Abstract
Traditional herbal drugs are widely used for the treatment of various diseases. Ellagic acid (EA) as an herbal polyphenol metabolite exists in many medicinal plants. EA has an important role against natural and chemical toxicities due to its antioxidant and anti-inflammatory properties. For this review, several search engines or databases such as PubMed, Scopus, the Web of Science, and Google Scholar were used, and the most relevant published papers till February 2022 were included.
The protective effects of EA against natural and chemical compounds are mediated through molecular mechanisms including scavenging of free radicals, modulation of proinflammatory cytokine synthesis, and reduction of lipid peroxidation. These properties make EA a highly fascinating compound that may contribute to different aspects of health; whereas, more studies are needed, especially on the pharmacokinetic profile of EA.
In this review, we selected articles that include the protective effect of EA against several synthetic and natural toxins such as aflatoxin, lipopolysaccharide, acrylamide, and rotenone.
Key Words: Antidote, Anti-oxidant, Chemical toxic agent, Ellagic acid, Natural toxin, Protective
Introduction
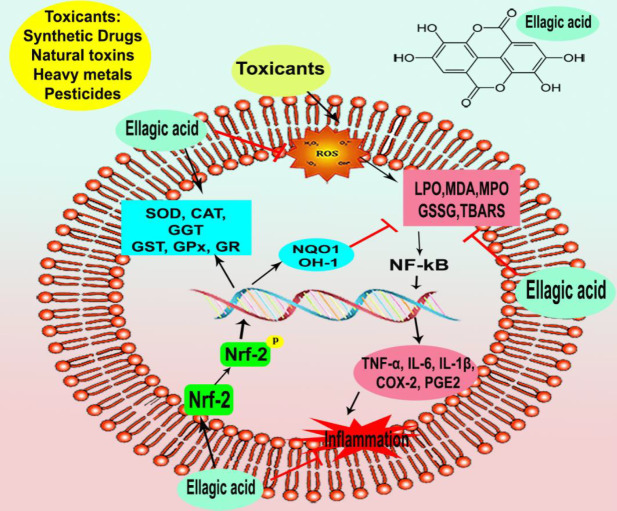
Ellagic acid (EA) (2,3,7,8-tetrahydroxy [1]-benzopyranol [5,4,3-cde] benzopyran-5,10-dione) C14H6O8; (Figure 1) belongs to the class of polyphenol extractives (tannins) that is isolated from many fruits, nutgalls, and plants such as raspberries, strawberries, grapes, pomegranate, black currants, longan seeds, and green tea (1). EA usually is conjugated with a glycoside moiety such as glucose, rhamnose, arabinose, or bounded in the form of ellagitannins that have astringent properties and act as a defense system against microbial and animal attacks (2). Free EA is commonly formed during food processing or in physiological conditions in the human gastrointestinal tract (3). EA, with a molecular mass of 302.197 g/mol, is an extremely thermostable molecule with low water solubility and moderate solubility in alcohol (4). Previous investigations have reported numerous pharmacological activities of EA such as antioxidant, anti-inflammatory, neuroprotective, nephroprotective, and hepatoprotective properties (5-8). The suggested mechanisms for EA protective effects are via activation of antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione-S-transferase (GST), and also modulation of various signaling pathways such as nuclear erythroid 2-related factor 2 (Nrf2), phosphoinositide 3-kinase and glycogen synthase kinase 3 beta (GSK-3β) (6, 9). Furthermore, it suppresses pro-inflammatory markers such as cyclooxygenase (COX-2) and nuclear factor-kappa B (NF-кB) (10-12). It has been indicated that EA is an efficient radical scavenger of OH•, methoxyl (OCH3•), and nitrogen dioxide (NO2•) in the body organs due to its antioxidant effects (13, 14). Previous in vivo and in vitro studies have indicated the ameliorative effects of EA against several malignancies including colorectal, breast, and prostate cancers, and also leukemia, lymphoma, and melanoma (10, 15). In this review, we aim to explain the antioxidant, anti-inflammatory, and ameliorative properties of EA against various types of toxins or toxic agents including pharmaceuticals, heavy metals, and pesticides (Figure 2).
Figure 1.
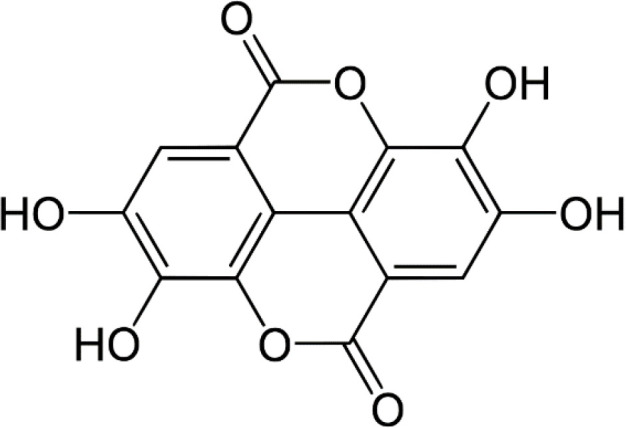
Chemical structure of ellagic acid
Figure 2.
Schematic diagram that explains ellagic acid’s anti-inflammatory and antioxidant properties against chemical agents and natural toxins
CAT: catalase; COX-2: cyclooxygenase 2; GGT: gamma-glutamyltransferase; GPx: glutathione peroxidase; GR: Glutathione reductase; GSSG: Glutathione disulfide; GST: glutathione-S-transferase, IL-6: interleukin-6; IL-1β: interleukin-1β; LPO: lipid peroxidation; MDA: malondialdehyde; MPO: myeloperoxidase; NF-κB: nuclear factor kappa-B; NQO1: NAD(P)H quinone oxidoreductase; Nrf2: nuclear erythroid 2-related factor 2; PGE2: prostaglandin-E2; SOD: superoxide dismutase; TBARS: thiobarbituric acid reactive substances; TNF-α: tumor necrosis factor-alpha
Method
For this review, we used several search engines or databases such as PubMed, Scopus, the Web of Science, and Google Scholar, and we selected the most relevant published papers till February 2022. The selected keywords were “ellagic acid”, “antioxidant”, “anti-inflammatory”, “protective”, “ameliorative”, “hepatoprotective”, “neuroprotective”, “nephroprotective”, and “cardioprotective”. Only articles written in English and published in peer-reviewed scientific journals were selected.
Protective effects of ellagic acid against adverse effects or toxicity of synthetic drugs
Cyclophosphamide
Cyclophosphamide (CPM) is an alkylating antineoplastic agent that is commonly administered for the treatment of various disorders including leukemias, myeloblastoma, breast cancer, malignant lymphomas, and ovarian carcinoma (16). CPM has a wide range of adverse effects, including nephrotoxicity, genotoxicity, and reproductive toxicity (17). EA administration (10 mg/kg, intraperitoneal (IP)) to rats significantly decreased liver damage indicator enzymes such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and gamma-glutamyltransferase (18). The antioxidant effect of EA against CPM-induced oxidative stress was manifested via the decrease in malondialdehyde (MDA), advanced oxidized proteins product contents, xanthine oxidase (XO) activities, elevated glutathione (GSH) concentrations, and CAT activity. Furthermore, EA treatment increased the B-cell lymphoma 2 (Bcl-2)/ Bcl-2-associated X protein (Bax) ratio and diminished the expression of CD15, involved in the extracellular adhesion and migration of cells, in the hepatocytes and lymphocytes which was induced via CPM (18). CPM-induced testicular and spermatozoa toxicity was reported to be associated with oxidative stress and apoptosis, a decrease in epididymal sperm content and motility, and significantly increased MDA levels in male rats. EA (2 mg/kg, PO) administration protected rats against the development of these detrimental effects, significantly increased the number of Bcl-2-positive cells, reduced the number of Bax-negative cells, and ameliorated the CPM-induced immunohistochemical damage (19). Treating rats with EA (2 mg/kg, PO) protects against CPM-induced lipid peroxidation and structural damages in spermatozoa and testicular tissue of rats (20). Administration of CPM to mice increased MDA and XO levels with depletion in GSH content, decreased antioxidant enzyme activities including GPx, glutathione reductase (GR), CAT, and quinone reductase, and induced DNA strand breaks in kidney tissue. EA supplementation in mice (100 mg/kg, PO) significantly suppressed the kidney serum toxicity markers including blood urea nitrogen, creatinine, and lactate dehydrogenase (LDH) which were induced by CPM (21). Moreover, EA (15 mg/kg, IP) treatment modulated enhancement of hydroxyproline level, lipid peroxidation (LPO), myeloperoxidase activity, nitric oxide (NO), and protein carbonyl generation in lungs of rats exposed to CPM. EA treatment restored the activity of antioxidant enzymes such as GPx and GR and reduced reactive oxygen species (ROS) generation in the lung tissue of rats exposed to CPM (22) (Table 1).
Table 1.
