Abstract
Study Objectives
Sleep disturbances, which can worsen during pregnancy, have been linked to inflammatory processes. This study tested the hypothesis that more pro-inflammatory diets during pregnancy are associated with a decrease in sleep quality and shorter sleep duration.
Methods
The Health in Pregnancy and Postpartum study promoted a healthy lifestyle in pregnant women with pre-pregnancy overweight or obesity (n = 207). Data from <16 weeks and 32 weeks gestation were used. Sleep was measured using BodyMedia’s SenseWear® armband. Diet was assessed using two 24-hr dietary recalls. Energy-density Dietary Inflammatory Index (E-DIITM) scores were calculated from micro and macronutrients. Linear mixed-effects models estimated the impact of the E-DII score on sleep parameters.
Results
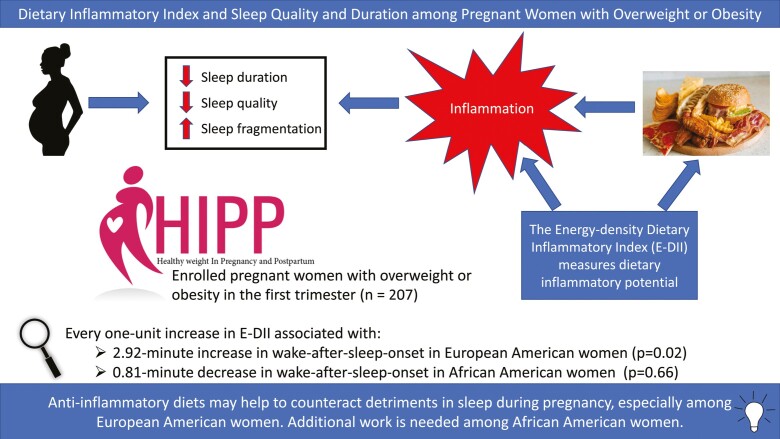
Women with more pro-inflammatory diets, compared to those with more anti-inflammatory diets, were more likely to be nulliparous (51% vs. 25%, p = 0.03), frequent consumers of fast food (29% vs. 10% consuming on 4–6 days during the previous week, p = 0.01), ever-smokers (21% vs. 6%, p = 0.02), and younger (mean age 29.2 vs. 31.3 years, p = 0.02). For every one-unit increase (i.e., more pro-inflammatory) in the E-DII score, sleep latency increased by 0.69 min (p < 0.01). Among European Americans only, every one-unit higher E-DII was associated with a 2.92-min longer wake-after-sleep-onset (p = 0.02).
Conclusion
An E-DII score that is 5 points lower (i.e., more anti-inflammatory) would equate to about 105 min of additional sleep per week among European American women. Anti-inflammatory diets may help to counteract detriments in sleep during pregnancy, especially among European American women. Additional work is needed among African American women.
Clinical Trials Identifier
Name: Promoting Health in Pregnancy and Postpartum (HIPP); URL: https://clinicaltrials.gov/ct2/show/NCT02260518; Registration Identifier: NCT02260518
Keywords: sleep, inflammation, diet, Dietary Inflammatory Index, pregnancy
Graphical Abstract
Graphical Abstract.
Statement of Significance.
Few studies have examined the impact of diet quality on sleep during pregnancy when dietary changes and concurrent changes in sleep and inflammatory responses occur. This study found that more pro-inflammatory diets are associated with longer sleep latency among all participants and longer wake-after-sleep-onset (time spent awake after falling asleep) among European-American women during pregnancy. Diet-associated inflammation at baseline (i.e. <16 weeks) was more strongly associated with outcomes than longitudinal changes in diet-associated inflammation during pregnancy. Thus, pre-pregnancy or very early pregnancy may be the ideal times to intervene on diet to enhance more healthy sustainable sleep patterns throughout pregnancy.
Introduction
A scoping review reported sleep duration was commonly less than 7 hr per night during pregnancy and that prevalence of short sleep increases across trimesters [1], which is concerning given adults aged 18–64 years should get between 7 and 9 hr of sleep per night [2]. Broadly speaking, sleep quality decreases during pregnancy [3] and one meta-analysis found the average Pittsburgh Sleep Quality Index (PSQI) score was 6.4 (95% confidence interval [95%CI] = 5.3–6.9) during pregnancy [4]. This is concerning given poor sleep quality, as measured by the PSQI, is defined as a score of >5 [5]. During pregnancy, the number of nighttime awakenings, snoring, and insomnia increases [6, 7]. Rates of restless leg syndrome, obstructive sleep apnea (OSA), and insomnia are higher among those who are pregnant [7, 8], while rapid-eye movement (REM) sleep is decreased compared to non-pregnant women. Concerningly, short sleep duration and poor sleep quality during pregnancy have been associated with pregnancy complications, maternal depression and anxiety, cesarean delivery, and adverse birth outcomes such as preterm birth, lower birthweight, and stillbirth [3, 7, 9, 10].
Inflammatory cytokines impact various aspects of sleep [11–15]. A meta-analysis of 72 studies found that short sleep (i.e. <7 hr) or long sleep (i.e. >8 hr), compared to 7–8 hr of sleep, was associated with elevated pro-inflammatory markers [16]. Various sleep disturbances or diagnosed sleep disorders, such as insomnia, have been associated with inflammation [11, 17, 18]. Interleukin (IL)-6 was found to increase during early pregnancy, decline somewhat during mid-pregnancy, and then increase again in late pregnancy [19]. It is interesting to note that some of the same adverse maternal and birth outcomes associated with poor sleep during pregnancy also are associated with chronically high levels of inflammatory markers, especially during early pregnancy [19, 20].
Diet is one of the strongest drivers of chronic inflammation [21]. Western-like dietary patterns characterized by high intakes of fats, ultra-processed and fried foods, and added sugar tend to be pro-inflammatory. Diets characterized by increased intake of fruits and vegetables, whole grains, herbs and spices, and fish (e.g. Mediterranean diets) are more anti-inflammatory [21]. The Dietary Inflammatory Index (DII®) was developed to quantify inflammatory potential of whole-diet and can be used across cuisines and with various dietary reporting tools [22, 23]. In addition to being associated with inflammatory biomarkers (now in over 41 studies) [24–27], the DII has been associated with a range of conditions related to chronic inflammation [28–30]. In relation to maternal and child health, associations have been observed between more pro-inflammatory diets and preterm birth (<34 weeks) [31], low birth weight (LBW, <2500 g) [31], spontaneous abortions [32], cesarean delivery [33], maternal inflammation (as measured by c-reactive protein [CRP] and IL-6) [34–36], gestational diabetes [34], and fetal acidosis [37].
Given the overlapping mechanisms of sleep, diet, and inflammation, we sought to explore the role of the inflammatory potential of diet on sleep during pregnancy. We hypothesized that women with more pro-inflammatory dietary changes during pregnancy would have greater adverse changes in sleep quality (as measured by sleep efficiency, wake-after-sleep onset [WASO], and sleep latency), and reduced sleep duration compared to women with more anti-inflammatory changes. In addition to sleep quality, sleep timing metrics (i.e. time-in-bed [TIB], bedtime and final wake time) were examined as outcomes. African Americans and European Americans have been found to have differences in expression of certain inflammatory cytokines and circadian clock genes [38–40], which may affect associations with sleep. Therefore, race was examined as a potential effect modifier in all models.
