Abstract
Study Objectives
Insomnia is associated with elevated levels of suicidal thoughts and behaviors. Emerging evidence suggests that cognitive-behavioral therapy for insomnia (CBTI) may reduce suicidal ideation (SI). However, the role of digital therapeutics in both the alleviation and prevention of SI remains unclear, and treatment mechanisms facilitating SI reductions have not been clearly identified.
Methods
A total of 658 adults with Diagnostic and Statistical Manual of Mental Disorders, 5th Edition insomnia disorder enrolled in a single-site randomized controlled trial evaluating the efficacy of digital CBTI relative to attention control. Outcomes were measured at pretreatment, posttreatment, and 1-year follow-up.
Results
Before treatment, 126 patients endorsed SI (19.1% prevalence). Among those with baseline SI, CBTI patients reported lower SI rates at posttreatment (30.0% vs 54.5%, p = .005) and 1-year follow-up (29.6% vs 46.8%, p = .042) relative to control. PRODCLIN analysis estimated that half of suicidolytic effects of CBTI were mediated through insomnia remission. Among those without baseline SI, CBTI did not directly prevent new onset SI. However, insomnia remitters reported lower rates of new-onset SI at posttreatment relative to non-remitters (1.5% vs 6.5%, p = .009). Mediation analysis supported a significant indirect effect wherein CBTI increased the likelihood of insomnia remission, which was associated with SI prevention (αβ = −3.20, 95% CI = −5.74 to −0.87).
Conclusion
Digital CBTI reduces insomnia symptoms, which promotes SI alleviation and prevention. For nonsuicidal patients, digital CBTI may serve as a highly accessible monotherapy for improving sleep, thereby reducing the risk for SI. For suicidal patients, digital CBTI may be appropriately administered as an adjunct treatment to support mainline intervention more directly targeting suicidogenic thoughts.
Keywords: CBT-I, CBTI, suicide, eHealth, digital health, prevention, sleep, depression
Graphical Abstract
Graphical Abstract.
Statement of Significance.
Individuals with insomnia report high rates of suicidal thoughts. Early evidence suggests that cognitive-behavioral therapy for insomnia (CBTI) may reduce suicidal thoughts in insomnia patients. Unfortunately, many individuals with insomnia do not have access to CBTI providers, thus creating a need for improving care access. Digital CBTI (delivered via an automated and interactive online program) is highly accessible and has been shown to be effective and safe for treating insomnia. However, it is unclear whether suicidolytic effects of CBTI are preserved in its digital format. Our trial showed that treating insomnia to remission with digital CBTI alleviates and prevents suicidal ideation. This study is the first to support treating insomnia as a potentially viable strategy in suicide prevention efforts.
Introduction
According to the Centers for Disease Control and Prevention (CDC), suicide was the 12th leading cause of death in the United States across all ages in 2020 [1]. CDC statistics show that suicide ranged between being the second and fourth leading cause of death for age groups within adolescence, young adulthood, and middle adulthood. To help curb suicide rates, researchers have identified demographic and clinical risk factors for suicidal thoughts and behaviors. The identification of modifiable factors associated with suicidality can provide targets for intervention to alleviate or even prevent suicidal thoughts and behaviors. Traditionally, these risk factors have centered on mood disorders, substance abuse disorders, and major physical health conditions as well as access to lethal means [2–6]. However, a burgeoning literature indicates that insomnia represents a critical—and historically overlooked—risk factor for suicidality [7, 8].
Meta-analytic data on sleep and suicidality reveal that insomnia is associated with an increased risk for suicidal ideation (SI), suicide attempts, and suicide [9, 10]. Critically, the association between insomnia and SI is significant and substantial even when adjusting for depression and other mental health conditions [11–13]. Notably, among depressed individuals, insomnia is a unique and robust indicator of SI intensity [14]. As insomnia prospectively predicts future SI [15–17], suicide-related outcomes have gained interest in clinical trials for insomnia.
An emerging literature suggests cognitive-behavioral therapy for insomnia (CBTI) decreases SI in patients with insomnia. In the first insomnia trial examining SI as an outcome, Manber and colleagues published data from 301 adults who participated in seven (90-minute) group CBTI sessions [18]. In this trial, 23% of patients endorsed SI before treatment, but SI rates decreased to 10% post-treatment. In replication of these study findings, Trockel et al. showed that SI rates decreased from 32% at pretreatment to 21% at posttreatment in 647 Veterans with insomnia who received in-person CBTI in an individual format [19]. In a randomized controlled trial (RCT), Pigeon and colleagues showed that brief CBTI (delivered face-to-face in primary care) reduced SI intensity in 54 Veterans with insomnia [20].
Not only does CBTI reduce SI, but the two latter trials also reported associations between insomnia alleviation and decreases in SI, thereby offering preliminary support that insomnia reduction may represent a key mechanism by which CBTI affects SI [19, 20]. In the Trockel study, each 7-point reduction in the insomnia severity index (ISI) during CBTI was associated with a 65% reduction in odds of SI.
Although the greatest support for insomnia therapeutics serving a role in suicide-risk management centers on CBTI, it is notable that a recent RCT evaluated the suicidolytic effects of zolpidem-CR (i.e. zolpidem extended release) in a sample of suicidal patients with major depression starting antidepressant medication [21]. In this trial, patients were randomized to either adjunct zolpidem-CR or placebo. Zolpidem-CR yielded a significant treatment effect on one of two SI indices. Importantly, SI reductions were associated with decreases in insomnia symptoms, thereby lending further preliminary support that insomnia alleviation may represent a viable treatment target to reduce suicide-related outcomes.
As scientific inquiry into the role of insomnia therapeutics in suicide management is still in its early stages, there remain several unknowns. First, as healthcare moves increasingly into the digital realm, it is important to determine whether digital CBTI has efficacy for reducing suicide-related outcomes. We know of only one clinical trial that published data on the effects of digital CBTI on SI [22]. In 2020, our team showed that 5.5% of pregnant patients with insomnia endorsed SI before treatment. After treatment, 0.0% of CBTI patients endorsed SI, whereas 6.7% of controls endorsed SI. While the treatment effect approached significance (p = .054) suggesting that digital CBTI may reduce SI, we were ultimately underpowered to detect significance in posttreatment SI rates in a sample of 91 patients.
Second, prior trials examining the suicidolytic effects of CBTI have included patients with and without baseline SI in a single sample. As a result, these studies have tested CBTI effects on alleviation (for patients with baseline SI) and prevention (for patients without baseline SI) simultaneously. However, examining CBTI's effects on SI separately in these subgroups may produce more nuanced knowledge regarding the potential effects of CBTI for alleviating and preventing SI, respectively.
Third, several studies have shown that reducing insomnia is associated with reductions in SI [19–21], which yields promising preliminary support for insomnia alleviation as a key treatment mechanism for reducing SI. Indeed, accumulating evidence from observational studies shows that nocturnal wakefulness increases risk of suicide [23, 24], and suicides most often occur at night [25]. Thus, interventions aimed to reduce nocturnal wakefulness may yield clinically meaningful effects on suicide-related outcomes. Yet, no RCT has empirically tested an indirect effect of CBTI on suicide outcomes as mediated by insomnia symptom alleviation, which would offer strong support for alleviating insomnia to reduce suicidality.
