Abstract
In this study, the levels of indicator PCBs congeners and PAHs compounds were reported in cow milk from selected areas in Dhaka, Bangladesh, and the potential human health risks were assessed. A total of 100 milk samples were collected and analyzed using gas chromatography-tandem mass spectrometry (GC-MS/MS). Method validation was performed using recovery performance, linearity, limit of detection (LOD), and limit of quantification (LOQ) assays. PCBs congeners, including PCB No. 52 (2,2´,5,5´-tetrachlorobiphenyl), PCB No. 101 (2,2´,4,5,5´-pentachlorobiphenyl), PCB No. 153 (2,2,4,4,5,5–hexachlorobiphenyl), and PCB No 209 (Decachloro-1,1′-biphenyl perchlorobiphenyl) were detected, whereas PCB No 28 (2,2 ´,4–trichlorobiphenyl), PCB No. 138 (2,2´,3,4,4´,5´–hexachlorobiphenyl), and PCB No 180 (2,2´,3,4,4´,5,5´–heptachlorobiphenyl) were not detected in the analyzed milk samples. Among the 16 PAHs compounds, benzo (a) anthracene and chrysene were detected in milk samples. The Σ hazard risk index (HI) of all detected PCBs congeners was below the limit set by the European Food Safety Authority, which indicates limited health risks to animals and humans in the study area. However, the presence of PCBs and PAHs in milk samples from industrial areas may negatively affect human health, and further detailed studies are required to ensure public health safety.
Keywords: Pesticides, Hexachlorocyclohexane, Heavy metals, Health hazards, POPs, GC-MS/MS
Graphical Abstract
1. Introduction
Biogenic and anthropogenic sources introduce pollutants into the environment. However, human activities are primarily responsible for their massive discharge into the atmosphere. Polychlorinated biphenyls (PCBs), hexachlorocyclohexane (HCH), polybrominated diphenyl ethers (PBDEs), polyaromatic hydrocarbons (PAHs), pesticides, and heavy metals are anthropogenic inorganic and organic pollutants that are dispersed throughout the atmosphere, hydrosphere, and lithosphere. They may be transformed into other compounds that are even more toxic to flora and fauna [1]. Because of their industrial uses, several contaminants, including PCBs and PAHs, are mixed with the environment, which is very hazardous and is retained in the environment, posing a severe threat to both public health and the ecosystem [2], [3], [4], [5], [6], [7], [8], [9].
PCBs are long-lasting contaminants that result from the defective combustion of organic and synthetic materials [10]. PCBs are synthetic organic compounds found in most industrial and consumer items. The production of PCBs was banned in the USA in 1977 [11], [12]. Because of their persistence and bioaccumulation, PCBs are categorized as persistent organic pollutants (POPs) [13]. The major concern is the presence of PCBs in consumer items such as plasticizers in paints, plastics, and rubber products. As PCBs accumulate in adipose tissue, they are typically found in animal-derived foods. PCB exposure is commonly caused by eating fish; however, other foods with lower PCB levels that are consumed more frequently, such as beef, dairy, and chicken products, also contribute to PCB exposure [11]. PCBs are causative agents of cancer, immune system diseases, and malfunctions in the reproductive, neurological, and other biological systems [14]. PCBs are widely used as pollutant markers because of their presence in biotic and abiotic conditions [15].
PAHs comprise hydrogen and carbon fused with two or more aromatic rings [16]. The characteristics of PAHs include a high melting point, low vapor pressure, and low aqueous solubility [17]. They are ubiquitous pollutants that are formed during food processing [18]. Normally, PAHs consisting of five or more rings are potentially hazardous to humans, which is a great concern for public health [19], [20], [21]. PAHs can be categorized based on the presence of aromatic rings [22]. LMW PAHs show acute toxicity, whereas HMW PAHs show high carcinogenicity [23]. PAHs are generally considered to be organic pollutants [22]. Because of the presence of PAHs in food, their control is necessary for public health safety [24]. Most PAHs are exposed to the human body through diet in different countries [19], [20], [21]. The major problem is that PAHs are not normally detected in raw foods [25].
PAHs can contaminate foods from environmental pollution or during food preparation and processing. Most gas-phase PAHs compounds are adsorbed in the particulate matter and finally accumulate in water and soil with heavy PAHs, which enter the food chain through vegetation and plant foods. Therefore, grazing cows can uptake PAHs from the soil through their feed and accumulate them in animal-origin foods [26]. In food, PAHs are present as a complex mixture of light and heavy compounds [27]. The other possible ways of accumulating PAHs through food consumption are smoking, drying, and fuel combustion [28]. Foods processed at high temperatures can also generate PAHs. According to one study, PAHs exposure in non-smokers occurs through food consumption [29]. PAHs have harmful effects on human health through carcinogenesis, mutagenesis, and immunosuppression. Several studies in animals and humans have demonstrated that PAH exposure is associated with increased cancer prevalence [28], [29], [30].
Several reports have demonstrated that products with high-fat content are important sources of human exposure to recognized toxins in the environment. Consequently, fatty foods, including meat, milk, and eggs, may contain significant amounts of POPs and other chemicals [30]. Harmful products, such as PCBs, can be consumed through the human diet. However, the principal sources of human exposure to lipophilic PCBs are fatty meals of animal origin [31], [32]. After discharge into the environment, these organic pollutants may accumulate in the soil and grasses. Milk and milk products are important sources of the human diet. Milk products also can be contaminated through PAHs. Therefore, a proper food management system should be established to ensure public health safety. PAHs are lipophilic and can accumulate in fats, particularly in foods of animal origin. Milk and dairy products are important sources of PAHs because milk can be contaminated by ingesting contaminated feed, grass from contaminated soil, and soil [33], [34], [35].
The impacts of PCBs and PAHs, PCBs and PBDEs are considered seriously because of their persistence nature in the environment [36]. Milk containing environmental pollutants is a major health threat. Therefore, monitoring milk samples is necessary to assess potential health risks. PCBs are used in different products, and PCB-containing waste is stored in landfills. It has been estimated that approximately 10% of the PCBs produced earlier still exist in the environment. Several by-products can be formed from PCBs through burning, which can be easily dissolved in water rather than evaporating. PCBs are fat-soluble and stored in animal fat through the food chain. Humans are mainly exposed to fat-rich foods. People can also have a small amount of PCBs through breathing contaminated air. PCBs have several effects on human health, including fertility, child growth, the immune system, and cancer development. The United States Environmental Protection Agency (USEPA) has identified 16 PAHs compounds as ‘priority pollutants and has been monitored extensively [37], [38]. The European Union (EU) listed 15 PAHs compounds in monitoring studies in 2005 [37]. Several compounds, including benzo (a) anthracene, chrysene, benzo (b) fluoranthene, benzo (k) fluoranthene, benzo (a) pyrene, indeno (1,2,3-cd)pyrene, benzo (ghi) pyrene, and dibenzo (ah) were identified as highly potential carcinogenic compounds compared to others [38]. PAHs such as BaA, Chr, BbF, and BaP are classified as mutagenic, genotoxic, and carcinogenic compounds by The International Agency for Research on Cancer (IARC) and Joint Food and Agriculture Organization/World Health Organization (FAO/WHO), Scientific Committee on Food (SCF), and European Food Safety authority (EFSA).
Although the presence of PCBs and PAHs in fat-rich foods, such as milk, is a major health concern, not much work has been done so far in Bangladesh. This study aimed to determine the levels of PCBs and PAHs in cow milk from selected areas in Bangladesh and assess possible human health risks.
2. Material and methods
2.1. Area of study
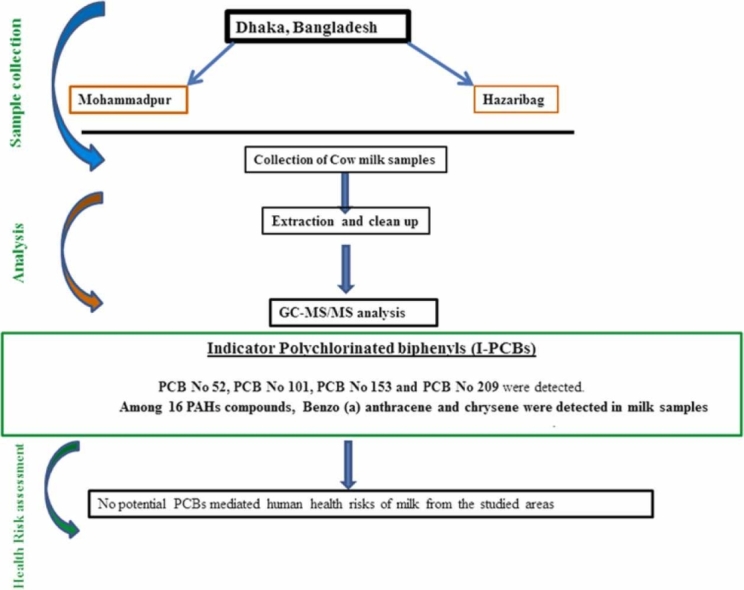
A total of 100 raw milk samples from 10 sampling sites were collected from the dairy firms of Mohammadpur and Hazaribag in Dhaka. The samples were collected between October 2020 and February 2021. Hazaribag is used in the leather industry. The site selection was based on industrial and agricultural activities. Many leather and tanning industries are located in Hazaribag. Mohammadpur is adjacent to the Turag River. The water of the Turag River is contaminated by industrial waste. Moreover, several waste dumping sites are located near both sampling areas, where the wastes are burned regularly. Milk samples were collected from dairy farms and local shops. Raw milk samples were used in this analysis. About 500 mL of milk samples were collected in plastic polythene bags, transported to the laboratory on ice box and stored at 4ºC and analyzed within 24 h of sample collection.
