Abstract
目的
探讨半乳糖凝集素-1(galectin-1)对肺腺癌细胞的影响及其机制。
方法
收集并比较肺腺癌组织和癌旁正常组织galectin-1的表达水平。qRT-PCR法检测肺腺癌细胞系A549、H1299与正常支气管上皮细胞细胞BEAS-2b中galectin-1的表达差异。通过si-RNA敲低galectin-1,分为对照组和si-RNA组,对照组转染同剂量NC-siRNA片段。通过qRT-PCR和Western blot检测galectin-1mRNA和蛋白水平的表达情况。CCK8、Transwell实验、划痕实验和流式细胞术检测敲低galectin-1后对肺腺癌细胞增殖能力、侵袭和迁移能力和细胞凋亡的情况。Western blot检测敲低galectin-1后凋亡相关蛋白BAX、BCL-2、Caspase3等蛋白和AKT、ERK二条通路蛋白的相对表达情况。
结果
galectin-1的mRNA在肺癌组织和肺腺癌细胞株中表达量明显升高(P < 0.05)。抑制galectin-1表达后,细胞增殖、迁移和侵袭等下降,凋亡率明显升高(P < 0.05)。在抑制galectin-1表达后, ERK信号通路磷酸化水平明显降低(P < 0.05)。
结论
galectin-1具有抑制肺腺癌细胞增殖、迁移、侵袭和促进其凋亡的作用,其机制可能与ERK通路的磷酸化激活水平有关。
Keywords: 肺腺癌, galectin-1, 增殖, 侵袭, 迁移, 凋亡, ERK信号通路
Abstract
Objective
To investigate the effect of galectin-1 on biological behaviors of lung adenocarcinoma cells and the underlying mechanism.
Methods
The expression levels of galectin-1 mRNA were detected in 8 pairs of lung adenocarcinoma tissues and adjacent normal tissues and in lung adenocarcinoma cell lines A549 and H1299 and normal bronchial epithelial cell line BEAS-2b using qRT-PCR. The effect of galectin-1 knockdown by RNA interference on the proliferation, invasion and migration abilities and apoptosis of lung adenocarcinoma cells were examined using CCK8 assay, Transwell assay, scratch assay and flow cytometry. Western blotting was performed to detect the expressions of apoptosis-related proteins BAX, BCL-2, and caspase-3 and the proteins involved in the AKT and ERK pathways.
Results
The mRNA expression of galectin-1 was significantly increased in lung cancer tissues and lung adenocarcinoma cell lines (P < 0.05). In lung adenocarcinoma cells, galectin-1 knockdown significantly inhibited cell proliferation, migration and invasion and obviously increased cell apoptosis rate (P < 0.05), causing also significant reduction of the phosphorylation level of ERK signaling pathway (P < 0.05).
Conclusion
Galectin-1 knockdown inhibits proliferation, migration, and invasion and promotes apoptosis of lung adenocarcinoma cells, and this effect is mediated probably by inhibition of the phosphorylation levels of the ERK pathway.
Keywords: lung adenocarcinoma, galectin-1, proliferation, invasion, migration, apoptosis, ERK signaling pathway
随着近些年来全球癌症患者数越来越多,中国癌症患者人数中肺癌病人的总患病率和癌症死亡率一直居高不下[1, 2]。每年大约180万肺癌患者,且每年近160万人死于肺癌,5年肺癌治疗患者的生存率为4%~17%[3]。根据肺癌的组织学分型大致可以将肺癌分为肺小细胞癌、鳞癌、腺癌和大细胞癌等4种组织学类型[4]。在肺癌的各种病理分型诊断中肺腺癌患者的肺癌占比几乎是最高的,大约占据到中国所有的肺癌病例总量的40% [5]。但目前对肺腺癌的发生发展及其转移的具体机制的探索还不够深入。因此,对肺腺癌发生发展、增殖迁移等方面的研究将有重要意义。
