Abstract
Neural oscillations are thought to play a central role in orchestrating activity states between distant neural populations. For example, during isometric contraction, 13–30 Hz beta activity becomes phase coupled between the motor cortex and the contralateral muscle. This and related observations have led to the proposal that beta activity and connectivity sustain stable cognitive and motor states – or the ‘status quo’ – in the brain. Recently, however, beta activity at the single-trial level has been shown to be short-lived – though so far this has been reported for regional beta activity in tasks without sustained motor demands. Here, we measured magnetoencephalography (MEG) and electromyography (EMG) in 18 human participants performing a sustained isometric contraction (gripping) task. If cortico-muscular beta connectivity is directly responsible for sustaining a stable motor state, then beta activity within single trials should be (or become) sustained in this context. In contrast, we found that motor beta activity and connectivity with the downstream muscle were transient. Moreover, we found that sustained motor requirements did not prolong beta-event duration in comparison to rest. These findings suggest that neural synchronisation between the brain and the muscle involves short ‘bursts’ of frequency-specific connectivity, even when task demands – and motor behaviour – are sustained.
Keywords: Beta, Burst, Neural oscillation, Movement
Highlights
-
•
Trial-average 13–30 Hz beta activity and connectivity with the muscle appear sustained during stable motor behaviour.
-
•
Single-trial beta activity and connectivity are short-lived, even when motor behaviour is sustained.
-
•
Sustained task demands do not prolong beta-event duration in comparison to resting state.
1. Introduction
Frequency-specific patterns of neural activity – often referred to as ‘neural oscillations’ – are ubiquitous in the brain and have been postulated to play a central role in orchestrating communication between remote neural populations (Buzsáki, 2009, Fries, 2005, Siegel et al., 2012, Thut et al., 2012, Varela et al., 2001, Vidaurre et al., 2018). Beta activity (13–30 Hz) is one prominent class of such frequency-specific brain activity (Jenkinson and Brown, 2011, Kilavik et al., 2013) and provides an ideal model system for studying long-range neural communication in humans (Schoffelen et al., 2005). During steady isometric muscle contraction, activity in the primate motor cortex is known to become phase-coupled with that of the contralateral muscle resulting in cortico-muscular coherence (CMC) at the beta frequency (Bourguignon et al., 2017, Brovelli et al., 2004, Conway et al., 1995, Salenius et al., 1997, Schoffelen et al., 2005). These and related findings, typically visualised in trial averages, have led to the proposal that beta activity may play an active role in ‘sustaining’ a stable motor state (Androulidakis et al., 2006, Baker, 2007, Gilbertson et al., 2005, Witham et al., 2011) – and this concept has been proposed to extend to sustaining the ‘status quo’ in the cognitive realm (Engel and Fries, 2010).
In parallel, the sustained nature of rhythmic beta activity is increasingly called into question by a rapidly growing number of reports that demonstrate and quantify that, at the level of single trials, beta activity may not be sustained, but instead occurs in short-lived, burst-like events (Bourguignon et al., 2017, Feingold et al., 2015, Heideman et al., 2020, Jones, 2016, Little et al., 2019, Lundqvist et al., 2016, Quinn et al., 2019, Shin et al., 2017, Tinkhauser et al., 2017, van Ede et al., 2018; see also: Jasper and Penfield, 1949; Murthy and Fetz, 1992, Murthy and Fetz, 1996). However, so far, this has been demonstrated and quantified primarily in tasks that did not directly require participants to sustain a measurably steady behavioural output. Instead, beta events have been noted in the resting state (Seedat et al., 2020), during preparatory periods of tasks requiring a single behavioural response (Heideman et al., 2020, Little et al., 2019, Rule et al., 2017, Shin et al., 2017), or during periods following behavioural responses (Feingold et al., 2015, Little et al., 2019). As these contexts may not involve or require a sustained neural process, they may not call for the expression of sustained beta rhythms and instead allow for the manifestation of beta as transient events.
Here, we ask whether motor beta activity and beta connectivity between the brain and the muscle are similarly short-lived during an isometric contraction task designed to yield a sustained motor output. Building on extensive prior work describing the sources of beta activity within the sensorimotor system (Gerloff et al., 2006, Witham et al., 2010), our central aim was to study the temporal features (i.e. the transient vs. sustained nature) of macroscopic beta activity and cortico-muscular connectivity, and their correspondence to sustained motor behaviour. We reasoned that, if cortico-muscular beta connectivity is directly and continuously responsible for sustaining steady motor contraction, then motor beta activity should be (or become) sustained – also in single trials – in this set-up.
2. Results
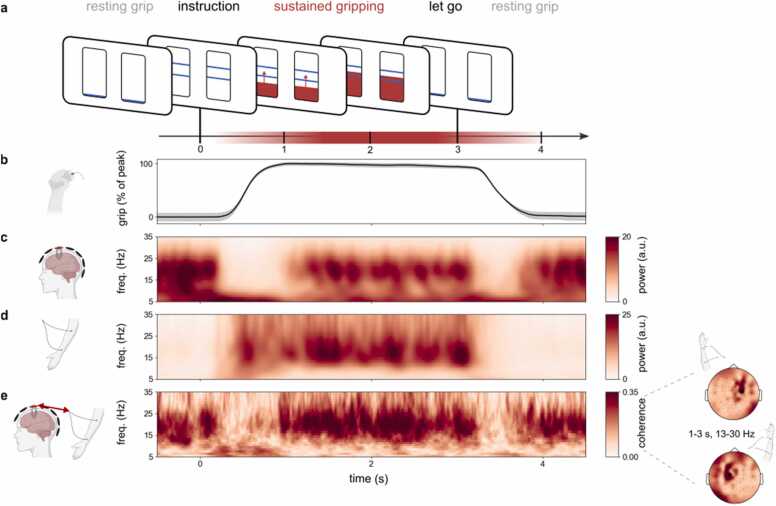
While participants (N = 18) performed a task with instructed periods of bimanual, isometric contraction, we measured brain activity (using magnetoencephalography (MEG)) and forearm muscle activity (using electromyography (EMG) from both forearms). Participants held a gripper device in each hand and a prompt instructed them to contract both grippers until they reached the force level indicated on the screen in front of them. They sustained the steady force output (on which they received real-time visual feedback) until the signal to release the grip, 3 s after grip instruction (Fig. 1a). As shown in the trial-average gripper data from a representative participant (Fig. 1b; see also Supplementary Figure 1 for the data from all participants), gripping stabilised approximately 1 s after grip instruction, and was held steady until the instruction to release the grip.
Fig. 1.