Methods
Study population and design
The Health in Pregnancy and Postpartum (HIPP) study was a randomized controlled trial, conducted in the Southeastern United States, originally designed to test whether a behavioral lifestyle intervention was effective in reducing excessive maternal gestational weight gain (GWG) and promoting postpartum weight loss among pregnant women who were overweight or obese before pregnancy [41]. Results from the HIPP intervention indicated a lower weight gain among overweight African American women in the intervention group compared to the standard care group. However, these results did not extend to obese African Americans or European Americans [42]. More complete details on the HIPP study protocol can be found elsewhere [41, 42]. This population is of importance given estimates indicating that in the United States between 44% and 50% of women who are pregnant have a pre-pregnancy BMI that is ≥25 kg/m2 (i.e. at least overweight) [43–45]. Participants in the HIPP study were recruited through prenatal clinics, as well through community and social media advertisements. The initial study eligibility criteria included being 18–44 years old, ability to speak English, having no plan to move within 18 months, being within the first 16 weeks of pregnancy, having a pre-pregnancy BMI of ≥25 kg/m2 and being either African American or European American. Women with contraindications to exercise during pregnancy were excluded from the study [41, 46, 47].
This study focused on the antenatal period and data obtained from baseline (≤16 weeks gestation) and 32 weeks gestation were used. The focus on the intervention arm was on healthy diet, active living and weight monitoring achieved through an in-depth counseling session, biweekly telephone counseling, behavioral podcasts, and access to a private Facebook page. The podcasts and handouts paralleled topics in the telephone counseling. The attention-only standard care arm continued to receive standard prenatal care from their providers, which may have included nutrition and physical activity-related information and services. They were also sent mailings and podcasts to match the duration and frequency of that provided to the intervention arm. However, the mailings and podcasts did not discuss weight, physical activity, or diet. Institutional Review Board approvals were granted by recruiting hospitals and our university.
Sleep ascertainment and sleep metric descriptions
Sleep was measured at both baseline and 32 weeks gestation using the validated BodyMedia’s SenseWear® armbands [48, 49] worn on the upper left arm, regardless of handedness. The device activates when its sensors contact skin. It uses triaxial accelerometry supplemented by two heat sensors, which include a thermistor-based skin surface sensor and a heat flux sensor, as well as a galvanic skin response sensor. Participants were asked to wear the armbands continuously for 8 days, except for water-based activities including swimming and showering (participants did keep a log for non-wear periods). For characterization of average nightly sleep metrics, a minimum of four nights of sleep data were needed [50, 51]. All armband data were analyzed using BodyMedia’s SenseWear® Professional software version 7.0 [52].
Sleep and wake were determined in 60-s epochs using proprietary algorithms to derive the sleep metrics. Sleep onset (i.e. bedtime) was defined as the first of three consecutive 60-s epochs identified as sleep with at least 10 consecutive minutes lying down. Sleep latency was the time between lying down and sleep onset. Wake-after-sleep-onset (WASO) was the summation of minutes awake (at least 2 min in length) after sleep onset until the final wake time. Nighttime sleep duration was the sum of all sleep-designated minutes during time-in-bed (TIB). TIB was defined as the period between the first minute spent lying down through to the final wake time. Final wake time was the first of 90 consecutive minutes spent awake. Sleep efficiency was nighttime sleep duration divided by the summation of the duration of sleep latency, sleep duration, and WASO. The research team has used these defined sleep metrics in previously published research [50, 51, 53].
Diet ascertainment and the DII
To obtain estimates of usual dietary intake at both time points during gestation, two 24-hr dietary recalls were obtained using the ASA24 developed by the National Cancer Institute. One recall occurred on a weekday and the other on a weekend day [54]. Of the possible 45 DII food parameters, 27 were used in this analysis. The participants’ reported intake of micro and macro nutrients per 1,000 kcals consumed was subtracted from the global mean (based on dietary intake from 11 populations around the world and converted to amount consumed per 1,000 kcal density units). This difference was then divided by the global standard deviation to create a z-score for each of the 27 food parameters. These z-scores were then converted to a proportion and then centered on 0 by doubling the proportion and subtracting 1. Next, this is multiplied by the article effect scores indicating the inflammatory effect of the food parameter (derived from peer-reviewed research from nearly 2,000 research articles) to obtain a DII value for each food parameter. These are then all summed to create the overall DII score on a per 1,000 kcal consumed basis (also known as the energy-adjusted DII or E-DII) score. E-DII scores can theoretically range from about −8 to +8, but generally ranges are narrower than that in practice (mainly ≈−5.5 to ≈+5.5). More negative values are more anti-inflammatory and more positive numbers are more pro-inflammatory. The details and nuances of calculating the DII have been described in detail elsewhere [22, 23].
Covariates
A range of self-report demographic characteristics were considered, including self-reported race; education; marital status; income; employment; age; type of insurance; Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) status; and number of children in the household. Important self-reported behavioral and reproductive factors included parity, vitamin usage, fast food consumption, and smoking status prior to pregnancy. For categorical covariates defined categories can be found in Table 1. A range of psychosocial questionnaires were used to provide important covariate information. Specific questionnaires important for this work included general social support as measured by the Medical Outcomes Study Social Support Survey [55], the Perceived Stress Scale [56], Social Support for Diet [57] and Social Support for Physical Activity [57]. Steps per day was assessed via BodyMedia’s SenseWear armband. Covariates were examined as possible confounders due to their potential to impact the DII, sleep metrics, or both.
Table 1.
