Abstract
Introduction
Sepsis is a life-threatening condition, and biomarkers are needed to diagnose sepsis fast and accurately. We aimed to perform this meta-analysis to investigate the diagnostic value of calprotectin on sepsis in critically ill patients.
Methods
The investigators searched MEDLINE, Embase, Web of Science and Cochrane Library. Studies were included if they assessed the diagnostic accuracy of serum calprotectin for sepsis in intensive care unit (ICU). We estimated its diagnostic value and explored the source of heterogeneity. The bivariate model and the hierarchical summary receiver operating characteristic (HSROC) curve were used in the meta-analysis.
Results
Six records assessing 821 patients were included in this meta-analysis. The pooled sensitivity, specificity, positive likelihood ratio (PLR), and diagnostic odds ratio (DOR) were separately as 0.77, 0.85, 5.20, 0.27, respectively. The Fagan's nomogram showed post‐test probabilities of 91% and 35% for positive and negative outcomes, respectively. Subgroup analysis indicated that sepsis definition could be a possible source of heterogeneity, but there’s no sufficient data to investigate sepsis-3 definition. Sensitivity analysis suggested that two studies could affect the stability of pooled results.
Conclusion
On the basis of our meta-analysis, calprotectin is a helpful marker for early diagnosis of sepsis on ICU admission.
Keywords: calprotectin, sepsis, diagnosis, meta-analysis, biomarker
Introduction
Sepsis is a leading cause of mortality and critical illness worldwide and is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection (Vincent et al., 2014; Singer et al., 2016; Fleischmann et al., 2016). The Sequential Organ Failure Assessment (SOFA) score helps to identify patients with sepsis and is an important part of the current sepsis definition. However, it is still difficult to prove infection fast and accurately at complicated medical situations, and diagnosis of sepsis is often delayed (Taylor et al., 2021). Delay in the diagnosis and treatment of sepsis increases mortality and the financial burden (Bui et al., 2022; Souza et al., 2022). Moreover, the lack of a generally accepted biomarker golden standard for sepsis is a common limitation in all sepsis studies. Including a uniform group of patients is a challenge, thus restricting the practicality and catholicity of the results.
Therefore, biomarkers may play a vital role in the timely diagnosis and management of sepsis. C-reactive protein (CRP) and procalcitonin (PCT) are widely used biomarkers, but their diagnostic accuracy has been challenged (Wu et al., 2017; Wu et al., 2018; Yeh et al., 2019). It is reported that surgery and trauma can interfere with the results even in patients without infection (Parli et al., 2018). They cannot readily distinguish infection from sterile inflammation. Therefore, biomarkers with high diagnostic sensitivity and specificity are indispensable.
Calprotectin is a calcium-binding protein that was originally found in neutrophils and was named major leukocyte protein L1. It is a 24-kDa heterodimer composed of light [myeloid-related protein 8 (MRP8)] and heavy (MRP14) chains (8 and 14 kDa), also named S100A8 and S100A9, which belong to the S100 protein family (Edgeworth et al., 1991). Recent findings showed that calprotectin is closely related to sepsis. Increased ascitic calprotectin is associated with spontaneous bacterial peritonitis, and its most common etiology of mortality is sepsis (Abdel-Razik et al., 2020). Moreover, calprotectin in the amniotic fluid is a strong predictor of neonatal sepsis (Delanghe and Speeckaert, 2015). It was also reported of diagnostic value in sepsis-associated encephalopathy and sepsis-induced acute kidney injury (Zhang et al., 2016; Liao et al., 2021). The serum calprotectin expression level was also found significantly increased in sepsis and had a high diagnostic value (Canani et al., 2011; Gao et al., 2015; Decembrino et al., 2015; Huang et al., 2016; Simm et al., 2016; Shams et al., 2017; Wirtz et al., 2020; Larsson et al., 2020; Pirr et al., 2021). However, the number of patients in each study is limited and most of the studies recruited patients from a single center, leading to a potential lack of generalizability of the results. Moreover, the results vary in previous studies (sepsis vs. non-sepsis controls: 42.5%–89% sensitivity, 56%–96% specificity).
In consequence, we aimed to perform this meta-analysis to synthesize the current evidence and investigate the diagnostic value of calprotectin on sepsis.
Materials and methods
Data sources and search strategy
We constructed a protocol of complete meta-analysis that adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards (Page et al., 2021). The protocol was registered with the PROSPERO database (registration number CRD42022304473).
The investigators searched MEDLINE, Embase, Web of Science, and Cochrane Library using the search term “(sepsis OR “bacterial infection” OR “systemic inflammatory response syndrome”) AND (calprotectin).” Two investigators (R-YG and Y-ZH) independently searched the databases from the establishment of these databases until January 2022 and reran the search before the final analysis. The research was done without language or publication period restrictions, and unpublished studies were not searched.
Study selection criteria
Studies that assessed the accuracy of serum calprotectin for differentiation between critically ill patients with sepsis and those without sepsis were included. Sepsis could be diagnosed using any recognized diagnostic criteria, and there are no restrictions on the types of study design eligible for inclusion. All retrieved articles were initially screened by title and abstract. Subsequently, the relevant ones were rescreened by full text. The selection was done independently by two investigators (R-YG and Y-ZH). If there were any discrepancies in the process of the study selection, they would turn to a third party. EndNote was used for recording decisions.
