Abstract
Study Objectives
The pedunculopontine tegmental (PPT) nucleus is implicated in many brain functions, ranging from sleep/wake control and locomotion, to reward mechanisms and learning. The PPT contains cholinergic, GABAergic, and glutamatergic neurons with extensive ascending and descending axonal projections. Glutamatergic PPT (PPTvGlut2) neurons are thought to promote wakefulness, but the mechanisms through which this occurs are unknown. In addition, some researchers propose that PPTvGlut2 neurons promote locomotion, yet even though the PPT is a target for deep brain stimulation in Parkinson’s disease, the role of the PPT in locomotion is debated. We hypothesized that PPTvGluT2 neurons drive arousal and specific waking behaviors via certain projections and modulate locomotion via others.
Methods
We mapped the axonal projections of PPTvGlut2 neurons using conditional anterograde tracing and then photostimulated PPTvGlut2 soma or their axon terminal fields across sleep/wake states and analyzed sleep/wake behavior, muscle activity, and locomotion in transgenic mice.
Results
We found that stimulation of PPTvGlut2 soma and their axon terminals rapidly triggered arousals from non-rapid eye movement sleep, especially with activation of terminals in the basal forebrain (BF) and lateral hypothalamus (LH). With photoactivation of PPTvGlut2 terminals in the BF and LH, this wakefulness was accompanied by locomotion and other active behaviors, but stimulation of PPTvGlut2 soma and terminals in the substantia nigra triggered only quiet wakefulness without locomotion.
Conclusions
These findings demonstrate the importance of the PPTvGluT2 neurons in driving various aspects of arousal and show that heterogeneous brain nuclei, such as the PPT, can promote a variety of behaviors via distinct axonal projections.
Keywords: pedunculopontine, NREM sleep, wake, locomotion, photostimulation
Graphical Abstract
Graphical Abstract.
Statement of Significance.
We show that photoactivation of glutamatergic neurons in the pedunculopontine tegmental (PPT) rapidly wakes mice from non-rapid eye movement sleep (but not rapid eye movement sleep) in a stimulation frequency-dependent manner. Photoactivation of axon terminals from glutamatergic PPT neurons in the basal forebrain (BF) and lateral hypothalamus (LH) trigger long-lasting wake periods with active behaviors, while stimulation of axon terminals in the substantia nigra (SN) produce only quiet wake without active behaviors. In addition, muscle activity increased during photostimulation of terminals in the BF and LH, but not during stimulation of terminals in the SN or the PPT cell soma, suggesting distinct pathways for regulation of arousal and muscle activation. These findings shed light on the mechanisms governing arousal and may further development of treatments for sleepiness or coma. These results also build on our understanding of the PPT in the regulation of muscle activity and locomotion which is important for optimizing deep brain stimulation in people with Parkinson’s disease.
Introduction
The pedunculopontine tegmental (PPT) nucleus is implicated in the control of sleep/wake states [1–4], motor function [5–7], cognition [8], learning [9, 10], and reward [11–13]. PPT neurons likely regulate these behaviors via axonal projections to many regions, including the basal forebrain (BF) [14–16], thalamus [17], hypothalamus [18], and midbrain [19–21], as well as descending projections to the pons, medulla, and spinal cord [22, 23].
The PPT is delineated by a cluster of cholinergic neurons just ventrolateral to the caudal periaqueductal gray [19, 24], but this nucleus is heterogeneous and also contains distinct yet intermingled populations of glutamatergic and GABAergic neurons [25, 26]. In a previous study, we found that selective chemoactivation of each of these three cell populations has different effects on sleep/wake regulation. Specifically, activation of cholinergic PPT (PPTChat) neurons suppressed deep non-rapid eye movement (NREM) sleep, whereas activation of glutamatergic neurons in the PPT (PPTvGluT2) increased wake for several hours, but the mechanisms through which this arousal occurs remain unknown [4].
Besides regulating sleep/wake states, the PPT is also implicated in locomotion and movement control. Some researchers consider the PPT part of the mesencephalic locomotor region (MLR) as electrical stimulation of this region triggers locomotion even in the absence of cortical input [5, 27–29]. In addition, photostimulation of glutamatergic neurons in and around the PPT can promote running in head-fixed mice [6, 7, 30]. Based on this link to motor control and neuropathology studies showing loss of PPT neurons in Parkinson’s disease (PD) [31], the PPT is a target for deep brain stimulation (DBS) in some PD patients [32]. However, researchers debate whether these locomotor effects are actually driven by neurons in the PPT or adjacent regions [33–35]. Further, the effects of DBS in PD patients are highly variable, with some patients showing great improvement in mobility, while others show little or no improvement [36–38]. Clinical studies highlight the importance of accurate DBS probe placement and suggest gait improvements are more likely at the dorsal edge of the PPT and in the adjacent cuneiform nucleus (CnF) [37, 39]. Indeed, several carefully targeted electrical stimulation studies [40–43], as well as chemogenetic and optogenetic studies stimulating PPTvGlut2 neurons in mice and cats show that activation of the PPT fails to initiate locomotion [4] and can actually halt ongoing movement [42, 44, 45], while CnF stimulation produces locomotion [6, 44–46].
We hypothesized that PPTvGlut2 neurons mainly drive arousal and that they promote specific waking behaviors via certain projections and modulate behaviors such as locomotion via others. To better define the functions of PPTvGlut2 neurons, we photostimulated PPTvGlut2 soma and specific PPTvGlut2 axon terminal fields across sleep/wake states and analyzed sleep/wake behavior, muscle activity, and locomotion using EEG, EMG, and video recordings.
Methods
All experimental procedures were performed in accordance with the National Institutes of Health Guidelines and were approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center.
Animals
We used 8- to 10-week-old male mice expressing cre recombinase (cre) in neurons producing the vesicular glutamate transporter-2 (vGluT2-cre mice [47]). In a previous study, we validated the eutopic recombination of cre in vGlut2 mRNA-containing PPT neurons using in situ hybridization [4]. In total, we used 59 vGluT2-cre mice which had been backcrossed to C57BL/6J for more than 6 generations. We included mice in the analysis if more than 75% of virally transduced neurons were inside the PPT boundary (as defined by the cholinergic neurons) and optical fibers were within 0.5 mm of the target coordinates (a total of 15 mice were excluded). Mice were housed singly on a 12:12 h light/dark cycle (lights on at 7 am) at an ambient temperature of 22 ± 1°C with food and water available ad libitum.
Study design
To map the projection targets of PPTvGluT2 neurons, we unilaterally injected the PPT of four vGluT2-cre mice with an AAV carrying cre-dependent Channelrhodopsin-2 and the fluorescent tag mCherry (AAV8-EF1α-DIO-hChR2[H134r]-mCherry, 1.5 × 1013 viral particles/mL, custom prep from UNC Vector Core, termed “AAV-ChR2-mCherry”). Four to six weeks later, we immunostained brain sections from these mice for mCherry to map the anterograde projections, using a scoring system of innervation similar to Agostinelli et al. [48]: - no innervation; + very low innervation; ++ moderate innervation; +++ dense innervation; ++++ very dense innervation. We analyzed three sections per animal for each structure, and all sections were analyzed by the same experimenter.
To assess the roles of PPTvGlut2 neurons in sleep/wake states and locomotion, we injected AAV-ChR2-mCherry bilaterally into the PPT of vGluT2-cre mice and placed optical fibers either above the PPT (n = 9 mice) or above the terminal fields of these neurons in the BF (n = 7), lateral hypothalamus (LH) (n = 6), dorsal thalamus (n = 7), or substantia nigra (SN) (n = 6). Three weeks after implantation of the optical fibers and EEG/EMG leads, we recorded sleep/wake behavior and other behaviors by video while photostimulating ChR2-expressing cell bodies or axon terminals.
Surgery
Similar to previously described surgical procedures and AAV injections [49], we deeply anesthetized mice with ketamine–xylazine (100/10 mg/kg, i.p.), placed them in a stereotactic frame, and injected 20 nL of AAV-ChR2-mCherry unilaterally (for projection mapping) or bilaterally (for photostimulation) into the PPT. For the photostimulation experiments, we implanted mice with bilateral optical fibers (200 µm diameter, NA 0.39, ThorLabs) 0.2 mm above their respective AAV target sites: above the PPT (coordinates from bregma: AP −4.5, L 1.1, DV 3.3 [50]), BF (AP −0.22, L 1.6, DV 5.0), LH (AP −1.58, L 1.0, DV 4.5), dorsal thalamus (AP −1.06, L 1.0, DV 2.4), or SN (AP −3.08, L 1.35, DV 4.35). In addition, we implanted all mice in the photostimulation groups with two stainless-steel screws (1.5 mm lateral to bregma and 1.5 mm lateral to lambda) for EEG recordings, as well as two multistranded stainless-steel wires (#AS131, Cooner Wire) under the extensor neck muscles for EMG recordings. EEG screws and EMG leads were connected to a small pin board which was affixed to the skull by dental cement. The AAV injection, and implantation of the optical fibers and headstage were all performed in the same surgery. For postoperative care, all mice received meloxicam SR (4 mg/kg, s.c.). A group of cre-negative control mice (WT, n = 5) received bilateral injections of AAV5-mCherry (with no ChR2; AAV5-CamKIIa-mCherry 4.9 × 1012 viral particles/mL; UNC Vector Core), EEG/EMG implants as well as optical fiber implants.
We recorded EEG/EMG signals as previously described [4] and simultaneously recorded infrared video. We scored sleep/wake behavior in 10-s epochs using SleepSign for Animals (Kissei Comtec) and conventional criteria for scoring wake, rapid eye movement (REM) sleep, and non-REM (NREM) sleep [51].
Photostimulation and EEG/EMG recordings
After 1 week of recovery from surgery plus 2 weeks of acclimation to the EEG/EMG cables and optical fibers, we recorded EEG/EMG with photostimulation. We applied blue light (473 nM wavelength) at 10–12 mW (light output at the fiber tip) using a DPSS laser (item# R471005FX, Laserglow Technologies, Canada).