半乳糖凝集素1(galectin-1)是属于可溶性糖类的蛋白质之一,广泛存在于自然界中[6]。它是一类β-半乳糖苷结合的哺乳动物类的凝集素,其相对分子质量大约是14 000[7]。Galectin-1以二聚体的形式存在,通过疏水相互作用维持疏水核心[8]。半乳糖凝集素可以分为嵌合型(由胶原蛋白和单个CRD重复结构域融合而成)、原型(仅含有单一的由130个残基组成的CRD,哺乳动物的Galectin一般属于原型,这其中就包括galectin-1)和串联重复型(通过短接头序列串联起来的两个同源CRD)3种分子亚型。Galectin-1作为半乳凝集素家族成员之一[9],属于小分子蛋白质的一种且在许多动物体内都有广泛的发现,主要分布于细胞外基质和细胞的胞浆中,在人类肿瘤组织病理学中也受到广泛的研究,它还被实验证明其在人类肿瘤基因的克隆生成工作中已发挥着相当重要的推动作用[10, 11]。Galectin-1可以与免疫细胞上的糖基化受体结合,触发肿瘤微环境中免疫细胞功能的抑制,有助于肿瘤的免疫逃逸[12]。Galectin-1具有促进炎症或T细胞介导的免疫反应[13]。半乳糖凝集素1的过表达被证明存在于多种肿瘤组织中,已经有临床数据证明半乳糖凝集素1与肿瘤增殖和血管生成相关[14]。在经典的霍奇金淋巴瘤中RS细胞通过AP1依赖性增强子选择性地过度表达galectin-1[15]。Galectin-1在头颈部癌患者中分泌明显增高,而且galectin-1高表达的患者具有更差的治疗反应和总体生存率[16-18]。Galectin-1通过参与EMT通路参与胃癌血管的生成,过表达galectin-1后可以促进胃癌细胞的增殖、迁移、侵袭和血管生成[19, 20]。Galectin-1还被证实与胰腺导管癌[21]、乳腺癌[22]、卵巢癌[23]、肝癌[24]、结直肠癌[25]和食管癌[26]等肿瘤的发生发展有紧密的联系。研究表明galectin-1通过α 6β4和Notch1/Jagged2传导机制介导肺癌细胞的迁移和侵袭,还有研究揭示galectin-1介导着肺癌的耐药和加速其进展[27, 28]。但其没有揭示galectin-1究竟通过何种传导机制介导肺腺癌细胞的增殖和凋亡等。因此,本研究旨在从基础实验的理论基础上探究galectin-1与肺腺癌的增殖和凋亡等行为是否有关,且通过何种信号传导通路影响这些生物学行为同时通过该信号传导通路是否同样也对肺腺癌细胞的侵袭和迁移是否产生影响。
1. 材料和方法
1.1. 实验试剂
正常支气管上皮细胞株BEAS-2b和支气管肺腺癌细胞株A549、H1299是直接采用来自于本实验室保存下来的细胞株,来源于由中科院上海细胞库。细胞培养应用于10%的胎牛的血清(四季青公司)培养基、RPMI 1640培养基和DMEM培养基(Gibco),细胞生长的环境为5%的CO2、37 ℃。实时荧光定量PCR所用的引物、小干扰RNA(si-RNA)(上海生工生物工程)。TRIzol、荧光定量的试剂盒、CCK8的试剂盒(biosharp),逆转录的试剂盒(碧云天)。Galectin-1和GAPDH一抗(Affinity);BAX、Bcl-2、AKT、p-Akt、ERK、p-ERK、β-actin一抗(武汉三鹰公司);caspase-3(ABclonal)。与其相对应的二抗(碧云天)。基质胶(Corning),transwell板(Labselect)。
1.2. 标本来源
8对配对肺腺癌标本和癌旁正常组织标本均收集于蚌埠医学院第一附属医院胸外科,癌组织收集肿瘤中心处,癌旁组织收集于距癌组织边缘5 cm以上处。收集的所有患者均已签署知情同意书,实验方案由蚌埠医学院伦理委员会审批(编号2019-024)。所有标本收集后均放置于-80 ℃冰箱保存,以便用于后续实验。
1.3. 实验方法
1.3.1. 细胞培养
细胞培养于含有约10%活胎牛血清的DMEM培养基和1640培养基瓶中,细胞培养箱适宜的培养操作条件通常为约37℃、5%以上的干燥二氧化碳。待细胞的生长密度接近90%用胰酶消化下来然后离心传代。
1.3.2. 细胞转染
细胞转染待细胞的培养密度为50%~ 80%时,加入2 mL的opti无血清培养基,随后取出2个1.5 mL无菌的EP管先各加入125 μL的opti无血清培养基,再各加入5 μL的Lip6000和siRNA室温静置5 min后将含有siRNA的培养液加入含有Lip6000的培养液中混匀室温静置5 min,之后将混匀的培养液加入到六孔板相应的孔内,后放回培养箱。等待4~6 h,然后切换到正常的培养基,48 h后进行相应的实验检测。
1.3.3. 实时荧光定量PCR(Real-time PCR)检测Galectin-1的mRNA的表达
从细胞和临床病理标本肺组织中提取总RNA(Trizol法),然后可以通过逆转录获得cDNA,再可以通过PCR进行扩增。用GAPDH作为内参。得到的数据用2-△△Ct去计算表达量。荧光定量法和PCR定量法测定的主要技术参数反应的温度条件一般设置为低于95 ℃时预冷变性温度5 min,10 s 95 ℃,30 s 60 ℃,40次循环。引物序列见表 1。
1.
Galectin-1、GAPDH引物序列
Primer sequence of galectin-1 and GAPDH
| Gene | Primer sequence |
| Galectin-1 | F: ACTAGGCGCTCACTGTTCTCTC R: CATCGCCCCACTTGATTTTGG |
| GAPDH | F: TCGCCAGCAACCTGAATCT R: CACACCTCTGCAACACTTCC |
1.3.4. Western blot
待实验中所示需处理的细胞贴壁密度达到了90%左右后就应该立即取出用胰酶先把细胞消化溶解下来,后再加蛋白裂解液拌匀后取出放置于冰上冷冻至少30 min,最后可进行离心获取总蛋白。利用BCA定量法测定蛋白浓度,电泳凝胶为12%的SDSPAGE。电泳时可先以80 V的电压电泳至少30 min,之后可调整电压调整为超过110 V时再重复电泳90 min。电泳完成后再进行转膜,转膜前PVDF膜要先用甲醇去激活,之后再以200 mA的恒定电流去进行转膜,转膜结束后先用封闭液封闭,再4摄氏度冰箱内一抗孵育过夜,洗膜(3次,8 min),然后室温二抗孵育2 h即可,洗膜(3次,8 min),最后进行曝光。
1.3.5. CCK8检测细胞增殖
接种5000个左右的细胞在96孔板里面,等细胞贴壁后进行转染实验,每组铺设6个复孔,共5块板,分别为24、48、72、96、120 h等时间点去检测在450 nm处波长的吸光度。在进行上机检测之前每个孔要加入10 μL的CCK8试剂。先震荡混匀,然后要放在培养箱中1h,之后再去酶标仪上机检测。
1.3.6. 划痕实验检测细胞迁移能力
将细胞铺在六孔板中,待到细胞密度长到60%左右根据Lip6000转染试剂的操作手册对A549和H1299细胞系转染siRNA,待细胞密度长到90%左右用200 μL的枪头去进行划痕实验,然后将细胞培养基变为无血清培养基,之后在0和24 h对划痕处进行拍照。
1.3.7. Transwell实验检测细胞迁移能力
待细胞长满后用胰酶将细胞给消化下来,然后用无血清培养基去重悬细胞。首先将无血清的细胞悬液先缓缓加入到transwell的上室中,然后将混有血清和细胞的培养基缓慢加入transwell下室。在细胞培养箱中放置36 h。之后再取出transwell板,用干棉签轻轻仔细揩拭去transwell上室中残留的残余的培养基物质和细胞,之后可以取出,我们先用多聚甲醛固定约30 min,最后再用结晶紫染色,晾干放置约30 min,然后取再用倒置式的光学显微镜上作显微镜观察并拍照。
