Abstract
Background:
Emotional disorder is an important indicator for assessing the quality of life (QOL) of people with epilepsy (PWE). Depression, somatic symptom disorder (SSD) and anxiety are among the most frequently occurring mental disorders and overlap with each other.
Objectives:
This study examines the overlap of these three emotional disorders and their effects separately and in combination on the QOL of PWE.
Design:
Cross-sectional study.
Data Sources and Methods:
Adults attending our epilepsy clinic between 1 July 2020 and 1 May 2022 were consecutively enrolled. They were screened for depression, SSD, and anxiety by structured interviews, and demographic, epilepsy-related and QOL indicators were collected. Multivariate analysis, propensity score matching (PSM) and stratified analysis were used to explore the effects of their respective and combined effects on QOL.
Results:
Among the 749 patients, 189 patients (25%) were diagnosed with depression, 183 patients (24%) were diagnosed with SSD, and 157 patients (21%) were diagnosed with anxiety. The frequency of occurrence of each emotional disorder together with other emotional disorders was higher than the frequency of occurrence of an emotional disorder alone. Depression, SSD, and anxiety all had an independent effect on QOL of PWE (p < 0.001). Depression had the greatest effect, followed by SSD, and then anxiety (β: multivariate analysis, −11.0 versus –7.8 versus –6.5; PSM, −14.7 versus –9.4 versus –6.8). The QOL of PWE decreased more significantly with the increasing number of comorbid emotional disorders (β: –12.1 versus –20.7 versus –23.0).
Conclusion:
It is necessary to screen for three emotional disorders, that is, depression, SSD, and anxiety, in PWE. Attention should be paid to people with multiple comorbid emotional disorders.
Keywords: comorbidity, emotional disorder, QOL, risk factors, seizure
Introduction
Epilepsy is one of the most common neurological diseases. Epidemiological studies have shown that approximately 70 million people of different ages worldwide suffer from epilepsy.1 In terms of global disability-adjusted life-years (DALYs), epilepsy ranked 20th globally among diseases in 2016 and ranked second among neurological diseases.2 The quality of life (QOL) of people with epilepsy (PWE) is significantly reduced.3,4 Studies have found that epilepsy-related factors have a limited impact on the QOL of PWE and that emotional disorders (anxiety and depression) have the most significant impact on this indicator.3–7 Therefore, emotional disorder is an important indicator for assessing the QOL of PWE. In the International League Against Epilepsy (ILAE) classification of the epilepsies issued in 2017, the ILAE calls for the inclusion of emotional disorders in the early diagnosis and early treatment of epilepsy.8 Subsequently, the ILAE Psychological Task Force released psychological treatment and medical treatment of co-morbid depression for PWE in 2018 and 2022, respectively.9,10
The three most common emotional disorders are anxiety, depression, and somatic symptom disorder (SSD).11–14 Co-morbid depression and anxiety are well documented in PWE in the literature. There is compelling evidence for a bidirectional relationship between epilepsy and depression: epilepsy was frequently followed by depression,15 and prior history of depression was a risk factor for subsequent unprovoked seizures.16 Shared pathophysiology of the two disorders, such as hippocampus,17 amygdala,18 and orbitofrontal cortex19 volume loss; alteration in neurotransmitter system;20 and hypothalamic–pituitary–adrenal axis dysregulation,21,22 may contribute to their interlinking relationship. SSD is a new definition for persistent distressing somatic symptoms from the 2013 DSM-5 (Diagnostic and Statistical Manual of Mental Disorder-5) and replaces somatoform disorders, which had vague diagnostic criteria that were difficult to apply clinically. The diagnosis of SSD must meet three criteria: (1) one or more somatic symptoms that make the individual feel distressed or cause significant damage to his or her daily life; (2) excessive thoughts, feelings, or behaviors related to somatic symptoms, or excessive worry related to health; and (3) although all somatic symptoms may not persist, the symptomatic state is persistent (typically longer than 6 months).23 From the perspective of the definition of SSD, its pathogenesis is largely derived from the body’s erroneous cognition of somatic symptoms. Although SSD may be accompanied by anxiety and depression, the basic treatment measures include psychotherapy, such as cognitive behavioral therapy and mindfulness therapy.24 Previous studies have consistently shown that individuals with SSD reported lower QOL compared to those without in general or outpatient and inpatient samples.25–28 Currently, no study has explored the effect of comorbid SSD on epilepsy people.
According to high-quality literature reviews, only a few studies used structured clinical interviews to diagnose depression and anxiety in PWE,29,30 precluding reliable estimates of the prevalence of these disorders. In addition, the relationship among the three emotional disorders in reality is not pure, and they can be independent of each other or overlap with each other. Their overlapping relationship is less well studied. Therefore, screening depression, anxiety, and SSD by structured interviews, and studying their overlapping relationship and effects separately and in combination on the QOL of PWE can help to develop more scientific screening and treatment protocols to improve patient outcomes.
In this study, PWE were screened for depression, SSD, and anxiety by structured interviews, and demographic, epilepsy-related and QOL indicators were collected. The aims of the study were as follows: (1) to clarify the prevalence of the three emotional disorders, either occurring alone or occurring at the same time; (2) to investigate how the three emotional disorders overlap; (3) to investigate the independent effects of each of the three emotional disorders on the QOL of PWE by controlling for confounding factors through multivariate analysis and propensity score matching (PSM); and (4) to explore, using stratified analysis, the impact of the combination of the three emotional disorders on QOL.
Method
Subjects
PWE who were treated in our epilepsy clinic between 1 July 2020 and 1 May 2022, were continuously enrolled.
Inclusion criteria
(1) People who were 18 years of age and older; (2) people who had been diagnosed with epilepsy for at least 1 year; (3) people with a score of 69 or higher on the Wide Range Achievement Test (WRAT; to ensure his or her reading ability); and (4) people who agreed to participate in this study, participated in structured interviews for three emotional disorders with doctors, and completed questionnaires.
Exclusion criteria
(1) People who lost the ability to provide self-assessments or refused to participate in the study; (2) people who were undergoing vagal nerve stimulation; and (3) people who were confirmed to have dementia or severe mental disorders, such as schizophrenia.
Methods
Data collection process
Continuous sampling was used. PWE who met the inclusion and exclusion criteria were invited to participate in this study when they were waiting at the neurology clinic. The purpose, content and process of the study were described, and the principle of voluntariness and privacy protection measures were explained. All participants who were willing to participate in the study signed an informed consent form. The participants who signed the informed consent form were invited to another room when they were waiting for treatment. First, they underwent self-report questionnaires to screen for depression, SSD, and anxiety, including Patient Health Questionnaire (PHQ-9), symptom-related psychological distress questionnaire (SSD-12) and Generalized Anxiety Disorder 7-item scale (GAD-7). Those with a total score higher than cutoff points of any one of the questionnaires, then underwent a structured clinical interview conducted by the psychiatrist investigator in accordance with the structured clinical interview for DSM-5, research version (SCID-5-RV) to confirm whether three emotional disorders were present. After the interview, the participants were treated at the neurology clinic. To rule out possible false-positives, neurologists distinguished SSD from adverse reactions to antiseizure medication (ASM) and seizures in general based on the relationship between the timing of the onset of somatic symptoms and the use and discontinuation of ASMs and based on whether the symptoms were episodic, transient, stereotypic in nature and occurred in post-ictal period. Then, the participants were asked to complete an Internet-based self-compiled questionnaire within 1 week. Demographic–sociological, epilepsy-related, mental state–related and QOL-related information were collected from the participants. After the questionnaire was submitted, the investigator confirmed the accuracy of the submitted questionnaire by telephone inquiry within 12 h. The data for participants whose key information was incorrect or lost or who could not be reached by phone were deleted from the samples. The comprehensive collection of epilepsy-related information, such as type of epilepsy, etiology, and drug-resistant epilepsy (DRE), was performed by two epilepsy specialists using each patient’s medical history and medical orders, epilepsy-related information gathered from the questionnaire, and electroencephalography (EEG), video EEG, and cranial magnetic resonance imaging (MRI) results in accordance with ILAE standards. Disagreements between the two parties were resolved through discussions until a consensus was reached.
