Abstract
Narcolepsy Type 1 (NT1), a sleep disorder with similar prevalence in both sexes, is thought to be due to loss of the hypocretin/orexin (Hcrt) neurons. Several transgenic strains have been created to model this disorder and are increasingly being used for preclinical drug development and basic science studies, yet most studies have solely used male mice. We compared the development of narcoleptic symptomatology in male vs. female orexin-tTA; TetO-DTA mice, a model in which Hcrt neuron degeneration can be initiated by removal of doxycycline (DOX) from the diet. EEG, EMG, subcutaneous temperature, gross motor activity, and video recordings were conducted for 24-h at baseline and 1, 2, 4, and 6 weeks after DOX removal. Female DTA mice exhibited cataplexy, the pathognomonic symptom of NT1, by Week 1 in the DOX(-) condition but cataplexy was not consistently present in males until Week 2. By Week 2, both sexes showed an impaired ability to sustain long wake bouts during the active period, the murine equivalent of excessive daytime sleepiness in NT1. Subcutaneous temperature appeared to be regulated at lower levels in both sexes as the Hcrt neurons degenerated. During degeneration, both sexes also exhibited the “Delta State”, characterized by sudden cessation of activity, high delta activity in the EEG, maintenance of muscle tone and posture, and the absence of phasic EMG activity. Since the phenotypes of the two sexes were indistinguishable by Week 6, we conclude that both sexes can be safely combined in future studies to reduce cost and animal use.
Keywords: sleep, sleep disorder, narcolepsy, REM sleep, NREM sleep, wakefulness, EEG, EMG, spectral analysis, subcutaneous temperature
Statement of Significance.
Although narcolepsy is a disorder that affects both men and women with similar frequency, most basic research and preclinical development studies of sleep have utilized male experimental subjects. The identification of the hypocretin/orexin (Hcrt) neuron loss as the likely cause of human narcolepsy has led to the development of transgenic mouse strains that model this disorder. Here, we compare the emergence of narcoleptic symptoms in male vs. female bigenic orexin-tTA; TetO-DTA mice, an inducible narcolepsy model in which degeneration of the Hcrt neurons can be triggered by dietary manipulation. We find that female mice develop the narcoleptic phenotype more rapidly than males but that both sexes are equally symptomatic by the end of the degeneration period.
Introduction
Narcolepsy, a lifelong illness characterized by excessive daytime sleepiness (EDS) and associated symptoms, is classified as type 1 or 2 (NT1 and NT2) based on the presence or absence of cataplexy and/or hypocretin/orexin deficiency, with up to 60% of patients with narcolepsy having NT1 [1–3]. Approximately 1 in 2000 people are diagnosed with narcolepsy [4–7] and more than 50% of patients report that their first symptoms occurred before 16 years of age [2, 8]. However, only 18% of patients with narcolepsy receive a diagnosis within 1 year of symptom onset and only 50% of patients with narcolepsy receive a diagnosis within 5 years [2, 9].
Conflicting reports exist regarding sex differences in the prevalence of narcolepsy. Epidemiological studies have indicated that narcolepsy is more common in men than in women [4, 6, 10]. When the research focus is restricted to NT1, however, either no sex difference is reported [5, 11–13] or the incidence is reported as higher in women [6]. Due to overall differences in access to health services, women are less likely to undergo polysomnographic assessment [14], so the existing literature may not be completely reliable. Nonetheless, cataplexy has been reported to be more common in women than in men [5, 15], although symptom severity may not differ between the sexes [13].
Since NT1 is a debilitating disorder, pharmacological management of symptoms is critical to maintain quality of life [16]. With the development of mouse models of narcolepsy that better reflect the loss of hypocretin/orexin (Hcrt) neurons that underlie this disorder [17], future drug development for narcolepsy will likely rely on such animal models [18]. Indeed, there has been increasing use on such models for both basic science and preclinical development studies in recent years [19–26]. In such studies, however, only male mice have typically been used, which is problematic for several reasons. First, use of a single sex ignores biology that is relevant to the at least half the human population, particularly since baseline differences in sleep have been documented in both humans and rodents [27]. Furthermore, a precedent already exists for a difference in recommended doses for a sleep medication in men vs. women [28]. Lastly, breeding of transgenic mice is expensive and time-consuming and to discard half of the offspring violates one of the 3R principles [29].
In the present study, we compare the development of narcolepsy symptoms in male and female bigenic orexin-tTA; TetO-DTA mice, an inducible narcolepsy model in which degeneration of the Hcrt neurons can be initiated by a simple dietary manipulation [30]. We find that female mice progress into the narcoleptic phenotype more rapidly than male mice but that both sexes are equally symptomatic by the end of the degeneration period. We also find a novel state that emerges in both sexes during Hcrt neuron degeneration that we call the “Delta State”, which is characterized by sudden, brief periods of movement cessation without a loss of muscle tone that is accompanied by high delta activity in the EEG while the eyes remain open.
Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee at SRI International and were conducted in accordance with the principles set forth in the Guide for Care and Use of Laboratory Animals.
Animals
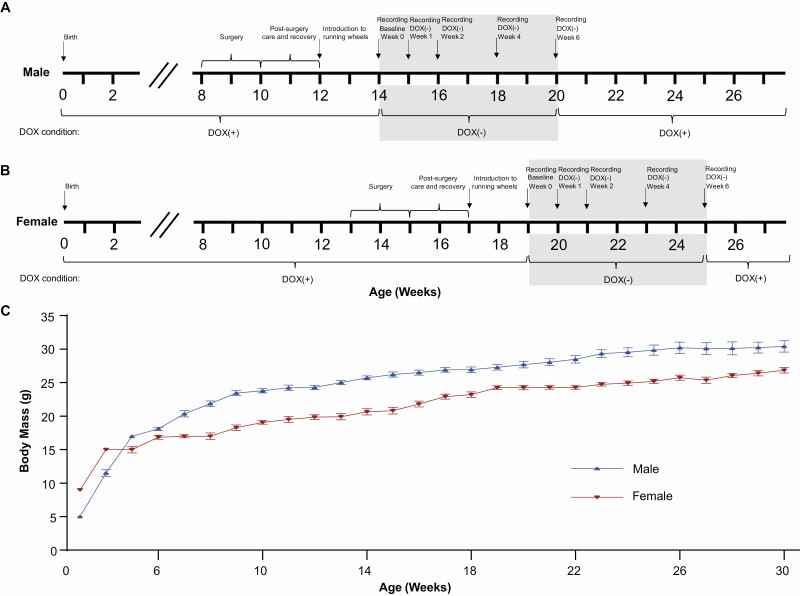
“DTA mice” (N = 7 males; N = 7 females) were the double transgenic offspring of orexin/tTA mice (C57BL/6-Tg(orexin/tTA)/Yamanaka) [30], which express the tetracycline transactivator (tTA) exclusively in Hcrt neurons [31], and B6.Cg-Tg(tetO-DTA)1Gfi/J mice [32] (JAX #008468), which express a diphtheria toxin A (DTA) fragment in the absence of dietary doxycycline (DOX). Both parental strains were from a C57BL/6J genetic background. Parental strains and offspring used for EEG/EMG recording were maintained on a diet (Envigo T-7012, 200 DOXycycline) containing doxycycline (DOX(+) condition) to repress transgene expression until neurodegeneration was desired. Hcrt neuron degeneration was initiated by substituting DOX(+) chow with normal rodent chow (DOX(-) condition). When the diet was changed from DOX(+) to DOX(-) condition, the animal’s cage was changed as well to prevent continued DOX exposure due to coprophagy. Mice were maintained on normal chow for 6 weeks and then DOX(+) chow was reintroduced to arrest any further neurodegeneration (Figure 1). All mice were maintained on a LD12:12 light:dark cycle at room temperature (22 ± 2oC; 50 ± 20% relative humidity), had access to food and water ad libitum, were weighed weekly.
Figure 1.
Time course of the experimental procedures for male (A) and female (B) orexin/tTA; TetO-DTA mice. Shaded area indicates the 6 week DOX(–) degeneration period. (C) Growth curves for male (N = 18) and female (N = 14) orexin/tTA; TetO-DTA mice. Values are mean ± SEM.
Surgical procedures
Because of the differential growth rate of males and females (Figure 1C), the two sexes of DTA mice underwent surgery at slightly different ages. To ensure that all mice were at least 20 g at the time of surgery (the minimum recommended body mass for this procedure), male mice were implanted at 9 ± 1 weeks of age and were 25.7 ± 0.5 g, whereas female DTA mice were implanted at 14 ± 1 weeks and were 20.7 ± 0.8 g. Mice were anesthetized with isoflurane and sterile telemetry transmitters (HD-X02 for males and F20-EET for females, Data Sciences Inc., St Paul, MN) were placed subcutaneously on the left dorsum. Biopotential leads were routed subcutaneously to the head and EMG leads were positioned in the right nuchal muscle. Cranial holes were drilled through the skull at -2.0 mm AP from bregma and 2.0 mm ML and on the midline at −1 mm AP from lambda. The two biopotential leads used as EEG electrodes were inserted into these holes and affixed to the skull with dental acrylic. The incision was closed with absorbable suture. Analgesia was managed with meloxicam (5 mg/kg, s.c.) and buprenorphine (0.05 mg/kg, s.c.) upon emergence from anesthesia and for the first day post-surgery. Meloxicam (5 mg/kg, s.c., q.d.) was continued for 2 d post-surgery.
EEG, EMG, activity, and subcutaneous temperature recording
Prior to data collection, DTA mice had 2 weeks post-surgical recovery and at least 2 weeks adaptation to running wheels. All mice then underwent a 24-h baseline (Week 0) recording in which digital videos, EEG, EMG, subcutaneous body temperature (Tsc), and gross motor activity were recorded via telemetry using Ponemah (DSI, St Paul, MN). Digital videos were recorded at 10 frames per second, 4CIF de-interlacing resolution; EEG and EMG were sampled at 500 Hz. Mice were then switched to normal chow (DOX(-) condition) to induce expression of the DTA transgene specifically in the Hcrt neurons and thereby initiate degeneration of these cells [30] and 24-h recordings were again conducted at 1, 2, 4, and 6 weeks in the DOX(-) condition (Figure 1A, B). To ensure that data collection occurred at the same phase of the estrus cycle for females, 24-h recordings occurred at 4 day intervals, e.g., on day 8 in the DOX(-) condition during Week 1, on day 16 DOX(-) during Week 2, on day 28 during Week 4, and on day 42 during Week 6. After completion of the recording at 6 wk, DOX chow was reintroduced to minimize further degeneration. Tail snips to confirm the DTA genotype were obtained at weaning and at the end of the study.
Subsequent to surgery, mice were housed individually in home cages with access to food, water, nestlets, and running wheels ad libitum. Room temperature, humidity, and lighting conditions (LD12:12; lights on at 06:00) were monitored continuously. Animals were inspected daily in accordance with AAALAC and SRI guidelines and body mass measurements were taken weekly.
