Abstract
Despite the prolongation of hospitalization, increase in morbidity, mortality and cost of care associated with both surgical site infections (SSIs) and antibiotic resistance, there are limited data on SSIs and antibiotic resistance to guide prevention strategies in Sierra Leone. This study assessed the burden of SSIs and related antibiotic resistance in the 34 Military Hospital (MH) and Makeni Government Hospital (MGH) located in two geographic regions of Sierra Leone using a prospective study design to collect data from adults aged 18 years or older. Of the 417 patients, 233 (55.9%) were enrolled in MGH. Most were women 294 (70.5%). The incidence rate of SSI was 5.5 per 1000 patient-days, and the cumulative incidence of SSI was 8.2%. Common bacteria isolated in MH were Escherichia coli (6,33.3%) and Pseudomonas aeruginosa (3,16.7%) and in MGH were P. aeruginosa (3,42.9%) and Proteus mirabilis (2,28.9%). Of the gram-negative bacteria, 40% were Extended-spectrum beta-lactamase-producing Enterobacteriaceae, 33% were Carbapenem-resistant P. aeruginosa and 10% were carbapenem-resistant Enterobacteriaceae. Although the incidence of SSIs in our study is lower than previously reported, the rate of antibiotic resistance reported in this study is high. Urgent action is needed to invest in the microbiology infrastructure to support SSI surveillance and prevention strategies.
Keywords: Antimicrobial resistance (AMR), Carbapenem-resistant Enterobacteriaceae (CRE), Extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL), Sierra Leone, Structured operational research initiative training (SORT IT) and Antibiotic resistance, Surgical site infection (SSI), Surgical site infections (SSIs)
Background
Owing to the complexity and substantial resources required to diagnose healthcare-associated infections (HAIs) and multi-drug resistance pathogens, their actual burden in developing countries is unknown.1,2 Global estimates of the incidence of HAIs in developed countries range from 1.2% to 5.2%.1 In developing countries, the pooled prevalence of HAIs is 10.1%.1 The prevalence of HAIs is 19.4% in Ethiopia, 14.8% in Tanzania and 14.3% in Nigeria.3–5 Cumulatively, the incidence of HAIs in the African region is between 2.5% and 14.8%, with most studies reporting assessments of surgical site infections (SSIs).6
Several risk factors underlie the development of SSIs. Laloto et al.7 reported in 2017 that older age, prolonged preoperative hospital stay and inappropriate antibiotic prophylaxis contribute to the development of SSIs. Other factors, such as comorbidities, smoking, high American Society of Anaesthesiologists (ASA) preoperative assessment scores and low compliance with hand hygiene, also contribute to the development of postoperative wound infections.8–10 Our previous research has shown that many of these risk factors exist in our environment. In these studies, there were high rates of inappropriate surgical antibiotic prophylaxis, low compliance to hand hygiene among healthcare workers and limited resources to support hand hygiene promotion activities in Sierra Leone.11–13
SSIs and antibiotic resistance have an intertwined relationship. Bacteria isolated from postoperative wounds are often resistant to multiple antibiotics and lead to prolonged hospital stay, worsened morbidity and mortality, and increased cost of care.7,14 Recognizing the enormous challenges caused by SSI, other HAIs and antibiotic resistance, the member states of the United Nations (UN) made commitments to tackle the problem in its 2015 General Assembly, taking into account that antimicrobial resistance is incorporated into the Sustainable Development Goals.15,16 Furthermore, in January 2022, the World Health Organization (WHO) Executive Board called on countries to prioritize the prevention of SSI and other infections at the health facility level as a means of strengthening health systems.17 Hence, to strengthen the local health system, surveillance and prevention strategies for SSIs and drug-resistant pathogens should be prioritized by all health facilities.
In Sierra Leone, the SSI data needed to identify existing gaps in surveillance and prevention are limited. This challenge has a tendency to disrupt the design and implementation of targeted improvement plans. Previous studies, conducted mainly in the capital of Sierra Leone, have reported SSIs prevalence rates between 7.3% and 11.5%, comparable to the global prevalence of SSIs.18–20 There is no such data to describe SSIs in different geographic regions of Sierra Leone. Understanding the burden of SSI and antibiotic resistance and its drivers will have important policy implications for implementing appropriate antibiotic prescribing practices and Infection Prevention and Control (IPC) activities.
In this study, we aimed to assess the burden of SSIs and associated antibiotic resistance in two hospitals in two geographic regions of Sierra Leone to provide information for local antibiotic stewardship and IPC. Specifically, in 2021, the study (1) determined the incidence of SSIs; (2) described the clinical characteristics of postoperative patients; (3) assessed the risk factors for SSIs; and (4) identified bacteria isolated from postoperative wounds and determined their antibiotic resistance patterns.
Methods
Study design and study population
The study employed a prospective observational design to collect data from adults 18 years or older who had surgeries in two hospitals in Sierra Leone.
Study settings
We selected two hospitals in different geographic regions of Sierra Leone for the study. Both hospitals serve large populations in their catchment areas, thereby providing quasi-tertiary healthcare services.
Of the two hospitals, the 34 Military Hospital (MH) is located in Freetown, Sierra Leone’s capital with a population of 1 million persons while Makeni Government Hospital (MGH) is a regional hospital located 170 km away from the capital with a population of 444,270 inhabitants, which is approximately 6.3% of the Sierra Leonean population.21 The two hospitals were selected because their location in different geographic settings provided an opportunity to understand the urban–rural differences in the burden of SSIs and antibiotic resistance in Sierra Leone.
The two hospitals are owned by the government of Sierra Leone with similar infrastructure and almost an equal capacity of 200 beds each. Both provide medical, surgical, paediatrics, obstetrics and gynaecology services.
Sampling method
With a confidence level of 95%, a margin of error of 3% and a proportion of patients with SSI of 13.85%,3 we calculated a minimum sample size of 183 patients per hospital using the Leslie Kish formula. However, we sequentially enrolled 417 surgical patients admitted to the surgical and the obstetrics and gynaecology wards of the two hospitals, from March 2021 through October 2021. Of these, 233 were recruited at MGH.