The present study is a secondary analysis of RCT data testing the efficacy of digital CBTI, relative to digital sleep education control, on SI. The sleep education control condition included psychoeducation on sleep as well as sleep hygiene tips. We selected sleep education as the control comparator due to the common role of sleep hygiene education serving as nonpharmacological treatment as usual in real-world clinical settings. Unique to this RCT, we separated the full sample into two subgroups: patients with and without baseline SI. We predicted that: (Hypothesis 1A) CBTI would alleviate SI relative to control in patients with baseline SI, and that (Hypothesis 1B) mediation analyses would support a significant indirect effect wherein CBTI predicts insomnia remission, which, in turn, predicts reduced SI. Similarly, we predicted that (Hypothesis 2A) CBTI would prevent SI relative to control in patients without baseline SI, and that (Hypothesis 2B) mediation analyses would support a significant indirect effect wherein CBTI predicts insomnia remission, which, in turn, predicts SI prevention.
Methods
Study setting and recruitment
Study data were obtained from the Sleep to PRevent Evolving Affective Disorders (SPREAD) trial (NCT02988375). The study setting was Henry Ford Health (HFH) in southeastern Michigan. HFH is centrally located in Metro Detroit and includes six hospitals and over 30 outpatient medical centers. Additionally, HFH owns a major health insurance company (Health Alliance Plan). Patients were recruited for HFH hospitals, outpatient clinics, and insurance patient registries. Recruitment occurred in 2016 and 2017 via internet-based methods including clinic databases, health system-wide email newsletters, and existing research databases (e.g. previous research patients who expressed interest in future studies). All study participants provided informed consent prior to participating. Study procedures were approved by the HFH institutional review board.
Study eligibility
Interested patients completed an online screening survey (Qualtrics, Provo, UT) that assessed for study eligibility. Inclusion criteria included Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) [26] diagnostic criteria for chronic insomnia disorder and being ≥18 years of age. Exclusion criteria included patient-reported sleep disorder diagnoses other than insomnia (e.g. restless legs and narcolepsy), untreated obstructive sleep apnea (OSA; treated OSA was OK), diagnosed bipolar disorder, diagnosed seizure disorder, and chronic depression symptoms; see Table 1.
Table 1.
Eligibility criteria
| Inclusion | Exclusion |
|---|---|
| 1. DSM-5 insomnia disorder | 1. Sleep disorders (other than insomnia), for example, restless legs syndrome, narcolepsy, central sleep apnea, and untreated obstructive sleep apnea (OSA; treated OSA was not exclusionary) |
| 2. Age 18 years or older | |
| 2. Bipolar disorder | |
| 3. Epilepsy or other seizure disorder | |
| 4. High depression chronicity |
Insomnia disorder was survey assessed and determined per DSM-5 criteria. Exclusionary items were reported by patients on an online screener. Specifically, patients were asked to report diagnosed sleep disorders, diagnosed bipolar, and diagnosed seizure disorders. Depression chronicity was operationalized as self-reported daily or near-daily depressed mood and anhedonia.
Study design and participants
This was a RCT with simple randomization into two parallel arms of either digital CBTI or digital sleep education control (see details below). Randomization was computerized and automated centrally through Qualtrics immediately after participants met eligibility criteria. A total of 1385 adults with insomnia disorder from Metro Detroit were enrolled and randomized to digital CBTI or control at a 2:1 ratio due to higher anticipated attrition for active versus control conditions as previously demonstrated in digital intervention studies [27]. A total of 658 patients engaged in CBTI or control treatment. Of these 658 patients, 139 (21.1%) identified as male, and 126 (19.1%) endorsed SI before CBTI. Most patients identified as non-Hispanic White or Black (92.7%). See Figure 1 for the enrollment flow chart and refer to the “Sample characteristics” of the Results.
Figure 1.
Study flow chart.
Interventions
Digital CBTI
Patients randomized to CBTI completed the Sleepio program via the internet (www.sleepio.com, Big Health Inc.). Sleepio is among several currently available digital CBTI programs and was selected for this study because it is evidence-based, standardized, fully automated, and has been tested in multiple RCTs comprising several thousand patients including several of our own trials [22, 28–37]. Patients received access for 12 weeks during which they could complete the six sessions; each session was unlocked on a weekly basis, and patients were advised to complete one session per week. The intervention covered behavioral components (sleep restriction, stimulus control), cognitive components (e.g. cognitive restructuring, paradoxical intention), progressive muscle relaxation, and sleep hygiene. Sessions were directed by an animated “virtual therapist” who reviews and guides the progress of the patient based on submitted sleep data. Participants were granted access to new sessions weekly (i.e. new sessions became available a week after completing a previous session).
Digital sleep education control
Patients randomized to the online sleep education condition received six weekly emails based on the National Institutes of Health guide to healthy sleep [38]. Information was provided on the basics of sleep regulation; relationships between sleep and health problems such as obesity, diabetes, and cardiovascular disease; effects of sleep-disruptive substances such as caffeine, nicotine, and alcohol; and tips on creating a sleep-conducive bedroom environment. Sleep education was selected as the control condition because psychoeducation and sleep hygiene are common in clinical practice, especially in primary care [39, 40], and also because they are commonly used as an attention control in clinical trials of insomnia. Importantly, neither sleep education nor sleep hygiene is considered an effective standalone treatment for insomnia [41]. We have utilized sleep education as our attention control in previous RCTs [22, 42].
Study outcomes
Study outcomes were assessed a week before treatment, 1 week after treatment, and then 1-year after their completed posttreatment assessment.
Suicidal ideation (SI) served as our primary end-point. SI was assessed using the Quick Inventory of Depressive Symptomatology, a 16-item self-report survey (QIDS-SR16), which measures symptoms over the prior 7 days [43]. Specifically, any endorsement of the “thoughts of death or suicide” item was operationalized as an endorsement of SI. We also used the QIDS-SR16 total score to measure overall depressive symptoms for descriptive purposes. Scores range from 0 to 27 with higher scores indicating greater depression. QIDS-SR16 ≥ 11 indicates moderate depression.
The ISI was used to assess insomnia symptoms, which measures symptoms over the prior 2 weeks [44]. Higher scores on the ISI reflect greater insomnia severity with ISI scores ≥ 11 indicating clinical symptom severity. In treatment studies, ISI scores ≤ 7 after treatment indicate insomnia remission [44].
Analysis plan
All statistical analyses were performed using SPSS 26 and R 4.2.1. All inferential tests were two-sided with a significance level of alpha = 0.05. We first presented descriptive data for pretreatment sample characteristics, including sociodemographic information and presenting clinical symptoms. We then split the sample into two subgroups: Patients who presented with pretreatment SI and those who denied SI before treatment. Before testing our hypotheses, we compared patients with and without pretreatment SI on sociodemographics and clinical symptoms for descriptive purposes.
