Abstract
Anurans are known to detect vibrations, but few studies explore relationships between vibrations and resultant behaviors. We studied the reaction of calling captive-bred male midwife toads (Alytes obstetricans) to the randomized playback of a vibrational crescendo stimulus train. We considered two sources of natural abiotic vibrational stimuli: rainfall and wind. Rainfall was expected to induce calling and wind was expected to inhibit it. Playback experiments with two synthetic tones (200 Hz and 300 Hz) tested the sensitivity to pure tones and could possibly reveal a hearing sensitivity trend between these frequencies. The toads did not increase call rate in response to rainfall vibrations and only one of the five wind stimulus levels caused a significant decrease in call rate. This limited response could be explained, because the tested toads came from a captive population, where emergence may not be mediated by rainfall vibrations. We found that A. obstetricans is highly sensitive to very low frequencies, which could explain the sensitivity observed to vibrational stimuli. Playback of a random crescendo stimulus train proves to be a valid approach for addressing behavioral questions. However, the use of a captive population may have been a limitation in the clarity of the results.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00359-022-01596-5.
Keywords: Seismic signals, Biotremology, Communication, Behavior, Hearing
Introduction
Anurans have an exquisite seismic sensor for the detection of vibrations (Narins and Lewis 1984). The anuran hearing system has been well-studied in recent years (Gerhardt and Huber 2002; Ryan 2001). However, comparatively little is known about behavioral responses to sensed substrate vibrations (Narins and Lewis 1984; Narins et al. 2007). The inner ear of anurans has a receptor organ for acoustic stimuli generally above 1000 Hz, the basilar papilla, that is homologous to that found in the hearing system of other higher vertebrates, such as birds and mammals. In addition to the basilar papilla, there are at least two other sensory organs in the anuran inner ear: the amphibian papilla and the sacculus (Simmons et al. 2006; Schoffelen et al. 2009). The amphibian papilla, an organ found exclusively in amphibians, is tuned to frequencies between 50 and 1000 Hz, whereas the sacculus is tuned to low frequencies between 20 and 300 Hz. These two organs are known to sense substrate-borne vibrations (Christensen Dalsgaard and Narins 1993; Lewis and Narins 1985) that may be related to behaviors of high selective value (Márquez et al. 2016).
This vibrational sensory system has been reported in a variety of contexts (Hill 2009), e.g., intra-specific signalling (Lewis and Narins 1985; Caldwell et al. 2010; Narins et al. 2018), prey detection (Solano 2016), predator avoidance (Warkentin 2005; Warkentin et al. 2017; Cohen et al. 2019), and detection of environmental cues (Halfwerk et al. 2016; Márquez et al. 2016). In the latter category, the authors showed that vibrations from wind and rain affected the behavior of species exposed to it. This means that, like other animals (Ben-Ari and Inbar 2014; Hill 2001), anurans use vibrational senses to process their surroundings (Halfwerk et al. 2016).
Given the importance of the hearing system in amphibian anurans, whose reproduction and survival depend on communication and the perception of the environment, abiotic vibrations could act not only by masking essential pieces of information but also in decision making. We choose as model species the midwife toads (Alytes obstetricans), because we have already evaluated their sensitivity to certain frequencies like the anthropogenic ones (Caorsi et al. 2019), so these further tests will allow us to better understand their vibrational hearing.
In particular, we studied the effect of abiotic vibrational noise (wind and rainfall) on the midwife toads using playback experiments to examine if they influence one of the most fundamental activities of anurans reproduction, the calling activity. Furthermore, we tested two synthetic tones (200 Hz and 300 Hz) derived from the peak frequencies of wind and rainfall. These playback stimuli allow us to explore the sensitivity of the toads to pure tones and to possibly detect a hearing sensitivity trend within these frequencies. We expect the wind to be an inhibitor of calling activity as active toads are less active on windy nights, possibly due to the desiccating effect of wind on toads (Oseen and Wassersug 2002; Steelman and Dorcas 2010; Brown 2013); while rainfall, on the other hand, is known to act as a trigger of emergence and activity in some species (Márquez et al. 2016) and is thus expected not to inhibit calling activity and eventually even increase calling rate in the study species.
