Abstract
Background:
Vascular mineralocorticoid receptor (MR) expression increases with age driving aging-associated vascular stiffness and hypertension. MR has two isoforms (1α, 1β) with distinct 5’-untranslated and promoter sequences (P1, P2), but the gene regulatory mechanisms remain unknown. We investigated mechanisms driving MR gene transcriptional regulation in aging human smooth muscle cells (SMC).
Methods:
MR was quantified in aortic tissue and primary human aortic SMC (HASMC) comparing adult and aged donors and adult HASMC treated with H2O2, to induce aging. Predicted transcription factor (TF) binding sites in the MR gene were validated using chromatin immunoprecipitations (ChIP) and reporter assays. The impact of TF inhibitors on MR isoforms and fibrosis gene target was examined.
Results:
Expression of both MR mRNA isoforms increased with donor age or H2O2 treatment in HASMCs. Hypoxia-inducible factor (HIF)1α and the inflammatory TF NFκB both increased with age in HASMCs and are predicted to bind MR promoters. H2O2 induced HIF1α and NFκB expression and DNA binding of HIF1α to the MR P1 promoter and of NFκB to both MR promoters in HASMCs. HIF1α inhibition decreased MR-1α isoform expression while NFκB inhibition decreased both MR isoforms. HIF1α, NFκB, and MR inhibition decreased the expression of a SMC-MR target gene implicated in vascular fibrosis. In human aortic tissues, expression of HIF1α and NFκB each positively correlated with donor age and MR expression (p<0.0001).
Conclusion:
These data implicate the inflammatory TF, NFκB, and oxidative stress-induced TF, HIF1α, in regulating SMC MR transcription in aging HASMCs which drives aging-related vascular stiffness and cardiovascular disease.
Keywords: Mineralocorticoid Receptor, Gene Regulation, HIF1α, NFκB, Aging, Oxidative Stress, vascular smooth muscle cells
Graphical Abstract
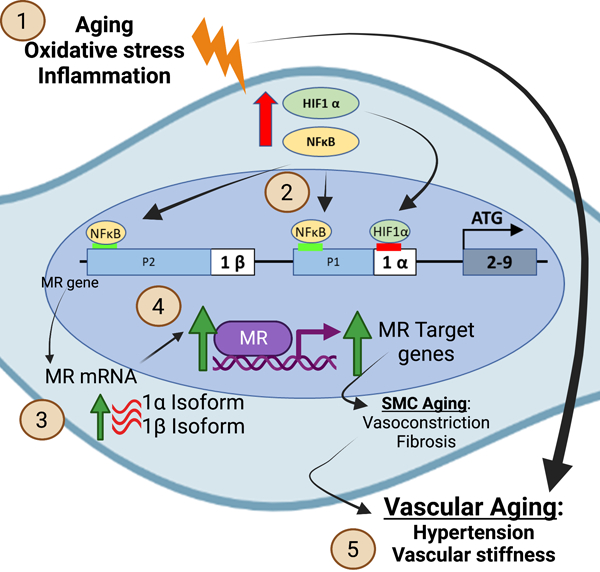
Introduction
The mineralocorticoid receptor (MR, NR3C2) was identified in 1987 to bind the hormone aldosterone, a critical regulator of blood pressure.1 Like all steroid receptors, MR functions as a ligand-activated transcription factor (TF) to coordinate gene expression across multiple tissues in response circulating hormones.2 The traditional MR ligand is aldosterone, produced by the adrenal gland to regulate renal electrolyte homeostasis and blood pressure. MR also binds glucocorticoids in tissues lacking the enzyme 11beta-hydroxysteroid dehydrogenase-2 (11βHSD2), which locally inactivates glucocorticoids.3 More recently, MR was found to be activated in a hormone-independent manner by angiotensin type-1 receptor signaling in vascular smooth muscle cells (SMC)4 and by Rac1 signaling in renal and cardiac cells.5 Activated MR translocates into the nucleus and binds to specific DNA sequences, known as mineralocorticoid-responsive elements (MRE), to regulate the expression of target genes, including connective tissue growth factor (CTGF), a regulator of tissue fibrosis.6,7 Thus, as MR can be activated by diverse ligands and signaling pathways, the level of MR protein expression can influence overall activity, independent of systemic aldosterone levels. Indeed, while MR antagonists are approved to treat hypertension and heart failure, these drugs have additional cardiovascular benefits that are independent of natriuresis, blood pressure, or serum aldosterone.8 However, little is known about the mechanism controlling expression of the MR gene.
MR is expressed in non-renal cells including endothelial cells, SMC, cardiomyocytes, neurons, macrophages, and adipocytes where it contributes to cardiovascular disease by a variety of mechanisms.8 Activation of MR in the heart (cardiomyocytes) and vasculature (endothelial cells and SMCs) contributes to cardiac fibrosis, stiffness and hypertrophy in response to cardiac injury,9,10 vascular stiffness, vasoconstriction and blood pressure elevation with aging,7,11–13 and endothelial dysfunction in the setting of obesity14,15 or atherosclerosis.16,17 Recent data support that MR expression itself is induced in situations of cardiovascular stress in which MR contributes to cardiovascular disease. For example, in human endothelial cells, MR expression is increased in females compared to males and contributes to sex differences in obesity-associated endothelial dysfunction.14,18 Multiple studies demonstrate that MR expression in SMC increases with advancing age in rodents and in humans.13,19 We have recently demonstrated that rising MR expression in aging SMC contributes to the aging-associated rise in blood pressure, vascular fibrosis and vascular stiffness via MR regulation of genes that contribute to vasoconstriction and fibrosis.7,11–13 For example, MR expression rises with age in the mouse aorta where it regulates expression of connective tissue growth factor (CTGF) and hence, SMC-specific deletion of MR prevents the increase in CTGF, vascular fibrosis, and vascular stiffness with aging. 7,11–13 Although these findings are consistent with cardiovascular benefits of MR antagonists that often accrue independent of elevated aldosterone levels, there is a remarkable paucity of data describing how transcription of the MR gene is regulated in situations of cardiovascular stress.
The NR3C2 gene coding for the MR is located on chromosome 4q31. The gene includes 10 exons, 8 coding exons that determine the single MR protein sequence, and 2 alternatively spliced first exons (1α and 1β). Thus, the NR3C2 gene encodes 2 mRNA isoforms that translate into the same functional MR protein and differ only in their 5’-untranslated region and regulator sequences.20 In 1995, Zennaro and colleagues reported that the 5’ flanking sequence of the MR includes two promoter regions (P1 and P2) that control expression of the two isoforms via distinct transcriptional regulators and further studies in mice suggested that these promoters may mediate tissue-specific isoform expression.20–23 Since that time, virtually no new data have become available regarding MR gene transcriptional regulation. However, the presence of two isoforms with distinct regulatory domains is highly conserved from humans to rodents suggesting that the potential to differentially regulate the two MR isoforms has implications for species survival.20
MR gene expression rises with age in SMC from mice and humans and globally regulates vascular gene expression to contribute to many aspects of vascular aging including rising blood pressure, vascular fibrosis and stiffness. Indeed, MR antagonism or SMC-specific deletion of the MR prevents the aging-associated rise in blood pressure, vascular stiffness, and expression of the fibrosis regulator CTGF.7,11–13 Thus, we investigated mechanisms regulating MR gene expression, specifically focusing on MR gene regulation in aging human SMC. We examined regulation of both MR isoforms via the P1 and P2 promoters using primary low passage human aortic smooth muscle cells (HASMCs) from young and old, male and female donors and used primary SMCs and cell lines to explore molecular mechanisms. We identify two transcription factors (HIF1α and NFκB) that differentially bind to and regulate the MR gene promoters and control transcription of the two MR isoforms in aging human SMC and show a correlation between these factors and MR levels in human aortic tissue across the aging spectrum.
Material and Methods
All data associated with this manuscript will be shared upon reasonable request to the corresponding author. See detailed methods in Online Methods Supplement.
Human Tissue and Cells
Primary human aortic SMC (HASMC) were cultured from de-identified human aortic tissue obtained post mortem from the NIH supported National Disease Research Interchange (NDRI) and hence the medical ethics committee of the participating center (Tufts Medical Center) deemed this research to be exempt from human subjects research requirements. As such, only age and sex information is available for the source of the tissue.
Cell Culture and Treatment
Primary HASMC (passage <10) and Pac1 (rat pulmonary artery SMC) cells were treated with 10µM H2O2 (Sigma Aldrich), spironolactone (Spiro, 1µM, Research plus), 10µM CAY10585 (CAY, HIF1α inhibitor, Abcam), 10µM Dimethyl-bisphenol A (DI, HIF1α inhibitor, Abcam), 10µM Parthenolide (PAR, NFκB inhibitor, Sigma), 10µM JSH23 (JSH, NFκB inhibitor, Abcam) or vehicle (DMSO) for 24 hours based on prior time course studies and cells were harvested for mRNA and protein quantification, luciferase assays and chromatin immunoprecipitation (ChIP) studies. This concentration of H2O2 was chosen based on published data showing that this concentration induces cell senescence and an aging phenotype while higher concentrations lead to cell death.24,25
Statistical Analysis
Values are presented as mean±SEM. Differences between two groups were analyzed using student t-test. Differences between multiple groups were analyzed by one-way ANOVA followed by Tukey post-hoc test. Pearson correlation coefficients were calculated to determine correlations in human aortic tissue. Statistical analyses were performed using GraphPad Software Inc. version 8. Statistical significance was set at p<0.05.