SI alleviation.
We first evaluated whether CBTI alleviated SI in patients who presented with pretreatment SI. Using a logistic model, we regressed posttreatment SI on the treatment condition. We then conducted a 2 × 2 chi-square to report SI rates for each treatment group for post hoc descriptive purposes. This process was then repeated for 1-year follow-up.
Next, we tested whether insomnia remission mediated the effects of CBTI on SI alleviation. Here, we describe mediation using the terms independent variable (IV), dependent variable (DV), and mediator (M). The direct effect (also known as the tau (τ) path) refers to the IV → DV effect; this path is reflected by the first logistic regression model described above wherein posttreatment SI was regressed onto treatment condition. This regression model determines that CBTI decreases odds SI relative to control. Although a direct effect of the CBTI (IV) on SI (DV) is not necessary for mediation, the determination of a direct effect is nevertheless informative.
We then tested the indirect (i.e. mediated) effect. For the alpha path (IV → M), we regressed insomnia remission onto treatment condition. This regression model determines whether CBTI increases the odds of insomnia remission relative to control. For the beta path (M → DV), we regressed posttreatment SI onto insomnia remission, while controlling for treatment condition. This model determines whether insomnia remission (M) is associated with SI outcomes (DV) while controlling for CBTI effects (IV). In this model, the effect of CBTI on SI becomes τʹ, which is the effect of the IV on the DV while controlling for the mediator.
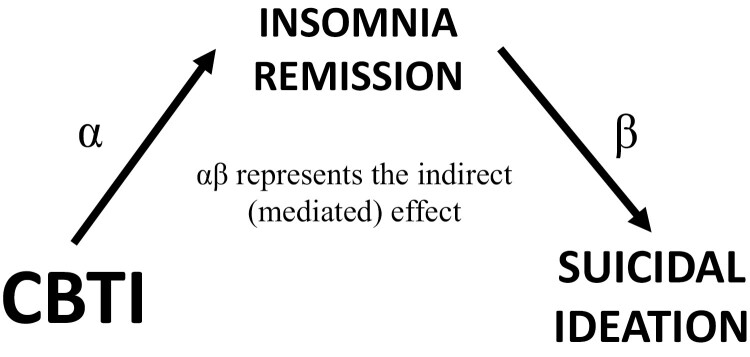
From these regression models, the product of the α and β parameter estimates represent the indirect (i.e. mediated) effect. The confidence intervals (CIs) of the indirect effect (αβ) were estimated using the PRODCLIN method in R 4.0.2 using the RMediation library [45]. This method does not assume a normal distribution, yields asymmetric CIs, and is more accurate than traditional significance tests. If the 95% CI for the indirect effect does not include zero, then significant mediation is inferred. Refer to Figure 2 for visual depiction of the mediation model. If the direct effect was significant, then we can estimate the proportion of the direct effect that is mediated by the indirect effect with the following equation: (τ − τʹ)/τ.
Figure 2.
Hypothesized mediation model wherein cognitive-behavioral therapy for insomnia (CBTI) effects on suicidal ideation (SI) are mediated by insomnia remission. α represents the effect of treatment on the mediator (IV → M). β represents the effect of the mediator on the outcome (M → DV) while controlling for the effects of treatment. The product of these parameters (αβ) represents the indirect effect of treatment on the outcome through the mediator (IV → M → DV). If the 95% CI of αβ does not overlap with zero, then mediation is inferred.
SI prevention.
These analyses were conducted in patients who denied SI at baseline. Otherwise, the analytic steps were the same as the SI alleviation analyses described above.
Per protocol and modified intent-to-treat analyses.
No study outcomes data were missing at posttreatment, and we retained 92.4% of patients at 1-year follow-up (n = 608/658). Due to attrition, all 1-year follow-up analyses were performed using two approaches: (1) per-protocol analysis and (2) intent-to-treat (ITT) using imputed data. Follow-up data were largely consistent between both approaches. Results reported below are for the per-protocol analysis, which presents group SI rates that are unaffected by data imputation. We report methodology and results related to the ITT approach for 1-year follow-up analyses in the Supplement.
Results
Sample characteristics
We observed a wide range of ages from 18 to 92 years. The sample was predominately female (78.9%), and patients who identified racially as non-Hispanic White (71.4%) and non-Hispanic Black (21.3%) were well-represented. Three-hundred-and-sixty-seven (55.8%) patients reported attaining a post-secondary degree.
Of the 658 patients in the study, 19.1% (n = 126/658) endorsed SI prior to treatment. Group comparisons showed that patients with pretreatment SI were more likely to identify as male (relative risk [RR] = 1.47) and reported an annual household income <$20 000 (RR = 1.99). Moreover, patients with SI reported greater depressive symptoms (Cohen’s d = 1.41) and younger age (Cohen’s d = 0.29). See Table 2 for comparisons of patients with and without SI before treatment. Notably, pretreatment SI rates did not differ by race or education level.
Table 2.
Pretreatment sample characteristics for the full sample and by pretreatment SI status
| Full sample | No baseline SI | Baseline SI | Test statistic, p-value, and effect size | |
|---|---|---|---|---|
| n = 658 | n = 532 | n = 126 | ||
| Age (M ± SD, range) | 45.03 ± 15.42 | 45.89 ± 15.22 | 41.37 ± 15.81 | t(656) = −2.98, p = .003, d = 0.29 |
| Male sex (n; %) | 139; 21.1% | 103; 19.4% | 36; 28.6% | χ2 = 5.19, p = .023, RR = 1.47 |
| Race (n;%) | ||||
| White | 470; 71.4% | 382; 71.8% | 88; 69.8% | χ2 = 0.18, p = .672 |
| Black | 140; 21.3% | 116; 21.8% | 24; 19.0% | Note: chi-square analysis compared SI rates between White and Black as they were the most well-represented groups. |
| Unreported | 20; 3.0% | 14; 2.6% | 6; 4.8% | |
| Asian | 12; 1.8% | 8; 1.5% | 4; 3.2% | |
| Multiracial | 12; 1.8% | 9; 1.7% | 3; 2.4 | |
| Native American | 4; 0.6% | 3; 0.6% | 1; 0.8% | |
| Education level (n;%) | χ2 = 2.11, p = .147 | |||
| No HS or GED (<4) | 15; 2.3% | 9; 1.7% | 6; 4.8% | Note: chi-square analysis compared SI rates between patients with and without college degrees. |
| HS or GED (4–7) | 276; 41.9% | 219; 42.2% | 57; 45.2% | |
| Associates (8) | 84; 12.8% | 72; 13.5% | 12; 9.5% | |
| Bachelors (9 & 10) | 172; 26.1% | 144; 27.1% | 28; 22.2% | |
| Graduate degree (11–12) | 111; 16.9% | 88; 16.5% | 23; 18.3% | |
| Poverty (<$20K) (n;%) | 140; 21.3% | 95; 17.9% | 45; 35.7% | χ2 = 19.39, p < .001, RR = 1.99 |
| ISI (M ± SD) | 17.79 ± 4.29 | 17.67 ± 4.35 | 18.29 ± 4.01 | t(656) = 1.46, p = .146 |
| QIDS-SR16 (M ± SD) | 10.77 ± 4.54 | 9.72 ± 4.07 | 15.21 ± 3.69 | t(656) = 13.88, p < .001, d = 1.41 |
SI, suicidal ideation over the past week, operationalized as endorsing the SI item on the quick inventory of depressive symptomatology, 16-item self-report version; M ± SD, mean and standard deviation; n, number of patients; HS, high school; GED, General Education Development; Associates, associates degree; Bachelors, bachelor's degree; ISI, insomnia severity index; QIDS-SR16, quick inventory of depressive symptomatology, 16-item self-report version; χ2, chi-square; RR, relative risk; d, Cohen’s d effect size.