1.3.8. Transwell实验检测细胞的侵袭能力
先用无血清的1640培养基以1∶8的比例去稀释基质胶,transwell的每个小室中加入50 μL稀释后的基质胶,摇匀后放入培养箱,待基质胶凝固约2 h。然后再用胰蛋白酶液溶解消化细胞,然后用无血清培养基去重悬细胞。首先将无血清的细胞悬液先缓缓加入到transwell的上室中,然后将混有血清和细胞的培养基缓慢加入transwell下室。在细胞培养箱中放置36 h。然后再将transwell板取出,用小棉签均匀小心滴擦去基质胶, 之后取出,先用多聚甲醛固定30 min,最后在再用结晶紫进行染色,晾干再保存至少30 min,然后再在用倒置式的光学显微镜上进行观察实验或拍照。
1.3.9. 细胞凋亡实验
先将已经培养好的细胞先用无EDTA的胰酶消化下来,再用培养过该细胞的混有凋亡细胞的培养基去终止消化,然后在用已经预混冷处理过了的PBS清洗2次。最后就可以加凋亡试剂,实验组和对照组每管加入5 μL的FITC和10 μL的PI染色液,避光室温下染色10 min,最后上流式机检测凋亡率。
1.4. 统计学方法
用GraphPad Prism8软件对实验数据进行统计学的分析,实验数据以均数±标准差的形式表示,两组数据组间使用独立样本t检验,多组数据使用单因素方差分析,P < 0.05表示差异有统计学意义,所有实验均是独立重复3次。
2. 结果
2.1. 肺腺癌组织galectin-1的mRNA的表达情况
PCR结果显示,肺腺癌患者组织中galectin-1的mRNA表达水平远高于癌旁正常肺组织,差异有统计学意义(P < 0.01,图 1)。
1.

8对配对的肺腺癌组织和癌旁正常组织galectin-1的mRNA的表达情况
Galectin-1 mRNA expression in 8 pairs of lung adenocarcinoma tissues and adjacent normal tissues. *P < 0.05; **P < 0.01; ***P < 0.001 vs normal tissues group.
2.2. 肺腺癌细胞株和人正常支气管上皮细胞株galectin-1 mRNA表达水平
PCR结果显示,肺腺癌细胞株H1299和A549的galectin-1mRNA表达水平明显高于正常人支气管上皮细胞BEAS-2b,差异有统计学意义(P < 0.01,图 2)
2.

肺腺癌细胞株和人正常支气管上皮细胞株galectin-1mRNA表达水平
Galectin-1 mRNA expression levels in lung adenocarcinoma cell lines and human normal bronchial epithelial cell lines. ***P < 0.001 vs BEAS-2b.
2.3. si-RNA对galectin-1的干扰效率
荧光定量PCR和蛋白印迹检测3条RNA序列对galectin-1的干扰,结果显示si-148这条序列对galectin-1的干扰效率最高(P < 0.01,图 3),后续实验均采用si-148这条序列的si-RNA进行。
3.

si-RNA对galectin-1的干扰效率
Interference efficiency of si-RNA for galectin-1 knockdown. A: Galectin-1 was efficiently knocked down in A549 cells. B: The expression of galectin-1 protein was down-regulated in A549 cells. C: Relative expression of Galectin-1 mRNA in A549 cells after knockdown. D: Galectin-1 was successfully knocked down in H1299 cells. E: Relative expression of galectin-1 protein was downregulated in H1299 cells. F: Relative expression of galectin-1 mRNA in H1299 cells after knockdown. *P < 0.05; **P < 0.01; ***P < 0.001 vs control group.
2.4. 敲低galectin-1表达后对肺腺癌细胞和正常支气管上皮细胞增殖能力的影响
利用CCK8实验检测干扰galectin-1表达后,实验组即是干扰galectin-1表达的相对与对照组的增殖能力明显降低(P < 0.01,图 4)。在正常支气管上皮细胞中实验组与对照组结果差异无统计学意义。
4.

干扰galectin-1后对肺腺癌细胞和正常支气管上皮细胞增殖能力的影响
Effects of galectin-1 interference on proliferation of the two lung adenocarcinoma cell lines A549 (A) and H1299 (B) and in BEAS-2b cells (C). *P < 0.05; **P < 0.01 vs si-RNA.
2.5. 干扰galectin-1的表达后对肺腺癌细胞和正常支气管上皮细胞的侵袭能力的影响
实验组细胞的侵袭能力明显下降,差异有统计学意义(P < 0.01,图 5)。在正常支气管上皮细胞中实验组与对照组结果无差异。
5.

干扰galectin-1的表达后对肺腺癌细胞和正常支气管上皮细胞的侵袭能力的影响
Effect of interference with galectin-1 expression on invasion ability of lung adenocarcinoma cells and BEAS-2b cells. A, B: Transwell assay of A549 cells (Original magnification: × 200). C, D: Transwell assay of H1299 cells (×200). E, F: Transwell assay of BEAS-2b cells (×200). **P < 0.01 vs control group.
2.6. 干扰galectin-1的表达后对肺腺癌细胞和支气管上皮细胞的体外迁移能力产生的影响
实验组的细胞迁移能力明显下降,差异具有统计学意义(P < 0.01,图 6)。在正常支气管上皮细胞中实验组与对照组结果差异无统计学意义(P>0.05)。
6.