Trial-average beta activity and connectivity appear sustained during sustained motor behaviour (gripping). a ) Schematic of a single trial. Before each trial, participants held the gripping devices in both hands (resting grip). At time 0, two horizontal lines, indicating the gripping strength, appeared on the bars on the screen, prompting participants to grip. Participants began gripping until they reached a steady grip at the indicated strength, which they sustained for ~2 s. The drop of the horizontal lines to the bottom of the bars indicated the end of a trial and the return to resting grip. b) Average grip output across trials and across left and right gripping devices in a representative participant, expressed as a percentage of the peak force in each trial. Shading indicates 95% confidence intervals. c) Time-frequency spectrum of trial-average activity in selected motor MEG channels in a representative participant. Selected MEG channels correspond to those with maximal cortico-muscular coherence during sustained gripping (see methods) as also indicated in the topographical distribution in Fig. 1e (black dots). d) Time-frequency spectrum showing trial-average EMG activity across both forearms in a representative participant. e) Time-frequency spectrum of cortico-muscular coherence (phase coupling) between the selected MEG channels and the contralateral forearm muscles. Topographies show coherence with the left and right forearms, averaged over the indicated time-frequency window in a representative participant.
2.1. Motor beta activity and cortico-muscular connectivity appear sustained during sustained motor behaviour
To zoom in on the sensorimotor beta activity of interest, we focused our analysis on bilateral MEG channels that each showed the maximal coherence with the contralateral forarm muscles (Methods for details). We focused on the signals that we directly measured at the sensor level because we were mainly interested in temporal (duration) parameters (rather than spatial attributes) of beta activity. Similar to previous reports of sensorimotor beta activity during isometric contraction tasks (e.g., Conway et al., 1995; Salenius et al., 1997; Schoffelen et al., 2005; Tomassini et al., 2020), beta power in the brain (Fig. 1c) and in the muscle (Fig. 1d) appeared particularly prominent and sustained during the period of stable gripping (from around 1–3 s after grip instruction) when averaged across trials. During this period, beta activity in the brain and muscle were also directly related, as revealed by pronounced phase coupling between signals recorded from MEG sensors and the contralateral muscle at the beta frequency band (cortico-muscular coherence; Fig. 1e for a representative participant; see Supplementary Figure 2 for average across all 18 participants).
Similar to previous reports (Salmelin and Hari, 1994), these data also showed that when participants’ grip force changed – either from the resting grip to the instructed level, or from the instructed level back to the resting grip – beta activity in the brain and its connectivity with the contralateral muscle, were relatively attenuated.
Because our focus in the current study was on the temporal dynamics of motor beta activity during sustained contraction, we focused all remaining analyses on the sustained gripping period. If the patterns of beta activity and connectivity appear similarly sustained in the single trials, the findings would be compatible with the putative role of motor beta in sustaining a steady output.
2.2. Beta activity is transient in single trials, despite sustained motor output
Trial averaging can lead to seemingly sustained activity even when the single-trial activity is short-lived (Jones, 2016, Lundqvist et al., 2016, Stokes and Spaak, 2016). We therefore next turned to the patterns of motor beta activity at the level of individual trials (Fig. 2).
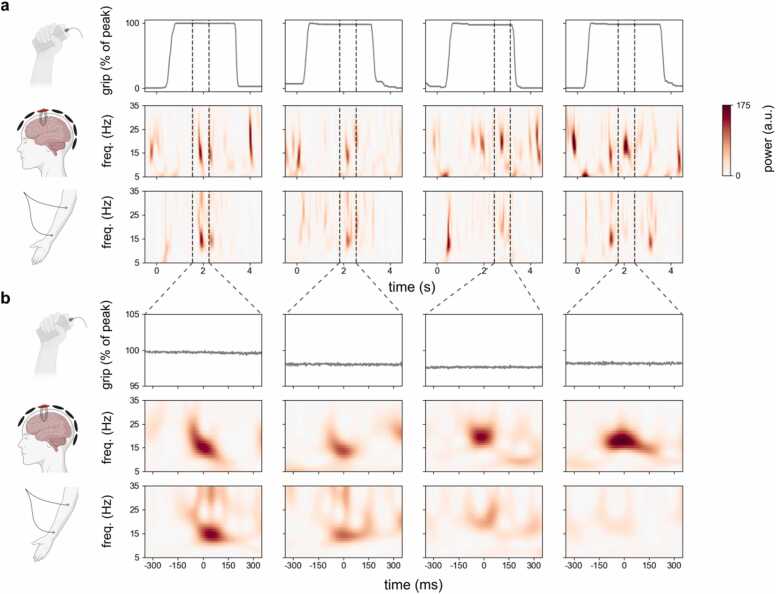
Fig. 2.
Beta activity is transient in single trials, despite sustained motor output. a) Gripper traces together with time-frequency spectra in brain and muscle in four example trials from the same participant whose trial-average data is shown in Fig. 1. b) Zoomed in view of the data in a, aligned to the occurrence of a beta event in the single trials above. Percentage of maximal grip output was defined relative to the whole epoch.
Participants’ grip-force traces were sustained throughout the gripping period in the majority of trials (see Fig. 2a for some representative trials, see also Supplementary Figure 3). We also found prominent periods of beta activity at the level of single trials, in both the brain (Fig. 2a, middle row) and the muscle (Fig. 2a, lower row). Critically, however, unlike the measured grip, beta activity appeared highly transient at the single-trial level in both the brain and the muscle. In fact, after exhaustive visual inspection of single trials, we found no trial with a clearly sustained period of pronounced motor beta activity (Supplementary Figure 3). Single-trial inspection (e.g. Fig. 2) also revealed the heterogeneity in the mean frequency and frequency spread of individual beta events in the context of isometric contraction. Nevertheless, here, our focus was on the temporal properties of beta events.
Fig. 2b shows the data aligned to prominent individual ‘beta events’ from Fig. 2a. These data revealed several important points, which we further describe and quantify below. First, periods of high beta power were often transient, lasting a few hundred milliseconds at most. Second, during contraction, periods of high beta power in the brain (middle row) were often accompanied by a similarly short-lasting period of beta activity in the downstream contralateral muscle (bottom row). Finally, transient beta activity appeared to occur despite sustained motor output, making it unlikely that the observed transient nature of beta was a direct consequence of (undesired) transient motor behaviour during our task (e.g. corrections in grip; see Supplementary Figure 4).
To quantify this pattern across all our data, we identified periods of high beta power (‘ON’ events) in the single-trial MEG data in the pre-defined MEG channels above the left and right motor cortices (as determined by the site with maximal CMC) and accordingly aligned the wavelet-transformed MEG, EMG and CMC data, as well as the grip output (Fig. 3).
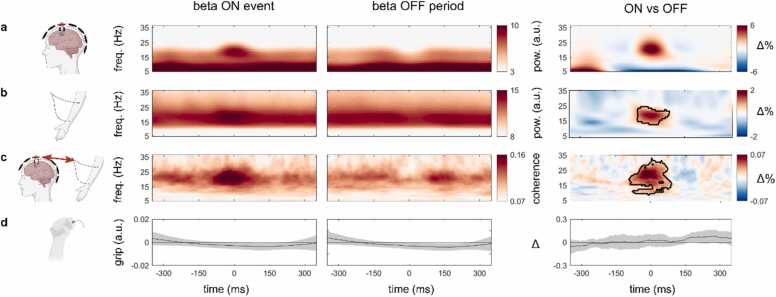
Fig. 3.
