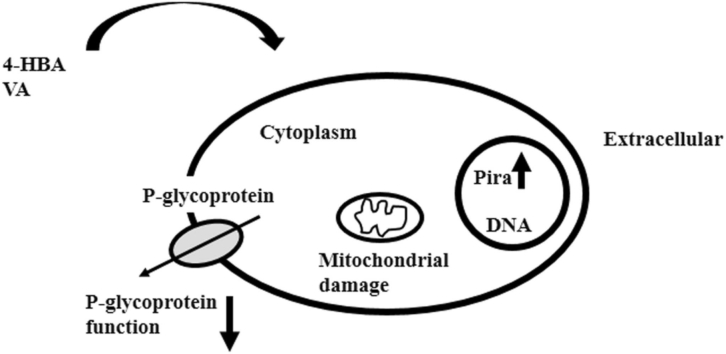
Abstract
The objective was to investigate the effect of 4-hydroxybenzoic acid (4-HBA) and 4-hydroxy-3-methoxybenzoic acid (Vanillic acid, VA) on p-glycoprotein (P-gp) activity in multidrug-resistant K562/Dox cancer cells. The cytotoxic and co-treatment with pirarubicin (Pira) were analyzed using a resazurin assay. A noninvasive functional spectrofluorometric technique was used to determine the kinetics of Pira uptake in living multidrug-resistant K562/Dox cancer cells. The three biological endpoints for determination of cellular energetic state included the activity of mitochondria, mitochondrial membrane potential (ΔΨm), and ATP levels. The results revealed that 4-HBA (10 mM) and VA (5 and 10 mM) statistically decreased cell viability in K562 and multidrug-resistant K562/Dox cancer cells. In ways consistent with that result, 4-HBA and VA (0.01, 0.1, 1, and 10 mM) could statistically decrease the IC50 of Pira in K562 and multidrug-resistant K562/Dox cancer cells at 48 and 72 h. The overall intracellular Pira concentration increased in 4-HBA- and VA-treated multidrug-resistant K562/Dox cancer cells when compared to control. The ratio of kai/ka0 in 4-HBA- and VA-treated multidrug-resistant K562/Dox cancer cells was significantly decreased when 4-HBA and VA concentration increased. The activity of mitochondria, ΔΨm, and ATP levels significantly reduced in multidrug-resistant K562/Dox cancer cells incubated with 0.01, 0.1, 1, and 10 mM 4-HBA and VA at all harvest time points. In conclusion, 4-HBA and VA were able to bring about cell death in multidrug-resistant K562/Dox cancer cell at high concentrations. The 4-HBA and VA could modify P-gp function via an impaired cellular energetic state, resulting in increased in intracellular drug concentration in multidrug-resistant K562/Dox cancer cells.
Keywords: 4-hydroxybenzoic acid, 4-hydroxy-3-methoxybenzoic acid, Multidrug resistance, P-glycoprotein, Pirarubicin, Cancer, Polyphenols, Vanillic acid
Graphical Abstract
Highlights
-
•
The 4-HBA and VA could exhibit anti-proliferative activity on human leukemic K562 cells and K562/Dox (P-glycoprotein-overexpression) cells.
-
•
The 4-HBA and VA could impair cellular energetic state in K562/Dox cancer cells
-
•
The 4-HBA and VA could modify P-glycoprotein function in K562/Dox cancer cells.
1. Introduction
Multidrug resistance is a key stumbling block to chemotherapy-based cancer treatment, and is frequently associated with P-gp expression in the plasma membrane which contains ATP-dependent transporters that effluxes anticancer drug out of cells, diminishing intracellular drug levels thereby allowing cancer cells to survive [1]. There are diverse pharmacological agents that inhibit the P-gp function. For example, verapamil, cyclosporine A, quinidine, tamoxifen, and toremifene can all have this effect. These compounds are limited due to their toxicity because the required dose to inhibit P-gp is in high serum concentrations [2]. Consequentially, P-gp modulators with less undesirable side effects are needed.
Natural products such as flavonoids have been reported to modulate P-gp and demonstrate anticancer activities in cancer cells [3], [4], [5]. These flavonoids include the following: Quercetin was discovered to inhibit P-gp function; this causes an enhancement in the intracellular concentration of doxorubicin and rhodamine 123 [6]. Similarly, Kaempferol also inhibited the efflux of rhodamine 123 and vinblastine in KB-V1 cells whenever P-gp was strongly expressed [7]. Apigenin could enhance the potential of doxorubicin in MES-SA/Dx5 cells that have overexpressed P-gp [8].
In addition, phenolic acids such as gallic acid, ellagic acid, and protocatechuic acid are also natural products which have been reported to enhance the efficacy of anticancer activities in various cancer cells [9], [10], [11]. However, few reports studied the effect of phenolic acids on the function of P-gp in multidrug resistant cells. Teng YN. et al. [12] examined the effects of caffeic acid and its mechanisms on P-gp. These authors showed that caffeic acid could uncompetitively and competitively inhibit rhodamine 123 and doxorubicin efflux, respectively. In addition, Muthusamy G. et al. [13] studied the effect of ferulic acid on P-gp-mediated drugs resistance in multidrug resistant cell lines. These authors found that ferulic acid inhibited P-gp function in drug resistant cell lines.
The 4-HBA and VA are members of polyphenols and phenolic acid derivatives. These compounds have been used in research area such as antioxidant activities and anti-inflammatory activities [14], [15], [16], [17], [18]. Even though they are less toxic compounds, they have anticancer activities in higher concentration [19], [20], [21]. Because of having anticancer activities based on previous studies, we would like to study whether these compounds can affect mitochondria function and P-gp function by impairing cellular energetic state. The current studies focused on 4-HBA and VA on the function of P-gp in multidrug resistant cancer cells due to their potential on anticancer activities in cancer cells [19], [21], [22]. Fig. 1 illustrates the chemical structures of 4-HBA and VA. Here, we report that 4-HBA and VA could regulate P-gp function in multidrug resistant cancer cells via cellular energetic damage, resulting in an increase in anticancer drug intracellular concentration.
Fig. 1.