Baseline population characteristics overall and by energy-density Dietary Inflammatory Index categories among the health in pregnancy and postpartum study
| Characteristic | All | EDII Category I (n = 52) | EDII Category II (n = 75) | EDII Category III (n = 80) | P-value |
|---|---|---|---|---|---|
| Race | 0.04 | ||||
| African American | 92 (44%) | 21 (40%) | 42 (56%) | 29 (36%) | |
| European American and Other | 115 (56%) | 31 (60%) | 33 (44%) | 51 (64%) | |
| Education | 0.36 | ||||
| <College graduate | 83 (40%) | 19 (37%) | 27 (36%) | 37 (46%) | |
| College graduate | 124 (60%) | 33 (63%) | 48 (64%) | 43 (54%) | |
| Currently married | |||||
| No | 69 (22%) | 11 (21%) | 29 (39%) | 29 (36%) | 0.09 |
| Yes | 138 (67%) | 41 (79%) | 46 (61%) | 51 (64%) | |
| Income | 0.91 | ||||
| <$35,000 | 61 (30%) | 13 (25%) | 21 (28%) | 27 (34%) | |
| $35,000–$49,000 | 28 (14%) | 7 (13%) | 10 (14%) | 11 (14%) | |
| $50,000–$74,999 | 40 (19%) | 9 (17%) | 16 (22%) | 15 (19%) | |
| $75,000+ | 77 (37%) | 23 (44%) | 27 (36%) | 27 (34%) | |
| WIC enrollment | 0.69 | ||||
| No | 161 (78%) | 42 (81%) | 56 (75%) | 63 (79%) | |
| Yes | 46 (22%) | 10 (19%) | 19 (25%) | 17 (21%) | |
| Full-time employment | 0.42 | ||||
| No | 79 (38%) | 18 (35%) | 26 (35%) | 35 (44%) | |
| Yes | 128 (62%) | 34 (65%) | 49 (65%) | 45 (56%) | |
| Insurance | 0.21 | ||||
| Private | 122 (59%) | 35 (67%) | 47 (63%) | 40 (51%) | |
| Medicaid | 64 (31%) | 11 (21%) | 23 (31%) | 30 (38%) | |
| Other Public Insurance | 20 (10%) | 6 (12%) | 5 (7%) | 9 (11%) | |
| Parity | 0.03 | ||||
| No previous live births | 87 (42%) | 13 (25%) | 33 (44%) | 41 (51%) | |
| 1 previous live birth | 78 (38%) | 23 (44%) | 30 (40%) | 25 (31%) | |
| 2+ previous live births | 42 (20%) | 16 (31%) | 12 (16%) | 14 (18%) | |
| Vitamin usage | 0.60 | ||||
| No | 104 (50%) | 23 (44%) | 39 (52%) | 42 (53%) | |
| Yes | 103 (50%) | 29 (56%) | 36 (48%) | 38 (48%) | |
| Fast food past 7 days | <0.01 | ||||
| None | 25 (12%) | 11 (21%) | 4 (5%) | 10 (13%) | |
| 1–3 days | 128 (62%) | 36 (69%) | 45 (60%) | 47 (59%) | |
| 4–6 days | 54 (26%) | 5 (10%) | 26 (35%) | 23 (29%) | |
| Ever smoke | 0.02 | ||||
| No | 180 (87%) | 49 (94%) | 68 (91%) | 63 (79%) | |
| Yes | 27 (13%) | 3 (6%) | 7 (9%) | 17 (21%) | |
| Children in house ≤5 years old | 0.03 | ||||
| 0 | 114 (55%) | 21 (40%) | 42 (56%) | 51 (64%) | |
| 1+ | 93 (45%) | 31 (60%) | 33 (44%) | 29 (36%) | |
| Children in house 6–17 years old | 0.71 | ||||
| 0 | 140 (68%) | 35 (67%) | 47 (63%) | 58 (73%) | |
| 1 | 43 (21%) | 10 (19%) | 19 (25%) | 14 (18%) | |
| 2+ | 24 (12%) | 7 (13%) | 9 (12%) | 8 (10%) | |
| General social support | 35.0 ± 5.1 | 36.5 ± 4.5 | 34.6 ± 5.0 | 34.5 ± 5.3 | 0.04 |
| Perceived stress scale | 4.7 ± 3.2 | 3.7 ± 2.6 | 4.6 ± 3.2 | 5.5 ± 3.4 | <0.01 |
| Social support for physical activity | 82.5 ± 14.3 | 85.7 ± 17.4 | 82.0 ± 12.9 | 81.2 ± 13.3 | 0.09 |
| Social support for diet | 82.1 ± 11.3 | 81.7 ± 12.4 | 82.8 ± 12.4 | 81.8 ± 9.6 | 0.99 |
| Average steps per day (min) | 5360 ± 2164 | 5714 ± 2367 | 4961 ± 1946 | 5512 ± 2318 | 0.82 |
| Average MVPA per day (min) | 36.7 ± 22.4 | 38.6 ± 20.4 | 34.9 ± 22.2 | 37.0 ± 23.4 | 0.79 |
| Average sedentary time per day (min) | 1159 ± 97 | 1140 ± 95.8 | 1169 ± 87.6 | 1161 ± 105.6 | 0.30 |
| Body mass index (kg/m2) | 33.6 ± 6.4 | 33.0 ± 5.6 | 32.7 ± 5.1 | 34.8 ± 7.8 | 0.08 |
| Age (years) | 29.8 ± 5.0 | 31.3 ± 4.2 | 29.5 ± 5.3 | 29.2 ± 5.1 | 0.02 |
Frequencies within EDII categories may not equal column totals due to missing data. Column percentages may not equal 100% due to rounding. For categorical covariates frequencies (percentages) were presented and p-values were obtained using chi-square tests.
For continuous covariates, means ± standard deviations were presented with use of ANOVAs to compare across DII density categories. DII range for the categories were as follows: Anti-inflammatory = <−1.0, Neutral = −1 to 0.99, and Pro-inflammatory ≥ 1.0. Abbreviations: EDII – Energy-Density Dietary Inflammatory Index; MVPA – moderate-to-vigorous physical activity; WIC – Special Supplemental Nutrition Program for Women, Infants, and Children.
Statistical analyses
Baseline descriptive statistics, including frequencies and percentages for categorical variables and means and standard deviations for continuous variables, were presented for the entire population and by DII categories (<–1.0 [anti-inflammatory], –1.0 to +1.0 [neutral], >+1.0 [pro-inflammatory]). There were few participants in the very anti-inflammatory (i.e. <–3.0) and very pro-inflammatory (i.e. >+3.0) groups. Therefore, these groups were combined with the moderately anti- (<–1.0) and moderately pro-inflammatory groups (>+1.0), respectively. ANOVAs, for continuous variables, and chi-square tests, for categorical covariates, were used to compare population characteristics across E-DII categories.
The dependent variables of interest included TIB, nighttime sleep duration, sleep efficiency, WASO, sleep latency, bedtime, and final wake time. All were treated as continuous metrics, and values represented the average daily values over the actigraph wear-time period. The E-DII served as the independent variable and was treated as continuous and categorical in separate models. All inferential analyses were based on linear mixed models with a random intercept. For model selection, candidate confounders were identified as covariates with a p-value ≤0.15 in bivariate analyses (i.e. dependent = E-DII + covariate). The final model retained all candidate confounders that either changed the beta coefficient of the E-DII substantially (by at least 10%) when removed or were statistically significant at the 0.05 level. Once final models were determined, the variance inflation factor was examined for potential multicollinearity; no violations were found. The shape and distribution of model residuals were examined; no violations of model assumptions were found.
There were two main inferential models. First, we used a stationary model where the outcome at a specific time point was modeled as a function of the E-DII at that time point. In the stationary model, the E-DII was fit as a continuous variable. Also, categorical E-DII was used to obtain LS means of the dependent variables with the main comparison focusing on the difference between anti-inflammatory and pro-inflammatory categories. Second, to investigate the impact of changes in the E-DII over time on changes in sleep over time, we estimated the impact of between-women (cross-sectional) and within-women (longitudinal) differences in E-DII. The cross-sectional effect corresponds to the impact of baseline E-DII on sleep, while the longitudinal effect corresponds to the impact of the change in E-DII (second time point minus baseline) on sleep. The coefficient for the longitudinal effect estimates the change in sleep outcomes per unit increase in change in E-DII from baseline. The stationary and cross-section/longitudinal models are equivalent if the cross-sectional and longitudinal coefficients are equal. This was tested via a contrast statement.
Interactions between race and the E-DII in any of the aforementioned models with a p-value of ≤0.15 were further examined through stratification. Given the parent study was an intervention trial, sensitivity analyses further adjusted for intervention status. No differences in results were observed (data not shown); hence, models without adjustment for intervention status were presented. Lastly, intervention status was investigated as an effect modifier in both the stationary effects and longitudinal analyses.
