Abstract
Non-clinical in vitro studies were conducted to investigate the characteristics of extracts from tobacco free nicotine pouches alongside a reference snus product and/or 1R6F reference cigarette. In vitro investigations were conducted in the Neutral Red Uptake (NRU) cytotoxicity assay, Bacterial Reverse Mutation (Ames) assay, and in vitro Mammalian Cell Micronucleus (ivMN) assay. These products were also investigated for their oral irritation potential in the EpiGingival™ 3D tissue model. Results from the Ames, in vitro Micronucleus and NRU assays indicated that the tested products were non-mutagenic, non-genotoxic and non-cytotoxic in contrast to results obtained for the 1R6F reference cigarette. Results from Complete Artificial Saliva (CAS) extracts from these products also failed to be classified as irritants (as measured using the MTT assay), in the EpiGingival™ 3D tissue model
Keywords: Smokeless, Nicotine pouch, In vitro, EpiGingival™, 3D tissue, Cytotoxicity, Micronucleus, Ames
Graphical Abstract
Highlights
-
•
Non-clinical in vitro studies on tobacco free nicotine pouch extracts.
-
•
Extracts were non-mutagenic, non-genotoxic and non-cytotoxic in contrast to results from the 1R6F reference cigarette.
-
•
Extracts were not classified as irritant (as measured using the MTT assay), in human 3D gingival epithelial cell cultures.
-
•
To our knowledge, this is the first study to assess the impact of nicotine pouch extracts on gingival organotypic cultures.
1. Introduction
Tobacco free nicotine pouch products represent a comparatively new product category offering additional choice to all tobacco product consumers, including cigarette smokers, snus users, and electronic cigarette vapers.
These products are very similar in appearance to Swedish style snus but differ significantly in that they do not contain tobacco. They may consist of plant-based fibers or filler materials (e.g., modified cellulose), additives, food grade flavorings, and pharmaceutical grade purity nicotine.
With the emergence of this product category, various guidelines and standards (e.g. [18], [19] have been developed defining certain safety and quality related requirements with the aim to establish a degree of uniformity across the product category. Additionally, some jurisdictions have or will issue product regulation e.g. Sweden.
These products are intended for oral use, are documented to have lower toxicant levels than marketed Swedish-style snus [1], [12] and have been suggested as offering reduced harm potential [1]. Given the current albeit limited data documenting reduced toxicant levels in comparison to Swedish style snus and noting that in 2019 the FDA authorized the marketing of eight Swedish style snus smokeless tobacco products through the modified risk tobacco product (MRTP) pathway, this assumption of reduced harm potential may not be entirely implausible.
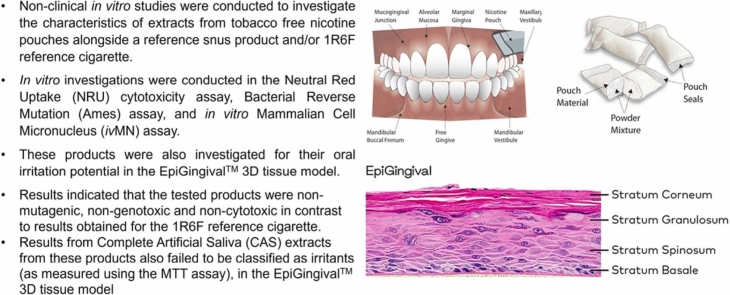
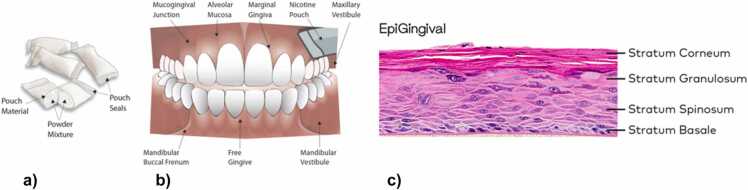
More recently, these products have been documented to have reduced in vitro biological activity and have been commonly tested in comparison to reference cigarette (1R6F), reference Swedish snus (CRP1 or 1.1), or commercially available Swedish style snus products. Bishop et al. [2] noted that tobacco-free nicotine pouches were significantly less biologically active than an equivalent reference snus product, whereas Yu et al. [6], more recently concluded that these products have a substantially reduced in vitro toxicological activity compared with traditional tobacco products. In the absence of standardized in vitro methods to evaluate these products East et al. [5] proposed a tiered testing strategy starting with screening ahead of commencing more standard regulatory assays. This publication evaluates the activity of these products as Complete Artificial Saliva (CAS) extracts in established regulatory assays (i.e. Ames, Neutral red uptake, in vitro micronucleus assays) and as these products are intended to be placed in the mouth under the lip, it also evaluates the products oral irritation potential in the EpiGingival™ 3D Tissue Model (see Fig. 1).
Fig. 1.
a) nicotine pouch product graphic b) oral cavity where pouch is usually placed c) EpiGingival™ 3D tissue model.
To our knowledge, this is the first study to assess the impact of nicotine pouch products on fully differentiated gingival organotypic cultures. All testing within this study was conducted at external independent contract research organizations using guideline protocols.
2. Method
2.1. Tested products
The products tested and the assays conducted in this study are shown in Table 1 alongside their respective sample codes.
Table 1.
Summary of products tested, and assays conducted.
| CODE | Sample |
Assay |
||
|---|---|---|---|---|
| Product name | Nicotine content | Regulatory assays (Ames, NRU, ivMN) | EpiGingivalTM 3D Tissue Model | |
| NP1 SM | Nordic Spirit Spearmint | 11.2 mga) | Yes | Yes |
| NP1 M | Nordic Spirit Mint | 9 mga) | Yes | Yes |
| NP1 M | Nordic Spirit Mint | 6 mga) | Yes | Yes |
| 1R6F | Kentucky Reference Cigarette | 0.7 mgb) | Yes | Yes |
| CRP1 | CORESTA snus Reference product | 8 mgc) | Yes | No |
a) per 650 mg of pouch, b) per cigarette (in smoke), c) per 1000 mg of pouch.
All nicotine pouch products were commercially available at the time of the study and were shipped to the independent research testing facilities, (batch numbers, expiry dates and certificates of analysis were also provided). Reference products i.e. 1R6F cigarette or CORESTA Swedish style snus product CRP1 were provided by the testing laboratories.