To assess the role of PPTvGlut2 neurons during NREM sleep, we photostimulated the PPT at 0.5–20 Hz (10-ms pulse width) for 10 s (pulse generator model # PCGU1000, Velleman Inc.) during the light period (between ZT 4 and ZT 9) after >30 s of stable NREM sleep (as assessed in real time by a human observer trained in recognizing mouse sleep/wake states). In some experiments, these responses to photostimulation were compared to 10-s periods of “Sham stimulation” during NREM sleep in which the laser remained off. We randomized the pulse frequencies for each photostimulation and applied each frequency at least 15 times. After each stimulation, mice were allowed to sleep though one full sleep cycle (NREM sleep, REM sleep [if it occurred], and Wake) with no stimulation to avoid any buildup of sleep pressure. We chose our stimulation frequency range (0.5–20 Hz) based on the in vivo discharge rate of putative glutamatergic PPT neurons, reported by Boucetta et al. [52].
To examine the effects of PPTvGlut2 neuron activation across all states, we then photostimulated PPTvGlut2 neurons at random times (regardless of sleep/wake state) 8 times per hour across the light period (5 Hz for 10 s). We chose 5 Hz as this consistently triggered awakenings, but the arousals were brief and unlikely to substantially disrupt homeostatic sleep drive. We analyzed the difference in percent time spent in each state for the 10 s prior to stimulation versus the 10 s after stimulation. To assess the effect of photostimulation on the latency to wake from NREM or REM sleep, we selected the first 11 bouts from each state during which stimulation started >15 s after the onset of either NREM sleep or REM sleep and recorded the latency from stimulation onset to wake.
To determine whether photostimulation of PPTvGlut2 soma and terminals affects muscle activity, we measured neck extensor EMG amplitude in the 10 s before and during the 10-s stimulation period (10 Hz stimulation frequency, 3 samples per mouse, 6–7 mice per cohort). We digitally filtered the raw EMG trace (bandstop filter: 50–65 Hz), converted all values to absolute values, and measured the difference between total EMG activity in the 10 s prior to stimulation and the 10 s of photostimulation.
Behavioral analysis
Photostimulation of PPTvGlut2 neurons often triggered wakefulness, and to assess whether this promoted specific behaviors, we analyzed the effects of photostimulation (10 Hz, 10 s, applied after 30 s of NREM sleep) of PPTvGlut2 soma or their axon terminals on mouse behavior. We specifically applied the photostimulation during NREM sleep to assess resulting wake behaviors, avoiding confounding effects of photostimulations that might occur during ongoing behaviors (e.g. grooming), which would be hard to interpret. The choice of 10 Hz was based on our observation that lower frequency stimulations often resulted in short wake responses from which animals quickly returned to sleep without engaging in observable wake behaviors other than short periods of quiet wake. Using the video recordings, we scored behaviors in 5-s intervals during the 10-s stimulation period and the 60 s after the stimulation in 11 trials for each mouse. We scored the behaviors as sleep, quiet wake (awake in the nest without gross motor movements), postural adjustments (awake and moving around within the nesting area), grooming, feeding, drinking, or locomotion (ambulation outside of nesting area).
In vitro electrophysiology
For in vitro electrophysiological recordings, we injected 30 nL of AAV-ChR2-mCherry into the PPT region of 1- to 2-week-old vGlut2-Cre mice (n = 5) and prepared brain slices 2 weeks later. We anesthetized mice with isoflurane (5% in oxygen) and transcardially perfused them with ice-cold N-methyl-d-glucamine (NMDG)-based solution containing (in mM): 100 NMDG, 2.5 KCl, 1.24 NaH2PO4, 30 NaHCO3, 25 glucose, 20 HEPES, 2 thiourea, 5 Na-l-ascorbate, 3 Na-pyruvate, 0.5 CaCl2, 10 MgSO4 (pH 7.3 with HCl when carbogenated with 95% O2 and 5% CO2; 310–320 mOsm). We prepared brain slices as previously described [53] and recorded the slices submerged and perfused (1.5–2 mL/min) with artificial CSF (ACSF; Na-based solution) that contained (in mM): 120 NaCl, 2.5 KCl, 1.3 MgCl2, 10 glucose, 26 NaHCO3, 1.24 NaH2PO4, 4 CaCl2, 2 thiourea, 1 Na-l-ascorbate, 3 Na-pyruvate (pH 7.3–7.4 when carbogenated with 95% O2 and 5% CO2; 310–320 mOsm). Recordings were performed at room temperature (18–20°C). We recorded ChR2-mCherry-expressing PPTvGlut2 neurons using a combination of fluorescence and infrared differential interference contrast microscopy using a fixed stage upright microscope (BX51WI, Olympus America Inc.) equipped with a Nomarski water immersion lens (Olympus 40×/0.8 NAW) and IR-sensitive CCD camera (ORCA-ER, Hamamatsu, Bridgewater, NJ). We recorded in whole-cell or cell-attached configurations using a Multiclamp 700B amplifier, a Digidata 1322A interface, and Clampex 9.0 software (Molecular Devices, Foster City, CA). We excluded from the analysis neurons with changes in input resistance greater than 10% over the recordings, and we corrected for junction potentials. We photostimulated the PPT using full-field light openings (~10 mW/mm2, 1 mm beam width) from a 5 W LUXEON blue light-emitting diode (470 nm wavelength; #M470L2-C4; Thorlabs, Newton, NJ) coupled to the epifluorescence pathway of the microscope. We used 10-s train stimulations with 10-ms light pulses at 0.5, 1, 5, 10, and 20 Hz. We recorded using a K-gluconate-based pipette solution, in whole-cell current clamp mode or in cell-attached configuration (Vh = 0 mV). The K-gluconate-based pipette solution contained (in mM): 120 K-gluconate, 10 KCl, 3 MgCl2, 10 HEPES, 2.5 K-ATP, 0.5 Na-GTP (pH 7.2 adjusted with KOH; 280 mOsm). In all the recordings, we added 0.5% biocytin in the pipette solution to mark the recorded neurons. After in vitro recordings, we fixed the recorded slices in 10% buffered formalin overnight and then incubated them for 12–24 h in streptavidin-conjugated Alexa Fluor AF-488 (green; 1:500; Invitrogen) to fluorescently label the biocytin-filled neurons. We imaged the biocytin-labeled neurons using a Zeiss LSM 5 Pascal confocal microscope using Zen 2009 software (Zeiss). We analyzed the in vitro recording data using Clampfit 10 (Molecular Devices), MiniAnalysis 6 (Synaptosoft, Leonia, NJ), and MatLab (MathWorks; Natick, MA). Figures were generated using Igor Pro 6 (WaveMetrics), Prism 7 (GraphPad, La Jolla, CA), and Photoshop (Adobe) software.
Immunohistochemistry
After the sleep recordings, we photostimulated the PPT for 2 h (20 Hz for 10 s every 30 s or sham stimulation) and then perfused mice with phosphate-buffered saline (PBS) and 10% formalin under deep ketamine–xylazine anesthesia (150/15 mg/kg). We harvested the brains, placed them in 10% formalin for 24 h at 4°C, and then cryoprotected them in PBS azide with 30% sucrose for another 24 h at 4°C. Then, we sectioned the brains into three series (30 µm) using a sliding microtome (Microm HM 440E, GMI) with a blade cleaned with RNAseZap (Life Technologies) and stored the sections in 1% formalin in DEPC-PBS.
To map the AAV injection sites and the location of optical fibers relative to the cholinergic neurons that define the PPT, we incubated one series of brain sections overnight in goat anti-ChAT antiserum (1:750; Millipore, #AB-144p). The next day, we incubated the slices in biotinylated donkey anti-goat IgG secondary antibody (1:500; Jackson ImmunoResearch, #705-065-147) for 1 h, followed by 2 h of incubation with a streptavidin-conjugated fluorescent antibody (1:1000; Alexa 488, Invitrogen, #S11224). We then scanned these sections using a slide-scanning fluorescence microscope (VS120, Olympus) and mapped the distribution of red mCherry-expressing neurons in relation to the green cholinergic neurons of the PPT; as shown below, the regions encompassed >75% of all mCherry-containing neurons.
To assess whether ChR2-expressing PPTvGlut2 neurons were activated by photostimulation, we immunostained another series of sections for fos using DAB, as described previously [54]. In brief, we incubated brain sections for 30 min in a 0.3% H2O2 solution before incubating them overnight with a rabbit anti-cFos primary antibody (1:5000; Calbiochem, #IC32). We then applied a biotinylated donkey anti-rabbit secondary antibody (1:500; Jackson ImmunoResearch, #711-065-152) for 1 h, followed by incubation with horseradish peroxidase-conjugated avidin–biotin complex (1:1000; Vector Labs, #PK-6100) for 1 h. We then labeled fos-containing nuclei black using 0.06% DAB solution with 0.01% hydrogen peroxide, 0.01% nickel ammonium sulfate, and 0.005% cobalt chloride. The next day, we incubated the same sections overnight with rabbit anti-DsRed primary antibody (1:500; Clontech, #632496). We used the same biotinylated donkey anti-rabbit secondary antibody and DAB solution (without nickel and cobalt) as before, resulting in a brown cytoplasmic labeling of mCherry tagged neurons. We digitized the sections using a slide-scanning microscope.