Results
Population characteristics
The study population was diverse (44% African American, 56% European Americans). Among participants, 60% were college educated, 67% were married, 56% had a household income over $50,000 with 62% working fulltime, and 58% had at least one previous live birth. At baseline (i.e. ≤16 weeks), the average age was 29.8 years (±5.0), average BMI was 33.6 kg/m2 (±6.4 with a range of 24.1–61.5 kg/m2), and average steps per day was 5360 (±2164). Compared to E-DII category I (anti-inflammatory), those in category III (pro-inflammatory), were more likely to be nulliparous (25% and 51%, respectively, p = 0.03), to have consumed fast food on 4–6 days over the previous 7 days prior to reporting (29% vs. 10%, respectively, p < 0.01), and to be ever-smokers (21% vs. 6%, respectively, p = 0.02, Table 1). Those with pro-inflammatory diets were younger compared to those with anti-inflammatory diets (29.2 vs. 31.3 years, respectively, p = 0.02). Those in the most pro-inflammatory category also had lower general social support (p = 0.04) and higher perceived stress (<0.01) compared to the other categories (Table 1). The mean E-DII score at baseline was 0.27 (±1.80) with a range from –4.71 to +4.75 (data not tabulated). Although the E-DII score decreased slightly during the study period (mean E-DII: 0.16 to -0.23) in the intervention arm with practically no change in the control arm (mean E-DII: 0.40 to 0.35), the difference in these changes was not statistically significant (p = 0.22, data not tabulated). About 35% of the full study population reduced their E-DII score (healthy change in direction), 40% stayed approximately the same, and 25% changed in the pro-inflammatory direction (data not tabulated).
Stationary effects models
In the stationary effects models, those with more pro-inflammatory diets had a longer mean sleep latency compared to those with more anti-inflammatory diets (19.7 min vs. 17.1 min, p = 0.01). Every 1-unit (equates to a full point increase, which is about 6.25% of the 16-point theoretical range from –8 to +8) increase in E-DII score was associated with a 0.70-minute higher sleep latency (p < 0.01). To put this in context, there is approximately a 9-to-10-point difference between a Standard American Diet (SAD) and a vegetable-based East Asian or Mediterranean diet [58, 59]. No other statistically significant findings were observed among the total population (Table 2). However, noteworthy interactions between the E-DII and race for WASO (p = 0.06) and sleep efficiency were observed (p = 0.15). Specifically, among European Americans, every 1-unit increase in the E-DII score was associated with a 2.92 (SE = 1.23, p = 0.02) minute greater WASO; whereas, no such observation was noted among African Americans (β = −0.81, SE = 1.84, p = 0.66). This also was reflected in the results for sleep efficiency. Every 1-unit increase in the E-DII led to a 0.64% (SE = 0.26, p = 0.02) lower sleep efficiency in European Americans, but no change was observed in African Americans (β = −0.09, SE = 0.37, p = 0.81, data not tabulated). The only statistically significant interaction between E-DII and intervention status was for sleep latency (p = 0.03). In the control arm, there was no association between the E-DII and sleep latency (β = −0.09, SE = 0.32, p = 0.79). Within the intervention group, for every unit higher E-DII score, sleep latency was higher by 1.10 min (SE = 0.27, p < 0.01, data not tabulated).
Table 2.
Stationary effects between the Dietary Inflammatory Index and sleep quantity and quality
| Sleep metric | Anti-inflammatory (n = 52) | Neutral (n = 75) | Pro-inflammatory (n = 80) | P-value: Anti vs. Pro | DII Continuous beta (SE) | P-value continuous |
|---|---|---|---|---|---|---|
| Time-in-bed (hr) | 8.5 (8.2–8.7) | 8.5 (8.3–8.7) | 8.6 (8.4–8.8) | 0.44 | 1.65 (1.88) | 0.38 |
| Sleep duration (hr) | 6.4 (6.1–6.7) | 6.4 (6.1–6.6) | 6.3 (6.1–6.6) | 0.71 | −0.01 (0.03) | 0.72 |
| Sleep efficiency (%) | 77.0 (75.3–78.8) | 76.4 (74.9–77.9) | 76.0 (74.9–77.9) | 0.28 | −0.35 (0.22) | 0.11 |
| WASO (min) | 83.7 (75.1–92.2) | 86.3 (79.0–93.6) | 89.0 (81.8–96.2) | 0.27 | 1.48 (1.04) | 0.16 |
| Sleep latency (min) | 17.1 (13.9–20.2) | 18.9 (16.0–21.8) | 19.7 (16.7–22.7) | 0.01 | 0.70 (0.20) | <0.01 |
| Bedtime (HH:MM, in PM) | 11:27 (11:05–11:50) | 11:30 (11:09–11:51) | 11:23 (11:04–11:43) | 0.66 | −1.39 (2.14) | 0.52 |
| Wake time (HH:MM, in AM) | 7:39 (7:22–57) | 7:43 (7:27–7:58) | 7:31 (7:16–7:46) | 0.36 | −1.59 (1.98) | 0.42 |
P-value Anti vs. Pro represents the p-value for the least square difference in outcomes between the Anti-Inflammatory group and the Pro-inflammatory group. DII Continuous Beta represents the beta coefficient for the continuous form of the DII. P-value Continuous represents the p-value for the continuous form of the DII. The DII was allowed to vary with time. DII range for the categories were as follows: Anti-inflammatory = <−1.0, Neutral = −1 to 0.99, and Pro-inflammatory ≥ 1.0. Adjustments: TIB – vitamin usage, WIC status, social support, steps per day, and sedentary time per day; Sleep Duration – vitamin usage, ever-smoke status, parity, race, insurance of mother and sedentary minutes per day; Sleep Efficiency – vitamin usage, race, mother’s insurance, physical activity social support from family and friends, and dietary social support from family and friends; WASO – mother’s insurance, dietary social support from family and friends, and steps per day; Sleep latency – children in household 5−17 years, race, physical activity social support from family and friends, dietary social support from family and friends, perceived stress, and sedentary time per day; Bedtime – fast food consumption, ever-smoke status, income, race, employment status, mother’s insurance, dietary social support from family and friends, social support, and moderate-to-vigorous physical activity per day; Wake time – parity, income, employment status, mother’s insurance, perceived stress, and steps per day. Abbreviations: DII – Dietary Inflammatory Index; SE – standard error; WASO – wake-after-sleep-onset.
Longitudinal analyses
In models that incorporated both longitudinal and cross-sectional effects, the change in E-DII score from baseline to follow-up was not associated with changes in any of the sleep metrics among the entire population after adjustment for a range of covariates. However, it should be noted that within these models, increasing baseline E-DII scores were associated with increased sleep latency (β = 1.03, SE = 0.23, p = <0.01, Table 3). This is confirmed by a statistically significant contrast (p = 0.01) between the cross-sectional (i.e. baseline effect) and longitudinal effect. Additionally, the interaction between the change in E-DII scores and race was not statistically significant in any of these models. The same was true for interactions with intervention status.
Table 3.