Bacterial Reverse Mutation (Ames), Neutral Red Uptake (NRU) and in vitro Micronucleus (ivMN) assays were conducted by an independent contract research organization in Canada (Labstat). Additionally, testing on gingival organotypic 3D cultures were completed at a Laboratory located in Skokie, Illinois, USA (Charles River Laboratories, Sara Hurtado (study director)).
2.2. Sample preparation
2.2.1. Combustible tobacco product
Combustible tobacco products for regulatory testing were conditioned according to ISO3402 (e.g. 22 ± 1 °C and 60 ± 3 % relative humidity for a minimum of 48 h but not exceeding 10 days) then, smoked on a rotary smoking machine and processed into cigarette smoke condensate samples. The smoking parameters are set out in Table 2. below.
Table 2.
Summary of study smoking parameters.
| Variable | ISO 3308 | HCI/ ISO 20778 |
|---|---|---|
| Puff Volume (mL) | 35 | 55 |
| Interval (sec) | 60 | 30 |
| Duration (sec) | 2 | 2 |
| Ventilation holes | Open | 100 % closed |
Condensate samples for Ames, NRU and ivMN (regulatory assays) were collected in accordance with ISO 3308 [11]. Condensate samples for EpiGingival™ 3D tissue testing was collected in accordance with the Health Canada Intense HCI regime (HCI/ISO 20778) defined by ISO 20778 [10].
In brief, TPM collection for the Ames, NRU, ivMN or 3D tissue testing was collected on pre-weighed Cambridge filter pads and extracted in an appropriate amount of solvent i.e. either DMSO or ethanol to obtain a TPM extract concentration of 10 mg TPM/mL DMSO (for Ames, NRU and ivMN), or 30 mg TPM/mL in ethanol for 3D tissue testing.
Note: For the Ames and micronucleus assays, the CRO tested a previously prepared, pooled sample of TPM extracts in DMSO from the 1R6F cigarette control smoked under HCI. This sample was in addition to the fresh TPM test extracts prepared under ISO for this study. Results from these pooled extracts were comparted to laboratory historical ranges to confirm the validity of assay results. For the NRU assay, the laboratory prepared fresh TPM samples smoked under HCI for comparison with historical laboratory ranges.
2.2.2. Preparation of smokeless products
Smokeless products were prepared by removal of non-tobacco material. The contents (smokeless product excluding pouch) were then ground and sieved through a 4 mm sieve. Non-tobacco material was then ground/scissor-cut and sieved separately and later recombined with the previously ground contents and mixed thoroughly.
2.2.3. Preparation of complete artificial saliva (CAS)
Complete Artificial Saliva (CAS) was prepared and stored in accordance with the methodology described by Chou and Que Hee, [3].
In brief, 1 L of sterile Type I water was added to a sterile glass container with a Teflon stir bar and heated to 60 °C using a hotplate. The appropriate amount of mucin (see Table 3) was then dissolved in the water, the heat was removed, and the solution allowed to cool, while continually stirring. As the solution cooled, all other components of the base solution were added in their documented amounts (see Table 3).
Table 3.
Summary of base solution components.
| Component | Source | CAS No. | Grams/L Type I Water |
|---|---|---|---|
| Mucin | Gastric Mucin III | 84082-64-4 | 2.70 |
| Potassium | Potassium chloride | 7447-40-7 | 0.95 |
| Sodium | Sodium chloride | 7647-14-5 | 1.40 |
| Calcium | Calcium chloride | 10043-52-4 | 0.27 |
| Phosphorus | Sodium dihydrogen phosphate | 7758-80-7 | 0.58 |
| Magnesium | Magnesium chloride | 7786-30-3 | 0.1 |
| Urea | Urea | 57-13-6 | 0.09 |
| Glucose | D-(+)-Glucose | 50-99-7 | 0.20 |
CAS preparation was completed by adding enzymes, to the base solution as per Table 4 below. e.g. for a 2000 mL volume of CAS, 1000 mL of the base solution was gently heated in a sterile container, to which 12.500 mL Alpha-amylase solution, 0.596 mL lysozyme solution, 16.000 mL of acid phosphatase solution and 970.904 mL type I water was added. The CAS solution was then mixed.
Table 4.
Summary of enzyme solution components.
| Component | Units per Liter of final solution |
|---|---|
| Alpha-amylase | 100,000 |
| Lysozyme | 700 |
| Acid Phosphatase | 4 |
2.2.4. Preparation of Smokeless extract for Ames, NRU, ivMN and 3D Tissue Assay
Ground product (2 ± 0.02 g) was added to 6 mL of CAS. The content was mixed by vortexing. The mixture was shaken at 60 rpm, 37 ± 0.5 ºC for 1 ± 0.1 h using the shaker incubator. The mixture was then transferred into centrifuge tube(s) and centrifuged for 15 min at room temperature. The liquid phase (supernatant) was then transferred into another container using a pipette. One mL of CAS was then pipetted into the test tube to wash the residue. The supernatant was then filtered through 5.0, 1.2, and then 0.45 µm nylon filters. The CAS extracts were then filter-sterilized using a sterile disposable 0.22 µm filter. An appropriate amount of filter-sterilized smokeless extract was then prepared for toxicology assays, chemistry analysis, determination of pH or osmolarity of extracts with tissue culture media.
2.2.5. Preparation of smokeless extract for EpiGingival™ 3D Tissue Model studies
Smokeless extract was prepared as a stock formulation in CAS at a target concentration of 30 % (w/v) on the day of the initial and confirmatory irritation assays. The top concentration of 30 % (w/v), was found to be the highest workable laboratory concentration.
Concentrations tested in the initial assays were 3.75 %, 7.5 %, 15 %, and 30 % (w/v), with lower concentrations being prepared by dilution of the concentrated stock in CAS. A dosing volume of 100 µL, was then applied to the apical surface of the tissue.
3. Bacterial Reverse Mutation (Ames) Assay
The Bacterial Reverse Mutation Assay, commonly referred to as the Ames assay, was utilized adopting the pre-incubation method and performed according to the requirements of Health Canada official test method T-501 [7], based on OECD Guideline No. 471 [16].