干扰galectin-1的表达后对肺腺癌细胞和正常支气管上皮细胞的体外迁移能力产生的影响
Effect of galectin-1 knockdown on in vitro migration of lung adenocarcinoma cells. A, B: Transwell assay of A549 cell migration (×200). C, D: Transwell assay of H1299 cell migration (× 200). E, F: Transwell assay of BEAS-2b cell migration (× 200). **P < 0.01 vs control group.
2.7. 划痕实验检测galectin-1对细胞的迁移能力的影响
实验组的细胞迁移能力明显下降,差异具有统计学意义(P < 0.01,图 7)。在正常支气管上皮细胞中实验组与对照组结果差异无统计学意义。
7.

划痕实验检测galectin-1对细胞的迁移能力的影响
Effect of galectin-1 knockdown on migration of A549 cells (A, B), H1299 cells (C, D) and BEAS-2b cells (E, F) detected by wound healing assay (×100). **P < 0.01 vs control group.
2.8. 干扰galectin-1表达后对肺腺癌细胞和正常支气管上皮细胞凋亡情况的影响
流式细胞术结果显示,与对照组相比,干扰galectin-1表达组的凋亡率明显上升,其差异有统计学意义(P < 0.01,图 8)。在正常支气管上皮细胞中实验组与对照组结果差异无统计学意义。
8.
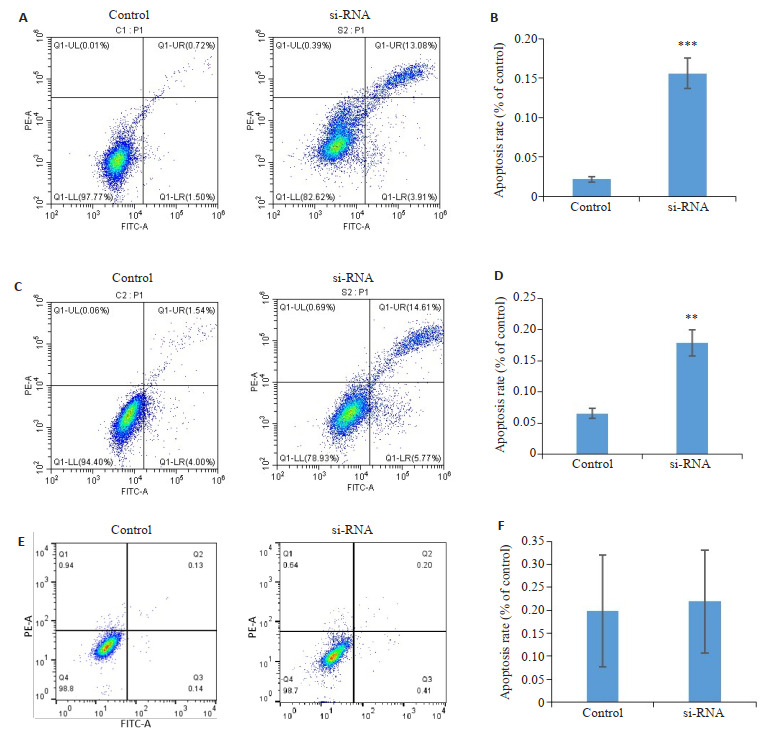
干扰galectin-1表达后对肺腺癌细胞凋亡情况的影响
Effects of galectin-1 knockdown on apoptosis of A549 cells (A, B), H1299 cells (C, D) and BEAS-2b cells (E, F) detected by annexinⅤ-FITC/ propidium iodide apoptosis assay. **P < 0.01; ***P < 0.001 vs control group.
2.9. 干扰galectin-1表达后BAX、Bcl-2、cleaved caspase3等凋亡相关蛋白的变化
干扰galectin-1表达后,促进细胞凋亡的相关蛋白BAX和cleaved caspase3的表达量增加,抑制细胞凋亡的相关蛋白Bcl-2的表达量下降,差异具有统计学意义(P < 0.01,图 9)。
9.

干扰galectin-1表达后BAX、Bcl-2、cleaved caspase3等凋亡相关蛋白的变化
Changes of BAX, Bcl-2, cleaved caspase-3 and other apoptosis-related proteins after galectin-1 knockdown. A: Western blotting of apoptosis-related proteins in A549 cells. B: Western blotting of apoptosis-related proteins in H1299 cells. C, D: Quantitative analysis of the protein expressions inA549 cells and H1299 cells, respectively. *P < 0.05; **P < 0.01 vs control group.
2.10. 干扰galectin-1表达后AKT、ERK等2条通路的激活情况的变化
干扰galectin-1表达后与对照组相比AKT信号通路的磷酸化水平无明显变化,ERK信号通路的磷酸化水平明显降低,差异具有统计学意义(P < 0.01,图 10)。
10.

干扰galectin-1表达后AKT、ERK等2条通路的激活情况的变化
Changes in the activation of Akt and ERK pathways after galectin-1 knockdown detected by Western blotting in A549 cells (A, C) and H1299 cells (B, D). **P < 0.01 vs control group.
