Abstract
VlsE, the variable surface antigen of Borrelia burgdorferi, contains a 26-amino-acid-long immunodominant invariable region, IR6. In the present study, three overlapping 14-mer peptides reproducing the sequence of IR6 were used as peptide-based enzyme-linked immunosorbent assay antigens to map this invariable region in infected monkeys, mice, and human Lyme disease patients. Antibodies of the two primate species appeared to recognize IR6 as a single antigenic determinant, while mouse antibodies recognized multiple epitopes within this region.
Borrelia burgdorferi sensu lato, the etiologic agent of Lyme disease (17), expresses a surface antigen, VlsE, which undergoes antigenic variation (21). Unlike other variable antigens, such as the variant surface glycoprotein of African trypanosomes (1, 5, 6, 20) and the variable major protein (Vmp) of Borrelia hermsii (3, 14, 18), which contain nonantigenic invariable portions, VlsE contains a 26-amino-acid-long invariable region (IR) which is immunodominant (11). This sequence (IR6), which remains unchanged during antigenic variation (21), is highly conserved among strains and genospecies of B. burgdorferi sensu lato (11) and may thus play a critical role in maintaining the physiologic function of VlsE. Anti-IR6 immunoglobulin G (IgG) antibody is readily detectable in both the early and late phases of B. burgdorferi infection in mice, monkeys, and humans (11, 12). However, since IR6 is exposed at the surface of the VlsE molecule but not at the surface of the spirochete (11), anti-IR6 antibody is likely not protective in vivo. On the other hand, the conservation and immunodominance of IR6 indicates that anti-IR6 antibody may have powerful diagnostic attributes. An enzyme-linked immunosorbent assay (ELISA) based on a peptide with the IR6 sequence which is both sensitive and specific for the serodiagnosis of Lyme disease has been developed (12).
In this study, we attempted to map linear B-cell epitopes within IR6 using sera from experimentally infected monkeys and mice and from humans clinically diagnosed with Lyme disease. A peptide-based ELISA was used.
The sequences of three overlapping 14-mer peptides were designed based on a consensus of IR6 sequences from strains B31 (21) and 297 (10) of B. burgdorferi sensu stricto and IP90 of Borrelia garinii (11) (Fig. 1). The three 14-mers were named C6N, C6M, and C6C. They were prepared using the fluorenylmethoxycarbonyl synthesis protocol (2). N-terminal conjugation to biotin was performed by the N-succinimidyl maleimide carboxylate method as per the instructions of the manufacturer (Molecular Probes, Eugene, Oreg.).
FIG. 1.
Diagrammatic illustration of the VlsE structure. VlsE consists of two invariable domains at the amino and carboxyl termini and one variable domain at the center (21). The variable domain contains six variable regions, VRI to VRVI, and six invariable regions, IR1 to IR6. The framed sequences show the IR6 sequences from strains B31 (21) and 297 (10) of B. burgdorferi sensu stricto and IP90 of B. garinii (11). Bold letters indicate amino acids unique to each strain. The consensus sequences of the three overlapping peptides used in this study are depicted below (C6N, C6M, and C6C). The C6 sequence is based on that of IR6 of IP90.
The peptide-based ELISA was performed as previously described (11). Briefly, 96-well ELISA plates were coated with streptavidin (Pierce Chemical Company, Rockford, Ill.) in coating buffer (0.1 M carbonate buffer, pH 9.2), followed by incubation with biotinylated peptides. Then antiserum and horseradish peroxidase-conjugated secondary antibody dilutions (goat anti-monkey IgG [γ chain specific; Kirkegaard & Perry Laboratories, Gaithersburg, Md.], anti-mouse IgG [heavy and light chain specific; Sigma Chemical Co., St. Louis, Mo.], or anti-human IgG [heavy and light chain specific; Pierce]) were applied. The antigen-antibody reaction was probed using a peroxidase substrate system (Kirkegaard & Perry), and optical density (OD) was measured at 450 nm. For competitive peptide-based ELISA, the biotinylated peptide C6 was applied to the ELISA plate which had been coated with streptavidin. Competitive inhibition of anti-C6 antibody was performed by adding 50 μl of 14-mer peptide(s) (C6N, C6M, C6C, or a combination of two or three of the peptides), each between 0 and 5,000 ng per well. An equal volume of serum diluted 1:100 with blocking solution (phosphate-buffered saline containing 0.1% Tween 20 and 5% nonfat dry milk, pH 7.4) was added to each well. The plate was shaken for 1 h. The remaining steps were performed as described for the peptide-based ELISA.