Data were collected by nurses trained in SSI assessment and antibiotic resistance. After admission, patients were recruited in the preoperative ward by collecting data on the ASA score,22 and preoperative skin preparation. In the postoperative ward, information on the intraoperative procedure and surgical wound was collected.
Patients were followed weekly by phone or hospital visit until they develop SSI or when the wound is completely healed. If the wound is still active, we continue to follow patients until 30 days after surgery. When a patient developed an SSI, wound swabs were collected under aseptic technique and sent to the laboratory for processing. All laboratory results were communicated to the clinicians managing the patients.
Definitions
Surgical wounds were classified according to the possibility of infection as clean, clean-contaminated and contaminated.23,24 While a clean wound is an uninfected operative wound in which no inflammation is encountered and the respiratory, alimentary, genital or uninfected urinary tracts are not entered, clean-contaminated wounds are operative wounds in which the respiratory, alimentary, genital or urinary tracts are entered under controlled conditions and without unusual contamination. A contaminated wound is an open, fresh, accidental wound.
A postoperative wound was clinically identified as SSI if it occurs within 30 days after the operative procedure with any of purulent discharge from the incision, local swelling, redness, pain or tenderness, wound abscess or fever.23,24
SSI were classified clinically into superficial (presence of signs and symptoms of infection at the site), deep (presence of at least purulent drainage from the deep incision, spontaneous dehisces or a deliberately opened deep incision, localized pain, tenderness or abscess) and organ/space (involves any part of the anatomy other than the incision, which was opened or manipulated during an operation and at least purulent drainage from a drain that is placed through a stab wound into the organ/space).
Laboratory procedure
On reaching the laboratory, wound swabs were streaked onto the chromogenic agar plate (CHROMagarTM orientation) and incubated aerobically at 37°C for 18–24 h. Where there was a bacterial growth, a colony was picked and streaked onto a Brain Heart Infusion (BHI) agar plate for purification and gram staining. All isolates were cultured twice to ensure they were pure but there wer no discordant results.
A VITEK 2 compact system (bioMérieux, France) was used for identification and antibiotic susceptibility testing of isolates from pure cultures. Using a DensiCHEK Plus turbidimeter (bioMérieux, France), a solution of bacteria in saline was prepared in polystyrene tubes (bioMérieux, France) to levels between 0.5 and 0.63 McFarland standard. Antibiotic susceptibility tests were performed by adding 145 μl (for gram-negative bacteria) or 280 μl (for gram-positive bacteria) of suspension into a new polystyrene tube as per the manufacturer’s instructions.
The isolate suspensions were loaded on the VITEK 2 compact system and incubated overnight at 37°C. All the culture and antibiotic susceptibility results generated were printed and dispatched to the research team.
Data collection, management and analysis
Data were exported from the Epi-collect platform to Microsoft Excel, cleaned, coded and transferred to STATA version 16 (StataCorp LLC) for analysis. Descriptive statistics, such as frequencies and percentages, were used to present the demographic and clinical characteristics of study participants.
Bivariate and multivariate Cox regression was used to identify factors associated with SSI.
Variables with p values <0.25 from a bivariate Cox regression model were fitted to a multivariate logistic regression model to identify predictors of SSI. Results are shown as crude hazard ratios and adjusted hazard ratios (aHR) with 95% confidence intervals (CIs) after bivariate analysis and multivariate analysis. P values less than 0.05 were considered statistically significant.
Results
Demographic characteristics of study participants
Of the 417 patients enrolled in this study, 233 (55.9%) were recruited in MGH. The majority were women (294,70.5%), and 197 (47.2%) were between the ages of 25 and 44 years. Details of the patients’ demographic characteristics are presented in Table 1.
Table 1.
Sociodemographic characteristics of the study participants (N = 417).
| Variables | Total N (%) |
MH N (%) |
MGH N (%) |
|---|---|---|---|
| Overall total | 417 (100) | 184 (44.1) | 233 (55.9) |
| Sex | |||
| Female | 294 (70.5) | 69 (37.5) | 225 (97.6) |
| Male | 123 (29.5) | 115 (62.5) | 8 (3.4) |
| Age (years) | |||
| <25 | 174 (41.7) | 52 (28.3) | 122 (52.4) |
| 25–44 | 197 (47.2) | 86 (48.4) | 108 (46.3) |
| 45–60 | 29 (7.0) | 24 (15.2) | 1 (0.4) |
| >60 | 17 (4.1) | 15 (8.1) | 2 (0.9) |
| Level of education | |||
| None | 70 (16.8) | 38 (20.7) | 32 (13.7) |
| Primary | 62 (14.9) | 30 (16.3) | 32 (13.7) |
| Secondary | 223 (53.5) | 83 (45.1) | 140 (60.1) |
| Tertiary | 62 (14.9) | 33 (17.9) | 29 (12.5) |
MGH, Makeni Government Hospital; MH, Military Hospital.
Clinical findings
Most patients had an ASA score of 2 or above (383,91.8%). The majority of the patients had obstetric surgery (232,55.6%), were admitted for less than 24 h before surgery (326,78.4%) and were placed on antibiotics preoperatively (400,96.1%) (Table 2).
Table 2.
Clinical characteristics of study participants (N = 417).