2.2. Chemicals and standards
A cocktail standard mix of PCBs congeners including PCB No 28, PCB No 52, PCB No 101, PCB No. 138, PCB No. 153, PCB No.180, and PCB No. 209 (PCB standard solution 7) and a cocktail mix of 16 PAHs compounds containing Napthalene, 2-methylnapthalene, 1-methylnapthalene, Acenapthylene, Acenapthalene, Fluorene, Phenanthrene, Anthracene, Fluranthene, Pyrene, Benzo (A) Anthacene, Chrysene, Benzo (B) Fluoranthene, Benzo (K) Fluranthene, Benzo (A) Pyrene, Indeno (1, 2, 3-CD) Pyrene and Dibenz (A, H) Anthracene brought from Sigma Aldrich (Darmstadt, Germany). Acetonitrile (Advent Chembio, India) and other chemicals including n-hexane, magnesium sulfate, sodium acetate, and NaCl were purchased from Merck (Darmstadt, Germany).
2.3. Extraction and cleanup
Sample extraction was performed using the quick, easy, cheap, effective, rugged, and safe (QuEChERS) method [39], [40], [41] with slight modifications for PCBs analysis. Samples were brought to normal temperature, and 5 mL of milk was transferred to a 50 mL polypropylene tube. After dilution with ultrapure water, the samples were extracted with 10 mL hexane: acetone (1:1, v/v). Anhydrous magnesium sulfate (4 g) and sodium chloride (1 g) were added to the mixture and shaken for 5 min at 175 rpm. Thereafter, the mixture was centrifuged at 3500 rpm for 5 min. Approximately 10 mL of the supernatant was poured into a glass tube, and the solution was dried using a nitrogen stream at 40ºC the solution was dried. Subsequently, the samples were cleaned, and the eluates were evaporated using a nitrogen stream at 40 °C. Finally, the extracts were dissolved in 100 µL of isooctane for GC-MS/MS analysis.
For PAHs analysis, samples were first homogenized, and 5 g of homogenized samples were placed in a conical tube and shaken for 1 min. A mixture of hexane and acetonitrile (1:1 v/v) was prepared, 15 mL of the mixture was added, and the mixture was shaken for a few seconds. Subsequently, magnesium sulfate (6 g) and sodium acetate (1.5 g) were added to the mix [42]. The solubility of the PAHs was reduced by adding NaCl to the mixture. The mixture was then centrifuged at 2500 g for 5 min. The supernatant was removed, and PAHs were isolated through solid-phase extraction (SPE). Subsequently, activated silica gel and 1 g of Na2SO4 were loaded onto a glass chromatographic column, and n-hexane was used. The concentrated extracts were dissolved in 5 mL of n-hexane, loaded into the column, and eluted with 50 mL of n-hexane. Finally, the eluents were concentrated and dissolved in n-hexane (0.5 mL of n-hexane for GC-MS/MS analysis [43].
2.4. Analysis of PCBs congeners and PAHs compounds in milk samples
PCBs congeners were analyzed using a gas chromatograph coupled with a MS (Thermo Scientific, USA). Analytical separations were performed using a Trace GOLD™ TG-5MS GC Column (0.25 mm × 0.25 µm X 0.25 m). Helium was used at a constant pressure (18.29 psi) at 1.6 mL/min flow rate. The injection port temperature was 250 °C, and the initial oven temperature was 60 °C, which was further increased to 220ºC at 5ºC/min heating ramp. The detector temperature was approximately 320 °C during the sample analysis. The sample injection volume was 2 µL.
For the PAHs analysis, helium was used as the carrier gas at 1.8 mL/min flow rate. The injection volume was 1 µL in split-less mode. The ion source and quadrupole temperatures were 280 °C and 180 °C, respectively, whereas the injector and transfer line temperatures were 280 °C and 310 °C, respectively. The column temperature was initially set at 35 °C for 1 min, then increased to 200 °C at a ramp rate of 25 °C/min, and finally ramped to 310 °C at 8 °C/min and kept for 3.5 min. Both of PCBs and PAHs analyte detection was carried out using MS at 70 eV ionization energy in selected ion monitoring (SIM) acquisition mode.
2.5. Method quality control
Working solutions were prepared by diluting standard stock solutions with n-hexane. For the linearity test, working solutions of several dilutions in the range of 50–200 µg/L were prepared and injected into the GC-MS/MS system. The LOQ and LOD were determined using the signal-to-noise ratio. Method validation was performed using a recovery performance evaluation. The recovery percentage was determined using the following equation:
| Pi= (Si/Ti) × 100Pi= (Si/Ti) × 100 | (1) |
Here, Pi is the recovery percentage, Si is the result of the control, and Ti is the percent recovery of the spiked samples.
2.6. Health risk assessment of PCBs congeners
Human exposure to chemicals in food is estimated using three different parameters: substance concentration in food (ng/kg lipid weight (l.w)), food consumption rate (kg), and population body weight (kg).
The estimated daily intake (EDI) is determined through the following equation:
| EDI = (DFC×FCC)/BW | (2) |
In this case, EDI is the estimated daily intake (g day−1), DFC indicates daily food consumption (kg/day), FFC indicates the food chemical concentration present in the analyzed food (mg/kg), and BW indicates the body weight (kg) of consumers. In this analysis, an average body weight of 70 kg/per adult person was considered for calculating the dietary exposure. The per capita milk consumption per day in the Bangladeshi population is 27.31 g [44].
2.7. Hazard Risk Index (HI) of PCBs congeners
The following formula was used for HI calculation:
| HI = EDI/ADI | (3) |
EDI: estimated daily intake of PCBs; ADI: acceptable daily intake through milk consumption. HI > 1 is considered a potential health hazard, and HI< 1 is considered safe for public health [45], [46].
2.8. Statistical analysis
Data were processed using mass spectrometry. The peak areas were considered for PCB quantification. Analytical data were collected and organized using Microsoft Excel. Mean concentration differences and standard deviations in milk samples, in accordance with the sampling sites, were determined using one-way ANOVA. Samples below the LOD values were considered to have a concentration of 0.0 for statistical analysis. Median, Interquartile range of detected analytes were calculated using Calculator Soup online software.
3. Results and discussion
3.1. Method performance
There are two common methods for the detection of PCBs in food samples: GC with an ionizing flame detector and MS [37], [47], and liquid chromatography with a fluorescence detector [48], [49], [50] are used. There are several methods for the detection of PAHs in milk products, including HPLC-FLD [51], [52], high-performance liquid chromatography–ultraviolet detection (HPLC-UV) [53], [54], [55], [56], [57] and GC-MS [35], [58], [59] or GC-MS/MS [24]. Because of its sensitivity and accuracy, we used GC-MS/MS in this study to determine PCBs and PAHs in cow milk. The chromatograms of the residual peaks of PCBs and PAHs are shown in Figs. 1 and 2, respectively. The method was validated based on recovery performance. Blank samples were adulterated with 50 ng/g and 100 ng/g PCBs congeners and PAHs compounds, respectively. Later, the adulterated samples were compared with those of the blank samples. In this study, the recovery rate of the PCBs were varied from 77.53% to 92.49%, while LOD and LOQ values were detected in the range of 0.016 ng/g to 0.031 ng/g l.w and 0.059 ng/g to 0.08 ng/g l.w, respectively. Table 1 lists the LOD, LOQ values, and % recoveries of each PCBs. In contrast, PAHs compounds were recovered in the range of 67.90–99.76%. The LOD and LOQ values were 0.3, 1.0, and 1.0, 4.0 respectively. The elution order, LOD, LOQ, and recovery rate of PAHs are shown in Table 2.
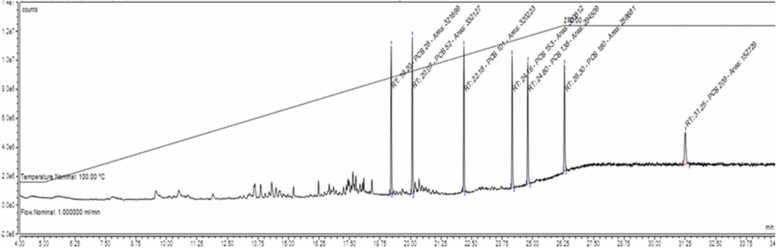
Fig. 1.
Chromatogram showing the peaks of PCBs congeners in standard solution. The elution order is as follows: 1: PCB 28; 2: PCB 52; 3: PCB 101; 4: PCB 153; 5: PCB 138; 6: PCB 180 and 7: PCB 209.