Data aligned to beta ON and OFF periods in the brain reveal transient beta in brain, muscle, and connectivity despite sustained motor output. Beta ON events and OFF periods were identified using Empirical Mode Decomposition (EMD) in the selected MEG channels during the ~1–3 s period of interest. After ON and OFF period detection, data were aligned and averaged across all trials and all participants (N = 18). Columns from left to right represent our four signals of interest (a-d) aligned to the central point of a beta ON event (left), a beta OFF period (middle) and the difference between them (right). a) MEG time-frequency spectrum, b) EMG time-frequency spectrum, c) CMC time-frequency spectrum and d) gripper signal. Shading indicates 95% confidence intervals. Black outlines in the right time-frequency maps indicate significant clusters.
To detect beta events, we used Empirical Mode Decomposition (EMD), a data-driven signal decomposition technique, which has the benefit of preserving high temporal resolution when exploring frequency-specific patterns of brain activity (Huang et al., 1998; see Methods and Supplementary Figure 5 for details). EMD allowed us not only to capture beta activity in the MEG signal with high temporal resolution, but also to successfully isolate it from other frequency bands (Supplementary Figure 6). We note, however, that the advantages of using EMD for beta-event detection were not a pre-requisite for obtaining our central results (as presented in Fig. 3). Similar results could be obtained when using a simple median split on beta frequency power for beta-event detection (Supplementary Figure 7).
To focus on the sustained gripping period, we only sampled beta events that occurred during the temporal window of interest (depicted in Supplementary Figure 1). For data alignment, we only considered ON events that lasted at least 50 ms (the duration of a single beta cycle at 20 Hz). On average, we detected 464.9 ± 114 [M±SD] usable ON events per participant. For comparison, we also identified ’OFF’ periods, defined as periods in-between the ON events. To compare neural activity surrounding all ON and OFF periods, we aligned our wavelet-transformed data to the centre time-point of all ON and OFF periods.
When averaging across all the identified beta ON events (Fig. 3, left column) and beta OFF periods (Fig. 3, middle column) across all trials and all participants, we confirmed that the identified beta ON events were short-lived. This was particularly clear when comparing ON vs OFF periods (Fig. 3, right column). Note how the ON vs. OFF comparison removes the background activity present in ON and OFF periods, including the 1/f component and therefore allows for focusing on the effect of interest. In line with our single-trial observations (Fig. 2), we further confirmed that beta ON events in the brain (Fig. 3a) were accompanied by a correspondingly short-lasting increase in beta activity in the muscle (Fig. 3b), and by a similarly time-limited pattern of beta connectivity between brain and muscle (Fig. 3c). The observed patterns did not critically depend on our use of EMD to define ON and OFF periods, as similar results were obtained when defining ON and OFF periods based on a simple median split of 13–30 Hz power time-courses (Supplementary Figure 7). Moreover, our results were consistent across all participants (Supplementary Figure 8).
Cluster-based permutation analyses (Maris and Oostenveld, 2007) confirmed significant, transient differences between ON vs OFF periods (as defined in the brain) in beta activity in the muscle (Fig. 3b third column; cluster p = 0.023) and in the connectivity between the brain and the muscle (Fig. 3c third column; cluster p = 0.0002). These clusters appeared in frequencies within the beta band and with durations corresponding to those of the beta ON events identified in the brain. Importantly, the significant clusters on the muscle and the brain-muscle connectivity were found despite the fact that these data were aligned to the beta ON events that we identified exclusively on the brain activity (to avoid double dipping, we did not statistically evaluate the ON-vs-OFF effect in the MEG data). A supplementary analysis confirmed that the connectivity associated with the identified bursts in the brain was predominantly contralateral (vs. ipsilateral), in line with the anatomy of the cortico-spinal tract (Supplementary Figure 9). Moreover, source localisation confirmed that beta events mapped onto sensorimotor cortical areas (Supplementary Figure 10).
The physiological difference measured in the brain and muscle, as well as in their connectivity, during beta ON periods did not appear to be driven by transient changes in grip output by the participants. Gripping-force differences between beta ON and OFF periods showed no clear modulation around the time of the identified ON/OFF periods and were never significantly different from zero (no clusters found; all uncorrected p > = 0.22). Moreover, locking the MEG, EMG and coherence signals around periods of small changes in grip revealed no systematic increases in beta power or CMC before, during or after minor grip adjustments (Supplementary Figure 4). Together, these findings suggest that cortical beta activity, and its connectivity with the contralateral forearm muscle, is transient, even when participants are sustaining a steady grip. This central pattern of results was observed in each of our two grip-strength conditions (Supplementary Figure 11).
2.3. Beta events are similar in duration during sustained motor output and rest
While beta ON events were short-lived in our task, it was possible that they were more sustained (i.e., longer lasting) than the transient beta events that have previously been reported in the absence of sustained behavioural output. To address this question, we used the same analysis pipeline to identify beta ON events in MEG recordings of the same participants during a separate resting-state recording on the same day. While neural activity patterns in task and rest may differ along many features, we focused specifically on the duration of beta events (Fig. 4) as this was the key variable pertaining to the investigation of the sustained vs. transient nature of beta activity during sustained behaviour.
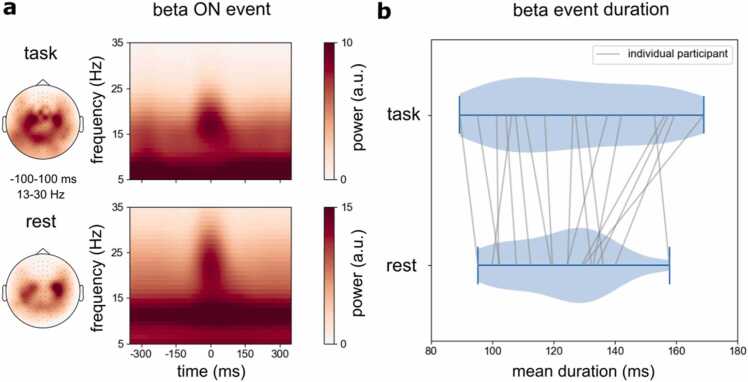
Fig. 4.
Comparison of the mean duration of beta events during sustained gripping and rest. a) MEG time-frequency spectra aligned to all beta ON periods during sustained gripping (task) and during resting state. Topographies show beta power (13–30 Hz) at the time of the detected ON events during gripping and rest. b) Violin plot showing the distribution of mean beta event durations during gripping (top) and resting state (bottom). Grey lines represent individual participants. A paired-sample t-test revealed no significant differences in mean burst duration between periods of sustained motor contraction and periods of rest (t17 = 1.52, p = 0.15, d = 0.36).
As shown in Fig. 4a, the beta events in the brain (as identified by our analysis) were similar in duration, as well as topography, during the sustained contraction task (top) and during the resting-state recording (bottom). When directly comparing the mean duration of all identified beta ON events per participant (Fig. 4b), no significant changes in beta event duration could be identified (t = 1.52, p = 0.15).
While no differences were observed in beta-event duration – the parameter of primary interest – between rest and gripping, task-relevant variation in other burst parameters were observed (see Supplementary Figure 12 for an overview, where we also show beta event parameters as a function of grip strength). Moreover, we found clear grip-related changes in beta activity and connectivity at the trial-average level (Fig. 1; Supplementary Figure 2).
