Abstract
Pharmacological studies have revealed the potential antidiabetic effects of bitter melon seeds (Momordica charantia) in animals and humans. However, the sub-chronic safety of bitter melon seeds remains elusive. This exploratory study aimed to assess the acute and sub-chronic toxicity of a bitter melon seed extract from supercritical carbon dioxide (scCO2) extraction in Wistar rats based on the Organization for Economic Co-operation and Development (OECD) guidelines No. 423 and 408. No mortality and toxicity were observed in rats treated with a single dose of the extract during the 14-day observation period. The median lethal dose (LD50) of the extract was considered greater than 2000 mg/kg body weight (BW). For the sub-chronic toxicity study, male and female rats were orally administered daily doses of 0, 250, 500, and 1000 mg/kg BW for 90 days. No mortality, morbidity, and abnormal pathological and biochemical alterations were observed. The no-observed-adverse-effect-level (NOAEL) of the bitter melon seed extract was greater than 1000 mg/kg BW. Accordingly, bitter melon seed extract from scCO2 extraction may be considered a non-toxic dietary ingredient.
Keywords: Momordica charantia, Bitter melon seeds, Acute toxicity, Sub-chronic toxicity, Supercritical carbon dioxide extraction
Graphical Abstract
Highlights
-
•
Pharmacological studies have revealed the potential hypoglycemic effect of bitter melon seed extracts.
-
•
The LD50 of the scCO2-based bitter melon seed extract was > 2000 mg/kg BW.
-
•
The NOAEL of the scCO2-based bitter melon seed extract in rats was > 1000 mg/kg BW.
1. Introduction
Bitter melon (Momordica charantia, Cucurbitacea) is a common vegetable and well-known ethnomedicinal herb in subtropical regions [1]. The whole plant of bitter melon (e.g., fruit pulp, leaves, and seeds) possesses several pharmacological effects, such as anti-diabetic, anti-hyperlipidemic, contraceptive, laxative, and anti-bacterial effects [2]. In particular, bitter melon is considered a complementary and alternative medicine (i.e., dietary supplement) for glycemic control [3], [4], [5], [6], [7]. According to the report by the International Diabetes Federation (IDF), the global diabetes prevalence in adults in 2021 is around 10% (537 million), and the number of patients is projected to increase to 643 million by 2030 [8]. Diabetes causes an increase in healthcare expenditures; for example, the total cost of diabetes diagnosis increased from $327 billion in 2017 to $245 billion in 2012 [9].
The hypoglycemic effects of bitter melon seed extracts have been demonstrated in rodents and patients with diabetes/prediabetes [10], [11], [12], [13], [14], [15], [16]. The bitter melon seeds mainly consist of (37.6%, dry basis) and protein (30.4%, dry basis), and their phytochemicals mainly contain flavonoids, phenolics, triterpenes (e.g., cucurbitacins), and saponins (e.g., charatin) [17], [18], [19], [20]. According to prior studies, charatin, polypeptide-p, polypeptide-k, momordins, mcIRBP-19, and associated derivatives (e.g., alpha-momorcharin) isolated from the seeds might serve as insulin-like compounds for glucose modulation in muscle and fat cells [1], [12], [13], [14], [15], [16], [21]. For instance, mcIRBP-19 interacts with the insulin receptor and enhances the expression of glucose transporter 4 (GLUT4) in 3T3-L1 adipocytes [21]. Nevertheless, bitter melon seeds are not officially recognized as a dietary ingredient by most countries owing to their possible adverse effects (e.g., hemolytic anemia, stomach pain, headache, abortion, and antifertility) [22]. Alpha-eleostearic acid and lectin purified from the seeds may induce apoptosis and cytotoxicity in leukemia cells [23], [24]. Bitter melon seed extracts with the major active component methoxy-phenyl oxime also demonstrated cytotoxicity in human tumor cells [25]. The immune, hepatic, and reproductive toxicity of bitter melon seed extracts was observed in zebrafish embryo and rodent models [26], [27], [28], [29], [30], [31]. Rats treated with alpha-momorcharin for five weeks had obvious immunogenicity, immunotoxicity, and liver lesions [26]. Several studies revealed that bitter melon seeds caused histological alterations in the prostate and testes of male rodents [29], [30], [31]. Nonetheless, to the best of our knowledge, the long-term safety of bitter melon seed extracts in rodent models is elusive.
In this study, we assessed the acute and sub-chronic oral toxicity of bitter melon seed extract from scCO2 extraction in rats in compliance with OECD guidelines No. 423 and 408 [32], [33]. This research may provide a scientific perspective on the safety of bitter melon seed supplements during the evaluation of novel foods.
2. Materials and methods
2.1. Preparation of the bitter melon seed extract
Ripe bitter melon was purchased from Xi'an, Shaanxi, China. The ripe yellow fruits were cleaned with running water and cut to gather the seeds manually, and the skin with pulp inside the fruit was disposed of. The red arils surrounding bitter melon seeds were removed. The processed seeds (230 kg) were washed with running and distilled water. Afterwards, the seeds were dehydrated using food dehydrators at 40 °C and ground using a blender. The dried bitter melon seeds were extracted using supercritical carbon dioxide (pressure: 26–27 MPa; temperature 50–53 ℃). Subsequently, the extract was evaporated by freeze-drying for 3 days.
2.2. Animal and dose administration
Wistar rats (Rattus norvegicus) were obtained from an animal breeding facility (Jai Research Foundation, JRF). Animals for the acute and sub-chronic toxicity studies were 10–11 and 7–8 weeks old, respectively. The animals were kept in an experimental room (temperature: 19–22 ℃; relative humidity: 55–66%; light cycle: 12 h light/dark) and acclimatized for a period of 5–15 days before the main studies. Rats were housed in groups of two rats/sex/cage during the study period in sterilized polypropylene rat cages. Feed (rodent diet, Envigo) and water were provided ad libitum. The study was approved by the institutional animal ethics committee of JRF.
The bitter melon seed extract was dissolved in reverse osmosis (RO) water. Individual dose volumes were adjusted according to the body weight and dose levels. All rats were administrated via oral gavage (day 0) using a BD 1 mL disposable syringe. The rats were fasted overnight prior to dosing until three hours post-dosing.
2.3. Acute toxicity study
The acute toxicity study complied with OECD guideline no. 423 [32]. The first set of three female rats was administered a single dose of 300 mg/kg body weight (BW) of the bitter melon seed extract. As no mortality was observed at this dose level, the second set of three female rats was administered the same dose of the extract. No mortality was observed at this dose level; therefore, the third set of three female rats was administered 2000 mg/kg BW of the extract. As no mortality was observed at this dose level, the fourth set of three female rats was administered the same dose of the extract. No mortality was observed at this dose. Accordingly, the endpoint was achieved and a further study was not required. The LD50 value was determined based on the principle that at least two doses result in mortality rates higher than 0% and lower than 100%.
2.4. Sub-chronic toxicity study
The sub-chronic toxicity study complied with OECD guideline no. 408 [33]. Forty male and forty female rats were randomly assigned into the control (0 mg/kg BW), low-dose (250 mg/kg BW), middle-dose (500 mg/kg BW), or high-dose (1000 mg/kg BW) group. The prepared dose formulations of the bitter melon seed extract in RO water were administered orally by gavage to rats for 90 days. During the experimental period, all rats were observed for signs of toxicity, morbidity, and mortality. Body weight was recorded weekly. Body weight and food consumption were measured weekly. Ophthalmological examinations were performed before and after the study. Neurobehavioral observations were performed weekly. A functional observational battery was performed during week 12 in the main groups. At the end of the treatment, clinical pathology evaluations were performed in all rats. Microscopic examination was performed using the tissues and organs in the control and high-dose groups.
