Abstract
Background:
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has tremendous implications for the management of patients with autoimmune conditions such as multiple sclerosis (MS) under immune therapies targeting CD20+ B cells (aCD20).
Objectives:
Here, we investigated humoral and cellular immune responses, including anti-spike titers, neutralization against SARS-CoV-2 wild-type (WT), delta, and omicron variant and T cell responses of aCD20-treated relapsing–remitting MS patients following SARS-CoV-2 vaccination compared with healthy controls.
Methods:
Blood samples were collected within 4–8 weeks following the second vaccination against SARS-CoV-2. Sera were analyzed for anti-SARS-CoV-2 spike antibodies and neutralization capacity against pseudovirus for wild-type (WT), delta, and omicron variant. Peripheral blood mononuclear cells (PBMCs) were stimulated with a SARS-CoV-2 peptide pool and analyzed via flow cytometry.
Results:
The aCD20-treated MS patients had lower anti-SARS-CoV-2-spike titers, which correlated with B cell repopulation. Sera of aCD20-treated patients had reduced capacity to neutralize WT, delta, and omicron pseudoviruses in vitro. On the contrary, PBMCs of aCD20-treated patients elicited higher frequencies of CD3+ T cells and CD4+ T cells and comparable response of cytotoxic T cells, while Th1 response was reduced following restimulation with SARS-CoV-2.
Conclusion:
In summary, aCD20-treated patients have a reduced humoral immune response, depending on B cell repopulation, in accordance with preserved cellular immune response, suggesting partial cellular protection against SARS-CoV-2.
Keywords: anti-CD20 therapy, multiple sclerosis, SARS-CoV-2 vaccination, T cell response
Introduction
Coronavirus-disease-2019 (COVID-19) induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a potentially life-threatening disease, having led to a global pandemic since early 2020. This raised concerns especially for vulnerable patient groups with impaired immunity, namely patients with chronic autoimmune conditions under highly active immunotherapy such as multiple sclerosis (MS). During the last 20 years, scientific progress led to the development of nearly 20 medications for MS therapy, including monoclonal antibodies targeting B cells via anti-CD20 (aCD20) such as rituximab or ocrelizumab.1,2 The aCD20-treated patients are at higher risk for hospitalization and a more severe course of COVID-19.3 Since the end of 2020, the first vaccine candidates received emergency use authorization, showing high efficacy against SARS-CoV-2 infection.4 Because B cells are crucial for the development of a humoral response, investigating the immune response following SARS-CoV-2 vaccination is of huge importance. First reports in 2021 showed reduced humoral response in aCD20-treated MS patients.5 However, T cells can exert long-lasting and robust immune responses, provide more longevity compared with neutralizing antibodies and are, in addition, less prone for escape mechanisms.6 We here analyzed humoral and T cell responses of MS patients under aCD20 therapy 4–8 weeks following a second vaccination against SARS-CoV-2. Data were corroborated by the analysis of the neutralization capacity of respective sera against the WT, delta, and currently circulating omicron variant of concern of SARS-CoV-2 in vitro. Those data will help to better stratify aCD20-treated patients regarding risk management and vaccination strategy.
Methods
Study design
The study was authorized by the local ethics committee of the Ruhr-University Bochum (20-6953-bio). Patients were recruited at the Department of Neurology, Ruhr-University Bochum, St. Josef-Hospital. We included n = 10 healthy age-matched controls and n = 34 B cell-depleted relapsing–remitting (RR) MS patients (n = 20 ocrelizumab, n = 14 off-label rituximab). All patients and healthy controls (HCs) provided written informed consent. The demographics of the study participants are presented in Table 1. Samples were collected within 4–8 weeks following the second vaccination against SARS-CoV-2.
Table 1.
Demographic and clinical characteristics of recruited MS patients and healthy controls.
| MS | HCs | |
|---|---|---|
| Number | 34 | 10 |
| Male (%) | 8 (24%) | 5 (50%) |
| Female (%) | 26 (76%) | 5 (50%) |
| Age ± SD (range) | 40.7 ± 10.6 (20–58) | 38.7 ± 15.4 (22–60) |
| Disease duration ± SD (range) | 10.1 ± 7.6 (1–29) | NA |
| EDSS ± SD ± SEM | 2.3 ± 1.4 ± 0.25 | NA |
| EDSS median (range) | 2 (0 to 6.5) | NA |
| Treatment | MS total 34 | HC total 10 |
| Ocrelizumab | 20 (59%) | – |
| Rituximab | 14 (41%) | – |
| No therapy | – | 10 (100%) |
| Vaccination | MS total 34 | HC total 10 |
| Biontech | 29 (85%) | 5 (50%) |
| Astrazeneca | 1 (3%) | – |
| Moderna | 1 (3%) | – |
| Mixed (Astrazeneca and Biontech) | 3 (9%) | 5 (50%) |
| Mean weeks between vaccination and blood sampling (95% CI lower, upper, range) | 6.529 (5.162, 7.896, 3–19) | 7 (4.545, 9.455, 3–14) |
| Mean weeks between aCD20 treatment and vaccination (95% CI lower, upper, range) | 23.68 (20.75, 26.6, 6–50) | – |
Age, disease duration, and EDSS are presented as mean.
CI, confidence interval; EDSS, Expanded Disability Status Scale; HC, healthy control; MS, multiple sclerosis; NA, not applicable; SD, standard deviation; SEM, standard error of the mean.