Results
Smooth Muscle Cell MR Expression Increases with Donor Age and Oxidative Stress in Primary Human SMC
As MR expression has been shown to increase with age in rodent vessels7,11,12 and more recently, in human SMCs from males,7 we first examined MR expression in primary low passage HASMCs from males and females comparing primary aortic SMC from “adult” (average age 40s, range 20–50s) versus “aged” (age>70) donors (Figures 1A and 1B) and in adult HASMCs (Figures 1C and 1D) treated with vehicle versus hydrogen peroxide (H2O2, 10µM for 24 hours), a previously identified inducer of an aging phenotype in cultured cells.24 In both aging models, cell senescence markers significantly increased with donor age or H2O2 treatment, as measured by senescence-associated β galactosidase (SAβ-gal) staining (Figure 1A and 1C) and p53 and p21 protein expression (Figure S1), confirming the aging phenotype in these two in vitro aging models. Consistent with prior studies, Figure 1B confirms a 2–3 fold increase in MR protein expression in SMC from aged compared with adult males and, for the first time, demonstrates a similar increase in HASMCs from aged females (Figure 1B). Similarly, induction of aging with H2O2 in adult HASMCs significantly increases MR protein (Figure 1D). Since primary human SMCs are not transfectable, we also confirmed the H2O2 induction of senescence markers and MR upregulation in Pac1 cells (Figure S2), a transfectable rat pulmonary artery cell line for use in mechanistic experiments. Co-treatment with the MR antagonist spironolactone with or without H2O2 showed no impact on senescence markers suggesting that MR upregulation is downstream of the senescence phenotype which is MR-independent (Figure S3). These data confirm that MR protein is upregulated in primary SMC from aging human males and females and support the use of H2O2-treated primary human SMC and Pac1 cells to explore molecular mechanisms for MR regulation in aging SMCs.
Figure 1. Mineralocorticoid receptor (MR) expression and cell senescence increase with age and oxidative stress in primary human aortic smooth muscle cells (HASMC).
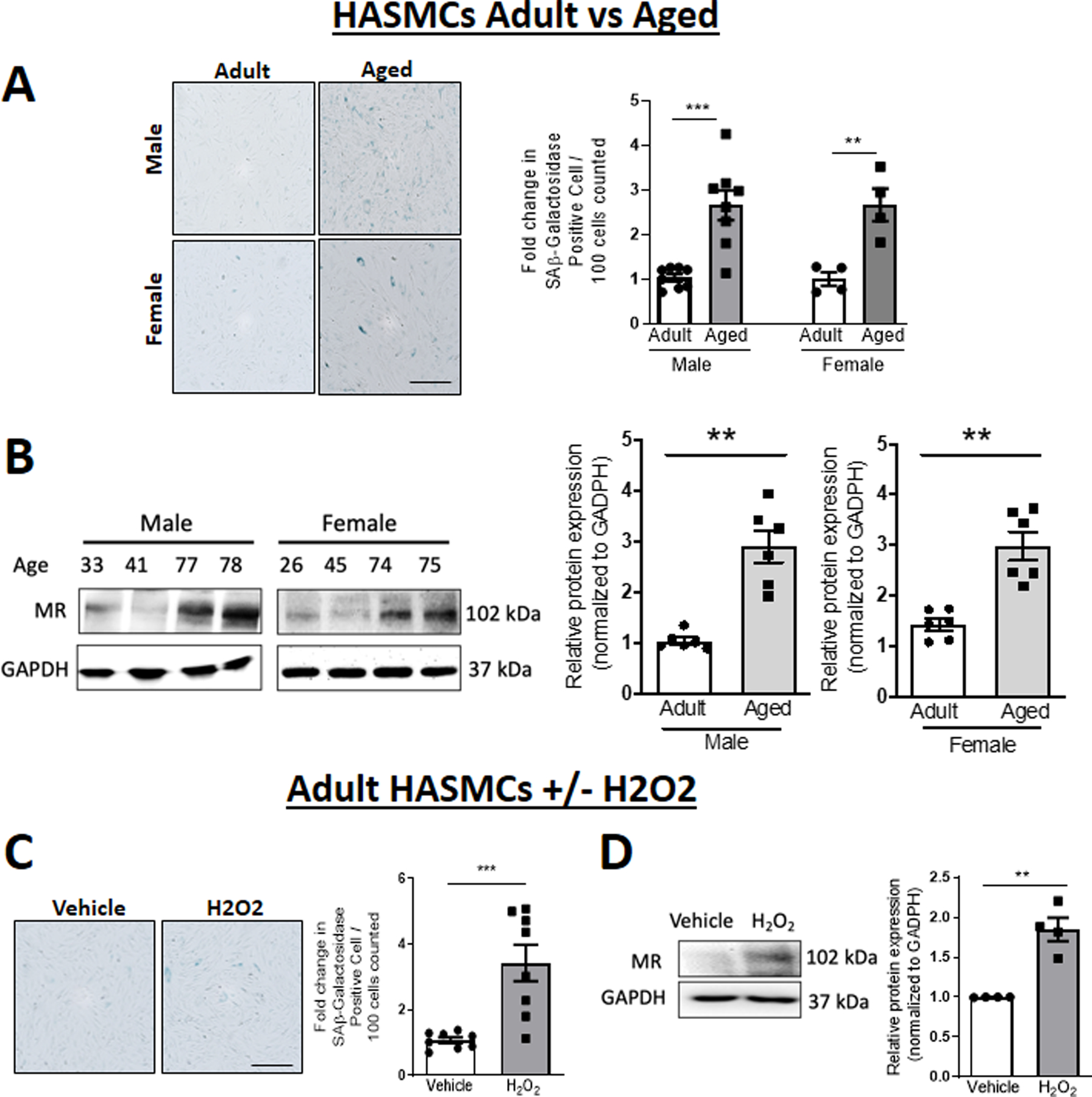
Comparisons between (A-B) primary HASMC from adult (average age 40s) versus aged (average age late 70s) donors or (C-D) adult HASMCs treated with vehicle versus H2O2 (10 µM for 24h). (A, C) Representative images and quantification of senescence-associated (SA) β-galactosidase staining. Scale Bar 40 μM N=4–8 per group. (B, D) Representative immunoblots and quantification of MR protein expression in cells from males and females, normalized to GAPDH. N=4–6 per group. Dot plots show the individual data points and bars indicate the mean with error bars indicating the SEM. Student’s t-Test, *p<0.05, **p< 0.01.
Both MR mRNA Isoforms Increase with Age and Oxidative Stress in Human SMC
The MR gene has two alternative first exons (1α and 1β) which alternatively splice to the common exon 2, containing the translational start site. This gene structure produces one MR protein derived from exons 2–9 with two different mRNA isoforms (isoform 1α and 1β), with distinct 5’-untranslated regions (Figure 2A). Each isoform is regulated by an isoform-specific promoter termed P1 and P2.20 For each promoter, we defined the transcriptional start site (TSS) as base pair “0” and number the bases in the promoter in the negative direction from the TSS and the bases in the first exon in the positive direction from the TSS (Figure 2A, middle). Each MR mRNA isoforms was quantified using exon 1-specific primers in the three in vitro SMC aging models. Both the 1α and 1β mRNA isoforms are significantly increased in HASMCs from adult compared to aged donors (Figure 2B) and in adult HASMCs (Figure 2C) and Pac1 cells (Figure 2D) treated with H2O2 compared to vehicle.
Figure 2. MR-1α and MR-1β Isoform Expression and MR P1 and P2 Promoter Activity Increase with Age in SMC.
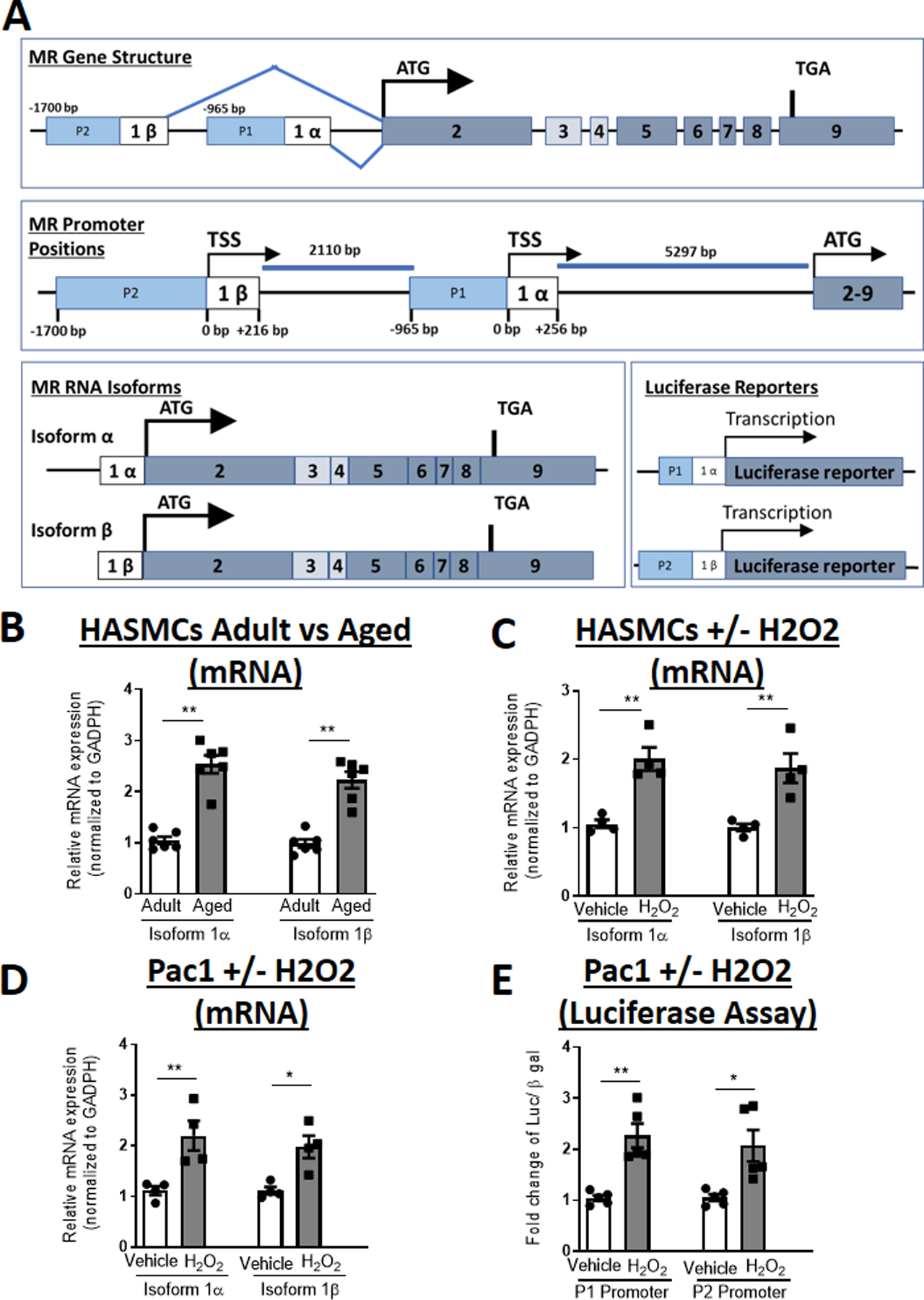
(A) Schematic representation of MR gene structure with the translational start site (ATG), two MR alternative first exons with the alternative transcriptional start sites (TSS) defined as base pair (BP) +1 relative to the 1α and 1β exons and the P1 and P2 promoters, alternatively spliced MR mRNA isoforms and luciferase reporters. Quantification of MR mRNA isoforms in: (B) primary human aortic SMC from adult (age 40s) and aged (age 70s) donors. N=6 per age group; (C) adult primary human aortic SMC or (D) Pac1 SMC line treated with vehicle versus hydrogen peroxide (H2O2) for 24 hours. N=4 experiments; (E) Quantification of MR promoter-reporter activity of the P1 or P2 promoters transfected into Pac1 SMC line and treated with vehicle versus hydrogen peroxide (H2O2) for 24 hours. N=5 experiments. Dot plots show the individual data points and bars indicate the mean with error bars indicating the SEM. Student’s t-Test, *p<0.05, **p< 0.01.
