Abstract
An estimated 6.1 million Americans live with cognitive impairment—a number that is expected to triple by 2050. Alzheimer disease (AD) is the most common cause of impairment. The development of blood-based biomarkers capable of detecting pathological changes of AD in living patients has the potential to revolutionize the diagnostic approach to cognitive impairment by enabling screening for AD using accessible, non-invasive measures of amyloid and tau neuropathology, with accuracy that increasingly approaches that seen with “gold standard” positron emission tomography and cerebrospinal fluid measures. Demand for biomarker testing is expected to intensify with the emergence of effective treatments for AD and related dementias. Clinicians in all fields must prepare to meet this demand. Primary care practitioners are well positioned to support dementia diagnosis and management, including the application and interpretation of biomarkers. This article reviews the current uses of AD biomarkers and the potential applications of emerging blood-based AD biomarkers in clinical practice.
Keywords: Alzheimer disease, blood biomarkers, amyloid, tau, dementia, mild cognitive impairment, screening
Introduction
An estimated 6.1 million Americans are living with cognitive impairment.1 These numbers are expected to triple by 2050,2 increasing demand on an already strained clinical workforce. Alzheimer disease (AD) accounts for the majority of cases of dementia. Historically, the diagnosis of symptomatic AD has relied upon detection of a characteristic profile of neurocognitive deficits that typically include prominent progressive short-term memory loss, with confirmation established through detection of cerebral amyloid plaques and tau tangles at death.3 This approach runs counter to patients’ and caregivers’ desire for timely diagnoses,4 and exemplifies the need for biological markers that can be reliably and safely measured, and used to advance the diagnosis and treatment of patients with symptomatic AD.
A biomarker is an “indicator of normal biological or pathologic processes, or responses to an exposure or intervention, including therapeutic interventions.”5 Biomarkers are not new in clinical practice. Practitioners routinely use biomarkers to diagnose and monitor patients with diabetes (serum hemoglobin A1C), hyperlipidemia (serum high-density lipoprotein/low-density lipoprotein, triglycerides, and cholesterol), prostate cancer (serum prostate-specific antigen), ovarian cancer (serum cancer antigen 125), and acute coronary syndromes (serum troponin). Neither are biomarkers new in AD. Neuroimaging- and cerebrospinal fluid-based biomarkers of amyloid and tau neuropathology have been used to quantify brain changes associated with AD in research participants for years.6,7 The extension of research biomarkers to clinical practice has the potential to improve the diagnostic evaluation of patients with cognitive impairment, allowing patients with symptomatic AD to be diagnosed earlier and with greater confidence.8 Furthermore, the emergence of less-invasive blood-based biomarkers may further expand the application of this technology, enabling screening to take place in primary care clinics, revolutionizing the diagnostic approach to cognitive impairment.
Primary care practitioners are well positioned to meet the rising demand for practitioners with expertise in dementia diagnosis and care. Recognizing this, we summarize the current uses of AD biomarkers in clinical practice and review the literature concerning emergent blood-based AD biomarkers. Special focus is paid to discussion of implications of emergent blood-based AD biomarkers in the primary care setting.
Studies included within this commentary were identified through a targeted search of the MEDLINE (1946-2022) database, including English-language studies in humans. There were no limits to publication date. The search strategies were created using a combination of keywords and standardized index terms, including MeSH, Embase/Emtree terms, and keywords such as Alzheimer disease, Alzheimer dementia, blood-based biomarkers, amyloid beta, Aβ42/Aβ40, phosphorylated-tau, p-tau, p-tau181, and p-tau217.
Addressing the Need
Primary care practitioners bear the brunt of the burden of assessment of patients with new memory complaints. Yet worryingly, a survey commissioned by the Alzheimer’s Association revealed that half of primary care practitioners believed that the medical profession was “not prepared for the expected increase in demand” for dementia care. The majority also expressed concern that there were not enough specialists to manage patient referrals.9 Limitations in training contribute to the dearth of appropriately trained practitioners. A survey of post-graduate medical trainees (residents) in the United States confirmed that post-graduate trainees received, on average, 8 h of formal training on AD and related dementias over the course of their primary care residency programs. As a result, 72% reported feeling “somewhat,” “not very” or “not at all prepared” to diagnose and manage patients with cognitive impairment.9 Compounding issues with training, primary care practitioners are stretched for time due to increasingly shortened office visits, which are consumed by increasingly complex patients. Collectively, these factors contribute to under-recognition and misdiagnoses of cognitive impairment in clinical practice (Figure 1). These challenges highlight a need that could be addressed by expanding training for primary care practitioners concerning the assessment, diagnosis, and management of patients with dementia—a task that will be advanced by specialized knowledge concerning the appropriate use of disease-specific biomarkers in clinical practice.
