Abstract
目的
构建慢性药物性肝损伤(DILI)复发风险预测模型,进而评价DILI复发风险与肝纤维化的关系。
方法
回顾性收集2017年1月~2022年1月在解放军总医院第五医学中心住院经肝活检证实的慢性DILI患者的临床资料,按照是否复发分为复发组(n=154)和无复发组(n=984)。根据Logistic单因素和多因素回归分析的结果构建相关风险预测模型,采用AUC值及Hosmer-Lemeshow检验评价模型的区分度及校准度,应用200次5、10、20折交叉验证法对模型进行验证。Spearman等级相关分析评价新建模型与纤维化的相关性,并绘制ROC曲线比较新建模型与APRI、FIB-4诊断肝纤维化的效能。
结果
共纳入慢性DILI患者1138例,平均年龄44.9±11.7岁,女性524例(46.0%),复发组较未复发组肝纤维化程度更重,复发组较未复发组肝纤维化程度更重,复发组中S0、S1、S2、S3、S4分别为1.9%、13.1%、42.2%、27.9%、14.9%,而未复发组分别为8.9%、43.5%、26.1%、17.1%、4.4%。Logistic多因素分析结果显示LSM≥13.7kPa(OR=4.35,95% CI:2.61~7.25,P < 0.001),CHE < 2500 U/L(OR=5.17,95% CI:2.13~12.53,P < 0.001),CHE 2500~5000 U/L(OR=4.07,95% CI:2.75~6.01,P < 0.001),AST > 2× ULN(OR=2.29,95% CI:1.38~3.80,P=0.001)是慢性DILI复发的危险因素。基于以上无创指标构建ACLS预测模型,AUC值为0.803(95% CI:0.78-0.83),Hosmer-Lemeshow拟合优度检验示χ2=7.73(P=0. 46),200次5、10、20折交叉验证显示平均AUC值为0.803,表明该模型稳定性良好。Spearman等级相关分析显示ACLS评分与肝纤维化程度呈正相关(rho=0.530,P < 0.001),ACLS模型诊断中度肝纤维化的最佳界值3分,AUC值为0.78(特异度72.7%,灵敏度73.3%),效能优于APRI和FIB-4(P < 0.001);诊断重度肝纤维化的界值为6分,AUC值为0.83(特异度75.7%,灵敏度72.7%),效能优于APRI(P < 0.001),但与FIB-4差异无统计学意义(P=0.38)。
结论
复发风险高的慢性DILI患者,肝纤维化程度更重,此类患者需要密切随访并适时采取积极的治疗。
Keywords: 慢性药物性肝损伤, 复发, 肝纤维化, 危险因素, 预测模型
Abstract
Objective
To construct a risk prediction model for relapse of chronic drug-induced liver injury (DILI) and explore the correlation between DILI relapse risk and liver fibrosis.
Methods
We retrospectively collected the clinical data of 1138 patients with chronic DILI hospitalized from January, 2017 to January, 2022, including 154 patients with and 984 without DILI relapse. Based on the results of univariable and multivariable logistic regression analyses, a risk prediction model for DILI relapse was constructed, evaluated for its discrimination and calibration using AUC value and Hosmer-Lemeshow test, and verified with a 200 times 5, 10 and 20 folds cross validation method. Spearman correlation analysis was used to evaluate the correlation between the new model and liver fibrosis, and its diagnostic efficiency for liver fibrosis was assessed by comparison with APRI and FIB-4 using ROC curve.
Results
The proportions of patients with S0, S1, S2, S3 and S4 liver fibrosis were 1.9%, 13.1%, 42.2%, 27.9% and 14.9% in the relapse group, respectively, as compared with 8.9%, 43.5%, 26.1%, 17.1% and 4.4% in the non-relapse group, respectively, showing severer liver fibrosis in patients with than those without DILI relapse. Multivariable logistic regression analysis identified LSM≥13.7 kPa (OR=4.35, 95%CI: 2.61-7.25, P < 0.001), CHE < 2500 U/L (OR=5.17, 95%CI: 2.13-12.53, P < 0.001), CHE of 2500-5000 U/L (OR=4.07, 95%CI: 2.75-6.01, P < 0.001), and AST > 2×ULN (OR=2.29, 95%CI: 1.38-3.80, P=0.001) as risk factors for relapse of chronic DILI. The ACLS model constructed based on these non-invasive indicators had an AUC value of 0.803 (95%CI: 0.78-0.83). The results of Hosmer-Lemeshow goodness of fit test (χ2=7.73, P=0.46) and the cross validation tests (average AUC of 0.803) all showed a good stability of the model. Spearman correlation analysis showed that ACLS score was positively correlated with the severity of liver fibrosis (rho=0.530, P < 0.001). At the optimal cut-off value of 3 points for diagnosing moderate liver fibrosis, the ACLS model had an AUC value of 0.78 (with specificity of 72.7% and sensitivity of 73.3%), demonstrating a better efficacy than that of APRI and FIB-4 (P < 0.001). At the cut-off value of 6 for severe liver fibrosis, the diagnostic efficacy of the model (AUC=0.83; specificity 75.7%, sensitivity 72.7%) was still better than that of APRI (P < 0.001) but comparable with that of FIB-4 (P=0.38).
Conclusion
The patients at high risks of chronic DILI relapse have severer liver fibrosis and should be followed up regularly for timely aggressive treatment.