Classification of arousal states
For all recordings, a video camera was placed lateral to each cage and optimized to enable characterization of cataplexy and sleep- and wake-associated behaviors (e.g., posture, position in cage relative to the nest, and eye state (open vs. closed)). EEG and EMG data collected during the first 6-h of the dark period (ZT13-ZT18) were used by expert scorers to classify 10-s recording epochs as wakefulness (W), non-Rapid Eye Movement (NREM) sleep, REM sleep, or cataplexy (C) using NeuroScore (DSI, St. Paul, MN) as in our previous studies [19, 20]. Criteria for cataplexy were ≥10 s of EMG atonia, theta-dominated EEG, and video-confirmed behavioral immobility preceded by ≥ 40 s of wakefulness [33]. Running wheel activity, determined from video recordings, was scored in 10-s epochs for the purpose of training Somnivore (see below). While analyzing these recordings, we recognized a novel state that we called the Delta State (DS). Criteria for scoring an epoch as DS were the sudden cessation of locomotion during W, the persistence of muscle tone but the absence of phasic EMG activity, and a synchronous, NREM-like EEG pattern while the eyes remained open and the mouse remained in a standing position. DS terminated abruptly and mice rapidly resumed ambulatory behaviors with typical Wake EEG patterns.
Application of a supervised machine learning model to assist in arousal state classification
To score the remaining 18-h of each 24-h recording, the manually scored 6-h period of each recording (ZT13-ZT18) was provided to Somnivore (ver. 1.0.70) [34] in EDF format to create a training dataset for each individual recording and for subsequent assessment of autoscoring accuracy (see below). From the manually scored 6-h recordings of each mouse, 100 10-s epochs of Wake and 100 epochs of running wheel activity were randomly selected to train a classifier. If a recording had fewer than 100 epochs of running wheel activity in the initial 6-h period, additional epochs were selected from the remaining 18-h recording. Since little NREM and REM occurred during the first 6-h of the active phase (ZT13-ZT18), training epochs for these states were manually selected from throughout the entire 24-h recording. For cataplexy, if the 6-h recordings contained fewer than 100 epochs, REM and cataplexy were trained as a single state and were manually separated post-autoscoring through application of the scoring rules described below. Cataplexy was never observed by expert manual scorers in week 0 recordings and was thus not included in the training dataset for recordings from this period. The selected training epochs for all states were then applied in a supervised machine learning model to automatically score sleep-wake states for the entire 24-h period.
Following initial autoscoring, agreement with the manually scored 6-h period was assessed and generalization of the autoscoring to the remaining 18-h of the recording was evaluated. An additional 5–25 epochs of states with low accuracy values were added to the training dataset and recordings were automatically scored again. Following this optimization phase, scoring rules were applied to the Somnivore-scored dataset to select potentially misidentified epochs of cataplexy and/or REM. Manual correction of the scoring occurred when autoscoring provided any of the following unlikely results:
- single epochs of REM
- epochs of REM preceded by W, cataplexy, or wheel running
- epochs of cataplexy preceded by NREM or REM
- prolonged or frequently occurring short bouts of cataplexy or REM
Finally, accuracy of autoscoring was evaluated by comparing the corrected autoscoring to the manual 6-h scoring with a function provided by the software that enables comparison of an F score for each state, a measure of precision and accuracy used to quantify algorithm generalization that was calculated as follows:
[34].
Accuracy of the autoscored data had high concordance with manual scoring (overall agreement = 93.13 ± 0.25%), with F scores for most states indicating a high level of accuracy.
Data analysis and statistics
Among the 7 female DTA mice implanted for this study, the quality of the recordings was problematic for one mouse on Week 0 and another female on Week 6. Due to the repeated-measures experimental design of this study, it was necessary to eliminate these two females from the data analysis; thus, statistical analyses are based on N = 7 males and N = 5 females. In the Results and Discussion below, however, these two mice are included for descriptive rather than quantitative statements regarding the presence or absence of cataplexy during Weeks 1-4.
For all states, data were analyzed as time spent in state per hour and cumulative time spent in each state. Sleep/wake architecture measures included the duration and the number of bouts for each state. A “bout” of a particular state was defined as 2 or more consecutive epochs of that state and ended with a single epoch of any other state.
The EEG power spectrum (0.5–100 Hz) during W, NREM, and REM was analyzed by fast Fourier transform algorithm on all artifact-free epochs. For spectral analyses, a minimum of 6 consecutive epochs of Wake or NREM and a minimum of 3 consecutive epochs of REM was required for inclusion in the analysis. For cataplexy and Delta State, there was no minimum number of epochs criterion; all epochs were included in spectral analyses. For each mouse, EEG power for W, NREM, and REM during the degeneration period was normalized to the 6-h average power per 0.122 Hz bin during the pre-degeneration baseline recording (Week 0). To determine whether any changes in EEG power occurred across the degeneration phase, we compared EEG power within each state across DOX(-) weeks to the DOX(+) condition. Since cataplexy did not occur during the baseline recording in the DOX(+) condition, Wake EEG power during Week 0 was used for normalization of cataplexy across degeneration weeks. EEG spectra for Wake and Cataplexy were analyzed in 0.122 Hz bins and in standard frequency bands rounded to the nearest half-Hz value (delta: 0.5–4 Hz, theta: 6–9 Hz, alpha: 9–12 Hz, beta: 12–30 Hz, low gamma: 30–60 Hz and high gamma: 60–100 Hz). Hourly averages of Tsc and gross activity determined from the telemetry transmitters were also analyzed.
Total time in states during the 12-h dark and 12-h light phases and across the 24-h period, REM/NREM ratios, mean bout durations, the number of bouts of each state, and the standard frequency EEG power bands were analyzed using 1-way repeated-measures analysis of variance (RM-ANOVA) followed by the posthoc Dunnett multiple comparisons test. Because the continuous wakefulness during the 12-h dark period during Week 0 in one female mouse resulted in a single long Wake bout duration, the nonparametric Friedman test was applied to analyze Wake bout duration in females. When RM-ANOVA was not possible data due to missing data (e.g., the absence of cataplexy and the Delta State during baseline Week 0 or the continuous wakefulness observed in the female DTA mouse mentioned above that resulted in the absence of other states for that individual during that hour), mixed-effect analysis was used. All other data analyses were by 2-way RM-ANOVA within each sex with condition (DOX(–) week) and time of day as factors. When 2-way RM-ANOVA indicated statistical significance, paired two-tailed t-tests were performed posthoc to determine specific differences. Statistics were calculated using the functions provided in the MATLAB statistics and machine learning toolbox and using GraphPad Prism (ver. 8.4.2). For the EEG spectra data, statistical comparisons were calculated only on the standard frequency bands.
Results
State amounts and the development of cataplexy
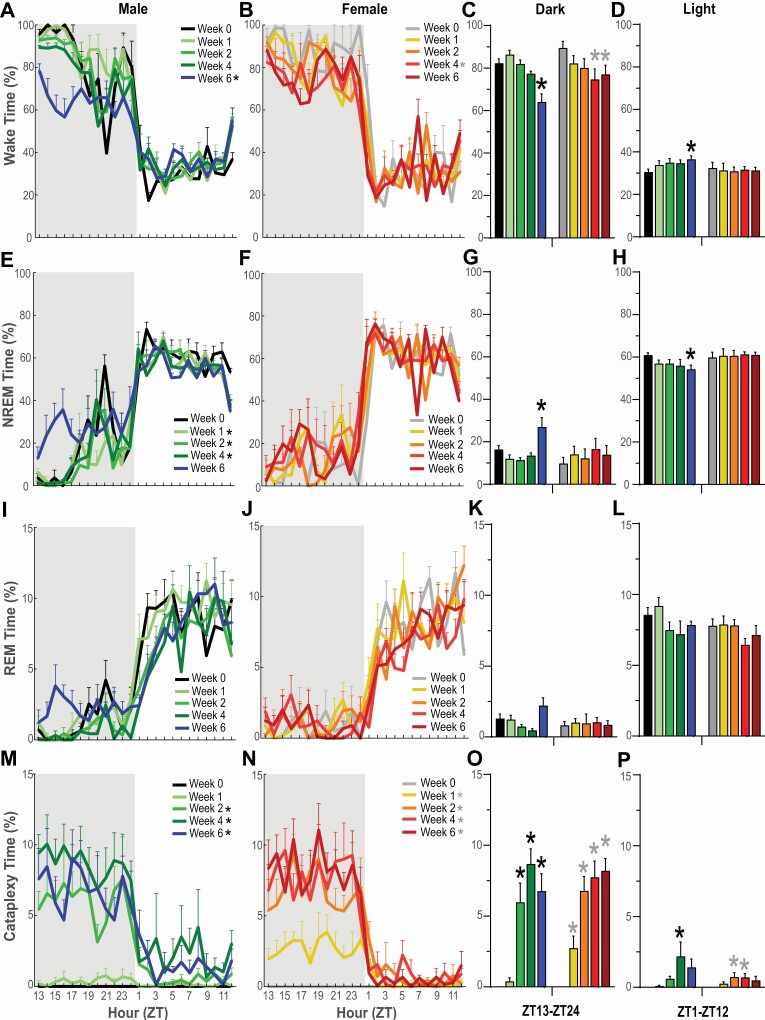
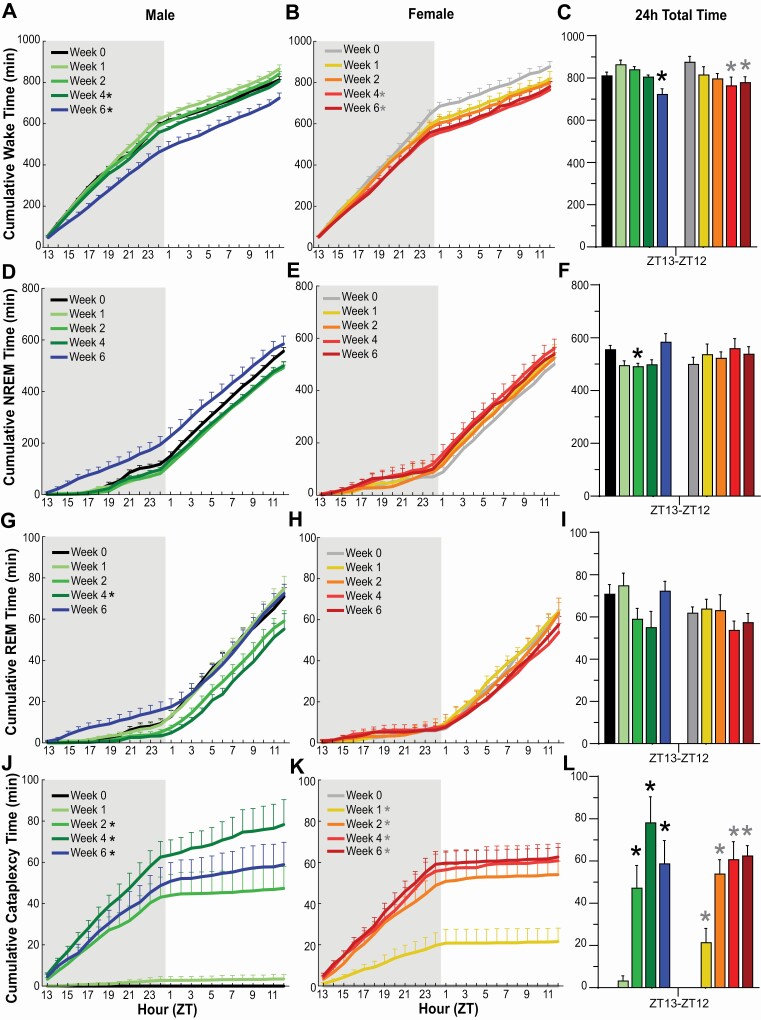
Relatively few changes were observed for hourly, total, and cumulative time spent in Wake, NREM, or REM during the 24-h L/D cycle across the 6 week degeneration period (Figures 2 and 3).
