Abstract
Although highly susceptible to orogastric candidiasis, T-cell receptor δ- and α-chain knockout mice, deficient in γδ and αβ T cells, respectively, were found to be resistant to disseminated candidiasis of endogenous origin and to acute systemic candidiasis (resulting from intravenous injection).
Several immunosuppressive and immunodeficiency-related conditions predispose humans to candidiasis (12, 13, 15). In general, animal and clinical studies suggest that T cells are important for resistance to mucosal candidiasis and to systemic candidiasis of endogenous origin. For instance, CD4 deficiencies in humans and T-cell defects in animal models, such as those found in SCID, athymic, major histocompatibility complex class I knockout (KO) mice and γδ- or CD4+-T-cell-depleted mice, have all been associated with increased susceptibility to mucosal candidiasis (1, 3, 5, 6, 8, 11). Some of these T-cell deficiencies, however, have been associated with hyperresistance to experimentally induced systemic candidiasis (1, 6, 8, 14), thus suggesting that T cells inhibit clearance and may even contribute to the pathology of acute systemic candidiasis. To better understand the roles of different T-cell subsets in resistance to orogastric and systemic candidiasis, mice with genetically engineered deficiencies in αβ or γδ T cells (i.e., T-cell receptor [TCR] α- and δ-chain KO mice, respectively) were used in this study. We now report that mice without αβ or γδ T cells are susceptible to orogastric candidiasis; however, neither T-cell subset appears to be required for murine resistance to acute systemic candidiasis or to systemic candidiasis of endogenous (alimentary tract) origin.
TCR δ and α-chain KO mice (Jackson Laboratories, Bar Harbor, Maine) and the corresponding C57BL/6 × L129 controls were derived into the germfree state at the University of Wisconsin Gnotobiote Laboratory in Madison (1), and their gnotobiotic and T-cell-deficient statuses were tested as previously described (10, 19). Germfree mice were orally swabbed with a suspension of 108 Candida albicans CFU/ml. Colonization of the alimentary tract by the fungus was confirmed 3 days later by culturing fecal contents on Sabouraud dextrose agar. At several time points following colonization with C. albicans, the mice were euthanized and their orogastric tissues—i.e., tongue, hard palate, esophagus, and stomach tissues—were fixed in 10% buffered formalin and stained with hematoxylin-eosin and Gomori's methenamine silver for histologic analysis (2). These mice were also tested for extraintestinal dissemination of C. albicans by culturing spleen, liver, kidney, and brain homogenates on Sabouraud dextrose agar. The susceptibility of T-cell-deficient and control mice to systemic candidiasis was assessed by determining the fungal burdens in spleen, liver, kidney, and brain tissues of mice inoculated intravenously with 104 CFU of C. albicans.
Between 10 and 30 days after colonization with C. albicans, both TCR α- and δ-chain KO mice had similar grades of mucosal infection (histopathology scores of 2.5, as defined by the presence of budding yeast cells and hyphal penetration) but different incidences (100 and 50%, respectively) of orogastric infection (Table 1; Fig. 1). In contrast to beige-athymic mice, which are deficient in phagocytic cells and thymus-educated T-cell and natural killer cell function (5), adult TCR α-chain KO mice, deficient in αβ T cells, and TCR δ-chain KO mice, deficient in γδ T cells, did not die when their gastrointestinal tracts were chronically colonized with C. albicans (30-day study). However, chronic colonization proved lethal for infant TCR α-chain KO mice. Ten 3-week-old TCR α-chain KO mice, born to and raised by two different C. albicans-monoassociated TCR α-chain KO mothers, became emaciated and died at about 21 days after birth. Their esophagi were occluded by C. albicans and squamous debris (Fig. 2).
TABLE 1.
