Abstract
Background
Checkpoint inhibitor-related pneumonitis (CIP) induced by immune checkpoint inhibitors (ICIs) is one of the most fatal immune-related adverse events (irAE). However, only limited data are available on rechallenge with ICIs after CIP. We evaluated the efficacy and safety of rechallenge after CIP in patients with advanced lung cancer to identify the potential populations that would benefit.
Methods
We conducted a multicenter retrospective study of advanced lung cancer patients who received further ICI treatment (rechallenge) or did not undergo re-administration after grade ≥1 CIP between May 2017 and May 2021. Progression-free survival (PFS) and overall survival (OS) were estimated from first or second ICI initiation to disease progression (PFS1 and PFS2, respectively), death, or last follow-up (OS1 and OS2, respectively). The recurrence of CIP and new irAEs in these patients after ICI rechallenge were calculated.
Results
Among 107 patients afflicted with CIP, 45 (42.1%) received ICI rechallenge. Multivariate analysis showed that severe grade (grades ≥3) and ground-glass opacity of pneumonitis lesions were negatively associated with rechallenge. Following rechallenge, 9 (20.0%) patients developed recurrent pneumonitis, and 11 (24.4%) developed a new irAE. Severe grade of CIP and poor performance status at initial CIP as well as levels of interleukin (IL)-6 and C-reactive protein (CRP), and absolute white blood cell and neutrophil counts at the time of ICI rechallenge were associated with a higher recurrence rate. The median (95% confidence interval) PFS1 and PFS2 were 17.9 (9.9–24.2) and 15.5 (5.5–25.6) months, respectively. The median (95% confidence interval) OS1 and OS2 were 23.5 (16.5–30.5) and 18.4 (10.1–26.7) months, respectively. Lower OS2 was observed in patients with severe grade of CIP and poor performance status at the initial CIP, recurrence of CIP, and in patients with high levels of CRP and IL-6 at rechallenge. Only IL-6 was found to affect OS2 on multivariate analysis.
Conclusions
ICI rechallenge following CIP may be a promising treatment for patients with advanced lung cancer, particularly in those with low-grade of CIP and good performance status at initial CIP, and low levels of IL-6 and CRP at the time of initial challenge. Prospective studies are needed for further verification.
Keywords: Checkpoint inhibitor-related pneumonitis (CIP), immune checkpoint inhibitors (ICIs), lung cancer, rechallenge
Introduction
Immune checkpoint inhibitors (ICIs), including those targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death ligand-1 (PD-L1), used alone or in combination therapy (including ICI + chemotherapy and dual immunotherapy) have markedly improved overall survival (OS) in patients with advanced lung cancer (1,2). However, ICIs are also associated with certain immune-related adverse events (irAEs) (3). Checkpoint inhibitor-related pneumonitis (CIP) is the most fatal irAE in patients receiving anti-PD-1/PD-L1 agents (4). In clinical studies the incidence of CIP is reportedly 3–5% (5), but is higher in real-world data, ranging from 10% to 19% (6-9). A meta-analysis demonstrated that non-small cell lung cancer (NSCLC) was more likely to be associated with all-grade or high-grade CIP than other cancers (10).
The official guidelines indicate patients with grade 1 CIP can resume ICI therapy on radiographic evidence of improvement or resolution of the pneumonitis episode; grade 2 CIP requires temporary discontinuation of ICIs until resolution to ≤ grade 1; and CIP of grade 3–4 requires permanent discontinuation of ICIs (11-13). These recommendations are based both on expert consensus and anecdotal experience because there is at present a lack of adequate clinical data on ICI retreatment after CIP improvement.
A recent study reported that the OS of irAE patients in the rechallenge group was longer than that in the non-rechallenge group (38.6 vs. 24.9 months) (14). A recent cohort of 144 NSCLC patients who were retreated with ICIs exhibited encouraging rechallenge efficacy, especially for patients who had an interruption of the first ICI treatment due to toxicity or following a clinical decision (treatment withdrawal in view of the long-term benefit achieved or the patient’s needs despite disease control and absence of toxicities), for those not receiving systemic treatment between the two ICIs, and for those with good Eastern Cooperative Oncology Group performance status (ECOG PS) at rechallenge (15). To date, evidence regarding effectiveness of retreatment following CIP is scarce. It is not clear which patients can benefit from rechallenge. Studies have shown that the recurrence rate of irAEs after ICI rechallenge varied between 39% and 55% for various types of cancer (16-18). Dolladille et al. found that the recurrence rate of CIP, colitis, and hepatitis were higher than that of other irAEs (19). Continuing ICIs without any suspension as well as resuming ICIs after suspension remain challenging, and more data would be helpful in selecting patients for rechallenge.
This study was designed to evaluate the safety and efficacy of rechallenge of ICIs in patients with advanced lung cancer and to identify the potential risk factors associated with the recurrence of CIP. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-732/rc).
Methods
Patient selection
This multicenter, retrospective, observational cohort study was conducted in 3 centers [First Affiliated Hospital of Guangzhou Medical University (FHGMU), Sun Yat-sen University Cancer Center (SYUC), and Shanghai Chest Hospital (SCH)]. Medical records of consecutive patients who received at least 1 dose of an ICI between May 2017 and May 2021 were reviewed. Patients with stage IV or unresectable stage III (including but not limited poor cardiopulmonary function, bulky nodal involvement, T4N2 disease or N3 disease, etc.) lung cancer [according to the 2015 World Health Organization Classification of Lung Tumors (20)] who experienced CIP were included in this study. Patients with other cancers, early lung cancer, or lacking complete data, were excluded. CIP was diagnosed by a multidisciplinary team that utilized the guidelines of the National Comprehensive Cancer Network, the American Society for Clinical Oncology, and the European Society for Medical Oncology (11-13). CIP was defined as new-onset infiltrates on chest imaging and/or clinical symptoms such as cough, expectoration, shortness of breath, or fever that were likely caused by ICIs, with other etiologies excluded. Enrolled patients were divided into two groups according to their subsequent re-exposure to ICIs: patients who received ICI therapy without interruption or who restarted ICI therapy after interruption (rechallenged group); and patients who did not undergo re-administration (non-rechallenged group). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2021-38). Individual consent for this retrospective analysis was waived. All participating hospitals/institutions were informed and agreed the study.
Data collection and study assessment
The following information was retrospectively collected from each patient’s medical record: demographics (age, sex), ECOG PS, ICI (type, duration of treatment, and outcomes), CIP data (time to CIP onset, symptoms, maximum CIP grade, imaging features, clinical type, management, and outcomes), and laboratory findings. All patients received follow-up from enrollment, through an electronic medical record system review and telephone follow-up. For patients with recurrent CIP or new irAEs during follow-up, the time and grade of occurrence were recorded. In the rechallenged group, the time from CIP occurrence to rechallenge and type of ICI used when treatment was restarted were also collected.
ECOG PS was evaluated prior to ICI treatment and at the time point of the most severe grade of pneumonitis. The severity of CIP was graded according to the Common Toxicity Criteria for Adverse Events (version 4.0). Based on the imaging findings, CIP lesions were divided into 5 subtypes: cryptogenic organizing pneumonitis (COP), ground-glass opacity (GGO), interstitial, hypersensitivity, and pneumonitis not otherwise specified (NOS) (6). CIP was classified into 3 types in terms of clinical factors: pure type (idiopathic, with or without autoimmune disease), induced type (i.e., having distinct etiologies, such as radiotherapy, cytomegalovirus infection, or Epstein-Barr virus reactivation), and mixed type (combined with infectious pneumonia, tumor progression or radiation-related pneumonitis) (21). Improvement of CIP was defined as an improvement of symptoms, reduction in oxygen demand, or improvement in the infiltrates. Conversely, worsening of CIP was defined as an exacerbation of symptoms, increased oxygen demand, or increased infiltration.
