Abstract
Renal injury is an important factor in the development of chronic kidney diseases that pathologically manifested as renal fibrosis and podocyte damage. In the disease state, renal fibroblasts lead to high expression levels of α-smooth muscle actin (α-SMA), while podocytes undergo epithelial–mesenchymal transition, leading to proteinuria. Celastrol, a bioactive compound in the medicinal plant Tripterygium wilfordii, was found to delay the progression of early diabetic nephropathy and attenuate renal fibrosis in mice with unilateral ureteral obstruction. However, its effect on the renal system in 5/6 nephrectomized (Nx) rats remains unknown. The aim of this study was to explore the protective effects of celastrol and its underlying mechanisms in 5/6 Nx rats. We found that 24 h proteinuria and levels of blood urea nitrogen, serum creatinine, triglycerides, serum P, renal index and cholesterol significantly increased (P < 0.05), while that of serum albumin decreased significantly in 5/6 Nx rats. After intervention with celastrol, 24 h proteinuria and levels of blood urea nitrogen, serum creatinine, triglycerides, serum P, renal index, and cholesterol significantly decreased, while that of serum albumin significantly increased. Renal tissue pathological staining and transmission electron microscopy showed that celastrol ameliorated kidney injury and glomerular podocyte foot injury and induced significant anti-inflammatory effects. Quantitative polymerase chain reaction (PCR) and western blotting results revealed that nephrin and NEPH1 expression levels were upregulated, whereas α-SMA and Col4a1 expression levels were downregulated in the celastrol group. Celastrol inhibited the expression of transforming growth factor (TGF)-β1/Smad3 signaling pathway-related molecules such as TGF-β1 and P-Smad3. In summary, celastrol contributes to renal protection by inhibiting the epithelial–mesenchymal transdifferentiation and TGF-β1/Smad3 pathways.
Keywords: Celastrol, 5/6 nephrectomized rats, epithelial–mesenchymal transdifferentiation, TGF-β1/Smad3
Impact Statement
Clinical studies have shown that hormones and immunosuppressants (angiotensin II receptor blockers or angiotensin-converting enzyme inhibitors) slow disease progression in chronic kidney disease (CKD).1,2 However, these agents are associated with various side effects. 3 Owing to this, effective treatment methods for CKD with reduced side effects are necessary. Celastrol, a bioactive compound in the medicinal plant Tripterygium wilfordii, was found to delay the progression of several diseases.4 –7 However, its effect on renal 5/6 nephrectomized (Nx) rats remains unknown. In this study, we developed a 5/6Nx rat model resembling primary chronic renal failure in humans and intervention with celastrol. Our results showed that celastrol can maintain podocyte homeostasis, reduce proteinuria, and improve renal function. In addition, the molecule displayed a significant anti-inflammatory effect, in turn minimizing the progression of glomerular sclerosis. Overall, our study provides new evidence for developing therapeutic strategies for CKD in clinical practice.
Introduction
Chronic kidney disease (CKD) is becoming increasingly prevalent because of its high incidence and mortality rates. In China, 132.3 million patients have CKD, accounting for a global prevalence of 9.1%, which is the largest in the world. 8 CKD presents with significant manifestations of renal fibrosis and podocytopathy. 9 Renal fibroblasts are the main source of extracellular matrix (ECM) and lead to high expression levels of α-smooth muscle actin (α-SMA). 10 Under pathological conditions, podocytes undergo epithelial–mesenchymal transition (EMT). During this process, the expression of podocyte phenotype molecules, such as nephrin and NEPH1, weakens or disappears, while the expression of mesenchymal phenotype molecules, such as α-SMA increases. 11 EMT is a critical early-stage process in podocyte injury that can lead to proteinuria. As the injury worsens, renal failure can occur. Therefore, effective intervention should be introduced to reduce proteinuria and ultimately delay renal failure. 12
Celastrol, a natural bioactive compound, extracted from the medicinal plant Tripterygium wilfordii, is widely used in many countries for several indications, including chronic inflammatory and immune disorders.13,14 It exhibits immunosuppressive, anti-inflammatory, and antioxidant activities.4,7,15 It delays the progression of early diabetic nephropathy and attenuates renal fibrosis in mice with unilateral ureteral obstruction.16,17 However, its effect on renal 5/6 nephrectomized (Nx) rats remains unknown.
Transforming growth factor (TGF)-β1 is the main mediator in the pathogenesis of CKD; therefore, inhibition of TGF-β1 and Smad3 phosphorylation can alleviate kidney fibrosis.18,19 Knocked out TGF-β1 or Smad3 attenuates renal fibrosis in mice. 20 Therefore, the TGF-β1/Smad3 pathway is a potential therapeutic target in CKD. In this study, we investigated the effects of celastrol and its potential mechanisms in alleviating renal injury in 5/6 Nx rats. Our study provides new evidence for developing therapeutic strategies for CKD in clinical practice.
