Abstract
Introduction
Head and neck cancers were generally characterized with many possible causes. Exposure to outdoor particulate matter affected to multiple organ systems but it was unknown whether which species in PM was an association with cancer incidence.
Objectives
The study aimed to examine the oral- oropharyngeal- laryngeal cancer incidence and accumulated air pollution-related cancers in the spatial patterns.
Methods
Observational study was conducted, and the Poisson log-linear models were used which were analyzed on subgroups-specific incidence rates by national references of Thailand and NASA’s database of aerosol diagnostics model (MERRA-2).
Results
With a significant influence on increasing of 1 μg/m3 black carbon, organic carbon, Dust-PM2.5, and SO4 were associated with increased cancer risk in 1.433 times (95%CI: 1.215–1.690), 1.272 times (95%CI: 1.139–1.420), 3.640 times (95%CI: 2.011–6.589), and 1.704 times (95%CI: 1.334–2.177), respectively.
Conclusion
This study indicated that oral-oropharyngeal-laryngeal cancer incidence could worsen because of adverse air pollution conditions. These issues should be addressed and the importance of the monitoring procedure for dust-PM2.5, sulfate, black carbon, and organic carbon should be emphasized.
Keywords: Incidence, Oral, Oropharyngeal and laryngeal cancer, Air pollution, Spatial epidemiology, Aerosol diagnostics model, MERRA-2
Graphical Abstract
Summary of PM2.5 and oral- oropharyngeal- laryngeal cancer development: PM2.5, which contains dust-PM2.5, sulfate, black carbon, and organic carbon, is typically spread to humans by aerosol substance. Following inhalation and even with food contamination, the pollutants are also associated with increased risk of oral, oropharyngeal and laryngeal cancer.
Highlights
-
•
Dust (DS), sea salt (SS), sulfate (SO4), black carbon (BC) and organic carbon (OC) are main fraction of PM2.5.
-
•
The routes of PM2.5 exposure include ingestion, inhalation, and dermal contact.
-
•
The relative risk of air quality and incidence rates of oral, oropharyngeal and laryngeal cancer was conducted.
-
•
Exposure to Dust, SO4, black carbon and organic carbon can increase the risk of developing cancer.
-
•
Every increase of 1μg/m3 in Dust concentrations were associated with 3.640 times in the risks of cancer.
1. Introduction
Thailand is increasingly facing environmental pollution, particularly air pollution. The concentration of PM2.5 across the Thailand capital city of Bangkok was also caused by vehicular exhausts and biomass burning [1]. A similar problem demonstrated in the Northern and the Northeastern regions [2], [3]. Diesel exhaust was the primary contributor of carbonaceous aerosols at exceeding levels during weekdays [4]. Atmospheric particulate matter in the southern is region composed of organic carbon, elemental carbon, water-soluble ionic species, and polycyclic aromatic hydrocarbons (PAHs). The average concentration of SO4 was the major ionic components [5]. It was also found that the five main air pollution types were sulfur dioxide (SO2), nitrogen dioxide (NO2), carbon monoxide (CO), ozone gas (O3), and dust particles up to 10 µm (PM10) [6]. These pollutants were found higher than normal limits within various regions of Thailand [7]. Diurnal variation of outdoor air pollution was the main result of human activities. The morning peak corresponding to road traffic emitted more particles at ground level, whereas the emissions from the burning of biomass and agricultural crop also influenced the accumulation of aerosol particles at higher atmospheric layer [8].
Multiple primary malignancies are a group of major diseases that were then affected [9], [10], [11]. Moreover, air pollution has a direct impact on both respiratory and cardiovascular diseases and the population morbidity rate [12]. Besides, increasing the amount of PM2.5 in the atmosphere contributes to higher population mortality [13], [14]. Furthermore, toxic aerosol elements in the air such as sulfur dioxide [15] and nickel [16] worsen the mortality rates. The amount and time of exposure to these substances also lead to higher mortality rates [17]. The pollutant is not only prevalent in chronic diseases, but also has a direct impact on acute respiratory diseases [10], [18]. The higher level of trace gaseous species and PM in haze episode significantly affected the requirement of the medical treatment [19].
The exposure of hazardous substances from air pollution to the body system would be harmful and may cause malignancies. Skin contact can cause skin cancer [20]. The presence of pollutants in food is a risk factor for tumors in the gastrointestinal tract [21]. Both the respiratory and gastrointestinal tract share a common pathway that is exposed to pollutants from air and food and has a direct impact on the occurrence of head and neck cancer [22], [23], [24]. Plants and vegetables grown from soil contaminated with heavy metals often lead to acute and chronic toxicity in humans [21], [25]. The same goes for the contamination of water which was recognized as, “The cycle of food and drinking water” [25], [26]. Moreover, chemical particles in the atmosphere would contaminate the entire food chain [27]. PAHs have been detected in various types of food, including soybean. Thai soybean products have unique fingerprints of PAHs. Fortunately, the level was within the acceptable range [28].
Inhaling carcinogenic pollutants from the air and chronic smoking are strongly associated with malignancies arising from oropharynx and laryngopharynx that are considered as a part of head and neck cancers [29], [30], [31], [32]. These referenced the primary cause more than any others that could lead to head and neck cancer. Unfortunately, many people have overlooked the possibilities of air pollution on malignancies [33].