3. Discussion
Our data confirm previous findings that beta activity varies with overall motor-task requirements (Kristeva-Feige et al., 2002, Baker, 2007, Brovelli et al., 2004, Gilbertson et al., 2005, Jenkinson and Brown, 2011, Kilavik et al., 2013, Kilner et al., 2000, Little et al., 2019). At the same time, they also reveal an important dissociation in duration. Beta activity, and connectivity with the downstream muscle, are manifest as transient events even when participants engage in sustained motor behaviour. Reinforcing the temporal dissociation, we found no obvious correspondence between the occurrence of a beta event – in the brain, the muscle, and their connectivity – and a change in overall force output surrounding the identified beta event. That is, while beta events identified in the brain had a clear correspondence in the pattern of muscle activity (see also Bourguignon et al., 2017; Novembre et al., 2019; Seedat et al., 2020; Tomassini et al., 2020), grip output was similarly sustained in periods with and without clear beta events.
This lack of a direct mirroring between beta activity and motor behaviour (for other examples see also Murthy and Fetz, 1996; Rule et al., 2017; van Ede et al., 2015) is unlikely to result from lack of sensitivity: we were able to identify and aggregate more than 8000 ON events, and the ON events were clearly distinguishable from OFF periods (in the brain, muscle, and connectivity). Furthermore, the gripper signals did show robust and pronounced changes in grip strength during the instructed grip periods, at the single-trial level.
A widely held theoretical position is that frequency-specific patterns of brain activity play a role in coordinating communication between distant neural populations (Buzsáki, 2009, Fries, 2005, Siegel et al., 2012, Varela et al., 2001). Our data reveal that synchronisation between the brain and the muscle entails short ‘bursts’ of beta-frequency connectivity that may last only a few cycles (see also Baker et al., 2014; Bourguignon et al., 2017; Vidaurre et al., 2018; Seedat et al., 2020). Our results further emphasize that this may be the case even when task demands call for sustained motor output – and that sustained task demands do not prolong beta-event duration in comparison to rest. In our view, however, this need not imply that the transient beta activity that we report here plays no role in sustaining neural communication and behaviour (i.e., while transient beta may not subserve or reflect motor behaviour directly, beta events may still be necessary).
Indeed, short ‘bursts’ of beta activity and connectivity may contribute to sustained activity indirectly, such as by probing the state of the periphery (Baker, 2007, Witham et al., 2011) and/or triggering a separate neural process that then implements sustained behaviour. Moreover, other kinds of connectivity (i.e. cortico-cortical and cortico-subcortical connectivity) may follow temporal patterns different from the CMC pattern observed in the present study and the same may be true for the wider sensorimotor network (cf. Brovelli et al., 2004; Crone et al., 1998; Miller et al., 2009, Miller et al., 2012; Witham et al., 2010). Relatedly, the present study measured muscle activity only from the forearms. Consequently, we cannot rule out the possibility that other task-relevant muscles (e.g. for arm or body posture) may have different beta-frequency connectivity (in terms of location, frequency spread…) with motor brain areas, or may be characterised by transient coupling at distinct times. Elucidating the full mechanisms through which beta activity may support sustained motor activity is beyond the scope of our aims or of any single study. However, we take a first important step in this enquiry by testing the prevalent hypothesis that sustained beta oscillations are required for sustained motor output. By revealing transient beta activity and cortico-muscular connectivity during sustained motor behaviour, our study paves the way for exciting new investigations of whether and how short beta events play a causal role in initiating and/or tuning stable motor behaviour.
At least one prior study has reported that beta activity in motor cortex may ‘emerge’ transiently in LFP measurements, even when neural firing rates in the same areas are sustained at the beta frequency (Rule et al., 2017). Their findings highlight that LFP signals provide complementary information to neural spiking. Accordingly, transient activity observed in our MEG measurements need not imply that there is no sustained beta rhythmicity in other aspects of neural activity to which our measurements were simply insensitive. Based on our results, we do not wish to argue that beta activity is never sustained or that there is only one pattern of beta activity and connectivity in the brain. Indeed, transient beta activity/connectivity may co-exist with sustained beta activity/connectivity. Perhaps, then, the most urgent question prompted by the current work pertains to how macroscopic beta activity and connectivity relate to other neural processes at the more granular level, and how these contribute to sustaining neural interactions and behaviour. At a minimum, our data reveal that the links between these respective processes may be far less straightforward than commonly assumed.
Here, we focused on beta activity in the human sensorimotor system during a simple sustained motor task. Related work in non-human primates has shown how beta (as well as gamma) activity in prefrontal cortex during working memory maintenance may be similarly transient (Lundqvist et al., 2016). This is another example of a transient neural activity pattern that occurs despite sustained task demands. However, unlike in our task, sustained demands in working-memory tasks can only be inferred indirectly by assuming that memory maintenance requires sustained activity (an assumption that is increasingly questioned, e.g. Lundqvist et al., 2016; Mongillo et al., 2008; Stokes, 2015). In contrast, by investigating beta activity in the human sensorimotor system, we were able to monitor the sustained process of interest (gripping) directly. Doing so, we have revealed that beta activity, as well as cortico-muscular connectivity, in the human sensorimotor system are transient, even while engaging in measurably sustained behaviour.
4. Methods
4.1. Participants
The present study had approval of the Oxfordshire Research Ethics Committee as part of the National Research Ethics Service (Reference number 12/SC/0650). Participants gave informed consent prior to participation and received a monetary compensation upon completion of the study. The data come from a cohort of 18 healthy participants (8 females) of advanced age (M = 69.6 years, SD = 4.66, range = 62–88) who participated as a control group in the context of a larger translational study (Zokaei et al., 2021). All participants except for two were right-handed. Participants spent an average of 16.06 years in education (SD: 2.95). Though the participant sample analysed here is on average older than a typical sample of undergraduate students, the participants understood the task without any trouble and were able to sustain their grip as instructed (Supplementary Figure 1).
4.2. Experimental setup
Brain activity was measured using the Elekta NeuroMag 306-channel MEG system at the Oxford Centre for Human Brain Activity (OHBA). Head position inside the scanner was monitored by means of a magnetic Polhemus FastTrack 3D system. Data were acquired with a sampling frequency of 1000 Hz and using a band-pass filter between 0.03 and 300 Hz. Electrocardiography (ECG), electrooculography (EOG) and bilateral electromyography (EMG) were acquired concurrently. EMG electrodes were placed over flexor digitorum superficialis (forearm) in a bipolar configuration, with a reference electrode on the lateral epicondyles of each arm (similar to Proudfoot et al., 2018; van Ede and Maris, 2013).
Participants were presented with visual stimuli on a 58 × 46 cm screen which was 120 cm away and which had a spatial resolution of 1280 × 1024 pixels. The task was programmed and implemented using Presentation (Neurobehavioral Systems, Inc., Berkeley, CA, www.neurobs.com). For the duration of the session, participants were seated upright in the MEG scanner with their arms resting on their laps, while holding the grippers on their resting hands. Participants’ responses on each trial were recorded using a bimanual fibre-optic gripping device that measured gripping strength and which was compatible with the MEG scanner (Grip Force, Force Fibre Optic Response Pad, Current Designs, USA).