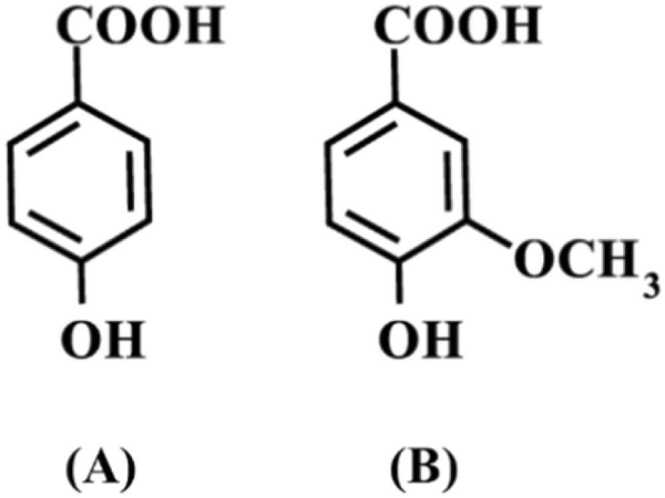
Chemical structure of 4-HBA (A) and VA (B).
2. Materials and methods
2.1. Chemicals
4-hydroxybenzoic acid (4-HBA), 4-hydroxy-3-methoxybenzoic acid (Vanillic acid, VA), Pirarubicin (Pira), Rhodamine B (Rho B), 3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyltetrazoliumbromide (MTT), and Resazurin were acquired from Sigma-Aldrich (St Louis, MO, USA).
2.2. Cancer cell lines
The cancer cell lines were multidrug-resistant K562/Dox (Overexpressed P-gp) cells and K562 cells lines. These cells were routinely cultured in a RPMI-1640 medium supplemented with 1 % penicillin/streptomycin and 10 % heat-inactivated fetal bovine serum in a cell culture incubator.
2.3. Cytotoxic and co-treatment assays
The cytotoxic and co-treatment assays were determined by using the resazurin assay in accordance with procedures obtained from prior reports [23], [24]. In brief, for cytotoxic assay, 5 × 104 cells/mL were treated with several doses of 4-HBA, VA, or Pira in a completed RPMI-1640 medium. For co-treatment assay, 5 × 104 cells/mL were treated with different Pira concentrations ranging from 10 to 5000 nM and fixed concentration of 4-HBA or VA (0.01, 0.1, 1, and 10 mM) in completed RPMI-1640 medium.
These treated cells were housed in a cell culture incubator. Afterward, the treated cells were given 100 µL of 0.1 mg/mL resazurin at 48 and 72 h later. A spectrofluorometer (Perkin Elmer LS55) was used to measure the emission of fluorescence at 590 nm (excited at 570 nm) after 4 h of incubation with resazurin.
The % cell viability was determined by the fluorescence emission intensity of treated cells at 590 nm divided by those of non-treated cells, and then in multiplies of one hundred. The Pira concentrations required to inhibit 50 % of cell viability (IC50) was obtained by plotting the % cell viability versus the Pira concentration. IC50 was measured at 48 and 72 h. In addition, the efficacy of 4-HBA and VA in increasing the efficacy of Pira on multidrug-resistant K562/Dox cancer cells was calculated as follows;.
α = [IC50(R)-IC50(RD)]/[IC50(R)-IC50(S)].
Where; IC50(R) and IC50(RD) was concentration of Pira that inhibits 50 % of multidrug-resistant K562/Dox cancer cell viability in the absence and presence of 4-HBA or VA, respectively.
IC50(S) was the concentration of Pira that inhibits 50 % of K562 cancer cell viability.
2.4. Determination of Pira uptake in living multidrug-resistant K562/Dox cancer cells
The kinetics of Pira uptake in living multidrug-resistant cancer cells was determined using a noninvasive functional spectrofluorometric (Perkin Elmer LS55) technique done according to directions mentioned in previous reports [25], [26]. Briefly, 2 × 106 cells/mL were treated with 4-HBA or VA in a HEPES-Na+ buffer, pH 7.25 at the temperature of 37 °C for 5 min and then it was followed by the addition of a 1 μM Pira concentration. The fluorescence emission intensity of Pira at 590 nm was functionally recorded when excited at 480 nm. Then, at the steady state, 5 µL of 4 % Triton X-100 solution was added to the cells, resulting in the state of equilibrium.
The parameters of Pira uptake in living multidrug-resistant K562/Dox cancer cells were calculated; ka was the active P-gp-mediated efflux coefficient for Pira, and Cn was the overall intracellular Pira concentration. In addition, the ratio of kai to ka0 could represent the potentiality of 4-HBA and VA to inhibit the P-gp function, kai and ka0 were the P-gp-mediated active efflux of Pira in the presence and absence of 4-HBA or VA, respectively.
2.5. Determination of cellular energetic state
The three biological endpoints for determination of cellular energetic state included activity of mitochondria, ΔΨm, and ATP levels.
2.5.1. Determination of activity of mitochondria
The assay was used to evaluate the mitochondrial activity based on directions indicated in a previous report [27]. Briefly, 5 × 105 cell/mL were treated with 0.01, 0.1, 1, and 10 mM 4-HBA or VA in a completed RPMI-1640 medium in a cell culture incubator for 5, 10, 30, 60, 120, and 240 min. The cells were then treated with 1 mM MTT. After 20 min, insoluble formazan was obtained and then was dissolved by DMSO. A spectrophotometer (Spectrophotometer, Shimadzu) was used to evaluate the absorbance (Abs.) at 560 nm. The absorption at 560 nm change indicates the activity of mitochondria. The % Abs. at 560 nm change in treated cells was calculated and compared to non-treated cells.
2.5.2. Determination of ΔΨm
A non-invasive functional spectrofluorometric approach was used to determine the ΔΨm based on previous reports [25], [27], [28]. Briefly, 2 × 106 cells/mL were treated with 0.01, 0.1, 1, and 10 mM 4-HBA or VA for 5, 10, 30, 60, 120, and 240 min in HEPES-Na+ buffer, pH 7.25 at a temperature of 37 °C and then this was followed by an addition of 40 nM concentration of Rho B. The fluorescence emission intensity of Rho B at 582 nm was recorded as a function of time when excited at 553 nm. After 20 min, 200 µM MTT was added to the cells, leading to a constant decline in Rho B fluorescence. The following equation was used to calculate ΔΨm;.
ΔΨm = −61.51 logVi - 258.46, mV.
Where; Vi was the initial rate of decrease in Rho B fluorescence, (dF/dt) × (CT/F0), nM/s (dF/dt was slope of the tangent to the curve F = f(t) after the addition of MTT.
CT was Rho B concentration.
F0 was Rho B fluorescent intensity.