Anti-SARS-CoV-2 spike antibody titer
Titers were evaluated using the Elecsys anti-SARS-CoV-2 S assay (range 0.4–2500 U/ml; Roche).
Cell isolation and cryopreservation
Blood collection for peripheral blood mononuclear cells (PBMCs) isolation was conducted using four 7.5 ml KABEVETTE® G EDTA tubes per patient. Blood residues in the EDTA tubes were transferred to the collecting tube by washing each tube with 7.5 ml phosphate-buffered saline (PBS). The 30 ml blood sample was slowly placed on top of 15 ml ROTI®Sep 1077 human density gradient (Carl Roth) in a 50 ml centrifuge tube. Density gradient centrifugation was performed at 800 g for 30 min without break. The interface containing the PBMCs was withdrawn from the gradient and washed twice with PBS at 1200 r/min for 10 min. The cell pellet was resuspended in 10 ml PBS and cell number was determined using trypan blue in an improved Neubauer chamber. A total (10–20)×106 cells were cryopreserved in 1 ml CTL-Cryo-ABC Freezing media Kit (ImmunoSpot®) according to the manufacturer instructions. The cryotubes were cooled down overnight in a MrFrosty (Sigma) at –80°C and stored at –80°C.
PBMC stimulation using SARS-CoV-2 peptide pool
PBMCs were thawed in a 37°C water bath and diluted in 10 ml Roswell Park Memorial Institute (RPMI) medium 1640 (Gibco) with GlutaMAX™ and 1% Penicillin/Streptomycin (Pen/Strep; both Thermofisher) and centrifuged at 1200 r/min for 10 min. The pellet was washed once with 10 ml PBS and resuspended in 1 ml OpTmizer™ CTS™ (Thermofisher). Cell number was determined using trypan blue in an improved Neubauer chamber. A total 80 µl per sample were seeded in duplicates in a round bottom 96-well plate (Sarstedt) at a concentration of 2.5×106 cells/ml. Cells were stimulated with 2 µg/ml PepMix SARS-CoV-2 (JPT) for 16–18 h together with Brefeldin A solution (BioLegend). The peptide mix consisted of a pool of 315 (158+157) peptides derived from a peptide scan (15 mers with 11 aa overlap) through Spike glycoprotein (Swiss-Prot ID: P0DTC2) of SARS-CoV-2. Additional wells in duplicates were seeded for unstained control, dead cell positive control and unstimulated control without PepMix SARS-CoV-2 for each treatment group.
Flow cytometry
B cell frequency
For analysis of B cells, cells were stained for flow cytometry against extracellular markers (CD45, CD19, CD3, CD8, CD16/56). Frequency of CD19+ cells was assessed following gating into the CD45+ lymphocyte population.
Analysis following SARS-CoV-2 peptide restimulation
After peptide stimulation for 16–18 h, the cells were stained for flow cytometry against extracellular markers (BioLegend, anti-CD3 clone: SK7 RRID: AB_2616890, anti-CD4 clone: SK3 RRID: AB_1937227, anti-CD8a clone: RPA-T8 RRID: AB_2629694) and intracellular markers (BioLegend, anti-IFN-γ clone: 4S.B3 RRID: AB_961357, anti-IL-2 clone: MQ1-17H12 RRID: AB_315096, anti-IL-4 clone: MP4-25D2 RRID: AB_315127). Antibody dilutions are presented in Supplementary Table 1. Extracellular staining was conducted together with viability stain Zombie Aqua™ Dye (BioLegend) for 30 min at 4°C. Fixation and permeabilization were performed with Fixation Buffer (BioLegend) and intracellular staining perm wash buffer (BioLegend) before intracellular staining according to the manufacturer’s instructions. Subsequently, for intracellular staining, cells were incubated for 30 min at room temperature. Flow cytometry was performed using a FACSCelesta™ Flow cytometer (BD) with High Throughput Sampler and FACSDiva™ Software (BD FACS Diva Software, Version 9.0). The gating strategy is shown in Figure S1. Flow cytometry data were analyzed with FlowJo™ (BD, version 10.7.1).
SARS-CoV-2 neutralization assay
The SARS-CoV-2 pseudoviruses were prepared as described previously.7 Briefly, sera were incubated for 30 min at 56°C to inactivate complement factors. Single-cycle VSV*ΔG(FLuc) pseudoviruses bearing the SARS-CoV-2 spike (D614G) protein8 or SARS-CoV-2 B.1.617.2 (Delta; EPI_ISL_1921353) spike in the envelope were incubated with quadruplicates of twofold serial dilutions from 1:20 to 1:2560 of immune sera in 96-well plates prior to infection of Vero E6 cells (1 × 104 cells/well) in Dulbecco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum (FBS; Life Technologies). At 18 h post infection, firefly luciferase (FLuc) reporter activity was determined as previously described9 using a CentroXS LB960 (Berthold) and the reciprocal antibody dilution causing 50% inhibition of the luciferase reporter was calculated as pseudovirus neutralization dose 50% (PVND50). Detection range is defined to be between 1:20 of above 1:2560.
Statistics
GraphPad Prism (version 9.2.0) was used for statistical analysis. Data were tested for Gaussian distribution using Kolmogorov–Smirnov test. Data were analyzed with Mann–Whitney U test, correlations were calculated using Spearman correlation.