Self-compiled questionnaire and assessment scale
Demographic data questionnaire
Self-compiled questionnaire. The collected indicators included name, gender, age, height, body weight, ethnicity, place of residence, education level, medical insurance, marital status, living conditions, occupation, personal monthly income, monthly household income, and physical activity.
Assessment scales
PHQ-9: This questionnaire consists of nine depression screening items and assesses the degree of distress caused by depressive symptoms in patients in the past 2 weeks. Each item is scored from 0 to 3 points, with a total score of 0–27 points. Previous studies have shown that a total score of 10 points is the optimal threshold for diagnosing depression.31–33 The higher is the total score, the more severe are the depressive symptoms.
SSD-12: The SSD-12, composed of 12 items, measured the three psychological sub-criteria of SSD (cognitive, affective, and behavioral aspects associated with bothersome somatic symptoms). Each item is scored from 0 (never) to 3 (very often) points, with a total score of 0–48 points. Previous study in China have shown that a total score of 16 is the optimal cutoff point for the diagnosis of SSD.34 The higher is the total score, the more severe are the SSD symptoms.
GAD-7: This scale consists of seven items related to generalized anxiety and assesses the degree of distress caused by anxiety symptoms in the past 2 weeks. Each item is scored from 0 to 3 points, with a total score of 0–21 points. Previous studies have shown that a total score of 10 is the optimal threshold for the diagnosis of generalized anxiety.31 The higher is the total score, the more severe are the anxiety symptoms.
Quality of Life in Epilepsy Inventory 31 (QOLIE-31): The scale has a total of 31 items in seven subscales.35 Each item is scored using the percentile system, and each item has a corresponding score for different options. When scoring, the subscale score is first calculated; the subscale score is equal to the sum of the scores of all the items in the subscale divided by the number of items in the subscale. The total scale score is then calculated; the total scale score is equal to the sum of the scores of each subscale from the previous step multiplied by the weight of that subscale. The higher is the total score, the better is QOL.
Diagnosis of three emotional disorders using structured interviews
In this study, we conducted structured clinical interviews for the diagnosis of depression, SSD, and anxiety based on the SCID-5-RV.
Evaluation of epilepsy-related information
Epilepsy-related information was collected using self-compiled questionnaires and expert assessments. The age of onset of epilepsy, disease duration, epilepsy-related past history, severity of epilepsy symptoms (expressed by score of the Chalfont-National Hospital Seizure Severity Scale, NHS3 scale), seizure frequency, and use of ASM were collected from the questionnaire. Frequent seizures were defined as seizure frequencies higher than once a month. Epilepsy-related EEG changes, epilepsy-related MRI lesions, status epilepticus,36,37 types of epilepsy,38 etiology of epilepsy,38 and drug-resistant epilepsy (DRE)39 were evaluated by two epilepsy specialists using ILAE criteria and the medical history of each patient in the medical order system, epilepsy-related information provided on the questionnaire, and conventional EEG, video EEG, and cranial MRI results. Disagreements between the two parties were resolved through discussions until a consensus was reached.
Outcome indicators
The outcome indicator was patient QOL, expressed as the QOLIE-31 score. The higher is the score, the better is the QOL.
Statistical analysis
SPSS 22.0 and R language (version 3.6.2) were used for data analyses. DataGraph 4.6 software was used to generate images.
Measurement data that conformed to a normal distribution are expressed as the mean ± standard deviation (mean ± SD), and measurement data that did not conform to a normal distribution are expressed as the median and interquartile range. Categorical data are expressed as frequency (%).
To facilitate statistical analyses, multicategory variables were reassigned to two-category variables: divorced or separated were coded as abnormal marriage conditions, and other marriage situations were coded as non-abnormal marriage conditions. A high school education or below were coded as low educational background, and other educational level were coded as non-low educational background. A monthly household income of less than 4000 yuan were coded as low monthly household income, and other monthly household income were coded as non-low monthly household income. Living alone, living in a nursing home, and living with other relatives were coded as abnormal living conditions, and other living situations were coded as non-abnormal living conditions.
In this study, two different statistical methods were used to control for confounding factors, and the independent effects of depression, anxiety, and SSD on the QOL of PWE were investigated. Method 1 was multivariate regression analysis; Method 2 was PSM.
Before multivariate analysis, univariate analysis was performed to screen variables that might have an impact on the QOL of PWE. The variables screened by univariate analysis that have an impact on the QOL of patients with epilepsy were used as the independent variable (X), and the QOLIE-31 score for PWE was used as the dependent variable (Y). The independent effect of three emotional disorders on the QOL of PWE was investigated, and the result are expressed as β [(95% confidence interval (CI)].
Before PSM, intergroup comparisons between depressed patients and nondepressed patients, patients with anxiety and patients without anxiety, and SSD patients and non-SSD patients were performed. The factors that were significantly different (p < 0.05) between groups were used as the matching variables, and the propensity score (PS) was obtained through the logistic regression formula. The nearest neighbor matching (NNM) method was used, and the caliper value was 0.02. The matching was performed at a ratio of 1:1 to obtain matched datasets for depression, anxiety, and SSD. Multivariate regression analysis was performed using the matched datasets to verify the independent effects of depression, anxiety, and SSD on the QOL of PWE
In addition, in this study, the patients were divided into the comorbid SSD and anxiety, comorbid SSD and depression, comorbidity anxiety and depression, and comorbid SSD and anxiety and depression groups on the basis of the overlap among the three emotional disorders. The effects of different combinations of depression, anxiety, and SSD on the QOL of PWE was investigated by stratified analysis. Using the number of comorbid emotional disorders, the patients were divided into the following groups: zero, one, two, and three comorbid emotional disorders. The impact of the number of comorbid emotional disorders on the QOL of PWE was investigated by stratified analysis.
Results
Description of the study population
Between 1 July 2020, and 1 May 2022, 890 PWE met the inclusion and exclusion criteria and were willing to participate in this study. A total of 787 questionnaires with complete information were collected, and the response rate was 88%. The questionnaire information submitted by 38 participants was inconsistent with the information confirmed by the investigator by telephone; therefore, they were excluded. Overall, 749 PWE were enrolled in this study.
The average age of the 749 subjects was 28.2 years (age range of 18–84 years), and 384 were males, accounting for 51.3% of the sample. A total of 355 participants (47.4%) lived in rural areas, 97 people (13.0%) did not have medical insurance, 123 participants (16.4%) lived alone or did not live with their family members, and 57 people (7.6%) were divorced or separated. Approximately 58.5% of PWE had a high school education or below, 24.3% were unemployed or out of school, 53.8% had no regular income, and 40.5% had a monthly household income of less than 4000 yuan. The average age of onset of epilepsy was 19.8 years, and the average disease duration was 8.9 years. A total of 68.1% of the people had epilepsy-related abnormalities on EEG, 44.3% had an abnormal cranial MRI, 24.2% had experienced status epilepticus during the course of disease, and 27.2% had a seizure frequency greater than once a month. The average NHS3 score for epilepsy severity was 7.1 points. According to the assessment by two epilepsy specialists, 79.2% of the people had focal epilepsy, 29.3% had causes of epilepsy that were structure related, and 4.5% of people had epilepsy due to hippocampal sclerosis (HS). A total of 46.7% of the people took at least two ASMs, and 33.7% had DRE (Table 1).
Table 1.