2.5. Clinical pathology
At the end of the treatment, blood samples were collected from rats under light isoflurane anesthesia by orbital plexus puncture using a fine heparinized capillary tube. Rats were fasted overnight (ad libitum supply of drinking water) before blood collection. Blood samples were collected for hematology (in vials containing 4% EDTA anticoagulant for whole blood), coagulation parameters (in vials containing 3.2% sodium citrate anticoagulant for plasma separation), and biochemical analysis (in plain vials for serum separation). The thyroid hormones, triiodothyronine (T3) and thyroxine (T4), were analyzed using liquid chromatography tandem-mass spectrometry (LC-MS/MS), and thyroid-stimulating hormone (TSH) levels were determined with ELISA.
2.6. Pathological examination
All rats were euthanized by carbon dioxide asphyxiation and subjected to full gross necropsy under the supervision of a veterinary pathologist. All rats were carefully examined for external abnormalities. The cranial, thoracic, and abdominal cavities were cut and opened, and a thorough examination of the organs was performed to detect abnormalities. All macroscopic abnormalities observed during necropsy were recorded.
Organs and tissues from both sexes were collected, weighed, and preserved. Adherent adipose tissue from the organs was removed via trimming, and the wet weights of the organs were recorded. All organs were preserved in 10% neutral buffered formalin solution, except for the testes and eyes, which were collected in modified Davidson’s fluid and Davidson’s fluid, respectively. The paired organs were weighed together, and the combined weights presented. The parathyroid and pituitary weights of the thyroid gland were determined after fixation. The organ weight ratio was determined as a percentage of terminal body weight.
Histopathological examination was carried out on the preserved organs and tissues of rats from the control and high-dose groups. The vital organs and tissue samples were processed, embedded, cut into 3–5 µm thick sections, and stained with hematoxylin and eosin.
2.7. Animal welfare
The acute and sub-chronic toxicity studies complied with the guidelines of the “Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International” and “Guidelines for Laboratory Animals Facility” issued by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India. Compliance with these guidelines ensured humane care of the animals throughout the experiment and further enhanced the well-being of animals, which subsequently promoted the quality of outcomes of the experiments for the advancement of biological knowledge relevant to humans and animals. The project proposals were approved by the “Institutional Animal Ethics Committee (IAEC),” JRF. The ethics proposal numbers for the acute and subchronic studies were JRF/IAEC/2020/471 and JRF/IAEC/2020/461, respectively.
2.8. Statistical analysis
Data were presented as the group mean and standard deviation. The Kolmogorov-Smirnov test or Shapiro–Wilk test was first employed for normality checks of all data [34]. Subsequently, based on the analysis of homogeneity of variance [F-test (comparison of two groups), Bartlett's test (comparison of three groups), Levene's test (comparison of three groups), or Brown–Forsythe test (comparison of three groups)], significant analyses of body weight, food consumption, absolute organ weight, relative organ weight, hematology, and clinical chemistry were determined using Student's t-test, Satterthwaite method, or analysis of variance (ANOVA) with Dunnett’s test [35]. Statistical significance was set at p < 0.05.
3. Results
3.1. Acute toxicity assessment of the bitter melon seed extract
The animals were orally administered a single dose of 300 or 2000 mg/kg BW of the bitter melon seed extract. The mean body weights of male and female rats in the low-and high-dose groups increased during the 14-day observation period (Fig. 1). The food consumption and water intake patterns of animals did not show significant changes (data not shown). Further, mortality, abnormal clinical signs, or obvious pathological lesions were not observed. Accordingly, the LD50 of the bitter melon seed extract in rats was greater than 2000 mg/kg BW.
Fig. 1.
Effect of the single administration of bitter melon seed extract on the body weight of rats (n = 6; mean value ± SEM).
3.2. Sub-chronic toxicity assessment of bitter melon seed extract
No mortality or morbidity was observed in this study. Clinical and neurobehavioral observations and ophthalmological examination of the skin, fur, mucous membranes, occurrence of secretions and excretions, and autonomic activity did not reveal any abnormal effects in the animals during the 90-day study period.
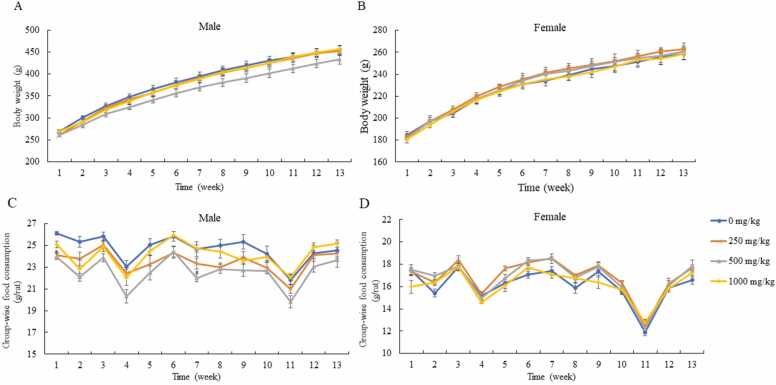
3.3. Influence of bitter melon seed extract on body weight and food consumption
There was no statistical difference in weekly body weight between the control and experimental groups for either sex (Fig. 2). The weight gain of male and female rats displayed similar trends. Food consumption by female rats in the experimental groups was comparable with that of females in the control group; however, feed intake by male rats in the low-dose group at week 1 and male rats in the middle-dose group at weeks 1 and 7 was significantly decreased relative to that consumed by rats in the control group.
Fig. 2.
Effects of the repeated administration of bitter melon seed extract on the body weight and food consumption of rats. (A, B) Body weight of both sexes (n = 10; mean value ± SEM). (C, D) Group-wise food consumption for both sexes (n = 5; mean value ± SEM).
3.4. Influence of bitter melon seed extract on biochemical indices
Hematological examination did not reveal any noticeable findings or treatment-associated effects in all experimental groups (Table 1). Table 2 shows the results of the biochemical parameters. The liver and kidney indexes in the experimental groups did not reveal any obvious findings except a significant increase in γ-glutamyltransferase (γ-GT) in female rats in the high-dose group. In male rats, the albumin/globulin (A/G) ratios in all treatment groups were significantly lower than those in the control group. In addition, there was no statistically significant difference in the levels of T3, T4, and TSH between the control and experimental groups (Table 3).
Table 1.
Results of the hematological analysis for rats (n = 10; mean value ± S.D.).
