Abstract
目的
探究苦参碱(Mat)联合LY294002对人髓系白血病K562细胞增殖、凋亡和细胞周期的影响及可能机制。
方法
采用CCK-8法检测不同浓度Mat单药和联合LY294002对K562细胞增殖的影响;将K562细胞分为对照组、Mat组、LY294002组和Mat+LY294002组,分别采用0.4 g/L Mat及10 μmol/L LY294002单独或联合干预48 h后,应用光学显微镜观察细胞形态变化,流式细胞术annexin Ⅴ-FITC/PI双标记检测细胞凋亡,PI单标记检测细胞周期变化,Western blot法检测Mat联合LY294002对K562细胞p-mTOR、p-PI3K、Akt、p-Akt、CyclinD1、Bcl-2、Caspase-9蛋白表达的影响。
结果
CCK-8结果显示,不同浓度Mat单药和联合LY294002均可抑制K562细胞的增殖,具有时间和浓度依赖性,且联合组增殖抑制率比单药组明显升高(P<0.01)。形态学检查显示,联合用药组比单药组凋亡表现更明显。流式细胞结果显示,与对照组及单药组相比,联合组显著促进细胞凋亡([42.50±2.63)%,P<0.01],显著增加了细胞周期G0/G1期细胞比率([57.23±1.44)%,P<0.01]。Western blot结果表明,两药联合后K562细胞p-mTOR、CyclinD1、p-PI3K、p-Akt、Bcl-2蛋白表达水平明显增高(P<0.01),Caspase-9蛋白表达水平下降(P<0.01)。
结论
Mat和LY294002联合应用可以增强对K562细胞生长抑制的协同作用,其机制可能是通过有效下调PI3K/Akt信号通路中的p-Akt表达,使p-mTOR、CyclinD1和Bcl-2蛋白表达下调,Caspase-9蛋白表达上调来实现的。
Keywords: 急性髓系白血病, 苦参碱, LY294002, 细胞凋亡, 细胞周期, Akt
Abstract
Objective
To investigate the effects of matrine combined with LY294002 on proliferation, apoptosis and cell cycle of human myeloid leukemia K562 cells and explore the underlying mechanism.
Methods
The effects of different concentrations of matrine alone and in combination with LY294002 on the proliferation of K562 cells were examined with CCK-8 assay. The changes in morphology of K562 cells were observed following treatment for 48 h with 0.4 g/L matrine and 10 μmol/L Y294002, either alone or in combination, and cell apoptosis was detected using flow cytometry with annexin V-FITC/PI double labeling; the changes in cell cycle was detected with PI labeling. Western blotting was performed to examine the effect of matrine combined with LY294002 on expressions of p-mTOR, p-PI3K, Akt, p-Akt, cyclinD1, Bcl-2 and caspase-9 in the cells.
Results
Treatment with different concentrations of matrine, both alone and in combination with LY294002, inhibited the proliferation of K562 cells in a time- and concentration-dependent manner. Compared with matrine treatment alone, the combined treatment caused more obvious morphological changes of the cells, significantly increased cell apoptosis (P < 0.01), and induced cell cycle arrest in G0/G1 (P < 0.01). Western blotting showed that the protein expression levels of p-mTOR, cyclinD1, p-PI3K, p-Akt and Bcl-2 in K562 cells increased while the expression level of caspase-9 decreased significantly after the combined treatment (P < 0.01).
Conclusion
Matrine combined with LY294002 produces a synergistic inhibitory effect on K562 cells possibly by down-regulating the p-Akt expression in PI3K/Akt signaling pathway, reducing the expressions of p-mTOR, cyclinD1 and Bcl-2, and increasing the expression of caspase-9.
Keywords: acute myeloid leukemia, matrine, LY294002, apoptotic, cell cycle, Akt