4.3. Stimuli and experimental procedure
Participants were asked to hold the gripping device in both hands for the duration of the experiment. The task consisted of 12 blocks (each separated by a 20-s rest period) with each 10 trials of bimanual gripping: a total of 120 trials per participant. Each gripping trial lasted 3 s and the whole task lasted around 10 min. At all points during the task, the screen showed two bars, each associated with one of the hand-held gripping devices (Fig. 1a). At time 0 of each trial, one of two potential grip strengths (low or moderate) was indicated by two perpendicular lines on the bars, with lower or higher positions representing low and moderate grip strengths. The low grip strength corresponded to approximately 12 Newtons and the moderate grip strength to approximately 17 Newtons. Indicated grip strength was always the same on both bars (i.e., for both hands). After appearance of the two horizontal lines, participants were asked to grip the grippers and sustain their grip until the horizontal lines dropped to the bottom of the bars, at which point they were to release grip (while continuing to hold the gripper devices in their hands). The horizontal lines that indicated the instructed grip strength were on the screen for 3 s. On average, allowing for rise time, participants’ grips were steady at the indicated strength for approximately of 2 s (Fig. 1b; Supplementary Figure 1). During sustained gripping, participants received real-time visual feedback of the strength with which they were gripping, with the aim of ensuring a stable, steady grip strength across the trial. The grip output was quantified as a percentage of the peak grip output per trial (and was always below the participants’ maximal possible contraction). For the purposes of the present study, low and moderate grip strength conditions were collapsed and analysed together. We confirmed that the critical patterns reported in the current study were similar in both grip-strength conditions.
Earlier in the session, the same participants completed a 10-minute resting-state MEG recoring with their eyes open. The same preprocessing and analysis pipeline was applied to the MEG signals recorded during the gripper task and during resting state.
4.4. Data pre-processing
Noisy MEG channels were detected and corrected using MaxFilter version 2.2.15 software (Elekta Neuromag). Other pre-processing steps were performed using FieldTrip toolbox (Oostenveld et al., 2011). Artifacts related to eye movements, blinks, and heartbeat were removed using independent-component analysis (ICA) and visual inspection of the detected components. An average of 1.3 components [SD: 1.1] were removed per participant.
We focused our analyses on planar gradiometer MEG channels. Pairs of gradiometers (two per position) were combined in the time domain using the ‘svd’ method in Fieldtrip. To focus our analysis on the temporal dynamics of beta activity in those MEG channels that best captured activity in the human cortico-muscular pathways, we first computed the average corticomuscular coherence (CMC; as in Schoffelen et al., 2005; Salenius et al., 1997) using the data from the full predefined 1–3 s period. CMC was calculated using Fourier-transformed EMG and MEG at each MEG channel for all participants as implemented in Fieldtrip. For further analysis, we chose the two MEG channels (one left and one right) that showed largest CMC in the group average. We selected the best channels based on the group average as there could be ambiguity when choosing the best channel for each participant individually (provided that CMC was not equally clear in each participant). The sustained vs. transient nature of beta activity was not considered when making this channel selection, as the full gripping period was subjected to our CMC analysis at this initial channel-selection stage.
All of the MEG analyses on this study, including those on resting-state recordings, were performed using the same two MEG channels of interest. As in the influential study by Schoffelen et al. (2005), we chose to perform our analyses on sensor-space MEG signals. This choice increased comparability to related studies that used EEG (e.g. Tomassini et al., 2020). We focused on the channels with the maximum CMC provided that invasive studies have shown that CMC is maximal over the contralateral primary sensorimotor cortex (e.g. Witham et al., 2010, Gerloff et al., 2006). We chose a single left and a single right channel to facilitate the isolation of beta activity using EMD. We confirmed that these channels captured primary sensorimotor cortex activity by showing that beta connectivity with the muscle at the time of bursts was predominantly contralateral, in line with the anatomny of the cortico-spinal tract (Supplementary Figure 9). All EMG analyses were performed on the raw EMG time-series.
Subsequently, noisy trials were identified and removed from the task recording. Data were cut-out into 6 s epochs beginning 1 s before the start of a trial and ending 5 s after. For each participant, the average grip strength change across trials was plotted and a temporal window of interest was identified by visual inspection (as marked in Supplementary Figure 1), based on where the grip appeared relatively stable in the trial average. The mean duration of this window was 2.2 s (SD: 0.2 s). The generalised extreme Studentized deviate test for outliers (Matlab: isoutlier) was performed for each trial and for each signal type (EMG, MEG, and gripper) during the selected temporal window of interest. Subsequently, bilateral MEG, EMG, and grip force signals were plotted for each of the identified outlier trials and those deemed noisy or unstable by visual inspection were eliminated from all subsequent analyses. An average of 1.5 trials were eliminated per participant (SD: 1.29, range: 0–4).
4.5. Spectral analyses
MEG and EMG time-series were converted into the frequency domain by means of convolving the signals with a complex 5-cycle Morlet wavelet. This was done from 5 to 40 Hz in steps of 1 Hz. Power was calculated as the squared magnitude of the wavelet-convolved data. Time-frequency spectra were subsequently epoched around the temporal windows of interest. This window of interest during which a constant level of sustained gripping was maintained was optimally suited for testing for a corresponding sustained level of motor beta activity. Time- and frequency resolved CMC was calculated as the consistency in phase-relation between the Morlet-convolved MEG and EMG signals (Schoffelen et al., 2005).
4.6. Detection of ON and OFF periods
Next, we identified periods of high and low beta-frequency (13–30 Hz) activity in the selected MEG channels during the temporal window of interest. To identify these periods with high sensitivity while preserving the excellent time resolution of our measurements, we used empirical mode decomposition (EMD) – though we confirmed that similar results could be obtained using a more basic thresholding approach (Supplementary Figure 7). EMD is an analysis method developed by Huang et al., 1998, Huang et al., 2003 for the decomposition of nonlinear and nonstationary time series, such as signals arising from brain activity (e.g. MEG). In contrast to conventional Fourier-based time-frequency transforms, EMD allows for higher temporal resolution, and it successfully isolates beta from other frequency bands (Supplementary Figure 6).
4.7. Empirical mode decomposition
All EMD analyses were performed using Python 3.7 Empirical Mode Decomposition toolbox v0.2.0 (https://emd.readthedocs.io/) together with custom scripts. EMD is based on the empirical (data-driven) identification of a set of intrinsic oscillatory modes in the signal-of-interest and on the decomposition of such signal into a finite set of Intrinsic Mode Functions (IMFs). The algorithm separates the signal x(t) into IMFs that fulfil two properties: 1) The number of local maxima in an IMF differs from the number of minima in the same IMF by one or zero, and 2) The mean value of an IMF is zero.