Figure 2.
Hourly percentage of time in Wakefulness, NREM sleep, REM sleep, and cataplexy in narcoleptic male (A, E, I, M) and female (B, F, J, N) orexin/tTA; TetO-DTA mice during baseline (Week 0) and Weeks 1, 2, 4, and 6 in the DOX(–) condition during which time the Hcrt neurons are expected to degenerate. Shaded area indicates the dark period of the 24-h cycle. * in the legend within each panel indicates a significant difference (p < 0.05) during that week relative to baseline Week 0 as determined by 2-way ANOVA. Panels C, G, K and O summarize the percentage of time that each sex spent in these states during the 12-h dark period whereas panels D, H, L and P summarize these data for both sexes during the 12-h light period. * above the bars in each panel indicate significance (p < 0.05) during that week relative to baseline Week 0 as determined by 1-way ANOVA or mixed-effect analysis followed by posthoc testing. Values are mean ± SEM.
Figure 3.
Cumulative amounts of Wakefulness, NREM sleep, REM sleep, and Cataplexy in narcoleptic male (A, D, G, J) and female (B, E, H, K) orexin/tTA; TetO-DTA mice during baseline (Week 0) and Weeks 1, 2, 4, and 6 in the DOX(–) condition during which time the Hcrt neurons are expected to degenerate. Shaded area indicates the dark period of the 24-h cycle. * in the legend indicates a significant difference (p < 0.05) during that week relative to baseline Week 0 as determined by 2-way ANOVA. Total amounts of Wakefulness (C), NREM sleep (F), REM sleep (I), and Cataplexy (L) in narcoleptic male and female orexin/tTA; TetO-DTA mice across the 24-h period. * above the bars in each panel indicates significance (p < 0.05) relative to baseline Week 0 as determined by 1-way ANOVA or mixed-effect analysis followed by posthoc testing. Values are mean ± SEM.
Wakefulness
In male DTA mice, 2-way ANOVA indicated a significant effect of condition on Wakefulness across the 24-h period (F(4, 24) = 10.01, p = 6.51 × 10–5; Figures 2A, 3C). Posthoc tests established that Wake decreased during Week 6 of the degeneration (p = 0.0028); this decrease was primarily due to a reduction in Wake during the first half of the dark phase. One-way ANOVA indicated that total time in Wake decreased in the 12-h dark phase during Week 6 (F(4, 24) = 13.27, p = 7.69 × 10–6; Figure 2C) but increased slightly in the 12-h light phase during the same week (p = 0.039, Dunnett’s multiple comparisons posthoc test; Figure 2D). Two-way ANOVA also indicated a significant effect of condition on cumulative time in Wakefulness in male DTA mice (F(4, 24) = 13.12, p = 8.41 × 10–6; Figure 3A).
In females, 2-way ANOVA also revealed a significant effect of condition on Wakefulness across the 24-h cycle (F(4,16)= 3.06, p = 0.047; Figure 2B); posthoc tests indicated that the reduced Wake during Week 4 (p = 0.024) was due primarily to a decrease in the dark phase that was also evident in Week 6 (Figure 2C). Two-way ANOVA also indicated a significant effect of condition on cumulative Wake time in female DTA mice (F(4,16)= 5.77, p = 0.005; Figure 3B). One-way ANOVA followed by Dunnett’s multiple comparison posthoc tests confirmed that Wake was reduced in females during both Week 4 (p = 0.019) and Week 6 (p = 0.046) relative to Week 0 (Figure 3C).
NREM sleep
Two-way ANOVA indicated a significant effect of condition on NREM sleep in male DTA mice across the 24-h period (F(4, 24) = 6.08, p = 0.002; Figure 2E); posthoc tests indicated decreased NREM during Week 1 (p = 0.027), Week 2 (p = 0.030) and Week 4 (p = 0.048) when compared to Week 0. During Week 6, posthoc tests indicated that NREM increased in the 12-h dark phase (p = 0.017; Figure 2G) but decreased in the 12-h light phase (p = 0.04; Figure 2H). One-way ANOVA followed by Dunnett’s multiple comparison posthoc tests confirmed that the 24-h amount of NREM sleep decreased during Week 2 (p = 0.044) relative to Week 0 (Figure 3F).
No effect of condition was found for NREM in females across the 6 week degeneration period (Figures 2F, G, H, and 3F).
REM sleep
No significant differences in REM sleep were observed in either males or females for percent time across the 24-h period or during the 12-h dark and 12-h light phases (Figures 2I-2L). However, 2-way ANOVA indicated a significant effect of condition on cumulative REM time in males (F(4, 24) = 3.93, p = 0.014; Figure 3G); posthoc tests indicated a significant difference between Week 0 and Week 4 (p = 0.005). Total REM time per 24-h period (Figure 3I) analyzed by 1-way ANOVA indicated significance in males (F(4, 24) = 2.875, p = 0.045) but Dunnett’s multiple comparison posthoc tests did not identify any week that differed from Week 0.
Cataplexy
The emergence of cataplexy during the degeneration period differed between male and female DTA mice (Figures 2M–2P, 3J–3L). Cataplexy was evident in all 7 female mice during the first week of the degeneration while only 3 of 7 males had discernible bouts. However, by Week 2 and for subsequent weeks, all males and females exhibited cataplexy. Two-way ANOVA indicated a significant effect of condition on cataplexy in both males (F(4, 24) = 24.54, p = 3.5 × 10–8; Figure 2M) and females (F(4, 16) = 50.66, p = 6.9 × 10–9; Figure 2N). In males, posthoc tests indicated a significant increase in cataplexy between Week 0 and Week 2 (p = 0.004), Week 4 (p = 0.0006) and Week 6 (p = 0.0015) and, in female DTA mice, between Week 0 and Week 1 (p = 0.03), Week 2 (p = 0.0012), Week 4 (p = 0.0019) and Week 6 (p = 0.0002). Cataplexy significantly increased across the 24-h recordings (Figure 3L) and cumulatively for Weeks 2-6 in males (F(4, 24) = 25.13, p = 2.81 × 10–8; Figure 3J) and for Weeks 1-6 in females (F(4, 16)= 43.45, p = 2.12 × 10–8; Figure 3K).
For both sexes, cataplexy was most evident in the dark (active) phase (Figure 2O vs. 2P). In males, total cataplexy time increased during the 12-h dark phase in Weeks 2–6 (F(4, 24) = 24.01, p = 4 × 10–8) and during the 12-h light phase in Week 4 (F(4, 24) = 2.81, p = 0.048) whereas, in females, total cataplexy time increased during the 12-h dark phase in Weeks 1–6 (F(4, 16) = 44.80, p = 2 × 10–8) and during the 12-h light phase in Weeks 2–4 (F(4, 16) = 3.79, p = 0.024).
Neither the NREM nor REM sleep latencies (measured from lights off) nor REM:NR ratios were significantly different from Week 0 for any degeneration week (data not shown).