Does CBTI alleviate SI?
To test CBTI's effects on alleviating SI, we first analyzed data from the 126 patients who endorsed SI prior to treatment, which involved comparing 60 CBTI patients to 66 controls.
Posttreatment.
Logistic regression showed that CBTI was associated with lower likelihood of SI after treatment relative to control (Odds Ratio [OR] = 0.36, 95% CI = 0.17 to 0.75, p = .006). Specifically, 30.0% (n = 18/60) CBTI patients endorsed SI after treatment, whereas 54.5% (n = 36/66) controls endorsed SI after treatment, thereby indicating that controls with pretreatment SI were nearly twice as likely to continue endorsing SI after treatment relative to CBTI patients with pretreatment SI (χ2 = 7.73, p = .005, RR =1.82; Table 3).
Table 3.
SI rates by treatment condition and insomnia remission status at posttreatment and 1-year follow-up
| CBTI | Control | Remission | Non-remission | |||
|---|---|---|---|---|---|---|
| Alleviating SI: Rates of SI at posttreatment and 1-year follow-up for patients who endorsed SI before treatment | ||||||
| Posttreatment | ||||||
| SI endorsed | 18/60; 30.0% | 36/66; 54.5% | χ2 = 7.73, p = .005, RR = 1.82 | 8/40; 20.0% | 46/86; 53.5% | χ2 = 12.50, p < .001, RR = 2.68 |
| 1-year follow-up | ||||||
| SI endorsed | 16/56; 29.6% | 29/62; 46.8% | χ2 = 4.13, p = .042, RR = 1.58 | 8/37; 21.6% | 37/81; 53.5% | χ2= 6.23, p = .013, RR = 2.12 |
| SI endorsed (ITT) | 17/60; 28.3% | 30/66; 45.5% | χ2 = 3.94, p = .047, RR = 1.61 | 8/43; 18.6% | 39/83; 47.0% | χ2 = 9.76, p = .002, RR = 2.53 |
| Preventing SI: Rates of SI at posttreatment and 1-year follow-up for patients who denied SI before treatment | ||||||
|---|---|---|---|---|---|---|
| Posttreatment | ||||||
| SI endorsed | 15/298; 5.0% | 10/234; 4.3% | χ2 = 0.17, p = .681 | 3/195; 1.5% | 22/337; 6.5% | χ2 = 6.87, p = .009, RR = 4.33 |
| 1-year follow-up | ||||||
| SI endorsed | 12/270; 4.4% | 15/220; 6.8% | χ2 = 1.31, p = .252 | 8/191; 4.2% | 19/299; 6.4% | χ2 = 1.05, p = .305 |
| SI endorsed (ITT) | 14/298; 4.7% | 18/234; 7.7% | χ2 = 2.08, p = .149 | 8/209; 3.8% | 24/323; 7.4% | χ2 = 2.91, p = .088 , RR = 1.95 |
SI, suicidal ideation over the past week, operationalized as endorsing the SI item on the quick inventory of depressive symptomatology, 16-item self-report version; Remission, insomnia severity index score ≤ 7; Non-remission, insomnia severity index score ≥ 8. One-year follow-up data are derived from per-protocol analysis, except when noted as ITT (intent-to-treat; these ITT results are reported in the Supplement). χ2, chi-square for comparing SI rates between treatment conditions and between remission statuses; RR, relative risk; CBTI, cognitive-behavioral therapy for insomnia.
One-year follow-up.
Logistic regression again showed that CBTI patients, relative to controls, were at lower odds of reporting SI a year after treatment (OR = 0.46, 95% CI = 0.21 to 0.98, p = .044). Specifically, 29.6% (n = 16/56) CBTI patients endorsed SI a year after treatment relative to 46.8% (n = 29/62) controls, thereby indicating that controls with pretreatment SI were 1.5 times as likely to continue endorsing SI a year after treatment relative to CBTI patients (χ2 = 4.13, p = .042, RR = 1.58).
Insomnia remission as a CBTI mechanism in SI alleviation
Alpha path: Treatment →Insomnia remission.
Logistic regression revealed that CBTI patients were at substantially greater odds of remitting from insomnia at posttreatment than controls (b = 2.57, SE = 0.50, OR = 13.08, 95% CI = 4.90 to 34.93, p < .001). Among CBTI patients with pretreatment SI, 56.7% (n = 34/60) of these patients remitted from insomnia at posttreatment. By comparison, just 9.1% (n = 6/66) of controls with pretreatment SI remitted from insomnia after treatment.
Beta path: Insomnia remission → SI.
Logistic regression revealed that insomnia remitters were at substantially lower odds of endorsing SI after treatment relative to non-remitters (b = −1.26, SE = 0.50, OR = 0.28, 95% CI = 0.11 to 0.76, p = .012). Notably, treatment condition was no longer significant after accounting for the effects of insomnia remission (b = −0.50, OR = 0.61, 95% CI = 0.26 to 1.40, p = .243). A post hoc chi-square analysis revealed that, irrespective of treatment condition, insomnia non-remitters were 2–3 times more likely to endorse SI relative to insomnia remitters; see Table 3 for SI rates.
Indirect path: Treatment → Insomnia remission → SI.
The PRODCLIN estimate of the indirect effect supported mediation (αβ = −3.30, SE = 1.59, 95% CI = −6.78 to −0.60) whereby CBTI increases odds of insomnia remission, which, in turn, decreases odds of endorsing SI after treatment (Figure 3, model 1). The estimated effect of CBTI on SI decreased from b = −1.03 (τ) in a bivariate model to b = −0.50 (τʹ) when controlling for insomnia remission. Thus, we estimated that insomnia remission mediates 51.5% of CBTI’s effect on SI reduction (τ/[τ − τʹ] = 1.03/[1.03 – 0.50]).
Figure 3.
Mediation results in suicidal ideation (SI) alleviation and SI prevention. α represents the effect of cognitive-behavioral therapy for insomnia (CBTI) on insomnia remission (IV → M). β represents the effect of insomnia remission on SI (M → DV), controlling for CBTI effects. αβ represents the indirect effect of CBTI on SI as mediated by insomnia remission.