IMFs are isolated through a ‘sifting’ process. In the first sifting step: 1) local minima and maxima of the raw signal are identified, 2) two envelopes connecting all of the maxima and all of the minima of the signal, respectively, are created, 3) the mean of the two envelopes is calculated, 4) this mean is subtracted from the raw signal, 5) if the product of the subtraction itself is not an IMF (as defined by the properties above), a new sifting process begins with the product of the subtraction as the new input signal.
To avoid mode mixing (the presence of components at different intrinsic frequencies in a single IMF; Deering and Kaiser, 2005), we used an adaptive version of the sift process similar to the one developed by Huang et al. (2009): masked EMD (mEMD). At each iteration, a masking signal at a specific frequency was summed with the input signal before extrema identification, ensuring that intermittent signals did not result in component splitting. After extrema identification, envelope interpolation and envelope average subtraction from the input signal, the mask was subtracted from the product signal to return the IMF and continue the sifting process. We chose frequency masks at the following frequencies: 125, 62, 31, 16, 8, 4 and 2 Hz (directing modes between the masks, such as between 31 and 16 Hz). To ensure comparability across participants, we imposed an upper bound on the number of IMFs that the sifting process would return; namely, 6. The IMF that best corresponded to what is commonly described as ‘beta’ was the 3rd IMF in all participants (Supplementary Figure 6), which was isolated using the 31 and 16 Hz masks (Quinn et al., 2021).
4.8. Beta cycle detection
Following identification of the beta mode (beta IMF), we identified periods of high and low beta activity. The outstanding temporal resolution of EMD enabled us to do so at the level of single beta cycles. We identified beta cycles in our signal based on the instantaneous phase and instantaneous amplitude of the beta IMF. We defined ‘good’ beta cycles as those: 1) beginning with an instantaneous phase value between 0 and pi/24 and ending within 2pi – pi/24 and 2pi, 2) with a phase differential bigger than 0 (no phase reversals) and 3) instantaneous amplitude above the 50th percentile of the beta IMF’s instantaneous amplitude for each channel, to ensure that only cycles with a sufficiently high amplitude were included. We defined ‘beta ON events’ as successive ‘good’ beta cycles.
4.9. ON and OFF period identification
We chose chains made of good beta cycles (ON events) that were at least 50 ms long (the estimated duration of a single beta cycle at 20 Hz) and that occurred at least 500 ms away from the beginning or end of the sustained gripping period identified separately for each participant (Supplementary Figure 1). For comparison, in the same gripping period, we also marked all moments in between the ON events as OFF periods. To ensure that we did not mix-up OFF periods with ON events that we later discarded because they were less than 50 ms long, we marked OFF periods before discarding these “ON events”. Both ON and OFF periods were identified exclusively on the MEG signals from the target channels. We found the central point of these ON and OFF periods and aligned the time- and frequency-resolved data for all ON and OFF periods to their middle point. Both the MEG and raw EMG signals were aligned to the central point of these ON and OFF periods (Fig. 3). We also calculated the percentage change between ON and OFF periods’ wavelet-derived time-frequency spectra as: ON-OFF. Prior to the comparison of CMC during ON and OFF periods, we randomly subsampled our data to calculate CMC with the same number of ON and OFF periods, provided that CMC is biased by the number of trials.
Although we had good reasons for using EMD (its high temporal resolution and ability to isolate the beta mode from other frequencies), we also confirmed that the results we obtained were not dependent on using EMD. To this end, we also found ON and OFF periods in the more conventional wavelet-convolved time-frequency spectra and identified ON events as those periods for which beta power (13–30 Hz) exceeded the median beta power for at least 50 ms. Highly similar results were observed (Supplementary Figure 7).
4.10. Detection of minor changes in grip
To investigate the possibility that beta events may be driven by small adjustments in grip in either direction (i.e. upwards or downwards), we calculated the differential of the gripper signal during the identified sustained contraction window in each trial. We then identified the maximum and the minimum of the grip signal differential per trial and locked the MEG, EMG and coherence data to these moments (Supplementary Figure 4).
5. Source localisation
Source localisation was performed as detailed by Quinn et al. (2019) and as implemented in the OHBA Software Library (OSL; Woolrich et al., 2011). The continuous sensor data were projected into an 8-mm grid in source space by means of a Linearly Constrained Minimum Variance (LCVM) vector beamformer. This beamformer combines signal from magnetometers and gradiometers. It uses principal component analysis (PCA) to regularize the data covariance matrix estimation and account for the reduction in dimensionality caused by Maxfilter. The beamformer weights were estimated in an 8-mm grid cast within the MNI152 brain.
At the end of the source reconstruction, we retrieved one time-series per voxel. We locked the reconstructed time-series to the times of beta ON and OFF periods, as identified in the sensor signal (Fig. 3). A 200-ms window around the beta ON and OFF periods was extracted, and we calculated the average beta power in the windows. This was performed separately for the left and right MEG channels. Beta power was estimated per voxel and z-scored per participant to account for the inter-participant variability. Beta power during OFF periods was subtracted from beta power during ON periods. The participant-averaged difference between ON and OFF beta power was plotted on a MNI152 T1 brain (see Supplementary Figure 10).
5.1. Statistical analyses
We focused our statistical analysis on the ON vs OFF comparison described above, separately for the EMG, CMC, and grip output measurements. We refrained from statistically evaluating the ON vs OFF comparison on the MEG data given that we used the MEG data itself to identify the ON events and OFF periods. We used cluster-based permutation testing (Maris and Oostenveld, 2007) as implemented in FieldTrip. This approach circumvents the multiple comparisons problem that stems from statistical evaluation of multi-dimensional data (in our case, data with a time and frequency dimension) by evaluating clusters of neighbouring samples under a single permutation distribution of the largest cluster. We performed 10,000 permutations with a clustering threshold of a univariate comparison at alpha = 0.05. We also compared the mean duration of beta events between steady contraction and rest. For this analysis, we extracted the mean event duration as the time from the beginning to the end of a ‘chain’ of consecutive beta cycles (as defined above) and used a paired samples t-test to compare mean duration of ON events between conditions of sustained contraction and resting state.
Acknowledgements
This research was funded by a Wellcome Trust PhD Studentship (102170/Z/13/Z) I.E.A., a James S. McDonnell Foundation Understanding Human Cognition Collaborative Award (220020448) and a Wellcome Trust Senior Investigator Award (104571/Z/14/Z) to A.C.N, an ERC Starting Grant from the European Research Council (MEMTICIPATION, 850636) to F.v.E., Wellcome Trust grants (106183/Z/14/Z, 215573/Z/19/Z) to M.W.W. and the New Therapeutics in Alzheimer’s Diseases (NTAD) study supported by UK MRC and the Dementia Platform UK to A.C.N. and M.W.W., and by the NIHR Oxford Health Biomedical Research Centre. The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z). For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. Figure schematics were created with BioRender.com.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pneurobio.2022.102281.
Contributor Information
Irene Echeverria-Altuna, Email: irene.echeverriaaltuna@psy.ox.ac.uk.
Anna C. Nobre, Email: kia.nobre@psy.ox.ac.
Appendix A. Supplementary material
Supplementary material.
.