Ten serum samples derived from each host species were used to epitope map IR6. Serum specimens were obtained from 10 rhesus monkeys (2- to 4-year-old Macaca mulatta), 9 of which had been inoculated by the bites of Ixodes scapularis nymphal ticks and 1 of which had been inoculated with needle and syringe. The ticks were themselves infected with either of the B. burgdorferi sensu stricto strains JD1 (15) and B31 (16). The needle-inoculated animal received JD1 organisms (15). Ten mice (6- to 8-week-old C3H/HeN mice; Jackson Laboratories, Bar Harbor, Maine) were infected either with B. burgdorferi sensu stricto strain Sh-2-82 (low passage number; a gift from Denee Thomas, University of Texas Health Science Center, San Antonio, Tex.) by subcutaneous needle inoculation with 108 spirochetes administered in 1 ml of BSK-H medium (Sigma) or with B. burgdorferi sensu stricto strain B31 by the bites of infected ticks. Serum samples were collected before and 4 to 6 weeks postinfection. Human serum samples were collected from 10 Lyme disease patients who had disease signs and symptoms that satisfied the Centers for Disease Control and Prevention clinical case definition (7). Five patients (A1 to A5) were diagnosed as having late Lyme arthritis, and five patients (N1 to N5) had late neuroborreliosis. Sera were kindly supplied by Allen Steere (New England Medical Center, Tufts University School of Medicine, Boston, Mass.).
Sera from 9 out of 10 infected monkeys did not contain detectable anti-14-mer peptide antibodies despite the presence of high levels of anti-C6 antibody in virtually all of the specimens (Fig. 2). Only monkey J200 showed a significant reactivity with C6C. These results indicate that this host species most likely recognizes the entire IR6 segment as a single antigenic determinant.
FIG. 2.
Reactivities of overlapping peptides with monkey antibody. Serum samples were collected from monkeys at 4 to 6 weeks postinoculation. Animals were infected by tick inoculation either with the JD1 strain of B. burgdorferi (J200, J415, J831, K205, and L131) or with the B31 strain (L457, L549, M021, and M581). Animal J748 was needle inoculated with JD1 spirochetes. Levels of antibody to peptides were assessed using a peptide-based ELISA. Goat anti-monkey IgG–peroxidase conjugate was used as the secondary antibody. All serum samples were diluted 1:200. The cutoff line was based on the mean + 3 standard deviations of the OD values for 10 prebleeds that were reacted individually with each of the overlapping peptides and C6.