Data extraction
Two investigators (R-YG and Y-ZH) independently extracted the data from individual reports, including first author, year of publication, study location, population setting, admission category (surgical or medical), sepsis definition, and sample size. We also recorded true positive (TP), false positive (FP), false negative (FN), true negative (FN), sensitivity, specificity, area under the curve (AUC), and optimal cutoff value of calprotectin for the diagnosis of sepsis. If there was any disagreement between the two reviewers in the process of data extraction, it was resolved by a third party. We contacted the corresponding authors when further information was needed. If no response was received after sending a reminder, the study would be excluded. The data extracted were recorded in an Excel spreadsheet.
Quality assessment
The quality assessment was done by two independent investigators using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool (Whiting et al., 2011). This evaluation includes the following domains: patient selection, index test, reference standard, and flow and timing.
Statistical analysis
Hierarchical models are increasingly being accepted as standard methods for the meta-analysis of diagnostic test accuracy studies (Lee et al., 2015). Therefore, the hierarchical summary receiver operating characteristic (HSROC) curve was computed, and the bivariate random effects model was used to determine the summary estimates of the sensitivity, specificity, diagnostic odds ratio (DOR), positive likelihood ratio (PLR), and negative likelihood ratio (NLR). Fagan’s nomogram was plotted to assess the utility of serum calprotectin for the diagnosis of sepsis.
Heterogeneity induced by threshold effect was calculated by testing Spearman correlation, and p < 0.05 represented significant threshold effects. The I² statistics was used to evaluate heterogeneity induced by a non-threshold effect. Different I² values reflected low (<30%), moderate (30%–50%), and high (>50%) degrees of heterogeneity.
Subgroup analyses were performed according to varied factors to identify the causes of heterogeneity. To examine the stability of the results, sensitivity analysis was also performed. It was analyzed with a method of reducing one article at a time, and the effect of a single study on the meta-analysis was evaluated. Publication bias was examined by Deeks’ funnel plot asymmetry test. All statistical analyses were performed using MetaDiSc (version 1.4) and STATA (version 15.1) software.
Results
Study selection and characteristics
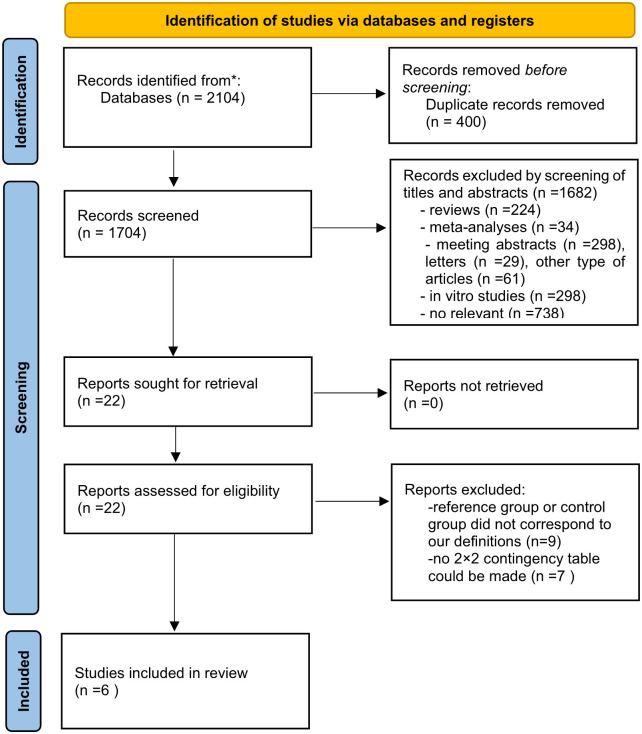
A PRISMA flow diagram summarizing the study selection process is presented in Figure 1 . A total of 2,104 related reports were initially obtained from the databases. After removing 2,082 animal or in vitro studies, duplicates, and irrelevant articles, a total of 22 full-text references were screened for eligibility. Among them, nine did not meet our selection criteria, and seven had insufficient data to construct a 2 × 2 contingency table. Finally, six records were included in our meta-analysis (Canani et al., 2011; Decembrino et al., 2015; Gao et al., 2015; Simm et al., 2016; Shams et al., 2017; Larsson et al., 2020). In two studies (Simm et al., 2016; Larsson et al., 2020), investigators reported the diagnostic accuracy separately for medical and surgical patients. The studies were divided into parts, but there were overlaps between different parts. Thus, we chose the datasets showing the highest Youden index for analysis. While in the subgroup analyses, we analyzed medical and surgical subgroups according to the admission category.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the study selection.
The characteristics of these studies are listed in Table 1 . All eligible studies were published between 2011 and 2020, and most were done in Europe. All of these studies were conducted in intensive care units (ICUs), and blood samples were taken on admission to the ICU. Three of them investigated adult patients, whereas the other three were done in neonatal ICUs. As sepsis criteria changed in these years, two studies used the American College of Chest Physicians/Society of Critical Care Medicine (ACCP/SCCM) definition for sepsis, one study used the sepsis-3 definition, and the neonatal studies diagnosed sepsis by either clinical signs or blood culture. The cutoff for calprotectin concentration varied, ranging from 1,300 to 38,300 ng/ml.
Table 1.