Materials and methods
Animal experiments
Specific pathogen-free male Sprague-Dawley rats (190–210 g; Hangzhou Medical College, Hangzhou, China) were housed at Hangzhou Medical College Laboratory Animal Center with a 12 h light/dark cycle. All experiments were approved by the Institutional Animal Care and Use Committee (approval no. ZJCLA-IACUC-20070015) and the experiments conformed to the ARRIVE guidelines. 21 Twenty-one rats were randomly assigned to the sham (n = 7) and the CKD (n = 14) groups. Rat modeling was performed as follows: all rats were intraperitoneally injected with 1.0% pentobarbital sodium (45 mg/kg body weight), and the total right Nx rat kidney and two-thirds of the left kidney were removed. The sham group served as the sham operation control group. Fourteen rats were randomly divided into the 5/6 Nx and celastrol groups 1 month after surgery. The intervention group was intragastrically administered with celastrol (3 mg/kg) once daily. Celastrol was purchased from Chengdu Refensi Biotechnology Co., Ltd. After 8 weeks of intervention, serum and cortical kidney tissue samples were collected from each group for follow-up analysis.
Biochemical analysis
After 8 weeks of intervention, 24-h urine specimens were collected from each rat in metabolic cages. Next, 24-h urinary protein excretion and serum creatinine (Scr), blood urea nitrogen (BUN), serum albumin (ALB), triglyceride (TG), and cholesterol (TC) levels were evaluated using an automated biochemical analysis instrument (Hitachi 7180) following the manufacturer’s instructions (Hitachi, Japan).
Pathological staining analysis
Renal cortex tissues were routinely fixed and embedded in paraffin. Paraffin-embedded sections (2–3 μm thickness) were subjected to hematoxylin and eosin (HE) and Masson’s trichrome staining. Kidney histology was viewed and analyzed using an Olympus BX53 microscope. The degree of sclerosis in each group was assessed using the glomerular sclerosis index (GSI). 22
Transmission electron microscopic analysis
Rat kidney cortex samples (1 mm3 in size) were fixed with 2.5% glutaraldehyde overnight, embedded, sectioned to 100 nm, and subjected to uranium and lead double staining. The ultrastructure of the renal tissue was observed under a JEM-1400 TEM (JEOL, Japan, Tokyo).
Tissue RNA extraction and quantitative polymerase chain reaction (qPCR)
RNA was extracted from kidney tissues using TRIzol reagent (TaKaRa, Japan), and cDNA was synthesized with a Primescript™ RT reagent kit (TaKaRa, Japan). Changes in the expression levels of IL2, IL6, CXCL10, NEPH1, nephrin, α-SMA, TGF-β1, Smad3, Col4a1, and GAPDH were quantified using SYBR® Green Master Mix (TaKaRa, Japan). Primer sequences are shown in Table 1.
Table 1.
Primers used for gene expression analyses.
| Primer name | Sequence (5′ to 3′) | Application |
|---|---|---|
| Rat-NEPH1-F | GGGCTGGCTCTGGGTATGGG | Real-time RT-PCR |
| Rat-NEPH1-R | GGCACTCATAGGAAGCGTCATCAG | Real-time RT-PCR |
| Rat-Nephrin-F | GTTGGTGGTCTTCTGCTGCTCTC | Real-time RT-PCR |
| Rat-Nephrin-R | CTTCTGCTGTGCTAACCGTGGAG | Real-time RT-PCR |
| Rat-α-SMA-F | GCGTGGCTATTCCTTCGTGACTAC | Real-time RT-PCR |
| Rat-α-SMA-R | CCATCAGGCAGTTCGTAGCTCTTC | Real-time RT-PCR |
| Rat-TGF-β1–F | GGCACCATCCATGACATGAACCG | Real-time RT-PCR |
| Rat-TGF-β1-R | GCCGTACACAGCAGTTCTTCTCTG | Real-time RT-PCR |
| Rat-Col4a1-F | GATTGTGGTGGCTCTGGCTGTG | Real-time RT-PCR |
| Rat-Col4a1-R | TCGTTCCAGGAAGTCCAGGTTCTC | Real-time RT-PCR |
| Rat-IL2-F | GCAGCGTGTGTTGGATTTGACTC | Real-time RT-PCR |
| Rat-IL2-R | TGGCTCATCATCGAATTGGCACTC | Real-time RT-PCR |
| Rat-IL-6-F | CCTTCTTGGGACTGATGT | Real-time RT-PCR |
| Rat-IL-6-R | TCCAGGTAGAAACGGAAC | Real-time RT-PCR |
| Rat-CXCL10-F | TGAAAGCGGTGAGCCAAAGAAGG | Real-time RT-PCR |
| Rat-CXCL10-R | CTGGGTAAAGGGAGGTGGAGAGAC | Real-time RT-PCR |
| Rat-GAPDH-F | ACCACAGTCCATGCCATCAC | Real-time RT-PCR |
| Rat-GAPDH-R | TCCACCACCCTGTTGCTGTA | Real-time RT-PCR |
F, forward primer; R, reverse primer.