Sleep/wake architecture measures
Wakefulness
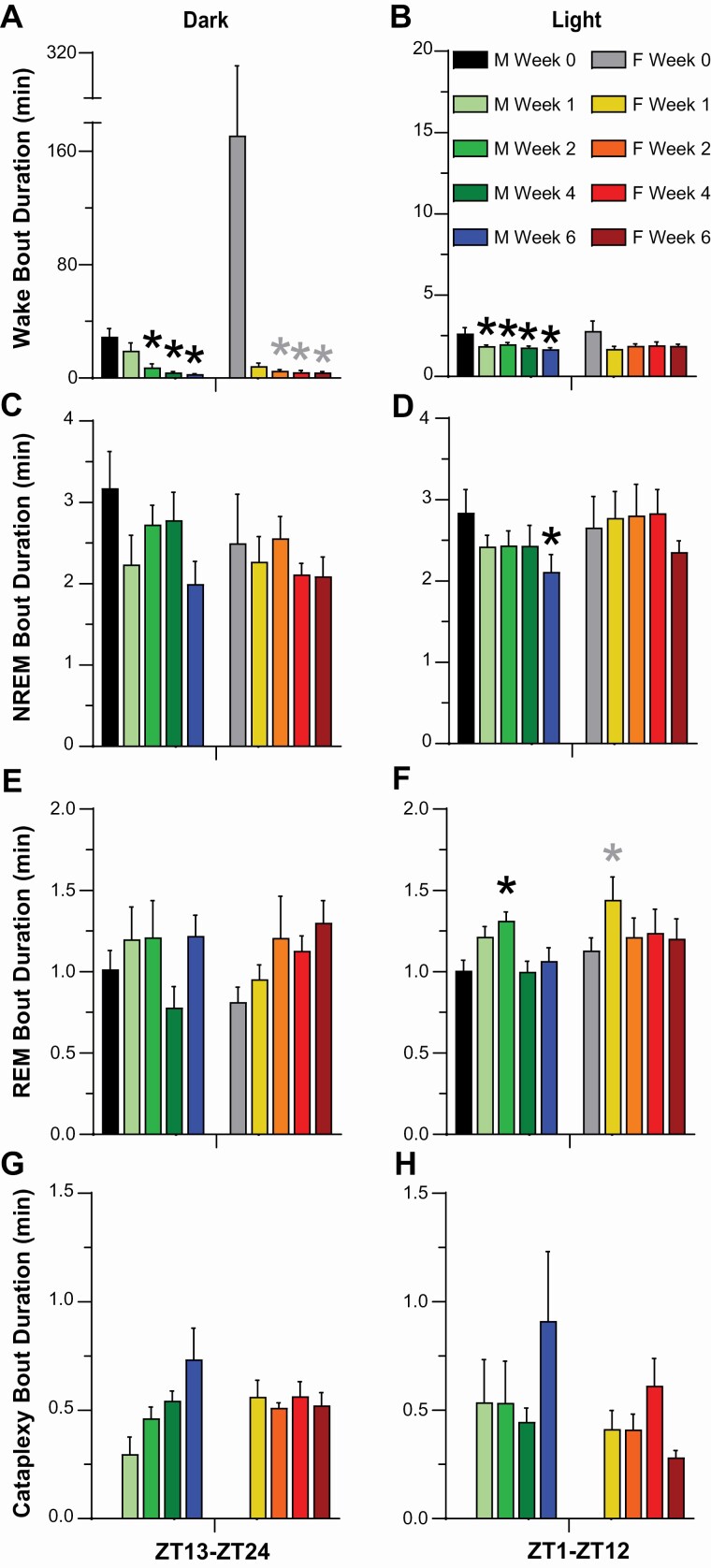
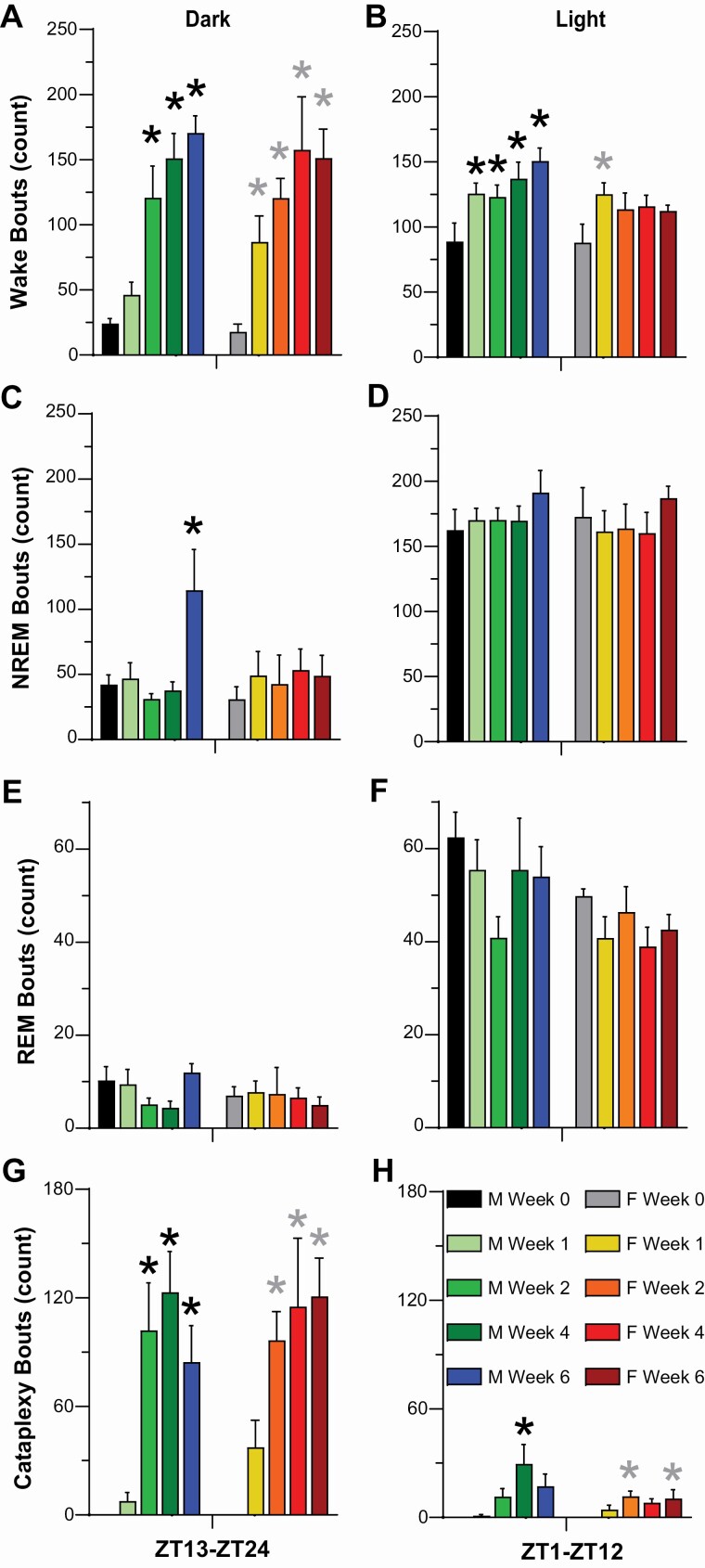
As indicated by both the progressive reduction in mean Wake bout duration (Figure 4A, 4B) and the increased number of Wake bouts (Figure 5A, 5B), Wakefulness became more fragmented in both female and male DTA mice during Hcrt neuron degeneration but the phenotype differed somewhat. Two-way ANOVA indicated a significant effect of condition on Wake bout duration in both males (F(4, 24) = 25.48, p = 2.46 × 10–8) and females (F(4, 16) = 35.87, p = 8.41 × 10–8; Figure 4A, 4B). Posthoc tests indicated significant decreases in Wake bout duration in males between Week 0 and Week 2 (p = 0.0004), Week 4 (p = 1.99 × 10–5) and Week 6 (p = 1.46 × 10–5) and, in females, between Week 0 and Week 1 (p = 0.003), Week 2 (p = 0.004), Week 4 (p = 0.003) and Week 6 (p = 0.004).
Figure 4.
Mean bout durations for Wakefulness (A, B), NREM sleep (C, D), REM sleep (E, F), and cataplexy (G, H), during the 12-h dark (left) and light (right) periods for narcoleptic male and female orexin/tTA; TetO-DTA mice during baseline (Week 0) and Weeks 1, 2, 4, and 6 in the DOX(–) condition during which time the Hcrt neurons are expected to degenerate. Values are mean +/– SEM. * above the bars in each panel indicate significance (p < 0.05) during that week relative to baseline Week 0 as determined by 1-way ANOVA or mixed-effect analysis followed by posthoc testing or the Friedman test, which was applied to analyze Wake bout duration in female mice due to the one female who did not sleep at all during the dark period.
Figure 5.
Number of bouts of Wakefulness (A, B), NREM sleep (C, D), REM sleep (E, F), and cataplexy (G, H), during the 12-h dark (left) and light (right) periods for narcoleptic male and female orexin/tTA; TetO-DTA mice during baseline (Week 0) and Weeks 1, 2, 4, and 6 in the DOX(–) condition during which time the Hcrt neurons are expected to degenerate. Values are mean ± SEM. * above the bars in each panel indicate significance (p < 0.05) during that week relative to baseline Week 0 as determined by 1-way ANOVA or mixed-effect analysis followed by posthoc tests.
During the 12-h dark phase, mean Wake bout duration (Figure 4A) decreased during Weeks 2–6 in both sexes (males: F(4, 24) = 8.56, p = 0.0002, 1-way ANOVA; females: p = 0.003, Friedman test), while the number of Wake bouts (Figure 5A) increased during Weeks 2–6 in males (F(4, 24) = 18.07, p = 5.8 × 10–7) and Weeks 1–6 in females (F(4, 16) = 11.53, p = 0.0001). Similarly in males during the 12-h light phase, mean Wake bout duration (Figure 4B) decreased (F(4, 24) = 6.62, p = 0.001) and the number of Wake bouts (Figure 5B) increased for Weeks 1–6 (F(4, 24) = 6.21, p = 0.001) whereas, for female mice, the number of Wake bouts increased during Week 1 (p = 0.043, Dunnett’s multiple comparisons posthoc test) without any significant change in bout duration.
NREM sleep
Two-way ANOVA indicated a significant effect of condition on NREM bout duration in males (F(4, 24) = 7.65, p = 0.0004; Figure 4C, 4D) but not in females; posthoc tests indicated significant decreases in NREM bout duration in males between Week 0 and Week 6 (p = 0.004). NREM bout duration decreased in the 12-h light phase (p = 0.02) in males (Figure 4D).
Two-way ANOVA also indicated a significant effect of condition on the number of NREM bouts in males (F(4, 24) = 3.64, p = 0.02; Figure 5C, 5D) but not for females; posthoc tests indicated a significant increase in the number of NREM bouts in males between Week 0 and Week 6 (p = 0.03). One-way ANOVA indicated that the number of NREM bouts increased during the 12-h dark phase (F(4, 24) = 4.79, p = 0.006) during Week 6 (Figure 5C) for male DTA mice.
REM sleep
Two-way ANOVA indicated a significant effect of condition on REM bout duration (Figure 4E, 4F) in both males (F(4, 24) = 11.76, p = 1.98 × 10–5) and females (F(4, 16) = 5.95, p = 0.004). Posthoc tests indicated a significant increase in REM bout duration in males between Week 0 and Week 2 (p = 0.02) and in females between Week 0 and Week 1 (p = 0.037) and Week 6 (p = 0.013). REM bout duration in the 12-h light phase (Figure 4F) increased in male mice (F(4, 24) = 4.32, p = 0.009) during Week 2 (p = 0.01) and in female mice (F(4, 16) = 3.08, p = 0.047) during Week 1 (p = 0.02).
There was no significant condition effect on the number of REM bouts (Figure 5E, 5F).
Cataplexy
Statistical analyses of Cataplexy bout duration were limited by the fact that Cataplexy was not observed during the baseline (Week 0) condition. Nonetheless, Cataplexy bout duration (Figure 4G, 4H) during the 12-h dark phase appeared to increase progressively with Hcrt neuron degeneration in male but not female DTA mice.
Two-way ANOVA indicated a significant effect of condition on the number of cataplexy bouts (Figure 5G, 5H) in both males (F(4, 24) = 17.27, p = 8.54 × 10–7) and females (F(4, 16) = 15.38, p = 2.41 × 10–5). Posthoc tests indicated a significant increase in the number of Cataplexy bouts in males between Week 0 and Week 2 (p = 0.008), Week 4 (p = 0.001), and Week 6 (p = 0.004) and in females between Week 0 and Week 2 (p = 0.002), Week 4 (p = 0.03), and Week 6 (p = 0.002). The number of cataplexy bouts increased in the 12-h dark phase (Figure 5G) for both sexes during weeks 2-6 (males: F(4, 24) = 13.42, p = 7.03 × 10–6; females: F(4, 16) = 13.07, p = 6.48 × 10–5) and during the 12-h light phase (Figure 5H) in Week 4 for males (F(4, 24) = 5.74, p = 0.002) and Weeks 2 and 6 for females (F(4, 16) = 3.84, p = 0.023).