Data availability
All data are available from the corresponding author SF upon reasonable request.
Results
Baseline characteristics
We included 34 MS patients with a mean age of 40.7 years and a disease duration of 10.1 ± 7.6 years (mean ± SD; Table 1) and n = 10 age-controlled controls (age 38.7 ± 15.4, HC). MS patients had a mean Expanded Disability Status Scale (EDSS) of 2.3 ± 1.4 (range, 0–6.5). About 56% of aCD20-treated patients were under therapy with ocrelizumab, 44% received off-label rituximab. The majority of MS patients had been vaccinated with a messenger RNA (mRNA) vaccine (85% Comirnaty, Pfizer/Biontech).
Anti-SARS-CoV-2-Spike antibody spike titer correlates with repopulating B cells
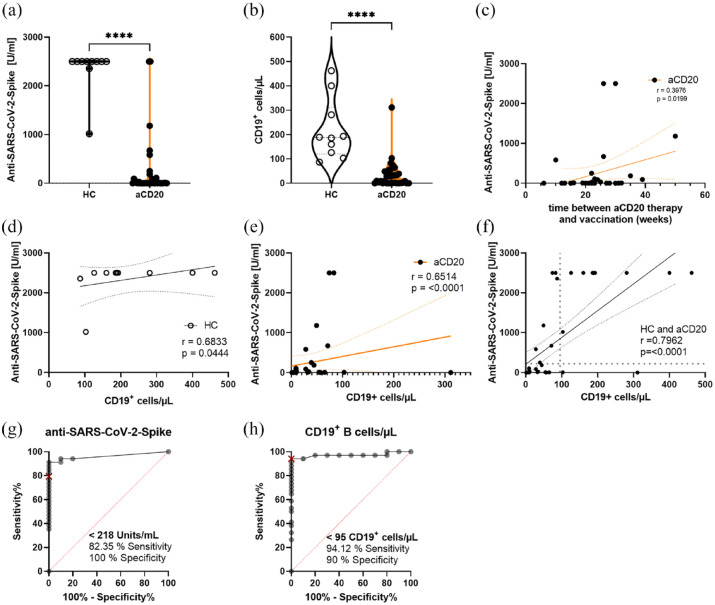
Anti-SARS-CoV-2-Spike antibodies were significantly lower in aCD20-treated MS patients compared with HCs [HC 2337 U/ml ± 147.3 (mean ± SEM); aCD20 243.1 U/ml ± 106.8; p < 0.0001; Figure 1(a)]. As expected, B cells were significantly less abundant in aCD20 patients (Figure 1(b)) and the anti-SARS-CoV-2-Spike titer was increasing depending on the time span between last aCD20 treatment and the vaccination (Figure 1(c)). The HCs showed low correlation of CD19+ B cells with the titer because 80% of the patients had a titer of 2500 U/ml at the maximum range (Figure 1(d)). On the contrary, in aCD20-treated patients, the anti-SARS-CoV-2-Spike titer correlated with the frequency of CD19+ B cells [r = 0.6974, p < 0.0001; Figure 1(e)]. We conducted a receiver operating characteristic curve (ROC) analysis for anti-SARS-CoV-2-Spike and CD19+ B cells/µL to determine a threshold above which B cell frequencies could be considered normal enough to produce a sufficient amount of anti-SARS-CoV-2-Spike antibodies (Figure 1(g) and (h)). Regarding the ROC analysis of anti-SARS-CoV-2-Spike titers, the clinically relevant level of over 200 U/ml had a sensitivity of 82.4% and a specificity of 100%. The threshold of the number of CD19+ cells/µL was 95 cells/µL with a sensitivity of 94.1% and a specificity of 90%. Considering these thresholds of the ROC analysis, most aCD20-treated patients did not have normal B cell numbers and anti-spike antibodies (Figure 1(f)).
Figure 1.
Anti-SARS-CoV-2-spike titer correlates with B cell repopulation. (a) anti-SARS-CoV-2-spike titers and (b) CD19+ B cells were lower in anti-CD20 (aCD20)-treated MS patients compared with healthy controls (HCs). (c) Dependence of anti-SARS-CoV-2-Spike titers on the weeks elapsed between the aCD20 therapy and preceding anti-SARS-CoV-2 vaccination. (d and e) Correlation of anti-SARS-CoV-2-Spike titers with CD19+ B cells/µL, showing a correlation in HCs and aCD20-treated MS patients. (f) Overall correlation of anti-SARS-CoV-2-Spike titers with CD19+ B cells/µL correlated strongly. Most of the aCD20-treated patients did not reach normal ranges according to the threshold lines determined via ROC analysis for (g) anti-SARS-CoV-2-Spike titers and (h) CD19+ B cells/µL. Data were analyzed with non-parametric, two-tailed Mann–Whitney test (a), non-parametric, two-tailed, Spearman-correlation (c–f) or ROC analysis (g and h) and are presented as mean with error; n = 36 aCD20, n = 10 HCs, ****p < 0.0001.
Age did not correlate with anti-SARS-CoV-2-spike antibodies (Figure S2(b) and (c)). Unsuspectedly, the time span between the last vaccination and the blood sampling did not influence the anti-SARS-CoV-2-Spike titer significantly (Figure S2(a)). The lacking significance could, however, be due to small sample sizes and be more prominent with more time elapsed since the last vaccination. Furthermore, we implemented a multiple linear regression to visualize the effect of time since vaccination, vaccine type, and age on the anti-SARS-CoV-2-Spike titer (Figure S3). The highest spike titers were found in patients with least time since mRNA vaccination, with age having no influence on the spike titer.