Protein extraction and western blotting
Rat kidney tissues were ground and lysed with RIPA lysis buffer (Beyotime, China) on ice for 30 min. Proteins were quantified using the BCA assay (Beyotime, China), separated by 6–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. transferred onto nitrocellulose membranes, blocked with 5% skimmed milk for 2 h, and incubated with a primary antibody overnight. The following antibodies were used: anti-nephrin (ab58968, Abcam); anti-NEPH1 (sc-373787, Santa); anti-Smad3 (66,516–1-Ig, ProteinTech); anti-P-Smad3 (P84022, Boster); anti-TGF-β1(21898–1-AP, ProteinTech); anti-GAPDH (60,004–1-Ig, ProteinTech). Subsequently, the membranes were washed with TBST and incubated with the corresponding infrared-labeled anti-rabbit/mouse IgG antibody (1:15,000). Signals were detected using an Odyssey CLx imaging system (LI-COR, USA).
Statistical analysis
Data are presented as mean ± inter-quartile range. Results were analyzed using IBM SPSS Statistics 19.0 and GraphPad 8.0. A non-parametric Kruskal–Wallis test for multiple comparisons was used for data analysis. Statistical significance was set at p ⩽ 0.05.
Results
Celastrol improved urinary protein excretion and renal function in 5/6 Nx rats
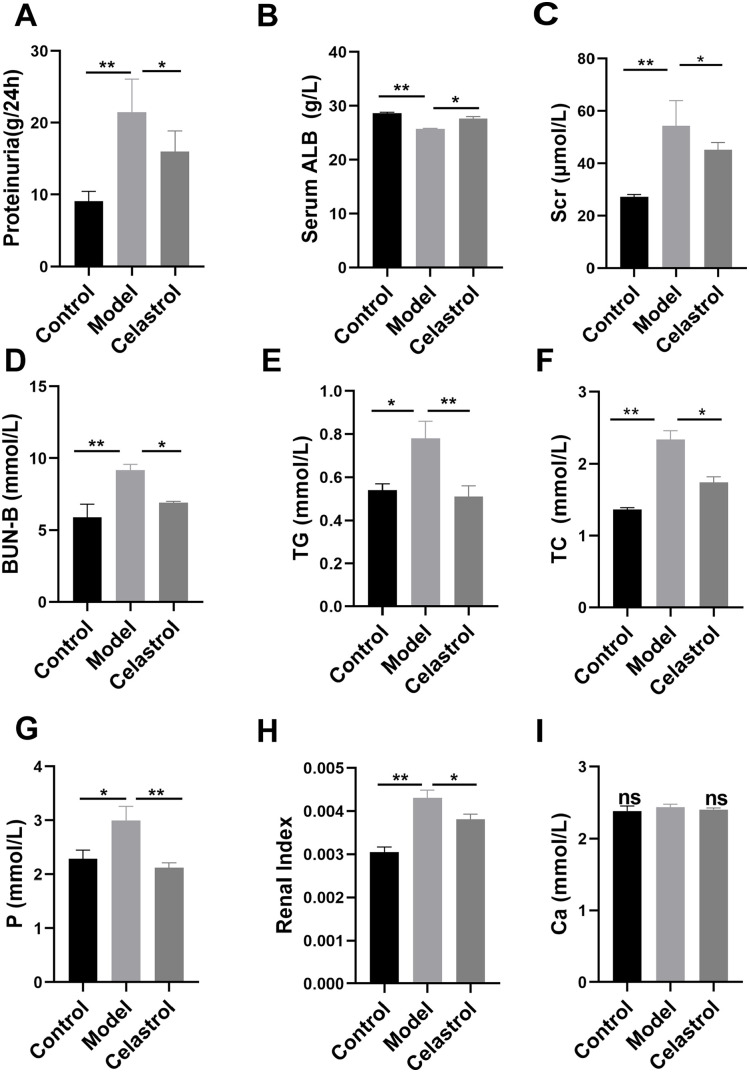
The 24-h urinary protein levels in the 5/6 Nx model group significantly increased, indicating that the CKD model was successfully established (Figure 1(A)). After celastrol treatment, the 24-h urinary protein levels in the celastrol group were lower than those in the model group (Figure 1(A)). In addition, serum ALB levels in the model group were significantly lower than those in the control group. The serum ALB levels in the celastrol group showed obvious improvement (Figure 1(B)). Therefore, celastrol attenuated urinary protein excretion. We further evaluated the changes in the renal function index and found that the Scr, BUN, TG, TC, serum P, and renal index levels in the celastrol group were significantly reduced compared to those in the model group (Figure 1(C) to (H)). However, there was no significant change in serum Ca levels among the three groups (Figure 1(I)). These results indicated that celastrol can improve kidney function.