Activity and subcutaneous temperature (Tsc) rhythms
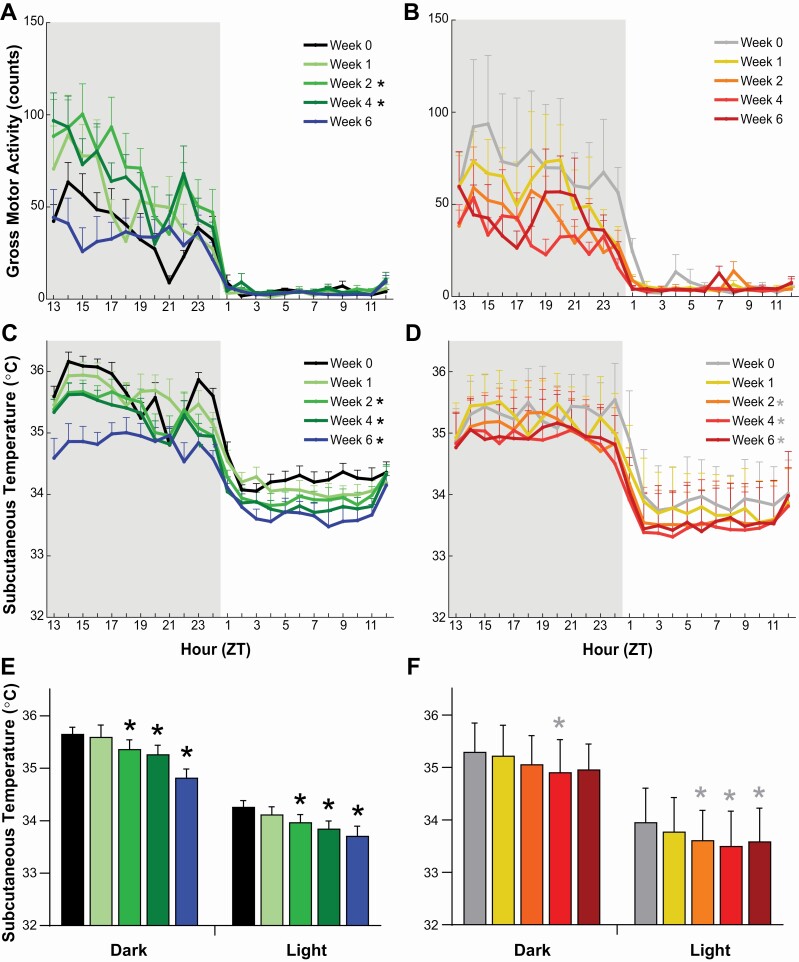
In males, 2-way ANOVA indicated a significant effect of condition on gross motor activity (F(4, 24) = 4.76, p = 0.006, Figure 6A) without a significant effect in females. Posthoc tests indicated a significant increase in gross motor activity in males between baseline Week 0 and degeneration Weeks 2 (p = 0.02) and 4 (p = 0.03) that returned to baseline levels by Week 6. The absence of a significant differences in gross activity in females was perhaps due to the high variation during Week 0 (Figure 6B).
Figure 6.
Gross motor activity (A, B) and subcutaneous temperature (C, D) in narcoleptic male (left) and female (right) orexin/tTA; TetO-DTA mice during baseline (Week 0) and weeks 1, 2, 4, and 6 in the DOX(–) condition during which time the Hcrt neurons are expected to degenerate. Progressive decline in mean subcutaneous temperature for males (E) and females (F) during the 12-h dark and 12-h light across the 6 week Hcrt neuron degeneration period. Values are mean ± SEM. * in the legend and above the bars indicates a significant difference (p < 0.05) during that week relative to baseline Week 0 as determined by 2-way ANOVA and 1-way ANOVA or mixed-effect analysis followed by posthoc tests.
Two-way ANOVA indicated a significant effect of condition on Tsc (Figure 6C–F) in both male (F(4, 24) = 28.25, p = 9.12 × 10–9) and female DTA mice (F(4, 16) = 7.86, p = 0.001). Posthoc tests indicated a significant decrease in Tsc in both sexes between Week 0 and Week 2 (males: p = 0.005; females: p = 0.045), Week 4 (males: p = 0.001; females: p = 0.015), and Week 6 (males: p = 0.0002; females: p = 0.014). This decrease occurred across the 24-h period and was indicative of an overall reduction of subcutaneous Tsc during both the dark and light phases. For males, mean Tsc declined significantly in both the dark (F(4, 24) = 19.18, p = 3.4 × 10–7) and light (F(4, 24) = 16.95, p = 1.01 × 10–6) phases during Weeks 2-6 (Figure 6E). For females, significant reductions in Tsc occurred during Week 4 in the 12-h dark phase (F(4, 16) = 3.51, p = 0.031) and for Weeks 2-6 during the 12-h light phase (F(4, 16) = 13.79, p = 4.7 × 10–5) (Figure 6F).
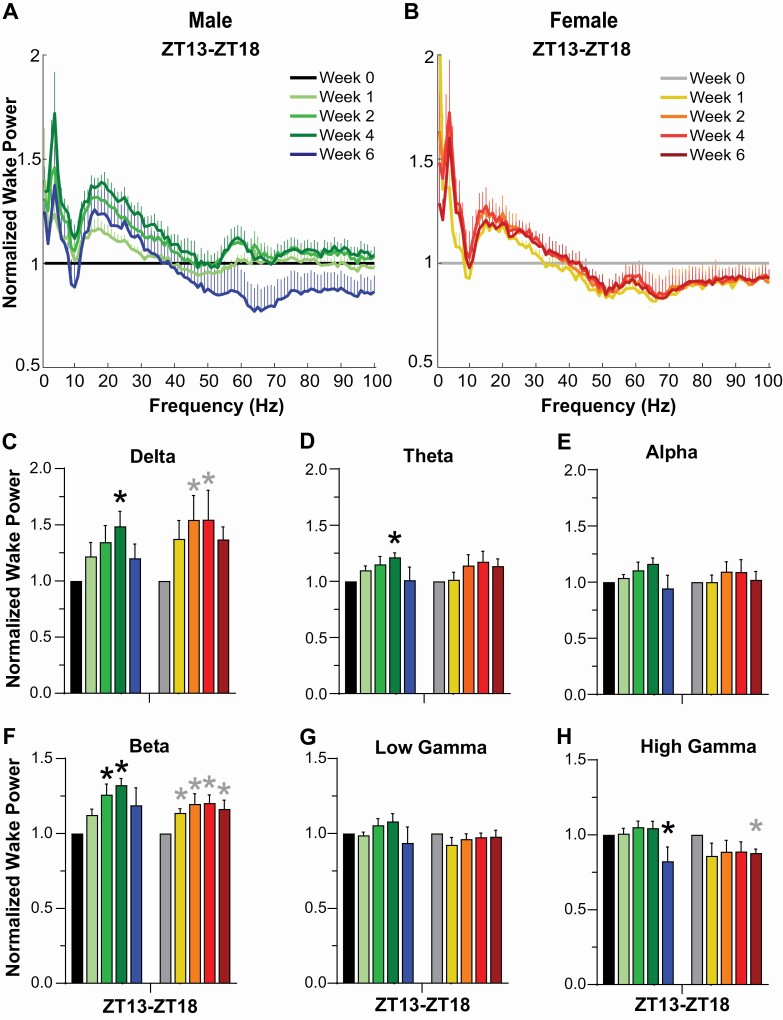
EEG power spectra
Spectral analyses were only performed on the first 6-h of the dark phase for each recording since this period was manually scored and care was taken to remove movement and other recording artifacts that could “contaminate” the EEG spectra. Analyses during this period revealed progressive changes in Wake EEG that were more evident in males than females (Figure 7). When compared to pre-degeneration Wake (normalized to Week 0), increased spectral power was observed from 2-9 Hz and 15-25 Hz for both sexes (Figure 7A, 7B). However, when EEG power was binned into the standard power bands, few statistical differences were found (Figure 7C–H). Posthoc tests indicated that Delta power increased in males during Week 4 (p = 0.011) and in females during Weeks 2 (p = 0.010) and 4 (p = 0.009), theta power increased in males during Week 4 (p = 0.007), and beta power increased in males during Weeks 2 (p = 0.013) and 4 (p = 0.002) and in females during Weeks 1 (p = 0.041), 2 (p = 0.004), 4 (p = 0.003), and 6 (p = 0.014). Since the F20-EET transmitters used in the female mice have an attenuated signal above 60 Hz, high gamma results for female mice (Figure 7H) are included for informational purposes only, although the statistical results obtained are consistent with the decline in high gamma observed in males during Week 6 (p = 0.044).
Figure 7.
Normalized EEG spectral power (0-100 Hz) during wakefulness for (A) male and (B) female orexin/tTA; TetO-DTA mice during baseline (Week 0) and Weeks 1, 2, 4, and 6 in the DOX(-) condition during which time the Hcrt neurons are expected to degenerate. Normalized Wake Power in male and female orexin/tTA; TetO-DTA mice in the (C) delta, (D) theta, (E) alpha, (F) beta, (G) low gamma and (H) high gamma bandwidths. Values are mean ± SEM. * above the bars in panels C-H indicate significance (p < 0.05) during that week relative to baseline Week 0 as determined by 1-way ANOVA or mixed-effect analysis followed by posthoc tests.
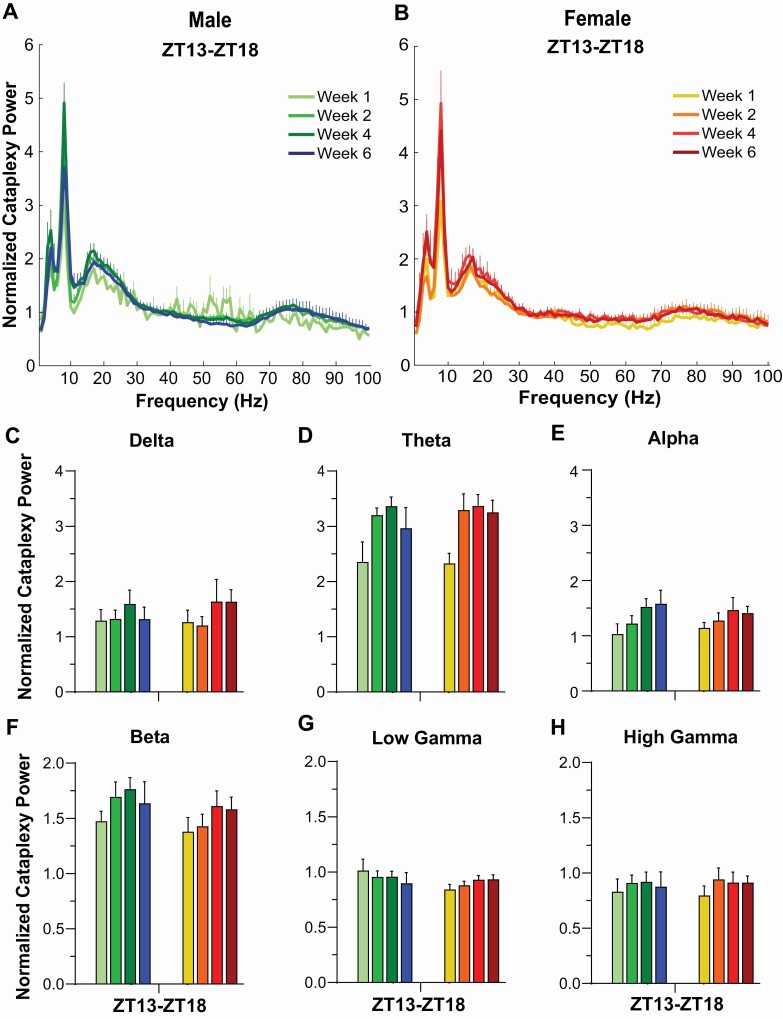
To evaluate EEG power during cataplexy, we normalized to the average Wake power during Week 0 (Figure 8). Increased power in the delta, theta, alpha, and beta ranges were observed with small changes in low and high gamma bands. Statistical analyses were not performed since no Cataplexy occurred during Week 0.