| Variables | Total N (%) |
MH N (%) |
MGH N (%) |
|---|---|---|---|
| Overall total | 417 (100) | 184 (44.1) | 233 (55.9) |
| ASA score | |||
| <2 | 34 (8.2) | 28 (15.2) | 6 (2.6) |
| ⩾2 | 383 (91.8) | 156 (84.8) | 227 (97.4) |
| Surgery | |||
| Obstetric | 232 (55.6) | 44 (23.9) | 188 (80.7) |
| Gynaecological | 24 (5.8) | 4 (2.2) | 20 (8.6) |
| Abdominal | 145 (34.8) | 122 (66.3) | 23 (9.9) |
| Non-abdominal | 16 (3.8) | 14 (7.6) | 2 (0.9) |
| Preoperative blood transfusion | |||
| No | 413 (99.0) | 183 (99.5) | 230 (98.7) |
| Yes | 4 (1.0) | 1 (0.5) | 3 (1.3) |
| Comorbidities | |||
| Absent | 385 (92.3) | 157 (85.3) | 228 (97.8) |
| Present | 32 (7.7) | 27 (14.7) | 5 (2.2) |
| Preoperative admission (h) | |||
| ⩽24 | 326 (78.4) | 108 (59.0) | 218 (93.6) |
| >24 | 90 (21.6) | 75 (41.0) | 15 (6.4) |
| Preoperative antibiotics | |||
| No | 16 (3.9) | 4 (2.2) | 12 (5.2) |
| Yes | 400 (96.1) | 180 (97.8) | 220 (94.8) |
| Surgery duration (min) | |||
| <120 | 408 (97.8) | 177 (96.2) | 231 (99.1) |
| ⩾120 | 9 (2.2) | 7 (3.8) | 2 (0.9) |
| Type of anaesthesia | |||
| Local/spinal | 177 (42.5) | 165 (89.7) | 12 (5.2) |
| General | 240 (57.5) | 19 (10.3) | 221 (94.8) |
| Type of suture | |||
| Vicryl | 233 (55.9) | 0 (0) | 233 (100) |
| Nylon | 184 (44.1) | 184 (100) | 0 |
| Intraoperative blood transfusion | |||
| No | 398 (95.4) | 182 (98.9) | 216 (92.7) |
| Yes | 19 (4.6) | 2 (1.1) | 17 (7.3) |
| Number of surgeons | |||
| ⩽2 | 409 (98.3) | 177 (96.2) | 232 (100) |
| >2 | 7 (1.7) | 7 (3.8) | 0 (0) |
| Number of scrub nurses | |||
| 1 | 397 (95.4) | 169 (92.3) | 228 (97.8) |
| >1 | 19 (4.6) | 14 (7.7) | 5 (2.2) |
| Number of anaesthetists | |||
| 1 | 233 (56.0) | 9 (4.9) | 224 (96.5) |
| >1 | 183 (44.0) | 175 (95.1) | 8 (3.5) |
| Intraoperative antibiotics | |||
| No | 384 (94.6) | 165 (90.2) | 219 (98.2) |
| Yes | 22 (5.4) | 18 (9.8) | 4 (1.8) |
| Type of wound | |||
| Clean | 387 (93.0) | 175 (95.6) | 212 (91.0) |
| Clean-contaminated/contaminated | 29 (7.0) | 8 (4.4) | 21 (9.0) |
| Postoperative antibiotics | |||
| No | 4 (1.0) | 3 (1.7) | 1 (0.4) |
| Yes | 410 (99.0) | 178 (98.3) | 232 (99.6) |
| Postoperative NG tube | |||
| No | 410 (98.6) | 179 (97.8) | 231 (99.1) |
| Yes | 6 (1.4) | 4 (2.2) | 2 (0.9) |
| Postoperative urinary catheter | |||
| No | 16 (3.8) | 1 (0.5) | 15 (6.4) |
| Yes | 401 (96.2) | 183 (99.5) | 218 (93.6) |
| Surgical drains use | |||
| No | 400 (95.9) | 168 (91.3) | 232 (99.6) |
| Yes | 17 (4.1) | 16 (8.7) | 1 (0.4) |
| Postoperative admission (h) | |||
| ⩽48 | 25 (6.0) | 14 (7.6) | 11 (4.7) |
| >48 | 392 (94.0) | 170 (92.4) | 222 (95.3) |
| Fasting blood glucose (mmol/litre) | |||
| <5.6 | 149 (35.7) | 28 (15.2) | 121 (51.9) |
| 5.6–6.9 | 185 (44.4) | 89 (48.4) | 96 (41.2) |
| ⩾7 | 83 (19.9) | 67 (36.4) | 16 (6.9) |
| Surgical site infection | |||
| Absent | 383 (91.8) | 164 (89.1) | 219 (94.0) |
| Present | 34 (8.2) | 20 (10.9) | 14 (6.0) |
ASA, American Society of Anaesthesiologists; MGH, Makeni Government Hospital; MH, Military Hospital; NG, nasogastric.
In 177 (42.5%) surgeries, patients were given local or spinal anaesthesia. Surgery was attended by more than two surgeons [7 (1.7%)], more than one scrub nurse [19 (4.6%)] or more than one anaesthesiologist [183 (44%)]. Most surgical wounds were clean [387 (93%)]. Intraoperatively, antibiotics were administered to 22 (5.4%) patients.
Postoperatively, antibiotics were prescribed for 410 (99%) patients. In total, 83 (19.9%) patients reported elevated fasting blood glucose in the diabetic range (Table 2).
Incidence and risk factors associated with SSIs
All 417 patients were followed up for 6227 person-days. During this period, 34 patients developed an SSI (cumulative SSI incidence of 8.2%). The incidence rate of SSI was 5.5 per 1000 patient-days (Table 2). Among the 34 patients with an SSI, 58.8% of them received care at MH.
In bivariate Cox regression analysis, preoperative admission for more than 24 h (p = 0.005), contaminated versus clean wounds (p < 0.001) and postoperative admission time >48 h (p = 0.022) were among the factors associated with the occurrence of SSI. Multivariate Cox regression showed that contaminated wounds had an almost sevenfold higher risk of SSI compared with clean wounds [aHR = 6.82, 95% CI (1.66–28.1); p = 0.008] (Table 3).
Table 3.