| Parameter | 0 mg/kg |
250 mg/kg |
500 mg/kg |
1000 mg/kg |
||||
|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Female | Male | Female | Male | Female |
| WBC (103/μL) | 5.12 ± 1.06 | 3.68 ± 1.00 | 5.96 ± 1.58 | 3.83 ± 1.24 | 5.49 ± 1.11 | 3.37 ± 0.80 | 6.21 ± 1.03 | 3.48 ± 0.92 |
| RBC (106/μL) | 9.35 ± 0.55 | 8.52 ± 0.29 | 9.16 ± 0.60 | 8.38 ± 0.25 | 9.09 ± 0.45 | 8.47 ± 0.44 | 9.27 ± 0.37 | 8.56 ± 0.17 |
| Hemoglobin (g/dL) | 16.38 ± 0.40 | 15.77 ± 0.39 | 16.54 ± 0.51 | 15.57 ± 0.39 | 16.44 ± 0.45 | 15.76 ± 0.51 | 16.38 ± 0.53 | 15.89 ± 0.38 |
| Hematocrit (%) | 46.26 ± 1.72 | 44.51 ± 0.80 | 46.26 ± 1.76 | 43.41 ± 0.91 | 45.55 ± 1.82 | 44.26 ± 1.53 | 46.42 ± 1.42 | 44.92 ± 1.21 |
| MCV (fL) | 49.59 ± 2.04 | 52.30 ± 1.26 | 50.58 ± 1.77 | 51.83 ± 1.34 | 50.15 ± 1.05 | 52.28 ± 1.56 | 50.12 ± 0.87 | 52.51 ± 0.91 |
| MCH (pg) | 17.57 ± 1.01 | 18.53 ± 0.47 | 18.12 ± 1.14 | 18.58 ± 0.53 | 18.09 ± 0.64 | 18.65 ± 0.95 | 17.69 ± 0.55 | 18.57 ± 0.44 |
| MCHC (g/dL) | 35.40 ± 0.95 | 35.46 ± 0.46 | 35.78 ± 1.19 | 35.87 ± 0.67 | 36.12 ± 0.73 | 35.60 ± 0.99 | 35.31 ± 0.57 | 35.37 ± 0.69 |
| Platelet (103/μL) | 978.20 ± 93.29 | 992.70 ± 121.02 | 953.40 ± 51.41 | 934.30 ± 63.56 | 943.60 ± 71.36 | 986.10 ± 64.71 | 998.10 ± 63.67 | 1018.50 ± 58.70 |
| Neutrophil (103/μL) | 1.37 ± 0.25 | 0.70 ± 0.13 | 1.73 ± 0.82 | 0.85 ± 0.31 | 1.40 ± 0.44 | 0.64 ± 0.16 | 1.38 ± 0.17 | 0.91 ± 0.43 |
| Lymphocyte (103/μL) | 3.32 ± 1.04 | 2.68 ± 0.81 | 3.75 ± 0.98 | 2.71 ± 1.00 | 3.63 ± 0.80 | 2.50 ± 0.62 | 4.34 ± 0.83 | 2.29 ± 0.65 |
| Monocyte (103/μL) | 0.17 ± 0.05 | 0.11 ± 0.04 | 0.19 ± 0.09 | 0.11 ± 0.03 | 0.17 ± 0.05 | 0.09 ± 0.04 | 0.17 ± 0.05 | 0.10 ± 0.05 |
| Eosinophil (103/μL) | 0.12 ± 0.04 | 0.10 ± 0.08 | 0.12 ± 0.04 | 0.07 ± 0.03 | 0.13 ± 0.04 | 0.06 ± 0.02 | 0.11 ± 0.03 | 0.08 ± 0.04 |
| Basophil (103/μL) | 0.08 ± 0.03 | 0.05 ± 0.03 | 0.11 ± 0.03 | 0.05 ± 0.02 | 0.09 ± 0.03 | 0.05 ± 0.03 | 0.10 ± 0.02 | 0.05 ± 0.02 |
| Retic count (×109/L) | 180.33 ± 28.01 | 176.35 ± 30.77 | 175.49 ± 28.06 | 189.49 ± 26.90 | 188.60 ± 34.45 | 187.58 ± 35.08 | 194.42 ± 34.00 | 175.43 ± 30.99 |
| PT (sec.) | 12.18 ± 0.53 | 9.58 ± 0.82 | 12.04 ± 0.38 | 10.26 ± 0.69 | 12.22 ± 0.57 | 10.59 ± 1.44 | 12.16 ± 0.63 | 9.82 ± 0.99 |
| APTT (sec.) | 16.26 ± 2.08 | 18.36 ± 1.32 | 17.46 ± 1.71 | 19.03 ± 1.58 | 16.70 ± 1.51 | 19.73 ± 1.31 | 16.45 ± 1.75 | 17.65 ± 2.08 |
WBC, white blood cells; RBC, red blood cells; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PT, prothrombin time; APTT, activated partial thromboplastin time.
Table 2.
Results of the biochemical analysis for rats (n = 10; mean value ± S.D.).
| Parameter | 0 mg/kg |
250 mg/kg |
500 mg/kg |
1000 mg/kg |
||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Glucose (mg/dL) | 119.41 ± 14.41 | 120.98 ± 14.69 | 115.37 ± 16.27 | 117.32 ± 17.66 | 118.99 ± 12.30 | 120.97 ± 23.20 | 124.30 ± 12.66 | 109.24 ± 18.14 |
| Total cholesterol (mg/dL) | 104.60 ± 19.49 | 85.37 ± 12.10 | 92.60 ± 15.08 | 81.86 ± 14.57 | 96.80 ± 16.92 | 79.91 ± 9.01 | 97.40 ± 11.72 | 88.18 ± 21.46 |
| Triglycerides (mg/dL) | 80.80 ± 21.91 | 42.83 ± 9.45 | 84.63 ± 43.97 | 40.60 ± 7.74 | 73.03 ± 21.21 | 47.67 ± 8.99 | 69.07 ± 29.71 | 43.23 ± 8.02 |
| Creatinine (mg/dL) | 0.65 ± 0.08 | 0.63 ± 0.04 | 0.64 ± 0.06 | 0.67 ± 0.05 | 0.64 ± 0.04 | 0.64 ± 0.03 | 0.66 ± 0.07 | 0.64 ± 0.04 |
| γ-GT (U/L) | 0.98 ± 1.18 | 0.32 ± 0.35 | 0.72 ± 0.62 | 1.30 ± 2.04 | 1.63 ± 3.43 | 0.52 ± 0.49 | 1.35 ± 1.35 | 0.69 ± 0.42 * |
| ALP (U/L) | 82.23 ± 16.90 | 31.24 ± 3.18 | 89.35 ± 14.23 | 36.43 ± 11.27 | 80.34 ± 10.20 | 40.69 ± 21.40 | 83.44 ± 17.56 | 35.63 ± 13.78 |
| ALT (U/L) | 57.89 ± 20.35 | 31.45 ± 5.41 | 61.87 ± 13.84 | 32.32 ± 6.61 | 67.78 ± 18.05 | 33.67 ± 6.19 | 56.64 ± 9.38 | 38.99 ± 8.52 |
| AST (U/L) | 133.66 ± 29.77 | 101.13 ± 13.83 | 127.31 ± 29.17 | 112.66 ± 34.18 | 124.87 ± 41.38 | 96.66 ± 20.66 | 139.31 ± 46.34 | 113.38 ± 21.69 |
| Calcium (mg/dL) | 10.86 ± 0.38 | 10.72 ± 0.18 | 10.71 ± 0.16 | 10.69 ± 0.22 | 10.85 ± 0.42 | 10.37 ± 0.68 | 10.86 ± 0.21 | 10.73 ± 0.45 |
| Phosphorus (mg/dL) | 5.44 ± 0.62 | 4.61 ± 0.73 | 5.75 ± 0.83 | 5.05 ± 0.82 | 5.48 ± 0.51 | 4.45 ± 0.66 | 5.66 ± 0.49 | 4.90 ± 1.11 |
| Total protein (g/dL) | 6.71 ± 0.27 | 7.00 ± 0.25 | 6.79 ± 0.28 | 6.89 ± 0.29 | 6.94 ± 0.32 | 6.94 ± 0.47 | 6.91 ± 0.24 | 7.17 ± 0.37 |
| Albumin (g/dL) | 3.92 ± 0.14 | 4.21 ± 0.10 | 3.88 ± 0.12 | 4.10 ± 0.22 | 3.96 ± 0.15 | 4.13 ± 0.35 | 3.94 ± 0.11 | 4.24 ± 0.23 |
| Globulin (g/dL) | 2.79 ± 0.18 | 2.79 ± 0.22 | 2.91 ± 0.17 | 2.79 ± 0.12 | 2.98 ± 0.18 | 2.81 ± 0.16 | 2.96 ± 0.17 | 2.93 ± 0.16 |
| A/G | 1.41 ± 0.07 | 1.52 ± 0.12 | 1.34 ± 0.04 * | 1.47 ± 0.07 | 1.33 ± 0.05 * | 1.47 ± 0.09 | 1.33 ± 0.07 * | 1.45 ± 0.06 |
| Urea (mg/dL) | 37.17 ± 4.29 | 43.04 ± 6.31 | 37.02 ± 5.43 | 48.91 ± 7.95 | 39.29 ± 3.96 | 44.98 ± 4.93 | 39.19 ± 4.89 | 45.24 ± 6.07 |
| BUN (mg/dL) | 17.36 ± 2.00 | 20.10 ± 2.94 | 17.29 ± 2.54 | 22.84 ± 3.71 | 18.35 ± 1.85 | 21.01 ± 2.30 | 18.30 ± 2.28 | 21.13 ± 2.84 |
| Total bilirubin (μmol/L) | 0.78 ± 0.34 | 0.58 ± 0.55 | 0.77 ± 0.18 | 0.84 ± 0.58 | 0.85 ± 0.22 | 0.57 ± 0.39 | 0.72 ± 0.32 | 0.29 ± 0.27 |
| HDL-C (mg/dL) | 19.63 ± 1.06 | 18.55 ± 2.99 | 17.83 ± 2.22 | 18.25 ± 3.20 | 18.42 ± 2.30 | 17.45 ± 2.09 | 18.19 ± 2.26 | 19.33 ± 5.06 |
| LDL-C (mg/dL) | 3.20 ± 1.06 | 2.91 ± 0.81 | 3.46 ± 1.58 | 3.17 ± 1.06 | 3.60 ± 0.89 | 3.62 ± 1.29 | 1.88 ± 0.15 | 3.23 ± 1.17 |
| Sodium (meq/L) | 144.54 ± 0.84 | 142.79 ± 0.61 | 144.21 ± 1.54 | 142.96 ± 1.11 | 144.13 ± 1.11 | 143.04 ± 1.39 | 147.10 ± 1.60 | 143.50 ± 0.91 |
| Potassium (meq/L) | 4.43 ± 0.22 | 4.29 ± 0.29 | 4.41 ± 0.22 | 4.25 ± 0.42 | 4.38 ± 0.25 | 4.18 ± 0.28 | 8.59 ± 1.49 * | 4.34 ± 0.34 |
| Chloride (meq/L) | 109.10 ± 1.11 | 107.69 ± 1.12 | 108.35 ± 1.47 | 108.01 ± 2.04 | 108.19 ± 0.99 | 107.72 ± 2.18 | 99.70 ± 1.49 | 108.62 ± 1.36 |
γ-GT, γ-glutamyltransferase; ALP, alkaline phosphatase; AST, aspartate transaminase; ALT, alanine aminotransferase; A/G, albumin/globulin; BUN, blood urea nitrogen; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. * p < 0.05; significance compared to the control group.