Summary of the included studies.
| Author | Year | Country | Population | Admission Category | Sepsis Definition | Sample | Cutoff (ng/ml) | Sample Size | AUC | TP | FP | FN | TN | Sensitivity % | Specificity % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Canani | 2011 | Italy | Neonatal | Medical | Blood culture/Clinical | Serum | 1,700 | 91 | NR | 55 | 1 | 7 | 28 | 89.0 | 96.0 |
| Decembrino | 2015 | Italy | Neonatal | Medical | Blood culture | Serum | 2,200 | 41 | 0.61 | 5 | 10 | 3 | 23 | 62.5 | 69.7 |
| Gao | 2015 | China | Adult | NR | ACCP/SCCM | Plasma | 3,128.8 | 298 | 0.9 | 188 | 8 | 38 | 64 | 83.1 | 88.5 |
| Larsson | 2020 | Sweden | Adult | Trauma | Sepsis-3 | Plasma | 1,300 | 109 | 0.79 | 62 | 10 | 15 | 22 | 81.0 | 70.0 |
| Larsson | 2020 | Sweden | Adult | Medical | Sepsis-3 | Plasma | 1,300 | 159 | 0.67 | 62 | 34 | 15 | 48 | 81.0 | 59.0 |
| Shams | 2017 | Iran | Neonatal | Medical | Clinical | Plasma | 38,300 | 80 | NR | 17 | 4 | 23 | 36 | 42.5 | 90.0 |
| Simm | 2016 | Sweden | Adult | Surgical | ACCP/SCCM | Plasma | 3,400 | 38 | 0.65 | 9 | 10 | 6 | 13 | 60.0 | 56.5 |
| Simm | 2016 | Sweden | Adult | Medical | ACCP/SCCM | Plasma | 2,700 | 22 | 0.95 | 13 | 1 | 2 | 6 | 86.7 | 85.7 |
AUC, area under the curve; TP, true positive; FP, false positive; FN, false negative; TN, true negative; NR, not reported; ACCP/SCCM, American College of Chest Physicians/Society of Critical Care Medicine; ELISA, enzyme-linked immunosorbent assay; PETIA, particle-enhanced turbidimetric immunoassay.
Quality assessment and publication bias
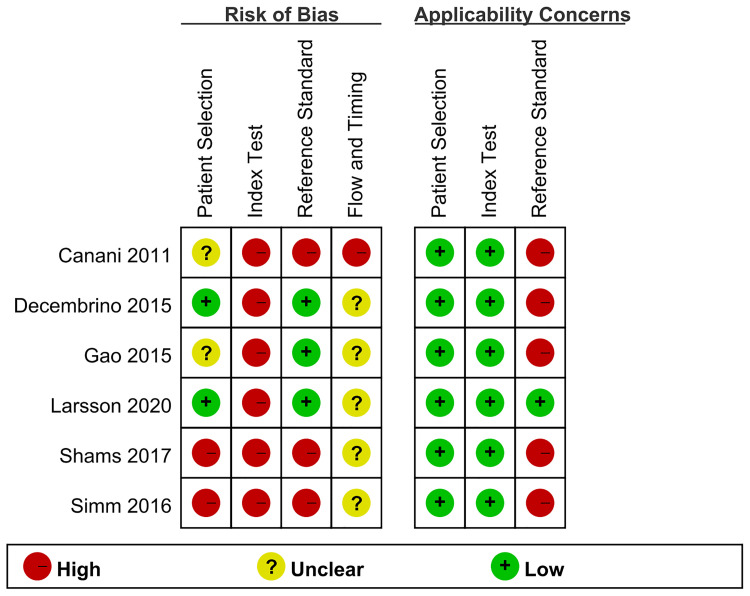
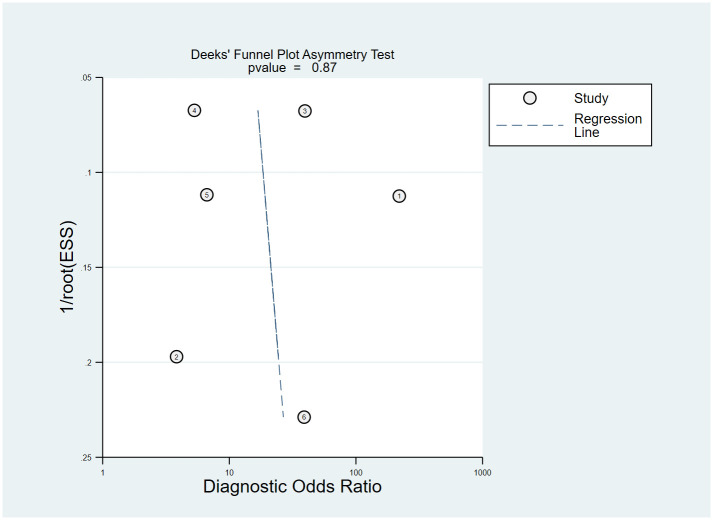
The QUADAS-2 tool was applied to evaluate the quality of the six studies, and the detailed results were shown in Figure 2 . Deeks’ funnel plot asymmetry test ( Figure 3 ) results indicated that no significant publication bias existed in this meta-analysis (p = 0.87).
Figure 2.
Risk of bias and applicability concerns of the included studies.
Figure 3.
Deeks’ funnel plot.