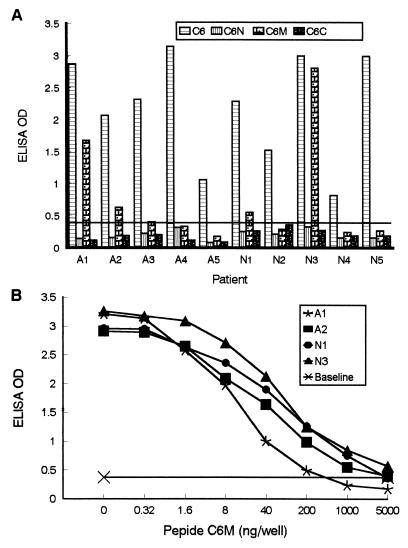
Four out of 10 human serum samples showed reactivity with the overlapping peptide C6M, but none contained detectable antibodies to C6N or C6C (Fig. 3A). To determine what proportion of anti-C6 antibody reactivity was contributed by antibody to C6M, a competitive peptide-based ELISA was performed. The reactivity of specific antibody with C6 in the four serum samples (A1, A2, N1, and N3) which reacted with C6M was fully inhibited by increasing concentrations of C6M (Fig. 3B). Even though serum N1 showed a very weak reactivity with C6M in the peptide-based ELISA (Fig. 3A), a high concentration of this peptide was necessary to fully inhibit the reactivity with C6 (Fig. 3B). The low reactivity with C6M in the peptide-based ELISA but full inhibition in the competitive ELISA may have resulted from human antibodies with a lower affinity for C6M than for C6. C6M may bind antibody as a partial epitope. No significant inhibition was observed when the peptides C6N and C6C were used in this assay (data not shown). Hence, the inhibition observed with the peptide C6M should be considered specific. Taken together, our results suggest that humans, like monkeys, recognize IR6 as a single epitope.
FIG. 3.
Epitope mapping with human antibody. (A) Reactivities of overlapping peptides with human antibody. Ten sera were collected from patients with late Lyme arthritis (A1 to A5) or late neuroborreliosis (N1 to N5). Levels of antibody to peptides were assessed using a peptide-based ELISA. Goat anti-human IgG–peroxidase conjugate was used as the secondary antibody. All serum samples were diluted 1:200. The cutoff line (baseline) value is the mean + 3 standard deviations of OD values for 10 human serum specimens collected from hospitalized patients in Louisiana, where Lyme disease is not endemic. For this purpose, the three overlapping peptides and C6 were individually used as an ELISA antigen. (B) Inhibition of human antibody reactivity with C6 by C6M. Amounts of C6M ranging from 0 to 5,000 ng per well were added to the C6-bound ELISA plate. Human serum diluted 1:100 was applied. The remaining steps were performed as described for panel A.
A different result was obtained when mouse sera were tested. High-level antibody responses to all three 14-mers were detected in several animals. Seven mice (mice 184, 219, 220, 224, 288, 289, and 290) had antibodies that reacted with the peptide C6N. While serum from the first four animals reacted weakly, that of the latter three reacted as strongly as with the complete C6 peptide (Fig. 4). Another set of three mice (mice 220, 288, and 290) had anti-C6M antibodies, and five mice (mice 184, 191, 224, 289, and 290) possessed anti-C6C antibodies (Fig. 4). Mouse 289 responded to both C6N and C6C but not to C6M, indicating that this animal processed at least two independent epitopes at the amino and carboxyl termini of IR6. Serum collected from mouse 288 reacted with both C6N and C6M, while serum from mouse 290 reacted with all three overlapping peptides. Competitive peptide-based ELISA revealed much more complicated antibody responses to IR6 in mice than in monkeys and humans. Even the combination of the three overlapping peptides failed to fully inhibit the reactivity of serum 290 with the C6 peptide (data not shown). The overlapping peptides C6N and C6M together also could not fully inhibit the reactivity of serum 288 with C6, although C6C did not react with this serum (data not shown). These results suggest that mice are able to recognize multiple epitopes within IR6.
FIG. 4.
Reactivities of overlapping peptides with mouse antibody. Serum samples were collected from mice at 4 to 6 weeks postinoculation. Animals were infected with either B. burgdorferi sensu stricto strain B31 (mice 184, 191, 194, and 196) by tick inoculation or Sh-2-82 (mice 219, 220, 224, 288, 289, and 290) by needle inoculation. Levels of antibody to peptides were assessed using a peptide-based ELISA. Goat anti-mouse IgG–peroxidase conjugate was used as the secondary antibody. Sera were diluted 1:200. The cutoff line was based on the mean + 3 standard deviations of OD values for all of the 10 prebleeds reacted individually with each overlapping peptide and C6.