Clinical responses were classified according to RECIST version 1.1. The overall response rate (ORR) in patients receiving the first and second ICI was defined as the percentage of patients achieving a complete response (CR) as well as partial response (PR). The disease control rate (DCR) corresponded to all patients with CR, PR and stable disease (SD) treated with the first or second ICI. Progression-free survival (PFS) from the first ICI (PFS1) was estimated as the duration from the first ICI administration to disease progression or death. The PFS under rechallenge (PFS2) was defined as the time from the initiation of the rechallenge to disease progression or death. OS under the first ICI (OS1) was defined as the time from the initiation of ICI to death due to any cause or the last follow-up date (1 July 2021), and OS2 was estimated from the initiation of the rechallenge.
Laboratory findings included the absolute white blood cell count (WBC), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), absolute eosinophil count (AEC), and the levels of interleukin (IL)-6 and -10, lactate dehydrogenase (LDH), albumin (ALB), C-reactive protein (CRP), and Krebs von den Lungen-6 (KL-6). The neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing the ANC by the ALC. These laboratory findings were collected at the initial CIP for each included case. In the rechallenged group, we also collected the same laboratory findings at the initiation of the rechallenge treatment.
Statistical analysis
Statistics are summarized as the frequency and percentage for categorical variables and as the median (range) for continuous variables. Independent-samples t-test or the Mann-Whitney U test was used for the continuous variables. Differences in the categorical variables were assessed using the Chi-square (χ2) or Fisher’s exact test. Spearman’s rank correlation was performed for the correlation analysis of clinical factors and the severity of initial CIP. Logistic univariate analysis was applied to identify which factors were associated with rechallenge. Variables with P<0.1 in the univariate analysis were analyzed in the multivariate logistic regression analysis.
Receiver operating characteristic (ROC) and area under curve (AUC) values were applied for exploring the value of laboratory findings to predict recurrent CIP in the rechallenged group. Youden’s index was used to determine the best cutoff value. Logistic univariate analysis was used to determine which factors were associated with recurrent CIP among the rechallenged patients. The sample size of the recurrent CIP patient group was too small to conduct multivariate analysis. The severity of initial and recurrent CIP was compared using the Wilcoxon signed-rank test.
The Kaplan-Meier method was used to estimate PFS and OS with a 95% confidence interval (CI), and the log-rank test was used to evaluate between-group differences. Cox regression was applied to calculate the hazard ratios (HRs) with a 95% CI of factors associated with PFS and OS. All P values were based on the two-sided hypothesis test, and P<0.05 was considered statistically significant. All analyses were conducted using IBM SPSS Statistics version 25 (Armonk, NY).
Results
Participants
There were 124 patients with CIP after ICI therapy at FHGMU (n=105), SYUC (n=15), and SCH (n=4). A total of 17 patients were excluded: 11 with other cancers, 2 with early lung cancer, and 4 lacking complete data. Ultimately, 107 patients were included in the study (Figure 1) and their characteristics are summarized in Table 1. The median age of all participants was 66 (range, 36–85) years. Squamous cell carcinoma was the most common tumor type (45.8%), followed by adenocarcinoma (32.7%) and small cell lung cancer (SCLC) (12.1%). Of the 107 patients, 19 (17.8%) had preexisting lung diseases (16 chronic obstructive pulmonary disease, 2 pulmonary fibrosis, and 1 pulmonary tuberculosis), and 33 (30.8%) had a prior history of radiotherapy (23 with thoracic radiotherapy, 7 with extrathoracic radiotherapy and 3 both with thoracic and extrathoracic radiotherapy). Among the 50 participants (46.7%) whose PD-L1 expression was determined, PD-L1 <1%, 1–49%, and ≥50% in 28 (26.2%), 15 (14.0%) and 7 (6.5%) patients, respectively. Initial ICI therapy was a PD-1 inhibitor in 100 patients (93.5%) and a PD-L1 inhibitor in 7 patients (6.5%).
Figure 1.
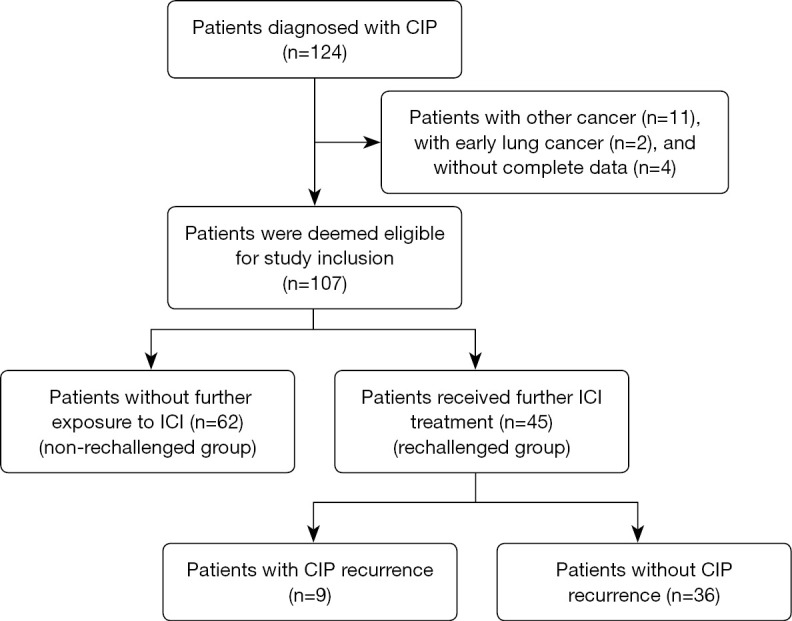
Flowchart showing study design and patient inclusion. CIP, checkpoint inhibitor-related pneumonitis; ICI, immune checkpoint inhibitor.
Table 1. Characteristics of advanced lung cancer patients treated with ICIs.
| Variable | All patients (n=107) | Rechallenge (n=45) | Non-rechallenge (n=62) | P value |
|---|---|---|---|---|
| Age (years), median [range] | 66 [36–85] | 65 [36–85] | 66 [43–85] | 0.58 |
| Sex, n (%) | 0.34 | |||
| Male | 92 (86.0) | 37 (82.2) | 55 (88.7) | |
| Female | 15 (14.0) | 8 (17.8) | 7 (11.3) | |
| Smoking status, n (%) | 0.29 | |||
| Current/former | 68 (63.6) | 28 (62.2) | 40 (64.5) | |
| Never | 39 (36.4) | 17 (37.8) | 22 (35.5) | |
| Histologic type, n (%) | 0.48 | |||
| Squamous | 49 (45.8) | 23 (51.1) | 26 (41.9) | |
| Adenocarcinoma | 35 (32.7) | 11 (24.4) | 24 (38.7) | |
| SCLC | 13 (12.1) | 6 (13.3) | 7 (11.3) | |
| Other | 10 (9.3) | 5 (11.1) | 5 (8.1) | |
| Preexisting lung disease, n (%) | 19 (17.8) | 8 (17.8) | 11 (17.7) | 0.99 |
| Chronic obstructive pulmonary disease | 16 (15.0) | 6 (13.3) | 10 (16.1) | 0.69 |
| Pulmonary fibrosis | 2 (1.9) | 2 (4.4) | 0 | 0.18 |
| Pulmonary tuberculosis | 1 (0.9) | 0 | 1 (1.6) | 1 |
| Radiation before CIP, n (%) | 33 (30.8) | 10 (22.2) | 23 (37.1) | 0.10 |
| Chest | 23 (21.5) | 6 (13.3) | 17 (27.4) | 0.080 |
| Non-chest | 7 (6.5) | 2 (4.4) | 5 (8.1) | 0.19 |
| Both | 3 (2.8) | 2 (4.4) | 1 (1.6) | 0.57 |
| ECOG PS, n (%) | 1 | |||
| 0–1 | 103 (96.3) | 43 (95.6) | 60 (96.8) | |
| ≥2 | 4 (3.7) | 2 (4.4) | 2 (3.2) | |
| PD-L1 expression status, n (%) | 0.40 | |||
| <1% | 28 (26.2) | 14 (31.1) | 14 (22.6) | |
| 1–49% | 15 (14.0) | 4 (8.9) | 11 (17.7) | |
| ≥50% | 7 (6.5) | 3 (6.7) | 4 (6.5) | |
| Unknown | 57 (53.3) | 24 (53.3) | 33 (53.2) | |
| Treatment lines, n (%) | 0.33 | |||
| 1 | 73 (68.2) | 33 (73.3) | 40 (64.5) | |
| ≥2 | 34 (31.8) | 12 (26.7) | 22 (35.5) | |
| ICI type, n (%) | 0.45 | |||
| Anti-PD-1 | 100 (93.5) | 41 (91.1) | 59 (95.2) | |
| Anti-PD-L1 | 7 (6.5) | 4 (8.9) | 3 (4.8) | |
| Treatment data, n (%) | 0.36 | |||
| Monotherapy | 23 (21.5) | 6 (13.3) | 17 (27.4) | |
| Combination with chemotherapy | 65 (60.7) | 30 (66.7) | 35 (56.5) | |
| Combination with anti-angiogenesis | 5 (4.7) | 2 (4.4) | 3 (4.8) | |
| Combination with chemotherapy and anti-angiogenesis | 14 (13.1) | 7 (15.6) | 7 (11.3) | |
| Best response, n (%) | 0.011* | |||
| Partial response | 45 (42.1) | 27 (60.0) | 18 (29.0) | |
| Stable disease | 47 (43.9) | 17 (37.8) | 30 (48.4) | |
| Progression of disease | 8 (7.5) | 1 (2.2) | 7 (11.3) | |
| Not evaluated | 7 (6.5) | 0 | 7 (11.3) | |
| ORR (%) (95% CI) | 45.0 (35.1–54.9) | 60.0 (45.1–74.9) | 32.7 (19.9–45.5) | 0.006* |
| Median PFS (months) (95% CI) | 8.8 (5.3–12.2) | 17.9 (11.2–24.6) | 7.0 (5.6–8.4) | 0.008* |
| Median OS (months) (95% CI) | 17.2 (10.5–24.0) | 23.5 (16.5–30.5) | 12.6 (9.4–15.7) | 0.024* |
*, P<0.05. ICI, immune checkpoint inhibitor; SCLC, small cell lung cancer; CIP, checkpoint inhibitor-related pneumonitis; ECOG PS, Eastern Cooperative Oncology Group performance status; PD-L1, programmed death ligand-1; PD-1, programmed cell death protein-1; ORR, overall response rate; CI, confidence interval; PFS, progression-free survival; OS, overall survival.