Table 3.
Results of thyroid hormone analysis for rats (n = 10; mean value ± S.D.).
| T3 (ng/mL) |
T4 (ng/mL) |
TSH (ng/mL) |
||||
|---|---|---|---|---|---|---|
| Group | Male | Female | Male | Female | Male | Female |
| 0 mg/kg | 0.497 ± 0.091 | 0.532 ± 0.089 | 66.648 ± 5.457 | 41.965 ± 6.793 | 1.200 ± 0.595 | 0.837 ± 0.524 |
| 250 mg/kg | 0.492 ± 0.085 | 0.556 ± 0.096 | 70.218 ± 10.685 | 47.374 ± 8.809 | 0.921 ± 0.301 | 1.484 ± 1.609 |
| 500 mg/kg | 0.509 ± 0.098 | 0.440 ± 0.150 | 75.909 ± 4.644 | 38.036 ± 13.254 | 1.048 ± 0.734 | 1.187 ± 0.683 |
| 1000 mg/kg | 0.507 ± 0.087 | 0.390 ± 0.180 | 73.361 ± 11.495 | 30.228 ± 12.351 | 1.139 ± 0.672 | 1.023 ± 0.433 |
T3, triiodothyronine; T4, thyroxine (T4); TSH, thyroid-stimulating hormone.
3.5. Influence of bitter melon seed extract on pathological parameters
Gross findings did not reveal abnormal alterations or palpable lesions in any of the animals (Tables S1 and S2). In female rats, the absolute and relative weights of the liver, heart, spleen, brain, and thymus were determined. The kidneys, adrenals, testes/ovaries, epididymides, seminal vesicles with coagulating glands and prostate, thyroid with parathyroid glands, and pituitary gland in the treatment groups were similar with those of the control group (Table 4). On the other hand, in male rats, the absolute weight of the thyroid with parathyroid in the high-dose group was significantly higher than that of the control group, but other organ weights in the treatment groups were similar with those in the control group.
Table 4.
Results of the absolute and relative organ weights of rats (n = 10; mean value ± S.D.).
| 0 mg/kg |
250 mg/kg |
500 mg/kg |
1000 mg/kg |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Organ | Absolute organ weight (g) | Relative organ weight (%) | Absolute organ weight (g) | Relative organ weight (%) | Absolute organ weight (g) | Relative organ weight (%) | Absolute organ weight (g) | Relative organ weight (%) | |
| Male | Liver | 11.624 ± 0.681 | 2.649 ± 0.124 | 11.688 ± 1.543 | 2.662 ± 0.177 | 11.167 ± 1.249 | 2.668 ± 0.164 | 11.733 ± 1.267 | 2.664 ± 0.246 |
| Heart | 1.178 ± 0.089 | 0.269 ± 0.024 | 1.167 ± 0.088 | 0.267 ± 0.019 | 1.133 ± 0.104 | 0.271 ± 0.012 | 1.165 ± 0.121 | 0.264 ± 0.021 | |
| Spleen | 0.632 ± 0.078 | 0.144 ± 0.014 | 0.622 ± 0.086 | 0.142 ± 0.014 | 0.621 ± 0.084 | 0.149 ± 0.019 | 0.664 ± 0.087 | 0.151 ± 0.016 | |
| Brain | 2.151 ± 0.109 | 0.491 ± 0.035 | 2.105 ± 0.092 | 0.482 ± 0.032 | 2.080 ± 0.109 | 0.499 ± 0.030 | 2.104 ± 0.110 | 0.478 ± 0.023 | |
| Thymus | 0.289 ± 0.059 | 0.066 ± 0.013 | 0.351 ± 0.084 | 0.080 ± 0.015 | 0.297 ± 0.040 | 0.071 ± 0.010 | 0.349 ± 0.062 | 0.080 ± 0.016 | |
| Kidneys | 2.327 ± 0.225 | 0.529 ± 0.0.34 | 2.333 ± 0.202 | 0.533 ± 0.032 | 2.223 ± 0.182 | 0.532 ± 0.024 | 2.357 ± 0.247 | 0.536 ± 0.056 | |
| Adrenals | 0.065 ± 0.005 | 0.015 ± 0.001 | 0.067 ± 0.012 | 0.015 ± 0.002 | 0.066 ± 0.008 | 0.016 ± 0.002 | 0.067 ± 0.010 | 0.015 ± 0.003 | |
| Testes | 4.035 ± 0.325 | 0.919 ± 0.069 | 3.863 ± 0.252 | 0.885 ± 0.072 | 3.983 ± 0.337 | 0.958 ± 0.102 | 3.960 ± 0.529 | 0.898 ± 0.100 | |
| Epididymides | 1.322 ± 0.099 | 0.301 ± 0.019 | 1.299 ± 0.120 | 0.297 ± 0.024 | 1.286 ± 0.072 | 0.309 ± 0.025 | 1.225 ± 0.193 | 0.278 ± 0.039 | |
| Seminal vesicles with coagulating glands and prostate | 2.184 ± 0.388 | 0.495 ± 0.069 | 2.291 ± 0.403 | 0.522 ± 0.073 | 2.309 ± 0.259 | 0.554 ± 0.065 | 2.349 ± 0.312 | 0.533 ± 0.059 | |
| Thyroid with parathyroid | 0.0216 ± 0.0029 | 0.0049 ± 0.0005 | 0.0234 ± 0.0038 | 0.0053 ± 0.0007 | 0.0216 ± 0.0025 | 0.0052 ± 0.0007 | 0.0254 ± 0.0029a | 0.0058 ± 0.0009 | |
| Pituitary gland | 0.0104 ± 0.0016 | 0.0024 ± 0.0003 | 0.0104 ± 0.0018 | 0.0024 ± 0.0003 | 0.0102 ± 0.0012 | 0.0025 ± 0.0002 | 0.0107 ± 0.0016 | 0.0024 ± 0.0003 | |
| Female | Liver | 7.015 ± 0.404 | 2.856 ± 0.267 | 7.305 ± 0.520 | 2.929 ± 0.252 | 7.157 ± 0.919 | 2.880 ± 0.173 | 7.174 ± 0.617 | 2.927 ± 0.200 |
| Heart | 0.836 ± 0.038 | 0.340 ± 0.017 | 0.837 ± 0.068 | 0.335 ± 0.028 | 0.848 ± 0.072 | 0.343 ± 0.032 | 0.820 ± 0.058 | 0.335 ± 0.020 | |
