Abstract
The zoopathogenic fungus Histoplasma capsulatum, like other eukaryotic aerobic microorganisms, requires iron for growth. Under conditions of low iron availability, the fungus secretes hydroxamates that function as siderophores (iron chelators). The experiments to be reported were designed to gather further information on the hydroxamate siderophores of H. capsulatum. The fungus was grown in a synthetic medium deferrated with the cationic exchange resin Chelex 100. Siderophores were detected after 4 days of incubation at 37°C in media containing 0.3 to 1.0 μM iron. The secretion was suppressed by 10 μM iron. The hydroxamates were purified by reverse-phase and size-exclusion chromatography. On the basis of ions observed during electrospray mass spectroscopy, five hydroxamate siderophores were tentatively identified: dimerum acid, acetyl dimerum acid, coprogen B, methyl coprogen B, and fusarinine (monomeric). A polyclonal antibody to dimerum acid was generated. This reagent cross-reacted with coprogen B and fusarinine. Thus, the antibody detects hydroxamates in all three families of siderophores excreted by H. capsulatum.
The pathogenic fungus Histoplasma capsulatum is a facultative intracellular parasite of the mononuclear phagocytes of an infected host and must obtain nutrients necessary for growth from that immediate environment. Like other eukaryotic aerobic microorganisms, H. capsulatum requires iron for growth. In the mammalian host, iron is bound and transported into the cell by transferrin. The iron released inside the cell is accumulated in a labile pool or bound to ferritin (7, 19, 20, 22). H. capsulatum might obtain its iron from the intermediate iron pool of the macrophage. That it does so is suggested by the fact that chloroquine, which alkalinizes endosomes and thereby prevents the release of iron from transferrin and ferritin, inhibits intracellular growth of H. capsulatum in human macrophages (17). Moreover, the use of iron chelators in macrophage cultures severely restricts the intracellular growth of the fungus (11, 18). When starved for iron in in vitro broth cultures, H. capsulatum secretes low-molecular-weight (Mr < 1,500) hydroxamates that function as siderophores (iron chelators) by sequestering ferric iron from the environment (1; K. F. Faull, D. H. Howard, R. Rafi, and A. Tiwari, Abstr. 98th Gen. Meet. Am. Soc. Microbiol. 1998, abstr. F-81, p. 266–267, 1998). The fact that iron in the labile pool is susceptible to chelation by the bacterial siderophore desferoxamine (22) makes the utilization of hydroxamates secreted by H. capsulatum in the capture of iron from that same intracellular pool a possibility to be explored. Since iron is essential, the acquisition of it must represent a factor important in the pathogenesis of histoplasmosis. Accordingly, we set out to gather information on the biology of the hydroxamate siderophores of H. capsulatum.
Initial studies involved the development of isolation and purification procedures for hydroxamate siderophores of H. capsulatum. Plasticware was used wherever possible. Glassware was soaked overnight in 6 N HNO3. The acid-treated glassware was rinsed extensively in double-distilled deionized water. Liquid shake cultures were grown in the synthetic medium devised by McVeigh and Morton (MM) (15). MM medium was depleted of iron by treatment with the cationic exchange resin Chelex 100 (BioRad Laboratories, Richmond, Calif.). Untreated MM medium contains approximately 1.0 μM Fe(III). After Chelex treatment, the medium (CMM) contains approximately 0.3 μM Fe(III). Iron levels were determined by the Carter method which incorporates a ferrozine-neocuproin reagent (2). H. capsulatum 505, used in many of our previous experiments, was employed in this work (11). In addition, strain G184A (ATCC 26027), obtained from W. E. Goldman (Washington University, St. Louis, Mo.), was studied. Two variants of G184A were included: G184A-R, the rough-colony parental (virulent) strain, and G184A-S, a smooth-colony (avirulent) variant of the R strain (9).