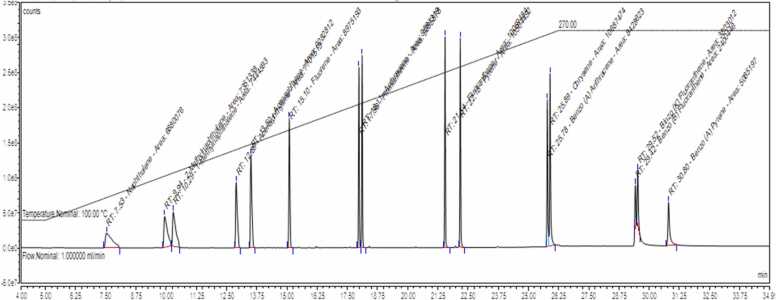
Fig. 2.
Chromatogram showing the peaks of PAHs compounds in standard solution.
Table 1.
The percent recoveries, LOQ and LOD values of PCBs congeners.
| PCBs | 50 ng/ g | 100 ng/ g | LOQ | LOD |
|---|---|---|---|---|
| PCB No 28 | 92.49 ± 5.73 | 81.49 ± 5.69 | 0.069 | 0.019 |
| PCB No 52 | 88.33 ± 6.42 | 88.32 ± 4.65 | 0.07 | 0.027 |
| PCB No 101 | 87.82 ± 4.86 | 90.21 ± 6.97 | 0.074 | 0.031 |
| PCB No. 138 | 92.35 ± 5.21 | 82.63 ± 4.04 | 0.061 | 0.019 |
| PCB No. 153 | 82.43 ± 6.33 | 77.53 ± 5.54 | 0.059 | 0.016 |
| PCB No 180 | 80.54 ± 4.98 | 83.23 ± 7.42 | 0.066 | 0.022 |
| PCB No 209 | 89.23 ± 5.75 | 83.33 ± 5.95 | 0.074 | 0.028 |
Table 2.
Elution order, limit of detection, limit of quantification and recovery rate of 16 PAHs compounds.
| Analyte | Elution Order | LOQ | LOD | % recovery | |
|---|---|---|---|---|---|
| (ng/g) | (ng/g) | 50 ng/g | 100 ng/g | ||
| Napthalene | 1 | 1 | 0.4 | 74.43 ± 4.48 | 67.98 ± 6.54 |
| 2-methylnapthalene | 2 | 1 | 0.3 | 76.9 ± 7.56 | 73.89 ± 9.65 |
| 1-methylnapthalene | 3 | 1 | 0.4 | 68.54 + 9.54 | 69.09 ± 5.78 |
| Acenapthylene | 4 | 1 | 0.4 | 75.09 ± 4.49 | 76.87 ± 9.56 |
| Acenapthalene | 5 | 1 | 0.3 | 70.56 ± 5.67 | 83.89 ± 8.85 |
| Fluorene | 6 | 1 | 0.3 | 68.98 ± 7.02 | 80.67 ± 9.07 |
| Phenanthrene | 7 | 2 | 0.5 | 70.78 ± 0.76 | 99.90 ± 7.54 |
| Anthracene | 8 | 2 | 0.5 | 95.98 ± 9.56 | 90.65 ± 6.67 |
| Fluranthene | 9 | 2 | 0.5 | 88.23 ± 3.89 | 85.76 ± 4.58 |
| Pyrene | 10 | 2 | 0.5 | 81.09 ± 8.98 | 80.65 ± 4.47 |
| Benzo (A) Anthacene | 11 | 3 | 0.9 | 76.67 ± 6.87 | 69.67 ± 7.98 |
| Chrysene | 12 | 3 | 0.8 | 67.90 ± 6.65 | 92.89 ± 7.67 |
| Benzo (B) Fluoranthene | 13 | 3 | 0.9 | 74.98 ± 7.89 | 76.98 ± 4.55 |
| Benzo (K) Fluranthene | 14 | 3 | 0.8 | 88.98 ± 9.09 | 66.89 ± 6.54 |
| Benzo (A) Pyrene | 15 | 3 | 0.8 | 92.87 ± 7.45 | 90.87 ± 5.66 |
| Indeno (1, 2, 3-CD) Pyrene | 16 | 4 | 0.9 | 99.76 ± 6.74 | 74.56 ± 9.77 |
| Dibenz (A, H) Anthracene | 17 | 4 | 0.8 | 92.90 ± 8.43 | 86.54 ± 5.90 |
3.2. Detection of PCB residues in milk
The levels of PCBs in cow milk samples collected from local firms in Dhaka, Bangladesh, were determined using GC-MS/MS. In this study, 100 milk samples were collected. PCBs residues including PCB No 52 (0.63 ± 0.21 ng/g l.w), PCB No 101 (0.52 ± 0.22 ng/g l.w), PCB No 153 (4.75 ± 0.41 ng/g l.w), and PCB No 202 (5.35 ± 0.87 ng/g l.w) were detected, whereas PCB No 28, PCB No 138, and PCB No 180 were not detected or detected below the detection limit (<LOD). The mean values, median, Interquartile range of detected PCBs congeners and Percentage of Positive samples are shown in Table 3, Table 4 respectively. Among the detected PCBs residues, PCB 209 was detected at the highest concentrations, followed by PCB 153, PCB 52, and PCB 101. The First quartile, second quartile, third quartile and Interquartile Range values of PCB 52 were 0.49, 0.59, 0.76 and 0.27 respectively. For PCB 101, the values were 0.415, 0.485, 0.615 and 0.2 respectively. The first quartile, second quartile, third quartile and Interquartile Range values of PCB 153 were 2.13, 2.87, 3.25 and 1.12 respectively. For PCB 209, the values were 2.945, 3.45, 3.71 and 0.765 respectively.
Table 3.
Mean concentration ± SD of detected PCBs in cow milk samples of Dhaka, Bangladesh.
| PCBs | Mean Concentration (ng/kg l.w) |
|---|---|
| PCB No 28 | ND |
| PCB No 52 | 0.63 ± 0.21 |
| PCB No 101 | 0.52 ± 0.22 |
| PCB No. 138 | ND |
| PCB No. 153 | 2.75 ± 0.41 |
| PCB No 180 | ND |
| PCB No 209 | 3.35 ± 0.87 |
* ND: Not detected
Table 4.
Median, Interquartile range of detected PCBs congeners and Percentage of Positive samples.
| PCB No 52 | % of Positive samples | PCB No 101 | % of Positive samples | PCB No. 153 | % of Positive samples | PCB No 209 | % of Positive samples |
|---|---|---|---|---|---|---|---|
| First Quartile | 70 | First Quartile | 80 | First Quartile | 70 | First Quartile | 50 |
| 0.49 | 0.415 | 2.13 | 2.945 | ||||
| Second Quartile | Second Quartile | Second Quartile | Second Quartile | ||||
| 0.59 | 0.485 | 2.87 | 3.45 | ||||
| Third Quartile | Third Quartile | Third Quartile | Third Quartile | ||||
| 0.76 | 0.615 | 3.25 | 3.71 | ||||
| Interquartile Range | Interquartile Range | Interquartile Range | Interquartile Range | ||||
| 0.27 | 0.2 | 1.12 | 0.765 | ||||
| Median = Q2 | Median = Q2 | Median = Q2 | Median = Q2 | ||||
| 0.59 | 0.485 | 2.87 | 3.45 | ||||
| Minimum | Minimum | Minimum | Minimum | ||||
| 0.46 | 0.39 | 1.78 | 2.68 | ||||
| Maximum | Maximum | Maximum | Maximum | ||||
| 0.97 | 0.77 | 3.68 | 3.76 | ||||
| Range | Range | Range | Range | ||||
| 0.51 | 0.38 | 1.9 | 1.08 |
3.3. Determination of PAHs compounds in milk
The mean values, median, interquartile range of detected PAHs analytes and Percentage of Positive samples of PAHs in the milk samples are presented in Table 5, Table 6 respectively. Among the 16 PAHs compounds, only benzo (a) anthracene and chrysene were detected. The concentrations of Benzo(a) anthracene and chrysene were 0.5497 ± 0.30 ng/g and 1.077 ± 0.878831 ng/g, respectively. The First quartile, second quartile, third quartile and Interquartile Range values of Benzo (A) Anthacene were 0.34, 0.435, 0.75 and 0.41 respectively. For Chrysene, the values were 0.465, 0.68, 1.275 and 0.81 respectively. Contaminated samples were detected from 2 of the 10 sampling sites. Samples collected from nearby industrial areas contained these two PAHs compounds, whereas samples from other sampling sites did not contain any PAHs compounds. The concentrations of other compounds were not detected in any samples. The absence of those compounds indicated low contamination rate in the studied area.
Table 5.
Detected PAHs compounds (ng/g) in Cow milk.
| Mean (ng/g) | |
|---|---|
| Napthalene | ND |
| 2-methylnapthalene | ND |
| 1-methylnapthalene | ND |
| Acenapthylene | ND |
| Acenapthalene | ND |
| Fluorene | ND |
| Phenanthrene | ND |
| Anthracene | ND |
| Fluranthene | ND |
| Pyrene | ND |
| Benzo (A) Anthacene | 0.5497 ± 0.304514 |
| Chrysene | 1.077 ± 0.878831 |
| Benzo (B) Fluoranthene | ND |
| Benzo (K) Fluranthene | ND |
| Benzo (A) Pyrene | ND |
| Indeno (1, 2, 3-CD) Pyrene | ND |
| Dibenz (A, H) Anthracene | ND |
* ND: Not detected
Table 6.