Longitudinal changes and baseline effects of the Dietary Inflammatory Index on various sleep parameters
| Sleep metric | β Change (SE) | P-value βChange | β Base (SE) | P-value βBase | P-value βChange vs. βBase |
|---|---|---|---|---|---|
| Time-in-bed (hr) | −0.01 (1.12) | 0.82 | 0.05 (0.03) | 0.20 | 0.24 |
| Sleep duration (hr) | −0.03 (0.04) | 0.52 | −0.00 (0.04) | 0.89 | 0.66 |
| Sleep efficiency (%) | −0.30 (0.27) | 0.27 | −0.44 (0.27) | 0.11 | 0.69 |
| WASO (min) | 1.58 (1.36) | 0.25 | 1.38 (1.30) | 0.29 | 0.90 |
| Sleep latency (min) | 0.19 (0.29) | 0.52 | 1.03 (0.23) | <0.01 | 0.01 |
| Bedtime (HH:MM, in PM) | −3.05 (2.61) | 0.24 | 1.22 (2.91) | 0.67 | 0.23 |
| Wake time (HH:MM, in AM) | −2.13 (2.44) | 0.38 | 1.18 (2.68) | 0.66 | 0.31 |
P-value βChange represents the p-value for the longitudinal change in DII score beta coefficient. P-value βBase represents the p-value for the baseline DII beta coefficient. P-value βChange vs. βBase represents the p-value for the contrast between βBase and βChange. The change in DII was defined the baseline DII minus the value at later time points. Adjustments: TIB – vitamin usage, race, social support, steps per day, and sedentary time per day; Sleep Duration – vitamin usage, income, race, social support, sedentary minutes per day, and steps per day; Sleep Efficiency – vitamin usage, income, race, and dietary social support from family and friends; WASO – mother’s insurance, dietary social support from family and friends, and steps per day; Sleep latency – children in household 5–17 years, physical activity, dietary social support from family and friends, perceived stress, and sedentary time per day; Bedtime – WIC status, income, race, dietary social support from family and friends, social support, and moderate-to-vigorous physical activity per day; Wake time – income, dietary social support from family and friends, perceived stress, and steps per day. Abbreviations: DII – Dietary Inflammatory Index; SE – standard error; WASO – wake-after-sleep-onset.
Discussion
This study set out to investigate the associations between the E-DII and sleep duration and quality during pregnancy. Overall, this study found that more pro-inflammatory diets were associated with longer sleep latency among all participants, although the results may not be of clinical significance, and increased WASO, but only among European Americans.
The current study found that higher E-DII scores were associated with longer sleep latency. Sleep latency in this study (adjusted values ranging from about 17 to 20 min per night) was higher than polysomnography-derived sleep latency observed in previous research [60], but less than self-report in other studies during pregnancy [61, 62]. A search of the National Library of Medicine database on terms involving “Sleep” AND “Latency” AND “Pregnancy” AND “Diet” revealed no publications examining the association between any sort of diet-related factor and sleep latency during pregnancy. In other populations, increased fruit and vegetable intake among young adults decreased sleep latency by about 4 min (95%CI = −8 to 0) [63]. In a diverse sample of women in the American Heart Association Go Red for Women cohort study, women with a sleep latency of 60+ min compared to those with ≤15 min had greater energy intake and lower intake of whole grains [64]. Although some of these previous reports indicate an association between diet and sleep latency, the literature is not consistent on this topic [53, 65]. While studies have reported conflicting findings, the biological link between the DII and sleep latency is strongly plausible. For example, tumor necrosis factor (TNF)-α and interleukin (IL)-1β are considered sleep-promoting cytokines [11, 12]; animal studies have shown their administration induces non-REM (NREM) sleep [13]. Some cytokines peak during slow-wave sleep (SWS), but IL-6 has been shown to peak during REM sleep with increasing amounts occurring throughout the night, along with soluble IL-6 receptor [14, 15]. It is conceivable that those with more pro-inflammatory diets have higher levels of pro-inflammatory cytokines. With chronically higher levels of pro-inflammatory cytokines, the circadian increase of these cytokines, which may be a cue for the body to begin the process of sleep initiation and for maintaining sleep [15, 66], may be blunted.
Other than sleep latency, results were null for the other measures of sleep quality and sleep duration among the study population. Overall, sleep efficiency in other studies during pregnancy ranged from around 74% to 86% [60, 62, 67–69], which encompasses sleep efficiency metrics from this study. Sleep duration was found to vary in past studies from about 5.4 hr per night to 7.4 hr per night; again, this encompasses sleep duration from this study [60, 62, 67–69]. Previously, in a study of 497 women who were 26–28 weeks pregnant, better sleep quality, as measured by the PSQI, was associated with healthier scores on the HEI. However, this association was attenuated and was no longer statistically significant after adjustment for anxiety [70]. Another study examined Spanish women during the 16th week of gestation (n = 150) and during the 34th week of gestation (n = 118). Those in the upper median of the Mediterranean Food Pattern score (i.e. indicating greater adherence to a Mediterranean diet) had lower PSQI global scores compared to those in the lower median at both 16 and 34 weeks gestation (p < 0.01) [71]. However, caution is warranted when comparing to these studies. Both of the previous studies used the PSQI; whereas the current study measured sleep objectively. In terms of diet quality, one of the previous studies used the HEI [70] and the other used a measure of Mediterranean diet adherence [71]. Although the HEI and E-DII are correlated (i.e. around −0.5 to −0.7) [72], that correlation is far from perfect indicating they do not measure the same aspects of diet. Presumably, this discrepancy is related to inflammation, given the focus of the E-DII [72]. Although Mediterranean diets tend to be anti-inflammatory [58], there are many healthy diets that would score poorly on Mediterranean dietary indices. Therefore, use of such a dietary index may apply only to select populations [22]. The null findings among the study population for sleep duration and quality metrics may relate to the higher average BMI of this group. It could be that factors related to obesity, other than diet, are stronger drivers of sleep duration and quality in this population. Related to this, information about OSA, which is strongly associated with overweight and obesity [73], was not obtained.
By subgroups, clinically and statistically significant results were found among European Americans, but not African Americans. Specifically, for every 1-unit higher E-DII score (i.e. more pro-inflammatory), European Americans spent about an additional 3 min awake per night. The findings agree with previous studies. Among police officers who worked primarily day shifts over a period of years, every 1-unit change in the E-DII score (i.e. becoming more pro-inflammatory) was associated with a 3.3-min increase in WASO (p < 0.01). The officers working day shifts were primarily European American [74]. Within the context of a healthy anti-inflammatory diet intervention of mostly overweight or obese women, those with more anti-inflammatory diet changes compared to those with more neutral or pro-inflammatory diet changes decreased WASO by about 26 min per night (p < 0.01) [53].
There is no clear reason why an association between diet and sleep exists for European Americans, but not African Americans. One explanation may be variations in inflammatory processes. Research has demonstrated that there is a G/C polymorphism at nucleotide −174 in the promoter region of the IL-6 gene that is associated with increased IL-6 production. The polymorphism is much more common in individuals of African descent than those with other geographical origins [38]. Although the mechanisms are complex as there are other polymorphisms that are in linkage disequilibrium with the polymorphism at −174 [75], it may partially explain why diet did not as strongly predict sleep among African-American women, because African-American women may already have elevated IL-6 levels [75]. Another mechanism may be vitamin D. Research has shown that vitamin D insufficiency is more common among African Americans than European Americans [76]. Vitamin D plays a role in immunomodulation and can help in reducing inflammation [77]. Given the link between sleep and inflammation and sleep and immune function, it is conceivable that potential vitamin D deficiencies impact sleep among African Americans. Social determinants of health may also be contributing to the null findings observed in the African American participants. The Weathering hypothesis describes the cumulative effect of racial discrimination and other life stresses such as low socioeconomic status, environmental pollution, increased risk of violence, and lack of access to healthy food and healthcare accumulate over the life course, leading to a biological age that out-paces chronological age [78, 79]. This, in turn, may affect maternal and childhood health outcomes, such as spontaneous abortions and increased inflammation, among African Americans [80]. Hence, there may be factors that are stronger drivers of inflammation and sleep changes than diet among African Americans compared to European Americans.
