxwell CL, Tantalo LC, Sahi SK, Marra CM. Neurosyphilis Treatment Outcomes After Intravenous Penicillin G Versus Intramuscular Procaine Penicillin Plus Oral Probenecid. Clin Infect Dis. 2020;71(2):267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harp C, Thanei GA, Jia X, et al. Development of an age-adjusted model for blood neurofilament light chain. Ann Clin Transl Neurol. 2022;9(4):444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramchandani MS, Litvack JR, Marra CM. Otosyphilis: A Review of the Literature. Sex Transm Dis. 2020;47(5):296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. Neurosyphilis in a clinical cohort of HIV-1-infected patients. Aids. 2008;22(10):1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marra CM, Maxwell CL, Smith SL, et al. Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J Infect Dis. 2004;189(3):369–76. [DOI] [PubMed] [Google Scholar]