Summarized data of EA protection against synthetic drugs’ adverse effects or toxicity
| Toxic agent | Dose/Concentration period and route of exposure | Dose/Concentration of EA treatment period and route of administration | In vivo | Results | Reference |
|---|---|---|---|---|---|
| CPM | 200 mg/kg, Single-dose, IP | 10 mg/kg for 5 days, IP | Male Wistar rats | Decreased MDA, AOPP, and XO levels and increased the activities of GSH and CAT. Diminished ROS, AST, ALT, ALP, and GGT in the liver. |
(18) |
| 15 mg kg, once a week, for 8 weeks, p.o | 2 mg/kg every day, for 8 weeks, p.o | Male Sprague-Dawley rats | Ameliorated epididymal sperm concentration and motility, and decreased MDA level and oxidative stress. Increased Bcl-2 and reduced Bax in testicular tissue. |
(19) | |
| 15 mg kg, once a week, for 8 weeks, p.o | 2 mg/kg, every day for 8 weeks, p.o | Male Sprague-Dawley rats | Improved the tail and total abnormality of sperm, plasma MDA level, erythrocyte SOD activity, and erythrocyte CAT activity in testicular tissue. | (20) | |
| 50 mg/kg, single dose, IP | 100 mg/kg, daily, for 7 days, p.o | Male Swiss albino mice | Decreased BUN, Cr, LDH MDA, and XO levels and increased GSH content, GPx, and CAT activities in the kidney. | (21) | |
| 150 mg/kg, single dose, IP | 15 mg/kg, daily, for 14 days, p.o | Male Wistar rats | Attenuated LPO, MPO, and NO production and protein carbonyl level. Increased GPx and reduced ROS generation in the lung. |
(22) | |
| CsA | 15 mg/kg, daily, for 21 days, IP | 10 mg/kg, daily, for 21 days, p.o | Male Sprague–Dawley rats | Ameliorated the weights of testes and ventral prostate, epididymal sperm concentration, motility, increased testicular tissue GSH and CAT, and decreased MDA levels. | (24) |
| 15 mg/kg, daily, for 21 days, s.c | 10 mg/kg, daily, for 21 days, p.o | Male Sprague–Dawley rats | Significantly increased the GSH level, and CAT activities, and decreased MDA level of kidney, liver, and heart tissues. | (25) | |
| 15 mg/kg, daily, for 30 days, p.o | 10 mg/kg, daily, for 30 days, s.c | Male albino rats | Reduced oxidative stress, LPO, and MDA levels and elevated CAT and Px activities and testicular GSH concentration. | (26) | |
| 25 mg/kg, daily, for 21 days, p.o | 50 mg/kg, daily, for 21 days, p.o | Male albino Wistar rats | Decreased the activities of serum hepatic enzymes such as AST, ALT, ALP and LDH, and LPO. | (27) | |
| Dox | 5 mg/kg, twice a week, for 2 weeks, IP | 10 mg/kg, daily, for 2 weeks, p.o | Male Sprague–Dawley rats | Improved testicular relative weight, sperm count, motility, serum testosterone, testicular glycogen. Decreased oxidative stress, and TNF-α. |
(29) |
| 20 mg/kg, single dose, IP | 1 g mixed with diet and given for 8 weeks, p.o | Male C57BL/6 mice | Reduced ROS, IL-6, IL-10 levels, MDA and XO activities, and monocyte chemoattractant protein-1 and TNF-α levels in the cardia. | (30) | |
| 5 mg/kg, twice a week, for 2 weeks, IP | 10 mg/kg, daily, for 14 days, p.o | Male Sprague–Dawley rats | Decreased MDA, TNF-𝛼, iNOS, caspase-3, and cholinesterase and increased GSH levels in the brain. | (31) | |
| Gentamicin | 100 mg/kg, single dose, IP | 10 mg/kg, daily for 10 days, p.o | Male Sprague Dawley rats | Decreased LPO and MDA and increased CAT, SOD enzyme activity, and GSH content in kidney tissue. Increased the ratio of Bcl-2/Bax and prevented MMP loss in the kidney. |
(33) |
| ISP | 100 mg/kg, daily, for 2 days, s.c | 15 mg/kg, daily, for 10 days, p.o | Male Wistar rats | Suppressed troponin-I, creatine kinase, LDH, C-reactive protein, and TBARS. Increased SOD, GST, and CAT and restored the GSH content in heart tissue. |
(36) |
| MTX | 20 mg/kg, single dose, IP | 10 mg/kg, daily, for 10 days, p.o | Male Wistar rats | Decreased MDA levels, Bcl-2/Bax ratio, cytochrome-c release, and caspase-3/9 and decreased mitochondrial outer membrane potential. Up-regulation of both Nrf2 and HO-1in the liver. |
(38) |
| 20 mg/kg, single dose, IP | 10 mg/kg, daily, for 5 days, p.o | Male Wistar rats | Reduced MDA, NO, and PGE2 levels and diminished the activity of MPO and XO, and increased GSH content level in the intestinal tissue. | (39) | |
| 20 mg/kg, single dose, IP | 10 mg/kg, daily, for 10 days, p.o | Male Wistar rats | Reduced AST, ALT, ALP, MDA, and LPO. Increased SOD, CAT, and GST, and restored the GSH content level in the liver. |
(40) | |
| Paracetamol | 500 mg/kg, single dose, p.o | 100 mg/kg, daily, for 7 days, p.o | Swiss albino mice | Suppressed liver marker enzymes (ALT, AST, and ALP) in serum and MDA levels. Increased CAT and SOD activity and GSH level in the liver. |
(42) |
| Sodium valproate | 400 mg/kg, daily for 7 days, p.o | 50 mg/kg, daily, for 10 days, p.o | Male Wistar rats | Protected the male rats' reproductive system against necrosis, atrophy in seminiferous tubules, multi-nucleated giant cell formation, and interstitial edema induced by sodium valproate. | (44) |
| STZ | 3 mg/kg single dose, ICV | 35 mg/kg, daily, for 4 weeks, p.o | Wistar rats | Decreased the TBARS and LDH content and depression of GSH levels and SOD and CAT activity in the brain of rats. Decreased AChE and LDH activity, TNF-α, and eNOS levels. |
(46) |
| 50 mg/kg, single dose, IP | 50 mg/kg, daily, for 21 days, p.o | Wistar albino rats | Reduced MDA, TOS, and NO levels Increased TAS level, CAT, and PON-1 activities in sciatic and brain tissue. |
(47) |
AChE: acetylcholinesterase; AOPP: advanced oxidized proteins products; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; Bax: Bcl-2-associated X protein; Bcl-2: B-cell lymphoma 2; BUN: blood urea nitrogen; CAT: catalase; CPM: Cyclophosphamide; Cr: creatinine; CsA: Cyclosporine A; EA: ellagic acid; Dox: doxorubicin; eNOS: endothelial nitric oxide synthetase; GSH: Glutathione; GST: glutathione-S-transferase; GPx: glutathione peroxidase; GGT: gamma-glutamyl transferase; ICV: intracerebroventricular; IL-6 or 10: interleukin-6/-10; ISP: isoproterenol; IP: intraperitoneal; iNOS: inducible nitric oxide synthase; LPO: lipid peroxidation; LDH: lactate dehydrogenase; MTX: Methotrexate; MDA: malondialdehyde; MPO: myeloperoxidase; MMP: mitochondrial membrane potential; NO: nitric oxide; Nrf2: nuclear erythroid 2-related factor 2; Px: peroxidase; PGE2: prostaglandin-E2; PON-1: paraoxonase; p.o: oral; ROS: reactive oxygen species; STZ: streptozotocin; SOD: superoxide dismutase; TBARS: thiobarbituric acid reactive substances; TNF-α: tumor necrosis factor-alpha; TAS: total antioxidant status; TOS: total oxidant status; XO: xanthine oxidase
Cyclosporine A
Cyclosporine A (CsA) is a common immunosuppressive agent that is widely used in candidates for solid-organ transplantation (23). Besides the beneficial effect of CsA, several related toxicities have been reported including reproductive toxicity, nephrotoxicity, hepatotoxicity, and cardiotoxicity through oxidative stress, autophagy, and depletion of antioxidant enzymes (23). In rats, CsA diminished the weights of testes and ventral prostate, epididymal sperm level, sperm motility, GSH, and CAT, and increased MDA level in testicular tissue, while EA (10 mg/kg, PO) treatment attenuated all the CsA-induced negative changes (24). Also, EA significantly ameliorated CsA-induced germinal cell necrosis, interstitial edema, spermatogenic arrest, and capillary congestion (24). EA treatment (10 mg/kg, PO) significantly increased the GSH level, GPx, and CAT activities and decreased the MDA content of the kidney, liver, and heart in rats exposed to CsA. Furthermore, CsA caused severe damage in the kidney, liver, and heart tissues which were noticeably ameliorated by EA supplementation (25). EA (10 mg/kg, subcutaneous (SC)) co-treatment with CsA improved CsA toxic side effects in testis and bone marrow tissues with a significant reduction in oxidative stress, LPO, and MDA levels. EA therapy elevated CAT and peroxidase activities and GSH concentration (26). CsA induced liver damage by increasing the serum hepatic enzymes such as AST, ALT, ALP, and LDH and elevating LPO markers such as thiobarbituric acid reactive substances (TBARS) and hydroperoxides. Administrations of EA (50 mg/kg, PO) in rats significantly decreased the activities of hepatic marker enzymes and also reduced the levels of TBARS and hydroperoxides (27) (Table 1).
Doxorubicin
Doxorubicin (Dox) is a valuable anticancer drug widely used in the treatment of various hematological and solid tumor malignancies. Although DOX is a very useful drug, its therapeutic use has been limited because of its acute and chronic cardiac toxicities and neurotoxicity (28). Exposure of rats to Dox declined testicular weight, testicular glycogen, sperm count and motility, and serum testosterone level. It increased the histopathological changes, oxidative stress, and TNF-α level, and administration of EA (10 mg/kg, PO) reversed these changes (29). Supplementation of Dox-treated mice with EA (1 g, PO) reduced ROS, interleukin-6 (IL-6), and IL-10 levels. Also, it decreased MDA and XO activity and monocyte chemoattractant protein-1 and TNF-α levels, and it enhanced GSH content and GPx, SOD, and CAT activity in heart tissue (30). Furthermore, Dox exposure increased caspase-3, nuclear factor kappa-B (NF-κB), p50, and p65 protein content, and EA administration down-regulated these inflammatory and apoptotic proteins (30). A previous study indicated that DOX elevated MDA, TNF-𝛼, inducible nitric oxide synthase (iNOS), caspase-3, and cholinesterase levels and significantly reduced GSH levels, monoamines including serotonin, dopamine, and norepinephrine in the brain; however, EA (10 mg/kg, PO) effectively protected rats against DOX-induced neurotoxicity by its antioxidant, anti-inflammatory, and antiapoptotic properties (31) (Table 1).
Gentamicin
Gentamicin is an aminoglycoside antibiotic discovered in 1963 and widely used in clinics against gram-negative bacillary infections especially those originating from Pseudomonas aeruginosa. Gentamicin accumulation in several organs induced toxicity such as nephrotoxicity and ototoxicity (32). EA (10 mg/kg, PO) co-treatment with gentamicin in rats significantly ameliorated oxidative stress markers including LPO and MDA, and increased CAT, SOD enzyme activity, and GSH content in the kidney tissue (33). Furthermore, they reported that EA administration increased the Bcl-2/Bax ratio and prevented mitochondrial membrane potential (MMP) loss in kidney tissue against gentamicin (33) (Table 1).
Isoproterenol
Isoproterenol (ISP), a synthetic β-adrenoceptor agonist, is commonly used in the treatment of cardiac dysfunction such as bradycardia, thioridazine-induced torsade de pointes, and heart block (34). ISP induces myocardial infarction (MI) through alteration in biochemical and histological changes, oxidative stress, LPO, and pro-inflammatory cytokines (35). EA administration (15 mg/kg, PO) in ISP-treated rats noticeably decreased levels of cardiac markers such as cardiac troponin-I, creatine kinase, LDH, C-reactive protein, TBARS, and lipid hydroperoxides (36). Also, EA displayed high potential activity to elevate the anti-peroxidative enzymes including SOD, GST, and CAT, and also restored the GSH content in ISP-induced myocardial infarction (36) (Table 1).
Methotrexate
Methotrexate (MTX) as an antimetabolite agent for cancer treatment could induce many adverse effects including renal dysfunction, myelosuppression, mucositis, hepatotoxicity, and in severe cases multiorgan failure (37). Administration of MTX in rats induced oxidative stress, increased MDA levels, Bax/Bcl-2 ratio, cytochrome-c release, and caspase-3/9 level, and decreased mitochondrial outer membrane potential; furthermore, it elevated the concentration of pro-inflammatory factors such as NF-ĸB and IL-6 that were decreased by EA (10 mg/kg, PO) administration. EA therapy considerably up-regulated both Nrf2 and HO-1 which were down-regulated in MTX-treated rats (38). EA (10 mg/kg, PO) treatment in rats significantly reduced MDA, NO, and prostaglandin-E2 levels and diminished the activity of myeloperoxidase, XO, and AD, and also increased GSH content level in the intestinal tissue of rats treated with MTX; moreover, EA administration protected the intestinal tissue against histopathological changes caused by MTX (39). Moreover, EA (10 mg/kg, PO) protected the liver against MTX-induced damage through its antioxidant properties by reducing the serum liver enzymes, including AST, ALT, and ALP (40). Also, EA treatment prevented the elevation of MDA and LPO by activating the antioxidant enzymes such as SOD, CAT, and GST, and also restoring the GSH content level in the liver of rats exposed to MTX (40) (Table 1).