急性髓系白血病(AML)是由于遗传变异导致的发生于髓系干细胞前体的血液系统恶性肿瘤,表现为原始或幼稚髓系细胞克隆性增生而正常造血受抑制[1]。AML治疗有分子靶向治疗、诱导分化治疗、联合化疗、造血干细胞移植等多种治疗方法,目前药物治疗仍是其治疗主要手段,药物不良反应、耐药性、微量残留白血病等限制其临床运用,故探寻新的、安全的抗癌方案具有重要的临床意义[2]。中医药是我国的宝贵财富,中药苦参具有悠久的药用历史,苦参碱(Mat)作为苦参的主要有效成分之一,具有抗炎、抗病毒、抗肝纤维化及抗肿瘤等多种生物活性。现代药理学研究指出,Mat能够通过多元化抑制PI3K/Akt/mTOR[3]、Akt/GSK3β/β-catenin[4]、PTEN/Akt[5]等信号转导通路的过度活化,多途径下调Akt活化,发挥抗恶性肿瘤的作用[6]。Akt异常过表达或活化已在许多癌症中被观察到,与癌细胞增殖和生存增加相关,靶向Akt可能是癌症预防和治疗的重要途径[7]。针对PI3K/Akt信号通路的分子靶向治疗是研究的热点[8],LY294002是PI3K/Akt抑制剂,也能下调Akt活化,抑制信号转导通路下游效应[9]。LY294002与Mat联合应用是否有协同作用,是否能减少药物用量,尚无文献报道。
本研究以具有急、慢性髓细胞白血病细胞特征的K562细胞为研究对象,通过Mat与LY294002联合作用,观察两者对K562细胞增殖、周期及凋亡的影响,进一步探讨其作用机制,以期为中西医结合治疗AML提供理论依据。
1. 材料和方法
1.1. 材料
1.1.1. 细胞株及培养
K562细胞株为蚌埠医学院肿瘤基础研究重点实验室冻存株,用RPMI 1640完全培养基(含10% FBS)于37 ℃、5% CO2、饱和湿度培养箱培养,取对数生长期细胞用于后继实验。
1.1.2. 实验试剂
小牛血清(Lonsera),RPMI 1640(Hyclone),苦参碱(成都瑞芬思生物科技有限公司),吖啶橙(Sigma),Wright染色液(Baso),CCK-8(Biomiky),Annexin V-FITC/PI试剂盒(凯基生物),青霉素-链霉素、RIPA裂解液、PMSF、BCA蛋白浓度测定试剂盒(碧云天),LY294002(APExBIO),β-actin、p-mTOR、p-PI3K、Akt、p-Akt、CyclinD1、Bcl-2、Caspase-9(ABclonal),HRP标记的羊抗兔IgG(Affinity Biosciences)。
1.1.3. 实验仪器
MultiSkan3酶标仪(Thermo),倒置荧光显微镜(Olympus),普通光学显微镜(Olympus),流式细胞仪(BD),垂直电泳仪(Bio-Rad)。
1.2. 方法
1.2.1. CCK-8比色法检测细胞增殖
取对数生长期细胞K562接种于96孔板,浓度设定为2×108/L,实验组中苦参碱浓度分别为0.2、0.4、0.8、1.6 g/L,联合LY294002的浓度为10 μmol /L,对照组不加药,空白孔不加药和不加细胞,均设3个平行孔,置于37 ℃,5% CO2培养箱孵育。在前期预实验中发现,联合使用药物12h内作用不显著,超过48 h细胞增殖抑制率进入平台期,故设计分别孵育24 h和48 h,孵育后,每孔加入10 μL CCK-8溶液,继续于培养箱内孵育2 h,用酶标仪测量各孔在波长450 nm处的吸光度值(A值),实验重复3次。细胞增殖抑制率(%)= [1(-A实验-A空白)(/A对照-A空白)]×100%。
1.2.2. 细胞形态学观察
取对数生长期状态良好的K562细胞接种于6孔板,实验分组:对照组、苦参碱(0.4 g/L)组、LY294002(10 μmol/L)组和联合组(终浓度为0.4 g/L的苦参碱+ 10 μmol/L LY294002)处理K562细胞,孵育48 h后,各取1 mL混匀的细胞悬液于24孔板中,静置0.5 h后,用倒置显微镜观察形态;各取1 mL混匀的细胞悬液,1500 r/min离心,5 min,弃上清,混匀细胞,涂片,自然干燥后,用Wright染色,观察细胞形态变化;各取2 mL混匀的细胞悬液,用PBS缓冲液清洗2次,用1 μg/mL吖啶橙(AO)染色,避光染色15 min,PBS洗3次,应用倒置荧光显微镜观察细胞形态。
1.2.3. Annexin V-FITC/PI双染色法细胞凋亡检测
细胞培养及实验分组同上,孵育48 h后,4℃离心去上清液,用冷PBS洗2次,离心尽去上清,轻弹起细胞,使其尽量散开,按照凋亡检测试剂盒说明书进行AnnexinⅤ-FITC/PI双标记染色,并设置空白对照组和单色对照组。1 h内用流式细胞仪测定完毕,采用FlowJo7.6软件分析细胞凋亡情况。
1.2.4. FCM测定细胞周期分布
细胞培养及实验分组同上,收集细胞,预冷PBS洗涤1次,预冷70%乙醇固定细胞12 h,离心去上清,加入预冷PBS洗涤后转入流式管,再离心去上清,弹起细胞,加入含有RNase A和PI的染色液0.5 mL,37 ℃避光温浴30 min,随后冰浴避光放置,于24 h内用流式细胞仪检测,应用FlowJo7.6软件分析细胞周期。
1.2.5. Western blot方法检测目的蛋白的表达
细胞培养及实验分组同上,分别收集各组的K562细胞至离心管中,预冷PBS洗涤细胞2次,转入EP管中,离心后尽去上清,加入适量的细胞裂解混合液,冰浴后裂解细胞提取总蛋白,BCA蛋白试剂盒测定蛋白浓度。各组蛋白样品上样量为30 μg,CyclinD1、Bcl-2、Caspase-9分子量小进行12% SDS-PAGE分离胶电泳,p-mTOR、p-PI3K、Akt、p-Akt分子量较大进行6% SDS-PAGE分离胶电泳。转移至PVDF膜上,室温封闭2 h后,β-actin一抗1∶100 000稀释,其它一抗1∶1000稀释;4 ℃孵育过夜;TBST洗膜3次;HRP标记的二抗37 ℃孵育2 h;再TBST洗膜3次;ECL显影,FluorChem M凝胶成像系统曝光显影。Image J图像分析软件检测条带灰度值,以β-actin为内参,计算:蛋白相对表达量=目的条带灰度/内参条带灰度。
1.3. 统计学处理
采用GraphPad Prism 6.0软件对数据进行统计绘图分析,组间比较采用单因素方差分析,P<0.05为差异有统计学意义。
2. 结果
2.1. Mat联合LY294002对K562细胞的增殖抑制作用
CCK-8法检测实验结果(图 1)分析,不同浓度的Mat对K562细胞的增殖均有抑制作用,且呈剂量时间依赖性。相同作用时间下,Mat联合LY294002对K562细胞的增殖抑制作用明显强于单药组(P<0.01)。联合用药作用48 h K562细胞半数抑制浓度(IC50)时,Mat浓度约为0.4 g/L。当Mat达到0.8 g/L以后联合用药组和单药组的细胞抑制率差别趋于减小(图 1),联合作用48 h后细胞增殖抑制率大于80%(图 1),相比较低浓度组表现为细胞数过少,且药物的毒副作用与剂量相关,联合用药的目的之一是减少单药的毒性。因此,以下将使用0.4 g/L作为Mat单药组,10 μmol/L作为LY294002单药组,0.4 g/L的Mat+10 μmol/L LY294002作为联合用药组,作用48 h进行实验。
1.