2.5.3. Determination of ATP in cells
The cellular ATP was measured in ways consistent with previous reports [27]. Briefly, 2 × 106 cells/mL were treated with 0.01, 0.1, 1, and 10 mM 4-HBA or VA for 30, 60, 120, 240 min in a HEPES-Na+ buffer, having a pH of 7.25 at a temperature of 37 °C. Cellular ATP samples were extracted by a single-step using boiling water (60 °C). Thin layer chromatography (TLC silica gel 60 F254, Merck, Germany) was used to purify the extracted ATP samples. The acetic acid: butanol: distilled water (1:4:5, v/v) solution was used as mobile phase. ATP standard was used as a reference. At fluorescence emission ranges of 390–500 nm (Excited at 360 nm), purified ATP samples were recorded. The % ATP change in treated cells was calculated as that compared to non-treated cells.
2.6. Statistical analysis
The analysis on experiment results was done as mean ± standard error of the mean (S.E.). To evaluate the statistical differences between control and treated samples, the student’s t-test and analysis of variance (ANOVA) methods were employed. A p-value less than 0.05 was considered statistically significant.
3. Results
3.1. Effect of 4-HBA, VA, and Pira on cell viability
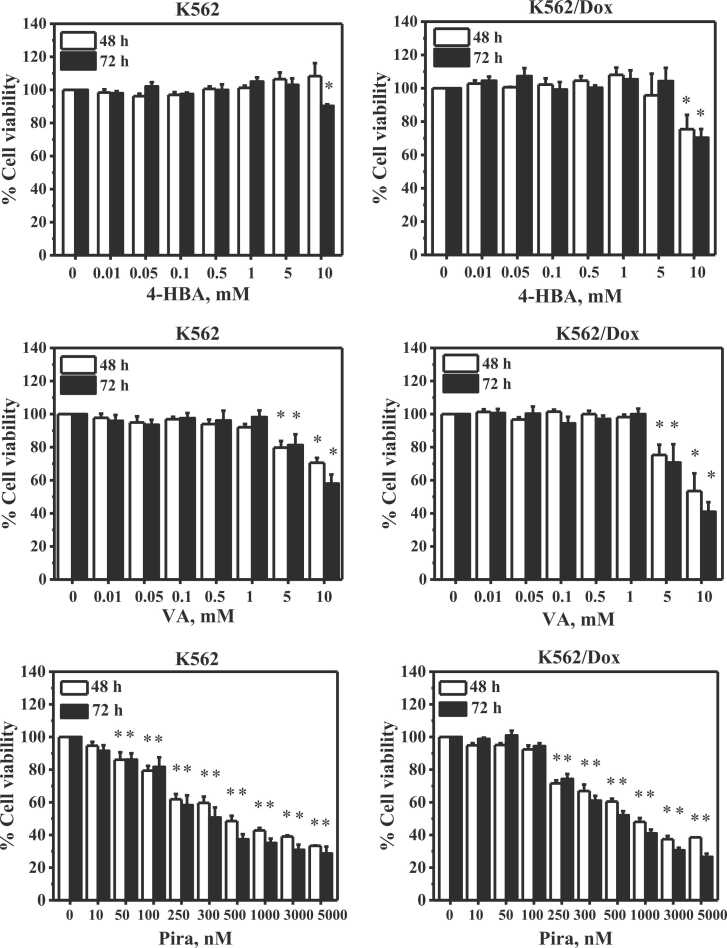
Fig. 2 shows the effects of 4-HBA, VA, and Pira on cell viability of K562 and multidrug-resistant K562/Dox cancer cells. At 48 h, 4-HBA did not exhibit any effect on the cell viability of K562 cancer cells, whereas 10 mM 4-HBA statistically reduced the cell viability of multidrug-resistant K562/Dox cancer cells to 75 %. At 72 h, 10 mM 4-HBA statistically decreased cell viability of K562 and multidrug-resistant K562/Dox cancer cells to 90 % and 70 %, respectively.
Fig. 2.
Effect of 4-HBA, VA and Pira on cell viability of K562 and multidrug-resistant K562/Dox cancer cells. * Significant difference at p < 0.05 when compared to control. n = 4.
VA (5 and 10 mM) statistically decreased cell viability of K562 cancer cells to 79 % and 70 % at 48 h and 81 % and 57 % at 72 h, respectively. Similarly, 5 and 10 mM VA statistically decreased cell viability of multidrug-resistant K562/Dox cancer cells to 75 % and 53 % at 48 h and 70 % and 40 % at 72 h, respectively.
Pira (range 50–5000 nM) statistically reduced cell viability of K562 cancer cells while 250−5000 nM Pira statistically reduced cell viability of multidrug-resistant K562/Dox cancer cells at 48 and 72 h. Such decrements were in a dose dependent manner.
3.2. Effect of co-treatment of 4-HBA and VA with Pira on cell viability
Table 1 shows the effect of co-treatment of 4-HBA and VA with Pira on cell viability of K562 and multidrug-resistant K562/Dox cancer cells. 4-HBA (0.01, 0.1, 1, and 10 mM) could statistically decrease the IC50 of Pira in K562 and multidrug-resistant K562/Dox cancer cells at 48 and 72 h. Similarly, VA (0.01, 0.1, 1, and 10 mM) could statistically decrease the IC50 of Pira in K562 and multidrug-resistant K562/Dox cancer cells at 48 and 72 h.
Table 1.
Pattern of cytotoxicity of Pira, Pira/4-HBA and Pira/VA on K562 and multidrug-resistant K562/Dox cancer cells. n = 4.
| Drugs | IC50, nM |
|||
|---|---|---|---|---|
| K562 |
Multidrug-resistant K562/Dox |
|||
| 48 h | 72 h | 48 h | 72 h | |
| Pira | 427.8 ± 58.4 | 302.4 ± 47.0 | 728.3 ± 90.5 | 511.2 ± 47.0 |
| Pira + 0.01 mM 4-HBA | 192.5 ± 25.2* | 126.3 ± 16.0* | 221.3 ± 68.0* | 227.5 ± 23.3* |
| Pira + 0.1 mM 4-HBA | 181.3 ± 20.6* | 141.3 ± 16.1* | 237.5 ± 73.8* | 182.5 ± 18.8* |
| Pira + 1 mM 4-HBA | 178.8 ± 27.0* | 133.8 ± 21.2* | 164.5 ± 36.7* | 126.3 ± 22.9* |
| Pira + 10 mM 4-HBA | 170.0 ± 25.2* | 132.5 ± 25.4* | 82.5 ± 12.3* | 83.8 ± 14.2* |
| Pira + 0.01 mM VA | 212.5 ± 37.6* | 123.8 ± 15.5* | 290.0 ± 53.2* | 206.3 ± 42.1* |
| Pira + 0.1 mM VA | 210.0 ± 20.9* | 140.0 ± 18.3* | 297.5 ± 47.2* | 185.0 ± 9.8* |
| Pira + 1 mM VA | 143.8 ± 34.5* | 128.8 ± 25.5* | 120.0 ± 20.8* | 147.0 ± 44.8* |
| Pira + 10 mM VA | 162.5 ± 34.2* | 86.3 ± 29.0* | 125.0 ± 9.8* | 121.0 ± 9.3* |
Note: IC50 are the Pira concentrations required to inhibit 50 % of cell viability. * Significantly different at p < 0.05 when compared to control.