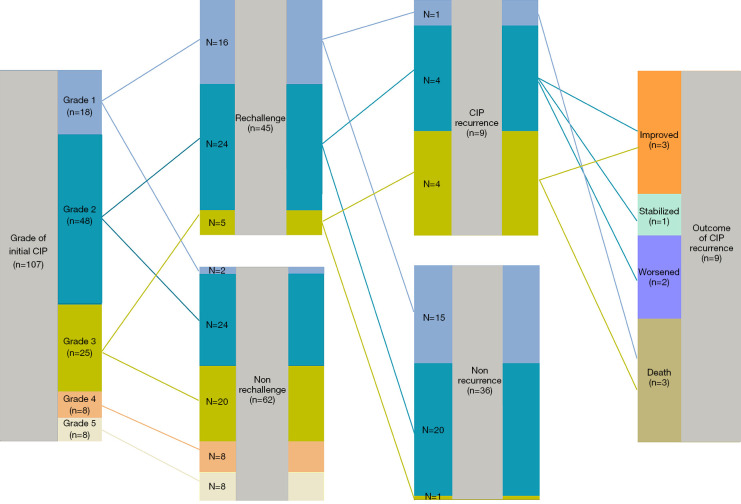
Of the 107 patients, 45 (42.1%) patients received further ICI treatment (with or without interruption) after initial CIP (rechallenged group), and 62 were not rechallenged (non-rechallenged group) (Figures 1,2). ICI combination therapy was the predominant treatment type in both groups, but accounted for a larger proportion (86.7%) of treatment in the rechallenged group (P=0.080) (Table 1).
Figure 2.
Flowchart of patients with immune checkpoint inhibitor rechallenge who developed CIP recurrence and their outcomes. CIP, checkpoint inhibitor-related pneumonitis.
Initial CIP and rechallenge
The median time from the onset of ICI therapy to first CIP was 3.4 months (range, 0.2–13.9 months); 13 patients (12.1%) had no symptoms. The main symptoms of CIP were cough (74.8%), shortness of breath (62.6%), and expectoration (57.0%). The severity of CIP was grade 1–2 in 66 patients (61.7%), grade 3–4 in 33 patients (30.8%), and grade 5 in 8 patients (7.5%). GGO was the predominant imaging lesion (49.5%), followed by interstitial pneumonitis (21.5%) and COP (18.7%). Among the 107 patients, 48 had pure-type CIP, 13 had induced-type CIP, and 46 were mixed-type CIP (Table 2).
Table 2. Patients’ characteristics at CIP.
| Variable | All patients (n=107) | Rechallenge (n=45) | Non-rechallenge (n=62) | P value |
|---|---|---|---|---|
| Duration of drug administration (months) | 3.4 (0.2–13.9) | 2.9 (0.7–13.9) | 4.1 (0.2–12.4) | 0.17 |
| Asymptomatic | 13 (12.1) | 11 (24.4) | 2 (3.2) | 0.001* |
| Symptoms | ||||
| Fever | 19 (17.8) | 5 (11.1) | 14 (22.6) | 0.13 |
| Cough | 80 (74.8) | 30 (66.7) | 50 (80.6) | 0.10 |
| Expectoration | 61 (57.0) | 23 (51.1) | 38 (61.3) | 0.29 |
| Shortness of breath | 67 (62.6) | 20 (44.4) | 47 (75.8) | 0.001* |
| ECOG PS | <0.001* | |||
| 0–1 | 35 (32.7) | 25 (55.6) | 10 (16.1) | |
| ≥2 | 72 (67.3) | 20 (44.4) | 52 (83.9) | |
| Grade | <0.001* | |||
| 1–2 | 66 (61.7) | 40 (88.9) | 26 (41.9) | |
| 3–4 | 33 (30.8) | 5 (11.1) | 28 (45.2) | |
| 5 | 8 (7.5) | 0 | 8 (12.9) | |
| Radiological features | 0.010* | |||
| COP | 20 (18.7) | 13 (28.9) | 7 (11.3) | |
| GGO | 53 (49.5) | 14 (31.1) | 39 (62.9) | |
| NSIP | 23 (21.5) | 11 (24.4) | 12 (19.4) | |
| NOS | 7 (6.5) | 4 (8.9) | 3 (4.8) | |
| Unknown | 4 (3.7) | 3 (6.7) | 1 (1.6) | |
| Clinical type | 0.002* | |||
| Pure | 48 (44.9) | 29 (64.4) | 19 (30.6) | |
| Induced | 13 (12.1) | 4 (8.9) | 9 (14.5) | |
| Mixed | 46 (43.0) | 12 (26.7) | 34 (54.8) | |
| Progression of disease at first CIP | 18 (16.8) | 3 (6.7) | 15 (24.2) | 0.017* |
| Combined with other irAEs | 23 (21.5) | 9 (20.0) | 14 (22.6) | 0.75* |
| Laboratory values at first CIP | ||||
| IL-6, pg/mL | 14.0 (1.9–309.7) | 8.2 (1.9–31.8) | 20.7 (2.3–309.7) | 0.19 |
| IL-10, pg/mL | 3.1(0.6–20.4) | 2.9 (0.6–5.2) | 3.2 (1.9–20.4) | 0.30 |
| LDH, U/L | 276.3 (149.0–512.7) | 250.0 (149.0–307.0) | 297.4 (185.3–512.7) | 0.08 |
| ALB, g/L | 34.2 (28.0–41.5) | 37.1 (33.5–41.5) | 32.8 (28.0–36.3) | 0.001* |
| CRP, mg/L | 32.3 (1.9–318.0) | 8.2 (1.9–31.8) | 103.9 (4.8–318.0) | 0.14 |
| KL-6, U/mL | 743 (222–9,358) | 245 (222–4,022) | 1192 (391–9,358) | 0.014* |
| WBC, K/μL | 8.0 (1.0–49.7) | 7.7 (1.0–22.9) | 8.8 (3.4–49.7) | 0.12 |
| ALC, K/μL | 0.7 (0.3–2.1) | 1.3 (0.4–2.1) | 0.5 (0.3–1.3) | <0.001* |
| AEC, K/μL | 0.1 (0–0.5) | 0.2 (0.1–0.5) | 0.1 (0–0.3) | <0.001* |
| ANC, K/μL | 7.0 (2.2–18.5) | 3.3 (2.2–9.8) | 8.4 (2.3–18.5) | 0.012* |
| NLR | 8.0 (1.3–46.3) | 5.5 (1.3–13.0) | 12.8 (2.5–46.3) | <0.001* |
| Systemic corticosteroid use | 78 (72.9) | 24 (53.3) | 54 (87.1) | <0.001* |
| Outcome of CIP | <0.001* | |||
| Recovery/improved | 82 (76.6) | 42 (93.3) | 40 (64.5) | |
| Stabilized | 10 (9.3) | 2 (4.4) | 8 (12.9) | |
| Worsened/death | 12 (11.2) | 0 | 12 (19.4) | |
| Unknown | 3 (2.8) | 1 (2.2) | 2 (3.2) | |
| Time to improvement (months) | 0.8 (0.1–7.6) | 0.9 (0.1–7.6) | 0.4 (0.1–3.9) | 0.008* |
| Recurrence of CIP | 17 (15.9) | 9 (20.0) | 8 (12.9) | 0.32 |
The data are shown as n (%) or median (range). *, P<0.05. CIP, checkpoint inhibitor-related pneumonitis; ECOG PS, Eastern Cooperative Oncology Group performance status; COP, cryptogenic organizing pneumonitis; GGO, ground glass opacities; NSIP, non-specific interstitial pneumonia; NOS, pneumonitis not otherwise specified; irAEs, immune-related adverse events; IL, interleukin; LDH, lactate dehydrogenase; ALB, albumin; CRP, C-reactive protein; KL-6, Krebs von den Lungen-6; WBC, absolute white blood cell count; ALC, absolute lymphocyte count; AEC, absolute eosinophil count; ANC, absolute neutrophil count; NLR, neutrophil-to-lymphocyte ratio.