In situ hybridization
We processed one series of sections through the PPT (n = 4 mice) for in situ hybridization for vGlut2 mRNA plus mCherry immunochemistry. After cutting the brains in RNase-free conditions, we mounted the sections on Superfrost Plus slides and dried them overnight at 4°C. We rewarmed the slides to room temperature and further dried them in an oven for 30 min at 60°C and then performed in situ hybridization (RNAscope Multiplex Flourescent Reagent Kit V2; #323100, Advanced Cell Diagnostics, Hayward, CA). We pretreated the sections with hydrogen peroxide for 20 min at room temperature, before performing the target retrieval reagent procedure by placing the slides in a steamer (at >99°C) for 5 min. Afterward we dehydrated the sections in 90% alcohol and air-dried them for 5 min. We then incubated the sections with protease reagent (Protease III) at 40°C for 30 min. Following a rinse with sterile water, we performed the hybridization step by incubating the sections in the RNAscope probe for vGlut2 (RNAscope Probe-Mm-Slc17a6-C1; Cat No. 319171, Advanced Cell Diagnostics) for 2 h at 40°C. Next, we performed three amplification steps at 40°C (AMP1-FL and AMP2-FL: 30 min each; AMP3-FL: 15 min) and later incubated the sections in HRP-C1 for 15 min. After that, we incubated the sections in TSA plus Fluorescein (# NEL741001, Perkin Elmer) for 30 min to visualize the vGlut2 mRNA (Channel 1 at 488 nm) followed by incubation in HRP blocker for 15 min at 40°C. Since this procedure partially reduces native mCherry fluorescence, we also immunostained sections for mCherry. As above, we incubated sections in rabbit anti-DsRed antibody (1:7500) followed by donkey anti-rabbit secondary antibody tagged with Alexa Fluor 555 (Thermo Fisher Scientific, #A-31572). We cover-slipped slides with Vectashield mounting medium (Vector Laboratories, Catalog # H-1200). We analyzed coexpression of mCherry and vGlut2 mRNA at four levels through the PPT.
AAV specificity and transduction efficiency
After labeling neurons expressing vGlut2 mRNA in green and mCherry-expressing neurons in red, we digitized the brain slices (VS120, Olympus) and assessed the numbers of neurons with red only and red plus green labeling to determine the specificity of viral transduction (n = 4 mice; bilateral cell counts in four levels of the PPT). Next, we used adjacent brain slices immunolabeled for ChAT to map the boundaries of the PPT (area of ChAT+ neurons) onto images with neurons labeled for vGlut2 and mCherry expression. We counted the number of red (vGlut2+) neurons within the PPT boundaries to assess the viral transduction efficiency within the PPT vGlut2 neuron population (using Microsoft PowerPoint).
Statistical analysis
For all experiments, we first assessed whether the data were normally distributed, using a Kolmogorov–Smirnov test, before applying parametric or nonparametric statistical tests. We used one-way analyses of variance (ANOVAs) to analyze the frequency of action potentials in relation to the stimulation frequency. For comparisons of percent wake elicited by different frequencies of photostimulation, we used a Friedman test, followed by Dunn’s multiple comparison tests. We used paired t-tests to assess the differences in percent time spent in each state with photoactivation of PPTvGlut2 soma and terminals at random times during the light period, as well as comparisons of latencies to wake from NREM sleep and REM sleep. We used Kruskal–Wallis tests to compare percent time spent in different states or behaviors with photostimulation across axonal fields. Lastly, we used Wilcoxon matched-pairs signed rank tests to determine differences in EMG tone during photoactivation. All statistical tests were conducted using Prism 7 (GraphPad, La Jolla, CA).
Results
Genetically targeted photoactivation of PPTvGlut2 neurons
Chemogenetic activation of PPTvGlut2 neurons in vGlut2-cre mice during the inactive period robustly increases wakefulness for 6–8 h [4]. As chemogenetics has poor temporal resolution and is unsuitable to study the role of PPTvGlut2 neurons during specific sleep/wake states or the functions of distinct axonal projections, we turned to optogenetics. We first verified eutopic expression of an adeno-associated viral vector (AAV) coding for cre-dependent channelrhodopsin and mCherry by microinjecting AAV-ChR2-mCherry into the PPT of four vGlut2-cre mice and labeling vGlut2 mRNA using RNAscope followed by immunolabeling for mCherry. In total we counted 1974 vGlut2+ neuronsof these, 826 expressed mCherry, while 65 mCherry-labeled neurons lacked vGlut2 mRNA. Overall, 92.7 ± 1.3% of mCherry+ neurons contained vGlut2 mRNA, and 41.8 ± 5% of glutamatergic neurons within the PPT expressed mCherry. These results demonstrate highly eutopic expression with good transduction efficiency (Supplementary Figure 1).
To test whether ChR2-expressing PPTvGlut2 neurons are activated by photostimulation, we photostimulated the PPT region with blue light (20 Hz; 10 s on, 20 s off) or sham stimulation (lasers turned off) for the 2 h prior to sacrifice (n = 3 in each group; ZT 6–8). We then immunolabeled sections for fos and mCherry and found fos expression in 59.7 ± 3% of mCherry-expressing PPT neurons after photostimulation compared to only 4.5 ± 2.5% after sham stimulation (Supplementary Figure 1, F and G).
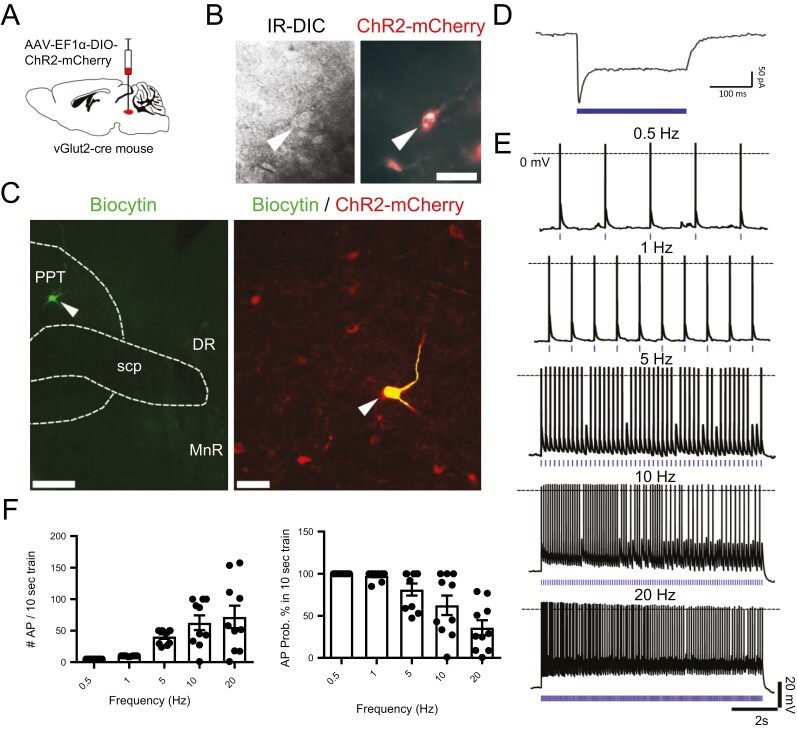
To assess the electrophysiological responses of ChR2-expressing PPTvGlut2 neurons, we studied their spontaneous activity and responses to photostimulation using in vitro brain slices. PPTvGlut2 neurons spontaneously fired at 2.25 ± 0.74 Hz (n = 4 cells). We then photostimulated PPTvGlut2 neurons at 0.5, 1, 5, 10, and 20 Hz (10 s train, 10 ms pulse duration; n = 10 neurons from five mice; Figure 1, A–E). Photostimulation increased the number of action potentials in a frequency-dependent fashion (F(4,45) = 9.845, p < 0.0001, one-way ANOVA). At 5 Hz and slower frequencies, PPTvGlut2 neurons entrained well to photostimulation (>81% probability to elicit an action potential), but the probability decreased to 62.6% at 10 Hz and 35.9% at 20 Hz (n = 10 neurons; Figure 1, E and F). With these higher frequency stimuli, neurons initially entrained at 10–20 Hz for the first 4–5 s, but then fired action potentials only at 5–10 Hz, suggesting that with sustained rapid photostimulation, PPTvGlut2 neurons are unable to trigger action potentials consistently.
Figure 1.
Photostimulation of PPTvGluT2 neurons drives action potentials in a frequency-dependent fashion. (A) Schematic of the experimental approach with AAV (AAV8-EF1α-DIO-ChR2-mCherry) injection into the PPT of vGlut2-cre mice. (B) Infrared (left) and fluorescent (right) visualization of transduced ChR2-mCherry-expressing neurons in brain slices. Scale bar is 50 µm. (C) Biocytin-labeled neuron (green) within the PPT coexpressing ChR2-mCherry (red) after in vitro recording and labeling with streptavidin-conjugated Alexa Fluor AF-488. Scale bar in left panel is 200 µm; scale bar in the right panel is 50 µm. DR, dorsal raphe nucleus; MnR, median raphe nucleus; scp, superior cerebellar peduncle. (D) In vitro voltage clamp recording of a PPTvGluT2 neuron expressing ChR2 during photostimulation with a 250-ms-long single pulse (holding potential: −70 mV; in blue: light-pulse stimulation). (E) In vitro current clamp recordings of PPTvGlut2 neurons expressing ChR2 with application of 10 ms light pulses (blue tick marks) in 10 s trains at 0.5, 1, 5, 10, and 20 Hz frequencies (dotted line: 0 mV membrane potential). (F) Photostimulation increases action potentials in a frequency-dependent fashion (left), but the probability of triggering an action potential falls off at higher frequencies (right). Bars represent mean ± SEM for each group (n = 10 neurons per stimulation frequency), and individual data points represent the mean values for individual neurons (calculated from three 10 s trains per stimulation frequency).