Luciferase reporter assays were used to examine the impact of aging induction with H2O2 on activity of the P1 and P2 promoters. Since primary HASMCs are resistant to transfection, Pac1 cells were used for these studies. Pac1 cells were co-transfected with luciferase reporter plasmids driven by either the MR P1 (−965 to −85 bp relative to exon-1α TSS) or P2 promoter sequence (−1673 to +216 relative to exon-1β TSS) as defined in Zennaro et al.22 Sixteen hours after transfection, cells were treated with H2O2 for 24 hours and luciferase activity quantified relative to β-galactosidase activity. H2O2 treatment significantly increased activity of both promoters (Figure 2E), suggesting that both promoters are sensitive to induction of a SMC aging phenotype.
HIF1α is Induced in Aging SMC and Binds to the MR P1 Promoter
To begin to explore the transcriptional regulators of MR expression in aging SMC, in silico analysis of the MR promoter and first exon sequences was performed using PROMO to identify predicted TF binding sites (TFBS) within these sequences. The MR P1 and P2 promoter and intron sequences contained 131 and 154 predicted TFBS, respectively, of which 112 TFs were common to both MR promoters. Since SMC MR induction with aging is conserved between rodents and humans, we limited to TFBS conserved from rodents to humans and based on the data in Figures 1–2, we honed in on TFs implicated in aging or oxidative stress. We further tested whether the predicted TFs are upregulated in aged compared to adult HASMCs. This analysis identified two potential MR-regulating transcription factors, HIF1α and NFκB that met these criteria. HIF1α expression was significantly increased in adult versus aged HASMCs in males and females (Figure 3A). PROMO software identified 4 evolutionarily conserved predicted HIF1α consensus sites, three potential regulators of MR-1α isoform, at positions −1651, +77 and +196 relative to MR-1α TSS, and one potential regulator of MR-1β isoform, at −413 relative to MR-1β TSS (Figure 3B). To examine HIF1α binding to these sites, HASMCs and Pac1 cells were treated with H2O2 and chromatin immunoprecipitation (ChIP)-qPCR was performed using HIF1α antibody and primers flanking the 4 predicted HIF1α binding sites. In both HASMCs (Figure 3C) and Pac1 cells (Figure S4), H2O2 treatment significantly enriched HIF1α binding compared to IgG control antibody only at the P1 promoter +77 HIF1α binding site.
Figure 3. HIF1α is induced by aging, binds to the MR gene P1 promoter region and regulates MR expression via the 1α isoform.
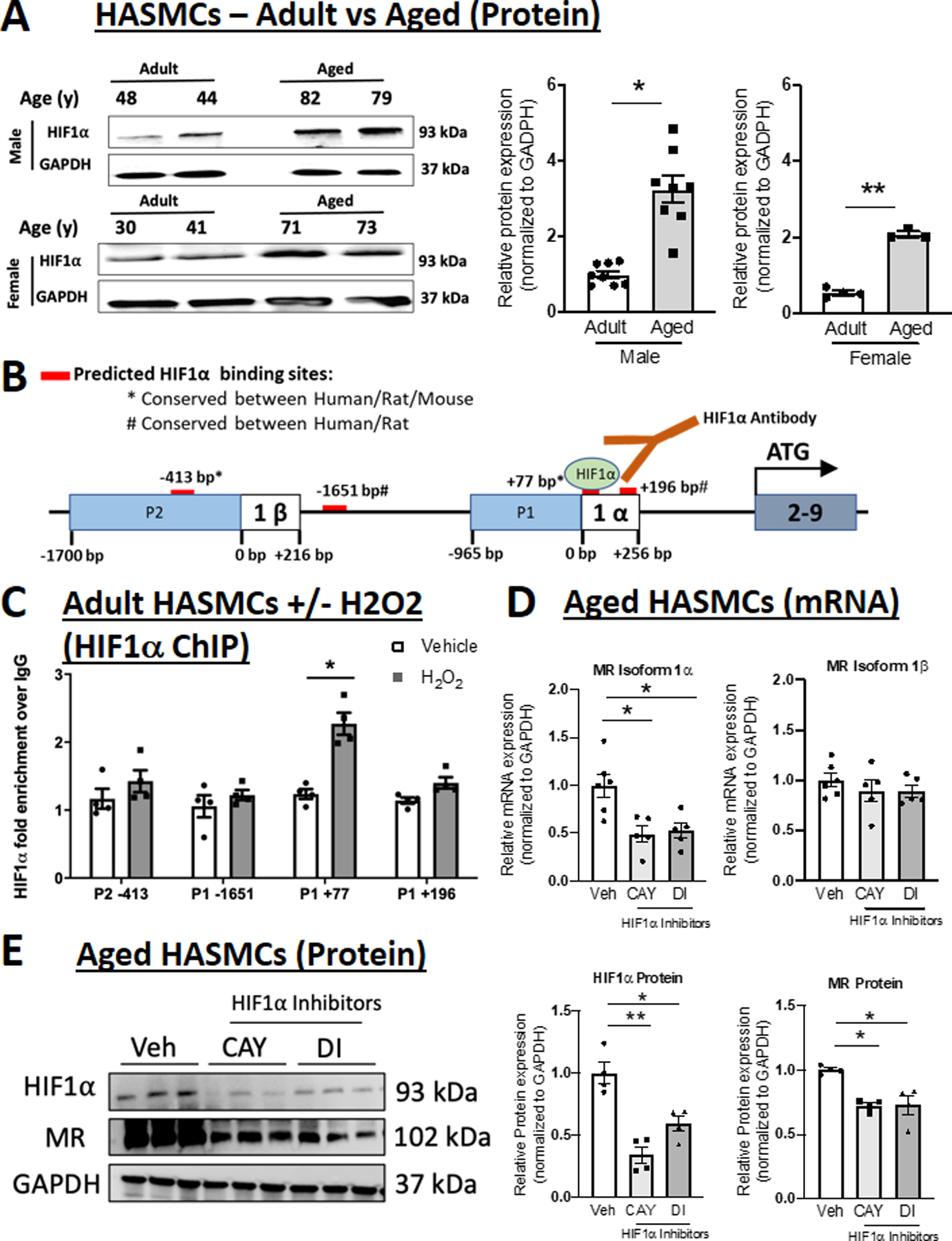
(A) Representative immunoblots and quantification of HIF1α protein expression comparing primary HASMC from adult (average age 40s) versus aged (average age late 70s) male and female donors. N=4–8 per sex and age group; (B) Schematic representation of the four predicted HIF1α binding sites (red bars) conserved between human and rodents (rat and/or mouse). (C) Chromatin immunoprecipitation (ChIP)-qPCR of chromatin isolated from HASMCs treated with H2O2 (grey bar) or vehicle for 24 hours using anti-HIF1α compared to control IgG followed by PCR with primers specific to the predicted P1 and P2 promoter HIF1α binding sites. (D) Quantification of MR mRNA isoforms in aged primary HASMCs treated with HIF1α inhibitors CAY10585 (CAY) and Dimethyl-bisphenol A (DI). N=5 experiments. (E) Representative immunoblots and quantification of HIF1α and MR protein expression in aged primary HASMCs treated with HIF1α inhibitors. N=4 for each group. Dot plots show the individual data points and bars indicate the mean with error bars indicating the SEM. One-way ANOVA with Tukey post hoc test, *p<0.05 **p<0.01.
HIF1α Specifically Regulates MR Isoform 1α and MR Protein Expression in Aged Smooth Muscle Cells
Next, HIF1α was inhibited in aged SMC using two different inhibitors, that decrease HIF1α activity by distinct mechanism of action,26,27 and the impact on MR isoform expression was measured. Both HIF1α inhibitors significantly decreased the MR-1α isoform with no impact on expression on the MR-1β isoform in aged HASMC (Figure 3D). Similarly, in the Pac1 SMC line, both HIF1α inhibitors prevented H2O2 induction of the MR-1α without significantly changing MR-1β isoform expression (Figure S5). These results are consistent with the findings in Figure 3C in which HIF1α binding is enriched only at the predicted HIF1α TFBS +77 bases from the MR-1α TSS. Conversely, in adult (non-aged) HASMC, HIF1α inhibitors did not modulate expression of either MR mRNA isoforms (Figure S6), consistent with HIF1α induction and a role in aged but not adult SMCs. Finally, we confirm that both HIF1α inhibitors significantly decreased HIF1α protein expression and this is associated with a significant 25% decrease in MR protein level in SMC from aged donors (Figure 3E) and in H2O2-treated Pac1 SMCs (Figure S7).