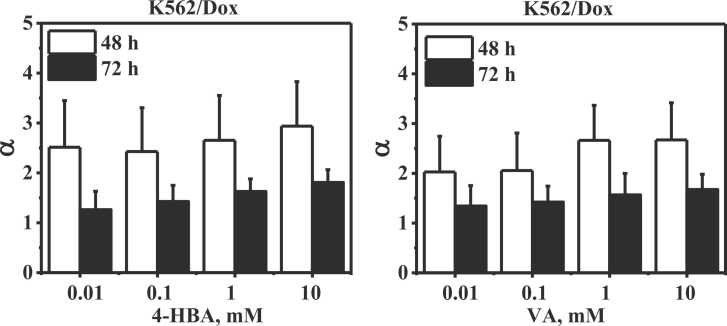
The efficacy (α) of 4-HBA and VA in reversing multidrug resistance phenomena and/or re-sensitizing the multidrug-resistant K562/Dox cancer cells to Pira are indicated in Fig. 3. The α values of 0.01, 0.1, 1, and 10 mM 4-HBA and VA were higher than 1 at 48 and 72 h, resulting in the IC50(RD) < IC50(S). This data demonstrated that 4-HBA and VA could be re-sensitizing the multidrug-resistant K562/Dox cancer cells to Pira.
Fig. 3.
Efficacy (α) of 4-HBA and VA to increase the cytotoxicity of Pira on multidrug-resistant K562/Dox cancer cells. n = 4.
3.3. Effect of 4-HBA and VA on Pira uptake in living multidrug-resistant K562/Dox cancer cells
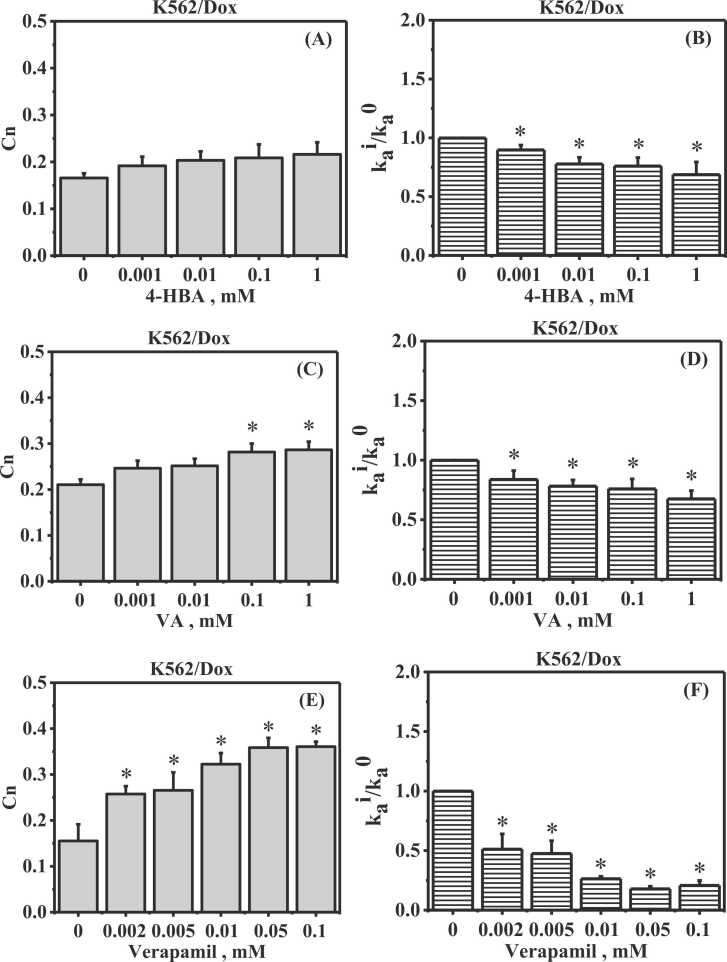
Fig. 4(A) and (B) show the effect of 4-HBA on Pira uptake in living multidrug-resistant K562/Dox cancer cells. The overall intracellular Pira concentration (Cn) increased in 4-HBA-treated multidrug-resistant K562/Dox cancer cells compared to control. The ratio of kai/ka0 values in 4-HBA-treated multidrug-resistant K562/Dox significantly decreased when a 4-HBA concentration increased.
Fig. 4.
Effect of 4-HBA and VA on Pira uptake in living multidrug-resistant K562/Dox cancer cells. Effect of 4-HBA on Cn (A) and kai/ka0 (B) and VA on Cn (C) and kai/ka0 (D) and verapamil on Cn (E) and kai/ka0 (F) in living multidrug-resistant K562/Dox cancer cells. Cn: overall intracellular Pira concentration, kai/ka0: the potentiality of 4-HBA, VA and verapamil to suppress the P-glycoprotein function. The ratio of kai/ka0 value equal to 1 defined as 4-HBA, VA and verapamil cannot inhibit the active efflux and the ratio of kai/ka0 value equal to 0 defined as 4-HBA, VA and verapamil can completely inhibit the active efflux. * Significantly different at p < 0.05 when compared to control. n = 4.
Fig. 4(C) and (D) show the effect of VA on Pira uptake in living multidrug-resistant K562/Dox cancer cells. Cn increased in VA-treated multidrug-resistant K562/Dox cancer cells when compared to control. Moreover, 0.1 and 1 mM VA could significantly increase Cn in treated multidrug-resistant K562/Dox cells. Furthermore, VA could significantly decrease kai/ka0 values in treated multidrug-resistant K562/Dox cells.
Fig. 4(E) and (F) show the effect of verapamil on Pira uptake in living multidrug-resistant K562/Dox cancer cells. Cn significantly increased in multidrug-resistant K562/Dox cancer cells treated with verapamil in a concentration dependent manner. The ratio of kai/ka0 values also significantly decreased in multidrug-resistant K562/Dox cancer cells when concentration of verapamil increased.
The results suggested that 4-HBA, VA, and verapamil could modify P-gp-mediated Pira transport into the multidrug-resistant K562/Dox cancer cells. However, 4-HBA and VA weakly exhibited the modification of P-gp function when compared to verapamil.