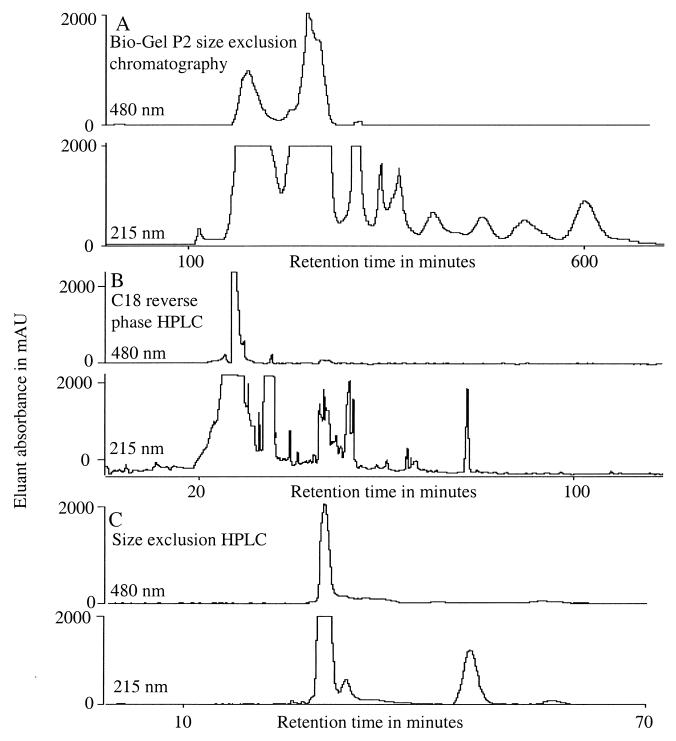
Large-volume liquid shake cultures in CMM medium were prepared in 500 ml of medium in 2-liter polypropylene flasks (Nalgene). Cells were harvested from 72-h cultures on glucose-cysteine blood agar (11) into phosphate-buffered saline (PBS), washed three times, and were resuspended in the same buffer. The medium was inoculated with 2 × 106 cells/ml. Strains UCLA 505, G184A-S, and G184A-R were used in these large-volume shake cultures. The cultures were incubated at 37°C on a rotary shaker for 14 days, after which a 1-ml sample was taken and centrifuged. The supernatant was tested for siderophores with the ferric perchlorate reagent (21). One-liter quantities were harvested by centrifugation and were frozen. Thawed samples were tested for hydroxamates by the Csáky method (21) and for sterility before subjection to extraction, purification, and characterization. Fifty-milliliter samples were reduced to dryness by lyophilization. The dried material was then redissolved in 5 ml of water and 2.5 ml of a FeCl3-HCl reagent (16.2 g FeCl3 · 6H2O in 500 ml of 0.05 N HCl) was added. The reddish solution was saturated with 4 g of ammonium sulfate, mixed, and left at 4°C overnight. The mixture was then centrifuged (10,000 × g, 10 min), and the supernatant was transferred to a clean test tube and extracted with benzyl alcohol according to the method described by Neilands (16). One milliliter of benzyl alcohol was added, the sample was mixed and centrifuged, and the benzyl alcohol was transferred to a clean test tube. The aqueous phase was extracted twice more in the same manner, and the benzyl alcohol extracts were pooled. The siderophores were reextracted into water by the method of Manulis et al. (14). Four milliliters of diethyl ether and 500 μl of water was added, the tube was mixed and centrifuged, and the aqueous layer (lower phase) was transferred to a clean tube. The remaining upper layer was extracted twice more with 500 μl of water (each time). In each extraction, the sample was mixed and centrifuged and the lower aqueous phases were removed and pooled. The final preparation was centrifuged once more, and any remaining ether was carefully removed. The sample was then dried in a vacuum centrifuge. The benzyl alcohol extraction proved to be a convenient and fast method for siderophore recovery from large volumes of culture filtrate. Visible inspection indicated excellent efficiency of extraction into the benzyl alcohol phase of the red color present in the culture filtrate after the addition of FeCl3. The final extract was sufficiently clean to allow further purification to be easily accomplished by size-exclusion and reverse-phase procedures (Fig. 1).
FIG. 1.
Chromatographic profiles obtained at 215- and 480-nm adsorption during the purification of siderophores from H. capsulatum culture filtrates.