TCR α- and δ-chain KO mice are susceptible to orogastric candidiasis after oral colonization with a pure culture of C. albicans
| Mouse strain | Histopathology scorea | Incidenceb |
|---|---|---|
| TCR δ KO | 2.5 | 50% (12/6) |
| TCR α KO | 2.5 | 100% (17/17) |
| Sev 129/C57B1/6 | 0 | 0% (0/12) |
C. albicans infections at the mucosal surfaces of infected mice were scored 10 to 30 days after colonization as previously described (2) from 0 (no lesions were detected) to 4 (confluent fungal invasion of the mucosal surface was observed). All of the mice that were scored as “infected” showed yeast and hyphal penetration in one or more tissues. If infected in more than one tissue, the individual organ scores were averaged. The mucosal tissue evaluated consisted of tongue, hard palate, esophagus, and stomach.
Numbers in parentheses are number of mice colonized/number infected.
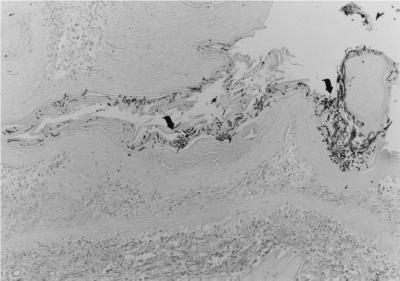
FIG. 1.
Grade 3 infection of stomach by C. albicans in an 11-week-old TCR δ-chain KO mouse that was colonized for 10 days. Arrows indicate hyphae. All adult mice used for this study were 8 to 15 weeks of age. Germfree mice were orally colonized by swabbing their mouths with 108 CFU of C. albicans as previously described (18). At least two or three longitudinal sections of tissues were stained and examined for each mouse. Gomori's methenamine silver stain; magnification, ×80.
FIG. 2.
Occlusion of esophagus by C. albicans in a 3-week-old TCR α-chain KO mouse that was colonized at birth. Gomori's methenamine silver stain; magnification, ×80.
Unfortunately, TCR δ-chain KO mice did not breed well under germfree or C. albicans-monoassociated conditions, so the role of γδ T cells in conferring protection to young animals is unclear. Nevertheless, it is interesting that two pups that were born to a C. albicans-colonized TCR δ-chain KO mother survived to adulthood and showed no indication of orogastric lesions when they were sacrificed at 5 months of age.
In summary, adult αβ- or γδ-T-cell-deficient mice can survive oral colonization with C. albicans; however, αβ T cells appear to be essential for protecting infant mice from the lethal effects of a natural C. albicans colonization obtained by contact with colonized mothers. Immunocompetent C57BL/6 × L129 controls showed no histologic evidence of orogastric candidiasis at the time points (30 days or at birth) examined in this study. In accordance with other studies (1–3, 5, 10–12, 14), the hyphae and budding yeast cells present on mucosal surfaces of either TCR-KO strain of mouse colonized by C. albicans suggest that both αβ and γδ T cells participate in host resistance to mucosal candidiasis.
No disseminated candidiasis was detected in the spleens, livers, kidneys, or brains of orally colonized immunocompetent or TCR-deficient mice, regardless of the grade of mucosal infection sustained. The latter data suggest that αβ and γδ T cells are not required for protection of mice from systemic candidiasis of endogenous origin. Paradoxically, the absence of CD8+ T cells or the lack of an major histocompatibility complex class I classical or nonclassical antigen-presenting pathway does lead to dissemination from the mucosal surface to internal organs (3). Other immune system defects associated with dissemination include concomitant defects in phagocytes, natural killer cells, and CD4+ T cells (i.e., bg/bg nu/nu mice [5]), depletion of phagocytes in SCID mice (9), and genetic abrogation of intracellular adhesion molecules which impair leukocyte infiltration and activation (7). T- and B-cell-deficient SCID mice with intact phagocytic function, although somewhat more susceptible to gastric candidiasis than immunocompetent controls, manifest resistance to disseminated candidiasis of endogenous origin and to acute systemic candidiasis (2, 14). Perhaps the disruption of the balance between T-cell subsets and phagocytic cell deficiencies lead to diminished resistance to disseminated candidiasis of endogenous origin.