Figure 1.
Celastrol improved urinary protein excretion and renal function in 5/6 Nx rats. The levels of 24-h proteinuria (g/24 h) and serum ALB (g/L), Scr (μmol/L), BUN (mmol/L), TG (mmol/L), TC (mmol/L), serum Ca (mmol/L), and serum P (mmol/L) were detected using a fully automated biosystem chemical analyzer (Hitachi 7180) (A–G, I). The levels of renal index in each group are shown in Figure 1(H). Data are presented as the mean ± inter-quartile range, *p < 0.05; **p < 0.01.
Celastrol-affected renal histopathology in 5/6 Nx rats
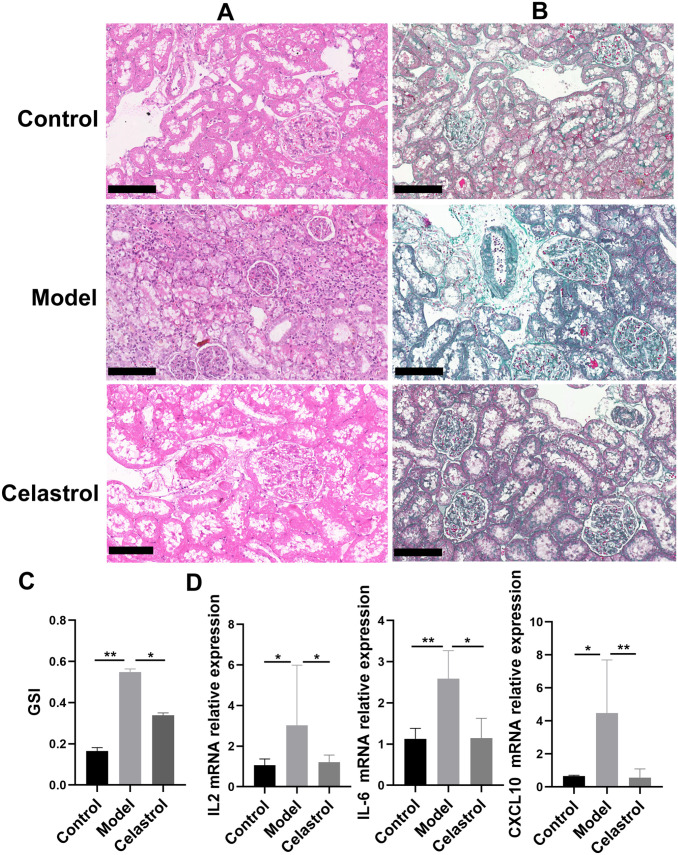
To assess histopathological changes in the kidney tissues, we performed HE and Masson’s trichome staining of kidney tissues. In the control group, the renal tissue structure was tightly arranged, the glomerular structure was clear, and the capillary loops opened well (Figure 2(A) and (B)). The model group displayed segmental sclerosis of the glomerulus, accompanied by marked thickening of the glomerular capillary basement membrane and hyperplasia of the mesangial matrix and focal renal fibrosis. Celastrol treatment also changed the GSI (Figure 2(C)). Moreover, celastrol treatment significantly reduced the levels of inflammatory factors IL2 and IL6, as well as chemokine factor CXCL10 (Figure 2(D)). Therefore, celastrol treatment can ameliorate renal pathological damage.
Figure 2.
Treatment with celastrol attenuated renal lesions and fibrosis caused by 5/6 nephrectomy. Renal histopathology in each group was subjected to HE and Masson’s trichome staining (A and B). Representative micrographs from each group are shown (BF, bright field, original magnification 200×, Bars = 50 μm). (C) Representative GSI rate for each group. (D) The levels of inflammatory factors IL2 and IL6, as well as chemokine CXCL10 in each group. (A color version of this figure is available in the online journal.)