NFκB Increases with Age, Binds to Both MR Gene Promoters and Regulates Expression of Both MR Isoforms in Aged Human SMC
The data thus far support that aging is associated with increased SMC expression of both MR mRNA isoforms but that rising HIF1α in aging SMC specifically regulates only the MR-1α isoform. Thus, we next focused on the pro-inflammatory TF NFκB, another TF implicated in aging and predicted to bind to conserved MR gene regulatory regions in both promoters. NFκB protein (detected using a specific antibody for p65)28 is also significantly increased in aged compared to adult HASMCs from male and female donors (Figure 4A). PROMO software identified 6 evolutionarily conserved predicted NFκB consensus sites, four potential regulators of MR-1α isoform, at positions −1790, −1437, −802, +711 relative to the 1α TSS, and two potential regulator of MR-1β isoform, at −1571 and −1083 relative to the MR-1β TSS (Figure 4B). HASMCs from adult donors and Pac1 cells were treated with H2O2 and ChIP-qPCR was performed using NFkB antibody and primers flanking each of the 6 predicted NFkB binding sites. In both HASMC (Figure 4C) and Pac1 cells (Figure S8A), H2O2 treatment induced significant enrichment of NFκB over IgG control antibody at P2 −1571 and P1 −802, indicating that NFκB binds to both MR promoters when aging is induced by H2O2 in SMC. Next, NFkB was inhibited with two different inhibitors with distinct mechanisms of action (PAR inhibits IκB thereby preventing release of NFκB from the cytoplasmic IκB complex29 and JSH, directly binds NFκB, preventing its interaction with DNA.30) NFkB inhibition significantly decreased mRNA expression of both MR-1α and MR-1β isoforms in aged HASMCs (Figure 4D) and Pac1 cells treated with H2O2 (Figure S8B), consistent with the ChIP results. Conversely, NFκB inhibitors did not modulate MR isoform expression in adult HASMCs, where NFκB and MR are not up-regulated (Figure S9). These data demonstrate that NFκB increases with aging in SMCs from males and females, binds to both the MR P1 and P2 gene promoters and regulates expression of both MR isoforms. Finally, we confirm that both NFκB inhibitors significantly decreased NFκB protein and this is associated with a greater than 50% decrease in MR protein in SMC from aged donors (Figure 4E) and prevents H2O2-induction of MR in Pac1 SMCs (Figure S10).
Figure 4. NFκB is induced with age, binds to the MR P1 and P2 promoters in response to oxidative stress, and regulates expression of both MR isoforms in human aortic smooth muscle cells (HASMC).
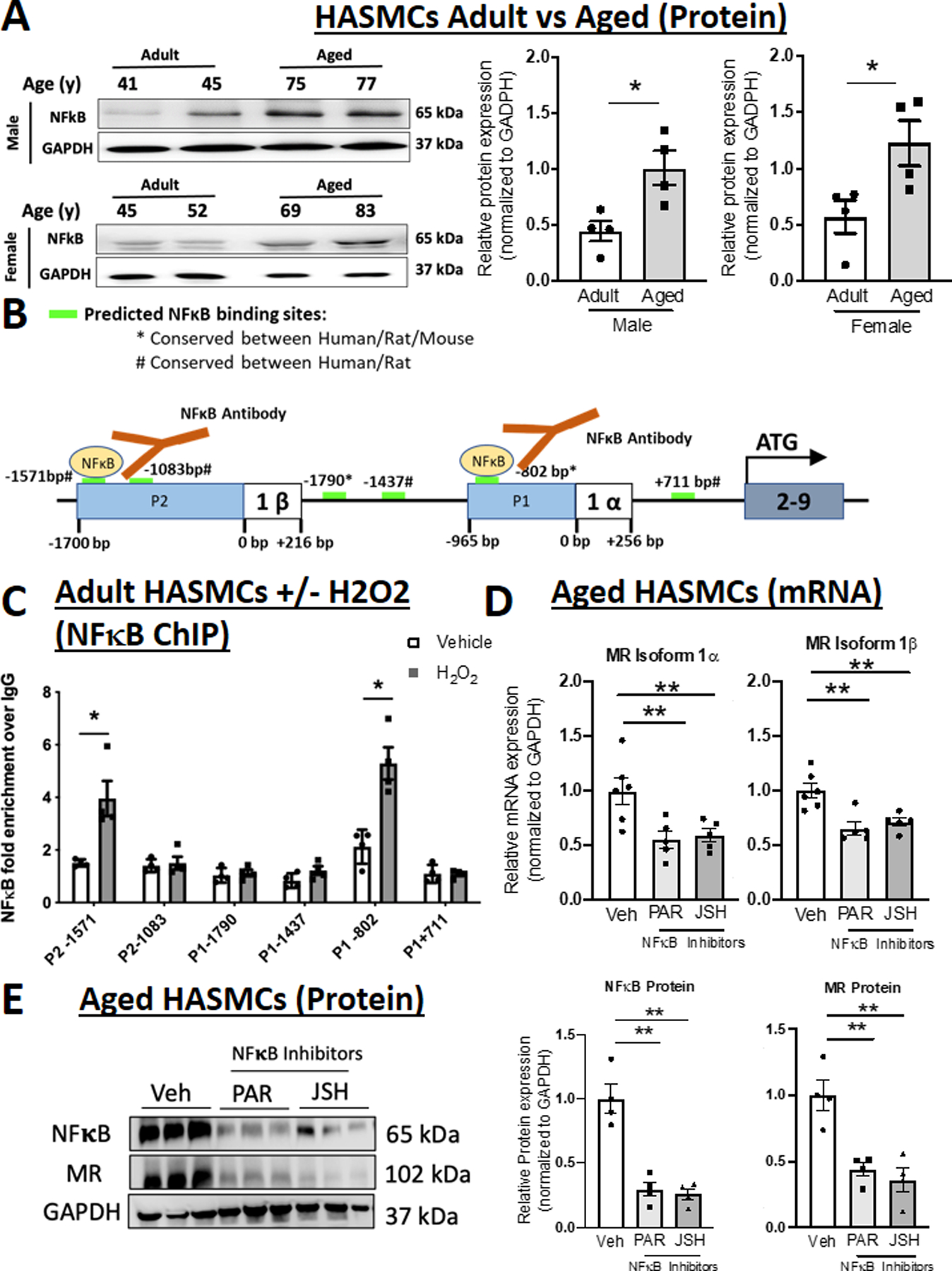
(A) Representative immunoblots and quantification of NFκB protein expression comparing primary HASMC from adult (average age 40s) versus aged (average age late 70s) male and female donors. N=4 for each sex and age group. (B) Schematic representation of the six predicted NFκB binding sites (green bars) conserved between human and rodents (rat and/or mouse). (C) Chromatin immunoprecipitation (ChIP) performed in chromatin isolated from HASMCs treated with vehicle (white bar) or H2O2 (grey bar) for 24 hours using anti-NFκB antibody compared to control IgG antibody and PCR with primers specific to the predicted P1 and P2 promoter NFκB binding sites. N=4 experiments. (D) Quantification of MR mRNA isoforms in aged primary HASMCs treated with two distinct NFκB inhibitors Parthenolide (PAR) and JSH23 (JSH). N=5 experiments. (E) Representative immunoblots and quantification of NFκB and MR protein expression in aged primary HASMCs treated with NFκB inhibitors. N=4. Dot plots show the individual data points and bars indicate the mean with error bars indicating the SEM. One-way ANOVA with Tukey post hoc test, **p<0.01.
HIF1α, NFκB and MR inhibition decrease SMC expression of the profibrotic MR target gene CTGF
The data thus far support that HIF1α and NFκB contribute to regulation of MR expression in aging human vascular SMCs. To explore if this contributes to expression of relevant MR target genes in SMC, the impact of HIF1α and NFκB inhibition on CTGF, a well validated and pathologically relevant SMC-MR gene targets,6,7 was measured in aged HASMCs and in H2O2-treated Pac1 cells. HIF1α and NFκB inhibition significantly reduce CTGF mRNA expression compared with vehicle in aged HASMCs and prevented H2O2-induction of CTGF in Pac1 cells (Figure 5A-B). Pac1 cells were also treated with H2O2 along with an MR antagonist. Spironolactone prevented H2O2-induction of CTGF in Pac1 cells (Figure 5C) supporting that MR is necessary for the induction of CTGF by H2O2 in SMCs.
Figure 5. HIF1α- and NFκB Contribute to Pro-fibrotic MR Target Gene Expression in SMCs and their Binding Sites are Necessary for MR Gene Promoter Regulation by Oxidative Stress.