Median, Interquartile range of detected PAHs analytes and Percentage of Positive samples.
|
Benzo (A) Anthacene |
Chrysene |
||
|---|---|---|---|
| First Quartile | % of Positive samples | First Quartile | % of Positive samples |
| 0.34 | 80 | 0.465 | 90 |
| Second Quartile | Second Quartile | ||
| 0.435 | 0.68 | ||
| Third Quartile | Third Quartile | ||
| 0.75 | 1.275 | ||
| Interquartile Range | Interquartile Range | ||
| 0.41 | 0.81 | ||
| Median = Q2 | Median = Q2 | ||
| 0.435 | 0.68 | ||
| Minimum | Minimum | ||
| 0.19 | 0.38 | ||
| Maximum | Maximum | ||
| 1.12 | 3.98 | ||
| Range | Range | ||
| 0.93 | 3.6 | ||
3.4. Health risk from the consumption milk from the studied area
The EDI and HI values of the PCBs are presented in Table 7. The HI values of PCBs No. 52, No. 101, 153, and 209 were 5.58 × 10−6, 4.61 × 10−6, 4.20 × 10−5, and 4.73 × 10−5, respectively. From the risk analysis, it can be concluded that the milk samples from the study area were safe for consumers, as the HI values were far below the risk level. However, milk samples containing PCBs residues for a longer time may cause health hazards. The results obtained from the method validation were satisfactory for the analysis of PCBs and PAHs. Table 3 shows the mean concentrations (ng/g l.w) of PCBs detected in milk samples. Among the 100 analyzed milk samples, PCB No. 52, 101, 153, and 209 were detected, whereas some PCBs, including PCB No. 28, 138, and PCB No. 180 showed values below the limit of detection. The PCBs concentrations detected in this study were lower than those reported in other studies [60], [61], [62]. Findings from Japan have indicated that intake of PCB-contaminated foods is toxic to mothers [63]. Milk processing at higher temperatures could lower PCBs concentrations, as it has been reported in several studies that pesticide concentration is reduced after thermal processing [64], [65], [66]. According to the regulation of 1259/2011, the Committee for European Communities has established a PCB reference limit of 40 ng/g fat [67] According to the regulation of 1259/2011, the committee for European communities has established a reference limit of PCBs of 40 ng/g fat [65]. As there have no established Maximum Residue Limits (MRLs) values for PCBs concentrations in cow milk in Bangladesh, therefore, no comparisons in PCBs in milk can be done in Bangladeshi perspective. Animal-derived foods (both terrestrial and aquatic) are significant components of the human diet because they supply essential minerals [68], [69], [70] for human health. Various researchers throughout the world have detected the presence of PAHs and PCBs in protein source foodstuffs [28], [71], [72], [73]. Higher concentration of chrysene (12.56 ± 19.17 ng/g) and small quantity of benz(a)anthracene (0.30 ± 0.46 ng/g) was detected in cow milk of southern Italy [74] while in Egypt, sum of 13 PAHs was detected in the range of 1.3–8.2 ng/g [75]. Similarly, in Mexico, the total amount of detected PAHs did not exceed the permissible limit [76]. The analysis of cow milk samples in California identified PCB-101, PCB-118, PCB-138 as dominant PCBs congeners [69]. However, the the sum of all PCB congeners were below than the permissible tolerance level. Very little amount of PCBs congeners were detected in Slovakia [77]. In another study from UK identified 118, 153, 138 and 180 as dominant PCBs congeners and the total values of all PCBs congeners were in the range of 3.4–16.4 ng/g milk fat [78]. From this analysis, it was also found that some of the milk samples contained higher concentrations of PCBs congeners, which might be due to taking contaminated feeds like contaminated grass by cows.
Table 7.
The Estimated daily intakes (ng/g), Hazard Risk Index (HI) values of detected PCBs congeners.
| PCBs | Mean concentration (ng/kg l.w) | EDI | ADI | HI |
|---|---|---|---|---|
| PCB No 52 | 0.63 ± 0.21 | 0.2232 | 40,000 ng/kg | 0.00000558 |
| PCB No 101 | 0.52 ± 0.22 | 0.184229 | 40,000 ng/kg | 0.00000461 |
| PCB No. 153 | 2.75 ± 0.41 | 1.682857 | 40,000 ng/kg | 4.20714E−05 |
| PCB No 209 | 3.35 ± 0.87 | 1.895429 | 40,000 ng/kg | 4.73857E−05 |
| Σ HI | 9.96472E-05 |
Animals are continually exposed to environmental toxins and are able to collect high concentrations of pollutants in their tissues, particularly fat tissues [79]. Therefore, the EU Scientific Committee on Food has fixed values for foods containing several contaminants. [67]. This analysis indicated that approximately 12% of the samples were contaminated with PCBs congeners. However, the concentration of PCBs detected in all milk samples was below the maximum level set by the EU Scientific Committee on Food. A study reported that if the soil is contaminated, it is possible to present PCBs residues in animal-derived foods, including milk, meat, and eggs [80]. While cattle take grass in an open field, they may take up soil contaminated with grass; as a result, contaminants such as PCBs accumulate in their body, and finally those contaminants are transferred to the human body through foods of animal origin.
Studies have reported that amyloidogenesis can be inhibited by polychlorinated biphenyls upon binding to transthyretin in the blood [81]. PCB exposure leads to neurobehavioral changes. PCBs particularly affect the neurotransmitter, dopamine. PCB associated with brominated flame-retardants (BFRs) induces the formation of reactive oxygen species (ROS) in neurons, which are involved in cell death. PCBs and BFRs also have negative effects on the immune system by producing ROS in neutrophils [82]. The endocrine systems of animals and humans can be disrupted by PCBs by controlling natural hormones [83]. PCBs congeners can affect mammalian oocyte maturation and embryonic development. PCBs can interfere with microtubules and alter ooplasm compartmentalization. Ovotoxicity occurred after a miscommunication between the germinal and somatic compartments. Coplanar PCBs can regulate gene expression through aryl hydrocarbon receptor [84]. PCBs can accelerate DNA damage, which can lead to breast cancer. Several chronic inflammatory diseases, such as cardiovascular disease, type 2 diabetes, obesity, hepatic disorders, endocrine dysfunction, and neurological deficits, can develop through PCB exposure [85], [86]. Because of their lipophilic nature, PAHs bind to the cell membranes. As a result, structural changes affect normal cell function [87]. PAHs distribution is mainly dependent on fatty acids in conjugation with triacylglycerides, free cholesterol, and phospholipids, and is later distributed through blood capillaries to tissues such as skeletal muscle tissue, adipose tissue, and liver [88], [89]. PAHs exposure can lead to lung cancer and intestinal diseases in smokers and nonsmokers, respectively [27], [90], [91]. PAHs exposure can lead to the development of cancers, including skin, lung, bladder, and gastrointestinal cancers, as well as damage to several organs such as the liver, kidney, and cataracts. Furthermore, the exposure PAHs causes gene mutation, damage of cells as well as cardiopulmonary related mortality [27], [92].
The metabolism of PAHs occurs through the cytochrome P450 peroxidase and aldo-keto reductase pathways. These pathways involve diol-epoxides and radical cations, which disrupt DNA and damage cells by binding to DNA and proteins. As a result, carcinogenic, mutagenic, immunosuppressive, and teratogenic damage occurs and tumors finally develop tumors [93], [94], [95], [96]. Benzo(a)pyrene (BaP) is associated with delayed hatching in zebrafish by altering the structure of the hatching enzyme (ZHE1). BaP toxicity also affects the skeletal regions of zebrafish by enhancing oxidative stress, apoptosis, and delaying embryonic development [97]. BaP can increase the weight of mice through b-adrenergic mediated stimulation of adipose tissues lipolysis [98]. Lower concentrations of chrysene can diminish cell viability in MIO-M1 cells as a result of apoptosis, whereas higher concentrations can lead to necrosis. Chrysene also alters mitochondrial function. Several studies have shown that PAH can form DNA adducts, leading to cell proliferation [99], [100], [101]. Animal cell culture studies have reported chrysene as a mutagenic, carcinogenic, and genotoxic compound [102], [103], [104]. In male mice, it was observed that immune function and CYP450 activity was altered due to the Chrysene activity [105]. Chrysene was also found to be toxic to rat liver epithelial cells and organisms collected from the sea [106], [107]. A study in C. chanos indicated that anthracene and benzo [a] pyrene can increase oxidative stress and are neurotoxic [108]. The number of cultured hamster cells was reduced after treatment with benzo (a)pyrenediones [109]. Almost similar result was observed with Hexachlorocyclohexane (HCH), an insecticide which significantly reduced the hatching and survivability rate of zebra fishes. Besides this effect, morphological deformities were also observed. Moreover, it induced oxidative stress [110].
PAHs are allocated throughout the environment and persist for a longer time [111]. As PAHs are lipophilic compounds they may contaminate accumulate on high fat containing foods like milk. After accumulation in adipose tissue, PAHs may be excreted in milk [48].