The proportion of severe grade (grades 3–5) along with poor ECOG PS score (≥2) at initial CIP was higher in the non-rechallenged group than in the rechallenged group (58.1% vs. 11.1%, P<0.001; 83.9% vs. 44.4%, P<0.001; respectively). GGO accounted for the highest proportion of lesions on imaging in the two groups, but was higher in the non-rechallenged group (62.9% vs. 31.1%, P=0.001). In the rechallenged group, the proportion of pure-type CIP was the highest, but in the non-rechallenged group the mixed type CIP was predominant. When pneumonitis occurred, the frequency of disease progression (PD) in the non-rechallenged group was higher than in the rechallenged group (24.2% vs. 6.7%, P=0.017) (Table 2).
The ALC, AEC, and ALB detected at initial CIP were statistically significantly lower in the non-rechallenged group than in the rechallenged group. Conversely, KL-6, ANC, and NLR were lower in the rechallenged group (Table 2). These peripheral blood parameters (ALC, ALB, KL-6, and NLR) were statistically associated with the severity of the first CIP episode (Table S1).
Of the 107 patients, 78 (72.9%) were treated with glucocorticoids (including methylprednisolone, prednisolone, and dexamethasone), including 24 in the rechallenged group and 54 in the non-rechallenged group (53.3% vs. 87.1%, P<0.001).
CIP recovered, improved, or stabilized in all patients in the rechallenged group, with an improvement rate of 93.3% versus 64.5% in the non-rechallenged group (P<0.001). The improvement time in the rechallenged group was longer than in the non-rechallenged group (0.9 vs. 0.4 months, P=0.008).
Multivariate analysis showed that severe grade of CIP [odds ratio (OR) =0.16, 95% CI: 0.04–0.73, P=0.018] and GGO (OR =0.27, 95% CI: 0.09–0.86, P=0.027) were significantly and independently associated with non-rechallenge of ICI (Table S2).
Safety of rechallenge
45 patients who were rechallenged with an ICI, of which 10 continued ICI treatment without interruption, and 35 re-initiated ICI therapy after an interruption. Most patients (88.9%) received the same ICI or ICI combination therapy. The median duration from initial CIP to ICI rechallenge was 0.8 months (range, 0–5.9 months).
Overall, 30 (66.7%) patients did not experience any subsequent irAEs, 9 (20.0%) had recurrence of CIP, and 11 (24.4%) developed a new irAE (Table 3, Figure 3A). Of the 9 patients who experienced CIP recurrence, the severity of CIP was grade 2 in 3 patients, grade 3 in 1 patient, grade 4 in 2 patients, and grade 5 in 3 patients. The second CIP episodes were more severe than the initial event. The median time from rechallenge to recurrent CIP was shorter than from the initial ICI to first CIP (41 vs. 113 days, P=0.007). Eventually, 3 patients died, 2 progressed, 1 stabilized, and 3 improved (Figure 2). Excluding the 12 patients with pneumonitis progression or death, 8 patients (16.0%) experienced recurrent pneumonitis in the non-rechallenged group during follow-up, of whom 3 patients (37.5%) died. The grade of recurrent pneumonitis was also higher than that of the initial pneumonitis episode in the non-rechallenged group. The 10 patients who did not have immunotherapy suspended after grade 1 initial CIP did not develop exacerbations or recurrent CIP.
Table 3. Factors associated with recurrence of pneumonitis in patients after ICI rechallenge.
| Variable | CIP recurrence (n=9) | CIP non-recurrence (n=36) | P value |
|---|---|---|---|
| Age (years), median [range] | 65 [55–75] | 66 [36–85] | 0.79 |
| Male, n (%) | 9 (100.0) | 28 (77.8) | 0.18 |
| Initial ICI type, n (%) | 0.57 | ||
| Anti-PD-1 | 9 (100.0) | 32 (88.9) | |
| Anti-PD-L1 | 0 | 4 (11.1) | |
| Initial treatment data, n (%) | 0.011 | ||
| Monotherapy | 2 (22.2) | 3 (8.3) | |
| Combination with chemotherapy | 2 (22.2) | 27 (75.0) | |
| Combination with anti-angiogenesis | 2 (22.2) | 1 (2.8) | |
| Combination with chemotherapy and anti-angiogenesis | 3 (33.3) | 5 (13.9) | |
| Median time to first CIP (months) (range) | 3.8 (1.5–5.3) | 2.6 (0.7–13.9) | 0.55 |
| ECOG PS (≥2) at first CIP, n (%) | 7 (77.8) | 13 (36.1) | 0.057 |
| Severe grade of first CIP, n (%) | 4 (44.4) | 1 (2.8) | 0.004* |
| Radiological features, n (%) | 0.24 | ||
| COP | 4 (44.4) | 9 (25.0) | |
| GGO | 1 (11.1) | 13 (36.1) | |
| NSIP | 4 (44.4) | 7 (19.4) | |
| NOS | 0 | 4 (11.1) | |
| Unknown | 0 | 3 (8.3) | |
| Clinical type, n (%) | 0.30 | ||
| Pure | 4 (44.4) | 25 (69.4) | |
| Induced | 1 (11.1) | 3 (8.3) | |
| Mixed | 4 (44.4) | 8 (22.2) | |
| Laboratory values at rechallenge, median (range) | |||
| IL-6, pg/mL | 25 (8.1–51.6) | 7.6 (1.3–49.1) | 0.007* |
| IL-10, pg/mL | 3.5 (1.4–22.0) | 2.2 (0.8–29.9) | 0.32 |
| LDH, U/L | 262.0 (165.6–2,111.3) | 227.1 (126.8–397.0) | 0.24 |
| ALB, g/L | 35.5 (25.9–39.0) | 37.0 (28.7–43.4) | 0.54 |
| CRP, mg/L | 32.3 (10.0–107.5) | 10.1 (1.2–188.2) | 0.032* |
| KL-6, U/mL | 1,730.5 (395.0–2,385.0) | 822.5 (386.0–2,283.0) | 0.29 |
| WBC, K/μL | 10.3 (4.9–16.4) | 6.9 (4.3–15.3) | 0.022* |
| ALC, K/μL | 1.8 (0.4–3.2) | 1.3 (0.6–3.2) | 0.69 |
| AEC, K/μL | 0.1 (0–0.5) | 0.1 (0–0.4) | 0.63 |
| ANC, K/μL | 7.2 (3.2–11.7) | 4.6 (1.7–12.4) | 0.030* |
| NLR | 3.6 (2.0–24.3) | 3.5 (0.7–8.5) | 0.46 |
| Rechallenge with same ICI or ICI combination, n (%) | 7 (77.8) | 33 (91.7) | 0.26 |
| Median duration from first CIP to rechallenge (months) (range) | 1.4 (0–4.4) | 0.8 (0–5.9) | 0.43 |
| Occurrence of new irAEs, n (%) | 5 (55.6) | 6 (16.7) | 0.028* |
| Hepatitis | 3 (33.3) | 5 (13.9) | 0.33 |
| Myocarditis | 3 (33.3) | 0 | 0.036* |
| Colitis | 0 | 1 (2.8) | 1 |
| Encephalitis | 0 | 1 (2.8) | 1 |
*, P<0.05. ICI, immune checkpoint inhibitor; PD-1, programmed cell death protein-1; PD-L1, programmed death ligand-1; CIP, checkpoint inhibitor-related pneumonitis; ECOG PS, Eastern Cooperative Oncology Group performance status; COP, cryptogenic organizing pneumonitis; GGO, ground glass opacities; NSIP, non-specific interstitial pneumonia; NOS, pneumonitis not otherwise specified; IL, interleukin; LDH, lactate dehydrogenase; ALB, albumin; CRP, C-reactive protein; KL-6, Krebs von den Lungen-6; WBC, absolute white blood cell count; ALC, absolute lymphocyte count; AEC, absolute eosinophil count; ANC, absolute neutrophil count; NLR, neutrophil-to-lymphocyte ratio; irAEs, immune-related adverse events.