Data synthesis
The HSROC curve for all included studies is depicted in Figure 4 , and the AUC was 0.88. The pooled sensitivity was 0.77 (95% CI 0.62–0.87), and the pooled specificity was 0.85 (95% CI 0.74–0.92). The pooled PLR was 5.20 (95% CI 2.75–9,84), the pooled NLR was 0.27 (95% CI 0.15–0.48), and the pooled DOR was 19.37 (95% CI 6.71–55.92). Fagan’s nomogram showed that with the pretest probability of 67%, the posttest probability reached 91% and 35% for the positive and negative tests, respectively ( Figure 5 ). These results confirmed the high diagnostic efficiency of serum calprotectin in the diagnosis of sepsis. Fagan’s nomogram comprehensively considers PLR and NLR and adjusts the likelihood ratios according to the prior probability of diagnosis for salivary biomarkers. It shows that serum calprotectin could be helpful in diagnosing sepsis.
Figure 4.
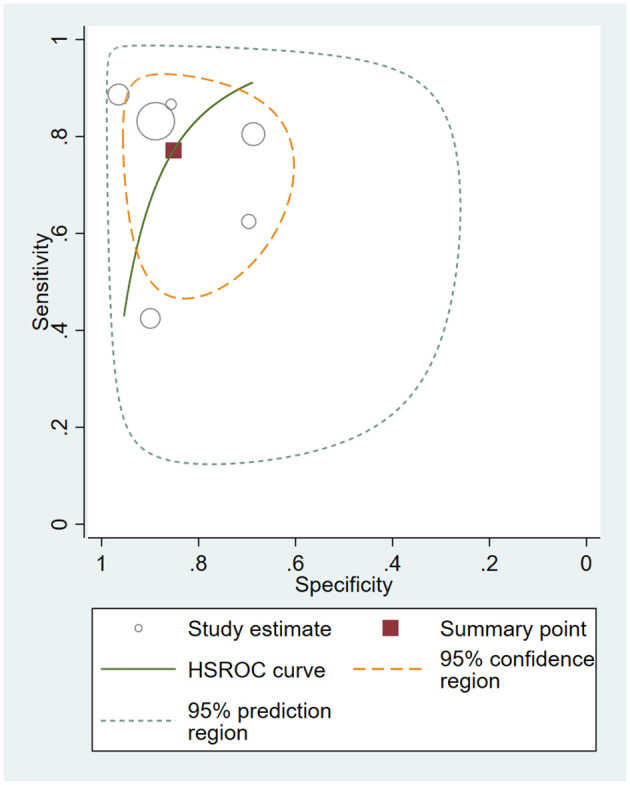
Hierarchical summary receiver operating characteristic (HSROC) curves for all included studies.
Figure 5.
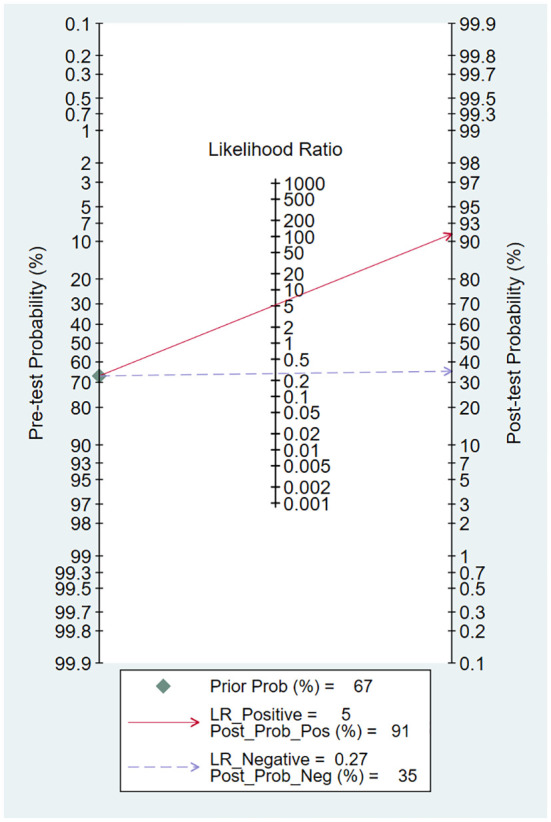
Fagan’s nomogram showing the posttest probability of sepsis.
We performed a heterogeneity analysis of the six studies. The I2 was 71.4% for DOR, indicating that substantial heterogeneity exists among them, while the threshold analysis p value was 0.54, giving no evidence of a threshold effect.
We then performed a subgroup analysis to explore the cause of heterogeneity. The subgroup analysis was based on admission category, population setting, and sepsis definition. There are five studies focused on patients treated by physicians and constituted the medical subgroup, while two studies focused on patients undergoing surgery and were termed the surgery subgroup. The pooled DOR was 12.23 (95% CI 3.82–39.15, I2 = 68.5%) and 4.53 (95% CI 1.01–20.38, I2 = 71.2%), respectively. According to the population setting, the six studies were divided into “adult” subgroup and “neonate” subgroup. Pooled DOR was 21.56 (95% CI 6.75–68.83, I2 = 64.5%) and 15.42 (95% CI 1.85–128.6, I2 = 80.5%), respectively. Similarly, two studies used the ACCP/SCCM definition for sepsis and they made up the “ACCP/SCCM” subgroup, while the other four made up the “others” subgroup. Pooled DOR was 39.53 (95% CI 18.19–85.89, I2 = 0.0%) and 12.16 (95% CI 3.37–43.87, I2 = 70.7%), respectively. The results of the subgroup analysis were shown in Table 2 .