Does CBTI prevent SI?
To test CBTI's effects on reducing risk for developing new-onset SI, we analyzed data from the 532 patients who denied SI prior to treatment, which involved comparing 298 CBTI patients to 234 controls.
Posttreatment.
Contrary to the alleviation results, logistic regression showed that posttreatment SI rates did not differ between patients in the CBTI and control conditions (OR = 1.19, 95% CI = 0.52 to 2.69, p = .681). See Table 3 for SI rates by treatment condition.
One-year follow-up.
Once again, logistic regression showed that CBTI was not directly associated with the prevention of SI a year after treatment relative to control (OR = 0.64, 95% CI = 0.29 to 1.39, p = .255). See Table 3 for SI rates by treatment condition.
Insomnia remission as a CBTI mechanism in SI prevention
Although CBTI did not produce a direct effect on SI prevention, it is possible that CBTI may nevertheless produce an indirect effect on SI prevention via insomnia remission. Indeed, significance testing of a mediation effect does not require a significant direct effect (indicated by the 95% CI of αβ) [46–49].
Alpha path: Treatment →Insomnia remission.
Logistic regression revealed that CBTI patients without pretreatment SI were at substantially greater odds of remitting from insomnia at posttreatment than control patients without pretreatment SI (b = 1.84, SE = 0.22, OR = 6.29, 95% CI = 4.13 to 9.59, p < .001). Among CBTI patients without pretreatment SI, 53.4% (n = 159/298) of these patients remitted from insomnia at posttreatment. By comparison, just 15.4% (n = 36/234) of controls without pretreatment SI remitted from insomnia after treatment.
Beta path: Insomnia remission → SI.
Logistic regression revealed that insomnia remitters without pretreatment SI were at substantially lower odds of endorsing new onset SI after treatment relative to non-remitters (b = −1.74, SE = 0.64, OR = 0.18, 95% CI = 0.05 to 0.62, p = .007). Specifically, 1.5% of insomnia remitters endorsed new SI after treatment relative to 6.5% of non-remitters (SI rates by insomnia remission status in Table 3). Treatment condition remained a nonsignificant predictor in this model (b = 0.64, OR = 1.89, 95% CI = 0.81 to 4.42, p = .140).
Indirect path: Treatment → Insomnia remission → SI.
The PRODCLIN estimate of the indirect effect supported mediation (αβ = −3.20, SE = 1.24, 95% CI = −5.74 to −0.87) whereby CBTI increases odds of insomnia remission, which, in turn, reduces odds of endorsing new SI after treatment for patients who denied SI prior to treatment (Figure 3, Model 2). As CBTI did not produce a direct effect on SI, the data suggest that CBTI prevents SI to the extent that it produces insomnia remission.
Discussion
The present study examined the effects of digital CBTI on the alleviation and prevention of SI in 658 adults with DSM-5 insomnia disorder. Among insomnia patients presenting to treatment with suicidal thoughts, digital CBTI yielded strong acute and long-lasting effects on SI. Importantly, our study offers novel findings that treating insomnia patients to remission prevents new-onset SI, which may support expanding the role of CBTI and other insomnia therapeutics in suicide management. Overall, these clinical trial findings suggest that digital CBTI can serve a role in alleviating and even preventing SI in insomnia patients and that insomnia remission represents a critical treatment mechanism by which CBTI mitigates suicide risk.
CBTI alleviates SI
Nearly 1 in 5 insomnia patients who presented to treatment in our study endorsed SI. This prevalence rate is consistent with prior treatment-seeking insomnia patient samples [18, 19], indicating that a substantial proportion of insomnia patients have suicidal thoughts that deserve clinical attention.
Our findings strongly supported the effectiveness of digital CBTI for reducing SI relative to a control condition. Among suicidal patients, we observed a 70% reduction in SI in the CBTI condition at posttreatment, which remained consistent a year later at a 72% reduction. In the control condition, we observed just a 45% reduction in SI at posttreatment, which slightly increased to a 53% reduction from baseline at follow-up. Moreover, we estimated that insomnia remission mediated approximately half of the effect of CBTI on SI, thereby identifying insomnia remission as a key treatment mechanism by which CBTI alleviates SI. Overall, the findings are highly consistent with prior studies supporting the effectiveness of CBTI on SI, and with prior preliminary evidence suggesting that insomnia reduction serves as an important treatment mechanism [18–20, 22].
Our study offers novel findings. First: prior studies have evaluated the effects of CBTI on SI in a combined sample of patients with and without SI at baseline. Thus, our study is the first to test SI alleviation specifically in suicidal patients. Second: prior studies supporting CBTI for suicide-related outcomes were face-to-face interventions in individual and group formats. Thus, our study is the first to demonstrate CBTI efficacy for alleviating and preventing SI using an eHealth digital delivery format. Even if face-to-face delivery formats are preferred, especially for suicidal patients, the expansion of these findings to the digital therapeutic format is likely to be a critical and growing element in future clinical practice given existing shortages of trained CBTI specialists [50].
CBTI prevents SI via insomnia remission
CBTI has gained significant interest for its potential role in mitigating the risk of future mental illness, particularly in regard to depression and anxiety, and has yielded promising results [51–56]. Given that insomnia increases risk for future SI [15–17], it is no wonder that targeting insomnia via CBTI has been identified as a potential method to reduce suicide-related outcomes in this at-risk population [57].
Our study results offer support for CBTI as a preventive measure against suicide for individuals struggling with insomnia, especially given that remission of insomnia was identified as a key factor in SI prevention. Although CBTI did not yield a direct effect on new-onset SI prevention at posttreatment or 1-year follow-up, we observed a robust indirect effect wherein CBTI increased the likelihood of insomnia remission, which, in turn, was associated with lower SI rates at posttreatment for insomnia remitters (1.5%) relative to insomnia non-remitters (6.5%; RR = 4.33). Data at 1-year follow-up were less clear with per-protocol analysis results showing no significant SI rate difference between remitters and non-remitters (4.2% vs 6.4%), whereas ITT analyses revealed a statistical trend to suggest that insomnia remission a year after treatment may be related to lower SI rates at 1-year follow-up (3.8% vs 7.4%).
A limitation that is important to highlight here is our limited power for testing prevention at 1-year follow-up. A post hoc power analysis revealed that our logistic regression analysis produced an OR = 0.496, probability of SI for untreated patients = 7.4% in the ITT analysis, alpha = 0.05, n = 532, and a binomial X distribution would have β = 0.40 power, which is below the recommended minimum of β = 0.80. Thus, our ITT result showing a statistical trend of p = .088 should be interpreted within the context of limited statistical power to detect prevention based on the observed effect size. Indeed, when evaluating SI rates for patients who denied SI before treatment, CBTI patients consistently endorsed new onset SI rates of 4%–5% at posttreatment and a year later. By comparison, control patients reported new SI rate of 4% at posttreatment then 7%–8% a year later. Taken together, we believe our study offers strong preliminary support for CBTI preventing SI through insomnia remission, and that insomnia remission may confer a degree of long-term protection. Future research utilizing a larger patient sample is needed to better understand the role of CBTI in the prevention of suicide-related outcomes.