The potential for mutagenicity was assessed in Salmonella typhimurium tester strains TA98, TA100, TA 102, TA1535, and TA1537 in the presence and absence of metabolic activation. In summary, tester strains were prepared in sterile pre-labelled reaction tubes by adding the required amount of phosphate buffer or S9 mix (Aroclor 1254-induced rat (Sprague Dawley) liver S9), the required amount of smokeless product (SP) CAS extract, cigarette TPM extract in DMSO, DMSO, CAS or positive control solution (see Table 5). After vortexing, the mixture was allowed to incubate with shaking for 20 ± 2 min at 37 ± 1 °C. Two milliliters of selective top agar were then added to each tube and the mixture overlaid onto the surface of 25 mL of minimal glucose bottom agar. After solidifying, the plates were inverted and incubated for 48–72 h at 37 ± 1 °C.
Table 5.
Summary of Ames assay positive controls.
|
S. typhimurium strain |
S9 Activation |
Positive Control |
Solvent | Concentration [µg/plate] |
|---|---|---|---|---|
| TA98 | No activation | 2-nitrofluorene | DMSO | 4.0 |
| TA98 | Rat liver | 2-aminoanthracene | DMSO | 2.0 |
| TA98 | Rat liver | Benzo[a]pyrene | Acetone | 5 |
| TA100 | No activation | Sodium azide | Type I Water | 1.0 |
| TA100 | Rat liver | 2-aminoanthracene | DMSO | 2.0 |
| TA102 | No activation | Mitomycin C | Type I Water | 0.5 |
| TA102 | Rat liver | 2-aminoanthracene | DMSO | 7.5 |
| TA1535 | No activation | Sodium azide | Type I Water | 1.0 |
| TA1535 | Rat liver | 2-aminoanthracene | DMSO | 4.0 |
| TA1537 | No activation | 9-aminoacridine | DMSO | 100.0 |
| TA1537 | Rat liver | 2-aminoanthracene | DMSO | 4.0 |
For the 1R6F reference cigarette, TPM extract dose levels of 0, 25, 50, 75, 100, 125, 250, 500 µg TPM/plate were tested. For each smokeless test item in CAS dose levels of 0, 0.71, 1.43, 2.86, 5.71, 8.57, 11.43, 14.29, 17.14, 22.86, 28.57 mg SP/plate were tested for replicate 1 and dose levels of 0, 0.71, 1.43, 2.86, 5.71, 11.43, 14.29, 28.57 mg SP/plate were tested for replicate 2 and 3.
The assays were completed in triplicate.
3.1. Data analysis
The test samples were evaluated as mutagenic if the results in any strains with or without S9 were shown to give a reproducible dose-dependent increase in the number of revertants and at least a two-fold increase in the number of revertants over concurrent solvent control at one or more dose concentrations [15].
All Ames assay results for the TPM extracts in DMSO and smokeless product extracts in CAS were subjected to the point rejection method for determining the linear portion of the dose-response curve.
4. Neutral Red Uptake (NRU) Assay
The NRU assay was performed to assess cytotoxicity. The assay was conducted following the requirements of the Health Canada official test method T-502 [8], Third Edition 2017-12-31. In brief, Chinese hamster ovary (CHO) cells were cultured in tissue culture media consisting of Ham’s F-12 media supplemented with 10 % fetal bovine serum and 100 units/mL penicillin and 100 µg/mL streptomycin mixture, as T-502. Cells derived from log phase cultures were plated in 96-well microtiter plates at a density of approximately 5 × 104 cells/mL. Prepared cells were then exposed to CAS extract of smokeless products, cigarette TPM extract in DMSO, DMSO, CAS or positive control (Sodium lauryl sulphate, 110 μg/mL) at 37 ± 1 °C for 24 h in a humidified 5 % CO2 incubator.
After 24 h, the medium with test item was removed from the plate and 200 µL of neutral red dye solution was added to each well. After 3 h of incubation, cells were fixed with a 1 % formalin solution. The dye absorbed by intracellular organelle lysosomes were then extracted by adding 200 µL of neutral red desorb solution (1 % v/v glacial acetic acid, 50 % v/v ethanol, 49 % v/v water). After brief agitation, the concentration of the dye in the supernatant of each well was determined by reading the optical density at 540 nm on a microplate reader.
4.1. Tested concentrations
Cigarette TPM extract in DMSO, was tested at dose levels of 10, 50, 75, 100, 120, 140, 160, 200 µg TPM/ mL. Additionally, following the results of dose range finding studies, 9 and 11.2 mg CAS extract of smokeless product was tested at dose levels of 1.43, 2.86, 5.71, 8.57, 11.43, 12.86, 14.29, 17.14 mg SP/mL. Whereas the 6 mg CAS extract of smokeless product including CRP 1 were tested at 1.43, 2.86, 5.71, 8.57, 11.43, 14.29, 15.71, 17.14 mg SP/mL.
Dose levels were chosen based on pH and osmolarity results. The assay was completed in triplicate.
4.2. Data analysis
The study endpoint for the NRU cytotoxicity assay is the IC50, (inhibitory concentration 50 %), which is the concentration of the test item that reduces the relative absorbance to 50 % of that of the vehicle control. IC50 was determined by fitting a non-linear sigmoidal model to the concentration-response curve and determining the concentration yielding a 50 % reduction (i.e. relative absorbance of 50 %).
Each IC50 statistic (i.e. response measure) has the unit “µg TPM/mL” for combustible test item in DMSO and “mg SP/mL” for the smokeless test item in CAS samples.
Relative absorbance (%) was calculated from the raw assay plate absorbance readings on an individual assay plate. The raw absorbance readings for each 96-well plate were blank-corrected and expressed relative to the vehicle control absorbance reading as described in the Health Canada test methodology.
All NRU cytotoxicity assay results were evaluated, to determine which dose results fit the non-linear regression model. All instances of excluded doses or results which did not fit the model were noted.
5. In vitro Micronucleus Assay (ivMN)
The ivMN assay was performed in accordance with Health Canada Official Method T-503 [9], which is based on the OECD Guideline No. 487 [17].
In summary, Chinese hamster ovary (CHO-WBL (IVGT)) cells were grown as a monolayer in tissue culture grade flasks. The average number of viable cells per mL of cell suspension was calculated using a hemocytometer. Each cell suspension was seeded into the required number of tissue culture flasks and flasks were incubated at 37 ± 2 °C in a humidified, 5 ± 1 % CO2 atmosphere for 24 ± 3 h.