Satellite-related technology provides many advantages. The high-resolution technology of satellites can be utilized to substitute air, water environment, and ground quality indication. NASA scientists can track aerial pollution trends for decades in various regions globally [34]. However, the association between these aerial pollution regions and population health has not yet been explored.
Spatial epidemiology is statistical science for analyzing the relationship between areas with incidences or mortalities from diseases. It also emphasizes the explanation and determination of geographic patterns and their relationship between diseases and various risk factors involved. Various regions have linked and analyzed the risk of disease [35], [36], [37] which leads to the optimal protection approach to provide a link for future medical scientists/ researchers who would like to evaluate the findings on pollution via satellite-related technology. This present study, therefore, examined the relation between oral, oropharyngeal and laryngeal cancer and the associated risks and accumulated pollution in various areas of Thailand, using the satellites to explore the number of toxic substances especially aerosols in ambient air.
2. Materials and methods
Observational research was performed on the study of.
-
(i)
Assessing oral, oropharyngeal and laryngeal cancer incidence rates of 30,720 eligible patients. We collected data of oral, oropharyngeal and laryngeal carcinoma from the Strategy and Planning Division, Ministry of Public Health’s database between January 1, 2017, and December 31, 2017 based on ICD-10 coding. The data displayed the incidence rates in 77 provinces (per 100,000 population) (Table 1). Thailand is located between the coordinates of 5.77434 latitudes to 20.43353 and longitudes of 97.96852–105.22908 [38]. Within the territory, Bangkok is the capital city. Thailand map representation of any kind of data was applied by Quantum Geographic Information System (QGIS) software [39].
-
(ii)
An environmental database
The database was collected from the Modern-Era Retrospective Analysis for Research and Applications version 2 (MERRA-2) by NASA’s Global Modeling and Assimilation Office (GMAO). The quantity of each of aerosol components (aerosol diagnostics model) was analyzed (Black Carbon Surface Mass Concentration or black carbon, Dust Surface Mass Concentration – PM2.5 or mineral dust, Organic Carbon Surface Mass Concentration or organic carbon, Sea Salt Surface Mass Concentration or sea-salt and SO4 Surface Mass Concentration or SO4). The presence of ambient air quality with details of both horizontal and vertical grids from 2010 to 2016 was computed on a cubed-sphere grid and the analysis algorithm model was attributed by controlling the variable for moisture used in recent versions of Gridpoint Statistical Interpolation analysis system (GSI) [40]. Highlights of the MERRA-2 system performed detailed data analysis every 3 h. This data was utilized as the accumulated dose for each substance.
-
(iii)
Carcinogenic risks associated with air quality
An investigation of spatial epidemiology associations for the relative risk of air quality and incidence rates of oral, oropharyngeal and laryngeal cancer in Thailand was conducted. The analysis correlations were separately analyzed by provinces.
2.1. Data processing and analysis
-
1.
The spatial patterns were characterized by their influence between place - air quality in accumulated dose and burden of the diseases- location was examined. The analysis was used to detect which substance risk factors most correlated with cancer cases throughout the year by the Poisson log-linear model. The correlation between the incidence rate ratios of cancer and the accumulated surface mass concentration of each substance was performed to confirm their spatial correlation.
-
2.
It is expected that different substances may become hybridizing various interactions to organ targets. The air quality profile from MERRA-2 of accumulated surface mass concentration was analyzed by the individual risk, except for the sea salt. The incidence rate ratio of oral, oropharyngeal and laryngeal cancer was estimated. This subsequent procedure was to compare the incidence rate ratio by each province. The low incidence rate area was used as comparable base with other provinces for estimation of the high-risk area.
2.2. Ethics approval and consent to participate
Data were obtained from two public domains that were opened for the public to use for noncommercial purposes. None of the variables or data used in this study allowed the identification of individuals. Confidentiality in this study was considered together with the privacy consideration, where relevant. The obligation to protect and promote the non-disclosure of information imparted in a relationship of trust lies at the core of the concept of confidentiality. The study was reviewed and approved by the Khon Kaen University Ethics Committee for Human Research (HE611183).
3. Results
Dust particles in our atmosphere are recognized by the suspension particles of solids and liquids in the air by the MERRA-2 system. These systems are necessary for performing the elements of the different tasks required such as black carbon, organic carbon, dust-PM2.5, SO4, and sea salt. The advantages of this system include a reanalysis and near-real-time climate analysis. The variables were chosen to evaluate the aerosol diagnostic series with ambient air quality from 2010 to 2016 (Table 1).
This study determined the average black carbon, organic carbon, sea salt, dust-PM2.5, and sulfate to be 8.211 μg/m3 (interquartile range [IQR]: 5.777 ± 10.058), 49.566 μg/m3 (interquartile range [IQR]: 47.318 ± 59.660), 119.374 μg/m3 (interquartile range [IQR]: 48.577 ± 180.296), 14.368 μg/m3 (interquartile range [IQR]: 12.343 ± 16.369), and 26.112 μg/m3 (interquartile range [IQR]: 22.842 ± 31.770), respectively.