3.4. Effect of 4-HBA and VA on cellular energetic state in multidrug-resistant K562/Dox cancer cells
3.4.1. Effect on activity of mitochondria
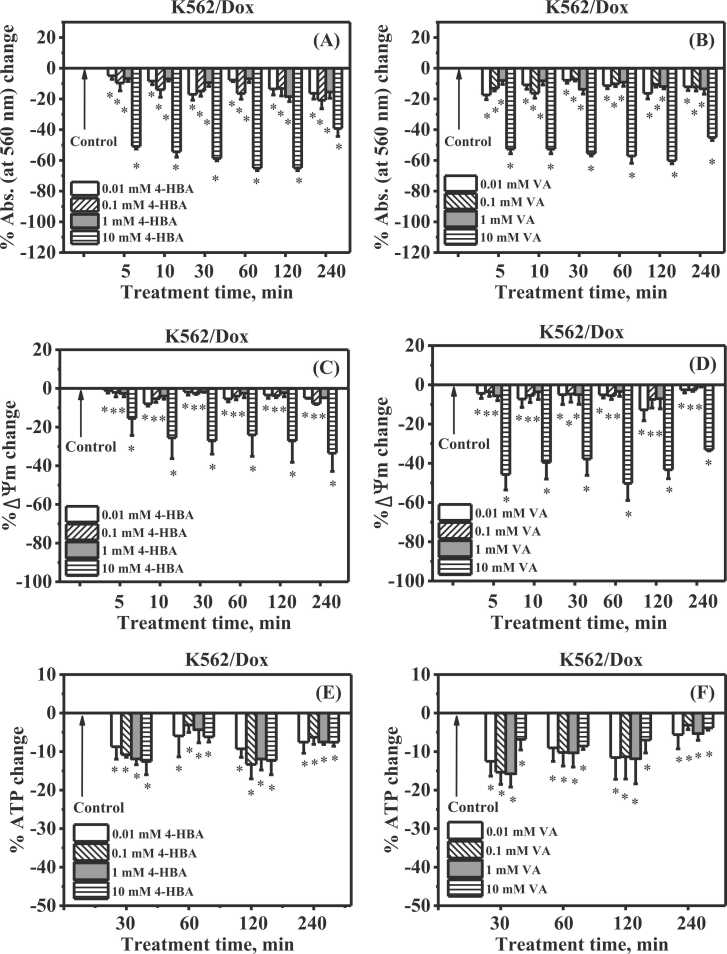
The activity of mitochondria in multidrug-resistant K562/Dox cancer cells was observed for the occurrence of insoluble formazan production. The Abs. at 560 nm was used to determine the formazan. The alteration of Abs. at 560 nm was referred to as mitochondrial activity. Fig. 5 (A) and (B) show % Abs. at 560 nm change of incubated multidrug-resistant K562/Dox cancer cells, compared with control cells at 5, 10, 30, 60, 120, and 240 min. At all harvest time points, the Abs. at 560 nm in multidrug-resistant K562/Dox cancer cells incubated with 0.01, 0.1, 1, and 10 mM 4-HBA had significantly decreased. Similarly, 0.01, 0.1, 1, and 10 mM VA could significantly decrease Abs. at 560 nm in multidrug-resistant K562/Dox cancer cells at all harvest time points. These results suggested that 4-HBA and VA had an effect on mitochondrial activity in multidrug-resistant K562/Dox cancer cells.
Fig. 5.
Effect of 4-HBA or VA cellular energetic state in multidrug-resistant K562/Dox cancer cells. Effect of 4-HBA (A) and VA (B) on % Abs. at 560 nm change, Abs. at 560 nm represents mitochondrial activity. Effect of 4-HBA (C) and VA (D) on % ΔΨm change. Effect of 4-HBA (E) and VA (F) on % ATP change. * Significantly different at p < 0.05 when compared to control. n = 4.
3.4.2. Effect on ΔΨm
Fig. 5(C) and (D) shows % ΔΨm change of incubated multidrug-resistant K562/Dox cancer cells, compared with control cells at 5, 10, 30, 60, 120, and 240 min. The % ΔΨm was significantly reduced in multidrug-resistant K562/Dox cancer cells incubated with 0.01, 0.1, 1, and 10 mM 4-HBA at all harvest time points. Similarly, 0.01, 0.1, 1, and 10 mM VA could significantly decrease % ΔΨm in multidrug-resistant K562/Dox cancer cells at all harvest time points. These results suggested that 4-HBA and VA had an effect on ΔΨm in multidrug-resistant K562/Dox cancer cells.
3.4.3. Effect on ATP levels
Fig. 5(E) and (F) show % ATP change of incubated multidrug-resistant K562/Dox cancer cells, compared with control cells at 5, 10, 30, 60, 120, and 240 min. The % ATP was considerably reduced in multidrug-resistant K562/Dox cancer cells incubated with 0.01, 0.1, 1, and 10 mM 4-HBA at all harvest time points. Similarly, 0.01, 0.1, 1, and 10 mM VA could significantly decrease % ATP in multidrug-resistant K562/Dox cancer cells at all harvest time points. These results suggested that 4-HBA and VA had an effect on ATP levels in multidrug-resistant K562/Dox cancer cells.
4. Discussion
There are several P-gp modulated compounds such as verapamil, CsA, SDZ PSC833, VX710, LY335979, and OC144-093 [29], [30], [31], [32], [33]. Waghray and Zhang [34] have shown how to sensitize anticancer agents in multidrug-resistant cancer cells. Natural products, flavonoids, and phenolic acids have also been revealed to have anticancer activities in cancer cells [12], [35], [36]. 4-HBA, a phenolic acid, has been shown to enhance the sensitivity of cancer cells to anticancer agents. Wang XN. et al. [21] studied the 4-HBA to sensitize breast cancer cells to anticancer agent-induced anticancer effects. The authors found that 4-HBA could successfully reverse breast cancer resistance and induced the anticancer effect which was associated with homeodomain interacting protein kinase-2. In addition, VA exhibited anticancer effects on the inhibition of hypoxia-inducible factor-1 expression induced by hypoxia in colon cancer cells [19].
