Abstract
Background
Moringa stenopetala (Baker f.) Cudof. and Mentha spicata L. are widely used in the traditional system of medicine for the treatment of diabetes, hypertension, digestive problems and various disorders. The leaves formulation of M. stenopetala and M. spicata herbal tea showed better antidiabetic and antihypertensive effects in rodent models. However, its long-term safety profile has not been investigated yet. Thus, this study investigated the subchronic (90 days) oral toxicity of the leaves formulation of M. stenopetala and M. spicata herbal tea in Wistar albino rats.
Methods
Four groups of rats (n = 10, with 5/sex/group) were randomly assigned into a control (vehicle) group and three test groups (559.36, 1118.72 and 2237.44 mg/kg, respectively). The three test groups received the herbal tea of M. stenopetala and M. spicata leaves blend daily for 90 days. The control group received distilled water. During the treatment period, clinical signs were observed daily, and food consumption and body weight changes of the rats were measured weekly. At the end of the experiment, macro-pathological, hematological and biochemical parameters were evaluated. Furthermore, histopathology of liver, kidney, heart, stomach and pancreas were examined.
Results
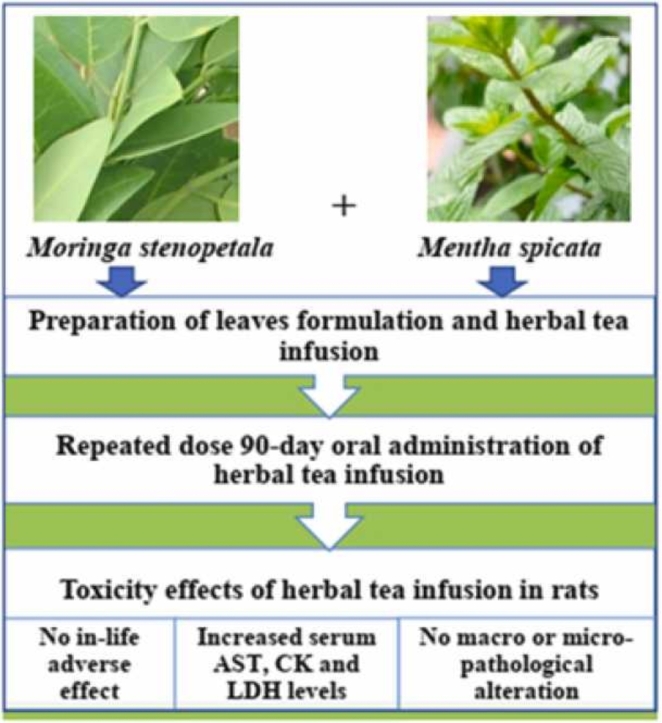
Subchronic oral administration of the herbal tea of M. stenopetala and M. spicata leaves blend did not result in death or significant toxicity signs in the treated group rats. Moreover, the herbal tea caused no significant changes on body weight, food intake, organ weight, hematological and biochemical parameters in either sex. However, the serum AST, CK and LDH levels were significantly elevated in rats treated with 2237.44 mg/kg of herbal tea in both sexes. There was no significant alteration in the histology of organs, only minor lesions in the liver, kidney and pancreas were observed.
Conclusion
The study results indicate that the herbal tea of M. stenopetala and M. spicata leaves blend is relatively safe/low toxic to rats in subchronic exposure. However, further preclinical (chronic, teratogenic, reproductive and developmental toxicity) studies in animals are required in order to have sufficient safety and toxicity profiles for its use in humans.
Abbreviations: BASO, Basophil; CK, Creatine kinase; EOSI, Eosinophil; EPHI, Ethiopian Public Health Institute; LYMP, Lymphocyte; LDH, Lactate Dehydrogenase; MONO, Monocyte; NEUT, Neutrophil; OECD, Organization for Economic Corporation and Development; PLT, Platelet; RH, Relative Humidity; TMMRD, Traditional and Modern Medicine Research Directorate
Keywords: Moringa stenopetala, Mentha spicata, Herbal formulation, Herbal tea, Subchronic toxicity
Graphical Abstract
Highlights
-
•
We evaluated the subchronic oral toxicity of herbal tea of M. stenopetala and M. spicata leaves blend in Wistar albino rats.
-
•
Toxic effects of the herbal tea on in-life, blood, gross necropsy and histopathological indices were investigated.
-
•
Serum levels of AST, CK and LDH were significantly elevated in both sexes of rats treated with high dose of the herbal tea.
1. Introduction
Medicinal plants have been used worldwide as therapeutic agents for centuries. In several African countries including Ethiopia, more than 80% of their population rely on medicinal plants for primary health care. In such developing countries, where standard health services are limited, medicinal plants are typically the preferred, inexpensive and accessible remedies for disease management [1], [2], [3].
Moringa stenopetala (Baker f.) Cudof. and Mentha spicata L. are known for their traditional medicinal and nutritional values [4], [5]. Moringa stenopetala is a member of the family Moringaceae. It is native to Southern Ethiopia and Northern Kenya. In southern Ethiopia, M. stenopetala is widely used as a therapeutic herb. People with diabetes, high blood pressure, asthma and stomach problems boil the leaves as tea and drink the extract to get relief [4], [6]. The leaves of M. stenopetala are reservoirs of beneficial phytomolecules such as alkaloids, flavonoids, phenolic compounds, glycosides, saponins and glucosinolates [7], [8]. Over the past several years, pharmacological studies have shown that the M. stenopetala leaves possess antihyperglycemic, antihypertensive, antihyperlipidemic and diuretic activities [10], [11], [9].
Mentha is a genus of aromatic herbs belonging to the family Lamiaceae. It is distributed all over the world, but it is native to temperate regions of Europe, western and central Asia [5]. In many parts of Ethiopia, Mentha spicata is widely used as a flavoring agent in various food products. Besides its culinary use, it is used as a therapeutic herb in the traditional system of medicine. The leaves of M. spicata are commonly prepared in the form of tea infusion and are used to cure high blood pressure, extended flow of menstruation, various digestive and respiratory disorders [12], [13], [14]. The leaves of this plant contain alkaloids, flavonoids, phenols, glycosides, steroids, and carvone [15], [16], [17]. The leaves were also reported to possess different pharmacological activities including antihyperglycemic, diuretic, antimicrobial, antioxidant and anti-inflammatory properties [16], [18], [19].
Nowadays, herbal formulations have attained a widespread acceptability and popularity, owing to the fact that the herbal mixtures possess some advantages which are not present in a single herb. Most of the herbal formulations commonly consist of one main (a primary) herb and one or more adjuvant herb [20]. Recently, herbal formulation containing leaves blend of Moringa stenopetala (primary herb) and Mentha spicata (an adjuvant herb) was developed in the Traditional and Modern Medicine Research Directorate (TMMRD) of Ethiopian Public Health Institute (EPHI). Such leaves formulation has shown good physicochemical (e.g., good organoleptic properties, excellent flow and solubility properties, optimal total phenolic and flavonoid contents, ash value and moisture content as well as better particle size, angle of repose, bulk and tapped density) and microbial quality properties than the individual herbs. Moreover, the leaves blend of these herbs showed better antihyperglycemic and antihypertensive effects on rodent models of hypertension and hyperglycemia. Furthermore, the combined herbal tea of M. stenopetala and M. spicata showed low toxic effects during acute administration in rodents [21]. However, the long-term safety profile of the herbal formulation of these herbs has not been investigated yet.
Even though any herbal medicines may be safe/low toxic in short-term exposure, their repeated or long-term usage may cause adverse effects. This was observed in long-term toxicity study of the leaf extract of Acacia ataxacantha, where the extract (at doses of 200 and 400 mg/kg/day) induced glomerular necrosis in the kidney, kupffer cells hyperplasia with hepatocellular necrosis in the liver and mucosal necrosis in the stomach [22]. Furthermore, various adverse effects (hematological and biochemical toxicity, organ toxicity, and teratogenic) have been reported with different mono and poly-herbal materials [23], [24], [25]. Therefore, toxicity studies are important to ensure the safety of medicinal herbs within a scientific platform to be used as therapeutics. This study was intended to evaluate the subchronic (90-days) toxicity of herbal tea of M. stenopetala and M. spicata leaves blend in male and female Wistar albino rats. The findings of this study may fill literature gap on the long-term safety profile of this herbal product and may also provide baseline data for further pre-clinical and clinical investigations.