Celastrol attenuated podocyte injury in 5/6 Nx rats
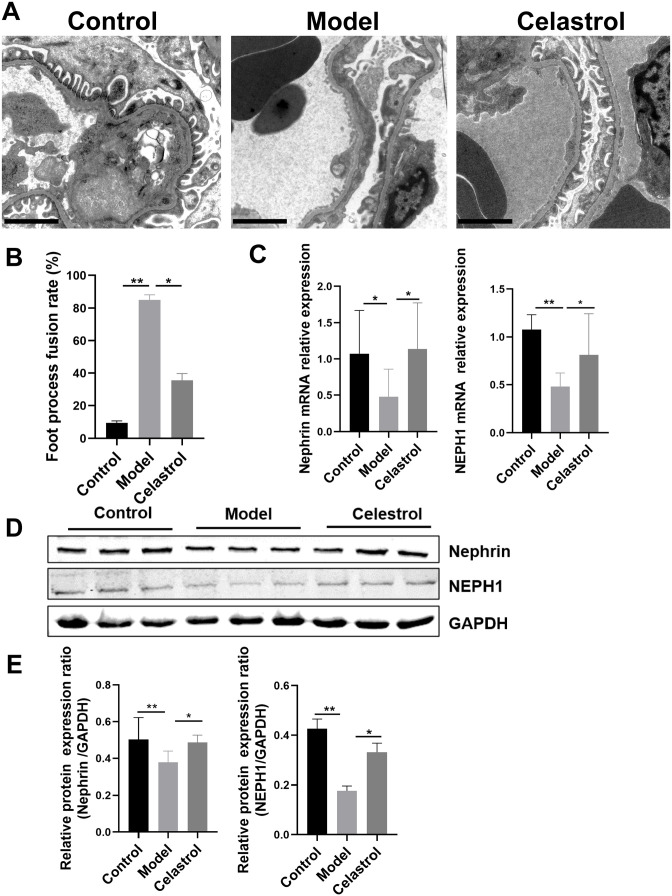
To further explore the effect of podocyte lesions, we used TEM to observe podocyte foot process fusion in each group. We found that the extensive fusion of podocyte foot processes became flat and fused, and it even disappeared in the 5/6 Nx model group. However, the foot process fusion rate decreased, and lesions were obviously reduced in the celastrol group (Figure 3(A) and (B)). We further assessed the expression of nephrin and NEPH1 in the podocyte slit diaphragm. qPCR and western blotting results suggested that the expression levels of nephrin and NEPH1 were significantly upregulated after celastrol intervention (Figure 3(C) to (E)). These results indicated that celastrol can protect against podocyte injury.
Figure 3.
Celastrol attenuated the podocyte injury in 5/6 Nx rats. (A and B) Podocyte foot process analyzed by TEM and representative micrographs in each group are shown (original magnification 10,000×, Bars = 2 μm, n = 7 animals in each group). (C) The mRNA expressions levels of nephrin and NEPH1 in rat kidney tissues of different groups were determined by qRT-PCR. The relative gene expression was normalized to that of GAPDH. (D and E) Representative western blots of nephrin and NEPH1 in each group. Data are presented as the mean ± interquartile range. *p < 0.05; **p < 0.01.
Celastrol treatment attenuated renal fibrosis and inhibited the TGF-β1/Smad3 signaling pathway in 5/6 Nx rats
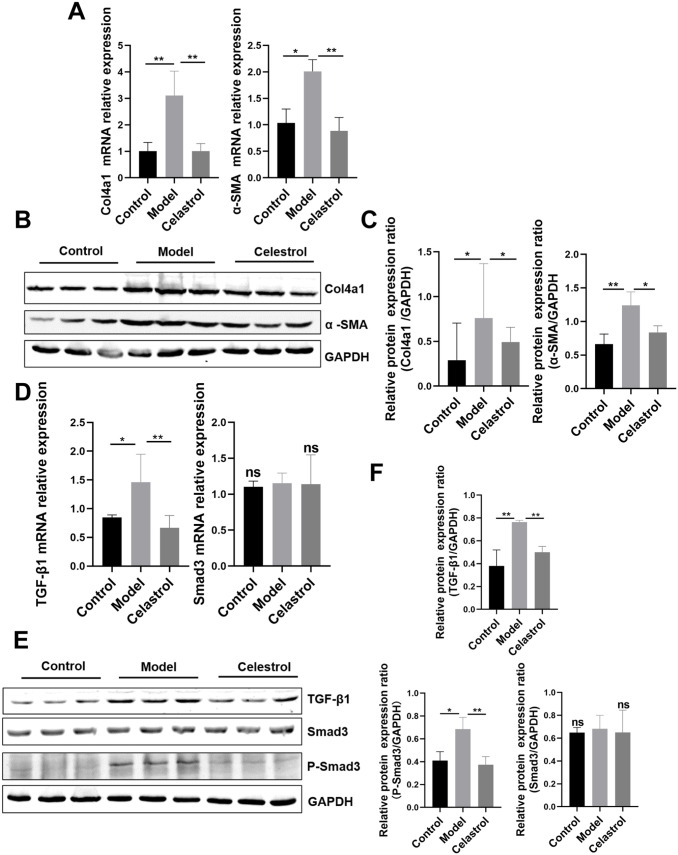
To further investigate the protective effect of celastrol on renal fibrosis in 5/6 Nx rats, we examined the expression levels of the fibrotic factors, α-SMA and Col4a1 by western blotting and qPCR. Celastrol effectively reversed the increase in α-SMA and Col4a1 levels compared with those observed in the model group (Figure 4(A) to (C)). We investigated the antifibrotic mechanism of celastrol in the TGF-β1/Smad3 pathway via western blot analysis. We found that the expression of TGF-β1 and phosphorylated Smad3 significantly increased in the model group. The levels of phosphorylated Smad3 and TGF-β1 were attenuated by celastrol treatment (Figure 4(D) to (F)). Therefore, celastrol may inhibit the activation of the TGF-β1/Smad3 pathway.