Mat和联合LY294002对K562细胞增殖抑制作用
EInhibitory effect of matrine alone and in combination with LY294002 on proliferation of K562 cells. **P < 0.01, compared with the single drug group, the combined medication group of the same time of action.
2.2. Mat与LY294002单用和联用对细胞形态的影响
K562细胞胞体多呈圆形、可见瘤状突起、胞核大而多为类圆形、含数个核仁(图 2B1和2C1)。当加入LY294002和/或苦参碱作用48 h后,可见干预组细胞生长明显变慢,且有细胞出现形态变化。0.4 g/L苦参碱作用48 h后,可见胞体变小,胞核变小,染色质密集(图 2A2、B2、C2)的凋亡表现;可见胞质中出现数个明显的空泡(图 2A2、B2),吖啶橙(AO)染色胞质中可见呈强桔黄色球状荧光(图 2C2),似为自噬小体;10 μM/L LY294002用48 h后,可见胞体变小、胞核变小、染色质密集(图 2A3、B3、C3),吖啶橙染色胞质中可见呈弱桔黄色球状荧光(图 2C3);LY294002与苦参碱联用K562细胞形态变化更加明显(图 2A4、B4、C4),且吖啶橙染色可见胞核呈致密浓染的黄绿色荧光,在少数细胞胞质中也可见到桔黄色球状荧光。
2.
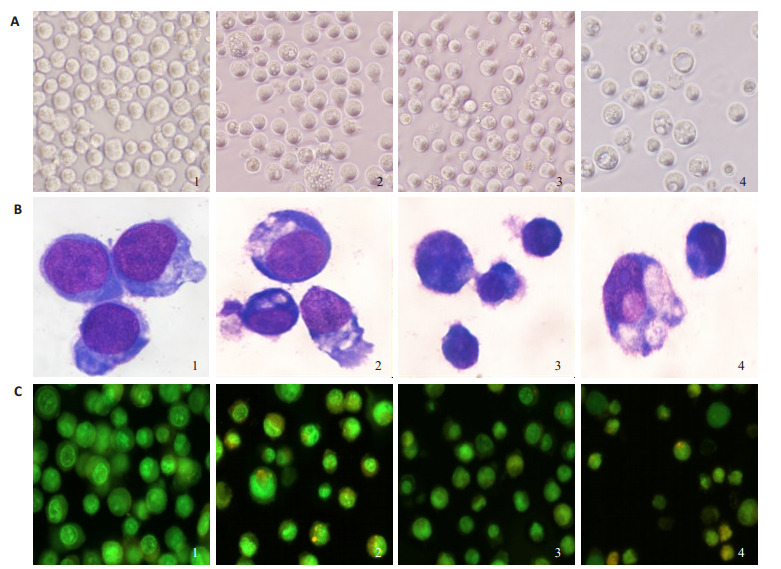
Mat联用LY294002对K562细胞形态的影响
Morphological changes of K562 cells treated with matrine alone or in combination with LY294002. A: Cell morphology observed under inverted microscope (Original magnification: × 200). B: Cell morphology observed with Wright staining under optical microscope (× 1000). C: Cell morphology observed with acridine orange (AO) staining under fluorescence microscope (×200). 1: Control group; 2: Matrine group; 3: LY294002 group; 4: Matrine +LY294002 group.
2.3. 苦参碱与LY294002单用和联用对细胞
凋亡的影响Mat、LY294002、两者联合作用于K562细胞48h后,早期凋亡与晚期凋亡的细胞总和分别为(13.78±1.64)%、(18.85±1.56)%、(42.50±2.63)%,与对照组(2.10±0.40)%相比,差异具有统计学意义(P<0.01,图 3)。
3.

Mat联合LY294002对K562细胞凋亡的影响
Effects of matrine combined with LY294002 on apoptosis of K562 cells detected by flow cytometry. **P < 0.01, ***P < 0.0001. LY: LY294002
2.4. Mat与LY294002单用和联用后K562细胞周期的影响
流式细胞术检测结果表明(表 1,图 4),药物处理K562细胞48 h后,Mat组和LY294002组G0/G1期细胞较对照组增加,差异有统计学意义(P<0.05,表 1);联合用药组G0/G1期细胞显著增加,S期显著减少,较对照组及单药组差异均有统计学意义(P<0.01,表 1)。
1.
