Abstract
目的
研究miR-607异常表达在肝细胞癌(HCC)中的临床意义及对肝癌细胞生长转移的影响。
方法
荧光定量PCR检测45对HCC及癌旁组织中miR-607表达,分析miR-607表达与患者临床病理特征间的相关性。通过转染miR-607 mimics提高HCC细胞(Huh-7和HCCLM3)内miR-607的表达水平。采用CCK-8、流式细胞仪、划痕愈合实验及transwell小室模型分别检测过表达miR-607对HCC细胞增殖、凋亡、迁移和侵袭的影响。双荧光素酶报告基因系统检测miR-607是否与潜在靶点TRPC5的mRNA 3'-非翻译区直接结合。Western blot检测过表达miR-607对HCC细胞内TRPC5、CCND1、MMP2表达及Akt磷酸化的影响。
结果
MiR-607在HCC组织及HCC细胞中的表达水平均降低(P < 0.05);miR-607低表达与肿瘤直径>5 cm、血管侵犯及较晚TNM分期(Ⅲ+Ⅳ)密切相关(P < 0.05)。过表达miR-607抑制HCC细胞增殖、迁移、侵袭并诱导细胞凋亡(P < 0.05)。双荧光素酶报告基因系统证实miR-607与TRPC5 mRNA 3'-非翻译区直接结合(P < 0.05)。过表达miR-607抑制HCC细胞内TRPC5、CCND1及MMP2表达并下调Akt的磷酸化水平(P < 0.05)。
结论
miR-607低表达与肝细胞癌恶性临床特征密切相关,miR-607可能通过下调TRPC5表达进而抑制Akt通路激活来发挥抗HCC生长转移作用。
Keywords: miR-607, 肝细胞癌, TRPC5, Akt通路, 转移
Abstract
Objective
To investigate the clinical implications of abnormal expression of miR-607 in hepatocellular carcinoma (HCC) and its influence on HCC cell proliferation and migration.
Methods
The expression of miR-607 in 45 pairs of HCC and adjacent tissues were detected with real-time PCR, and the correlation between miR-607 expression and clinicopathological features of the patients was analyzed. The effects of transfection with miR-607 mimics on proliferation, apoptosis, migration and invasion of two HCC cell lines (Huh-7 and HCCLM3) were evaluated using CCK-8 assay, flow cytometry, wound-healing assay and Transwell assay. A dual-luciferase reporter system was used to detect the direct binding between miR-607 and 3'-UTR of TRPC5 mRNA. Western blotting was used to measure the expressions of TRPC5, CCND1, MMP2 and phosphorylated Akt in the HCC cells.
Results
The expression of miR-607 was significantly decreased in HCC tissues (P=0.029) and HCC cell lines (P < 0.05). In HCC patients, a low expression of miR-607 was associated with a larger tumor size (>5 cm, P=0.031), vascular invasion (P=0.027) and advanced TNM stages (Ⅲ + Ⅳ, P=0.015). In the two HCC cell line, overexpression of miR-607 significantly inhibited cell proliferation, migration, and invasion and enhanced cell apoptosis (P < 0.05). The results of dualluciferase reporter assay confirmed that TRPC5 was a direct target of miR- 607 in HCC cells. Overexpression of miR-607 significantly inhibited the expressions of TRPC5, CCND1, and MMP2 and suppressed Akt phosphorylation in HCC cells (P < 0.05).
Conclusion