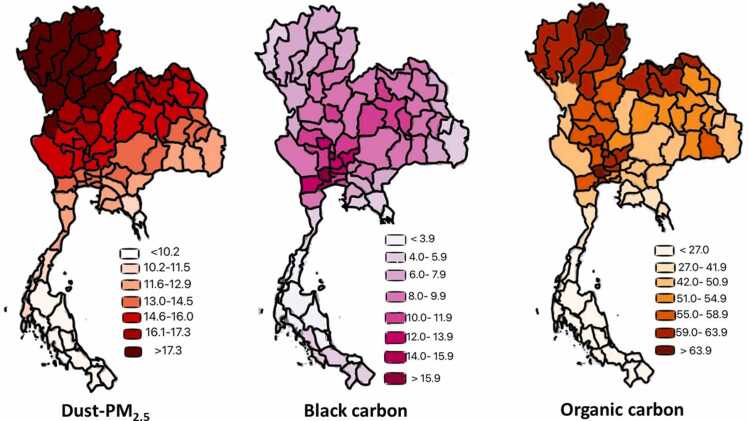
The number of new cases of cancer (cancer incidence) was 31.859 per 100,000 people per year (based on 2017 cases). Fig. 1 displays the incidence rate of oral, oropharyngeal and laryngeal cancer in selected regions by spatial distribution as a scale for the various locations. The incidence rates were mostly concentrated in some provinces of the Northern, Northeastern, Southern and the Central regions of Thailand. The results of this study indicated the largest percentage of cancer incidence in three provinces. For research purposes, the distribution of pollution intensity in Thailand between 2010 and 2016 is presented in Fig. 2, Fig. 3. They demonstrate the natural arrangement of the geographical distribution in accumulated dose which estimate the exposure of Dust– PM2.5, Black Carbon, Organic Carbon, and Sulfate Surface Mass Concentration across the different provinces. The maps were adjusted on an identical grid thus enabling a quantitative comparison between them. The correlation between these maps was relatively low (light color), emitted small amounts of pollution and dark color for large amounts of high pollution. Organic carbon and dust-PM2.5 in a grid resolution of 0.50 × 0.6250 and 72 hybrid-eta levels from the surface to 0.01hPa were displayed more clustered pattern with higher values in the Northern, Northeastern and the Central regions, whereas black carbon and sulfate were densely compacted in the Northeastern and the Central regions of Thailand. The visual and geographical analysis of the map system revealed overall information of ambient air quality and the incidences of cancer. Results from the analysis showed that 12 of the 77 provinces had highest accumulated concentration level of dust-PM2.5, indicating a poorest degree of collinearity among certain predictors within census categories. Whereas, sulfate, organic carbon, and black carbon were found highest in 7, 5, and 1 province, respectively.
Fig. 1.
Thailand geographic map and the oral, oropharyngeal and laryngeal cancer incidence (per 100,000 population) in the year 2017.
Fig. 2.
Geographic accumulated Dust– PM2.5, Black Carbon and Organic Carbon Surface Mass Concentration (μg/m3) between 2010 and 2016.
Fig. 3.
Geographic accumulated Sulfate and Sea Salt Surface Mass Concentration (μg/m3). The results of this study indicated that there were three provinces had the largest percentage of cancer incidence.
The risk-exceedance probabilities were shown in Table 2. Dust-PM2.5 showed strong associations with cancer risk. Minor associated risk reduced in SO4, black carbon, and organic carbon, respectively. A significant influence on increasing of 1 μg/m3 Dust-PM2.5, SO4 black carbon, and organic carbon were associated with increased cancer risk in 3.640 times (95%CI: 2.011–6.589), 1.704 times (95%CI: 1.334–2.177), 1.433 times (95%CI: 1.215–1.690), and 1.272 times (95%CI: 1.139–1.420), respectively.
4. Discussion
The measurement of ambient air quality is a standard procedure used to measure various substances in the atmosphere such as carbon monoxide, lead, nitrogen dioxide, ozone, sulfur dioxide, and PM which contain various sizes of dust and moisture particles. PM10 is inhalable particles approximately 10 micrometers in circumference or smaller. PM2.5 is a tiny inhalable particle, generally 2.5 micrometers in circumference or smaller. Ground stations were built for collecting polluted data to a variety of users and monitoring. So far as reasonably practicable, there was insufficient station in each country. For instance, some technocrats were the choice to change the procedures with high capacity of data collection. This present study focused on the process of gathering and analyzing data by the Modern-Era Retrospective Analysis for Research and Applications, Version 2 (MERRA-2) manufactured by NASA’s Global Modeling and Assimilation Office (GMAO). These methods are advantageous for understanding risk factors involving geographic locations that were affected by the incidences of oral, oropharyngeal, and laryngeal cancer in Thailand from various locations in 2017. The definite effect-time of more than 5 years of exposure to air pollution was shown to increase mortality of respiratory disease, lung cancer, colon cancer and cardiovascular disease [41], [42], [43]. The developed design of this study is also possible to confirm with the cancer association regarding risk factors in various areas for more than 5 years of exposure; pollution detection in general. A common method of assessment for air pollution is using a continuous ambient air monitoring ground station. However, some provinces in Thailand provide insufficient data for effective measurements [44]. For this reason, MERRA-2 models may be a tool that can monitor all areas simultaneously. Quality information between two resources, the MERRA-2 PM2.5 was underestimated compared with ground measurements, partly due to the bias in the MERRA-2 Aerosol Optical Depth (AOD) assimilation [45]. On the other hand, gathering pollution data by the ground station was suitable for local monitoring and too difficult to elaborate all areas or for large scale. Most installations required expensive equipment and complex units [46] which is why air quality monitoring throughout the country by MERRA-2 would be the most appropriate measurement method.