Keywords: chronic drug-induced liver injury, relapse, liver fibrosis, risk factors, prediction model
近年来,药物性肝损伤(DILI)的发病率逐年升高[1],一项全国性回顾性研究显示DILI年发病率约为23.80/10万人[2]。文献报道显示8%~20%的DILI患者可发展为慢性,其中部分患者表现为反复发作,进而加剧了肝纤维化的进程,甚至引起肝硬化或肝衰竭,严重影响患者的生活质量[3-7]。有学者将DILI患者反复出现肝酶异常的现象称为延长恢复或复发,但相关研究多集中在探索DILI延长恢复或复发的危险因素[8-10]。一项基于肝活检的大样本研究发现肝组织学的炎症程度与慢性DILI的复发风险有关,推测可能的机制是反复的炎症损伤和修复促使肝纤维化程度加重,从而影响此类患者的预后[11]。鉴于肝纤维化是预测慢性DILI不良结局的重要因素,因此,如何评价慢性DILI与肝纤维化的关系显得尤为重要。肝活检是诊断DILI的重要手段,不仅可以准确区分肝纤维化分期,还可以帮助判断预后,但其临床应用价值因有创性及潜在并发症风险受到一定限制[12]。无创预测模型如肝硬度检测(LSM)、APRI、FIB-4等已广泛应用于肝纤维化的评价,有学者将LSM与APRI联合用于诊断慢性DILI的显著肝纤维化,显示出较高的效能[13]。目前尚不清楚慢性DILI的复发是否与肝纤维化有关,本研究旨在探索慢性DILI复发与肝纤维化的关系,构建相关风险预测模型,并评估模型的临床应用价值,以期为改善此类患者的预后提供一定的帮助。
1. 资料和方法
1.1. 研究对象
回顾性选取2017年1月~2022年1月在解放军总医院第五医学中心住院的慢性DILI患者,所有患者均随访超过1年。具体纳入标准:符合《药物性肝损伤诊疗指南(2015版)》中慢性药物性肝损伤的诊断标准[1];接受肝活检;RUCAM评分>6分;年龄>18岁。排除标准:合并病毒感染及其他类型肝脏疾病(如:自身免疫性肝病、酒精性肝病、吉尔伯特综合征、非酒精性脂肪性肝病、原发性胆汁硬化综合征等);具有恶性肿瘤病史;严重的心脏、肾脏或其他脏器的原发疾病或精神系统疾病;与自身免疫性肝病无法区别的慢性DILI;怀孕或哺乳期。本研究获得解放军总医院伦理委员会批准(批号:2019024D)。
1.2. 数据收集
收集患者确诊慢性DILI时的一般资料(包括性别、年龄、体质量指数、用药史及合并症)及实验室指标[包括ALT、AST、TBIL、γ-谷氨酰转肽酶(GGT)、血小板(PLT)、凝血酶原时间(PT)、胆碱酯酶(CHE)等]作为基线数据,并计算谷草转氨酶与血小板比率指数(APRI)、肝纤维化4因子指数(FIB-4)、R值。具体公式如下:APRI=(AST/正常值上限× 100)/PLT;FIB-4=(年龄× AST)÷(PLT×ALT1/2);R=(ALT实测值/40)(/ALP实测值/150),临床分型分别为胆汁淤积型(R≤2)、肝细胞损伤型(R≥5)、混合型(2 < R < 5)三型[1]。采用FibroTouch(无锡海斯凯尔)测量LSM值,根据LSM诊断DILI肝纤维化S1、S2、S3和S4期的界值,将LSM分为 < 7.2 kPa、7.2~ 8.5 kPa、8.5~12.3 kPa、12.3~13.7kPa及≥13.7 kPa五组[14]。
1.3. 肝组织学检查
采用16G活检针进行超声引导下经皮肝活检,要求肝组织长度≥15 mm,至少包括11个汇管区[15],10%中性甲醛固定,常规石蜡包埋,连续切片,并行HE及网状纤维和(或)Masson染色。由2名经验丰富的病理医师进行双盲法阅片,根据Scheuer评分系统[16]将肝脏纤维化分为S0、S1、S2、S3、S4五期。
1.4. 研究终点
符合慢性DILI诊断的患者均随访超过1年,其中,经保肝药物治疗后1月内连续2次检测肝酶正常,此后1年以内再次出现至少1次的血清ALT或AST>1.5倍正常值上限(ULN)[9]或ALP>1.1×ULN[10]者(需排除病毒性肝炎、自身免疫性肝病、酒精或非酒精性脂肪性肝病等病因)[17]判定为慢性DILI复发,肝酶复常1年后再次异常的患者不属于复发。
1.5. 统计学方法
采用R语言4.1.0版本进行数据统计分析。正态分布计量资料以均数±标准差表示,组间比较采用独立样本t检验;非正态分布计量资料以M(Q1~Q3)表示,组间比较采用Mann-Whitney U检验;计数资料以例(%)表示,两组比较采用χ2检验。采用Spearman秩相关进行等级变量相关性分析。通过Logistic多因素逐步回归模型筛选慢性DILI复发的危险因素,根据各因素危险比(OR值)赋予相应分值,以构建一种新的评分模型便于临床使用。采用AUC值及Hosmer-Lemeshow检验评价模型的区分度及校准度,应用200次5、10、20折交叉验证法进一步划分训练集和测试集对模型进行验证。使用Medcalc软件绘制ROC曲线,根据AUC值确定诊断界值,并计算相应灵敏度、特异度。P < 0.05为差异有统计学意义。
2. 结果
2.1. 一般资料
共纳入慢性DILI患者1138例,平均年龄44.9± 11.7岁,女性524例(46.0%),男性614例(54.0%),984例(86.5%)判定为无复发,154例(13.5%)为复发。复发组较未复发组肝纤维化程度更重,复发组中S0、S1、S2、S3、S4分别为1.9%、13.1%、42.2%、27.9%、14.9%,而未复发组分别为8.9%、43.5%、26.1%、17.1%、4.4%。两组的年龄、PT、LSM、TBIL、AST、GGT、CHE及纤维化分期差异有统计学意义(P < 0.05),而性别、体质量指数、合并症、临床分型、用药史、PLT、ALT及ALP差异无统计学意义(表 1)。
1.