Materials and methods
Location and study species
The playback experiments were completed between in June and July 2020 in the Amphibian Recovery Center of the Sierra de Guadarrama National Park, where a captive population of midwife toads (Alytes obstetricans, Alytidae) is established for the reintroduction of the toads in their natural habitats (Martín-Beyer et al. 2011). This anuran species is widespread across Europe but has suffered substantial declines throughout its range and population size, especially due to chytridiomycosis and ranavirosis (e.g., Bosch et al. 2001, 2021). The genus is known for its remarkable reproductive behavior, with males carrying the eggs entwined around their hind legs on land for about a month, from fertilization to hatching. Males typically call in open spaces, while tending occurs mainly underground. During this period the eggs are kept under adequate temperature and moisture conditions, away from predation or infection by fungi, and finally, the mature egg masses are released at the shore of a body of water (Márquez and Verrell 1991; Márquez 1995; Márquez and Bosch 1995).
We tested a total of 15 male radio-tagged individuals that were temporarily housed in the outdoor enclosure of the Center. Animals are housed indoors in winter to avoid low temperatures and to allow them to breed year-round and are kept in outdoor enclosures from May to September. After each complete experiment, the tested animal was identified and returned to its indoor terrarium. This population was selected for testing, because travel between regions was forbidden in Spain during the 2020 field season due to the COVID-19 pandemic and the captive population was within the limits of the region of Madrid. We verified that every animal was healthy by observation when measuring its length and weight after the test. We ensured that every animal had the minimum stress possible, minimizing handling and transportation times. Every individual tested was left 24 h in the open enclosure before testing. Experiments started after sunset and the emission of stimuli started when the focal male seemed at the peak of its vocal activity.
Playback stimuli
For the experiments, we constructed a set of 9 playback crescendo stimuli trains: rainfall, wind in five different intensities (5 km/h, 6 km/h, 7 km/h, 8 km/h, 9 km/h), a pure tone of 200 Hz, a pure tone of 300 Hz, and a silence stimulus (control). All stimuli lasted 2 min and were modified by applying a linear ‘fade-in’ amplitude filter from 0% to 100%, using Audacity 2.0.2 (SourceForge, Carnegie Mellon University, Pittsburgh, PA, USA) Before applying the ‘fade-in’ amplitude filter, the stimuli were peak normalized to 100% with some exceptions: the amplitude of the 5 wind stimuli was scaled, so that the highest wind level (9 km/h) was peak normalized at 100% but the rest kept their relatively lower amplitudes. Therefore, the highest peaks of the 4 lower wind stimuli did not reach 100%. The advantage of using fade-in stimuli is that only the maximum intensity of each stimulus had to be adjusted to the conditions when re-cording in the field. This was adjusted using exactly the same equipment and recording level in the playbacks as was used in the recording of the wind playback and ensuring recording level did not saturate. As for rainfall the maximum level was adjusted to the maximum level of the higher wind speed.
Rainfall vibrations were recorded in Puente Ajolí, (5 m.a.s.l. El Rocío, Doñana, Huelva 37º08′01862 N 6º27′44.98″W) in 2012 with an Oyo ONE geophone vertically protected by a foam-covered structure to prevent the direct impact of the drops on the geophone. The stimulus was the same that was used in Márquez et al. (2016). To obtain the spectral components of the rainfall vibration, recordings, FFTs (1,024 points, sampling rate 48 kHz, 61.9 Hz bandwidth) were made using Audacity 2.0.2 and Raven Pro 2.5 (Lab of Ornithology, Cornell University, Ithaca, NY, USA). The recordings obtained had slightly irregular flat spectra toward the lower end of the spectrum. Based on this acoustic signature, a 2-min synthetic vibration stimulus was generated with Audacity 2.0.2 by low-pass filtering broadband noise (100% amplitude).
Wind vibrations were recorded in Puerto de Morcuera (1700 masl 40º50′32.76″N, 3º50′10.912 W, Madrid) in July 2020 in a mountain grassland habitat similar to the habitat of the extant natural population of midwife toads in Peñalara, and in the near vicinity. Vibrations were recorded with an Oyo ONE geophone protected (vertically and laterally) by a foam-covered structure to prevent the direct impact of the wind on the geophone, while the wind speed was monitored with a Bresser (model 7002510) portable weather station.
Both for rainfall and wind vibrations, the geophone was connected to a custom-made amplifier which in turn was connected to the input of a Sound Devices 744 T recorder.
The pure tones of 200 and 300 Hz were included to test if there was a detection bias in the range considered. 200 Hz was an approximate central frequency for the wind stimuli and 300 Hz was an approximate central frequency for the rainfall stimuli. Synthetic stimuli were synthesized with Audacity software, and natural stimuli were edited and played back with the same software too. An online randomizer (https://www.random.org/lists/) was used to generate the random sequence of the stimuli. A single rainfall track was used and a single wind track was used for each of the five wind intensity levels.