A low expression of miR-607 in HCC is associated with poor clinicopathological phenotypes of HCC. Overexpression of miR-607 inhibits HCC growth and metastasis possibly by down- regulating TRPC5, which causes Akt signaling inactivation.
Keywords: miR-607, hepatocellular carcinoma, TRPC5, Akt signal pathway, metastasis
肝细胞癌(HCC)具有生长速度快、易发生脉管侵犯和肝内外转移的特点,目前已成为我国致死率最高的恶性肿瘤[1]。MicroRNA(miRNA, miR)是一种长度小于22个碱基的单链非编码RNA,miRNAs的异常表达在HCC发生发展中发挥关键作用[2]。MiR-607位于人类10号染色体,最早在抗埃博拉病毒相关miRNA中[3]被发现,随后miR-607被发现与神经精神疾病[4, 5]及多种癌症[6, 7]的进展密切相关。胰腺癌中,血清miR-607低表达患者出现淋巴结转移、肝脏转移和周围神经侵犯比例更高、预后更差[8],体外过表达miR-607能够抑制胰腺癌细胞增殖和迁移[9],提示miR-607在胰腺癌中具备液体活检标志物价值并能发挥抗癌作用。目前,仅通过测序发现HCC患者血清外泌体miR-607表达水平降低[10],但HCC组织中miR-607的表达和具体生物学功能尚缺乏研究。因此,本研究拟通过探讨miR-607在HCC中异常表达的临床意义和调节HCC生长转移的功能机制,以期明确miR-607在HCC疾病进展中的作用。
1. 资料和方法
1.1. 资料
收集2019年1月1日~2021年1月1日于安康市人民医院普通外科行手术切除的HCC组织及对应癌旁组织标本45例,包括男性29例,女性16例,年龄50~77岁,中位年龄63岁。所有患者术前均未接受过靶向、介入、放化疗等辅助治疗并且均签署知情同意书,实验方案由西安交通大学第一附属医院伦理委员会审核批准(批号:2021伦审科字第347号)。MicroRNA模拟物(mimics,miR-607及阴性对照)、microRNA PCR扩增引物试剂盒(miR-607及U6)、野生型及突变型TRPC5 mRNA 3'-非翻译区(3'-UTR)双荧光素酶报告基因系统由广州锐博生物科技有限公司提供。LO2、HepG2、Hep3B、Huh-7及HCCLM3细胞由西安交通大学第一附属医院肝胆外科实验室保存。DMEM液体培养基及胎牛血清(Gibco)。RNA提取试剂Trizol、转染试剂LipofectamineTM 3000及RT-PCR试剂盒(Invitrogen)。SYBR Green实时荧光定量PCR试剂盒(BIO-RAD)。TRPC5、Akt、Ser473磷酸化Akt、CCND1、MMP2及GAPDH一抗(proteintech)。CCK-8试剂盒、流式细胞仪凋亡检测试剂盒(上海生工生物有限公司)。TRPC5引物(上游5'-GTTCTAGGTTTCATTTGGGGTGAGA- 3';下游5'-ACATTTCCCATTCCTCCCTTGG-3)、' GA PDH引物(上游5'-CCAGGGCTGCTTTTAACTCT-3';下游5'-GGACTCCACGACGTACTCA-3)' 购自上海生工生物有限公司。Transwell小室(CORNING),人工基质胶(B&D)。
1.2. 方法
1.2.1. 细胞培养与传代
正常人永生化肝细胞LO2及肝癌细胞HepG2、Hep3B、Huh-7及HCCLM3细胞株培养于含10%胎牛血清的DMEM培养基中,置于细胞培养箱中,在37 ℃、5% CO2及饱和湿度环境下进行培养传代。稳定传代2~3代后,取对数生长期细胞进行后续实验。
1.2.2. qRT-PCR检测miR-607及TRPC5 mRNA表达
使用Trizol试剂按操作指南提取临床标本组织或体外培养细胞中的总RNA。定量后,每份组织样品或细胞样品取等量RNA配制20 μL的逆转录反应体系合成cDNA。cDNA稀释10倍后取5 μL配制实时荧光定量PCR体系,按如下条件进行PCR反应:95 ℃预变性30 s,1循环;95 ℃变性5 s,60 ℃退火延伸30 s,40循环。以U6 RNA为miR-607内参,以GAPDH为TRPC5的内参,采用2-△△Ct法计算miR-607及TRPC5 mRNA的相对表达量。
1.2.3. miR-607 mimics细胞转染
HCC细胞以过夜培养达50%~70%之密度接种于6孔板中。miR-607 mimics转染分组:阴性对照组(miR-ctrl)加入100 pmoL/孔的阴性对照mimics及5 μL转染试剂,miR-607组(miR-607)加入100 pmoL/孔的miR-607 mimics及5 μL转染试剂;以无血清DMEM培养基调整终体积为2 mL/孔。按后续实验要求确认转染时间。
1.2.4. CCK-8检测细胞增殖
分别收集转染24、48 h及72 h后的HCC细胞,制备密度为1×105/mL的无血清DMEM基细胞悬液,以200 μL/孔接种于96孔板。加入10 μL/孔CCK-8 solution试剂后放置于细胞培养箱内避光培养2 h。于酶标仪上测定各孔450 nm的吸光度A450 nm。每个样本独立重复实验3次。
1.2.5. 流式细胞仪检测凋亡
取转染72 h后的HCC细胞,用1×Binding Buffer制备密度为1×106/mL的细胞悬液,每管加入500 μL细胞悬液、5 μLAnnexin V-FITC和10 μLPropidium Iodide。混匀后,室温下避光反应10 min,进行流式细胞仪检测。每组样本独立重复实验3次。