Figure 1.
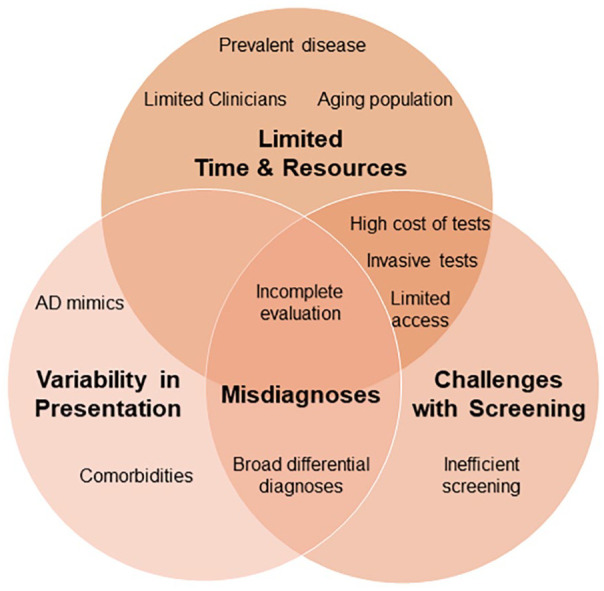
Diagnostic challenges in Alzheimer disease. The accurate diagnosis of Alzheimer disease requires a detailed history, physical examination and testing to rule-in Alzheimer disease and rule-out other causes of cognitive impairment. The initial workup for cognitive complaints typically includes a standardized mental status assessment (Montreal Cognitive Assessment, Mini-Mental Status Examination), screening for B12 deficiency and thyroid dysfunction, and often neuroimaging (brain magnetic resonance imaging or computerized tomography). Limited time and resources, variability in the clinical presentation and inefficacies in screening challenge accurate and efficient recognition of cognitive impairment in clinical practice and contribute to underdiagnoses and misdiagnoses of symptomatic Alzheimer disease.
AD Biomarkers in Clinical Practice
The optimal evaluation of patients with cognitive impairment necessitates that a detailed history be obtained from the patient and a reliable collateral source (eg, spouse, adult child, other family member, or friend), and integrated together with examination findings and bedside tests of cognition.3 Routine blood tests and neuroimaging should be leveraged, when appropriate, to exclude other causes of impairment.3 Primary care practitioners already possess these skills, and routinely accomplish these tasks amidst a busy schedule, seeking specialist opinions when necessary.
The development of biomarkers of amyloid and tau has made it possible to reliably measure AD neuropathology during life, taking the standard clinical assessment to the next level. Longitudinal studies establish a relationship between changes in markers of cerebral amyloid and tau neuropathology, and the timing and severity of cognitive symptoms, and findings at brain autopsy.10-12 Decreases in cerebrospinal fluid levels of amyloid are one of the earliest measurable findings in patients with AD, with changes paralleling the formation and accumulation of amyloid plaques within the brain (measured by amyloid positron emission tomography [PET]).7,13 The accumulation of cerebral amyloid in persons without cognitive complaints defines the preclinical or presymptomatic phase of AD, which typically spans 10 to 20 years (Figure 2).14 Symptoms of cognitive impairment emerge in lockstep with the accumulation of tau neuropathology (measured via tau-PET imaging or cerebrospinal fluid measures of total- and phopsphorylated181-tau) and neuronal degeneration—events that mark the onset of the shorter “clinical” or symptomatic stage of AD.10 The clinical stage of AD may be mild at first (“mild cognitive impairment”), with progression to dementia and death occurring over a variable period often lasting 8 to 12 years. These findings suggest that AD neuropathology accumulates gradually over time, leading to neuronal failure, degeneration, and ultimately cognitive impairment, and death.15
Figure 2.