Table 2.
Results of the subgroup analysis.
| Study | Subgroup | n | DOR | 95% CI | I2 (%) |
|---|---|---|---|---|---|
| Six studies | 6 | 16.08 | 5.33-48.50 | 78.3 | |
| Admission category | Medical | 5 | 12.23 | 3.82-39.15 | 68.5 |
| Population setting | Adult | 3 | 18.18 | 3.80-86.96 | 84.2 |
| Neonate | 3 | 15.42 | 1.85-128.60 | 80.5 | |
| Sepsis definition | ACCP/SCCM | 2 | 39.53 | 18.19-85.89 | 0.0 |
| Others | 4 | 10.54 | 2.95-37.00 | 73.3 |
DOR, diagnostic odds ratio; ACCP/SCCM, American College of Chest Physicians/Society of Critical Care Medicine.
In the sensitivity analysis, two studies (Canani et al., 2011; Gao et al., 2015) could affect the stability of the pooled results. Omitting these studies, the pooled sensitivity decreased to 0.71 (95% CI 0.51–0.85) and the pooled specificity was 0.77 (95% CI 0.63–0.87). The pooled PLR was 3.07 (95% CI 2.03-4.64), the pooled NLR was 0.38 (95% CI 0.23–0.64), and the pooled DOR was 8.02 (95% CI 4.07–15.80). The HSROC for the four studies was shown in Figure 6 .
Figure 6.
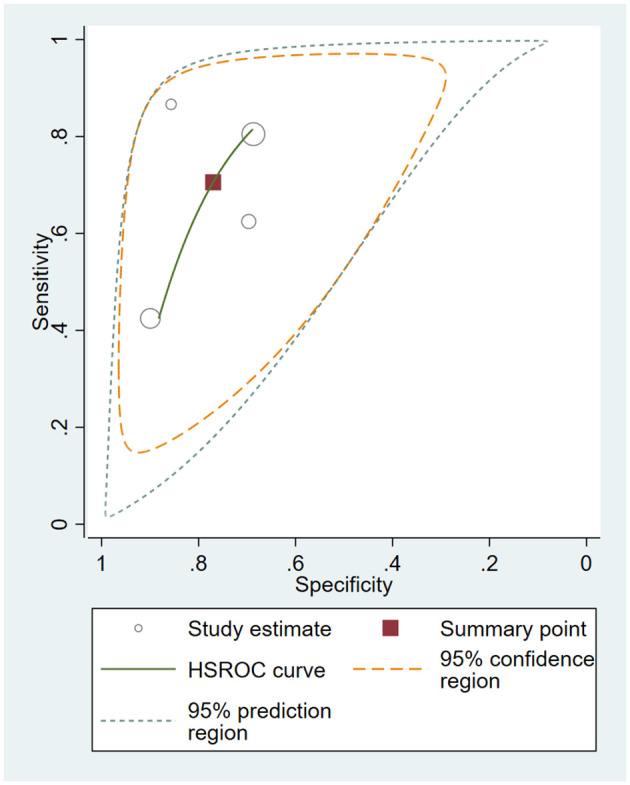
Hierarchical summary receiver operating characteristic (HSROC) curves for four studies.
Discussion
Sepsis is a life-threatening condition caused by a dysregulated host response to infection. Sepsis alters innate and adaptive immune responses and thus hampers immune homeostasis (Delano and Ward, 2016). We now diagnose sepsis with the help of the SOFA score, but methods diagnosing infection are still limited. As a result, there is a great need for early and reliable diagnostic biomarkers for sepsis.
Many studies have suggested that PCT is a biomarker for the early diagnosis of sepsis. However, different pathogens are believed to induce varied levels of PCT, and viral infection or ssystemic inflammatory response syndrome caused by noninfectious diseases can elevate the PCT level in critically ill septic patients (Parli et al., 2018). Several meta-analyses of its diagnostic accuracy in sepsis have been published (Wacker et al., 2013; Ruan et al., 2018; Tan et al., 2019; Li et al., 2021). They reported pooled sensitivities ranging from 0.71 to 0.81, pooled specificities ranging from 0.76 to 0.79, and AUC ranging from 0.80 to 0.87. According to our study, calprotectin is a useful biomarker for its better specificity and similar sensitivity.
Calprotectin is a protein secreted extracellularly from neutrophils and monocytes or is released into pus after cell disruption or death (Rammes et al., 1997; Boussac and Garin, 2000; Voganatsi et al., 2001). The soluble form of calprotectin was found in plasma, urine, body secretions, intestinal fluid, and feces (Pillay et al., 1998). Recently, it has gained great attention for participating in many types of infectious and inflammatory diseases, including inflammatory bowel disease, myocardial infarction, rheumatological diseases, and sepsis (Morrow et al., 2008; Sipponen and Kolho, 2015; Guo et al., 2016; Tydén et al., 2017). Calprotectin regulates immunity and participates in the inflammatory response and antimicrobial activities (Edgeworth et al., 1991; van Zoelen et al., 2009). As a result, calprotectin plays an important role in sepsis-induced alterations in the immune system.
Our results indicated that serum calprotectin on ICU admission could be a practical marker of sepsis. To the best of our knowledge, this is the first meta-analysis reporting the diagnostic value of calprotectin for sepsis on ICU admission, and the results are promising. Although heterogeneity existed, we performed a subgroup analysis and a sensitivity analysis to reduce it.