| Spleen | 0.440 ± 0.039 | 0.179 ± 0.021 | 0.454 ± 0.042 | 0.182 ± 0.020 | 0.450 ± 0.063 | 0.182 ± 0.026 | 0.425 ± 0.055 | 0.174 ± 0.023 | |
| Brain | 1.969 ± 0.073 | 0.801 ± 0.055 | 1.981 ± 0.048 | 0.794 ± 0.044 | 1.916 ± 0.114 | 0.776 ± 0.064 | 1.907 ± 0.083 | 0.780 ± 0.048 | |
| Thymus | 0.308 ± 0.033 | 0.125 ± 0.011 | 0.337 ± 0.073 | 0.135 ± 0.028 | 0.326 ± 0.064 | 0.132 ± 0.027 | 0.303 ± 0.054 | 0.123 ± 0.020 | |
| Kidneys | 1.552 ± 0.076 | 0.631 ± 0.033 | 1.587 ± 0.075 | 0.636 ± 0.045 | 1.514 ± 0.144 | 0.611 ± 0.038 | 1.507 ± 0.093 | 0.615 ± 0.040 | |
| Adrenals | 0.076 ± 0.009 | 0.031 ± 0.005 | 0.081 ± 0.004 | 0.033 ± 0.003 | 0.080 ± 0.014 | 0.032 ± 0.004 | 0.078 ± 0.009 | 0.032 ± 0.003 | |
| Ovaries | 0.089 ± 0.010 | 0.036 ± 0.005 | 0.095 ± 0.022 | 0.038 ± 0.008 | 0.104 ± 0.011 | 0.042 ± 0.003 | 0.092 ± 0.017 | 0.038 ± 0.006 | |
| Uterus with cervix | 0.628 ± 0.130 | 0.255 ± 0.049 | 0.708 ± 0.114 | 0.284 ± 0.049 | 0.742 ± 0.166 | 0.304 ± 0.084 | 0.641 ± 0.151 | 0.262 ± 0.064 | |
| Thyroid with parathyroid | 0.0159 ± 0.0019 | 0.0065 ± 0.0007 | 0.0165 ± 0.0023 | 0.0066 ± 0.0011 | 0.0166 ± 0.0026 | 0.0067 ± 0.0011 | 0.0149 ± 0.0015 | 0.0061 ± 0.0009 | |
| Pituitary gland | 0.0164 ± 0.0020 | 0.0067 ± 0.0010 | 0.0159 ± 0.0022 | 0.0064 ± 0.0010 | 0.0150 ± 0.0016 | 0.0061 ± 0.0008 | 1.0151 ± 0.0020 | 0.0062 ± 0.0008 | |
p < 0.05; significant compared to the control group.
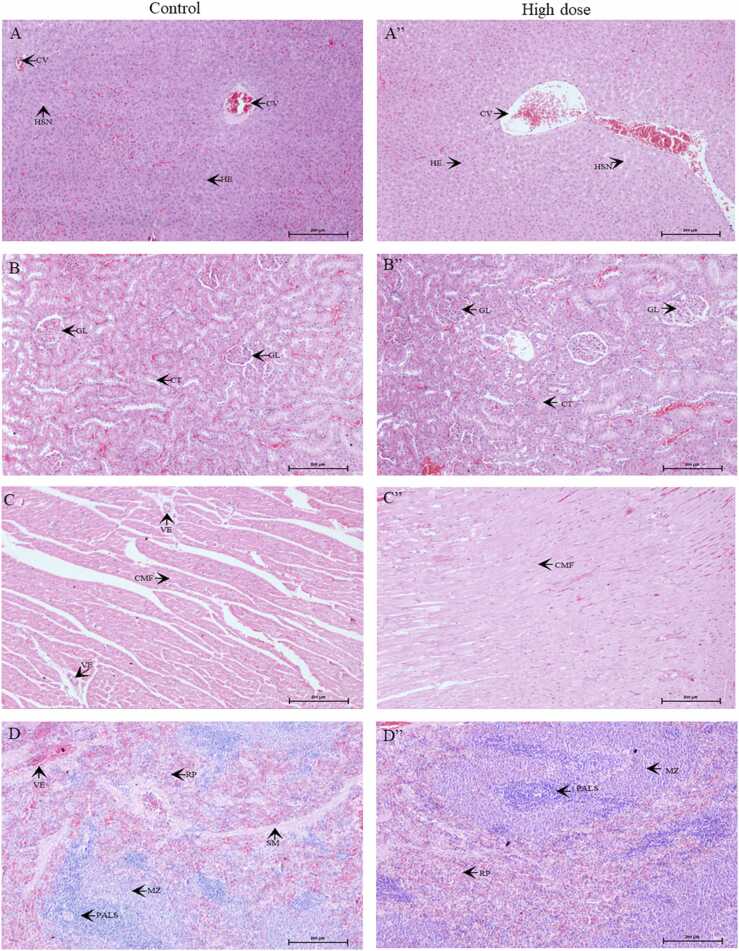
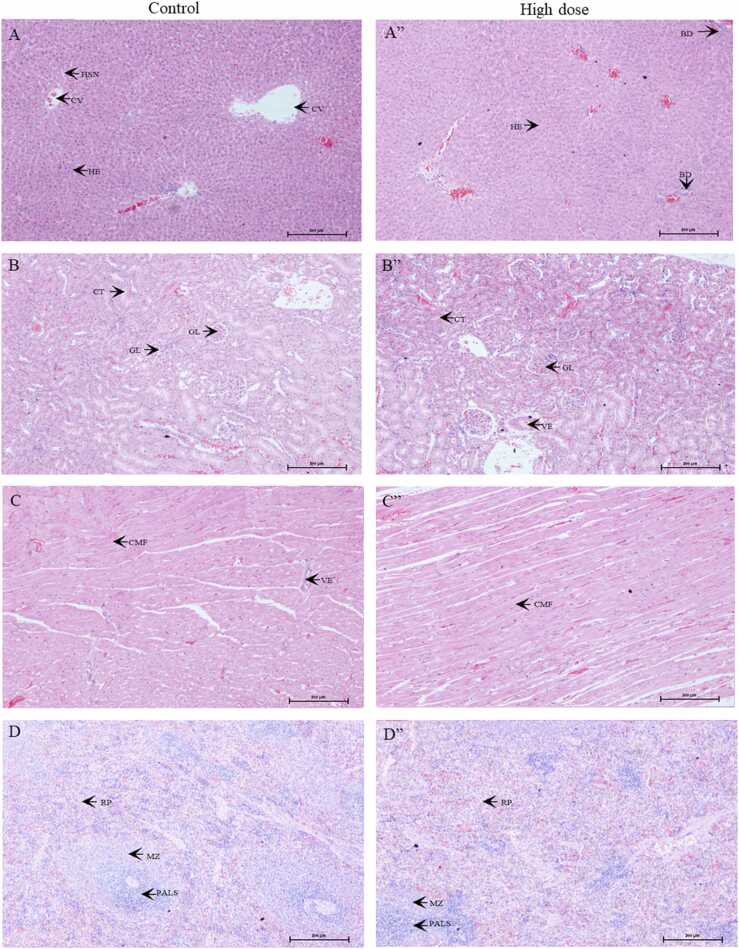
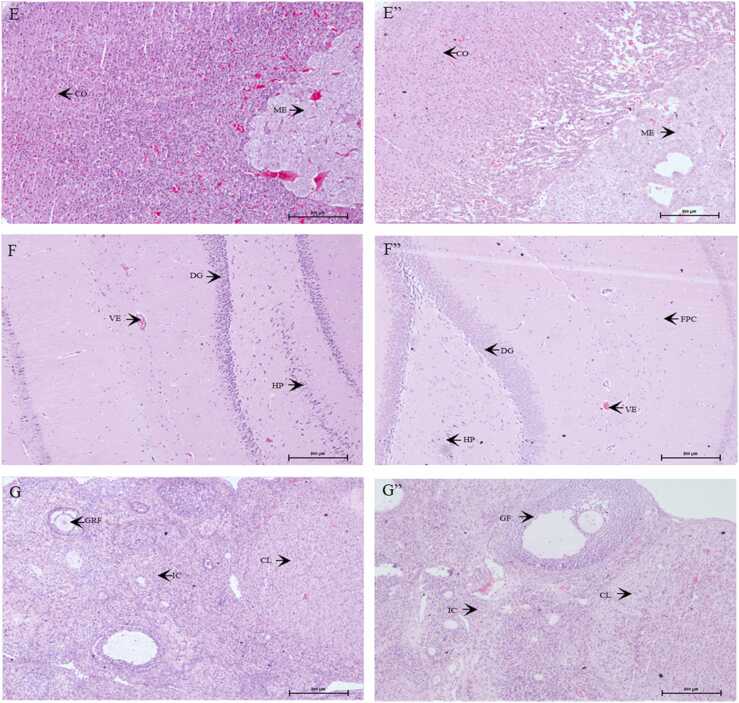
The microscopic lesions observed in various organs were of low occurrence, non-specific, and insignificant (Tables S1, S2). These lesions were considered spontaneous or incidental in nature, and were not related to treatment with the test item. Fig. 3, Fig. 4 show the histopathological results of vital organs in men and women. There were no treatment-related histopathological changes in the liver, kidneys, heart, spleen, adrenals, brain, or testes/ovaries in the control and high-dose groups. Non-specific lesions of the kidneys, heart, and lungs in male rats and adrenals, kidneys, liver, and spleen in female rats were incidentally observed in the control or high-dose groups; however, these lesions were not associated with treatment-related effects or dose-related toxicity. As a result, the no observed adverse effect level (NOAEL) of male and female rats was greater than 1000 mg/kg BW.
Fig. 3.
Microscopic sections of the liver (A, A”), kidneys (B, B”), heart (C, C”), spleen (D, D”), adrenals (E, E”), brain (F, F”), and testes (G, G”) in male rats. A-F represent the results of the control group. A”- F” represent the results of the high dose group. CV: central vein, HSN: hepatic sinusoids; HE: hepatocytes; GL: glomerulus; CT: convoluted tubules; CMF: cardiomyofiber; VE: vessel; PALS: periarteriolar lymphoid sheath; MZ: marginal zone, RP: red pulp; SM: smooth muscles; ME: medulla; CO: cortex; DG: dentate gyrus; HP: hippocampus; FPC: fronto parietal cortex; ST: seminiferous tubules, LC: Leydig cells; GC: germ cells. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Microscopic sections of the liver (A, A”), kidneys (B, B”), heart (C, C”), spleen (D, D”), adrenals (E, E”), brain (F, F”), and ovaries (G, G”) in female rats. A-F represent the results of the control group. A”- F” represent the results of the high dose group. CV: central vein, HSN: hepatic sinusoids; HE: hepatocytes; BD: bile duct; GL: glomerulus; CT: convoluted tubules; VE: vessel; CMF: cardiomyofiber; PALS: periarteriolar lymphoid sheath; MZ: marginal zone, RP: red pulp; ME: medulla; CO: cortex; DG: dentate gyrus; HP: hippocampus; FPC: fronto parietal cortex; CL: corpus luteum; IC: interstitial cells; GF: graafian follicle; GRF: growing follicle. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