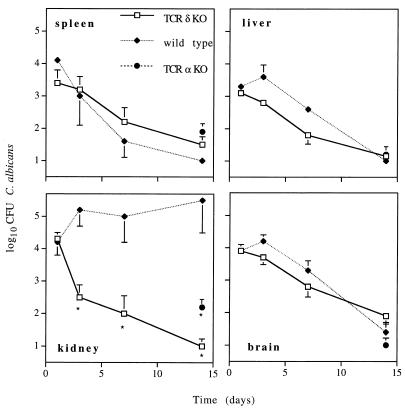
Both TCR δ- and α-chain KO mice eliminated C. albicans from the spleen, liver, kidney, and brain by day 14 after intravenous challenge with 104 C. albicans CFU, which is in sharp contrast with the 105 C. albicans CFU per g of dry tissue we observed in intravenously challenged immunocompetent controls (Fig. 3). The latter results suggest that resistance to acute systemic candidiasis is independent of αβ and γδ T cells. Our data are consistent with previous studies with patients and animal models which have noted that defects in innate, T-cell-independent immune responses, such as those associated with chronic granulomatous disease or myeloperoxidase deficiencies, predispose humans to systemic, but not mucosal, candidiasis (13, 15, 16). Also, in view of the resistance of T-cell-deficient mice (references 3, 6, and 8 and this study) and in light of the increased susceptibility of B-cell-deficient (18), phagocyte-deficient (4, 5, 9), and thymically reconstituted nude mice (17) to systemic candidiasis, it is likely that B cells and/or phagocytes, rather than αβ and γδ T cells, play an important and dominant role in host resistance to acute systemic candidiasis. In fact, our data suggest that T cells may contribute to immunopathology in this particular presentation of the disease, because T-cell-competent mice were more susceptible to renal candidiasis than T-cell-deficient (TCR α- or δ-chain KO) mice (Fig. 3).
FIG. 3.
Susceptibility of genetically engineered αβ- and γδ-T-cell-deficient mice to acute systemic candidiasis. Immunocompetent (wild-type) and TCR α-chain KO (TCR α-KO) or δ-chain KO (TCR δ-KO) mice were inoculated intravenously with 104 CFU of C. albicans. C. albicans-inoculated mice were sacrificed at days 1, 3, 7 (wild type and TCR δ-KO), and 14 (wild type, TCR δ-KO, and TCR α-KO) days after infection, and the numbers of CFU in the liver, spleen, kidney, and brain were determined. Data are expressed as mean log10 CFU of C. albicans per gram (dry weight) of tissue ± standard error of the mean and represent three separate experiments with a minimum of six mice per time interval. ∗, P < 0.05 as determined by Student's t-test. Organs from which no viable C. albicans organisms were recovered were assigned a value of 1.
Our experiments with these murine models support clinical observations that T-cell deficiencies are associated with susceptibility to orogastric candidiasis. Novel to this study was the observation that both T-cell subsets independently participated in host immunity to orogastric candidiasis while their presence during acute systemic challenge exacerbated murine susceptibility to renal candidiasis.
Acknowledgments
We thank Donna Brackett for secretarial preparation of the manuscript and R. D. Wagner for help in collecting some of the tissues for histological analysis. We also thank JoAnne Croft and the staff of the Gnotobiote Laboratory at the University of Wisconsin—Madison for maintaining the animals used in this study.