Paracetamol
Paracetamol (acetaminophen) is the most used analgesic and antipyretic medicine around the world (41). Chronic administration of paracetamol could induce several toxicities including hepatotoxicity, cardiotoxicity, nephrotoxicity, and neurotoxicity (41). Paracetamol in rats induced hepatic damage by increasing the activities of marker enzymes (ALT, AST, and ALP) in serum and MDA levels in the liver, and also reducing CAT and SOD activity and GSH level; however, EA (100 mg/kg, PO) administration prevented the liver damage induced with paracetamol (42) (Table 1).
Sodium valproate
Sodium valproate is a widely used antiepileptic drug with a wide-ranging activity and mechanism of action. Sodium valproate is a well-tolerated medicine in the patient, but it causes several health problems including hepatotoxicity and hyperammonemia encephalopathy (43). Sodium valproate produced reproductive toxicity and associated histological changes in male rats through the significant suppression of sperm count and motility which ameliorated via EA (50 mg/kg, PO) treatment (44). Furthermore, EA co-treatment with sodium valproate protected the male rats’ reproductive system against necrosis, seminiferous tubule atrophia, formation of the multinucleated giant cell, interstitial edema with congestion, reduction in germinal cell count, and compromised spermatogenesis (44) (Table 1).
Streptozocin
Streptozocin or streptozotocin is a glucosamine-nitrous-urea compound that first was introduced for treating metastatic pancreatic islet cell cancer, and in experimental models, it is used to induce insulin-dependent diabetes mellitus, non-insulin-dependent diabetes mellitus, and Alzheimer’s disease (AD) (45). EA administration (35 mg/kg, PO) to rats that received intracerebroventricular streptozotocin prevented them from the loss of cognitive abilities; it decreased the content of TBARS and elevated the level of GSH, SOD, and CAT activity in the brain tissue. Furthermore, EA administration decreased acetylcholinesterase and LDH activity, and TNF-α and eNOS levels in the brain (46). A similar study suggests that EA (50 mg/kg, PO) supplementation in rats treated with streptozocin significantly reduced MDA content, total oxidant status, oxidative stress index, and NO levels, and reverses levels of total antioxidant status, CAT, and paraoxonase activities in the brain tissue (47) (Table 1).
The protective effects of ellagic acid against natural toxins
Natural toxins usually are poisonous secondary metabolites that are produced by living organisms, which are typically not harmful to the organisms themselves but can impact human or animal health when consumed. Some plant-based foods may also be toxic if they are not processed or cooked appropriately before consumption. Besides, some toxins have been reported as valuable molecules for drug development.
Aflatoxin B1
Aflatoxins belong to mycotoxins and are produced by Aspergillus flavus and Aspergillus parasiticus. They are found in spoiled foodstuff, milk, meat, and eggs of animals who were fed aflatoxin-contaminated foods. Among them, AFB1 is the most hazardous type (48). Its exposure can cause nausea, vomiting, abdominal pain, and convulsion, and its chronic exposure can also lead to several health disorders like hepatotoxicity, immunotoxicity, and teratogenicity (49). EA (300 mg/kg, IP) prevented AFB1 genotoxicity in the bone marrow and lung cells (50). Treating Salmonella with AFB1 leads to mutagenicity and EA pretreatment diminished it by building an extracellular complex with AFB1 and reducing the interaction of AFB1 with the bacteria (51) (Table 2).
Table 2.
Summarized data of EA protection against natural toxins
| Toxic agent | Dose/Concentration period and route of exposure | Dose/Concentration of EA treatment period and route of administration | In vitro/In vivo | Results | Reference |
|---|---|---|---|---|---|
| AFB1 | 4 mg/kg, single dose, IP | 300 mg/kg, two doses, IP | Male albino Wistar rats | Prevented the genotoxicity of AFB1 in the bone marrow. | (50) |
| ConA | 20 mg/kg, single dose, i.v | 200 mg/kg, single dose, p.o | Male BALB/c mice | Decreased the expression of TLR2 and TLR4 mRNA and proteins in the liver. Decreased NF-κB and expression of proinflammatory cytokines such as TNF-α, IL-6, and IL-1β. |
(53) |
| Glutamate | 60 mg/kg, daily, for 30 days, p.o | 20 mg/kg, daily, for 30 days, p.o | Male albino rats | Increased SOD, GPx, GRx, and CAT and diminished MPO and XO. Elevated testosterone hormone levels enhanced male reproductive capacity, and inhibited histological and ultrastructure testicular damage. |
(57) |
| LPS | 50 μg/kg, single dose, IP | 20 mg/kg, single dose, 1 hr before exposure | Male BALB/c mice | Reduced hepatic MDA content, TNF-α, and serum ALT and AST levels. increased expression of Nrf2 and HO-1. |
(60) |
| Ethanol | 7.9 g/kg, daily for 45 days, p.o | 90 mg/kg, daily for 45 days, p.o | Female albino rats | Decreased TBARS, hydroperoxides, NO, ALT, AST, LHD, and ALP, and enhanced GSH level, SOD, and GST. | (65) |
| 7.9 g/kg, daily for 45 days, p.o | 90 mg/kg, daily for 45 days, p.o | Female albino Wistar rats | Improved body weight, restoring antioxidant status, modulating micronutrients, and attenuating lipid levels. Reduced AST, ALT, and ALP. |
(65) | |
| 100 mM for 24 hr | 100 μM, before exposure | hepatic HepG2 cells line | Reduced NO, TGF-β1, and SR-B1. | (66) | |
| Nicotine | 5 mg/kg, daily for 15 days, IP | 60 mg/kg, daily for 15 days, IP | Sprague-Dawley rats | Increased body weight, total GSH, GPx, and SOD activities, and decreased MDA and NO levels in the kidney | (69) |
AFB1: Aflatoxin B1; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CAT: catalase; ConA: concanavalin A; EA: ellagic acid; GRx: Glutaredoxins; GPx: glutathione peroxidase; IL-6: interleukin-6; IL-1β; interleukin-1β; IP: intraperitoneal, i.v: intravenous; LPS: Lipopolysaccharide; MDA: malondialdehyde; MPO: myeloperoxidase; NF-κB: nuclear factor kappa-B; Nrf2: nuclear erythroid 2-related factor 2; p.o: oral; SOD: superoxide dismutase; TLR: toll-like receptor; TNF-α: tumor necrosis factor-alpha; XO: xanthine oxidase
Concanavalin A
Concanavalin A (ConA), a Ca2+/Mn2+-dependent and mannose/glucose-binding lectin from Jack bean seeds, has a potent anti-cancer effect (52). ConA induced hepatitis by activating toll-like receptor (TLR) signaling pathways (52). EA (200 mg/kg, PO) pretreatment diminished the plasma levels of aminotransferases (ALT and AST) and liver necrosis in ConA-induced hepatitis (53). Also, EA significantly decreased TLR2 and 4 protein and mRNA expression in liver tissue. Also, EA supplementation decreased the expression of NF-κB and the expression of proinflammatory cytokines including TNF-α, IL-6, and interleukin-1β (IL-1β) in ConA-treated mice (53) (Table 2).
Glutamate
Glutamate, a non-essential amino acid, is a principal excitatory neurotransmitter in the brain. Animal experimental studies have shown it has the potential to induce testicular and neural toxicity (54). Monosodium glutamate which is used as a food additive in commercial foods could be considered an important source of exposure to the high level of glutamate (55), although, at the physiological level, the blood-brain barrier may restrict the passage of glutamate from the blood into the brain (56). Glutamate exposure in rats significantly reduced testicular antioxidant biomarkers including SOD, GPx, GRx, and CAT, and increased myeloperoxidase and XO activity. EA (20 mg/kg, PO) elevated testosterone hormone content, improved histological and ultrastructure testicular damages, and inhibited the redox state in male rats (57) (Table 2).
Lipopolysaccharide
Lipopolysaccharide (LPS) is the main component of the outer membrane of Gram-negative bacteria (58). It is the major reason for a severe systemic inflammatory response syndrome that is called sepsis (59). In the liver, it induced the activation of macrophages, the NF-κB pathway, and the production of inflammatory mediators including TNF-α and IL-6 which lead to hepatic failure (60). EA (20 mg/kg, PO) supplementation in mice exposed to LPS reduced hepatic MDA content, TNF-α, and serum ALT and AST levels. The inhibition of NF-κB with EA was found to be related to an increase in the activation of Nrf2/HO-1 signaling pathways in the liver tissue (60) (Table 2).
Ethanol
Ethanol toxicity can occur by ingestion of a large quantity of ethanol in the form of beverage ethanol, mouthwash, cologne, and cough medicine (61). Ethanol abuse is one of the most important world health issues that is associated with organ dysfunction and diseases such as hepatitis, liver cirrhosis, cardiomyopathy, and brain disorders (62). The chronic consumption of ethanol leads to inflammation in the liver tissue by enhancing the production of inflammatory mediators such as transforming growth factor-β1, TNF-α, and ROS which contribute to hepatocyte dysfunction, necrosis, apoptosis, and fibrosis (63, 64). EA (90 mg/kg, daily for 45 days, PO) co-administration in rats with ethanol (7.9 g/kg, daily for 45 days, PO) meaningfully improved the status of antioxidants and decreased TBARS, hydroperoxides, NO, protein carbonyl content, and liver marker enzymes including ALT, AST, LHD, and ALP, and also, enhanced GSH level and SOD activity in the liver (65). EA (90 mg/kg, PO) supplementation to rats inhibited alcohol-induced toxicity by improving body weight, restoring antioxidant status (SOD, CAT and GPx, and GSH level), and reduction of lipid content in the circulation; furthermore, EA diminished liver marker enzymes including gamma-glutamyltransferase, AST, ALT, and ALP (65). Ethanol-induced toxicity in HepG2 cells is accompanied by elevation of NO and transforming growth factor-beta production, which EA (100 μM) administration regulated NO and transforming growth factor-beta production in ethanol-stimulated HepG2 cells (66) (Table 2).
Nicotine
Nicotine with a 1-2 hr half-life is the primary component of tobacco. It is highly addictive with liability for widespread abuse. It is metabolized primarily in the liver and then excreted by the kidneys (67). Nicotine causes uterine vasoconstriction and placenta damage by enhancing the MDA level and LPO (68). Nicotine treatment in rats significantly decreased body weight, total GSH, and SOD activities, and increased MDA and NO levels in the kidney tissue which are reversed with EA (60 mg/kg, IP) co-treatment (69) (Table 2).
Protective effects of ellagic acid against the toxicity of pesticides
Malathion
Malathion (MAL), a known synthetic organophosphate pesticide, is widely used in agriculture, industry, and veterinary medicine (70). Chronic MAL exposure is associated with several organ toxicities such as neurotoxicity, cardiotoxicity, hepatotoxicity, and immunotoxicity in both humans and animals (71). Exposure to fish with MAL caused oxidative stress, significantly increased MDA, and diminished the activities of SOD and CAT in the liver, kidney, and gills which were reversed by EA administration (72) (Table 3).
Table 3.