Figure 4.
Celastrol treatment attenuated renal fibrosis and inhibited the TGF-β1/Smad3 signaling pathway in 5/6Nx rats: (A) RT-time PCR revealed that celastrol reversed the increase in mRNA expression levels of α-SMA and Col4a1. (B and C) The protein expression levels of α-SMA and Col4a1 were evaluated using western blot and quantitative analysis. (D) The mRNA expressions levels of Smad3 and TGF-β1 in different kidney tissues. (E and F) The protein expression levels of TGF-β1, Smad3 and P-Smad3 were evaluated using western blot and representative western blot analyses. Data are presented as the mean ± inter-quartile range, *p < 0.05; **p < 0.01 compared with the sham control group.
Discussion
In this study, we investigated the effects of celastrol on kidney injury in 5/6 Nx rats. Our results indicated that celastrol could improve renal function by lowering the levels of BUN, Scr, and 24 h proteinuria. The kidney injury caused by 5/6 nephrectomy was relieved following a significant reduction in α-SMA and Col4a1 expression levels. Further experiments showed the inhibitory effects of celastrol on the EMT and the TGF-β1/Smad3 signaling pathways. Therefore, our research provides a theoretical basis for the future clinical treatment strategies in treating CKD.
Celastrol has been widely explored because of its anti-inflammatory, antitumor and immunosuppressive effects. It has therapeutic activity against various nephropathies, including cisplatin-induced nephrotoxicity in mice, diabetic nephropathy in a rat model and acute renal injury in a mouse model.4 –6,23 It can prevent rat diabetic nephropathy renal injury by regulating the MAPK/NF-κB pathway and inflammatory response. 24 It also protects against cisplatin-induced acute kidney injury in mice by regulating NF-κB and mitochondrial function. 25 Herein, we also assessed the therapeutic effects of celastrol in a 5/6 Nx rat model. Interestingly, our results were the same as those in a previous study performed in a 5/6 Nx rat model, 26 thereby verifying the protective effect of celastrol in kidney disease models.
Renal fibrosis is characterized by abnormal and excessive ECM deposition, which exacerbates the severity of kidney injury. 27 We established a 5/6 Nx rat model, and successfully induced tubular fibrosis. Celastrol significantly alleviated this process by downregulating α-SMA, TGF-β1, and Col4a1. CKD progression is associated with maintenance of podocyte filtration membrane integrity. 28 End-stage renal disease and glomerular fibrosis are linked to podocyte EMT, and TGF-β1/Smad3 signaling contributes to EMT in podocytes.29,30 Celastrol protected podocytes from EMT, increased nephrin and NEPH1 expression, enhanced the foot process fusion rate, and reduced proteinuria.
The activation of TGF-β1/Smad3 signaling exacerbates renal fibrosis in diverse kidney injury models. 31 It also promotes podocyte EMT and plays a role in the etiology of CKD.32 –34 The inhibition of TGF-β1/Smad3 signaling leads to improved outcomes. Herein, the TGF-β1/Smad3 pathway was overactivated in the 5/6 Nx rat model, as evidenced by the significantly increased levels of TGF-β1 and P-Smad3. Nonetheless, celastrol reduced these effects and improved renal injuries such as mesangial enlargement, matrix accumulation, proteinuria, and glomerular basement membrane thickening. Overall, the findings implied that the renal protective effects of celastrol may be related to its inhibition of the TGF-β1/Smad3 signaling pathway.
Although our study demonstrated the inhibitory effects of celastrol on TGF-β1/Smad3 activation and TGF-β1/ Smad3-induced EMT, certain limitations are noted: (1) the number of the animals used was small (not more than 10 per group). (2) The dose of celastrol was not set to provide a concentration gradient. (3) It would be more convincing if experiments employing deficient or knockout mice were performed to determine the mechanism of celastrol in inhibiting TGF-β1/Smad3 activation.