2. Materials and methods
2.1. Plant materials collection and identification
Moringa stenopetala (Baker f.) Cudof. fresh leaves were collected from Arba-Minch University experimental site, Arba-Minch town, Ethiopia (6°01'59" N and 37° 32'59" E). Fresh leaves of M. spicata L. were collected from Wondo Genet Medicinal and Aromatic Plant Research Center, Wondo Genet town, Ethiopia (7°1′ N and 38° 35' E). The leaves of M. stenopetala and M. spicata were identified and deposited at the Herbarium Unit of the TMMRD of EPHI, Addis Ababa, Ethiopia with voucher numbers of Mst-011 and Msp-012 respectively.
2.2. Preparations of leaves formulation and tea infusion
The leaves of both plants were washed with distilled water, dried under shade at room temperature, cut into small pieces manually, and then pulverized individually with an electric grinder using a mesh size of 3 mm to produce coarse powder of the leaves. The coarse powder of each herb was then sieved with different mesh size (5 mm-212 µm) to obtain an appropriate particle size of 212 µm granules. The granules of M. stenopetala and M. spicata were weighed individually and then they were uniformly mixed (in ratio of 85:15 w/w respectively) for 10 min using an electric blender taking into consideration of 212 µm for particle sizes. The granules of these herbs were homogenized to visually acceptable levels [21].
The amount of leaves blend (formulation) to be measured was determined based on the total weight of rats in each dosage group. The different doses (559.36, 1118.72 and 2237.44 mg/kg body weight) selected for the present study were chosen based on pervious efficacy and pre-clinical studies [11], [19], [21], [26]. The measured leaves blend, for each dosage group, was kept on filter paper in a funnel placed glass beaker and a cup (100 ml) of boiled distilled water (94.5 °C) was poured over it. The herbal tea infusion was then cooled at room temperature and administered in a dosing volume of 2 ml/100 g body weight via intra-gastric tube [11], [21].
2.3. Experimental animals and ethical statement
Healthy drug naïve male and female Wistar albino rats (Rattus norvegicus, 8–9 weeks old and 150 −170 g body weight) were obtained from the Animal Breeding Unit of EPHI. Throughout the experiment, all rats were accommodated in the environmentally controlled animal room of the TMMRD of EPHI (with 22 + 30 C, 30 – 70% RH and with 12 h light - dark cycle). The rats were kept in clean plastic cages with wood shavings as a bedding material (n = 5/sex/cage) and allowed free access to standard pelleted diet and drinking water. The bedding was changed twice a week. Drinking water was kept in a clean polypropylene bottle with stainless steel sipper tube that was cleaned on daily basis. Prior to testing, all rats were acclimatized for a week to the laboratory conditions.
Care, accommodation and use of the animals were in accordance with the European Union guidelines Directive 2010/63/EU [27]. For euthanasia, the American Veterinary Medical Association guidelines were used [28]. The study procedures were reviewed and approved by institutional review board of the College of Health Sciences, Addis Ababa University (protocol number: AAUMF-010/18/ANAT) in compliance with the Organization for Economic Co-Operation and Development (OECD) guideline test number 408, for testing of chemicals on repeated dose 90-days oral toxicity study in rodents [29]. For reporting the experiment, the ARRIVE guidelines were used [30]. The experiment was conducted in the laboratories of EPHI and Saint Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia.
2.4. Experimental design
Twenty male and twenty female rats were randomly distributed into four groups, each group with 10 rats of five males and five females. The first (negative control) group received distilled water. The second, third and the fourth groups received the herbal tea infusion of M. stenopetala and M. spicata leaves formulation at doses of 559.36, 1118.72 and 2237.44 mg/kg respectively. The leaves formulation tea infusion was administered via intragastric tube, once a day for 90 consecutive days [29].
2.4.1. In-life observations and measurements
During the treatment period, the animals were inspected twice a day, usually at the beginning and at end of each day, for mortality and signs of severe pain and distress including excessive weight loss, severe dehydration, self-induced trauma, and labored respiration. General clinical observation was made once a day, especially after dosing, using an individual clinical score sheet. Changes in general body condition (emaciated, thin or obese), integumentary (abnormal skin, coat/fur), ophthalmic (abnormal size, color, position of the eyeball, eyelids), motor activities (atypical movement, gait), autonomic or physiologic activities (abnormal salivation, respiratory pattern, dehydration) and behavioral patterns (abnormal interaction) were evaluated. Body weight of each rat was measured before dosing, thereafter once a week and finally at necropsy. Food consumption was measured once a week per cage (n = 5/sex).
2.4.2. Hematological and serum biochemical analyses
After the last administration (90th day), all rats were fasted for 12 hours (overnight) and then anesthetized by pentobarbital (150 mg/kg of body weight) via intraperitoneal injection [28]. From each anesthetized rat blood sample (4–6 cc) was collected via cardiac puncture and placed into two separate test tubes: a test tube with anti-coagulant (di-potassium salt of ethylene-diamine tetra-acetic acid) and a plane test tube. Blood sample in a tube with anti-coagulant was analysed using Automated Hematology Analyzer (Symex-XT, 1800i, Japan) for determining values of total white blood cell count (WBC), total red blood cell count (RBC), blood platelet (PLT) count, hemoglobin (HGB), hematocrit (HCT), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), relative lymphocyte count (LYMP%), relative monocyte count (MONO%), relative neutrophil count (NEUT%), relative eosinophil count (EOSI%) and relative basophil count (BASO%). Blood sample in a plane tube was allowed to clot for one hour at room temperature and then centrifuged for ten minutes using an electrical centrifuge at 3000 rpm to obtain serum. Then serum was analysed using Automated Clinical Chemistry Analyzer (Huma Star 80, Germany) and values of alanine transaminase (ALT), alkaline phosphate (ALP), aspartate aminotransferase (AST), creatine kinase (CK), triglyceride (TG), lactate dehydrogenase (LDH), creatinine, urea, uric acid, total protein, total bilirubin, total cholesterol, amylase and glucose were recorded.
2.4.3. Gross necropsy and organ weight
At the end of the treatment period (on the 91st day), all surviving rats were subjected to external gross necropsy for changes on body condition; skin and coat/fur; eye ball and eyelid and for abnormal discharges from natural orifices. After exsanguination, they were sacrificed and dissected to examine gross lesions in subcutaneous tissue (edema, discoloration and hematoma); superficial lymph nodes (abnormal shape, size, color and consistency) and the major body cavities (abnormal position of viscera, excessive fluid/blood). The vital thoracic and peritoneal viscera were harvested, cleaned from any adherent tissues and were examined for macroscopic changes: size, location, color, shape, consistency, surface appearance, appearance of cut surface and free margins. Furthermore, visceral organs (liver, kidney, heart, pancreas and stomach) were weighed.
2.4.4. Histopathology of major organs
Potential target sites from the liver, kidney, heart, pancreas and stomach were collected for histopathological examinations. Tissue processing was performed as follows: tissue samples were fixed in 10% neutral buffered formalin solution for 24 h; washed in running tap water overnight; dehydrated with increasing concentrations of alcohol (30%, 50%, 70% and 90% ethanol for 2 h in each, followed with 100% ethanol for 2 h X3, and 100% ethanol overnight); cleared in xylene (for 2 h X2); infiltrated in two changes of melted paraffin wax (wax I for 2 and ½ hours and wax II overnight); embedded in melted paraffin wax and sliced with a thickness of 4–5 µm using a rotary microtome. The paraffin ribbons were laid onto the surface of water bath (40 °C) and were mounted on the slides. In order to firmly attach the ribbons onto the slides, the slides with the ribbons were placed in an oven (45–50°C) for 20–25 min [31], [32].