1.2.6. 划痕愈合实验检测细胞迁移
取转染24 h后的HCC细胞,以过夜培养达80%~90%之密度接种于6孔板中。过夜培养后用无菌200 μL移液器枪头沿培养孔中线划过制作初始划痕。以无血清DMEM培养基清洗培养孔以洗净悬浮细胞。每孔再次加入无血清DMEM培养基2 mL,将6孔板置于培养箱中继续培养48 h。在显微镜下拍照记录划痕剩余距离,计算细胞迁移情况。
1.2.7. Transwell小室模型检测细胞侵袭
用无血清DMEM培养基按1:8比例稀释50 mg/L的基质胶100 μL/孔覆盖transwell小室上室面,于37 ℃细胞培养箱内风干1 h制成侵袭小室模型。取转染48 h后的HCC细胞用无血清DMEM培养基重悬,以2×105/mL的密度加入100 μL/孔的细胞悬液。24孔细胞培养板内加入750 μL/孔含10%胎牛血清的DMEM培养基,建立细胞侵袭检测模型。培养完成后,PBS清洗小室、4%多聚甲醛固定,并用0.1%的结晶紫染色10 min。擦除上室面未成功穿过小室膜的细胞,显微镜下观察并计数下室面细胞数目。
1.2.8. 生物信息学分析
使用Targetscan网站(<a href="https://www.targetscan.org/" target="_blank">https://www.targetscan.org/</a>)确定miR-607与潜在靶基因TRPC5 mRNA 3'-UTR区的结合位点。
1.2.9. 双荧光素酶报告基因系统
Huh-7及HCCLM3细胞分别以1×105/孔种于24孔板中过夜培养,每种细胞均设置4个分组Wt-TRPC5质粒+ miR- ctrl mimics;Mut-TRPC5质粒+miR-ctrl mimics;Wt-TRPC5质粒+ miR-607 mimics;Mut-TRPC5质粒+miR-607 mimics。采用LipofectamineTM 3000将混合物转染入特定细胞中培养48 h后,按双荧光素酶检测试剂说明书进行荧光强度测定。
1.2.10. Western blot
提取转染48 h后的HCC细胞总蛋白并调齐所有样品的蛋白浓度。采用垂直电泳法,利用4%~10% SDS-PAGE凝胶按相对分子质量分离总蛋白。采用湿转法,按100 V转印2 h之条件将分离后的蛋白转移至NC膜上,5% BSA室温封闭NC膜1 h。裁取目的蛋白所在的位置条带,按1:1000比例配制TRPC5、Akt、Ser473磷酸化Akt、CCND1、MMP2及GAPDH一抗,4 ℃孵育过夜。PBS洗去未结合一抗后采用1:5000稀释的HRP标记山羊抗兔二抗室温孵育NC膜1 h。于暗室内,ECL法曝光目的蛋白,以条带灰度强度计算蛋白相对表达量。
1.3. 统计学分析
采用SPSS22.0统计软件进行分析,连续性资料以均数±标准差表示,两组间比较采用两独立样本t检验。非连续性资料结果采用Pearson卡方检验,以P < 0.05表示差异具有统计学意义。
2. 结果
2.1. miR-607在肝细胞癌中表达及临床意义
分析45对HCC及癌旁组织的miR-607表达,HCC组织中miR-607的平均表达水平低于对应癌旁组织(t=2.214,P=0.029,图 1A)。以HCC组织中miR-607平均表达量为截断值,将45例肝细胞癌患者分为miR-607高表达组(n=16)和miR-607低表达组(n=29)。miR-607低表达与肿瘤直径>5 cm(χ2=4.640,P=0.031)、血管侵犯(χ2=4.865,P=0.027)及较晚的TNM分期(Ⅲ + Ⅳ,χ2=5.940,P=0.015)密切相关(表 1)。在体外培养细胞中,与正常人永生化肝细胞LO2相比,所检测HCC细胞系中miR-607的表达水平均降低(HepG2:P=0.044,Hep3B:P=0.041,Huh-7:P=0.037,HCCLM3:P=0.031;图 1B)。选择Huh-7及HCCLM3细胞进行mimics转染以开展下一步实验,结果显示,与miR-ctrl组相比,转染miR-607 mimics提高了两种HCC细胞内miR-607的表达水平(Huh-7:P < 0.001,图 1C;HCCLM3:P=0.003,图 1D)。
1.
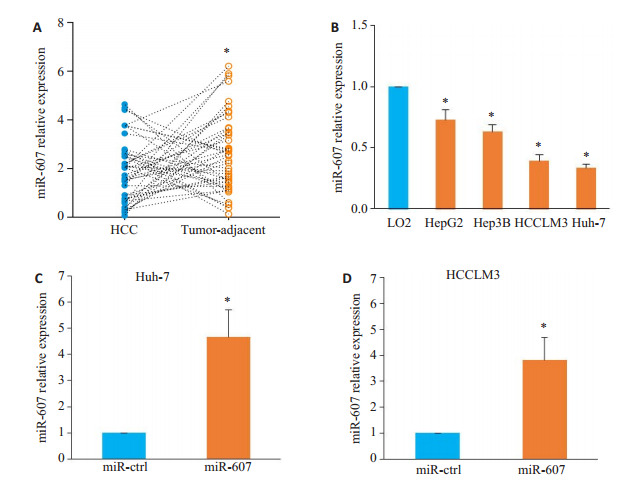
miR-607在HCC中的表达以及在Huh-7和HCCLM3细胞中过表达miR-607
Expression of miR-607 in HCC and overexpression of miR-607 in Huh-7 and HCCLM3 cells. A: Down-regulated expression of miR-607 in HCC tissues (*P < 0.05 vs tumor-adjacent tissues). B: miR-607 expression is lower in the 4 HCC cell lines (HepG2, Hep3B, Huh-7, and HCCLM3) than in LO2 cells (*P < 0.05 vs LO2). C, D: Transfection of miR-607 mimics increases miR-607 expression in Huh-7 (C) and HCCLM3 (D) cells (*P < 0.05 vs miR-ctrl group).