Although we cannot fully exclude the possibility that the antibody response to 14-mer portions of IR6 was exacerbated in mice because of the mode of inoculation (needle versus tick inoculation) or the strain of spirochete used (B31 versus Sh-2-82), we do not believe that these factors may fully explain the difference we observed in the responses to these sequences in the host species studied. The mode of inoculation may influence the antibody response because a higher dose of spirochetes is likely received by needle- than by tick-inoculated animals, and immune-suppressive substances are inoculated by the tick concomitantly with spirochetes (19). All of the six needle-inoculated mice that received the Sh-2-82 strain responded to one or more of the 14-mers, yet half of the four tick-inoculated mice also responded to C6C and one responded weakly to C6N. Monkey J748, which was needle inoculated, did not respond to any of the 14-mer peptides. As to spirochete strain differences, it is possible that spirochetes of the Sh-2-82 strain express a higher level of VlsE than that expressed by B31 organisms in vivo and may thus facilitate antipeptide responses. This we cannot exclude, but the fact that none of the four tick-inoculated monkeys that received the B31 strain responded to 14-mers whereas two of the four B31 tick-inoculated mice did underscores host species difference as the major reason for the differential responsiveness to fragments of IR6 that we found.
Most B-cell epitopes of native protein antigens are discontinuous in that they encompass regions which are separate from each other in the primary sequence of the polypeptide chain but are brought together on the native molecule by protein folding (4). Our results suggest that IR6 may function as a single epitope, probably nonlinear in view of its length, in primate species, human or nonhuman, but that IR6 may behave as a collection of multiple overlapping linear epitopes in mice. Analysis of the secondary structure of IR6 by either the Chou-Fasman (8) or the Robson-Garnier (9) algorithms (MacVector 4.1; Eastman Chemical Co., New Haven, Conn.) predicted an α-helix comprised of an 11-mer sequence [AA(I or M)(A or V)LRGMAKD] regardless of whether the B31, 297, or IP90 IR6 sequence was used in the calculation. The 11-amino-acid segment is located at the center of IR6 and is fully included in the C6M peptide (Fig. 1). In humans, this helix may serve as the epitope core, for when C6M was used as a competitor, it was able to fully inhibit the reactivity of human serum antibody with C6, albeit at a high concentration of the peptide (Fig. 3). The sequences immediately flanking the α-helix probably contribute to the binding affinity either by allowing the α-helix to “grow” or by contributing some tertiary structure to it, since human antibody yielded a higher ELISA OD with C6 than with C6M. The remarkable antigenicity of IR6 in several host species argues against the presence of additional, discontinuous regions of VlsE as contributors to the native antigenicity of IR6. In fact, all experimentally infected mice and monkeys we have tested thus far (10 mice and 10 monkeys), most human patients with early B. burgdorferi infections (117 of 138), and all patients with a late, clinically well defined infection (we tested 59) had detectable anti-IR6 antibodies (11, 12). Thus, while the totality of IR6 appears to be required to express the full antigenicity of this region, it is unlikely that other VlsE portions are required as well. Our studies have uncovered striking differences in the antibody responses to the invariable regions of VlsE in different host species. We had noted previously that mouse antibodies recognized IR2, IR4, and IR6 but that monkey and human antibodies reacted essentially only with IR6 (13). The present study revealed that primate host species recognize IR6 in toto but that mice appear to detect multiple linear and overlapping epitopes within this region. If VlsE plays a role in the host-microbe interplay of B. burgdorferi, these differences in B-cell antigen recognition patterns could contribute to explain, once that role has become clear, differences in the natural histories of murine and primate Lyme disease. At a more general level, this finding illustrates that B-cell epitope mapping is host dependent.
Acknowledgments
This work was supported by grants AI35027 and RR00164 from the National Institutes of Health and by a grant from SmithKline Beecham Biologicals.