Several PAHs compounds exist; however, only 16 PAHs compounds have been studied so far in most of the studies. These results indicate that environmental pollution plays an important role in the contamination of milk by PAHs [112]. There are several ways to contaminate food. PAHs can contaminate vegetables and fruits in contaminated soil. Fish and seafood can be contaminated by polluted oceans. Cattles can take PAHs along with grasses (sometimes soil from grasses) from contaminated soils in industrial areas. Several studies have shown that milk products contain a remarkable amount of contaminants such as PAHs [113]. Different foods can generate variable levels of PAHs depending on several factors, including duration of heat treatment, temperature, types of fuel used during food production, fat content in food, and smoke utilization during food preparation. Among dairy products, the highest concentration of PAHs is generated in smoked cheese because of the smoke used during preparation and the fat content; environmental toxic elements are bio-accumulated in milk and milk products, transferred to the human body, and finally influence human health. Pasteurized milk samples contain lower PAHs than raw and UHT milk samples because of the differences in fat content [87]. Other studies on milk and milk products also showed similar results [58]. In several other studies, naphthalene, acenaphthylene, acenaphthene, fluorene, and benzo (b) fluoranthene were detected in milk samples [33], [51], [58]. In our study, we detected only benzo (a) anthracene and chrysene at lower concentrations than in other studies performed in several countries worldwide. However, further studies are recommended to ensure public health safety.
4. Conclusion
This study investigated the presence of PCBs congeners and PAHs compounds in milk samples from several parts of Dhaka, Bangladesh using GC-MS/MS. The presence of PCBs in milk samples indicates the presence of environmental pollution. There are several other sources of PCBs contamination and there is a possibility of food contamination through PCBs congeners. The majority of PAHs compounds were not detected in milk samples. Very low amounts of benzo (a) anthracene and chrysene were detected in samples from industrial areas, where more human activities were observed. Therefore, proper detection and monitoring are required to ensure the safety of foods from animal sources. Detected values below the recommended levels indicate that the milk consumed by the population is free from toxicological risks. However, long-term exposure may result in significant exposure and cause a high contamination risk. Therefore, further studies are needed to establish specific MRL values for the Bangladeshi population.
CRediT authorship contribution statement
G.M.M. Anwarul Hasan: Conceptualization, Investigation, Methodology, Data curation, Formal analysis, Writing − original draft. Md. Aftab Ali Shaikh: Conceptualization, Funding acquisition, Project administration, Writing − review & editing. Mohammed A. Satter: Data curation, Formal analysis, Funding acquisition, Investigation, Resources, Software, Supervision, Validation. Md. Sabir Hossain: Methodology, Software, Supervision, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors would like to thank Institute of Food Science and Technology, Bangladesh Council of Scientific and Industrial Research (BCSIR) for financial support and research facilities.
Conflicts of Interest
The authors declare no competing interests.
References
- 1.Mansour S.A. Chemical pollutants threatening food safety and security: an overview. Adv. Food Prot. 2011:73–117. doi: 10.1007/978-94-007-1100-6_6. [DOI] [Google Scholar]
- 2.Papagiannis I., Kagalou I., Leonardos J., Petridis D., Kalfakakou V. Copper and zinc in four freshwater fish species from Lake Pamvotis (Greece) Environ. Int. 2004;30(3):357–362. doi: 10.1016/j.envint.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Sankar T.V., Zynudheen A.A., Anandan R., Nair P.V. Distribution of organochlorine pesticides and heavy metal residues in fish and shellfish from Calicut region, Kerala, India. Chemosphere. 2006;65(4):583–590. doi: 10.1016/j.chemosphere.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 4.Sharma R.K., Agrawal M., Marshall F. Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol. Environ. Saf. 2007;66(2):258–266. doi: 10.1016/j.ecoenv.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Ingelido A.M., Abballe A., Di Domenico A., Fochi I., Iacovella N., Saragosa A., De Felip E. Levels and profiles of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and polychlorinated biphenyls in feedstuffs and milk from farms in the vicinity of incineration plants in Tuscany, Italy. Arch. Environ. Contam. Toxicol. 2009;57(2):397–404. doi: 10.1007/s00244-008-9262-y. [DOI] [PubMed] [Google Scholar]
- 6.Turrio-Baldassarri L., Alivernini S., Carasi S., Casella M., Fuselli S., Iacovella N., Battistelli C.L. PCB, PCDD and PCDF contamination of food of animal origin as the effect of soil pollution and the cause of human exposure in Brescia. Chemosphere. 2009;76(2):278–285. doi: 10.1016/j.chemosphere.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Rahman M.M., Asaduzzaman M., Naidu R. Consumption of arsenic and other elements from vegetables and drinking water from an arsenic-contaminated area of Bangladesh. J. Hazard. Mater. 2013;262:1056–1063. doi: 10.1016/j.jhazmat.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 8.Lorenzi V., Ghidini S., Angelone B., Ferretti E., Menotta S., Fedrizzi G., Bertocchi L. Three years of monitoring of PCDD/F, DL-PCB and NDL-PCB residues in bovine milk from Lombardy and Emilia Romagna regions (Italy): Contamination levels and human exposure assessment. Food Control. 2016;68:45–54. doi: 10.1016/j.foodcont.2016.03.034. [DOI] [Google Scholar]
- 9.Ferrante M.C., Fusco G., Monnolo A., Saggiomo F., Guccione J., Mercogliano R., Clausi M.T. Food contamination by PCBs and waste disposal crisis: evidence from goat milk in Campania (Italy) Chemosphere. 2017;186:396–404. doi: 10.1016/j.chemosphere.2017.07.144. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X., Dupuis K.T., Aly N.A., Zhou Y., Smith F.B., Tang K., Baker E.S. Utilizing ion mobility spectrometry and mass spectrometry for the analysis of polycyclic aromatic hydrocarbons, polychlorinated biphenyls, polybrominated diphenyl ethers and their metabolites. Anal. Chim. Acta. 2018;1037:265–273. doi: 10.1016/j.aca.2018.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsharkawy E.E., Sharkawy A.A., Aly W.A. The Risk Profile of Pesticide and PCB Residues in Imported Meat Consumed in Egypt. Asian Basic Appl. Res. J. 2020:17–36. [Google Scholar]
- 12.Toxicological profile for Polychlorinated biphenyls (PCBs), 2000. [PubMed]
- 13.Kabir E.R., Rahman M.S., Rahman I. A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 2015;40(1):241–258. doi: 10.1016/j.etap.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter D.O. Exposure to and health effects of volatile PCBs. Rev. Environ. Health. 2015;30(2):81–92. doi: 10.1515/reveh-2014-0074. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi N., Bhavsar S.P., Reiner E.J., Chen T., Morse D., Arhonditsis G.B., Drouillard K.G. Evaluation and interconversion of various indicator PCB schemes for∑ PCB and dioxin-like PCB toxic equivalent levels in fish. Environ. Sci. Technol. 2015;49(1):123–131. doi: 10.1021/es503427r. [DOI] [PubMed] [Google Scholar]
- 16.IARC IARC Monographs on the evaluation of carcinogenic risks to humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Work. Group Eval. Carcinog. Risks Hum. 2010;92:33–771. [PMC free article] [PubMed] [Google Scholar]
- 17.Masih J., Singhvi R., Kumar K., Jain V.K., Taneja A. Seasonal variation and sources of polycyclic aromatic hydrocarbons (PAHs) in indoor and outdoor air in a semi-arid tract of northern India. Aerosol Air Qual. Res. 2012;12:515–525. [Google Scholar]
- 18.Agerstad M.J., Skog K. Review genotoxicity of heatprocessed foods. Mutat. Res. 2005;vol. 574:156–172. doi: 10.1016/j.mrfmmm.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 19.European Commission Scientific Committee on Foods . European Commission Scientific Committee on Foods,; Brussels, Belgium: 2002. Opinion of the Scientific Committee on Food on the Risks to Human Health of Polycyclic Aromatic Hydrocarbons in Food. [Google Scholar]
- 20.FAO/WHO Expert Committee on Food Additives, Meeting & World Health Organization, Evaluation of Certain Food Additives: Sixty-Fourth Meeting, 2005.