The aCD20-treated patients developed fewer neutralizing antibodies against the WT, delta, and omicron variant of SARS-CoV-2
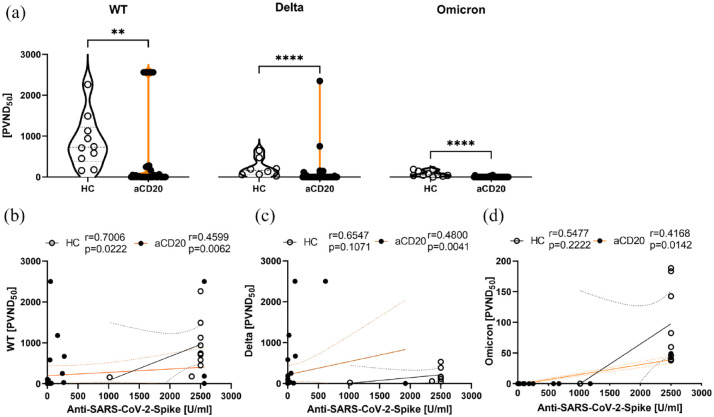
The participants’ sera were tested for their capacity to reduce the SARS-CoV-2-virus variants’ infection rate in vitro using a neutralization assay against WT, delta, and omicron variant of SARS-CoV-2. The capacity of respective sera to reduce the infectivity of mammalian cells using WT, delta, and omicron pseudoviruses was calculated as 50% inhibition (ND50) of a luciferase reporter virus. Sera of anti-CD20-treated MS patients had significantly less neutralizing effect on WT (p < 0.01), delta (p < 0.0001), and omicron variants (p < 0.0001) of SARS-CoV-2 compared with HCs (Figure 2(a)). We also analyzed whether there was an association of anti-SARS-CoV-2 spike titers and neutralization capacity of respective sera. In HCs, there was a significant correlation of anti-SARS-CoV-2 spike and WT neutralization (r = 0.70, p = 0.02), but not for delta and omicron, whereas aCD20-treated patients titer correlated with the neutralization capacity of all tested variants (Figure 2(b)–(d)).
Figure 2.
Neutralization of wild-type, delta, and omicron variants in vitro. (a) Neutralization of SARS-CoV-2 pseudoviruses wild-type (WT), delta, and omicron following 18 h of incubation. Shown is the luciferase reporter activity, indicating 50% inhibition (PVND50). (b–d) Correlation of anti-SARS-CoV-2 spike and neutralization capacity. Data were analyzed with non-parametric, two-tailed Mann–Whitney test (a) or non-parametric, two-tailed Spearman-correlation (b–d) and are presented as mean with error; n = 34 aCD20, n = 10 HCs; (a) **p < 0.01; ****p < 0.0001.
Higher frequency of CD3+ T cells and CD4+ T helper cells following SARS-CoV-2 peptide stimulation in aCD20-treated MS patients
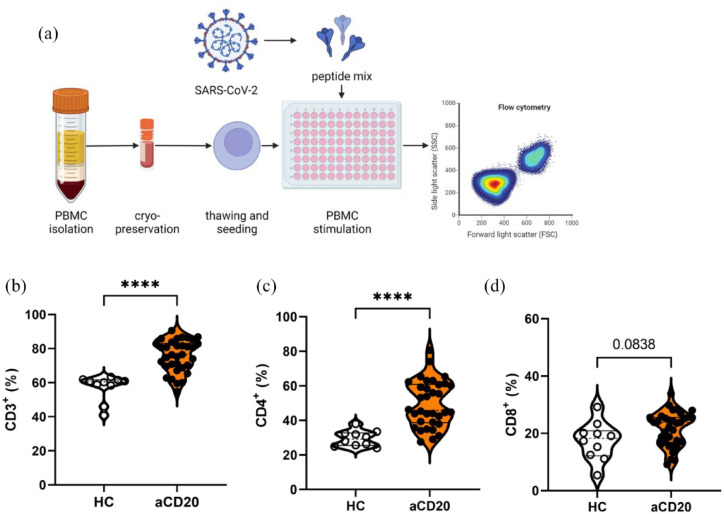
Because aCD20-treated MS patients revealed a restricted humoral immune response, we proceeded to evaluate cellular immune response following stimulation with a peptide pool of SARS-CoV-2 [pool of 315 (158 + 157) peptides derived from Spike glycoprotein (Swiss-Prot ID: P0DTC2); Figure 3(a)]. The aCD20-treated MS patients revealed a higher frequency of overall CD3+ T cells than HCs [HCs 56.3% ± 2.4%; aCD20 75.8% ± 1.6% (mean ± SEM); p < 0.0001; Figure 3(b)]. Frequencies of CD4+ T helper cells (Th cells) were also higher in aCD20-treated MS patients compared with HCs (HCs 29.6% ± 1.4%; aCD20 49.5% ± 2.3%; p < 0.0001). The CD8+ cytotoxic T cells showed a trend to be increased in aCD20 compared with HCs [HCs 17.3 ± 2.1; aCD20 21.6 ± 1.0; p = 0.0838; Figure 3(c)]. The frequency of T cells, Th cells, and cytotoxic T cells was neither in HCs nor in aCD20 dependent on the anti-SARS-CoV-2-Spike titer (Figure S4). In line with this finding, cellular immune response of T cells, Th cells, and cytotoxic T cells did not correlate with neutralization capacity of respective sera except for delta neutralization and frequencies of CD4+ Th cells in aCD20 [aCD20 r = 0.34, p = 0.0491; Figure S5(e)].