In the subgroup analysis, calprotectin showed well diagnostic value in both “surgical” and “medical” subgroups, indicating that serum calprotectin could be a potential biomarker to identify sepsis from noninfectious inflammatory disease. Heterogeneity still existed, so admission category was not the main cause of heterogeneity. We then analyzed if the population setting could affect the results, since it was reported that serum calprotectin levels were massively elevated after birth then slowly decreased during the first month of life (Pirr et al., 2017). However, both “neonate” and “adult” subgroups showed a high diagnostic efficacy, suggesting that the population setting was not the main cause of heterogeneity. Research conducted by Gao et al. (2015) and Simm et al. (2016) used the ACCP/SCCM definition for sepsis, and we discovered no heterogeneity in this subgroup. The sepsis definition may be a cause of heterogeneity. Over the past decades, a substantial amount of research improved the recognition and treatment of sepsis, and criteria changed during the process. In 2016, sepsis-3 was developed to further refine it, with an increased focus on recognizing organ dysfunction in the context of infection (Singer et al., 2016). The knowledge on the syndrome is still improving. There is a call for more research to investigate this promising diagnostic marker using a new definition.
Sensitivity analysis showed that studies conducted by Gao et al. (2015) and Canani et al. (2011) could result in heterogeneity, although they demonstrated an excellent diagnostic value of calprotectin in sepsis. Gao et al. (2015) did not report if the sample was enrolled consecutively or randomly, and whether there were patients lost to follow-up was not reported. As for the study by Canani et al. (2011), the sample size of 91 patients was small, and there were 28 patients excluded because of incomplete clinical data. Moreover, they selected neonates with blood culture-proven sepsis and those with the presence of a typical clinical picture of sepsis; this could lead to misclassification. These limitations could cause heterogeneity.
Our meta-analysis has several limitations. First, this meta-analysis included only six studies, and seven other studies associated with this topic were excluded because of insufficient data. Second, significant heterogeneity exists between these studies. As a result, we performed a subgroup analysis and a sensitivity analysis. In the subgroup analysis, sepsis definition turned out to be a source of heterogeneity, but there are no sufficient data to investigate the sepsis-3 definition. The broad and variable definition of sepsis is a common limitation in all sepsis biomarker studies, and the heterogeneities affected the results. Third, all of the studies included selected the test threshold to optimize sensitivity and specificity; it may lead to overestimation of test performance. However, there is no commonly accepted calprotectin threshold for sepsis. This limitation is still inevitable.
Conclusions
In conclusion, serum calprotectin on ICU admission plays an important role in the timely diagnosis of sepsis. However, further studies are required to confirm this finding.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Author contributions
R-YG contributed to study design, search strategy, screening of studies, data extraction, and statistical analysis, as well as writing the manuscript. H-MJ contributed to screening of studies and data extraction, as well as assisted in editing the manuscript. Y-ZH contributed to search strategy, screening of studies, and data extraction. B-SQ performed the quality assessment and PY synthesized the data. XKZ and WXL contributed in revising the manuscript. L-FH conceived of the study, contributed to study design and provided general supervision. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdel-Razik A., Abdelsalam M., Gad D. F., Abdelwahab A., Tawfik M., Elzehery R., et al. (2020). Recurrence of spontaneous bacterial peritonitis in cirrhosis: novel predictors. Eur. J. Gastroenterol. Hepatol. 32 (6), 718–726. doi: 10.1097/MEG.0000000000001578 [DOI] [PubMed] [Google Scholar]
- Boussac M., Garin J. (2000). Calcium-dependent secretion in human neutrophils: a proteomic approach. Electrophoresis 21 (3), 665–672. doi: [DOI] [PubMed] [Google Scholar]
- Bui M. H., Khuong Q. L., Le P. A., Nguyen T. A., Doan Q. H., Duong T. D., et al. (2022). Cost of postoperative sepsis in Vietnam. Sci. Rep. 12 (1), 4876. doi: 10.1038/s41598-022-08881-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canani R. B., Terrin G., Passariello A., Manguso F., Salvia G., Rapacciuolo L., et al. (2011). Serum calprotectin: An antimicrobial peptide as a new marker for the diagnosis of sepsis in very low birth weight newborns. Clin. Dev. Immunol. 2011. doi: 10.1155/2011/291085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decembrino L., De Amici M., Pozzi M., De Silvestri A., Stronati M. (2015). Serum calprotectin: A potential biomarker for neonatal sepsis. J. Immunol. Res. 2015, 147973. doi: 10.1155/2015/147973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanghe J. R., Speeckaert M. M. (2015). Translational research and biomarkers in neonatal sepsis. Clin. Chim. Acta 451 (Pt A), 46–64. doi: 10.1016/j.cca.2015.01.031 [DOI] [PubMed] [Google Scholar]