Experimental design
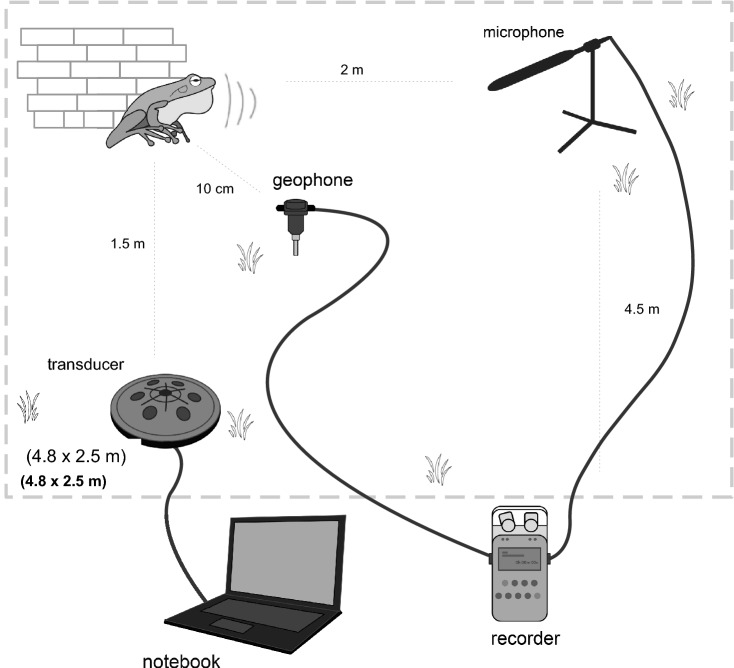
Experiments were carried out for 15 days during male calling activity, starting from 10 p.m., from the 8th of June to the 20th of July 2020. Alytes obstetricans males were calling in an open-air enclosure area with a small accumulation of bricks on a corner, which the toads used as a refuge and from which they called. Toads were individually marked with radio tags. Each toad was only tested once and was returned to its indoor terrarium after reading the tag after the test. One experiment was completed per night. One to three toads were housed in the enclosure but tests were started when only one individual was calling. Occasionally a second non-focal toad would start calling during the test. This was noted and considered in the statistical analyses. Playback vibrations were generated with a portable computer connected to a Kenwood KAC-5205 amplifier (with all filters switched off) and a “Clark Synthesis TST429 Platinum tactile-sound transducer” buried 10 cm below ground 1.5 m away from the bricks. To monitor the intensities of vibrations reaching the toads we placed an Oyo ONE geophone about 10 cm from the refuge that housed the animals during the experiment. The geophone was connected to a custom-made amplifier with a fixed gain of 30db. To record the intensities and number of calls of the toads we used a microphone (Audio Technica AT3032) placed approximately 2 m away from the calling male. The microphone and geophone were connected to a Sound Devices 744 T recorder with 4 channels. Playback was not equalized. The spectral components of the recorded signals by the geophone were only visually inspected at the time of playback and were compared to the emitted natural stimuli to verify that they were no major spectral degradation effects. The emitted stimuli were well within the ranges of the emission equipment (transducer and amplifier). The experimenters were located 4.5 m away from the enclosure, remaining motionless during the playback (Fig. 1).
Fig. 1.
Experimental setup in the field. The enclosure was 4.8 × 2.5 m wide. The microphone was placed 2 m away from the tested individual and the geophone was placed 10 cm away from it. The transducer was placed at a variable distance, but always more than 1.5 m away from the focal calling individual. Observers were located 4,5 m away from the enclosure
Test Protocol
Our test was based on the observation of a single calling toad before and during the emission of a crescendo stimulus train, a random sequence of stimuli separated by silence with each stimulus being linearly increasing in amplitude (“crescendo”, or Fade-in 0–100), similar to the technique used in Caorsi et al. (2019), Fig. 2. We emitted a playback train with 9 different stimuli to every individual (a total of 38 min). Every experiment started at 10 pm (sunset time, approximately). Each stimulus was 2 min long, with 2 min of silence before (pre-stimulus) and after (post-stimulus). The stimuli were presented in random order being different for each toad. Before each trial, we measured the temperature and the relative humidity.
Fig. 2.