两组患者一般资料的比较
Comparison of basic characteristics of patients with drug-induced liver injury (DILI) between relapse group and non-relapse group [n (%)]
| Characteristics | Total (n=1138) | Non-relapse (n=984) | Relapse (n=154) | P |
| BMI: Body mass index; DM: Diabetes mellitus; PT: Prothrombin time; PLT: Platelet; LSM: Liver stiffness measurement; CHE: Cholinesterase; ALT: Alanine aminotransferase; AST: Aspartate transaminase; TBIL: Total bilirubin; ALP: Alkaline phosphatase; GGT: Gamma-glutamyltransferase; ULN: Upper limit of normal range (CHE: 5000 U/L; TBIL: 20.5 μmol/L; ALT: 40 U/L; AST: 40 U/L; GGT: 50 U/L; ALP: 150 U/L). | ||||
| Female (%) | 524(46.0) | 448(45.5) | 76(49.4) | 0.425 |
| Age (year, %) | 0.003 | |||
| ≤44 | 520(45.7) | 468(47.6) | 52(33.8) | |
| 44-60 | 531(46.7) | 447(45.4) | 84(54.5) | |
| > 60 | 87(7.6) | 69(7.0) | 18(11.7) | |
| BMI (kg/m2, %) | 0.918 | |||
| < 24 | 625(54.9) | 541(55.0) | 84(54.5) | |
| 24-28 | 405(35.6) | 351(35.7) | 54(35.1) | |
| ≥28 | 108 (9.5) | 92(9.3) | 16(10.4) | |
| Comorbidity (%) | 0.460 | |||
| None | 999(87.8) | 869(88.3) | 130 (84.4) | |
| DM | 38(3.3) | 30(3.1) | 8(5.2) | |
| Hypertension | 90(7.9) | 76(7.7) | 14 (9.1) | |
| DM & Hypertension | 11(1.0) | 9 (0.9) | 2(1.3) | |
| Pattern (%) | 0.157 | |||
| Cholestatic | 587(51.6) | 517(52.5) | 70(45.5) | |
| Hepatocellular | 236(20.7) | 196(19.9) | 40(25.9) | |
| Mixed | 315(27.7) | 271(27.6) | 44(28.6) | |
| Offending agent (%) | 0.092 | |||
| Herbal | 279(35.1) | 243(36.3) | 36(28.8) | |
| Synthetic drugs | 312(39.3) | 264(39.5) | 48(38.4) | |
| Mixed | 203(25.6) | 162(24.2) | 41(32.8) | |
| PT (s, %) | < 0.001 | |||
| ≥14.3 | 66(5.8) | 43(4.4) | 23(14.9) | |
| < 14.3 | 1072 (94.2) | 941(95.6) | 131 (85.1) | |
| PLT (×109/L, %) | 0.323 | |||
| ≥100 | 1053 (92.5) | 914(92.9) | 139 (90.3) | |
| < 100 | 85(7.5) | 70(7.1) | 15 (9.7) | |
| LSM (kPa, %) | < 0.001 | |||
| < 7.2 | 500(43.9) | 476(48.4) | 24(15.6) | |
| 7.2-8.5 | 103 (9.1) | 94(9.5) | 9(5.9) | |
| 8.5-12.3 | 192(16.9) | 165(16.8) | 27(17.5) | |
| 12.3-13.7 | 42(3.7) | 35(3.6) | 7(4.5) | |
| ≥13.7 | 301(26.4) | 214(21.7) | 87(56.5) | |
| CHE (U/L, %) | < 0.001 | |||
| ≥5000 | 861(75.7) | 799(81.2) | 62(40.3) | |
| 2500-5000 | 251(22.0) | 169(17.2) | 82(53.2) | |
| < 2500 | 26(2.3) | 16(1.6) | 10 (6.5) | |
| TBIL (μmol/L, %) | < 0.001 | |||
| ≤1×ULN | 825(72.5) | 742(75.4) | 83(53.9) | |
| 1-2×ULN | 163(14.3) | 128(13.0) | 35(22.7) | |
| > 2×ULN | 150(13.2) | 114(11.6) | 36(23.4) | |
| ALT (U/L, %) | 0.213 | |||
| ≤1×ULN | 380(33.4) | 338(34.3) | 42(27.2) | |
| 1-2×ULN | 280(24.6) | 240(24.4) | 40(26.0) | |
| > 2×ULN | 478(42.0) | 406(41.3) | 72(46.8) | |
| AST (U/L, %) | < 0.001 | |||
| ≤1×ULN | 403(35.4) | 377(38.3) | 26(16.9) | |
| 1-2×ULN | 367(32.3) | 317(32.2) | 50(32.5) | |
| > 2×ULN | 368(32.3) | 290(29.5) | 78(50.6) | |
| GGT (U/L, %) | 0.001 | |||
| ≤1×ULN | 316(27.8) | 292(29.7) | 24(15.6) | |
| 1-2×ULN | 265(23.3) | 227(23.1) | 38(24.7) | |
| > 2×ULN | 557(48.9) | 465(47.2) | 92(59.7) | |
| ALP (U/L, %) | 0.222 | |||
| ≤1×ULN | 764(67.1) | 670(68.1) | 94(61.0) | |
| 1-2×ULN | 313(27.5) | 263(26.7) | 50(32.5) | |
| > 2×ULN | 61(5.4) | 51(5.2) | 10 (6.5) | |
| Fibrosis stage (%) | < 0.001 | |||
| S0 | 91(8.0) | 88(8.9) | 3(1.9) | |
| S1 | 448(39.4) | 428(43.5) | 20(13.1) | |