Treatment mechanisms for reducing SI: Insomnia is a good start
Prior clinical trials offered preliminary support for alleviating insomnia as a key mechanism by which insomnia treatment (psychotherapy, pharmacotherapy) reduces SI [19–21]. The present study added to these findings by showing that insomnia remission mediates the effects of CBTI on SI alleviation and prevention. Importantly, prior studies show that nocturnal wakefulness represents a robust risk factor for suicide [23, 24], and that suicides most often occur at night [25]. Future research taking a more granular approach is needed to evaluate whether insomnia remission in CBTI reflects the removal of a robust risk factor—i.e. nocturnal wakefulness—to reduce risk for SI. In other words, it is possible that insomnia remission is a suicidolytic mechanism to the extent to which it facilitates the reduction of nocturnal wakefulness. As insomnia produces effects on suicide-related outcomes independent of depression and other mental health conditions [11–13], insomnia therapeutics may be combined with other independent treatments (e.g. for depression, substance abuse, borderline personality disorder, etc.) to address multiple independent SI risk factors.
Despite the large effects of insomnia remission on suicide-related outcomes, we must emphasize that non-sleep-related risk factors (e.g. mental health symptoms and cognitive-emotional dysregulation) of course remain critical to suicide intervention. Important to highlight is that the interplay between sleep-related factors (e.g. insomnia) and non-sleep-related factors (e.g. cognitive-emotional dysregulation) substantially amplifies SI risk. Indeed, we have shown that SI rates are highest when patients endorse both insomnia and high levels of nocturnal perseverative thinking (e.g. worry and rumination) [58, 59]. Unfortunately, CBTI exerts modest influence on non-sleep-related SI risk factors such as depression and perseverative thinking [22, 52, 60, 61]. By extension, it is unsurprising that only insomnia remission was identified as a mechanism by which CBTI affected SI in the present study (see Supplementary Materials for exploration of changes in insomnia, depression, and perseverative thinking as potential treatment mechanisms for SI). Future studies should evaluate whether enhanced therapeutic approaches targeting both insomnia symptoms and perseverative thinking may maximize therapeutic benefits for suicide-related outcomes (e.g. lower rates of SI, lower levels of SI intensity, less likelihood of attempts) in insomnia patients.
Clinical implications and future directions
Role of CBTI in suicide-risk reduction.
Although CBTI significantly reduced SI among patients who initially presented to treatment with suicidal thoughts, many of these CBTI patients (28%–30%) continued endorsing SI up to a year after treatment, thereby indicating that standalone CBTI is insufficient for many suicidal patients with insomnia. Indeed, CBTI does not adequately address all relevant suicide-risk factors as discussed above. Thus, we believe that CBTI, regardless of delivery format, is most appropriately delivered as an adjunct therapy to other mental health interventions for suicidal patients presenting with insomnia.
Role of CBTI in preventing suicidal thoughts and behaviors.
Our data suggest that CBTI prevents the new development of SI by helping a large number of patients to fully remit from insomnia. Larger-scale studies are needed to examine more comprehensively the preventive effects of CBTI on suicidal thoughts and behaviors, including SI intensity, planning, intent, and behaviors. Accumulating evidence support clinical initiatives to deploy CBTI more broadly to poorly sleeping individuals—especially those with other suicide-risk factors, like depression—to mitigate suicide in high-risk groups.
Limitations
Study results should be interpreted in light of important methodological limitations. We have already addressed the lack of statistical power for detecting SI prevention effects a year after treatment in the CBTI prevents SI via insomnia remission section above. Along these lines, our assessment of SI focused on a single week prior to assessment, and insomnia symptom assessment focused on a 2-week period. These limited assessment windows can be considered both a strength and a limitation of this study. As a strength: prior studies have assessed insomnia and SI over the past several months or years. In these studies, it is unclear whether endorsement of both factors represents co-occurrence or not (e.g. insomnia may have been present for the past week, whereas SI may have been present a month earlier). In our study, the confined assessment windows suggest that these experiences co-occurred. As a limitation, our limited assessment windows preclude detection of any SI that occurred after treatment but earlier than a week before follow-up assessment, which would not have been captured in our study. Future studies evaluating suicide-related outcomes should consider more frequent assessments. Future insomnia clinical trials utilizing shorter intervals for assessing SI (momentary, daily, weekly) may capture more granular insights into how rapidly improvement in insomnia affects SI [62], and how long these effects may last after treatment. In addition, the present study only assessed SI presence. More comprehensive assessment of suicide-related outcomes (SI intensity, suicide planning, intent, attempts, deaths) would yield greater insights into the potential benefits of digital CBTI for suicidal thoughts and behaviors. We encourage researchers to include more comprehensive suicide outcomes assessment in insomnia clinical trials going forward.
Other key study limitations center on generalizability. Firstly, men were underrepresented, which is common in clinical trials of insomnia intervention. Even so, we showed that male insomnia patients were more likely to endorse SI relative to female insomnia patients, which replicates sex differences in the broader population [63]. Important to emphasize is that research also shows that men are more likely to attempt and die by suicide than women [64, 65]. Thus, suicide-focused insomnia studies should consider taking measures to enhance recruitment of male insomnia patients; this is especially important as women are more likely to endorse and seek treatment for insomnia [66, 67]. Moreover, only non-Hispanic White and non-Hispanic Black racial groups were well-represented in our study, which limits generalizability to other racial groups. Future RCTs evaluating the role of insomnia therapeutics in suicide management would benefit from recruiting a more racially and ethnically diverse sample.
Conclusions
Evidence supporting the use of insomnia therapeutics, and especially CBTI, in suicide-risk management is growing. Our study suggests that digital CBTI can be beneficial for reducing and preventing SI, and that insomnia remission plays a key role in these effects. Future research is needed to evaluate how to best incorporate insomnia treatment in suicide management efforts, both for suicidal patients and for broader prevention initiatives. In addition, while standard CBTI produces treatment effects on SI, augmenting CBTI to yield stronger effects on other suicide-related risk factors such as depression and perseverative thinking may improve its impact on suicide-related outcomes.
Supplementary Material
Acknowledgments
We would like to thank Colin Espie, Chris Miller, and Alasdair Henry for their support. This report is dedicated to VP, mostly because we love you but also a little because you would not want me dedicating this to you. Slava Ukraini!
Clinical Trial Information: Sleep to Prevent Evolving Affecting Disorders. NCT02988375. https://clinicaltrials.gov/ct2/show/NCT02988375.
Contributor Information
David A Kalmbach, Thomas Roth Sleep Disorders and Research Center, Henry Ford Health System, Detroit, MI, USA.
Philip Cheng, Thomas Roth Sleep Disorders and Research Center, Henry Ford Health System, Detroit, MI, USA.
Brian K Ahmedani, Center for Health Policy and Health Services Research, Henry Ford Health System, Detroit, MI, USA.