In conclusion, we developed a 5/6 Nx rat model resembling primary chronic renal failure in humans. After an 8-week intervention with celastrol, the levels of renal function indicators and the degree of renal pathological damage were alleviated. Nephrin and NEPH1 levels were predominantly upregulated, and the podocyte foot process fusion rate was decreased following celastrol treatment. The protective mechanism of celastrol was mediated by the downregulation of TGF-β1 and P-Smad3 expression. These results indicated that celastrol can maintain podocyte homeostasis, reduce the level of proteinuria, inflammation and chemokine factors, as well as improve renal function, thereby minimizing the progression of glomerular sclerosis. Therefore, celastrol may act as a therapeutic agent for preventing podocyte EMT in CKD.
Footnotes
Authors’ Contributions: TYW and YRC designed the experiments and analyzed the data, WF authored or reviewed drafts of the paper and approved the final draft. TXL performed the TEM experiments. ZHQ performed the animal modeling experiment. LY performed biochemical detection experiments. All authors approved submission of the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Natural Science Foundation of Zhejiang Province (grant no. LQ21H290005 and Y20H050004); the Public Welfare Technology Application Research Program of Zhejiang Province (grant no. LGC21H290002); and Project of Administration of Traditional Chinese Medicine of Zhejiang Province (grant no. 2022ZA122).
ORCID iDs: Yue-Wen Tang  https://orcid.org/0000-0002-6855-2907
https://orcid.org/0000-0002-6855-2907
Ru-Chun Yang  https://orcid.org/0000-0002-6567-4837
https://orcid.org/0000-0002-6567-4837
References
- 1. Sinha AD, Agarwal R. Clinical pharmacology of antihypertensive therapy for the treatment of hypertension in CKD. Clin J Am Soc Nephrol 2019;14:757–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mårup FH, Peters CD, Christensen JH, Birn H. Can patiromer allow for intensified renin-angiotensin-aldosterone system blockade with losartan and spironolactone leading to decreased albuminuria in patients with chronic kidney disease, albuminuria and hyperkalaemia? An open-label randomised controlled trial: MorphCKD. BMJ Open 2022; 12:e057503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Teplan V, Valkovsky I, Teplan V, Jr, Stollova M, Vyhnanek F, Andel M. Nutritional consequences of renal transplantation. J Ren Nutr 2009; 19:95–100 [DOI] [PubMed] [Google Scholar]
- 4. Boran T, Gunaydin A, Jannuzzi AT, Ozcagli E, Alpertunga B. Celastrol pretreatment as a therapeutic option against cisplatin-induced nephrotoxicity. Toxicol Res 2019;8:723–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chu C, He W, Kuang Y, Ren K, Gou X. Celastrol protects kidney against ischemia-reperfusion-induced injury in rats. J Surg Res 2014; 186:398–407 [DOI] [PubMed] [Google Scholar]
- 6. Nie Y, Fu C, Zhang H, Zhang M, Xie H, Tong X, Li Y, Hou Z, Fan X, Yan M. Celastrol slows the progression of early diabetic nephropathy in rats via the PI3K/AKT pathway. BMC Complemen Med Ther 2020;20:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tong S, Zhang L, Joseph J, Jiang X. Celastrol pretreatment attenuates rat myocardial ischemia/ reperfusion injury by inhibiting high mobility group box 1 protein expression via the PI3K/Akt pathway. Biochem Biophys Res Commun 2018;497:843–9 [DOI] [PubMed] [Google Scholar]
- 8. Tong S, Zhang L, Joseph J, Jiang X. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020;395:709–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Charles C, Ferris AH. Chronic kidney disease. Prim Care 2020;47:585–95 [DOI] [PubMed] [Google Scholar]
- 10. Strutz F, Zeisberg M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J Am Soc Nephrol 2006;17:2992–8 [DOI] [PubMed] [Google Scholar]
- 11. Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 2010;21:212–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strutz FM. EMT and proteinuria as progression factors. Kidney Int 2009;75:475–81 [DOI] [PubMed] [Google Scholar]
- 13. Chen SR, Dai Y, Zhao J, Lin L, Wang Y, Wang Y. A mechanistic overview of triptolide and celastrol, natural products from Tripterygium wilfordii Hook F. Front Pharmacol 2018;9:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu J, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U. Treatment of obesity with celastrol. Cell 2015;161:999–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu S, Feng Y, He W, Xu W, Xu W, Yang H, Li X. Celastrol in metabolic diseases: progress and application prospects. Pharmacol Res 2021; 167:105572. [DOI] [PubMed] [Google Scholar]