This study benefited from several strengths. First, 44% of this population were African Americans, an underrepresented group in research. This allowed for stratified analyses by self-identified race. Given the unique relationship between inflammation processes and sleep, use of the E-DII has advantages over other dietary indices. Sleep was measured objectively using validated BodyMedia SenseWear Armband®. Lastly, it was possible to explore changes over time given the study’s longitudinal design. Despite its strengths, there were limitations to this work that should be considered. The E-DII calculation included only 27 out of 45 possible food parameters. Although an even lower number of food parameters was predictive of inflammation [81], missing food parameters may have been important given changes in nutritional needs during pregnancy. It also should be noted that range of E-DII scores in this study was considerably narrower than what we typically find in most studies; around −5.5 to +5.5. Data on sleep states (e.g. REM, NREM,) and sleep stages (e.g. N1, N2, SWS), primarily obtained through polysomnography, were not available; hence, it is not possible to speak to changes in sleep architecture. Also, sleep was assessed only twice (i.e. once in two different trimesters). More transient changes that may require more frequent sleep monitoring during pregnancy cannot be assessed in this study. We did not have information related to inflammatory markers, discrimination, stress, or vitamin D levels to further investigate potential mechanisms possibly driving racial differences in findings. Results may be generalizable only to overweight/obese women who are pregnant and not all women who are pregnant. Lastly, it should be noted that statistically significant findings were observed only in the stationary effects model (analogous to cross-sectional associations), not the longitudinal models. Hence, causality cannot be inferred. In fact, there is potential for reverse causation. Potential mechanisms for reverse causation include short sleep duration and poor sleep quality being associated with increased energy intake, including fat; low nutrient intake levels; decreased food variety; an increase in ghrelin, increasing hunger; and consumption of high-sugar foods and drinks to stay awake during the daytime [82–85].
The key finding was that for every one-unit higher E-DII score WASO was higher by about 3 min among European-American women. Taken in context, a 5-point difference in E-DII scores between women, which is essentially half the difference between a SAD and either classic Mediterranean or macrobiotic diet [58, 59], would equate to 15 less minutes of sleep per night, or 105 min per week. That additional 105 min of sleep loss per week may lead to or exacerbate sleep debt. Interestingly, these results were observed only in the stationary effects models, but not in models examining longitudinal changes in diet and sleep during pregnancy. It is possible that physiological changes, such as hormonal changes, during pregnancy are primary drivers of changes in sleep quality during pregnancy. Hence, no association between changes in diet and changes in sleep over time were observed. This may speak to the importance of a healthy diet prior to pregnancy to help ensure healthy sleep during pregnancy. Because there is great potential benefit, but little risk in adopting an anti-inflammatory diet, it is intriguing to consider incorporating anti-inflammatory dietary interventions as an important component of comprehensive sleep hygiene interventions for women who are pregnant. A systematic review on sleep interventions during pregnancy found no interventions that incorporated diet quality change [86]. Investigating the combined impact of diet and other behavior changes is a further step in addressing the degradation of sleep quality during pregnancy.
Contributor Information
Michael D Wirth, College of Nursing, University of South Carolina, Columbia, SC, United States; Department of Epidemiology and Biostatistics, Arnold School of Public Health, University of South Carolina, Columbia, SC, United States; Cancer Prevention and Control Program, University of South Carolina, Columbia, SC, United States.
Jihong Liu, Department of Epidemiology and Biostatistics, Arnold School of Public Health, University of South Carolina, Columbia, SC, United States.
McKenzie K Wallace, School of Nursing, University of Pittsburgh, Pittsburgh, PA, United States.
Alexander C McLain, Department of Epidemiology and Biostatistics, Arnold School of Public Health, University of South Carolina, Columbia, SC, United States.
Gabrielle M Turner-McGrievy, Department of Health Promotion, Education, and Behavior, Arnold School of Public Health, University of South Carolina, Columbia, SC, United States.
Jean E Davis, College of Nursing, University of South Carolina, Columbia, SC, United States.
Nicole Ryan, Department of Exercise Science, Arnold School of Public Health, University of South Carolina, Columbia, SC, United States.
James R Hébert, Department of Epidemiology and Biostatistics, Arnold School of Public Health, University of South Carolina, Columbia, SC, United States; Cancer Prevention and Control Program, University of South Carolina, Columbia, SC, United States.
Data Access
The data underlying this article will be shared on reasonable request to the study PI, Dr Jihong Liu.
Funding
The Health in Pregnancy and Postpartum (HIPP) study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R01HD078407. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health. Dr Wallace was supported under an National Institute of Nursing Research, National Institutes of Health training grant (T32NR009759). Dr James Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company licensing the right to his invention of the Dietary Inflammatory Index (DII®) from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings.
Non-Financial Disclosure Statement
Dr Liu reported grants from the National Institutes of Health during the conduct of this study. In addition to his University of South Carolina appointments, Dr Michael Wirth was an employee of CHI. There are no other conflicts to report.
References
- 1. Ladyman C, et al. Sleep health in pregnancy: a scoping review. Sleep Med Clin. 2018;13(3):307–333. doi: 10.1016/j.jsmc.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 2. Hirshkowitz M, et al. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. doi: 10.1016/j.sleh.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 3. Pengo MF, et al. Sleep in women across the life span. Chest. 2018;154(1):196–206. doi: 10.1016/j.chest.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sedov ID, et al. Sleep quality during pregnancy: a meta-analysis. Sleep Med Rev. 2018;38:168–176. doi: 10.1016/j.smrv.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 5. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 6. Sedov ID, et al. Insomnia symptoms during pregnancy: a meta-analysis. J Sleep Res. 2021;30(1):e13207. doi: 10.1111/jsr.13207 [DOI] [PubMed] [Google Scholar]
- 7. Christian LM, et al. Maternal sleep in pregnancy and postpartum part I: mental, physical, and interpersonal consequences. Curr Psychiatry Rep. 2019;21(3):20. doi: 10.1007/s11920-019-0999-y [DOI] [PubMed] [Google Scholar]
- 8. Dunietz GL, et al. Restless legs syndrome and sleep-wake disturbances in pregnancy. J Clin Sleep Med. 2017;13(7):863–870. doi: 10.5664/jcsm.6654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johns EC, et al. Sleep disordered breathing in pregnancy: a review of the pathophysiology of adverse pregnancy outcomes. Acta Physiol (Oxf). 2020;229(2):e13458. doi: 10.1111/apha.13458 [DOI] [PubMed] [Google Scholar]
- 10. Lu Q, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev. 2021;58:101436. doi: 10.1016/j.smrv.2021.101436 [DOI] [PubMed] [Google Scholar]
- 11. Kapsimalis F, et al. Cytokines and pathological sleep. Sleep Med. 2008;9(6):603–614. doi: 10.1016/j.sleep.2007.08.019 [DOI] [PubMed] [Google Scholar]