References
- Androulidakis A.G., Doyle L.M.F., Gilbertson T.P., Brown P. Corrective movements in response to displacements in visual feedback are more effective during periods of 13–35 Hz oscillatory synchrony in the human corticospinal system. Eur. J. Neurosci. 2006;24(11):3299–3304. doi: 10.1111/j.1460-9568.2006.05201.x. [DOI] [PubMed] [Google Scholar]
- Baker A.P., Brookes M.J., Rezek I.A., Smith S.M., Behrens T., Probert Smith P.J., Woolrich M. Fast transient networks in spontaneous human brain activity. eLife. 2014;3 doi: 10.7554/eLife.01867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S.N. Oscillatory interactions between sensorimotor cortex and the periphery. Curr. Opin. Neurobiol. 2007;17(6):649–655. doi: 10.1016/j.conb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon M., Piitulainen H., Smeds E., Zhou G., Jousmäki V., Hari R. MEG insight into the spectral dynamics underlying steady isometric muscle contraction. J. Neurosci. 2017;37(43):10421–10437. doi: 10.1523/JNEUROSCI.0447-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovelli A., Ding M., Ledberg A., Chen Y., Nakamura R., Bressler S.L. Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc. Natl. Acad. Sci. USA. 2004;101(26):9849–9854. doi: 10.1073/pnas.0308538101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the Brain. Oxford University Press; 2009. Rhythms of the Brain. [DOI] [Google Scholar]
- Conway B.A., Halliday D.M., Farmer S.F., Shahani U., Maas P., Weir A.I., Rosenberg J.R. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J. Physiol. 1995;489(3):917–924. doi: 10.1113/jphysiol.1995.sp021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone N.E., Miglioretti D.L., Gordon B., Sieracki J.M., Wilson M.T., Uematsu S., Lesser P.R. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronisation. Brain. 1998;121(12):2271–2299. doi: 10.1093/brain/121.12.2271. https://doi.org/10.1093./brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- Deering, R., & Kaiser, J.F. (2005). The use of a masking signal to improve empirical mode decomposition. Proceedings. (ICASSP ’05). IEEE International Conference on Acoustics, Speech, and Signal Processing, 2005., 4, iv/485-iv/488 Vol. 4. 10.1109/ICASSP.2005.1416051. [DOI]
- Engel A.K., Fries P. Beta-band oscillations — signalling the status quo? Curr. Opin. Neurobiol. 2010;20(2):156–165. doi: 10.1016/J.CONB.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Feingold J., Gibson D.J., Depasquale B., Graybiel A.M. Bursts of beta oscillation differentiate postperformance activity in the striatum and motor cortex of monkeys performing movement tasks. Proc. Natl. Acad. Sci. USA. 2015;112(44):13687–13692. doi: 10.1073/pnas.1517629112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Gerloff C., Braun C., Staudt M., Hegner Y.L., Dichgans J., Krageloh-Mann I. Coherent corticomuscular oscillations originate from primary motor cortex: evidence from patients with early brain lesions. Hum. Brain Mapp. 2006;27:789–798. doi: 10.1002/hbm.20220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson T., Lalo E., Doyle L., di Lazzaro V., Cioni B., Brown P. Existing motor state is favored at the expense of new movement during 13–35 Hz oscillatory synchrony in the human corticospinal system. J. Neurosci. 2005;25(34):7771–7779. doi: 10.1523/JNEUROSCI.1762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heideman S.G., Quinn A.J., Woolrich M.W., van Ede F., Nobre A.C. Dissecting beta-state changes during timed movement preparation in Parkinson’s disease. Prog. Neurobiol. 2020;184(November 2019) doi: 10.1016/j.pneurobio.2019.101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N.E., Shen Z., Long S.R., Wu M.C., Snin H.H., Zheng Q., Yen N.C., Tung C.C., Liu H.H. The empirical mode decomposition and the Hubert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. A: Math., Phys. Eng. Sci. 1998;454(1971):903–995. doi: 10.1098/rspa.1998.0193. [DOI] [Google Scholar]
- Huang N.E., Wu M.-L.C., Long S.R., Shen S.S.P., Qu W., Gloersen P., Fan K.L. A confidence limit for the empirical mode decomposition and Hilbert spectral analysis. Proc. R. Soc. Lond. Ser. A: Math., Phys. Eng. Sci. 2003;459(2037):2317–2345. doi: 10.1098/rspa.2003.1123. [DOI] [Google Scholar]
- Huang N.E., Wu Z., Long S.R., Arnold K.C., Chen X., Blank K. On instantaneous frequency. Adv. Adapt. Data Anal. 2009;1(2):177–229. doi: 10.1142/S1793536909000096. [DOI] [Google Scholar]
- Jasper H., Penfield W. Electrocorticograms in man: Effect of voluntary movement upon the electrical activity of the precentral gyrus. Arch. Für Psychiatr. Und Nervenkrankh. 1949;183(1–2):163–174. doi: 10.1007/BF01062488. [DOI] [Google Scholar]
- Jenkinson N., Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011;34(12):611–618. doi: 10.1016/j.tins.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Jones S.R. When brain rhythms aren’t ‘rhythmic’: implication for their mechanisms and meaning. Curr. Opin. Neurobiol. 2016;40:72–80. doi: 10.1016/j.conb.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilavik B.E., Zaepffel M., Brovelli A., MacKay W.A., Riehle A. The ups and downs of beta oscillations in sensorimotor cortex. Exp. Neurol. 2013;245:15–26. doi: 10.1016/j.expneurol.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Kilner J.M., Baker S.N., Salenius S., Hari R., Lemon R.N. Human cortical muscle coherence is directly related to specific motor parameters. J. Neurosci. 2000;20(23):8838–8845. doi: 10.1523/jneurosci.20-23-08838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristeva-Feige R., Fritsch C., Timmer J., Lucking C.H. Effects of attention and precision of exerted force on beta range EEG-EMG synchronization during maintained motor contraction task. Clin. Neurophysiol. 2002;113:124–131. doi: 10.1016/s1388-2457(01)00722-2. https://doi.org.10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- Little S., Bonaiuto J., Barnes G., Bestmann S. Human motor cortical beta bursts relate to movement planning and response errors. PLoS Biol. 2019;17(10):1–30. doi: 10.1371/journal.pbio.3000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist M., Rose J., Herman P., Brincat S.L.L., Buschman T.J.J., Miller E.K.K. Gamma and beta bursts underlie working memory. Neuron. 2016;90(1):152–164. doi: 10.1016/j.neuron.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Miller K.J., Zanos S., Fetz E.E., den Nijs M., Ojemann J.G. Decoupling the cortical power spectrum reveals real-time representation of individual finger movements in humans. J. Neurosceicne. 2009;29(10):3132–3137. doi: 10.1523/jneurosci.5506-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K.J., Hermes D., Honey C.J., Hebb A.O., Ramsey N.F., Knight R.T., Ojemann J.G., Fetz E.E. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comput. Biol. 2012;8(9) doi: 10.1371/journal.pcbi.1002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongillo G., Barak O., Tsodyks M. Science. N.Y.; New York: 2008. Synaptic theory of working memory; pp. 1543–1546. [DOI] [PubMed] [Google Scholar]