Tissue culture flasks were then prepared, and the presence of CHO cells was confirmed in accordance with T-503. CAS extract of smokeless product (SP) or cigarette TPM extract in DMSO was prepared to defined concentrations following dose range finding studies (µg TPM/mL or mg SP/mL), depending on the treatment schedule. Negative control solutions of DMSO in growth media were prepared to the following concentrations of 20 µL/mL for short-term exposure, schedules (i) and (ii) 10 µL/mL for long-term exposure, schedule (iii). Negative control solutions of CAS in growth media were prepared to concentration of 100 µL/mL for all three schedules.
In the absence of metabolic activation, colchicine and mitomycin C were positive controls, whereas cyclophosphamide was used in the presence of metabolic activation (see Table 6). DMSO or CAS in growth media were used as solvent control in all treatment schedules.
Table 6.
Summary of ivMN assay positive controls.
| Treatment Schedule | S9 Metabolic Activation | Positive Control | Aneugen / Clastogen | Concentration [µg/mL] |
|---|---|---|---|---|
| Schedule (i) | No activation | Mitomycin C (MMC) | clastogen | 2 |
| Schedule (i) | No activation | Colchicine | aneugen | 2 |
| Schedule (ii) | Rat liver | Cyclophosphamide (CP) | clastogen | 7.5 |
| Schedule (iii) | No activation | Mitomycin C (MMC) | clastogen | 0.5 |
| Schedule (iii) | No activation | Colchicine | aneugen | 0.5 |
5.1. Treatment schedules
Cells were exposed to growth medium containing each test sample (positive control, untreated control, solvent control (DMSO or CAS), CAS extract of smokeless product or TPM extract in DMSO) in three treatment schedules: short-term exposure without (schedule i) or with (schedule ii) metabolic activation and a long-term exposure without metabolic activation (schedule iii).
For the short-term exposure, the cell culture was treated with test samples for 3 h ± 15 min without or with 9-mix containing Aroclor 1254-induced rat liver homogenate. After removal of the test sample, the cells were incubated for 27 h ± 1 h. For the long-term exposure, the cells were incubated with test sample for 30 h ± 1 h in the absence of metabolic activation. All exposures were conducted at 37 ± 2 °C in a humidified, 5 ± 1 % CO2 atmosphere.
5.2. Tested concentrations
Reference cigarette TPM extract in DMSO was tested at 0, 75, 100, 150, 200 µg TPM/ mL in schedule i and ii i.e. without and with S9 and at 0, 12.5, 25, 50, 75 µg TPM/mL in schedule iii (long-term in the absence of S9). Additionally, further to the results from preliminary dose range finding (DRF) studies each test item smokeless extract in CAS, was tested at: 0, 5.71, 8.57, 11.43, 14.29, 17.14 mg SP/mL in schedules i, ii and iii.
After exposing cells to the test articles and incubating the flasks, cells were then detached from the surface of each flask and counted. Cytotoxicity was measured by calculating the number of viable cells (relative increase in cell count) using a hemocytometer and trypan blue.
Cells were fixed and slides were prepared using a cytospin centrifuge. Cells were subsequently stained with Acridine Orange. Following slide preparation, 1000 randomly selected cells were scored for the presence of micronuclei, (1000 cells/slide, duplicate culture) using fluorescence microscopy. Duplicate flasks were assayed at each dose level for each test item i.e. TPM extract in DMSO, smokeless extract in CAS, solvent or positive control.
Micronucleated cells per 2000 cells were scored and micronucleus (MN) cell frequency (%MN) was calculated. Experiments were conducted in triplicate.
The test articles were deemed to give a positive genotoxic response if:
-
1)
There was a statistically significant increase (α = 0.01) in the mean frequency of micronuclei for at least one concentration compared to the solvent control using the Dunnett’s test.
-
2)
There was a concentration-related increase in the number of micronuclei per 1000 scored cells over the range tested.
-
3)
The mean frequency of micronuclei at any TPM or smokeless extract in CAS exceeds the historical DMSO or CAS solvent control
A test item was regarded as genotoxic in the replicate assay if all criteria were met.
6. EpiGingival™ 3D tissue model
3D tissue models are a highly reproducible, non-animal technique that may be utilized in screening for the irritation potential of oral products [13]. Tissue irritation is measured as function of cell viability and is determined using the MTT (3-[4,5-dimethylthiazol-2-yl]−2,5- diphenyl tetrazolium bromide) assay. The assay measures the extent of MTT reduction by monitoring a color change i.e. metabolically active cells reduce the yellow MTT tetrazolium salt to a purple formazan dye and as such, this is a measure of cell viability. Therefore, inhibition of cell growth and/or cell death will decrease the amount of MTT being reduced, and cytotoxicity is expressed as a concentration-dependent decrease in MTT reduction to formazan in response to test chemical exposure.
6.1. Tissue properties
EpiGingival™ tissues were purchased from MatTek Corporation (Ashland, MA USA) as part of the EpiGIN-100 Kit. The gingival tissue (designated GIN-100) was 9–13 layers thick and cornified at the apical surface. The tissues express cytokeratin’s 13 and 14.1. The GIN-100 tissue model consists of a partial thickness model of cell layers containing stratum distendum, and basal cells. Tissues were stored refrigerated (2–8 °C) for up to 72 h prior to dosing.
6.2. Tissue equilibration
One day prior to dosing, tissues were equilibrated and then aliquoted into each well of sterile 6-well plates (0.9 mL/well). Plates were then labeled with the tissue type and date and placed into a humidified incubator at 36–38 °C and 4–6 % CO2.
6.3. Irritation Assay
Following the equilibration period, the assay media was aspirated from each well (or tissues were transferred to fresh plates), and the media was replaced with 0.9 mL/well of pre-warmed, fresh assay media.
The vehicle control, positive control, or test article was then applied to the apical surface of the tissue (100 µL/tissue). Plates were then returned to the incubator for incubation periods of 4-h or overnight (18–24-h).
Smokeless test article was tested at concentrations of 3.75 %, 7.5 %, 15 %, and 30 % (w/v) and TPM extract was tested at 0.625 %, 1.25 %, 2.50 %, and 5.00 % (v/v) in duplicate tissues (i.e. initial and confirmatory tests). Stock concentrations of 30 % (w/v) in CAS were prepared fresh on each day of testing with lower concentrations prepared by dilution of the stock.