Our results are consistent among the cancer studies with the strong link between air quality and oral- oropharyngeal- laryngeal cancer development. However, based on our analysis such an approach is inhibited by considerable problems with data availability. Specific of some datasets such as individual-level risk factors, uncertainties in exposure, lifestyle, occupation risks, smoking habits, underlying diseases, and genetic predisposition were not collected. We designed the common methods for studying relationships between the spatially non-random cancer incidence rates and spatial patterns of the accumulated long-term exposure to air pollutants, and manipulated the data attributed availability by a Bayesian hierarchical regression modeling framework.
To our knowledge, this is the first study to investigate the relevance of specific chemical components of PM substances and upper aerodigestive tract cancers using advanced technological data recorders. The study site (Fig. 1), Northern and Northeastern, shows the dense location of cancer incidence (dark pink). In comparison with the accumulated pollutants, Dust– PM2.5 showed the same direction in color tone which explains the higher correlation than other pollutants. Agriculture was the principal occupation in Northern and Northeastern of Thailand. Major crops were rice, corn, and sugarcane. After harvesting, they burned the grass to quickly prepare the farmland for the next sowing. It contributed to smog [47], [48]. Dust-PM2.5 was the most potentially carcinogenic correlation, followed by sulfate, black carbon, and organic carbon, respectively. There is an excess risk of cancer incidence in areas with high aerosol levels. There were some of the important local-level characterizations of violence dynamics, which are complicated by spatio-temporal scales of variation in influencing factors that drive diversity of the cancer incidence.
Uncertainties in the perception of their complex effects limit our knowledge about PM composition to health affected. Understanding of factorization of composition in PM was linked to identify and quantify the types of PM sources. The information of influences in geographic regions to specific sources of PM was essential in future efforts to reduce levels of aerosol pollutants and the overall burden on cancer. A significant influence on increasing of 1 μg/m3 Dust-PM2.5 was associated with increased cancer risk in 3.6 times, whereas black carbon, organic carbon, and SO4 were associated with increased cancer risk in 1.2–1.7 times. This determined that not only the concentration of PM affects the organ system but the amount of each composition could influence in varying degree.
Epidemiologic studies over the past 40 years displayed the effect of the general ambient air pollution; the burning of fossil fuels from automobiles and industrial plants releases emissions throughout the day resulted in significantly higher rates of lung cancer [49]. It is also possible that prolonged exposure (many years) can promote the rate of cancer [50] and the examination of miRNAs discovered that it is associated with non-small cell lung cancer [51]. Ambient Air is not only a single toxic aerosol substance with many forms. According to studies in rodents which indicated the atmosphere with ozone (O3), or nitrogen dioxide (NO2) was related to inflammation in the respiratory tract and lung function [52], [53]. Research gathered from several countries displayed risk factors that cause lung cancer as a result of pollution such as black smoke concentration, NO2, PM2.5, PM10, and SO2 [54], [55], [56], [57], [58]. The next generation of pollutants-driven disease prediction models will be most focused on the interaction of each chemical pollutant with another in PM or even another aerosol to cancer effects.
In the matter of head and neck cancer, the instances of the disease differ in various areas, such as oral cancer found in India, nasopharyngeal cancer discovered in Hong Kong, pharyngeal and laryngeal cancers distributed worldwide. The primary contributing factors that appear to increase the risk of cancer are smoking, drinking, and betel nut chewing [59]. It is important to note that Thailand’s national anti-smoking campaign affected the behavior trends. Prevalence of smoking in Thailand dropped from 42.1% in 2010 to 38.8% in 2016 [60]. The highest prevalence of smoking was recorded from the Northeast and the South regions [61]. However, the incidence of tongue and oral, oropharyngeal cancer in males increased between 2013 and 2015 [62]. This reversal tendency of primary risk and cancer prevalence could be attributed to risk factors. Future studies, therefore, should assess correlation, including synergistic effects between each intrinsic and extrinsic cancer risk which may impact future policy measures for cancer prevention strategies. A study regarding the impact of pollution on these group’s cancers is also less common compared to lung cancer [49], [50], [51]. If we think about the path that the air passes through the lungs, these organs will have the same impact. Negative environmental impacts are more dangerous than most people conceive. However, some studies found that PM10 and pollution are directly related to the occurrence of laryngeal cancers as well as lung cancer [63], [64]. In this regard, it is similar to passive smoking. These factors are commonly neglected [65]. PM is harmful in the atmosphere with the ability to affect both lung cancer and laryngeal cancer [66]. Primary factors that affect the high level of aerosol in Thailand, mainly due to the rising population problems and lack of pollution control such as automobiles, industrial plants, and agricultural practices that focus on burning to harvest or preparation for the next season. This research, coupled with these precious air quality monitoring technologies, is a step and opportunity for better air quality in Thailand.