Summarized data of EA protection against toxicity of pesticides
| Toxic agent | Dose/Concentration period and route of exposure | Dose/Concentration of EA treatment period and route of administration | In vitro/In vivo | Results | Reference |
|---|---|---|---|---|---|
| Malathion | 0.5 mg/L for 14 days, dissolved in water | Diet contains EA in aqua solution | Cyprinus carpio (fish) | Diminished MDA and increased SOD, CAT, and GSH-Px, activities in the liver and kidney. | (72) |
| Paraquat | 45 mg/kg single dose, IP | 85 mg/kg, 1 hr after exposure, p.o | Female albino Wistar rats | Improved kidney tissue structure histologically and increased TAS and TOS. | (74) |
| 100 μM for 72 hr | 80 μM for 72 hr | Human lung carcinoma, A549 cells | Decreased ROS, LPO, and LDH and up-regulated Nrf2, HO-1, and NQO1. | (75) | |
| Phosalone | 0.11 mM for 24 hr | 100 nM for 24 hr | Rat embryonic fibroblast | Suppressed ROS and LPO and inflammatory cytokines (TNF-α, IL-1β, IL-6, and NF-κB). | (77) |
| Rotenone | 1 mg/kg, daily for 5 weeks, s.c | 100 mg/kg, daily for 5 weeks, p.o | Wild-type C57BL/6J male mice | Activated the Nrf2/HO-1 signaling pathways in the brain. | (80) |
CAT: catalase; EA: ellagic acid; GST: glutathione-S-transferase; GSH-Px: Glutathione peroxidase; IL-6: interleukin-6; IL-1β: interleukin-1β; IP: intraperitoneal; LPO: lipid peroxidation; LDH: lactate dehydrogenase; MDA: malondialdehyde; NF-κB: nuclear factor kappa-B; NQO1: NAD(P)H quinone oxidoreductase; Nrf2: nuclear erythroid 2-related factor 2; p.o: oral; ROS: reactive oxygen species; s.c: subcutaneous; SOD: superoxide dismutase; TAS: total antioxidant status; TNF-α: tumor necrosis factor-alpha; TOS: total oxidant status
Paraquat
Paraquat (PQ) is an efficient and widely used herbicide for destroying weeds that may decrease crop yields, however, PQ is highly toxic through generation of ROS, LPO, and oxidation of GSH to glutathione disulfide (73). The protective effects of EA (85 mg/kg, PO) against PQ-induced nephrotoxicity were shown to be mediated via improving tissue structure and increasing the total antioxidant status (74). The cell viability was decreased and ROS, LPO, and LDH increased in the A549 cell line by PQ treatment, and these effects were reversed by EA pretreatment. Moreover, EA (80 μM) significantly up-regulated the level of Nrf2, HO-1, and NAD(P)H quinone oxidoreductase that protects the A549 cell line against PQ toxicity (75) (Table 3).
Phosalone
Phosalone is an organophosphate pesticide that is widely used around the world. Phosalone induced several toxicities in organs including the liver, brain, kidney, and heart by enhancing ROS and LPO which leads to DNA fragmentation (76). Phosalone in rat embryonic fibroblast (REF) cells decreased cell viability and increased oxidative stress markers (ROS and LPO) and inflammatory cytokines (TNF-α, IL-1β, IL-6, and NF-κB) (77). However, EA (100 nM) pretreatment protected cells by suppressing free radicals and ROS formation, decreasing the expression and protein levels of p38 and p53, and reduction of inflammatory factors including TNF-α, IL-1β, IL-6, and NF-κB (77). Induction of NF-κB leads to p53 activation which causes cell arrest in G1/S (78) (Table 3).
Rotenone
Rotenone, a known widely used insecticide, is a potent mitochondrial complex I inhibitor that induces neurotoxicity and causes neurodegenerative disorders such as Parkinson’s disease (79). EA (100 mg/kg, PO) supplementation in mice protected them against rotenone neurotoxicity via activation of the Nrf2/HO-1 signaling pathway. Nrf2 mediates the expression of HO-1 and NAD(P)H quinone oxidoreductase gene in astrocytes and prevented neuronal damage related to superoxide (80) (Table 3).
Protective effects of ellagic acid against heavy metals toxicity
Aluminum
Aluminum (Al) is easily accumulated in the brain, bone, liver, and kidney of mammalian tissues and increases the risk of health disorders (81). Administration of EA (60 mg/kg, PO) ameliorated hepatic dysfunction, dyslipidemia, hepatic histological alterations, reduced liver MDA and protein carbonyl content levels, and elevated liver CAT, GPx, and SOD activity and GSH content in Al toxicants (82). Furthermore, EA administration decreased serum level of total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), and very-low-density lipoprotein cholesterol, and increased the high-density lipoprotein cholesterol (HDL-C) level in AlCl3 treated rats (82) (Table 4).
Table 4.
Summarized data of EA protection against heavy metals
| Toxic agent | Dose/Concentration period and route of exposure | Dose/Concentration of EA treatment period and route of administration | In vitro/In vivo | Results | Reference |
|---|---|---|---|---|---|
| Aluminum | 20 mg/kg, daily, for 8 weeks, p.o | 60 mg/kg, daily for 8 weeks, p.o | Male albino rats | Reduced liver MDA levels and modulated elevation of liver CAT, GPx, and SOD activity and GSH level. | (82) |
| Arsenic | 10 mg/kg, daily, for 8 days, p.o | 40 mg/kg, daily, for 11 days, p.o | Wistar rats | Decreased the inflammatory markers (IL-1β, TNF-α, and INF-γ) and apoptotic markers in the brain, down-regulated Bax and up-regulated Bcl-2 | (86) |
| 10 mg/kg, daily, for 21 days, p.o | 30 mg/kg, daily, for 14 days, p.o | Male Wistar albino rats | Diminished MDA and NO levels and inflammation markers (IL-1β and TNF-α), and increased TAC, GPx, and GSH levels in the brain. | (87) | |
| 10 mg/kg, daily, for 21 days, p.o | 30 mg/kg, daily, for 14 days, p.o | Male Wistar rats | Decreased AST, CK-MB, LDH, and cTnI; and decreased MDA and NO with the increase of GSH level and activities of CAT, SOD, and GPx in the cardia. | (88) | |
| 500 µM for 24 hr | 75 µM, 1 hr prior to exposure | Chinese hamster V79 cells | Diminished the generation of ROS and DNA fragmentation. | (89) | |
| 10 mg/kg daily for 21 days, p.o | 30 mg/kg daily for 14 days, p.o | Male Wistar rats | Increased TAC, GSH level, and the activity of antioxidant enzymes (SOD, CAT, and GPx) in testicular tissue. Suppressed MDA, IL-1β, TNF-α, and NO. |
(90) | |
| Arsenic trioxide | 2 µM for 24 hr | 20 µM, 1 hr prior to exposure for 24 hr | SH-SY5Y human neuroblastoma cell line | Attenuated MMP loss and released cytochrome c and DNA damage. | (92) |
| 5 mg/kg, daily, for 10 days, IP | 30 mg/kg, daily, for 10 days, p.o | Male Wistar rats | Decreased MDA and increased GSH, and GPx activity, and attenuated QTc prolongation in the heart. | (93) | |
| Iron | 100 mg/kg, for 5 alternative days, IP | 8 mg/kg, daily, for 21 days, p.o | Swiss albino male mice | Suppressed ROS generation and serum marker levels (ALP, ALT, AST, and LDH) and increased antioxidant enzymes (SOD and CAT). Decreased the Bax/Bcl-2 ratio and inhibited the caspase-3 activation. |
(95) |
ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; Bax: Bcl-2-associated X protein; Bcl-2: B-cell lymphoma 2; CAT: catalase; CK-MB: Creatine kinase-MB; GPx: glutathione peroxidase; GSH: Glutathione; IL-1β; interleukin-1β; INF-γ: Interferon-gamma; LDH: lactate dehydrogenase; MDA: malondialdehyde; MMP: mitochondrial membrane potential; NO; nitric oxide; p.o: oral; ROS: reactive oxygen species; SOD: superoxide dismutase; TAC: total antioxidant capacity; TNF-α: tumor necrosis factor-alpha
Arsenic
Arsenic (As) is a known toxic element that is present in the air, water, and soil. Based on the experimental studies, it has toxic effects on vital organs such as the kidney, brain, and liver (83, 84). The toxicity of As significantly enhanced the generation of free hydroxyl radicals, superoxide anions, dimethyl arsenic peroxy radical, dimethyl arsenic radical, and nitric oxide (85). Treating rats with EA (40 mg/kg, PO) during As exposure significantly decreased the inflammatory (IL-1β, TNF-α, and INF-γ) and apoptotic markers; EA attenuated MMP and ROS generation (86). Sodium arsenate (SA) in the rat brain increased oxidative stress elements (MDA, NO, and PC) level and inflammatory markers (IL-1β and TNF-α), and decreased the total antioxidant capacity, GPx, and GSH content; these symptoms were reversed following EA administration (30 mg/kg, PO) (87). EA administration also reduced the plasma cardiac markers levels (AST, CK-MB, LDH, and cTnI) in SA-treated rats. Moreover, the heart of rats treated with SA displayed an increase in MDA and NO levels with a reduction in GSH content and activity of CAT, SOD, and GPx that were reversed by EA (30 mg/kg, PO) administration (88). Moreover, SA treatment in rats significantly diminished white blood cells, red blood cells, hemoglobin, hematocrit, and platelets and increased mean corpuscular volume and mean corpuscular hemoglobin; these all were attenuated with EA therapy (88).
Pretreatment of the normal mammalian V79 cell lines with EA (75 µM) before As exposure significantly diminished the generation of ROS and DNA fragmentation (89). In rats, SA noticeably reduced levels of serum testosterone, total antioxidant capacity, GSH level, and the activity of antioxidant enzymes (SOD, CAT, and GPx) in testicular tissue which increased with EA (30 mg/kg, PO) supplementation. Furthermore, they showed SA enhanced the levels of MDA, IL-1β, TNF-α, and NO that were suppressed by EA (90).
Overall, EA due to antioxidant and anti-inflammatory properties protected the body against As toxicity (Table 4).
Arsenic trioxide
Arsenic trioxide (As2O3) is an effective drug in treating acute promyelocytic leukemia due to its substantial anticancer effect (91). As2O3 could cause cardiotoxicity, QTc prolongation, torsades de pointes, endothelial dysfunction, and sudden death (91). As2O3 treated SH-SY5Y human neuroblastoma cell lines significantly indicated a decrease in cell viability via increasing MMP and releasing cytochrome c and inducing DNA damages via generation of ROS which was reversed with EA (20 µM) pretreatment (92). EA (30 mg/kg, PO) protected against As2O3 (5 mg/kg, daily, for 10 days, IP) cardiac toxicity. In this study, EA decreased MDA content and increased GSH and GPx activity, and diminished QTc interval prolongation (93) (Table 4).
Iron
Iron is an important nutrient that is crucial for various functions such as oxygen transport, cell respiration, and DNA synthesis; however, extra iron accumulation in the liver leads to the generation of oxidative stress and hepatotoxicity (94). Iron overload in mice increased liver iron content, ROS, and levels of ALP, ALT, AST, and LDH. It decreased antioxidant enzyme activity (SOD, GST, and CAT), and induced liver damage, fibrosis, and hepatotoxicity (95). EA (8 mg/kg, PO) supplementation prevented iron overload toxicity and reversed these changes in the mice’s liver tissue. Furthermore, EA protected the liver of mice against iron overload-induced apoptosis by a decrease in the Bax/Bcl-2 ratio and inhibiting caspase-3 activation (95) (Table 4).