Tissue staining was carried out as follows: the tissue sections on the slides were deparaffinized with xylene (for 5 min X3), rehydrated in decreasing concentrations of alcohol (100%, 100%, 90%, 80% and 70% ethanol for 3 min in each) and rinsed in distilled water for 5 min. After washing, the tissue sections were stained with Harris hematoxylin for 5–7 min; washed with running tap water for 3–6 min; decolorized in acid alcohol for 3–5 s; rinsed with running tap water for 1 min; immersed in sodium carbonate solution for 30 s; washed in running tap water for 30 s and counterstained with eosin Y for 1 min. Then, the stained sections were dehydrated by increasing concentrations of ethanol (70%, 80%, 90% and 100% for 3 min in each); cleared in two changes of xylene for 5 min in each; mounted with Dibutylphthalate polystyrene xylene; covered with cover slips and examined using a compound light microscope [31], [32]. The representative photomicrographs were captured by a digital camera fitted with the microscope (Nicon Coolpix-5000, Germany).
2.5. Statistical analysis
Statistical Package for Social Sciences (SPSS) version 22 software was used for data analysis. For equality of variances, Levene’s homogeneity and for normality of means, Bonferroni normality tests were conducted. If the assumptions were met, one-way analysis of variance (ANOVA) followed by Turkey (to check difference between the study groups) and Dunnett’s (to determine difference between control and treated groups) post hoc tests were performed. Sex-dependent variation was analysed by two-way ANOVA. Results were presented as mean + standard deviation (M + SD). P < 0.05 was considered to be statistically significant.
3. Results
3.1. Effects the herbal tea infusion on mortality and clinical signs
During the 90-days repeated oral administration, no mortality or severe clinical signs were observed in any of the treated groups, in both sexes. However, minor clinical signs (mild diarrhea and mild piloerection) were observed in a few rats of all study groups, during the first two days after dosing. These mild signs did not affect the general health status of the animals and were considered as common findings for Wistar albino rats.
3.2. Effects the herbal tea infusion on body weight and food intake
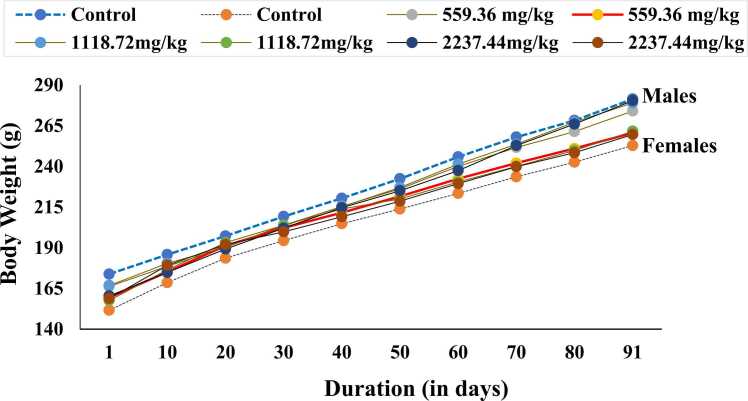
Throughout the subchronic experiment, body weight showed gradual increment for all study groups of both sexes (Fig. 1). However, there was a different pattern among the male and female groups. The results showed that in male rats, the weekly body weight gain was lower in the treated groups (559.36, 1118.72 and 2237.44 mg/kg) in comparison with the control group throughout the study period. The final body weight changes of the male groups were 107.60 + 6.93 g (62%) with the controls, 107.80 + 12.03 g (65%) at 559.36 mg/kg, 112.20 + 3.11 g (67%) at 1118.72 mg/kg and 120.00 + 8.66 g (75%) at 2237.44 mg/kg groups in comparison with their respective day-1 (initial) body weights. The final weight increment was higher in the treated than in the control males.
Fig. 1.
Body weight changes of the rats by sex, dosage group and time in subchronic toxicity study. Data expressed as mean ± SD; n = 5 animals/sex/group.
In females, the weekly body weight change was higher in the treated than in the controls over the study period of 13 weeks. When compared with the control females, the final weight gain was lower at 2237.44 mg/kg group, higher at 1118.72 mg/kg group, but no change at 559.36 mg/kg group. The differences were not statistically significant for any of the treated groups in both sexes. Nevertheless, there was a significant sex-related difference. The weekly and final body weight changes were significantly higher (P < 0.05) in male than in the female rats. The body weight data of the control and treated groups are presented in Table S1.
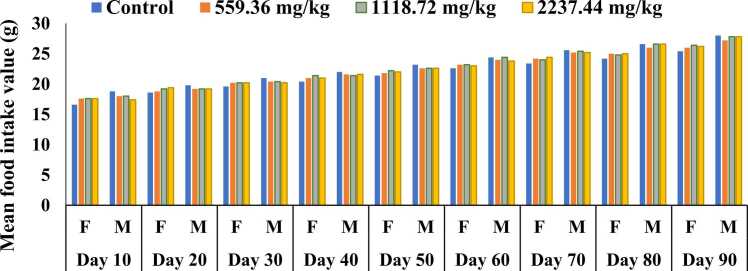
No statistically significant variation was observed in the amount of food consumption between the control and treated groups for male and female rats during the course of the experiment (Fig. 2). Nevertheless, sex-related significant difference was observed in the amount of food consumption. Throughout the study period, the amount of food consumption was significantly higher in male than in the female groups (P < 0.05). The food consumption data of all study groups at an interval of 10 days are presented in Table S2.
Fig. 2.
Food consumption of the rats by sex, dosage group and time in subchronic toxicity study.
Data are mean ± SD; n = 5 rats/sex/group.
3.3. Effects of the herbal tea infusion on hematological parameters
Hematological parameters of the experimental groups are presented in Table 1. Subchronic (90-day) oral administration of the herbal tea infusion did not induce significant adverse effects in the hematological parameters of the test groups including the highest dosage group, 2237.44 mg/kg/day, in both sexes. The hematological indices in the treated groups were only marginally varied from the control group values in either sex (P > 0.05). However, there was sex-dependent variation in the hematological parameters. In male rats, the values of RBCs, HGB, HCT, MCHC and NEUT were significantly higher, while the WBCs, MCH and MCV values were significantly lower, when compared with the values of the female rats (P < 0.05).
Table 1.
Hematological parameters data by dosage group and sex in subchronic toxicity study.
