Abstract
The 12.4 kDa fungal immunomodulatory protein from Ganoderma microsporum (GMI) has bioactivity in vitro and in vivo. This study assessed the safety of GMI derived from engineered Pichia pastoris in Sprague-Dawley rats as a dietary supplement and food ingredient by evaluating subchronic toxicity, teratology, and mutagenicity. The oral gavage administration of 10 mL GMI at 0, 50, 75, or 100 mg GMI/kg body weight/day assayed for 91 consecutive days showed no mortality or moribundity. There were no test article-related findings in animal observations/measurements: cageside observation, detailed clinical observations, body weights, feed consumption, ophthalmic examinations, functional observation battery, clinical chemistry, hematology, coagulations, urinalysis, or terminal necropsy (gross or histopathology findings) suggesting that GMI has no subchronic toxicity. The teratology toxicity study of pregnant female rats orally administered GMI at 0, 50, 75, or 100 mg/kg body weight/day throughout organogenesis (gestation date 6–18) showed no mortality, moribundity, and no test article-related finding to dam or fetus. GMI genotoxicity was not observed by mutagenicity studies of Salmonella typhimurium, in vitro chromosome aberrations, and an in vivo micronucleus test in mice. Overall, no observed-adverse-effect level (NOAEL) was determined for GMI based on the subchronic and teratology studies at 100 mg/kg body weight/day.
Keywords: Fungal immunomodulatory protein, Ganoderma microsporum, Food ingredient, Oral safety, Toxicity, GMI
Graphical Abstract
Highlights
-
•
The NOAEL in 90-day oral toxicity study in rats was 100 mg GMI / kg body weight / day - the highest dose tested.
-
•
The NOAEL in teratology study in rats was 100 mg GMI / kg body weight / day - the highest dose tested.
-
•
GMI was not genotoxic per the standard battery for genotoxicity testing.
-
•
The safety of human ingestion of GMI was noted as the new dietary ingredient in US and the food ingredient in Taiwan.
1. Introduction
Ganoderma microsporum (NCBI:txid34462) was firstly identified and reported in 1989 [1]. Decades later, the fungal immunomodulatory protein (FIP) identified from G. microsporum was characterized in 2009 [2], annotated as FIP-gmi in NCBI databank (GenBank: GP714988.1), and named Ganoderma microsporum immunomodulatory protein (GMI) in this work. Ling Zhi-8 (LZ-8) is the first FIP family member isolated from the mycelium extract of Ganoderma lucidum [3]. Since its discovery, FIP family proteins are commonly identified in various groups of edible mushrooms [4] including LZ-9 [5] from G. lucidum, FIP-gts [6] from G. tsugae, FIP-gsi [7] from G. sinense, FIP-tve [8] from Trametes versicolor, FIP-pcp [9] from Poria cocos, FIP-fve [10] from Flammulina velutipes, FIP-vvo [11] from Volvariella volvacea, FIP-aca [12] from Anthodia camphorate, FIP-lrh [13] from Lignosus rhinocerotis, and others. All proteins in the FIP family have a molecular weight of approximately 13 kDa with 110–114 amino acids and have an immunoglobulin-like tertiary structure [14]. GMI belongs to the FIP protein family and GMI shares high sequence, structural and functional similarities to other proteins in the FIP group [15], [16].
Ganoderma spp. (also known as Reishi or Lingzhi) has been used in Asian countries for centuries as a dietary supplement described to improve the efficacy of the immune system for over 2 millennia [17]. Ganoderma spp. is now marketed as a dietary supplement in the Unites States of America and the European Union [18]. Polysaccharides, triterpenoids, alkaloids, and proteins extracted from Ganoderma spp. mycelium, the fruiting body (mushroom), and spores were characterized and resulted in a broad-spectrum bioactivity such as immunomodulatory activities, anti-inflammatory, antiviral, anti-diabetes, antioxidant, and antitumor. [19]. FIP activates macrophages and T lymphocytes alone or with the major bioactive polysaccharide in Ganoderma spp. [20]. The content of LZ-8 in G. lucidum or FIP-fve in F. velutipes (enoki, velvet shank, golden needle mushroom or winter mushroom) is 15 or 90 mg per kg wet weight in the fresh mycelium or fruiting body, respectively [3], [10]. The ingestion of natural FIP and GMI is believed to be safe since it is a natural product traditionally used from the Ganoderma mushroom. However, the extraction process for natural FIP enrichment is a bottle neck in its economic feasibility.
Functional or bioactive proteins are used in food industry for the food processing or as part of food ingredients. These proteins were traditionally isolated from various microorganisms and not well-adapted or characterized to the commercial use because of the modern food process condition and the safety concern [21]. The recombinant protein technology has made a more efficient market use of these bioactive protein through the protein engineering or molecular evolution under the well-defined condition such as food processing enzymes - amylase, lipase, and aminopeptidase et. al. - and food color additive - leghemoglobin [22], [23]. The lessons learnt from those commercially available recombinant proteins used in the food industry were adopted in the pursuit of using GMI as a dietary supplement [24], [25]. The yeast Pichia pastoris is nontoxigenic/nonpathogenic according to the qualified presumption of safety (QPS) list from the European Food Safety Authority [26] and is used in the recombinant expression of US Food and Drug Administration (FDA)-approved proteins that are “generally recognized as safe” (GRAS) [27], [29], [28]. Genomic engineering of P. pastoris to express fip-gmi results in 1–2 g/L of protein with the purity not less than 50% [2]. GMI enrichment free from viable cells of the production organism and recombinant DNA is achieved using tangential flow filtration technology [30], [31].
The objective of this study was to assess the suitability of standardized GMI from engineered P. pastoris as a new dietary ingredient (NDI) for food application. The safety assessment was evaluated for potential genotoxicity and systemic toxicity through in vitro and in vivo studies using Salmonella typhimurium (strain TA98, TA100, TA102, TA1535, and TA1537), Chinese hamster ovary (CHO) cells, CD-1® ICR mice, and Sprague-Dawley (SD) rats, respectively. Results indicated the safe use of GMI under the conditions tested.
2. Materials and methods
2.1. Test article
The test article, GMI (provided by MycoMagic Biotechnology Co., Ltd, Taiwan), was the test article obtained from engineered P. pastoris fermentation and filtrate recovery using a micro-/ultra-filtration-based recovery process with food- or pharmaceutical-grade materials. The resulting GMI as the protein concentrate is formulated with phosphate buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.4) and stored at − 20°C or below at a concentration described in the toxicology studies (frozen liquid). The GMI was analyzed for GMI concentration, appearance, residual transgene, heavy metals (cadmium, lead, mercury, copper, and arsenic), and screened using a microbial limit test (total aerobic plate count, total yeast/molds, coliform, Escherichia coli, Staphylococcus aureus, Salmonella, and Listeria monocytogenes) to determine its suitability as a food ingredient. The GMI specifications and batch analysis for lots used in this study are presented in Supplemental Table S1.
2.2. Test facility, test system and study design
Genetic toxicity studies were conducted at the Preclinical Testing Center of Level Biotechnology Inc. (Taipei, Taiwan). All research was performed in compliance with the “Good Laboratory Practice for Nonclinical Laboratory Studies,” Title 21 Code of Federal Regulations Part 58, the “Good Laboratory Practice for Non-clinical Laboratory Studies,” Ministry of Health and Welfare, Taiwan, the “OECD Principles on Good Laboratory Practice,” TAF OECD GLP Compliance, No. 1, and the “Regulations for Application of Health Food Permit,” Taiwan Food and Drug Administration outlined by the Ministry of Health and Welfare, Taiwan. General toxicity studies were conducted at Pharmaron Inc. (Beijing, China). All research was performed in compliance with the “Good Laboratory Practice for Nonclinical Laboratory Studies,” Title 21 Code of Federal Regulations Part 58, the “OECD Principles on Good Laboratory Practice,” TAF OECD GLP Compliance, No. 1, and the “China State Food and Drug Administration Good Laboratory Practice regulations”. During all the in vivo studies described below, the GMI concentrations, stability, and homogeneity were analyzed and evaluated in the test facility using the same method in the compliance with the “Good Laboratory Practice for Nonclinical Laboratory Studies,” Title 21 Code of Federal Regulations Part 58, and the “OECD Principles on Good Laboratory Practice,” TAF OECD GLP Compliance, No. 1.
2.2.1. Subchronic study - a 13-week oral gavage toxicity and toxicokinetic (TK) study with a 4-week recovery period
As previously described [32], the single dose acute toxicity of GMI and the 14-day repeat dose toxicity in CD® SD rats (Beijing Vital River Laboratory Animal Technology, Co. Ltd. China) was conducted by oral gavage at a dose volume of 10 mL/kg for the dose level selection in the subchronic study. Seven-eight weeks old SD rats were randomized into 4 groups with 3 rats at each dose level at 25, 50, 100, or 149.8 mg/kg of both genders for all groups in the single dose acute toxicity study; more naïve rats were also randomized into 4 groups with 5 rats at each dose level at 0, 50, 100, or 149.8 mg/kg of both genders for all groups in the 14-day repeat dose toxicity study. The subchronic study was then conducted under the same test facility. One hundred and forty-two SD rats were placed into four groups: group 1, vehicle (PBS) using 36 rats (20 were sacrificed on day 92, 10 were not treated after day 91 and sacrificed on day 120, and six were involved in the TK study); group 2, GMI dosage at 50 mg/kg body weight using 32 rats (20 were sacrificed on day 92, 12 were involved in the TK study); group 3, as for group 2 except the GMI dosage was 75 mg/kg; group 4, as for group 2 except the GMI dosage was 100 mg/kg and 42 rats were used (20 were sacrificed on day 92, 10 were not treated after day 91 and sacrificed on day 120, and 12 were involved in the TK study) (Supplementary information for further tabulated details). All experiments involved an equal proportion of males and females. The test article (GMI) or vehicle (PBS) was administered once daily for 91 consecutive days via oral gavage at a dose volume of 10 mL/kg. The following parameters were evaluated: cageside and detailed clinical observations, body weights and body weight changes, food consumption, ophthalmology, functional observational battery (FOB), clinical pathology (clinical chemistry, hematology, coagulation and urinalysis), absolute and relative organ weights, gross pathology, microscopic pathology and toxicokinetics. Ophthalmology examinations were performed using a pen light, an indirect ophthalmoscope and a slit lamp. The eyes of each animal were examined following administration of one drop of mydriasis reagent, tropicamide, purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). FOB observations and tests included the home-cage, hand-held, open-field, elicited responses, and physiological measurement (body temperature). TK animals in all groups had blood samples for serum TK collected at: 0, 0.5, 1, 2, 4, 8, and 24 h postdose on Day 1 and Day 91. Serum samples were stored frozen until analysis. Serum concentrations of the test article, GMI, were determined using a validated enzyme-linked immunosorbent assay (ELISA) method. The quantification range of GMI is 10–200 ng/mL (Low Limit of Quantification, LLOQ, nominal concentration at 10 ng/mL). Quantitative results were analyzed by Provantis version 7.0.3 (which is integrated with the industry standard SAS statistical analysis tool) using one-way analysis of variance (ANOVA) followed by Dunnett’s method. A probability (p-value) below 0.05 (two-tailed test) was used as the critical level of significance for all tests.
2.2.2. Teratology study - an embryo-fetal development and TK study
As previous described [33], naïve female rats were mated with male rats of the same source and strain. The day on which a copulatory plug was observed in situ or on the tray was considered day 0 of presumed gestation (GD 0). One hundred and forty mated female SD rats were assigned into four groups for dosing: group 1, vehicle (PBS) using 25 female rats in the main study and 4 female rats in the TK study; group 2–4, GMI at 50, 75, 100 mg/kg body weight, respectively using 25 female rats in the main study and 12 female rats in the TK study for each group. Vehicle or GMI was administered daily to the animals by oral gavage from GD 6 to GD 18 at a dose volume of 10 mL/kg. All surviving animals in the main study were sacrificed on GD 21 (Supplementary information for further tabulated details). The following parameters were evaluated: cageside and detailed clinical observations, body weights and body weight changes, food consumption, gross pathology, ovarian and uterine parameters, fetal examinations (body weight, sex, crown-rump length, gross external, visceral and skeletal alterations), and toxicokinetics. The reproductive tract was dissected from the abdominal cavity. The ovaries and gravid uterus including the cervix were weighted (paired organs were weighed together). The uterus was opened and examined. The fetuses were removed from the uterus and placed in appropriate containers. The ovaries and uterus were examined for number and distribution of corpora lutea, implantation sites, placentae (size, color or shape), live and dead fetuses, and early and late resorptions. Uteri that appeared non-gravid were further examined by ammonium sulfide staining to confirm the absence of implantation sites. Fetuses were examined for gender and external abnormalities and the crown-rump length was measured. At least one-half of the fetuses (odd numbered) in each litter were fixed in modified Davidson’s fixative. Each fetus was examined for visceral abnormalities using a micro-dissection technique. The head, thoracic cavity and abdominal cavity were subsequently examined. The remaining fetuses (even numbered, approximately one-half of the fetuses in each litter) were fixed in 95% ethanol, and double stained with Alcian Blue solution and Alizarin Red solution which were all purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). After staining, the skull, sternum, scapula, clavicle, appendicular skeleton, rib, and vertebral column were examined. TK animals in all groups had blood samples for serum TK collected at: 0, 0.5, 1, 2, 4, 8, and 24 h postdose on GD 6 and GD 18. Serum samples were handled as described in the Section 2.2.1. Quantitative results were analyzed as stated above.