流式细胞仪检测药物作用48 h后K562细胞的周期变化
Cell cycle changes detected by flow cytometry after treatment for 48 h with matrine alone or in combination with LY294002 (Mena±SD, n=3)
| Group | Cell cycle distribution (%) | ||
| G0/G1 | S | G2/M | |
| *P<0.05 vs control group; △ P<0.01 vs Mat group; #P<0.01 vs LY294002 group. | |||
| Control | 29.17±1.15 | 55.23±1.33 | 15.51±1.27 |
| Matrine | 38.11±0.86* | 50.85±1.10 | 10.97±1.12* |
| LY294002 | 42.94±1.64*△ | 44.75±2.32* | 12.19±1.78* |
| Mat+LY294002 | 57.23±1.44*△# | 33.89±2.09*△# | 8.37±1.05* |
4.
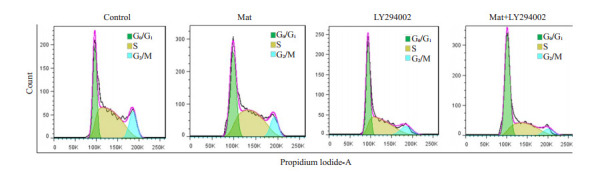
流式细胞仪检测药物作用48 h后K562细胞的周期分布
Cell cycle distributions detected by flow cytometry after treatment for 48 h with matrine alone or in combination with LY294002.
2.5. Mat与LY294002单用和联用对K562细胞周期和细胞凋亡蛋白表达的影响
Western blot法对凋亡相关蛋白的表达量进行检测结果显示(图 5),各药物处理组的G0/G1期周期蛋白CyclinD1和凋亡相关蛋白Bcl-2蛋白表达量减少,凋亡相关蛋白Caspase-9的表达量增加,与对照组相比差异有统计学意义(P<0.05),且Mat(0.4 g/L)和LY294002(10 μmol/L)联合组蛋白的表达变化与单药组相比差异也有统计学意义,即两者共同作用下蛋白的表达变化更明显降(P<0.01)。
5.

药物作用48 h后对K562细胞周期蛋白和细胞凋亡蛋白表达水平
Expression levels of cyclins and apoptotic proteins in K562 cells after treatment for 48 h with matrine alone or in combination with LY294002 detected by Western blotting. *P < 0.05, **P < 0.01, ***P < 0.001 vs control group; #P < 0.01, ##P < 0.001 vs Mat group and LY294002 group. LY: LY294002.
2.6. Mat与LY294002单用和联用对K562细胞PI3K/Akt通路相关蛋白表达的影响
Western blot实验结果表明(图 6),K562细胞内AKT蛋白的表达各组差异不明显。单独应用Mat(0.4 g/L)或LY294002(10 μmol /L),p-mTOR、p-PI3K、p-Akt蛋白的表达与对照组相比有下降,而联合用药组与对照组和单药组比较,下降均有显著性差异(P<0.01),p-Akt下调更为明显(P<0.001)。
6.

药物作用48 h后对K562细胞PI3K/AKT通路相关蛋白表达水平的影响
Expression level of proteins of PI3K/AKT pathway in K562 cells after treatment for 48 h with matrine alone or in combination with LY294002 detected by Western blotting. *P < 0.05, **P < 0.01, ***P < 0.001 vs control group; #P < 0.01, ##P < 0.001 vs Mat group and LY294002 group.
3. 讨论
随着人们对AML发病机制认识提高和对其细胞遗传学、分子异常及治疗靶点的识别,推动了靶向治疗、药物有效组合治疗、个体化治疗和新药研发等。目前,从中草药中提取的活性化合物用于治疗肿瘤受到广泛的关注与研究[10, 11]。中药苦参碱(Mat)属四环喹嗪啶类生物碱,分子式为C15H24N2O,具有抗病毒、抗肿瘤、免疫调节等多种生理及药理学活性[12]。多项研究表明[10],苦参碱对白血病、肝癌[13, 14]、肺癌[15]、乳腺癌[16]宫颈癌[17][18]等多种肿瘤细胞均有抗癌活性,能够通过“多靶点-多途径”发挥抗肿瘤作用[19]。Mat浓度不同生物学侧重不同,低浓度(<0.1 g/L)主要促进白血病细胞分化和自噬性保护;中浓度(>0.1 g/L)主要阻滞细胞周期,促进细胞自噬性死亡和凋亡,从而抑制细胞生长;高深度(>2.0 g/L)主要使细胞坏死[20]。本研究以K562细胞为研究对象,发现Mat(0.2 g/L~1.6 g/L)可以抑制白血病细胞株K562细胞增殖,且作用呈剂量和时间依赖性关系。在单药和联合用药中,可见细胞凋亡与自噬共存于同一个细胞内。最近研究证实[10],自噬和凋亡两条通路可受某些共同因子和成分的作用,两者间存在着交叉反应,也可以互相重叠地发挥功能;在某些条件下,自噬为凋亡所需,此时自噬通常先于凋亡,进而启动凋亡,促进细胞死亡。我们也发现以0.4 g/LMat处理K562细胞形态学观察可见自噬与凋亡现象共存,并可见到两种现象共存于同一细胞,我们推测可能存在细胞自噬与凋亡机制串话促进了细胞凋亡。
Mat虽然生物活性多样,但体内达到抗肿瘤治疗浓度有毒副作用,所以临床一般不作为单剂量应用于肿瘤治疗,多与细胞毒性化疗药物联合使用,提高治疗效果[22, 23]。虽然细胞毒性化疗药物在AML治疗中占有重要地位,并且联合Mat已有研究[23],但是有部分AML对细胞毒性化疗药物有原发性和继发性耐药,也有少数患者由于身体原因不能耐受细胞毒性化疗药物,而且AML经治疗缓解后几乎皆存在微小残留白血病细胞的问题,对这一部分患者,Mat联合分子靶向药物治疗,具有重要的治疗价值。急性白血病分子靶向治疗是白血病治疗的热点,Mat是否与分子靶向药物协同作用,需要实验验证。AML发生是一个多步骤的过程,它通过改变癌蛋白的表达和转录因子,激活细胞增殖和侵袭,抑制凋亡和分化等过程,能够靶向其中一个或多个过程的药物应该是癌症防治的理想药物[24]。PI3K/Akt是备受关注的信号通路,在包括急慢性白血病、淋巴瘤和多发性骨髓瘤在内的多种恶性肿瘤组织中被异常激活[25]。PI3K能磷酸化下游底物二磷酸磷脂酰肌醇(PIP2)成为三磷酸磷脂酰肌醇(PIP3),PIP3能促进3-磷脂酰肌醇依赖性蛋白激酶(PDK1)磷酸化Akt,激活的Akt能激活下游靶基因,如及mTOR、Bcl-2、Cyclin D1等,进而参与控制细胞的增殖、凋亡、自噬、迁移及血管生成等生物学行为。LY294002是PI3K的抑制剂,能够透过细胞膜,可通过和ATP竞争结合PI3K催化结构域,降低分子Akt的磷酸化水平,抑制肿瘤细胞增殖[9]。以往研究已证实[26],10 μmol/L LY294002单药能够促进恶性肿瘤肺癌细胞A549的自噬和凋亡,本研究形态学显示LY294002作用于髓性白血病细胞K562亦有促进自噬和凋亡现象,流式细胞仪检测也证实能促细胞凋亡阻细胞周期,发挥抑制K562增殖作用。