1.
miR-607异常表达在肝细胞癌中的临床意义
Correlation between miR-607 expression and clinicopathological features of HCC patients
| Clinical features | miR-607 expression | χ2 | P | ||
| Low (n=29) | High (n=16) | ||||
| Gender | Male | 19 | 10 | 0.041 | 0.840 |
| Female | 10 | 6 | |||
| Age (year) | < 60 | 8 | 9 | 3.604 | 0.058 |
| ≥60 | 21 | 7 | |||
| HBsAg | Negative | 7 | 6 | 0.896 | 0.344 |
| Positive | 22 | 10 | |||
| Liver cirrhosis | Absent | 9 | 9 | 2.732 | 0.098 |
| Present | 20 | 7 | |||
| Tumor size (cm) | ≤5 | 7 | 9 | 4.640 | 0.031 |
| > 5 | 22 | 7 | |||
| Vascular invasion | No | 10 | 11 | 4.865 | 0.027 |
| Yes | 19 | 5 | |||
| Edmondson-steiner grading | Ⅰ+Ⅱ | 11 | 10 | 2.501 | 0.114 |
| Ⅲ+Ⅳ | 18 | 6 | |||
| Serum AFP level (ng/mL) | < 400 | 13 | 11 | 2.371 | 0.124 |
| ≥400 | 16 | 5 | |||
| TNM stage | Ⅰ+Ⅱ | 9 | 11 | 5.940 | 0.015 |
| Ⅲ+Ⅳ | 20 | 5 | |||
2.2. 过表达miR-607抑制HCC细胞增殖并诱导凋亡
与miR-ctrl组相比,miR-607组Huh-7细胞的增殖活力在转染48 h后明显下降(P=0.037),并且在转染72 h后仍可检测出差异(P=0.023,图 2A)。同时,转染72 h后通过流式细胞仪分析发现,miR-607组细胞凋亡比例高于转染miR-ctrl组(P=0.005,图 2B)。过表达miR-607可抑制HCCLM3的增殖活力(48 h:P=0.045,72 h:P=0.025;图 2C)并促进细胞凋亡发生(P=0.004,图 2D)。
2.
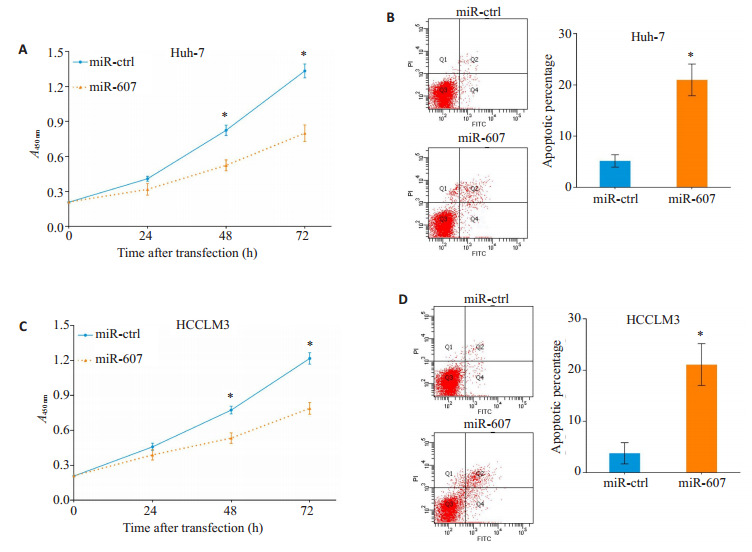
过表达miR-607抑制HCC细胞增殖并促进凋亡
Overexpression of miR-607 inhibits proliferation and promotes apoptosis in HCC cells. A, C: Overexpression of miR-607 inhibits proliferation of Huh-7 (A) and HCCLM3 (C) cells. B, D: Overexpression of miR-607 promotes apoptosis of Huh-7 (B) and HCCLM3 (D) cells. *P < 0.05 vs miR-ctrl group.
2.3. 过表达miR-607抑制HCC细胞迁移和侵袭
细胞划痕愈合试验发现,与miR-ctrl组相较,miR- 607组中Huh-7细胞的迁移距离缩短(P=0.011,图 3A)。Transwell侵袭小室结果显示,miR-ctrl组Huh-7细胞穿膜数量少于miR-607组Huh-7细胞穿膜数量(P=0.005,图 3B)。过表达miR-607具有抑制HCCLM3细胞迁移(P=0.023,图 3C)和侵袭(P=0.003,图 3D)的效应。
3.
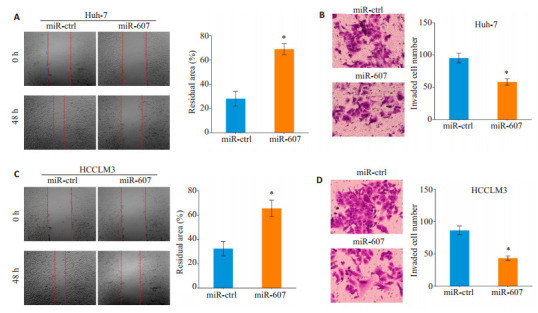
过表达miR-607抑制HCC细胞迁移和侵袭
Overexpression of miR-607 inhibits migration and invasion of HCC cells. A, C: Overexpression of miR-607 inhibits migration of Huh-7 (A) and HCCLM3 (C) cells. B, D: Overexpression of miR-607 inhibits invasion of Huh-7 (B) and HCCLM3 (D) cells. *P < 0.05 vs miR-ctrl group.