| Hemograms | Sex | Experimental groups |
|||
|---|---|---|---|---|---|
| Control | 559.36 mg/kg | 1118.72 mg/kg | 2237.44 mg/kg | ||
| WBC (x103/µL) | F | 6.38 + 1.41 | 7.93 + 1.04 | 7.91 + 1.04 | 7.80 + 0.47 |
| M | 7.11 + 0.66 | 6.55 + 0.80 | 7.10 + 0.65 | 7.86 + 0.82 | |
| RBC (x106/µL) | F | 7.81 + 0.09 | 8.03 + 0.42 | 8.02 + 0.61 | 7.77 + 0.31 |
| M* | 8.79 + 0.40 | 9.04 + 0.17 | 8.78 + 0.39 | 8.65 + 0.17 | |
| PLT (x103/µL) | F | 988.00 + 100.95 | 952.20 + 94.00 | 957.20 + 48.70 | 961.60 + 32.70 |
| M | 1026.20 + 4.59 | 975.40 + 38.10 | 1025.80 + 44.27 | 956.00 + 64.34 | |
| HGB (g/dL) | F | 15.09 + 0.13 | 15.86 + 0.52 | 15.45 + 0.77 | 15.46 + 0.59 |
| M* | 16.59 + 0.78 | 16.62 + 0.43 | 16.58 + 0.77 | 16.25 + 0.34 | |
| HCT (%) | F | 43.38 + 0.33 | 45.53 + 1.61 | 45.52 + 1.61 | 44.55 + 1.58 |
| M* | 46.23 + 1.66 | 46.38 + 2.25 | 45.22 + 1.35 | 46.23 + 0.72 | |
| MCH (pg) | F* | 19.35 + 0.11 | 19.30 + 0.08 | 19.29 + 0.08 | 19.31 + 0.70 |
| M | 18.90 + 0.08 | 18.81 + 0.150 | 18.89 + 0.08 | 18.78 + 0.12 | |
| MCHC (g/dL) | F | 34.80 + 0.21 | 34.22 + 0.64 | 34.20 + 0.64 | 34.68 + 0.18 |
| M* | 35.88 + 0.37 | 35.38 + 0.42 | 35.88 + 0.37 | 35.34 + 0.52 | |
| MCV (fL) | F* | 55.57 + 0.65 | 56.42 + 1.30 | 56.41 + 1.30 | 57.43 + 1.30 |
| M | 52.58 + 0.50 | 53.09 + 0.30 | 52.57 + 0.50 | 53.10 + 0.97 | |
| LYMP (%) | F* | 5.22 + 1.10 | 6.45 + 1.72 | 6.45 + 1.73 | 6.75 + 0.51 |
| M | 4.93 + 0.30 | 4.41 + 1.04 | 4.94 + 0.31 | 5.11 + 1.30 | |
| NEUT (%) | F | 0.85 + 0.22 | 1.30 + 0.10 | 1.32 + 0.12 | 1.13 + 0.57 |
| M* | 1.95 + 0.39 | 1.48 + 0.52 | 1.76 + 0.30 | 1.61 + 0.68 | |
| MONO (%) | F* | 0.29 + 0.07 | 0.30 + 0.05 | 0.31 + 0.07 | 0.24 + 0.04 |
| M | 0.14 + 0.07 | 0.17 + 0.15 | 0.14 + 0.07 | 0.12 + 0.07 | |
| EOSI (%) | F | 0.08 + 0.02 | 0.07 + 0.01 | 0.07 + 0.01 | 0.08 + 0.01 |
| M | 0.08 + 0.00 | 0.08 + 0.03 | 0.08 + 0.00 | 0.08 + 0.02 | |
| BASO (%) | F* | 0.02 + 0.01 | 0.03 + 0.01 | 0.03 + 0.01 | 0.02 + 0.02 |
| M | 0.02 + 0.01 | 0.01 + 0.00 | 0.01 + 0.01 | 0.02 + 0.01 | |
Values are presented as M + SD/E; One/two -way ANOVA followed by a Dennett’s post hoc test; P < 0.05, was considered statistically significant; *Significant p-value, between male and female groups; n = 5 animals/sex/group.
3.4. Effect of the herbal tea infusion on biochemical parameters
Table 2 shows serum values of biochemical parameters in the control and treated groups. Serum analysis showed no significant variation in the biochemical parameters (except for AST, LDH and CK) of the treated groups in comparison with the control groups (P > 0.05). In both male and female groups, the AST, LDH and CK levels were significantly elevated at 2237.44 mg/kg/day group, as compared with the control group values (P < 0.05). Furthermore, some biochemical parameters displayed sex-related variations. In female rats, total cholesterol, TG, total protein and urea levels were significantly lower, while the ALP value was significantly higher, when compared with the values of the male rats (P < 0.05).
Table 2.
Biochemical parameters data by dosage group and sex during subchronic study.
| Biochemicals | Sex | Experimental groups |
|||
|---|---|---|---|---|---|
| Control | 559.36 mg/kg | 1118.72 mg/kg | 2237.44 mg/kg | ||
| ALP (IU/L) | F | 80.50 + 15.53 | 66.60 + 8.94 | 72.80 + 4.88 | 80.90 + 19.38 |
| M* | 80.10 + 7.59 | 86.20 + 4.82 | 86.80 + 6.13 | 89.60 + 4.51 | |
| ALT (IU/L) | F | 78.72 + 11.02 | 68.84 + 11.07 | 84.84 + 17.23 | 84.01 + 15.36 |
| M | 65.83 + 5.48 | 75.19 + 8.23 | 76.23 + 12.12 | 78.24 + 7.16 | |
| AST (IU/L) | F* | 135.15 + 17.42 | 146.30 + 9.42 | 151.10 + 12.80 | 167.48 + 5.69★ |
| M | 127.20 + 12.03 | 131.62 + 9.81 | 138.00 + 16.62 | 155.62 + 11.49✢ | |
| CK (IU/L) | F | 491.80 + 104.75 | 522.00 + 79.26 | 536.20 + 64.97 | 640.60 + 86.96★ |
| M | 544.60 + 28.87 | 567.00 + 70.30 | 570.40 + 65.42 | 694.20 + 93.03✢ | |
| LDH (U/L) | F | 1002.2 + 263.31 | 1091.2 + 77.51 | 1106.2 + 66.18 | 1249.4 + 77.41★ |
| M | 843.8 + 274.64 | 1090.4 + 90.06 | 1015.6 + 90.10 | 1262.2 + 186.81✢ | |
| TG (mg/DL) | F | 62.48 + 4.61 | 56.05 + 5.21 | 63.97 + 3.90 | 59.80 + 5.81 |
| M* | 64.74 + 8.95 | 63.74 + 3.47 | 62.82 + 6.00 | 61.64 + 3.87 | |
| HDLC (mg/DL) | F* | 49.21 + 0.72 | 47.17 + 3.98 | 50.86 + 2.57 | 52.45 + 6.34 |
| M | 36.46 + 2.30 | 39.46 + 2.38 | 37.54 + 1.34 | 38.24 + 0.87 | |
| Urea (mg/dl) | F* | 46.00 + 2.59 | 44.32 + 3.49 | 40.72 + 2.09 | 47.08 + 15.52 |
| M | 39.26 + 1.13 | 38.48 + 3.68 | 38.88 + 1.28 | 38.84 + 6.33 | |
| Uric acid (mg/dl) | F | 1.40 + 0.20 | 1.68 + 0.49 | 1.72 + 0.54 | 1.33 + 0.42 |
| M | 1.74 + 0.57 | 1.66 + 0.51 | 1.54 + 0.32 | 1.60 + 0.50 | |
| Creatinine (mg/dl) | F | 0.38 + 0.01 | 0.40 + 0.01 | 0.41 + 0.02 | 0.38 + 0.02 |
| M | 0.36 + 0.01 | 0.41 + 0.10 | 0.38 + 0.05 | 0.38 + 0.04 | |
| Albumin (mg/dL) | F* | 4.67 + 0.11 | 4.47 + 0.11 | 4.45 + 0.11 | 4.46 + 0.20 |
| M | 4.17 + 0.11 | 4.24 + 0.15 | 4.18 + 0.07 | 4.32 + 0.14 | |
| Glucose (mg/dl) | F | 168.51 + 6.36 | 180.45 + 5.77 | 171.25 + 7.03 | 162.25 + 32.60 |
| M | 180.47 + 25.24 | 167.66 + 6.38 | 165.27 + 14.70 | 161.60 + 4.82 | |
| Amylase (U/L) | F | 2391.6 + 135.57 | 2326.4 + 90.15 | 2324.2 + 92.33 | 2255.4 + 124.03 |
| M | 2218.4 + 73.61 | 2298.8 + 76.82 | 2319.6 + 107.66 | 2327.0 + 93.11 | |
| Total bilirubin (mg/dl) | F | 0.08 + 0.03 | 0.10 + 0.04 | 0.07 + 0.03 | 0.08 + 0.02 |
| M | 0.09 + 0.02 | 0.07 + 0.01 | 0.07 + 0.01 | 0.07 + 0.01 | |
| Total protein (mg/dL) | F* | 6.32 + 0.03 | 6.46 + 0.22 | 6.46 + 0.23 | 6.17 + 0.33 |
| M | 5.81 + 0.26 | 6.07 + 0.17 | 5.85 + 0.30 | 5.86 + 0.08 | |
| Total cholesterol (mg/DL) | F* | 55.50 + 4.28 | 57.53 + 5.30 | 57.53 + 5.10 | 51.41 + 6.65 |
| M | 45.46 + 2.69 | 44.53 + 3.27 | 43.46 + 2.31 | 42.62 + 1.08 | |
Values are presented as M + SD; One/two -way ANOVA followed by a Dennett’s post hoc test; P < 0.05 was considered statistically significant; *Significant p-value, between male and female groups; ✢Significant p-value, between control and treated groups of males; ★Significant p-value, between control and treated groups of females.