| S2 | 322(28.3) | 257(26.1) | 65(42.2) | |
| S3 | 211(18.5) | 168(17.1) | 43(27.9) | |
| S4 | 66(5.8) | 43(4.4) | 23(14.9) | |
2.2. 慢性DILI复发模型的建立与评价
将LSM及其他单因素分析P < 0.05的指标纳入Logistic多因素分析,结果显示:LSM≥13.7 kPa(OR= 4.35,95% CI:2.61-7.25,P < 0.001),CHE < 2500 U/L(OR= 5.17,95% CI:2.13-12.53,P < 0.001),CHE 2500-5000 U/L(OR=4.07,95% CI:2.75-6.01,P < 0.001),AST>2×ULN(OR=2.29,95% CI:1.38-3.80,P=0.001)是慢性DILI复发的危险因素(表 2)。根据LSM、CHE及AST的OR值赋予相应分值,构建一种新的评分模型,称为ACLS评分模型,范围为0~12分(表 3)。ACLS模型的AUC值为0.803(95% CI:0.77-0.84),Hosmer-Lemeshow拟合优度检验示χ2=7.73,P=0. 46。200次5、10、20折交叉验证显示平均AUC值为0.803,进一步证实模型的稳健性良好(表 4)。依据ROC界值将患者分为3个危险等级:0~ 3分为低风险,预测复发风险 < 5%;3~6为中风险,复发风险5~30%;6~12分为高风险,复发风险>30%,按照上述3风险分组患者实际的复发占比分别为1.9%(8/424),10.1%(33/326)和29.1%(113/388)。
2.
慢性DILI复发高危因素的Logistic单因素及多因素回归分析
Univariable and multivariable logistic regression analyses of high-risk factors of relapse of chronic DILI
| Characteristic | Univariable | Multivariable | |||
| OR (95% CI) | P | OR (95% CI) | P | ||
| Age (year) | |||||
| 44-60 | 1.69(1.17-2.45) | 0.005 | |||
| > 60 | 2.35(1.30-4.25) | 0.005 | |||
| PT≥14.33 (s) | 3.84(2.24-6.58) | < 0.001 | |||
| CHE (U/L) | |||||
| 2500-5000 | 6.25(4.32-9.05) | < 0.001 | 4.07(2.75-6.01) | < 0.001 | |
| < 2500 | 8.05(3.51-18.50) | < 0.001 | 5.17(2.13-12.53) | < 0.001 | |
| AST (U/L) | |||||
| 1-2×ULN | 2.29(1.39-3.76) | 0.001 | 1.53(0.90-2.61) | 0.119 | |
| > 2×ULN | 3.90(2.44-6.24) | < 0.001 | 2.29(1.38-3.80) | 0.001 | |
| TBIL (μmol/L) | |||||
| 1-2×ULN | 2.44(1.58-3.79) | < 0.001 | |||
| > 2×ULN | 2.82(1.82-4.38) | < 0.001 | |||
| LSM (kPa) | |||||
| 7.2-8.5 | 1.90(0.86-4.22) | 0.115 | 1.67(0.74-3.80) | 0.220 | |
| 8.5-12.3 | 3.25(1.82-5.78) | < 0.001 | 2.49(1.36-4.53) | 0.003 | |
| 12.3-13.7 | 3.97(1.60-9.85) | 0.003 | 2.43(0.93-6.32) | 0.070 | |
| ≥13.7 | 8.06(4.99-13.03) | < 0.001 | 4.35(2.61-7.25) | < 0.001 | |
| GGT (U/L) | |||||
| 1-2×ULN | 2.04(1.19-3.49) | 0.010 | |||
| > 2×ULN | 2.41(1.50-3.86) | < 0.001 | |||
3.
ACLS模型评分表
Score table of the ACLS model in predicting relapse risk of chronic DILI
| Indicators | Point |
| LSM: Liver stiffness measurement; AST: Aspartate transaminase; CHE: Cholinesterase; ULN: Upper limit of normal range (risk stratification of ACLS model: 0-3 points was low risk; 3-6 points was moderate risk; more than 6 points was high risk). | |
| LSM (kPa) | |
| < 7.2 | 0 |
| 7.2-8.5 | 1 |
| 8.5-12.3 | 2 |
| 12.3-13.7 | 3 |
| ≥13.7 | 4 |
| AST (U/L) | |
| ≤1×ULN | 0 |
| 1-2×ULN | 2 |
| > 2×ULN | 3 |
| CHE (U/L) | |
| ≥5000 | 0 |
| 2500-5000 | 4 |
| < 2500 | 5 |
4.
200次5、10、20折交叉验证的AUC值
AUC values of 200 times 5, 10 and 20 folds cross validation
| Cross validation | Minimum | Q25 | Median | mean | Q75 | Maximum |
| AUC: Area under the ROC curve. | ||||||
| 5-fold | 0.674 | 0.780 | 0.804 | 0.803 | 0.827 | 0.899 |
| 10-fold | 0.571 | 0.767 | 0.805 | 0.803 | 0.842 | 0.953 |
| 20-fold | 0.385 | 0.755 | 0.809 | 0.803 | 0.859 | 1.000 |
2.3. ACLS风险模型与肝纤维化的关系
为进一步评估ACLS风险模型与肝纤维化的关系,以肝组织学作为肝纤维化的诊断标准将肝纤维化分为无肝纤维化(S0)、轻度肝纤维化(S1)、中度肝纤维化(S2-3)和重度肝纤维化(S4),结果显示,伴随慢性DILI复发风险的增高,肝纤维化程度逐渐加重。Spearman等级相关分析表明ACLS模型评分与肝纤维化程度呈正相关(rho=0.530,P < 0.001,图 1)。进一步绘制ACLS模型、APRI及FIB-4诊断肝纤维化的ROC曲线,结果表明ACLS模型诊断中度肝纤维化的最佳界值3分,AUC值为0.78(特异度72.7%,灵敏度73.3%),效能优于APRI和FIB-4(P < 0.001);诊断重度肝纤维化的界值为6分,AUC值为0.83(特异度75.7%,灵敏度72.7%),效能优于APRI(P < 0.001),但与FIB-4差异无统计学意义(P=0.38,表 5)。
1.