Characteristics of participants.
| Variables | Mean ± SD /n (%) |
|---|---|
| Age, years, mean ± SD | 28.2 ± 12.1 |
| Sex, male, n (%) | 384 (51.3) |
| BMI, mean ± SD | 22.7 ± 4.5 |
| Ethnic minorities, n (%) | 45 (6.0) |
| Without medical insurance, n (%) | 97 (13.0) |
| Lived in rural areas, n (%) | 355 (47.4) |
| Divorced or separated, n (%) | 57 (7.6) |
| Abnormal living conditions, n (%) | 123 (16.4) |
| Unemployed or out of school, n (%) | 182 (24.3) |
| No regular income, n (%) | 403 (53.8) |
| Low monthly household income (less than 4,000 yuan), n (%) | 303 (40.5) |
| Low educational background (high school education or below, n (%) | 438 (58.5) |
| Physical activity in winter, n (%) | 472 (63.0) |
| Physical activity in summer, n (%) | 498 (66.5) |
| Age of onset of seizure, mean ± SD | 19.8 ± 13.0 |
| Disease duration, mean ± SD | 8.9 ± 8.1 |
| Epilepsy-related history, n (%) | |
| Asphyxia at birth | 88 (11.7) |
| Febrile convulsion | 149 (19.9) |
| Severe traumatic brain injury | 100 (13.4) |
| Encephalitis | 61 (8.1) |
| Craniotomy | 49 (6.5) |
| Family history | 62 (8.3) |
| Epilepsy-related EEG changes, n (%) | 510 (68.1) |
| Epilepsy-related abnormalities on MRI, n (%) | 332 (44.3) |
| Status epilepticus, n (%) | 181 (24.2) |
| Epilepsy severity, NHS3, mean ± SD | 7.1 ± 6.5 |
| Frequent seizures, n (%) | 204 (27.2) |
| Types of epilepsy, focal, n (%) | 593 (79.2) |
| Etiology of epilepsy, n (%) | |
| Structure etiology | 219 (29.3) |
| Hippocampal sclerosis | 34 (4.5) |
| Traumatic brain injury | 22 (2.9) |
| Cerebral tumor | 11 (1.5) |
| Cerebrovascular disease | 7 (0.9) |
| Perinatal causes | 9 (1.2) |
| Infectious etiology | 57 (7.6) |
| Genetic etiology | 66 (8.8) |
| Immune etiology | 8 (1.1) |
| Unknown etiology | 450 (60.1) |
| At least two ASMs, n (%) | 350 (46.7) |
| DRE, n (%) | 251 (33.7) |
| Emotional disorders, n (%) | |
| Depression | 189 (25.2) |
| SSD | 183 (24.4) |
| Anxiety | 157 (21.0) |
| QOLIE-31 score | 60.6 ± 16.9 |
BMI, body mass index; DRE, drug-resistant epilepsy; EEG, electroencephalography; MRI, magnetic resonance imaging; NHS, national hospital seizure severity; QOLIE, quality of life in epilepsy inventory; SD, standard deviation; SSD, somatic symptom disorder.
Overlap of the three emotional disorders
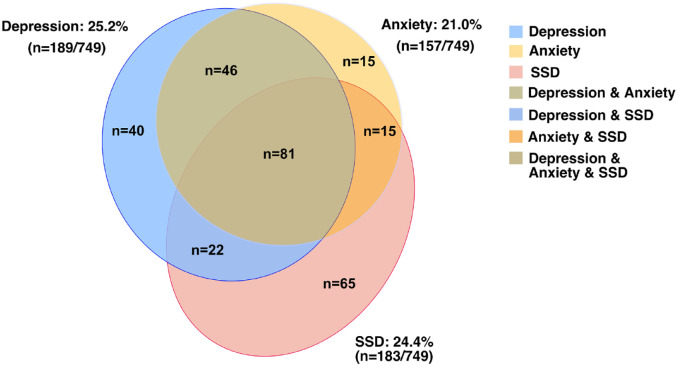
Among the 749 PWE, 189 people (25%) were diagnosed with depression, 183 patients (24%) were diagnosed with SSD, and 157 patients (21%) were diagnosed with anxiety. The overlap of these three emotional disorders is shown in Figure 1. The frequency of occurrence of each emotional disorder together with other emotional disorders was higher than the frequency of occurrence of a single emotional disorder: 79% (149/189) of the depression PWE had anxiety, SSD, or both; 64% (118/183) of the SSD PWE had depression, anxiety, or both; and 90% (142/157) of the anxiety PWE had depression, SSD, or both.
Figure 1.
Venn diagram shows high overlap of depression, SSD, and anxiety in PWE.
Among the 749 PWE, 189 people (25%) were diagnosed with depression, 183 patients (24%) were diagnosed with SSD, and 157 patients (21%) were diagnosed with anxiety. According to the overlap of the three emotional disorders, 40 patients with comorbid depression, 65 patients with comorbid SSD, 15 patients with comorbid anxiety, 15 patients with comorbid SSD and anxiety, 46 patients with comorbid depression and anxiety, 22 patients with comorbid SSD and depression, and 81 patients with comorbid SSD and anxiety and depression. According to the number of comorbid emotional disorders, there were 120 PWE with one comorbid emotional disorder, 83 PWE with two comorbid emotional disorders, and 81 PWE with three comorbid emotional disorders.
According to the overlap of the three emotional disorders, there were 465 PWE with comorbidities without emotional disorders, 40 patients with comorbid depression, 65 patients with comorbid SSD, 15 patients with comorbid anxiety, 15 patients with comorbid SSD and anxiety, 46 patients with comorbid depression and anxiety, 22 patients with comorbid SSD and depression, and 81 patients with comorbid SSD and anxiety and depression.
According to the number of comorbid emotional disorders, there were 465 PWE with zero comorbid emotional disorders, 120 PWE with one comorbid emotional disorder, 83 PWE with two comorbid emotional disorders, and 81 PWE with three comorbid emotional disorders.
Intergroup comparisons between patients with emotional disorder and those without
In our sample, the factors that were significantly different between participants with depression and those without included sex (male, 43.4% versus 53.9%, p = 0.012), lived in rural areas (58.7% versus 43.6%, p < 0.001), unemployed or out of school (35.4% versus 20.5%, p < 0.001), no regular income (63.0% versus 50.7%, p = 0.003), low monthly household income (less than 4000 yuan, 52.9% versus 36.2%, p < 0.001), low educational background (high school education or below, 70.9% versus 54.3%, p < 0.001), febrile convulsion (25.9% versus 17.9%, p = 0.016), encephalitis (12.2% versus 6.8%, p = 0.019), epilepsy-related abnormalities on MRI (51.3% versus 42.0%, p = 0.025), infectious etiology (13.2% versus 5.7%, p < 0.001), epilepsy severity (NHS3, 9.1 ± 6.9 versus 6.4 ± 6.2, p < 0.001), frequent seizures (more than once a month, 38.1% versus 23.6%, p < 0.001), and DRE (45.5% versus 29.7%, p < 0.001; Supplemental Table 1, before PSM)
Compared with participants without SSD, those with SSD were more likely to live in rural areas (57.4% versus 44.2%, p = 0.002) and be unemployed or out of school (30.6% versus 22.3%, p = 0.022) with low monthly household income (51.4% versus 36.9%, p < 0.001) and low educational background (67.8% versus 55.5%, p = 0.03). People with SSD had significantly more focal epilepsy (86.3% versus 76.9%, p = 0.006), frequent seizures (39.3% versus 23.3%, p < 0.001), and DRE (48.9% versus 28.8%, p < 0.001), showing significantly higher NHS3 total scores (9.2 ± 6.6 versus 6.4 ± 6.3, p < 0.001) than those without, with higher percentage of HS etiology (7.7% versus 3.5%, p = 0.02), status epilepticus (32.8% versus 21.4%, p = 0.02) and epilepsy-related abnormalities on MRI (51.4% versus 42.0%, p = 0.027; Supplemental Table 2, before PSM). The top three most common symptoms were memory impairment, headache, and dizziness (87.4%, 79.2%, and 78.1%, respectively). General system was the most frequently affected system in people with SSD (Supplemental Table 4).