Bivariate and multivariable Cox regression of factors associated with SSIs.
| Variables | Surgical site infection | Crude HR (CI) |
p | Adjusted HR (CI) |
p | |
|---|---|---|---|---|---|---|
| Yes, n (%) 34 (8.2) |
No, n (%) 383 (91.8) |
|||||
| Hospital study site | ||||||
| MH | 20 (58.8) | 164 (42.8) | 1 | 1 | ||
| MGH | 14 (41.2) | 219 (57.2) | 0.52 (0.27–1.05) | 0.067 | 0.84 (0.10–6.96) | 0.874 |
| Sex | ||||||
| Female | 22 (64.7) | 272 (71.0) | 1 | |||
| Male | 12 (35.3) | 111 (29.0) | 1.34 (0.66–2.71) | 0.412 | ||
| Age (years) | ||||||
| <25 | 10 (29.4) | 164 (42.8) | 1 | 1 | ||
| 26–44 | 15 (44.1) | 182 (47.5) | 1.50 (0.67–3.34) | 0.320 | 1.44 (0.59–3.48) | 0.421 |
| 45–65 | 6 (17.7) | 23 (6.0) | 3.91 (1.42–10.8) | 0.008 | 2.78 (0.66–11.76) | 0.163 |
| >65 | 3 (8.8) | 14 (3.7) | 4.71 (1.29–17.2) | 0.019 | 3.66 (0.72–18.71) | 0.119 |
| ASA | ||||||
| <2 | 2 (5.9) | 32 (8.4) | 1 | |||
| ⩾2 | 32 (94.1) | 351 (91.6) | 1.48 (0.35–6.13) | 0.599 | ||
| Site of surgery | ||||||
| Obstetric | 12 (35.3) | 220 (57.4) | 1 | 1 | ||
| Gynaecological | 1 (2.9) | 23 (6.0) | 1.00 (0.13–7.71) | 0.999 | 0.39 (0.04–3.69) | 0.416 |
| Abdominal | 18 (52.9) | 127 (33.2) | 2.59 (1.25–5.39) | 0.011 | 0.84 (0.25–2.78) | 0.773 |
| Non-abdominal | 3 (8.8) | 13 (3.4) | 3.51 (0.99–12.47) | 0.053 | 1.31 (0.25–6.95) | 0.755 |
| Comorbidities | ||||||
| Absent | 29 (85.3) | 357 (92.9) | 1 | 1 | ||
| Present | 5 (14.7) | 27 (7.1) | 2.00 (0.77–5.16) | 0.154 | 1.51 (0.49–4.67) | 0.476 |
| Preoperative admission (h) | ||||||
| ⩽24 | 19 (55.9) | 307 (80.4) | 1 | 1 | ||
| >24 | 15 (44.1) | 75 (19.6) | 2.61 (1.33–5.14) | 0.005 | 1.27 (0.51–3.19) | 0.610 |
| Preoperative antibiotics | ||||||
| No | 2 (5.9) | 14 (3.7) | 1 | |||
| Yes | 32 (94.1) | 368 (96.3) | 0.47 (0.11–1.97) | 0.303 | ||
| Surgery duration (min) | ||||||
| <120 | 31 (91.2) | 377 (98.4) | 1 | 1 | ||
| ⩾120 | 3 (8.8) | 6 (1.6) | 2.68 (0.82–8.82) | 0.104 | 0.44 (0.07–2.74) | 0.379 |
| Type of anaesthesia | ||||||
| Local/spinal | 16 (47.1) | 161 (42.0) | 1 | |||
| General | 18 (52.9) | 222 (58.0) | 0.81 (0.41–1.58) | 0.533 | ||
| Number of surgeons | ||||||
| ⩽2 | 30 (88.2) | 379 (99.2) | 1 | 1 | ||
| >2 | 4 (11.8) | 3 (0.8) | 6.1 (2.13–17.41) | 0.001 | 1.49 (0.24–9.19) | 0.669 |
| Number of scrub nurses | ||||||
| 1 | 29 (85.3) | 368 (96.3) | 1 | 1 | ||
| >1 | 5 (14.7) | 14 (3.7) | 3.03 (1.15–7.94) | 0.024 | 1.26 (0.31–5.18) | 0.745 |
| Number of anaesthetists | ||||||
| 1 | 14 (41.2) | 219 (57.3) | 1 | 1 | ||
| >1 | 20 (58.8) | 163 (42.7) | 1.91 (0.96–3.78) | 0.064 | 1.07 (0.13–8.75) | 0.946 |
| Intraoperative antibiotics | ||||||
| No | 31 (96.9) | 353 (94.4) | 1 | |||
| Yes | 1 (3.1) | 21 (5.6) | 0.57 (0.08–4.23) | 0.588 | ||
| Type of wound | ||||||
| Clean | 25 (73.5) | 362 (94.8) | 1 | 1 | ||
| Clean-contaminated/contaminated | 9 (26.5) | 20 (5.2) | 4.32 (2.02–9.26) | <0.001 | 6.82 (1.66–28.1) | 0.008 |
| Postoperative antibiotics | ||||||
| No | 0 (0) | 4 (1.0) | 1 | |||
| Yes | 34 (100) | 376 (99.0) | 0.82 (0.04–15.64) | 0.898 | ||
| Postoperative NG tube | ||||||
| No | 31 (91.2) | 379 (99.2) | 1 | 1 | ||
| Yes | 3 (8.8) | 3 (0.8) | 3.74 (1.12–12.51) | 0.032 | 0.31 (0.04–2.15) | 0.235 |
| Urinary catheter | ||||||
| No | 2 (5.9) | 14 (3.7) | 1 | |||
| Yes | 32 (94.1) | 369 (96.3) | 0.59 (0.14–2.49) | 0.477 | ||
| Surgical drains use | ||||||
| No | 30 (88.2) | 370 (96.6) | 1 | 1 | ||
| Yes | 4 (11.8) | 13 (3.4) | 2.97 (1.04–8.42) | 0.041 | 1.47 (0.31–6.91) | 0.624 |
| Postoperative admission (h) | ||||||
| ⩽48 | 5 (14.7) | 20 (5.2) | 1 | 1 | ||
| >48 | 29 (85.3) | 363 (94.8) | 0.33 (0.13–0.85) | 0.022 | 0.33 (0.11–1.00) | 0.050 |
ASA, American Society of Anaesthesiologists; CI, confidence interval; HR, hazard ratio; MGH, Makeni Government Hospital; MH, Military Hospital.
Bacteria isolates from postoperative wounds and patterns of antibiotic resistance
In total, wound swabs were collected from 32 (94%) of 34 SSI patients; 20 (100%) in MH and 12 (86%) in MGH. Bacterial growth was reported in 18 (90%) and 7 (58%) swabs collected from MH and MGH, respectively.
Common bacteria isolated in MH were Escherichia coli [6 (33.3%)] and Pseudomonas aeruginosa [3 (16.7%)]. P. aeruginosa [3 (42.9%)] and Proteus mirabilis [2 (28.9%)] were the common bacteria isolated in MGH (Table 4).