2.2.3. Genetic toxicology study
2.2.3.1. Bacterial reverse mutation test (Ames test)
As previously described [34], [35], Salmonella typhimurium strains TA98, TA100, TA102, TA1535, TA1537 were used in the plate incorporation method in the presence or absence of a S9 metabolic activation system performed in triplicate. These Salmonella typhimurium strains are mutant for the biosynthesis of histidine amino acid. Thus, they are unable to grow and form colonies in the plate with medium lacking histidine. When these strains treated with test articles, which are mutagenic causes a reversal of mutation in strains, which enables colonies to grow on a media lacking in histidine. Firstly, the cytotoxicity and solubility were determined in a preliminary experiment using TA100. 2-nitrofluorene, sodium azide, mitomycin C, 9-aminoacridine, and benzo(a)pyrene were used as positive controls for assays with or without metabolic activation. S9 fraction (Aroclor 1254-induced; Moltox, Boone, USA) containing active liver enzymes were used to mimic the hepatic metabolic activation system. A cytotoxic effect was concluded when a decrease of below 0.5-fold was observed in revertant colonies over the negative control, and a loss of bacterial lawn, or pin colonies appeared. A potential mutagen had an increase in revertants over the negative control (2-fold for TA98, TA100, and TA102; and 3-fold for TA1535 and TA1537). If the test article was considered a potential mutagen, the data was further analyzed by ANOVA and Dunnett’s test to evaluate the difference between the negative/vehicle control group and the test article group. If the data was statistically significant, the dose-related response was further evaluated in numbers of revertant colonies on the test article groups as compared to the negative/vehicle controls. If a dose-related response was confirmed, the test article was considered a mutagen.
2.2.3.2. In vitro mammalian chromosomal aberration test
As previously described [36],epithelial-like CHO cells (CHO-K1) with a modal chromosome number of 20 ± 2 (Food Industry Research and development Institute - BCRC / NHRI Cell Bank, BCRC 60006, Hsinchu City, Taiwan) were selected for the GMI cytotoxicity assay using the MTT (3-[4,5-dimethylthiazol-2-yl]−2,5 diphenyl tetrazolium bromide) assay for cell viability. Cells were grown in Ham’s F-12 medium (Biological Industries) with 2 mM L-glutamine, 100 U/mL penicillin and streptomycin, and 10% FBS in a humidified atmosphere containing 5 ± 1% CO2 at 37 ± 1 °C. Cell morphology examination using a microscope (Leica, TYP 090-135.001) equipped with CCD image system (QImaging, MicroPublisher 3.3RTV) was first evaluated to choose the treatment concentrations: the highest testing concentration without cytotoxic effects was used as the greatest value tested. S9 fraction with cofactor was used to mimic the metabolic activation system as mentioned above. The culture medium was used as the negative control. Each test was performed in duplicate and incubated for the short- or long-term (3–6 or 18–22 h, respectively). The positive controls included mitomycin C for short- and long-term treatment without S9 activation and benzo(a)pyrene for the short-term treatment with S9 activation. At least 200 well-spread metaphase cells with (20 ± 2) centromeres were scored for all treatment and concurrent control groups. Structural chromosome aberrations, including chromosome breakage and exchange, chromatid breakage and exchange, and other abnormalities (polyploidy) were scored and recorded by photographing. The Fisher’s exact test was used for statistical analysis (p < 0.05). If more than two significant doses exist, the test article resulted in chromosome aberration in CHO-K1 cells. If only one significant dose existed, the Cochran–Armitage trend test (C-A test) was used for dose-dependent analysis. The test article was considered to display genotoxicity when the dose-dependent analysis was positive.
2.2.3.3. In vivo mammalian erythrocyte micronucleus test
As previously described [37], fifty CD-1® (ICR) mice (BioLASCO Taiwan Co., Ltd, SPF grade, 6 weeks-old) were quarantined and acclimated for 7 days before dosing. The following experimental layout was used with ten animals (five male, five female for each experiment): group 1, PBS; group 2, positive control (cyclophosphamide) at 80 mg/kg body weight; group 3, GMI at 87.5 mg/kg; group 4, GMI at 175 mg/kg; group 5, GMI at 350 mg/kg. Test article (GMI) or vehicle (PBS) was administered via oral gavage at a dose volume of 20 mL/kg. Cyclophosphamide was the positive control and was administered intraperitoneally. The following parameters were evaluated: clinical observations, body weights and body weight changes, polychromatic erythrocytes percentage (PCE%), and micronucleus frequency (MN‰PCE). At least 1000 erythrocytes were counted to determine PCE% and at least 2000 PCEs were observed to determine MN‰PCE. Data were presented as mean ± standard deviation. The MN‰PCE of the concurrent negative control (vehicle) group was within the historical data and below 3‰. The MN‰PCE of the concurrent positive control (cyclophosphamide) group was more than double that of the concurrent negative control group. Poisson distribution was used for statistical analysis (p < 0.05). If there were significant differences between the testing groups and the concurrent negative control group, the C-A test was used for dose-dependent analysis. The test article was considered to display genotoxicity when the dose-dependent analysis was positive. If the PCE percentage of the testing group was 50% less than negative control, this indicated that the test article inhibited erythropoiesis.
3. Results
3.1. Test article in-study stability and dose formulation analysis
Neither the production strain (engineered P. pastoris) nor its recombinant DNA (transgene) were detected in the final GMI (Supplemental Table S1). The concentration of dosing solutions in all studies was confirmed to within ± 15% of the nominal concentrations. In addition, no GMI was detected in the vehicle (PBS) formulation samples.
3.2. Genotoxicity studies
3.2.1. Bacterial reverse mutation test (Ames test)
GMI did not cause an increase in the number of revertant colonies in S. typhimurium strains TA98, TA100, TA102, TA1535 and TA1537 and no precipitation occurred at 3.125, 6.25, 12.5, 25 and 50 μL/plate (i.e. 0.055, 0.109, 0.219, 0.438, 0.875 mg GMI/plate) in the presence and/or absence of S9 metabolic activation mixture (data not shown). The study was considered as acceptable since revertant colonies in the positive control group were more than two-fold (TA98, TA100, and TA102) and three-fold (TA1535 and TA1537) that of the negative control groups and the number of revertant colonies in the negative control groups of each strain was within the range of the test facility historic control data (data not shown). Therefore, GMI was not considered a potential mutagen to S. typhimurium under the testing system.
3.2.2. In vitro chromosomal aberration assay
Cell viability in CHO-K1 cells following short-term exposure (3–6 h) without S9 metabolic activation at 19.5, 39.1, 78.1, 156.3, 312.5, 625, 1250, 2500, and 5000 μg/mL GMI was 107.24 ± 0.26%, 102.24 ± 2.55%, 98.75 ± 2.98%, 99.02 ± 2.64%, 83.03 ± 0.48%, 79.83 ± 1.70%, 73.41 ± 0.27%, 59.81 ± 0.82%, and 33.80 ± 0.23%, respectively compared with the vehicle (PBS) control. The same experiment with S9 metabolic activation showed viability values of 101.52 ± 2.11%, 95.07 ± 3.87%, 86.61 ± 3.74%, 86.64 ± 2.66%, 78.18 ± 0.77%, 70.89 ± 3.77%, 80.9 ± 2.97%, 74.86 ± 1.23%, and 55.93 ± 1.19%, respectively. Meanwhile, cell viability for a long-term exposure (18–22 h) without S9 metabolic activation at 1.2, 2.4, 4.9, 9.8, 19.5, 39.1, and 78.1 μg/mL GMI was 99.6 ± 11.53%, 101.65 ± 10.19%, 100.49 ± 9.80%, 78.06 ± 1.38%, 33.05 ± 0.37%, 3.34 ± 0.16%, and 2.35 ± 0.16%, respectively. The following GMI testing concentrations which showed no cytotoxicity (45 ± 5% cell viability) were then used in the chromosome aberration assay: 625, 1250, and 2500 µg/mL in 3-hour treatments without S9 metabolic activation; 1250, 2500, and 5000 µg/mL in 3-hour treatments with S9 metabolic activation; and 2.4, 4.9, and 9.8 µg/mL in 18-hour term treatments without S9 metabolic activation. There was no statistical difference (p value > 0.05) in the number of cells with structural chromosome aberrations with or without metabolic activation compared to the vehicle (PBS) in the short-term or long-term treatment (data not shown). Therefore, GMI was negative for the induction of structural and numerical chromosome aberration in CHO-K1 cells.
3.2.3. In vivo mammalian erythrocyte micronucleus test
There were no significant differences in mean body weights between the groups, no abnormal clinical symptoms observed, and no mortalities. The PCE percentage did not decrease in all treatment groups compared to the vehicle (PBS) indicating that GMI did not inhibit erythropoiesis (Fig. 1A&B). The validity of the experiment was confirmed since the PCE percentage of the positive control group (cyclophosphamide) decreased in both females and males at 48 h indicating that erythropoiesis inhibition occurred. In addition, there were no statistically significant increases in the frequency of micronuclei in mice at any GMI dosage tested (87.5, 175 or 350 mg/kg) compared to the vehicle (PBS). Meanwhile, cyclophosphamide significantly increased micronucleus frequency (MN‰PCE) (Fig. 1C&D). Therefore, GMI did not inhibit erythropoiesis or induce chromosomal damage in ICR mice erythrocytes.
Fig. 1.
Dose related changes in mouse polychromatic erythrocytes (PCE) at 48 and 72 h following oral administration of GMI to (A) male mice and (B) female mice. The percentage of PCE was presented as mean ± SD. In vivo mammalian micronucleus frequency (MN ‰PCE) following oral administration of GMI to (C) male mice and (D) female mice. The MN ‰PCE was presented as mean ± SD. Note: Negative control (NC); Positive control (PC); Body weight (bw); Phosphate buffered saline (PBS);#p < 0.05, significantly different from controls.
3.3. Subchronic toxicity study
The single dose acute toxicity and the 14-day repeat dose toxicity of GMI up to 149.8 mg/kg body weight did not cause any mortality or moribundity in SD rats and there was no test article-related finding. Therefore, dose levels of 0, 50, 75, 100 mg/kg were selected for the subchronic toxicity study. In conclusion, repeat oral administration of GMI once daily for 91 consecutive days up to 100 mg GMI/kg were well tolerated with no clear test article-related changes. Thus, the no-observed-adverse-effect level (NOAEL) of GMI treatment for 91 consecutive days in rats was 100 mg GMI/kg/dose.
3.3.1. Animal observations/measurements
Evaluated animal observations/measurements included cageside observations, detailed clinical observations, body weight, food consumption, ophthalmology and FOB.
3.3.1.1. Cageside observations and detailed clinical observations
Cageside observations included moribundity, general health, and signs of toxicity while detailed clinical observations included changes in the skin, fur, eyes, mucous membranes, surface lymph node, respiratory system, circulatory system, autonomic and central nervous system, and somatomotor activity and behavior pattern. There was no test article-related mortality during the study. One female administered PBS and one male administered 100 mg GMI/kg/dose were found dead on days 15 and 49, respectively. Macroscopic observations of the dead female rat at 0 mg GMI/kg/dose included blood clots in the thoracic cavity and subcutaneous tissue (right axilla) and red discoloration of all lung lobes which correlated with minimal congestion by microscopic analysis. Although a cause of death was undetermined by microscopic examination, the macroscopic observations suggested hemorrhage as the cause of death. Macroscopic observations of the dead male rat at 100 mg GMI/kg/dose included foam in the trachea, clear fluid in the thoracic cavity, and dark red discoloration of all lung lobes. Lung discoloration correlated microscopically with marked edema and fibrin, and mild hemorrhage in the alveoli with marked neutrophil infiltrates. These findings were consistent with gavage error as the cause of death. All remaining rats survived to their scheduled necropsies. Alopecia, scab and score were not considered to be test article-related due to their occurrence in control animals and low incidence.
3.3.1.2. Body weight and food consumption
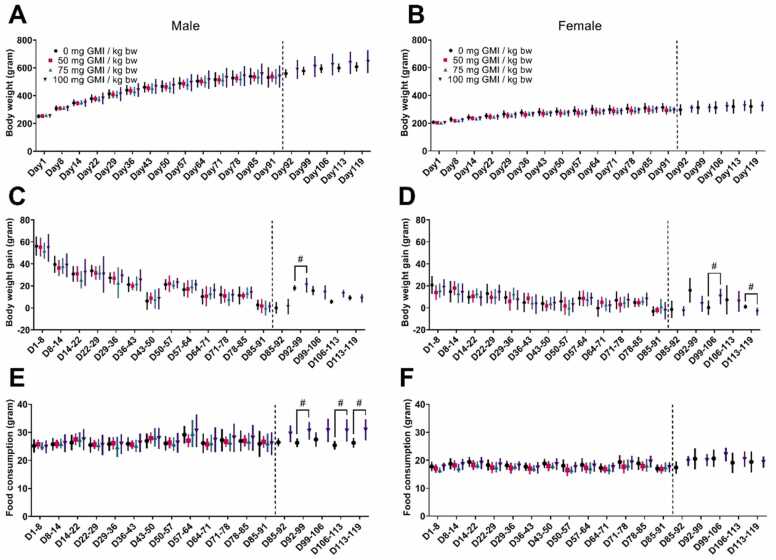
There was no test article-related effect on body weights (Fig. 2A&B) or body weight changes (Fig. 2C&D) or on food consumption (Fig. 2E&F).
Fig. 2.
Mean body weight of (A) male and (B) female, mean body weight changes of (C) male and (D) female, and the average food consumption of (E) male and (F) female rats following oral administration at different dose level of GMI for 91 days (13 weeks) or 119 days (4 weeks recovery period). Note:#p < 0.05, significantly different from controls.
3.3.1.3. Ophthalmology and FOB
There was no test article-related effect on ophthalmology parameters and FOB parameters. In addition, there was no test article-related effect on homecage observations, hand-held observations, and body temperature at week 13. The incidences of removal and reactivity to handling, and miosis in GMI treated groups were comparable to the control (PBS) group at week 13. Fast respiration and tiptoe gait separately observed in one male rat at 100 mg GMI/kg/dose for each symptom and in several groups of GMI treated females were comparable to the control group at week 13. The incidences of high arousal in males at 100 mg GMI/kg/dose were comparable to the control group at week 13. Meanwhile, 11 of 15 females (73.3%) had high arousal with the same dosage at week 13 compared with 6 of 14 females (42.9%) in the control group which fell to 40% (2 of 5 female rats) at week 17 which was comparable to the control group. The relationship of this finding to the test article treatment requires further experimentation. There was no test article-related effect on elicited response at week 13 and the incidences of auditory reactivity and tail pinch in GMI treated groups were comparable to the control.