This current study investigated the anticancer activities of 4-HBA and VA in K562 and multidrug-resistant K562/Dox cancer cells and their potential to modify P-gp-mediated Pira transport in multidrug-resistant K562/Dox cancer cells which have an overexpression of P-gp. Both K562 and K562/Dox cancer cells are leukemia cell lines. These cells are widely used as a highly sensitive in vitro studies and mostly used in multidrug resistance studies. The K562 cell was used as drug sensitive phenotype where P-gp was not overexpressed in these cells and use as a control to compare the effectiveness of drugs. The K562/Dox was the P-gp overexpression drug resistant phenotype in which P-gp function could be detected by using P-gp inhibitors or modulators. The current results showed that Pira inhibited cell viability in a nanomolar range of concentration in ways that were consistent with other reports [37], [38], [39] while 4-HBA and VA inhibited cell viability in a millimolar range of concentration in both K562 and multidrug-resistant K562/Dox cancer cells. Moreover, VA could inhibit cell viability in both of these cancer cells more than 4-HBA. It could be suggested that 4-HBA and VA weakly exhibited anticancer activity in K562 and multidrug-resistant K562/Dox cancer cells, in comparison to Pira. Of note, 4-HBA and VA alone showed higher efficacy in multidrug-resistant K562/Dox than K562 cells.
Pira is a well-known multidrug resistance compound [40]. Co-treatment using Pira with several concentrations of 4-HBA or VA could decrease the IC50 for both K562 and multidrug-resistant K562/Dox cancer cells. The efficacy (α) values of 4-HBA and VA were higher than 1. In another words, the co-treatment of Pira with 4-HBA and VA showed that the IC50 of multidrug-resistant K562/Dox cells was less than that of K562 cells. These results suggested that 4-HBA and VA could be re-sensitizing the multidrug-resistant K562/Dox cancer cells to Pira. Taken together, the results implied that 4-HBA and VA could be used as a multidrug resistance modulator.
To understand the mode of action of 4-HBA and VA and increments of Pira in multidrug resistant cells, we investigated the ability of 4-HBA and VA to inhibit the kinetics of P-gp-mediated efflux Pira out of the cells. Our results showed the ratio kai/ka0 values were significantly less than 1 in treated multidrug-resistant K562/Dox cancer cells at 0.001-1 mM 4-HBA and VA. This suggested that 4-HBA and VA could inhibit P-gp efflux Pira out of the multidrug resistant cells. Thus, we proposed that 4-HBA and VA decreased the P-gp function leading to an increase intracellular Pira concentration. Consequently, the multidrug resistant cells showed re-sensitizing to Pira. Of note, higher than 1 mM concentration of 4-HBA and VA could not lead to a determination of the ratio kai/ka0 values in multidrug-resistant K562/Dox cancer cells because these concentrations quenched fluorescence emission of Pira.
In addition, we also investigated how 4-HBA and VA inhibited the P-gp function in multidrug-resistant cells. Our findings showed that 4-HBA and VA significantly decreased the ΔΨm and mitochondrial function of treated cells when compared to control. The determination of mitochondrial activity in the current study could reveal mitochondrial succinate dehydrogenase (SDH) activity. The MTT was primarily reduced to formazan crystals by the SDH. The formazan was analyzed spectrophotometrically (Abs. at 560 nm) after dissolution in DMSO. Hence, we proposed that 4-HBA and VA could inhibit SDH activity.
Due to the change of ΔΨm and mitochondrial function, there could be an effect on ATP production in cells [26]. Our results showed that ATP levels decreased in 4-HBA- and VA- treated cells. Of note, P-gp is an ATP-binding-cassette protein transporter. P-gp function is an energy-dependent efflux pump for chemotherapeutic agents [41]. Hence, we proposed that 4-HBA and VA induced cellular energetic damage had resulted in deceased ATP levels. Consequently, the function of P-gp decreased in multidrug-resistant cells and intracellular drug accumulation increased, and ultimately multidrug-resistant cells died.
Of note, the methoxylated substitutions in the chemical structure of polyphenol compounds had been shown to be beneficial for the inhibitory activity on P-gp in KB/MDR1 cells [42] and on the fluorescein transport in Caco-2 cell monolayers [43]. The Cn values of VA-treated cells appear to be higher than 4-HBA-treated cells in multidrug-resistant K562/Dox cells. This might be due to methoxylated substitution in the chemical structure of VA (Fig. 1). However, the structure activity relationships need to be further investigated in future studies.
5. Conclusion
Taken together, the current study suggested that 4-HBA and VA were able to bring about cell death in K562 and multidrug-resistant K562/Dox cancer cell at high concentrations and that there were anticancer effects possibly via inducing energetic state damage and modification of P-gp function. Although the current study implies that there are alternative multidrug-resistant compounds that can re-sensitize Pira in multidrug-resistant cancer treatment at the cellular level, the anticancer activities should be evaluated at in vivo level.
CRediT authorship contribution statement
Ohnmar Myint: Methodology, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. Sakornniya Wattanapongpitak: Methodology, Investigation. Suchart Kothan: Resources, Writing – review & editing. Chatchanok Udomtanakunchai: Resources, Writing – review & editing. Singkome Tima: Resources, Writing – review & editing. Montree Tungjai: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Ohnmar Myint would like to thank the Ph.D. degree program in biomedical sciences, Faculty of Associated Medical Sciences, Chiang Mai University, under the CMU Presidential Scholarship. This research was partially supported by Chiang Mai University and the Faculty of Associated Medical Sciences, Chiang Mai University. The authors would like to thank the Department of Radiologic Technology, Faculty of Associated Medical Sciences, Chiang Mai University for their supports.