Playback scheme (crescendo stimulus train) showing the 9 playback stimuli (total 38 min) presented to each animal in random order. Triangles indicate the increase of the amplitude of the vibration emission within the 2-min treatment
Data extraction
For every stimulus, pre-stimulus and post-stimulus we measured the following acoustic characteristics and measurements: total number of calls, mean dominant frequency of the last 5 calls, and mean fundamental frequency of the last 5 calls. In addition, we determined for each experiment: the total number of specimens in the enclosure, the total number of specimens calling in the enclosure, background noise, time before hearing the first specimen singing, duration of a call of a specimen before turning silent during the playback of a stimulus, duration of the 1° call group defined as a regular calling, duration of the 2° call group defined as a regular calling, duration of the 3° call group defined as a regular calling and mean duration of all regular call groups. We define regular calling an interrupted call group using Koehler et al. (2017) terminology. These variables represent the duration of the calling bouts of the calls during playback tests. They were recorded to spot toads with low calling activity in case they should not be included in the analyses but we did not exclude any test based on these parameters. The analysis was carried out with the software Raven Pro on an Acer Nitro 5 notebook. The FFT window size used was 2048, passband filter 0.5–10 kHz.
Statistical analysis
To test the effect of vibration stimuli on the calling activity of the focal individuals, we used a general linear mixed-effects model (LMM, Baayen 2008), as the experiment followed a repeated measured design within individuals. Thus, we included the type of playback stimulus (9 levels) as fixed factor, and individual (15 levels) as random factor. Since environmental temperature and the presence of other calling males may influence the calling activity of the focal males, we included two additional factors as covariates to account for these effects, namely, air temperature (measured every time at the beginning of any experiment) and the maximum number of non-focal males exhibiting calling activity during the playback test. The air temperature was previously z-transformed to be centred and scaled. The response variable of the LMM was estimated as the difference in call rate (number of calls per minute) between the 2-min experimental period (stimulus) and an average of five 2-min silence periods (pre-stimulus and post-stimulus of a given treatment and the entire silence stimulus of a given individual). This variable represents the behavioral response of the focal males to the vibration stimuli in comparison with their behavior during periods without stimuli. The response variable follows a symmetric distribution and hence we fitted a LMM with Gaussian error structure and identity link using the function lmer of the R package lme4 (Bates et al. 2014). The interaction terms and random slopes were not included to reduce model complexity. To test the effect of the stimulus order regardless the kind of vibration, we fitted a similar LMM but replacing type of stimulus by the position of every stimulus in each experimental sequence as fixed effect. The model structure and fitting procedure remained identical. Visual diagnostics (Q–Q plots, residuals plotted against fitted values, etc.) revealed no obvious deviations from the canonical assumptions of linearity, normally distributed and homogenous residuals, and the absence of influential observations in both LMMs. Variance inflation factor (Field 2005) was applied to a standard linear model (excluding the random effect) using the function vif of the R package car (Fox and Weisberg 2011) and indicated no collinearity (VIF < 1.29). The LMMs were fitted using the Maximum Likelihood (rather than Restricted Maximum Likelihood; Bolker et al. 2008) and model inference established by full-null model comparisons (null model comprising without the fixed effect) with a likelihood ratio test using the R function anova (test = “Chisq”; Dobson 2002; Forstmeier and Schielzeth 2011). The effect of covariates was based on additional likelihood ratio tests, comparing the full with respective reduced models using the R function drop1 (Barr 2013). Confidence intervals for model estimates and fitted values were derived using the function bootMer of the R package lme4, using 1000 parametric bootstraps and bootstrapping over the random effects. These intervals were used for post-hoc pairwise comparisons. Finally, we calculated conditional and marginal coefficients of determination with the function r.squaredGLMM of the R package MuMIn (Barton 2022). Significance level was set at 5%. All statistical analysis and figures were performed using R v 3.6.3 (R Core Team 2020).