3.5. Effects of the herbal tea infusion on gross necropsy and organ weight
Treatment with the herbal tea infusion did not result in changes on the external and internal organs of the treated animals. The liver, kidneys, heart, panaceas and stomach did not show significant weight differences (P > 0.05) among the control and treated groups in both sexes (Table S3). However, sex-dependent variation was noted in the absolute weight of the liver and kidney. These weight changes were significantly higher in male than in the female rats (P < 0.05).
3.6. Effect of the herbal tea infusion on histology of organs
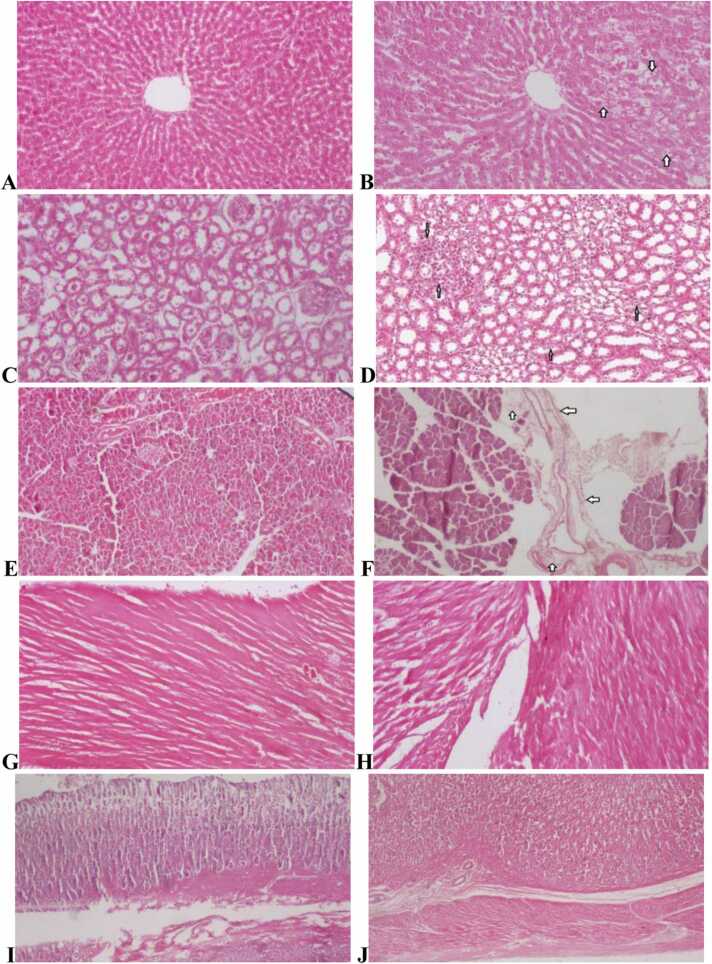
Repeated dose (90 days) oral administration of herbal tea of M. stenopetala and M. spicata leaves blend did not induce remarkable pathological changes in the stomach, pancreas, heart, kidney and liver (Fig. 3). Only minor lesions were observed in the liver, kidney and pancreas tissues of a few rats both in the control and treated groups. In the liver, mild hydrophic changes (small cellular vacuoles) were found around the central veins and portal areas (Fig. 3B), but the general hepatic architecture was normal. In the kidney, mild-focal inflammation (infiltration of leukocytes) was observed around the glomeruli and renal tubules (Fig. 3D), but the lesion did not distort the renal histologic appearance, in which the glomeruli, Bowman’s capsules, urinary spaces, proximal and distal convoluted tubules were intact. In the pancreas, mild peri-pancreatic inflammation (neutrophil infiltration) was found around the excretory ducts (Fig. 3F). However, the pancreatic acini, islets of Langerhans, excretory ducts and the interstitial septa were normal. In terms of severity and frequency of occurrence, the liver, kidney and pancreas histopathological findings did not show significant variations between the control and treated groups in both sexes. Histopathological data of the studied organs are presented in Table S4.
Fig. 3.
Histopathology of the liver, kidneys, heart, panaceas and stomach in subchronic toxicity study (H & E stained). (A) Normal histology of the liver from controls (x10); (B) Mild hydropic changes of the liver at 2237.44 mg/kg (x10); (C) Normal histology of the kidney from control group (x20); (D) Mild-focal inflammation of the kidney at 2237.44 mg/kg group (x20); (E) Normal architecture of the pancreas from control rats (x10); (F) Mild inflammation of the pancreas at 2237.44 mg/kg group (x20); (G) Normal cardiac myocytes appearance from control group (x10); (H) Normal cardiac architecture at 2237.44 mg/kg group (x20); (I) Normal histology appearances of the stomach from control rat (x20); and (J) Normal microscopic appearances of the stomach at 2237.44 mg/kg group (x20).
4. Discussion
All over the world, particularly in developing nations, medicinal plants and herbal products have long been used to fight various human diseases. In many rural parts of the developing nations, medicinal plants are the most easily accessible and affordable health resources [2], [3]. It is generally assumed that medicinal herbs and their products are safe, non-toxic and without side effects. However, researches have proven that not all medicinal plants are safe and without of adverse effects. Various adverse effects have been reported with the administration of different herbs including systemic toxic effects of Acacia ataxacantha [22] and Caralluma dalzielii [23] as well as developmental toxic effects of Achyranthes aspera [25]. The adverse effects can result from inherent toxic effect of the active principle or long-term use or overdosing. Therefore, toxicological and pharmacological properties of medicinal herbs should be scientifically evaluated to warrant the outstanding quality and safety for patients’ use [33].
The leaves of M. stenopetala [10], [11] and M. spicata [19] have been studied and have demonstrated antidiabetic and diuretic activities on animal models. However, the leaves formulation of M. stenopetala and M. spicata has shown better antihyperglycemic and antihypertensive effects than the individual herbs [21]. Individually, each herb in this formulation is safe, but the combined safety profile of these herbs is not investigated. Hence, the present study evaluated the subchronic toxicity potential of the herbal tea of M. stenopetala and M. spicata leaves blend in rats. In this study, treatment-related toxicity effects on in-life, hematological, biochemical, macro-pathological and histopathological parameters were investigated.
Mortality, severe clinical signs, loss of body weight and altered food intake are crucial indicators of test compound-related in-life adverse effects in laboratory animals [34]. During the treatment period, no herbal tea-related deaths nor severe clinical signs were observed up to the maximum dose of 2237.44 mg/kg/day.
Throughout the experimental period, body weight and food intake were not altered in any of the treated groups in comparison with the control group in both sexes. The normal food consumption in the treated groups suggests that the herbal tea infusion might not have resulted in any alteration in dietary intake or diet metabolism. Over the course of the study, there was also a normal gradual weight increment in all test groups of both sexes. The progressive weight increment could be attributed to the nutritional constituents of the herbal tea infusion and thus its oral administration up to a dose of 2237.44 mg/kg for 90 days did not affect negatively the normal growth of the studied rats. The present results are in agreement with toxicity studies of the leaves extract of M. stenopetala [35], [9] and Moringa oleifera [36], which revealed non-toxic effects of the extracts on in-life parameters in rodents. Other studies also reported the presence of several nutritive and phytochemical constituents in the leaves of M. stenopetala [37] and Mentha spicata [15] that are important for normal growth and development.