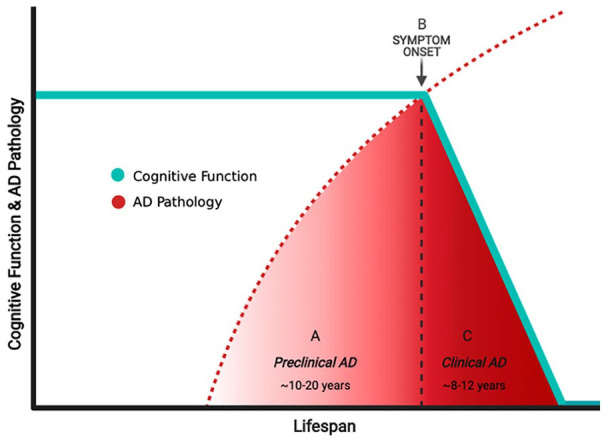
The relationship between Alzheimer disease (AD) pathology and cognitive function across the lifespan. Accrual of AD neuropathology begins decades before the emergence of cognitive complaints, identified as the “preclinical” (or presymptomatic) period (A). Declines in cognitive function attributable to AD neuropathology herald the onset of “clinical” (or symptomatic) AD (B), a period that commonly lasts between 8 and 12 years (C). The protracted “preclinical” period presents an ideal time during which treatments could be provided to at-risk individuals to prevent or reverse the accrual of AD neuropathology and delay the onset or progression of clinical AD.
Although AD biomarkers have mainly been applied in the research setting, clinical applications are becoming clearer. In a US-wide study of patients with mild cognitive impairment or atypical dementia, access to amyloid-PET imaging improved diagnostic confidence, and influenced clinical management in the majority of patients.8 Patients and caregivers also indicate that they want access to AD biomarkers—particularly when biomarker results may improve access to resources.4,16 The demand for biomarkers is likely to increase even more with the rising prospect of disease-modifying therapies for AD17-21 and expansion of clinical trials evaluating investigational agents designed to prevent cognitive impairment in patients at high risk of developing dementia due to AD.22,23
Established AD biomarkers include PET-neuroimaging and cerebrospinal fluid measures. However, PET-based measures are costly, are seldom covered by insurers, and require access to specialized neuroimaging infrastructure and expertise.24 These limitations render them inaccessible to most people. Although cerebrospinal fluid biomarkers are more accessible—with commercial laboratories across the United States providing quantitative measures of amyloid, total tau, and phosphorylated tau25-27—diagnostic lumbar punctures are perceived as invasive, and require skilled operators to perform.28 As a result, these biomarker measures are unlikely to be widely applied outside of academic and subspecialty settings, presenting obvious challenges to wide dissemination and application in clinical practice. The emergence of blood-based measures of amyloid and tau pathology promises to change this.
A Blood-Based Revolution
Blood-Based Measures of Cerebral Amyloid
The advent of immunoprecipitation mass spectrometry assays capable of measuring minute amounts of isoforms in blood accelerated the development of blood-based measures of cerebral amyloid. Aβ40 is the most common variant of amyloid found in the brain, and plasma levels are relatively consistent across populations. Aβ42, on the other hand, is more prone to aggregation and predominates in amyloid plaques that define AD.29 Ratios of these isoforms (Aβ42/Aβ40) in cerebrospinal fluid are highly specific for the presence of AD neuropathology, with decreases in the ratio associated with increases in cerebral amyloid plaque deposition.7,30,31 Only recently were these findings replicated in blood-based measures.
In 2017, a group at Washington University in St. Louis (Saint Louis, MO) successfully demonstrated that decreases in plasma Aβ42/Aβ40 differentiated amyloid “positive” versus “negative” individuals, determined using “gold standard” cerebrospinal fluid and amyloid-PET biomarkers.32 Furthermore, participants who had a negative amyloid-PET at baseline but abnormal plasma Aβ42/Aβ40 measures had a 15-fold greater risk of converting to amyloid-PET “positive” status across follow-up than patients with normal plasma amyloid levels.33 These findings suggest that plasma biomarkers may represent an earlier measure of cerebral amyloid status in preclinical patients.
These findings have been reproduced in additional studies engaging hundreds of participants with and without cognitive impairment, which cumulatively affirm the ability of blood-based measures of amyloid to reliably discriminate between individuals with and without clinically significant cerebral amyloidosis with excellent sensitivity and specificity (receiver operating characteristics demonstrate an area under the curve of 0.85-0.97).32-37 These advances culminated in FDA-approval of a blood test that quantifies plasma Aβ42/Aβ40 levels (approved for clinical use in the US; October 2020). Its suggested use is for symptomatic individuals only, with testing limited to a select commercial providers.38,39 Studies defining the utility of this test in broad populations of patients are needed to inform the generalizability of research findings and practical limitations.