The factors that were significantly different between participants with anxiety and those without were the same as factors that were significantly different between participants with depression and those without, except for type of epilepsy. People with anxiety had significantly more focal epilepsy (86.6% versus 76.9%, p = 0.006) than those without (Supplemental Table 3, before PSM).
Propensity score matching
Through PSM, 92 pairs were successfully matched in the epilepsy comorbid depression group and the non-comorbid depression group; 145 pairs were successfully matched in the epilepsy comorbid SSD group and non-comorbid SSD group; and 79 pairs were successfully matched in the epilepsy comorbid anxiety group and non-comorbid anxiety group. After matching, all baseline characteristics were balanced between groups (p > 0.05). Comparisons of baseline characteristics between the epilepsy comorbid depression group and the non-comorbid depression group, between the epilepsy comorbid SSD group and the non-comorbid SSD group, and between the epilepsy comorbid anxiety group and the non-comorbid anxiety group after PSM are shown in Supplemental Tables 1–3, respectively.
QOL of epilepsy people before and after PSM
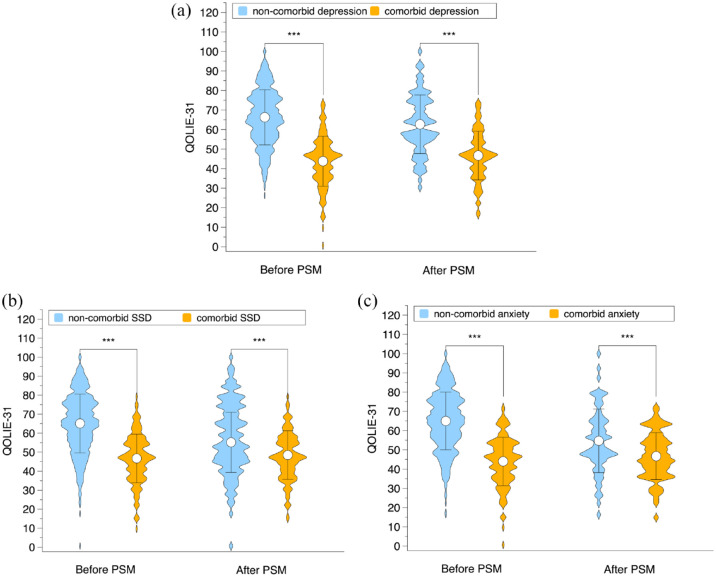
Before PSM, the QOLIE-31 score for people in the epilepsy comorbid depression group was significantly lower than that for people in the epilepsy non-comorbid depression group (mean ± SD: 43.8 ± 12.8 versus 66.3 ± 14.1, p < 0.001). After PSM, the gap between the two groups’ scores narrowed but remained statistically significant [mean ± SD: 46.7 ± 12.5 versus 62.7 ± 15.0, p < 0.001; Figure 2(a)].
Figure 2.
QOL of epilepsy people before and after PSM. (a) Before PSM, the QOLIE-31 score for people in the epilepsy comorbid depression group was significantly lower than that for people in the epilepsy non-comorbid depression group (43.8 ± 12.8 versus 66.3 ± 14.1, p < 0.001). After PSM, the gap between the two groups’ scores narrowed but remained statistically significant (46.7 ± 12.5 versus 62.7 ± 15.0, p < 0.001). (b) Before PSM, the QOLIE-31 score for people in the epilepsy comorbid SSD group was significantly lower than that for people in the epilepsy non-comorbid SSD group (46.7 ± 12.8 versus 65.1 ± 15.5, p < 0.001); After PSM, the gap between the two groups’ scores narrowed but remained statistically significant (48.45 ± 12.78 versus 55.17 ± 15.86, p < 0.001). (c) Before PSM, the QOLIE-31 score for people in the epilepsy comorbid anxiety group was significantly lower than that for people in the epilepsy non-comorbid anxiety group (44.0 ± 12.6 versus 65.0 ± 15.0, p < 0.001). After PSM, the gap between the two groups’ scores narrowed but remained statistically significant (46.7 ± 12.2 versus 54.7 ± 16.5, p < 0.001). PSM, propensity score matching.
Before PSM, the QOLIE-31 score for people in the epilepsy comorbid SSD group was significantly lower than that for people in the epilepsy non-comorbid SSD group (mean ± SD: 46.7 ± 12.8 versus 65.1 ± 15.5, p < 0.001); After PSM, the gap between the two groups’ scores narrowed but remained statistically significant [mean ± SD: 48.45 ± 12.78 versus 55.17 ± 15.86, p < 0.001; Figure 2(b)].
Before PSM, the QOLIE-31 score for people in the epilepsy comorbid anxiety group was significantly lower than that for people in the epilepsy non-comorbid anxiety group (mean ± SD: 44.0 ± 12.6 versus 65.0 ± 15.0, p < 0.001). After PSM, the gap between the two groups’ scores narrowed but remained statistically significant [mean ± SD: 46.7 ± 12.2 versus 54.7 ± 16.5, p < 0.001; Figure 2(c)].
Univariate and multivariate analyses of the QOL of epilepsy people before PSM
As seen in Table 2, the univariate analysis showed that the indicators related to a reduction in QOL were body mass index (BMI, p = 0.033), ethnic minorities (p = 0.026), living in rural areas (p < 0.001), unemployed (p < 0.001), no income (p = 0.008), low monthly household income (p < 0.001), low education level (p < 0.001), previous history of febrile seizures (p = 0.003), history of encephalitis (p < 0.001), history of craniotomy (p < 0.001), abnormal EEG (p = 0.003), abnormal cranial MRI (p < 0.001), history of status epilepticus (p = 0.002), severe epilepsy symptoms (p < 0.001), frequent seizures (p < 0.001), etiology of infection (p < 0.001), taking at least two ASMs (p < 0.001), DRE (p < 0.001), SSD (p < 0.001), anxiety (p < 0.001), and depression (p < 0.001). The protective factors for QOL included summer/winter physical exercise (p < 0.001), generalized epilepsy (p = 0.009), and unknown etiology (p = 0.003).
Table 2.
Univariate analyses of the QOL of epilepsy people before PSM.