Table 4.
Bacterial isolates from postoperative wounds.
| Bacterial isolate | MH | MGH | ||
|---|---|---|---|---|
| N = 18 | % | N = 7 | % | |
| Escherichia coli | 6 | 33.3 | 1 | 14.3 |
| Klebsiella pneumoniae ssp pneumonia | 0 | 0.0 | 0 | 0.0 |
| Klebsiella oxytoca | 1 | 5.6 | 0 | 0.0 |
| Pseudomonas aeruginosa | 3 | 16.7 | 3 | 42.9 |
| Enterobacter cloacae complex | 1 | 5.6 | 0 | 0.0 |
| Enterococcus gallinarum | 1 | 5.6 | 0 | 0.0 |
| Acinetobacter baumannii complex | 1 | 5.6 | 0 | 0.0 |
| Sphingomonas paucimobilis | 1 | 5.6 | 0 | 0.0 |
| Staphylococcus haemolyticus | 1 | 5.6 | 0 | 0.0 |
| Rhizobium radiobacter | 0 | 0.0 | 1 | 14.3 |
| Proteus mirabilis | 0 | 0.0 | 2 | 28.6 |
| Kocuria kristinae | 1 | 5.6 | 0 | 0.0 |
| Achromobacter xylosoxidans | 1 | 5.6 | 0 | 0.0 |
| Stenotrophomonas maltophilia | 1 | 5.6 | 0 | 0.0 |
MGH, Makeni Government Hospital; MH, Military Hospital.
Less common gram-negative bacilli such as Achromobacter xylosoxidans, Stenotrophomonas maltophilia and Sphingomonas paucimobilis were isolated mostly in MH. Rhizobium radiobacter, another less commonly found gram-negative bacillus, was isolated in MGH. Two isolates of the gram-positive cocci such as Staphylococcus haemolyticus and the rare emerging pathogen, K. kristinae, were isolated in MH.
Patterns of antibiotic resistance of bacteria isolates from postoperative wounds
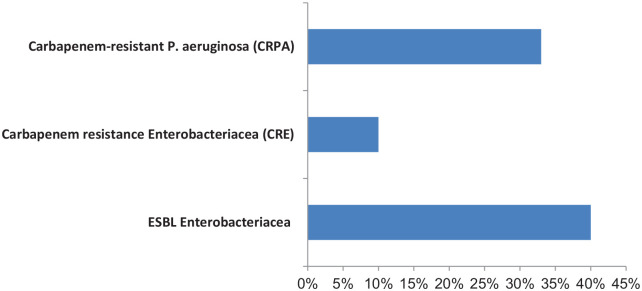
Of all the gram-negative bacilli isolated from post-operative wounds, 40% were Extended-spectrum β-lactamase (ESBL) producing Enterobacteriaceae, 33% were Carbapenem-resistant P. aeruginosa (CRPA) and 10% were Carbapenem-resistant Enterobacteriaceae (CRE) (Figure 1). Many bacteria were sensitive to piperacillin/tazobactam, amikacin, cefotetan, imipenem and meropenem (Table 5).
Figure 1.
Patterns of antibiotic resistance of bacteria isolates from postoperative wounds.
Table 5.
Antibiotic resistance profile of gram-negative bacteria isolates.
| Antibiotics | Resistance rate (%)a | ||||||
|---|---|---|---|---|---|---|---|
|
E. coli
N = 7 |
S. paucimobilis
N = 1 |
P. aeruginosa
N = 6 |
A. Baumannii
N = 1 |
K. oxytoca
N = 1 |
P. mirabilis
N = 2 |
S. maltophilia
N = 1 |
|
| Imipenem | 0.0 | 0.0 | 33.3 | 0.0 | 0.0 | — | — |
| Meropenem | 0.0 | 0.0 | 33.3 | — | 0.0 | 50.0 | — |
| Ampicillin | 100.0 | 100.0 | — | 100.0 | — | — | — |
| Ampicillin/sulbactam | 86.0 | 81.8 | — | 0.0 | — | — | — |
| Ciprofloxacin | 86.0 | 81.8 | 83.3 | 0.0 | 100.0 | 100.0 | — |
| Levofloxacin | 86.0 | 54.5 | 66.7 | 0.0 | 100.0 | 100.0 | — |
| Amikacin | 0.0 | 9.1 | 0.0 | 0.0 | 0.0 | 0.0 | — |
| Gentamicin | 86.0 | 63.0 | 83.3 | 0.0 | 100.0 | 100.0 | — |
| Tobramycin | 43.0 | 72.0 | 83.3 | 0.0 | 100.0 | 100.0 | — |
| Aztreonam | 86.0 | 63.0 | — | — | 100.0 | — | — |
| Co-trimoxazole | 100 | 72.0 | — | 0.0 | 100.0 | — | 0.0 |
| Nitrofurantoin | 0.0 | 13.3 | — | 100.0 | 0.0 | — | — |
| Ceftriaxone | 100.0 | 90.9 | — | 100.0 | 100.0 | 100 | — |
| Cefuroxime | 100.0 | 100.0 | — | 100.0 | 100.0 | — | — |
| Cefuroxime axetil | 100.0 | 100.0 | — | 100.0 | 75.0 | — | — |
| Cefotetan | 0.0 | 0.0 | — | 100.0 | 0.0 | 50.0 | — |
| Cefazolin | 100.0 | 81.8 | — | 100.0 | 100.0 | — | — |
| Ceftazidime | 100.0 | 100.0 | 33.3 | 100.0 | 100.0 | 100.0 | — |
| Cefepime | 100.0 | 100.0 | 25.0 | 100.0 | 100.0 | 100.0 | — |
| Piperacillin/tazobactam | 14.3 | 9.1 | 16.7 | 0.0 | 100.0 | 50.0 | — |
Percentages = number of resistant antibiotics/total number of antibiotics tested.
(—) indicates not tested.