Results
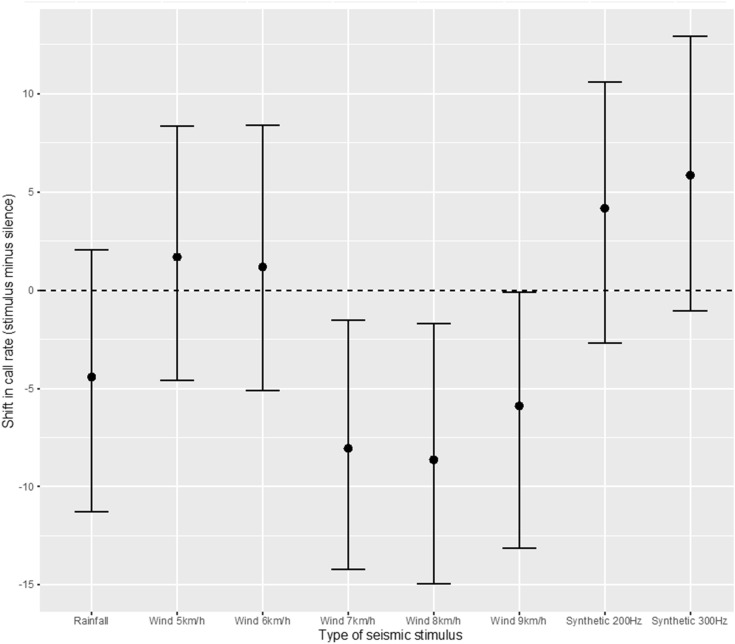
The full-null model comparison showed that the type of stimulus significantly influenced the call rate of the focal males (χ2 = 27.1, df = 7, p < 0.001). Specifically, calling activity increased with synthetic vibrations of 200 and 300 Hz, whereas it decreased when males were exposed to vibration stimuli associated with intense wind (7–9 km/h; Fig. 3). However, wide confidence intervals prevent us from establishing clear differences among stimuli. According to the model estimates, only pure tones of 200 and 300 Hz elicited a consistent (positive) response (Table 1). According to a visual inspection using predicted (fitted) values, only wind stimuli at higher intensity (7–9 km/h) showed a consistent (negative) response, with wind stimuli at 7 and 8 km/h evoking a significantly different shift in call rate than pure tone of 300 Hz (Fig. 3). No evidence of rainfall and weak wind vibrations having an effect on call rate was found, as indicated by their 95% confidence intervals including zero (Table 1; Fig. 3). Similarly, temperature and neighbour calling males did not influence calling behavior of the focal males (χ2 = 1.95, df = 1, p = 0.16; χ2 = 1.56, df = 1, p = 0.21, respectively).
Fig. 3.
Effect of vibrational stimuli on call rate, measured as difference in number of calls per minute between the 2-min experimental period (stimulus) and an average of five 2-min silence periods (pre-stimulus and post-stimulus of a given treatment and the entire silence stimulus of a given individual). Points are mean predicted (fitted) values of shift in call rate, corresponding to the additive effect of each coefficient when taking into account the covariates included in the model. Bars represent 95% confidence intervals for each type of stimulus as obtained with the R function bootMer applied on the fitted regression model. The dashed line indicates no change in the call rate. We considered the effect of a stimulus in call rate as significant if the estimate and corresponding 95% confidence interval did not include zero. Shifts in call rate larger than 0 represent a positive effect (i.e., stimulus increasing calling activity). Shifts in call rate below 0 indicate a negative effect (i.e., stimulus decreasing calling activity). In addition, using confidence intervals for post-hoc pairwise comparisons, we considered the effect of a stimulus as significantly different from another stimulus if their confidence intervals did not overlap
Table 1.
Model estimates of the effect of the vibration stimuli on call rate of the midwife toad
| Fixed effects | Estimate | SE | CI.lb | CI.ub |
|---|---|---|---|---|
| (intercept) Rainfall | –4.414 | 3.393 | –11.818 | 1.789 |
| Wind 5 km/h | 6.107 | 3.810 | –0.900 | 13.754 |
| Wind 6 km/h | 5.606 | 3.813 | –2.105 | 13.391 |
| Wind 7 km/h | –3.634 | 3.813 | –10.977 | 4.197 |
| Wind 8 km/h | –4.213 | 3.810 | –11.541 | 3.839 |
| Wind 9 km/h | –1.480 | 3.810 | –8.848 | 5.826 |
| Synthetic 200 Hz | 8.587 | 3.810 | 1.353 | 16.549 |
| Synthetic 300 Hz | 10.267 | 3.810 | 2.685 | 18.553 |
| Nb, calling males | 3.087 | 2.385 | –1.461 | 8.000 |
| Temperature | –3.106 | 2.117 | –7.049 | 1.054 |
The standard error of the estimates (“SE”) and the lower and upper bounds of the 95% confidence intervals (“CI.lb” and “CI-ub”) are shown
The fixed factors explained less than a quarter of the total variance (R2m = 0.234), while both fixed and random factors of the model accounted for less than half (R2c = 0.459). The largest proportion of the variability on the response variable that was not explained by fixed factors is due to the “residual variance” (71%), meaning that the differences among the focal individuals (“random variance”, 29%) were less relevant, and factors other than individuals and/or our predictor and covariates should account for this unexplained variability in call rate.
Regardless of the type of vibration, the order of stimuli within the experimental sequence had no effect on calling activity of the focal males (χ2 = 8.3, df = 8, p = 0.40), with all stimulus orders showing no significantly different shifts in call rate (Fig. 4). The actual distribution of the order of presentation of the randomly chosen sequences during the experiments indicated that some stimuli, such as rainfall and wind 8 km/h, were presented at the onset of the experiment more often than other stimuli.
Fig. 4.