REFERENCES
- 1.Barbet A F, McGuire T C. Crossreacting determinants in variant-specific surface antigens of African trypanosomes. Proc Natl Acad Sci USA. 1978;75:1989–1993. doi: 10.1073/pnas.75.4.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barony G, Merrifield R B. The peptides: analysis, synthesis, & biology. New York, N.Y: Academic Press; 1980. pp. 3–285. [Google Scholar]
- 3.Barstad P A, Coligan J E, Raum M G, Barbour A G. Variable major proteins of Borrelia hermsii. Epitope mapping and partial sequence analysis of CNBr peptides. J Exp Med. 1985;161:1302–1314. doi: 10.1084/jem.161.6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin D C. B-cell epitopes: fact and fiction. In: Aledort L M, et al., editors. Inhibitors to coagulation factors. New York, N.Y: Plenum Press; 1995. pp. 95–108. [Google Scholar]
- 5.Borst P. Molecular genetics of antigenic variation. Immunol Today. 1991;12:A29–A33. doi: 10.1016/S0167-5699(05)80009-X. [DOI] [PubMed] [Google Scholar]
- 6.Borst P, Cross G A M. Molecular basis for trypanosome antigenic variation. Cell. 1982;29:291–303. doi: 10.1016/0092-8674(82)90146-5. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Case definition for infectious conditions under public health surveillance. Morbid Mortal Weekly Rep. 1997;46:20. [Google Scholar]
- 8.Chou P Y, Fasman G D. Empirical predictions of protein conformations. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- 9.Garnier J, Osguthorpe D J, Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978;120:97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- 10.Kawabata H, Myouga F, Inagaki Y, Murai N, Watanabe H. Genetic and immunological analyses of Vls (VMP-like sequences) of Borrelia burgdorferi. Microb Pathog. 1998;24:155–166. doi: 10.1006/mpat.1997.0183. [DOI] [PubMed] [Google Scholar]
- 11.Liang F T, Alvarez A L, Gu Y, Nowling J M, Ramamoorthy R, Philipp M T. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J Immunol. 1999;163:5566–5573. [PubMed] [Google Scholar]
- 12.Liang F T, Steere A C, Marques A R, Johnson B J B, Miller J N, Philipp M T. Sensitive and specific serodiagnosis of Lyme disease by ELISA with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J Clin Microbiol. 1999;37:3990–3996. doi: 10.1128/jcm.37.12.3990-3996.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang F T, Philipp M T. Analysis of antibody response to invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi. Infect Immun. 1999;67:6702–6706. doi: 10.1128/iai.67.12.6702-6706.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier J T, Simon M I, Barbour A G. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell. 1985;41:403–409. doi: 10.1016/s0092-8674(85)80013-1. [DOI] [PubMed] [Google Scholar]
- 15.Philipp M T, Aydintug M K, Bohm R P, Jr, Cogswell F B, Dennis V A, Lanners H N, Lowrie R C, Jr, Roberts E D, Conway M D, Karaçorlu M, Peyman G A, Gubler D J, Johnson B J B, Piesman J, Gu Y. Early and early disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infect Immun. 1993;61:3047–3059. doi: 10.1128/iai.61.7.3047-3059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philipp M T, Lobet Y, Bohm R P, Jr, Roberts E D, Dennis V A, Gu Y, Lowrie R C, Jr, Desmons P, Duray P H, England J, Hauser P, Piesman J, Xu K. The outer surface protein A (OspA) vaccine against Lyme disease: efficacy in the rhesus monkey. Vaccine. 1997;15:1872–1884. doi: 10.1016/s0264-410x(97)00133-3. [DOI] [PubMed] [Google Scholar]
- 17.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 18.Stoenner H G, Dodd T, Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982;156:1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urioste S, Hall L R, Telford III S R, Titus R G. Saliva of the Lyme disease vector, Ixodes dommini, blocks cell activation by a nonprostaglandin E2-dependent mechanism. J Exp Med. 1994;180:1077–1085. doi: 10.1084/jem.180.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van der Ploeg L H T, Gottesdiener K, Lee M G S. Antigenic variation in African trypanosomes. Trends Genet. 1992;8:452–457. doi: 10.1016/0168-9525(92)90330-7. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J R, Hardham J M, Barbour A G, Norris S J. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]