Figure 3.
Flow cytometry analysis of T cell populations. (a) PBMCs were stimulated with SARS-CoV-2 peptide pool. (b) Higher frequencies of CD3+ T cells and (c) CD4+ T helper cells in aCD20-treated MS patients compared with HCs. (d) CD8+ T cells did not differ. Data were analyzed with non-parametric, two-tailed Mann–Whitney test and are presented showing median and quartiles; n = 34 aCD20, n = 10 HCs; ****p < 0.0001.
To evaluate the effect of aging on cellular immune response, we correlated overall CD3+ T cells with age. In aCD20-treated patients, there was a trend that the percentage of T cells in the blood was dependent on the age with a younger age accounting for a higher percentage of T cells (r = –0.3362, p = 0.0519; Figure S6). In HCs, we observed the same trend, which also lacked significance (r = –0.4985, p = 0.1456).
T helper cell populations following SARS-CoV-2 peptide stimulation in MS patients under anti-CD20 therapy
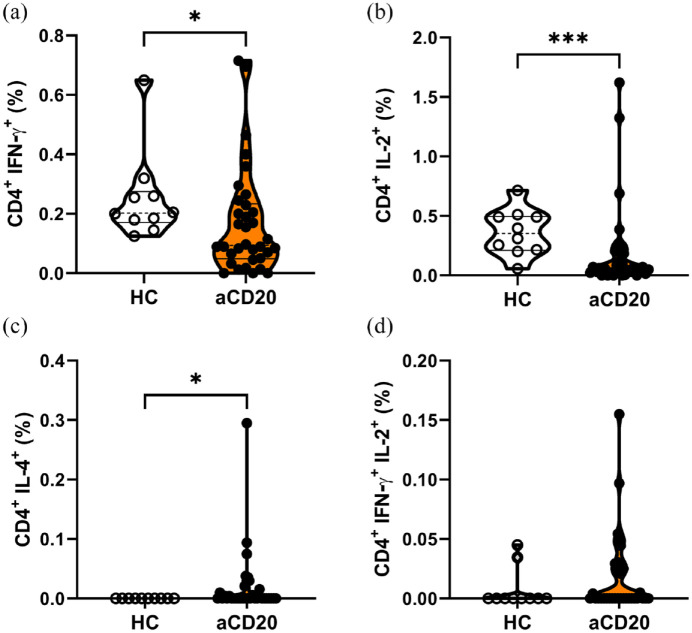
We further analyzed subpopulations of CD4+ Th cells between respective groups. The type 1 helper T (Th1) cells expressing both CD4 and IFN-γ were significantly less abundant in aCD20-treated MS patients than in HCs [HCs 0.25% ± 0.05%; aCD20 0.17% ± 0.03% (mean ± SEM); p < 0.05; Figure 4(a)]. In addition, memory CD4+ Th cells, characterized by the expression of IL-2, were lower in aCD20 after restimulation [HCs 0.36% ± 0.06%; aCD20 0.19% ± 0.06% (mean ± SEM); p < 0.001; Figure 4(b)]. In contrast, IL-4 expressing CD4+ Th cells were significantly more abundant in PBMCs of aCD20-treated MS patients than in HCs (Figure 4(c)). IFN-γ and IL-2 double positive CD4+ Th cells did not significantly differ between both groups, although there was a trend toward higher frequency in aCD20-treated MS patients (Figure 4(d)). The Th cell subpopulations did not correlate with anti-SARS-CoV-2-spike titer (Figure S7).
Figure 4.
The aCD20-treated patients express reduced frequencies of CD4+ IFN-γ+ (Th1) and CD4+ IL-2+ T helper cells. Frequencies of CD4+ T cells expressing (a) IFN-γ or (b) IL-2 were significantly lower in aCD20-treated patients than in HCs. (c) Higher frequencies of IL-4 expressing CD4+ T helper cells were present in aCD20 compared with HCs. (d) Double expression of IFN-γ with IL-2 showed no differences between groups. Data were analyzed with non-parametric, two-tailed Mann–Whitney test and are presented showing median and quartiles; n = 34 aCD20, n = 10 HCs; *p < 0.05; ***p < 0.001.