- 21.Nisbet I.C., LaGoy P.K. Toxic equivalency factors (TEFs) for polycyclic aromatic hydrocarbons (PAHs) Regul. Toxicol. Pharmacol. 1992;vol. 16(3):290–300. doi: 10.1016/0273-2300(92)90009-x. [DOI] [PubMed] [Google Scholar]
- 22.Plaza-Bolaños P., Frenich A.G., Vidal J.L.M. Polycyclic aromatic hydrocarbons in food and beverages. Analytical methods and trends. J. Chromatogr. A. 2010;vol. 1217(41):6303–6326. doi: 10.1016/j.chroma.2010.07.079. [DOI] [PubMed] [Google Scholar]
- 23.WITT G. Polycyclic aromatic hydrocarbons in water and sediment of the Baltic Sea. Mar. Pollut. Bull. 1995;Vol. 31:237–248. [Google Scholar]
- 24.Veyrand B., Brosseaud A., Sarcher L., et al. Innovative method for determination of 19 polycyclic aromatic hydrocarbons in food and oil samples using gas chromatography coupled to tandem mass spectrometry based on an isotope dilution approach. J. Chromatogr. A. 2007;vol. 1149(2):333–344. doi: 10.1016/j.chroma.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization . WHO,; Geneva, Switzerland: 1998. Selected Non-Heterocyclic PolycyclicAromatic Hydrocarbons. Environmental Health Criteia No. 202. [Google Scholar]
- 26.Garcia L.P., Gonçalves B.L., Panho G., Scussel V.M. Hidrocarbonetos policíclicos aromáticosemalimentos:Uma revisão. Pubvet. 2014 doi: 10.22256/pubvet.v8n19.1788. [DOI] [Google Scholar]
- 27.Purcaro G., Moret S., Conte L.S. Overview on polycyclic aromatic hydrocarbons: Occurrence, legislation and innovative determination in foods. Talanta. 2013:105. doi: 10.1016/j.talanta.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 28.Hamidi E.N., Hajeb P., Selamat J., Razis A.F.A. Polycyclic aromatic hydrocarbons (PAHs) and their bioaccessibility in meat: A tool for assessing human cancer risk. Asian Pac. J. Cancer Prev. 2016;17:15–23. doi: 10.7314/APJCP.2016.17.1.15. [DOI] [PubMed] [Google Scholar]
- 29.Rengarajan T., Rajendran P., Nandakumar N., Lokeshkumar B., Rajendran P., Nishigaki I. Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac. J. Trop. Biomed. 2015;5:S1691–S2221. doi: 10.1016/S2221-1691(15)30003-4. [DOI] [Google Scholar]
- 30.Domingo J.L. Concentrations of environmental organic contaminants in meat and meat products and human dietary exposure: A review. Food Chem. Toxicol. 2017;107:20–26. doi: 10.1016/j.fct.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 31.Fadaei H., Watson A., Place A., Connolly J., Ghosh U. Effect of PCB bioavailability changes in sediments on bioaccumulation in fish. Environ. Sci. Technol. 2015;49(20):12405–12413. doi: 10.1021/acs.est.5b03107. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert J.M., Baduel C., Li Y., Reichelt-Brushett A.J., Butcher P.A., McGrath S.P., Christidis L. Bioaccumulation of PCBs in liver tissue of dusky Carcharhinus obscurus, sandbar C. plumbeus and white Carcharodon carcharias sharks from south-eastern Australian waters. Mar. Pollut. Bull. 2015;101(2):908–913. doi: 10.1016/j.marpolbul.2015.10.071. [DOI] [PubMed] [Google Scholar]
- 33.Grova N., Feidt C., Cr_epineau C., Laurent C., Lafargue P.E., Hachimi A., Rychen G. Detection of polycyclic aromatic hydrocarbon levels in milk collected near potential contamination sources. J. Agric. Food Chem. 2002;50:4640–4642. doi: 10.1021/jf0201071. [DOI] [PubMed] [Google Scholar]
- 34.Environmental Protection Agency (EP 2006). 〈http://www.epa.gov/superfund/programs/clp/svtarget.htm〉.
- 35.Lee S.Y., Lee J.Y., Shin H.S. Evaluation of chemical analysis method and determination of polycyclic aromatic hydrocarbons content from seafood and dairy products. Toxicol. Res. 2015;31:265–271. doi: 10.5487/TR.2015.31.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdel-Shafy H.I., Mansour M.S. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016;25(1):107–123. doi: 10.1016/j.ejpe.2015.03.011. [DOI] [Google Scholar]
- 37.Plaza-Bolanõs P., Frenich A.G., Vidal J.L.M. Polycyclic aromatic hydrocarbons in food and beverages. Anal. Methods Trends J. Chromatogr. A. 2010;1217:6303–6326. doi: 10.1016/j.chroma.2010.07.079. [DOI] [PubMed] [Google Scholar]
- 38.Viegas O., Novo P., Pinto E., et al. Effect of charcoal types and grilling conditions on formation of heterocyclic aromatic amines (HAs) and polycyclic aromatic hydrocarbons (PAHs) in grilled muscle foods. Food Chem. Toxicol. 2012;50:2128–2134. doi: 10.1016/j.fct.2012.03.051. [DOI] [PubMed] [Google Scholar]
- 39.Sapozhnikova Y., Simons T., Lehotay S.J. Evaluation of a ast and simple sample preparation method for polybrominated diphenyl ether (PBDE) flame retardants and dichlorodiphenyltrichloroethane (DDT) pesticides in fish for analysis by ELISA compared with GC-MS/MS. J. Agric. Food Chem. 2015;63(18) doi: 10.1021/jf505651g. 4429±34. Epub 2015/02/04. [DOI] [PubMed] [Google Scholar]
- 40.Sapozhnikova Y., Lehotay S.J. Multi-class, multi-residue analysis of pesticides, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, polybrominated diphenyl ethers and novel flame retardants in fish using fast, low-pressure gas chromatography-tandem mass spectrometry. Anal. Chim. Acta. 2013;758 doi: 10.1016/j.aca.2012.10.034. 80±92. Epub 2012/12/19. [DOI] [PubMed] [Google Scholar]
- 41.Zacs D., Rjabova J., Viksna A., Bartkevics V. Method development for the simultaneous determination of polybrominated, polychlorinated, mixed polybrominated/chlorinated dibenzo-p-dioxins and dibenzofurans, polychlorinated biphenyls and polybrominated diphenyl ethers in fish. Chemosphere. 2015;118:72–80. doi: 10.1016/j.chemosphere.2014.06.032. Epub 2014/07/12. [DOI] [PubMed] [Google Scholar]
- 42.Gratz S., Mohrhaus A., Gamble B., Gracie J., Jackson D., Ciolino L., Mccauley H., Schneider G., Crockett D., Krol W., et al. Screen for the Presence of Polycyclic Aromatic Hydrocarbons in Select Seafoods Using LC-Fluorescence. FDA Lab. Inf. Bull. 2010;4475:1–39. [Google Scholar]
- 43.Adekunle A.S., Oyekunle J.A.O., Ojo O.S., Maxakato N.W., Olutona G.O., Obisesan O.R. Determination of Polycyclic Aromatic Hydrocarbon Levels of Groundwater in Ife North Local Government Area of Osun State, Nigeria. Toxicol. Rep. 2017;4:39–48. doi: 10.1016/j.toxrep.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.BBS : Preliminary Report on Households Income and Expenditure Survey 2016 0–2, 2017.
- 45.US EPA (United States Environmental Protection Agency) Guidelines for the health risk assessment of chemical mixtures Fed. Regist. 1986;51(185):34014–34025. [Google Scholar]
- 46.US EPA (United States Environmental Protection Agency) Integrated Risk Information System US EPA, Washington, 2007.
- 47.Dobrinas S., Soceanu A., Popescu V., Coatu V. Polycyclic aromatic hydrocarbons and pesticides in milk powder. J. Dairy Res. 2016;83(2):261–265. doi: 10.1017/S0022029916000169. [DOI] [PubMed] [Google Scholar]
- 48.García Londoño V.A., Reynoso C.M., Resnik S. Polycyclic aromatic hydrocarbons in milk powders marketed in Uruguay. Food Addit. Contam.: Part B. 2017;10(4):284–291. doi: 10.1080/19393210.2017.1349191. [DOI] [PubMed] [Google Scholar]
- 49.Fasano E., Esposito F., Scognamiglio G., Cocchieri Amodio R., Cirillo T. Detection of polycyclic aromatic hydrocarbons in smoked buffalo mozzarella cheese produced in Campania Region, Italy. J. Sci. Food Agric. 2016;96(5):1704–1708. doi: 10.1002/jsfa.7275. [DOI] [PubMed] [Google Scholar]
- 50.Girelli A.M., Sperati D., Tarola A.M. Determination of polycyclic aromatic hydrocarbons in Italian milk by HPLC with fluorescence detection. Food Addit. Contam.: Part A. 2014;31(4):703–710. doi: 10.1080/19440049.2013.878959. [DOI] [PubMed] [Google Scholar]
- 51.Kishikawa N., Wada M., Kuroda N., Akiyama S., Nakashima K. Determination of polycyclic aromatic hydrocarbons in milk samples by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B. 2003;789:257–264. doi: 10.1016/s1570-0232(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 52.Santonicola S., Albrizio S., Murru N., Ferrante M.C., Mercogliano R. Study on the occurrence of polycyclic aromatic hydrocarbons in milk and meat/fish based baby food available in Italy. Chemosphere. 2017;184:467–472. doi: 10.1016/j.chemosphere.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 53.Lin D., Zhu L. Polycyclic aromatic hydrocarbons: pollution and source analysis of a black tea. J. Agric. Food Chem. 2004;52:8268–8271. doi: 10.1021/jf048636n. [DOI] [PubMed] [Google Scholar]