3.3.2. Clinical pathology
Clinical pathology data (clinical chemistry, hematology, coagulation and urinalysis) showed no test article-related alterations at any time point (Table 1, Table 2, Table 3). There was an increased incidence of hematuria in GMI treated males and females compared to the PBS control. These changes are considered spurious and unrelated to the test article in the absence of correlating microscopic findings in the urinary tract (urinary bladder and kidney) or clinical chemistry parameters (blood urea nitrogen and creatinine).
Table 1.
Clinical Chemistry of rats following oral administration of GMI over 13-week period.
| Male |
Female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Dose level | 0 mg GMI / kg bw | 50 mg GMI / kg bw | 75 mg GMI / kg bw | 100 mg GMI / kg bw | 0 mg GMI / kg bw | 50 mg GMI / kg bw | 75 mg GMI / kg bw | 100 mg GMI / kg bw | |
| Day 15 | ALT | U/L | 44.3 ± 4.7 | 48 ± 6.5 | 46.3 ± 7.2 | 47.5 ± 9.5 | 40.5 ± 10.5 | 35.7 ± 3.6 | 34.9 ± 8.4 | 42 ± 14.8 |
| ALP | U/L | 229.8 ± 36.2 | 232.6 ± 56 | 232 ± 51.2 | 203.7 ± 53.1 | 103.3 ± 20.6 | 109.4 ± 27.8 | 106.8 ± 30 | 110.1 ± 24.7 | |
| GGT | U/L | 2.45 ± 0.07 | ||||||||
| TP | g/L | 68.09 ± 2.76 | 65.36 ± 2.8 | 65.94 ± 2.23 | 66.83 ± 3.23 | 69.19 ± 3.5 | 66.6 ± 3.63 | 69.38 ± 4.41 | 67.99 ± 3.93 | |
| BUN | mmol/L | 6.616 ± 1.049 | 6.443 ± 1.314 | 7.092 ± 1.049 | 6.721 ± 0.955 | 5.236 ± 0.674 | 5.764 ± 1.052 | 5.45 ± 0.543 | 5.691 ± 0.622 | |
| CRE | μmol/L | 27.1 ± 2.7 | 26.8 ± 2.3 | 31.8 ± 7.5 | 28.8 ± 8.3 | 27.7 ± 3.4 | 31.8 ± 4.1* | 28.9 ± 3.6 | 27.3 ± 2.7 | |
| GLU | mmol/L | 5.977 ± 0.512 | 5.965 ± 0.569 | 5.759 ± 0.334 | 5.94 ± 0.534 | 5.576 ± 0.579 | 5.48 ± 0.33 | 5.407 ± 0.628 | 5.389 ± 0.71 | |
| Na | mmol/L | 146.17 ± 1.45 | 145.06 ± 1.04 | 146.86 ± 1.63 | 147.73* ± 1.48 | 142.73 ± 1.28 | 142.39 ± 1.07 | 142.99 ± 1.22 | 143.33 ± 1.39 | |
| K | mmol/L | 6.528 ± 0.273 | 6.288 ± 0.314 | 6.358 ± 0.33 | 6.62 ± 0.31 | 5.749 ± 0.314 | 5.761 ± 0.415 | 5.632 ± 0.336 | 5.622 ± 0.35 | |
| Cl | mmol/L | 102.25 ± 1.01 | 101.89 ± 0.94 | 102.97 ± 1.03 | 103.68 ± 0.93** | 100.51 ± 0.92 | 100.54 ± 1.21 | 101.14 ± 1.56 | 101.66 ± 1.6 | |
| Day 45 | ALT | U/L | 36.6 ± 5.8 | 40.1 ± 4.8 | 37.7 ± 6.3 | 38.4 ± 6.3 | 37.1 ± 9.2 | 33.9 ± 4 | 49.3 ± 49.4 | 35.4 ± 7.7 |
| ALP | U/L | 137.3 ± 25.1 | 136.1 ± 28.5 | 140.2 ± 25.2 | 121.7 ± 33 | 62.9 ± 16.6 | 68.7 ± 15.6 | 65.8 ± 17.8 | 64.8 ± 12.5 | |
| GGT | U/L | |||||||||
| TP | g/L | 75.31 ± 3 | 72.95 ± 3.45 | 74.75 ± 2.96 | 74.34 ± 1.68 | 80.91 ± 6.23 | 80.6 ± 5.78 | 79.28 ± 5.67 | 75.72 ± 4.94* | |
| BUN | mmol/L | 5.883 ± 0.757 | 5.585 ± 0.272 | 5.947 ± 0.558 | 6.103 ± 0.646 | 5.428 ± 0.657 | 5.781 ± 0.757 | 5.355 ± 0.442 | 5.722 ± 0.67 | |
| CRE | μmol/L | 29.1 ± 2.3 | 29 ± 3.9 | 29.7 ± 2.6 | 28.3 ± 2.1 | 32.1 ± 2.8 | 32.8 ± 2.9 | 32.7 ± 3.3 | 33.1 ± 3.1 | |
| GLU | mmol/L | 6.16 ± 0.499 | 6.03 ± 0.628 | 5.792 ± 0.456 | 5.9 ± 0.47 | 6.261 ± 0.535 | 6.476 ± 0.349 | 6.481 ± 0.438 | 6.166 ± 0.925 | |
| Na | mmol/L | 145.15 ± 1.81 | 144.69 ± 1.29 | 145.35 ± 1.7 | 145.65 ± 0.87 | 143.86 ± 1.36 | 143.08 ± 1.39 | 143.62 ± 1.55 | 143.15 ± 1.34 | |
| K | mmol/L | 6.344 ± 0.3 | 6.134 ± 0.408 | 6.241 ± 0.392 | 6.317 ± 0.369 | 5.77 ± 0.416 | 5.73 ± 0.372 | 5.441 ± 0.393 | 5.551 ± 0.375 | |
| Cl | mmol/L | 101.61 ± 1.34 | 102.1 ± 1.16 | 101.79 ± 1.06 | 102.19 ± 0.73 | 101.71 ± 1.28 | 101.38 ± 0.91 | 101.4 ± 1.22 | 102.13 ± 1.49 | |
| Day 92 | ALT | U/L | 32.6 ± 6.3 | 36.7 ± 16.2 | 32 ± 6.8 | 32.7 ± 5.1 | 35.6 ± 12.1 | 37.1 ± 29.1 | 62.6 ± 77.6 | 27.2 ± 14.6 |
| AST | U/L | 87 ± 14.3 | 90.4 ± 20.4 | 87.2 ± 10.2 | 86.4 ± 6.3 | 80.4 ± 18.6 | 83.4 ± 28.9 | 128.5 ± 129.4 | 80.4 ± 17.2 | |
| ALP | U/L | 76.3 ± 15.1 | 72.1 ± 15.9 | 77.3 ± 14.9 | 69.2 ± 13.5 | 30.3 ± 6.6 | 36.6 ± 9.3 | 34.5 ± 7.3 | 35.8 ± 9.6 | |
| GGT | U/L | 2.4 | ||||||||
| CK | U/L | 147.6 ± 88.4 | 161.4 ± 42 | 160.2 ± 53.1 | 155.9 ± 76.7 | 179 ± 184.6 | 100.5 ± 39.1 | 121.3 ± 85.7 | 169.2 ± 143.6 | |
| LDH | U/L | 293.19 ± 263.57 | 285.76 ± 80.63 | 303.48 ± 168.28 | 312.4 ± 193.5 | 251.11 ± 156.51 | 184.01 ± 111.01 | 220.49 ± 174.28 | 310.72 ± 316.53 | |
| TP | g/L | 66.08 ± 2.82 | 66.09 ± 2.12 | 65.81 ± 3.62 | 66.84 ± 2.97 | 77.48 ± 4.39 | 73.14 ± 6.69 | 74.81 ± 4.05 | 72.69 ± 6.5 | |
| ALB | g/L | 45.7 ± 2.73 | 45.27 ± 2.02 | 45.56 ± 2.05 | 45.09 ± 1.99 | 58.62 ± 5.48 | 53.77 ± 6.32 | 56.79 ± 5.02 | 53.1 ± 5.82 | |
| GLO | g/L | 20.38 ± 1.01 | 20.82 ± 1.95 | 20.25 ± 2.22 | 21.76 ± 2.43 | 18.86 ± 2.83 | 19.37 ± 1.5 | 18.02 ± 3.58 | 19.59 ± 3.26 | |
| A/G | Ratio | 2.249 ± 0.186 | 2.195 ± 0.259 | 2.269 ± 0.22 | 2.094 ± 0.254 | 3.211 ± 0.821 | 2.786 ± 0.347 | 3.302 ± 0.898 | 2.783 ± 0.56 | |
| TBIL | μmol/L | 0.647 ± 0.261 | 0.601 ± 0.196 | 0.777 ± 0.316 | 0.516 ± 0.275 | 1.176 ± 0.544 | 1.487 ± 0.327 | 1.35 ± 0.58 | 1.168 ± 0.482 | |
| BUN | mmol/L | 5.736 ± 0.707 | 5.713 ± 0.49 | 5.59 ± 0.562 | 5.672 ± 0.455 | 5.58 ± 0.579 | 5.864 ± 0.961 | 5.318 ± 1.022 | 5.665 ± 0.88 | |
| CRE | μmol/L | 30.6 ± 4.9 | 29.4 ± 3.1 | 29.1 ± 6 | 27.7 ± 3.5 | 32.3 ± 4.8 | 31.9 ± 2.6 | 31.8 ± 4.1 | 31.1 ± 4.3 | |
| BUN/C | Ratio | 21.576 ± 2.177 | 22.377 ± 3.19 | 22.659 ± 4.951 | 23.644 ± 2.981 | 20.042 ± 3.791 | 20.974 ± 3.065 | 19.097 ± 3.057 | 20.808 ± 2.009 | |
| GLU | mmol/L | 8.916 ± 1.349 | 9.253 ± 1.403 | 9.302 ± 1.46 | 8.49 ± 1.118 | 8.718 ± 2.029 | 7.06 ± 0.747 | 7.091 ± 1.323 | 7.493 ± 1.942 | |
| CHO | mmol/L | 2.019 ± 0.299 | 1.821 ± 0.479 | 1.949 ± 0.249 | 1.804 ± 0.307 | 1.963 ± 0.687 | 2.185 ± 0.309 | 2.155 ± 0.613 | 1.711 ± 0.595 | |
| TG | mmol/L | 0.544 ± 0.331 | 0.558 ± 0.585 | 0.472 ± 0.198 | 0.508 ± 0.256 | 0.336 ± 0.244 | 0.173 ± 0.042* | 0.192 ± 0.096 | 0.187 ± 0.09 | |
| Na | mmol/L | 143.84 ± 2.49 | 144.57 ± 1.48 | 146.16 ± 1.56* | 146.52 ± 2.35* | 144.48 ± 2.25 | 144.61 ± 2.36 | 145.64 ± 3.1 | 144.21 ± 2.72 | |
| K | mmol/L | 9.766 ± 2.004 | 9.782 ± 1.813 | 8.48 ± 1.344 | 8.914 ± 2.375 | 7.058 ± 0.724 | 7.451 ± 0.824 | 7.087 ± 1.467 | 7.8 ± 1.365 | |
| Cl | mmol/L | 103.57 ± 1.17 | 104.16 ± 0.91 | 104.4 ± 0.98 | 104.39 ± 1.6 | 103.61 ± 0.87 | 104.29 ± 1.04 | 103.21 ± 1.63 | 103.33 ± 1.32 | |
| Ca | mmol/L | 2.889 ± 0.154 | 2.885 ± 0.122 | 2.908 ± 0.122 | 2.927 ± 0.091 | 2.982 ± 0.168 | 2.916 ± 0.16 | 2.958 ± 0.092 | 2.935 ± 0.205 | |
| P | mmol/L | 3.43 ± 0.29 | 3.48 ± 0.39 | 3.35 ± 0.37 | 3.16 ± 0.34 | 2.63 ± 0.31 | 2.71 ± 0.35 | 2.55 ± 0.36 | 2.78 ± 0.33 | |
| Day 120 | ALT | U/L | 30.6 ± 6.9 | 30.4 ± 3.8 | 75.2 ± 84.8 | 29.8 ± 7.1 | ||||
| AST | U/L | 81.4 ± 7 | 83.6 ± 9 | 200 ± 243.9 | 81.4 ± 4.8 | |||||
| ALP | U/L | 64.6 ± 9.1 | 59.4 ± 22.1 | 34.4 ± 16.9 | 28.2 ± 4.9 | |||||
| GGT | U/L | |||||||||
| CK | U/L | 187.2 ± 39.3 | 157.6 ± 60.8 | 137.6 ± 54.2 | 149 ± 36.8 | |||||
| LDH | U/L | 510.04 ± 136.79 | 331.94 ± 206.58 | 325.3 ± 168.47 | 319.84 ± 95.4 | |||||
| TP | g/L | 65.38 ± 2.28 | 65.88 ± 1.91 | 75.74 ± 9.16 | 72.22 ± 3.66 | |||||
| ALB | g/L | 45 ± 2.09 | 44.4 ± 1.16 | 58.4 ± 6.77 | 55.2 ± 4.22 | |||||
| GLO | g/L | 20.38 ± 0.38 | 21.48 ± 2.29 | 17.34 ± 3.78 | 17.02 ± 1.95 | |||||
| A/G | Ratio | 2.208 ± 0.094 | 2.092 ± 0.286 | 3.462 ± 0.619 | 3.286 ± 0.521 | |||||
| TBIL | μmol/L | 0.954 ± 0.33 | 0.71 ± 0.196 | 1.576 ± 1.02 | 1.822 ± 0.91 | |||||
| BUN | mmol/L | 6.564 ± 0.702 | 7.262 ± 0.587 | 6.108 ± 0.616 | 6.604 ± 1.562 | |||||
| CRE | μmol/L | 30 ± 7.3 | 31.8 ± 3.9 | 33.2 ± 1.8 | 35.6 ± 3.5 | |||||
| BUN/C | Ratio | 25.792 ± 5.056 | 26.228 ± 2.624 | 21.008 ± 2.283 | 21.112 ± 3.945 | |||||
| GLU | mmol/L | 8.094 ± 0.662 | 8.88 ± 1.194 | 8.98 ± 2.229 | 7.782 ± 1.072 | |||||
| CHO | mmol/L | 2.036 ± 0.352 | 2.098 ± 0.229 | 2.236 ± 0.925 | 1.532 ± 0.547 | |||||
| TG | mmol/L | 0.934 ± 0.466 | 0.872 ± 0.607 | 0.516 ± 0.196 | 0.312 ± 0.056 | |||||
| Na | mmol/L | 144.06 ± 2.67 | 143.02 ± 1.97 | 143.42 ± 1.19 | 144.64 ± 2.08 | |||||
| K | mmol/L | 8.182 ± 2.064 | 10.424 ± 1.824 | 7.254 ± 1.26 | 7.212 ± 0.879 | |||||
| Cl | mmol/L | 103.84 ± 0.96 | 103.62 ± 0.45 | 102.7 ± 1.45 | 104.76 ± 1.56 | |||||
| Ca | mmol/L | 2.774 ± 0.059 | 2.868 ± 0.083 | 2.988 ± 0.168 | 2.798 ± 0.056* | |||||
| P | mmol/L | 3.04 ± 0.26 | 3.38 ± 0.3 | 2.32 ± 0.36 | 1.92 ± 0.15 | |||||
Note: Alanine aminotransferase (ALT); Aspartate aminotransferase (AST); Alkaline phosphatase (ALP); Gamma glutamyl transferase (GGT); Creatine kinase (CK); Lactate dehydrogenase (LDH); Total protein (TP); Albumin (ALB); Globulin (GLO); A/G ratio (A/G); Total bilirubin (TBIL); Blood urea nitrogen (BUN); Creatinine (CRE); BUN/Creatinine ratio (BUN/C); Glucose (GLU); Cholesterol (CHO); Triglycerides (TG); Sodium (Na); Potassium (K); Chloride (Cl); Calcium (Ca);
Phosphorus (P); The values of GGT below detection limit (GGT: 2.3 U/L) was excluded from the statistical analysis. * p < 0.05, significantly different from controls; ** p < 0.01, significantly different from controls
Table 2.