Figure 8.
Normalized EEG spectral power (0–100 Hz) during cataplexy for (A) male and (B) female orexin/tTA; TetO-DTA mice during baseline (Week 0) and Weeks 1, 2, 4, and 6 in the DOX(–) condition during which time the Hcrt neurons are expected to degenerate. Normalized EEG power during cataplexy in male and female orexin/tTA; TetO-DTA mice in the (C) delta, (D) theta, (E) alpha, (F) beta, (G) low gamma and (H) high gamma bandwidths.
EEG power in NREM and REM was not evaluated due to the paucity of these states during the ZT13-18 time period that met the criterion of 6 consecutive epochs of Wake or NREM and 3 consecutive epochs of REM, as described in the Methods section.
A novel high EEG delta state during wakefulness
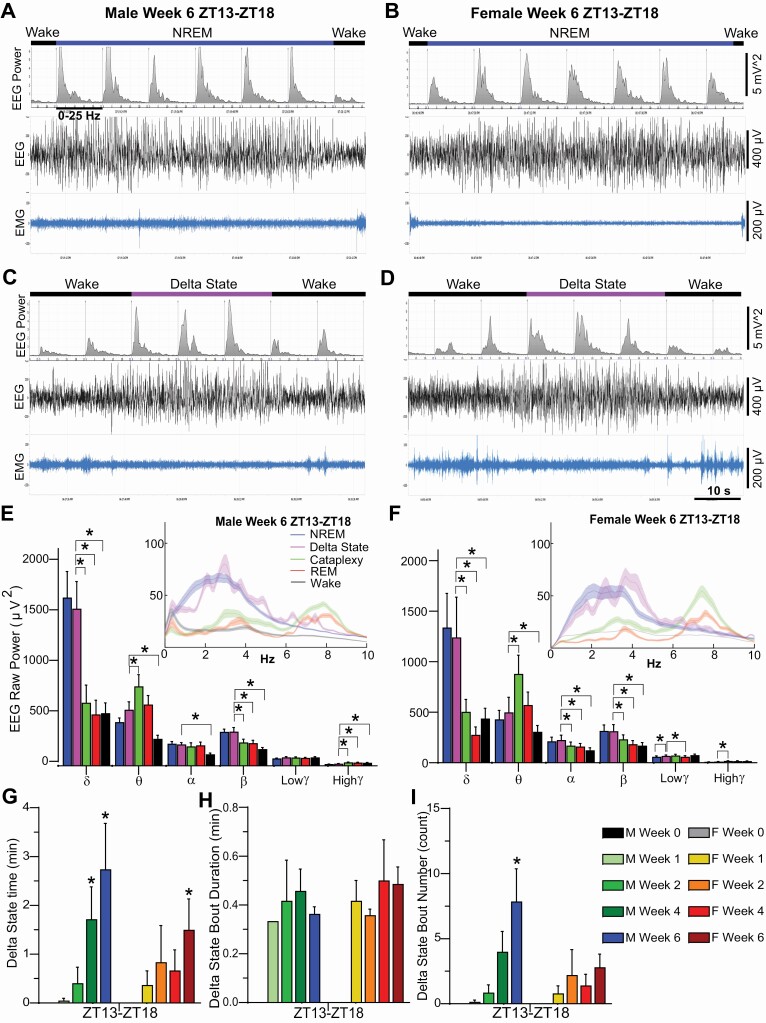
Manual scoring of the EEG records revealed a novel state which we labeled as Delta State (DS; Figure 9). DS only occurred following the initiation of the degeneration process and became more prevalent as the degeneration proceeded. DS was observed during Wake, typically during active Wake, and was characterized by a sudden cessation of movement with an absence of phasic EMG activity. Differing from cataplexy, muscle tone was not lost and video recordings revealed postural maintenance. Concurrently, the EEG becomes synchronized with high delta activity as in NREM sleep. Without video recordings, these episodes would be difficult to distinguish from NREM. However, the behavior of the mice was distinctly different from that of a mouse entering sleep. Typical behavior that precedes sleep, such as lying down or curling up into their nest, did not occur. The mice were typically ambulatory but suddenly stopped, the EEG became synchronized with high Delta, and no phasic EMG activity occurred. Videos revealed that the eyes remained open. Within 1-6 epochs, the mouse suddenly returned to its ambulatory behavior, a Wake-like EEG reappeared and phasic EMG activity was evident (Figure 9A–D, Supplementary Videos S1 & S2).
Figure 9.
Example EEG spectrograms (7 10-sec epochs), EEG and EMG traces illustrating typical transitions from Wake to NREM sleep back to Wake over a 70-sec period in (A) male and (B) female orexin/tTA; TetO-DTA mice during Week 6 in the DOX(-) condition. Example EEG spectrograms (7 10-sec epochs), EEG and EMG traces illustrating typical transitions from Wake to the Delta State back to Wake over a 70-sec period in (C) male and (D) female orexin/tTA; TetO-DTA mice during Week 6 in the DOX(–) condition. Raw EEG power during the first 6-h of the dark period for the delta (0.5–4 Hz), theta (6–9 Hz), alpha (9–12 Hz), beta (12–30 Hz), low gamma (30–60 Hz) and high gamma (60–100 Hz) bandwidths during NREM, the Delta State, Cataplexy, REM and Wake during Week 6 in the DOX(–) condition for a male (E) and a female (F) orexin/tTA; TetO-DTA mouse. Insets show raw power in the 0–10 Hz range, plotted as a rolling average of three adjacent 0.122 Hz bins. (G) Total time in the Delta State during the first 6-h of the dark period during baseline (Week 0) and Weeks 1, 2, 4, and 6 in the DOX(–) condition during which time the Hcrt neurons are expected to degenerate. (H) Mean Delta State bout duration and (I) number of Delta State bouts during the first 6-h of the dark period during baseline (Week 0) and Weeks 1, 2, 4 and 6 in the DOX(–) condition. Values in panels E–I are mean ± SEM. * above the bars in panels E–I indicate significance (p < 0.05) as determined by 1-way ANOVA or mixed-effects analysis followed by posthoc tests. For E and F, spectral power for DS within each bandwidth was compared to all other states by 1-way ANOVA or mixed-effects analysis followed by posthoc tests.
Both male and female mice exhibited DS which progressively increased as the degeneration progressed (Figure 9G–I). EEG power spectra during DS were similar to the NREM power spectra (Figure 9E,F). In both sexes, delta power was greater in DS compared to W, REM or C (male: F(4, 24) = 24.3, p = 4.0 × 10–8; female: F(4, 14) = 10.47, p = 0.0004), spectral power in the theta, alpha and beta bands was greater in DS than in W (male: theta F(4, 24) = 20.71, p = 1.7 × 10–7; alpha F(4, 24) = 10.22, p = 5.6 × 10–5; beta F(4, 24) = 25.45, p = 2 × 10–8; female: theta F(4, 14) = 14.46, p = 7.08 × 10–5; alpha F(4, 14) = 18.18, p = 1.96 × 10–5; beta F(4, 14) = 16.19, p = 3.79 × 10–5), and beta power was also greater in DS than in cataplexy and REM sleep (male: F(4, 24) = 25.45, p = 2 × 10–8; female: F(4, 14) = 16.19, p = 3.79 × 10–5). On the other hand, theta power in DS was lower than during cataplexy in both sexes (male: F(4, 24) = 20.71, p = 1.7 × 10–7; female: F(4, 14) = 14.46, p = 7.08 × 10–5). No significant differences in any of the frequency bands were found between DS and NREM, except for low gamma power in females which was greater in DS than in NREM sleep (F(4, 14) = 9.98, p = 0.0005). Therefore, the EEG power spectra of DS was clearly different from that found during REM, cataplexy or Wake and most resembled that of NREM sleep.
Discussion
Although the consequences of dietary DOX withdrawal and subsequent Hcrt neuron degeneration has been well-documented in male orexin-tTA; TetO-DTA mice [30], similar information for female mice of this strain has been lacking. This is not unusual as, with some notable exceptions [35–42], female mice have been rarely used in sleep studies [27, 43]. The primary symptoms of human narcolepsy that can be assessed in a mouse model of this disorder are excessive daytime sleepiness (EDS), nocturnal sleep disruption, and cataplexy, the pathognomonic symptom of NT1.
We find that female DTA mice exhibit cataplexy sooner after DOX removal than their male counterparts: by Week 1, all female mice exhibited cataplexy whereas only 3 of 7 male mice showed this behavior. In contrast, all mice of both sexes exhibited cataplexy by Week 2 and cataplexy levels were similar in both sexes by Week 6. As described for males [30], cataplexy in females primarily occurred in the dark (active) phase, although some cataplexy also occurs during the light (inactive) phase in both sexes (Figure 2O vs. 2P). In males, mean cataplexy bout duration during the dark phase increased with time post-DOX removal as noted previously [30, 41], but this relationship was not evident for female DTA mice. The amount of cataplexy during the 12-h dark phase has previously been reported to be similar in males and females [41], results that are congruent with our findings in Weeks 2–6. However, the same study reported that female DTA mice exhibit more cataplexy bouts in the 12-h dark period than males [41], which differs from our results (Figure 5G). Inter-laboratory differences in substrain, housing conditions (presence or absence of a running wheel), or scoring of cataplexy may have a role here as the mean number of cataplexy bouts during the dark phase (Figure 5G) is more than twice the number reported previously for female DTA mice [41]. The more rapid emergence of cataplexy in females along with the absence of the progressive increase of cataplexy duration observed in males suggests that Hcrt neurons may degenerate faster in female than in male DTA mice. Alternatively, the neural circuits dependent upon input from Hcrt neurons may be more sensitive in females than males. However, the earlier appearance of cataplexy in female DTA mice could also be due to a sex difference in tetracycline pharmacokinetics and should be explored further.
The progressive decrease in Wake Bout Duration and increased number of wake bouts in both male and female DTA mice are indicative of disrupted sleep/wake architecture as Hcrt neuron degeneration proceeds. Both sexes exhibited 6–7 fold more wake bouts during the dark in Week 6 compared to baseline, indicating an inability to sustain wakefulness that was also reflected in the progressive reduction in mean wake bout duration. Together, these two parameters mirror EDS in people with narcolepsy for whom daytime naps are not only common, but a necessity for some individuals. It is interesting to note here that, although cataplexy bout duration in female DTA mice reaches a plateau by Week 1, wake bout duration progressively decreases with weeks after DOX removal. These results indicate that, in females, a more limited disruption of Hcrt neurotransmission results in cataplexy whereas the severity of EDS symptomatology may be more dependent on the extent of Hcrt neurotransmission disruption.