- 54.Lin D., Tu Y., Zhu L. Concentrations and health risk of polycyclic aromatic hydrocarbons in tea. Food Chem. Toxicol. 2005;43:41–48. doi: 10.1016/j.fct.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 55.Corredera L., Bayarri S., P_erez-Arquillu_e C., L_azaro R., Molino F., Herrera A. Multiresidue determination of carcinogenic polycyclic aromatic hydrocarbons in honey by solid-phase extraction and highperformance liquid chromatography. J. Food Protein. 2011;74:1692–1699. doi: 10.4315/0362-028X.JFP-11-140. [DOI] [PubMed] [Google Scholar]
- 56.Liu W., Qi J., Yan L., Jia Q., Yu C. Application of poly (butyl methacrylate-co-ethylene glycol dimethacrylate) monolith microextraction coupled with high performance liquid chromatography to the determination of polycyclic aromatic hydrocarbons in smoked meat products. J. Chromatogr. B. 2011;879:3012–3016. doi: 10.1016/j.jchromb.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 57.Kumari R., Chaturvedi P., Ansari N.G., Murthy R.C., Patel D.K. Optimization and validation of an extraction method for the analysis of polycyclic aromatic hydrocarbons in chocolate candies. J. Food Sci. 2012;77:34–40. doi: 10.1111/j.1750-3841.2011.02488.x. [DOI] [PubMed] [Google Scholar]
- 58.Aguinaga N., Campillo N., Vi~nas P., Hernandez-Cordoba M. Determination of 16 polycyclic aromatic hydrocarbons in milk and related products using solid-phase microextraction coupled to gas chromatography-mass spectrometry. Anal. Chim. Acta. 2007;596:285–290. doi: 10.1016/j.aca.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Sanagi M.M., Loh S.H., Wan Ibrahim W.A., Hasan M.N., Hassan Y. Determination of polycyclic aromatic hydrocarbons in fresh milk by hollow fiber liquid-phase microextraction–gas chromatography mass spectrometry. J. Chromatogr. Sci. 2013;51:112–116. doi: 10.1093/chromsci/bms113. [DOI] [PubMed] [Google Scholar]
- 60.Costabeber I.H., Coelho A.N., Schwanz T.G., Weis G.C.C., Carpilovsky C.K. Levels of polychlorinated biphenyls (PCBs) in whole milk powderand estimated daily intake for a population of children. Ciência Rural. 2018:48. [Google Scholar]
- 61.Ramos L., Torre M., Marina M.L. Gas chromatography determination of polychlorinated biphenyls in powdered and liquid soybean milks. J. Chromatogr. A. 1998;815(2):272–277. doi: 10.1016/S0021-9673(98)00504-4. [DOI] [PubMed] [Google Scholar]
- 62.Heck M.C., dos Santos J.S., Junior S.B., Costabeber I., Emanuelli T. Estimation of children exposure to organochlorine compounds through milk in Rio Grande do Sul, Brazil. Food Chem. 2007;102(1):288–294. doi: 10.1016/j.foodchem.2006.05.019. [DOI] [Google Scholar]
- 63.Eguchi A., Otake M., Hanazato M., Suzuki N., Matsuno Y., Nakaoka H., Mori C. Assessment of questionnaire-based PCB exposure focused on food frequency in birth cohorts in Japan. Environ. Sci. Pollut. Res. 2017;24(4):3531–3538. doi: 10.1007/s11356-016-8119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abou-Arab A.A.K. Behavior of pesticides in tomatoes during commercial and home preparation. Food Chem. 1999;65(4):509–514. [Google Scholar]
- 65.Balinova A.M., Mladenova R.I., Shtereva D.D. Effects of processing on pesticide residues in peaches intended for baby food. Food Addit. Contam. 2006;23(9):895–901. doi: 10.1080/02652030600771715. [DOI] [PubMed] [Google Scholar]
- 66.Yang A.G., Shim K.H., Choi O.J., Park J.H., Do J.A., Oh J.H., Shim J.H. Establishment of the Korean total diet study (TDS) model in consideration to pesticide intake. Korean J. Pestic. Sci. 2012;16(2):151–162. doi: 10.1016/j.aca.2018.02.054. [DOI] [Google Scholar]
- 67.EUROPEAN COMMISSION (EC), 2011, No 1259/2011 of 2 December, amending Regulation (EC) No 1881/2006 as regards maximumlevels for dioxins, dioxin-like PCBs and non dioxin-like PCBs in foodstuffs. Official Journal of the European Union, L320, 18–23, Available from: 〈https://www.fsai.ie/uploadedFiles/Reg1259_2011.pdf〉. Accessed: May 4, 2018.
- 68.Martínez M., Angulo R., Pozo R., Jodral M. Organochlorine pesticides in pasteurized milk and associated health risks. Food Chem. Toxicol. 1997;35(6):621–624. doi: 10.1016/S0278-6915(97)00028-8. [DOI] [PubMed] [Google Scholar]
- 69.Chen X., Lin Y., Dang K., Puschner B. Quantification of polychlorinated biphenyls and polybrominated diphenyl ethers in commercial cows’ milk from california by gas chromatography–triple quadruple mass spectrometry. PloS One. 2017;12(1) doi: 10.1371/journal.pone.0170129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Storelli M.M., Stuffler R.G., Marcotrigiano G.O. Polycyclic aromatic hydrocarbons, polychlorinated biphenyls, chlorinated pesticides (DDTs), hexachlorocyclohexane, and hexachlorobenzene residues in smoked seafood. J. Food Prot. 2003;66(6):1095–1099. doi: 10.4315/0362-028X-66.6.1095. [DOI] [PubMed] [Google Scholar]
- 71.Tamakawa, K., 2004, Polycyclic aromatic hydrocarbons in food. Handbook of Food Analysis Second Edition: Residues and Other Food Component Analysis, 2(2), 1449–1483. https://doi.org/10.1201/9781351072946–10.
- 72.Wang L., Liu A., Zhao Y., Mu X., Huang T., Gao H., Ma J. The levels of polycyclic aromatic hydrocarbons (PAHs) in human milk and exposure risk to breastfed infants in petrochemical industrialized Lanzhou Valley, Northwest China. Environ. Sci. Pollut. Res. 2018;25(17):16754–16766. doi: 10.1007/s11356-018-1799-3. [DOI] [PubMed] [Google Scholar]
- 73.Lee Y.N., Lee S., Kim J.S., Patra J.K., Shin H.S. Chemical analysis techniques and investigation of polycyclic aromatic hydrocarbons in fruit, vegetables and meats and their products. Food Chem. 2019;277:156–161. doi: 10.1016/j.foodchem.2018.10.114. [DOI] [PubMed] [Google Scholar]
- 74.Di Bella C., Traina A., Giosuè C., Carpintieri D., Lo Dico G.M., Bellante A., Ferrantelli V. Heavy metals and PAHs in meat, milk, and seafood from Augusta area (Southern Italy): contamination levels, dietary intake, and human exposure assessment. Front. Public Health. 2020;8:273. doi: 10.3389/fpubh.2020.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rawash E.S.A., Mohamed G.G., Souaya E.R., Khalil L.H., El-Chaghaby G.A., El-Gammal M.H. Distribution and health hazards of polycyclic aromatic hydrocarbons in Egyptian Milk and dairy-based products. Beverages. 2018;4(3):63. [Google Scholar]
- 76.Gutiérrez R., Vega S., Ortiz R., Pérez J.J., Schettino B. Presence of PAHs in milk of industrial farms from Tizayuca, Hidalgo, Mexico. J. Environ. Sci. Health, Part B. 2015;50(5):317–321. doi: 10.1080/03601234.2015.1000166. [DOI] [PubMed] [Google Scholar]
- 77.Toman R., Pšenková M., Tančin V. Polychlorinated biphenyls in cow’s milk, feed and soil in selected areas of Slovakia. Acta Fytotech. Et. Zootech. 2020;23:4. [Google Scholar]
- 78.Sewart A., Jones K.C. A survey of PCB congeners in UK cows' milk. Chemosphere. 1996;32(12):2481–2492. doi: 10.1016/0045-6535(96)00141-5. [DOI] [PubMed] [Google Scholar]
- 79.Authman M.M., Zaki M.S., Khallaf E.A., Abbas H.H. Use of fish as bio-indicator of the effects of heavy metals pollution. J. Aquac. Res. Dev. 2015;6(4):1–13. doi: 10.4172/2155-9546.1000328. [DOI] [Google Scholar]
- 80.Weber R., Herold C., Hollert H., Kamphues J., Blepp M., Ballschmiter K. Reviewing the relevance of dioxin and PCB sources for food from animal origin and the need for their inventory, control and management. Environ. Sci. Eur. 2018;30(1):1–42. doi: 10.1186/s12302-018-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Purkey H.E., Palaninathan S.K., Kent K.C., Smith C., Safe S.H., Sacchettini J.C., Kelly J.W. Hydroxylated polychlorinated biphenyls selectively bind transthyretin in blood and inhibit amyloidogenesis: rationalizing rodent PCB toxicity. Chem. Biol. 2004;11(12):1719–1728. doi: 10.1016/j.chembiol.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 82.Fonnum F., Mariussen E., Reistad T. Molecular mechanisms involved in the toxic effects of polychlorinated biphenyls (PCBs) and brominated flame retardants (BFRs) J. Toxicol. Environ. Health, Part A. 2006;69(1–2):21–35. doi: 10.1080/15287390500259020. [DOI] [PubMed] [Google Scholar]