Blood-Based Measures of Tau Neuropathology
Since 2018, studies have shown that blood-based measures of phosphorylated-tau (p-tau) correlate with cerebrospinal fluid and PET measures of amyloid and tau neuropathology, and with symptomatic disease progression.40-48 From a diagnostic perspective, measures of p-tau217 isomers appear to hold the greatest promise. Independent studies support its sensitivity and specificity for AD,40,42,43,45 while a head-to-head study established superiority of p-tau217 measured by mass spectrometry for the diagnosis of symptomatic AD over 9 other immunoassays, with near-perfect ability to discriminate participants with AD (area under the curve of 0.95).48 Commercial blood-based markers of tau neuropathology are not yet available but are expected in the near future.
Increases in plasma p-tau217 levels may precede changes in tau-PET, and even amyloid-PET.42,43,46 Thus, elevations in plasma p-tau217 may mark the earliest stages of “preclinical” or “asymptomatic” AD. Although the ability to identify asymptomatic patients at-risk of developing cognitive impairment offers little-to-no clinical benefit at the present time, the prospect of effective disease-modifying therapies for AD justifies continued research in this area.
Clinical Applications of Blood-Based Biomarkers
Expanded access to blood-based biomarkers of amyloid plaques and tau tangles promise to revolutionize dementia diagnosis and care, facilitating surveillance and early detection of AD in susceptible individuals and increasing the demand for biomarker testing in symptomatic individuals. Clinicians in all fields must prepare to meet this demand. This will require appropriate training in the proper use of these tests and interpretation of results, in addition to the development of standardized protocols and frameworks to guide AD biomarker testing in practice. The appropriate use guidelines developed for clinical research provide an excellent starting point for clinical guidelines.49
While patients (and providers) often focus on the potential benefits of testing, there are practical downsides that warrant consideration.50,51 Knowledge of one’s amyloid or tau status, and therefore likely risk of AD, may influence access to healthcare and insurability (specifically, long-term care, disability, and life insurance), as well as decisions concerning employment, driving, and independent living. Findings in one patient may also have implications for other family members (eg, future risk of AD, insurability, etc.).52 Informed decisions concerning AD biomarker testing will necessitate a clear discussion and documentation of reasons for biomarker testing (ie, perceived benefits), potential risks, and alternatives. As in other areas of controversial testing (eg, genetic testing), adequate resources should be offered to patients who elect to undergo testing, and health care providers must be equipped and prepared to deliver these resources.
Future Directions
The ability to objectively measure disease-specific brain changes through a simple blood draw represents a milestone in AD diagnosis and evaluation. Beyond implications for clinical care, the validation of blood-based biomarkers of AD will allow widespread screening of cognitively normal community-dwelling individuals, with the potential to identify individuals at the highest risk of developing symptomatic AD who may benefit from participation in clinical trials of putative AD-modifying therapies designed to prevent the onset of cognitive impairment.24 The possibility that serial blood-based measures may reflect cerebral amyloid and tau pathology also raises the potential that biomarkers may serve as interim markers of drug efficacy, accelerating discovery and evaluation of medications designed to slow, arrest, or clear amyloid plaques and tau tangles.19 Access to efficacious therapies for AD will prompt substantial investment in AD research. This investment will, in turn, support further diagnostic and therapeutic advances, catalyzing the discovery and validation of better biomarkers and biomarker panels, and further increasing patient and caregiver interest and demand for testing.53 Already work is underway to refine techniques capable of measuring nano-sized membrane-bound extracellular vesicles that are released by all living cells and used to shuttle molecular cargo across the blood-brain barrier. Early work with nanoparticle tracking analysis and cryo-electron microscopy suggests that it may be possible to measure vesicles contents, including proteins, mRNA, lipids, and other nucleic acids.54 Although speculative, progress in this area may further inform the diagnosis, staging, and monitoring of neurodegenerative diseases, representing another substantial step toward a blood-based diagnostic revolution.54,55
Conclusions
Rising demand coupled with the declining capacity of dementia specialists threatens to further widen the gap in dementia care. Primary care practitioners with experience in dementia diagnosis and care have a vital role to play in addressing patients’ questions, concerns, and requests for non-invasive testing, and supporting interpretation of test results on a patient-by-patient basis. Providers with this requisite expertise are needed to stand in the gap while training programs are developed, implemented, and scaled to meet demand.