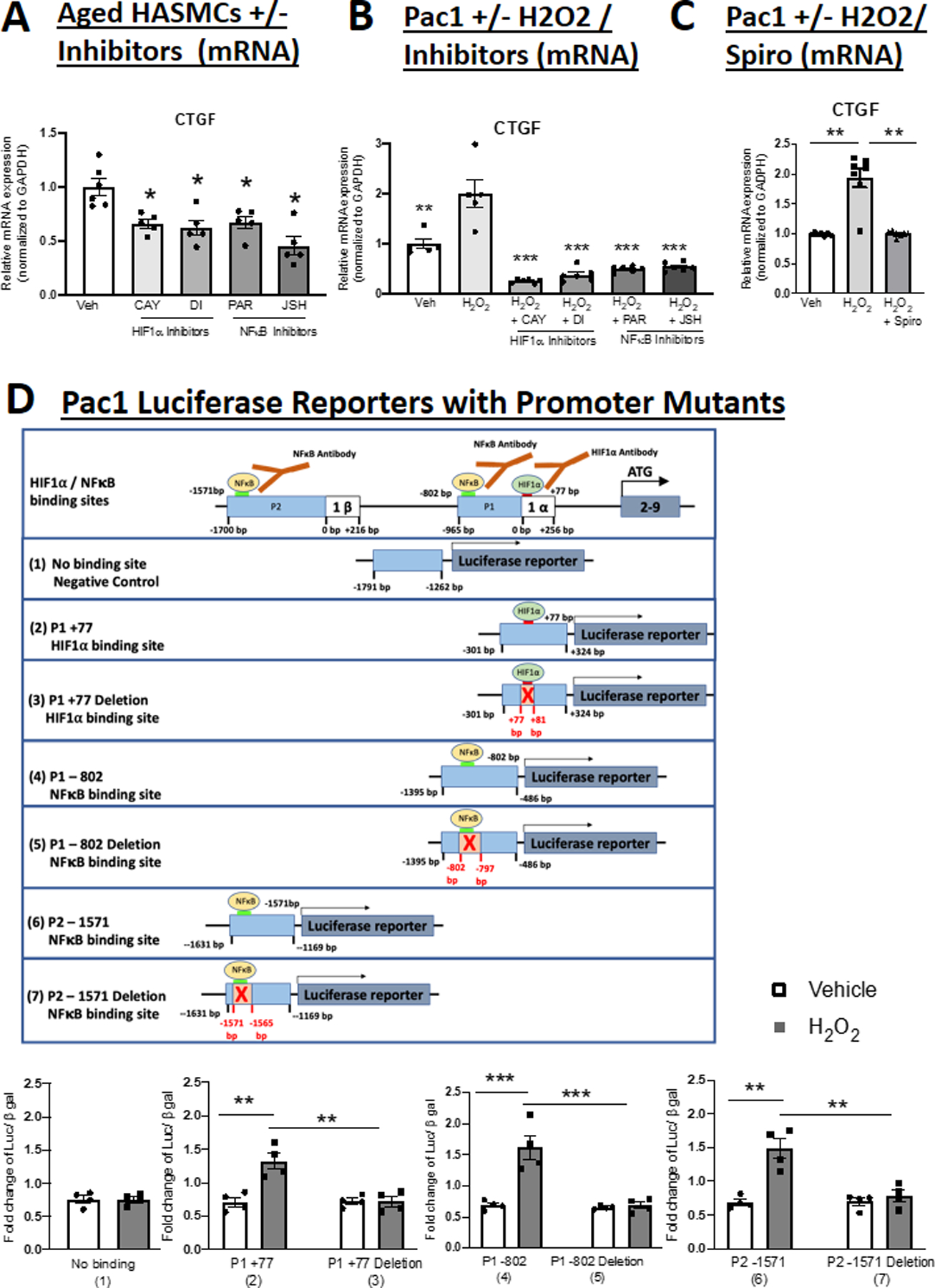
Quantification of Connective Tissue Growth Factor (CTGF) mRNA expression in; (A) Aged HASMCs treated with HIF1α or NFκB inhibitors; and (B) Pac1 SMCs treated with H2O2 and HIF1α inhibitors or NFκB inhibitors. N=5. (C) Pac1 SMCs treated with H2O2 and spironolactone (Spiro). N=7. (D) Schematic representation of luciferase constructed reporters containing regions of the MR P1 or P2 promoter containing a predicted HIF1α binding site that did not support transcription factor binding (1) or sites that bound HIF1α (2) or NFκB (4, 6) or the same constructs with the HIF1α (3) or NFκB (5, 7) sites deleted. Quantification of reporter activity in transfected Pac1 SMCs treated with vehicle versus hydrogen peroxide (H2O2) for 24 hours. N=4 experiments. Dot plots show the individual data points and bars indicate the mean with error bars indicating the SEM. One-way ANOVA with Tukey post hoc teste. (A) *p<0.05 versus Vehicle, (B) **p<0.01, ***p< 0.001 versus H2O2, (C) **p<0.01 versus H2O2, (D) **p<0.01, ***p<0.001.
HIF1α and NFκB binding sites are necessary for MR promoter induction by H2O2 in SMC.
To test whether the HIF1α and NFκB binding sites are necessary for MR promoter activity in SMCs, reporters were designed with MR promoter regions containing each of the putative MR-regulatory binding sites intact and also with the HIF1α and NFκB binding sites specifically deleted and confirmed by sequencing. As a negative control, a construct containing a predicted HIF1α binding site that did not show binding in response to H2O2 by ChIP was also included (Figure 5D top). Pac1 cells were transfected with each construct and treated with vehicle or H2O2. H2O2 significant increased luciferase activity of the three reporters containing the binding sites for HIF1α and NFκB observed by ChIP, but not the HIF1α site that did not show enrichment by ChIP. When each specific HIF1α or NFκB binding site was deleted, the H2O2 induction of reporter activity was lost (Figure 5D, bottom), confirming that these specific binding sites are required for induction of MR promoter activity.
Correlations Between HIF1α and NFκB Expression and MR Protein Level in Human Aortic Tissue with Advancing Age
Finally, we measured the protein level of MR and the transcription factors implicated in MR gene regulation in human aortic tissue. Protein was isolated from aortic tissue (predominantly composed of SMCs) from 7 male and 27 female individuals ranging in age from 14–86 years and MR, HIF1α and NFκB proteins were quantified. MR expression positively correlated with age as did the level of HIF1α and NFκB (P<0.0001 for all, Figure 6A-C). There was also a significant positive correlation in human vessels between the level of MR protein and the level of both HIF1α and NFκB (p<0.0001, Figure 6D-E), supporting the concept that these TFs may be driving rising MR expression in the aging human aorta.
Figure 6. Correlations between age and mineralocorticoid receptor (MR) expression with HIF1α and NFκB expression in human aortic tissue.
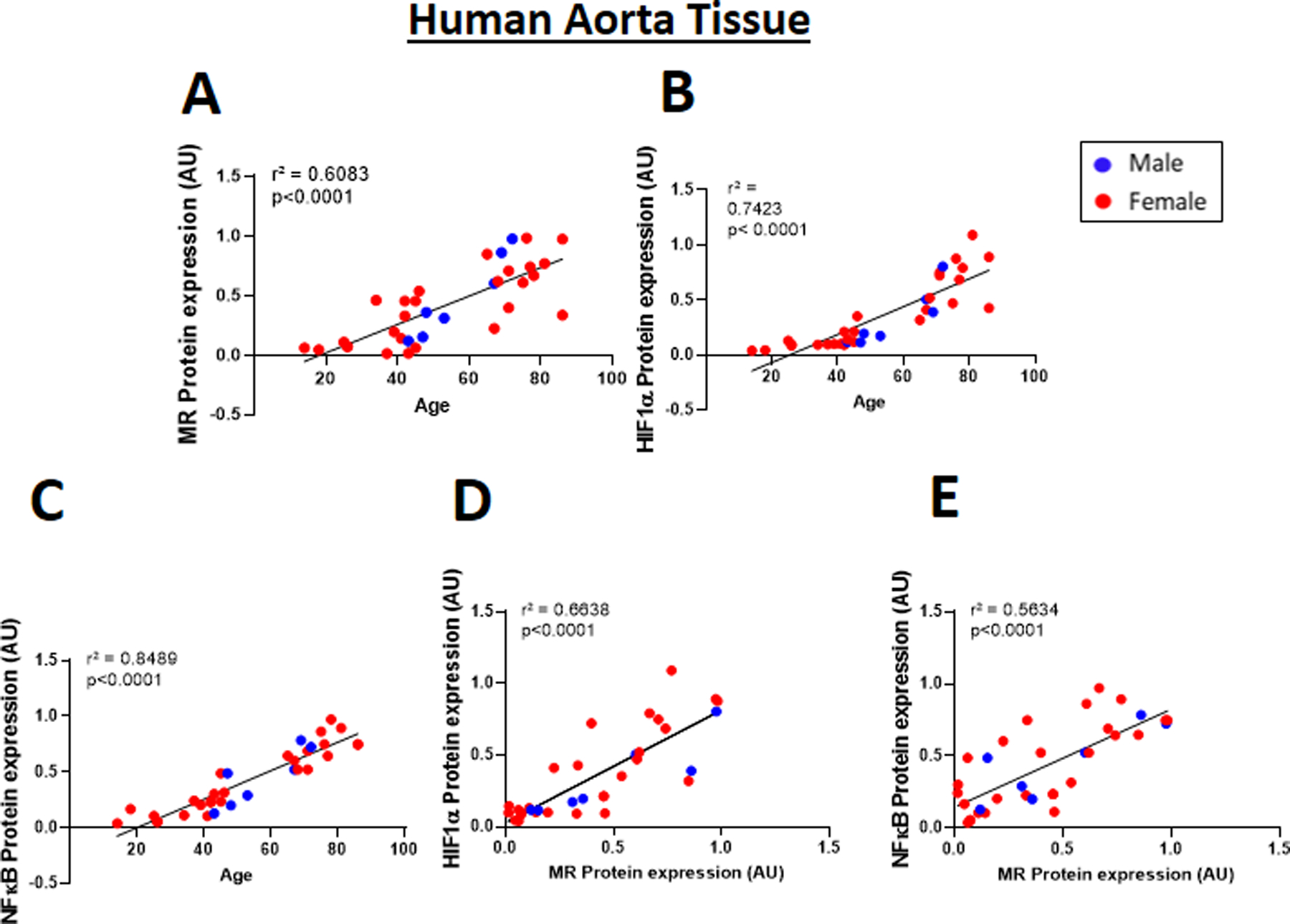
Immunoblotting was performed in aortic tissue from 34 male (blue dots) and female (red dots) donors ranging from 14–86 years old. Correlation between increasing age and (A) MR, (B) HIF1α and (C) NFκB in human aortic tissue. Correlation between MR protein level and the transcription factors (D) HIF1α and (E) NFκB in human aortic tissue. Pearson correlation coefficients and the degree of significance are indicated in each graph.