| Variables | QOLIE-31 β (95% CI) |
p-value |
|---|---|---|
| Age | −0.0 (−0.1, 0.1) | 0.633 |
| Sex | −1.4 (−3.8, 1.0) | 0.264 |
| BMI | −0.3 (−0.6, −0.0) | 0.033 |
| Ethnic minorities | −5.8 (−10.8, −0.7) | 0.026 |
| Without medical insurance | 0.4 (−3.2, 4.1) | 0.807 |
| Lived in rural areas | −6.2 (−8.6, −3.9) | < 0.001 |
| Divorced or separated | −1.4 (−5.9, 3.2) | 0.556 |
| Abnormal living conditions | 0.9 (−2.4, 4.2) | 0.585 |
| Unemployed or out of school | −9.1 (−11.8, −6.3) | < 0.001 |
| No regular income | −3.3 (−5.7, −0.9) | 0.008 |
| Low monthly household income (less than 4000 yuan) | −8.7 (−11.0, −6.3) | < 0.001 |
| Low educational background (high school education or below | −7.4 (−9.8, −5.0) | < 0.001 |
| Physical activity in winter | 4.9 (2.4, 7.4) | < 0.001 |
| Physical activity in summer | 4.5 (2.0, 7.1) | < 0.001 |
| Age of onset of seizure | 0.0 (−0.1, 0.1) | 0.567 |
| Disease duration | −0.1 (−0.3, 0.0) | 0.085 |
| Epilepsy-related history | ||
| Asphyxia at birth | −2.2 (−6.0, 1.5) | 0.247 |
| Febrile convulsion | −4.5 (−7.6, −1.5) | 0.003 |
| Severe traumatic brain injury | −0.6 (−4.1, 3.0) | 0.749 |
| Encephalitis | −7.4 (−11.8, −3.0) | < 0.001 |
| Craniotomy | −8.8 (−13.6, −3.9) | < 0.001 |
| Family history | −1.8 (−6.1, 2.6) | 0.432 |
| Epilepsy-related EEG changes | −3.9 (−6.4, −1.3) | 0.003 |
| Epilepsy-related abnormalities on MRI | −4.4 (−6.8, −2.0) | < 0.001 |
| Status epilepticus | −4.4 (−7.2, −1.6) | 0.002 |
| Epilepsy severity, NHS3 | −0.7 (−0.8, −0.5) | < 0.001 |
| Frequent seizures | −10.5 (−13.1, −7.9) | < 0.001 |
| Types of epilepsy, general | 4.0 (1.0, 6.9) | 0.009 |
| Etiology of epilepsy | ||
| Structure etiology | −2.5 (−5.1, 0.2) | 0.068 |
| Hippocampal sclerosis | 0.3 (−5.5, 6.1) | 0.92 |
| Traumatic brain injury | 1.2 (−5.9, 8.4) | 0.733 |
| Cerebral tumor | −9.1 (−19.1, 1.0) | 0.077 |
| Cerebrovascular disease | 7.1 (−5.5, 19.7) | 0.268 |
| Perinatal causes | 3.3 (−7.8, 14.4) | 0.563 |
| Infectious etiology | −8.3 (−12.9, −3.8) | < 0.001 |
| Genetic etiology | −0.2 (−4.5, 4.1) | 0.928 |
| Immune etiology | 4.0 (−7.8, 15.7) | 0.507 |
| Unknown etiology | 3.7 (1.2, 6.2) | 0.003 |
| At least two ASMs | −7.7 (−10.1, −5.3) | < 0.001 |
| DRE | −10.2 (−12.7, −7.8) | < 0.001 |
| Depression | −22.5 (−24.8, −20.2) | < 0.001 |
| SSD | −18.4 (−20.9, −15.9) | < 0.001 |
| Anxiety | −21.0 (−23.6, −18.4) | < 0.001 |
BMI, body mass index; CI, confidence interval; DRE, drug-resistant epilepsy; EEG, electroencephalography; MRI, magnetic resonance imaging; NHS, national hospital seizure severity; QOLIE, quality of life in epilepsy inventory; SSD, somatic symptom disorder. Value in bold, p ≤ 0.05.
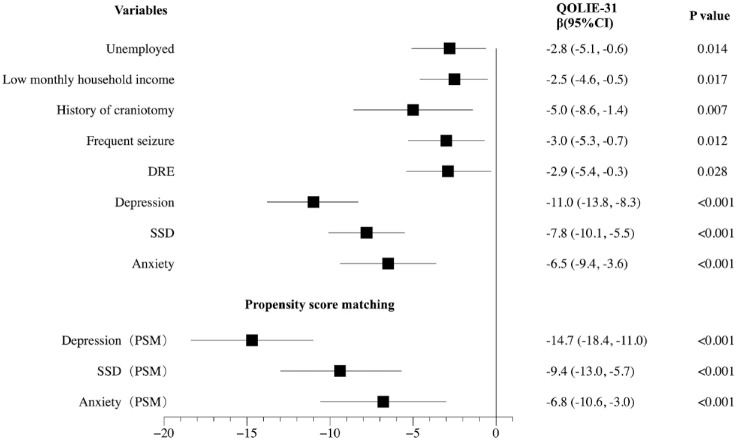
Multivariate analysis revealed that unemployed [β (95% CI): –2.8 (−5.1, −0.6), p = 0.014], low monthly household income [β (95% CI): –2.5 (−4.6, −0.5), p = 0.017], history of craniotomy [β (95% CI): –5.0 (−8.6, −1.4), p = 0.007], frequent seizures [β (95% CI): –3.0 (−5.3, −0.7), p = 0.012], DRE [β (95% CI): –2.9 (−5.4, −0.3), p = 0.028], depression (95% CI): –11.0 (−13.8, −8.3), p < 0.001), SSD (95% CI): –7.8 (−10.1, −5.5), p < 0.001), and anxiety (95% CI): –6.5 (−9.4, −3.6), p < 0.001) were independent indicators of the QOL of PWE.
In our model, the majority of demographic factors are not independent indicators of the QOL, except for unemployed and low monthly household income. Although BMI, ethnic minorities, living in rural areas, no income, and low educational level were associated with QOL in univariate analysis. As for epilepsy-related factors, only history of craniotomy, frequent seizures, and DRE were retained in multivariate analysis. Psychological features have the most significant impact on QOL than other demographic and epilepsy-related factors. Depression had the greatest effect with regard to reducing QOLIE-31 scores, followed by SSD and anxiety (β: –11.0 versus –7.8 versus –6.5). The detailed results of the multivariate analysis of the QOL of PWE are shown in Figure 3.
Figure 3.
Multivariate analyses of the QOL of epilepsy people before PSM and effects of depression, anxiety, and SSD on the QOL of PWE after PSM.
Multivariate analysis revealed that unemployed [β (95% CI): –2.8 (−5.1, −0.6), p = 0.014], low monthly household income [β (95% CI): –2.5 (−4.6, −0.5), p = 0.017], history of craniotomy [β (95% CI): –5.0 (−8.6, −1.4), p = 0.007], frequent seizures [β (95% CI): –3.0 (−5.3, −0.7), p = 0.012], DRE [β (95% CI): –2.9 (−5.4, −0.3), p = 0.028], depression (95% CI): –11.0 (−13.8, −8.3), p < 0.001), SSD (95% CI): –7.8 (−10.1, −5.5), p < 0.001), and anxiety (95% CI): –6.5 (−9.4, −3.6), p < 0.001) were independent indicators of the QOL of PWE. After PSM, the multivariate analysis results indicated that depression, SSD, and anxiety all had an independent effect on QOL in PWE (all p < 0.001). Moreover, depression had the greatest effect in reducing QOLIE-31 scores, followed by SSD and anxiety (β: –14.7 versus –9.4 versus –6.8).
Effects of depression, anxiety, and SSD on the QOL of PWE after PSM
After PSM, in the datasets for the epilepsy comorbid depression group and the non-comorbid depression group, the epilepsy comorbidity SSD group and the non-comorbidity SSD group, and the epilepsy comorbid anxiety group and the non-comorbid anxiety group, after controlling for all factors associated with QOLIE-31 scores in the univariate analysis, the multivariate analysis results indicated that depression, SSD, and anxiety all had an independent effect on QOL in PWE (all p < 0.001). Moreover, depression had the greatest effect in reducing QOLIE-31 scores, followed by SSD and anxiety (β: –14.7 versus –9.4 versus –6.8).
The effect of the combination of different emotional disorders and the number of comorbid emotional disorders on the QOL of PWE
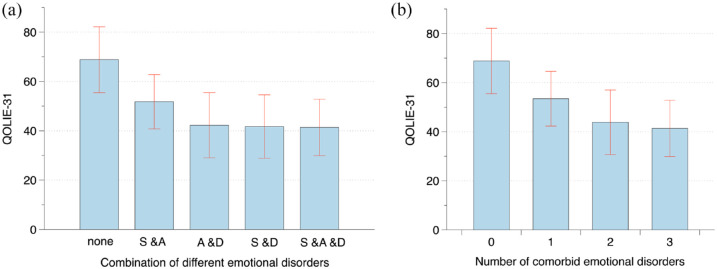
As shown in Figure 4(a), compared with those for people without comorbid emotional disorders, the QOLIE-31 scores for comorbid SSD and anxiety people, comorbid anxiety and depression people, comorbid SSD and depression people, and comorbid SSD and anxiety and depression people gradually decreased (mean ± SD: 68.8 ± 13.3 versus 51.8 ± 11.0 versus 42.3 ± 13.2 versus 41.7 ± 12.9 versus 41.4 ± 11.5). The difference between groups was statistically significant (p < 0.001).
Figure 4.