Photoactivation of PPTvGlut2 neuron soma increases NREM to wake transitions
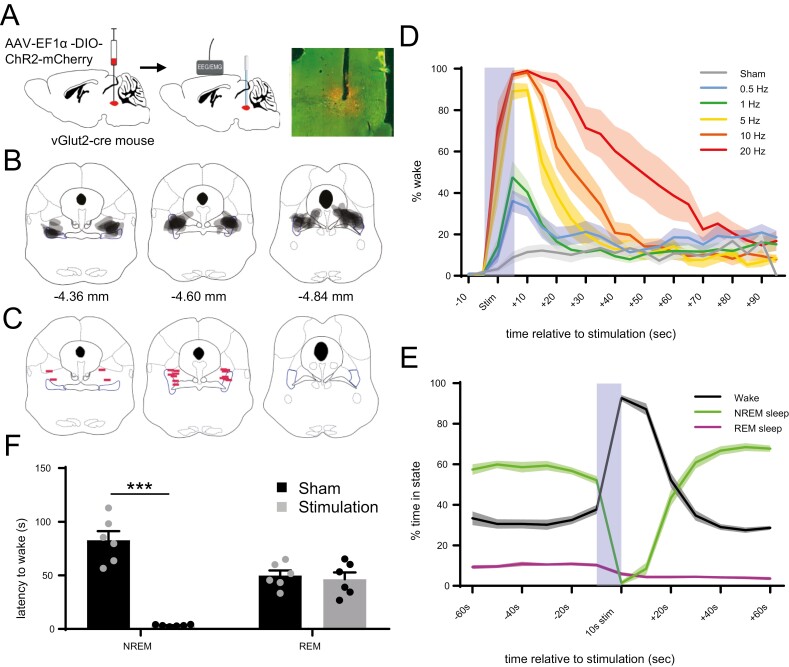
To assess whether activation of PPTvGlut2 neurons affects sleep–wake states, we expressed ChR2 in PPTvGluT2 neurons and implanted bilateral optical fibers above the PPT as well as EEG/EMG leads (Figure 2, A–C). We first applied photostimulation after 30 s of stable NREM sleep. Photostimulation at 0.5–20 Hz for 10 s increased wakefulness in a frequency-dependent manner, with an increased total amount of wakefulness (F(6, 9) = 37.25, p < 0.0001, Friedman ANOVA, followed by Dunn’s multiple comparison tests, n = 9 mice) and longer duration wake bouts with higher frequencies (F(6,9), 20.92, p = 0.0008; Figure 2, D, Supplementary Figures 2 and 6). Photostimulation did not increase wakefulness in a negative control group of mice (n = 5) expressing only mCherry in PPTvGlut2 neurons (Supplementary Figure 3).
Figure 2.
Photoactivation of PPTvGluT2 soma increases NREM to wake transitions. (A) Schematic of the experimental approach with bilateral injections of AAV (AAV8-EF1α-DIO-ChR2-mCherry) into the PPT of vGlut2-cre mice (left), implantation of optical fibers above the PPT and EEG/EMG leads affixed to the skull (middle), and sample AAV expression (in red) with choline acetyltransferase staining (in green) and optical fiber tract. (B) Gray-scale map of transduced neurons in all nine mice with anterior–posterior atlas coordinates [50]. (C) Location of optical fiber tips. (D) Photostimulation for 10 s (light blue bar) of PPTvGlut2 neurons during NREM sleep triggers rapid awakenings in a frequency-dependent fashion. During “Sham” stimulation, the laser remains off. Lines represent mean ± SEM for the group (n = 9 mice, each with 11 stimulation trials per frequency). (E) Photostimulation at random times during the light period increases wake, reduces NREM sleep, and slightly reduces REM sleep (5 Hz photostimulation for 10 s; 8 times per hour during the light period). Lines represent mean ± SEM for the group (n = 6 mice, with 83–96 stimulations per mouse). (F) Latency to wake from NREM sleep or REM sleep after 5 Hz photostimulation or sham stimulation. Bars represent mean + SEM for each group (n = 6 mice with 10 trials per condition). ***p < 0.001 using unpaired t-test.
To assess the effects of PPTvGlut2 neuron activation across sleep/wake states, we then photostimulated at random times (5 Hz for 10 s, 8 times per hour) during the light period. This also severely decreased the percent of time spent in NREM sleep (52.1 ± 2% NREM sleep in the 10 s before stimulation versus 8.5 ± 3% in the 10 s after stimulation; p < 0.0001, paired t-test, n = 6 mice), and it strongly increased wakefulness (37.7 ± 2.3% vs. 87.1 ± 2.9%, p < 0.0001; Figure 2, E). However, while photoactivation significantly reduced REM sleep (10.2 ± 0.8% vs. 4.4 ± 0.7%, p = 0.0061), in absolute terms REM sleep was affected much less than NREM sleep. We did not observe any obvious effects when stimulation occurred during wakefulness, but this was not analyzed in detail.
To further quantify the effects of photoactivation of PPTvGlut2 neurons on NREM sleep and REM sleep, we measured the latency to wakefulness when photostimulation (10 s at 5 Hz, or sham) randomly occurred during NREM sleep or REM sleep. With photostimulation during NREM sleep, the latency to wake was much shorter than after sham stimulation (3.3 ± 0.3 vs. 82.7 ± 3.3 s, p < 0.0003, paired t-test; Figure 2, F). In contrast, photoactivation during REM sleep did not alter the latency to wake (46.5 ± 6.3 s with photostimulation vs. 49.8 ± 4.7 s after sham stimulation; p < 0.59). These results indicate that photoactivation of PPTvGlut2 neurons rapidly triggers arousals from NREM sleep but has little effect on REM sleep.
Afferent and efferent axonal projections of PPTvGlut2 neurons
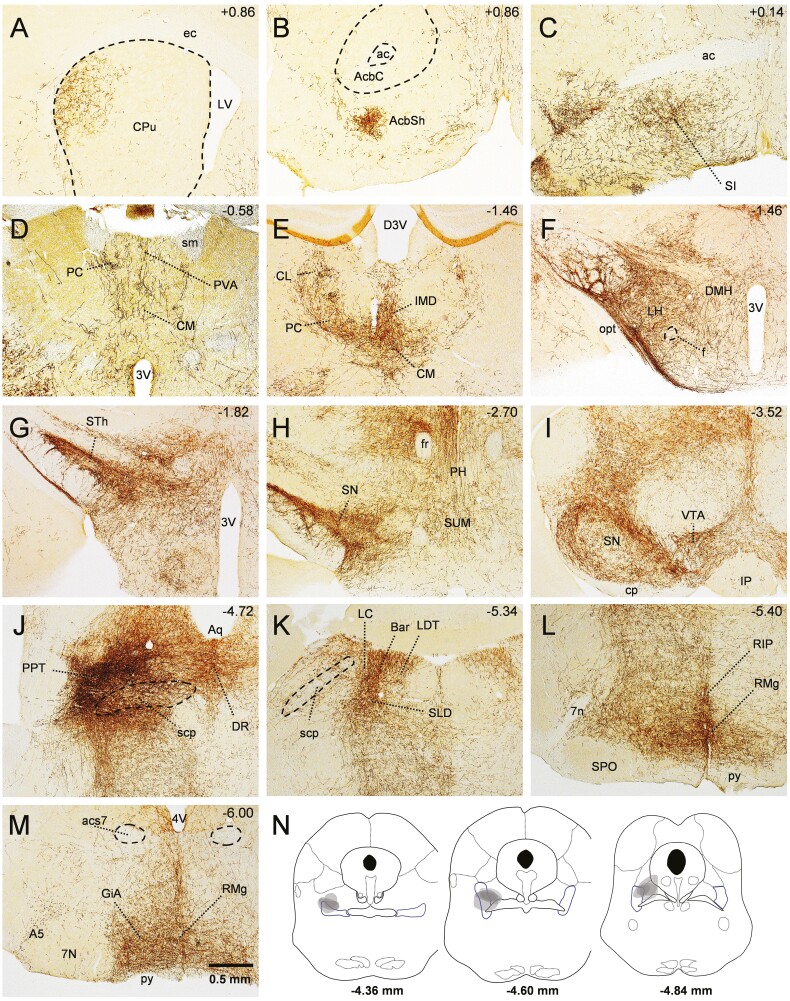
To map the axonal projections of PPTvGlut2 neurons, we injected the PPT unilaterally with AAV-ChR2-mCherry (n = 4 mice) and traced mCherry-labeled axons throughout the brain (Figure 3, Supplementary Table 1). We found that 70%–90% of PPTvGlut2 neuronal projections are ipsilateral. PPTvGlut2 neurons moderately innervated the lateral-dorsal part of the caudate–putamen (as previously described [21]) and ventral parts of the nucleus accumbens shell. Innervation was moderate to dense throughout the BF, with heavy innervation of the substantia innominata (Figure 3, A–C). Axon terminals were moderate to dense in several thalamic nuclei, particularly the central medial thalamic nucleus, paracentral nucleus, and the intermediodorsal nucleus (Figure 3, D and E). In the hypothalamus, PPTvGlut2 neurons densely innervated the LH and to a lesser extent the dorsomedial nucleus of the hypothalamus, supramammillary nucleus and tuberomammillary nucleus. In agreement with previous tracing studies, projections were especially dense in the subthalamic nucleus, SN, and ventral tegmental area (Figure 3, F–I) [45, 55, 56]. Interestingly, while the inferior colliculus was mostly devoid of fibers from PPTvGlut2 neurons, the contralateral superior colliculus showed a moderately dense innervation. In the pons, we observed very dense innervation of the locus coeruleus, Barrington’s nucleus, dorsal raphe nucleus as well as the sublaterodorsal nucleus. PPTvGlut2 neurons densely innervated the ventrolateral periaqueductal gray and lightly innervated the lateral and medial parts of the parabrachial nucleus (Figure 3, J and K). We also found dense to very dense innervation of ventral medullary areas such as the raphe magnus, and gigantocellular reticular nucleus (alpha part), the latter being an important relay for motor control (Figure 3, L and M) [57].
Figure 3.
Axonal projections of PPTvGluT2 neurons. (A–M) PPTvGluT2 neurons innervate a variety of brain regions, with heavy projections to the substantia innominate (SI), intralaminar nuclei of the thalamus, LH, subthalamic nucleus (STh), SN, Barrington’s nucleus (Bar), and raphe magnus (RMg). mCherry-expressing cell soma and axons are labeled in brown. Anterior–posterior atlas coordinates [50] are shown in upper right corner of each panel. 3V, 3rd ventricle; 4V, 4th ventricle; 7n, facial nerve; 7N, facial nucleus; A5, A5 noradrenaline cells; ac, anterior commissure; AcbC, nucleus accumbens core; AcbSh, nucleus accumbens shell; acs7, accessory facial nerve; Aq, aqueduct; CL, centrolateral thalamic nucleus; CM, central medial thalamic nucleus; cp, cerebral peduncle; CPu, caudate/putamen nucleus; DMH, dorsomedial hypothalamus; D3V, dorsal 3rd ventricle; DR, dorsal raphe nucleus; ec, external capsule; f, fornix; fr, fasciulus retroflexus; GiA, gigantocellular reticular nucleus alpha part; IMD, intermediodorsal thalamic nucleus; IP, interpeduncular nucleus; LC, locus coeruleus; LDT, laterodorsal tegmental nucleus; LV, lateral ventricle; opt, optic tract; PC, paracentral thalamic nucleus; PH, posterior hypothalamic nucleus; PVA, anterior part of paraventricular thalamic nucleus; py, pyramidal tract; RIP, raphe interpositus nucleus; RMg, raphe magnus nucleus; scp, superior cerebellar peduncle; SI, substantia innominata; sm, stria medullaris; SPO, superior paraolivary nucleus; STh, subthalamic nucleus; SUM, supramammillary nucleus; VTA, ventral tegmental area. See also Supplementary Table 1. (N) Map of injection sites for tracing study.