Hematology values of rats following oral administration of GMI over 13-week period.
| Parameters | Dose level | Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 mg GMI / kg bw | 50 mg GMI / kg bw | 75 mg GMI / kg bw | 100 mg GMI / kg bw | 0 mg GMI / kg bw | 50 mg GMI / kg bw | 75 mg GMI / kg bw | 100 mg GMI / kg bw | |||
| Day 15 | RBC | 10^6/μL | 7.929 ± 0.339 | 7.816 ± 0.297 | 7.902 ± 0.357 | 8.014 ± 0.419 | 7.952 ± 0.355 | 8.059 ± 0.226 | 7.961 ± 0.333 | 8.06 ± 0.405 |
| HCT | Percentage | 47.78 ± 1.99 | 46.69 ± 1.82 | 47.07 ± 2.76 | 47.47 ± 1.77 | 44.95 ± 1.41 | 45.71 ± 1.04 | 45.64 ± 1.14 | 45.73 ± 1.9 | |
| HGB | g/dL | 15.73 ± 0.5 | 15.44 ± 0.56 | 15.71 ± 0.78 | 15.69 ± 0.55 | 15.43 ± 0.48 | 15.84 ± 0.32 | 15.64 ± 0.36 | 15.56 ± 0.68 | |
| MCV | fL | 60.28 ± 1.85 | 59.76 ± 0.84 | 59.53 ± 2.31 | 59.3 ± 1.73 | 56.6 ± 2.11 | 56.75 ± 1.58 | 57.39 ± 1.62 | 56.76 ± 1.19 | |
| MCH | pg | 19.87 ± 0.5 | 19.78 ± 0.38 | 19.91 ± 0.65 | 19.6 ± 0.61 | 19.42 ± 0.67 | 19.66 ± 0.55 | 19.67 ± 0.55 | 19.31 ± 0.55 | |
| MCHC | g/dL | 32.95 ± 0.57 | 33.09 ± 0.44 | 33.43 ± 0.55 | 33.07 ± 0.3 | 34.34 ± 0.51 | 34.68 ± 0.44 | 34.27 ± 0.44 | 34.05 ± 0.54 | |
| ABRETIC | 10^9/L | 264.07 ± 29.09 | 261.06 ± 31.82 | 242.78 ± 18.93 | 262.66 ± 46.29 | 162.77 ± 31.04 | 180.86 ± 37.66 | 153.3 ± 34.3 | 197.49 ± 32.69* | |
| WBC | 10^3/μL | 11.263 ± 3.069 | 12.201 ± 1.814 | 9.402 ± 1.511 | 9.327 ± 1.611 | 11.371 ± 2.472 | 10.646 ± 1.947 | 9.629 ± 4.071 | 10.197 ± 2.433 | |
| ABNEUT | 10^3/μL | 1.233 ± 0.324 | 2.053 ± 0.924** | 1.454 ± 0.404 | 1.257 ± 0.209 | 1.588 ± 1.09 | 1.593 ± 0.58 | 1.539 ± 0.716 | 1.976 ± 0.887 | |
| ABLYMP | 10^3/μL | 9.517 ± 2.824 | 9.557 ± 1.574 | 7.49 ± 1.277 | 7.63 ± 1.52 | 9.282 ± 1.755 | 8.59 ± 1.883 | 7.669 ± 3.398 | 7.744 ± 1.814 | |
| ABMONO | 10^3/μL | 0.293 ± 0.08 | 0.343 ± 0.139 | 0.273 ± 0.091 | 0.264 ± 0.03 | 0.278 ± 0.09 | 0.235 ± 0.07 | 0.211 ± 0.101 | 0.247 ± 0.128 | |
| ABBASO | 10^9/L | 0.025 ± 0.016 | 0.023 ± 0.013 | 0.017 ± 0.014 | 0.013 ± 0.008 | 0.023 ± 0.011 | 0.021 ± 0.008 | 0.019 ± 0.017 | 0.016 ± 0.007 | |
| ABEOS | 10^3/μL | 0.098 ± 0.049 | 0.113 ± 0.029 | 0.084 ± 0.019 | 0.087 ± 0.035 | 0.084 ± 0.035 | 0.099 ± 0.027 | 0.107 ± 0.033 | 0.119 ± 0.053 | |
| PLT | 10^3/μL | 1249.9 ± 235.6 | 1211.7 ± 102.9 | 1254.3 ± 173.6 | 1335.5 ± 209.5 | 1316.5 ± 121 | 1298.8 ± 162.8 | 1248.9 ± 169.5 | 1298.4 ± 233.7 | |
| MPV | fL | 8.18 ± 0.18 | 8.13 ± 0.24 | 8.32 ± 0.12 | 8.24 ± 0.2 | 8.32 ± 0.24 | 8.39 ± 0.2 | 8.64 ± 0.21 | 8.77 ± 0.4** | |
| Day 45 | RBC | 10^6/μL | 8.823 ± 0.329 | 8.822 ± 0.37 | 8.974 ± 0.29 | 9.089 ± 0.386 | 8.151 ± 0.391 | 8.078 ± 0.508 | 8.066 ± 0.24 | 8.187 ± 0.235 |
| HCT | Percentage | 48.88 ± 1.9 | 48.44 ± 1.96 | 49.15 ± 1.77 | 49.33 ± 1.98 | 44.75 ± 1.91 | 44.81 ± 2.41 | 45.03 ± 0.98 | 45.18 ± 1.36 | |
| HGB | g/dL | 16.44 ± 0.55 | 16.38 ± 0.63 | 16.52 ± 0.51 | 16.57 ± 0.66 | 15.32 ± 0.63 | 15.41 ± 0.82 | 15.47 ± 0.36 | 15.42 ± 0.53 | |
| MCV | fL | 55.41 ± 1.42 | 54.93 ± 1.24 | 54.81 ± 2.35 | 54.29 ± 1.34 | 54.93 ± 1.87 | 55.52 ± 1.11 | 55.89 ± 1.09 | 55.2 ± 1.03 | |
| MCH | pg | 18.64 ± 0.53 | 18.58 ± 0.47 | 18.44 ± 0.74 | 18.23 ± 0.44 | 18.79 ± 0.66 | 19.09 ± 0.54 | 19.16 ± 0.32 | 18.84 ± 0.47 | |
| MCHC | g/dL | 33.63 ± 0.7 | 33.81 ± 0.34 | 33.67 ± 0.42 | 33.57 ± 0.33 | 34.22 ± 0.5 | 34.38 ± 0.48 | 34.3 ± 0.55 | 34.13 ± 0.5 | |
| ABRETIC | 10^9/L | 177.25 ± 21.22 | 179.43 ± 22.84 | 184.03 ± 25.54 | 193.25 ± 25.51 | 138.54 ± 36.84 | 147.61 ± 31.92 | 137.1 ± 35.67 | 138.24 ± 39.88 | |
| WBC | 10^3/μL | 11.311 ± 3.749 | 11.592 ± 1.641 | 10.338 ± 1.606 | 11.264 ± 2.127 | 9.561 ± 1.917 | 8.4 ± 2.538 | 8.177 ± 3.257 | 8.925 ± 2.52 | |
| ABNEUT | 10^3/μL | 1.296 ± 0.451 | 1.552 ± 0.441 | 1.568 ± 0.649 | 1.542 ± 0.802 | 1.275 ± 0.601 | 0.942 ± 0.423 | 1.169 ± 0.55 | 1.862 ± 1.582 | |
| ABLYMP | 10^3/μL | 9.358 ± 3.326 | 9.319 ± 1.194 | 8.137 ± 1.52 | 9.026 ± 1.895 | 7.797 ± 1.638 | 7.092 ± 2.146 | 6.637 ± 2.623 | 6.597 ± 2.039 | |
| ABMONO | 10^3/μL | 0.354 ± 0.114 | 0.386 ± 0.104 | 0.342 ± 0.079 | 0.385 ± 0.121 | 0.255 ± 0.078 | 0.173 ± 0.067 | 0.183 ± 0.106 | 0.218 ± 0.099 | |
| ABBASO | 10^9/L | 0.028 ± 0.017 | 0.026 ± 0.01 | 0.023 ± 0.007 | 0.023 ± 0.01 | 0.016 ± 0.007 | 0.01 ± 0.007 | 0.01 ± 0.01 | 0.012 ± 0.007 | |
| ABEOS | 10^3/μL | 0.129 ± 0.047 | 0.161 ± 0.046 | 0.146 ± 0.039 | 0.132 ± 0.048 | 0.101 ± 0.028 | 0.1 ± 0.043 | 0.102 ± 0.035 | 0.143 ± 0.075 | |
| PLT | 10^3/μL | 1144.3 ± 135.7 | 1145.7 ± 91.5 | 1155.5 ± 146.6 | 1219.1 ± 146.9 | 1225.7 ± 128 | 1122.2 ± 134.3 | 1115 ± 116.3 | 1186.5 ± 188.2 | |
| MPV | fL | 8.11 ± 0.19 | 8.3 ± 0.13* | 8.18 ± 0.1 | 8.2 ± 0.22 | 8.14 ± 0.13 | 8.13 ± 0.24 | 8.42 ± 0.23* | 8.3 ± 0.25 | |
| Day 92 | RBC | 10^6/μL | 8.947 ± 0.446 | 8.902 ± 0.342 | 8.876 ± 0.482 | 8.915 ± 0.463 | 7.967 ± 0.443 | 7.828 ± 0.534 | 7.938 ± 0.285 | 8.059 ± 0.336 |
| HCT | Percentage | 48.22 ± 2.31 | 48.11 ± 2.39 | 48.09 ± 3.25 | 47.15 ± 1.85 | 44.92 ± 3.05 | 44 ± 2.55 | 45.38 ± 1.88 | 44.96 ± 1.99 | |
| HGB | g/dL | 15.77 ± 0.65 | 15.56 ± 0.66 | 15.58 ± 0.82 | 15.24 ± 0.57 | 14.9 ± 0.72 | 14.7 ± 0.78 | 15.04 ± 0.49 | 14.82 ± 0.42 | |
| MCV | fL | 53.92 ± 1.79 | 54.03 ± 1.06 | 54.17 ± 2.23 | 52.94 ± 1.43 | 56.39 ± 2.78 | 56.26 ± 1.69 | 57.16 ± 1.26 | 55.79 ± 1.69 | |
| MCH | pg | 17.63 ± 0.58 | 17.47 ± 0.27 | 17.59 ± 0.74 | 17.09 ± 0.55 | 18.72 ± 0.89 | 18.81 ± 0.68 | 18.95 ± 0.31 | 18.4 ± 0.5 | |
| MCHC | g/dL | 32.7 ± 0.66 | 32.33 ± 0.4 | 32.46 ± 0.59 | 32.28 ± 0.31 | 33.21 ± 0.86 | 33.41 ± 0.55 | 33.14 ± 0.61 | 32.98 ± 0.65 | |
| ABRETIC | 10^9/L | 149.3 ± 15.93 | 162.03 ± 26.49 | 152.35 ± 37.18 | 142.55 ± 22.82 | 129.89 ± 29.17 | 135.54 ± 27.34 | 145.85 ± 26.66 | 134.23 ± 33.03 | |
| WBC | 10^3/μL | 12.803 ± 2.745 | 14.684 ± 2.118 | 13.843 ± 3.831 | 13.218 ± 3.021 | 10.817 ± 2.097 | 11.264 ± 3.753 | 10.696 ± 4.498 | 11.314 ± 2.654 | |
| ABNEUT | 10^3/μL | 1.99 ± 1.448 | 1.579 ± 0.291 | 2.095 ± 1.609 | 2.271 ± 1.547 | 1.087 ± 0.263 | 1.665 ± 1.016 | 1.31 ± 0.412 | 1.182 ± 0.501 | |
| ABLYMP | 10^3/μL | 9.976 ± 1.947 | 12.149 ± 1.829 | 10.595 ± 2.117 | 9.944 ± 2.007 | 9.176 ± 1.962 | 8.971 ± 3.109 | 8.721 ± 4.146 | 9.41 ± 2.286 | |
| ABMONO | 10^3/μL | 0.472 ± 0.171 | 0.504 ± 0.149 | 0.63 ± 0.493 | 0.58 ± 0.348 | 0.297 ± 0.068 | 0.33 ± 0.105 | 0.362 ± 0.121 | 0.387 ± 0.123 | |
| ABBASO | 10^9/L | 0.062 ± 0.017 | 0.07 ± 0.009 | 0.069 ± 0.024 | 0.059 ± 0.01 | 0.026 ± 0.007 | 0.036 ± 0.025 | 0.039 ± 0.03 | 0.033 ± 0.016 | |
| ABEOS | 10^3/μL | 0.126 ± 0.039 | 0.197 ± 0.06** | 0.168 ± 0.033 | 0.166 ± 0.059 | 0.086 ± 0.043 | 0.099 ± 0.04 | 0.114 ± 0.069 | 0.149 ± 0.101 | |
| PLT | 10^3/μL | 1022.2 ± 98.8 | 1145.9 ± 111.5 | 1106 ± 119.9 | 1176.3 ± 173.5* | 1111.9 ± 104.1 | 1058.2 ± 197.7 | 1000.4 ± 168.1 | 1169.3 ± 151.2 | |