临床研究讲明,PI3K/Akt通路与其他信号通路存在串扰,Akt也可以通过负反馈机制被重新激活,单独使用通路抑制剂只有适度的抗白血病作用[27]。本研究设想通路抑制剂LY294002与具有多种生物活性的中药Mat联合应用,或许能起到强化治疗和减少药物用量的作用。本研究根据相关文献报道[28]及预实验采用10 μmol/L LY294002和各浓度Mat联用作用于K562 24 h和48 h后,绘制增殖抑制曲线,显示联合用药细胞的增殖抑制率明显低于单药组,药物联合显示出协同效应。形态学观察发现,两单药组细胞数目减少,可见细胞胞体变小,胞核固缩,药物联合组表现更为明显。FCM检测细胞周期和凋亡以解释这些结果的可能原因,结果表明,两种单药组细胞凋亡率和G0/G1期阻滞细胞的比例均高于对照组,联合组细胞凋亡率和G0/G1期阻滞率高于单药组,表明苦参碱和LY294002通过促进细胞凋亡和阻滞细胞周期进程协同抑制K562细胞的生长。
进一步研究了苦参碱和LY294002联合作用增强抑制K562细胞增殖的分子机制。PI3K、Akt这两个分子是PI3K/Akt信号通路最关键的两个分子,它们相互作用的过程是这一通路的核心步骤[8]。PI3K可以被多个上游通路激活,PI3K相关激酶家族成员在Akt丝氨酸473位点将其磷酸化,活化的Akt通过磷酸化抑制或增强靶蛋白的活性,从而调节多种类型的下游通路[29]。活化的Akt通过磷酸化TSC2切入mTOR通路,阻止TSC1/2对小G蛋白Rheb的负调控,从而间接激活mTOR复合物1 (mTORC1)来促进细胞生长,抑制细胞自噬和凋亡。活化的Akt可以通过下游多种途径发挥抑制凋亡的作用,活化的Akt磷酸化Bcl-2家族成员BAD,使BAD与14-3-3结合而阻止BAD与Bcl-2/Bcl-XL结合,从而保持Bcl-2/Bcl-XL的抑制凋亡作用[30]。Bcl-2是目前研究最广泛的抑制凋亡蛋白之一,能与促凋亡蛋白BAX形成异源二聚体,阻断凋亡信号,促进细胞的存活和增殖[31]。活化的Akt还能抑制蛋白水解酶Caspase-9的活性从而阻止凋亡级联反应的启动,发挥抑制凋亡作用。Akt的激活还可以使GSK-3β磷酸化而失活,从而激活c-myc和Cyclin D1等信号分子,促进细胞的生长与存活。Cyclin D1为调控细胞由G1期进入S期的关键蛋白,其表达水平降低,细胞生长缓慢[32]。LY294002是PI3K的抑制剂,间接抑制Akt活化,Mat能通过多条通路抑制Akt活性和抑制细胞增殖促进凋亡。本研究发现,Mat和LY294002组单药组PI3K和Akt的磷酸化水平下调,同时mTOR的磷酸化修饰水平下调,Bcl-2和CyclinD1的表达均下调,Caspase-9的表达均上调。苦参碱联合LY294002的治疗效果较单一药物治疗有显著性差异(P<0.01),特别是p-Akt(P<0.001),两者联用起到了生物学上的协同和增强效应。
综上所述,在体外联合运用Mat和LY294002能显著抑制K562细胞系增殖和促进K562细胞周期阻滞及细胞凋亡。其可能原因之一是加强了抑制PI3K/Akt通路中Akt的活化,从而抑制下游事件的发生,导致K562细胞的增殖抑制和凋亡增加。因此有多种抗肿瘤活性的中药与靶点信号通路抑制剂联合应用理论上应能成为AML患者治疗的新方向,本实验也为临床中西医联合用药提供实验基础。
Funding Statement
国家大学生创新训练项目(202010367049);安徽省教育厅重点科学项目(KJ2021A0691)
References
- 1.Pelcovits A, Niroula R. Acute myeloid leukemia: a review. R I Med J: 2013. 2020;103(3):38–40. [Pelcovits A, Niroula R. Acute myeloid leukemia: a review[J]. R I Med J: 2013, 2020, 103(3): 38-40.] [PubMed] [Google Scholar]
- 2.Yang X, Wang JX. Precision therapy for acute myeloid leukemia. J Hematol Oncol. 2018;11(1):3. doi: 10.1186/s13045-017-0543-7. [Yang X, Wang JX. Precision therapy for acute myeloid leukemia[J]. J Hematol Oncol, 2018, 11(1): 3.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hao YM, Zhang N, Wei NN, et al. Matrine induces apoptosis in acute myeloid leukemia cells by inhibiting the PI3K/Akt/mTOR signaling pathway. Oncol Lett. 2019;18(3):2891–6. doi: 10.3892/ol.2019.10649. [Hao YM, Zhang N, Wei NN, et al. Matrine induces apoptosis in acute myeloid leukemia cells by inhibiting the PI3K/Akt/mTOR signaling pathway[J]. Oncol Lett, 2019, 18(3): 2891-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai M, Cai Z, Chen N, et al. Matrine suppresses stemness of hepatocellular carcinoma cells by regulating β-catenin signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39(10):1239–45. doi: 10.12122/j.issn.1673-4254.2019.10.17. [Dai M, Cai Z, Chen N, et al. Matrine suppresses stemness of hepatocellular carcinoma cells by regulating β-catenin signaling pathway[J]. Nan Fang Yi Ke Da Xue Xue Bao, 2019, 39(10): 1239-45.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li QL, Zhang SY, Wang MY, et al. Downregulated miR-21 mediates matrine-induced apoptosis via the PTEN/Akt signaling pathway in FTC-133 human follicular thyroid cancer cells. Oncol Lett. 2019;18(4):3553–60. doi: 10.3892/ol.2019.10693. [Li QL, Zhang SY, Wang MY, et al. Downregulated miR-21 mediates matrine-induced apoptosis via the PTEN/Akt signaling pathway in FTC-133 human follicular thyroid cancer cells[J]. Oncol Lett, 2019, 18(4): 3553-60.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.黄 思玲, 周 喜汉, 杨 焕珍, et al. 苦参碱在肝癌治疗中的生物学和逆转多药耐药作用机制研究进展. 山东医药. 2021;61(15):107–10. doi: 10.3969/j.issn.1002-266X.2021.15.027. [黄思玲, 周喜汉, 杨焕珍, 等. 苦参碱在肝癌治疗中的生物学和逆转多药耐药作用机制研究进展[J]. 山东医药, 2021, 61(15): 107-10.] [DOI] [Google Scholar]