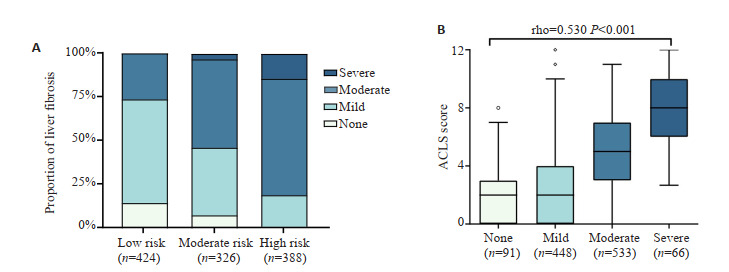
ACLS评分与肝纤维化程度
Relationship between ACLS score and liver fibrosis progression. A: Proportion of liver fibrosis in different risk groups. B: Relationship between ACLS score and liver fibrosis.
5.
不同模型诊断中度及重度肝纤维化的效能比较
Comparison of different models for diagnostic efficiency for moderate and severe hepatic fibrosis
| Model | Cut off value | AUC (95% CI) | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Youden index |
| AUC: Area under the ROC curve; CI: Confidence interval; PPV: Positive predictive value; NPV: Negative predictive value; APRI: Aspartate aminotransferase-to-platelet ratio index; FIB4: Fibrosis index based on four factors. | |||||||
| Moderate fibrosis | |||||||
| ACLS | 3 | 0.78 (0.76-0.81) | 73.29 | 72.73 | 74.90 | 71.00 | 0.46 |
| APRI | 0.79 | 0.70 (0.68-0.73) | 64.94 | 67.35 | 68.80 | 63.40 | 0.32 |
| FIB-4 | 1.53 | 0.72 (0.70-0.75) | 69.12 | 65.68 | 69.10 | 65.70 | 0.35 |
| Severe fibrosis | |||||||
| ACLS | 6 | 0.83 (0.80-0.85) | 72.73 | 75.65 | 15.50 | 97.80 | 0.48 |
| APRI | 0.76 | 0.74 (0.71-0.76) | 89.39 | 51.68 | 10.20 | 98.80 | 0.41 |
| FIB-4 | 2.94 | 0.80 (0.78-0.83) | 77.27 | 75.93 | 16.50 | 98.20 | 0.53 |
3. 讨论
慢性DILI临床表现不典型,且部分患者存在肝酶反复异常,肝纤维化持续进展的现象,甚至快速进展为肝硬化或者肝衰竭[18]。本课题组前期研究发现慢性DILI患者仍有11.8%的复发率[17],早期准确预测患者的复发风险,评估其与肝纤维化的关系,有助于筛选高危人群,提早积极干预(如避免重复应用肝损伤药物)或指导药物治疗,以提高患者的生存质量,改善预后。此外,临床预测模型可以将多因素分析结果可视化,个体量化患者的复发风险,可操作性强,有助于制定临床决策。
本研究为进一步评估慢性DILI复发与肝纤维化的关系,将LSM作为重要参数纳入分析,结果显示LSM、CHE及AST是影响慢性DILI患者预后的危险因素。近年来,越来越多的研究报道表明LSM与慢性肝病的不良预后有关,可用于预测肝脏相关事件(失代偿、肝癌、死亡等)的发生风险[19-21],本研究亦发现复发患者较未复发患者肝纤维化程度更重,尤其是LSM≥13.7 kPa更为显著(56.5% vs 21.7%),与LSM < 7.2 kPa患者相比,其复发风险升高4.35倍。多项研究报道CHE是反映蛋白合成功能和判断肝病预后的重要指标[22, 23],亦有研究发现CHE活性与肝组织炎症活动度及纤维化程度呈显著负相关[24],本研究将CHE纳入模型更有利于评估慢性DILI患者的预后。近期研究发现AST可用于预测慢性DILI生化学未缓解的风险,可能由于肝脏中AST主要分布于线粒体,持续线粒体损伤AST升高则更为显著[11],这与我们的研究结果类似。我们采用上述三个无创指标构建了慢性DILI复发风险预测模型(ACLS评分),能够一定程度上反映复发与肝纤维化的关系,结果显示区分度及校准度良好。交叉验证是一种评估模型泛化能力的方法,有研究显示通过k次试验取评价指标的平均值能够较好的评估模型的综合性能[25, 26],本研究分别采用200次5折、10折、20折的交叉验证法对模型进行了深度评价,结果进一步证实了ACLS模型的效能。当评分小于3分时,患者复发风险相对较低,可适当减少随访次数,节约医疗支出;当评分大于6分时,患者复发风险相对较高,应密切随访并适时采取积极的治疗措施,例如激素治疗。有研究提示部分慢性DILI患者接受激素联合复方甘草酸苷治疗可获得良好的生化学(肝酶正常)及组织学(肝纤维化改善)应答[27],从而改善此类患者的预后,明确了使用激素治疗适应征及疗法,而本研究可以精准的判断启动激素治疗的时机,为慢性DILI患者的精准治疗提供了一体化诊疗路径。
目前,肝活检仍然是评价肝纤维化的金标准,但由于其有创性及高费用等缺点,在实际临床实践中开展大规模肝活检几乎是无法实现的。近年来,采用无创检测方法评价肝纤维化的已成为一种流行趋势,APRI、FIB-4及LSM既往多用于评价慢性乙型、丙型肝炎及酒精或非酒精性脂肪性肝病等疾病的肝纤维化程度,然而较少用于DILI肝纤维化的诊断[28-30]。有研究报道,单独采用APRI和FIB-4在慢性肝病患者中的诊断准确性较差[31-33],而LSM被指南推荐用于肝纤维化的诊断,同样适用DILI患者,且具有良好的诊断效能[1, 21]。有研究将LSM与CHE、APRI联合并构建了LAC模型,用于评估慢性DILI的肝纤维化程度,其结果证实LAC评分与病理结果高度一致,可以部分替代肝活检[13]。本研究以肝活检作为肝纤维化的评价标准,进一步评估了慢性DILI的复发风险与肝纤维化的关系,结果表明复发风险与肝纤维化程度呈正相关(rho=0.530,P < 0.001)。进一步地,我们通过对比分析ACLS模型与APRI、FIB-4的曲线下面积,发现其诊断慢性DILI中度或重度肝纤维化分期最佳界值分别为3分和6分。相较于肝活检,我们的模型具有无创、简便、可重复性强等优势,并且将慢性DILI复发与肝纤维化相结合,这是本研究的新颖之处。
综上,基于LSM、CHE及AST构建的风险预测模型不仅可用于个体化预测慢性DILI患者的复发风险,并且与肝纤维化程度高度相关,临床实用性强,为实现慢性DILI患者的分层管理与风险预警提供了简便可行的量化工具,值得进一步应用推广。
Biography
邓亚,在读硕士研究生,E-mail: 1418267450@qq.com
Funding Statement
首都临床特色应用研究特色课题(Z181100001718034);菊梅肝胆病防治能力建设专项基金重点项目(2018JM12603003)