5. Conclusion
From obvious relationships between oral, oropharyngeal, and laryngeal cancer with ambient air quality based on the MERRA-2 model, environmental factor detectors, NASA’s technology, play a vital part in the management of health. This study indicated that oral, oropharyngeal, and laryngeal cancer incidence could worsen because of adverse air pollution conditions. These issues should be addressed and the importance of the monitoring procedure for dust-PM2.5, sulfate, black carbon, and organic carbon should be emphasized. It is also important to limit chronic exposure to air pollution as much as possible, the changes will provide everyone with quality living.
Funding
This work was supported by the Chronic Kidney Disease Prevention in the Northeast of Thailand (CKDNET) for assistance with statistical analysis and the Research Affairs, Faculty of Medicine, Khon Kaen University, Thailand for funding (IN62323). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
CRediT authorship contribution statement
Kriangsak Jenwitheesuk: Conceptualization, Methodology, Writing – review & editing. Udomlack Peansukwech: Conceptualization, Visualization, Methodology, Software, Data curation, Writing – review & editing. Kamonwan Jenwitheesuk: Conceptualization, Methodology, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We acknowledge the MODIS mission scientists who are associated with NASA’s personnel for pollution information and the Strategy and Planning Division, Ministry of Public Health of Thailand, for the cancer database used in this research effort. The authors wish to thank assistant professor Isaraporn Thepwongsa and Dr. Radhakrishnan Muthukumar for assistance with the English-language presentation. We also thank Saksri and Supaporn Jenwitheesuk for the final assembled figure demonstration of the manuscript.
Authors' contributions
All authors conceived and planned the project. UP performed the analytic calculations and performed the numerical simulations. KJ and KJ took the lead in writing the manuscript. All authors provided critical feedback and help.
Handling Editor: Dr. Lawrence Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.04.015.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.ChooChuay C., Pongpiachan S., Tipmanee D., Suttinun O., Deelaman W., Wang Q., et al. Impacts of PM2. 5 sources on variations in particulate chemical compounds in ambient air of Bangkok, Thailand. Atmos. Pollut. Res. 2020;11:1657–1667. [Google Scholar]
- 2.Pengchai P., Chantara S., Sopajaree K., Wangkarn S., Tengcharoenkul U., Rayanakorn M. Seasonal variation, risk assessment and source estimation of PM 10 and PM10-bound PAHs in the ambient air of Chiang Mai and Lamphun, Thailand. Environ. Monit. Assess. 2009;154:197–218. doi: 10.1007/s10661-008-0389-0. [DOI] [PubMed] [Google Scholar]
- 3.Pongpiachan S., Tipmanee D., Khumsup C., Kittikoon I., Hirunyatrakul P. Assessing risks to adults and preschool children posed by PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) during a biomass burning episode in Northern Thailand. Sci. Total Environ. 2015;508:435–444. doi: 10.1016/j.scitotenv.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Pongpiachan S., Kositanont C., Palakun J., Liu S., Ho K.F., Cao J. Effects of day-of-week trends and vehicle types on PM2. 5-bounded carbonaceous compositions. Sci. Total Environ. 2015;532:484–494. doi: 10.1016/j.scitotenv.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 5.ChooChuay C., Pongpiachan S., Tipmanee D., Deelaman W., Suttinun O., Wang Q., et al. Long-range transboundary atmospheric transport of polycyclic aromatic hydrocarbons, carbonaceous compositions, and water-soluble ionic species in southern Thailand. Aerosol Air Qual. Res. 2020;20:1591–1606. [Google Scholar]
- 6.Greenpeace. Pollution of small dust not more than 2.5 microns (PM2.5) of the city in Thailand between January-June 2017 [Internet]. 2018 [cited 2018 Oct 10]. Available from: 〈https://bit.ly/2qz8Fh5〉 (in Thai).
- 7.Pongpiachan S., Hattayanone M., Cao J. Effect of agricultural waste burning season on PM2.5-bound polycyclic aromatic hydrocarbon (PAH) levels in Northern Thailand. Atmos. Pollut. Res. 2017;8:1069–1080. [Google Scholar]
- 8.Pongpiachan S., Ho K., Cao J. Effects of biomass and agricultural waste burnings on diurnal variation and vertical distribution of OC/EC in Hat-Yai City, Thailand. Asian J. Appl. Sci. 2014;7:360–374. [Google Scholar]
- 9.Chang C.C., Chung Y.H., Liou C.B., Lee Y.C., Weng W.L., Yu Y.C., et al. Influence of residential environment and lifestyle on multiple primary malignancies in Taiwan. Asian Pac. J. Cancer Prev. 2015;16:3533–3538. doi: 10.7314/apjcp.2015.16.8.3533. [DOI] [PubMed] [Google Scholar]
- 10.Jiřík V., Machaczka O., Miturová H., Tomášek I., Šlachtová H., Janoutová J., et al. Air pollution and potential health risk in ostrava region - a review. Cent. Eur. J. Public Health. 2016;24:S4–S17. doi: 10.21101/cejph.a4533. [DOI] [PubMed] [Google Scholar]