For TPM extract, the concentrations tested were the limits of solvent compatibility with the test system. Additionally, the TPM doses corresponded to TPM concentrations of 0.188, 0.375, 0.75, and 1.5 mg/mL.
The positive control was sodium lauryl sulfate (SLS), tested at 0.5 % (w/v) and 0.15 % (w/v) for the 4-h and overnight (18–24-h) treatments, respectively. The vehicle/negative control was CAS or ethanol/PBS.
6.4. MTT addition
Approximately 15 min before each dosing period was complete, MTT solution was pipetted into a 24-well plate (300 µL/well). After exposure of the tissue samples to the test material, any remaining liquid within the cell culture insert was decanted or aspirated off. Each culture insert was then rinsed with DPBS (Dulbecco's Phosphate-Buffered Saline) to remove any residual test material with any excess liquid being shaken off prior to placing the insert in the MTT containing 24-well plate. The tissue samples in the 24-well plate were then returned to the incubator for 2–3 h at 36–38 °C, 4–6 %CO2.
6.5. MTT extraction
After incubation with MTT each insert was removed and placed in a pre-labeled 24-well extraction plate. The cell culture inserts were then immersed with 2.0 mL of extractant solution per well to completely cover the cell culture insert. After the extraction period, the liquid within each insert was decanted back into the well from which it was taken (i.e. the solution was mixed with the extractant in the well).
6.6. Data collection
The extractant solution was then pipetted to ensure through mixing of the extraction solutions. Next, 200 µL of the mixed extraction solution was added to the designated wells of a clear flat bottom 96-well microtiter plate. The optical density of the extracted samples was then measured at 570 nm, (subtracting out a background reading for all samples at 650 nm and using 200 µL of extractant as a blank).
A dose response curve for each treatment time was then constructed, if the relative viability at any test article concentration was < 50 % of the vehicle control. Using a semi-log scale, the % viability (linear y axis) was plotted versus the dosing concentration (log x axis). The EC50 values would then be determined from the test article concentration-response, using a Hill function which is a four-parameter logistic mathematical model relating the concentration of the test article or positive control to the response (typically following a sigmoidal shape).
A decrease in cell viability, below 50 % of the vehicle control was used as a predictor of irritation [13].
7. Nicotine quantification in CAS smokeless extracts (Ames, NRU and ivMN Assays)
Two grams of ground product was lyophilized for 48 h and then ground further using a bench-top grinder and a #40 screen (40 sections/square inch). Twenty-five milligrams of ground product were then extracted with 1.0 mL of a methanolic KOH solution containing an internal standard (e.g. 4,4-dipyridyl dihydrochloride or 4,4-dipyridyl hydrate) in an ultrasonic bath for 3 h followed by 0.5 h of shaking on a wrist-action shaker. The mixture was then centrifuged at low speed to separate any solids from the solution. The supernatant was then transferred to an autosampler vial and analyzed by gas chromatography (GC) using a CAM fused silica capillary column with a polyethylene glycol (PEG) stationary phase that has been specifically base-deactivated for volatile amines analysis. Quantification was achieved using an internal standard calibration by comparing the Thermionic Specific Detector (TSD) response of the analytes in the samples against a five-point calibration of alkaloids in the standards.
For CAS extract, 100 µL was diluted with 900 µL of Type I water. This diluted extract was then mixed with 1000 µL of Alkaloids extraction solution containing internal standard and with solution of K2CO3 in dilute aqueous ammonia.
Limits of Detection (LOD) and Limits of Quantification (LOQ) for nicotine in liquid extracts were 18.75 and 62.5 µg/mL respectively.
8. Results
8.1. Ames Mutagenicity Assay
The mutagenic response for each test item i.e. TPM extract in DMSO or smokeless extracts in CAS for each replicate assay was evaluated, followed by an overall assessment of the mutagenic response across all three replicate assays.
The 1R6F TPM extract in DMSO was mutagenic in strains TA98 (+S9) and TA1537 (+S9). Additionally, the 1R6F TPM extract was only found to be mutagenic in one replicate assay for strain TA98 (-S9). Given the number of replicates that were mutagenic, the 1R6F test item was deemed overall non-mutagenic in strain TA98 (-S9). Furthermore, the overall assay response for 1R6F was considered non-mutagenic for all other strains (TA100 (+S9), TA100 (-S9), TA102 (+S9), TA102 (-S9), TA1535 (+S9), TA1535 (-S9), TA1537 (-S9)) under the testing conditions.
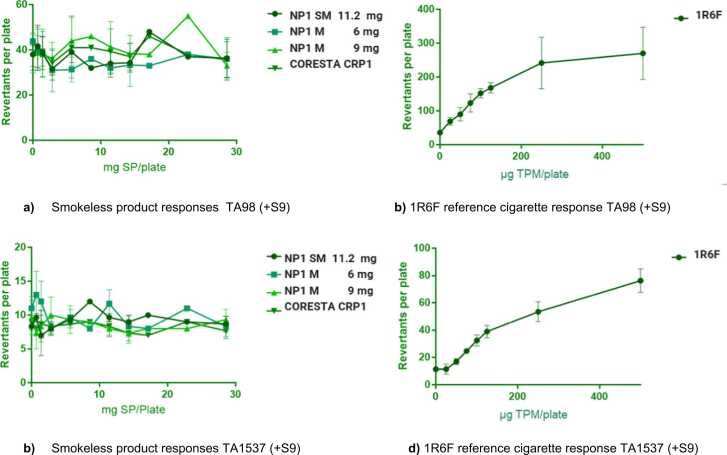
All smokeless product CAS extract in all three replicate assays were non-mutagenic in all strains with and without metabolic activation, see Fig. 2. (a–d).
Fig. 2.
Bacterial Reverse Mutation (Ames) Assay responses in strains TA 98 (+S9) and TA 1537 (+S9). Data points in each plot represent the mean values ± SD from 3 replicate assays.
Precipitation was not evident within the culture media used for this project or on any assay plate. There was however evidence of Ames toxicity (i.e. background lawn not comparable to negative control) at the higher TPM or SP concentrations for each assay i.e. for 1R6F reference cigarette above 125 ug TPM plate and greater than or equal to 11.43 mg SP/plate.