The dried extract was redissolved in 3 ml of water and was loaded onto a size-exclusion column (Biorad Bio-Gel P2, 200/400 mesh, 395 by 28 mm, column volume of 243 ml, suspended in water). The column was then eluted with water at 0.5 ml/min. The absorbance of the eluate was monitored at 215 and 480 nm, and the eluate was collected in 5-ml fractions. Size-exclusion chromatography on Bio-Gel P2 of the benzyl alcohol extract from culture filtrates of H. capsulatum typically revealed two broad peaks in the 480-nm absorption trace of the column effluent eluting between 100 and 330 min, superimposed on a much more complex trace at 215 nm absorption (Fig. 1A). The first of the two peaks appeared yellow while the second appeared red. Following treatment with additional ferric chloride, the yellow peak changed to red, indicating that the loss of the iron from the ligand had occurred during size-exclusion chromatography in aqueous buffer. These peaks were combined for the C18 reverse-phase high-pressure liquid chromatography (HPLC) column. The pooled fractions were dried in a vacuum centrifuge and then redissolved in water and injected onto a C18 reverse-phase HPLC column (Keystone Scientific Aquasil, 250 by 10 mm, 5-μm particle size, 100-Å-diameter pore size) equilibrated in 0.1% trifluoroacetic acid in water. The column was eluted at 3.0 ml/min for 10 min with equilibration solvent and then at the same flow rate with an increasing linear gradient of acetonitrile containing 0.1% trifluoroacetic acid (0.75%/min) over 134 minutes. The absorbance of the eluate was monitored at 215 and 480 nm, and 3-ml fractions were collected.
When the 480-nm absorbing peaks were separately chromatographed on C18 reverse-phase HPLC, the peaks of red coloration emerged in the effluent between 10 and 35 min (Fig. 1B). The 215-nm trace emerging from this chromatogram was more complex, revealing the elution of many other compounds which did not absorb significantly in the visable region (Fig. 1B). Individual fractions were kept separate, dried in a vacuum centrifuge, and then redissolved in 0.1% formic acid in water. The solution was then injected (50 μl/run) onto an HPLC size-exclusion column (TosoHaas G2500PWxl, 300 by 7.8 mm, 6-μm particle size) equilibrated in 0.1% formic acid in water. The column was eluted at 0.5 ml/min isocratically with equilibration solvent, and the absorbance of the eluant was monitored at 215 and 480 nm, and 0.5-ml/min fractions were collected. Separated fractions were dried in a vacuum centrifuge for examination by mass spectrometry. When the reverse-phase-purified peaks were chromatographed on size-exclusion HPLC, individually resolved peaks were often superimposed as single peaks of 215-nm absorption (Fig. 1C), which indicated the general cleanliness of the preparations.
A Perkin-Elmer Sciex (Thornill, Canada) API III triple quadrupole mass spectrometer was calibrated by flow injection of a mixture of polypropylene glycol 425, 1,000, and 2,000 (3.3 × 10−5, 1 × 10−4, and 2 × 10−3 M, respectively) in water-methanol (1/1, vol/vol) containing 2 mM ammonium formate and 0.1% acetronitrile. Spectra were obtained by scanning at instrument conditions sufficient to resolve the isotopes of the polypropylene glycol-NH4+ singly charged ion at m/z 906 with 40% valley. For data acquisition, a 0.3-Da step size was used, and spectra were recorded with an orifice voltage of 70 V. Dried samples were redissolved in water-acetronitrile-formic acid (50/50/0.1, all by volume) to an estimated concentration of about 20 pmol/μl and were introduced into the ion source in 10-μl aliquots in a stream of the same solvent entering the source at 10 μl/min.