Discussion
In summary, these data reveal novel mechanisms by which the human MR gene is regulated, focusing specifically on SMC in the aging human vasculature, where MR has been previously shown to be upregulated to drive the aging phenotype, including CTGF expression, vascular fibrosis and stiffness.31 We demonstrate for the first time that: (1) MR expression increases with age in primary, low passage, human aortic SMC from both male and females donors; (2) Both MR isoforms (isoform 1α and isoform 1β) are expressed and increase with age in primary HASMCs; (3) HIF1α, a transcription factor induced by hypoxia and oxidative stress, is increased in aged HASMCs and in human aortic tissue; (4) HIF1α contributes to SMC MR gene regulation with aging and oxidative stress, binding at +77 relative to the 1α promoter and contributing specifically to MR P1 promoter regulation and MR-1α isoform expression in aged SMCs; (5) NFκB, a pro-inflammatory transcription factor, also increases with age in HASMCs and human aortic tissue, binding to two sites to regulate both the MR P1 and P2 promoters and transcription of both MR isoforms (1α and 1β) in aged SMCs; (6) HIF1α and NFκB contribute to regulation of the pro-fibrotic MR target gene CTGF in aged HASMCs; and (7) HIF1α and NFκB protein expression each positively correlate with advancing age and with MR expression in human aortic tissue. Overall, these results are consistent with the model summarized in the graphical abstract: In the aging vasculature, a situation of increased oxidative stress and inflammation, expression of HIF1α and NFκB are increased in smooth muscle cells. HIF1α binds to the MR P1 promoter to induce transcription of MR-1α isoform and NFκB binds to both the P1 and P2 promoters to induce both mRNA isoforms resulting in increased vascular SMC MR protein and activity to drive target gene expression that contributes to vascular aging. By providing the first new information about MR gene regulation in over 2 decades, these results support a mechanism for rising MR in the aging vasculature where SMC MR has been shown in multiple studies to promote vascular constriction, fibrosis, calcification and stiffness thereby contributing to hypertension, cardiac failure and other common disorders of aging.7,11–14,32 These findings set the stage for future studies examining MR gene regulation in other cell types and in other conditions in which MR activity contributes to disease progression and to determine the physiological importance of the distinct regulation of the two MR isoforms.
The human MR cDNA was cloned in 19871 and the gene structure and two alternatively spliced isoforms were described in 1995.20 Early characterization of the 5’ regulatory regions had shown differential hormonal regulation of the two alternative promoters that regulate two different mRNA isoforms both of which translate into the same MR protein.22 Yet, despite its critical role in blood pressure control and ample data in the past 25 years implicating MR in cardiovascular disease, no studies have explored the transcriptional regulation of the MR isoforms in cardiovascular cells and tissues.33 Although aldosterone levels do not rise with age, SMC MR has been implicated in many aging-associated cardiovascular disorders including vascular fibrosis and stiffness,7,12,13 hypertension,11 atherosclerosis,16 and heart failure.13,15,34 This evolving central role for vascular MR in aging pathology raises the importance of understanding how the MR is upregulated with age. Increased MR expression has been demonstrated in aging vascular SMC in mouse and rat models and in human cells.7,19 Here we confirmed that MR expression increases with age in primary human SMC and in human aortic tissue from both males and females and show for the first time that both MR isoforms are expressed in HASMCs and increase with donor age. We identify the transcription factors HIF1α and NFκB as MR gene regulators in aged human SMCs. This study specifically used primary low passage human cells, rather than animal models, to more faithfully model human vascular aging and to explore regulation of the human gene, but it is notable that the identified TFBS are conserved in rodents. Moreover, ample data already implicates SMC MR in vascular aging in mice. 7,11–13 Furthermore, confirmation in whole human aorta of the positive correlation between MR expression with age, HIF1α, and NFκB expression, supports that the culture conditions faithfully model MR expression in the intact human vessel.
HIF1α is a transcriptional factor that regulates genes involved in the adaptive response to hypoxia. As such, it regulates genes involved in angiogenesis and physiological processes necessary for vascular homeostasis, such as cell energy metabolism, growth, survival, and migration.35 HIF1α itself is regulated at the level of protein stability, with increased stability induced not only by hypoxia, but also a variety of other factors that increase with age including superoxide, hydrogen peroxide, platelet derived growth factor ligands and TNFα.35 As such, HIF1α protein levels have been also shown to be increased in conditions associated with cardiovascular disease including hypertension, atherosclerosis, vascular stiffness and vascular aging,36,37 all conditions to which SMC MR is known to contribute. Indeed previous studies demonstrated the direct role of HIF1α in vascular remodeling.38 While a recent study showed that HIF1α negatively regulates adrenal steroidogenesis enzymes,39 a role in regulation of MR itself has not been previously explored. Here we confirm that HIF1α protein level is increased in SMC with increasing age or oxidative stress and demonstrate for the first time that HIF1α binds the MR gene near the P1 promoter, that this binding site is required for MR promoter regulation by oxidative stress, and that HIF1α contributes to MR-1α isoform, MR protein, and MR target gene expression in aged human SMC. In addition to describing a new mechanism for MR gene regulation, the results provide a potential explanation for how HIF1α contributes to vascular remodeling, by upregulation of SMC-MR, a driver of vascular fibrosis and stiffness via regulation of vascular stiffness genes including CTGF, collagen-1, integrin-α5 and MMP2.4,7,13 One study recently suggested beneficial effects of HIF1α inhibitors in pulmonary vascular SMC cells, supporting therapeutic potential for HIF1α inhibitors in vascular remodeling in pulmonary arterial hypertension.40 The data in this manuscript expand the potential for HIF1α inhibitors to be targets to prevent vasculature aging disorders, although further studies are needed to test this possibility.
NFκB was also identified as a regulator of MR in aging SMC. NFκB is a transcription factor that coordinates the inflammatory response by regulating genes involved in leukocyte recruitment, immune cell function, and activation of inflammasomes.41 In addition to its role in the acute immune response to infection, NFκB has been implicated in chronic inflammatory diseases, including atherosclerosis. Ample literature demonstrates a role for NFκB in endothelial cells in vascular inflammation.42 NFκB is activated in endothelial cells by oxidative stress, inflammatory cytokines and lipids and regulates expression of pro-inflammatory factors including leukocyte adhesion molecules, pro-oxidant enzymes, and inflammatory mediators (TNFα, MCP1) to promote the vascular aging phenotype in endothelial cells.42–45 MR is also expressed in endothelial cells where it contributes to endothelial dysfunction, vascular inflammation, and atherosclerosis in a sex-specific manner.16,46 Here we demonstrate that NFκB expression is increased with age in male and female human aortic SMCs and aortic tissue from aged compared with the adult donors, expanding the potential role of NFκB to SMC aging. We further show that NFκB binds to the MR gene at two different locations in P1 and P2, that those binding sites are necessary for MR promoter regulation, and that NFκB contributes to both MR-1α and MR-1β mRNA, MR protein, and MR target gene expression in SMC. Whether MR is also regulated by NFκB in aging endothelial cells remains to be explored. Prior studies showed that aldosterone, via the MR, induces an inflammatory response in SMC via activation of the canonical NFκB pathway.47,48 However regulation of MR itself by NFκB has not been previously demonstrated. This new finding could provide a mechanism for chronic inflammation in which NFκB activation in the acute setting increases vascular MR expression, which in turn enhances NFκB-mediated inflammatory responses, preventing resolution of inflammation in the aging vasculature, a mechanism that warrants further exploration.
Oxidative stress and inflammation are common pathways that are induced in vascular aging, atherosclerosis, obesity, hypertension and heart failure.49–51 MR has been shown to contribute to the adverse cardiovascular consequences of all of these conditions, independent of aldosterone level, which has long been a conundrum. This study provides a novel mechanism linking cardiovascular risk factors to enhanced MR activity in SMC to promote cardiovascular disease (see graphical abstract). Specifically, in the aging vasculature, increased oxidative stress and inflammation induce expression and activation of the transcription factors HIF1α and NFκB. Both transcription factors bind to the MR gene in its regulatory regions and induce transcription of the MR, with HIF1α specifically contributing to MR-1α isoform expression and NFκB regulating both the MR-1α and MR-1β mRNA. This results in increased SMC-MR protein expression and activity, independent of changes in aldosterone. Prior studies show that increased SMC-MR activity in the aging vasculature contributes to vasoconstriction,11 vascular calcification32 and fibrosis7,13 leading to rising blood pressure and vascular stiffness, both of which associate with adverse cardiovascular outcomes.52 We show that HIF1α specifically regulates the MR-1α isoform while NFκB regulates both MR promoters. As such, HIF1a inhibition only decreases the MR-1α isoform resulting in a modest (25%) decrease in MR protein while NFκB inhibition decreases both MR isoforms with a greater impact (>50% decline) on MR protein. This supports the potential for the two MR isoforms to be differentially regulated under different conditions. Future studies are needed to address regulation of each MR isoform by HIF1α, NFκB, and likely other transcription factors in cell type-, disease state-, and developmental stage-specific situations.
In this study we identified a new mechanism by which MR is induced in conditions of oxidative stress and inflammation. These findings, combined with our previously published in vivo data using SMC-MR deficient mice, show that induction of MR in SMC contributes to vascular fibrosis and stiffness with aging and regulates expression of fibrosis markers such as CTGF.7,11–13 However, it is clear that there are also MR-independent mechanisms of SMC aging, as evidenced by the lack of impact of MR antagonism on oxidative stress-induced SMC senescence. Thus, oxidative stress and inflammation contribute to the vascular aging phenotype by MR-dependent and also by MR-independent mechanisms that warrant further study.
This study has several limitations that we have attempted to mitigate. Cell culture studies are limited by changes in cellular function when grown in vitro and without surrounding cells. We attempt to mitigate this limitation by using only low passage primary cells cultured in gelatin-coated plates and by comparing key findings in cultured cells to results in whole human tissues. The primary model of aging used here is in vivo natural aging in humans, achieved by comparing primary low passage SMC derived from aged versus adult donors. We used H2O2-induced senescence as a secondary model that allows us to induce aging in vitro to study mechanism. Each model has limitations that are mitigated by the use of multiple approaches. The human tissue sample size is relatively small and spans a large age range which may contribute to why the human data correlations are not very strong. In addition to differences by sex or age, many other factors surely influence aortic gene expression including comorbid conditions, cause of death, or medication use but this clinical history is unknown for these de-identified samples. While this limits the conclusions that can be drawn from the results, this limitation is partially mitigated by integrating the human tissue results with the mechanistic data from cells. All of the mechanistic studies are performed in vitro without corresponding in vivo data. Since there is not an antibody that specifically identifies mouse MR, the protein quantification and ChIP studies cannot be performed in vivo using mice. However, these results are consistent with the substantial published literature demonstrating increased vascular MR mRNA expression in aging mice and rats7,11,12,19 and demonstrating an important role for rising SMC-MR in vascular aging phenotypes in vivo including vascular fibrosis and stiffness mediated by MR transcriptional regulation of CTGF. Moreover, results in primary low passage human cells are likely more relevant to the human aging phenotype that we hope to understand. Finally, here we focused on transcription factors identified by in silico analysis that are already linked to aging and oxidative stress but cannot rule out a role for additional TFs in regulating MR which can be explored in the future.