| MPV | fL | 8.37 ± 0.41 | 8.36 ± 0.2 | 8.36 ± 0.27 | 8.23 ± 0.25 | 8.21 ± 0.55 | 8.19 ± 0.37 | 8.35 ± 0.26 | 8.38 ± 0.51 | |
| Day 120 | RBC | 10^6/μL | 8.446 ± 0.484 | 8.766 ± 0.773 | 7.906 ± 0.285 | 7.784 ± 0.194 | ||||
| HCT | Percentage | 45.12 ± 2.31 | 46.66 ± 2.91 | 44 ± 2.89 | 44.52 ± 1.34 | |||||
| HGB | g/dL | 14.66 ± 0.69 | 15.12 ± 0.99 | 14.42 ± 0.62 | 14.86 ± 0.4 | |||||
| MCV | fL | 53.44 ± 1.52 | 53.32 ± 1.55 | 55.6 ± 1.78 | 57.2 ± 0.91 | |||||
| MCH | pg | 17.38 ± 0.63 | 17.26 ± 0.47 | 18.22 ± 0.18 | 19.08 ± 0.26** | |||||
| MCHC | g/dL | 32.48 ± 0.35 | 32.42 ± 0.26 | 32.84 ± 0.75 | 33.38 ± 0.18 | |||||
| ABRETIC | 10^9/L | 181.58 ± 20.2 | 188.28 ± 18.22 | 151.8 ± 53.15 | 164.76 ± 32.04 | |||||
| WBC | 10^3/μL | 15.288 ± 3.156 | 14.46 ± 3.252 | 9.974 ± 1.275 | 10.014 ± 4.723 | |||||
| ABNEUT | 10^3/μL | 2.314 ± 1.118 | 2.114 ± 0.751 | 1.682 ± 0.232 | 2.006 ± 2.024 | |||||
| ABLYMP | 10^3/μL | 11.954 ± 2.176 | 11.37 ± 2.341 | 7.434 ± 1.402 | 7.272 ± 2.519 | |||||
| ABMONO | 10^3/μL | 0.6 ± 0.146 | 0.616 ± 0.198 | 0.522 ± 0.096 | 0.408 ± 0.233 | |||||
| ABBASO | 10^9/L | 0.058 ± 0.029 | 0.058 ± 0.015 | 0.038 ± 0.008 | 0.04 ± 0.02 | |||||
| ABEOS | 10^3/μL | 0.176 ± 0.046 | 0.12 ± 0.02* | 0.118 ± 0.056 | 0.136 ± 0.059 | |||||
| PLT | 10^3/μL | 1117.8 ± 203.6 | 1201.4 ± 214.3 | 1044.4 ± 208.4 | 964.4 ± 104.1 | |||||
| MPV | fL | 8.54 ± 0.15 | 8.44 ± 0.6 | 8.5 ± 0.39 | 8.26 ± 0.39 | |||||
Note: Erythrocyte count (RBC); Hematocrit (HCT); Hemoglobin (HGB); Mean corpuscular volume (MCV); Mean corpuscular hemoglobin (MCH); Mean corpuscular hemoglobin concentration (MCHC); Reticulocyte count (ABRETIC); Leukocyte count (WBC); Leukocyte differential count - Neutrophil (ABNEUT), Lymphocyte (ABLYMP), Monocyte (ABMONO), Basophil (ABBASO), Eosinophil (ABEOS); Platelet count (PLT); Mean Platelet Volume (MPV); * p < 0.05, significantly different from controls; ** p < 0.01, significantly different from controls.
Table 3.
Coagulation values of rats following oral administration of GMI over 13-week period.
| Male |
Female |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 mg GMI / kg bw | 50 mg GMI / kg bw | 75 mg GMI / kg bw | 100 mg GMI / kg bw | 0 mg GMI / kg bw | 50 mg GMI / kg bw | 75 mg GMI / kg bw | 100 mg GMI / kg bw | |||
| Day 92 | APTT | seconds | 17.94 ± 1.84 | 18.24 ± 2.75 | 18.9 ± 1.54 | 18.01 ± 1.84 | 16.93 ± 2.26 | 17.67 ± 2.68 | 17.38 ± 3.32 | 16.47 ± 2.06 |
| PT | seconds | 22.47 ± 0.94 | 22.5 ± 2.09 | 23.15 ± 1.48 | 23.41 ± 1.01 | 23.71 ± 1.14 | 24.81 ± 1.01 | 23.94 ± 1.77 | 24.81 ± 1.38 | |
| Day 120 | APTT | seconds | 16.72 ± 0.83 | 15.32 ± 1.45 | 16.54 ± 1.71 | 16.5 ± 3 | ||||
| PT | seconds | 22.2 ± 0.89 | 21.96 ± 1.5 | 23.44 ± 1.07 | 24.04 ± 0.92 | |||||
Note: Activated partial thromboplastin Time (APTT); Prothrombin time (PT)
3.3.3. Necropsy and microscopic examination
Macroscopic necropsy examination included the external surface of the body, all orifices, cranial cavity, external surface of the brain, the thoracic, abdominal, and pelvic cavities and their viscera, cervical areas, carcass, and genitalia. There were no test article-related organ weight changes (Table 4, Table 5), macroscopic observations (gross pathology), and microscopic findings (histopathology).
Table 4.
Relative organ weights (%Brain weight) of rats following oral administration of GMI over 13-week period.
| Dose level | 0 mg GMI / kg bw | 50 mg GMI / kg bw | 75 mg GMI / kg bw | 100 mg GMI / kg bw | |||
|---|---|---|---|---|---|---|---|
| Gender | Male | ||||||
| Day 92 | Adrenals | %BrainWt | Percentage | 2.802 ± 0.448 | 3.102 ± 0.399 | 2.633 ± 0.382 | 2.969 ± 0.34 |
| Heart | %BrainWt | Percentage | 84.234 ± 41.455 | 71.004 ± 6.342 | 73.881 ± 7.687 | 73.613 ± 8.108 | |
| Kidneys | %BrainWt | Percentage | 161.574 ± 19.5 | 166.504 ± 17.517 | 160.051 ± 23.083 | 176.726 ± 21.629 | |
| Liver | %BrainWt | Percentage | 609.397 ± 117.226 | 615.078 ± 53.411 | 599.924 ± 61.646 | 601.618 ± 88.67 | |
| Spleen | %BrainWt | Percentage | 34.3 ± 5.316 | 34.913 ± 3.594 | 33 ± 5.014 | 32.031 ± 2.53 | |
| Thymus | %BrainWt | Percentage | 14.187 ± 4.229 | 16.929 ± 4.035 | 12.381 ± 2.455 | 13.26 ± 1.527 | |
| Thyroids | %BrainWt | Percentage | 1.755 ± 0.448 | 1.464 ± 0.45 | 1.532 ± 0.415 | 1.531 ± 0.36 | |
| Testes | %BrainWt | Percentage | 211.199 ± 33.874 | 220.712 ± 13.608 | 209.282 ± 18.723 | 230.127 ± 17.027 | |
| Gender | Female | ||||||
| Day 92 | Adrenals | %BrainWt | Percentage | 3.854 ± 0.875 | 3.758 ± 0.304 | 3.5 ± 0.655 | 3.668 ± 1.104 |
| Heart | %BrainWt | Percentage | 55.039 ± 6.693 | 52.471 ± 6.626 | 49.894 ± 7.973 | 49.457 ± 4.227 | |
| Kidneys | %BrainWt | Percentage | 104.083 ± 9.997 | 100.058 ± 8.068 | 103.153 ± 7.087 | 101.59 ± 8.928 | |
| Liver | %BrainWt | Percentage | 399.417 ± 43.746 | 365.135 ± 32.308 | 384.751 ± 42.618 | 382.148 ± 33.334 | |
| Spleen | %BrainWt | Percentage | 26.86 ± 4.482 | 24.153 ± 4.302 | 27.305 ± 5.09 | 26.497 ± 3.743 | |
| Thymus | %BrainWt | Percentage | 12.717 ± 2.059 | 12.353 ± 2.158 | 13.133 ± 2.739 | 12.785 ± 2.978 | |
| Thyroids | %BrainWt | Percentage | 1.332 ± 0.188 | 1.103 ± 0.235 | 1.136 ± 0.272 | 1.146 ± 0.185 | |
| Uterus | %BrainWt | Percentage | 36.496 ± 11.512 | 33.777 ± 6.261 | 36.596 ± 6.225 | 37.051 ± 15.019 | |
| Ovaries | %BrainWt | Percentage | 5.608 ± 0.691 | 4.242 ± 1.089* | 4.184 ± 0.938** | 5.093 ± 1.047 | |
| Dose level | 0 mg GMI / kg bw | 100 mg GMI / kg bw | 0 mg GMI / kg bw | 100 mg GMI / kg bw | |||
| Gender | Male | Female | |||||
| Day 120 | Adrenals | %BrainWt | Percentage | 2.95 ± 0.419 | 2.83 ± 0.251 | 4.146 ± 0.783 | 3.704 ± 0.577 |
| Heart | %BrainWt | Percentage | 77.234 ± 5.609 | 78.956 ± 11.678 | 52.856 ± 8.456 | 51.666 ± 6.567 | |
| Kidneys | %BrainWt | Percentage | 163.452 ± 23.718 | 172.44 ± 19.584 | 111.854 ± 9.058 | 104.106 ± 6.808 | |
| Liver | %BrainWt | Percentage | 712.462 ± 65.724 | 762.856 ± 127.778 | 453.45 ± 81.526 | 409.356 ± 54.828 | |
| Spleen | %BrainWt | Percentage | 39.834 ± 4.532 | 44.136 ± 6.435 | 25.066 ± 2.663 | 26.918 ± 5.063 | |
| Thymus | %BrainWt | Percentage | 14.014 ± 0.975 | 12.486 ± 2.093 | 9.542 ± 3.042 | 11.506 ± 3.321 | |
| Thyroids | %BrainWt | Percentage | 1.654 ± 0.173 | 1.632 ± 0.445 | 1.154 ± 0.164 | 1.214 ± 0.28 | |
| Testes | %BrainWt | Percentage | 215.51 ± 19.833 | 233.476 ± 13.934 | |||
| Uterus | %BrainWt | Percentage | 39.75 ± 4.209 | 33.686 ± 8.075 | |||
| Ovaries | %BrainWt | Percentage | 4.844 ± 1.758 | 5.358 ± 1.671 | |||
Note: Weight (Wt); Body weight (bw); *p < 0.05, significantly different from controls; ** p < 0.01, significantly different from controls.
Table 5.
Relative organ weights (%Body weight) of rats following oral administration of GMI over 13-week period.