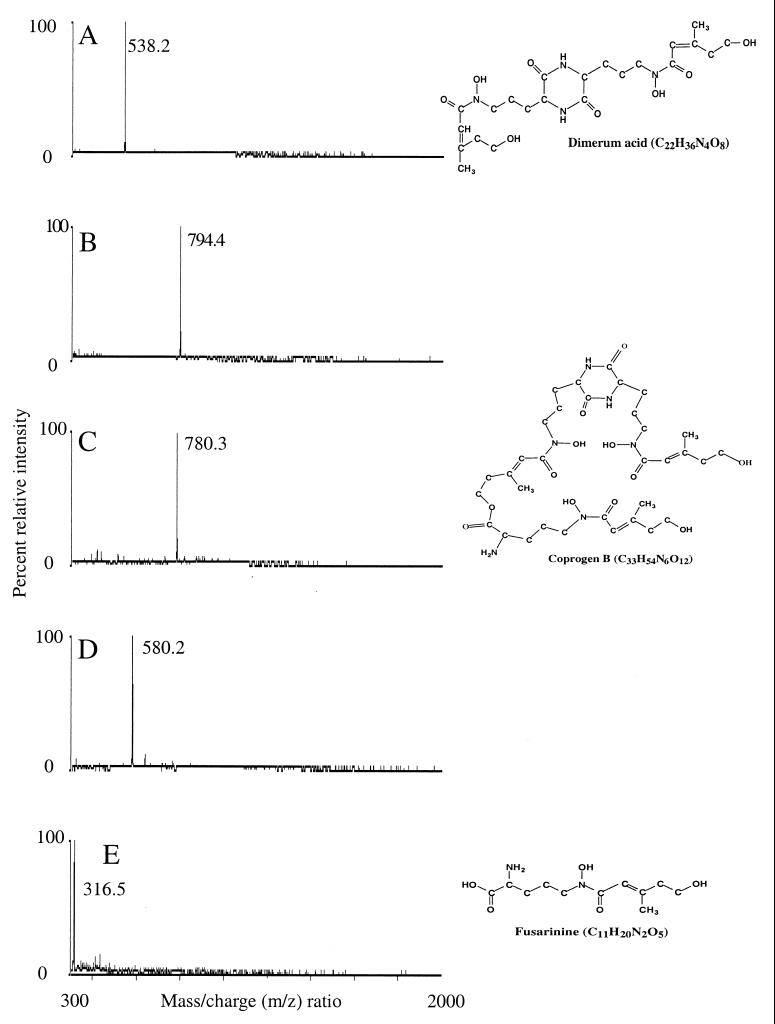
Electrospray mass spectrometry of the HPLC size-exclusion-purified peaks generally revealed spectra containing one or a few dominant ions in the m/z 300 to 800 region. No significant ions were seen from any samples between m/z 800 and 2000. In the large-volume liquid shake cultures, the ion at m/z 538 was an intense signal obtained from all strains examined and appeared to be the only siderophore present in these cultures (Table 1). Small-volume cultures were initiated to study siderophore synthesis in greater detail. Forty milliliters of CMM, or in some experiments MM, was contained in 300-ml sidearm flasks. Cultures were incubated on a rotary shaker at 37°C. Siderophores were detected in as little as 4 days, and a greater diversity of molecular species was observed. On the basis of ions observed during electrospray mass spectroscopy, five hydroxamate siderophores were identified: dimerum acid, acetyl dimerum acid, coprogen B, methyl coprogen B, and fusarinine (monomeric) (Table 1). These same results were obtained from filtrates of small-volume cultures of G184A-S (data not shown). The strain G184G-R was not used in the small-volume culture work. The unique spectrum of each of these hydroxamates produced from the clean hydroxamate fractions (Fig. 1C) are shown in Fig. 2. Samples of the pure siderophores, whose spectra are shown in Fig. 2, had stimulatory activity in the Aureobacterium flavescens JG-9 (ATCC 25091) bioassay (21). The relative frequency of occurrence of the siderophores in all of the experiments was dimerum acid greater than fusarinine which was greater than methyl coprogen B which was greater than coprogen B which was greater than acetyl dimerum acid. The major hydroxamate formed was dimerum acid which could achieve levels of 180 mg/liter (estimated from an average molar extinction coefficient of 3,000 [10, 24] read at 480 nm). The hydroxamates formed by H. capsulatum belong to three of the four families of hydroxamates formed by fungi (6).
TABLE 1.
Retention times and proposed assignments of siderophores in culture filtrates from H. capsulatum
| Culture vol | Fungal strain | Observed ion (m/z) | Proposed assignmenta | Expected ion (m/z) | Chromatography retention times (min) in:
|
||
|---|---|---|---|---|---|---|---|
| Bio-Gelb | Reverse-phase-C18c | Size exclusionc | |||||
| Large | 505, G184A-R, and G184A-S | 538.2d | Dimerum acid | 538.2 | 220–300 | 28–28.3 (4) | 31.0–33.6 (3) |
| Small | 505 | 316.5 | Fusarinine (monomeric) | 316.5 | 200–300 | 3.8 | 15.2 |
| 505 | 538.2 | Dimerum acid | 538.2 | 220–300 | 27.9 | 27.2 | |
| 505 | 580.2 | Acetyldimerum acide | 580.2 | 200–290 | 35.2 | Not done | |
| 505 | 780.3 | Coprogen Be | 780.3 | 220–245 | 35.9 | 19.15 | |
| 505 | 794.4 | Methyl coprogen B | 794.4 | 200–240 | 37.1–37.5 (2) | 19.3–19.5 (2) | |
All in the ferrated state.
