Abstract
Milk and dairy products are the most important nutritional foods among all age groups. Aflatoxin M1 (AFM1) contaminates milk and makes its consumption potentially dangerous. Infants are mostly at risk because they are typically fed as many as six and more times per day, which is indeed a disquieting concern. This study aimed at evaluating AFM1 levels especially above international (European Food Safety Authority, EFSA) (0.05 µg/kg) and local (Ghana Standards Authority, GSA) (0.5 µg/kg) standards and cancer risks associated with the ingestion of raw cow milk (n = 120) sampled from Southern Ghana (Greater Accra, Volta, Western and Eastern Regions). AFM1 were measured with High-Performance Liquid Chromatography with a Fluorescence Detector (HPLC-FLD). Risk assessments were also conducted using models prescribed by the Joint FAO/WHO Expert Committee on Additives (JECFA). Out of the 120 samples analyzed for AFM1, 67 (55.8%) tested positive, 63 (52.5%) exceeded the limits of EFSA and were between the range 0.06 ± 0.001–3.52 ± 0.5 µg/kg whereas 50(41.7%) within the range of 0.50 ± 0.03–3.52.01 ± 0.5 µg/kg exceeded GSA limits. Risk assessments of AFM1 for infants, toddlers, children, adolescents, and adults ranged between 0.06 and 2.03 ng/kg bw/day, 197.04–6666.67, 0–0.0323 ng aflatoxins/kg bw/day and 1.94 × 10-3- 0.07 cases/100,000 person/yr respectively for Estimated Daily Intake (EDI), Margin of Exposure (MOE), Average Potency, and Cancer Risks. It was concluded that the consumption of raw milk posed adverse health effects on all age categories studied for the regions investigated. The use of raw cow milk may cause some problems and endanger the health of people of different age groups due to noncompliance with prescribed regulatory limits.
Keywords: Aflatoxin M1 (AFM1), Toxigenic fungi, Raw milk, Mycotoxins, Ghana, HPLC-FLD
Graphical Abstract
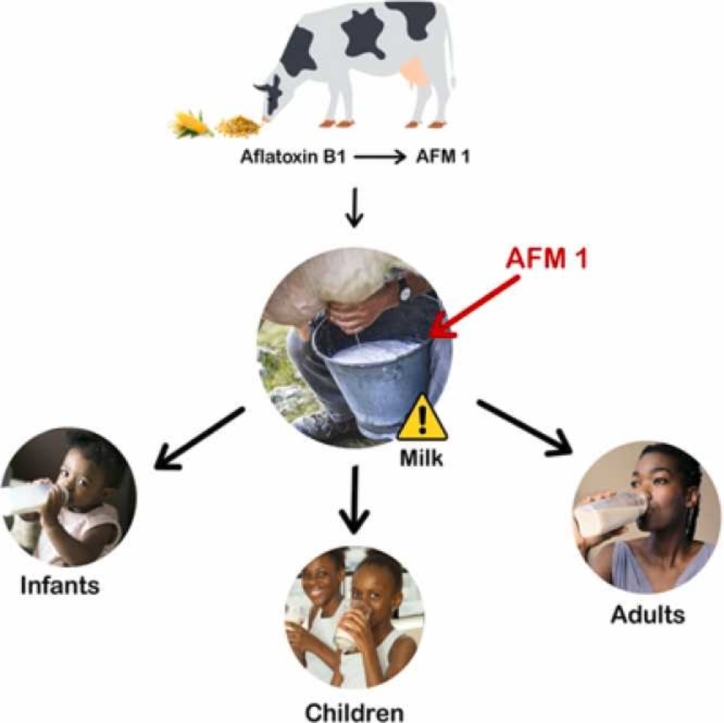
Highlights
-
•
Overall 55.8% were positive for AFM1 in 120 raw cow milk samples analyzed in Southern Ghana.
-
•
41.7% and 52.5% were recorded as contaminated raw cow milk samples exceeding permissible limits.
-
•
Risk assessment on raw cow milk samples revealed a public health concern.
-
•
Exposure to AFM1 and vulnerability principally result from the consumption of fungi infected.
1. Introduction
Milk is a superb source of nutrients, being an aqueous colloidal suspension containing countless macro-and micronutrients that are essential for the growth, supply of energy, reproduction, maintenance and repair, appetite satisfaction and maintenance of human health [91], [74]. In spite of the health benefits of milk, mycotoxins are one probable group of natural food contaminants which contaminate milk and render it unsafe for consumption when ingested, which may cause ailments and pose adverse health effects.