Protective effect of ellagic acid against synthetic toxicants
6-Hydroxydopamine
6-Hydroxydopamine (6-OHDA) is a neurotoxic agent and plays an important role in preclinical research to induce Parkinson’s disease model. 6-OHDA is uptaken by dopamine or noradrenaline membrane transporters due to its structural similarity with endogenous catecholamines (96). 6-OHDA causes neuronal damage by the generation of oxidative stress and ROS formation (96). EA (50 mg/kg, PO) supplementation reduced 6-OHDA brain toxicity. It decreased MDA, ROS, and DNA fragmentation. Also, this study demonstrated the protective effect of EA against 6-OHDA via activation of the ERβ/Nrf2/HO-1 cascade (97). Moreover, Farbood Y et al. study showed that injection of 6-OHDA into the right medial forebrain bundle in rats increased levels of TNF-α and IL-1β, which ameliorated with EA (50 mg/kg, PO) treatment (98) (Table 5).
Table 5.
Summarized data of EA protection against synthetic toxicants
| Toxic agent | Dose/Concentration period and route of exposure | Dose/Concentration of EA treatment period and route of administration | In vitro / In vivo | Results | Reference |
|---|---|---|---|---|---|
| 6-OHDA | 5 μl microinjection into the left striatum | 50 mg/kg, daily for 1 week, p.o | Male Wistar rats | Decreased MDA and ROS formation, and DNA fragmentation activated ERβ/Nrf2/HO-1 cascade |
(97) |
| 16 μg/2 μl injection into the right MFB | 50 mg/kg, daily for 10 days, p.o | Male Wistar rats | Decreased the levels of TNF-α and IL-1β in MFB. | (98) | |
| Acrylamide | 20 mg/kg, daily for 30 days, p.o | 30 mg/kg, daily for 30 days, p.o | Male Wistar rats | Diminished MDA, NO, Il-β, and TNF-α, and enhanced GSH, SOD, GPx, and CAT in brain tissue. | (100) |
| CCl 4 | 1.5 ml/kg, twice a week for 4 weeks, IP | 10 mg/kg, 5 times a week for 8 weeks, IP | Male Wistar albino rats | Decreased Bcl-2 and NF-kB expression and the MDA level. Up-regulated caspase-3 and Nrf-2 |
(102) |
| 2 ml/kg, 3 times a week for 6 weeks, IP | 15 mg/kg, daily for 5 weeks, p.o | C57BL/6 male mice | Decreased ROS formation and angiogenesis by VEGF and VEGFR2, and the caspase‑3 activity. Decreased serum levels of ALT, AST, and albumin. |
(103) | |
| Dichloroacetate | 32 mM, incubated for 144 hr | 20 mM, incubated for 144 hr | Zebrafish | Suppressed the developmental abnormalities and significantly reduced levels of NO in zebrafish embryos. | (105) |
| TCDD | 46 ng/kg, daily for 90 days, p.o | 1 mg/kg, daily for 90 days, p.o | Female Sprague-Dawley rats | Diminished LPO and DNA SSBs in the brain. | (107) |
| 46 ng/kg, daily for 90 days, p.o | 1 mg/kg, daily for 90 days, p.o | Female Sprague-Dawley rats | Significantly increased SOD, CAT, and GPx activities, as well as GSH levels. | (108) | |
| 15 µg/kg for 21 days, p.o | 10 mg/kg daily for 21 days, p.o | Male albino Wistar rats | Attenuated antioxidant enzyme activities (SOD, GSH, GPx, and CAT), and CYP1A1 and ATPase enzyme activities. | (109) | |
| 100 ng/kg daily for 8 weeks, p.o | 2 mg/kg daily for 8 weeks, p.o | Male Sprague-Dawley rats | Enhanced sperm motility and concentration, SOD and CAT activity, and GSH content level, and increased MDA and LPO levels. | (110) |
6-OHDA: 6-hydroxydopamine; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; Bcl-2: B-cell lymphoma 2; CAT: catalase; CCl4: Carbon tetrachloride; ERβ: Estrogen receptor beta; GSH: Glutathione; GST: glutathione-S-transferase; GPx: glutathione peroxidase; IL-1β; interleukin-1β; IP: intraperitoneal; LPO: lipid peroxidation; LDH: lactate dehydrogenase; MDA: malondialdehyde; MFB: mid frontal brain; NF-κB: nuclear factor kappa-B; NO; nitric oxide; Nrf2: nuclear erythroid 2-related factor 2; p.o: oral; ROS: reactive oxygen species; SOD: superoxide dismutase; SR-B1: Scavenger receptor class B type 1; SSBs: single-strand breaks; TGF-β1: Transforming growth factor-beta; TNF-α: tumor necrosis factor-alpha; TCDD: 2,3,7,8-tetrachlorodibenzo-p-dioxin; TBARS: thiobarbituric acid reactive substances; VEGF: vascular endothelial growth factor; VEGFR2: vascular endothelial growth factor receptor 2
Acrylamide
Acrylamide is considered a neurotoxic agent (99). Treating rats with acrylamide increased the generation of MDA, NO, Il-β, and TNF-α and decreased GSH, SOD, GPx, and CAT in the brain tissue; co-treatment with EA (30 mg/kg, PO) prevented these changes (100). Also, acrylamide exposure causes neuron and glial cell necrosis in the brain cortex and EA provided a protective effect against them (Table 5).
Carbon tetrachloride
Carbon tetrachloride (CCl4), a known hepatotoxic agent, has been used as an effective solvent and cleaning agent in industrial manufacturers (101). Exposure to CCl4 leads to liver injury and increases levels of GDH, ALP, ALT, and AST (101). EA (10 mg/kg, IP) protected the rat liver against CCl4 toxicity (102). CCl4 exposure induces cirrhosis through the activation of ROS formation and angiogenesis by expressing vascular endothelial growth factor and vascular endothelial growth factor receptor 2, and increasing caspase-3 activity. These aforementioned effects were noticeably reversed by EA administration (103). EA (15 mg/kg, PO) supplementation also effectively diminished the plasma levels of ALT, AST, and albumin, and significantly suppressed the gene expression of iNOS and collagen I in cirrhosis mice (103) (Table 5).
Dichloroacetate
Dichloroacetate (DCA) is mainly formed during the chlorination process of drinking water. It has been shown that DCA has toxic effects on various organs and can induce liver cancer (104). DCA caused developmental abnormalities and significantly enhanced levels of superoxide anion and NO in zebrafish embryos. EA (20 mM) protected against these changes (105) (Table 5).
2,3,7,8-Tetrachlorodibenzo-p-dioxin
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) formed as an unwanted product in waste burning or as a side product in organic synthesis, is one of the most toxic and representative compounds of dioxins and its toxicity is mediated by the aryl hydrocarbon receptor (106). TCDD administration to rats meaningfully produced superoxide anion, LPO, and DNA single-strand breaks in the brain, and EA treatment (1 mg/kg, PO) reversed these changes (107). In the rat brain, EA (1 mg/kg, PO) significantly increased SOD and CAT activities (108). EA (100 mg/kg, PO) supplementation also protected against TCDD-induced nephrotoxicity in rats by improving antioxidant enzymes activity (SOD, GSH, GPx, and CAT), CYP1A1, and ATPase enzymes activity (Na+/K+-ATPase, and Mg2+-ATPase) with a significant decrease in Ca2+-ATPases activity (109). The membrane-bound enzymes such as Na+/K+-ATPase, Mg2+-ATPase, and Ca2+-ATPase are responsible for the transport of sodium/potassium, magnesium and calcium ions by inhibition of which TCDD causes nephrotoxicity (109). TCDD (100 ng/kg daily for 8 weeks, PO) induced testicular toxicity, decreased sperm concentration and motility, and the testis levels of SOD, CAT, and GSH. Also, it increased testicular MDA and LPO levels. EA (2 mg/kg daily for 8 weeks, PO) administration reversed all these defects (110). Moreover, EA supplementation protected testicular tissue against TCDD-induced histopathological changes such as degeneration, desquamation, disorganization, reduction in germinal cells, interstitial edema, and congestion (110) (Table 5).
Metabolism, safety, and dose translation to humans
Unlike food containing EA, free EA is mainly absorbed in the stomach and can be detected in the plasma 2 hours after intake. Urolithin derivatives are the main EA-derived metabolites that are produced by human intestinal microbiota, and after metabolizing phase 1 and 2, urolithin A and B conjugates are the main detectable forms in plasma, urine, and some tissues in humans. Enterohepatic circulation contributes to their long-time persistence in plasma and urine (up to 48-72 hr) (3, 111). However, individual variability in response to dietary EA due to different types of metabotypes associated with different types of gut microbiota should be taken into account (112).
No adverse effect have been reported in humans with EA when this compound was consumed as a nutrition supplement or as a part of the diet (3). However, based on animal studies, a high portion of ellagitannins, as a complex form of EA, induces antinutritional effects because they inhibit β-galactosidase and bind with some dietary proteins, fibers, and minerals that may result in malabsorption (113). The toxicological effects of this compound have not been thoroughly investigated. The LD50 (lethal dose for 50% of the population) of EA in the rat after IP administration has been reported to be 630 mg/kg. In a 90-day sub-chronic study, EA caused no observable changes in male rats, while a slight reduction in body weight of female rats was observed. Based on this study, the no-observed-effect level (NOEL) was estimated to be 3011 mg/kg b.w./day for males and less than 778 mg/kg b.w./day for female rats. Meanwhile, the no-observed-adverse-effect level (NOAEL) was estimated at 3254 mg/kg b.w./day for female rats (114).
Dose translation from animals to humans is based on an indirect formulation which is normalized by body surface area (BSA): Human equivalent dose (mg/kg) = Animal Dose (mg/kg)×(animal Km/human Km), where Km value results from body weight (kg) divided by BSA (m2). Therefore, 50 mg/kg in mice whose weight and BSA are respectively 0.02 kg and 0.007 m2 means about 4 mg/kg for a 60 kg adult human with 1.6 m2 BSA (115).
In this regard, for starting a clinical trial in healthy adult volunteers based on animal studies, the human equivalent dose is calculated using the BSA normalization of the safe animal dose (115).
Conclusion
Taken together, several available studies on EA indicate the protective effects of this natural product against the adverse effects of many toxins or toxic agents.