A range of retention times is given for the Bio-Gel peaks because they are broad, and the indicated range represents the region that was pooled for the next chromatographic step.
Data are presented as the range of retention times recorded on different days with different preparations (number of different experiments) or as the retention time observed on a single chromatogram.
This component comprised the bulk of the second peak of 480-nm absorption eluting from the Bio-Gel P2 column and was present as the predominant component in all filtrates examined.
Tentative assignment.
FIG. 2.
Electrospray mass spectra of purified putative siderophores from culture filtrates of H. capsulatum. (A) Dimerum acid (C22H34O8N4Fe, singly charged), (B) methyl coprogen B, (C) coprogen B (C33H52O12N6Fe, singly charged), (D) acetyldimerum acid, and (E) fusarinine (monomeric C11H20N2O5Fe, singly charged). All the compounds were in the ferrated form. Schematic representations of the uncharged forms of dimerum acid, coprogen B, and fusarinine are presented with their elemental compositions on the right side of the figure.
On the basis of the clarity of the electrospray mass spectra of the final purified samples, it appears that the sequential combination of Bio-Gel P2 size-exclusion, C18 reverse-phase, and HPLC size-exclusion chromatographies is sufficient for purification of siderophores in the culture filtrates for tentative structural assignments by mass spectrometry. It also appears that the similarity in retention times of many of the compounds found in the culture filtrates (Table 1) means that caution must be exercised when attempting tentative identification on the basis of chromatographic behavior alone. On the other hand, the unique masses of the various siderophores make electrospray mass spectrometry of purified samples a convenient method for their recognition (Fig. 2). Our selection of reverse-phase packing deserves a comment. The highly polar water-soluble nature of many siderophores results in minimal retention on most C18 reverse-phase packings. The selection of a packing containing polar functions (hydroxyl groups) provided extra retention of siderophores and permitted sample loading with the column equilibrated in 100% aqueous buffer.
In the small-volume liquid shake cultures, siderophores were detected after 4 days of incubation, which is the early-stationary phase for H. capsulatum under these growth conditions. Varying the starting pH from 5.5 to 7.0 did not affect the quantity or type of siderophores obtained. The relative order of occurrence was the same as given above. Dimerum acid was the predominant hydroxamate observed under all conditions studied. Siderophore synthesis was consistently observed in the presence of levels of iron ranging from 0.3 to 1.0 μM and was completely suppressed at 10 μM. There were no quantitative or qualitative differences in siderophores produced at the two iron concentrations. The growth of H. capsulatum was never inhibited by more than 10 to 20% over iron replete cultures, yet siderophores were consistently excreted in the low-iron-concentration medium. The relatively good growth probably reflected iron storage by the cells grown on blood agar. Nevertheless, the iron reserves of the fungus were exhausted during incubation in MM or CMM media, and siderophores were characteristically formed.
H. capsulatum forms representatives of three of the four families of hydroxamate siderophores synthesized by zoopathogenic fungi (Table 1 and Fig. 2). Examples of the ferrochrome family of siderophores were not detected. The ester group of coprogen B (Fig. 2) can be hydrolyzed at alkaline pH (pH 9) to yield 1 mol of dimerum acid and 1 mol of trans-fusarinine. However, purified coprogen B from our work did not yield the hydrolysis products when subjected to a repetition of the extraction and purification procedures. Thus, the molecules revealed by mass spectroscopy appear to be fermentation products and not artifacts of the purification procedure. It is not known whether H. capsulatum utilizes hydrolysis to generate dimerum acid, but this seems unlikely because the pH of the cultures (final pH of culture filtrates ranged from 7.2 to 7.5) did not reach the pH at which hydrolysis optimally takes place (24).