REFERENCES
- 1.Balish E, Balish M J, Salkowski C A, Lee K W, Bartizal K F. Colonization of congenitally athymic, gnotobiotic mice by Candida albicans. Appl Environ Microbiol. 1984;47:647–652. doi: 10.1128/aem.47.4.647-652.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balish E, Jensen J, Warner T, Brekke J, Leonard H. Mucosal and disseminated candidiasis in gnotobiotic SCID mice. J Med Vet Mycol. 1993;31:143–154. doi: 10.1080/02681219380000161. [DOI] [PubMed] [Google Scholar]
- 3.Balish E, Vazquez-Torres F A, Jones-Carson J, Wagner R D, Warner T. Importance of β2-microglobulin in murine resistance to mucosal and systemic candidiasis. Infect Immun. 1996;64:5092–5097. doi: 10.1128/iai.64.12.5092-5097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantorna M T, Balish E. Mucosal and systemic candidiasis in congenitally immunodeficient mice. Infect Immun. 1990;58:1093–1100. doi: 10.1128/iai.58.4.1093-1100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantorna M T, Balish E. Role of CD4+ lymphocytes in resistance to mucosal candidiasis. Infect Immun. 1991;59:2447–2455. doi: 10.1128/iai.59.7.2447-2455.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cutler J E. Acute systemic candidiasis in normal and congenitally thymic-deficient (nude) mice. J Reticuloendothel Soc. 1976;19:121–124. [PubMed] [Google Scholar]
- 7.Davis S L, Hawkins E P, Mason E O, Jr, Smith C W, Kaplan S L. Host defenses against disseminated candidiasis are impaired in intercellular adhesion molecule 1-deficient mice. J Infect Dis. 1996;174:435–439. doi: 10.1093/infdis/174.2.435. [DOI] [PubMed] [Google Scholar]
- 8.Jensen J, Warner T, Balish E. Resistance of SCID mice to Candida albicans administered intravenously or colonizing the gut: role of polymorphonuclear leukocytes and macrophages. J Infect Dis. 1993;167:912–919. doi: 10.1093/infdis/167.4.912. [DOI] [PubMed] [Google Scholar]
- 9.Jensen J, Warner T, Balish E. The role of phagocytic cells in murine resistance to disseminated candidiasis in granulocytopenic mice. J Infect Dis. 1994;170:900–905. doi: 10.1093/infdis/170.4.900. [DOI] [PubMed] [Google Scholar]
- 10.Jones-Carson J, Vazquez-Torres F A, Balish E. B cell-independent selection of memory T cells after mucosal immunization with Candida albicans. J Immunol. 1997;158:4328–4335. [PubMed] [Google Scholar]
- 11.Jones-Carson J, Vazquez-Torres A, van der Heyde H C, Warner T, Wagner R D, Balish E. γδ T cell-induced nitric oxide production enhances resistance to mucosal candidiasis. Nat Med. 1995;1:552–557. doi: 10.1038/nm0695-552. [DOI] [PubMed] [Google Scholar]
- 12.Klein R S, Harris C A, Small C B, Moll B, Lesser M, Friedland G H. Oral candidiasis in high-risk patients as the initial manifestation of acquired immunodeficiency syndrome. N Engl J Med. 1984;311:354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 13.Lehrer R I. Measurement of candidacidal activity of specific leukocyte types in mixed cell populations. I. Normal, myeloperoxidase-deficient, and chronic granulomatous disease neutrophils. Infect Immun. 1970;2:42–47. doi: 10.1128/iai.2.1.42-47.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narayanan R, Joyce W A, Greenfield R A. Gastrointestinal candidiasis in a murine model of severe combined immunodeficiency syndrome. Infect Immun. 1991;59:2116–2119. doi: 10.1128/iai.59.6.2116-2119.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oh M K, Rodey C E, Good R A, Chilgren R A, Quie P G. Defective candidacidal capacity of polymorphonuclear leukocytes in chronic granulomatous disease of childhood. J Pediatr. 1969;75:300–303. doi: 10.1016/s0022-3476(69)80403-8. [DOI] [PubMed] [Google Scholar]
- 16.Parry M F, Root R K, Metcalf J A, Delaney K K, Kaplow L S, Richar W J. Myeloperoxidase deficiency: prevalence and clinical significance. Ann Intern Med. 1981;95:293–301. doi: 10.7326/0003-4819-95-3-293. [DOI] [PubMed] [Google Scholar]
- 17.Rogers T J, Balish E, Manning D D. The role of thymus-dependent cell-mediated immunity in resistance to experimental disseminated candidiasis. J Reticuloendothel Soc. 1976;29:291–298. [PubMed] [Google Scholar]
- 18.Wagner R D, Vazquez-Torres A, Jones-Carson J, Warner T, Balish E. B cell knockout mice are resistant to mucosal and systemic candidiasis of endogenous origin but susceptible to experimental systemic candidiasis. J Infect Dis. 1996;174:589–597. doi: 10.1093/infdis/174.3.589. [DOI] [PubMed] [Google Scholar]
- 19.Yale C E, Balish E. Blood and serum chemistry values of gnotobiotic beagles. Lab Anim Sci. 1976;26:633–639. [PubMed] [Google Scholar]