Acknowledgments
GS Day serves as a topic editor on dementia for DynaMed Plus (EBSCO Industries, Inc), consultant for Parabon Nanolabs Inc, and as clinical director for the Anti-NMDA Receptor Encephalitis Foundation. He holds stock in ANI Pharmaceuticals Inc and has provided record review and expert medical testimony on legal cases pertaining to management of Wernicke encephalopathy.
Footnotes
Author Contributions: M Paczysnki participated in study design; acquisition and interpretation of data; drafting and revision of the manuscript. GS Day participated in study design; acquisition and interpretation of data; revision and finalization of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: GS Day receives research/grant support from the National Institutes of Health for studies focused on biomarkers in dementia (K23AG064029).
ORCID iD: Gregory S. Day  https://orcid.org/0000-0001-5133-5538
https://orcid.org/0000-0001-5133-5538
References
- 1. Manly JJ, Jones RN, Langa KM, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 Health and Retirement Study harmonized cognitive assessment protocol project. JAMA Neurol. Published online October 24, 2022. doi: 10.1001/jamaneurol.2022.3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alzheimer’s Association. 2021. Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17(3):327-406. doi:10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- 3. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armstrong MJ, Gronseth GS, Day GS, Rheaume C, Alliance S, Mullins CD. Patient stakeholder versus physician preferences regarding amyloid PET testing. Alzheimer Dis Assoc Disord. 2019;33(3):246-253. doi: 10.1097/WAD.0000000000000311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cagney DN, Sul J, Huang RY, Ligon KL, Wen PY, Alexander BM. The FDA NIH biomarkers, endpoints, and other tools (BEST) resource in neuro-oncology. Neuro Oncol. 2018;20(9):1162-1172. doi: 10.1093/neuonc/nox242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frisoni GB, Boccardi M, Barkhof F, et al. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. Lancet Neurol. 2017;16(8):661-676. doi: 10.1016/S1474-4422(17)30159-X [DOI] [PubMed] [Google Scholar]
- 7. Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59(3):512-519. doi: 10.1002/ana.20730 [DOI] [PubMed] [Google Scholar]
- 8. Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321(13):1286-1294. doi: 10.1001/jama.2019.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alzheimer’s Association. 2020. Alzheimer’s disease facts and figures. Alzheimers Dement. Published online March 10, 2020. doi:10.1002/alz.12068 [Google Scholar]
- 10. Gordon BA, Friedrichsen K, Brier M, et al. The relationship between cerebrospinal fluid markers of Alzheimer pathology and positron emission tomography tau imaging. Brain. 2016;139(8):2249-2260. doi: 10.1093/brain/aww139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brier MR, Gordon B, Friedrichsen K, et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer's disease. Sci Transl Med. 2016;8(338):338ra66. doi: 10.1126/scitranslmed.aaf2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang L, Benzinger TL, Su Y, et al. Evaluation of Tau imaging in staging Alzheimer disease and revealing interactions between β-amyloid and tauopathy. JAMA Neurol. 2016;73(9):1070-1077. doi: 10.1001/jamaneurol.2016.2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Palmqvist S, Mattsson N, Hansson O. Cerebrospinal fluid analysis detects cerebral amyloid-β accumulation earlier than positron emission tomography. Brain. 2016;139(4):1226-1236. doi: 10.1093/brain/aww015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jack CR, Jr., Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jack CR, Jr, Holtzman DM. Biomarker modeling of Alzheimer's disease. Neuron. 2013;80(6):1347-1358. doi: 10.1016/j.neuron.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mozersky J, Hartz S, Linnenbringer E, et al. Communicating 5-year risk of Alzheimer’s disease dementia: development and evaluation of materials that incorporate multiple genetic and biomarker research results. J Alzheimers Dis. 2021;79(2):559-572. doi: 10.3233/JAD-200993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zetterberg H, Bendlin BB. Biomarkers for Alzheimer's disease-preparing for a new era of disease-modifying therapies. Mol Psychiatry. 