3. 讨论
本研究中我们通过si-RNA敲低肺腺癌细胞系A549和H1299中galectin-1的表达后,其增殖能力明显下降,细胞的侵袭和迁移能力也明显下降,细胞的凋亡率明显上升。上述实验结果表明,galectin-1在肺腺癌细胞系的增殖、侵袭、迁移和凋亡等功能的调控中发挥着重要的作用。
细胞凋亡由基因调控,在促凋亡蛋白如BAX、抑凋亡蛋白Bcl-2和凋亡调控因子caspase3等共同参与下细胞的有序性死亡[29, 30]。细胞凋亡的失调可导致多种疾病的发生,有超过50%的肿瘤在凋亡机制上失调,凋亡的异常常导致本应死亡的细胞存留了下来,这其中包括有一些突变的细胞增殖失控从而导致了肿瘤的发生。Caspase蛋白作为细胞凋亡的启动者和执行者,是细胞凋亡的关键酶在细胞凋亡的过程中发挥着至关重要的作用。细胞凋亡过程中caspase3被激活转化为有活性的cleaved-caspase3,从而促进细胞凋亡,促进细胞体内蛋白质的降解,使细胞走向不可逆的死亡,caspase3被激活是细胞进入凋亡阶段的标志[31]。BAX和Bcl-2是相互拮抗的两种凋亡蛋白,正常情况下二者以适当的比例存在,在细胞发生凋亡后,BAX比例增加,Bcl-2比例下降,在BAX表达增加后可以激活caspase3蛋白加速进入凋亡过程[32-34]。本实验我们通过si-RNA敲低galectin-1表达后,cleaved-caspase3和BAX表达增加,Bcl-2表达下降,提示galectin-1与肺腺癌细胞caspase3/ BAX/Bcl-2凋亡信号通路有关。
细胞外信号调控激酶(ERK)途径是丝裂原活化蛋白激酶(MAPK)超家族的成员,ERK信号通路是研究最活跃的信号转导通路之一同时也是MAPK信号通路中最主要最经典的通路,以磷酸化的形式激活[35, 36]。有研究发现ERK信号通路的激活是通过p-ERK将信号传导到细胞核与靶基因结合,再参与调控下游靶基因表达从而参与细胞增殖、凋亡、分化、代谢、侵袭、迁移和转录的调控[37-39]。例如在乳腺癌的研究中发现GPR19通过ERK途径调控上皮间充质转化从而促进乳腺癌细胞的迁移[40]。同时ERK可以作为一个调控凋亡的关键因子在神经元细胞中发挥作用[41]。ERK可能通过治疗剂的类型、细胞的特异性以及ERK的激活状态来影响来影响细胞的增殖或凋亡的[42]。本研究发现在敲低galectin-1的表达后,与对照组相比ERK信号通路中的磷酸化水平明显降低。由此可得galectin-1的敲低影响着肺腺癌细胞ERK磷酸化水平的表达。
综上所述,本实验结果表明galectin-1可能通过改变ERK信号通路的磷酸化激活水平来调控肺腺癌细胞的增殖、侵袭、迁移和凋亡。Galectin-1的异常增高可能是肺腺癌细胞发生发展的重要机制之一,galectin-1有望可能成为干预肺腺癌临床治疗的关键靶标。但是我们仅仅是敲低了galectin-1表达后,肺腺癌细胞中信号通路蛋白ERK的磷酸化水平降低,关于Galectin-1如何具体的调控ERK磷酸化水平的具体机制还需要进一步的探究。
Biography
陈文邦,在读硕士研究生,E-mail: 18271649362@163.com
Funding Statement
安徽省教育厅自然科学重点项目(KJ2019A0340)
Contributor Information
陈 文邦 (Wenbang CHEN), Email: 18271649362@163.com.
张 雷 (Lei ZHANG), Email: 13855282388@139.com.
References
- 1.Schwartz AG, Cote ML. Epidemiology of lung cancer. Adv Exp Med Biol. 2016;893(1):21–41. doi: 10.1007/978-3-319-24223-1_2. [Schwartz AG, Cote ML. Epidemiology of lung cancer[J]. Adv Exp Med Biol, 2016, 893(1): 21-41.] [DOI] [PubMed] [Google Scholar]
- 2.Hoy H, Lynch T, Beck M. Surgical treatment of lung cancer. Crit Care Nurs Clin North Am. 2019;31(3):303–13. doi: 10.1016/j.cnc.2019.05.002. [Hoy H, Lynch T, Beck M. Surgical treatment of lung cancer[J]. Crit Care Nurs Clin North Am, 2019, 31(3): 303-13.] [DOI] [PubMed] [Google Scholar]
- 3.Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8. [Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments[J]. Lancet, 2017, 389(10066): 299-311.] [DOI] [PubMed] [Google Scholar]
- 4.Ruiz-Cordero R, Devine WP. Targeted therapy and checkpoint immunotherapy in lung cancer. Surg Pathol Clin. 2020;13(1):17–33. doi: 10.1016/j.path.2019.11.002. [Ruiz-Cordero R, Devine WP. Targeted therapy and checkpoint immunotherapy in lung cancer[J]. Surg Pathol Clin, 2020, 13(1): 17-33.] [DOI] [PubMed] [Google Scholar]
- 5.Denisenko TV, Budkevich IN, Zhivotovsky B. Cell death-based treatment of lung adenocarcinoma. Cell Death Dis. 2018;9(2):117. doi: 10.1038/s41419-017-0063-y. [Denisenko TV, Budkevich IN, Zhivotovsky B. Cell death-based treatment of lung adenocarcinoma[J]. Cell Death Dis, 2018, 9(2): 117.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasmatzi E, Papadionysiou C, Monastirli A, et al. Galectin 1 in dermatology: current knowledge and perspectives. Acta Dermatovenerol Alp Pannonica Adriat. 2019;28(1):27–31. [Pasmatzi E, Papadionysiou C, Monastirli A, et al. Galectin 1 in dermatology: current knowledge and perspectives[J]. Acta Dermatovenerol Alp Pannonica Adriat, 2019, 28(1): 27-31.] [PubMed] [Google Scholar]
- 7.Goud NS, Soukya PSL, Ghouse M, et al. Human galectin-1 and its inhibitors: privileged target for cancer and HIV. Mini Rev Med Chem. 2019;19(16):1369–78. doi: 10.2174/1389557519666190304120821. [Goud NS, Soukya PSL, Ghouse M, et al. Human galectin-1 and its inhibitors: privileged target for cancer and HIV[J]. Mini Rev Med Chem, 2019, 19(16): 1369-78.] [DOI] [PubMed] [Google Scholar]
- 8.López-Lucendo MF, Solís D, André S, et al. Growth-regulatory human galectin-1:crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding. J Mol Biol. 2004;343(4):957–70. doi: 10.1016/j.jmb.2004.08.078. [López-Lucendo MF, Solís D, André S, et al. Growth-regulatory human galectin-1: crystallographic characterisation of the structural changes induced by single-site mutations and their impact on the thermodynamics of ligand binding[J]. J Mol Biol, 2004, 343(4): 957-70.] [DOI] [PubMed] [Google Scholar]