- 7.Song MQ, Bode AM, Dong ZG, et al. AKT as a therapeutic target for cancer. Cancer Res. 2019;79(6):1019–31. doi: 10.1158/0008-5472.CAN-18-2738. [Song MQ, Bode AM, Dong ZG, et al. AKT as a therapeutic target for cancer[J]. Cancer Res, 2019, 79(6): 1019-31.] [DOI] [PubMed] [Google Scholar]
- 8.Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside. Semin Cancer Biol. 2019;59:125–32. doi: 10.1016/j.semcancer.2019.07.009. [Alzahrani AS. PI3K/Akt/mTOR inhibitors in cancer: at the bench and bedside[J]. Semin Cancer Biol, 2019, 59: 125-32.] [DOI] [PubMed] [Google Scholar]
- 9.Mayer IA, Arteaga CL. The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med. 2016;67:11–28. doi: 10.1146/annurev-med-062913-051343. [Mayer IA, Arteaga CL. The PI3K/AKT pathway as a target for cancer treatment[J]. Annu Rev Med, 2016, 67: 11-28.] [DOI] [PubMed] [Google Scholar]
- 10.王 梦涵, 司 佩茹, 莫 菲, et al. 中药抗肿瘤的研究进展. 西北药学杂志. 2021;36(4):687–92. doi: 10.3969/j.issn.1004-2407.2021.04.034. [王梦涵, 司佩茹, 莫菲, 等. 中药抗肿瘤的研究进展[J]. 西北药学杂志, 2021, 36(4): 687-92.] [DOI] [Google Scholar]
- 11.王 卫东, 何 小花, 杨 庆敏, et al. 治疗急性髓系白血病的中药活性成分研究进展. 时珍国医国药. 2021;32(6):1444–9. doi: 10.3969/j.issn.1008-0805.2021.06.46. [王卫东, 何小花, 杨庆敏, 等. 治疗急性髓系白血病的中药活性成分研究进展[J]. 时珍国医国药, 2021, 32(6): 1444-9.] [DOI] [Google Scholar]
- 12.Li X, Tang ZW, Wen L, et al. Matrine: a review of its pharmacology, pharmacokinetics, toxicity, clinical application and preparation researches. J Ethnopharmacol. 2021;269:113682. doi: 10.1016/j.jep.2020.113682. [Li X, Tang ZW, Wen L, et al. Matrine: a review of its pharmacology, pharmacokinetics, toxicity, clinical application and preparation researches[J]. J Ethnopharmacol, 2021, 269: 113682.] [DOI] [PubMed] [Google Scholar]
- 13.Zhang PP, Zhang F, Zhu K, et al. Matrine exerted an anti-tumor effect on acute myeloid leukemia via the lncRNA LINC01116/miR-592-mediated JAK/STAT pathway inactivation. Neoplasma. 2022;69(1):123–35. doi: 10.4149/neo_210802N1083. [Zhang PP, Zhang F, Zhu K, et al. Matrine exerted an anti-tumor effect on acute myeloid leukemia via the lncRNA LINC01116/miR-592-mediated JAK/STAT pathway inactivation[J]. Neoplasma, 2022, 69 (1): 123-35.] [DOI] [PubMed] [Google Scholar]
- 14.Tetik Vardarl\u0131 A, Düzgün Z, Erdem C, et al. Matrine induced G0/G1 arrest and apoptosis in human acute T-cell lymphoblastic leukemia (T-ALL) cells. Bosn J Basic Med Sci. 2018;18(2):141–9. doi: 10.17305/bjbms.2017.2457. [Tetik Vardarl\u0131 A, Düzgün Z, Erdem C, et al. Matrine induced G0/G1 arrest and apoptosis in human acute T-cell lymphoblastic leukemia (T-ALL) cells[J]. Bosn J Basic Med Sci, 2018, 18(2): 141-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu GY, Cao C, Deng ZH, et al. Effects of matrine in combination with cisplatin on liver cancer. Oncol Lett. 2021;21(1):66. doi: 10.3892/ol.2020.12327. [Hu GY, Cao C, Deng ZH, et al. Effects of matrine in combination with cisplatin on liver cancer[J]. Oncol Lett, 2021, 21(1): 66.