Contributor Information
邓 亚 (Ya DENG), Email: 1418267450@qq.com.
纪 冬 (Dong JI), Email: jidg302@126.com.
References
- 1.于 乐成, 茅 益民, 陈 成伟. 药物性肝损伤诊治指南. https://www.cnki.com.cn/Article/CJFDTOTAL-GBSY201702039.htm. 实用肝脏病杂志. 2017;20(2):257–74. [于乐成, 茅益民, 陈成伟. 药物性肝损伤诊治指南[J]. 实用肝脏病杂志, 2017, 20(2): 257-74.] [Google Scholar]
- 2.Shen T, Liu YX, Shang J, et al. Incidence and etiology of druginduced liver injury in mainland China. Gastroenterology. 2019;156(8):2230–41.e11. doi: 10.1053/j.gastro.2019.02.002. [Shen T, Liu YX, Shang J, et al. Incidence and etiology of druginduced liver injury in mainland China[J]. Gastroenterology, 2019, 156(8): 2230-41.e11.] [DOI] [PubMed] [Google Scholar]
- 3.Fontana RJ, Hayashi PH, Gu JZ, et al. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology. 2014;147(1):96–108.e4. doi: 10.1053/j.gastro.2014.03.045. [Fontana RJ, Hayashi PH, Gu JZ, et al. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset[J]. Gastroenterology, 2014, 147(1): 96-108.e4.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang QL, Huang A, Wang JB, et al. Chronic drug-induced liver injury: updates and future challenges. Front Pharmacol. 2021;12:627133. doi: 10.3389/fphar.2021.627133. [Wang QL, Huang A, Wang JB, et al. Chronic drug-induced liver injury: updates and future challenges[J]. Front Pharmacol, 2021, 12: 627133.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashby K, Zhuang W, González-Jimenez A, et al. Elevated bilirubin, alkaline phosphatase at onset, and drug metabolism are associated with prolonged recovery from DILI. J Hepatol. 2021;75(2):333–41. doi: 10.1016/j.jhep.2021.03.021. [Ashby K, Zhuang W, González-Jimenez A, et al. Elevated bilirubin, alkaline phosphatase at onset, and drug metabolism are associated with prolonged recovery from DILI[J]. J Hepatol, 2021, 75(2): 333-41.] [DOI] [PubMed] [Google Scholar]
- 6.Church RJ, Kullak-Ublick GA, Aubrecht J, et al. Candidate biomarkers for the diagnosis and prognosis of drug-induced liver injury: an international collaborative effort. Hepatology. 2019;69(2):760–73. doi: 10.1002/hep.29802. [Church RJ, Kullak-Ublick GA, Aubrecht J, et al. Candidate biomarkers for the diagnosis and prognosis of drug-induced liver injury: an international collaborative effort[J]. Hepatology, 2019, 69 (2): 760-73.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo Re V III, Haynes K, Forde KA, et al. Risk of acute liver failure in patients with drug-induced liver injury: evaluation of hy's law and a new prognostic model. Clin Gastroenterol Hepatol. 2015;13(13):2360–8. doi: 10.1016/j.cgh.2015.06.020. [Lo Re V III, Haynes K, Forde KA, et al. Risk of acute liver failure in patients with drug-induced liver injury: evaluation of hy's law and a new prognostic model[J]. Clin Gastroenterol Hepatol, 2015, 13(13): 2360-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.黄 昂, 孙 颖, 邹 正升. 药物性肝损伤慢性化的研究近况. 临床肝胆病杂志. 2020;36(3):501–4. doi: 10.3969/j.issn.1001-5256.2020.03.004. [黄昂, 孙颖, 邹正升. 药物性肝损伤慢性化的研究近况[J]. 临床肝胆病杂志, 2020, 36(3): 501-4.] [DOI] [Google Scholar]