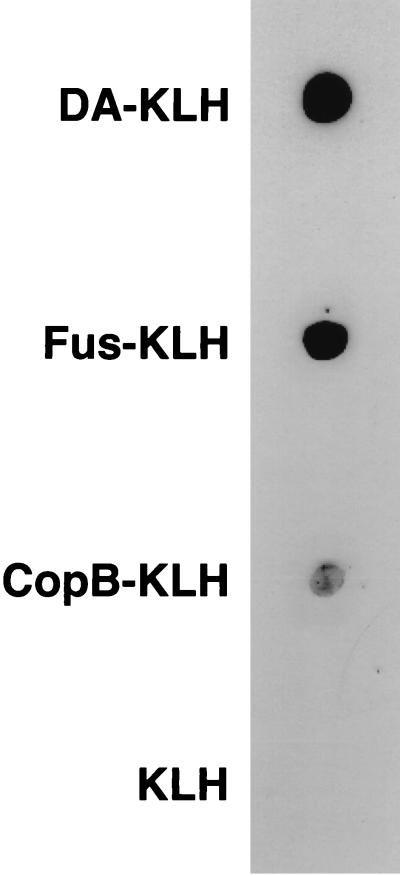
A polyclonal antiserum to dimerum acid was generated. A purified specimen of dimerum acid (DA) (Fig. 2) was conjugated to bovine serum albumin (BSA). The procedure was that given in the insert of an Imject Immunogen EDC Conjugation Kit from Pierce (Rockford, Ill.). The DA-BSA conjugate was used to immunize a rabbit. The concentration of DA used was 180 μg/ml. The specimen had been saturated with iron during the earlier purification procedure. Fractions containing the DA were, therefore, orange as they came off the column, indicating the attachment of DA to the BSA. The orange-colored fractions were pooled. Keyhole limpet hemocyanin (KLH) was the conjugation protein used in the preparation of the test antigen (DA-KLH). Specific anti-siderophore antibodies were produced following immunization of a 6-month-old New Zealand white male rabbit with a BSA conjugate of DA (DA-BSA) as follows. DA-BSA (100 μg) in 1.5 ml of PBS, pH 7.2, was combined with an equal volume of complete Freund's adjuvant (Calbiochem-Novabiochem Co., La Jolla, Calif.). The suspension was divided into 3 equal volumes (0.5 ml) which were injected at one subcutaneous and two intramuscular sites. After 1 month, the animal received a boost immunization by the same routes with the same preparation of 100 g of DA-BSA in 1.5 ml of PBS combined with an equal volume of incomplete Freund's adjuvant (Calbiochem-Novabiochem Co.). Two weeks after boost immunization, the animal was bled from the central ear artery, and the blood was processed into serum. The serum was stored at −20°C until used. The reaction of this antiserum with dimerum acid attached to a heterologous protein carrier (KLH) is shown in Fig. 3. Detection of specific anti-siderophore antibodies against DA, fusarinine, and methyl coprogen B was determined as follows. Aliquots (10 μl) containing approximately 7 μg of the conjugates of DA-KLH, fusarinine-KLH, and methyl coprogen B-KLH, as well as a nonconjugated KLH control, were spotted and dried onto strips of nitrocellulose (Schleicher and Schuell, Keene, N.H.). The strips were incubated for 1 h in anti-DA-BSA serum diluted 1:1,000 in PBS containing 5% nonfat dry milk (Carnation Co., Los Angeles, Calif.) and 0.1% Tween 20 (Sigma Chemical Co., St. Louis, Mo.). The strips were then washed several times in PBS and incubated for an additional hour in goat anti-rabbit immunoglobulin conjugated to horseradish perioxidase (Amersham, Little Chalfont, Buckinghamshire, England) diluted 1:2,500 in 5% nonfat dry milk–0.1% Tween 20. Antibody-antigen binding was detected by using the enhanced chemiluminescence system of Amersham (Western blotting protocol manual, Amersham). The results are shown in Fig. 3. The signal to methyl coprogen B-KLH was weaker because about 10-fold less material was used due to a limited supply. Thus, hydroxamates in each of the families produced by H. capsulatum were detected by the rabbit antiserum.
FIG. 3.
Dot blot analysis of immune serum and conjugated siderophores.
The levels of iron sensed as deficient varies among the fungi. For example, 1.6 μM is a deficient level for Neurospora (3), but the level must be much lower (<0.1 μM) before Saccharomyces responds to the deficiency (13). From our work it was observed that H. capsulatum forms siderophores at levels of iron ranging from 0.3 to 1.0 μM. The level of iron in the Salmonella-containing vacuoles of epithelial cells has been estimated to be 1.0 μM (4), a level that is sensed as deficient by H. capsulatum in our work. We are not aware of similar work with macrophages which would be more relevant to our interests. The antiserum generated in our work may help to detect hydroxamates in cell cultures of the fungus. Such detection has been accomplished with the wood-rotting fungus Geophyllum trabeum and with chrysobactin (8, 12). Both of these siderophores are phenolic. The antibodies we obtained are the first we are aware of to hydroxamate type siderophores. However, the antibody prepared to chrysobactin cross-reacted weakly with the hydroxamates, rhodotorulic acid, and ferrichrome (12).