In this study, bitter melon seed extract was prepared by scCO2 extraction rather than aqueous or organic extraction. scCO2 is a green and nontoxic solvent for separating hydrophobic components from liquid solutions [36]. The bitter melon seed extract contained 29.88% of protein, its lipid content was less than 8%, and the levels of mcIRBP-19, charantin, vicine, and cucurbitacin B were 969 ppm, 645.48 ppm, 21.82 mg/g, and 0.94 mg/g, respectively, in this extract (data not shown). Charantin is a peptide that resembles insulin; however, its toxicity remains elusive [37]. Vicine may cause favism in individuals with genetically inherited deficiencies in glucose-6-phosphate dehydrogenase (G6PD) [38]. According to a prior study, 50–150 mg/kg BW of vicine exerted good anti-inflammatory effects and no hepatotoxic activity in rats. Further, the LD50 of vicine was 2100 mg/kg BW in rats [39]. The dose levels of 300 and 2000 mg/kg/BW of the bitter melon seed extract represented 6.55 and 43.64 mg/kg BW in this acute toxicity study; thus, the utilized dosages appeared within the safe range [40]. Moreover, the LD50 of cucurbitacin B was 1.1 mg/kg BW in mice [41]. The bioavailability of a chemical administered intraperitoneally was approximately 6-fold higher than that administered orally; thus, we deduced that cucurbitacin B had a minimal impact in this study [42].
No obvious findings of mortality or abnormal clinical signs were observed in the acute toxicity study. The oral LD50 of the extract was greater than 2000 mg/kg BW. According to the Globally Harmonized System of Classification, bitter melon seed extract is classified as category 5 or unclassified [32]. Abalaka et al. and Husna et al. reported that the LD50 values of the ethanolic extracts of bitter melon were 1200 and < 2000 mg/kg BW, respectively, in rats [31], [34]. In addition, a methanolic extract of bitter melon seeds was found to have a lethal effect in zebrafish embryos [28]. These results were not consistent with our observations; this discrepancy might be in part due to the differences in the extraction method, the bitter melon used, and the study model.
In the repeated-dose 90-day study, there was no statistically significant difference in body weight between both sexes in the control and treatment groups. The decline in food intake in male rats was marginal, dose-independent, and inconsistent; therefore, the change in food consumption was not considered a treatment-related effect. Hematological parameters in animals enable the identification of concordant target organ toxicities in humans [43]. The hematological parameters in all animals were within the reference ranges, and there were no noticeable changes in hematological parameters between the control and treatment groups. Liver and kidney functions are essential for survival and play important roles in detoxification in animals [44]. Although a significant increase in γ-GT was noted in female rats in the high-dose group, this phenomenon was not caused by treatment-related effects due to lack of consistency between sexes and the absence of concomitant effects on ALT, AST, and total bilirubin. This outcome might be explained by the presence of hepatotoxins in bitter melon seeds [27]. Tennekoon et al. observed elevated γ-GT and ALP levels in rats after oral treatment with a bitter melon seed extract; however, no noticeable histopathological changes were observed in the liver [27]. The kidney function indices in all animals did not show any abnormalities. The decrease in A/G ratios in male rats in the treatment groups was still within the historical reference ranges (male: 1.01 −2.43; female: 1.08 −3.54) and might not be considered treatment-related effects, owing to the absence of similar findings in female rats. The thyroid gland is a vital endocrine system that secretes thyroid hormones that modulate the basal metabolic rate and affect diverse bodily functions [45]. The extract did not interfere with the function of the thyroid gland, as the levels of TSH, T3, and T4 in both sexes in the treatment groups were comparable to those in the control groups. To our knowledge, this is the first study to report the thyroid toxicity of bitter melon seeds.