During toxicity studies, administration of medicinal herbs or test compounds at toxic doses often result in significant changes in blood parameters, since they are the most sensitive targets for adverse effects of foreign chemical compounds [38], [39]. In this study, hematological parameters of the treated rats did not show statistically significant alterations when compared with the control rats in either sex. This may suggest that repeated dose oral administration of the herbal tea infusion up to the maximum dose of 2237.44 mg/kg did not induce adverse effects on the hematological parameters of the rats. The hematology results in this study are in line with earlier toxicity studies of the leaves extract of Moringa oleifera [36], the leaves extract of M. stenopetala [40] and green coffee bean extract of Coffea arabica [41] in rats, which revealed that subchronic administration of the extracts with different doses produced no significant alteration on hematological parameters.
In the present serum analysis, the levels of AST, LDH and CK were significantly elevated (P < 0.05) in rats treated with 2237.44 mg/kg of the herbal tea infusion when compared with the values of the control rats in both sexes. The serum biochemical alterations were often accompanied by anatomic-pathological changes of organs. Since, injured organs release their contents into circulation, which could change the normal contents of their biomarkers in the blood plasma [38], [39].
AST may be elevated due to liver diseases, muscular disorders (muscle injury, recent heart attack) or other diseases/factors (excess alcohol ingestion). Thus, additional biochemical and histopathological data are required to identify the source of elevation. AST is not as liver-specific as ALT. In liver injury, both AST and ALT are elevated, thus ALT and AST are usually analysed together. In the absence of liver injury and ALT elevation, the elevation of AST may lead to muscular injury. The muscular injury is confirmed by serum levels of additional enzymes (LDH and CK) and by histopathology of the cardiac and/or skeletal muscle. High level of LDH may indicate presence of damaged tissues/cells in the liver, cardiac and skeletal muscles [39], [42].
Most serum CK originates from skeletal and cardiac muscles and its elevated serum level can result from muscular injuries. Severe stress and prolonged exercise can also elevate serum CK values. Elevations of AST with increased CK and LDH in the absence of liver disease increase the accuracy of diagnosis of muscular injury [39], [43]. In myocardial injury, AST and CK show significant increase, while ALT exhibits a slight increment [42]. Nevertheless, the increments in serum AST, LDH and CK were not accompanied by significant macro-pathological and histopathological alterations in the liver and heart sections of rats that received 2237.44 mg/kg of herbal tea infusion. This may indicate subchronic administration of herbal tea of M. stenopetala and M. spicata leaves blend at a high dose only altered some functions of the liver and the heart. Otherwise, it takes a chronic (> 90 days) administration to detect remarkable alterations in the liver and heart.
On the other hand, serum biochemical values can also be altered by various factors such as severe stress, prolonged exercise, limited blood sampling, some physiological disorders (e.g., ascites), and infections. For instance, daily handling of rodents can cause severe stress and the stress response give rise to high serum CK [39], [42]. Similar biochemical findings were observed with repeated dose oral administration of Caesalpinia volkensii leaves extract [44] and Coffea arabica green bean extract [41] in rats.
Repeated dose (90-days) oral administration of the herbal tea infusion did not induce macro-pathological lesions or changes in the external and internal viscera of any of the treated groups. During the microscopic evaluation, histological tissues of the organs (liver, kidney, heart, pancreas and stomach) in the treated groups showed no significant pathological lesions. Only minor lesions such as mild hydropic changes of the liver, and mild inflammation of the kidney and pancreas were observed in a few rats of all study groups including the control.
Mild hydrophic change (small vacuolation) is an acute reversible, transient and nonlethal cellular injury and is characterized by intracytoplasmic accumulation of water or cellular swelling [45], [46]. In the present study, the mild hydropic lesion did not alter the hepatocytes, as the liver-specific enzymes (ALT and ALP) in the treated groups were statistically comparable with the control group in both sexes. ALT is liver-specific enzyme and is the most sensitive indicator of liver damage [39].
An inflammation is the process that occurs when an animal tries to remove an injurious agent and/or to repair the damaged tissue and is characterized by infiltration of inflammatory cells (neutrophils) in the injured tissue/organ [45], [46]. In the current study, the mild inflammation in the kidney and pancreas did not distort cellular components and functions of these organs, as the kidney biomarkers (urea, creatinine, uric acid) and pancreatic enzyme (amylase) remained normal for all treated groups in either sex. Furthermore, the aforementioned hepatic, renal and pancreas lesions did not show dose or sex-related changes. These histopathological findings may represent normal anatomic variability, background lesions or spontaneous histopathological changes that are observed in the population of Wistar albino rats [47], [48].
In this study, body weight, food consumption, organs weight (liver and kidney), hemograms (RBCs, HGB, HCT, MCHC) and biochemicals (total cholesterol, TG, total protein, urea) showed sex-dependent variations. These parameters were significantly lower in the female than in the male groups. The sex-dependent findings might be associated with male and female differences in their response to the herbal tea treatment. Several factors are responsible for the sex-differences in drug metabolism including: gonadal steroids, growth hormones, fatty acids and various enzymes [49], [50]. For instance, cytochrome P450 (CYP) is one of the main enzymes involved in drug metabolism. CYP isoforms mediated reactions facilitate the metabolism and excretion of xenobiotics/drugs. The male-female differences in CYP expression or activity can be due to sex hormones, growth hormones, genetics, or due to enzyme inducers in the drug/chemical. In rats, the male-female differences in CYP expression could also exceed 10–100-fold, thus some isoforms appear to be sex-specific. It has been reported that female rats have less total CYP (by 10–30%) as compared with the total CYP in male rats [49], [51]. This sex-specific difference causes many xenobiotics/drugs having longer half-lives and slower clearance in female rats than in male rats. The slower drug metabolism and clearance in female rats produces higher tissue concentrations of xenobiotics that may induce adverse effects in target organ. Therefore, males and females differ in their response to drug treatment [49], [51], which support the current sex-dependent findings. Similar sex-dependent results were observed with toxicity studies in rodents [23], [52].
5. Conclusion
Repeated (90-days) administration of the herbal tea of M. stenopetala and M. spicata leaves formulation did not result in mortality or severe toxicity signs. Administration of the herbal tea caused no significant toxic effects on body weight gain, food consumption, organ weight, hematological and biochemical parameters. However, the serum AST, CK and LDH levels were significantly elevated in rats treated with 2237.44 mg/kg/day of herbal tea. There was no significant alteration in the histology of organs, only minor pathological changes were detected in the liver, kidney and pancreas. Long-term (> 90 days) toxicity study should be carried out to elucidate the inconsistency between the biochemical and histopathological findings. In general, the present study demonstrated that herbal tea of M. stenopetala and M. spicata leaves formulation has a relatively safe subchronic safety profile. However, further preclinical (chronic, teratogenic, reproductive and developmental toxicity) studies in animals are required in order to have sufficient safety and toxicity profiles for its use in humans.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, not-for-profit sectors. Nevertheless, EPHI provided material and chemical support, but had no roles in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
CRediT authorship contribution statement
Abdu Hassen Musa: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Validation, and Writing – original draft. Asfaw Debella: Conceptualization, Methodology, Resources, Supervision, Validation, Writing – review & editing. Girmai Gebru, Eyasu Makonnen: Supervision, Methodology, Resources, Validation, Writing – review & editing. Mesfin Asefa: Supervision, Methodology, Resources, Validation, Writing – review & editing. Abinet Admas: Investigation, Methodology, Validation, Supervision, Methodology, Resources, Validation, Writing – review & editing. Abiy Abebe: Investigation, Methodology, Validation, Supervision, Methodology, Resources, Validation, Writing – review & editing. Boki Lengiso: Investigation, Methodology, Validation, Supervision, Methodology, Resources, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to extend our great appreciation and thanks to the EPHI, Addis Ababa University and Saint Paul’s Hospital Millennium Medical College for their support. We would like to acknowledge Mrs Yewubdar Haile for her contribution in the animal care.