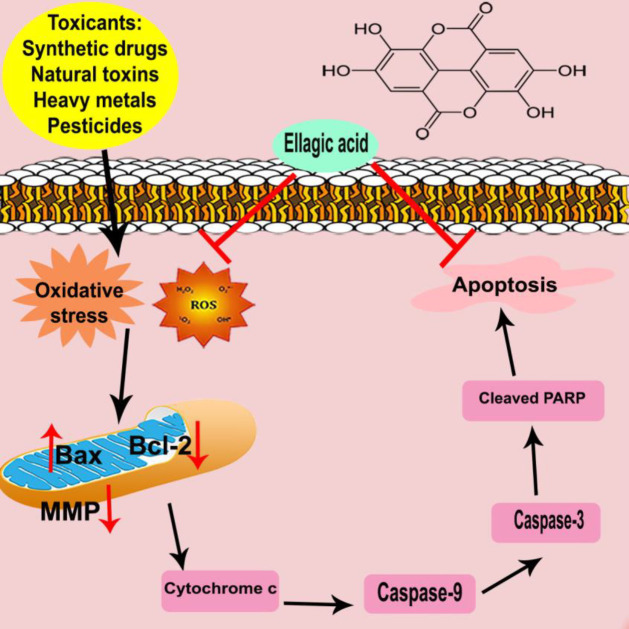
In this review, we summarized findings implicate the antidotal/protective effects of EA against some types of chemical drugs, natural toxins, pesticides, heavy metals, and synthetic compounds in vivo and in vitro. Based on the gathered data, the ameliorative effects of EA are via several mechanisms including (i) activation of the antioxidant response through the Nrf2/HO-1 signaling pathway; (ii) inhibition of pro-inflammatory agents, such as iNOS, COX-2, and cytokines by inhibiting nuclear factor-kappa B (NF-ĸB); (iii) alteration of several growth factors expression, as transforming growth factor-beta (TGF-α), vascular endothelial growth factor, and vascular endothelial growth factor receptor; (iv) modulation of several cell survival and cell-cycle genes such Bcl-2, Bax, caspase-3 and tumor suppressors (p53); and (v) regulation of kinases, like phosphoinositide 3-kinase (PI3-K) and GSK-3β. Also, it protects against DNA damage and provides an extracellular complex with some toxins, and may reduce their interaction with cells (Figure 3).
Figure 3.
Schematic diagram of ellagic acid anti-apoptotic effects against chemical and natural toxins
Bax: Bcl-2-associated X protein; Bcl-2: B-cell lymphoma 2; MMP: mitochondrial membrane potential; ROS: reactive oxygen species
Altogether, it needs more work, in particular, randomized clinical trials, to confirm the effects and also consider any probable unwanted effects. To the best of our knowledge, no clinical trial has been done in this area and further research should be done to reveal more about these mechanisms in humans due to linking the bench to the bed, the ultimate goal of researchers.
Authors’ Contributions
KN and MR wrote the manuscript; HH designed the study; All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Acknowledgment
The authors declare that there was no financial support.
References
- 1.Garcia-Nino WR, Zazueta C. Ellagic acid: Pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol Res. 2015;97:84–103. doi: 10.1016/j.phrs.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Evtyugin DD, Magina S, Evtuguin DV. Recent advances in the production and applications of ellagic acid and its derivatives. A review. Molecules. 2020;25:2745–2764. doi: 10.3390/molecules25122745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muthukumaran S, Tranchant C, Shi J, Ye X, Xue SJ. Ellagic acid in strawberry (Fragaria spp ): Biological, technological, stability, and human health aspects. Food Qual Saf. 2017;1:227–252. [Google Scholar]
- 4.Rios JL, Giner RM, Marin M, Recio MC. A pharmacological update of ellagic acid. Planta Med. 2018;84:1068–1093. doi: 10.1055/a-0633-9492. [DOI] [PubMed] [Google Scholar]
- 5.Ceci C, Lacal PM, Tentori L, De Martino MG, Miano R, Graziani G. Experimental evidence of the antitumor, antimetastatic and antiangiogenic activity of ellagic acid. Nutrients. 2018;10:1756–1778. doi: 10.3390/nu10111756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansouri Z, Dianat M, Radan M, Badavi M. Ellagic acid ameliorates lung inflammation and heart oxidative stress in elastase-induced emphysema model in rat. Inflammation. 2020;43:1143–1156. doi: 10.1007/s10753-020-01201-4. [DOI] [PubMed] [Google Scholar]
- 7.Javaid N, Shah MA, Rasul A, Chauhdary Z, Saleem U, Khan H, et al. Neuroprotective effects of ellagic acid in alzheimer’s disease: Focus on underlying molecular mechanisms of therapeutic potential. Curr Pharm Des. 2021;27:3591–3601. doi: 10.2174/1381612826666201112144006. [DOI] [PubMed] [Google Scholar]
- 8.Aishwarya V, Solaipriya S, Sivaramakrishnan V. Role of ellagic acid for the prevention and treatment of liver diseases. Phytother Res. 2021;35:2925–2944. doi: 10.1002/ptr.7001. [DOI] [PubMed] [Google Scholar]
- 9.Yuce A, Atessahin A, Ceribasi AO, Aksakal M. Ellagic acid prevents cisplatin-induced oxidative stress in liver and heart tissue of rats. Basic Clin Pharmacol Toxicol. 2007;101:345–349. doi: 10.1111/j.1742-7843.2007.00129.x. [DOI] [PubMed] [Google Scholar]
- 10.Shakeri A, Zirak MR, Sahebkar A. Ellagic acid: A logical lead for drug development? Curr Pharm Des. 2018;24:106–122. doi: 10.2174/1381612823666171115094557. [DOI] [PubMed] [Google Scholar]
- 11.Zeb A. Ellagic acid in suppressing in vivo and in vitro oxidative stresses. Mol Cell Biochem. 2018;448:27–41. doi: 10.1007/s11010-018-3310-3. [DOI] [PubMed] [Google Scholar]
- 12.El-Shitany NA, El-Bastawissy EA, El-desoky K. Ellagic acid protects against carrageenan-induced acute inflammation through inhibition of nuclear factor kappa B, inducible cyclooxygenase and proinflammatory cytokines and enhancement of interleukin-10 via an antioxidant mechanism. Int Immunopharmacol. 2014;19:290–299. doi: 10.1016/j.intimp.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 14.Alfei S, Marengo B, Zuccari G. Oxidative stress, antioxidant capabilities, and bioavailability: Elagic acid or urolithins? Antioxidants (Basel) 2020;9:707–737. doi: 10.3390/antiox9080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell C, Hawthorne S. Ellagic acid, pomegranate and prostate cancer -a mini review. J Pharm Pharmacol. 2008;60:139–144. doi: 10.1211/jpp.60.2.0001. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed AR, Hombal SM. Cyclophosphamide (Cytoxan) A review on relevant pharmacology and clinical uses. J Am Acad Dermatol. 1984;11:1115–1126. doi: 10.1016/s0190-9622(84)80193-0. [DOI] [PubMed] [Google Scholar]
- 17.Ghobadi E, Moloudizargari M, Asghari MH, Abdollahi M. The mechanisms of cyclophosphamide-induced testicular toxicity and the protective agents. Expert Opin Drug Metab Toxicol. 2017;13:525–536. doi: 10.1080/17425255.2017.1277205. [DOI] [PubMed] [Google Scholar]
- 18.Stojiljkovic N, Ilic S, Stojanovic N, Jankovic-Velickovic L, Stojnev S, Kocic G, et al. Nanoliposome-encapsulated ellagic acid prevents cyclophosphamide-induced rat liver damage. Mol Cell Biochem. 2019;458:185–195. doi: 10.1007/s11010-019-03541-8. [DOI] [PubMed] [Google Scholar]
- 19.Turk G, Ceribasi AO, Sakin F, Sonmez M, Atessahin A. Antiperoxidative and anti-apoptotic effects of lycopene and ellagic acid on cyclophosphamide-induced testicular lipid peroxidation and apoptosis. Reprod Fertil Dev. 2010;22:587–596. doi: 10.1071/RD09078. [DOI] [PubMed] [Google Scholar]
- 20.Ceribasi AO, Turk G, Sonmez M, Sakin F, Atessahin A. Toxic effect of cyclophosphamide on sperm morphology, testicular histology and blood oxidant-antioxidant balance, and protective roles of lycopene and ellagic acid. Basic Clin Pharmacol Toxicol. 2010;107:730–736. doi: 10.1111/j.1742-7843.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- 21.Rehman MU, Tahir M, Ali F, Qamar W, Lateef A, Khan R, et al. Cyclophosphamide-induced nephrotoxicity, genotoxicity, and damage in kidney genomic DNA of Swiss Albino mice: The protective effect of ellagic acid. Mol Cell Biochem. 2012;365:119–127. doi: 10.1007/s11010-012-1250-x. [DOI] [PubMed] [Google Scholar]
- 22.Saba , Khan S, Parvez S, Chaudhari B, Ahmad F, Anjum S, et al. Ellagic acid attenuates bleomycin and cyclophosphamide-induced pulmonary toxicity in Wistar rats. Food Chem Toxicol. 2013;58:210–219. doi: 10.1016/j.fct.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 23.Wu Q, Wang X, Nepovimova E, Wang Y, Yang H, Kuca K. Mechanism of cyclosporine A nephrotoxicity: Oxidative stress, autophagy, and signalings. Food Chem Toxicol. 2018;118:889–907. doi: 10.1016/j.fct.2018.06.054. [DOI] [PubMed] [Google Scholar]
- 24.Turk G, Sonmez M, Ceribasi AO, Yuce A, Atessahin A. Attenuation of cyclosporine A-induced testicular and spermatozoal damages associated with oxidative stress by ellagic acid. Int Immunopharmacol. 2010;10:177–182. doi: 10.1016/j.intimp.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Yuce A, Atessahin A, Ceribasi AO. Amelioration of cyclosporine A-induced renal, hepatic and cardiac damages by ellagic acid in rats. Basic Clin Pharmacol Toxicol. 2008;103:186–191. doi: 10.1111/j.1742-7843.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- 26.Abdul-Hamid M, Abdella EM, Galaly SR, Ahmed RH. Protective effect of ellagic acid against cyclosporine A-induced histopathological, ultrastructural changes, oxidative stress, and cytogenotoxicity in albino rats. Ultrastruct Pathol. 2016;40:205–221. doi: 10.1080/01913123.2016.1203854. [DOI] [PubMed] [Google Scholar]
- 27.Pari L, Sivasankari R. Effect of ellagic acid on cyclosporine A-induced oxidative damage in the liver of rats. Fundam Clin Pharmacol. 2008;22:395–401. doi: 10.1111/j.1472-8206.2008.00609.x. [DOI] [PubMed] [Google Scholar]
- 28.Pugazhendhi A, Edison T, Velmurugan BK, Jacob JA, Karuppusamy I. Toxicity of doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018;200:26–30. doi: 10.1016/j.lfs.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 29.Georgy GS, Maher OW. Ellagic acid and rosmarinic acid attenuate doxorubicin-induced testicular injury in rats. J Biochem Mol Toxicol. 2017;31:e21937. doi: 10.1002/jbt.21937. [DOI] [PubMed] [Google Scholar]
- 30.Lin MC, Yin MC. Preventive effects of ellagic acid against doxorubicin-induced cardio-toxicity in mice. Cardiovasc Toxicol. 2013;13:185–193. doi: 10.1007/s12012-013-9197-z. [DOI] [PubMed] [Google Scholar]
- 31.Rizk HA, Masoud MA, Maher OW. Prophylactic effects of ellagic acid and rosmarinic acid on doxorubicin-induced neurotoxicity in rats. J Biochem Mol Toxicol. 2017;31:e21977. doi: 10.1002/jbt.21977. [DOI] [PubMed] [Google Scholar]
- 32.Chen C, Chen Y, Wu P, Chen B. Update on new medicinal applications of gentamicin: evidence-based review. J Formos Med Assoc. 2014;113:72–82. doi: 10.1016/j.jfma.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Sepand MR, Ghahremani MH, Razavi-Azarkhiavi K, Aghsami M, Rajabi J, Keshavarz-Bahaghighat H, et al. Ellagic acid confers protection against gentamicin-induced oxidative damage, mitochondrial dysfunction and apoptosis-related nephrotoxicity. J Pharm Pharmacol. 2016;68:1222–1232. doi: 10.1111/jphp.12589. [DOI] [PubMed] [Google Scholar]