Effect of the order of presentation of the stimuli on call rate. Points are mean predicted (fitted) values of shift in call rate, and bars are 95% confidence intervals for each stimulus order. The dashed line indicates no change in the call rate (see further details in Fig. 3)
Discussion
Among the environmental stimuli, the only significant observed effect on calling rate is limited to the stimulus two of the high levels of wind (7 and 8 km/h) which, as predicted, inhibits calling behavior and thus alters its call rate. The remaining high wind speed (9 km/h) also inhibits the calling rate but the effect is not significant (Table 1 and Fig. 3). The two lighter wind speeds (5 km/h and 6 km/h) increased slightly, not significantly, calling rate.
The toads did not react to rainfall vibrations by increasing their call rate. Contrary to expectations, it could be related to the fact that the tested toads came from a captive population, where emergence to the surface is not mediated by rainfall vibrations. This effect has been observed in other species in their natural habitat (Márquez et al. 2016). The vibrations were presented in random order to the toads. Each test had its specific sequence. The random sequences used presented rainfall in the first position on several occasions (3 of 15 toads) but the first stimulus presented did not have a substantially larger inhibitory effect (Fig. 4). On the other hand, the single frequency vibrations (pure tones) increased call rate significantly. This result was not expected and opens many questions about how wide the frequency band of the stimulus has to be to elicit call inhibition and whether airborne stimulation (auditory) may play a role.
Anuran auditory neurons have a clear frequency selectivity that characterizes many frog species by their neural tuning curves, or frequency-threshold curves (Dijk et al. 2011; Zakon and Wilczynski 1988). In the focal species, a study measured the sensitivity of the torus semicircularis auditory midbrain to frequencies from 100 to 5000 Hz. It revealed regions of high sensitivity in the low-frequency range, between approximately 100–500 Hz and, in the high-frequency range, between approximately 1200–2400 Hz. The best thresholds in the lower frequency range reached values of approximately 40 dB SPL, occurring at the lowest audio frequency tested (100 Hz), whereas those in the high-frequency range were between 40 and 50 dB SPL (Mohneke and Schneider 1979; Penna et al. 2015). Thus, A. obstetricans is highly sensitive to very low frequencies, which could explain the sensitivity observed to vibrational stimuli (10 Hz and 100 Hz) in previous experiments in nature (Caorsi et al. 2019), and, to a lesser extent, in the present study.
The experimental setup of the playback of the crescendo stimulus trains appears to be a valid approach for addressing behavioral questions. The limitation to one exemplar of each natural stimulus may affect the power of the statistical tests applied but it is difficult to increase the number of exemplars per test without increasing excessively the duration of the playback test. Based on the natural calling pattern of the species, the first 2 h after sunset are the best time window to run the tests. On the other hand, the lack of an effect of presentation order (Fig. 4) appears to support this approach.
The use of a captive population which is kept in an environment with a high level of anthropogenic vibrations (terrarium maintenance and feeding, aerators of adjacent aquaria and vibration from a nearby road), may have affected the response of the toads to the stimuli if there is habituation. This may explain the limitation in the clarity of the results, although the model used yielded results with factors that explained a sizeable percentage of the variance. If naturally occurring populations of toads tested in the field respond differently, this remains to be demonstrated.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Access and study permits were granted by Parque Nacional de la Sierra de Guadarrama, Spain. We are grateful to Dr. Richard G. Bowker who reviewed the English version of the manuscript and to two anonymous reviewers who provided valuable comments on the study. We thank Roger Mundry for providing R functions to compute GLMM confidence intervals and model assumptions. DL also acknowledges a postdoctoral grant provided by Comunidad de Madrid (2020-T1/AMB-20636, Programa de Atracción de Talento investigador, Spain).
Author contributions
RM and JFB conceptualized the study. JL and RM collected data and wrote the main manuscript text. VZ-C prepared figures 1 and 2. DL and JB made the statistical analysis and made figures 3, 4 and 5. All authors reviewed the manuscript. All authors contributed to the preparation of the tests.