The present study provides limited evidence that the nocturnal sleep disruption characteristic of people with narcolepsy is reflected in DTA mice. In male DTA mice, there was a reduction of mean NREM bout duration during the light phase in Week 6 (Figure 4D) and there is a comparable nonsignificant trend in females, but the number of NREM bouts is constant in both sexes as Hcrt neuron degeneration proceeds (Figure 5D). Transient increases in mean REM bout duration occur during the light phase in both sexes as Hcrt neuron degeneration proceeds (Figure 4F) but these changes are not sustained.
As mentioned above and as illustrated by the large variation in wake bout duration in females (Figure 4A), one female DTA mouse remained awake throughout the entire dark (active) phase during baseline Week 0. Female C57BL/6 mice are known to spend less time awake during the dark period than males [35, 39] but, even in comparison to the female mice reported in those studies, this DTA female mouse is an outlier. Although the estrus cycle is reported to have minimal effects on sleep parameters relative to background strain [42], to control for any effect of the estrus cycle on sleep/wake, we conducted the video/EEG/EMG recordings at 4 day intervals. The sustained wakefulness of this female at baseline contrasts with the more typical pattern observed from this same individual during the dark phase in the Week 1, 2, 4, and 6 recordings during which this female should have been at the same stage of the estrus cycle. Moreover, none of the other mice recorded simultaneously with this mouse during Week 0 showed excessive wakefulness during the dark phase.
Reduced Tsc rhythm as Hcrt degeneration proceeds
The Tsc rhythm reported by the subcutaneously-placed DSI transmitters clearly shows a decline in the Tsc rhythm as Hcrt degeneration proceeds. Although this reduction is most evident in males (Figure 6C, E), ANOVA indicated that this decline was significant compared to the baseline (Hcrt neuron intact) condition in both sexes from Weeks 2–6. A study of human narcoleptics (conducted prior to the distinction between NT1 and NT2) that selected experimental subjects on the basis of short REM sleep onset found that rectal Tb was higher in people with narcolepsy at night than in control subjects, which was attributed to the disturbed nocturnal sleep of the patients [44]. Similarly, prepro-orexin knockout (KO) mice have a higher core Tb during sleep than wild type (WT) mice, which was attributed to either a deficiency in the heat loss or sustained activity of heat production mechanisms [45]. On the other hand, Hcrt/orexin neuron-ablated mice failed to exhibit the increased Tb that occurs in WT and KO mice in response to handling stress [46], implicating a colocalized Hcrt neuron neurotransmitter in thermoregulatory responses. Taken together, these data suggest a role for Hcrt neurons, although not the peptides themselves, in thermoregulation. Since mice of different strains and sexes prefer temperatures between 26–29°C [47] but our experiments were conducted at an ambient temperature of 22 ± 2°C, the DTA mice may have been unable to maintain Tsc at the baseline level while Hcrt neuron degeneration was proceeding. Human narcoleptics show an abnormal distal to proximal skin temperature gradient while both awake and asleep, suggesting a role for Hcrt/orexin neurons in skin temperature regulation [48]. Furthermore, manipulation of distal to proximal skin temperature improved nocturnal sleep in people with narcolepsy [49].
EEG spectra and delta state
The EEG spectra for waking and cataplexy reported in Figures 7 and 8 are based on human-scored EEG/EMG/video recordings collected from the first 6-h of the dark period (ZT13-18) and excluded epochs that contain artifacts that could otherwise contaminate the analyses. Consistent with the concept of narcolepsy as an arousal state boundary disorder, the waking EEG spectra in both sexes of DTA mice are characterized by progressively elevated delta, theta, and beta frequencies as the Hcrt neurons degenerate (Figure 7). Whereas some of these increases may be transient, the increased beta activity in female DTA mice persists at least as long as 6 weeks post-DOX. The waking EEG spectra show further evidence of a slower progression of the development of the narcoleptic phenotype in males compared to females. The normalized wake power between 0.5–40 Hz for Week 1 is distinctly different from Weeks 2–6 in males but not in females (Figure 7A, B).
For the EEG spectra during cataplexy, statistical analyses compared to the DOX(+) condition could not be performed since no cataplexy occurred on Week 0; consequently, our interpretation of the EEG spectra during cataplexy is qualitative (Figure 8). Overall, the EEG spectra in cataplexy was very similar between males and females and also showed a similar progression, with lower levels of theta (Figure 8D) and beta (Figure 8F) power in Week 1 than in subsequent weeks.
Careful analysis of the EEG, EMG, and video recordings by our expert scorers revealed a unique state that we called the DS, which was observed in both sexes but only while the Hcrt neurons were degenerating. DS events were typically ~30 sec in duration, ranging from 1 to 6 10-sec epochs, and were both preceded and followed by active Wake. Although the mice ceased movement during DS as in cataplexy, muscle tone was maintained but phasic EMG activity was absent. The EEG was synchronized with high delta activity as in NREM sleep but, as evident from the video recordings which were essential to recognize DS, the mice remain standing with their eyes open. The delta power during DS in both sexes was greater than that observed in Wake, REM, and Cataplexy and the theta power was less than that observed during REM sleep (Figure 9E, F). DS was distinguished from Wake not only by the increased delta power but spectral power in the theta, alpha, and beta bands was greater in both sexes during DS compared to Wake. DS has some similarities to “delta-theta sleep” which we recently described in mice in which both the Hcrt and melanin-concentrating hormone (MCH) neurons were ablated [50]. While we find increased delta in DS, we did not find increased theta as has been reported for delta-theta sleep [50]. However, methodological differences, such as different electrode configurations, could be a confound to preclude a direct comparison between DS and delta-theta sleep. Unfortunately, we did not follow this cohort of mice beyond 6 weeks DOX(–), so we do not know whether DS is a transitional state that only occurs while the Hcrt neurons are degenerating or whether this state occurs throughout the remainder of the mouse’s life.
DS resembles a previously reported state that has been labeled as “sleep attack” [51]. However, during DS, mice do not lose muscle tone or assume a sleeping position; they remain upright and the eyes remain open. Since the term “sleep attack” suggests the animals are asleep, the behavioral characteristics of DS do not resemble sleep. Consequently, we have used the term “delta state”. Further studies are required to determine whether “sleep attacks” and “delta state” are the same or different states. Although the DS state bears some resemblance to both “sleep attacks” and microsleeps, the exact analog of DS in humans remains to be unequivocally identified. Nonetheless, DS appears to be another manifestation of the arousal state boundary instability that characterizes narcolepsy in both humans and animal models of this disorder.
Use of Somnivore
In this study, we utilized the machine learning-based (ML) program Somnivore [34] to aid in scoring the remaining 18-h of the 24-h recordings for both male and female mice. Somnivore has been validated in a variety of eutherian mammals including birds [52], mice [34, 53], rats [34], and humans [34] and has also been used in another mouse model of narcolepsy, the prepro-orexin KO mouse [34]. The use of automated scoring systems for classification of arousal states is becoming increasingly widespread and has recently been implemented in studies of DTA mice [41]. Once a user has been trained in Somnivore, a 24-h EEG/EMG recording of a DTA mouse can be scored in 20–30 min. Thus, this tool and others like it hold the promise of greatly expediting sleep studies in rodents, in particular, although trained users must always be vigilant to monitor the raw recordings for unusual states such as DS.
Limitations of the present study
Although our study is a thorough characterization of male vs. female DTA mice, it is not without its limitations. First, our conclusions are based on a relatively small N for each sex which limits some statistical analyses yet, by and large, the results appear robust. As indicated above, we have utilized a ML-based algorithm to score the last 18-h of 24-h recordings, including cataplexy. Since video-based information is yet to be incorporated into this algorithm, the identification of cataplexy during the last 18-h was based on a training set of epochs scored as cataplexy by expert scorers who utilized the videos collected during the first 6-h of the 24-h recording. Nonetheless, all epochs scored as cataplexy by Somnivore were re-inspected by expert scorers who had access to the video recordings for epochs classified by Somnivore as cataplexy. Although this quality control step was absolutely crucial to the entire process, it was somewhat laborious. Despite such efforts, our study did not replicate the results of a recent publication [41] which claimed that REM sleep was elevated in male DTA mice during the dark phase. Although there are many procedural differences between these two studies that could account for this difference, one possible source that cannot be ignored is our use of a ML algorithm to score the second half of the dark phase.
While the DTA model, an inducible narcoleptic model that allows post-pubertal degeneration of the Hcrt neurons, provides a substantial advantage over other constitutive models in which developmental compensation can occur, the etiology of narcoleptic disorder is somewhat controversial. Although genetic, environmental, and serological studies link narcolepsy to an autoimmune mechanism [3, 54], a recent study has suggested that the Hcrt neurons remain intact in human narcolepsy and that this disorder may instead be due to epigenetic silencing of Hcrt, dynorphin, and NARP peptide expression in the Hcrt neurons [55]. As the etiology of this disorder becomes better understood, the validity of various animal models of narcolepsy should be reassessed, and other models that may be more reflective of the disorder developed.
Conclusions and Perspective
Although female DTA mice develop cataplexy, fragmented wakefulness, and EEG spectral changes more rapidly than their male counterparts, there appear to very few differences between the two sexes by 6 weeks DOX(–). Accordingly, we advocate for use of both sexes of this unique mouse model of narcolepsy to ensure that information relevant to the entire human population is acquired and that the goal of reducing the use of the number of animals can be achieved. Furthermore, to expedite sleep studies, we find that a ML-based algorithm can be utilized with high confidence by expert scorers as long as they remain vigilant for atypical EEG states. Lastly, we have identified a unique state called DS that resembles sleep attacks that occur in human narcolepsy.
As suggested above, the earlier occurrence of cataplexy, as well as the absence of the progressive increase of cataplexy duration in females, suggests a topic for future research. Do the Hcrt neurons degenerate faster in female than in male DTA mice and, if so, is this difference due to a sex difference in the rate of metabolism of tetracycline? Or are the neural circuits in females more sensitive to loss of Hcrt input than in males? Further definition of the DS, including its similarities and differences with delta-theta sleep, as well as identification of the neural substrates that underlie this state is likely to be a productive future research area.
Supplementary Material
Acknowledgments
We thank Haley Courtney, Laure Alexandre and Sahin Ozsoy for technical assistance and Chihung (Jeffrey) Hung for his comments on the distinction between the Delta State and Delta-Theta Sleep described in [50].
Contributor Information
Yu Sun, Biosciences Division, SRI International, Menlo Park, CA, USA.
Ryan Tisdale, Biosciences Division, SRI International, Menlo Park, CA, USA.
Sunmee Park, Biosciences Division, SRI International, Menlo Park, CA, USA.
Shun-Chieh Ma, Biosciences Division, SRI International, Menlo Park, CA, USA.
Jasmine Heu, Biosciences Division, SRI International, Menlo Park, CA, USA.