Figure 3.
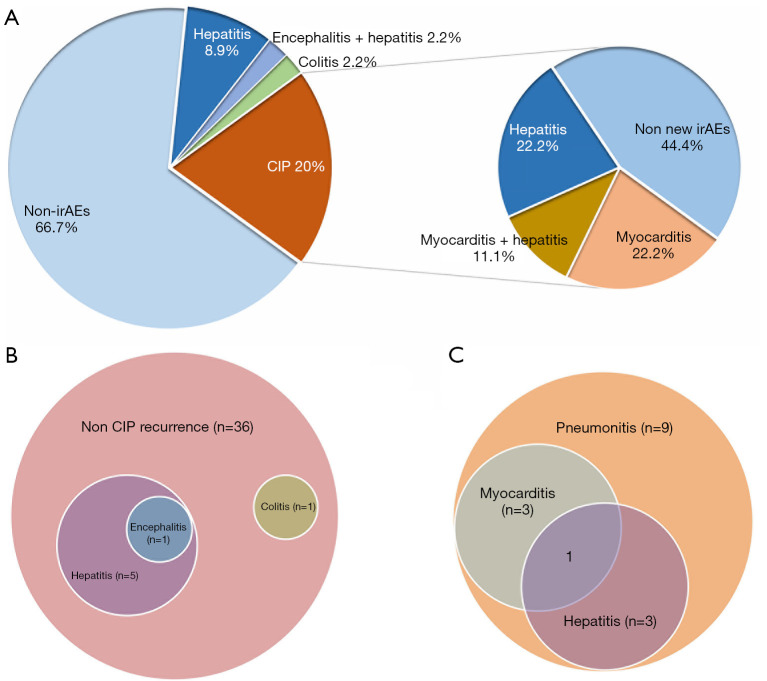
Pie chart showing the distribution irAEs in the rechallenged group (A). Venn diagram of irAEs distribution in patients without CIP recurrence (B) and with CIP recurrence (C). irAEs, immune-related adverse events; CIP, checkpoint inhibitor-related pneumonitis.
A total of 11 patients experienced a new irAE, all of which were grade 1–2, except for 2 cases of myocarditis. New irAEs post-rechallenge were most commonly hepatitis; 6/36 patients (16.7%) without recurrent pneumonitis developed other irAEs (Figure 3B), and 5/9 patients (55.6%) with recurrent pneumonitis and other irAE complications (Figure 3C).
Three treatment-related deaths occurred. In one case, a patient treated with anti-PD-1 combined with bevacizumab initially developed grade 3 CIP (mixed type) and recovered after 20 days. The disease progressed and the patient was treated with a PD-L1 inhibitor combined with chemotherapy, but 14 days later redeveloped CIP, which resulted in death. In the second patient, grade 1 CIP occurred after receiving anti-PD-1 combined with chemotherapy, and the original treatment was continued for 3 courses. The patient developed CIP and myocarditis and eventually died. In the third patient, after receiving the PD-1 inhibitor plus chemotherapy, grade 3 CIP occurred and immunotherapy was suspended. After further multi-line anti-tumor therapy, the disease progressed. The patient was treated with anti-PD-1 and anti-CTL-4 therapy, but 1 week later, CIP and myocarditis developed, which led to death. All 3 patients had a history of chronic obstructive pulmonary disease, and the third case had a pneumothorax at initial CIP.
Factors associated with CIP recurrence
The characteristics of patients with recurrent CIP in the rechallenged group are summarized in Table 3. The frequency of grade 3–4 initial CIP in the recurrence cohort was significantly higher than in the non-recurrence cohort (44.4% vs. 2.8%, P=0.004). Similarly, the proportion of patients with ECOG PS ≥2 at initial CIP was higher in the recurrence cohort than in the non-recurrence cohort (77.8% vs. 36.1%, P=0.057). Upon univariate analysis, severe grade of CIP (OR =28.0, 95% CI: 2.6–303.5, P=0.006) and poor PS (OR =6.2, 95% CI: 1.1–34.3, P=0.037) at initial CIP were significantly associated with CIP recurrence (Table 4).
Table 4. Univariate logistic regression analysis for the risk factors of recurrence of CIP.
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Grade 3–4 of first CIP | 28.0 | 2.6–303.5 | 0.006* |
| ECOG PS (≥2) of first CIP | 6.2 | 1.1–34.3 | 0.037* |
| Clinical type of first CIP | |||
| Pure | Reference | ||
| Induced | 2.1 | 0.2–25.3 | 0.57 |
| Mixed | 3.1 | 0.6–15.5 | 0.16 |
| Laboratory values at rechallenge | |||
| IL-6 (≥8.08 pg/mL) | 12.0 | 1.2–115.4 | 0.031* |
| CRP (≥30.62 mg/L) | 10.5 | 1.4–78.1 | 0.022* |
| WBC (≥8.61 K/μL) | 12.0 | 2.1–68.6 | 0.005* |
| ANC (≥6.75 K/μL) | 13.5 | 2.4–76.8 | 0.003* |
*, P<0.05. ANC, absolute neutrophil count; CI, confidence interval; CIP, checkpoint inhibitor-related pneumonitis; CRP, C-reactive protein; ECOG PS, Eastern Cooperative Oncology Group performance status; IL, interleukin; OR, odds ratio; WBC, absolute white blood cell count.
Notably, patients with CIP recurrence had significantly higher peripheral blood levels of IL-6, CRP, WBC and ANC at ICI rechallenge than those without CIP recurrence. ROC curve analysis was performed to determine the best threshold for IL-6, CRP, WBC, and ANC levels for the prediction of CIP recurrence: 8.08 pg/mL for IL-6 (AUC =0.83, P=0.017), 30.62 mg/L for CRP (AUC =0.75, P=0.062), 8.61 K/µL for WBC (AUC =0.73, P=0.091), and 6.75 K/µL for ANC (AUC =0.73, P=0.091) (Figure S1). Univariate logistic regression analysis revealed that IL-6 (OR =12.0, P=0.031), CRP (OR =10.5, P=0.022), WBC (OR =12.0, P=0.005), and ANC (OR =13.5, P=0.003) at the time of ICI rechallenge were significantly associated with CIP recurrence (Table 4).