Funding
Partial financial support was received from “Torno Subito” scholarship from Lazio Region in Italy (JDL).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Baayen RH. Analyzing linguistic data. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- Barr DJ. Random effects structure for testing interactions in linear mixed-effects models. Front Psychol. 2013;4:328. doi: 10.3389/fpsyg.2013.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton K (2022) MuMIn: multi-model inference. R package version 1.46.0. R-Forge. https://CRAN.R-project.org/package=MuMIn. Accessed 12 Oct 2022
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2014;67(1):1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Ben-Ari M, Inbar M. Aphids link different sensory modalities to accurately interpret ambiguous cues. Behav Ecol. 2014;25(3):627–632. doi: 10.1093/beheco/aru033. [DOI] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol. 2008;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Bosch J, Martínez-Solano I, García-París M. Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of Central Spain. Biol Conserv. 2001;97:331–337. doi: 10.1016/S0006-3207(00)00132-4. [DOI] [Google Scholar]
- Bosch J, Mora-Cabello de Alba A, Marquínez S, Price SJ, Thumsová B, Bielby J. Long-term monitoring of amphibian populations of a national park in Northern Spain reveals negative persisting effects of Ranavirus, but not Batrachochytrium dendrobatidis. Front Vet Sci. 2021;8:64549. doi: 10.3389/fvets.2021.645491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DJ. Predictive models for calling and movement activity of the endangered Houston toad. Am Midl Nat. 2013;169:303–321. doi: 10.1674/0003-0031-169.2.303. [DOI] [Google Scholar]
- Caldwell MS, Johnston GR, McDaniel JG, Warkentin KM. Vibrational signaling in the agonistic interactions of red-eyed treefrogs. Curr Biol. 2010;20:1012–1017. doi: 10.1016/j.cub.2010.03.069. [DOI] [PubMed] [Google Scholar]
- Caorsi V, Guerra V, Furtado R, Llusia D, Miron L, Borges-Martins M, Both C, Narins P, Meenderink S, Márquez R. Anthropogenic substrate-borne vibrations impact anuran calling. Sci Rep. 2019 doi: 10.1038/s41598-019-55639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen Dalsgaard J, Narins PM. Sound and vibration sensitivity of VIIIth nerve fibers in the frogs Leptodactylus albilabris and Rana pipiens pipiens. J Comp Physiol A. 1993;172:653–662. doi: 10.1007/BF00195391. [DOI] [PubMed] [Google Scholar]
- Cohen KL, Piacentino ML, Warkentin KM. Two types of hatching gland cells facilitate escape-hatching at different developmental stages in red-eyed treefrogs, Agalychnis callidryas (Anura: Phyllomedusidae) Biol J Linn Soc Lond. 2019;126:751–767. doi: 10.1093/biolinnean/bly214. [DOI] [Google Scholar]
- Dijk P, van Mason MJ, Schoffelen RLM, Narins PM, Meenderink SWF. Mechanics of the frog ear. Hear Res. 2011;273:46–58. doi: 10.1016/j.heares.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson AJ. An introduction to generalized linear models. Boca Raton: Chapman & Hall/CRC; 2002. [Google Scholar]
- Field A. Discovering Statistics using SPSS. London: Sage Publications; 2005. [Google Scholar]
- Forstmeier W, Schielzeth H. Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behav Ecol Sociobiol. 2011;65(1):47–55. doi: 10.1007/s00265-010-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S. An R companion to applied regression. Thousand Oaks: Sage Publications; 2011. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic communication in insects and anurans. Common problems and diverse solutions. Chicago: University of Chicago Press; 2002. [Google Scholar]
- Halfwerk W, Ryan MJ, Wilson PS. Wind- and rain-induced vibrations impose different selection pressures on multimodal signaling. Am Nat. 2016;188:279–288. doi: 10.1086/687519. [DOI] [PubMed] [Google Scholar]
- Hill PSM. Vibration and animal communication: a review. Am Zool. 2001;41(5):1135–1142. doi: 10.1093/icb/41.5.1135. [DOI] [Google Scholar]
- Hill PSM. How do animals use substrate-borne vibrations as an information source? Sci Nat. 2009;96:1355–1371. doi: 10.1007/s00114-009-0588-8. [DOI] [PubMed] [Google Scholar]
- Koehler J, Jansen M, Rodriguez A, Kok PJ, Toledo LF, Emmrich M, Vences M. The use of bioacoustics in anuran taxonomy: theory, terminology, methods and recommendations for best practice. Zootaxa. 2017;4251(1):1–124. doi: 10.11646/ZOOTAXA.4251.1.1. [DOI] [PubMed] [Google Scholar]