- 34.Mehdizadeh R, Parizadeh MR, Khooei AR, Mehri S, Hosseinzadeh H. Cardioprotective effect of saffron extract and safranal in isoproterenol-induced myocardial infarction in Wistar rats. Iran J Basic Med Sci. 2013;16:56–63. [PMC free article] [PubMed] [Google Scholar]
- 35.Anandan R, Mathew S, Sankar TV, Viswanathan Nair PG. Protective effect of n-3 polyunsaturated fatty acids concentrate on isoproterenol-induced myocardial infarction in rats. Prostaglandins Leukot Essent Fatty Acids. 2007;76:153–158. doi: 10.1016/j.plefa.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Kannan MM, Quine SD. Ellagic acid ameliorates isoproterenol induced oxidative stress: evidence from electrocardiological, biochemical and histological study. Eur J Pharmacol. 2011;659:45–52. doi: 10.1016/j.ejphar.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 37.Howard SC, McCormick J, Pui CH, Buddington RK, Harvey RD. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471–1482. doi: 10.1634/theoncologist.2015-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebrahimi R, Sepand MR, Seyednejad SA, Omidi A, Akbariani M, Gholami M, et al. Ellagic acid reduces methotrexate-induced apoptosis and mitochondrial dysfunction via up-regulating Nrf2 expression and inhibiting the IkBalpha/NFkB in rats. Daru. 2019;27:721–733. doi: 10.1007/s40199-019-00309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Boghdady NA. Protective effect of ellagic acid and pumpkin seed oil against methotrexate-induced small intestine damage in rats. Indian J Biochem Biophys. 2011;48:380–387. [PubMed] [Google Scholar]
- 40.Mehrzadi S, Mehrabani M, Malayeri AR, Bakhshayesh M, Kalantari H, Goudarzi M. Ellagic acid as a potential antioxidant, alleviates methotrexate-induced hepatotoxicity in male rats. Acta Chir Belg. 2019;119:69–77. doi: 10.1080/00015458.2018.1455419. [DOI] [PubMed] [Google Scholar]
- 41.McCrae JC, Morrison EE, MacIntyre IM, Dear JW, Webb DJ. Long-term adverse effects of paracetamol: A review. Br J Clin Pharmacol. 2018;84:2218–2230. doi: 10.1111/bcp.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girish C, Koner BC, Jayanthi S, Ramachandra Rao K, Rajesh B, Pradhan SC. Hepatoprotective activity of picroliv, curcumin and ellagic acid compared to silymarin on paracetamol induced liver toxicity in mice. Fundam Clin Pharmacol. 2009;23:735–745. doi: 10.1111/j.1472-8206.2009.00722.x. [DOI] [PubMed] [Google Scholar]
- 43.Macfarlane A, Greenhalgh T. Sodium valproate in pregnancy: What are the risks and should we use a shared decision-making approach? BMC Pregnancy Childbirth. 2018;18:200–210. doi: 10.1186/s12884-018-1842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girish C, Shweta O, Raj V, Balakrishnan S, Varghese RG. Ellagic acid modulates sodium valproate induced reproductive toxicity in male Wistar rats. Indian J Physiol Pharmacol. 2014;58:416–422. [PubMed] [Google Scholar]
- 45.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–546. [PubMed] [Google Scholar]
- 46.Kumar M, Bansal N. Ellagic acid prevents dementia through modulation of PI3-kinase-endothelial nitric oxide synthase signalling in streptozotocin-treated rats. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:987–1001. doi: 10.1007/s00210-018-1524-2. [DOI] [PubMed] [Google Scholar]
- 47.Uzar E, Alp H, Cevik MU, Firat U, Evliyaoglu O, Tufek A, et al. Ellagic acid attenuates oxidative stress on brain and sciatic nerve and improves histopathology of brain in streptozotocin-induced diabetic rats. Neurol Sci. 2012;33:567–574. doi: 10.1007/s10072-011-0775-1. [DOI] [PubMed] [Google Scholar]
- 48.Hamid AS, Tesfamariam IG, Zhang Y, Zhang ZG. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: geographical distribution, mechanism of action and prevention. Oncol Lett. 2013;5:1087–1092. doi: 10.3892/ol.2013.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rushing BR, Selim MI. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem Toxicol. 2019;124:81–100. doi: 10.1016/j.fct.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 50.Kumar A, Tyagi YK, Seema , Ponnan P, Rohil V, Prasad AK, et al. Ellagic acid peracetate is superior to ellagic acid in the prevention of genotoxicity due to aflatoxin B1 in bone marrow and lung cells. J Pharm Pharmacol. 2007;59:81–86. doi: 10.1211/jpp.59.1.0011. [DOI] [PubMed] [Google Scholar]
- 51.Loarca-Pina G, Kuzmicky PA, de Mejia EG, Kado NY. Inhibitory effects of ellagic acid on the direct-acting mutagenicity of aflatoxin B1 in the Salmonella microsuspension assay. Mutat Res. 1998;398:183–187. doi: 10.1016/s0027-5107(97)00245-5. [DOI] [PubMed] [Google Scholar]
- 52.Li WW, Yu JY, Xu HL, Bao JK. Concanavalin A: A potential anti-neoplastic agent targeting apoptosis, autophagy and anti-angiogenesis for cancer therapeutics. Biochem Biophys Res Commun. 2011;414:282–286. doi: 10.1016/j.bbrc.2011.09.072. [DOI] [PubMed] [Google Scholar]
- 53.Lee JH, Won JH, Choi JM, Cha HH, Jang YJ, Park S, et al. Protective effect of ellagic acid on concanavalin A-induced hepatitis via toll-like receptor and mitogen-activated protein kinase/nuclear factor kappaB signaling pathways. J Agric Food Chem. 2014;62:10110–10117. doi: 10.1021/jf503188c. [DOI] [PubMed] [Google Scholar]
- 54.Cynober L. Metabolism of dietary glutamate in adults. Ann Nutr Metab. 2018;73 Suppl 5:5–14. doi: 10.1159/000494776. [DOI] [PubMed] [Google Scholar]
- 55.Niaz K, Zaplatic E, Spoor J. Extensive use of monosodium glutamate: A threat to public health? EXCLI J. 2018;17:273–278. doi: 10.17179/excli2018-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernstrom JD. Monosodium glutamate in the diet does not raise brain glutamate concentrations or disrupt brain functions. Ann Nutr Metab. 2018;73:43–52. doi: 10.1159/000494782. [DOI] [PubMed] [Google Scholar]
- 57.Hamza RZ, Al-Baqami NM. Testicular protective effects of ellagic acid on monosodium glutamate-induced testicular structural alterations in male rats. Ultrastruct Pathol. 2019;43:170–183. doi: 10.1080/01913123.2019.1671569. [DOI] [PubMed] [Google Scholar]
- 58.Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45:e66. doi: 10.1038/emm.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Punder K, Pruimboom L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front Immunol. 2015;6:223–223. doi: 10.3389/fimmu.2015.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu L, Deng WS, Liu Y, Jiang CH, Sun LC, Sun XF, et al. Ellagic acid protects lipopolysaccharide/D-galactosamine-induced acute hepatic injury in mice. Int Immunopharmacol. 2014;22:341–345. doi: 10.1016/j.intimp.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 61.LaHood AJ, Kok SJ. StatPearls Publishing Copyright © 2021. StatPearls Publishing LLC; 2021. Ethanol Toxicity. StatPearls. Treasure Island (FL) [PubMed] [Google Scholar]
- 62.Heier C, Xie H, Zimmermann R. Nonoxidative ethanol metabolism in humans-from biomarkers to bioactive lipids. IUBMB Life. 2016;68:916–923. doi: 10.1002/iub.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandal P, Pritchard MT, Nagy LE. Anti-inflammatory pathways and alcoholic liver disease: role of an adiponectin/interleukin-10/heme oxygenase-1 pathway. World J Gastroenterol. 2010;16:1330–1336. doi: 10.3748/wjg.v16.i11.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rezaee-Khorasany A, Razavi BM, Taghiabadi E, Tabatabaei Yazdi A, Hosseinzadeh H. Effect of crocin, an active saffron constituent, on ethanol toxicity in the rat: Histopathological and biochemical studies. Iran J Basic Med Sci. 2020;23:51–62. doi: 10.22038/IJBMS.2019.37133.8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Devipriya N, Srinivasan M, Sudheer AR, Menon VP. Effect of ellagic acid, a natural polyphenol, on alcohol-induced prooxidant and antioxidant imbalance: a drug dose dependent study. Singapore Med J. 2007;48:311–318. [PubMed] [Google Scholar]
- 66.Sohn E-H, Koo HJ, Jang S-A, Namkoong S, Lim JD, Kang SCJM, et al. Protective effects of ellagic acid on ethanol-induced toxicity in hepatic HepG2 cells. Mol Cell Toxicol. 2013;9:249–256. [Google Scholar]
- 67.Siqueira LM, Committee On Substance USE, Prevention Nicotine and tobacco as substances of abuse in children and adolescents. Pediatrics. 2017;139:e20163436. doi: 10.1542/peds.2016-3436. [DOI] [PubMed] [Google Scholar]
- 68.Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20:115–126. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- 69.Akkoyun HT, Karadeniz A. Investigation of the protective effect of ellagic acid for preventing kidney injury in rats exposed to nicotine during the fetal period. Biotech Histochem. 2016;91:108–115. doi: 10.3109/10520295.2015.1078910. [DOI] [PubMed] [Google Scholar]
- 70.Mohammadzadeh L, Abnous K, Razavi BM, Hosseinzadeh H. Crocin-protected malathion-induced spatial memory deficits by inhibiting TAU protein hyperphosphorylation and antiapoptotic effects. Nutr Neurosci. 2020;23:221–236. doi: 10.1080/1028415X.2018.1492772. [DOI] [PubMed] [Google Scholar]
- 71.Badr AM. Organophosphate toxicity: updates of malathion potential toxic effects in mammals and potential treatments. Environ Sci Pollut Res Int. 2020;27:26036–26057. doi: 10.1007/s11356-020-08937-4. [DOI] [PubMed] [Google Scholar]
- 72.Ural MS, Yonar ME, Mise Yonar S. Protective effect of ellagic acid on oxidative stress and antioxidant status in Cyprinus carpio during malathion exposure. Cell Mol Biol (Noisy-le-grand) 2015;61:58–63. [PubMed] [Google Scholar]
- 73.Dinis-Oliveira RJ, Duarte JA, Sanchez-Navarro A, Remiao F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38:13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 74.Silfeler I, Alp H, Dorum BA, Nacar E, Arslan S, Uygur V. Protective effect of ellagic acid on paraquat-induced kidney hazards in rats. Iran J Kidney Dis. 2017;11:23–28. [PubMed] [Google Scholar]
- 75.Kim YS, Zerin T, Song HY. Antioxidant action of ellagic acid ameliorates paraquat-induced A549 cytotoxicity. Biol Pharm Bull. 2013;36:609–615. doi: 10.1248/bpb.b12-00990. [DOI] [PubMed] [Google Scholar]