- Delano M. J., Ward P. A. (2016). Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J. Clin. Invest. 126 (1), 23–31. doi: 10.1172/JCI82224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgeworth J., Gorman M., Bennett R., Freemont P., Hogg N. (1991). Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J. Biol. Chem. 266 (12), 7706–7713. doi: 10.1016/S0021-9258(20)89506-4 [DOI] [PubMed] [Google Scholar]
- Fleischmann C., Scherag A., Adhikari N. K., Hartog C. S., Tsaganos T., Schlattmann P., et al. (2016). Assessment of global incidence and mortality of hospital-treated sepsis. Curr. Estimates Limitations Am. J. Respir. Crit. Care Med. 193 (3), 259–272. doi: 10.1164/rccm.201504-0781OC [DOI] [PubMed] [Google Scholar]
- Gao S., Yang Y., Fu Y., Guo W., Liu G. (2015). Diagnostic and prognostic value of myeloid-related protein complex 8/14 for sepsis. Am. J. Emergency Med. 33 (9), 1278–1282. doi: 10.1016/j.ajem.2015.06.025 [DOI] [PubMed] [Google Scholar]
- Guo Q., Zha X., Li C., Jia Y., Zhu L., Guo J., et al. (2016). Serum calprotectin–a promising diagnostic marker for adult-onset still's disease. Clin. Rheumatol. 35 (1), 73–79. doi: 10.1007/s10067-015-3108-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Li J., Han Y., Zhao S., Zheng Y., Sui F., et al. (2016). Serum calprotectin expression as a diagnostic marker for sepsis in postoperative intensive care unit patients. J. Interferon Cytokine Res. 36 (10), 607–616. doi: 10.1089/jir.2016.0037 [DOI] [PubMed] [Google Scholar]
- Larsson A., Tydén J., Johansson J., Lipcsey M., Bergquist M., Kultima K., et al. (2020). Calprotectin is superior to procalcitonin as a sepsis marker and predictor of 30-day mortality in intensive care patients. Scandinavian J. Clin. Lab. Invest. 80 (2), 156–161. doi: 10.1080/00365513.2019.1703216 [DOI] [PubMed] [Google Scholar]
- Lee J., Kim K. W., Choi S. H., Huh J., Park S. H. (2015). Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: A practical review for clinical researchers-part II. Stat. Methods Meta-Analysis. Korean J. Radiol. 16 (6), 1188–1196. doi: 10.3348/kjr.2015.16.6.1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y. L., Zhou X. Y., Ji M. H., Qiu L. C., Chen X. H., Gong C. S., et al. (2021). S100A9 upregulation contributes to learning and memory impairments by promoting microglia M1 polarization in sepsis survivor mice. Inflammation 44 (1), 307–320. doi: 10.1007/s10753-020-01334-6 [DOI] [PubMed] [Google Scholar]
- Li Q., Zheng S., Zhou P. Y., Xiao Z., Wang R., Li J. (2021). The diagnostic accuracy of procalcitonin in infectious patients after cardiac surgery: a systematic review and meta-analysis. J. Cardiovasc. Med. (Hagerstown) 22 (4), 305–312. doi: 10.2459/JCM.0000000000001017 [DOI] [PubMed] [Google Scholar]
- Morrow D. A., Wang Y., Croce K., Sakuma M., Sabatine M. S., Gao H., et al. (2008). Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the pravastatin or atorvastatin evaluation and infection therapy: Thrombolysis in myocardial infarction (PROVE IT-TIMI 22) trial. Am. Heart J. 155 (1), 49–55. doi: 10.1016/j.ahj.2007.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M., McKenzie J., Bossuyt P., Boutron I., Hoffmann T., Mulrow C., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PloS Med. 18 (3), e1003583. doi: 10.1371/journal.pmed.1003583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parli S. E., Trivedi G., Woodworth A., Chang P. K. (2018). Procalcitonin: Usefulness in acute care surgery and trauma. Surg. Infect. (Larchmt) 19 (2), 131–136. doi: 10.1089/sur.2017.307 [DOI] [PubMed] [Google Scholar]
- Pillay S. N., Asplin J. R., Coe F. L. (1998). Evidence that calgranulin is produced by kidney cells and is an inhibitor of calcium oxalate crystallization. Am. J. Physiol. 275 (2), F255–F261. doi: 10.1152/ajprenal.1998.275.2.F255 [DOI] [PubMed] [Google Scholar]
- Pirr S., Dauter L., Vogl T., Ulas T., Bohnhorst B., Roth J., et al. (2021). S100A8/A9 is the first predictive marker for neonatal sepsis. Clin. Transl. Med. 11 (4), e338. doi: 10.1002/ctm2.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirr S., Richter M., Fehlhaber B., Pagel J., Härtel C., Roth J., et al. (2017). High amounts of S100-alarmins confer antimicrobial activity on human breast milk targeting pathogens relevant in neonatal sepsis. Front. Immunol. 8, 1822. doi: 10.3389/fimmu.2017.01822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammes A., Roth J., Goebeler M., Klempt M., Hartmann M., Sorg C. (1997). Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J. Biol. Chem. 272 (14), 9496–9502. doi: 10.1074/jbc.272.14.9496 [DOI] [PubMed] [Google Scholar]