- 11.Wong C.M., Tsang H., Lai H.K., Thomas G.N., Lam K.B., Chan K.P., et al. Cancer mortality risks from long-term exposure to ambient fine particle. Cancer Epidemiol. Biomark. Prev. 2016;25:839–845. doi: 10.1158/1055-9965.EPI-15-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunekreef B., Beelen R., Hoek G., Schouten L., Bausch-Goldbohm S., Fischer P., et al. Effects of long-term exposure to traffic-related air pollution on respiratory and cardiovascular mortality in the Netherlands: the NLCS-AIR study. Res. Rep. Health Eff. Inst. 2009;139:5–71. discussion 73-89. [PubMed] [Google Scholar]
- 13.Krewski D., Burnett R., Jerrett M., Pope C.A., Rainham D., Calle E., et al. Mortality and long-term exposure to ambient air pollution: ongoing analyses based on the American Cancer Society cohort. J. Toxicol. Environ. Health A. 2005;68:1093–1109. doi: 10.1080/15287390590935941. [DOI] [PubMed] [Google Scholar]
- 14.Krewski D., Jerrett M., Burnett R.T., Ma R., Hughes E., Shi Y., et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res. Rep. Health Eff. Inst. 2009;140:5–114. discussion 115-36. [PubMed] [Google Scholar]
- 15.Krewski D., Burnett R.T., Goldberg M.S., Hoover B.K., Siemiatycki J., Jerrett M., et al. Overview of the reanalysis of the Harvard Six Cities Study and American Cancer Society study of particulate air pollution and mortality. J. Toxicol. Environ. Health A. 2003;66:1507–1551. doi: 10.1080/15287390306424. [DOI] [PubMed] [Google Scholar]
- 16.Tsai K.Y., Su C.C., Chiang C.T., Tseng Y.T., Lian I.B. Environmental heavy metal as a potential risk factor for the progression of oral potentially malignant disorders in central Taiwan. Cancer Epidemiol. 2017;47:118–124. doi: 10.1016/j.canep.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Abrahamowicz M., Schopflocher T., Leffondré K., du Berger R., Krewski D. Flexible modeling of exposure-response relationship between long-term average levels of particulate air pollution and mortality in the American Cancer Society study. J. Toxicol. Environ. Health A. 2003;66:1625–1654. doi: 10.1080/15287390306426. [DOI] [PubMed] [Google Scholar]
- 18.Goswami P., Baruah J. Quantitative assessment of relative roles of drivers of acute respiratory diseases. Sci. Rep. 2014;4:6532. doi: 10.1038/srep06532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pongpiachan S., Paowa T. Hospital out-and-in-patients as functions of trace gaseous species and other meteorological parameters in Chiang-Mai, Thailand. Aerosol Air Qual. Res. 2014;15:479–493. [Google Scholar]
- 20.Cohen A.J., Ross Anderson H., Ostro B., Pandey K.D., Krzyzanowski M., Künzli N., et al. The global burden of disease due to outdoor air pollution. J. Toxicol. Environ. Health A. 2005;68:1301–1307. doi: 10.1080/15287390590936166. [DOI] [PubMed] [Google Scholar]
- 21.Islam Eu, Yang X., He Z., Mahmood Q. Assessing potential dietary toxicity of heavy metals in selected vegetables and food crops. J. Zhejiang Univ. Sci. B. 2007;8:1–13. doi: 10.1631/jzus.2007.B0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roshandel G., Semnani S., Malekzadeh R., Dawsey S.M. Polycyclic aromatic hydrocarbons and esophageal squamous cell carcinoma. Arch. Iran. Med. 2012;15:713–722. [PMC free article] [PubMed] [Google Scholar]
- 23.Lin W.C., Lin Y.P., Wang Y.C., Chang T.K., Chiang L.C. Assessing and mapping spatial associations among oral cancer mortality rates, concentrations of heavy metals in soil, and land use types based on multiple scale data. Int. J. Environ. Res. Public Health. 2014;11:2148–2168. doi: 10.3390/ijerph110202148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong I.C.K., Ng Y.K., Lui V.W.Y. Cancers of the lung, head and neck on the rise: perspectives on the genotoxicity of air pollution. Chin. J. Cancer. 2014;33:476–480. doi: 10.5732/cjc.014.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waseem A., Arshad J., Iqbal F., Sajjad A., Mehmood Z., Murtaza G. Pollution status of Pakistan: a retrospective review on heavy metal contamination of water, soil, and vegetables. BioMed. Res. Int. 2014;2014 doi: 10.1155/2014/813206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ismail A., Riaz M., Akhtar S., Ismail T., Amir M., Zafar-ul-Hye M. Heavy metals in vegetables and respective soils irrigated by canal, municipal waste and tube well waters. Food Addit. Contam. Part B Surveill. 2014;7:213–219. doi: 10.1080/19393210.2014.888783. [DOI] [PubMed] [Google Scholar]
- 27.Pandey J., Pandey U. Accumulation of heavy metals in dietary vegetables and cultivated soil horizon in organic farming system in relation to atmospheric deposition in a seasonally dry tropical region of India. Environ. Monit. Assess. 2009;148:61–74. doi: 10.1007/s10661-007-0139-8. [DOI] [PubMed] [Google Scholar]
- 28.Pongpiachan S. A preliminary study of using polycyclic aromatic hydrocarbons as chemical tracers for traceability in soybean products. Food Control. 2015;47:392–400. [Google Scholar]