Discussion
We recorded an incidence rate of SSIs of 5.5 per 1000 patients-days and a cumulative SSIs incidence of 8.2%. The incidence of SSIs reported in this study is lower than the previously reported incidence of 11.5% in secondary and tertiary care settings in Sierra Leone.18 Similarly, only 7% of surgical wounds in this study were clean-contaminated or contaminated, compared with previously reported rates of more than 40% of clean-contaminated and contaminated wounds.18 These variations in the clinical characteristics may explain the differences in the incidence of SSIs reported in these studies. Furthermore, the incidence of SSIs reported in this study is lower than the African average of 16%.25 Sierra Leone is one of the countries that suffered the most from the 2014 to 2016 Ebola virus disease epidemic.26 In response to this epidemic, the government established the National IPC Policy in 2015.27 Since then, the IPC programme has been supporting hospitals to strengthen IPC services. The improvement in IPC services may explain the relatively lower incidence of SSI in our study. Nevertheless, because SSIs can have dire consequences for patients and their caregivers, including costs and worsening morbidity and mortality,7 long-term planning is required to sustain ongoing efforts.
More than 96% of patients in this study received an antibiotic during the preoperative and postoperative periods. One might think that this may have provided protection against the development of SSIs. However, there was no statistical association between the occurrence of SSI and antibiotic use. The lack of protection against SSIs by the prescribed antibiotics could be explained by the fact that, in these hospital settings, the wrong antibiotics are used at the wrong time for surgical prophylaxis.28
Risk factors for SSI in this study are older age, abdominal surgeries, duration of preoperative admission time more than 24 h, the presence of more than two surgeons or one scrub nurse, contaminated wounds, placement of nasogastric (NG) tube or surgical drain, the presence of comorbidities and surgery duration. This pattern is similar to the studies in sub-Saharan Africa18,25,29 and elsewhere in Israel30 and the United States.31 These findings may be a reflection of late presentation for care or perhaps it could be explained by the inappropriate use of surgical drains and NG tubes in these hospitals. Although not statistically significant, the difference in risk factor burden could also explain the higher incidence of SSI in MH than in MGH.
A variety of bacteria were isolated from postoperative wounds, most of which were gram-negative bacilli. This is not uncommon in this environment and in Africa as a whole. Similar patterns of bacterial isolates in postoperative wounds were reported in our previous study18 and several other studies in Africa.7,32 As normal flora common to the skin, Staphylococcus aureus and other gram-positive cocci have traditionally been the most common bacteria isolated from postoperative wounds.33 The reason for the changes in the pattern of bacteria isolates is unclear, but an important message from this finding is that guidelines on surgical antibiotic prophylaxis should take into consideration the local evidence of bacteria isolates and antibiotic resistance patterns.
Several unusual bacteria are isolated from the postoperative wounds of patients in MH. These include gram-negative bacilli such as A. xylosoxidans, S. maltophilia and S. paucimobilis, as well as the rare emerging gram-positive cocci, K. kristinae. A. xylosoxidans often causes disease in people with cystic fibrosis but has been isolated from the postoperative wound of a patient after ventral hernia repair.34 S. paucimobilis has been isolated in ophthalmic surgery.35 In a recent study, K. kristinae was isolated from the postoperative wound of a patient in a tertiary hospital in Sierra Leone.18
Antibiotic resistance is a global health problem, as it continues to increase at unparallel scale.36 In this study, most of the bacteria from postoperative wounds are either ESBL-producing Enterobacteriaceae or are CRPA similar to previous studies conducted in Sierra Leone18,37 and Egypt.33 Less commonly, some isolates are CRE.
This study has limitations and strengths. Although the evidence generated by this study is not nationally representative, it provides evidence of SSIs and associated antibiotic resistance in two geographic regions of Sierra Leone. The small number of bacterial isolates obtained from postoperative wounds of patients in this study makes the antibiotic resistance pattern not truly representative.
Conclusion
Although the incidence of SSI recorded in our study is lower than previously reported, the rate of antibiotic resistance in this study is high. Urgent action is needed to invest in improving the microbiology infrastructure to support SSIs surveillance and prevention strategies.
Acknowledgments
We acknowledge the support provided by the Chinese Medical Expert Group at the 34 Military Hospital. We acknowledge the cooperation of the staff and patients and patients’ relatives at the hospitals where the study was conducted.
Footnotes
ORCID iD: Sulaiman Lakoh  https://orcid.org/0000-0002-7639-0004
https://orcid.org/0000-0002-7639-0004
Contributor Information
Sulaiman Lakoh, Department of Medicine, College of Medicine and Allied Health Sciences, University of Sierra Leone, New England, Freetown, Sierra Leone; Ministry of Health and Sanitation, Government of Sierra Leone, New England, Freetown, Sierra Leone; Sustainable Health Systems Sierra Leone, Freetown, Sierra Leone; Infectious Disease Research Network, Freetown, Sierra Leone.
Le Yi, Tropical Infectious Disease Prevention and Control Center, Freetown, Sierra Leone.
James B.W. Russell, College of Medicine and Allied Health Sciences, University of Sierra Leone, Freetown, Sierra Leone Ministry of Health and Sanitation, Government of Sierra Leone, Freetown, Sierra Leone.
Juling Zhang, Department of Clinical Laboratory, The Fifth Medical Center of Chinese PLA General Hospital, Beijing, China.
Stephen Sevalie, College of Medicine and Allied Health Sciences, University of Sierra Leone, Freetown, Sierra Leone; Sustainable Health Systems Sierra Leone; 34 Military Hospital, Freetown, Sierra Leone.
Yongkun Zhao, Tropical Infectious Disease Prevention and Control Center, Freetown, Sierra Leone.
Joseph Sam Kanu, College of Medicine and Allied Health Sciences, University of Sierra Leone, Freetown, Sierra Leone; Ministry of Health and Sanitation, Government of Sierra Leone, Freetown, Sierra Leone.
Peng Liu, Department of Emergency Medicine, The Fifth Medical Center of Chinese PLA General Hospital, Beijing, China.
Sarah K. Conteh, Ministry of Health and Sanitation, Government of Sierra Leone, Freetown, Sierra Leone
Christine Ellen Elleanor Williams, College of Medicine and Allied Health Sciences, University of Sierra Leone, Freetown, Sierra Leone; Ministry of Health and Sanitation, Government of Sierra Leone, Freetown, Sierra Leone.