Edward L Peterson, Department of Public Health Services, Henry Ford Health System, Detroit, MI, USA; Department of Epidemiology, Henry Ford Health System, Detroit, MI, USA.
Anthony N Reffi, Thomas Roth Sleep Disorders and Research Center, Henry Ford Health System, Detroit, MI, USA.
Chaewon Sagong, Thomas Roth Sleep Disorders and Research Center, Henry Ford Health System, Detroit, MI, USA.
Grace M Seymour, Thomas Roth Sleep Disorders and Research Center, Henry Ford Health System, Detroit, MI, USA.
Melissa K Ruprich, Thomas Roth Sleep Disorders and Research Center, Henry Ford Health System, Detroit, MI, USA.
Christopher L Drake, Thomas Roth Sleep Disorders and Research Center, Henry Ford Health System, Detroit, MI, USA.
Funding
Robert Wood Johnson Foundation. Dr. Cheng’s effort was supported by the National Heart, Lung, and Blood Institute (K23HL138166).
Disclosure Statement
Financial Disclosure: PC has received research support from Harmony Biosciences. CLD has received research support from Apnimed Inc., Harmony Biosciences, Proctor & Gamble, Jazz Pharmaceuticals, Eisai Pharmaceuticals, and Axsome Therapeutics Inc. and has served on speaker’s bureau for Harmony Biosciences. No other financial interests exist.
Nonfinancial disclosure: The authors have no nonfinancial conflicts to disclose.
References
- 1. CDC. National Center for Health Statistics Mortality Data on CDC WONDER. US Department of Health and Human Services, CDC; 2021. https://wonder.cdc.gov/Deaths-by-Underlying-Cause.html. Accessed July 29, 2022. [Google Scholar]
- 2. Hall RC, et al. Suicide risk assessment: a review of risk factors for suicide in 100 patients who made severe suicide attempts: evaluation of suicide risk in a time of managed care. Psychosomatics. 1999;40(1):18–27. doi: 10.1016/S0033-3182(99)71267-3. [DOI] [PubMed] [Google Scholar]
- 3. Pompili M, et al. Substance abuse and suicide risk among adolescents. Eur Arch Psychiatry Clin Neurosci. 2012;262(6): 469–485. [PubMed] [Google Scholar]
- 4. Haney EM, et al. Suicide Risk Factors and Risk Assessment Tools: a Systematic Review. Washington, DC: Department of Veterans Affairs (US); 2012. [PubMed] [Google Scholar]
- 5. Lynch FL, et al. Substance use disorders and risk of suicide in a general US population: a case control study. Addict Sci Clin Pract. 2020;15(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boggs JM, et al. General medical, mental health, and demographic risk factors associated with suicide by firearm compared with other means. Psychiatr Serv. 2018;69(6):677–684. doi: 10.1176/appi.ps.201700237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ahmedani BK, et al. Major physical health conditions and risk of suicide. Am J Prev Med. 2017;53(3):308–315. doi: 10.1016/j.amepre.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Owen-Smith AA, et al. The mediating effect of sleep disturbance on the relationship between nonmalignant chronic pain and suicide death. Pain Pract. 2019;19(4):382–389. doi: 10.1111/papr.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pigeon WR, et al. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73(9):e116011734–e1160e1167. doi: 10.4088/jcp.11r07586. [DOI] [PubMed] [Google Scholar]
- 10. Malik S, et al. The association between sleep disturbances and suicidal behaviors in patients with psychiatric diagnoses: a systematic review and meta-analysis. Syst Rev. 2014;3(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chakravorty S, et al. Sleep duration and insomnia symptoms as risk factors for suicidal ideation in a nationally representative sample. Prim Care Companion CNS Disord. 2015;17(6):23281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tubbs AS, et al. Surviving the long night: the potential of sleep health for suicide prevention. Sleep Med Rev. 2019;44:83–84. doi: 10.1016/j.smrv.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vargas I, et al. Insomnia symptoms and suicide-related ideation in US Army service members. Behav Sleep Med. 2020;18(6):820–836. doi: 10.1080/15402002.2019.1693373. [DOI] [PubMed] [Google Scholar]
- 14. McCall WV, et al. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Med. 2010;11(9):822–827. doi: 10.1016/j.sleep.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allan NP, et al. Insomnia and suicidal ideation and behaviors in former and current US service members: does depression mediate the relations? Psychiatry Res. 2017;252:296–302. doi: 10.1016/j.psychres.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 16. Bernert RA, et al. Association of poor subjective sleep quality with risk for death by suicide during a 10-year period: a longitudinal, population-based study of late life. JAMA Psychiatry. 2014;71(10):1129–1137. doi: 10.1001/jamapsychiatry.2014.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pigeon WR, et al. Insomnia as a precipitating factor in new onset mental illness: a systematic review of recent findings. Curr Psychiatry Rep. 2017;19(8):1–11. [DOI] [PubMed] [Google Scholar]
- 18. Manber R, et al. CBT for insomnia in patients with high and low depressive symptom severity: adherence and clinical outcomes. J Clin Sleep Med. 2011;7(6):645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trockel M, et al. Effects of cognitive behavioral therapy for insomnia on suicidal ideation in veterans. Sleep. 2015;38(2):259–265. doi: 10.5665/sleep.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pigeon WR, et al. Brief CBT for insomnia delivered in primary care to patients endorsing suicidal ideation: a proof-of-concept randomized clinical trial. Transl Behav Med. 2019;9(6):1169–1177. doi: 10.1093/tbm/ibz108. [DOI] [PubMed] [Google Scholar]
- 21. McCall WV, et al. Reducing suicidal ideation through insomnia treatment (REST-IT): a randomized clinical trial. Am J Psychiatry. 2019;176(11):957–965. doi: 10.1176/appi.ajp.2019.19030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kalmbach DA, et al. A randomized controlled trial of digital cognitive behavioral therapy for insomnia in pregnant women. Sleep Med. 2020;72:82–92. doi: 10.1016/j.sleep.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Perlis ML, et al. Nocturnal wakefulness as a previously unrecognized risk factor for suicide. J Clin Psychiatry. 2016; 77(6):e72612828–e7261e733. doi: 10.4088/jcp.15m10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tubbs AS, et al. Nocturnal and morning wakefulness are differentially associated with suicidal ideation in a nationally representative sample. J Clin Psychiatry. 2021;82(6):36963. [DOI] [PubMed] [Google Scholar]
- 25. Tubbs AS, et al. Relationship of nocturnal wakefulness to suicide risk across months and methods of suicide. J Clin Psychiatry. 2020;81(2):12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American Psychiatric Association. Sleep-Wake Disorders. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 27. Christensen H, et al. Adherence in internet interventions for anxiety and depression: systematic review. J Med Internet Res. 2009;11(2):e13e1194. doi: 10.2196/jmir.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheng P, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med. 2018;1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng P, et al. Depression prevention via digital CBT for insomnia: a randomized controlled trial. Sleep. 2019;42(10). doi: 10.1093/sleep/zsz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pillai V, et al. The anxiolytic effects of cognitive behavior therapy for insomnia: preliminary results from a web-delivered protocol. J Sleep Med Disord. 2015;2(2):1017. [PMC free article] [PubMed] [Google Scholar]