8.2. Neutral Red Uptake Assay
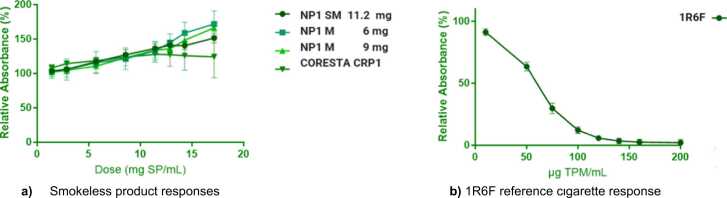
The cytotoxic response for each test item, in each replicate assay was evaluated, followed by an overall assessment of the cytotoxic response across all three replicate assays. The 1R6F test item elicited concentration-related decreases in neutral red uptake for TPM extracts in DMSO over the dose ranges tested (i.e. 0–200 µg TPM/mL), with at least a 50 % reduction in neutral red absorbance compared to the vehicle control. The test item was considered cytotoxic over the dose ranges tested with a mean IC50 of 59.0 µg TPM/mL and a standard error of 1.7 µg TPM/mL. The test item assay response for smokeless product extract in CAS all showed relative absorbance means greater than 50 % for all dose levels and assay plates. Hence, IC50 values could not be calculated under the testing conditions, see Fig. 3.
Fig. 3.
Neutral Red Uptake (NRU) Assay Results. Data points in each plot represent the mean values ± SD from 3 replicate assays.
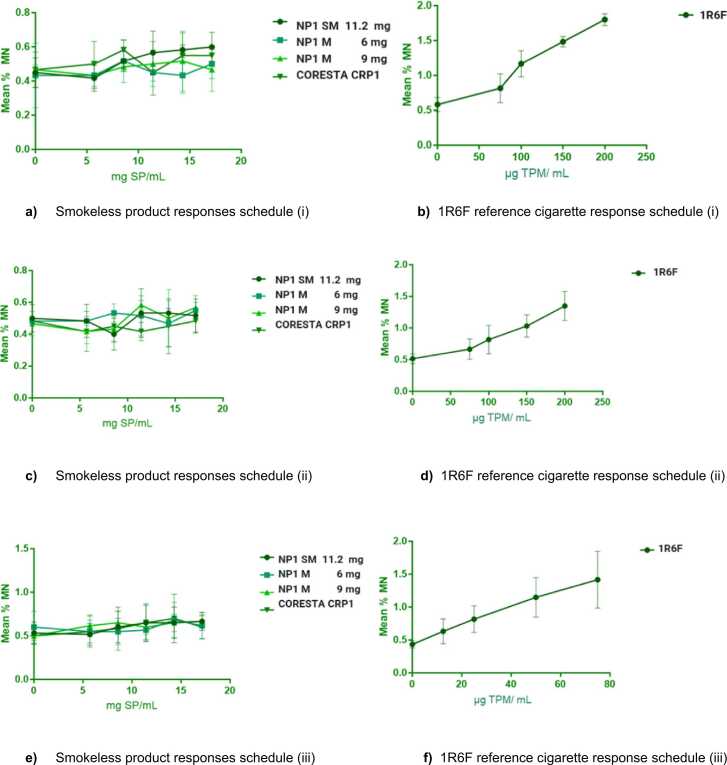
8.3. In vitro Micronucleus Assay
The genotoxic response of each test item in each replicate assay was evaluated, followed by an overall assessment of the genotoxic response across all three replicate assays. In both schedules (i) and (iii), the 1R6F reference cigarette had 2 of 3 replicates deemed as genotoxic. Based on the number of replicates that were genotoxic and the historical solvent control range, results were overall ‘genotoxic’ under the testing conditions. Additionally, 1R6F results in schedule (ii) indicated that all 3 replicates were genotoxic under the testing conditions. Hence, the overall assay response for 1R6F TPM extract in DMSO was considered genotoxic in all three treatment schedules.
For all treatment schedules (i), (ii) and (iii), all smokeless test items in CAS in all three replicate assays were non-genotoxic, see Fig. 4.
Fig. 4.
:In Vitro Mammalian Cell Micronucleus (ivMN) assay results. Data points in each plot represent the mean values ± SD from 3 replicate assays.
8.4. Gingival organotypic cultures EpiGingivalTM 3D tissue model
Smokeless products were tested at 3.75 %, 7.5 %, 15 %, and 30 % w/v in CAS (the maximum feasible concentration documented by the testing facility). Whereas 1R6F TPM was tested at 0.625 %, 1.25%, 2.50 %, and 5.00 % v/v, corresponding to TPM concentrations of 0.188, 0.375, 0.75, and 1.5 mg/mL).
No decrease in cell viability was noted in either the short (4-h) or extended overnight (18–24 h) treatments for any of the test articles (see Table 7). Additional information is also shown in supplementary Tables S1–S16.
Table 7.
Summary of results from the MTT viability assay for 1R6F and smokeless products extracts in the EpiGingival™ 3D Tissue Model (4-h and extended overnight Treatment). Values represent average viability calculated from 3 wells per tissue at a given concentration, (duplicate tissues were tested).