Umu Barrie, Infectious Disease Research Network, Freetown, Sierra Leone.
Mohamed Gbessay Sheku, Ministry of Health and Sanitation, Government of Sierra Leone, Freetown, Sierra Leone.
Mohamed Boie Jalloh, College of Medicine and Allied Health Sciences, University of Sierra Leone, Freetown, Sierra Leone; 34 Military Hospital, Freetown, Sierra Leone.
Olukemi Adekanmbi, Department of Medicine, College of Medicine, University of Ibadan, Ibadan, Nigeria; Department of Medicine, University College Hospital, Ibadan, Nigeria.
Darlinda F. Jiba, Ministry of Health and Sanitation, Government of Sierra Leone, Freetown, Sierra Leone
Matilda N. Kamara, College of Medicine and Allied Health Sciences, University of Sierra Leone, Freetown, Sierra Leone
Gibrilla F. Deen, College of Medicine and Allied Health Sciences, University of Sierra Leone, Freetown, Sierra Leone Ministry of Health and Sanitation, Government of Sierra Leone, Freetown, Sierra Leone.
Joseph Chukwudi Okeibunor, World Health Organization Regional Office for Africa, Brazzaville, Congo.
George A. Yendewa, Department of Medicine, Case Western Reserve University School of Medicine, Cleveland, OH, USA Division of Infectious Diseases and HIV Medicine, University Hospitals Cleveland Medical Center, Cleveland, OH, USA; Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Xuejun Guo, Tropical Infectious Disease Prevention and Control Center, Freetown, Sierra Leone.
Emmanuel Firima, Clinical Research Unit, Department of Medicine, Swiss Tropical and Public Health Institute, Basel, Switzerland; University of Basel, Basel, Switzerland; SolidarMed, Old Europa, Lesotho; Centre for Multidisciplinary Research and Innovation, Abuja, Nigeria.
Declarations
Ethics approval and consent to participate: Ethical approval was obtained from the Sierra Leone Ethics and Scientific Review Committee of the Ministry of Health and Sanitation, Government of Sierra Leone. An information sheet was provided to all participants and was read aloud for participants unable to read. Before participating in the study, each participant provided written informed consent.
Consent for publication: Not applicable.
Author contributions: Sulaiman Lakoh: Conceptualization; Formal analysis; Methodology; Writing – original draft.
Le Yi: Data curation; Resources.
James B.W. Russell: Conceptualization; Supervision.
Juling Zhang: Data curation.
Stephen Sevalie: Data curation; Supervision.
Yongkun Zhao: Data curation.
Joseph Sam Kanu: Conceptualization.
Peng Liu: Data curation.
Sarah K. Conteh: Conceptualization.
Christine Ellen Elleanor Williams: Writing – original draft.
Umu Barrie: Data curation; Methodology; Writing – original draft.
Mohamed Gbessay Sheku: Data curation; Supervision.
Mohamed Boie Jalloh: Data curation.
Olukemi Adekanmbi: Writing – review & editing.
Darlinda F. Jiba: Methodology.
Matilda N. Kamara: Writing – original draft.
Gibrilla F. Deen: Methodology; Supervision.
Joseph Chukwudi Okeibunor: Funding acquisition; Writing – review & editing.
George A. Yendewa: Supervision; Writing – review & editing.
Xuejun Guo: Funding acquisition; Resources.
Emmanuel Firima: Formal analysis; Methodology; Writing – original draft.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Structured Operational Research and Training Initiative (SORT IT), a global partnership coordinated by TDR, the Special Programme for Research and Training in Tropical Diseases at the World Health Organization; the Chinese Medical Expert Group at the 34 Military Hospital in Freetown, Sierra Leone
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: E.F. receives his salary from the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie (Grant No. 801076), through SSPH+ Global PhD Fellowship Programme in Public Health Sciences (GlobalP3HS). G.A.Y. reports salary support from the National Institutes of Health/AIDS Clinical Trials Group under Award Nos. 5UM1AI068636-15, 5UM1AI069501-09 and AI068636(150GYD212), and consultancy fees from Pfizer. All other authors do not have any conflict of interest.
Availability of data and materials: The data supporting this article are available in the repository of University of Sierra Leone and will be made available on request to the corresponding authors when required.
References
- 1. World Health Organization. Report on the burden of endemic health care-associated infection worldwide: clean care is safer care, 2011, https://apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng.pdf (accessed 20 March 2022).
- 2. Rothe C, Schlaich C, Thompson S. Healthcare-associated infections in Sub-Saharan Africa. J Hosp Infect 2013; 85: 257–267. [DOI] [PubMed] [Google Scholar]
- 3. Ali S, Birhane M, Bekele S, et al. Healthcare associated infection and its risk factors among patients admitted to a tertiary hospital in Ethiopia: longitudinal study. Antimicrob Resist Infect Control 2018; 7: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gosling R, Mbatia R, Savage A, et al. Prevalence of hospital-acquired infections in a tertiary referral hospital in Northern Tanzania. Ann Trop Med Parasitol 2003; 97: 69–73. [DOI] [PubMed] [Google Scholar]
- 5. Abubakar U. Point-prevalence survey of hospital acquired infections in three acute care hospitals in Northern Nigeria. Antimicrob Resist Infect Control 2020; 9: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bagheri Nejad S, Allegranzi B, Syed SB, et al. Health-care-associated infection in Africa: a systematic review. Bull World Health Organ 2011; 89: 757–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Legesse Laloto T, Hiko Gemeda D, Abdella SH. Incidence and predictors of surgical site infection in Ethiopia: prospective cohort. BMC Infect Dis 2017; 17: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raouf M, Ghazal T, Kassem M, et al. Surveillance of surgical-site infections and antimicrobial resistance patterns in a tertiary hospital in Alexandria, Egypt. J Infect Dev Ctries 2020; 14: 277–283. [DOI] [PubMed] [Google Scholar]
- 9. Olowo-Okere A, Ibrahim YKE, Olayinka BO, et al. Epidemiology of surgical site infections in Nigeria: a systematic review and meta-analysis. Niger Postgrad Med J 2019; 26: 143–151. [DOI] [PubMed] [Google Scholar]
- 10. Allegranzi B, Aiken AM, Zeynep Kubilay N, et al. A multimodal infection control and patient safety intervention to reduce surgical site infections in Africa: a multicentre, before-after, cohort study. Lancet Infect Dis 2018; 18: 507–515. [DOI] [PubMed] [Google Scholar]
- 11. Lakoh S, Adekanmbi O, Jiba DF, et al. Antibiotic use among hospitalized adult patients in a setting with limited laboratory infrastructure in Freetown Sierra Leone, 2017-2018. Int J Infect Dis 2020; 90: 71–76. [DOI] [PubMed] [Google Scholar]
- 12. Lakoh S, Firima E, Williams CEE, et al. An intra-COVID-19 assessment of hand hygiene facility, policy and staff compliance in two hospitals in Sierra Leone: is there a difference between regional and capital city hospitals? Trop Med Infect Dis 2021; 6: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lakoh S, Maruta A, Kallon C, et al. How well are hand hygiene practices and promotion implemented in Sierra Leone? A cross-sectional study in 13 public hospitals. Int J Environ Res Public Health 2022; 19: 3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Badia JM, Casey AL, Petrosillo N, et al. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect 2017; 96: 1–15. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization. Global action plan on antimicrobial resistance. WHO Press, 2015, https://www.who.int/publications/i/item/9789241509763 (accessed 27 October 2022). [DOI] [PubMed]
- 16. United Nations. Sustainable development goals, https://sdgs.un.org/goals (accessed 24 April 2022).