- 29.Sasco A.J., Secretan M.B., Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45(Suppl 2):S3–S9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- 30.Giraldi L., Leoncini E., Pastorino R., Wünsch-Filho V., de Carvalho M., Lopez R., et al. Alcohol and cigarette consumption predict mortality in patients with head and neck cancer: a pooled analysis within the International Head and Neck Cancer Epidemiology (INHANCE) Consortium. Ann. Oncol. 2017;28:2843–2851. doi: 10.1093/annonc/mdx486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beynon R.A., Lang S., Schimansky S., Penfold C.M., Waylen A., Thomas S.J., et al. Tobacco smoking and alcohol drinking at diagnosis of head and neck cancer and all-cause mortality: Results from head and neck 5000, a prospective observational cohort of people with head and neck cancer. Int. J. Cancer. 2018;143:1114–1127. doi: 10.1002/ijc.31416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Y.Q., Xue W.Q., Shen G.P., Tang L.L., Zeng Y.X., Jia W.H. Household inhalants exposure and nasopharyngeal carcinoma risk: a large-scale case-control study in Guangdong, China. BMC Cancer. 2015;15:1022. doi: 10.1186/s12885-015-2035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calderón-Garcidueñas L., Delgado R., Calderón-Garcidueñas A., Meneses A., Ruiz L.M., De La Garza J., et al. Malignant neoplasms of the nasal cavity and paranasal sinuses: a series of 256 patients in Mexico City and Monterrey. Is air pollution the missing link? Otolaryngol. Head Neck Surg. 2000;122:499–508. doi: 10.1067/mhn.2000.103080. [DOI] [PubMed] [Google Scholar]
- 34.K. Northon, New NASA satellite maps show human fingerprint on global air quality [Internet]. Washington: National Aeronautics and Space Administration [updated 2017 Aug 7; cited 2018 Nov 15]. Available from: 〈https://go.nasa.gov/1RNP304〉.
- 35.Lippi C.A., Stewart-Ibarra A.M., Muñoz Á.G., Borbor-Cordova M.J., Mejía R., Rivero K., et al. The social and spatial ecology of dengue presence and burden during an outbreak in Guayaquil, Ecuador, 2012. Int. J. Environ. Res. Public Health. 2018;15:827. doi: 10.3390/ijerph15040827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siraj A.S., Rodriguez-Barraquer I., Barker C.M., Tejedor-Garavito N., Harding D., Lorton C., et al. Spatiotemporal incidence of Zika and associated environmental drivers for the 2015-2016 epidemic in Colombia. Sci. Data. 2018;5 doi: 10.1038/sdata.2018.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang N., Mengersen K., Kimlin M., Zhou M., Tong S., Fang L., et al. Lung cancer and particulate pollution: a critical review of spatial and temporal analysis evidence. Environ. Res. 2018;164:585–596. doi: 10.1016/j.envres.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 38.latitudelongitude.org [Internet]. Thailand latitude and longitude [updated 2015; cited 2018 Nov 15]; [about 1 screens]. Available from: 〈https://www.latitudelongitude.org/th/〉.
- 39.QGIS Development Team. QGIS Geographic Information System [Internet]. [updated 2013; cited 2018 Nov 15]. Available from: 〈https://www.qgis.org/en/site/〉.
- 40.Gelaro R., McCarty W., Suárez M.J., Todling R., Molod A., Takacs L., et al. The modern-era retrospective analysis for research and applications, version 2 (MERRA-2) J. Clim. 2017;30:5419–5454. doi: 10.1175/JCLI-D-16-0758.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pun V.C., Kazemiparkouhi F., Manjourides J., Suh H.H. Long-term PM2.5 exposure and respiratory, cancer, and cardiovascular mortality in older US adults. Am. J. Epidemiol. 2017;186:961–969. doi: 10.1093/aje/kwx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenwitheesuk K., Peansukwech U., Jenwitheesuk K. Construction of polluted aerosol in accumulation that affects the incidence of lung cancer. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenwitheesuk K., Peansukwech U., Jenwitheesuk K. Accumulated ambient air pollution and colon cancer incidence in Thailand. Sci. Rep. 2020;10:17765. doi: 10.1038/s41598-020-74669-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greenpeace. Air quality situation of Thailand [Internet]. [updated 2019; cited 2021 Feb 1]. Available from: 〈https://www.greenpeace.org/thailand/story/2176/thailand-aqi-rank〉.
- 45.He L., Lin A., Chen X., Zhou H., Zhou Z., He P. Assessment of MERRA-2 Surface PM2.5 over the Yangtze River Basin: ground-based verification, spatiotemporal distribution and meteorological dependence. Remote Sens. 2019;11:460. (in Thai) [Google Scholar]
- 46.Snyder E.G., Watkins T.H., Solomon P.A., Thoma E.D., Williams R.W., Hagler G.S., et al. The changing paradigm of air pollution monitoring. Environ. Sci. Technol. 2013;47:11369–11377. doi: 10.1021/es4022602. Epub 2013 Oct 3. [DOI] [PubMed] [Google Scholar]
- 47.Facts and details. Agruculture in Thailand: history, land use, indebted farmers, irrigation and food industries [Internet]. 2014 [cited 2022 Apr 7]. Available from: 〈https://factsanddetails.com/southeast-asia/Thailand/sub5_8h/entry-3319.html〉.
- 48.Think global health. The tangled problem of sugarcane burning in Thailand [Internet]. 2022 [cited 2022 Apr 7]. Available from: 〈https://www.thinkglobalhealth.org/article/tangled-problem-sugarcane-burning-thailand〉.