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu HL, Lu Q, Lu Q, et al. Matrine regulates proliferation, apoptosis, cell cycle, migration, and invasion of non-small cell lung cancer cells through the circFUT8/miR-944/YES1 axis. Cancer Manag Res. 2021;13:3429–42. doi: 10.2147/CMAR.S290966. [Zhu HL, Lu Q, Lu Q, et al. Matrine regulates proliferation, apoptosis, cell cycle, migration, and invasion of non-small cell lung cancer cells through the circFUT8/miR-944/YES1 axis[J]. Cancer Manag Res, 2021, 13: 3429-42.] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Du JK, Li JW, Song DB, et al. Matrine exerts anti-breast cancer activity by mediating apoptosis and protective autophagy via the AKT/mTOR pathway in MCF-7 cells. Mol Med Rep. 2020;22(5):3659–66. doi: 10.3892/mmr.2020.11449. [Du JK, Li JW, Song DB, et al. Matrine exerts anti-breast cancer activity by mediating apoptosis and protective autophagy via the AKT/mTOR pathway in MCF-7 cells[J]. Mol Med Rep, 2020, 22 (5): 3659-66.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.郑 荣芳, 刘 伟, 张 芸中, et al. 苦参碱调控Wnt/β-catenin途径抑制宫颈癌细胞系Caski细胞侵袭迁移. https://www.cnki.com.cn/Article/CJFDTOTAL-NJYK202101007.htm. 南京医科大学学报: 自然科学版. 2021;41(1):35–40. [郑荣芳, 刘伟, 张芸中, 等. 苦参碱调控Wnt/β-catenin途径抑制宫颈癌细胞系Caski细胞侵袭迁移[J]. 南京医科大学学报: 自然科学版, 2021, 41(1): 35-40.] [Google Scholar]
- 19.Lin YD, He FM, Wu L, et al. Matrine exerts pharmacological effects through multiple signaling pathways: a comprehensive review. Drug Des Devel Ther. 2022;16:533–69. doi: 10.2147/DDDT.S349678. [Lin YD, He FM, Wu L, et al. Matrine exerts pharmacological effects through multiple signaling pathways: a comprehensive review[J]. Drug Des Devel Ther, 2022, 16: 533-69.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.张 明发, 沈 雅琴. 苦参碱类生物碱抗人红白血病K562细胞药理作用的研究进展. https://www.cnki.com.cn/Article/CJFDTOTAL-YWPJ201901036.htm. 药物评价研究. 2019;42(1):223–9. [张明发, 沈雅琴. 苦参碱类生物碱抗人红白血病K562细胞药理作用的研究进展[J]. 药物评价研究, 2019, 42(1): 223-9.] [Google Scholar]
- 21.Nikoletopoulou V, Markaki M, Palikaras K, et al. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833(12):3448–59. doi: 10.1016/j.bbamcr.2013.06.001. [Nikoletopoulou V, Markaki M, Palikaras K, et al. Crosstalk between apoptosis, necrosis and autophagy[J]. Biochim Biophys Acta, 2013, 1833(12): 3448-59.] [DOI] [PubMed] [Google Scholar]
- 22.Liao XZ, Tao LT, Liu JH, et al. Matrine combined with cisplatin synergistically inhibited urothelial bladder cancer cells via down-regulating VEGF/PI3K/Akt signaling pathway. Cancer Cell Int. 2017;17:124. doi: 10.1186/s12935-017-0495-6. [Liao XZ, Tao LT, Liu JH, et al. Matrine combined with cisplatin synergistically inhibited urothelial bladder cancer cells via down-regulating VEGF/PI3K/Akt signaling pathway[J]. Cancer Cell Int, 2017, 17: 124.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.晁 荣, 胡 晓燕, 朱 生东, et al. 苦参碱联合化疗药物对人急性髓系白血病HL-60细胞增殖及侵袭力的影响. 世界中医药. 2019;14(11):2910–3. doi: 10.3969/j.issn.1673-7202.2019.11.019. [晁荣, 胡晓燕, 朱生东, 等. 苦参碱联合化疗药物对人急性髓系白血病HL-60细胞增殖及侵袭力的影响[J]. 世界中医药, 2019, 14(11): 2910-3.] [DOI] [Google Scholar]
- 24.Molica M, Breccia M, Foa R, et al. Maintenance therapy in AML: the past, the present and the future. Am J Hematol. 2019;94(11):1254–65. doi: 10.1002/ajh.25620. [Molica M, Breccia M, Foa R, et al. Maintenance therapy in AML: the past, the present and the future[J]. Am J Hematol, 2019, 94(11): 1254-65.] [DOI] [PubMed] [Google Scholar]