- Ruan L., Chen G. Y., Liu Z., Zhao Y., Xu G. Y., Li S. F., et al. (2018). The combination of procalcitonin and c-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: a meta-analysis and systematic review. Crit. Care 22 (1), 316. doi: 10.1186/s13054-018-2236-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams S. F., Boskabadi H., Keramati M. R., Ayatollahi H., Shakeri S., Sheikhi M., et al. (2017). Evaluation of immature neutrophil ratio and calprotectin level for the diagnosis of neonatal sepsis. Iranian J. Neonatol. 8 (3), 19–24. doi: 10.22038/ijn.2017.19345.1220 [DOI] [Google Scholar]
- Simm M., Söderberg E., Larsson A., Castegren M., Nilsen T., Eriksson M., et al. (2016). Performance of plasma calprotectin as a biomarker of early sepsis: A pilot study. Biomarkers Med. 10 (8), 811–818. doi: 10.2217/bmm-2016-0032 [DOI] [PubMed] [Google Scholar]
- Singer M., Deutschman C. S., Seymour C. W., Shankar-Hari M., Annane D., Bauer M., et al. (2016). The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 315 (8), 801–810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipponen T., Kolho K. L. (2015). Fecal calprotectin in diagnosis and clinical assessment of inflammatory bowel disease. Scand. J. Gastroenterol. 50 (1), 74–80. doi: 10.3109/00365521.2014.987809 [DOI] [PubMed] [Google Scholar]
- Souza D. C., Jaramillo-Bustamante J. C., Céspedes-Lesczinsky M., Quintero E. M. C., Jimenez H. J., Jabornisky R., et al. (2022). Challenges and health-care priorities for reducing the burden of paediatric sepsis in Latin America: a call to action. Lancet Child Adolesc. Health 6 (2), 129–136. doi: 10.1016/S2352-4642(21)00341-2 [DOI] [PubMed] [Google Scholar]
- Tan M., Lu Y., Jiang H., Zhang L. (2019). The diagnostic accuracy of procalcitonin and c-reactive protein for sepsis: A systematic review and meta-analysis. J. Cell Biochem. 120 (4), 5852–5859. doi: 10.1002/jcb.27870 [DOI] [PubMed] [Google Scholar]
- Taylor S. P., Anderson W. E., Beam K., Taylor B., Ellerman J., Kowalkowski M. A. (2021). The association between antibiotic delay intervals and hospital mortality among patients treated in the emergency department for suspected sepsis. Crit. Care Med. 49 (5), 741–747. doi: 10.1097/CCM.0000000000004863 [DOI] [PubMed] [Google Scholar]
- Tydén H., Lood C., Gullstrand B., Jönsen A., Ivars F., Leanderson T., et al. (2017). Pro-inflammatory S100 proteins are associated with glomerulonephritis and anti-dsDNA antibodies in systemic lupus erythematosus. Lupus 26 (2), 139–149. doi: 10.1177/0961203316655208 [DOI] [PubMed] [Google Scholar]
- van Zoelen M. A., Vogl T., Foell D., Van Veen S. Q., van Till J. W., Florquin S., et al. (2009). Expression and role of myeloid-related protein-14 in clinical and experimental sepsis. Am. J. Respir. Crit. Care Med. 180 (11), 1098–1106. doi: 10.1164/rccm.200810-1552OC [DOI] [PubMed] [Google Scholar]
- Vincent J. L., Marshall J. C., Namendys-Silva S. A., François B., Martin-Loeches I., Lipman J., et al. (2014). Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir. Med. 2 (5), 380–386. doi: 10.1016/S2213-2600(14)70061-X [DOI] [PubMed] [Google Scholar]
- Voganatsi A., Panyutich A., Miyasaki K. T., Murthy R. K. (2001). Mechanism of extracellular release of human neutrophil calprotectin complex. J. Leukoc. Biol. 70 (1), 130–134. doi: 10.1189/jlb.70.1.130 [DOI] [PubMed] [Google Scholar]
- Wacker C., Prkno A., Brunkhorst F. M., Schlattmann P. (2013). Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect. Dis. 13 (5), 426–435. doi: 10.1016/S1473-3099(12)70323-7 [DOI] [PubMed] [Google Scholar]
- Whiting P. F., Rutjes A. W., Westwood M. E., Mallett S., Deeks J. J., Reitsma J. B., et al. (2011). QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155 (8), 529–536. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- Wirtz T. H., Buendgens L., Weiskirchen R., Loosen S. H., Haehnsen N., Puengel T., et al. (2020). Association of serum calprotectin concentrations with mortality in critically ill and septic patients. Diagnostics (Basel) 10 (11). doi: 10.3390/diagnostics10110990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. C., Lan H. M., Han S. T., Chaou C. H., Yeh C. F., Liu S. H., et al. (2017). Comparison of diagnostic accuracy in sepsis between presepsin, procalcitonin, and c-reactive protein: a systematic review and meta-analysis. Ann. Intensive Care 7 (1), 91. doi: 10.1186/s13613-017-0316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Yang H., Kang Y. (2018). Comparison of diagnostic accuracy among procalcitonin, c-reactive protein, and interleukin 6 for blood culture positivity in general ICU patients. Crit. Care 22 (1), 339. doi: 10.1186/s13054-018-2269-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C. F., Wu C. C., Liu S. H., Chen K. F. (2019). Comparison of the accuracy of neutrophil CD64, procalcitonin, and c-reactive protein for sepsis identification: a systematic review and meta-analysis. Ann. Intensive Care 9 (1), 5. doi: 10.1186/s13613-018-0479-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. N., Wang X. H., Wu L., Huang L., Zhao C. G., Peng Q. Y., et al. (2016). Diagnostic and predictive levels of calcium-binding protein A8 and tumor necrosis factor receptor-associated factor 6 in sepsis-associated encephalopathy: A prospective observational study. Chin. Med. J. (Engl) 129 (14), 1674–1681. doi: 10.4103/0366-6999.185860 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.