Effectiveness of rechallenge
The overall median follow-up for this analysis was 15.9 months. The ORR was higher in the rechallenged group than in the non-rechallenged group (60.0% vs. 32.7%, P=0.006). In addition, longer PFS (17.9 vs. 7.0 months; HR, 0.50, 95% CI: 0.30–0.84; P=0.010) and OS (23.5 vs. 12.6 months; HR, 0.51, 95% CI: 0.28–0.92; P=0.027) were achieved in the rechallenged group compared with the non-rechallenged group (Table 1, Figure S2).
After the first ICI treatment, 27 achieved PR (60.0%), 17 had SD (37.8%) and 1 had PD (2.2%). The ORR was 60.0%, and the DCR was 97.8%. With ICI rechallenge, 9 achieved PR (20.0%), 33 had SD (73.3%) and 3 had PD (6.7%) (Figure S3).
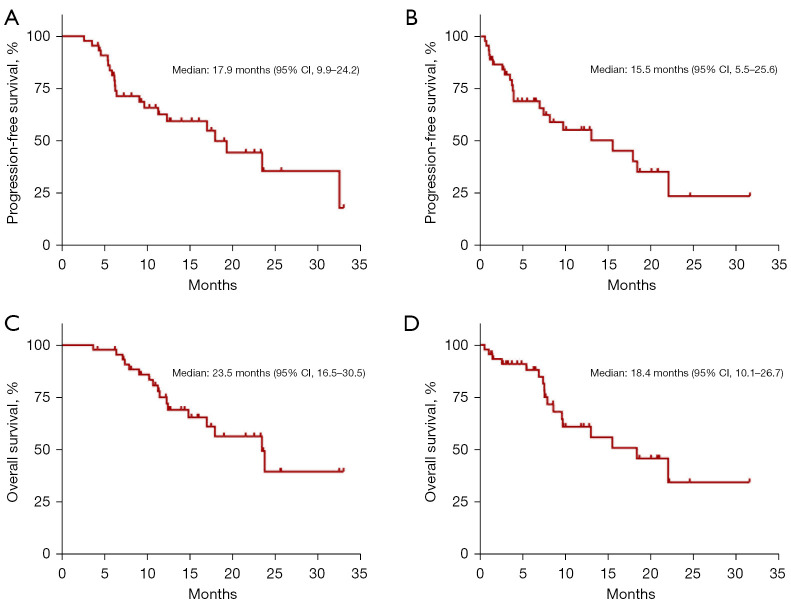
On analysis, 21 patients displayed progression or death after rechallenge therapy. The median PFS1 and PFS2 were 17.9 months (95% CI: 9.9–24.2) and 15.5 months (95% CI: 5.5–25.6), respectively (Figure 4). According to the univariate analysis, the median PFS2 was even longer in patients with grades 1–2 initial CIP (HR: 0.20, 95% CI: 0.06–0.62; P=0.005), ECOG PS of 0–1 at initial CIP (HR: 0.24, 95% CI: 0.10–0.57; P=0.001), low levels of IL-6 (HR: 0.33, 95% CI: 0.13–0.84; P=0.020) and ANC at rechallenge (HR: 0.33, 95% CI: 0.14–0.82; P=0.017), and those without CIP recurrence (HR: 0.28, 95% CI: 0.11–0.73; P=0.009) (Table 5).
Figure 4.
Kaplan-Meier survival curves of PFS and OS of patients who received ICI rechallenge treatment. (A) PFS from the first ICI treatment (PFS1). (B) PFS from the ICI rechallenge treatment (PFS2). (C) OS from the first ICI treatment (OS1). (D) OS from ICI rechallenge treatment (OS2). PFS, progression-free survival; OS, overall survival; ICI, immune checkpoint inhibitor.
Table 5. Cox proportional hazards regression analysis of clinical factors associated with PFS2 and OS2 in the rechallenged group.
| Variable | PFS2 | OS2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age ≥65 years | 1.47 (0.64–3.39) | 0.36 | – | – | 0.93 (0.26–3.30) | 0.91 | – | – | |||
| Male vs. female | 1.02 (0.34–3.08) | 0.97 | – | – | 1.59 (0.60–4.23) | 0.36 | – | – | |||
| Smoking (current or former vs. never) | 1.47 (0.64–3.39) | 0.37 | – | – | 1.08 (0.41–2.81) | 0.88 | – | – | |||
| Grade 1–2 of first CIP | 0.20 (0.06–0.62) | 0.005* | 0.70 (0.14–3.45) | 0.66 | 0.12 (0.04–0.39) | <0.001* | 1.57 (0.22–11.04) | 0.65 | |||
| ECOG PS (0–1) of first CIP | 0.24 (0.10–0.57) | 0.001* | 0.49 (0.12–1.98) | 0.32 | 0.19 (0.07–0.57) | 0.003* | 0.52 (0.12–2.26) | 0.38 | |||
| Clinical type of first CIP | |||||||||||
| Pure | Reference | Reference | |||||||||
| Induced | 1.93 (0.62–6.03) | 0.26 | – | – | 1.63 (0.44–6.11) | 0.47 | – | – | |||
| Mixed | 1.23 (0.46–3.26) | 0.68 | – | – | 1.84 (0.61–5.58) | 0.28 | – | – | |||
| GGO vs. non-GGO at first CIP | 0.99 (0.42–2.37) | 0.98 | – | – | 0.96 (0.35–2.64) | 0.93 | – | – | |||
| Treatment line of second ICI | 1.42 (0.60–3.32) | 0.42 | – | – | 0.91 (0.32–2.59) | 0.86 | – | – | |||
| Laboratory values at rechallenge | |||||||||||
| IL-6 (<8.08 pg/mL) | 0.33 (0.13–0.84) | 0.020* | 0.44 (0.13–1.47) | 0.18 | 0.15 (0.05–0.51) | 0.002* | 0.06 (0.01–0.58) | 0.015* | |||
| CRP (<30.62 mg/L) | 0.57 (022–1.49) | 0.26 | – | – | 0.24 (0.08–0.70) | 0.010* | 0.55 (0.09–3.19) | 0.50 | |||
| WBC (<8.61 K/μL) | 0.52 (0.20–1.33) | 0.17 | – | – | 0.62 (0.20–1.97) | 0.42 | – | – | |||
| ANC (<6.75 K/μL) | 0.33 (0.14–0.82) | 0.017* | 0.60 (0.12–3.05) | 0.54 | 0.57 (0.19–1.70) | 0.31 | – | – | |||
| CIP recurrence (no vs. yes) | 0.28 (0.11–0.73) | 0.009* | 1.03 (0.18–5.97) | 0.98 | 0.19 (0.06–0.57) | 0.003* | 0.98 (0.12–8.20) | 0.98 | |||
*, P<0.05. PFS2, progression-free survival from the rechallenge; OS2, overall survival from the rechallenge; ANC, absolute neutrophil count; CI, confidence interval; CIP, checkpoint inhibitor-related pneumonitis; CRP, C-reactive protein; ECOG PS, Eastern Cooperative Oncology Group performance status; GGO, ground-glass opacity; HR, hazard ratio; IL, interleukin; ICI, immune checkpoint inhibitor; WBC, absolute white blood cell count.
The median OS1 was 23.5 months (95% CI: 16.5–30.5) and the median OS2 was 18.4 months (95% CI, 10.1–26.7) (Figure 4), with 17 deaths. In the univariate analysis, the median OS2 differed significantly according to the severity of the initial CIP (1–2 vs. ≥3: 22.1 vs. 1.5 months; HR: 0.12, 95% CI: 0.04–0.39; P<0.001), ECOG PS at the initial CIP (0–1 vs. ≥2: 22.1 vs. 7.6 months; HR: 0.19, 95% CI: 0.07–0.57; P=0.003), IL-6 at rechallenge (<8.08 vs. ≥8.08 pg/mL: 15.5 vs. 7.6 months; HR: 0.15, 95% CI: 0.05–0.51), CRP at rechallenge (<30.62 vs. ≥30.62 mg/L: NR vs. 7.4 months; HR: 0.24, 95% CI: 0.08–0.70), and CIP recurrence (no vs. yes: 22.1 vs. 7.4 months; HR: 0.19, 95% CI: 0.06–0.57). After entering all these factors into the multivariate analysis, only low IL-6 levels at the rechallenge initiation was observed to have a favorable association with OS2 (Table 5).