| Dose level | 0 mg GMI / kg bw | 50 mg GMI / kg bw | 75 mg GMI / kg bw | 100 mg GMI / kg bw | |||
|---|---|---|---|---|---|---|---|
| Gender | Male | ||||||
| Day 92 | Adrenals | % BW | Percentage | 0.0126 ± 0.0025 | 0.0134 ± 0.0019 | 0.0118 ± 0.0014 | 0.0131 ± 0.0018 |
| Brain | % BW | Percentage | 0.4527 ± 0.0647 | 0.435 ± 0.0294 | 0.4515 ± 0.0357 | 0.4482 ± 0.0468 | |
| Heart | % BW | Percentage | 0.3889 ± 0.2455 | 0.3075 ± 0.0168 | 0.3325 ± 0.0339 | 0.328 ± 0.0304 | |
| Kidneys | % BW | Percentage | 0.7215 ± 0.0475 | 0.7239 ± 0.0873 | 0.7225 ± 0.1185 | 0.7857 ± 0.0657 | |
| Liver | % BW | Percentage | 2.6952 ± 0.1827 | 2.6625 ± 0.1163 | 2.69 ± 0.1616 | 2.6623 ± 0.1501 | |
| Spleen | % BW | Percentage | 0.1533 ± 0.0186 | 0.1515 ± 0.0142 | 0.148 ± 0.0174 | 0.143 ± 0.012 | |
| Thymus | % BW | Percentage | 0.0647 ± 0.0257 | 0.0733 ± 0.0172 | 0.0557 ± 0.0109 | 0.0592 ± 0.0068 | |
| Thyroids | % BW | Percentage | 0.0079 ± 0.0024 | 0.0062 ± 0.002 | 0.0069 ± 0.002 | 0.0069 ± 0.0019 | |
| Testes | % BW | Percentage | 0.9471 ± 0.1456 | 0.9596 ± 0.0854 | 0.9452 ± 0.1199 | 1.0301 ± 0.1126 | |
| Gender | Female | ||||||
| Day 92 | Adrenals | % BW | Percentage | 0.0262 ± 0.0062 | 0.0274 ± 0.0035 | 0.0256 ± 0.006 | 0.0262 ± 0.0077 |
| Brain | % BW | Percentage | 0.6889 ± 0.0696 | 0.7282 ± 0.065 | 0.7235 ± 0.0782 | 0.7129 ± 0.0516 | |
| Heart | % BW | Percentage | 0.3758 ± 0.0298 | 0.379 ± 0.0293 | 0.3563 ± 0.0328 | 0.3514 ± 0.0258 | |
| Kidneys | % BW | Percentage | 0.7133 ± 0.0646 | 0.7254 ± 0.0489 | 0.7442 ± 0.0739 | 0.7246 ± 0.0846 | |
| Liver | % BW | Percentage | 2.728 ± 0.152 | 2.6472 ± 0.2035 | 2.7723 ± 0.3433 | 2.7142 ± 0.1944 | |
| Spleen | % BW | Percentage | 0.1831 ± 0.0223 | 0.1739 ± 0.0191 | 0.1966 ± 0.0393 | 0.1882 ± 0.0242 | |
| Thymus | % BW | Percentage | 0.0873 ± 0.0142 | 0.0893 ± 0.014 | 0.0931 ± 0.011 | 0.0904 ± 0.0177 | |
| Thyroids | % BW | Percentage | 0.0091 ± 0.0018 | 0.0079 ± 0.0014 | 0.0081 ± 0.002 | 0.0081 ± 0.0017 | |
| Uterus | % BW | Percentage | 0.2483 ± 0.0672 | 0.249 ± 0.0627 | 0.2641 ± 0.0477 | 0.2671 ± 0.1147 | |
| Ovaries | % BW | Percentage | 0.0389 ± 0.0069 | 0.0305 ± 0.007* | 0.0297 ± 0.0045* | 0.0361 ± 0.0073 | |
| Dose level | 0 mg GMI / kg bw | 100 mg GMI / kg bw | 0 mg GMI / kg bw | 100 mg GMI / kg bw | |||
| Gender | Male | Female | |||||
| Day 120 | Adrenals | % BW | Percentage | 0.0112 ± 0.0008 | 0.0102 ± 0.0011 | 0.0282 ± 0.0033 | 0.025 ± 0.0054 |
| Brain | % BW | Percentage | 0.395 ± 0.0408 | 0.3736 ± 0.0434 | 0.692 ± 0.094 | 0.6678 ± 0.068 | |
| Heart | % BW | Percentage | 0.3038 ± 0.0233 | 0.2914 ± 0.02 | 0.36 ± 0.0243 | 0.3416 ± 0.0134 | |
| Kidneys | % BW | Percentage | 0.6422 ± 0.0812 | 0.6394 ± 0.0507 | 0.7694 ± 0.0767 | 0.6936 ± 0.0605 | |
| Liver | % BW | Percentage | 2.7988 ± 0.2029 | 2.808 ± 0.2355 | 3.08 ± 0.2039 | 2.7142 ± 0.2752* | |
| Spleen | % BW | Percentage | 0.1574 ± 0.0254 | 0.1632 ± 0.0177 | 0.172 ± 0.0144 | 0.1802 ± 0.0429 | |
| Thymus | % BW | Percentage | 0.0554 ± 0.0063 | 0.047 ± 0.0113 | 0.0642 ± 0.0118 | 0.075 ± 0.0151 | |
| Thyroids | % BW | Percentage | 0.0064 ± 0.0005 | 0.006 ± 0.0014 | 0.008 ± 0.0016 | 0.0078 ± 0.0013 | |
| Testes | % BW | Percentage | 0.845 ± 0.0252 | 0.8704 ± 0.0973 | |||
| Uterus | % BW | Percentage | 0.2732 ± 0.0297 | 0.2226 ± 0.0453 | |||
| Ovaries | % BW | Percentage | 0.0326 ± 0.0103 | 0.0362 ± 0.0138 | |||
Note: Body weight (BW or bw); *p < 0.05, significantly different from controls.
3.3.4. Toxicokinetics and bioanalysis
GMI concentrations of TK samples were all below the lower limit of quantification (LLOQ) in serum. Thus, the toxicokinetic parameters could not be calculated in this study.
3.4. Teratology study
The pregnancy rate was between 92% (23/25) and 96% (24/25) in SD rats. Female rats were caesarean-sectioned and examined for gross lesions collected at necropsy for potential evaluation. There was no test article-related mortality or moribundity, and no effects to the dam or fetus throughout the study. Therefore, the NOAEL of GMI for maternal toxicity and embryo-fetal developmental toxicity was taken as 100 mg GMI/kg/dose.
3.4.1. Animal observations/measurements
Animal observations/measurements included cageside observations, detailed clinical observations, body weight, and food consumption.
3.4.1.1. Cageside observations and detailed clinical observations
The types of observations paralleled that of 3.3.1.1 with all animals surviving to their scheduled sacrifice. Alopecia was observed from all study groups including the control with comparable incidence and is considered unrelated to the test article treatment. Staining and haircoat piloerection observed in one female given 50 mg GMI/kg on GD21 and rale noted in one female given 100 mg GMI/kg on GD15 are considered random findings.
3.4.1.2. Body weight and food consumption
There was no test article-related effect on body weights (Fig. 3A), body weight changes (Fig. 3B), and food consumption (Fig. 3C).
Fig. 3.
Mean body weight (A), mean body weight changes (B), and the average food consumption (C) of pregnant rats following oral administration of GMI at different dose level of GMI during gestation. Note:#p < 0.05, significantly different from controls.
3.4.2. Microscopic examination
There were no test article-related gross pathology findings in the thoracic, abdominal and pelvic cavities and their viscera.
3.4.2.1. Ovarian and uterine examination
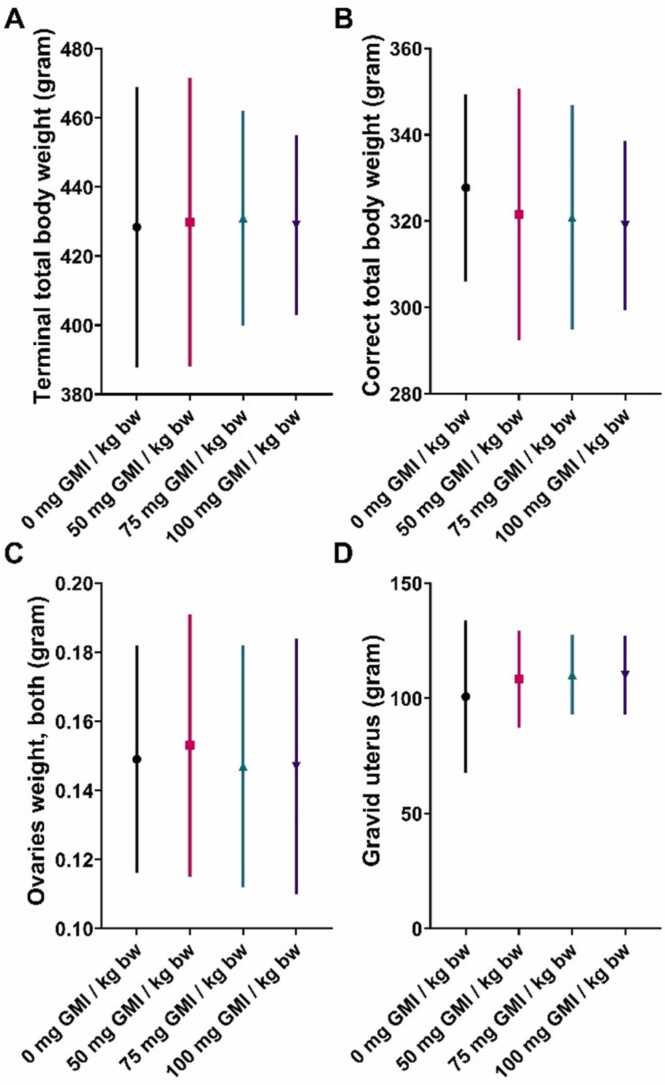
The gravid uterine weights and ovary weights were comparable among all study groups (Fig. 4). There were no statistically significant differences between treated and control (PBS) groups in the numbers of corpora lutea, implantations, sex ratio, live fetuses, dead fetuses, fetal deaths, embryonic resorptions, early resorptions, pre-/post-implantation loss, mean fetal weight or crown-rump length (Table 6).
Fig. 4.
Maternal terminal body weight (A), correct total body weight (B), both ovaries weight (C), and gravid uterus weight (D) of pregnant rats following oral administration of GMI at different dose level of GMI during gestation.
Table 6.
External, visceral, skeletal, ossification examination of fetuses.
| Dose level |
|||||
|---|---|---|---|---|---|
| Parameters | Classification | 0 mg GMI /kg bw | 50 mg GMI /kg bw | 75 mg GMI /kg bw | 100 mg GMI /kg bw |
| No. of fetuses (litters) examined | 325(21) | 365(24) | 349(23) | 358(23) | |
| No. of fetuses (litters) with abnormalities | 2(2) | 3(2) | 2(2) | 0(0) | |
| % fetuses (litters) with abnormalities | 0.6%(9.5%) | 0.8%(8.3%) | 0.6%(8.7%) | 0.0%(0.0%) | |
| No. of fetuses (litters) with variations | 1(1) | 3(2) | 1(1) | 0(0) | |
| % fetuses (litters) with variations | 0.3%(4.8%) | 0.8%(8.3%) | 0.3%(4.3%) | 0.0%(0.0%) | |
| No. of fetuses (litters) with Malformations | 1(1) | 0(0) | 1(1) | 0(0) | |
| % fetuses (litters) with Malformations | 0.3%(4.8%) | 0.0%(0.0%) | 0.3%(4.3%) | 0.0%(0.0%) | |
| External abnormalities | |||||
| Trunk/head, subcutaneous hemorrhage | Variation | 1(1) | 3(2) | 1(1) | 0(0) |
| General, small | Malformation | 1(1) | 0(0) | 1(1) | 0(0) |
| No. of fetuses (litters) examined | 167(21) | 189(24) | 181(23) | 186(23) | |
| No. of fetuses (litters) with abnormalities | 9(7) | 8(5) | 9(6) | 10(7) | |
| % fetuses (litters) with abnormalities | 5.4% (33.3%) | 4.2% (20.8%) | 5.0% (26.1%) | 5.4% (30.4%) | |
| No. of fetuses (litters) with variations | 8(7) | 8(5) | 8(5) | 9(6) | |
| % fetuses (litters) with variations | 4.8% (33.3%) | 4.2% (20.8%) | 4.4% (21.7%) | 4.8% (26.1%) | |
| No. of fetuses (litters) with Malformations | 1(1) | 0(0) | 1(1) | 1(1) | |
| % fetuses (litters) with Malformations | 0.6% (4.8%) | 0.0% (0.0%) | 0.5% (4.3%) | 0.5%(4.3%) | |
| Visceral abnormalities | |||||
| Renal pelvic, dilated | Variation | 7(6) | 8(5) | 8(5) | 9(6) |
| Diaphragm, thin | Variation | 1(1) | 0(0) | 0(0) | 0(0) |
| Lung, abnormal lobation | Malformation | 1(1) | 0(0) | 0(0) | 1(1) |
| Lung, Lobe fused | Malformation | 0(0) | 0(0) | 1(1) | 0(0) |
| No. of fetuses (litters) examined | 158 (21) | 176(24) | 168(23) | 172(23) | |
| No. of fetuses (litters) with abnormalities | 23(13) | 23(10) | 21(11) | 24(15) | |
| % fetuses (litters) with abnormalities | 14.6%(61.9%) | 13.1%(41.7%) | 12.5%(47.8%) | 14.0%(65.2%) | |
| No. of fetuses (litters) with variations | 23(13) | 23(10) | 21(11) | 24(15) | |
| % fetuses (litters) with variations | 14.6%(61.9%) | 13.1%(41.7%) | 12.54%(47.8%) | 14.0%(65.2%) | |
| No. of fetuses (litters) with Malformations | 0(0) | 1(1) | 0(0) | 0(0) | |
| % fetuses (litters) with Malformations | 0.0%(0.0%) | 0.6%(4.2%) | 0.0%(0.0%) | 0.0%(0.0%) | |
| Skeletal abnormalities | |||||
| Interparietal, incomplete ossification | Variation | 0(0) | 1(1) | 0(0) | 0(0) |
| sternebrae,incomplete ossification | Variation | 0(0) | 0(0) | 3(2) | 3(3) |
| Sternebrae, unossified | Variation | 4(3) | 4(2) | 4(3) | 1(1) |
| costal cartillage, interrupted | Variation | 0(0) | 0(0) | 3(3) | 0(0) |
| Thoracic centrum, bipartite ossification | Variation | 19(10) | 19(9) | 15(9) | 20(13) |
| Lumbar arch, absent | Malformation | 0(0) | 1(1) | 0(0) | 0(0) |
| Litters examined | 21 | 24 | 23 | 23 | |
| Fetuses examined | 158 | 176 | 168 | 172 | |
| Vertebrae | |||||
| Cervical | Mean±SD | 7 ± 0 | 7 ± 0 | 7 ± 0 | 7 ± 0 |
| Thoracic | Mean±SD | 13 ± 0 | 13 ± 0 | 13 ± 0 | 13 ± 0 |
| Lumbar | Mean±SD | 6 ± 0 | 6 ± 0 | 6 ± 0 | 6 ± 0 |
| Sacral | Mean±SD | 4 ± 0 | 4 ± 0 | 4 ± 0 | 4 ± 0 |
| Caudal | Mean±SD | 5.6 ± 0.5 | 5.3 ± 0.6 | 5.4 ± 0.7 | 5.3 ± 0.5 |
| Ribs(Pairs) | Mean±SD | 13 ± 0 | 13 ± 0 | 13 ± 0 | 13 ± 0 |
| Sternum | Mean±SD | 6 ± 0.1 | 6 ± 0.1 | 6 ± 0 | 6 ± 0 |
| Metacarpal | Mean±SD | 4 ± 0 | 4 ± 0 | 4 ± 0 | 4 ± 0 |
| Metatarsal | Mean±SD | 4.6 ± 0.3 | 4.5 ± 0.3 | 4.5 ± 0.4 | 4.6 ± 0.3 |
3.4.3. Fetal examinations (body weight, sex, crown-rump length, gross external, visceral and skeletal alterations)
3.4.3.1. External abnormalities and sex
There were no statistically significant differences observed among the crown rump length or other abnormalities for the four dose groups in a fetal external examination (Table 6). Subcutaneous hemorrhage of the trunk/head observed in the control (PBS) group and 50–75 mg GMI/kg/dose group and the general small body size observed in the control group and 75 mg GMI/kg/dose group were considered random findings without statistical significance.