An increase in the absolute weight of the thyroid gland in male rats in the high-dose group did not occur in female rats, which may be attributed to non-treatment-related effects. Pathological examination did not reveal any significant findings, and microscopic lesions were considered spontaneous and incidental. The results correlated with the outcomes of biochemical parameters. Importantly, pathological examination did not reveal histomorphological alterations in the seminiferous tubules and prostate of male rats observed in previous reproductive studies [29], [30], [31]. Regarding the teratogenic effect and miscarriage caused by bitter melon seeds, we conducted a prenatal developmental toxicity study based on the OECD guideline No. 414 to clarify the concerns of the sample [46]. The results of body weight, weight gain, reproductive parameters (e.g., live/dead fetuses, sex ratio, anogenital distance), fetal examination (i.e., external and visceral observations), organ weight, and thyroid hormone analysis in all treatment groups (250, 500, and 1000 mg/kg BW of the bitter melon seed extract from scCO2 extraction) were comparable with those of the control group. The toxic effects of bitter melon seed extract, especially hepatotoxicity, nephrotoxicity, and reproductive toxicity, were not observed in this study. The NOAEL of bitter melon seed extract was greater than 1000 mg/kg BW, which is equivalent to 100–200-fold of the recommended daily dosage of the product (300–600 mg/day for adults). The detailed chemical composition of bitter melon seeds is unclear. Further, most toxicity studies on bitter melon seeds were conducted using extracts instead of their specific components. To clearly identify the toxicity of the sample, we will conduct further studies to profile the explicit chemical composition and then perform a toxicity analysis. Notably, although this pioneering research does not discover the developmental toxicity of the bitter melon seed extract for pregnant rats, we do not encourage pregnant women to use such a product because the safety of bitte melon seeds is required more further investigation. We believe that these findings may provide a different perspective on the novel food evaluation of bitter melon seed extracts.
5. Conclusion
This study revealed the acute and sub-chronic toxicity of bitter melon seed extract from scCO2 extraction in rats. There was no significant difference in mortality, morbidity, biochemical parameters, and abnormal tissue alterations in all animals examined. The oral LD50 of the extract was greater than 2000 mg/kg BW. Accordingly, the extract is regarded as a practically non-toxic substance. The NOAEL of the extract was greater than 1000 mg/kg BW. Collectively, these results suggest that the bitter melon seed extract is safe for long-term use.
CRediT authorship contribution statement
Wan-Yu Chung: Project administration, Data curation, Manuscript preparation. Sudhakar Jadhav: Study implementation, Data analysis, Software. Pang-Kuei Hsu: Funding acquisition, Manuscript preparation. Chen-Meng Kuan: Project administration, Data analysis, Manuscript preparation and revision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are truly grateful to the Toxicology Department of the Jai Research Foundation for providing technical support.
Handling Editor: Lawrence Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.04.024.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Joseph B., Jini D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 2013;3:93–102. [Google Scholar]
- 2.Saini R.K., Assefa A.D., Keum Y.-S. Fatty acid and carotenoid composition of bitter melon (Momordica charantia L.) seed arils: a potentially valuable source of lycopene. J. Food Meas. Charact. 2017;11:1266–1273. [Google Scholar]
- 3.Chang C.I., Cheng S.Y., Nurlatifah A.O., Sung W.W., Tu J.H., Lee L.L., Cheng H.L. Bitter melon extract yields multiple effects on intestinal epithelial cells and likely contributes to anti-diabetic functions. Int. J. Med. Sci. 2021;18:1848–1856. doi: 10.7150/ijms.55866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sani F.A., Atiku M.K., Imam A.A. Effect of oral administration of aqueous leaf extract of Momordica charantia (bitter melon) on serum glucose, and lipid profile in alloxan-induced diabetic rats. Bayero J. Pure Appl. Sci. 2015;8:170–174. [Google Scholar]
- 5.Krawinkel M.B., Ludwig C., Swai M.E., Yang R.-Y., Chun K.P., Habicht S.D. Bitter gourd reduces elevated fasting plasma glucose levels in an intervention study among prediabetics in Tanzania. J. Ethnopharmacol. 2018;216:1–7. doi: 10.1016/j.jep.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 6.DiNardo M.M., Gibson J.M., Siminerio L., Morell A.R., Lee E.S. Complementary and alternative medicine in diabetes care. Curr. Diab. Rep. 2012;12:749–761. doi: 10.1007/s11892-012-0315-2. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed I., Adeghate E., Cummings E., Sharma A.K., Singh J. Beneficial effects and mechanism of action of Momordica charantia juice in the treatment of streptozotocin-induced diabetes mellitus in rat. Mol. Cell. Biochem. 2004;261:63–70. doi: 10.1023/b:mcbi.0000028738.95518.90. [DOI] [PubMed] [Google Scholar]
- 8.IDF Diabetes Atlas. Available at: 〈https://diabetesatlas.org/〉. (Accessed: 4 February 2022).
- 9.The Cost of Diabetes. Available at: 〈https://www.diabetes.org/resources/statistics/cost-diabetes〉. (Accessed: 4 February 2022).
- 10.Ali L., Khan A.K., Mamun M.I., Mosihuzzaman M., Nahar N., Nur-e-Alam M. B. Rokeya. Studies on hypoglycemic effects of fruit pulp, seed, and whole plant of Momordica charantia on normal and diabetic model rats. Planta Med. 1993;59:408–412. doi: 10.1055/s-2006-959720. [DOI] [PubMed] [Google Scholar]
- 11.Lo H.-Y., Ho T.-Y., Lin C., Li C.-C., Hsiang C.-Y. Momordica charantia and its novel polypeptide regulate glucose homeostasis in mice via binding to insulin receptor. J. Agric. Food Chem. 2013;61:2461–2468. doi: 10.1021/jf3042402. [DOI] [PubMed] [Google Scholar]
- 12.Hsu P.-K., Pan F.F.-C., Hsieh C.-S. mcIRBP-19 of bitter melon peptide effectively regulates diabetes mellitus (DM) patients’ blood sugar levels. Nutrients. 2020;12:1252. doi: 10.3390/nu12051252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y.-S., Wu N.-Y., Kornelius E., Huang C.-N., Yang N.-C. A randomized, double-blind, placebo-controlled trial to evaluate the hypoglycemic efficacy of the mcIRBP-19-containing Momordica charantia L. fruit extracts in the type 2 diabetic subjects. Food Nutr. Res. 2022;66:3685. doi: 10.29219/fnr.v66.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efird J.T., Choi Y.M., Davies S.W., Mehra S., Anderson E.J., Katunga L.A. Potential for improved glycemic control with dietary Momordica charantia in patients with insulin resistance and pre-diabetes. Int. J. Environ. Res. Public Health. 2014;11:2328–2345. doi: 10.3390/ijerph110202328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sirn Y.Y., Lok L.C., Weng P.H., Ahmad Z., Hakim M.N. Anti-diabetic activity of polypeptide-K isolated from Momordica charantia: a retrospective study of 142 cases. Biomed. Pharmacol. J. 2021;14:2004–2015. [Google Scholar]
- 16.Raman A., Lau C. Anti-diabetic properties and phytochemistry of Momordica charantia L. (Cucurbitaceae) Phytomedicine. 1996;2:349–362. doi: 10.1016/S0944-7113(96)80080-8. [DOI] [PubMed] [Google Scholar]