The mechanisms of iron acquisition by pathogenic fungi are quite diverse (6). The first mechanism of such acquisition studied in H. capsulatum was by means of hydroxamate siderophores (1). The present studies augment that initial observation by depicting a diversity of siderophores, outlining a procedure for their characterization, and describing the generation of a polyclonal antibody that promises to be useful in detecting these compounds produced intracellularly. Recently, three additional mechanisms of iron gathering used by H. capsulatum have been described: (i) a high affinity ferric reductase, (ii) low-molecular-weight reductant(s), and (iii) the utilization of holotransferrin as an iron source (M. M. Timmerman and J. B. Woods, Abstr. 99th Gen. Meet. Am. Soc. Microbiol. 1999, abstr. F-11, p. 297–298, 1999).
In our work, the earliest that siderophores were detected was 4 days after inoculation of the medium. In the case of H. capsulatum under these growth conditions, this represents early-stationary phase and would be comprised of about 108 to 5 × 108 cells/ml. It is not known if the time of appearance was due to the relative insensitivity of the FeCl3-detecting procedure (>50 μM equivalents of desferrioxamine required for detectable color) or whether cell density is important to the signal required for siderophore synthesis. In a recent report, a newly described siderophore-like activity in Legionella pneumophila was only observed in bacterial cultures of relatively high density (M. R. Liles and N. P. Cianciotto, Abstr. 99th Gen. Meet. Am. Soc. Microbiol. 1999, abstr. B/D-201, p. 68, 1999). It will be important to explore the role of cell density in siderophore synthesis in light of recent observations on quorum sensing among certain bacteria and in H. capsulatum (5, 23; L. G. Eissenberg and W. E. Goldman, Abstr. 99th Gen. Meet. Am. Soc. Microbiol. 1999, abstr. F-101, p. 315, 1999).
Acknowledgments
This work was supported, in part, by funds from USPHS Grant AI 40011-2 from the NIAID of the National Institutes of Health and an equipment grant from the W. M. Keck Foundation.
We are grateful to J. B. Neilands (University of California, Berkeley) and to P. Szaniszlo (University of Texas, Austin) for providing the samples of siderophores that we used to develop our techniques of isolation and purification of such compounds from H. capsulatum. We acknowledge M. L. Zeuthen's (Mt. St. Mary's College, Los Angeles, Calif.) development of the A. flavescens JG-9 (ATCC 25091) bioassay and Joyce Wong's work on the Carter Method for iron quantification. We are grateful to David Blanco for his supervision of the immunization procedure and for the evaluation of the immune serum so produced. The formulae inserted in Fig. 2 were drawn on a computer with CSC ChemDraw Plus 3.0.1 (Cambridge Scientific Computing, Inc.) by Houman Langroodi. We thank Lois F. Howard for a careful rendering of the manuscript.