2021;26(1):296-308. doi: 10.1038/s41380-020-0721-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cummings J. The role of biomarkers in Alzheimer’s disease drug development. Adv Exp Med Biol. 2019;1118:29-61. doi: 10.1007/978-3-030-05542-4_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Day GS, Scarmeas N, Dubinsky R, et al. Aducanumab use in symptomatic Alzheimer disease evidence in focus: a report of the AAN guidelines subcommittee. Neurology. 2022;98(15):619-631. doi: 10.1212/WNL.0000000000200176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shcherbinin S, Evans CD, Lu M, et al. Association of amyloid reduction after donanemab treatment with tau pathology and clinical outcomes: the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurol. 2022;79:1015-1024. doi: 10.1001/jamaneurol.2022.2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9(2):197-210. doi: 10.14283/jpad.2022.30 [DOI] [PubMed] [Google Scholar]
- 22. Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med. 2014;6(228):1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rafii MS, Sperling RA, Donohue MC, et al. The AHEAD 3-45 study: design of a prevention trial for Alzheimer's disease. Alzheimers Dement. Published online August 15, 2022. doi: 10.1002/alz.12748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the amyloid imaging task force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's association. Alzheimers Dement. 2013;9(1):e-1-e16. doi: 10.1016/j.jalz.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quest Diagnostics.Beta-amyloid 42/40 ratio, CSF. Quest Diagnostics Incorporated. 2022. Accessed October 31, 2022. https://testdirectory.questdiagnostics.com/test/test-detail/94627/beta-amyloid-4240-ratio-csf?p=r&cc=MASTER
- 26. Mayo Clinic Laboratories. Alzheimer Disease Evaluation, Spinal Fluid. Mayo Clinic. 2022. Accessed October 31, 2022. https://www.mayocliniclabs.com/test-catalog/Overview/607273 [Google Scholar]
- 27. Athena Diagnostics. ADmark® Alzheimer's Evaluation. Athena Diagnostics, Incorporated. 2022. Accessed October 31, 2022. https://www.athenadiagnostics.com/view-full-catalog/a/admark-reg;-alzheimer-s-evaluation
- 28. Day GS, Rappai T, Sathyan S, Morris JC. Deciphering the factors that influence participation in studies requiring serial lumbar punctures. Alzheimers Dement (Amst). 2020;12(1):e12003. doi: 10.1002/dad2.12003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer’s disease. Alzheimers Res Ther. 2019;11(1):34. doi: 10.1186/s13195-019-0485-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lewczuk P, Esselmann H, Otto M, et al. Neurochemical diagnosis of Alzheimer’s dementia by CSF Abeta42, Abeta42/Abeta40 ratio and total tau. Neurobiol Aging. 2004;25(3):273-281. doi: 10.1016/S0197-4580(03)00086-1 [DOI] [PubMed] [Google Scholar]
- 31. Slaets S, Le Bastard N, Martin JJ, et al. Cerebrospinal fluid Aβ1-40 improves differential dementia diagnosis in patients with intermediate p-tau181P levels. J Alzheimers Dis. 2013;36(4):759-767. doi: 10.3233/JAD-130107 [DOI] [PubMed] [Google Scholar]
- 32. Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13(8):841-849. doi: 10.1016/j.jalz.2017.06.2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schindler SE, Bollinger JG, Ovod V, et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93(17):e1647-e1659. doi: 10.1212/WNL.0000000000008081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 2018;554(7691):249-254. doi: 10.1038/nature25456 [DOI] [PubMed] [Google Scholar]
- 35. Giudici KV, de Souto Barreto P, Guyonnet S, Li Y, Bateman RJ, Vellas B. Assessment of plasma amyloid-β42/40 and cognitive decline among community-dwelling older adults. JAMA Netw Open. 2020;3(12):e2028634. doi: 10.1001/jamanetworkopen.2020.28634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Janelidze S, Teunissen CE, Zetterberg H, et al. Head-to-head comparison of 8 plasma amyloid-β 42/40 assays in Alzheimer disease. JAMA Neurol. 2021;78(11):1375-1382. doi: 10.1001/jamaneurol.2021.3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chong JR, Ashton NJ, Karikari TK, et al. Blood-based high sensitivity measurements of beta-amyloid and phosphorylated tau as biomarkers of Alzheimer's disease: a focused review on recent advances. J Neurol Neurosurg Psychiatry. 2021;92(11):1231-1241. doi: 10.1136/jnnp-2021-327370 [DOI] [PubMed] [Google Scholar]