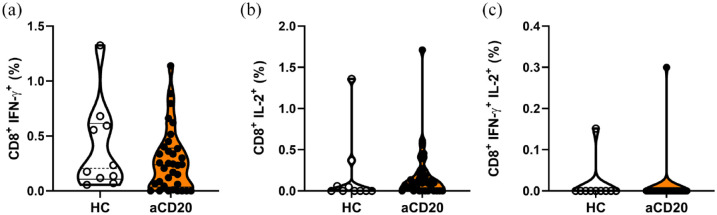
Subpopulations of cytotoxic T cells expressing IFN-γ and IL-2 remained unaltered following SARS-CoV-2 peptide stimulation between HCs and aCD20
We further analyzed subpopulations of CD8+ cytotoxic T cells regarding the expression of IFN-γ and IL-2. The CD8+ T cells expressing IFN-γ were abundant with a comparable frequency in aCD20-treated MS patients as in HCs [HCs 0.39% ± 0.13%; aCD20 0.27% ± 0.05% (mean ± SEM); Figure 5(a)]. This was also the case for CD8+ T cells expressing IL-2 and IFN-γ and IL-2. In aCD20-treated MS patients, anti-SARS-CoV-2-Spike titers correlated with Tc1 cell percentages (r = 0.3585; p = 0.0373; Figure S8). In HCs, this correlation was not present. Anti-SARS-CoV-2-spike titers did not correlate with cytotoxic T cell subsets expressing IL-2 or IFN-γ together with IL-2 (Figure S8).
Figure 5.
Cytotoxic T cell subpopulations were not differently expressed between HCs and aCD20 after restimulation. (a) IFN-γ expression in cytotoxic T cell population did not differ between aCD20 and HCs. (b) Frequencies of IL-2 expressing cytotoxic T cells in aCD20-treated MS patients outlined a trend toward higher expression compared with HCs (p = 0.1316). (c) IFN-γ and IL-2 double-positive cytotoxic T cells showed no significant differences between aCD20 and HCs. Data were analyzed with non-parametric, two-tailed Mann–Whitney test and are presented showing median and quartiles; n = 34 aCD20, n = 10 HCs.
To assess whether the type of vaccination might have influenced the cellular immune response, we performed a subanalysis. We could not detect differences depending on the type of vaccination [mRNA versus ChAdOx1 (Vaxzevria); Figure S9]. There was, however, only a low number of participants who were vaccinated with ChAdOx1.
Discussion
The SARS-CoV-2 pandemic has profound implications for the management of patients with chronic disorders, especially patients with chronic immune diseases in need of a permanent immunomodulatory or immunosuppressive therapy, such as patients with MS, rheumatoid arthritis, or transplant patients. The treatment of MS patients has seen huge progress during the last 20 years, offering a broad armamentarium of medications for different severities and MS courses.1,10 Early during the pandemic, there was a debate regarding the risk of MS patients to develop a severe course of COVID-19. Richter et al.11 and others showed that MS per se is not associated with a higher risk for severe COVID-19. There are, however, subgroups of patients at higher risk. Data from 657 suspected and 1683 confirmed COVID-19 MS patients under disease-modifying therapy showed that older age, progressive MS phenotype, and higher disability are associated with worse COVID-19 outcomes.3 Moreover, treatment with aCD20 therapy with ocrelizumab and rituximab was associated with higher rates of hospitalization [adjusted OR (aOR) = 1.75, 95% CI = 1.29–2.38; aOR = 2.76, 95% CI = 1.87–4.07] and ICU admission (aOR = 2.55, 95% CI = 1.49–4.36; aOR = 4.32, 95% CI = 2.27–8.23); and for rituximab, with artificial ventilation (aOR = 6.15, 95% CI = 3.09–12.27).3
The development of vaccines against SARS-CoV-2 raised the question whether especially patients under B cell depletion might be at risk to develop only a reduced humoral and cellular immune response due to the lack of B cells, important for co-stimulation. While there is evidence that B cell–depleted MS patients are at higher risk for a severe course of COVID-19,3 there are only anecdotal reports of patients under B cell–depleting therapy vaccinated against SARS-CoV-2 with breakthrough infection.12 Hence, at the time we conducted this study, it remained unclear how vaccination against SARS-CoV-2 might influence this risk. In the meantime, data from the ALITHIOS ofatumumab study reported breakthrough infection in 1.5% of vaccinated patients (7 out of 476).13 In a cohort characterized by us, we showed that patients under ofatumumab treatment with three SARS-CoV-2 vaccinations had a mild or moderate COVID course following breakthrough infection (4 out of 10 patients).14
We here demonstrate that patients under B cell depletion show a reduced humoral immune response by measuring anti-SARS-CoV-2 spike antibodies, in line with a reduced capacity to neutralize WT, delta, and omicron pseudovirus in vitro. Development of anti-SARS-CoV-2 spike antibodies correlated strongly with repopulation of B cells. On the contrary, aCD20-treated patients exhibited a stronger cellular immune response following SARS-CoV peptide stimulation compared with HC. This was mainly characterized by higher frequencies of overall CD3+ T cells and CD4+ Th cells, while Th1 cells were lower and Tc1 cells were comparable with HCs.
The first report raising questions regarding the development of a proper immune response in aCD20-treated patients was shown in early 2021 in MS patients treated in Israel.5 Those data showed that patients under B cell–depleting therapy indeed develop only in 22.7% of the cases a humoral immune response against SARS-CoV-2 spike 29.5–55 days after the second vaccine dose.5 Apart from MS, there are numerous reports having shown impaired humoral immune response in immunosuppressed patients such as in rheumatoid arthritis, systemic lupus erythematosus, and vasculitis under different immunosuppressive therapies including rituximab.15 We showed in a group of patients treated with a low-dose anti-CD20 regimen using ofatumumab over 2–4 months that humoral immune response is impaired also with this low-dose regimen, while cellular immune response is not affected.14 Using the IFN-γ enzyme-linked immunosorbent spot (ELISpot) assay, preserved cellular immune response in B cell–depleted patients with impaired humoral response was demonstrated in a group of rituximab-treated rheumatology patients.16 Apostolidis et al.17 showed a reduced spike-specific and receptor-binding domain (RBD)-specific antibody and memory B cell responses in most patients in a group of n = 20 MS patients under aCD20 therapy. Moreover, the group showed that CD8 T cell induction was augmented with preserved Th1 cell priming.17 In the meantime, during the execution of our study, several groups confirmed that aCD20-treated MS patients have a robust T cell response;18,19 those data are in line with our findings, showing that aCD20-treated patients present a strong response of CD3+ and CD4+ T cells.