- 9.Bacigalupo ML, Carabias P, Troncoso MF. Contribution of galectin-1, a glycan-binding protein, to gastrointestinal tumor progression. World J Gastroenterol. 2017;23(29):5266–81. doi: 10.3748/wjg.v23.i29.5266. [Bacigalupo ML, Carabias P, Troncoso MF. Contribution of galectin-1, a glycan-binding protein, to gastrointestinal tumor progression[J]. World J Gastroenterol, 2017, 23(29): 5266-81.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito K, Stannard K, Gabutero E, et al. Galectin-1 as a potent target for cancer therapy: role in the tumor microenvironment. Cancer Metastasis Rev. 2012;31(3/4):763–78. doi: 10.1007/s10555-012-9388-2. [Ito K, Stannard K, Gabutero E, et al. Galectin-1 as a potent target for cancer therapy: role in the tumor microenvironment[J]. Cancer Metastasis Rev, 2012, 31(3/4): 763-78.] [DOI] [PubMed] [Google Scholar]
- 11.Zhang PF, Li KS, Shen YH, et al. Galectin-1 induces hepatocellular carcinoma EMT and sorafenib resistance by activating FAK/PI3K/AKT signaling. Cell Death Dis. 2016;7(4):e2201. doi: 10.1038/cddis.2015.324. [Zhang PF, Li KS, Shen YH, et al. Galectin-1 induces hepatocellular carcinoma EMT and sorafenib resistance by activating FAK/PI3K/AKT signaling[J]. Cell Death Dis, 2016, 7(4): e2201.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang YY, Wang HC, Zhao JW, et al. Immunosuppressive roles of galectin-1 in the tumor microenvironment. Biomolecules. 2021;11(10):1398. doi: 10.3390/biom11101398. [Huang YY, Wang HC, Zhao JW, et al. Immunosuppressive roles of galectin-1 in the tumor microenvironment[J]. Biomolecules, 2021, 11(10): 1398.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou FC, Chen HY, Kuo CC, et al. Role of galectins in tumors and in clinical immunotherapy. Int J Mol Sci. 2018;19(2):430. doi: 10.3390/ijms19020430. [Chou FC, Chen HY, Kuo CC, et al. Role of galectins in tumors and in clinical immunotherapy[J]. Int J Mol Sci, 2018, 19(2): 430.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Astorgues-Xerri L, Riveiro ME, Tijeras-Raballand A, et al. Unraveling galectin-1 as a novel therapeutic target for cancer. Cancer Treat Rev. 2014;40(2):307–19. doi: 10.1016/j.ctrv.2013.07.007. [Astorgues-Xerri L, Riveiro ME, Tijeras-Raballand A, et al. Unraveling galectin-1 as a novel therapeutic target for cancer[J]. Cancer Treat Rev, 2014, 40(2): 307-19.] [DOI] [PubMed] [Google Scholar]
- 15.Juszczynski P, Ouyang J, Monti S, et al. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci USA. 2007;104(32):13134–9. doi: 10.1073/pnas.0706017104. [Juszczynski P, Ouyang J, Monti S, et al. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma[J]. Proc Natl Acad Sci USA, 2007, 104(32): 13134-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillenwater A, Xu XC, El-Naggar AK, et al. Expression of galectins in head and neck squamous cell carcinoma. Head Neck. 1996;18(5):422–32. doi: 10.1002/(SICI)1097-0347(199609/10)18:5<422::AID-HED5>3.0.CO;2-7. [Gillenwater A, Xu XC, El-Naggar AK, et al. Expression of galectins in head and neck squamous cell carcinoma[J]. Head Neck, 1996, 18 (5): 422-32.] [DOI] [PubMed] [Google Scholar]
- 17.Valach J, Fík Z, Strnad H, et al. Smooth muscle actin-expressing stromal fibroblasts in head and neck squamous cell carcinoma: increased expression of galectin-1 and induction of poor prognosis factors. Int J Cancer. 2012;131(11):2499–508. doi: 10.1002/ijc.27550. [Valach J, Fík Z, Strnad H, et al. Smooth muscle actin-expressing stromal fibroblasts in head and neck squamous cell carcinoma: increased expression of galectin-1 and induction of poor prognosis factors[J]. Int J Cancer, 2012, 131(11): 2499-508.] [DOI] [PubMed] [Google Scholar]
- 18.Nambiar DK, Aguilera T, Cao HB, et al. Galectin-1-driven T cell exclusion in the tumor endothelium promotes immunotherapy resistance. J Clin Invest. 2019;129(12):5553–67. doi: 10.1172/JCI129025. [Nambiar DK, Aguilera T, Cao HB, et al. Galectin-1-driven T cell exclusion in the tumor endothelium promotes immunotherapy resistance[J]. J Clin Invest, 2019, 129(12): 5553-67.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You XL, Wang YJ, Wu J, et al. Galectin-1 promotes metastasis in gastric cancer through a sphingosine-1-phosphate receptor 1-dependent mechanism. Cell Physiol Biochem. 2018;51(1):11–30. doi: 10.1159/000495157. [You XL, Wang YJ, Wu J, et al. Galectin-1 promotes metastasis in gastric cancer through a sphingosine-1-phosphate receptor 1-dependent mechanism[J]. Cell Physiol Biochem, 2018, 51(1): 11-30.] [DOI] [PubMed] [Google Scholar]