Meghan Haire, Biosciences Division, SRI International, Menlo Park, CA, USA.
Giancarlo Allocca, Somnivore Pvt. Ltd., Bacchus Marsh, VIC, Australia.
Akihiro Yamanaka, Department of Neuroscience II, Research Institute of Environmental Medicine, Nagoya University, Japan; Department of Neural Regulation, Nagoya University Graduate School of Medicine, Japan.
Stephen R Morairty, Biosciences Division, SRI International, Menlo Park, CA, USA.
Thomas S Kilduff, Biosciences Division, SRI International, Menlo Park, CA, USA.
Funding
This research was supported by National Institute of Neurological Diseases and Stroke R01 NS098813 and R01 NS103529 to T.S.K.
Disclosure Statements
Financial Disclosure: Giancarlo Allocca is a founder and owner of Somnivore, Pty. Ltd.
Non-financial Disclosure: none.
An earlier version of this manuscript is available at https://www.biorxiv.org/content/10.1101/2021.10.13.463880v1.
References
- 1. Scammell TE. Narcolepsy. N Engl J Med. 2015; 373 (27): 2654–2662. [DOI] [PubMed] [Google Scholar]
- 2. Thorpy MJ, et al. Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 2014;15(5):502–507. [DOI] [PubMed] [Google Scholar]
- 3. Szabo ST, et al. Neurobiological and immunogenetic aspects of narcolepsy: implications for pharmacotherapy. Sleep Med Rev. 2019;43:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silber MH, et al. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 2002;25(2):197–202. doi: 10.1093/sleep/25.2.197. [DOI] [PubMed] [Google Scholar]
- 5. Ohayon M, et al. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology. 2002;58(12):1826–1833. [DOI] [PubMed] [Google Scholar]
- 6. Longstreth WT, Jr. et al. Prevalence of narcolepsy in King County, Washington, USA. Sleep Med. 2009; 10 (4): 422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Longstreth WT, Jr., et al. The epidemiology of narcolepsy. Sleep. 2007; 30 (1): 13–26. doi: 10.1093/sleep/30.1.13. [DOI] [PubMed] [Google Scholar]
- 8. Maski K, et al. Listening to the patient voice in narcolepsy: diagnostic delay, disease burden, and treatment efficacy. J Clin Sleep Med. 2017;13(3):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morrish E, et al. Factors associated with a delay in the diagnosis of narcolepsy. Sleep Med. 2004;5(1):37–41. [DOI] [PubMed] [Google Scholar]
- 10. Ohayon MM, et al. Frequency of narcolepsy symptoms and other sleep disorders in narcoleptic patients and their first-degree relatives. J Sleep Res. 2005; 14 (4): 437–445. [DOI] [PubMed] [Google Scholar]
- 11. Heier MS, et al. Prevalence of narcolepsy with cataplexy in Norway. Acta Neurol Scand. 2009;120(4):276–280. [DOI] [PubMed] [Google Scholar]
- 12. Khatami R, et al. The European Narcolepsy Network (EU-NN) database. J Sleep Res. 2016;25(3):356–364. [DOI] [PubMed] [Google Scholar]
- 13. Luca G, et al. Clinical, polysomnographic and genome-wide association analyses of narcolepsy with cataplexy: a European Narcolepsy Network study. J Sleep Res. 2013;22(5):482–495. [DOI] [PubMed] [Google Scholar]
- 14. Auer M, et al. Gender-specific differences in access to polysomnography and prevalence of sleep disorders. J Womens Health (Larchmt) 2018;27(4):525–530. [DOI] [PubMed] [Google Scholar]
- 15. Scheer D, et al. Prevalence and incidence of narcolepsy in a US health care claims database, 2008-2010. Sleep 2019;42(7). doi: 10.1093/sleep/zsz091. [DOI] [PubMed] [Google Scholar]
- 16. Thorpy MJ, et al. Update on the pharmacologic management of narcolepsy: mechanisms of action and clinical implications. Sleep Med. 2020;68:97–109. [DOI] [PubMed] [Google Scholar]
- 17. Tisdale RK, et al. Animal models of narcolepsy and the hypocretin/orexin system: Past, present, and future. Sleep 2021;44(6). doi: 10.1093/sleep/zsaa278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thorpy MJ. Recently approved and upcoming treatments for narcolepsy. CNS Drugs. 2020; 34 (1): 9–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Black SW, et al. GABAB agonism promotes sleep and reduces cataplexy in murine narcolepsy. J Neurosci Off J Soc Neurosci. 2014;34(19):6485–6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Black SW, et al. Trace amine-associated receptor 1 agonists as narcolepsy therapeutics. Biol Psychiatry. 2017;82:623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmidt C, et al. The norepinephrine reuptake inhibitor reboxetine is more potent in treating murine narcoleptic episodes than the serotonin reuptake inhibitor escitalopram. Behav Brain Res. 2016;308:205–210. [DOI] [PubMed] [Google Scholar]
- 22. Irukayama-Tomobe Y, et al. Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models. Proc Natl Acad Sci USA. 2017;114(22):5731–5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sagawa Y, et al. Wake-promoting effects of ONO-4127Na, a prostaglandin DP1 receptor antagonist, in hypocretin/orexin deficient narcoleptic mice. Neuropharmacology. 2016; 110 (Pt A): 268–276. [DOI] [PubMed] [Google Scholar]
- 24. Fujiki N, et al. Specificity of direct transition from wake to REM sleep in orexin/ataxin-3 transgenic narcoleptic mice. Exp Neurol. 2009;217(1):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nirogi R, et al. Samelisant (SUVN-G3031), a potent, selective and orally active histamine H3 receptor inverse agonist for the potential treatment of narcolepsy: pharmacological and neurochemical characterisation. Psychopharmacology (Berl) 2021;238(6):1495–1511. [DOI] [PubMed] [Google Scholar]
- 26. Anaclet C, et al. Orexin/hypocretin and histamine: distinct roles in the control of wakefulness demonstrated using knock-out mouse models. J Neurosci Off J Soc Neurosci. 2009;29(46):14423–14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dib R, et al. review of the current state of knowledge on sex differences in sleep and circadian phenotypes in rodents. Neurobiol Sleep Circadian Rhythms 2021;11:100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Food and Drug Administration US. Risk of next-morning impairment after use of insomnia drugs; FDA requires lower recommended doses for certain drugs containing zolpidem (Ambien, Ambien CR, Edluar, and Zolpimist). In: Administration USFaD, ed. Washington, DC, 2013. [Google Scholar]
- 29. Festing MF, et al. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002; 43 (4): 244–258. [DOI] [PubMed] [Google Scholar]
- 30. Tabuchi S, et al. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci Off J Soc Neurosci. 2014;34(19):6495–6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tabuchi S, et al. Influence of inhibitory serotonergic inputs to orexin/hypocretin neurons on the diurnal rhythm of sleep and wakefulness. Sleep 2013;36(9):1391–1404. doi: 10.5665/sleep.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee P, et al. Conditional lineage ablation to model human diseases. Proc Natl Acad Sci USA. 1998;95(19):11371–11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scammell TE, et al. A consensus definition of cataplexy in mouse models of narcolepsy. Sleep. 2009;32(1):111–116. [PMC free article] [PubMed] [Google Scholar]
- 34. Allocca G, et al. Validation of “somnivore”, a machine learning algorithm for automated scoring and analysis of polysomnography data. Front Neurosci. 2019;13:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paul KN, et al. Diurnal sex differences in the sleep-wake cycle of mice are dependent on gonadal function. Sleep 2006;29(9):1211–1223. doi: 10.1093/sleep/29.9.1211. [DOI] [PubMed] [Google Scholar]
- 36. Franken P, et al. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions. Proc Natl Acad Sci USA. 2006;103(18):7118–7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ehlen JC, et al. Sex chromosomes regulate nighttime sleep propensity during recovery from sleep loss in mice. PLoS One. 2013; 8 (5): e62205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sare RM, et al. Sleep duration in mouse models of neurodevelopmental disorders. Brain Sci 2020;11(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koehl M, et al. Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep 2006;29(9):1224–1231. doi: 10.1093/sleep/29.9.1224. [DOI] [PubMed] [Google Scholar]
- 40. Huitron-Resendiz S, et al. Effects of withdrawal from chronic intermittent ethanol exposure on sleep characteristics of female and male mice. Alcohol Clin Exp Res. 2018;42(3):540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coffey AA, et al. The impacts of age and sex in a mouse model of childhood narcolepsy. Front Neurosci. 2021;15:644757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Koehl M, et al. Sleep in female mice: a strain comparison across the estrous cycle. Sleep 2003;26(3):267–272. [DOI] [PubMed] [Google Scholar]
- 43. Mong JA, et al. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci Off J Soc Neurosci. 2011;31(45):16107–16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mosko SS, et al. The 24-hour rhythm of core temperature in narcolepsy. Sleep 1983;6(2):137–146. doi: 10.1093/sleep/6.2.137. [DOI] [PubMed] [Google Scholar]
- 45. Mochizuki T, et al. Elevated body temperature during sleep in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2006;291(3):R533–R540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang W, et al. Orexin neurons are indispensable for stress-induced thermogenesis in mice. J Physiol. 2010; 588 (Pt 21): 4117–4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gaskill BN, et al. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS One. 2012; 7 (3): e32799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fronczek R, et al. Altered skin-temperature regulation in narcolepsy relates to sleep propensity. Sleep 2006;29(11):1444–1449. doi: 10.1093/sleep/29.11.1444. [DOI] [PubMed] [Google Scholar]
- 49. Fronczek R, et al. Manipulation of skin temperature improves nocturnal sleep in narcolepsy. J Neurol Neurosurg Psychiatry. 2008;79(12):1354–1357. [DOI] [PubMed] [Google Scholar]
- 50. Hung CJ, et al. Dual orexin and MCH neuron-ablated mice display severe sleep attacks and cataplexy. Elife 2020;9:e54275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu M, et al. Orexin gene transfer into zona incerta neurons suppresses muscle paralysis in narcoleptic mice. J Neurosci Off J Soc Neurosci. 2011;31(16):6028–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. van Hasselt SJ, et al. Seasonal variation in sleep homeostasis in migratory geese: a rebound of NREM sleep following sleep deprivation in summer but not in winter. Sleep 2021;44(4). doi: 10.1093/sleep/zsaa244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clark JW, et al. Manipulation of rapid eye movement sleep via orexin and GABAA receptor modulators differentially affects fear extinction in mice: effect of stable versus disrupted circadian rhythm. Sleep 2021;44(9). doi: 10.1093/sleep/zsab068. [DOI] [PubMed] [Google Scholar]
- 54. Bassetti CLA, et al. Narcolepsy–clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol. 2019;15(9):519–539. [DOI] [PubMed] [Google Scholar]
- 55. Seifinejad A, et al. 2021.. Narcolepsy with cataplexy is caused by epigenetic silencing of hypocretin neurons. bioRxiv. 2021.2009.2021.461046. Preprint: not peer reviewed. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.