Perspectives
Despite these limitations, this study provides novel insights with important implications. MR is well known to play a critical role in aging-associated cardiovascular disorders including hypertension, vascular stiffness and heart failure and hence MR antagonist drugs exert cardiovascular benefits in those conditions. However, those benefits appear independent of the level of the MR ligand aldosterone, and this study provides a potential explanation for this clinical conundrum. In SMC of the aging vasculature, MR activity may be driven by the level of MR expression, yet our understanding of the mechanism of MR gene regulation has advanced very little since the gene structure was uncovered in 1995. This study shows for the first time that expression of both the MR isoforms increase in human SMCs with age and identifies the oxidative stress and inflammation driven transcription factors HIF1α and NFκB as novel regulators of MR gene expression. These findings may also be relevant to our understanding of MR gene regulation in other cell types and in other conditions that are driven by oxidative stress and inflammation, such as obesity and atherosclerosis, in which MR contributes adverse cardiovascular outcomes. Since SMC MR has been shown to contribute to rising blood pressure with age, enhanced understanding MR gene regulation uncovers potential new targets to reduce MR expression, which may be used, alone or in combination with MR antagonists, as anti-aging and antihypertensive therapy to prevent or treat aging-associated cardiovascular diseases.
Supplementary Material
Novelty and Relevance.
1. What Is New?
Both MR isoforms are expressed and increase with age in human aortic SMC from male and female donors.
HIF1α expression is increased in aged HASMCs and in human aortic tissue and contributes MR-1α isoform expression.
The inflammatory transcription factor NFκB binds to both the MR P1 and P2 gene promoters and regulates expression of both MR isoforms in aging SMC.
HIF1α and NFκB protein expression correlate with age and with MR expression in human aortic tissue.
2. What Is Relevant?
These data help explain how MR activity may be modulated to drive adverse cardiovascular outcomes independent of its ligand aldosterone.
This study identifies a mechanistic link between oxidative stress and inflammation and enhanced vascular MR gene transcription and MR target gene expression as a potential additional mechanism for hypertension with aging.
3. Clinical/Pathophysiological Implications?
MR activation contributes to hypertension, vascular stiffness and heart failure thus understanding how the gene is regulated has important clinical implications.
These mechanistic insights provide novel potential targets to regulate MR expression as additional mechanisms to target common cardiovascular disorders including hypertension and heart failure.
SOURCES OF FUNDING
This work was supported by a grant from the National Institutes of Health, HL119290 to I.Z.J and the America Heart Association, postdoctoral fellowship, 903910 to J.I.
Nonstandard Abbreviations and Acronyms
- MR
Mineralocorticoid Receptor
- HIF1α
Hypoxia-inducible Factor 1-alpha
- NFκB
Nuclear Factor Kappa B
- CTGF
Connective Tissue Growth Factor
- SMC
Smooth Muscle Cell
- HASMCs
Human Aortic Smooth Muscle Cell
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
SUPPLEMENTAL MATERIAL
Supplemental Methods
Supplemental References
Supplemental Table S1
Major Resources Table
Supplemental Figures S1–10
References:
- 1.Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 1987;237(4812):268–275. doi: 10.1126/SCIENCE.3037703 [DOI] [PubMed] [Google Scholar]
- 2.Parker MG. Mechanism of steroid hormone action. Curr Opin Cell Biol doi: 10.1016/0955-0674(93)90016-j [DOI]
- 3.Chapman K, Holmes M, Seckl J. 11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiol Rev 2013;93(3):1139–1206. doi: 10.1152/PHYSREV.00020.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circulation Research 2005;96(6):643–650. doi: 10.1161/01.RES.0000159937.05502.d1 [DOI] [PubMed] [Google Scholar]
- 5.Nagase M, Fujita T. Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nat Rev Nephrol 2013;9(2):86–98. doi: 10.1038/NRNEPH.2012.282 [DOI] [PubMed] [Google Scholar]
- 6.Newfell BG, Iyer LK, Mohammad NN, McGraw AP, Ehsan A, Rosano G, Huang PL, Mendelsohn ME, Jaffe IZ. Aldosterone regulates vascular gene transcription via oxidative stress-dependent and -independent pathways. Arterioscler Thromb Vasc Biol 2011;31(8):1871–1880. doi: 10.1161/ATVBAHA.111.229070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibarrola J, Kim SK, Lu Q, DuPont J, Creech A, Sun Z, Hill MA, Jaffe JD, Jaffe IZ. Smooth muscle mineralocorticoid receptor as an epigenetic regulator of vascular aging. Cardiovasc Res Published online January 10, 2022.. doi: 10.1093/CVR/CVAC007 [DOI] [PMC free article] [PubMed]
- 8.Jaisser F, Farman N. Emerging Roles of the Mineralocorticoid Receptor in Pathology: Toward New Paradigms in Clinical Pharmacology. Pharmacol Rev 2016;68(1):49–75. doi: 10.1124/PR.115.011106 [DOI] [PubMed] [Google Scholar]
- 9.Kuster GM, Kotlyar E, Rude MK, Siwik DA, Liao R, Colucci WS, Sam F. Mineralocorticoid receptor inhibition ameliorates the transition to myocardial failure and decreases oxidative stress and inflammation in mice with chronic pressure overload. Circulation 2005;111(4):420–427. doi: 10.1161/01.CIR.0000153800.09920.40 [DOI] [PubMed] [Google Scholar]
- 10.Galuppo P, Bauersachs J. Mineralocorticoid receptor activation in myocardial infarction and failure: recent advances. Eur J Clin Invest 2012;42(10):1112–1120. doi: 10.1111/J.1365-2362.2012.02676.X [DOI] [PubMed] [Google Scholar]
- 11.McCurley A, Pires P, Bender S, Aronovitz M, Zhao M, Metzger D, Chambon P, Hill M, Dorrance A, Mendelsohn M, Jaffe I. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 2012;18(9):1429–1433. doi: 10.1038/NM.2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DuPont J, McCurley AT, Davel AP, McCarthy J, Bender SB, Hong K, Yang Y, Yoo J, Aronovitz M, Baur WE, Christou DD, Hill MA, Jaffe IZ. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight 2016;1(14). doi: 10.1172/JCI.INSIGHT.88942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SK, McCurley AT, DuPont JJ, Aronovitz M, Moss ME, Stillman IE, Karumanchi SA, Christou DD, Jaffe IZ. Smooth Muscle Cell-Mineralocorticoid Receptor as a Mediator of Cardiovascular Stiffness With Aging. Hypertension 2018;71(4):609–621. doi: 10.1161/HYPERTENSIONAHA.117.10437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davel AP, Lu Q, Moss ME, Rao S, Anwar IJ, DuPont JJ, Jaffe IZ. Sex-Specific Mechanisms of Resistance Vessel Endothelial Dysfunction Induced by Cardiometabolic Risk Factors. J Am Heart Assoc 2018;7(4). doi: 10.1161/JAHA.117.007675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, de Marco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR. Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circulation Research 2016;118(6):935–943. doi: 10.1161/CIRCRESAHA.115.308269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elizabeth Moss M, Lu Q, Iyer SL, Engelbertsen D, Marzolla V, Caprio M, Lichtman AH, Jaffe IZ. Endothelial Mineralocorticoid Receptors Contribute to Vascular Inflammation in Atherosclerosis in a Sex-Specific Manner. Arterioscler Thromb Vasc Biol 2019;39(8):1588–1601. doi: 10.1161/ATVBAHA.119.312954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Man JJ, Lu Q, Elizabeth Moss M, Carvajal B, Baur W, Garza AE, Freeman R, Anastasiou M, Ngwenyama N, Adler GK, Alcaide P, Jaffe IZ. Myeloid Mineralocorticoid Receptor Transcriptionally Regulates P-Selectin Glycoprotein Ligand-1 and Promotes Monocyte Trafficking and Atherosclerosis. Arterioscler Thromb Vasc Biol 2021;41(11):2740–2755. doi: 10.1161/ATVBAHA.121.316929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faulkner JL, Kennard S, Huby AC, Antonova G, Lu Q, Jaffe IZ, Patel VS, Fulton DJR, Belin De Chantemèle EJ. Progesterone Predisposes Females to Obesity-Associated Leptin-Mediated Endothelial Dysfunction via Upregulating Endothelial MR (Mineralocorticoid Receptor) Expression. Hypertension 2019;74(3):678–686. doi: 10.1161/HYPERTENSIONAHA.119.12802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krug A, Allenhöfer L, Monticone R, Spinetti G, Gekle M, Wang E, Lakatta E. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathwa. Hypertension 2010;55(6):1476–1483. doi: 10.1161/HYPERTENSIONAHA.109.148783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zennaro MC, Keightley MC, Kotelevtsev Y, Conway GS, Soubrier F, Fuller PJ. Human mineralocorticoid receptor genomic structure and identification of expressed isoforms. Journal of Biological Chemistry 1995;270(36):21016–21020. doi: 10.1074/jbc.270.36.21016 [DOI] [PubMed] [Google Scholar]