Handling Editor: Lawrence Lash
References
- 1.Nanayakkara A.K., Vogel P.D., Wise J.G. Prolonged inhibition of p-glycoprotein after exposure to chemotherapeutics increases cell mortality in multidrug resistant cultured cancer cells. PLoS One. 2019;14(6):e0217940.. doi: 10.1371/journal.pone.0217940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansal T., Jaggi M., Khar R.K., Talegaonkar S. Emerging significance of flavonoids as p-glycoprotein inhibitors in cancer chemotherapy. J. Pharm. Pharm. Sci. 2009;12(1):46–78. doi: 10.18433/j3rc77. [DOI] [PubMed] [Google Scholar]
- 3.G. B M.F., D S.P.C., MJ U.F. Overcoming multidrug resistance: flavonoid and terpenoid nitrogen-containing derivatives as ABC transporter modulators. Molecules. 2020;25(15) doi: 10.3390/molecules25153364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye Q., Liu K., Shen Q., Li Q., Hao J., Han F., Jiang R.W. Reversal of multidrug resistance in cancer by multi-functional flavonoids. Front Oncol. 2019;9:487. doi: 10.3389/fonc.2019.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdallah H.M., Al-Abd A.M., El-Dine R.S., El-Halawany A.M. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: a review. J. Adv. Res. 2015;6(1):45–62. doi: 10.1016/j.jare.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z., Huang C., Ma T., Jiang L., Tang L., Shi T., Zhang S., Zhang L., Zhu P., Li J., Shen A. Reversal effect of quercetin on multidrug resistance via FZD7/β-catenin pathway in hepatocellular carcinoma cells. Phytomedicine. 2018;43:37–45. doi: 10.1016/j.phymed.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 7.Limtrakul P., Khantamat O., Pintha K. Inhibition of p-glycoprotein function and expression by kaempferol and quercetin. J. Chemother. 2005;17(1):86–95. doi: 10.1179/joc.2005.17.1.86. [DOI] [PubMed] [Google Scholar]
- 8.Angelini A., Di Ilio C., Castellani M.L., Conti P., Cuccurullo F. Modulation of multidrug resistance p-glycoprotein activity by flavonoids and honokiol in human doxorubicin- resistant sarcoma cells (MES-SA/DX-5): implications for natural sedatives as chemosensitizing agents in cancer therapy. J. Biol. Regul. Homeost. Agents. 2010;24(2):197–205. [PubMed] [Google Scholar]
- 9.Maruszewska A., Tarasiuk J. Antitumour effects of selected plant polyphenols, gallic acid and ellagic acid, on sensitive and multidrug-resistant leukaemia HL60 cells. Phytother. Res. 2019;33(4):1208–1221. doi: 10.1002/ptr.6317. [DOI] [PubMed] [Google Scholar]
- 10.Yip E.C., Chan A.S., Pang H., Tam Y.K., Wong Y.H. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biol. Toxicol. 2006;22(4):293–302. doi: 10.1007/s10565-006-0082-4. [DOI] [PubMed] [Google Scholar]
- 11.Abotaleb M., Liskova A., Kubatka P., Büsselberg D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules. 2020;10(2) doi: 10.3390/biom10020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teng Y.N., Wang C.C.N., Liao W.C., Lan Y.H., Hung C.C. Caffeic acid attenuates multi-drug resistance in cancer cells by inhibiting efflux function of human p-glycoprotein. Molecules. 2020;25(2) doi: 10.3390/molecules25020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muthusamy G., Balupillai A., Ramasamy K., Shanmugam M., Gunaseelan S., Mary B., Prasad N.R. Ferulic acid reverses ABCB1-mediated paclitaxel resistance in MDR cell lines. Eur. J. Pharm. 2016;786:194–203. doi: 10.1016/j.ejphar.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S., Prahalathan P., Raja B. Antihypertensive and antioxidant potential of vanillic acid, a phenolic compound in L-NAME-induced hypertensive rats: a dose-dependence study. Redox Rep. 2011;16(5):208–215. doi: 10.1179/1351000211Y.0000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy K.K., Ravinder T., Kanjilal S. Synthesis and evaluation of antioxidant and antifungal activities of novel ricinoleate-based lipoconjugates of phenolic acids. Food Chem. 2012;134(4):2201–2207. doi: 10.1016/j.foodchem.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 16.Itoh A., Isoda K., Kondoh M., Kawase M., Kobayashi M., Tamesada M., Yagi K. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a-induced liver injury. Biol. Pharm. Bull. 2009;32(7):1215–1219. doi: 10.1248/bpb.32.1215. [DOI] [PubMed] [Google Scholar]
- 17.Cho J.Y., Moon J.H., Seong K.Y., Park K.H. Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci. Biotechnol. Biochem. 1998;62(11):2273–2276. doi: 10.1271/bbb.62.2273. [DOI] [PubMed] [Google Scholar]
- 18.Winter A.N., Brenner M.C., Punessen N., Snodgrass M., Byars C., Arora Y., Linseman D.A. Comparison of the neuroprotective and anti-inflammatory effects of the anthocyanin metabolites, protocatechuic acid and 4-hydroxybenzoic acid. Oxid. Med. Cell Longev. 2017;2017:6297080. doi: 10.1155/2017/6297080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong J., Zhou S., Yang S. Vanillic acid suppresses HIF-1α expression via inhibition of mTOR/p70S6K/4E-BP1 and Raf/MEK/ERK pathways in human colon cancer HCT116 cells. Int. J. Mol. Sci. 2019;20(3) doi: 10.3390/ijms20030465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velli S.K., Thiruvengadam D. Vanillic acid inhibits lung carcinogenesis by modulates glycoprotein abnormalities, membrane-bound enzymes, and inflammatory markers. Int. J. Pharm. Pharm. Sci. 2020;12(3):83–88. [Google Scholar]
- 21.Wang X.N., Wang K.Y., Zhang X.S., Yang C., Li X.Y. 4-hydroxybenzoic acid (4-HBA) enhances the sensitivity of human breast cancer cells to adriamycin as a specific HDAC6 inhibitor by promoting HIPK2/p53 pathway. Biochem. Biophys. Res. Commun. 2018;504(4):812–819. doi: 10.1016/j.bbrc.2018.08.043. [DOI] [PubMed] [Google Scholar]
- 22.Sitarek P., Skała E., Toma M., Wielanek M., Szemraj J., Skorski T., Białas A.J., Sakowicz T., Kowalczyk T., Radek M., Wysokińska H. T. Śliwiński, Transformed root extract of Leonurus sibiricus induces apoptosis through intrinsic and extrinsic pathways in various grades of human glioma cells. Pathol. Oncol. Res. 2017;23(3):679–687. doi: 10.1007/s12253-016-0170-6. [DOI] [PubMed] [Google Scholar]
- 23.Supawat B., Moungthong P., Chanloi C., Jindachai N., Tima S., Kothan S., Udomtanakunchai C., Tungjai M. Effects of gadolinium-based magnetic resonance imaging contrast media on red blood cells and K562 cancer cells. J. Trace Elem. Med. Biol. 2020;62 doi: 10.1016/j.jtemb.2020.126640. [DOI] [PubMed] [Google Scholar]