- 12. Obal F, Jr, et al. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8:d520–d550. doi: 10.2741/1033 [DOI] [PubMed] [Google Scholar]
- 13. Krueger JM, et al. Humoral links between sleep and the immune system: research issues. Ann N Y Acad Sci. 2003;992:9–20. doi: 10.1111/j.1749-6632.2003.tb03133.x [DOI] [PubMed] [Google Scholar]
- 14. Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019;19(11):702–715. doi: 10.1038/s41577-019-0190-z [DOI] [PubMed] [Google Scholar]
- 15. Redwine L, et al. Effects of sleep and sleep deprivation on interleukin-6, growth hormone, cortisol, and melatonin levels in humans. J Clin Endocrinol Metab. 2000;85(10):3597–3603. doi: 10.1210/jcem.85.10.6871 [DOI] [PubMed] [Google Scholar]
- 16. Irwin MR, et al. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80(1):40–52. doi: 10.1016/j.biopsych.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kheirandish-Gozal L, et al. Obstructive sleep apnea and inflammation: proof of concept based on two illustrative cytokines. Int J Mol Sci. 2019;20(3):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slavish DC, et al. Insomnia symptoms are associated with elevated C-reactive protein in young adults. Psychol Health. 2018;33(11):1396–1415. doi: 10.1080/08870446.2018.1500577 [DOI] [PubMed] [Google Scholar]
- 19. Ferguson KK, et al. Longitudinal profiling of inflammatory cytokines and C-reactive protein during uncomplicated and preterm pregnancy. Am J Reprod Immunol. 2014;72(3):326–336. doi: 10.1111/aji.12265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalagiri RR, et al. Inflammation in complicated pregnancy and its outcome. Am J Perinatol. 2016;33(14):1337–1356. doi: 10.1055/s-0036-1582397 [DOI] [PubMed] [Google Scholar]
- 21. Ahluwalia N, et al. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metabolism. 2013;39(2):99–110. doi: 10.1016/j.diabet.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 22. Hebert JR, et al. Perspective: The Dietary Inflammatory Index (DII)-lessons learned, improvements made, and future directions. Adv Nutr. 2019;10(2):185–195. doi: 10.1093/advances/nmy071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shivappa N, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696. doi: 10.1017/S1368980013002115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shivappa N, et al. Association between the Dietary Inflammatory Index (DII) and urinary enterolignans and C-reactive protein from the National Health and Nutrition Examination Survey-2003-2008. Eur J Nutr. 2019;58(2):797–805. doi: 10.1007/s00394-018-1690-5 [DOI] [PubMed] [Google Scholar]
- 25. Wirth MD, et al. The Dietary Inflammatory Index is associated with elevated white blood cell counts in the National Health and Nutrition Examination Survey. Brain Behav Immun. 2018;69:296–303. doi: 10.1016/j.bbi.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Na W, et al. Dietary inflammatory index and its relationship with high-sensitivity C-reactive protein in Korean: data from the health examinee cohort. J Clin Biochem Nutr. 2018;62(1):83–88. doi: 10.3164/jcbn.17-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marx W, et al. The dietary inflammatory index and human health: an umbrella review of meta-analyses of observational studies. Adv Nutr. 2021;12(5):1681–1690. doi: 10.1093/advances/nmab037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li D, et al. Dose-response relation between dietary inflammatory index and human cancer risk: evidence from 44 epidemiologic studies involving 1,082,092 participants. Am J Clin Nutr. 2018;107(3):371–388. doi: 10.1093/ajcn/nqx064 [DOI] [PubMed] [Google Scholar]
- 29. Ruiz-Canela M, et al. The role of dietary inflammatory index in cardiovascular disease, metabolic syndrome and mortality. Int J Mol Sci. 2016;17(8):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang J, et al. Dietary inflammatory index and depression: a meta-analysis. Public Health Nutr. 2018;22(4): 654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ishibashi M, et al. Effect of proinflammatory diet before pregnancy on gestational age and birthweight: the Japan Environment and Children’s Study. Matern Child Nutr. 2020;16(2):e12899. doi: 10.1111/mcn.12899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vahid F, et al. Association between Maternal Dietary Inflammatory Index (DII) and abortion in Iranian women and validation of DII with serum concentration of inflammatory factors: case-control study. Appl Physiol Nutr Metab. 2017;42(5):511–516. doi: 10.1139/apnm-2016-0274 [DOI] [PubMed] [Google Scholar]
- 33. McCullough LE, et al. Maternal inflammatory diet and adverse pregnancy outcomes: Circulating cytokines and genomic imprinting as potential regulators? Epigenetics. 2017;12(8):688–697. doi: 10.1080/15592294.2017.1347241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Z, et al. Association between dietary inflammatory index and gestational diabetes mellitus risk in a prospective birth cohort study. Nutrition. 2021;87-88:111193. doi: 10.1016/j.nut.2021.111193 [DOI] [PubMed] [Google Scholar]
- 35. Yang Y, et al. Relationship between dietary inflammatory index, hs-CRP level in the second trimester and neonatal birth weight: a cohort study. J Clin Biochem Nutr. 2020;66(2):163–167. doi: 10.3164/jcbn.19-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wallace MK, et al. Longitudinal assessment of relationships between health behaviors and IL-6 in overweight and obese pregnancy. Biol Res Nurs. 2021;23(3):481–487. doi: 10.1177/1099800420985615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kyozuka H, et al. Dietary inflammatory index during pregnancy and the risk of intrapartum fetal asphyxia: The Japan Environment and Children’s Study. Nutrients. 2020;12(11):3482. doi: 10.3390/nu12113482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fishman D, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102(7):1369–1376. doi: 10.1172/JCI2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith MR, et al. Racial differences in the human endogenous circadian period. PLoS One. 2009;4(6):e6014. doi: 10.1371/journal.pone.0006014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gillespie SL, et al. Adaptation of the inflammatory immune response across pregnancy and postpartum in Black and White women. J Reprod Immunol. 2016;114:27–31. doi: 10.1016/j.jri.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilcox S, et al. A randomized controlled trial to prevent excessive gestational weight gain and promote postpartum weight loss in overweight and obese women: Health In Pregnancy and Postpartum (HIPP). Contemp Clin Trials. 2018;66:51–63. doi: 10.1016/j.cct.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu J, et al. A behavioral lifestyle intervention to limit gestational weight gain in pregnant women with overweight and obesity. Obesity (Silver Spring). 2021;29(4):672–680. doi: 10.1002/oby.23119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Long SE, et al. Longitudinal associations of pre-pregnancy BMI and gestational weight gain with maternal urinary metabolites: an NYU CHES study. Int J Obes (Lond). 2022; 46(7): 1332–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krishnan S, et al. Bone mass accrual in first six months of life: impact of maternal diabetes, infant adiposity, and cord blood adipokines. Calcif Tissue Int. 2022;111(3): 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mischkot BF, et al. Maternal and infant hospitalization costs associated with maternal pre-pregnancy body mass index in California, 2007-2011. J Matern Fetal Neonatal Med. 2020;35(23): 4451–4460. [DOI] [PubMed] [Google Scholar]
- 46. ACOG Committee Opinion No. 650. Physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol. 2015;126(6):e135–e142. [DOI] [PubMed] [Google Scholar]
- 47. Canadian Society for Exercise Physiology. PARmed-X for Pregnancy: Physical Activity Readiness Medical Examination. https://csep.ca/2021/05/27/get-active-questionnaire-for-pregnancy/. Accessed March 9, 2022.