- 21.Zennaro MC, Farman N, Bonvalet JP, Lombès M. Tissue-specific expression of α and β messenger ribonucleic acid isoforms of the human mineralocorticoid receptor in normal and pathological states. Journal of Clinical Endocrinology and Metabolism 1997;82(5):1345–1352. doi: 10.1210/jcem.82.5.3933 [DOI] [PubMed] [Google Scholar]
- 22.Zennaro MC, le Menuet D, Lombès M. Characterization of the human mineralocorticoid receptor gene 5’-regulatory region: evidence for differential hormonal regulation of two alternative promoters via nonclassical mechanisms. Mol Endocrinol 1996;10(12):1549–1560. doi: 10.1210/MEND.10.12.8961265 [DOI] [PubMed] [Google Scholar]
- 23.le Menuet DL, Viengchareun S, Penfornis P, Walker F, Zennaro MC, Lombès M. Targeted oncogenesis reveals a distinct tissue-specific utilization of alternative promoters of the human mineralocorticoid receptor gene in transgenic mice. J Biol Chem 2000;275(11):7878–7886. doi: 10.1074/JBC.275.11.7878 [DOI] [PubMed] [Google Scholar]
- 24.Caldini R, Chevanne M, Mocali A, Tombaccini D, Paoletti F. Premature induction of aging in sublethally H2O2-treated young MRC5 fibroblasts correlates with increased glutathione peroxidase levels and resistance to DNA breakage. Mech Ageing Dev 1998;105(1–2):137–150. doi: 10.1016/S0047-6374(98)00085-2 [DOI] [PubMed] [Google Scholar]
- 25.del Mar Rivas-Chacón L, Yanes-Díaz J, de Lucas B, Riestra-Ayora JI, Madrid-García R, Sanz-Fernández R, Sánchez-Rodríguez C. Preventive Effect of Cocoa Flavonoids via Suppression of Oxidative Stress-Induced Apoptosis in Auditory Senescent Cells. Antioxidants (Basel) 2022;11(8):1450. doi: 10.3390/ANTIOX11081450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta SS, Sharp R, Hofferek C, Kuai L, Dorn GW, Wang J, Chen M. NIX-Mediated Mitophagy Promotes Effector Memory Formation in Antigen-Specific CD8 + T Cells. Cell Rep 2019;29(7):1862–1877.e7. doi: 10.1016/J.CELREP.2019.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu N, Jiang D, Huang E, Liu X, Li R, Liang X, Kim SH, Chen X, Gao JL, Zhang H, Zhang W, Kong YH, Zhang J, Wang J, Shui W, Luo X, Liu B, Cui J, Rogers MR, Shen J, Zhao C, Wang N, Wu N, Luu HH, Haydon RC, He TC, Huang W. BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. J Cell Sci 2013;126(Pt 2):532–541. doi: 10.1242/JCS.114231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maguire O, Collins C, O’Loughlin K, Miecznikowski J, Minderman H. Quantifying nuclear p65 as a parameter for NF-κB activation: Correlation between ImageStream cytometry, microscopy, and Western blot. Cytometry A 2011;79(6):461–469. doi: 10.1002/CYTO.A.21068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saadane A, Masters S, DiDonato J, Li J, Berger M. Parthenolide inhibits IkappaB kinase, NF-kappaB activation, and inflammatory response in cystic fibrosis cells and mice. Am J Respir Cell Mol Biol 2007;36(6):728–736. doi: 10.1165/RCMB.2006-0323OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbero G, Castro MV, Villanueva MB, Quezada MJ, Fernández NB, DeMorrow S, Lopez-Bergami P. An Autocrine Wnt5a Loop Promotes NF-κB Pathway Activation and Cytokine/Chemokine Secretion in Melanoma. Cells 2019;8(9). doi: 10.3390/CELLS8091060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorini S, Kim SK, Infante M, Mammi C, la Vignera S, Fabbri A, Jaffe IZ, Caprio M. Role of Aldosterone and Mineralocorticoid Receptor in Cardiovascular Aging. Front Endocrinol (Lausanne) 2019;10. doi: 10.3389/FENDO.2019.00584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaffe IZ, Tintut Y, Newfell BG, Demer LL, Mendelsohn ME. Mineralocorticoid receptor activation promotes vascular cell calcification. Arteriosclerosis, Thrombosis, and Vascular Biology 2007;27(4):799–805. doi: 10.1161/01.ATV.0000258414.59393.89 [DOI] [PubMed] [Google Scholar]
- 33.Grossmann C, Almeida‐Prieto B, Nolze A, Alvarez de la Rosa D. Structural and molecular determinants of mineralocorticoid receptor signalling. Br J Pharmacol Published online December 12, 2021.. doi: 10.1111/BPH.15746 [DOI] [PubMed]
- 34.Buonafine M, Bonnard B, Jaisser F. Mineralocorticoid Receptor and Cardiovascular Disease. Am J Hypertens 2018;31(11):1165–1174. doi: 10.1093/AJH/HPY120 [DOI] [PubMed] [Google Scholar]
- 35.López‐Lázaro M HIF-1: hypoxia-inducible factor or dysoxia-inducible factor? FASEB J 2006;20(7):828–832. doi: 10.1096/FJ.05-5168HYP [DOI] [PubMed] [Google Scholar]
- 36.Alique M, Sánchez-López E, Bodega G, Giannarelli C, Carracedo J, Ramírez R. Hypoxia-Inducible Factor-1α: The Master Regulator of Endothelial Cell Senescence in Vascular Aging. Cells 2020;9(1). doi: 10.3390/CELLS9010195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng S, Fragiadaki M, Souilhol C, Ridger V, Evans PC. Response by Feng et al to Letter Regarding Article, “Mechanical Activation of Hypoxia-Inducible Factor 1α Drives Endothelial Dysfunction at Atheroprone Sites.” Arterioscler Thromb Vasc Biol 2017;37(12):e199–e200. doi: 10.1161/ATVBAHA.117.310341 [DOI] [PubMed] [Google Scholar]
- 38.Qi D, Wei M, Jiao S, Song Y, Wang X, Xie G, Taranto J, Liu Y, Duan Y, Yu B, Li H, Shah YM, Xu Q, Du J, Gonzalez FJ, Qu A. Hypoxia inducible factor 1α in vascular smooth muscle cells promotes angiotensin II-induced vascular remodeling via activation of CCL7-mediated macrophage recruitment. Cell Death Dis 2019;10(8). doi: 10.1038/S41419-019-1757-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watts D, Stein J, Meneses A, Bechmann N, Neuwirth A, Kaden D, Krüger A, Sinha A, Alexaki VI, Perez-Rivas Luis Gustavo, Kircher S, Martinez A, Theodoropoulou M, Eisenhofer G, Peitzsch M, El-Armouche A, Chavakis T, Wielockx B. HIF1α is a direct regulator of steroidogenesis in the adrenal gland. Cell Mol Life Sci 2021;78(7):3577–3590. doi: 10.1007/S00018-020-03750-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arai MA, Sakuraba K, Makita Y, Hara Y, Ishibashi M. Evaluation of Naturally Occurring HIF-1 Inhibitors for Pulmonary Arterial Hypertension. Chembiochem 2021;22(18):2799–2804. doi: 10.1002/CBIC.202100223 [DOI] [PubMed] [Google Scholar]
- 41.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther 2017;2. doi: 10.1038/SIGTRANS.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donato AJ, Pierce GL, Lesniewski LA, Seals DR. Role of NFkappaB in age-related vascular endothelial dysfunction in humans. Aging 2009;1(8):678–680. doi: 10.18632/AGING.100080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 2007;100(11):1659–1666. doi: 10.1161/01.RES.0000269183.13937.E8 [DOI] [PubMed] [Google Scholar]
- 44.Silver AE, Beske SD, Christou DD, Donato AJ, Moreau KL, Eskurza I, Gates PE, Seals DR. Overweight and obese humans demonstrate increased vascular endothelial NAD(P)H oxidase-p47(phox) expression and evidence of endothelial oxidative stress. Circulation 2007;115(5):627–637. doi: 10.1161/CIRCULATIONAHA.106.657486 [DOI] [PubMed] [Google Scholar]
- 45.Rossman MJ, LaRocca TJ, Martens CR, Seals DR. Vascular Aging: Healthy lifestyle-based approaches for successful vascular aging. Journal of Applied Physiology 2018;125(6):1888. doi: 10.1152/JAPPLPHYSIOL.00521.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caprio M, Newfell BG, la Sala A, Baur W, Fabbri A, Rosano G, Mendelsohn ME, Jaffe IZ. Functional mineralocorticoid receptors in human vascular endothelial cells regulate intercellular adhesion molecule-1 expression and promote leukocyte adhesion. Circ Res 2008;102(11):1359–1367. doi: 10.1161/CIRCRESAHA.108.174235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu CJ, Wang QQ, Zhou JL, Liu HZ, Hua F, Yang HZ, Hu ZW. The mineralocorticoid receptor-p38MAPK-NFκB or ERK-Sp1 signal pathways mediate aldosterone-stimulated inflammatory and profibrotic responses in rat vascular smooth muscle cells. Acta Pharmacol Sin 2012;33(7):873–878. doi: 10.1038/APS.2012.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leroy V, de Seigneux S, Agassiz V, Hasler U, Rafestin-Oblin ME, Vinciguerra M, Martin PY, Féraille E. Aldosterone activates NF-kappaB in the collecting duct. J Am Soc Nephrol 2009;20(1):131–144. doi: 10.1681/ASN.2008020232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pastori D, Carnevale R, Pignatelli P. Is there a clinical role for oxidative stress biomarkers in atherosclerotic diseases? Intern Emerg Med 2014;9(2):123–131. doi: 10.1007/S11739-013-0999-6 [DOI] [PubMed] [Google Scholar]
- 50.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 2005;25(1):29–38. doi: 10.1161/01.ATV.0000150649.39934.13 [DOI] [PubMed] [Google Scholar]
- 51.Siti HN, Kamisah Y, Kamsiah J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul Pharmacol 2015;71:40–56. doi: 10.1016/J.VPH.2015.03.005 [DOI] [PubMed] [Google Scholar]
- 52.Hansen TW, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006;113(5):664–670. doi: 10.1161/CIRCULATIONAHA.105.579342 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