Activation of PPTvGlut2 axon terminals differentially increases NREM to wake transitions
To investigate the downstream targets through which PPTvGlut2 neurons trigger arousals from NREM sleep, we photostimulated PPTvGlut2 terminals in four brain regions implicated in sleep/wake control that are densely innervated by PPTvGlut2 neurons: BF (substantia innominata), LH, dorsal/intralaminar thalamus (TH), and SN. We bilaterally injected AAV-ChR2-mCherry into the PPT of vGluT2-cre mice and implanted optical fibers bilaterally above the different target regions (Supplementary Figure 4). We first photostimulated PPTvGlut2 terminal fields after at least 30 s of NREM sleep using 10 s trains of light pulses at 0.5–20 Hz. Photoactivation of axon terminals in the BF, LH, and SN consistently woke mice from NREM sleep in a frequency-dependent manner producing greater amounts of wake (BF: F(6,7) = 27.87, p < 0.0001; LH: F(6,6) = 21.81, p = 0.0006; SN: F(6,6) = 19.33, p = 0.0017; Figure 4, A–C, Supplementary Figures 6, A and 7). Photostimulation also produced longer wake bouts in a frequency-dependent manner in the BF and LH (BF: F(6,7) = 20.82, p = 0.0009; LH: F(6,6) = 14.83, p = 0.0111), but not in the SN (Supplementary Figure 6, B). In the TH, photoactivation did not increase the percent wake time or the duration of wake bouts in a stimulation frequency-dependent manner (Supplementary Figure 6, A and B).
Figure 4.
Photoactivation of distinct PPTvGluT2 axon terminals differentially triggers awakenings. (A–D) Photoactivation during NREM sleep of PPTvGluT2 axon terminals in the BF, LH, thalamus, and SN triggers rapid transitions to wake. Lines represent mean + SEM for the group (n = 6–7 mice per group, each with 11 stimulation trials per frequency). (E–H) 5 Hz Photoactivation at random times in the light period of PPTvGluT2 axon terminals increases wake and reduces NREM sleep but has little effect on REM sleep. Lines represent mean + SEM for each group (n = 6–7 mice in each group, with 84–96 stimulations per mouse).
To examine the effects of PPTvGlut2 projections across sleep/wake states, we photostimulated these terminal fields at random intervals during the light period (5 Hz, 10 s trains, 8 times per hour). Photostimulation of terminals in the BF, LH, TH, and SN significantly increased time in wake (BF: p = 0.0015, n = 7 mice; LH: p = 0.0008, n = 6 mice; TH: p = 0.0013; n = 7 mice; SN: p = 0.0005, n = 6 mice, paired t-tests) and decreased time in NREM sleep (BF: p = 0.0014; LH: p = 0.0011; TH: p = 0.0021; SN: p = 0.0008; Figure 4, E–H). As with PPTvGlut2 soma stimulation, the effects on REM sleep were minimal (BF: p = 0.1250; LH: p = 0.1432; TH: p = 0.0014; SN: p = 0.0171). Photostimulation also shortened the latency to wake from NREM sleep for all terminal fields (BF: p = 0.0033; LH: p = 0.0015; SN: p < 0.0032), except in the TH (TH: p = 0.3692, n = 7 mice, paired t-tests; Figure 5, A). Compared to sham stimulation, photostimulation did not alter the latency to wake from REM sleep for any terminal field (BF: p = 0.3441; LH: p = 0.2156; TH: p = 0.5293; SN: p = 0.4314; Figure 5, B). Photostimulation during wake also did not produce any obvious changes in wake behavior, though this was not analyzed in detail.
Figure 5.
Photostimulation of PPTvGluT2 soma and axon terminals quickly wakes mice from NREM sleep but not REM sleep. (A) Compared to sham stimulation, the latency to wake from NREM sleep is much shorter with 5 Hz photostimulation of PPTvGlut2 soma or axon terminals in the BF, LH, and SN, but not the thalamus. Bars represent mean + SEM for each group (n = 6–7 mice per group with 10 trials per condition). **p < 0.01; ***p < 0.001; ****p < 0.0001 using unpaired t-test. (B) Photostimulation does not shorten the latency to wake from REM sleep.
Photostimulation of specific PPTvGlut2 terminals produces distinct behaviors
To investigate whether photostimulation of PPTvGlut2 terminals drives specific behaviors, we analyzed videos of mouse behavior after photostimulation (10 Hz for 10 s applied after 30 s of NREM sleep). With sham stimulation, mice generally remained asleep, but during the 60 s after photostimulation of PPTvGlut2 soma or of PPTvGluT2 terminals in the BF and LH, mice spent more time in quiet wakefulness and other behaviors (collectively termed “wake behaviors”) (Kruskal–Wallis test p = 0.0052; Figure 6 and Supplementary Figure 8, A). Much of this time was spent in quiet wakefulness, but photostimulation of terminals in the BF and LH increased “active behaviors” including grooming, feeding and locomotion around the cage compared to stimulation of terminals in the SN (p = 0.0052; Figure 6 and Supplementary Figure 8, B). In addition, photoactivation of PPTvGluT2 terminals in the LH increased locomotion compared to soma sham stimulation (p = 0.0085; Figure 6, G). These findings suggest that activation of some PPTvGlut2 terminals promotes arousal with active behaviors, whereas others do not facilitate and may even inhibit movement even though the mice are awake.
Figure 6.
Photostimulation of specific PPTvGluT2 terminal fields produces distinct patterns of behavior. (A–F) Percent time spent in specific behaviors after 10 Hz photostimulation during NREM sleep. Panel A shows behaviors after sham stimulation of PPTvGlut2 soma for comparison. Behaviors were scored by analysis of 10 s epochs of video (n = 6–7 mice per group with 11 trials per mouse). (G) Percent time spent in locomotion during the 60 s after photostimulation. Stimulation of terminals in the LH increased locomotion compared to sham soma stimulation. *p < 0.05.
Role of PPTvGlut2 neurons in muscle tone
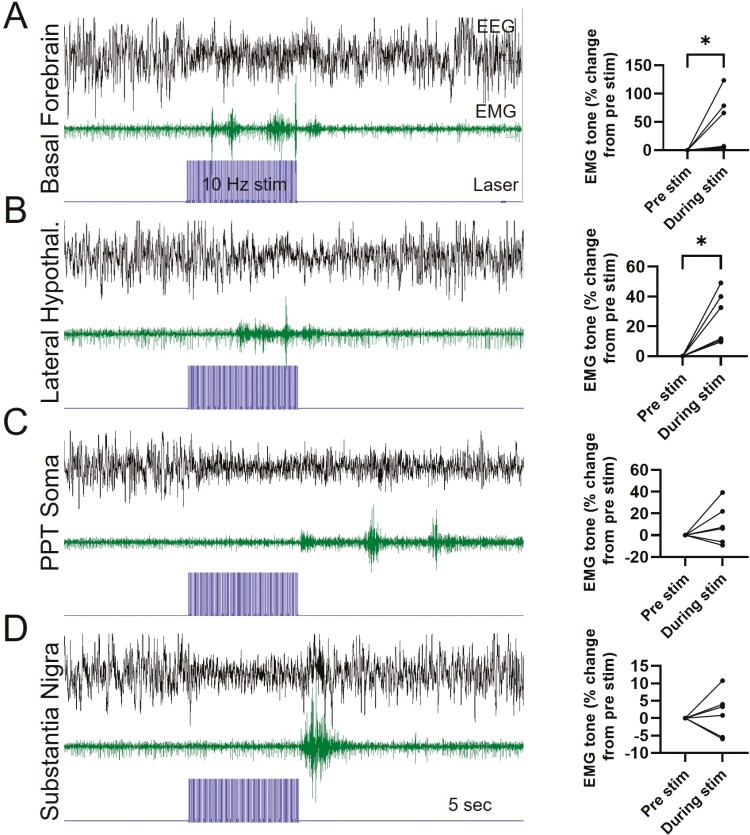
To further examine whether PPTvGlut2 neurons influence muscle tone, we assessed neck extensor EMG activity in relation to photoactivation of PPTvGlut2 neurons and axon terminals. Photoactivation of terminals in the BF and LH (10 Hz, 10-ms pulse width) during established NREM sleep produced a wake EEG (i.e. lower amplitude, faster rhythms), usually with increased EMG activity during the 10-s stimulation period (Figure 7). However, while activation of PPTvGlut2 soma and terminals in the SN produced EEG signs of wakefulness during the 10-s stimulation, the EMG signal showed little or no increase during the stimulation period, but at the end of the 10-s photoactivation, the mice usually had an abrupt burst of EMG activity. To quantify this phenomenon, we measured EMG signal amplitude during the stimulation period relative to the baseline period just prior. Photostimulation of PPTvGlut2 terminals in the BF and LH consistently increased EMG activity during the photostimulation period (BF: p = 0.0313; LH: p = 0.0313, Wilcoxon matched-pairs signed rank test, Figure 7), but overall, EMG tone did not increase with stimulation of PPTvGlut2 soma or axon terminals in the SN. These results further suggest that PPTvGlut2 projections to the SN do not facilitate muscle activity.
Figure 7.
Role of PPTvGluT2 neurons in muscle tone. (A–D) Representative traces of EEG and neck extensor EMG activity with 10 s (10 Hz) photostimulation (blue trace). Photoactivation of PPTvGluT2 soma or terminals in BF, LH, and SN consistently triggers wake (low amplitude, fast EEG), and EMG tone usually increases during photostimulation of PPTvGluT2 terminals in the BF and LH (BF: p = 0.03; LH: p = 0.03, Wilcoxon matched-pairs signed rank test). *p < 0.05.