Mycotoxins are natural toxins from fungi which contaminate a wide range of foodstuffs and render them potentially dangerous.
Mycotoxins represent one of the main global foodborne risks for human health and are considered an important issue in the situation of food safety, due to their acute and chronic toxic effects on animals and humans [11], [7].
Aflatoxins are mycotoxins produced by strains of Aspergillus flavus and A. parasiticus. Rao et al. [107] highlighted that they are comparatively the most known mycotoxins owing to their ubiquitous nature and persistence of Aspergillus sp. in the environment. Just about 4.5 billion people globally are at risk of disproportionate exposure to aflatoxins, which account for 4.6–28.2% of all cases of hepatocellular carcinoma [3]. Aflatoxins occur as five different types; aflatoxins B1, B2, G1, G2, and M1 produced primarily in cow milk by cows eating contaminated silage.
AFM1 contamination of milk and milk products at the global level is well established and has been reported in many countries [54]. AFM1 is a hydroxylated metabolite of AFB1 [15], that is, excreted in milk in the mammary glands of both humans and lactating animals [31], [32]. AFB1 is reported to have a significant association with AFM1 and so it is possible to predict the outcome of AFM1 with knowledge of AFB1 [68]. Various studies in recent decades have been committed to reducing the bioavailability of AFB1 thereby reducing the levels of AFM1 in lactating dairy cows [68]. Approximately 0.3–6.2% of AFB1 is converted into metabolized AFM1 and excreted in milk, depending on factors such as the genetics of the animals, seasonal variation, the milking process and environmental conditions [106]. In spite of AFM1’s comparatively lower of about 10 times its carcinogenic potential as AFB1, it still can be a probable health hazard to humans, especially children, considering their high milk consumption, lower body weight, high metabolic rate, and incomplete development of excretory organs [66].
Globally, studies have also shown that the presence of AFM1 in milk and dairy products is an important health issue because in many countries, every age group regularly consumes these products in their daily diet [32], especially in developing countries [86]. Children and especially infants are among the human population, who require milk for proper development during growth and often consume milk in greater quantities [85]. Besides milk consumption, their propensity of exposure to aflatoxins is further increased through the consumption of complementary feeds prepared from cereals and legumes [5], [56], which are foodstuffs prone to contamination by aflatoxigenic fungi (Aspergillus species). These complementary foods are formulated with milk again or consumed with milk.
AFM1 are dispersed universally but are commonly found in tropical or subtropical climates where there is an extreme range of temperature, rainfall, and humidity [30]. AFM1 contamination can also occur during the processing and storage of milk and its products. However, it has the ability to withstand numerous ordinary methods of food treatment such as cooking, sterilization, and freezing, hence its appearance in milk and dairy products [83]. Novel methods for minimizing the occurrence of AFM1 in milk by engaging yeast and lactic acid bacteria (LAB) microbial extracts as well as lactic acid fermentation to ferment a variety of traditional foods have shown aptitude for aflatoxin degradation, nevertheless are yet to be completely discovered [27], [53], [71].
Additionally, prolonged consumption of aflatoxins is well known to cause impaired immune function, teratogenic mutagenic disability [97], [35]. Aflatoxins have been reported to be associated with marasmus and kwashiorkor in children [99] and present some neurotoxic and neuro-immunotoxic effects of aflatoxin B1 as reported by Richard et al. [88]. Most importantly, aflatoxins have been reported to work concomitantly to worsen the risk of hepatocellular carcinoma (HCC), which is acknowledged to be the fifth most frequently occurring cancer in the world [44], [64]. A previous study by Liu and Wu [65] estimated that about 5–28% of global HCC cases are attributable to aflatoxin exposure, which occurs mainly through diet.
In view of these predicaments posed by mycotoxins (aflatoxins), limits are usually set by countries; eg. Europe (EFSA= 0.05 µg/kg) and other African countries such as Ghana (GSA= 0.5 µg/kg) to regulate the permissible levels to safeguard consumers from HCC attributable to AFM1 and some other negative health effects. In addition to setting regulatory limits for mycotoxins, it is also imperative to conduct health risk assessments in the population due to dietary exposure. A low-dose extrapolation approach introduced by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) in 1997 and the margin of exposure (MOE) method proposed at the 64th JECFA meeting in 2005 [16] were both recommended and have been widely used worldwide [100], [48] to assess the risk of dietary exposure to mycotoxins.