QOLIE-31 score of people with epilepsy who comorbid different emotional disorders and different number of emotional disorders. (a) Compared with those for people without comorbid emotional disorders, the QOLIE-31 scores for comorbid SSD and anxiety people, comorbid anxiety and depression people, comorbid SSD and depression people, and comorbid SSD and anxiety and depression people gradually decreased (mean ± SD: 68.8 ± 13.3 versus 51.8 ± 11.0 versus 42.3 ± 13.2 versus 41.7 ± 12.9 versus 41.4 ± 11.5). The difference between groups was statistically significant (p < 0.001). (b) Compared with that of epilepsy people without comorbid emotional disorders (zero), the QOL of epilepsy people with one, two, and three comorbid emotional disorders gradually decreased (mean ± SD: 68.8 ± 13.3 versus 53.5 ± 11.1 versus 43.8 ± 13.2 versus 41.4 ± 11.5), and the difference between groups was statistically significant (p < 0.001). S & A, SSD and anxiety; A & D, anxiety and depression; S & D, SSD and depression; S & A & D, SSD and anxiety and depression.
As shown in Figure 4(b), compared with that of epilepsy people without comorbid emotional disorders (zero), the QOL of epilepsy people with one, two, and three comorbid emotional disorders gradually decreased (mean ± SD:68.8 ± 13.3 versus 53.5 ± 11.1 versus 43.8 ± 13.2 versus 41.4 ± 11.5), and the difference between groups was statistically significant (p < 0.001).
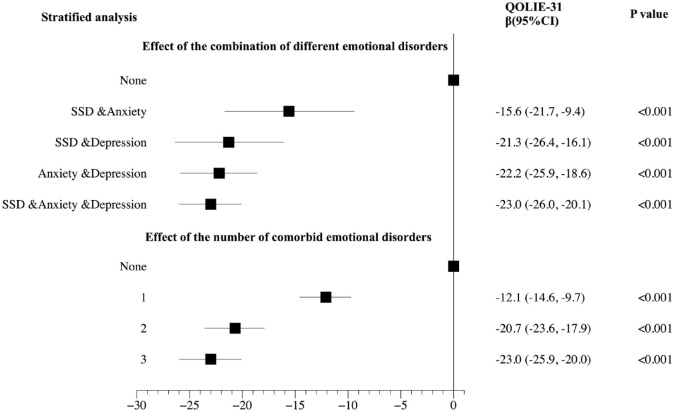
As shown in Figure 5, the stratified analysis showed that comorbid SSD and anxiety, comorbid SSD and depression, comorbid anxiety and depression, and comorbid SSD and anxiety and depression, all had independent effects on QOL (all p < 0.001). In addition, their effect of reducing QOLIE-31 scores increased sequentially (β: –15.6 versus –21.3 versus –22.2 versus –23.0). One, two, and three comorbid emotional disorders all had independent effects on the QOL of epilepsy people (all p < 0.001). Moreover, the effect on reducing the QOLIE-31 score was greater with the increasing number of comorbidities (β: –12.1 versus –20.7 versus –23.0).
Figure 5.
The effect of the combination of different emotional disorders and the number of comorbid emotional disorders on the QOL of people with epilepsy.
The stratified analysis showed that comorbid SSD and anxiety, comorbid SSD and depression, comorbid anxiety and depression, and comorbid SSD and anxiety and depression all had independent effects on QOL (all p < 0.001). In addition, their effect of reducing QOLIE-31 scores increased sequentially (β: –15.6 versus –21.3 versus –22.2 versus –23.0). One, two, and three comorbid emotional disorders, all had independent effects on the QOL of epilepsy people (all p < 0.001). Moreover, the effect on reducing the QOLIE-31 score was greater with the increasing number of comorbidities (β: –12.1 versus –20.7 versus –23.0).
Discussion
This study included 749 PWE with a disease course of at least 1 year. For the first time, the prevalence and overlap of depression, SSD, and anxiety were investigated using screening questionnaires preliminarily followed by structured diagnostic interviews in PWE. PSM and stratified analysis were used to explore the effects of their respective and combined effects on QOL. The results showed that the prevalence of these three disorders accounted for approximately one-fourth of epilepsy patients; the frequency of occurrence of each emotional disorder together with other emotional disorders was higher than the frequency of occurrence of an emotional disorder alone; depression, SSD, and anxiety were all independent indicators of the QOL of PWE. Depression had the greatest effect, followed by SSD, and then anxiety. The QOL of PWE decreased more significantly with the increasing number of comorbid emotional disorders.
Our study confirmed that emotional disorder is a major factor affecting the QOL of PWE and that demographic and epilepsy-related factors have a limited impact on QOL. This is consistent with the results of previous studies.3,4 This suggests that to improve the QOL of PWE, it is necessary to pay attention to the screening and treatment of emotional disorders in the management of epilepsy.
This study provides a basis for screening for depression, SSD, and anxiety in PWE. First, the prevalence of the three types of emotional disorders is relatively high in PWE, approximately one-quarter for each. This is comparable to the prevalence of depression29,30 and anxiety30 in PWE based on high-quality literature reviews. Second, all three have independent effects on the reduction in QOL, and screening helps to identify individuals who may benefit from anti-anxiety and anti-depression medication and psychotherapy. For epilepsy people with comorbid SSD, disease health education and psychotherapy, such as cognitive behavioral therapy, and mindfulness therapy24 can be beneficial. The results from this study can help to develop a personalized diagnosis and treatment plan for each patient. Finally, the higher was the number of comorbid emotional disorders, the more significant was the reduction in the QOL of PWE. In particular, the reduction in QOL in epilepsy people with two or more comorbid emotional disorders was approximately twice that in epilepsy people with one comorbid emotional disorder. This finding suggests that clinicians must identify epilepsy people with two or more comorbid emotional disorders.
The results from this study confirmed, for the first time, the relative magnitude of the impact of the three emotional disorders on the QOL of patients with epilepsy. Regardless of whether the analysis occurred before or after PSM, depression had the greatest effect on the reduction in QOL, followed by SSD, and then anxiety. Similar results have also been reported in other studies. Löwe et al.40 conducted a multicenter cross-sectional study that included 291 patients in 15 primary care clinics in the United States to clarify the independent and overlapping effects of SSD, anxiety, and depression on physical function impairment. The results indicated that for QOL, patients with depression, anxiety, and SSD had 17.8, 11.4, and 13.5 additional days of disability, respectively. For the SF-20 general health dimension, depression alone, anxiety alone, and SSD alone contributed 2.3%, 0%, and 7.1% variance, respectively. Combining the results for the different combinations of the three emotional disorders and the effect of different numbers of comorbidities on the QOL of patients with epilepsy in this study can guide clinicians in the stratified management of different patients.
As previously stated in the ‘Introduction’ section, the reasons for co-morbid anxiety and depression in epilepsy are well described in the literature. However, the reasons for co-morbid SSD remains to be clarified. One possible explanation for this is that they share similar brain structure and function change. Neuroimaging studies have shown volume loss in bilateral amygdala,41 prefrontal, cingulate, and insular cortex,42 and greater functional connectivity within the sensorimotor network, default mode network, and salience network43 in SSD patients. These structures are associated with emotion processing, recognition, and cognitive control that are main basis clinical presentation of SSD, and also constitute important nodes in epilepsy network.