Discussion
To investigate the roles of PPTvGlut2 neurons in driving arousal and other behaviors, we photostimulated PPTvGlut2 soma and axon terminal fields across sleep/wake states. We find that stimulation of PPTvGlut2 soma triggers frequency-dependent arousals from NREM sleep, but it has little effect on REM sleep. In addition, stimulation of PPTvGlut2 axon terminals in the BF and LH elicits sustained wake responses often accompanied by locomotion and other active behaviors, whereas stimulation of terminals in the TH and SN elicits brief periods of quiet wake. Moreover, muscle activity is low during stimulation of PPTvGlut2 soma and axon terminals in the SN despite a wake EEG pattern, suggesting that PPTvGlut2 projections to the SN do not lead to muscle activation and initiation of locomotion. Overall, these findings demonstrate the importance of PPTvGlut2 neurons in driving various aspects of arousal and show that specific PPTvGlut2 projections can drive distinct behaviors.
PPT stimulation and arousal
Many researchers consider the PPT a key element in the ascending arousal system (AAS), driving cortical activation and behavioral arousal either through thalamocortical projections [58, 59] or via the BF [60]. Lesions of the AAS in cats or monkeys can produce deep NREM sleep or coma [61, 62], while electrical stimulation produces strong wake responses [63]. Importantly, electrical stimulation of the PPT region can wake animals even from deep anesthesia [2], but it was unclear which PPT neurons drive cortical arousals. For many years, it was thought that the arousal response was mainly driven by cholinergic PPT neurons (PPTChat) with their widespread connections to thalamic targets [64]. However, our lab recently demonstrated that chemogenetic activation of PPTChat neurons produces lighter NREM sleep, but it does not increase wakefulness. Instead, chemoactivation of PPTvGlut2 neurons produced sustained wake for 5–7 h. Here, we now show that the wake response driven by PPTvGlut2 neurons critically depends on the stimulation frequency, with higher pulse frequencies eliciting quicker and longer-lasting arousals. Moreover, we show that photostimulation of specific terminal fields of PPTvGlut2 neurons differentially affects several aspects of arousals, such as the percent of wakefulness for the post stimulation period and duration of wake bouts. Importantly, these differences in arousal efficacy between axonal fields were not a reflection of axon terminal density in the specific regions (e.g. the density of PPTvGluT2 terminals in SN was actually higher than in BF or LH), nor a function of optical fiber distance to the stimulation area (we excluded animals from the analysis if fibers tips were >0.5 mm above the target area). We therefore suggest that the PPT mainly drives wakefulness via distinct, ascending, glutamatergic pathways to the BF, LH, and other regions.
PPTvGlut2 neurons trigger arousals from NREM sleep, but have little effect on REM sleep and wake
Photoactivation of PPTvGlut2 neurons and terminals consistently woke mice from NREM sleep, but in general, the effects on REM sleep were minimal, with occasional, slight reductions in REM sleep and no shortening of the latency to wake from REM sleep. Although photostimulation of PPTvGlut2 neurons and terminals did not substantially decrease REM sleep, we also did not observe an increase in REM sleep durations despite sizable projections of PPTvGlut2 neurons to the brainstem REM-on center in the sublaterodorsal nucleus (SLD). The PPT has historically been viewed as a crucial regulator of REM sleep, with studies showing projections of cholinergic PPT neurons to the SLD [65] and injections of cholinergic agonists into the PPT area leading to an REM-like state [66–68]. However, our data suggest that photoactivation of PPTvGlut2 neurons has no obvious effect on REM sleep. Possibly, the slight reductions in REM sleep that we observed with photoactivation of PPTvGlut2 neurons and terminals in the SN and TH occurred during pre-REM transitional periods, disrupting the initiation of REM sleep. These distinct effects on NREM and REM sleep were unexpected as arousals are elicited equally well from NREM sleep and REM sleep with photoactivation of orexin neurons in the LH [69, 70], noradrenergic neurons in the LC [71], and dopaminergic neurons in the VTA [72]. However, other wake-promoting systems also wake mice from NREM sleep but not REM sleep; for example, REM sleep is unaffected by photostimulation of wake-promoting GABAergic and neurotensin-producing neurons in the LH [73–75], GABAergic neurons in the bed nucleus of the stria terminalis [76], and cholinergic neurons in the BF [77–79]. Most likely, activity in the PPTvGlut2 neurons and these other populations is compatible with REM sleep, and these populations may even help regulate aspects of REM sleep. In support of this idea, unit recordings show that most PPTvGlut2 neurons are active during wake and REM sleep [52] as are cholinergic neurons in the BF and GABAergic neurons in the LH [73, 77].
Distinct behaviors from specific PPTvGlut2 projections
As the PPTvGlut2 neurons project extensively throughout the CNS and likely influence many behaviors, we assessed whether specific axonal projections drive distinct behaviors. Quiet wake was common after photoactivation of PPTvGlut2 soma and axon terminals, and these arousals were most robust with photostimulation of axon terminals in the BF and LH. These strong awakenings are consistent with prior research demonstrating that cells in these target regions can potently promote wake [69, 71, 79, 80] and are necessary for wake as large lesions of the BF or LH can produce coma [60, 81, 82]. Photoactivation of PPTvGlut2 terminals in the BF and LH also increased locomotion and grooming, possibly via BF cholinergic neurons [83] and LH neurons producing GABA or orexin/hypocretin [84, 85]. In contrast, photostimulation in the TH and SN produced only brief periods of quiet wake without much behavioral response, in line with prior work suggesting these regions are less essential for driving wake [60]. Importantly, these different behavioral responses with photostimulation of various PPTvGlut2 terminals suggests that either signals do not backpropagate to cell soma in the PPT or that different target areas are innervated by distinct populations of PPTvGlut2 neurons. Similarly, in a study of the wake-promoting properties of dopaminergic neurons in the VTA (VTADA), Eban-Rothschild et al. [72] also showed distinct effects on wake generation by photoactivation of different VTADA axon terminals. In their study, photoactivation of VTADA axon terminals in the NAc accounted for most of the wake-promoting effect of VTADA neuron soma, while photoactivation of axonal projections to the mPFC did not elicit wakefulness at all.
PPTvGlut2 stimulation and locomotor control
Whether PPT neurons initiate or modulate locomotion is debated [33–35, 45], yet this question is of crucial importance as the PPT is a target for DBS in some patients with PD. Several studies suggest that the PPT is part of the MLR, a brainstem region where electrical stimulation elicits locomotion in a stimulation intensity-dependent manner, even in decerebrate animals [5, 28, 29, 42]. Moreover, recent work by Lee, Roseberry, and colleagues suggests that photostimulation of PPTvGlut2 neurons may initiate locomotion [6, 7, 30], while PPTChat neurons can modulate the speed of locomotion [30]. However, both Lee et al. and Roseberry et al. used large volumes of AAV (500 and 300–500 nL, respectively), which transfected many neurons outside the PPT (e.g. in the lateral lemniscus, retrorubal nucleus, motor trigeminal nucleus, inferior colliculus, and cuneiform nucleus), making it difficult to determine if these effects are driven by PPTvGlut2 neurons or glutamatergic neurons in adjacent nuclei. Several studies highlight the locomotor-promoting roles of neurons medial to the PPT and in the cuneiform nucleus just dorsal to the PPT [42, 44, 45, 86, 87] while stimulation of the actual PPT can inhibit locomotion [40–42, 44, 45, 88]. With these divergent findings from animal research, it is not surprising that DBS in the PPT region of PD patients yields mixed results [36–38].
In our experiments, we microinjected 25 nL of AAV, and included mice for analysis only if >75% of transfected neurons were within the boundaries of the cholinergic PPT neurons to ensure that the observed results can be attributed to the PPT nucleus. We found that during photoactivation of PPTvGlut2 soma or their terminals in the SN, the EEG was consistent with wake, but mice did not move. Similarly, EMG tone remained low during photostimulation of these sites, and often increased just afterwards. On the other hand, EMG activity was often increased during photostimulation of PPTvGlut2 terminals in the BF and LH, and active behaviors occurred after photostimulation of these regions. This suggests that the axonal projections to the BF and LH can trigger active behaviors, but active behaviors are less common with stimulation of PPTvGlut2 cell soma perhaps because their projections to the SN may suppress movement. Of clinical relevance, we agree with several prior studies that PPT soma activation is insufficient to initiate locomotion [40, 42, 44, 45, 88], and so the PPT may not be a good target for DBS in PD patients [34, 35, 87].
Technical limitations
These experiments highlight the role of PPTvGlut2 neurons in promoting arousal, but some methodological issues should be kept in mind. First, photostimulation is nonphysiological and activates many PPTvGlut2 neurons simultaneously. The PPT is a heterogeneous nucleus with different afferent and efferent connections along the rostral–caudal axis as well as heterogeneity of neurons in terms of morphology and firing patterns during different brain states [52, 89–92]. It is therefore possible that certain subsets of PPTvGluT2 neurons are naturally active during specific behaviors. In other words, rhythmic and synchronous optogenetic activation may not reflect typical PPT activity during natural sleep/wake states or locomotion. However, if the PPT is considered for DBS in PD, then these results are relevant as DBS would similarly drive a variety of PPT neurons. Future work should clarify the roles of potential subsets of glutamatergic neurons in different parts of the PPT and adjacent regions.
In theory, photoactivation of axon terminals could trigger antidromic action potentials that could stimulate the cell soma and entire axonal tree, making it difficult to study specific projections. However, this seems unlikely in these experiments as stimulation of terminal fields produced different behavioral responses; activation of PPTvGlut2 axon terminals in the BF and LH often produced arousal and behaviors such as locomotion and grooming which were not seen with photoactivation in the TH or SN. In addition, PPTvGlut2 neurons may innervate local PPT GABAergic and/or cholinergic neurons which could explain some of the differences between photostimulation of PPTvGlut2 soma and their terminals. Another consideration is that subsets of PPTvGlut2 neurons may project to different targets. A recent study reported that intermingled glutamatergic neurons in the MLR (including the PPT) can be separated by their projection targets and specific behaviors they promote [93]. Future work could investigate this possibility with retrogradely transported dyes/beads injected into each axon terminal field to assess whether projections originate from the same PPTvGlut2 neurons or from different subpopulations.