REFERENCES
- 1.Burt W R. Identification of coprogen B and its breakdown products from Histoplasma capsulatum. Infect Immun. 1982;35:990–996. doi: 10.1128/iai.35.3.990-996.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine) Anal Biochem. 1971;40:450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- 3.Ernst J, Winkelmann G. Enzymatic release of iron from sideramines in fungi. NADH: sideramine oxidoreductase in Neurospora crassa. Biochim Biophys Acta. 1977;500:27. doi: 10.1016/0304-4165(77)90043-5. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-del Portillo F, Foster J W, Maguire M E, Finlay B B. Characterization of the microenvironment of Salmonella typhimurium within MDCK epithelial cells. Mol Microbiol. 1992;6:3289–3292. doi: 10.1111/j.1365-2958.1992.tb02197.x. [DOI] [PubMed] [Google Scholar]
- 5.Hastings J W, Greenberg E P. Quorum sensing: the explanation of a curious phenomenon reveals a common characteristic of bacteria. J Bacteriol. 1999;181:2667–2668. doi: 10.1128/jb.181.9.2667-2668.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard D H. The acquisition, transport and storage of iron by pathogenic fungi. Clin Microbiol Rev. 1999;12:394–404. doi: 10.1128/cmr.12.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs A. Low molecular weight intracellular iron transport compounds. Blood. 1977;50:433–439. [PubMed] [Google Scholar]
- 8.Jellison J, Chandhoke V, Goodell B, Fekete F A. This isolation and immunolocalization of iron-binding compounds produced by Geophyllum trabeum. Appl Biotechnol Microbiol. 1991;35:805–809. [Google Scholar]
- 9.Klimpel K R, Goldman W E. Isolation and characterization of spontaneous avirulent variants of Histoplasma capsulatum. Infect Immun. 1987;55:528–533. doi: 10.1128/iai.55.3.528-533.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konetschny-Rapp S, Huschka H-G, Winkelmann G, Jung G. High-performance liquid chromatography of siderophores from fungi. Biometals. 1988;1:9–17. doi: 10.1007/BF01128012. [DOI] [PubMed] [Google Scholar]
- 11.Lane T E, Wu Hsieh B A, Howard D H. Iron limitation and the gamma interferon-mediated antihistoplasma state of murine macrophages. Infect Immun. 1991;59:2274–2278. doi: 10.1128/iai.59.7.2274-2278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee C, Buyer J S, Okonya J F, Miller M J. Synthesis of optically pure chrysobactin in immunoassay developments. Biometals. 1996;9:377–383. doi: 10.1007/BF00140607. [DOI] [PubMed] [Google Scholar]
- 13.Lesuisse E, Labbe P. Reductive iron assimilation in Saccharomyces cerevisiae. In: Winkelmann G, Winge D R, editors. Metal ions in fungi. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 149–178. [Google Scholar]
- 14.Manulis S, Kashman Y, Barash I. Identification of siderophores and siderophore-mediated uptake of iron in Stemphylium botryosum. Phytochemistry. 1987;26:1317–1320. [Google Scholar]
- 15.McVeigh I, Morton K. Nutritional studies on Histoplasma capsulatum. Mycopathol Mycol Appl. 1965;25:294–308. doi: 10.1007/BF02049917. [DOI] [PubMed] [Google Scholar]
- 16.Neilands J B. A crystalline organo-iron pigment from a rust fungus (Ustilago sphaerogena) J Am Chem Soc. 1952;74:4846–4847. [Google Scholar]
- 17.Newman S L, Gootee L, Brunner G, Deepe G S., Jr Chloroquine induces human macrophage killing of Histoplasma capsulatum by limiting the availability of iron and is therapeutic in a mouse model of histoplasmosis. J Clin Investig. 1994;93:1422–1429. doi: 10.1172/JCI117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman S L, Gootee L, Stroobart V, van der Goot H, Boelaert J R. Inhibition of growth of Histoplasma yeast cells in human macrophages by the iron chelator VUF 8514 and comparison of VUF 8514 with deferoxamine. Antimicrob Agents Chemother. 1995;39:1824–1829. doi: 10.1128/aac.39.8.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunez M T, Galte V, Watkins J A, Glass J. Mobilization of iron from endocytic vesicles—the effects of acidification and reduction. J Biol Chem. 1990;265:6688–6692. [PubMed] [Google Scholar]
- 20.Payne S M. Iron acquisition in microbial pathogenesis. Trends Microbiol. 1994;1:66–69. doi: 10.1016/0966-842x(93)90036-q. [DOI] [PubMed] [Google Scholar]
- 21.Payne S M. Detection, isolation and characterization of siderophores. In: Clark V L, Bovail P M, editors. Bacterial pathogenesis, part A. New York, N.Y: Academic Press; 1994. pp. 331–344. [Google Scholar]
- 22.Roberts S, Bomford A. Chelation of transferrin iron by desferrioxamine in K562 cells—the partition of iron between ferrioxamine and ferritin. Biochem J. 1988;254:869–875. doi: 10.1042/bj2540869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strauss E. A symphony of bacterial voices. Science. 1999;284:1302–1304. doi: 10.1126/science.284.5418.1302. [DOI] [PubMed] [Google Scholar]
- 24.Winkelmann G. Kinetics, energetics, and mechanisms of siderophore iron transport in fungi. In: Barton L L, editor. Iron chelation in plants and soil microorganisms. New York, N.Y: Academic Press, Inc.; 1993. pp. 219–239. [Google Scholar]