2.4. miR-607靶向TRPC5抑制Akt通路激活
在Starbase、miRDB、TargetScan数据库进行生物信息学检索发现,经典瞬时受体电位通道5(TRPC5)可能是miR-607的潜在靶点之一(图 4A)。采用双荧光素酶报告基因系统检测证实,miR-607能够与HCC细胞内TRPC5 mRNA的野生型3'-UTR区直接结合(Huh-7:P=0.004,HCCLM3:P=0.011;图 4B)。PCR及Western blot检测显示,过表达miR-607下调HCC细胞内TRPC5 mRNA(Huh-7:P < 0.001,HCCLM3:P=0.002;图 4C)及蛋白质(Huh-7:P=0.001,HCCLM3:P=0.013;图 4D)的表达水平。
4.
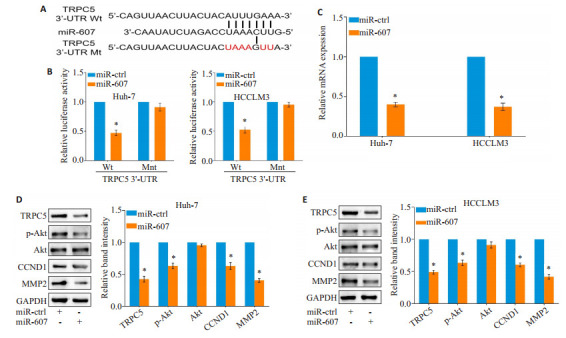
miR-607靶向TRPC5抑制Akt通路激活
miR-607 inhibits Akt pathway activation through targeting TRPC5. A: miR-607 was predicted to bind to TRPC5 mRNA 3'-UTR region by bioinformatics analysis. B: Overexpression of miR-607 inhibits luciferase activity of wild-type (Wt) but not mutant-type (Mut) 3'-UTR vectors of TRPC5 in HCC cells. C: Overexpression of miR-607 decreases TRPC5 mRNA expression in HCC cells. D, E: Total expressions of TRPC5, CCND1, MMP2 and phosphorylated Akt are decreased in Huh7 (D) and HCCLM3 (E) cells. *P < 0.05 vs miR-ctrl group.
与miR-ctrl组相比,过表达miR-607的HCC细胞内Akt的磷酸化水平下调(Huh-7:P=0.034,HCCLM3:P=0.041);同时,增殖相关分子CCND1(Huh-7:P=0.026,HCCLM3:P=0.029)及侵袭相关分子MMP2(Huh-7:P=0.004,HCCLM3:P=0.001)的表达水平降低(图 4D、E)。
3. 讨论
MicroRNA异常表达与肿瘤生长转移[11, 12]和患者预后[13]密切相关,其中miR-607在非小细胞肺癌中表达下调并提示预后不良[14];慢性淋巴细胞白血病中miR-607通过下调WNT信号通路受体FZD3表达发挥细胞周期阻滞和凋亡诱导作用[15]。本研究发现,miR-607在HCC组织中的表达水平显著下调,miR-607低表达与肿瘤体积增大、血管侵犯和较晚的临床分期等肿瘤生长转移特征密切相关;体外实验表明miR-607可以显著抑制HCC细胞的增殖、迁移、侵袭,并诱导细胞凋亡的发生。肿瘤中MicroRNA的表达下调大多与长链非编码RNA、环状RNA等其他内源性RNA的分子海绵的吸附效应有关[16]。宫颈癌中miR-607被lncRNATP73-AS1吸附降解,导致其对下游靶点CCND2的抑制作用减弱,引起肿瘤发生凋亡抵抗和侵袭转移[17]。在HCC中发现lncRNATP73-AS1表达显著升高[18],并促进HCC增殖侵袭[19]和放疗抵抗[20],上述结果从侧面揭示了本研究所发现的miR-607低表达原因。
TRPC5是一种以同源四聚体形式锚定于细胞膜上的Ca2+离子通道[21],开放后可介导细胞Ca2+内流[22]。据报道,TRPC5作为促癌因子在肺癌[23]、胃癌[24]中表达升高并与肿瘤生长转移和患者不良预后相关。生物信息学分析推断TRPC5可能是miR-607的潜在靶点之一。我们的研究通过双荧光素酶报告基因证实miR-607可与TRPC5的mRNA 3'-UTR区直接结合,PCR及Western blot结果也表明miR-607能下调TRPC5表达,说明TRPC5是miR-607的靶基因之一。
在肺鳞癌中,miR-607通过下调钙激活核苷酸酶1表达抑制肺癌细胞增殖侵袭[25],提示Ca2+信号参与了miR-607的抑癌过程。研究证实,Ca2+信号异常活动是TRPC5发挥促癌作用的主要机制。结肠癌中TRPC5介导的Ca2+内流可激活HIF-1α/Twist轴促进上皮间质转化发生[26]。乳腺癌细胞中TRPC5的通过Ca2+依赖转录因子NFATC3诱导多药耐药蛋白1表达将细胞内阿霉素泵至胞外,诱导原发性耐药形成[27]。在HCC中,通道受体异常表达引起的Ca2+内流能激活PI3K/Akt通路来促进HCC生长和转移[28]。据此线索,本研究发现过表达miR-607能够削弱Akt的磷酸化并下调CCND1、MMP2等Akt通路下游效应分子的表达,初步揭示了miR-607抗肿瘤的潜在生物学机制。由于目前尚未能在裸鼠荷瘤模型中进一步验证miR-607的抗癌活性及体内作用机制,因此尽管本研究在体外分子过程方面进行了较为深入的探索,但其结果仍有一定的局限性,需要在未来的工作中进一步完善。
综上所述,HCC中低表达miR-607与肿瘤恶性临床特征密切相关。MiR-607通过下调TRPC5表达进而抑制Akt通路激活来发挥抗HCC生长转移作用。调控miR-607表达具有靶向治疗肝细胞癌潜力。
Biography
李超,博士,助理研究员,博士后,E-mail: lca03326@btch.edu.cn
Funding Statement
国家自然科学基金(81773128);陕西省自然科学基础研究计划(2021JQ-397);北京市医院管理中心“青苗”计划(QML20210903)
Supported by National Natural Science Foundation of China (81773128)
Contributor Information
李 超 (Chao LI), Email: lca03326@btch.edu.cn.