- 49.Cohen A.J., Pope C.A., bSpeizer F.E. Ambient air pollution as a risk factor for lung cancer. Salud Publica Mex. 1997;39:346–355. doi: 10.1590/s0036-36341997000400012. [DOI] [PubMed] [Google Scholar]
- 50.Tsoi C.T., Tse L.A. Professional drivers and lung cancer: a systematic review and meta-analysis. Occup. Environ. Med. 2012;69:831–836. doi: 10.1136/oemed-2012-100666. [DOI] [PubMed] [Google Scholar]
- 51.Pan H.L., Wen Z.S., Huang Y.C., Cheng X., Wang G.Z., Zhou Y.C., et al. Down-regulation of microRNA-144 in air pollution-related lung cancer. Sci. Rep. 2015;5:14331. doi: 10.1038/srep14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Neill C.A., van der Vliet A., Eiserich J.P., Last J.A., Halliwell B., Cros C.E. s Oxidative damage by ozone and nitrogen dioxide: synergistic toxicity in vivo but no evidence of synergistic oxidative damage in an extracellular fluid. Biochem. Soc. Symp. 1995;61:139–152. doi: 10.1042/bss0610139. [DOI] [PubMed] [Google Scholar]
- 53.Chitano P., Rado V., Di Stefano A., Papi A., Boniotti A., Zancuoghi G., et al. Effect of subchronic in vivo exposure to nitrogen dioxide on lung tissue inflammation, airway microvascular leakage, and in vitro bronchial muscle responsiveness in rats. Occup. Environ. Med. 1996;53:379–386. doi: 10.1136/oem.53.6.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beelen R., Hoek G., van den Brandt P.A., Goldbohm R.A., Fischer P., Schouten L.J., et al. Long-term exposure to traffic-related air pollution and lung cancer risk. Epidemiology. 2008;19:702–710. doi: 10.1097/EDE.0b013e318181b3ca. [DOI] [PubMed] [Google Scholar]
- 55.Cesaroni G., Badaloni C., Gariazzo C., Stafoggia M., Sozzi R., Davoli M., et al. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ. Health Perspect. 2013;121:324–331. doi: 10.1289/ehp.1205862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dockery D.W., Pope C.A., Xu X., Spengler J.D., Ware J.H., Fay M.E., et al. An association between air pollution and mortality in six U.S. cities. New Engl. J. Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 57.Jerrett M., Burnett R.T., Ma R., Pope C.A., Krewski D., Newbold K.B., et al. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology. 2005;16:727–736. doi: 10.1097/01.ede.0000181630.15826.7d. [DOI] [PubMed] [Google Scholar]
- 58.Heinrich J., Thiering E., Rzehak P., Krämer U., Hochadel M., Rauchfuss K.M., et al. Long-term exposure to NO2 and PM10 and all-cause and cause-specific mortality in a prospective cohort of women. Occup. Environ. Med. 2013;70:179–186. doi: 10.1136/oemed-2012-100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sankaranarayanan R., Masuyer E., Swaminathan R., Ferlay J., Whelan S. Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Res. 1998;18:4779–4786. [PubMed] [Google Scholar]
- 60.Statista. Prevalence of smoking for males in Thailand from 2010 to 2016 2019 [Internet]. [updated 2018; cited 2021 Feb 1]. 〈https://www.statista.com/statistics/732857/thailand-male-smoking-rate/〉.
- 61.Lim A., McNeil D. Modelling for demographic and regional prevalence and trends of smoking in Thai males. Southeast Asian J. Trop. Med. Public Health. 2016;47:309–317. [PubMed] [Google Scholar]
- 62.Arunpraphan S. The trends of oral cavity and pharyngeal cancer incidence in Thailand. 2004-2015. Dent. Public Health J. 2019;24:55–67. [Google Scholar]
- 63.García-Pérez J., Pollán M., Boldo E., Pérez-Gómez B., Aragonés, Lope V., et al. Mortality due to lung, laryngeal and bladder cancer in towns lying in the vicinity of combustion installations. Sci. Total Environ. 2009;407:2593–2602. doi: 10.1016/j.scitotenv.2008.12.062. [DOI] [PubMed] [Google Scholar]
- 64.Yanagi Y., Assunção J.V., Barrozo L.V. The impact of atmospheric particulate matter on cancer incidence and mortality in the city of São Paulo, Brazil. Cad. Saude Publica. 2012;28:1737–1748. doi: 10.1590/s0102-311x2012000900012. [DOI] [PubMed] [Google Scholar]
- 65.Mallis A., Jelastopulu E., Mastronikolis N.S., Naxakis S.S., Kourousis C., Papadas T.A. Laryngeal cancer and passive smoking: the neglected factor? Eur. Arch. Otorhinolaryngol. 2011;268:727–731. doi: 10.1007/s00405-010-1403-z. [DOI] [PubMed] [Google Scholar]
- 66.Pereira F.A.C., Assunção J.V., Saldiva P.H.N., Pereira L.A.A., Mirra A.P., Braga A.L.F. Influence of air pollution on the incidence of respiratory tract neoplasm. J. Air Waste Manag. Assoc. 2005;55:83–87. doi: 10.1080/10473289.2005.10464603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.