- 17.Jia S., Shen M., Zhang F., Xie J. Recent advances in Momordica charantia: functional components and biological activities. Int. J. Mol. Sci. 2017;18:2555. doi: 10.3390/ijms18122555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horax R., Hettiarachchy N., Kannan A., Chen P. Proximate composition and amino acid and mineral contents of Mormordica charantia L. pericarp and seeds at different maturity stages. Food Chem. 2010;122:1111–1115. [Google Scholar]
- 19.Padmashree A., Sharma G.K., Semwal A.D., Bawa A.S. Studies on the antioxygenic activity of bitter gourd (Momordica charantia) and its fractions using various in vitro models. J. Sci. Food Agric. 2011;91:776–782. doi: 10.1002/jsfa.4251. [DOI] [PubMed] [Google Scholar]
- 20.Kesari P., Pratap S., Dhankhar P., Dalal V., Mishra M., Singh P.K., Chauhan H., Kumar P. Structural characterization and in-silico analysis of Momordica charantia 7S globulin for stability and ACE inhibition. Sci. Rep. 2020;10:1160. doi: 10.1038/s41598-020-58138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo H.-Y., Li C.-C., Ho T.-Y., Hsiang C.-Y. Identification of the bioactive and consensus peptide motif from Momordica charantia insulin receptor-binding protein. Food Chem. 2016;204:298–305. doi: 10.1016/j.foodchem.2016.02.135. [DOI] [PubMed] [Google Scholar]
- 22.Basch E., Gabardi S., Ulbricht C. Bitter melon (Momordica charantia): a review of efficacy and safety. Am. J. Health. Syst. Pharm. 2003;60:356–359. doi: 10.1093/ajhp/60.4.356. [DOI] [PubMed] [Google Scholar]
- 23.Kobori M., Ohnishi-Kameyama M., Akimoto Y., Yukizaki C., Yoshida M. Alpha-eleostearic acid and its dihydroxy derivative are major apoptosis-inducing components of bitter gourd. J. Agric. Food Chem. 2008;56:10515–10520. doi: 10.1021/jf8020877. [DOI] [PubMed] [Google Scholar]
- 24.Licastro F., Franceschi C., Barbieri L., Stirpe F. Toxicity of Momordica charantia lectin and inhibitor for human normal and leukaemic lymphocytes. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1980;33:257–265. doi: 10.1007/BF02899186. [DOI] [PubMed] [Google Scholar]
- 25.Chipps E.S., Jayini R., Ando S., Protzman A.D., Muhi M.Z., Mottaleb M.A., Malkawi A., Islam M.R. Cytotoxicity analysis of active components in bitter melon (Momordica charantia) seed extracts using human embryonic kidney and colon tumor cells. Nat. Prod. Commun. 2012;7:1203–1208. [PubMed] [Google Scholar]
- 26.Meng Y., Liu B., Lei N., Zheng J., He Q., Li D., Zhao X., Shen F. Alpha-momorcharin possessing high immunogenicity, immunotoxicity and hepatotoxicity in SD rats. J. Ethnopharmacol. 2012;139:590–598. doi: 10.1016/j.jep.2011.11.057. [DOI] [PubMed] [Google Scholar]
- 27.Tennekoon K.H., Jeevathayaparan S., Angunawala P., Karunanayake E.H., Jayasinghe K.S. Effect of Momordica charantia on key hepatic enzymes. J. Ethnopharmacol. 1994;44:93–97. doi: 10.1016/0378-8741(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 28.Khan M.F., Abutaha N., Nasr F.A., Alqahtani A.S., Noman O.M., Wadaan M.A.M. Bitter gourd (Momordica charantia) possess developmental toxicity as revealed by screening the seeds and fruit extracts in zebrafish embryos. BMC Complement. Altern. Med. 2019;19:184. doi: 10.1186/s12906-019-2599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tumkiratiwong P., Ploypattarapinyo R., Pongchairerk U., Thong-Asa W. Reproductive toxicity of Momordica charantia ethanol seed extracts in male rats. Iran J. Reprod. Med. 2014;12:695–704. [PMC free article] [PubMed] [Google Scholar]
- 30.Patil S.A., Patil S.B. Toxicological studies of Momordica charantia Linn seed extracts in male mice. Int. J. Morphol. 2011;29:1212–1218. [Google Scholar]
- 31.Boetse Y.O., Ikechukwu D.F., Olugbenga O.A., Ayodele O.A., Caramel N.C. Histomorphological alterations in the prostate gland and epithelium of seminiferous tubule of Sprague-Dawley rats treated with methanolic extract of Momordica charantia seeds. Iran. J. Med. Sci. 2011;36:266–272. [PMC free article] [PubMed] [Google Scholar]
- 32.OECD, Test No. 423: Acute oral toxicity – acute toxic class method, OECD Guidelines for the Testing of Chemicals, Organization for Economic Cooperation and Development, Paris, France, 2002.
- 33.OECD. Test No. 408: Repeated dose 90-day oral toxicity study in rodents, OECD Guidelines for the Testing of Chemicals. Organization for Economic Cooperation and Development, Paris, France, 2018.
- 34.Kobayashi K., Pillai K.S., Sakuratani Y., Suzuki M., Jie W. Do we need to examine the quantitative data obtained from toxicity studies for both normality and homogeneity of variance? J. Environ. Biol. 2008;29:47–52. [PubMed] [Google Scholar]
- 35.Carneiro M.L.B., Lopes C.A.P., Miranda-Vilela A.L., Joanitti G.A., da Silva I.C.R., Mortari M.R., de Souza A.R., Báo S.N. Acute and subchronic toxicity of the antitumor agent rhodium (II) citrate in Balb/c mice after intraperitoneal administration. Toxicol. Rep. 2015;2:1086–1100. doi: 10.1016/j.toxrep.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Díaz-Reinoso B., Moure A., Domínguez H., Parajó J.C. Supercritical CO2 extraction and purification of compounds with antioxidant activity. J. Agric. Food Chem. 2006;54:2441–2469. doi: 10.1021/jf052858j. [DOI] [PubMed] [Google Scholar]
- 37.Lalèyè O.A.F., Ahissou H., Assogba M.F., Azando V.B.E., Olounladé A.P., Agbo’Saga K.F., Lalèyè A. In vivo hypoglycemic activity and acute oral toxicity of ethanolic and aqueous leaves extract of Momordica charantia Linn (Cucurbitaceae) from Benin. J. Chem. Pharm. Res. 2015;7:376–385. [Google Scholar]
- 38.Khazaei H., Purves R.W., Hughes J., Link W., O'Sullivan D.M., Schulman A.H., Björnsdotter E., Geu-Flores F., Nadzieja M., Andersen S.U., et al. Eliminating vicine and convicine, the main anti-nutritional factors restricting faba bean usage. Trends Food Sci. Technol. 2019;91:549–556. [Google Scholar]
- 39.Hussein P.M. Anti-inflammatory effect of natural heterocycle glucoside vicine obtained from Vicia faba L. its aglucone (divicine) their effect on some oxidative stress biomarkers in Albino rats. Free Radicals and Antioxidants. 2012;2:44–54. [Google Scholar]
- 40.Metcalf R.L. Coevolutionary adaptations of rootworm beetles (Coleoptera: Chrysomelidae) to cucurbitacins. J. Chem. Ecol. 1986;12:1109–1124. doi: 10.1007/BF01638999. [DOI] [PubMed] [Google Scholar]
- 41.Kaushik U., Aeri V., Mir S.R. Cucurbitacins - an insight into medicinal leads from nature. Pharmacogn Rev. 2015 -;9(17):12–18. doi: 10.4103/0973-7847.156314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al Shoyaib A., Archie S.R., Karamyan V.T. Intraperitoneal route of drug administration: should it be used in experimental animal studies? Pharm. Res. 2019;37:12. doi: 10.1007/s11095-019-2745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson H., Betton G., Robinson D., Thomas K., Monro A., Kolaja G., Lilly P., Sanders J., Sipes G., Bracken W., Dorato M., Van Deun K., Smith P., Berger B., Heller A. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000;32:56e67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 44.Petterino C., Paolo B. Toxicology of various anticoagulant rodenticides in animals. Vet. Hum. Toxicol. 2001;43:353–360. [PubMed] [Google Scholar]
- 45.Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18:141–144. doi: 10.1089/thy.2007.0266. [DOI] [PubMed] [Google Scholar]
- 46.W.-Y. Chung, H. Nath, P.-K. Hsu, C.-M. Kuan. Assessment of Prenatal Developmental Toxicity Study of Bitter Melon Seed Extract from Supercritical Carbon Dioxide (scCO2) Extraction in Wistar Rats. PLOS ONE (submitted).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material