Handling Editor: Dr. Lawrence Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.03.043.
Contributor Information
Abdu Hassen Musa, Email: ahdu97@yahoo.com.
Asfaw Debella Hagos, Email: asfawdebella@gmail.com.
Girmai Gebru Dimsu, Email: girmai.gebru4@gmail.com.
Eyasu Makonnen Eshetu, Email: eyasumakonnen@yahoo.com.
Mesfin Asefa Tola, Email: mesatp@yahoo.com.
Abinet Admas, Email: admasabinet@gmail.com.
Abiy Abebe Gelagle, Email: abiyabg@yahoo.com.
Boki Lengiso Tullu, Email: bokilenjiso@gmail.com.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Birhan W., Giday M., Teklehaymanot T. The contribution of traditional healers’ clinics to public health care system in Addis Ababa, Ethiopia: a cross-sectional study. J. Ethnobiol. Ethnomed. 2011;7(1):1–7. doi: 10.1186/1746-4269-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enioutina E.Y., Salis E.R., Job K.M., Gubarev M.I., Krepkova L.V., Sherwin C.M. Herbal medicines: challenges in the modern world. Part 5. status and current directions of complementary and alternative herbal medicine worldwide. Expert Rev. Clin. Pharm. 2017;10:327–338. doi: 10.1080/17512433.2017.1268917. [DOI] [PubMed] [Google Scholar]
- 3.James P.B., Wardle J., Steel A., Adams J. Traditional, complementary and alternative medicine use in Sub-Saharan Africa: a systematic review. BMJ Glob. Health. 2018;3:1–18. doi: 10.1136/bmjgh-2018-000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mekonnen Y., Gessesse A. Documentation on the uses of Moringa stenopetala and its possible antileishmanial and antifertility effects. SINET: Ethiop. J. Sci. 1998;21(2):287–295. doi: 10.4314/sinet.v21i2.18126. [DOI] [Google Scholar]
- 5.Lawrence B.M., editor. Mint: the genus Mentha. CRC Press; 2006. [DOI] [Google Scholar]
- 6.Edwards S., Tadesse, M., Demissew, S., Hedberg, I., 2000. Flora of Ethiopia and Eritrea, Vol. 2, part1: Magnoliaceae to Flacourtiaceae. Uppsala, Sweden. 〈http://www.worldcat.org〉.
- 7.Mengistu M., Abebe Y., Mekonnen Y., Tolessa T. In vivo and in vitro hypotensive effect of aqueous extract of Moringa stenopetala. J. Afr. Health Sci. 2012;12(4):545–551. PMCID: PMC3598298; PMID: 23515422. [PMC free article] [PubMed] [Google Scholar]
- 8.Geleta B., Makonnen E., Debella A. Toxicological evaluations of the crude extracts and fractions of Moringa stenopetala leaves in liver and kidney of rats. J. Cytol. Histol. 2016;7(1):2–6. doi: 10.4172/2157-7099.1000383. [DOI] [Google Scholar]
- 9.Sileshi T., Makonnen E., Debella A., Tesfaye B. Antihyperglycemic and subchronic toxicity study of Moringa stenopetala leaves in mice. J. Coast. Life Med. 2014;2(3):214–221. doi: 10.12980/JCLM.2.2014C300. [DOI] [Google Scholar]
- 10.Geleta B., Makonnen E., Debella A., Tadele A. In vivo antihypertensive and antihyperlipidemic effects of the crude extracts and fractions of Moringa stenopetala (Baker.) Cufod. leaves in rats. Front. Pharmacol. 2016;7(97):1–10. doi: 10.3389/fphar.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fekadu N., Basha H., Meresa A., Degu S., Girma B., Geleta B. Diuretic activity of the aqueous crude extract and hot tea infusion of Moringa stenopetala (Baker f.) Cufod. leaves in rats. J. Exp. Pharmacol. 2017;9:73–80. doi: 10.2147/JEP.S133778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belayneh A., Asfaw Z., Demissew S., Bussa N.F. Medicinal plants potential and use by pastoral and agro-pastoral communities in Erer Valley of Babile Wereda, Eastern Ethiopia. J. Ethnobiol. Ethnomed. 2012;8(42):1–10. doi: 10.1186/1746-4269-8-42. https://doi.org/10.1186/1746-4269-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassahun B.M., Egata D.F., Lulseged T., Yosef W.B., Tadesse S. Variability in agronomic and chemical characteristics of spearmint (Mentha spicata L.) Genotypes in Ethiopia. Int. J. Adv. Biol. Biomed. Res. 2014;2(10):2704–2711. 〈http://www.ijabbr.com/article_10002.html〉 [Google Scholar]
- 14.Banjaw D.T., Dikir W., Gebre A., Geja W., Tsegaye D., Molla Y. Aromatic and medicinal plants in Wondo Genet Agricultural Research Center Botanical Garden, South Ethiopia. Med. Aroma Plants. 2016;5(6):1–3. doi: 10.4172/2167-0412.1000278. [DOI] [Google Scholar]
- 15.Shahbazi Y. Chemical composition and In Vitro antibacterial activity of Mentha spicata essential oil against common food-borne pathogenic bacteria. J. Pathog. 2015;2015:2–5. doi: 10.1155/2015/916305. Article ID 916305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snoussi M., Noumi E., Trabelsi N., Flamini G., Papetti A., De Feo V. Mentha spicata essential oil: chemical composition, antioxidant and antibacterial activities against planktonic and biofilm cultures of Vibrio spp. Strains. Molecules. 2015;20(8):14402–14424. doi: 10.3390/molecules200814402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahmi F., Khodir M., Mohamed C., Pierre D. Aromatic and medicinal plants - back to nature. InTechOpen; 2017. Chemical Composition and Biological Activities of Mentha Species; pp. 48–55. [DOI] [Google Scholar]
- 18.Tyagi A.K., Malik A. Antimicrobial potential and chemical composition of Mentha piperita oil in liquid and vapor phase against food spoiling microorganisms. Food Control. 2011;22(11):1707–1714. doi: 10.1016/j.foodcont.2011.04.002. [DOI] [Google Scholar]
- 19.Bayani M., Ahmadi-hamedani M., Javan A.J. Study of hyperglycemic, hypo-cholesterolemic and antioxidant activities of IranianMentha spicataleaves aqueous extract in diabetic rats. Iran. J. Pharm. Res. 2017;16:75–82. PMCID: PMC5963648; PMID: 29844778. [PMC free article] [PubMed] [Google Scholar]
- 20.Kasthuri O.R., Ramesh B. Toxicity studies on leaf extracts of Alternanthera brasiliana (L.) Kuntze and Alternanthera bettzickiana (Regel.) Voss. J. Appl. Pharm. Sci. 2018;8(10):082–089. doi: 10.7324/JAPS.2018.81011. [DOI] [Google Scholar]
- 21.Debella, A., 2021. Moringa stenopetala leaves and herbal adjuvants blend medicinal tea granule package for supportive care of pre-diabetes and pre-hypertension. In Proceeding of Dissemination Workshop of Multisectoral Interdisciplinary Research Findings on Moringa stenopetala, Arba-Minch University, Ethiopia, 25–28 February 2021; pp. 50–51. Available online at http://ephi.gov.et.