- 24.Myint O., Wattanapongpitak S., Supawat B., Kothan S., Udomtanakunchai C., Tima S., Tungjai M. Protein binding of 4-hydroxybenzoic acid and 4-hydroxy-3-methoxybenzoic acid to human serum albumin and their anti-proliferation on doxorubicin-sensitive and doxorubicin-resistant leukemia cells. Toxicol. Rep. 2021;8:1381–1388. doi: 10.1016/j.toxrep.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Supawat B., Udomtanakunchai C., Kothan S., Tungjai M. The effects of iodinated radiographic contrast media on multidrug-resistant K562/Dox cells: Mitochondria impairment and p-glycoprotein inhibition. Cell Biochem. Biophys. 2019;77(2):157–163. doi: 10.1007/s12013-019-00868-3. [DOI] [PubMed] [Google Scholar]
- 26.Reungpatthanaphong P., Mankhetkorn S. Modulation of multidrug resistance by artemisinin, artesunate and dihydroartemisinin in K562/adr and GLC4/adr resistant cell lines. Biol. Pharm. Bull. 2002;25(12):1555–1561. doi: 10.1248/bpb.25.1555. [DOI] [PubMed] [Google Scholar]
- 27.Aye K.T., Wattanapongpitak S., Supawat B., Kothan S., Udomtanakunchai C., Tima S., Pan J., Tungjai M. Gallic acid enhances pirarubicin‑induced anticancer in living K562 and K562/Dox leukemia cancer cells through cellular energetic state impairment and p‑glycoprotein inhibition. Oncol. Rep. 2021;46(4):227. doi: 10.3892/or.2021.8178. [DOI] [PubMed] [Google Scholar]
- 28.Tungjai M., Phathakanon N., Rithidech K.N. Effects of medical diagnostic low-dose X rays on human lymphocytes: Mitochondrial membrane potential, apoptosis and cell cycle. Health Phys. 2017;112(5) doi: 10.1097/HP.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z.L., Hirano T., Tanaka S., Onda K., Oka K. Persistent reversal of p-glycoprotein-mediated daunorubicin resistance by tetrandrine in multidrug-resistant human T lymphoblastoid leukemia MOLT-4 cells. J. Pharm. Pharm. 2003;55(11):1531–1537. doi: 10.1211/0022357022115. [DOI] [PubMed] [Google Scholar]
- 30.Dantzig A.H., Shepard R.L., Cao J., Law K.L., Ehlhardt W.J., Baughman T.M., Bumol T.F., Starling J.J. Reversal of p-glycoprotein-mediated multidrug resistance by a potent cyclopropyldibenzosuberane modulator, LY335979. Cancer Res. 1996;56(18):4171–4179. [PubMed] [Google Scholar]
- 31.Boesch D., Muller K., Pourtier-Manzanedo A., Loor F. Restoration of daunomycin retention in multidrug-resistant P388 cells by submicromolar concentrations of SDZ PSC 833, a nonimmunosuppressive cyclosporin derivative. Exp. Cell Res. 1991;196(1):26–32. doi: 10.1016/0014-4827(91)90452-z. [DOI] [PubMed] [Google Scholar]
- 32.Germann U.A., Shlyakhter D., Mason V.S., Zelle R.E., Duffy J.P., Galullo V., Armistead D.M., Saunders J.O., Boger J., Harding M.W. Cellular and biochemical characterization of VX-710 as a chemosensitizer: reversal of p-glycoprotein-mediated multidrug resistance in vitro. Anticancer Drugs. 1997;8(2):125–140. doi: 10.1097/00001813-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Newman M.J., Rodarte J.C., Benbatoul K.D., Romano S.J., Zhang C., Krane S., Moran E.J., Uyeda R.T., Dixon R., Guns E.S., Mayer L.D. Discovery and characterization of OC144-093, a novel inhibitor of p-glycoprotein-mediated multidrug resistance. Cancer Res. 2000;60(11):2964–2972. [PubMed] [Google Scholar]
- 34.Waghray D., Zhang Q. Inhibit or evade multidrug resistance p-glycoprotein in cancer treatment. J. Med. Chem. 2018;61(12):5108–5121. doi: 10.1021/acs.jmedchem.7b01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nabekura T., Kawasaki T., Furuta M., Kaneko T., Uwai Y. Effects of natural polyphenols on the expression of drug efflux transporter p-glycoprotein in human intestinal cells. ACS Omega. 2018;3(2):1621–1626. doi: 10.1021/acsomega.7b01679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takara K., Fujita M., Matsubara M., Minegaki T., Kitada N., Ohnishi N., Yokoyama T. Effects of propolis extract on sensitivity to chemotherapeutic agents in HeLa and resistant sublines. Phytother. Res. 2007;21(9):841–846. doi: 10.1002/ptr.2165. [DOI] [PubMed] [Google Scholar]
- 37.Frezard F., Garnier-Suillerot A. Comparison of the membrane transport of anthracycline derivatives in drug-resistant and drug-sensitive K562 cells. Eur. J. Biochem. 1991;196(2):483–491. doi: 10.1111/j.1432-1033.1991.tb15840.x. [DOI] [PubMed] [Google Scholar]
- 38.Marbeuf-Gueye C., Ettori D., Priebe W., Kozlowski H., Garnier-Suillerot A. Correlation between the kinetics of anthracycline uptake and the resistance factor in cancer cells expressing the multidrug resistance protein or the p-glycoprotein. Biochim Biophys. Acta. 1999;1450(3):374–384. doi: 10.1016/s0167-4889(99)00060-9. [DOI] [PubMed] [Google Scholar]
- 39.Borrel M.N., Pereira E., Fiallo M., Garnier-Suillerot A. Mobile ionophores are a novel class of p-glycoprotein inhibitors. The effects of ionophores on 4′-O-tetrahydropyranyl-adriamycin incorporation in K562 drug-resistant cells. Eur. J. Biochem. 1994;223(1):125–133. doi: 10.1111/j.1432-1033.1994.tb18973.x. [DOI] [PubMed] [Google Scholar]
- 40.Garnier-Suillerot A., Marbeuf-Gueye C., Salerno M., Loetchutinat C., Fokt I., Krawczyk M., Kowalczyk T., Priebe W. Analysis of drug transport kinetics in multidrug-resistant cells: implications for drug action. Curr. Med. Chem. 2001;8(1):51–64. doi: 10.2174/0929867013373967. [DOI] [PubMed] [Google Scholar]
- 41.Tarasiuk J., Garnier-Suillerot A. Kinetic parameters for the uptake of anthracycline by drug-resistant and drug-sensitive K562 cells. Eur. J. Biochem. 1992;204(2):693–698. doi: 10.1111/j.1432-1033.1992.tb16683.x. [DOI] [PubMed] [Google Scholar]
- 42.Xia M., Fang Y., Cao W., Liang F., Pan S., Xu X. Vol. 24. Basel; Switzerland: 2019. Quantitative structure-activity relationships for the flavonoid-mediated inhibition of p-glycoprotein in KB/MDR1 cells; p. 1661. (Molecules). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Konishi Y., Kubo K., Shimizu M. Structural effects of phenolic acids on the transepithelial transport of fluorescein in caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2003;67(9):2014–2017. doi: 10.1271/bbb.67.2014. [DOI] [PubMed] [Google Scholar]