- Lewis ER, Narins PM. Do frogs communicate with seismic signals? Science. 1985;227:187 LP–189. doi: 10.1126/science.227.4683.187. [DOI] [PubMed] [Google Scholar]
- Márquez R. Female choice in the midwife toads (Alytes obstetricans and A. cisternasii) Behavior. 1995;132(1–2):151–161. doi: 10.1163/156853995X00342. [DOI] [Google Scholar]
- Márquez R, Bosch J. Advertisement calls of the midwife toads Alytes (Amphibia, Anura, Discoglossidae) in continental Spain. J Zool Syst Evol Res. 1995;33(3–4):185–192. doi: 10.1111/j.1439-0469.1995.tb00224.x. [DOI] [Google Scholar]
- Márquez R, Verrell P. The courtship and mating of the Iberian midwife toad Alytes cisternasii (Amphibia: Anura: Discoglossidae) J Zool. 1991;225(1):125–139. doi: 10.1111/j.1469-7998.1991.tb03806.x. [DOI] [Google Scholar]
- Márquez R, Beltrán JF, Llusia D, Penna M, Narins PM. Synthetic rainfall vibrations evoke toad emergence. Curr Biol. 2016;26(24):R1270–R1271. doi: 10.1016/j.cub.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Martín-Beyer B, Fernández-Beaskoetxea S, García G, Bosch J (2011) Re-introduction program for the common midwife toad and Iberian frog in the Natural Park of Peñalara in Madrid, Spain: can we defeat chytridiomycosis and trout introductions? Digital.CSIC. https://digital.csic.es/handle/10261/124177. Accessed 10 Oct 2021
- Mohneke R, Schneider H. Effect of temperature upon auditory thresholds in two anuran species, Bombina v. variegata and Alytes o. obstetricans (Amphibia, Discoglossidae) J Comp Physiol A. 1979;130:9–16. doi: 10.1007/BF02582969. [DOI] [Google Scholar]
- Narins PM, Lewis ER. The vertebrate ear as an exquisite seismic sensor. J Acoust Soc Am. 1984;76:1384–1387. doi: 10.1121/1.391455. [DOI] [PubMed] [Google Scholar]
- Narins PM, Feng AS, Fay RR, Popper AN. Hearing and sound communication in amphibians. New York: Springer; 2007. [Google Scholar]
- Narins PM, Meenderink SWF, Tumulty JP, Cobo-Cuan A, Márquez R. Plant-borne vibrations modulate calling behavior in a tropical amphibian. Curr Biol. 2018;28:R1333–R1334. doi: 10.1016/j.cub.2018.10.056. [DOI] [PubMed] [Google Scholar]
- Oseen KL, Wassersug RJ. Environmental factors influencing calling in sympatric anurans. Oecologia. 2002;133:616–625. doi: 10.1007/s00442-002-1067-5. [DOI] [PubMed] [Google Scholar]
- Penna M, Velásquez NA, Bosch J. Dissimilarities in auditory tuning in midwife toads of the genus Alytes (Amphibia: Anura) Biol J Linn Soc Lond. 2015;116:41–51. doi: 10.1111/bij.12563. [DOI] [Google Scholar]
- R Core Team . R: a language and environment for statistical computing. Vienna: R foundation for statistical computing; 2020. [Google Scholar]
- Ryan MJ. Recent advances in anuran communication. Washington: Smithsonian Institution Press; 2001. [Google Scholar]
- Schoffelen RLM, Segenhout JM, Dijk P. Tuning of the tectorial membrane in the basilar papilla of the northern leopard frog. J Assoc Res Otolaryngol. 2009;10:309–320. doi: 10.1007/s10162-009-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DD, Sebastiaan WF, Pantelis NV. Hearing and sound communication. NewYork: Springer; 2006. Anatomy, physiology, and function of auditory end-organs in the frog inner ear. [Google Scholar]
- Solano LAR. Foraging behavior with possible use of substrate-borne vibrational cues for prey localization in Atelopus laetissimus (Ruiz-Carranza, Ardila-Robayo, and Hernández-Camacho, 1994) Herpetol Notes. 2016;9:191–195. [Google Scholar]
- Soorae PS. Global re-introduction perspectives: more case studies from around the globe. Abu Dhabi: IUCN/SSC re-introduction specialist group and environment agency; 2011. [Google Scholar]
- Steelman CK, Dorcas ME. Anuran calling survey optimization: developing and testing predictive models of anuran calling activity. J Herpetol. 2010;44:61–68. doi: 10.1670/08-329.1. [DOI] [Google Scholar]
- Warkentin KM. How do embryos assess risk? Vibrational cues in predator-induced hatching of red-eyed treefrogs. Anim Behav. 2005;70:59–71. doi: 10.1016/j.anbehav.2004.09.019. [DOI] [Google Scholar]
- Warkentin KM, Diaz JC, Güell BA, Jung J, Kim SJ, Cohen KL. Developmental onset of escape-hatching responses in red-eyed treefrogs depends on cue type. Anim Behav. 2017;129:103–112. doi: 10.1016/j.anbehav.2017.05.008. [DOI] [Google Scholar]
- Zakon HH, Wilczynski W. The evolution of the amphibian auditory system. New York: Wiley; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.