|
Values represent average viability calculated from 3 wells per tissue at a given concentration, (duplicate tissues were tested) | |||||
|---|---|---|---|---|---|
| Product | Dose Level (%, v/v) or (%,w/v) SP |
Average Viability (%) 4-h Treatment (Initial Irritation assay) |
Average Viability (%) 4-h Treatment (Confirmatory irritation assay) |
Average Viability (%) 18-h Treatment (Initial Irritation assay) |
Average Viability (%) 18-h Treatment (Confirmatory irritation assay) |
| 1R6F | 0.625 | 102 | 97 | 108 | 113 |
| 1.25 | 135 | 95 | 103 | 112 | |
| 2.50 | 101 | 91 | 99 | 110 | |
| 5.00 | 98 | 100 | 100 | 111 | |
| VC | 100 µL | 100 | 100 | 100 | 100 |
| SLS | 0.5 or 0.15 | 24 | 28 | 6 | 18 |
| NP1 M 6 mg | 3.75 | 97 | 98 | 103 | 99 |
| 7.5 | 92 | 102 | 107 | 93 | |
| 15 | 97 | 93 | 97 | 126 | |
| 30 | 101 | 98 | 86 | 76 | |
| VC | 100 µL | 100 | 100 | 100 | 100 |
| SLS | 0.5 or 0.15 | 15 | 31 | 21 | 14 |
| NP1 M 9 mg | 3.75 | 101 | 91 | 106 | 93 |
| 7.5 | 98 | 88 | 107 | 83 | |
| 15 | 100 | 91 | 95 | 83 | |
| 30 | 102 | 86 | 88 | 92 | |
| VC | 100 µL | 100 | 100 | 100 | 100 |
| SLS | 0.5 or 0.15 | 29 | 22 | 8.4 | 5 |
| NP1 SM 11.2 mg | 3.75 | 97 | 105 | 95 | 97 |
| 7.5 | 102 | 94 | 95 | 104 | |
| 15 | 97 | 91 | 93 | 81 | |
| 30 | 81 | 81 | 79 | 75 | |
| VC | 100 µL | 100 | 100 | 100 | 100 |
| SLS | 0.5 or 0.15 | 20 | 27 | 12 | 9 |
VC = 5 % Ethanol in Phosphate Buffered Saline (1R6F only) or VC = Vehicle Control; Complete Artificial Saliva
SLS - Sodium Lauryl Sulfate (%, w/v).
Data from the positive controls were comparable to expected historical control ranges and as such, demonstrated the validity and sensitivity of the test system for detecting irritation.
9. Discussion/ conclusion
Researchers conducting in vitro studies on nicotine pouch or smokeless products have documented extractions using various media. For a summary see Bishop et al. (2020) and more recently Yu et al. [6].
This study extracted smokeless products (including pouch material) in CAS as a means to generate a more physiologically relevant extract. Results showed that extracts from smokeless products of increasing nicotine strength resulted in a proportional increase in extract concentration thus demonstrating no matrix saturation. Additionally, extracted nicotine amounts in CAS were in line with those from similar strength nicotine products extracted in PBS [6], see Table 8.1 Additional information is also available in supplementary Table S17.
Table 8.
Overview of extracted smokeless nicotine content mg/mL solvent.
| Product extracted | mg nicotine/mL solvent |
|---|---|
| Values represent the mean of four replicates ± SD | |
| NP1 SM (CAS) 11.2 mg | 4.21 ± 0.45 |
| NP1 M (CAS) 9 mg | 3.22 ± 0.39 |
| NP1 M (CAS) 6 mg | 2.05 ± 0.31 |
| CRP1 (CAS) 8 mg | 2.67 ± 0.24 |
| Product extracted(as taken from Yu et al. [6] | mg nicotine/mL solvent |
| Values represent the mean of three replicates ± SD | |
| TFNP (PBS) 10.1 mg | 2.51 ± 0.15 |
| TFNP (PBS) 5.8 mg | 1.7 ± 0.07 |
| Snus (PBS) 10.9 mg | 2.59 ± 0.10 |
PBS (phosphate-buffered saline); CAS (Complete Artificial Saliva); SD (standard deviation).
Results from the Ames, ivMN and NRU assay showed that all tested smokeless product extracts were non-mutagenic, non-genotoxic and non-cytotoxic. Additionally, as all positive and negative control assay results were found to meet the acceptance criteria in the relevant standards, all results were deemed to be reflective of the characteristics of the tested products. These results contrasted with results from the 1R6F reference cigarette which was shown to be mutagenic in the Ames assay (strains TA98 (+S9) and TA1537 (+S9)), cytotoxic in the NRU assay and genotoxic in the ivMN assay.
One perceived irregularity recorded in the NRU assay showed relative absorbance values increasing above 100 % for the smokeless products, though this response has been previously reported in the literature with the evaluation of e-cigarettes/ e-liquids [4], [14]. It should be noted, however, that the top doses in this assay were chosen following dose-range finding studies where pH and osmolarity did not differ by more than approximately 20 % compared to the vehicle control + media and values were also noted to be within a physiological range thereby removing this as a confounding factor.
In the EpiGingival™ 3D tissue model testing, it was not technically feasible to obtain a CAS extract concentration above 30 %. However the tested nicotine extract concentrations from these studies correlated well and were in line with mean values of nicotine extraction data (in human use) with a range 1.952 – 2.325 mg (unpublished data).
Initially, tissue testing treatment conditions were set at 60 and 120 min (to reflect extreme product use), but due to the lack of response, a longer (overnight) exposure was adopted as previously documented by Zanetti et al. [20] and as a worst-case scenario. All smokeless and smoked samples failed to show irritancy (as measured using the MTT assay) i.e. upon test article treatment all tissue viabilities were > 50 % of the concurrent vehicle control tissues for both short and overnight treatments. Additionally, data from the positive controls were comparable to expected historical control ranges and yielded expected results thus confirming the validity and sensitivity of the test system for detecting irritation. Future studies will include the use of EpiOral tissues (MatTek ORL-200) as documented by Zanetti et al. [20] as others have documented success in discriminating between various products using this 3D tissue model.
The results from this study showed that the tested smokeless product extracts were less biologically active than cigarette TPM in the regulatory assays although debate remains on the best approach to compare between different product categories i.e. per nicotine basis or daily consumption. Additionally, the tested products were not classified as irritants.
To our knowledge, this is the first study to assess the impact of nicotine pouch products on fully differentiated gingival organotypic cultures.
CRediT authorship contribution statement
Jacqueline Miller-Holt: Conceptualization, Methodology, Validation, Writing – original draft, Writing – review & editing, Funding acquisition, Project administration. Irene Baskerville-Abraham: Methodology, Writing – review & editing, Validation, Funding acquisition, Project administration. Masanori Sakimura: Methodology, Writing – review & editing, Validation. Toshiro Fukushima: Conceptualization, Methodology, Validation, Writing – review & editing. Andrea Puglisi: Visualization, Writing – review & editing. Jeremie Gafner: Funding acquisition, Methodology, Writing – review & editing, Validation.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: JT International SA funded the project, and all authors were employees of Japan Tobacco Group at the time of the study. Nordic spirit tobacco-free nicotine pouches are manufactured and supplied by JT International.