- 38. C2N Diagnostics. PrecivityAD: Advanced Diagnostics in Alzheimer’s Disease. C2N Diagnostics; 2021 Accessed March 5, 2021. https://static1.squarespace.com/static/5f5f9aa2954456537a28b45f/t/5fa016b103e56c25d65e3662/1604327090503/PrecivityAD_Patient_Flyer.pdf
- 39. Quest Diagnostics. Assessing Alzheimer's Risk Starts with a Single Blood Test. Quest Diagnostics Incorporated;2022. Accessed October 31, 2022. https://images.health.questdiagnostics.com/Web/QuestDiagnosticsIncorporated/%7B1ec646f8-1169-480d-83f9-94cb2b6b1559%7D_QUEST_AD-Detect%E2%84%A2_Brochure.PDF?elqTrackId=1d700e7835f9467f971b9d9e1af8d5c2&elqaid=460&elqat=2 [Google Scholar]
- 40. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P-tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020;26(3):379-386. doi: 10.1038/s41591-020-0755-1 [DOI] [PubMed] [Google Scholar]
- 41. Moscoso A, Grothe MJ, Ashton NJ, et al. Longitudinal associations of blood phosphorylated tau181 and neurofilament light chain with neurodegeneration in Alzheimer disease. JAMA Neurol. 2021;78(4):396-406. doi: 10.1001/jamaneurol.2020.4986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324(8):772-781. doi: 10.1001/jama.2020.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Janelidze S, Berron D, Smith R, et al. Associations of plasma phospho-tau217 levels with tau positron emission tomography in early Alzheimer disease. JAMA Neurol. 2021;78(2):149-156. doi: 10.1001/jamaneurol.2020.4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brickman AM, Manly JJ, Honig LS, et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement. 2021;17(8):1353-1364. doi: 10.1002/alz.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thijssen EH, La Joie R, Strom A, et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol. 2021;20(9):739-752. doi: 10.1016/S1474-4422(21)00214-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. De Meyer S, Vanbrabant J, Schaeverbeke JM, et al. Phospho-specific plasma p-tau181 assay detects clinical as well as asymptomatic Alzheimer's disease. Ann Clin Transl Neurol. 2022;9(5):734-746. doi: 10.1002/acn3.51553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pichet Binette A, Palmqvist S, Bali D, et al. Combining plasma phospho-tau and accessible measures to evaluate progression to Alzheimer’s dementia in mild cognitive impairment patients. Alzheimers Res Ther. 2022;14(1):46. doi: 10.1186/s13195-022-00990-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Janelidze S, Bali D, Ashton NJ, et al. Head-to-head comparison of 10 plasma phospho-tau assays in prodromal Alzheimer's disease. Brain. Published online September 10, 2022. doi: 10.1093/brain/awac333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers Dement. Published online July 31, 2022. doi: 10.1002/alz.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Musiek ES, Morris JC. Possible consequences of the approval of a disease-modifying therapy for Alzheimer disease. JAMA Neurol. 2021;78(2):141-142. doi: 10.1001/jamaneurol.2020.4478 [DOI] [PubMed] [Google Scholar]
- 51. Karlawish J. Addressing the ethical, policy, and social challenges of preclinical Alzheimer disease. Neurology. 2011;77(15):1487-1493. doi: 10.1212/WNL.0b013e318232ac1a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jayadev S. Genetics of Alzheimer disease. Continuum. 2022;28(3):852-871. doi: 10.1212/CON.0000000000001125 [DOI] [PubMed] [Google Scholar]
- 53. Mozersky J, Roberts JS, Rumbaugh M, et al. Spillover: the approval of new medications for Alzheimer's disease dementia will impact biomarker disclosure among asymptomatic research participants. J Alzheimers Dis. Published online April 8, 2022. doi: 10.3233/JAD-220113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee S, Mankhong S, Kang JH. Extracellular vesicle as a source of Alzheimer's biomarkers: opportunities and challenges. Int J Mol Sci. 2019;20(7):1728. doi: 10.3390/ijms20071728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Picca A, Guerra F, Calvani R, Coelho-Junior HJ, Bucci C, Marzetti E. Circulating extracellular vesicles: friends and foes in neurodegeneration. Neural Regen Res. 2022;17(3):534-542. doi: 10.4103/1673-5374.320972 [DOI] [PMC free article] [PubMed] [Google Scholar]