- 31. Freeman D, et al. The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis. Lancet Psychiatry. 2017;4(10):749–758. doi: 10.1016/S2215-0366(17)30328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Espie CA, et al. Effect of digital cognitive behavioral therapy for insomnia on health, psychological well-being, and sleep-related quality of life: a randomized clinical trial. JAMA Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Espie CA, et al. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;35(6):769–781. doi: 10.5665/sleep.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bostock S, et al. Sleep and productivity benefits of digital cognitive behavioral therapy for insomnia: a randomized controlled trial conducted in the workplace environment. J Occup Environ Med. 2016;58(7):683–689. doi: 10.1097/JOM.0000000000000778. [DOI] [PubMed] [Google Scholar]
- 35. Barnes CM, et al. Helping employees sleep well: effects of cognitive behavioral therapy for insomnia on work outcomes. J Appl Psychol. 2017;102(1):104–113. doi: 10.1037/apl0000154. [DOI] [PubMed] [Google Scholar]
- 36. McGrath ER, et al. Sleep to lower elevated blood pressure: a randomized controlled trial (SLEPT). Am J Hypertens. 2017;30(3):319–327. doi: 10.1093/ajh/hpw132. [DOI] [PubMed] [Google Scholar]
- 37. Felder JN, et al. Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psychiatry. 2020;77(5):484–492. doi: 10.1001/jamapsychiatry.2019.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. National Institutes of Health. Your guide to healthy sleep. Birmingham, AL: South Med Assoc; 2011. [Google Scholar]
- 39. Irish LA, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23–36. doi: 10.1016/j.smrv.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Grandner MA, et al. Insomnia in primary care: misreported, mishandled, and just plain missed. J Clin Sleep Med. 2017;13(8):937–939. doi: 10.5664/jcsm.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morgenthaler T, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29(11):1415–1419. doi: 10.1093/sleep/29.11.1415. [DOI] [PubMed] [Google Scholar]
- 42. Drake CL, et al. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2). doi: 10.1093/sleep/zsy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rush AJ, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 44. Morin CM, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tofighi D, et al. RMediation: an R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43(3):692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao X, et al. Reconsidering Baron and Kenny: myths and truths about mediation analysis. J Cons Res. 2010;37(2):197–206. doi: 10.1086/651257. [DOI] [Google Scholar]
- 47. Rucker DD, et al. Mediation analysis in social psychology: current practices and new recommendations. Soc Pers Psychol Compass. 2011;5(6):359–371. doi: 10.1111/j.1751-9004.2011.00355.x. [DOI] [Google Scholar]
- 48. Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr. 2009;76(4):408–420. doi: 10.1080/03637750903310360. [DOI] [Google Scholar]
- 49. MacKinnon DP, et al. Equivalence of the mediation, confounding and suppression effect. Prev Sci. 2000;1(4):173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thomas A, et al. Where are the behavioral sleep medicine providers and where are they needed? A geographic assessment. Behav Sleep Med. 2016;14(6):687–698. doi: 10.1080/15402002.2016.1173551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheng P, et al. Depression prevention via digital cognitive behavioral therapy for insomnia: a randomized controlled trial. Sleep. 2019;42(10). doi: 10.1093/sleep/zsz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cheng P, et al. Depression prevention in digital cognitive behavioral therapy for insomnia: is rumination a mediator? J Affect Disord. 2020;273:434–441. [DOI] [PubMed] [Google Scholar]
- 53. Felder JN, et al. Randomized controlled trial of digital cognitive behavior therapy for prenatal insomnia symptoms: effects on postpartum insomnia and mental health. Sleep. 2022;45(2). doi: 10.1093/sleep/zsab280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lin Y-H, et al. The direct effect of cognitive behavioral therapy for insomnia on depression prevention and the mediation effect via insomnia remission. JAMA Psychiatry. 2022;79(5):514–515. doi: 10.1001/jamapsychiatry.2022.0149. [DOI] [PubMed] [Google Scholar]
- 55. Dietch JR, et al. Insomnia and cognitive arousal are important potential targets to reduce perinatal depression risk. Sleep. 2021;44(6). doi: 10.1093/sleep/zsab091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Irwin MR, et al. Prevention of incident and recurrent major depression in older adults with insomnia: a randomized clinical trial. JAMA Psychiatry. 2022;79(1):33–41. doi: 10.1001/jamapsychiatry.2021.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hamilton JL, et al. Reducing suicidality through insomnia treatment: critical next steps in suicide prevention. Am Psychiatric Assoc. 2019;176(11):897–899. [DOI] [PubMed] [Google Scholar]
- 58. Kalmbach DA, et al. Depression and suicidal ideation in pregnancy: exploring relationships with insomnia, short sleep, and nocturnal rumination. Sleep Med. 2020;65:62–73. doi: 10.1016/j.sleep.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kalmbach DA, et al. Nocturnal cognitive hyperarousal, perinatal-focused rumination, and insomnia are associated with suicidal ideation in perinatal women with mild to moderate depression. Sleep Med. 2021;81:439–442. doi: 10.1016/j.sleep.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kalmbach DA, et al. Examining patient feedback and the role of cognitive arousal in treatment on-response to digital cognitive-behavioral therapy for insomnia during pregnancy. Behav Sleep Med. 2022;20(2):143–163. doi: 10.1080/15402002.2021.1895793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kalmbach DA, et al. Treating insomnia improves depression, maladaptive thinking, and hyperarousal in postmenopausal women: comparing cognitive-behavioral therapy for insomnia (CBTI), sleep restriction therapy, and sleep hygiene education. Sleep Med. 2019;55:124–134. doi: 10.1016/j.sleep.2018.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Franklin JC, et al. Risk factors for suicidal thoughts and behaviors: a meta-analysis of 50 years of research. Psychol Bull. 2017;143(2):187–232. doi: 10.1037/bul0000084. [DOI] [PubMed] [Google Scholar]
- 63. Freeman A, et al. A cross-national study on gender differences in suicide intent. BMC Psychiatry. 2017;17(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Nock MK, et al. Prevalence of and risk factors for suicide attempts versus suicide gestures: analysis of the National Comorbidity Survey. J Abnorm Psychol. 2006;115(3):616–623. doi: 10.1037/0021-843X.115.3.616. [DOI] [PubMed] [Google Scholar]
- 65. Nock MK, et al. Suicide and suicidal behavior. Epidemiol Rev. 2008;30(1):133–154. doi: 10.1093/epirev/mxn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Morin CM, et al. Epidemiology of insomnia: prevalence, course, risk factors, and public health burden. Sleep Med Clin. 2013;8(3):281–297. [DOI] [PubMed] [Google Scholar]
- 67. Trauer JM, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. doi: 10.7326/M14-2841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.