- 20.You XL, Liu QH, Wu J, et al. Galectin-1 promotes vasculogenic mimicry in gastric cancer by upregulating EMT signaling. J Cancer. 2019;10(25):6286–97. doi: 10.7150/jca.33765. [You XL, Liu QH, Wu J, et al. Galectin-1 promotes vasculogenic mimicry in gastric cancer by upregulating EMT signaling[J]. J Cancer, 2019, 10(25): 6286-97.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian D, Lu ZP, Xu QC, et al. Galectin-1-driven upregulation of SDF-1 in pancreatic stellate cells promotes pancreatic cancer metastasis. Cancer Lett. 2017;397:43–51. doi: 10.1016/j.canlet.2017.03.024. [Qian D, Lu ZP, Xu QC, et al. Galectin-1-driven upregulation of SDF-1 in pancreatic stellate cells promotes pancreatic cancer metastasis[J]. Cancer Lett, 2017, 397: 43-51.] [DOI] [PubMed] [Google Scholar]
- 22.Dalotto-Moreno T, Croci DO, Cerliani JP, et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 2013;73(3):1107–17. doi: 10.1158/0008-5472.CAN-12-2418. [Dalotto-Moreno T, Croci DO, Cerliani JP, et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease[J]. Cancer Res, 2013, 73(3): 1107-17.] [DOI] [PubMed] [Google Scholar]
- 23.Masoodi M, Shah ZA, Beigh AH, et al. Galectin-1 as a predictive biomarker in ovarian cancer. J Ovarian Res. 2021;14(1):123. doi: 10.1186/s13048-021-00874-1. [Masoodi M, Shah ZA, Beigh AH, et al. Galectin-1 as a predictive biomarker in ovarian cancer[J]. J Ovarian Res, 2021, 14(1): 123.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davuluri GVN, Chen CC, Chiu YC, et al. Autophagy drives galectin-1 secretion from tumor-associated macrophages facilitating hepato-cellular carcinoma progression. Front Cell Dev Biol. 2021;9:741820. doi: 10.3389/fcell.2021.741820. [Davuluri GVN, Chen CC, Chiu YC, et al. Autophagy drives galectin-1 secretion from tumor-associated macrophages facilitating hepato-cellular carcinoma progression[J]. Front Cell Dev Biol, 2021, 9: 741820.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng KY, Jiang SS, Lee YW, et al. Stromal galectin-1 promotes colorectal cancer cancer-initiating cell features and disease dissemination through SOX9 and β-catenin: development of niche-based biomarkers. Front Oncol. 2021;11:716055. doi: 10.3389/fonc.2021.716055. [Peng KY, Jiang SS, Lee YW, et al. Stromal galectin-1 promotes colorectal cancer cancer-initiating cell features and disease dissemination through SOX9 and β-catenin: development of niche-based biomarkers[J]. Front Oncol, 2021, 11: 716055.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui YB, Yan M, Wu W, et al. ESCCAL-1 promotes cell-cycle progression by interacting with and stabilizing galectin-1 in esophageal squamous cell carcinoma. NPJ Precis Oncol. 2022;6(1):12. doi: 10.1038/s41698-022-00255-x. [Cui YB, Yan M, Wu W, et al. ESCCAL-1 promotes cell-cycle progression by interacting with and stabilizing galectin-1 in esophageal squamous cell carcinoma[J]. NPJ Precis Oncol, 2022, 6 (1): 12.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu YL, Wu CY, Hung JY, et al. Galectin-1 promotes lung cancer tumor metastasis by potentiating integrin α6β4 and Notch1/Jagged2 signaling pathway. Carcinogenesis. 2013;34(6):1370–81. doi: 10.1093/carcin/bgt040. [Hsu YL, Wu CY, Hung JY, et al. Galectin-1 promotes lung cancer tumor metastasis by potentiating integrin α6β4 and Notch1/Jagged2 signaling pathway[J]. Carcinogenesis, 2013, 34(6): 1370-81.] [DOI] [PubMed] [Google Scholar]
- 28.Chung LY, Tang SJ, Sun GH, et al. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin Cancer Res. 2012;18(15):4037–47. doi: 10.1158/1078-0432.CCR-11-3348. [Chung LY, Tang SJ, Sun GH, et al. Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2[J]. Clin Cancer Res, 2012, 18(15): 4037-47.] [DOI] [PubMed] [Google Scholar]
- 29.Swerdlow S, Distelhorst CW, et al. Bcl 2 regulated calcium signals as common mediators of both apoptosis and autophagy. Dev Cell. 2007;12(2):178–9. doi: 10.1016/j.devcel.2007.01.008. [Swerdlow S, Distelhorst CW. Bcl 2 regulated calcium signals as common mediators of both apoptosis and autophagy[J]. Dev Cell, 2007, 12 (2): 178-9.] [DOI] [PubMed] [Google Scholar]
- 30.Moshrefi M, Spotin A, Kafil HS, et al. Tumor suppressor p53 induces apoptosis of host lymphocytes experimentally infected by Leishmania major, by activation of Bax and caspase-3:a possible survival mechanism for the parasite. Parasitol Res. 2017;116(8):2159–66. doi: 10.1007/s00436-017-5517-8. [Moshrefi M, Spotin A, Kafil HS, et al. Tumor suppressor p53 induces apoptosis of host lymphocytes experimentally infected by Leishmania major, by activation of Bax and caspase-3: a possible survival mechanism for the parasite[J]. Parasitol Res, 2017, 116(8): 2159-66.] [DOI] [PubMed] [Google Scholar]