Currently, a variety of emotional disorder screening scales that have been tested for reliability and validity have been developed for use by non-psychiatrists, laying the foundation for the implementation of emotional disorder screening for PWE. These include depression screening scales, that is, the NDDI-E,44 BDI-II,45 and PHQ-9;31–33,46 anxiety screening scales, that is, the GAD-731,46 and SAS;47 SSD screening scale, that is, the SSD-12;48,49 somatic symptom scale-8;50 PHQ-15 for screening somatoform disorders;51 and Health Anxiety Scale-6.52
According to ILAE, clinical practice recommendations based on quality of evidence, psychological and medical treatments were proven to produce a clinically meaningful improvement on QOL in PWE who had comorbid mental health problems. Psychological interventions, including cognitive behavioral therapy and mindfulness-based therapies, received a GRADE recommendation of STRONG for depressive symptoms and a GRADE recommendation of WEAK for anxiety symptoms. These interventions should be incorporated into comprehensive epilepsy care.9 For patients with mild depression, psychological interventions are first-line treatments. As for patients with moderate to severe depression, selective serotonin reuptake inhibitors (SSRIs) are first-choice medications (Level B), and venlafaxine appears legitimate (Level C) when patients are partially or non-responding to SSRIs. The drug treatment should be continued for at least 6 months or even longer until depressive symptoms have subsided.10
There is evidence that social factors, such as stigma, also contribute to poor QOL in PWE.53,54 In patients with psychological symptoms, difficulties in emotion regulation may involve perceived stigma,55 and perceived stigma increases psychological symptoms in return,54 which illustrates the interrelatedness of psychological and social issues. Interventions for stigma and other social issues in epilepsy receive a GRADE recommendation of STRONG. Social/communication skills and social activation were suggested to address internal factors contributing to stigma.9
This study has certain limitations. This was a cross-sectional study. Although PSM analysis was used to minimize the differences between groups and multivariate analysis was used to control confounding factors, the natural advantages of a randomized controlled study with regard to controlling bias and confounding factors were not realized. Moreover, further studies are needed to validate our results.
Conclusion
It is necessary to screen for three emotional disorders, that is, depression, SSD, and anxiety, in PWE. Attention should be paid to people with multiple comorbid emotional disorders.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864221138147 for The overlapping relationship among depression, anxiety, and somatic symptom disorder and its impact on the quality of life of people with epilepsy by Sisi Shen, Zaiquan Dong, Qi Zhang, Jing Xiao, Dong Zhou and Jinmei Li in Therapeutic Advances in Neurological Disorders
Acknowledgments
Not applicable.
Footnotes
ORCID iD: Jinmei Li  https://orcid.org/0000-0002-7411-8269
https://orcid.org/0000-0002-7411-8269
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Sisi Shen, Department of Neurology, West China Hospital, Sichuan University, Chengdu, China.
Zaiquan Dong, Mental Health Centre, West China Hospital, Sichuan University, Chengdu, China.
Qi Zhang, Department of Neurology, West China Hospital, Sichuan University, Chengdu, China.
Jing Xiao, Department of Neurology, West China Hospital, Sichuan University, Chengdu, China.
Dong Zhou, Department of Neurology, West China Hospital, Sichuan University, No. 37 GuoXue Alley, Chengdu 610041, China.
Jinmei Li, Department of Neurology, West China Hospital, Sichuan University, No. 37 GuoXue Alley, Chengdu 610041, China.
Declarations
Ethics approval and consent to participate: This study was approved by the Ethics Committee of the West China Hospital (Nos. 2020-1303). Written informed consent was obtained from all participants in this study.
Consent for publication: All authors have read and approved the submitted manuscript.
Author contributions: Sisi Shen: Data curation; Formal analysis; Investigation; Software; Writing – original draft.
Zaiquan Dong: Investigation; Validation; Writing – original draft.
Qi Zhang: Investigation.
Jing Xiao: Investigation.
Dong Zhou: Conceptualization; Funding acquisition; Methodology; Resources; Supervision; Writing – review & editing.
Jinmei Li: Conceptualization; Funding acquisition; Methodology; Resources; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Jinmei Li is supported by grant from the Science & Technology Department of Sichuan Province (grant no. 2019YFH0145), National Natural Science Foundation of China (grant no. 81571272) and the 1.3.5 project for disciplines of excellence and Brain Science project of West China Hospital, Sichuan University, (grant no. ZYJC21001); Dong Zhou is supported by grant from the Science & Technology Department of Sichuan Province (grant no. 2019YFH0196) and National Natural Science Foundation of China (grant/award no. 8187017).
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and material: The data used to support the findings of this study are available from the corresponding author upon request.
References
- 1. Ngugi AK, Bottomley C, Kleinschmidt I, et al. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia 2010; 51: 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet 2017; 390: 1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tracy JI, Dechant V, Sperling MR, et al. The association of mood with quality of life ratings in epilepsy. Neurology 2007; 68: 1101–1107. [DOI] [PubMed] [Google Scholar]
- 4. Stevanovic D, Jancic J, Lakic A. The impact of depression and anxiety disorder symptoms on the health-related quality of life of children and adolescents with epilepsy. Epilepsia 2011; 52: e75–e78. [DOI] [PubMed] [Google Scholar]
- 5. Sajobi TT, Jette N, Fiest KM, et al. Correlates of disability related to seizures in persons with epilepsy. Epilepsia 2015; 56: 1463–1469. [DOI] [PubMed] [Google Scholar]
- 6. Terman SW, Hill CE, Burke JF. Disability in people with epilepsy: a nationally representative cross-sectional study. Epilepsy Behav 2020; 112: 107429. [DOI] [PubMed] [Google Scholar]
- 7. Tombini M, Assenza G, Quintiliani L, et al. Epilepsy and quality of life: what does really matter. Neurol Sci 2021; 42: 3757–3765. [DOI] [PubMed] [Google Scholar]
- 8. Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia 2017; 58: 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michaelis R, Tang V, Goldstein LH, et al. Psychological treatments for adults and children with epilepsy: evidence-based recommendations by the International League Against Epilepsy Psychology Task Force. Epilepsia 2018; 59: 1282–1302. [DOI] [PubMed] [Google Scholar]
- 10. Mula M, Brodie MJ, de Toffol B, et al. ILAE clinical practice recommendations for the medical treatment of depression in adults with epilepsy. Epilepsia 2022; 63: 316–334. [DOI] [PubMed] [Google Scholar]
- 11. Löwe B, Levenson J, Depping M, et al. Somatic symptom disorder: a scoping review on the empirical evidence of a new diagnosis. Psychol Med 2021; 52: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Waal MW, Arnold IA, Eekhof JA, et al. Somatoform disorders in general practice: prevalence, functional impairment and comorbidity with anxiety and depressive disorders. Br J Psychiatry 2004; 184: 470–476. [DOI] [PubMed] [Google Scholar]
- 13. Philbrick JT, Connelly JE, Wofford AB. The prevalence of mental disorders in rural office practice. J Gen Intern Med 1996; 11: 9–15. [DOI] [PubMed] [Google Scholar]
- 14. Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994; 272: 1749–1756. [PubMed] [Google Scholar]
- 15. Rashid H, Upadhyay AD, Pandey RM, et al. Point prevalence of depression in persons with active epilepsy and impact of methodological moderators: a systematic review and meta-analysis. Epilepsy Behav 2021; 125: 108394. [DOI] [PubMed] [Google Scholar]
- 16. Forsgren L, Nyström L. An incident case-referent study of epileptic seizures in adults. Epilepsy Res 1990; 6: 66–81. [DOI] [PubMed] [Google Scholar]