The Th1 cells producing IFN-γ and IL-2 are considered as a subpopulation of cells with superior protective cellular capacity.20 The cytotoxic T cell response is fast, potent, and detectable 10–12 days after a prime vaccination.4 On the contrary, a prime vaccination only leads to a limited presence of class-switched B cells that could produce S1-specific IgG, while the second booster vaccination mobilizes antigen-specific memory B cells to the periphery after boost.21 In addition, the T cell response is broader, targeting multiple SARS-CoV-2 variants of concern following BNT162b2 mRNA vaccination due to conservation of T cell epitopes on SARS-CoV-2 variants, whereas some variants can partially escape humoral immunity.22 A stable and fully functional CD8+ T cell response is already mobilized 1 week after prime vaccination with the mRNA vaccine BNT162b2, when circulating CD4+ T cells and neutralizing antibodies are still weakly detectable.21 The longevity of this protective T cell immunity induced by mRNA vaccination remains unclear. Patients who recovered from SARS following infection during the outbreak in 2003 possess indeed long-lasting memory T cells that are reactive to the N protein of SARS-CoV 17 years later.23 Moreover, these T cells displayed robust cross-reactivity to the N protein of SARS-CoV-2, suggesting that beta-coronaviruses induce multi-specific and long-lasting T cell immunity against the structural N protein.23 Memory B cells on the contrary are not durable in coronavirus infection and disappear within less than 3 years following infection.6
In summary, our study provides evidence that patients under B cell–depleting therapy have a reduced humoral response and that the development of an anti-SARS-CoV-2 titer depends on the reconstitution of B cells, in line with reduced capacity to neutralize WT, delta, and omicron variant of SARS-CoV-2. Moreover, we show that B cell depletion leads to a shift of cellular immune response, characterized by stronger overall T cell and CD4+ T cell response with comparable cytotoxic CD8+ T cells. Those data altogether support that, if possible, aCD20-treated patients should be vaccinated or boostered during the reconstitution of B cells. Long-term studies are required to address the question whether vaccination in aCD20-treated patients might be protective against the development of COVID-19 in real-world cohorts and to understand dynamics of T cell response over time.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864221141505 for Preserved T-cell response in anti-CD20-treated multiple sclerosis patients following SARS-CoV-2 vaccination by Simon Faissner, Neele Heitmann, Ricarda Rohling, Ulas Ceylan, Marielena Bongert, Carlos Plaza-Sirvent, Corinna Marheinecke, Xiomara Pedreiturria, Ilya Ayzenberg, Kerstin Hellwig, Ingo Schmitz, Stephanie Pfaender and Ralf Gold in Therapeutic Advances in Neurological Disorders
Acknowledgments
We thank Gert Zimmer, Institute for Virology and Immunology, Switzerland and Department of Infectious Diseases and Pathobiology (DIP), Vetsuisse Faculty, University of Bern, Switzerland and Stefan Pöhlmann and Markus Hoffmann, Infection Biology Unit, German Primate Center – Leibniz Institute for Primate Research, Göttingen, Germany, Faculty of Biology and Psychology, Georg-August-University Göttingen, Göttingen, Germany, for providing the WT und Delta plasmid.
Footnotes
ORCID iDs: Simon Faissner  https://orcid.org/0000-0002-3412-762X
https://orcid.org/0000-0002-3412-762X
Ralf Gold  https://orcid.org/0000-0002-7223-3052
https://orcid.org/0000-0002-7223-3052
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Simon Faissner, Department of Neurology, Ruhr-University Bochum, St. Josef-Hospital, Gudrunstrasse 56, 44791 Bochum, Germany.
Neele Heitmann, Department of Neurology, Ruhr-University Bochum, Bochum, Germany.
Ricarda Rohling, Department of Neurology, Ruhr-University Bochum, Bochum, Germany.
Ulas Ceylan, Department of Neurology, Ruhr-University Bochum, Bochum, Germany.
Marielena Bongert, Department of Neurology, Ruhr-University Bochum, Bochum, Germany.
Carlos Plaza-Sirvent, Department of Molecular Immunology, Ruhr-University Bochum, Bochum, Germany.
Corinna Marheinecke, Department of Molecular & Medical Virology, Ruhr-University Bochum, Bochum, Germany.
Xiomara Pedreiturria, Department of Neurology, Ruhr-University Bochum, Bochum, Germany.
Ilya Ayzenberg, Department of Neurology, Ruhr-University Bochum, Bochum, Germany.
Kerstin Hellwig, Department of Neurology, Ruhr-University Bochum, Bochum, Germany.
Ingo Schmitz, Department of Molecular Immunology, Ruhr-University Bochum, Bochum, Germany.
Stephanie Pfaender, Department of Molecular & Medical Virology, Ruhr-University Bochum, Bochum, Germany.