3.4.3.2. Body weights and identification
The body weight of each live fetus was individually identified with the litter number and uterine distribution. There were no statistically significant differences between treated and control groups in number of mean fetal weight.
3.4.3.3. Visceral examination
There were no statistically significant differences observed among the four dose groups in a fetal visceral examination (Table 6). Dilated renal pelvises were found in all study groups including the controls with comparable incidence so this symptom is considered unrelated to the test article treatment. Other visceral variations/malformations including thin diaphragms and abnormal lobation of the lungs were unrelated to the test article due to their low incidence with no observed dose-response relationship.
3.4.3.4. Skeletal examination
There were no statistically significant differences observed among the four dose groups in the average numbers of skeletal variation or malformation (Table 6). No statistically significant or biologically important differences were observed among the four dose groups in the average number of ossification sites per fetus for the vertebrae (cervical, thoracic, lumbar, sacral and caudal), ribs, sternum, metacarpals or metatarsals. The alterations in a few fetuses were not considered test article-related due to the lack of dose response and/or statistical significance. The skeletal variations included incomplete ossification of interparietal segment (50 mg GMI/kg/dose group) and sternebrae (75/100 mg GMI/kg/dose group), unossified sternebrae (control and all treated groups), interrupted costal cartilage (75 mg GMI/kg/dose group), and bipartite ossification of the thoracic centrum (control and all treated groups). The skeletal malformation included an absence of the lumbar arch (50 mg GMI/kg/dose group).
3.4.4. Toxicokinetics and bioanalysis
GMI concentrations of TK samples in the serum were all below the LLOQ. Thus, the toxicokinetic parameters could not be calculated in this study.
4. Discussion
Proteins have been consumed for thousands of years and are important building blocks for our body. They are considered nutraceutical and are used as dietary supplements or as food additives for added flavor [38]. Nonetheless, the protein with noted bioactivity or odor might not be abundant in the original diet source. For example, leghemoglobin is present in legume root nodules at 0.1–0.3% fresh weight and has a unique meat flavor [22]. It remains a challenge to bridge functional and useful proteins from the laboratory bench to the table.
Although the protein content in the mushroom is limited, their nutritional/medicinal benefits are well-studied [39], [40]. The FIP family (PF09259) was first identified from Ling-Zhi (Reishi or G. lucidum) in 1989 [3], then structurally annotated in 2003 [41], [42], [16]. The FIP from G. microsporum (i.e. GMI) used in the present study is manufactured using an engineered P. pastoris GMI overexpression strain [2]. The recombinant GMI could be standardized and well quality controlled for further comprehensive studies from the protein characterization to its pharmacological effects in the different cell or animal models. A 2.0 Å structure of GMI was solved and GMI showed a dimer:dimer quaternary structure with non-covalent interactions through the N-terminal helices (PDB ID 3KCW, [16]). Furthermore, GMI with the same quality were evaluated in the platform for the anti-tumor activity and immunomodulation under immune disorder/disease model, and GMI showed a variety of bioactivities in the application for oncology [45], [46], [47], [48], [49], [50], [51], [52], [44], [43], immuno-oncology [53], [54], [55], [56], [57], and immunology [59], [60], [62], [63], [64], [61], [58], [65]. Although these promising findings are attractive for the further clinical application, the quality and the safety are always the major concerns. Thus, the primary consideration in this work is focus on the safety for the use of this protein manufactured using a modern technology, recombinant protein, in the dietary supplement.
The oral gavage of GMI at the dose volume of up to 10 mL/kg/day (i.e. 100 mg GMI/kg/day) had no significant biological difference compared with the PBS control in male and female SD rats for 13-weeks and pregnant rats from gestation day 6–18. The potential risk of allergenicity and toxicity were evaluated through a literature search, sequence homology comparison to known allergens and toxins, and sensitivity to pepsin digestion using simulated gastric fluid in accordance with the CODEX Alimentarius Commission 2003 guidelines for genetically modified foods and the European Food Safety Authority (EFSA) guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed [66]. There were no findings in the scientific literature related to the allergenicity or toxicity of GMI derived from P. pastoris. The protein was not resistant to in vitro digestion under either simulated gastric fluid or intestinal fluid [67], [68]. Furthermore, the GMI primary amino acid sequence contains no significant homology to any known allergens or toxins via analysis in the AlergenOnline database (www.allergenonline.org) [69]. Together, there was no evidence to indicate any significant risk of dietary allergy or toxicity to consumers using GMI as a dietary supplement and/or in food products containing the GMI.
5. Conclusions
Daily oral gavage administration of GMI from engineered P. pastoris at levels of up to 10 mL/kg/day (i.e. 100 mg GMI/kg/day) to SD rats for 13-weeks (subchronic toxicity study) or during the embryo-fetal development (teratology study) were non-toxic. In addition, GMI was not genotoxic in all studies including the bacterial reverse mutation test (Ames test), in vitro chromosomal aberration assay, and in vivo mammalian erythrocyte micronucleus test. These systemic toxicity and reproductive health studies established a NOAEL of 100 mg GMI/kg/day for both genders of rats which was the maximum dose administered. The acceptable daily intake (ADI) is calculated by introducing a safety factor of 100 followed by the recommendation of the Code of Federal Regulations (Code of Federal Regulations, 2021). Thus, the proposed ADI for GMI is 100th of 100 mg/kg/day or 1 mg/kg/day.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Hsu-Yuan Fu and Ruey-Shyang Hseu are employees of MycoMagic Biotechnology Co., Ltd.
Acknowledgements and funding details
The work was supported by MycoMagic Biotechnology Co., Ltd (New Taipei City, Taiwan). (study sponsor). The sponsor co-designed the study and coordinated interactions with contract research organizations (CROs) staff and regulatory authorities. The CROs took charge of study operation in compliance with the Good Laboratory Practice for Non-clinical Laboratory Studies (FDA, 21 CFR, Part 58), the Good Laboratory Practice for Non-clinical Laboratory Studies (Ministry of Health and Welfare, R.O.C., 3rd ed., 2006), the OECD Principles on Good Laboratory Practice (TAF OECD GLP Compliance, No. 1, 1997), the Regulations for Application of Health Food Permit (TFDA, 2016) outlined by the Ministry of Health and Welfare, Taiwan, R.O.C, and the China State Food and Drug Administration Good Laboratory Practice regulations (GLP, September, 2003). Authors thanks the acknowledge letter without objection (AKL) in 2020 with the reference number 1133 from US FDA for the premarket safety notification for the use of GMI as a new dietary ingredient (NDI). Authors thank Chi-Jui Lee and Hui-Chang Wang, Ph.D. (MycoMagic Biotech. Co., Ltd.) for their helpful inputs toward this study. Authors thank Ada H.C. Kung, Ph.D., MBA, DABT (Expedient Solutions Pharmaceutical & Biotechnology Consulting Corp., Taipei, Taiwan) as an independent consulting toxicologist for her assistance in the study design and the preparation of this manuscript.
Handling Editor: Dr. Lawrence Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.05.013.
Contributor Information
Hsu-Yuan Fu, Email: fuh@mycomagic.com.tw.
Ruey-Shyang Hseu, Email: rshseu@ntu.edu.tw.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
References
- 1.Hseu R.-S., Chen Z.-C., Wang H. Ganoderma microsporum, a new species on weeping willow in Taiwan. Mycotaxon. 1989;35:35–40. [Google Scholar]
- 2.T.-L. Lin, Immunomodulatory protein cloned from Ganoderma microsporum (U.S. Patent No. 7,601,808 B2), 2009. U.S. Patent and Trademark Office. 〈https://patft.uspto.gov/netahtml/PTO/srchnum.htm〉.
- 3.Kino K., Yamashita A., Yamaoka K., Watanabe J., Tanaka S., Ko K., Shimizu K., Tsunoo H. Isolation and characterization of a new immunomodulatory protein, ling zhi-8 (LZ-8), from Ganoderma lucidium. J. Biol. Chem. 1989;264:472–478. PMID: 2909532. [PubMed] [Google Scholar]
- 4.Ejike U.C., Chan C.J., Okechukwu P.N., Lim R.L.H. New advances and potentials of fungal immunomodulatory proteins for therapeutic purposes. Crit. Rev. Biotechnol. 2020;40:1172–1190. doi: 10.1080/07388551.2020.1808581. [DOI] [PubMed] [Google Scholar]
- 5.Peek H.W., Halkes S.B., Tomassen M.M., Mes J.J., Landman W.J. In vivo screening of five phytochemicals/extracts and a fungal immunomodulatory protein against colibacillosis in broilers. Avian Pathol. 2013;42:235–247. doi: 10.1080/03079457.2013.780121. [DOI] [PubMed] [Google Scholar]
- 6.Lin W.H., Hung C.H., Hsu C.I., Lin J.Y. Dimerization of the N-terminal amphipathic alpha-helix domain of the fungal immunomodulatory protein from Ganoderma tsugae (Fip-gts) defined by a yeast two-hybrid system and site-directed mutagenesis. J. Biol. Chem. 1997;272:20044–20048. doi: 10.1074/jbc.272.32.20044. [DOI] [PubMed] [Google Scholar]
- 7.Han F., Liu Y., Guo L.Q., Zeng X.L., Liu Z.M., Lin J.F. Heterologous expression of the immunomodulatory protein gene from Ganoderma sinense in the basidiomycete Coprinopsis cinerea. J. Appl. Microbiol. 2010;109:1838–1844. doi: 10.1111/j.1365-2672.2010.04811.x. [DOI] [PubMed] [Google Scholar]
- 8.Li F., Wen H., Zhang Y., Aa M., Liu X. Purification and characterization of a novel immunomodulatory protein from the medicinal mushroom Trametes versicolor. Sci. China Life Sci. 2011;54:379–385. doi: 10.1007/s11427-011-4153-2. [DOI] [PubMed] [Google Scholar]
- 9.Chang H.-H., Sheu F. A novel fungal immunomodulatory protein (PCP) isolated from Poria cocos activates mouse peritoneal macrophage involved in toll-like receptor 4. FASEB J. 2007;21 doi: 10.1021/jf9011399. A738-A738. [DOI] [Google Scholar]
- 10.Ko J.L., Hsu C.I., Lin R.H., Kao C.L., Lin J.Y. A new fungal immunomodulatory protein, FIP-fve isolated from the edible mushroom, Flammulina velutipes and its complete amino acid sequence. Eur. J. Biochem. 1995;228:244–249. PMID: 7705335. [PubMed] [Google Scholar]
- 11.Hsu H.C., Hsu C.I., Lin R.H., Kao C.L., Lin J.Y. Fip-vvo, a new fungal immunomodulatory protein isolated from Volvariella volvacea. Biochem. J. 1997;323(Pt 2):557–565. doi: 10.1042/bj3230557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang C.Y., Cheng T.J., Chang F.R., Wang H.Y., Kan W.C., Li S.L., Huang L.H., Chen Y.C., Tsai W.C., Huang C.H., Cheng C.H., Lee G.Y., Shyue S.W., Chen Y.P., Lin K.C., Chuu J.J. Macrophage mediated anti-proliferation effects of Anthodia camphorata non-polysaccharide based extracts on human hepatoma cells. Biosci. Biotechnol. Biochem. 2011;75:624–632. doi: 10.1271/bbb.100559. [DOI] [PubMed] [Google Scholar]
- 13.Pushparajah V., Fatima A., Chong C.H., Gambule T.Z., Chan C.J., Ng S.T., Tan C.S., Fung S.Y., Lee S.S., Tan N.H., Lim R.L. Characterisation of a new fungal immunomodulatory protein from tiger milk mushroom, Lignosus rhinocerotis. Sci. Rep. 2016;6:30010. doi: 10.1038/srep30010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y., Bastiaan-Net S., Wichers H.J. Current understanding of the structure and function of fungal immunomodulatory proteins. Front. Nutr. 2020;7:132. doi: 10.3389/fnut.2020.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin J., Chen H., Bai Y., et al. Ganoderma immunomodulatory proteins: mushrooming functional FIPs. Appl. Microbiol. Biotechnol. 2022;106:2367–2380. doi: 10.1007/s00253-022-11839-9. [DOI] [PubMed] [Google Scholar]
- 16.M.-Y. Wu, M.-F. Hsu, C.-S. Huang, H.-Y. Fu, A.H. Wang, R.-S. Hseu, C.-T. Huang, C.-S. Yang, A 2.0 Å Structure of the Fungal Immunomodulatory Protein GMI from Ganoderma microsporum, 2007.