Among the patients who were rechallenged after initial grade 1 CIP, the median OS2 of patients continuing ICI without interruption was longer than that of patients who resumed ICI after interruption (22.1 vs. 15.5 months, P=0.97).
Discussion
This multicenter, retrospective study investigated 107 lung cancer patients with CIP, of whom 45 were rechallenged with an ICI and 62 were not. We found that severe grade and GGO at time of the first CIP were negatively associated with the ICI rechallenge outcome. Among the rechallenged patients, 9 (20.0%) developed recurrent CIP. The recurrence rate was associated with the grade 3–4 of CIP and ECOG PS ≥2 of the first CIP and with the levels of IL-6, CRP, WBC, and ANC at rechallenge. Most patients achieved a good response and curative effect following the first or rechallenge treatment with ICI. To our knowledge, our study is the most extensive characterization of ICI rechallenge after CIP in lung cancer patients.
On multivariate analysis, a severe grade of the first CIP was significantly and independently associated with non-rechallenge with ICI. For grade 3–4 CIP, the major guidelines do not recommend rechallenge (11-13). GGO was also negatively correlated with ICI rechallenge. One study showed that the survival of patients with GGO was significantly shortened (22), which may partly explain the reduced frequency of patients with GGO restarting ICI. Another possible reason was that the majority of patients with GGO were severe cases, leading clinicians to be cautious in choosing rechallenge. In the present study, the proportion of mixed-type CIP in the rechallenged group was lower than in the non-rechallenged group, and the mixed-type CIP was a negative factor for rechallenge in the univariate analysis results. Mixed-type CIP is pneumonitis with infection or PD or radiation pneumonitis, most of which are grade 2–4, with high severity (21), which reduces the opportunity for rechallenge. Interestingly, disease progression following the initial CIP episode may affect the choice of ICI rechallenge, because pneumonitis combined with disease progression complicate the patient’s condition, leading to clinicians to cautiously assess ICI rechallenge.
A CIP recurrence rate of 20.0% was observed in the rechallenged group. A study that included patients with ICI rechallenge after discontinuation for grade ≥2 irAEs, including 18 CIP, showed that 6 (33.3%) of these 18 patients had recurrent CIP (16). Another study showed that the recurrence rate of the same irAEs after rechallenge was 28.8%, and for CIP the rate was higher, reaching 34% (19). The lower recurrence rate in our study may be related to the inclusion criteria of the rechallenge group, which consisted of patients who did not had ICI suspended but continued treatment. In our study, 8 patients experienced recurrent pneumonitis in the non-rechallenged group. Asher et al. reported that among 9 patients with CIP, 7 were rechallenged, and of these 3 did experience recurrent CIP, while 3 were not rechallenged but experienced recurrent CIP (23). In our rechallenged group, recurrent CIP was more severe than the initial CIP, which was consistent with the findings of Asher et al. (23). In addition, the median time interval from rechallenge to CIP recurrence was shorter than from the first ICI to first CIP. One possible explanation for more severe and earlier CIP recurrence is that the first CIP caused lung damage, even when imaging ostensibly indicated recovery. Many studies have shown that people with underlying lung disease are more likely to develop CIP (24-26). Another important factor is that the initial CIP produces memory T cells (27), resulting in a faster activation of immune cells when ICI is rechallenged.
Patients with grade 1–2 CIP were generally selected for rechallenge after oncology, respiratory and radiology evaluations. Unusually, 5 patients in the study were rechallenged after grade 3 CIP. The decision to rechallenge ICI despite grade 3 CIP was made by a multidisciplinary team due to the patients’ high tumor burden and progression on multi-line treatment with no other available therapeutic option. Of these 5 patients, 4 experienced recurrent CIP and 2 died and 2 improved. We found that in both cases of death the patient had underlying lung disease, while the other 3 did not. Upon univariate logistic regression analysis, severe grade was related to recurrence of CIP. Similarly, poor ECOG PS at first CIP was associated with CIP recurrence. ECOG PS significantly correlated with the severity of initial CIP (Spearman’s rank correlation coefficient =0.55, P<0.001). Therefore, ICI rechallenge after grade ≥3 CIP should always be considered with caution, especially for patients with underlying lung disease.
We found that the IL-6, CRP, WBC and ANC levels at rechallenge were significantly higher in the patients with CIP recurrence compared with those without. On univariate analysis, these peripheral blood parameters at rechallenge were identified as risks factors for CIP recurrence. IL-6, CRP, WBC, and ANC have been reported to be inflammatory factors (28,29). A recent study analyzed CRP values at the time of diagnosis of 10 irAEs, revealing that, compared with patients with a CRP level less than the upper level of normal (ULN), patients with an elevated CRP (>2-fold ULN) had a higher risk of recurrence (P=0.054) (30). We hypothesize that these patients were rechallenged in an inflammatory state, and the cytokine levels were still very high, so the threshold for the occurrence of the second CIP may have been quite low. Upon ICI rechallenge, immune cells may have been rapidly activated and produced various cytokines that led to a cascade of reactions and the recurrence of CIP. Thus, rechallenge may not be indicated in patients when they are in an inflammatory state, even if they have recovered from the first CIP.
In this study, 10 patients with grade 1 initial CIP continued ICI treatment without suspension, and none developed exacerbation of CIP or recurrent CIP. The median OS at rechallenge seemed to indicate a net advantage over patients who resumed treatment with ICI after suspension. Therefore, for patients with grade 1 CIP and in good condition and with fewer lesions on imaging studies, ICI may not need to be suspended. However, more clinical data are needed to confirm these findings.
This study also demonstrated that patients in the rechallenged group had significantly higher ORR, as well as longer PFS and OS than those in the non-rechallenged group. However, there were more patients with grade ≥3 CIP in the non-challenged group. A severe CIP grade is reportedly associated with a poor prognosis (31). In our cohort, patients who experienced favorable outcomes with the initial ICI treatment also achieved similar favorable outcomes on rechallenge in terms of PFS (17.9 vs. 15.5 months) and OS (23.5 vs. 18.4 months). Xu et al. reported recently that median PFS for ICI rechallenge after disease progression was 6.8 months (95% CI: 5.8–7.8 months), but scant data are available in the literature to indicate the efficacy of ICI rechallenge after CIP (32).
The univariate analysis findings showed that an initial CIP of grade 1–2, good ECOG PS at first CIP, and non-recurrence of CIP were associated with favorable PFS and OS on rechallenge with ICIs. In addition, CIP recurrence was associated with the severity of both CIP and ECOG PS. A previous study showed that grade 1–2 CIP was associated with increased ICI efficacy, but that outcome evaluation did not involve an assessment of the efficacy of the rechallenge (31). In a report on patients with NSCLC who were rechallenged with ICI, the ECOG PS at rechallenge was independently associated with PFS and OS at rechallenge (14); however, the ECOG PS in our study was collected only at the initial CIP episode. Thus, the rather limited benefits of rechallenge in patients with severe grade or poor ECOG PS at the initial CIP should discourage physicians at this point from adopting rechallenge in this population.
In addition, elevated levels of IL-6 and CRP were associated with poor OS. CRP is an acute-phase protein induced by IL-6 in the liver (33). Indeed, in the multivariate analysis, only the IL-6 level was shown to independently influence OS at rechallenge. A previous clinical study we performed IL-6 to be an independent risk factor related to the clinical outcomes of initial ICI treatment in patients with lung cancer (34). A recent study in patients with irAEs also demonstrated that the CRP level decreased after receiving tocilizumab, an IL-6 inhibitor, resulting in clinical improvement in 27/34 patients (35). Thus, the application of IL-6 inhibitors may improve the effects of ICI on rechallenge and may reduce the recurrence rate in patients with high IL-6 levels.