Ralf Gold, Department of Neurology, Ruhr-University Bochum, Bochum, Germany.
Declarations
Ethics approval and consent to participate: The study was authorized by the local ethics committee of the Ruhr-University Bochum (20-6953-bio). Patients were recruited at the Department of Neurology, Ruhr-University Bochum, St. Josef-Hospital. All patients and healthy controls provided written informed consent.
Consent for publication: Not applicable.
Author contributions: Simon Faissner: Conceptualization; Data curation; Formal analysis; Funding acquisition; Methodology; Project administration; Resources; Supervision; Visualization; Writing – original draft; Writing – review & editing.
Neele Heitmann: Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft.
Ricarda Rohling: Data curation; Investigation; Methodology; Writing – review & editing.
Ulas Ceylan: Methodology; Writing – review & editing.
Marielena Bongert: Investigation; Writing – review & editing.
Carlos Plaza-Sirvent: Data curation; Formal analysis; Writing – review & editing.
Corinna Marheinecke: Investigation; Writing – review & editing.
Xiomara Pedreiturria: Investigation; Writing – review & editing.
Ilya Ayzenberg: Formal analysis; Writing – review & editing.
Kerstin Hellwig: Formal analysis; Writing – review & editing.
Ingo Schmitz: Formal analysis; Methodology; Writing – review & editing.
Stephanie Pfaender: Data curation; Formal analysis; Writing – review & editing.
Ralf Gold: Conceptualization; Funding acquisition; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a grant from the Rose foundation T298/28150/2016.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: All data are available from the corresponding author upon reasonable request.
References
- 1. Faissner S, Gold R. Efficacy and safety of the newer multiple sclerosis drugs approved since 2010. CNS Drugs 2018; 32: 269–287. [DOI] [PubMed] [Google Scholar]
- 2. Faissner S, Gold R. Efficacy and safety of multiple sclerosis drugs approved since 2018 and future developments. CNS Drugs 2022; 36: 803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology 2021; 97: e1870–e1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord 2021; 14: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hellerstein M. What are the roles of antibodies versus a durable, high quality T-cell response in protective immunity against SARS-CoV-2? Vaccine X 2020; 6: 100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zettl F, Meister TL, Vollmer T, et al. Rapid quantification of SARS-CoV-2-neutralizing antibodies using propagation-defective vesicular stomatitis virus pseudotypes. Vaccines 2020; 8: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kinast V, Plociennikowska A, Bracht T, et al. C19orf66 is an interferon-induced inhibitor of HCV replication that restricts formation of the viral replication organelle. J Hepatol 2020; 73: 549–558. [DOI] [PubMed] [Google Scholar]
- 10. Faissner S, Plemel JR, Gold R, et al. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat Rev Drug Discov 2019; 18: 905–922. [DOI] [PubMed] [Google Scholar]
- 11. Richter D, Faissner S, Bartig D, et al. Multiple sclerosis is not associated with an increased risk for severe COVID-19: a nationwide retrospective cross-sectional study from Germany. Neurol Res Pract 2021; 3: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peeters M, Verbruggen L, Teuwen L, et al. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open 2021; 6: 100274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cross AH, Delgado S, Habek M, et al. COVID-19 outcomes and vaccination in people with relapsing multiple sclerosis treated with ofatumumab. Neurol Ther 2022; 11: 741–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Faissner S, Heitmann N, Plaza-Sirvent C, et al. Immune response in ofatumumab treated multiple sclerosis patients after SARS-CoV-2 vaccination. Front Immunol 2022; 13: 980526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferri C, Ursini F, Gragnani L, et al. Impaired immunogenicity to COVID-19 vaccines in autoimmune systemic diseases. High prevalence of non-response in different patients’ subgroups. J Autoimmun 2021; 125: 102744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis 2021; 80: 1345–1350. [DOI] [PubMed] [Google Scholar]
- 17. Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021; 27: 1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Madelon N, Lauper K, Breville G, et al. Robust T cell responses in anti-CD20 treated patients following COVID-19 vaccination: a prospective cohort study. Clin Infect Dis 2022; 75: e1037–e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gadani SP, Reyes-Mantilla M, Jank L, et al. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. eBioMedicine 2021; 73: 103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKinstry KK, Alam F, Flores-Malavet V, et al. Memory CD4 T cell-derived IL-2 synergizes with viral infection to exacerbate lung inflammation. PLoS Pathog 2019; 15: e1007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oberhardt V, Luxenburger H, Kemming J, et al. Rapid and stable mobilization of CD8(+) T cells by SARS-CoV-2 mRNA vaccine. Nature 2021; 597: 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Geers D, Shamier MC, Bogers S, et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol 2021; 6: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020; 584: 457–462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864221141505 for Preserved T-cell response in anti-CD20-treated multiple sclerosis patients following SARS-CoV-2 vaccination by Simon Faissner, Neele Heitmann, Ricarda Rohling, Ulas Ceylan, Marielena Bongert, Carlos Plaza-Sirvent, Corinna Marheinecke, Xiomara Pedreiturria, Ilya Ayzenberg, Kerstin Hellwig, Ingo Schmitz, Stephanie Pfaender and Ralf Gold in Therapeutic Advances in Neurological Disorders
Data Availability Statement
All data are available from the corresponding author SF upon reasonable request.