- 17. World Health Organization. Infection prevention and control: report by the director-general, 2022, https://apps.who.int/gb/ebwha/pdf_files/EB150/B150_12-en.pdf (accessed 24 April 2022).
- 18. Lakoh S, Yi L, Sevalie S, et al. Incidence and risk factors of surgical site infections and related antibiotic resistance in Freetown, Sierra Leone: a prospective cohort study. Antimicrob Resist Infect Control 2022; 11: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Di Gennaro F, Marotta C, Pisani L, et al. Maternal caesarean section infection (MACSI) in Sierra Leone: a case-control study. Epidemiol Infect 2020; 148: e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chu K, Maine R, Trelles M. Cesarean section surgical site infections in sub-Saharan Africa: a multi-country study from Medecins Sans Frontieres. World J Surg 2015; 39: 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sierra Leone Statistics. 2015 population and housing census summary of final results, 2015, https://www.statistics.sl/images/StatisticsSL/Documents/final-results_-2015_population_and_housing_census.pdf [Google Scholar]
- 22. Saklad M. Grading of patients for surgical procedures. Anesthesiol 1941; 2: 281–284. [Google Scholar]
- 23. Plachouras D, Lepape A, Suetens C. ECDC definitions and methods for the surveillance of healthcare-associated infections in intensive care units [published correction appears in Intensive Care Med 2018]. Intensive Care Med 2018; 44: 2216–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. CDC/NHSN surveillance definition of healthcare-associated infection and criteria for specific types of infections in the acute care setting, 2013, http://www.socinorte.com/wp-content/uploads/2013/03/Criterios-de-IN-2013.pdf (accessed 27 October 2022). [DOI] [PubMed]
- 25. Ngaroua Ngah JE, Bénet T, Djibrilla Y. Incidence des infections du site opératoire en Afrique sub-saharienne: revue systématique et méta-analyse [Incidence of surgical site infections in sub-Saharan Africa: systematic review and meta-analysis]. Pan Afr Med J 2016; 24: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Omilabu SA, Salu OB, Oke BO, et al. The West African ebola virus disease epidemic 2014–2015: a commissioned review. Niger Postgrad Med J 2016; 23: 49–56. [DOI] [PubMed] [Google Scholar]
- 27. GoSL. National infection prevention and control policy, 2016, www.afro.who.int/publications/national-infection-prevention-and-control-guidelines-2016 (accessed 10 January 2022).
- 28. Lakoh S, Kanu JS, Conteh SK, et al. High levels of surgical antibiotic prophylaxis: implications for hospital-based antibiotic stewardship in Sierra Leone. Antimicrob Stewardship Healthc Epidemiol 2022; 2: e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shiferaw WS, Aynalem YA, Akalu TY, et al. Surgical site infection and its associated factors in Ethiopia: a systematic review and meta-analysis. BMC Surg 2020; 20: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoffman T, Shitrit P, Chowers M. Risk factors for surgical site infections following open versus laparoscopic colectomies: a cohort study. BMC Surg 2021; 21: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Waltz PK, Zuckerbraun BS. Surgical site infections and associated operative characteristics. Surg Infect (Larchmt) 2017; 18: 447–450. [DOI] [PubMed] [Google Scholar]
- 32. Ouedraogo S, Kambire JL, Ouedraogo S, et al. Surgical site infection after digestive surgery: diagnosis and treatment in a context of limited resources. Surg Infect (Larchmt) 2020; 21: 547–551. [DOI] [PubMed] [Google Scholar]
- 33. Humphreys H, Becker K, Dohmen PM, et al. Staphylococcus aureus and surgical site infections: benefits of screening and decolonization before surgery. J Hosp Infect 2016; 94: 295–304. [DOI] [PubMed] [Google Scholar]
- 34. Gupta V, Nirkhiwale S, Gupta P, et al. Achromobacter xylosoxidans mesh related infection: a case of delayed diagnosis and management. J Infect 2012; 64: e1–e5. [DOI] [PubMed] [Google Scholar]
- 35. Mitra S, Padhi TR, Basu S, et al. Unusual microbiological presentations in polymicrobial post-operative endophthalmitis and their clinical correlations. Int Ophthalmol 2019; 39: 2143–2148. [DOI] [PubMed] [Google Scholar]
- 36. Murray CJL, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022; 399: P629–P655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lakoh S, Li L, Sevalie S, et al. Antibiotic resistance in patients with clinical features of healthcare-associated infections in an urban tertiary hospital in Sierra Leone: a cross-sectional study. Antimicrob Resist Infect Control 2020; 9: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]