- 76.Amniattalab A, Razi M. Effect of phosalone on testicular tissue and in vitro fertilizing potential. Int J Fertil Steril. 2015;9:93–106. doi: 10.22074/ijfs.2015.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baeeri M, Momtaz S, Navaei-Nigjeh M, Niaz K, Rahimifard M, Ghasemi-Niri SF, et al. Molecular evidence on the protective effect of ellagic acid on phosalone-induced senescence in rat embryonic fibroblast cells. Food Chem Toxicol. 2017;100:8–23. doi: 10.1016/j.fct.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 78.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 79.Xiong N, Long X, Xiong J, Jia M, Chen C, Huang J, et al. Mitochondrial complex I inhibitor rotenone-induced toxicity and its potential mechanisms in Parkinson’s disease models. Crit Rev Toxicol. 2012;42:613–632. doi: 10.3109/10408444.2012.680431. [DOI] [PubMed] [Google Scholar]
- 80.Wei YZ, Zhu GF, Zheng CQ, Li JJ, Sheng S, Li DD, et al. Ellagic acid protects dopamine neurons from rotenone-induced neurotoxicity via activation of Nrf2 signalling. J Cell Mol Med. 2020;24:9446–9456. doi: 10.1111/jcmm.15616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumar V, Gill KD. Aluminium neurotoxicity: neurobehavioural and oxidative aspects. Arch Toxicol. 2009;83:965–978. doi: 10.1007/s00204-009-0455-6. [DOI] [PubMed] [Google Scholar]
- 82.Salem AM, Mohammaden TF, Ali MAM, Mohamed EA, Hasan HF. Ellagic and ferulic acids alleviate gamma radiation and aluminium chloride-induced oxidative damage. Life Sci. 2016;160:2–11. doi: 10.1016/j.lfs.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 83.Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, et al. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 84.Arnold LL, Eldan M, van Gemert M, Capen CC, Cohen SM. Chronic studies evaluating the carcinogenicity of monomethylarsonic acid in rats and mice. Toxicology. 2003;190:197–219. doi: 10.1016/s0300-483x(03)00165-3. [DOI] [PubMed] [Google Scholar]
- 85.Barchowsky A, Dudek EJ, Treadwell MD, Wetterhahn KE. Arsenic induces oxidant stress and NF-kappa B activation in cultured aortic endothelial cells. Free Radic Biol Med. 1996;21:783–790. doi: 10.1016/0891-5849(96)00174-8. [DOI] [PubMed] [Google Scholar]
- 86.Firdaus F, Zafeer MF, Anis E, Ahmad M, Afzal M. Ellagic acid attenuates arsenic induced neuro-inflammation and mitochondrial dysfunction associated apoptosis. Toxicol Rep. 2018;5:411–417. doi: 10.1016/j.toxrep.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goudarzi M, Amiri S, Nesari A, Hosseinzadeh A, Mansouri E, Mehrzadi S. The possible neuroprotective effect of ellagic acid on sodium arsenate-induced neurotoxicity in rats. Life Sci. 2018;198:38–45. doi: 10.1016/j.lfs.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 88.Goudarzi M, Fatemi I, Siahpoosh A, Sezavar SH, Mansouri E, Mehrzadi S. Protective effect of ellagic acid against sodium arsenite-induced cardio- and hematotoxicity in rats. Cardiovasc Toxicol. 2018;18:337–345. doi: 10.1007/s12012-018-9446-2. [DOI] [PubMed] [Google Scholar]
- 89.Roy M, Sinha D, Mukherjee S, Paul S, Bhattacharya RK. Protective effect of dietary phytochemicals against arsenite induced genotoxicity in mammalian V79 cells. Indian J Exp Biol. 2008;46:690–697. [PubMed] [Google Scholar]
- 90.Mehrzadi S, Bahrami N, Mehrabani M, Motevalian M, Mansouri E, Goudarzi M. Ellagic acid: A promising protective remedy against testicular toxicity induced by arsenic. Biomed Pharmacother. 2018;103:1464–1472. doi: 10.1016/j.biopha.2018.04.194. [DOI] [PubMed] [Google Scholar]
- 91.Vineetha VP, Raghu KG. An overview on arsenic trioxide-induced cardiotoxicity. Cardiovasc Toxicol. 2019;19:105–119. doi: 10.1007/s12012-018-09504-7. [DOI] [PubMed] [Google Scholar]
- 92.Firdaus F, Zafeer MF, Waseem M, Anis E, Hossain MM, Afzal M. Ellagic acid mitigates arsenic-trioxide-induced mitochondrial dysfunction and cytotoxicity in SH-SY5Y cells. J Biochem Mol Toxicol. 2018;32:e22024. doi: 10.1002/jbt.22024. [DOI] [PubMed] [Google Scholar]
- 93.Hemmati AA, Olapour S, Varzi HN, Khodayar MJ, Dianat M, Mohammadian B, et al. Ellagic acid protects against arsenic trioxide-induced cardiotoxicity in rat. Hum Exp Toxicol. 2018;37:412–419. doi: 10.1177/0960327117701986. [DOI] [PubMed] [Google Scholar]
- 94.Recalcati S, Gammella E, Cairo G. Dysregulation of iron metabolism in cancer stem cells. Free Radic Biol Med. 2019;133:216–220. doi: 10.1016/j.freeradbiomed.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 95.Shendge AK, Basu T, Panja S, Chaudhuri D, Mandal N. An ellagic acid isolated from Clerodendrum viscosum leaves ameliorates iron-overload induced hepatotoxicity in Swiss albino mice through inhibition of oxidative stress and the apoptotic pathway. Biomed Pharmacother. 2018;106:454–465. doi: 10.1016/j.biopha.2018.06.133. [DOI] [PubMed] [Google Scholar]
- 96.Simola N, Morelli M, Carta AR. The 6-hydroxydopamine model of Parkinson’s disease. Neurotox Res. 2007;11:151–167. doi: 10.1007/BF03033565. [DOI] [PubMed] [Google Scholar]
- 97.Baluchnejadmojarad T, Rabiee N, Zabihnejad S, Roghani M. Ellagic acid exerts protective effect in intrastriatal 6-hydroxydopamine rat model of Parkinson’s disease: Possible involvement of ERbeta/Nrf2/HO-1 signaling. Brain Res. 2017;1662:23–30. doi: 10.1016/j.brainres.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 98.Farbood Y, Sarkaki A, Dolatshahi M, Taqhi Mansouri SM, Khodadadi A. Ellagic acid protects the brain against 6-Hydroxydopamine induced neuroinflammation in a rat model of Parkinson’s disease. Basic Clin Neurosci. 2015;6:83–89. [PMC free article] [PubMed] [Google Scholar]
- 99.Tabeshpour J, Mehri S, Abnous K, Hosseinzadeh H. Neuroprotective effects of thymoquinone in acrylamide-induced peripheral nervous system toxicity through MAPKinase and apoptosis pathways in rat. Neurochem Res. 2019;44:1101–1112. doi: 10.1007/s11064-019-02741-4. [DOI] [PubMed] [Google Scholar]
- 100.Goudarzi M, Mombeini MA, Fatemi I, Aminzadeh A, Kalantari H, Nesari A, et al. Neuroprotective effects of ellagic acid against acrylamide-induced neurotoxicity in rats. Neurol Res. 2019;41:419–428. doi: 10.1080/01616412.2019.1576319. [DOI] [PubMed] [Google Scholar]
- 101.Teschke R. Liver injury by carbon tetrachloride intoxication in 16 patients treated with forced ventilation to accelerate toxin removal via the lungs: a clinical report. Toxics. 2018;6:25–56. doi: 10.3390/toxics6020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aslan A, Gok O, Erman O, Kuloglu T. Ellagic acid impedes carbontetrachloride-induced liver damage in rats through suppression of NF-kB, Bcl-2 and regulating Nrf-2 and caspase pathway. Biomed Pharmacother. 2018;105:662–669. doi: 10.1016/j.biopha.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 103.Ding Y, Wang L, Song J, Zhou S. Protective effects of ellagic acid against tetrachloride-induced cirrhosis in mice through the inhibition of reactive oxygen species formation and angiogenesis. Exp Ther Med. 2017;14:3375–3380. doi: 10.3892/etm.2017.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stacpoole PW, Martyniuk CJ, James MO, Calcutt NA. Dichloroacetate-induced peripheral neuropathy. Int Rev Neurobiol. 2019;145:211–238. doi: 10.1016/bs.irn.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 105.Williams FE, Sickelbaugh TJ, Hassoun E. Modulation by ellagic acid of DCA-induced developmental toxicity in the zebrafish (Danio rerio) J Biochem Mol Toxicol. 2006;20:183–190. doi: 10.1002/jbt.20135. [DOI] [PubMed] [Google Scholar]
- 106.Pohjanvirta R, Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: effects, mechanisms, and animal models. Pharmacol Rev. 1994;46:483–549. [PubMed] [Google Scholar]
- 107.Hassoun EA, Vodhanel J, Abushaban A. The modulatory effects of ellagic acid and vitamin E succinate on TCDD-induced oxidative stress in different brain regions of rats after subchronic exposure. J Biochem Mol Toxicol. 2004;18:196–203. doi: 10.1002/jbt.20030. [DOI] [PubMed] [Google Scholar]
- 108.Hassoun EA, Vodhanel J, Holden B, Abushaban A. The effects of ellagic acid and vitamin E succinate on antioxidant enzymes activities and glutathione levels in different brain regions of rats after subchronic exposure to TCDD. J Toxicol Environ Health A. 2006;69:381–393. doi: 10.1080/15287390500246431. [DOI] [PubMed] [Google Scholar]
- 109.Vijaya Padma V, Kalai Selvi P, Sravani S. Protective effect of ellagic acid against TCDD-induced renal oxidative stress: Modulation of CYP1A1 activity and antioxidant defense mechanisms. Mol Biol Rep. 2014;41:4223–4232. doi: 10.1007/s11033-014-3292-5. [DOI] [PubMed] [Google Scholar]
- 110.Sonmez M, Turk G, Ceribasi AO, Sakin F, Atessahin A. Attenuating effect of lycopene and ellagic acid on 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced spermiotoxicity and testicular apoptosis. Drug Chem Toxicol. 2011;34:347–356. doi: 10.3109/01480545.2011.557382. [DOI] [PubMed] [Google Scholar]
- 111.Tomás-Barberan FA, Espín JC, García-Conesa MT, Quideau S. Chemistry and Biology of Ellagitannins. 2009. Bioavailability and metabolism of ellagic acid and ellagitannins; pp. 273–297. [Google Scholar]
- 112.Cortes-Martin A, Selma MV, Tomas-Barberan FA, Gonzalez-Sarrias A, Espin JC. Where to look into the puzzle of polyphenols and health? The postbiotics and gut microbiota associated with human metabotypes. Mol Nutr Food Res. 2020;64:e1900952. doi: 10.1002/mnfr.201900952. [DOI] [PubMed] [Google Scholar]
- 113.Ismail T, Calcabrini C, Diaz AR, Fimognari C, Turrini E, Catanzaro E, et al. Ellagitannins in cancer chemoprevention and therapy. Toxins. 2016;8:151–172. doi: 10.3390/toxins8050151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tasaki M, Umemura T, Maeda M, Ishii Y, Okamura T, Inoue T, et al. Safety assessment of ellagic acid, a food additive, in a subchronic toxicity study using F344 rats. Food Chem Toxicol. 2008;46:1119–1124. doi: 10.1016/j.fct.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 115.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]