- 83.McKinney J.D., Waller C.L. Polychlorinated biphenyls as hormonally active structural analogues. Environ. Health Perspect. 1994;102(3):290–297. doi: 10.1289/ehp.94102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pocar P., Brevini T.A., Antonini S., Gandolfi F. Cellular and molecular mechanisms mediating the effect of polychlorinated biphenyls on oocyte in vitro maturation. Reprod. Toxicol. 2006;22(2):242–249. doi: 10.1016/j.reprotox.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 85.Gupta P., Thompson B.L., Wahlang B., Jordan C.T., Zach Hilt J., Hennig B., Dziubla T. The environmental pollutant, polychlorinated biphenyls, and cardiovascular disease: a potential target for antioxidant nanotherapeutics. Drug Deliv. Transl. Res. 2018;8(3):740–759. doi: 10.1007/s13346-017-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carpenter D.O. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev. Environ. Health. 2006;21(1):1–24. doi: 10.1515/reveh.2006.21.1.1. [DOI] [PubMed] [Google Scholar]
- 87.Naccari C., Cristani M., Giofrè F., Ferrante M., Siracusa L., Trombetta D. PAHs concentration in heat-treated milk samples. Food Res. Int. 2011;44(3):716–724. [Google Scholar]
- 88.Ifegwu O.C., Anyakora C. 1st ed. Vol. 72. Elsevier,; Amsterdam, The Netherlands: 2015. Polycyclic Aromatic Hydrocarbons: Part I. Exposure; pp. 277–304. (Advances in Clinical Chemistry). [DOI] [PubMed] [Google Scholar]
- 89.Katona B.W., Lynch J.P. Physiology of the Gastrointestinal Tract. Elsevier,; Amsterdam, The Netherlands: 2018. Mechanisms of gastrointestinal malignancies; pp. 1615–1642. [Google Scholar]
- 90.Haschek W.A., Rousseaux C.G., Wallig M.A. Fundamentals of Toxicologic Pathology. 2nd ed. Academic Press,; San Diego, CA, USA: 2010. Nomenclature: Terminology for morphologic alterations; pp. 67–80. [Google Scholar]
- 91.Kim K.-H., Jahan S.A., Kabir E., Brown R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013:60. doi: 10.1016/j.envint.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 92.Zelinkova Z., Wenzl T. The occurrence of 16 EPA PAHs in food—A Review. Polycycl. Aromat. Compd. 2015;35:248–284. doi: 10.1080/10406638.2014.918550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Agency for Toxic Substances and Disease Registry, Toxicological Profile for Polycyclic Aromatic Hydrocarbons; U.S. Department of Health and Human Services: Atlanta, GA, USA, 1995; pp. 1–455. Available online: 〈https://www.atsdr.cdc.gov/toxprofiles/tp69.pdf〉 (accessed on 12 April 2021). [PubMed]
- 94.Bernardo D.L., Barros K.A., Silva R.C., Pavão A.C. Carcinogenicity of polycyclic aromatic hydrocarbons. Quim. Nova. 2016;39:789–794. [Google Scholar]
- 95.Das D.N., Panda P.K., Naik P.P., Mukhopadhyay S., Sinha N., Bhutia S.K. Phytotherapeutic approach: A new hope for polycyclic aromatic hydrocarbons induced cellular disorders, autophagic and apoptotic cell death. Toxicol. Mech. Methods. 2017;27:1–17. doi: 10.1080/15376516.2016.1268228. [DOI] [PubMed] [Google Scholar]
- 96.Gao P., da Silva E., Hou L., Denslow N.D., Xiang P., Ma L.Q. Human exposure to polycyclic aromatic hydrocarbons: Metabolomics perspective. Environ. Int. 2018;119:466–477. doi: 10.1016/j.envint.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 97.Grover P.L. 1st ed. Vol. 2. CRC Press,; Boca Raton, FL, USA: 2019. pp. 1–218. (Chemical Carcinogens and DNA). [Google Scholar]
- 98.Elfawy H.A., Anupriya S., Mohanty S., Patel P., Ghosal S., Panda P.K., Patnaik S. Molecular toxicity of Benzo (a) pyrene mediated by elicited oxidative stress infer skeletal deformities and apoptosis in embryonic zebrafish. Sci. Total Environ. 2021;789 doi: 10.1016/j.scitotenv.2021.147989. [DOI] [PubMed] [Google Scholar]
- 99.Irigaray P., Ogier V., Jacquenet S., Notet V., Sibille P., Méjean L., Yen F.T. Benzo [a] pyrene impairs β‐adrenergic stimulation of adipose tissue lipolysis and causes weight gain in mice: A novel molecular mechanism of toxicity for a common food pollutant. FEBS J. 2006;273(7):1362–1372. doi: 10.1111/j.1742-4658.2006.05159.x. [DOI] [PubMed] [Google Scholar]
- 100.Falahatpisheh M., Kerzee J., Metz R., Donnelly K., Ramos K. Inducible cytochrome P450 activities in renal glomerular mesangial cells: biochemical basis for antagonistic interactions among nephrocarcinogenic polycyclic aromatic hydrocarbons. J. Carcinog. 2004;3:12. doi: 10.1186/1477-3163-3-12. [PMID: 15315710] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vakharia D.D., Liu N., Pause R., Fasco M., Bessette E., Zhang Q.Y., Kaminsky L.S. Polycyclic aromatic hydrocarbon/metal mixtures: effect on PAH induction of CYP1A1 in human HEPG2 cells. Drug Metab. Dispos. 2001;29:999–1006. [PMID: 11408366] [PubMed] [Google Scholar]
- 102.Heidel S.M., MacWilliams P.S., Baird W.M., Dashwood W.M., Buters J.T., Gonzalez F.J., Larsen M.C., Czuprynski C.J., Jefcoate C.R. Cytochrome P4501B1 mediates induction of bone marrow cytotoxicity and preleukemia cells in mice treated with 7,12-dimethylbenz[a]anthracene. Cancer Res. 2000;60:3454–3460. [PMID: 10910056] [PubMed] [Google Scholar]
- 103.Cheung Y.L., Gray T.J., Ioannides C. Mutagenicity of chrysene, its methyl and benzo derivatives, and their interactions with cytochromes P-450 and the Ah-receptor; relevance to their carcinogenic potency. Toxicology. 1993;81:69–86. doi: 10.1016/0300-483x(93)90157-n. [PMID: 8396278] [DOI] [PubMed] [Google Scholar]
- 104.Hoffmann D., Bondinell W.E., Wynder E.L. Carcinogenicity of methylchrysenes. Science. 1974;183:215–216. doi: 10.1126/science.183.4121.215. [PMID: 4808861] [DOI] [PubMed] [Google Scholar]
- 105.Mahadevan B., Luch A., Atkin J., Nguyen T., Sharma A.K., Amin S., Baird W.M. Investigation of the genotoxicity of dibenzo[c,p]chrysene in human carcinoma MCF-7 cells in culture. Chem. Biol. Inter. 2006;164:181–191. doi: 10.1016/j.cbi.2006.09.015. [PMID: 17094953] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peden-Adams M.M., Liu J., Knutson S., Dancik J., Bryant K., Bodine A.B., Dickerson R.L. Alterations in immune function and CYP450 activity in adult male deer mice (Peromyscus maniculatus) following exposure to benzo[a]pyrene, pyrene, or chrysene. J. Toxicol. Environ. Health A. 2007;70:1783–1791. doi: 10.1080/15287390701384643. [PMID: 17934950] [DOI] [PubMed] [Google Scholar]
- 107.Machala M., Svihalkova-Sindlerova L., Pencikova K., Krcmar P., Topinka J., Milcova A., Novakova Z., Kozubik A., Vondracek J. Effects of methylated chrysenes on AhR-dependent and -independent toxic events in rat liver epithelial cells. Toxicology. 2008;247:93–101. doi: 10.1016/j.tox.2008.02.008. [PMID: 18407395] [DOI] [PubMed] [Google Scholar]
- 108.Karacik B., Okay O.S., Henkelmann B., Bernhoft S., Schramm K.W. Polycyclic aromatic hydrocarbons and effects on marine organisms in the Istanbul Strait. Environ. Int. 2009;35:599–606. doi: 10.1016/j.envint.2008.11.005. [PMID: 19128832] [DOI] [PubMed] [Google Scholar]
- 109.Palanikumar L., Kumaraguru A.K., Ramakritinan C.M., Anand M. Biochemical response of anthracene and benzo [a] pyrene in milkfish Chanos chanos. Ecotoxicol. Environ. Saf. 2012;75:187–197. doi: 10.1016/j.ecoenv.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 110.Singh K., Verma S.K., Patel P., Panda P.K., Sinha A., Das B., Ray L. Hydoxylated β-and δ-Hexacholorocyclohexane metabolites infer influential intrinsic atomic pathways interaction to elicit oxidative stress-induced apoptosis for bio-toxicity. Environ. Res. 2022 doi: 10.1016/j.envres.2022.113496. [DOI] [PubMed] [Google Scholar]
- 111.Lorentzen R.J., Lesko S.A., McDonald K., Ts’o P.O. Toxicity of metabolic benzo (a) pyrenediones to cultured cells and the dependence upon molecular oxygen. Cancer Res. 1979;39(8):3194–3198. [PubMed] [Google Scholar]
- 112.Howsam M., Jones K.C., Ineson P. PAHs associated with the leaves of three deciduous tree species. I—concentrations and profiles. Environ. Pollut. 2000;vol. 108(3):413–424. doi: 10.1016/s0269-7491(99)00195-5. [DOI] [PubMed] [Google Scholar]
- 113.Raza N., Kim K.H. Quantification techniques for important environmental contaminants in milk and dairy products. Trends Anal. Chem. 2018;98:79–94. [Google Scholar]