姜 业臻 (Yezhen JIANG), Email: jyzbhh@163.com.
References
- 1.中华人民共和国国家卫生健康委员会 原发性肝癌诊疗指南(2022年版) 肿瘤防治研究. 2022;49(3):251–76. doi: 10.3971/j.issn.1000-8578.2022.03.0001. [中华人民共和国国家卫生健康委员会. 原发性肝癌诊疗指南(2022年版)[J]. 肿瘤防治研究, 2022, 49(3): 251-76.] [DOI] [Google Scholar]
- 2.叶 静静, 徐 文琴, 陈 天兵. 肝细胞癌中促癌miRNA调控网络分析与验证. https://www.j-smu.com/CN/10.12122/j.issn.1673-4254.2022.01.05. 南方医科大学学报. 2022;42(1):45–54. doi: 10.12122/j.issn.1673-4254.2022.01.05. [叶静静, 徐文琴, 陈天兵. 肝细胞癌中促癌miRNA调控网络分析与验证[J]. 南方医科大学学报, 2022, 42(1): 45-54.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golkar Z, Battaria R, Pace DG, et al. Inhibition of Ebola virus by antiEbola miRNAs in silico. J Infect Dev Ctries. 2016;10(6):626–34. doi: 10.3855/jidc.7127. [Golkar Z, Battaria R, Pace DG, et al. Inhibition of Ebola virus by antiEbola miRNAs in silico[J]. J Infect Dev Ctries, 2016, 10(6): 626-34.] [DOI] [PubMed] [Google Scholar]
- 4.Reinbold CS, Forstner AJ, Hecker J, et al. Analysis of the influence of microRNAs in lithium response in bipolar disorder. Front Psychiatry. 2018;9:207–18. doi: 10.3389/fpsyt.2018.00207. [Reinbold CS, Forstner AJ, Hecker J, et al. Analysis of the influence of microRNAs in lithium response in bipolar disorder[J]. Front Psychiatry, 2018, 9: 207-18.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen AN, Zhong LL, Ju KJ, et al. Bioinformatics analysis of a functional ANGPT1 variant that interferes with miR-607 and its association with susceptibility and outcome of ischemic stroke in a Han population. Ther Clin Risk Manag. 2021;17:1045–52. doi: 10.2147/TCRM.S328964. [Chen AN, Zhong LL, Ju KJ, et al. Bioinformatics analysis of a functional ANGPT1 variant that interferes with miR-607 and its association with susceptibility and outcome of ischemic stroke in a Han population[J]. Ther Clin Risk Manag, 2021, 17: 1045-52.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Y, Chen Z, Zhou Z, et al. Silencing of long non-coding RNA LINC00607 prevents tumor proliferation of osteosarcoma by acting as a sponge of miR-607 to downregulate E2F6. Front Oncol. 2020;10:584452–60. doi: 10.3389/fonc.2020.584452. [Zheng Y, Chen Z, Zhou Z, et al. Silencing of long non-coding RNA LINC00607 prevents tumor proliferation of osteosarcoma by acting as a sponge of miR-607 to downregulate E2F6[J]. Front Oncol, 2020, 10: 584452-60.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu B, Xue X, Lin S, et al. LncRNA LINC00115 facilitates lung cancer progression through miR-607/ITGB1 pathway. Environ Toxicol. 2022;37(1):7–16. doi: 10.1002/tox.23367. [Wu B, Xue X, Lin S, et al. LncRNA LINC00115 facilitates lung cancer progression through miR-607/ITGB1 pathway[J]. Environ Toxicol, 2022, 37(1): 7-16.] [DOI] [PubMed] [Google Scholar]
- 8.Jiang D, Yuan X, Ni J, et al. Low serum miR-607 level as a potential diagnostic and prognostic biomarker in patients of pancreatic ductal adenocarcinoma: a preliminary study. Can J Gastroenterol Hepatol. 2021;2021:8882129–36. doi: 10.1155/2021/8882129. [Jiang D, Yuan X, Ni J, et al. Low serum miR-607 level as a potential diagnostic and prognostic biomarker in patients of pancreatic ductal adenocarcinoma: a preliminary study[J]. Can J Gastroenterol Hepatol, 2021, 2021: 8882129-36.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lou C, Zhao J, Gu Y, et al. LINC01559 accelerates pancreatic cancer cell proliferation and migration through YAP-mediated pathway. J Cell Physiol. 2020;235(4):3928–38. doi: 10.1002/jcp.29288. [Lou C, Zhao J, Gu Y, et al. LINC01559 accelerates pancreatic cancer cell proliferation and migration through YAP-mediated pathway[J]. J Cell Physiol, 2020, 235(4): 3928-38.] [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Zhang C, Zhang P, et al. Serum exosomal microRNAs combined with alpha-fetoprotein as diagnostic markers of hepatocellular carcinoma. Cancer Med. 2018;7(5):1670–9. doi: 10.1002/cam4.1390. [Wang Y, Zhang C, Zhang P, et al. Serum exosomal microRNAs combined with alpha-fetoprotein as diagnostic markers of hepatocellular carcinoma[J]. Cancer Med, 2018, 7(5): 1670-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.