- 25.Nepstad I, Hatfield KJ, Grønningsæter IS, et al. The PI3K-Akt-mTOR signaling pathway in human acute myeloid leukemia (AML) cells. Int J Mol Sci. 2020;21(8):2907. doi: 10.3390/ijms21082907. [Nepstad I, Hatfield KJ, Grønningsæter IS, et al. The PI3K-Akt-mTOR signaling pathway in human acute myeloid leukemia (AML) cells[J]. Int J Mol Sci, 2020, 21(8): 2907.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao Y, Yin H, Zhu C, et al. Matrine inhibits proliferation and promotes autophagy and apoptosis in non-small cell lung cancer cells by deactivating PI3K/AKT/mTOR pathway. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39(7):760–5. doi: 10.12122/j.issn.1673-4254.2019.07.02. [Hao Y, Yin H, Zhu C, et al. Matrine inhibits proliferation and promotes autophagy and apoptosis in non-small cell lung cancer cells by deactivating PI3K/AKT/mTOR pathway[J]. Nan Fang Yi Ke Da Xue Xue Bao, 2019, 39(7): 760-5.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ersahin T, Tuncbag N, Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 2015;11(7):1946–54. doi: 10.1039/C5MB00101C. [Ersahin T, Tuncbag N, Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway[J]. Mol Biosyst, 2015, 11(7): 1946-54.] [DOI] [PubMed] [Google Scholar]
- 28.耿 英华, 武 文娟, 李 骏, et al. LY294002增强K562细胞对柔红霉素敏感性的研究. 中国实验血液学杂志. 2020;28(1):110–8. doi: 10.19746/j.cnki.issn1009-2137.2020.01.019. [耿英华, 武文娟, 李骏, 等. LY294002增强K562细胞对柔红霉素敏感性的研究[J]. 中国实验血液学杂志, 2020, 28(1): 110-8.] [DOI] [PubMed] [Google Scholar]
- 29.Darici S, Alkhaldi H, Horne G, et al. Targeting PI3K/Akt/mTOR in AML: rationale and clinical evidence. J Clin Med. 2020;9(9):2934. doi: 10.3390/jcm9092934. [Darici S, Alkhaldi H, Horne G, et al. Targeting PI3K/Akt/mTOR in AML: rationale and clinical evidence[J]. J Clin Med, 2020, 9(9): 2934.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, Yao SK. JNK-bcl-2/bcl-xL-bax/bak pathway mediates the crosstalk between matrine-induced autophagy and apoptosis via interplay with beclin 1. Int J Mol Sci. 2015;16(10):25744–58. doi: 10.3390/ijms161025744. [Yang J, Yao SK. JNK-bcl-2/bcl-xL-bax/bak pathway mediates the crosstalk between matrine-induced autophagy and apoptosis via interplay with beclin 1[J]. Int J Mol Sci, 2015, 16(10): 25744-58.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Aguanno S, del Bufalo D. Inhibition of anti-apoptotic bcl-2 proteins in preclinical and clinical studies: current overview in cancer. Cells. 2020;9(5):1287. doi: 10.3390/cells9051287. [D'Aguanno S, del Bufalo D. Inhibition of anti-apoptotic bcl-2 proteins in preclinical and clinical studies: current overview in cancer[J]. Cells, 2020, 9(5): 1287.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren CX, Wu CL, Yang CQ, et al. Vitamin C affects G0/G1 cell cycle and autophagy by downregulating of cyclin D1 in gastric carcinoma cells. Biosci Biotechnol Biochem. 2021;85(3):553–61. doi: 10.1093/bbb/zbaa040. [Ren CX, Wu CL, Yang CQ, et al. Vitamin C affects G0/G1 cell cycle and autophagy by downregulating of cyclin D1 in gastric carcinoma cells[J]. Biosci Biotechnol Biochem, 2021, 85(3): 553-61.] [DOI] [PubMed] [Google Scholar]