- 22.Abbasa M.Y., Ejiofora J.I., Yakubu M.I. Acute and chronic toxicity profiles of the methanol leaf extract of Acacia ataxacantha D.C (Leguminosae) in Wistar rats. Bull. Fac. Pharm. Cairo Univ. 2018;56(2018):185–189. doi: 10.1016/j.bfopcu.2018.09.001. [DOI] [Google Scholar]
- 23.Ugwah-Oguejiofor C.J., Okoli C.O., Ugwah M.O., Umaru M.L., Ogbulie C.S., et al. Acute and sub-acute toxicity of aqueous extract of aerial parts of Caralluma dalzielii N. E. Brown in mice and rats. Heliyon. 2019;5(1) doi: 10.1016/j.heliyon.2019.e01179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roome T., Qasim M., Aziz S., Dar Farooq A., Razzaq A., Ali S.F. Assessment of acute, sub-acute, chronic and genotoxicity of polyherbal formulation DCD-684 in mice. Pak. J. Pharm. Sci. 2021;34(4):1485–1498. doi: 10.36721/PJPS.2021.34.4.SUP.1485-1498.1. [DOI] [PubMed] [Google Scholar]
- 25.Teshome D., Tiruneh C., Berhanu L., Berihun G., Belete Z.W. Developmental toxicity of ethanolic extracts of leaves of Achyranthes aspera, Amaranthaceae in rat embryos and fetuses. J. Exp. Pharmacol. 2021;2021(13):555–563. doi: 10.2147/JEP.S312649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abed AL-Ani K.A.M. Primary phytochemical identification and some biochemical parameters study of ethanolic extract of Mentha spicata leaves in mice. J. Chem. Pharm. Res. 2016;8(7):818–822. 〈http://www.jocpr.com〉 (Available at) [Google Scholar]
- 27.European Union (EU), 2010. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the EU L 276/33; pp. 1–47. 〈https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079〉.
- 28.American Veterinary Medical Association (AVMA), 2020. The AVMA Guidelines for the euthanasia of animals: 2020 Edition. American Veterinary Medical Association. 1931 N, Meacham Road, Schaumburg, IL 60173. Version 2020.0.1 22–63. 〈http://www.avma.org〉.
- 29.Organization for Economic Co-Operation and Development (OECD), 2018. Guidelines for the testing of chemicals. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents. PP: 1–10. 〈http://www.oecd.org〉.
- 30.Percie du Sert N., Hurst V., Ahluwalia A., Alam S., Avey M.T., Baker M., et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18(7) doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slaoui M., Fiette L. Histopathology procedures: from tissue sampling to histopathological evaluation. Methods Mol. Biol. 2011;691:69–82. doi: 10.1007/978-1-60761-849-2_4. [DOI] [PubMed] [Google Scholar]
- 32.Suvarna S.K., Layton C., Bancroft J.D. Bancroft’s Theory and Practice of Histological Techniques. Eighth Ed. Elsevier Limited; Nottingham and Sheffield, UK: 2019. 〈http://www.elsevier.com〉 [Google Scholar]
- 33.Chanda I., Parekh J., Vaghasiya Y., Dave R., Baravalia Y., Nair R. Medicinal plants from traditional use to toxicity assessment. Int. J. Pharm. Sci. Res. 2015;6(7):2652–2670. doi: 10.13040/IJPSR.0975-8232.6(7).2652-70. [DOI] [Google Scholar]
- 34.Nohynek G.J. Taylor & Francis; 2002. Presenting toxicology results: how to evaluate data and write reports; pp. 44–156.〈http://www.taylorandfrancis.com〉 [Google Scholar]
- 35.Musa A.H., Debella A., Gebru G., Makonnen E., Mekonnen Y., Kumar P.K. Subchronic toxicity studies of butanol fraction of leaves of Moringa stenopetala in experimental rats. Int. J. Curr. Med. Pharm. Res. 2015;1(10):171–175. 〈http://www.journalcmpr.com〉 [Google Scholar]
- 36.Ajayi T.O., Olanrewaju M.J., Oloruntoba A.C. Toxicological evaluation of Moringa oleifera Lam seeds and leaves in Wistar rats. Pharmacogn. Commun. 2016;6(2):100–111. doi: 10.5530/pc.2016.2.8. [DOI] [Google Scholar]
- 37.Yisehak K., Solomon M., Tadelle M. Contribution of Moringa (Moringa stenopetala) a highly nutritious vegetable tree, for food security in south Ethiopia. Asian J. Appl. Sci. 2011;4(5):477–488. doi: 10.3923/ajaps.2011.477.488. [DOI] [Google Scholar]
- 38.Harrill A.H., Eaddy J.S., Rose K., Cullen J.M., Ramanathan L., Wanaski S., et al. Liver biomarker and in vitro assessment confirm the hepatic origin of aminotransferase elevations lacking histopathological correlate in beagle dogs treated with GABAA receptor antagonist NP260. Toxicol. Appl. Pharmcol. 2014;277:131–137. doi: 10.1016/j.taap.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Everds E.N. Evaluation of clinical pathology data: correlating changes with other study data. Toxicol. Pathol. 2015;43(1):90–97. doi: 10.1177/0192623314555340. [DOI] [PubMed] [Google Scholar]
- 40.Musa A.H., Debella A., Gebru G., Makonnen E., Mekonnen Y., Vata P.K. Biochemical and hematological study on butanol fraction of leaves of Moringa Stenopetala in experimental rats. IOSR. J. Pharm. 2016;6(5):64–68. 〈http://www.iosrphr.org〉 [Google Scholar]
- 41.Venkatakrishna K., Sudeep H.V., Shyamprasad K. Acute and sub-chronic toxicity evaluation of a standardized green coffee bean extract in Wistar albino rats. SAGE Open Med. 2021;9(6):1–12. doi: 10.1177/2050312120984885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whalan J.E. Springer International Publishing; 2015. A Toxicologist’s Guide to Clinical Pathology in Animals: Hematology, Clinical Chemistry, Urinalysis; pp. 27–31. [DOI] [Google Scholar]
- 43.Sadeq AL-Janabi A.A.H., Ali Z.Q., Noree Z.M. Lactate dehydrogenase as an indicator of liver, muscular and cancer diseases. J. Coast. Life Med. 2015;3(7):543–546. doi: 10.12980/JCLM.3.2015J5-54. [DOI] [Google Scholar]
- 44.Musila M.N., Ngai D.N., Mbiri J.W., Njagi S.M., Mbinda W.M., Ngugi M.P. Acute and subchronic oral toxicity study of methanolic extract of Caesalpinia volkensii (Harms) J. Drug Metab. Toxicol. 2017;8(1):1–8. doi: 10.4172/2157-7609.1000222. [DOI] [Google Scholar]
- 45.Underwood J.C.E. General and Systemic Pathology. Fourth Ed. Churchill Livingstone; Edinburgh: 2000. 〈http://www.studentconsult.com/content/default.cfm?ISBN=0443073341&ID〉 [Google Scholar]
- 46.Kumar, V., Abbas, A., Aster, J.C., 2017. Robbins basic pathology (10th ed.). Elsevier Health Science Division. pp. 10–308. 〈http://www.studentconsult.com/content/default.cfm?ISBN=0721692745&ID=P001〉.
- 47.McInnes E.F. Saunders, Elsevier,; Edinburgh: 2012. Background Lesions in Laboratory Animals: A Color Atlas. [DOI] [Google Scholar]
- 48.McInnes J.F. In: First ed., McInnes Elizabeth., editor. John Wiley & Sons Ltd; 2017. Pathology for Toxicologists: Principles and Practices of Laboratory Animal Pathology for Study Personnel; pp. 1–22. [DOI] [Google Scholar]
- 49.Kedderis G., Mugford C. Sex-dependent metabolism of xenobiotics. Drug Metab. Rev. 1998;30(3):441–498. doi: 10.3109/03602539808996322. [DOI] [PubMed] [Google Scholar]
- 50.Leming S., Baitang N. Sex differences in the expression of drug metabolizing and transporter genes in human liver. J. Drug Metab. Toxicol. 2012;3:3–12. doi: 10.4172/2157-7609.1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gochfeld M. Sex differences in human and animal toxicology: Toxicokinetics. Toxicol. Pathol. 2017;45(1):172–189. doi: 10.1177/0192623316677327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fonseca A.G., Sena L.L., Dantas F.R., Fernandes J.M., Zucolotto S.M., et al. In Vivo and In Vitro toxicity evaluation of hydroethanolic extract of Kalanchoe brasiliensis (Crassulaceae) leaves. J. Toxicol. 2018;2018:1–9. doi: 10.1155/2018/6849765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material