- Murthy V.N., Fetz E.E. Coherent 25- To 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc. Natl. Acad. Sci. USA. 1992;89(12):5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy V.N., Fetz E.E. Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. J. Neurophysiol. 1996;76(6):3949–3967. doi: 10.1152/jn.1996.76.6.3949. [DOI] [PubMed] [Google Scholar]
- Novembre G., Pawar V.M., Kilintari M., Bufacchi R.J., Guo Y., Rothwell J.C., Iannetti G.D. The effect of salient stimuli on neural oscillations, isometric force, and their coupling. NeuroImage. 2019;198(January):221–230. doi: 10.1016/j.neuroimage.2019.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational intelligence and neuroscience. 2011;2011 doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot M., van Ede F., Quinn A., Colclough G.L., Wuu J., Talbot K., Benatar M., Woolrich M.W., Nobre A.C., Turner M.R. Impaired corticomuscular and interhemispheric cortical beta oscillation coupling in amyotrophic lateral sclerosis. Clin. Neurophysiol. 2018;129(7):1479–1489. doi: 10.1016/j.clinph.2018.03.019. [DOI] [PubMed] [Google Scholar]
- Quinn A.J., Lopes-dos-Santos V., Dupret D., Nobre A.C., Woolrich M. EMD: empirical mode decomposition and hilbert-huang spectral analyses in python. J. Open Source Softw. 2021;6(59):2977. doi: 10.21105/joss.02977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn A.J., van Ede F., Brookes M.J., Heideman S.G., Nowak M., Seedat Z.A., Vidaurre D., Zich C., Nobre A.C., Woolrich M.W. Unpacking transient event dynamics in electrophysiological power spectra. Brain Topogr. 2019;32(6):1020–1034. doi: 10.1007/s10548-019-00745-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rule M.E., Vargas-Irwin C.E., Donoghue J.P., Truccolo W. Dissociation between sustained single-neuron spiking and transient β-LFP oscillations in primate motor cortex. J. Neurophysiol. 2017;117(4):1524–1543. doi: 10.1152/jn.00651.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salenius S., Portin K., Kajola M., Salmelin R., Hari R. Cortical control of human motoneuron firing during isometric contraction. J. Neurophysiol. 1997;77(6):3401–3405. doi: 10.1152/jn.1997.77.6.3401. [DOI] [PubMed] [Google Scholar]
- Salmelin R., Hari R. Spatiotemporal characteristics of rhythmic neuromagnetic activity related to thumb movement. Neuroscience. 1994;60(94):537–550. doi: 10.1016/0306-4522(94)90263-1. 〈http://www.sciencedirect.com/science/article/pii/0306452294902631〉 [DOI] [PubMed] [Google Scholar]
- Schoffelen J.M., Oostenveld R., Fries P. Neuronal coherence as a mechanism of effective corticospinal interaction. Science. 2005;308(5718):111–113. doi: 10.1126/science.1107027. [DOI] [PubMed] [Google Scholar]
- Seedat Z.A., Quinn A.J., Vidaurre D., Liuzzi L., Gascoyne L.E., Hunt B.A.E., O’Neill G.C., Pakenham D.O., Mullinger K.J., Morris P.G., Woolrich M.W., Brookes M.J. The role of transient spectral ‘bursts’ in functional connectivity: a magnetoencephalography study. NeuroImage. 2020;209(January) doi: 10.1016/j.neuroimage.2020.116537. [DOI] [PubMed] [Google Scholar]
- Shin H., Law R., Tsutsui S., Moore C.I., Jones S.R. The rate of transient beta frequency events predicts behavior across tasks and species. ELife. 2017;6:1–31. doi: 10.7554/eLife.29086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M., Donner T.H., Engel A.K. Spectral fingerprints of large-scale neuronal interactions. Nat. Rev. Neurosci. 2012;13(2):121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- Stokes M., Spaak E. The importance of single-trial analyses in cognitive neuroscience. Trends Cogn. Sci. 2016;20(7):483–486. doi: 10.1016/j.tics.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Stokes M.G. 'Activity-silent' working memory in prefrontal cortex: a dynamic coding framework. Trends in cognitive sciences. 2015;19(7):394–405. doi: 10.1016/j.tics.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G., Miniussi C., Gross J. The functional importance of rhythmic activity in the brain. Curr. Biol. 2012;22(16):PR658–R663. doi: 10.1016/j.cub.2021.06.061. [DOI] [PubMed] [Google Scholar]
- Tinkhauser G., Pogosyan A., Tan H., Herz D.M., Kühn A.A., Brown P. Beta burst dynamics in Parkinson’s disease off and on dopaminergic medication. Brain. 2017;140(11):2968–2981. doi: 10.1093/brain/awx252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini A., Maris E., Hilt P., Fadiga L., D’Ausilio A. Visual detection is locked to the internal dynamics of cortico-motor control. PLoS Biol. 2020;18(10) doi: 10.1371/journal.pbio.3000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F., Maris E. Somatosensory demands modulate muscular beta oscillations, independent of motor demands. J. Neurosci. 2013;33(26):10849–10857. doi: 10.1523/JNEUROSCI.5629-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F., Quinn A.J., Woolrich M.W., Nobre A.C. Neural oscillations: sustained rhythms or transient burst-events? Trends Neurosci. 2018;41(7):415–417. doi: 10.1016/j.tins.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F., Winner T., Maris E. Touch automatically upregulates motor readiness in humans. J. Neurophysiol. 2015;114(6):3121–3130. doi: 10.1152/jn.00504.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela, F., Lachaux, J.P., Rodriguez, E., & Martinerie, J. (2001). The brainweb: Phase synchronization and large-scale integration. [DOI] [PubMed]
- Vidaurre D., Hunt L.T., Quinn A.J., Hunt B.A.E., Brookes M.J., Nobre A.C., Woolrich M.W. Spontaneous cortical activity transiently organises into frequency specific phase-coupling networks. Nat. Commun. 2018;9(1):2987. doi: 10.1038/s41467-018-05316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witham C.L., Riddle C.N., Baker M.R., Baker S.N. Contributions of descending and ascending pathways to corticomuscular coherence in humans. J. Physiol. 2011;589(15):3789–3800. doi: 10.1113/jphysiol.2011.211045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witham C.L., Wang M., Baker S.N. Corticomuscular coherence between motor cortex, somatosensory areas and forearm muscles in the monkey. Front. Syst. Neurosci. 2010;4(38) doi: 10.3389/fnsys.2010.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M., Hunt L., Groves A., Barnes G. MEG beamforming using Bayesian PCA for adaptive data covariance matrix regularization. Neuroimage. 2011;57(4):1466–1479. doi: 10.1016/j.neuroimage.2011.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zokaei N., Quinn A.J., Hu M.T., Husain M., van Ede F., Nobre A.C. Reduced cortico-muscular beta coupling in Parkinson’s disease predicts motor impairment. Brain Commun. 2021;3(3) doi: 10.1093/braincomms/fcab179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.