Intense photostimulation could heat the brain or wake mice via nonspecific effects of the light. We think this problem is unlikely in our experiments as photostimulation did not trigger arousals in control mice expressing just mCherry in PPTvGluT2 neurons. In addition, we used brief (10 s), infrequent photostimulations minimizing the risk of light-induced heating of the brain. We also coated optical fibers and painted the head implants with black nail polish to avoid any light leakage that might influence sleep–wake states or other behaviors.
To limit our injections to the PPT, we used a very small volume of AAV (25 nL), and the resulting viral transduction efficiency of PPTvGluT2 neurons was moderate (~30% of all PPTvGlut2 neurons expressed mCherry). A higher transduction efficiency might produce stronger responses, but activation of ~30% of target neurons is typical of many optogenetic studies [94, 95] and is generally sufficient. Prior studies used much larger volumes (~500 nL) [6, 30], but these large injections also transduced many glutamatergic neurons in adjacent regions.
We used a number of different stimulation parameters to optogenetically drive PPTvGlut2 neurons or their terminal fields. With a range of frequencies (0.5–20 Hz), based on previous in vivo recordings [52], we first assessed the frequency dependence of soma stimulation on sleep–wake transitions and determined that a frequency of 5 Hz was ideal to investigate sleep–wake transitions in terminal fields while avoiding ceiling-effects at higher (e.g. 20 Hz) frequencies. However, to assess terminal field specific wake behaviors and EMG activation patterns, we used higher frequency (10 Hz) photostimulations to produce more sustained wakefulness, allowing enough time to assess behavioral responses.
Conclusions and Future Directions
We find that photoactivation of PPTvGlut2 neurons promotes arousals from NREM sleep but has little effect on REM sleep. Interestingly, the resulting behaviors after awakening from NREM sleep depend on which PPTvGluT2 axons are stimulated as photostimulation of terminals in the BF and LH often triggers behaviors such as locomotion and grooming whereas stimulation of terminals in the SN does not. This supports the many functions ascribed to the PPT over the last 30 years, ranging from control of cognition, reward behavior, motor functions, and sleep/wake behavior. Future research should assess the effects of loss of PPTvGlut2 function on these behaviors and should also examine descending projections to the brainstem and spinal cord which may impact locomotion. This could require a detailed investigation of neurochemical subtypes of PPTvGlut2 neurons and their potentially different effects on behavior. Defining the role of specific PPTvGluT2 populations will lead to a better understanding of the mechanisms regulating arousal and should ultimately help identify novel targets for the treatment of sleep/wake disorders including PD.
Supplementary Material
Acknowledgments
We thank Dr Carrie Mahoney for useful discussions and Megan Steele, Adam Joyal, and Thanh Trinh for technical support.
Contributor Information
Daniel Kroeger, Department of Neurology, Beth Israel Deaconess Medical Center and Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA; Department of Anatomy, Physiology, and Pharmacology, Auburn University, Auburn, AL, USA.
Jack Thundercliffe, Department of Neurology, Beth Israel Deaconess Medical Center and Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA.
Alex Phung, Department of Neurology, Beth Israel Deaconess Medical Center and Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA.
Roberto De Luca, Department of Neurology, Beth Israel Deaconess Medical Center and Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA.
Carolyn Geraci, Department of Neurology, Beth Israel Deaconess Medical Center and Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA.
Samuel Bragg, Department of Neurology, Beth Israel Deaconess Medical Center and Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA.
Kayleen J McCafferty, Department of Anatomy, Physiology, and Pharmacology, Auburn University, Auburn, AL, USA.
Sathyajit S Bandaru, Department of Neurology, Beth Israel Deaconess Medical Center and Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA.
Elda Arrigoni, Department of Neurology, Beth Israel Deaconess Medical Center and Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA.
Thomas E Scammell, Department of Neurology, Beth Israel Deaconess Medical Center and Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA.
Funding
This study was funded by NIH grants HL095491 and HL149630 (T.E.S.), as well as T32 HL007901 (D.K).
Authors’ Contributions
Conceptualization: D.K. and T.E.S.; experiments: D.K., J.T., A.P., R.D.L., C.G., S.B., and S.S.B.; manuscript: D.K., R.D.L., E.A., and T.E.S.
Disclosure Statement
None declared.
References
- 1. Steriade M, et al. Network modulation of a slow intrinsic oscillation of cat thalamocortical neurons implicated in sleep delta waves: cortically induced synchronization and brainstem cholinergic suppression. J Neurosci. 1991;11(10):3200–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seigneur J, et al. Cholinergic action on cortical glial cells in vivo. Cereb Cortex. 2006;16(5):655–668. [DOI] [PubMed] [Google Scholar]
- 3. Mena-Segovia J, et al. Cholinergic modulation of midbrain dopaminergic systems. Brain Res Rev. 2008;58(2):265–271. [DOI] [PubMed] [Google Scholar]
- 4. Kroeger D, et al. Cholinergic, glutamatergic, and GABAergic neurons of the pedunculopontine tegmental nucleus have distinct effects on sleep/wake behavior in mice. J Neurosci. 2017;37(5):1352–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garcia-Rill E, et al. Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res Bull. 1987;18(6):731–738. [DOI] [PubMed] [Google Scholar]
- 6. Caggiano V, et al. Midbrain circuits that set locomotor speed and gait selection. Nature. 2018;553(7689):455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee AM, et al. Identification of a brainstem circuit regulating visual cortical state in parallel with locomotion. Neuron. 2014;83(2):455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson JA, et al. Activity in mouse pedunculopontine tegmental nucleus reflects action and outcome in a decision-making task. J Neurophysiol. 2013;110(12):2817–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Syed A, et al. Pedunculopontine tegmental nucleus lesions impair probabilistic reversal learning by reducing sensitivity to positive reward feedback. Neurobiol Learn Mem. 2016;131:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bortolanza M, et al. Functional disconnection of the substantia nigra pars compacta from the pedunculopontine nucleus impairs learning of a conditioned avoidance task. Neurobiol Learn Mem. 2010;94(2):229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kobayashi Y, et al. Reward prediction error computation in the pedunculopontine tegmental nucleus neurons. Ann N Y Acad Sci. 2007;1104:310–323. [DOI] [PubMed] [Google Scholar]
- 12. Norton ABW, et al. Independent neural coding of reward and movement by pedunculopontine tegmental nucleus neurons in freely navigating rats. Eur J Neurosci. Published online 2011;33(10):1885–1896. doi: 10.1111/j.1460-9568.2011.07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoo JH, et al. Activation of pedunculopontine glutamate neurons is reinforcing. J Neurosci. 2017;37(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woolf NJ, et al. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986;16(5):603–637. [DOI] [PubMed] [Google Scholar]
- 15. Scarnati E, et al. The organization of nucleus tegmenti pedunculopontinus neurons projecting to basal ganglia and thalamus: a retrograde fluorescent double labeling study in the rat. Neurosci Lett. 1987;79(1–2):11–16. [DOI] [PubMed] [Google Scholar]
- 16. Dautan D, et al. Segregated cholinergic transmission modulates dopamine neurons integrated in distinct functional circuits. Nat Neurosci. 2016;19:10251–11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steriade M, et al. Projections of cholinergic and non-cholinergic neurons of the brainstem core to relay and associational thalamic nuclei in the cat and macaque monkey. Neuroscience. 1988;25(1):47–67. [DOI] [PubMed] [Google Scholar]
- 18. Hallanger AE, et al. Ascending projections from the pedunculopontine tegmental nucleus and the adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;274(4):483–515. [DOI] [PubMed] [Google Scholar]
- 19. Saper CB, et al. Projections of the pedunculopontine tegmental nucleus in the rat: evidence for additional extrapyramidal circuitry. Brain Res. 1982;252(2):367–372. [DOI] [PubMed] [Google Scholar]
- 20. Jackson A, et al. Nucleus tegmenti pedunculopontinus: efferent connections with special reference to the basal ganglia, studied in the rat by anterograde and retrograde transport of horseradish peroxidase. Neuroscience. 1983;10(3):725–765. [DOI] [PubMed] [Google Scholar]
- 21. Assous M, et al. Pedunculopontine glutamatergic neurons provide a novel source of feedforward inhibition in the striatum by selectively targeting interneurons. J Neurosci. 2019;39(24):4727–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skinner R, et al. Locomotor projections from the pedunculopontine nucleus to the medioventral medulla. Neuroreport. 1990;1(3 & 4):207–210. [DOI] [PubMed] [Google Scholar]
- 23. VanderHorst VGJM, et al. The organization of the brainstem and spinal cord of the mouse: relationships between monoaminergic, cholinergic, and spinal projection systems. J Chem Neuroanat. 2006;31(1):2–36. [DOI] [PubMed] [Google Scholar]
- 24. Armstrong DM, et al. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983;216(1):53–68. [DOI] [PubMed] [Google Scholar]
- 25. Mena-Segovia J, et al. GABAergic neuron distribution in the pedunculopontine nucleus defines functional subterritories. J Comp Neurol. 2009;515(4):397–408. [DOI] [PubMed] [Google Scholar]
- 26. Wang HL, et al. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29(2):340–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garcia-Rill E, et al. Chemical activation of the mesencephalic locomotor region. Brain Res. 1985;330(1):43–54. [DOI] [PubMed] [Google Scholar]
- 28. Garcia-Rill E, et al. The mesencephalic locomotor region. I. Activation of a medullary projection site. Brain Res. 1987;411(1):1–12. [DOI] [PubMed] [Google Scholar]
- 29. Shik ML, et al. Control of walking and running by means of electrical stimulation of the mesencephalon. Electroencephalogr Clin Neurophysiol. 1969;26(5):549–556. [PubMed] [Google Scholar]
- 30. Roseberry TK, et al. Cell-type-specific control of brainstem locomotor circuits by basal ganglia. Cell. 2016;164(3):526–537. doi: 10.1016/j.cell.2015.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hirsch EC, et al. Proc Natl Acad Sci USA. 1987;84:5976–5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mazzone P, et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson’s disease. Neuroreport. 2005;16(17):1877–1881. [DOI] [PubMed] [Google Scholar]