- 48. Fruin ML, et al. Validity of a multi-sensor armband in estimating rest and exercise energy expenditure. Med Sci Sports Exerc. 2004;36(6):1063–1069. doi: 10.1249/01.mss.0000128144.91337.38 [DOI] [PubMed] [Google Scholar]
- 49. Shin M, et al. The validity of Actiwatch2 and SenseWear armband compared against polysomnography at different ambient temperature conditions. Sleep Sci. 2015;8(1):9–15. doi: 10.1016/j.slsci.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wirth MD, et al. Association between actigraphic sleep metrics and body composition. Ann Epidemiol. 2015;25(10):773–778. doi: 10.1016/j.annepidem.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wirth MD, et al. Association of markers of inflammation with sleep and physical activity among people living with HIV or AIDS. AIDS Behav. 2015;19(6):1098–1107. doi: 10.1007/s10461-014-0949-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Crimarco A, et al. Baseline markers of inflammation, lipids, glucose, and Dietary Inflammatory Index scores do not differ between adults willing to participate in an intensive inflammation reduction intervention and those who do not. Nutr Health. 2019;25(1):9–19. doi: 10.1177/0260106018800645 [DOI] [PubMed] [Google Scholar]
- 53. Wirth MD, et al. Changes in dietary inflammatory potential predict changes in sleep quality metrics, but not sleep duration. Sleep. 2020;43(11):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Subar AF, et al. The Automated Self-Administered 24-hour Dietary Recall (ASA24): a resource for researchers, clinicians and educators from the National Cancer Institute. J Acad Nutr Dietetics. 2012;112(8):1134–1137. doi: 10.1016/j.jand.2012.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moser A, et al. The eight-item modified Medical Outcomes Study Social Support Survey: psychometric evaluation showed excellent performance. J Clin Epidemiol. 2012;65(10):1107–1116. doi: 10.1016/j.jclinepi.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cohen S, Williamson G.. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, eds. The Social Psychology of Health. Newbury Park, CA: Sage; 1988: 31–68. [Google Scholar]
- 57. Sallis JF, et al. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16(6):825–836. doi: 10.1016/0091-7435(87)90022-3 [DOI] [PubMed] [Google Scholar]
- 58. Turner-McGrievy G, et al. Examining commonalities and differences in food groups, nutrients, and diet quality among popular diets. Clin Nutr ESPEN. 2021;41:377–385. doi: 10.1016/j.clnesp.2020.10.017 [DOI] [PubMed] [Google Scholar]
- 59. Steck SE, et al. The Dietary Inflammatory Index: a new tool for assessing diet quality based on inflammatory potential. The [Academy of Nutrition and Dietetics] Digest. 2014;49(3):1–9. [Google Scholar]
- 60. Izci-Balserak B, et al. Changes in sleep characteristics and breathing parameters during sleep in early and late pregnancy. J Clin Sleep Med. 2018;14(7):1161–1168. doi: 10.5664/jcsm.7216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Christian LM, et al. Sleep quality across pregnancy and postpartum: effects of parity and race. Sleep Health. 2019;5(4):327–334. doi: 10.1016/j.sleh.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Flanagan EW, et al. Identification of changes in sleep across pregnancy and the impact on cardiometabolic health and energy intake in women with obesity. Sleep Med. 2021;77:120–127. doi: 10.1016/j.sleep.2020.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jansen EC, et al. Changes in fruit and vegetable consumption in relation to changes in sleep characteristics over a 3-month period among young adults. Sleep Health. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zuraikat FM, et al. Measures of poor sleep quality are associated with higher energy intake and poor diet quality in a diverse sample of women from the go red for women strategically focused research network. J Am Heart Assoc. 2020;9(4):e014587. doi: 10.1161/JAHA.119.014587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hudson JL, et al. Adults who are overweight or obese and consuming an energy-restricted healthy US-style eating pattern at either the recommended or a higher protein quantity perceive a shift from “poor” to “good” sleep: a randomized controlled trial. J Nutr. 2020;150(12):3216–3223. doi: 10.1093/jn/nxaa302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Krueger JM, et al. Sleep and cytokines. Sleep Med Clin. 2007;2(2):161–169. doi: 10.1016/j.jsmc.2007.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu B, et al. Relationships between objective sleep parameters and inflammatory biomarkers in pregnancy. Ann N Y Acad Sci. 2020;1473(1):62–73. doi: 10.1111/nyas.14375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liset R, et al. Sleep, evening light exposure and perceived stress in healthy nulliparous women in the third trimester of pregnancy. PLoS One. 2021;16(6):e0252285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Reid KJ, et al. Sleep during pregnancy: the numom2b pregnancy and sleep duration and continuity study. Sleep. 2017;40(5):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. van Lee L, et al. Sleep and dietary patterns in pregnancy: findings from the GUSTO Cohort. Int J Environ Res Public Health. 2017;14(11):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Flor-Alemany M, et al. Influence of dietary habits and Mediterranean diet adherence on sleep quality during pregnancy. The GESTAFIT Project. Nutrients. 2020;12(11):3569. doi: 10.3390/nu12113569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wirth MD, et al. Anti-inflammatory Dietary Inflammatory Index scores are associated with healthier scores on other dietary indices. Nutr Res. 2016;36(3):214–219. doi: 10.1016/j.nutres.2015.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pavlova MK, et al. Sleep disorders. Am J Med. 2019;132(3):292–299 [DOI] [PubMed] [Google Scholar]
- 74. Wirth MD, et al. Longitudinal and cross-sectional associations between the dietary inflammatory index and objectively and subjectively measured sleep among police officers. J Sleep Res. 2022;31(4): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Berger FG. The interleukin-6 gene: a susceptibility factor that may contribute to racial and ethnic disparities in breast cancer mortality. Breast Cancer Res Treat. 2004;88(3):281–285. doi: 10.1007/s10549-004-0726-0 [DOI] [PubMed] [Google Scholar]
- 76. Harris SS. Vitamin D and African Americans. J Nutr. 2006;136(4):1126–1129. doi: 10.1093/jn/136.4.1126 [DOI] [PubMed] [Google Scholar]
- 77. Cyprian F, et al.. Immunomodulatory effects of vitamin D in pregnancy and beyond. Front Immunol. 2019;10:2739. doi: 10.3389/fimmu.2019.02739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethn Dis. 1992;2(3):207–221. [PubMed] [Google Scholar]
- 79. Geronimus AT, et al. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–833. doi: 10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Frazier T, et al. Weathering the storm: a review of pre-pregnancy stress and risk of spontaneous abortion. Psychoneuroendocrinology. 2018;92:142–154. doi: 10.1016/j.psyneuen.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 81. Shivappa N, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 2014;17(8):1825–1833. doi: 10.1017/S1368980013002565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Soltanieh S, et al. Effect of sleep duration on dietary intake, desire to eat, measures of food intake and metabolic hormones: a systematic review of clinical trials. Clin Nutr ESPEN. 2021;45:55–65. doi: 10.1016/j.clnesp.2021.07.029 [DOI] [PubMed] [Google Scholar]
- 83. Taheri S, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Van Cauter E, et al. Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol. 2008;159(Suppl 1) :S59–S66. doi: 10.1530/EJE-08-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. St-Onge MP, et al. Effects of diet on sleep quality. Adv Nutr. 2016;7(5):938–949. doi: 10.3945/an.116.012336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bacaro V, et al. Interventions for sleep problems during pregnancy: a systematic review. Sleep Med Rev. 2020;50:101234. doi: 10.1016/j.smrv.2019.101234 [DOI] [PubMed] [Google Scholar]