- 31.Jung J, Kim HY, Maeng J, et al. Interaction of translationally controlled tumor protein with Apaf-1 is involved in the development of chemoresistance in HeLa cells. BMC Cancer. 2014;14:165. doi: 10.1186/1471-2407-14-165. [Moshrefi M, Spotin A, Kafil HS, et al. Tumor suppressor p53 induces apoptosis of host lymphocytes experimentally infected by Leishmania major, by activation of Bax and caspase-3: a possible survival mechanism for the parasite[J]. Parasitol Res, 2017, 116(8): 2159-66.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong FF, Wu CL, Sun XF, et al. Effect of Buyang Huanwu decoction combined with edaravone on the apoptosis of neuron and expression of Bcl-2 and Bax cerebral ischemia-reperfusion in mice. Chin J Mod Appl Pharm. 2016;33(11):1392–6. [Jung J, Kim HY, Maeng J, et al. Interaction of translationally controlled tumor protein with Apaf-1 is involved in the development of chemoresistance in HeLa cells[J]. BMC Cancer, 2014, 14: 165.] [Google Scholar]
- 33.Xie TF, Wang Ly, Liu L, et al. Berberine inhibits growth and metastasis of laryngeal carcinoma cells by regulating PI3K/Akt pathway. Chin J New Drugs Clin Rem. 2020;39(1):43–8. [Zhong FF, Wu CL, Sun XF, et al. Effect of Buyang Huanwu decoction combined with edaravone on the apoptosis of neuron and expression of Bcl-2 and Bax cerebral ischemia-reperfusion in mice[J]. Chin J Mod Appl Pharm, 2016, 33(11): 1392-6.] [Google Scholar]
- 34.Ren LP, Li XJ, Jin SJ, et al. Influence of sophoridine on proliferation and caspase-3/bcl-2/bax signaling pathway of human pancreatic cancer cell line Capan-1 cells. Chin J Mod Appl Pharm. 2017;34(3):325–8. [Xie TF, Wang Ly, Liu L, et al. Berberine inhibits growth and metastasis of laryngeal carcinoma cells by regulating PI3K/Akt pathway[J]. Chin J New Drugs Clin Rem, 2020, 39(1): 43-8.] [Google Scholar]
- 35.Peng S, Zhang Y, Zhang JN, et al. ERK in learning and memory: a review of recent research. Int J Mol Sci. 2010;11(1):222–32. doi: 10.3390/ijms11010222. [Ren LP, Li XJ, Jin SJ, et al. Influence of sophoridine on proliferation and caspase-3/bcl-2/bax signaling pathway of human pancreatic cancer cell line Capan-1 cells[J]. Chin J Mod Appl Pharm, 2017, 34 (3): 325-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li YP, Yang Q. Effect of PD98059 on chemotherapy in patients with colorectal cancer through ERK1/2 pathway. J BUON. 2019;24(5):1837–44. [Peng S, Zhang Y, Zhang JN, et al. ERK in learning and memory: a review of recent research[J]. Int J Mol Sci, 2010, 11(1): 222-32.] [PubMed] [Google Scholar]
- 37.Sugiura R, Satoh R, Takasaki T. ERK: a double-edged sword in cancer. ERK-dependent apoptosis as a potential therapeutic strategy for cancer. Cells. 2021;110(110):2509. doi: 10.3390/cells10102509. [Li YP, Yang Q. Effect of PD98059 on chemotherapy in patients with colorectal cancer through ERK1/2 pathway[J]. J BUON, 2019, 24 (5): 1837-44.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo YJ, Pan WW, Liu SB, et al. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19(3):1997–2007. doi: 10.3892/etm.2020.8454. [Sugiura R, Satoh R, Takasaki T. ERK: a double-edged sword in cancer. ERK-dependent apoptosis as a potential therapeutic strategy for cancer[J]. Cells, 2021, 10(10): 2509.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lavoie H, Gagnon J, Therrien M. ERK signalling: a master regulator of cell behaviour, life and fate. Nat Rev Mol Cell Biol. 2020;21(10):607–32. doi: 10.1038/s41580-020-0255-7. [Guo YJ, Pan WW, Liu SB, et al. ERK/MAPK signalling pathway and tumorigenesis[J]. Exp Ther Med, 2020, 19(3): 1997-2007.] [DOI] [PubMed] [Google Scholar]
- 40.Rao A, Herr DR. G protein-coupled receptor GPR19 regulates E-cadherin expression and invasion of breast cancer cells. Biochim Biophys Acta Mol Cell Res. 2017;1864(7):1318–27. doi: 10.1016/j.bbamcr.2017.05.001. [Lavoie H, Gagnon J, Therrien M. ERK signalling: a master regulator of cell behaviour, life and fate[J]. Nat Rev Mol Cell Biol, 2020, 21 (10): 607-32.] [DOI] [PubMed] [Google Scholar]
- 41.Cheung ECC, Slack RS. Emerging role for ERK as a key regulator of neuronal apoptosis. Sci STKE. 2004;2004(251):PE45. doi: 10.1126/stke.2512004pe45. [Rao A, Herr DR. G protein-coupled receptor GPR19 regulates E-cadherin expression and invasion of breast cancer cells[J]. Biochim Biophys Acta Mol Cell Res, 2017, 1864(7): 1318-27.] [DOI] [PubMed] [Google Scholar]
- 42.Bostan M, Petric\u0103-Matei GG, Ion G, et al. Cisplatin effect on head and neck squamous cell carcinoma cells is modulated by ERK1/2 protein kinases. Exp Ther Med. 2019;18(6):5041–51. doi: 10.3892/etm.2019.8139. [Cheung ECC, Slack RS. Emerging role for ERK as a key regulator of neuronal apoptosis[J]. Sci STKE, 2004, 2004(251): PE45.] [DOI] [PMC free article] [PubMed] [Google Scholar]