- 17. Hecimovic H, Santos J, Price JL, et al. Severe hippocampal atrophy is not associated with depression in temporal lobe epilepsy. Epilepsy Behav 2014; 34: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elst LT, Groffmann M, Ebert D, et al. Amygdala volume loss in patients with dysphoric disorder of epilepsy. Epilepsy Behav 2009; 16: 105–112. [DOI] [PubMed] [Google Scholar]
- 19. Butler T, Blackmon K, McDonald CR, et al. Cortical thickness abnormalities associated with depressive symptoms in temporal lobe epilepsy. Epilepsy Behav 2012; 23: 64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hooper A, Paracha R, Maguire J. Seizure-induced activation of the HPA axis increases seizure frequency and comorbid depression-like behaviors. Epilepsy Behav 2018; 78: 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mazarati AM, Shin D, Kwon YS, et al. Elevated plasma corticosterone level and depressive behavior in experimental temporal lobe epilepsy. Neurobiol Dis 2009; 34: 457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mazarati AM, Pineda E, Shin D, et al. Comorbidity between epilepsy and depression: role of hippocampal interleukin-1beta. Neurobiol Dis 2010; 37: 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dimsdale, JE. American Psychiatric Association. Somatic symptom and related disorders. In: Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association, 2013, pp. 309–315. [Google Scholar]
- 24. Henningsen P, Zipfel S, Sattel H, et al. Management of functional somatic syndromes and bodily distress. Psychother Psychosom 2018; 87: 12–31. [DOI] [PubMed] [Google Scholar]
- 25. Cao J, Wei J, Fritzsche K, et al. Prevalence of DSM-5 somatic symptom disorder in Chinese outpatients from general hospital care. Gen Hosp Psychiatry 2020; 62: 63–71. [DOI] [PubMed] [Google Scholar]
- 26. Liao SC, Ma HM, Lin YL, et al. Functioning and quality of life in patients with somatic symptom disorder: the association with comorbid depression. Compr Psychiatry 2019; 90: 88–94. [DOI] [PubMed] [Google Scholar]
- 27. Hüsing P, Löwe B, Toussaint A. Comparing the diagnostic concepts of ICD-10 somatoform disorders and DSM-5 somatic symptom disorders in patients from a psychosomatic outpatient clinic. J Psychosom Res 2018; 113: 74–80. [DOI] [PubMed] [Google Scholar]
- 28. Voigt K, Wollburg E, Weinmann N, et al. Predictive validity and clinical utility of DSM-5 somatic symptom disorder: prospective 1-year follow-up study. J Psychosom Res 2013; 75: 358–361. [DOI] [PubMed] [Google Scholar]
- 29. Fiest KM, Dykeman J, Patten SB, et al. Depression in epilepsy a systematic review and meta-analysis. Neurology 2013; 80: 590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scott AJ, Sharpe L, Hunt C, et al. Anxiety and depressive disorders in people with epilepsy: a meta-analysis. Epilepsia 2017; 58: 973–982. [DOI] [PubMed] [Google Scholar]
- 31. Snijkers JTW, van den Oever W, Weerts ZZRM, et al. Examining the optimal cutoff values of HADS, PHQ-9 and GAD-7 as screening instruments for depression and anxiety in irritable bowel syndrome. Neurogastroenterol Motil 2021; 33: e14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fischer F, Levis B, Falk C, et al. Comparison of different scoring methods based on latent variable models of the PHQ-9: an individual participant data meta-analysis. Psychol Med. Epub ahead of print 22 February 2021. DOI: 10.1017/s0033291721000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neupane D, Levis B, Bhandari PM, et al. Selective cutoff reporting in studies of the accuracy of the patient health questionnaire-9 and Edinburgh postnatal depression scale: comparison of results based on published cutoffs versus all cutoffs using individual participant data meta-analysis. Int J Methods Psychiatr Res 2021; 30: e1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li T, Wei J, Fritzsche K, et al. Validation of the Chinese version of the somatic symptom disorder-B criteria scale for detecting DSM-5 somatic symptom disorders: a multicenter study. Psychosom Med 2020; 82: 337–344. [DOI] [PubMed] [Google Scholar]
- 35. Cramer JA, Perrine K, Devinsky O, et al. Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia 1998; 39: 81–88. [DOI] [PubMed] [Google Scholar]
- 36. Blume WT, Lüders HO, Mizrahi E, et al. Glossary of descriptive terminology for ictal semiology: report of the ILAE task force on classification and terminology. Epilepsia 2001; 42: 1212–1218. [DOI] [PubMed] [Google Scholar]
- 37. Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus-report of the ILAE task force on classification of status epilepticus. Epilepsia 2015; 56: 1515–1523. [DOI] [PubMed] [Google Scholar]
- 38. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia 2017; 58: 522–530. [DOI] [PubMed] [Google Scholar]
- 39. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia 2010; 51: 1069–1077. [DOI] [PubMed] [Google Scholar]
- 40. Löwe B, Spitzer RL, Williams JB, et al. Depression, anxiety and somatization in primary care: syndrome overlap and functional impairment. Gen Hosp Psychiatry 2008; 30: 191–199. [DOI] [PubMed] [Google Scholar]
- 41. Atmaca M, Sirlier B, Yildirim H, et al. Hippocampus and amygdalar volumes in patients with somatization disorder. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1699–1703. [DOI] [PubMed] [Google Scholar]
- 42. Valet M, Gündel H, Sprenger T, et al. Patients with pain disorder show gray-matter loss in pain-processing structures: a voxel-based morphometric study. Psychosom Med 2009; 71: 49–56. [DOI] [PubMed] [Google Scholar]
- 43. Perez DL, Barsky AJ, Vago DR, et al. A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci 2015; 27: e40–e50. [DOI] [PubMed] [Google Scholar]
- 44. Gilliam FG, Barry JJ, Hermann BP, et al. Rapid detection of major depression in epilepsy: a multicentre study. Lancet Neurol 2006; 5: 399–405. [DOI] [PubMed] [Google Scholar]
- 45. Jones JE, Hermann BP, Woodard JL, et al. Screening for major depression in epilepsy with common self-report depression inventories. Epilepsia 2005; 46: 731–735. [DOI] [PubMed] [Google Scholar]
- 46. Castaldelli-Maia JM, Marziali ME, Lu Z, et al. Investigating the effect of national government physical distancing measures on depression and anxiety during the COVID-19 pandemic through meta-analysis and meta-regression. Psychol Med 2021; 51: 881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Olatunji BO, Deacon BJ, Abramowitz JS, et al. Dimensionality of somatic complaints: factor structure and psychometric properties of the self-rating anxiety scale. J Anxiety Disord 2006; 20: 543–561. [DOI] [PubMed] [Google Scholar]
- 48. Toussaint A, Löwe B, Brähler E, et al. The Somatic symptom disorder – B criteria scale (SSD-12): Factorial structure, validity and population-based norms. J Psychosom Res 2017; 97: 9–17. [DOI] [PubMed] [Google Scholar]
- 49. Kop WJ, Toussaint A, Mols F, et al. Somatic symptom disorder in the general population: associations with medical status and health care utilization using the SSD-12. Gen Hosp Psychiatry 2019; 56: 36–41. [DOI] [PubMed] [Google Scholar]
- 50. Gierk B, Kohlmann S, Kroenke K, et al. The somatic symptom scale-8 (SSS-8): a brief measure of somatic symptom burden. JAMA Intern Med 2014; 174: 399–407. [DOI] [PubMed] [Google Scholar]
- 51. Körber S, Frieser D, Steinbrecher N, et al. Classification characteristics of the patient health questionnaire-15 for screening somatoform disorders in a primary care setting. J Psychosom Res 2011; 71: 142–147. [DOI] [PubMed] [Google Scholar]
- 52. Fergus TA, Kelley LP, Griggs JO. Examining the Whiteley Index-6 as a screener for DSM-5 presentations of severe health anxiety in primary care. J Psychosom Res 2019; 127: 109839. [DOI] [PubMed] [Google Scholar]
- 53. Baker GA, Brooks J, Buck D, et al. The stigma of epilepsy: a European perspective. Epilepsia 2000; 41: 98–104. [DOI] [PubMed] [Google Scholar]
- 54. Jacoby A, Snape D, Baker GA. Epilepsy and social identity: the stigma of a chronic neurological disorder. Lancet Neurol 2005; 4: 171–178. [DOI] [PubMed] [Google Scholar]
- 55. Tombini M, Assenza G, Quintiliani L, et al. Depressive symptoms and difficulties in emotion regulation in adult patients with epilepsy: association with quality of life and stigma. Epilepsy Behav 2020; 107: 107073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864221138147 for The overlapping relationship among depression, anxiety, and somatic symptom disorder and its impact on the quality of life of people with epilepsy by Sisi Shen, Zaiquan Dong, Qi Zhang, Jing Xiao, Dong Zhou and Jinmei Li in Therapeutic Advances in Neurological Disorders