- 17.Wachtel-Galor S., Yuen J., Buswell J.A., Benzie I.F.F. In: Herbal Medicine: Biomolecular and Clinical Aspects. Benzie I.F.F., Wachtel-Galor S., editors. CRC Press/Taylor & Francis; Boca Raton, FL: 2011. Ganoderma lucidum (Lingzhi or Reishi): a medicinal mushroom. Chapter 9. PMID: 22593926. [Google Scholar]
- 18.Gunnels T., Creswell M., McFerrin J., Whittall J.B. The ITS region provides a reliable DNA barcode for identifying reishi/lingzhi (Ganoderma) from herbal supplements. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu K.D., Cheng K.C. From nutraceutical to clinical trial: frontiers in Ganoderma development. Appl. Microbiol. Biotechnol. 2018;102:9037–9051. doi: 10.1007/s00253-018-9326-5. [DOI] [PubMed] [Google Scholar]
- 20.Yeh C.-H., Chen H.-C., Yang J.-J., Chuang W.-I., Sheu F. Polysaccharides PS-G and protein LZ-8 from Reishi (Ganoderma lucidum) exhibit diverse functions in regulating murine macrophages and T lymphocytes. J. Agric. Food Chem. 2010;58:8535–8544. doi: 10.1021/jf100914m. [DOI] [PubMed] [Google Scholar]
- 21.Olempska-Beer Z.S., Merker R.I., Ditto M.D., DiNovi M.J. Food-processing enzymes from recombinant microorganisms--a review. Regul. Toxicol. Pharmacol. 2006;45:144–158. doi: 10.1016/j.yrtph.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Fraser R.Z., Shitut M., Agrawal P., Mendes O., Klapholz S. Safety evaluation of soy leghemoglobin protein preparation derived from Pichia pastoris, intended for use as a flavor catalyst in plant-based meat. Int. J. Toxicol. 2018;37:241–262. doi: 10.1177/1091581818766318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silano V., Barat Baviera J.M., Bolognesi C., Brüschweiler B.J., Cocconcelli P.S., Crebelli R., Gott D.M., Grob K., Lampi E., Mortensen A., Rivière G., Steffensen I.L., Tlustos C., Van Loveren H., Vernis L., Zorn H., Herman L., Marcon F., Bernasconi G., Gomes A., Liu Y., Chesson A. Safety evaluation of the food enzyme phospholipase C from a genetically modified Komagataella phaffii (strain PRF) Efsa J. 2019;17 doi: 10.2903/j.efsa.2019.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duman-Özdamar Z.E., Binay B. Production of industrial enzymes via Pichia pastoris as a cell factory in bioreactor: current status and future aspects. Protein J. 2021;40:367–376. doi: 10.1007/s10930-021-09968-7. [DOI] [PubMed] [Google Scholar]
- 25.Hanlon P., Sewalt V. GEMs: genetically engineered microorganisms and the regulatory oversight of their uses in modern food production. Crit. Rev. Food Sci. Nutr. 2021;61:959–970. doi: 10.1080/10408398.2020.1749026. [DOI] [PubMed] [Google Scholar]
- 26.Ricci A., Allende A., Bolton D., Chemaly M., Davies R., et al. Update of the list of QPS‐recommended biological agents intentionally added to food or feed as notified to EFSA 7: suitability of taxonomic units notified to EFSA until September 2017. Efs2. 2018;16 doi: 10.2903/j.efsa.2018.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad M., Hirz M., Pichler H., Schwab H. Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biotechnol. 2014;98:5301–5317. doi: 10.1007/s00253-014-5732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roohvand F., Shokri M., Abdollahpour-Alitappeh M., Ehsani P. Biomedical applications of yeast- a patent view, part one: yeasts as workhorses for the production of therapeutics and vaccines. Expert Opin. Ther. Pat. 2017;27:929–951. doi: 10.1080/13543776.2017.1339789. [DOI] [PubMed] [Google Scholar]
- 29.Roohvand F., Ehsani P., Abdollahpour-Alitappeh M., Shokri M., Kossari N. Biomedical applications of yeasts - a patent view, part two: era of humanized yeasts and expanded applications. Expert Opin. Ther. Pat. 2020;30:609–631. doi: 10.1080/13543776.2020.1781816. [DOI] [PubMed] [Google Scholar]
- 30.France T.C., Kelly A.L., Crowley S.V., O’Mahony J.A. Cold microfiltration as an enabler of sustainable dairy protein ingredient innovation. Foods. 2021;10:2091. doi: 10.3390/foods10092091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salunke P., Marella C., Metzger L.E. Microfiltration and ultrafiltration process to produce micellar casein and milk protein concentrates with 80% crude protein content: partitioning of various protein fractions and constituents. Dairy. 2021;2:367–384. doi: 10.3390/dairy2030029. [DOI] [Google Scholar]
- 32.OECD . OECD Publishing; Paris: 2018. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents. OECD Guidelines for the Testing of Chemicals, Section 4. [DOI] [Google Scholar]
- 33.OECD . OECD Publishing; Paris: 2018. Test No. 414: Prenatal Developmental Toxicity Study. OECD Guidelines for the Testing of Chemicals, Section 4. [DOI] [Google Scholar]
- 34.Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 35.OECD . OECD Publishing; Paris: 2020. Test No. 471: Bacterial Reverse Mutation Test. OECD Guidelines for the Testing of Chemicals, Section 4. [DOI] [Google Scholar]
- 36.OECD . OECD Publishing; Paris: 2014. Test No. 473: In Vitro Mammalian Chromosomal Aberration Test. [DOI] [Google Scholar]
- 37.OECD . OECD Publishing; Paris: 2014. Test No. 474: Mammalian Erythrocyte Micronucleus Test. [DOI] [Google Scholar]
- 38.Li-Chan E.C.Y. Bioactive peptides and protein hydrolysates: research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015;1:28–37. doi: 10.1016/j.cofs.2014.09.005. [DOI] [Google Scholar]
- 39.Braaksma A., Schaap D. Protein analysis of the common mushroom Agaricus bisporus. Postharvest Biol. Technol. 1996;7:119–127. doi: 10.1016/0925-5214(95)00034-8. [DOI] [Google Scholar]
- 40.Chang S.T., Buswell J.A. Mushroom nutriceuticals. World J. Microbiol. Biotechnol. 1996;12:473–476. doi: 10.1007/BF00419460. [DOI] [PubMed] [Google Scholar]
- 41.Huang L., Sun F., Liang C., He Y.X., Bao R., Liu L., Zhou C.Z. Crystal structure of LZ‐8 from the medicinal fungus Ganoderma lucidium. Protein Struct. Funct. Bioinform. 2009;75:524–527. doi: 10.1002/prot.22346. [DOI] [PubMed] [Google Scholar]
- 42.Paaventhan P., Joseph J.S., Seow S.V., Vaday S., Robinson H., Chua K.Y., Kolatkar P.R. A 1.7 Å structure of Fve, a member of the new fungal immunomodulatory protein family. J. Mol. Biol. 2003;332:461–470. doi: 10.1016/s0022-2836(03)00923-9. [DOI] [PubMed] [Google Scholar]
- 43.Chiu L.Y., et al. Combination treatment of Src inhibitor Saracatinib with GMI, a Ganoderma microsporum immunomodulatory protein, induce synthetic lethality via autophagy and apoptosis in lung cancer cells. J. Cell. Physiol. 2020:1–10. doi: 10.1002/jcp.29924. [DOI] [PubMed] [Google Scholar]
- 44.Chiu L.-Y.Y., et al. Immunomodulatory protein from Ganoderma microsporum induces pro-death autophagy through Akt-mTOR-p70S6K pathway inhibition in multidrug resistant lung cancer cells. PLoS One. 2015;10:1–23. doi: 10.1371/journal.pone.0125774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsin I.L., et al. GMI, an immunomodulatory protein from Ganoderma microsporum, induces autophagy in non-small cell lung cancer cells. Autophagy. 2011;7:873–882. doi: 10.4161/auto.7.8.15698. [DOI] [PubMed] [Google Scholar]
- 46.Hsin I.L., et al. Inhibition of lysosome degradation on autophagosome formation and responses to GMI, an immunomodulatory protein from Ganoderma microsporum. Br. J. Pharmacol. 2012;167:1287–1300. doi: 10.1111/j.1476-5381.2012.02073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsin I.L., et al. GMI, an immunomodulatory protein from Ganoderma microsporum, potentiates cisplatin-induced apoptosis via autophagy in lung cancer cells. Mol. Pharm. 2015;12:1534–1543. doi: 10.1021/mp500840z. [DOI] [PubMed] [Google Scholar]
- 48.Hsin I.-L., et al. GMI, a fungal immunomodulatory protein from Ganoderma microsporum, induce apoptosis via β-catenin suppression in lung cancer cells. Environ. Toxicol. 2018;33:955–961. doi: 10.1002/tox.22582. [DOI] [PubMed] [Google Scholar]
- 49.Huang S.Y., et al. Ganoderma microsporum immunomodulatory protein induces apoptosis and potentiates mitomycin C-induced apoptosis in urinary bladder urothelial carcinoma cells. J. Cell. Biochem. 2018;119:4592–4606. doi: 10.1002/jcb.26616. [DOI] [PubMed] [Google Scholar]
- 50.Lin C.H., et al. A new immunomodulatory protein from Ganoderma microsporum inhibits epidermal growth factor mediated migration and invasion in A549 lung cancer cells. Process Biochem. 2010;45:1537–1542. doi: 10.1016/j.procbio.2010.06.006. [DOI] [Google Scholar]
- 51.Lin T.Y., Hua W.J., Yeh H., Tseng A.J. Functional proteomic analysis reveals that fungal immunomodulatory protein reduced expressions of heat shock proteins correlates to apoptosis in lung cancer cells. Phytomedicine. 2021;80 doi: 10.1016/j.phymed.2020.153384. [DOI] [PubMed] [Google Scholar]
- 52.Lu C.Te, et al. Ganoderma immunomodulatory protein and chidamide down-regulate integrin-related signaling pathway result in migration inhibition and apoptosis induction. Phytomedicine. 2018;51:39–47. doi: 10.1016/j.phymed.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 53.Hsin I., Chiu L., Ou C., Wu W., Sheu G. CD133 inhibition via autophagic degradation in pemetrexed-resistant lung cancer cells by GMI, a fungal immunomodulatory protein from Ganoderma microsporum. Br. J. Cancer. 2020 doi: 10.1038/s41416-020-0885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee P.H., Hsieh P.L., Liao Y.W., Yu C.C. Inhibitory effect of GMI, an immunomodulatory protein from Ganoderma microsporum, on myofibroblast activity and proinflammatory cytokines in human fibrotic buccal mucosal fibroblasts. Environ. Toxicol. 2018;33(1):32–40. doi: 10.1002/tox.22489. [DOI] [PubMed] [Google Scholar]
- 55.Lin C.-H.H., et al. GMI, a Ganoderma immunomodulatory protein, down-regulates tumor necrosis factor α-induced expression of matrix metalloproteinase 9 via NF-κB pathway in human alveolar epithelial A549 cells. J. Agric. Food Chem. 2010;58:12014–12021. doi: 10.1021/jf103068w. [DOI] [PubMed] [Google Scholar]
- 56.Lu C., et al. Inhibition of proliferation and migration of melanoma cells by ketoconazole and Ganoderma immunomodulatory proteins. Oncol. Lett. 2019;891–897(2019) doi: 10.3892/ol.2019.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang T.Y., et al. GMI ablates cancer stemness and cisplatin resistance in oral carcinomas stem cells through IL-6/Stat3 signaling inhibition. Oncotarget. 2017;8:70422–70430. doi: 10.18632/oncotarget.19711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chao M.-W., Liao C.-W., Lin C.-H., Tseng C.-Y. Immunomodulatory protein from Ganoderma microsporum protects against oxidative damages and cognitive impairments after traumatic brain injury. Molecular and Cellular Neuroscience. 2022;120 doi: 10.1016/j.mcn.2022.103735. https://www.sciencedirect.com/science/article/pii/S1044743122000410 [DOI] [PubMed] [Google Scholar]
- 59.Chen W.-Y., et al. Anti-inflammatory and neuroprotective effects of fungal immunomodulatory protein involving microglial inhibition. Int. J. Mol. Sci. 2018;19:1–18. doi: 10.3390/ijms19113678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li C.-H., et al. The protective role of GMI, an immunomodulatory protein from Ganoderma microsporum, on 5-fluorouracil-induced oral and intestinal mucositis. Integr. Cancer Ther. 2019;18 doi: 10.1177/1534735419833795. 1534735419833795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu H.-J., Li C.-H., Kang Y.-T., Wu C.-M., Wu C.-H., Ko J.-L., Wu M.-F. Efficacy of GMI, a fungal immunomodulatory protein, for head and neck cancer patients with chemotherapy-related oral mucositis: an open-labeled prospective single-arm study. Medicine. 2022;101(16):e29185. doi: 10.1097/MD.0000000000029185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peng K.T., et al. GMI, an immunomodulatory peptide from Ganoderma microsporum, restrains periprosthetic joint infections via modulating the functions of myeloid-derived suppressor cells and effector T cells. Int. J. Mol. Sci. 2021;22(13):6854. doi: 10.3390/ijms22136854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teo W.H., Lo J.F., Fan Y.N., Huang C.Y., Huang T.F. Ganoderma microsporum immunomodulatory protein, GMI, promotes C2C12 myoblast differentiation in vitro via upregulation of Tid1 and STAT3 acetylation. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0244791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tseng C.-Y., et al. The Effect of Ganoderma microsporum immunomodulatory proteins on alleviating PM2.5-induced inflammatory responses in pregnant rats and fine particulate matter-induced neurological damage in the offsprings. Sci. Rep. 2019;9:6854. doi: 10.1038/s41598-019-38810-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yeh H., Vo D.N.K., Lin Z.-H., Ho H. P.T., Hua W.-J., Qiu W.-L., Tsai M.-H., Lin T.-Y. GMI, a protein from Ganoderma microsporum, induces ACE2 degradation to alleviate infection of SARS-CoV-2 Spike-pseudotyped virus. Phytomedicine. 2022 doi: 10.1016/j.phymed.2022.154215. https://www.sciencedirect.com/science/article/abs/pii/S094471132200294X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.EFSA Panel on Genetically Modified Organisms (GMO) Scientific opinion on guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed use. EFSA J. 2011;9(6):2193. doi: 10.2903/j.efsa.2011.2193. [DOI] [Google Scholar]
- 67.Ou C.-C., Hsiao Y.-M., Wang W.-H., Ko J.-L., Lin M.-Y. Stability of fungal immunomodulatory protein, FIP-gts and FIP-fve, in IFN-γ production. Food Agric. Immunol. 2009;20:319–332. doi: 10.1080/09540100903247688. [DOI] [Google Scholar]
- 68.Reyes T.F., Chen Y., Fraser R.Z., Chan T., Li X. Assessment of the potential allergenicity and toxicity of Pichia proteins in a novel leghemoglobin preparation. Regul. Toxicol. Pharmacol. 2021;119 doi: 10.1016/j.yrtph.2020.104817. [DOI] [PubMed] [Google Scholar]
- 69.M. Abdelmoteleb, C. Zhang, B. Furey, M. Kozubal, H. Griffiths, M. Champeaud, R.E. Goodman, Evaluating potential risks of food allergy of novel food sources based on comparison of proteins predicted from genomes and compared to www. AllergenOnline. org. Food Chem. Toxicol. 147 (2021), 111888. DOI: 10.1016/j.fct.2020.111888. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material