周 晓霞, 邓 洁, 张 维, et al. miR-600通过抑制HIF-1α信号通路降低宫颈癌细胞的增殖能力. https://www.j-smu.com/CN/10.12122/j.issn.1673-4254.2021.02.07. 南方医科大学学报. 2021;41(2):210–5. doi: 10.12122/j.issn.1673-4254.2021.02.07. [周晓霞, 邓洁, 张维, 等. miR-600通过抑制HIF-1α信号通路降低宫颈癌细胞的增殖能力[J]. 南方医科大学学报, 2021, 41(2): 210-5.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Wang Z, Chen S, et al. microRNA-552 promotes hepatocellular carcinoma progression by downregulating WIF1. Int J Mol Med. 2018;42(6):3309–17. doi: 10.3892/ijmm.2018.3882. [Li C, Wang Z, Chen S, et al. microRNA-552 promotes hepatocellular carcinoma progression by downregulating WIF1[J]. Int J Mol Med, 2018, 42(6): 3309-17.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.刘 绍华, 温 莹浩, 全 兵, et al. 高表达miR-3682-3p是肝细胞癌患者的不良预后因素. https://www.j-smu.com/CN/10.12122/j.issn.1673-4254.2021.12.19. 南方医科大学学报. 2021;41(12):1885–91. doi: 10.12122/j.issn.1673-4254.2021.12.19. [刘绍华, 温莹浩, 全兵, 等. 高表达miR-3682-3p是肝细胞癌患者的不良预后因素[J]. 南方医科大学学报, 2021, 41(12): 1885-91.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mo WL, Deng LJ, Cheng Y, et al. Circular RNA hsa_circ_0072309 promotes tumorigenesis and invasion by regulating the miR-607/FTO axis in non-small cell lung carcinoma. Aging: Albany NY. 2021;13(8):11629–45. doi: 10.18632/aging.202856. [Mo WL, Deng LJ, Cheng Y, et al. Circular RNA hsa_circ_0072309 promotes tumorigenesis and invasion by regulating the miR-607/FTO axis in non-small cell lung carcinoma[J]. Aging: Albany NY, 2021, 13(8): 11629-45.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia L, Wu LL, Bao J, et al. Circular RNA circ-CBFB promotes proliferation and inhibits apoptosis in chronic lymphocytic leukemia through regulating miR-607/FZD3/Wnt/β-catenin pathway. Biochem Biophys Res Commun. 2018;503(1):385–90. doi: 10.1016/j.bbrc.2018.06.045. [Xia L, Wu LL, Bao J, et al. Circular RNA circ-CBFB promotes proliferation and inhibits apoptosis in chronic lymphocytic leukemia through regulating miR-607/FZD3/Wnt/β-catenin pathway[J]. Biochem Biophys Res Commun, 2018, 503(1): 385-90.] [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen BG, Ro S. microRNAs and 'sponging' competitive endogenous RNAs dysregulated in colorectal cancer: potential as noninvasive biomarkers and therapeutic targets. Int J Mol Sci. 2022;23(4):2166–75. doi: 10.3390/ijms23042166. [Jorgensen BG, Ro S. microRNAs and 'sponging' competitive endogenous RNAs dysregulated in colorectal cancer: potential as noninvasive biomarkers and therapeutic targets[J]. Int J Mol Sci, 2022, 23(4): 2166-75.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Xue B, Wang S, et al. Long non-coding RNA TP73 antisense RNA 1 facilitates the proliferation and migration of cervical cancer cells via regulating microRNA-607/cyclin D2. Mol Med Rep. 2019;20(4):3371–8. doi: 10.3892/mmr.2019.10572. [Zhang H, Xue B, Wang S, et al. Long non-coding RNA TP73 antisense RNA 1 facilitates the proliferation and migration of cervical cancer cells via regulating microRNA-607/cyclin D2[J]. Mol Med Rep, 2019, 20(4): 3371-8.] [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Huang ZB, Liao CJ, et al. LncRNA TP73-AS1/miR-539/MMP-8 axis modulates M2 macrophage polarization in hepatocellular carcinoma via TGF-β1 signaling. Cell Signal. 2020;75:109738–47. doi: 10.1016/j.cellsig.2020.109738. [Chen J, Huang ZB, Liao CJ, et al. LncRNA TP73-AS1/miR-539/MMP-8 axis modulates M2 macrophage polarization in hepatocellular carcinoma via TGF-β1 signaling[J]. Cell Signal, 2020, 75: 109738-47.] [DOI] [PubMed] [Google Scholar]