- 9.Fontana RJ, Hayashi PH, Barnhart H, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110(10):1450–9. doi: 10.1038/ajg.2015.283. [Fontana RJ, Hayashi PH, Barnhart H, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury[J]. Am J Gastroenterol, 2015, 110(10): 1450-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medina-Caliz I, Robles-Diaz M, Garcia-Muñoz B, et al. Definition and risk factors for chronicity following acute idiosyncratic druginduced liver injury. J Hepatol. 2016;65(3):532–42. doi: 10.1016/j.jhep.2016.05.003. [Medina-Caliz I, Robles-Diaz M, Garcia-Muñoz B, et al. Definition and risk factors for chronicity following acute idiosyncratic druginduced liver injury[J]. J Hepatol, 2016, 65(3): 532-42.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CY, Deng Y, Li P, et al. Prediction of biochemical nonresolution in patients with chronic drug-induced liver injury: a large multicenter study. Hepatology. 2022;75(6):1373–85. doi: 10.1002/hep.32283. [Wang CY, Deng Y, Li P, et al. Prediction of biochemical nonresolution in patients with chronic drug-induced liver injury: a large multicenter study[J]. Hepatology, 2022, 75(6): 1373-85.] [DOI] [PubMed] [Google Scholar]
- 12.Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264–81.e4. doi: 10.1053/j.gastro.2018.12.036. [Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease[J]. Gastroenterology, 2019, 156(5): 1264-81.e4.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li ZB, Chen DD, He QJ, et al. The LAC score indicates significant fibrosis in patients with chronic drug-induced liver injury: a large biopsy-based study. Front Pharmacol. 2021;12:734090. doi: 10.3389/fphar.2021.734090. [Li ZB, Chen DD, He QJ, et al. The LAC score indicates significant fibrosis in patients with chronic drug-induced liver injury: a large biopsy-based study[J]. Front Pharmacol, 2021, 12: 734090.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.纪 冬, 熊 艺茹, 韩 萍, et al. 瞬时弹性成像对药物性肝损伤肝纤维化分期的诊断效能. https://www.cnki.com.cn/Article/CJFDTOTAL-JFJY201908012.htm. 解放军医学杂志. 2019;44(8):690–4. [纪冬, 熊艺茹, 韩萍, 等. 瞬时弹性成像对药物性肝损伤肝纤维化分期的诊断效能[J]. 解放军医学杂志, 2019, 44(8): 690-4.] [Google Scholar]
- 15.Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology. 2009;49(3):1017–44. doi: 10.1002/hep.22742. [Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy[J]. Hepatology, 2009, 49(3): 1017-44.] [DOI] [PubMed] [Google Scholar]
- 16.中华医学会感染病学分会, 中华医学会肝病学分会 慢性乙型肝炎防治指南(2019年版. 中华传染病杂志. 2019;37(12):711–36. doi: 10.3760/cma.j.issn.1000-6680.2019.12.003. [中华医学会感染病学分会, 中华医学会肝病学分会. 慢性乙型肝炎防治指南(2019年版[) J]. 中华传染病杂志, 2019, 37(12): 711-36.] [DOI] [Google Scholar]
- 17.邓 亚, 王 春艳, 谭 文辉, et al. 慢性药物性肝损伤复发的高危因素分析. https://www.cnki.com.cn/Article/CJFDTOTAL-JFJY202109014.htm. 解放军医学杂志. 2021;46(9):928–34. [邓亚, 王春艳, 谭文辉, 等. 慢性药物性肝损伤复发的高危因素分析[J]. 解放军医学杂志, 2021, 46(9): 928-34.] [Google Scholar]
- 18.陈 国凤, 纪 冬. 重视药物性肝损伤的临床研究. https://www.cnki.com.cn/Article/CJFDTOTAL-YXYZ201909003.htm. 医学研究杂志. 2019;48(9):1-4, 61. [陈国凤, 纪冬. 重视药物性肝损伤的临床研究[J]. 医学研究杂志, 2019, 48(9): 1-4, 61.] [Google Scholar]
- 19.王俊娜. LSM预测肝脏相关事件及全因死亡率: 系统回顾与非线性剂量反应meta分析[D]. 重庆: 重庆医科大学, 2018.