- 16. Tang M, Cao X, Zhang K, Li Y, Zheng QY, Li GQ, He QH, Li SJ, Xu GL, Zhang KQ. Celastrol alleviates renal fibrosis by upregulating cannabinoid receptor 2 expression. Cell Death Dis 2018;9:601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu P, Zhang J, Wang Y, Shen Z, Wang C, Chen DQ, Qiu X. The active compounds and therapeutic target of Tripterygium wilfordii Hook F. in attenuating proteinuria in diabetic nephropathy: a review. Front Med 2021;8:747922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gifford CC, Tang J, Costello A, Khakoo NS, Nguyen TQ, Goldschmeding R, Higgins PJ, Samarakoon R. Negative regulators of TGF-β1 signaling in renal fibrosis; pathological mechanisms and novel therapeutic opportunities. Clin Sci 2021;135:275–303 [DOI] [PubMed] [Google Scholar]
- 19. Zheng ZC, Zhu W, Lei L, Liu XQ, Wu YG. Wogonin ameliorates renal inflammation and fibrosis by inhibiting NF-κB and TGF-β1/Smad3 signaling pathways in diabetic nephropathy. Drug Des Devel Ther 2020; 14:4135–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zeng Z, Wang Q, Yang X, Ren Y, Jiao S, Zhu Q, Guo D, Xia K, Wang Y, Li C, Wang W. Qishen granule attenuates cardiac fibrosis by regulating TGF-β /Smad3 and GSK-3β pathway. Phytomed Int J Phytother Phytopharmacol 2019;62:152949. [DOI] [PubMed] [Google Scholar]
- 21. Percie du, Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Wurbel H. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Br J Pharmacol 2020;177:3617–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wan F, Yang RC, Shi YP, Tang YW, Tang XL, Zhu XL, Li YG, Wang YJ. The protective effect of Phellinus linteus decoction on podocyte injury in the kidney of FSGS rats. BMC Complemen Alter Med 2019; 19:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu X, Jia M, Fu Y, Zhang P, Zhang Z, Lin Q. Novel low-toxic derivative of celastrol maintains protective effect against acute renal injury. ACS Omega 2018;3:2652–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang M, Chen Y, Yang MJ, Fan XR, Xie H, Zhang L, Nie YS, Yan M. Celastrol attenuates renal injury in diabetic rats via MAPK/NF-κB pathway. Phytother Res 2019;33:1191–8 [DOI] [PubMed] [Google Scholar]
- 25. Yu X, Meng X, Xu M, Zhang X, Zhang Y, Ding G, Huang S, Zhang A, Jia Z. Celastrol ameliorates cisplatin nephrotoxicity by inhibiting NF-κB and improving mitochondrial function. Ebiomedicine 2018;36:266–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang X, Chen A, Liang Q, Dong Q, Fu M, Liu X, Wang S, Li Y, Ye Y, Lan Z, Ou JS, Lu L, Yan J. Up-regulation of heme oxygenase-1 by celastrol alleviates oxidative stress and vascular calcification in chronic kidney disease. Free Rad Biol Med 2021;172:530–40 [DOI] [PubMed] [Google Scholar]
- 27. Ma TT, Meng XM. TGF-β/smad and renal fibrosis. Adv Exp Med Biol 2019;1165:347–64 [DOI] [PubMed] [Google Scholar]
- 28. Garg P. A review of podocyte biology. Am J Nephrol 2018;47(Suppl. 1): 3–13 [DOI] [PubMed] [Google Scholar]
- 29. Chen YT, Jhao PY, Hung CT, Wu YF, Lin SJ, Chiang WC, Lin SL, Yang KC. Endoplasmic reticulum protein TXNDC5 promotes renal fibrosis by enforcing TGF-β signaling in kidney fibroblasts. J Clin Invest 2021; 131:e143645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang D, Zhao T, Zhao Y, Yin Y, Huang Y, Cheng Z, Wang B, Liu S, Pan M, Sun D, Wang Z, Zhu G. PPARγ mediates the anti-epithelial-mesenchymal transition effects of FGF1(ΔHBS) in chronic kidney diseases via inhibition of TGF-β1/SMAD3 signaling. Front Pharmacol 2021;12:690535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feng J, Xie L, Kong R, Zhang Y, Shi K, Lu W, Jiang H. RACK1 silencing attenuates renal fibrosis by inhibiting TGF-β signaling. Int J Mol Med 2017;40:1965–70 [DOI] [PubMed] [Google Scholar]
- 32. Meng XM, Nikolic -Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 2016;12:325–38 [DOI] [PubMed] [Google Scholar]
- 33. Jin Z, Gu C, Tian F, Jia Z, Yang J. NDRG2 knockdown promotes fibrosis in renal tubular epithelial cells through TGF-β1/Smad3 pathway. Cell Tissue Res 2017;369:603–10 [DOI] [PubMed] [Google Scholar]
- 34. Wu W, Wang Y, Li H, Chen H, Shen J. Buyang Huanwu Decoction protects against STZ-induced diabetic nephropathy by inhibiting TGF-β/Smad3 signaling-mediated renal fibrosis and inflammation. Chinese Med 2021;16:118. [DOI] [PMC free article] [PubMed] [Google Scholar]