This study evaluated the efficacy and safety of rechallenge in CIP patients and may help to screen for those would benefit from rechallenge. Nevertheless, this study has several limitations that should be considered. First, it was a real-world retrospective study. Second, due to the small sample size of CIP recurrence, multivariate analysis of the CIP recurrence rate was not carried out. Third, the practice standards of various institutions in this study may have been different and thus have affected both the data and its interpretation.
In conclusion, in study 20.0% of CIP patients with advanced lung cancer underwent CIP recurrence after ICI rechallenge. CIP recurrence of was associated with the grade (≥3) of CIP and ECOG PS (≥2) at the time of the initial CIP, and the IL-6, CRP, WBC and ANC levels at ICI rechallenge. Immunotherapy rechallenge had a good outcome in certain d CIP patients, particularly those with low-grade CIP and good ECOG PS at the first CIP, and low levels of IL-6 and CRP at rechallenge, as well as those without CIP recurrence. The conditions for rechallenge need to be further evaluated in prospective clinical trials.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This study was supported by National Key R&D Program of China (No. 2021YFC2301101), the Fundamental and Applied Fundamental Research Project of City-School (Institute) Joint Funding Project, Guangzhou Science and Technology Bureau (Nos. 202102010345, and 202102010357), Wu Jieping Medical Foundation (No. 320.6750.2020-19-8), and State Key Laboratory of Respiratory Disease-The Independent Project (No. SKLRD-Z-202206).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2021-38). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. All participating hospitals/institutions were informed and agreed the study.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-732/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-732/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-732/coif). YS serves as an unpaid editorial board member of Translational Lung Cancer Research from September 2020 to August 2022. MJ received lecturer fees, aboard fees and travel grants from AstraZeneca, Roche, Pfizer, Novartis, Takeda, MSD, Boehringeringelheim. The other authors have no conflicts of interest to declare.
References
- 1.Wagner G, Stollenwerk HK, Klerings I, et al. Efficacy and safety of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer (NSCLC): a systematic literature review. Oncoimmunology 2020;9:1774314. 10.1080/2162402X.2020.1774314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melosky B, Cheema PK, Brade A, et al. Prolonging Survival: The Role of Immune Checkpoint Inhibitors in the Treatment of Extensive-Stage Small Cell Lung Cancer. Oncologist 2020;25:981-92. 10.1634/theoncologist.2020-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139-48. 10.1016/j.ejca.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 4.Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:1721-8. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suresh K, Naidoo J, Lin CT, et al. Immune Checkpoint Immunotherapy for Non-Small Cell Lung Cancer: Benefits and Pulmonary Toxicities. Chest 2018;154:1416-23. 10.1016/j.chest.2018.08.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. 10.1200/JCO.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer 2018;125:150-6. 10.1016/j.lungcan.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 8.Cui P, Huang D, Wu Z, et al. Association of immune-related pneumonitis with the efficacy of PD-1/PD-L1 inhibitors in non-small cell lung cancer. Ther Adv Med Oncol 2020;12:1758835920922033. 10.1177/1758835920922033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suresh K, Voong KR, Shankar B, et al. Pneumonitis in Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Immunotherapy: Incidence and Risk Factors. J Thorac Oncol 2018;13:1930-9. 10.1016/j.jtho.2018.08.2035 [DOI] [PubMed] [Google Scholar]
- 10.Ma K, Lu Y, Jiang S, et al. The Relative Risk and Incidence of Immune Checkpoint Inhibitors Related Pneumonitis in Patients With Advanced Cancer: A Meta-Analysis. Front Pharmacol 2018;9:1430. 10.3389/fphar.2018.01430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714-68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119-42. 10.1093/annonc/mdx225 [DOI] [PubMed] [Google Scholar]
- 14.Albandar HJ, Fuqua J, Albandar JM, et al. Immune-Related Adverse Events (irAE) in Cancer Immune Checkpoint Inhibitors (ICI) and Survival Outcomes Correlation: To Rechallenge or Not? Cancers (Basel) 2021;13:989. 10.3390/cancers13050989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gobbini E, Toffart AC, Pérol M, et al. Immune Checkpoint Inhibitors Rechallenge Efficacy in Non-Small-Cell Lung Cancer Patients. Clin Lung Cancer 2020;21:e497-510. 10.1016/j.cllc.2020.04.013 [DOI] [PubMed] [Google Scholar]
- 16.Allouchery M, Lombard T, Martin M, et al. Safety of immune checkpoint inhibitor rechallenge after discontinuation for grade ≥2 immune-related adverse events in patients with cancer. J Immunother Cancer 2020;8:e001622. 10.1136/jitc-2020-001622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simonaggio A, Michot JM, Voisin AL, et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol 2019;5:1310-7. 10.1001/jamaoncol.2019.1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol Res 2018;6:1093-9. 10.1158/2326-6066.CIR-17-0755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolladille C, Ederhy S, Sassier M, et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol 2020;6:865-71. 10.1001/jamaoncol.2020.0726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 21.Lin X, Deng H, Chen L, et al. Clinical types of checkpoint inhibitor-related pneumonitis in lung cancer patients: a multicenter experience. Transl Lung Cancer Res 2021;10:415-29. 10.21037/tlcr-20-1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe S, Ota T, Hayashi M, et al. Prognostic significance of the radiologic features of pneumonitis induced by anti-PD-1 therapy. Cancer Med 2020;9:3070-7. 10.1002/cam4.2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asher N, Marom EM, Ben-Betzalel G, et al. Recurrent Pneumonitis in Patients with Melanoma Treated with Immune Checkpoint Inhibitors. Oncologist 2019;24:640-7. 10.1634/theoncologist.2018-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibaki R, Murakami S, Matsumoto Y, et al. Association of immune-related pneumonitis with the presence of preexisting interstitial lung disease in patients with non-small lung cancer receiving anti-programmed cell death 1 antibody. Cancer Immunol Immunother 2020;69:15-22. 10.1007/s00262-019-02431-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaguchi T, Shimizu J, Hasegawa T, et al. Pre-existing pulmonary fibrosis is a risk factor for anti-PD-1-related pneumonitis in patients with non-small cell lung cancer: A retrospective analysis. Lung Cancer 2018;125:212-7. 10.1016/j.lungcan.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 26.Galant-Swafford J, Troesch A, Tran L, et al. Landscape of Immune-Related Pneumonitis in Cancer Patients with Asthma Being Treated with Immune Checkpoint Blockade. Oncology 2020;98:123-30. 10.1159/000503566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suresh K, Naidoo J, Zhong Q, et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J Clin Invest 2019;129:4305-15. 10.1172/JCI128654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun 2018;70:61-75. 10.1016/j.bbi.2018.02.013 [DOI] [PubMed] [Google Scholar]
- 29.Ley K, Hoffman HM, Kubes P, et al. Neutrophils: New insights and open questions. Sci Immunol 2018;3:eaat4579. 10.1126/sciimmunol.aat4579 [DOI] [PubMed] [Google Scholar]
- 30.Lauwyck J, Beckwée A, Santens A, et al. C-reactive protein as a biomarker for immune-related adverse events in melanoma patients treated with immune checkpoint inhibitors in the adjuvant setting. Melanoma Res 2021;31:371-7. 10.1097/CMR.0000000000000748 [DOI] [PubMed] [Google Scholar]
- 31.Tone M, Izumo T, Awano N, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non-small cell lung cancer. Thorac Cancer 2019;10:2006-12. 10.1111/1759-7714.13187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Z, Hao X, Yang K, et al. Immune checkpoint inhibitor rechallenge in advanced or metastatic non-small cell lung cancer: a retrospective cohort study. J Cancer Res Clin Oncol 2022;148:3081-9. 10.1007/s00432-021-03901-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eklund CM. Proinflammatory cytokines in CRP baseline regulation. Adv Clin Chem 2009;48:111-36. 10.1016/S0065-2423(09)48005-3 [DOI] [PubMed] [Google Scholar]
- 34.Lin X, Deng H, Yang Y, et al. Peripheral Blood Biomarkers for Early Diagnosis, Severity, and Prognosis of Checkpoint Inhibitor-Related Pneumonitis in Patients With Lung Cancer. Front Oncol 2021;11:698832. 10.3389/fonc.2021.698832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract 2019;25:551-7. 10.1177/1078155217745144 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as