- 20.吕 日英, 李 仕雄, 向 文耀. FibroTouch联合ALB、ALP、AFU对肝硬化的诊断价值及预后评价. https://www.cnki.com.cn/Article/CJFDTOTAL-TDYX202004013.htm. 现代医学. 2020;48(4):484–7. [吕日英, 李仕雄, 向文耀. FibroTouch联合ALB、ALP、AFU对肝硬化的诊断价值及预后评价[J]. 现代医学, 2020, 48(4): 484-7.] [Google Scholar]
- 21.European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–64. doi: 10.1016/j.jhep.2015.04.006. [European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis[J]. J Hepatol, 2015, 63(1): 237-64.] [DOI] [PubMed] [Google Scholar]
- 22.刘 光伟, 叶 军, 薛 冬英, et al. 慢性肝病患者血清胆碱酯酶水平及其临床意义. https://www.cnki.com.cn/Article/CJFDTOTAL-GBSY200606012.htm. 实用肝脏病杂志. 2006;9(6):352–3. [刘光伟, 叶军, 薛冬英, 等. 慢性肝病患者血清胆碱酯酶水平及其临床意义[J]. 实用肝脏病杂志, 2006, 9(6): 352-3.] [Google Scholar]
- 23.王 肖引. 血清CHE、TBA与ADA水平变化在肝病患者临床诊断及预后判断中的应用价值. https://www.cnki.com.cn/Article/CJFDTOTAL-HYYX201706070.htm. 医药论坛杂志. 2017;38(6):168–9. [王肖引. 血清CHE、TBA与ADA水平变化在肝病患者临床诊断及预后判断中的应用价值[J]. 医药论坛杂志, 2017, 38(6): 168-9.] [Google Scholar]
- 24.侯 长利, 刘 晓壮, 王 健生. 血清胆碱酯酶与慢性肝病病理学的关系. https://www.cnki.com.cn/Article/CJFDTOTAL-LCGD200501020.htm. 临床肝胆病杂志. 2005;21(1):45. [侯长利, 刘晓壮, 王健生. 血清胆碱酯酶与慢性肝病病理学的关系[J]. 临床肝胆病杂志, 2005, 21(1): 45.] [Google Scholar]
- 25.梁 子超, 李 智炜, 赖 铿, et al. 10折交叉验证用于预测模型泛化能力评价及其R软件实现. https://www.cnki.com.cn/Article/CJFDTOTAL-JTYY202004001.htm. 中国医院统计. 2020;27(4):289–92. [梁子超, 李智炜, 赖铿, 等. 10折交叉验证用于预测模型泛化能力评价及其R软件实现[J]. 中国医院统计, 2020, 27(4): 289-92.] [Google Scholar]
- 26.Dutz A, Lühr A, Agolli L, et al. Development and validation of NTCP models for acute side-effects resulting from proton beam therapy of brain tumours. Radiother Oncol. 2019;130:164–71. doi: 10.1016/j.radonc.2018.06.036. [Dutz A, Lühr A, Agolli L, et al. Development and validation of NTCP models for acute side-effects resulting from proton beam therapy of brain tumours[J]. Radiother Oncol, 2019, 130: 164-71.] [DOI] [PubMed] [Google Scholar]
- 27.Wang JB, Huang A, Wang YJ, et al. Corticosteroid plus glycyrrhizin therapy for chronic drug-or herb-induced liver injury achieves biochemical and histological improvements: a randomised openlabel trial. Aliment Pharmacol Ther. 2022;55(10):1297–310. doi: 10.1111/apt.16902. [Wang JB, Huang A, Wang YJ, et al. Corticosteroid plus glycyrrhizin therapy for chronic drug-or herb-induced liver injury achieves biochemical and histological improvements: a randomised openlabel trial[J]. Aliment Pharmacol Ther, 2022, 55(10): 1297-310.] [DOI] [PubMed] [Google Scholar]
- 28.何 自倩, 周 明姣, 李 永忠. 实时剪切波弹性成像在诊断乙肝病毒肝纤维化患者中的价值. https://www.cnki.com.cn/Article/CJFDTOTAL-FZYX202101024.htm. 分子影像学杂志. 2021;44(1):122–6. [何自倩, 周明姣, 李永忠. 实时剪切波弹性成像在诊断乙肝病毒肝纤维化患者中的价值[J]. 分子影像学杂志, 2021, 44(1): 122-6.] [Google Scholar]
- 29.纪 冬, 陈 国凤. 肝纤维化无创诊断研究进展及其临床应用. https://www.cnki.com.cn/Article/CJFDTOTAL-CRBX201303022.htm. 传染病信息. 2013;26(3):190–4. [纪冬, 陈国凤. 肝纤维化无创诊断研究进展及其临床应用[J]. 传染病信息, 2013, 26(3): 190-4.] [Google Scholar]
- 30.付 懿铭, 纪 冬, 熊 艺茹, et al. 肝脏弹性检测对非酒精性脂肪性肝炎肝纤维化分期的诊断效能. https://www.cnki.com.cn/Article/CJFDTOTAL-JFJY201908009.htm. 解放军医学杂志. 2019;44(8):671–5. [付懿铭, 纪冬, 熊艺茹, 等. 肝脏弹性检测对非酒精性脂肪性肝炎肝纤维化分期的诊断效能[J]. 解放军医学杂志, 2019, 44(8): 671-5.] [Google Scholar]
- 31.Rong GH, Chen YP, Yu ZJ, et al. Synergistic effect of BiejiaRuangan on fibrosis regression in patients with chronic hepatitis B treated with entecavir: a multicenter, randomized, double-blind, placebo-controlled trial. J Infect Dis. 2022;225(6):1091–9. doi: 10.1093/infdis/jiaa266. [Rong GH, Chen YP, Yu ZJ, et al. Synergistic effect of BiejiaRuangan on fibrosis regression in patients with chronic hepatitis B treated with entecavir: a multicenter, randomized, double-blind, placebo-controlled trial[J]. J Infect Dis, 2022, 225(6): 1091-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji D, Chen Y, Shang QH, et al. Unreliable estimation of fibrosis regression during treatment by liver stiffness measurement in patients with chronic hepatitis B. Am J Gastroenterol. 2021;116(8):1676–85. doi: 10.14309/ajg.0000000000001239. [Ji D, Chen Y, Shang QH, et al. Unreliable estimation of fibrosis regression during treatment by liver stiffness measurement in patients with chronic hepatitis B[J]. Am J Gastroenterol, 2021, 116 (8): 1676-85.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong XQ, Wu Z, Zhao H, et al. Evaluation and comparison of thirty noninvasive models for diagnosing liver fibrosis in Chinese hepatitis B patients. J Viral Hepat. 2019;26(2):297–307. doi: 10.1111/jvh.13031. [Dong XQ, Wu Z, Zhao H, et al. Evaluation and comparison of thirty noninvasive models for diagnosing liver fibrosis in Chinese hepatitis B patients[J]. J Viral Hepat, 2019, 26(2): 297-307.] [DOI] [PubMed] [Google Scholar]