- 33. Garcia-Rill E, et al. Focus on the pedunculopontine nucleus. Consensus review from the May 2018 brainstem society meeting in Washington, DC, USA. Clin Neurophysiol. 2019;130(6):925–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Albin RL, et al. Targeting the pedunculopontine nucleus in Parkinson’s disease: time to go back to the drawing board. Mov Disord. 2018;33(12):1871–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang SJ, et al. Dissecting brainstem locomotor circuits: converging evidence for cuneiform nucleus stimulation. Front Syst Neurosci. 2020;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tubert C, et al. The pedunclopontine nucleus and Parkinson’s disease. Neurobiol Dis. 2019;128:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferraye MU, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson’s disease. Brain. 2010;133(1):205–214. [DOI] [PubMed] [Google Scholar]
- 38. Thevathasan W, et al. Pedunculopontine nucleus deep brain stimulation in Parkinson’s disease: a clinical review. Mov Disord. 2018;33(1):10–20. [DOI] [PubMed] [Google Scholar]
- 39. Goetz L, et al. Deep brain stimulation of the pedunculopontine nucleus area in Parkinson disease: MRI-based anatomoclinical correlations and optimal target. Clin Neurosurg. 2019;84(2):506–518. [DOI] [PubMed] [Google Scholar]
- 40. Kelland MD, et al. Pedunculopontine tegmental nucleus-induced inhibition of muscle activity in the rat. Behav Brain Res. 1989;34(3):213–234. [DOI] [PubMed] [Google Scholar]
- 41. Gut NK, et al. Deep brain stimulation of different pedunculopontine targets in a novel rodent model of parkinsonism. J Neurosci. 2015;35(12):4792–4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takakusaki K, et al. Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems. J Neural Transm. 2016;123(7):695–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takakusaki K. Functional neuroanatomy for posture and gait control. J Mov Disord. 2017;10(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Josset N, et al. Distinct contributions of mesencephalic locomotor region nuclei to locomotor control in the freely behaving mouse. Curr Biol. 2018;28(6):884–901.e3. [DOI] [PubMed] [Google Scholar]
- 45. Dautan D, et al. Cholinergic midbrain afferents modulate striatal circuits and shape encoding of action strategies. Nat Commun. 2020;11(1):1739. doi: 10.1038/s41467-020-15514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Opris I, et al. Activation of brainstem neurons during mesencephalic locomotor region-evoked locomotion in the cat. Front Syst Neurosci. 2019;13:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vong L, et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71(1):142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Agostinelli LJ, et al. Basal forebrain subcortical projections. Brain Struct Funct. 2019;224(3):1097–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kroeger D, et al. Ventrolateral periaqueductal gray mediates rapid eye movement sleep regulation by melanin-concentrating hormone neurons. Neuroscience. 2019;406:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Franklin K, et al. The Mouse Brain in Stereotaxic Coordinates. 3rd ed. Amsterdam/London: Academic Press; 2008. [Google Scholar]
- 51. Mochizuki T, et al. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24(28):6291–6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boucetta S, et al. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J Neurosci. 2014;34(13):4708–4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Anaclet C, et al. Genetic activation, inactivation, and deletion reveal a limited and nuanced role for somatostatin-containing basal forebrain neurons in behavioral state control. J Neurosci. 2018;38(22):5168–5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kroeger D, et al. Galanin neurons in the ventrolateral preoptic area promote sleep and heat loss in mice. Nat Commun. 2018;9(1):4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lavoie B, et al. Pedunculopontine nucleus in the squirrel monkey: cholinergic and glutamatergic projections to the substantia nigra. J Comp Neurol. 1994;344(2):232–241. [DOI] [PubMed] [Google Scholar]
- 56. Futami T, et al. Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci Res. 1995;21(4):331–342. [DOI] [PubMed] [Google Scholar]
- 57. Karlsson K, et al. Active medullary control of atonia in week-old rats. Neuroscience. 2005;130(1):275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Magoun HW. An ascending reticular activating system in the brain stem. AMA Arch Neurol Psychiatry. 1952;2(67):167–171. [DOI] [PubMed] [Google Scholar]
- 59. Steriade M. Arousal: revisiting the reticular activating system. Science. 1996;272(5259):225–226. [DOI] [PubMed] [Google Scholar]
- 60. Fuller P, et al. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519(5):933–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lindsley DB, et al. Behavioral and EEG changes following chronic brain stem lesions in the cat. Electroencephalogr Clin Neurophysiol. 1950;2(1–4):483–498. [DOI] [PubMed] [Google Scholar]
- 62. French JD, et al. Effects of chronic lesions in central cephalic brain stem of monkeys. AMA Arch Neurol Psychiatry. 1952;5(68):591–604. [DOI] [PubMed] [Google Scholar]
- 63. Moruzzi G, et al. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1(1–4):455–473. [PubMed] [Google Scholar]
- 64. Steriade M, et al. Cholinergic and noradrenergic modulation of the slow (approximately 0.3 Hz) oscillation in neocortical cells. J Neurophysiol. 1993;70(4):1385–1400. [DOI] [PubMed] [Google Scholar]
- 65. Quattrochi JJ, et al. Mapping neuronal inputs to REM sleep induction sites with carbachol-fluorescent microspheres. Science. 1989;os-2(57):341–342. [DOI] [PubMed] [Google Scholar]
- 66. Baghdoyan HA, et al. Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res. 1984;306(1–2):39–52. [DOI] [PubMed] [Google Scholar]
- 67. Shiromani PJ, et al. Pontine neuronal response to local cholinergic infusion: relation to REM sleep. Brain Res. 1986;386(1–2):20–31. [DOI] [PubMed] [Google Scholar]
- 68. Kubin L. Carbachol models of REM sleep: recent developments and new directions. Arch Ital Biol. 2001;139:147–168. [PubMed] [Google Scholar]
- 69. Adamantidis AR, et al. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Carter ME, et al. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J Neurosci. 2009;29(35):10939–10949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Carter ME, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13(12):1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Eban-Rothschild A, et al. VTA dopaminergic neurons regulate ethologically relevant sleep–wake behaviors. Nat Neurosci. 2016;19:1–14. doi: 10.1038/nn.4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Venner A, et al. An inhibitory lateral hypothalamic-preoptic circuit mediates rapid arousals from sleep. Curr Biol. 2019;29(24):4155–4168.e5. [DOI] [PubMed] [Google Scholar]
- 74. Herrera CG, et al. Hypothalamic feedforward inhibition of thalamocortical network controls arousal and consciousness. Nat Neurosci. 2016;19(2):290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Naganuma F, et al. Lateral hypothalamic neurotensin neurons promote arousal and hyperthermia. PLoS Biol. 2019;17(3):1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kodani S, et al. Excitation of GABAergic neurons in the bed nucleus of the stria terminalis triggers immediate transition from non-rapid eye movement sleep to wakefulness in mice. J Neurosci. 2017;37(30):7164–7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Xu M, et al. Basal forebrain circuit for sleep-wake control. Nat Neurosci. 2015;18(11):1641–1647. doi: 10.1038/nn.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Han Y, et al. Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr Biol. 2014;24(6):693–698. [DOI] [PubMed] [Google Scholar]
- 79. Ozen Irmak S, et al. Basal forebrain cholinergic modulation of sleep transitions. Sleep. 2014;37(12):1941–1951. doi: 10.5665/sleep.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Anaclet C, et al. Basal forebrain control of wakefulness and cortical rhythms. Nat Commun. 2015;6:8744. doi: 10.1038/ncomms9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nauta WJH. Hypothalamic regulation of sleep in rats. An experimental study. J Neurophysiol. 1946;9:285–316. [DOI] [PubMed] [Google Scholar]
- 82. Gerashchenko D, et al. Effects of hypocretin-saporin injections into the medial septum on sleep and hippocampal theta. Brain Res. 2001;913(1):106–115. [DOI] [PubMed] [Google Scholar]
- 83. Harrison TC, et al. Calcium imaging of basal forebrain activity during innate and learned behaviors. Front Neural Circuits. 2016;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Karnani MM, et al. Role of spontaneous and sensory orexin network dynamics in rapid locomotion initiation. Prog Neurobiol. 2020;187:101771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kosse C, et al. Orexin-driven GAD65 network of the lateral hypothalamus sets physical activity in mice. Proc Natl Acad Sci USA. 2017;114(17):4525–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sherman D, et al. Anatomical location of the mesencephalic locomotor region and its possible role in locomotion, posture, cataplexy, and parkinsonism. Front Neurol. 2015;6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fougère M, et al. Optogenetic stimulation of glutamatergic neurons in the cuneiform nucleus controls locomotion in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA. 2021;118(43):e2110934118. doi: 10.1073/pnas.2110934118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lai YY, et al. Muscle tone suppression and stepping produced by stimulation of midbrain and rostral pontine reticular formation. J Neurosci. 1990;10(8):2727–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Roš H, et al. Distinct types of non-cholinergic pedunculopontine neurons are differentially modulated during global brain states. Neuroscience. 2010;170(1):78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Martinez-Gonzalez C, et al. Topographical organization of the pedunculopontine nucleus. Front Neuroanat. 2011;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Boucetta S, et al. Activity profiles of cholinergic and intermingled GABAergic and putative glutamatergic neurons in the pontomesencephalic tegmentum of urethane-anesthetized rats. J Neurosci. 2009;29(14):4664–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Petzold A, et al. Decoding brain state transitions in the pedunculopontine nucleus: cooperative phasic and tonic mechanisms. Front Neural Circuits. 2015;9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ferreira-Pinto MJ, et al. Functional diversity for body actions in the mesencephalic locomotor region. Cell. 2021;184(17):4564–4578.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mahoney CE, et al. GABAergic neurons of the central amygdala promote cataplexy. J Neurosci. 2017;37(15):3995–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Vetrivelan R, et al. Melanin-concentrating hormone neurons specifically promote rapid eye movement sleep in mice. Neuroscience. 2016;336:102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.