- 19.Ma CX, Gao WC, Tian L. LncRNA TP73-AS1 promotes malignant progression of hepatoma by regulating microRNA-103. Eur Rev Med Pharmacol Sci. 2019;23(11):4713–22. doi: 10.26355/eurrev_201906_18052. [Ma CX, Gao WC, Tian L. LncRNA TP73-AS1 promotes malignant progression of hepatoma by regulating microRNA-103[J]. Eur Rev Med Pharmacol Sci, 2019, 23(11): 4713-22.] [DOI] [PubMed] [Google Scholar]
- 20.Song W, Zhang JJ, Xia QX, et al. Down- regulated lncRNA TP73-AS1 reduces radioresistance in hepatocellular carcinoma via the PTEN/Akt signaling pathway. Cell Cycle. 2019;18(22):3177–88. doi: 10.1080/15384101.2019.1671089. [Song W, Zhang JJ, Xia QX, et al. Down- regulated lncRNA TP73-AS1 reduces radioresistance in hepatocellular carcinoma via the PTEN/Akt signaling pathway[J]. Cell Cycle, 2019, 18(22): 3177-88.] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Song K, Wei M, Guo W, et al. Structural basis for human TRPC5 channel inhibition by two distinct inhibitors. Elife. 2021;10:e63429–38. doi: 10.7554/eLife.63429. [Song K, Wei M, Guo W, et al. Structural basis for human TRPC5 channel inhibition by two distinct inhibitors[J]. Elife, 2021, 10: e63429-38.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YY, Yu MF, Zhao XX, et al. Paracetamol inhibits Ca2+ permeant ion channels and Ca2+ sensitization resulting in relaxation of precontracted airway smooth muscle. J Pharmacol Sci. 2020;142(2):60–8. doi: 10.1016/j.jphs.2019.07.007. [Chen YY, Yu MF, Zhao XX, et al. Paracetamol inhibits Ca2+ permeant ion channels and Ca2+ sensitization resulting in relaxation of precontracted airway smooth muscle[J]. J Pharmacol Sci, 2020, 142(2): 60-8.] [DOI] [PubMed] [Google Scholar]
- 23.纪 竹青, 钱 龙, 杜 楠, et al. TrpC5在肺鳞癌中的表达及其临床意义. https://www.cnki.com.cn/Article/CJFDTOTAL-TDYX201801013.htm. 现代医学. 2018;46(1):46–50. [纪竹青, 钱龙, 杜楠, 等. TrpC5在肺鳞癌中的表达及其临床意义[J]. 现代医学, 2018, 46(1): 46-50.] [Google Scholar]
- 24.余 苏云, 邓 蕊, 王 爱云, et al. 基于TRPC5探讨山奈酚抗胃癌转移的作用及机制. https://www.cnki.com.cn/Article/CJFDTOTAL-YLBS202110063.htm. 中国药理学与毒理学杂志. 2021;35(10):753–63. [余苏云, 邓蕊, 王爱云, 等. 基于TRPC5探讨山奈酚抗胃癌转移的作用及机制[J]. 中国药理学与毒理学杂志, 2021, 35(10): 753-63.] [Google Scholar]
- 25.Qiao G, Wang HB, Duan XN, et al. The effect and mechanism of miR-607/CANT1 axis in lung squamous carcinoma. Anticancer Drugs. 2021;32(7):693–702. doi: 10.1097/CAD.0000000000001045. [Qiao G, Wang HB, Duan XN, et al. The effect and mechanism of miR-607/CANT1 axis in lung squamous carcinoma[J]. Anticancer Drugs, 2021, 32(7): 693-702.] [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Zhu Y, Dong Y, et al. Overexpression of TrpC5 promotes tumor metastasis via the HIF-1α-Twist signaling pathway in colon cancer. Clin Sci: Lond. 2017;131(19):2439–50. doi: 10.1042/CS20171069. [Chen Z, Zhu Y, Dong Y, et al. Overexpression of TrpC5 promotes tumor metastasis via the HIF-1α-Twist signaling pathway in colon cancer[J]. Clin Sci: Lond, 2017, 131(19): 2439-50.] [DOI] [PubMed] [Google Scholar]
- 27.Zou Y, Liu ZX, Zhou YN, et al. TRPC5 mediates TMZ resistance in TMZ-resistant glioblastoma cells via NFATc3-P-gp pathway. Transl Oncol. 2021;14(12):101214–25. doi: 10.1016/j.tranon.2021.101214. [Zou Y, Liu ZX, Zhou YN, et al. TRPC5 mediates TMZ resistance in TMZ-resistant glioblastoma cells via NFATc3-P-gp pathway[J]. Transl Oncol, 2021, 14(12): 101214-25.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dou CW, Zhou ZY, Xu QR, et al. Hypoxia-induced TUFT1 promotes the growth and metastasis of hepatocellular carcinoma by activating the Ca2+/PI3K/AKT pathway. Oncogene. 2019;38(8):1239–55. doi: 10.1038/s41388-018-0505-8. [Dou CW, Zhou ZY, Xu QR, et al. Hypoxia-induced TUFT1 promotes the growth and metastasis of hepatocellular carcinoma by activating the Ca2+/PI3K/AKT pathway[J]. Oncogene, 2019, 38(8): 1239-55.] [DOI] [PubMed] [Google Scholar]