Handling Editor: Lawrence Lash
Footnotes
Study data in mg nicotine/mL solvent to aid comparison with published literature.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.06.008.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Azzopardi D., Liu C., Murphy J., Chemical characterization of tobacco-free "modern" oral nicotine pouches and their position on the toxicant and risk continuums, Drug Chem Toxicol., 2021 May 25, 1–9. 〈 10.1080/01480545.2021.1925691〉. [DOI] [PubMed]
- 2.Bishop E., East N., Bozhilova S., Santopietro S., Smart D., Taylor M., Meredith S., Baxter A., Breheny D., Thorne D., Gaca M., An approach for the extract generation and toxicological assessment of tobacco-free ‘modern’ oral nicotine pouches, Food Chem Toxicol, 145, 2020, 111713. 〈 10.1016/j.fct.2020.111713〉. [DOI] [PubMed]
- 3.Chou Chin, Que Hee Shane. Bioassay-driven analysis of chewing tobacco extracts. Environmental Toxicology and Chemistry. 1994;13(7):1177–1186. [Google Scholar]
- 4.Daniel Gianluca Cudazzo, Smart J., McHugh Damian, Vanscheeuwijck Patrick. Lysosomotropic-related limitations of the BALB/c 3T3 cell-based neutral red uptake assay and an alternative testing approach for assessing e-liquid cytotoxicity. Toxicol. Vitr. 2019;61 doi: 10.1016/j.tiv.2019.104647. [DOI] [PubMed] [Google Scholar]
- 5.East N., E. Bishop, D. Breheny, M. Gaca, D. Thorne, A screening approach for the evaluation of tobacco-free ‘modern oral’ nicotine products using Real Time Cell Analysis, Toxicol. Rep., . 8,2021, 481–488. https://doi.org/10.1016/j.toxrep.2021.02.014. [DOI] [PMC free article] [PubMed]
- 6.Fan Yu, Kathryn Rudd, Sarah Jean Pour, Edgar Trelles Sticken, Ole Dethloff, Roman Wieczorek, Thomas Nahde, Liam Simms, Fiona Chapman, Lukasz Czekala, Matthew Stevenson, Grant O′Connell, 2022. Preclinical Assessment of tobacco-free nicotine pouches demonstrates reduced in vitro toxicity compared with tobacco snus and combustible cigarette smoke, Appl. Vitro Toxicol., http://doi.org/10.1089/aivt.2021.0020.
- 7.Health Canada, Official Method T-501, Bacterial Reverse Mutation Assay for Mainstream Tobacco Smoke, 2017. 〈https://open.canada.ca/data/en/dataset/670ef382–02c6–4943-80b6–2c589e6ea48a〉.
- 8.Health Canada, Official Method T-502, Neutral Red Uptake Assay for Mainstream Tobacco Smoke, 2017. 〈https://open.canada.ca/data/en/dataset/670ef382–02c6–4943-80b6–2c589e6ea48a〉.
- 9.Health Canada, Official Method T-503, In Vitro Micronucleus Assay for Mainstream Tobacco Smoke, 2017. 〈https://open.canada.ca/data/en/dataset/670ef382–02c6–4943-80b6–2c589e6ea48a〉.
- 10.ISO 20778 ,2018, ISO 20778, Routine Analytical Cigarette Smoking Machine — Definitions and Standard Conditions with an Intense Smoking Regime, International Organization for Standardization, Geneva, Switzerland.
- 11.ISO:3308, 2012, ISO 3308:2012 Routine Analytical Cigarette-smoking Machine — Definitions and Standard Conditions, International Organization for Standardization, Geneva, Switzerland.
- 12.Jablonski J.J., Cheetham A.G., Martin A.M. Market survey of modern oral nicotine products: determination of select HPHCs and Comparison to traditional smokeless tobacco products. Separations. 2022;9:65. doi: 10.3390/separations9030065. [DOI] [Google Scholar]
- 13.Klausner M., Ayehunie S., Breyfogle BA, Wertz PW, Bacca L., Kubilus J. , Organotypic human oral tissue models for toxicological studies, Toxicol In Vitro, 2007 Aug, 21(5), 938–49. 〈 10.1016/j.tiv.2007.01.024〉. [DOI] [PubMed]
- 14.Misra M., Leverette RD, Cooper BT, Bennett MB, Brown SE. , Comparative in vitro toxicity profile of electronic and tobacco cigarettes, smokeless tobacco and nicotine replacement therapy products: e-liquids, extracts and collected aerosols, Int, J, Environ, Res, Public Health, 2014 Oct 30,11(11),11325–11347. 〈 10.3390/ijerph111111325〉. [DOI] [PMC free article] [PubMed]
- 15.Neal F. Cariello, Walter W. Piegorsch, The Ames test: the two-fold rule revisited, Mut. Res.Genet. Toxicol., 36 (1–2),1996, 23–31. 〈 10.1016/S0165-1218(96)90044-0〉. [DOI] [PubMed]
- 16.OECD Guideline No. 471, OECD. Test No. 471: Bacterial Reverse Mutation Test, OECD, Paris, France, 2020.
- 17.OECD Guideline No. 487, 2016, Test No. 487: in vitro mammalian cell micronucleus test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, 〈 10.1787/9789264264861-en〉. [DOI]
- 18.PAS 8877:2022,Tobacco-free Oral Nicotine Pouches – Composition, Manufacture and Testing –Specification, The British Standards Institution 2022. Published by BSI Standards Limited 2022. ISBN 978 0 539 17496 0, ICS 65.160.
- 19.Swedish Institute for Standards (SIS):TS 72:2020, Nicotine Containing Tobacco Free Oral Products – Safety and Quality Related Requirements.
- 20.Zanetti Filippo, Alain Sewer, Bjoern Titz, Walter K., Schlage, Anita R. Iskandar, Athanasios Kondylis, Patrice Leroy, Emmanuel Guedj, Keyur Trivedi, Ashraf Elamin, Florian Martin, Stefan Frentzel, Nikolai V. Ivanov, Manuel C. Peitsch, Julia Hoeng , Assessment of a 72-hour repeated exposure to Swedish snus extract and total particulate matter from 3R4F cigarette smoke on gingival organotypic cultures, Food Chem. Toxico., 125, 2019, 252–270. 〈 10.1016/j.fct.2018.12.056〉. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material