In Ghana, insufficient research has been done on the prevalence of AFM1 in milk and milk products. To the best of our knowledge, this work is the only one with the objective of determining the levels, dietary intake assessment, and cancer risk involved with the ingestion of AFM1 through raw cow milk in southern Ghana. The outcome of this research will be expedient in informing policy makers on where to focus regarding food safety, particularly fungal intoxication of foods. Again, in adopting international legislations on food quality parameters. Lastly, contamination of raw cow milk with AFM1 should be considered a high priority for Ghana’s mycotoxin risk management actions.
2. Materials and methods
2.1. Chemicals and standards
The analytical standard of AFM1 was supplied by Sigma-Aldrich (St. Louis, MO, USA). All solvents used for the preparation of the mobile phase were HPLC grade and obtained from Merck (Darmstadt, Germany). All homogenized mixtures and eluates were filtered through Whatman no. 4 and 0.45 mm membrane filters, respectively (Whatman plc, Maidstone, UK). De-ionized water was obtained with a Millipore Elix Essential purification system (Bedford, MA, USA). EASI-extracted AFM1 immunoaffinity columns (stored at 4 °C until use) were supplied by R-Biopharm, Rhone limited and used for SPE and cleanup.
2.2. Preparation of standard solutions
A mother stock solution (0.1 μg/mL) was prepared from a standard solution of AFM1 (0.993 μg/mL in acetonitrile) and stored with care in a freezer. A working stock solution of 0.01 μg/mL was diluted step by step with the combined solution (acetonitrile/water, 75/25, v/v) to prepare a sequence of working solutions which were stored in vials below 4 ºC for the calibration curve. Calibration solutions of 0.02 μg/kg, 0.04 μg/kg, 0.06 μg/kg, 0.08 μg/kg, and 0.10 μg/kg were used. Samples with AFM1 amount above the calibration range were diluted and dilution factors applied for quantification as outlined by EN ISO 14501:2007 [101].
3. Materials
A total of one hundred and twenty (n = 120) raw cow milk samples were used in this study. Thirty (30) raw cow milk samples were obtained from local markets in each region; Greater Accra, Volta Region, Western and Eastern Regions of Ghana (Table 1) between the period of March and July 2021. Milk samples were stored in coolers with ice packs and transported to the laboratory and analyzed for AFM1.
Table 1.
Geographical location and some attributes of the origins of raw cow milk samples.
| Region | No. of samples | Agro-ecological zones | Rainfall (mm) | Temperature (oC) | Coordinates |
|---|---|---|---|---|---|
| Greater Accra | 30/120 | Coastal Savannah | 800–1000 | 26.6 | 5.8143°N, 0.0747° E |
| Volta | 30/120 | Coastal Savannah/Deciduous Forest | 1000–1400 | 26.2 | 6.5781°N, 0.4502° W |
| Western | 30/120 | Evergreen | 1800–2000 | 25.9 | 5.3902°N, 2.1450°W |
| Eastern | 30/120 | Deciduous Forest | 1400–1900 | 25.9 | 6.2374°N, 0.4502°W |
3.1. Preparation of samples
After warming at about 37 ºC in a water bath, the samples were centrifuged at 2000 g to separate the fat layers and then filtered. The prepared test portion of 50 mL was transferred into a syringe barrel attached to AFM1 immunoaffinity column and passed at a slow steady flow rate of 1–2 mL/min. The columns were then washed with 20 mL deionized water and air was passed through the columns to dryness. AFM1 was eluted with 4 mL pure acetonitrile by allowing it to be in contact with the column for not less than 60 s. The eluate was evaporated to dryness using a gentle streams of nitrogen. The residue was dissolved in 500 µl of mobile phase and filtered using a membrane filter before injected into HPLC for quantification (EN ISO 14501:2007) [101].
4. Instrumentation
Agilent high performance liquid chromatography system (HPLC 1260 infinity series) with a quaternary pump and fluorescence detection was used for AFM1 quantification analysis and was carried out as per the method given by EN ISO 14501:2007 [101]. Data acquisition and quantification was done using Chem station (Open Lab edition). The Agilent HPLC equipped with a fluorescence detector was set at an excitation wavelength of 360 nm and an emission wavelength of 440 nm and the column compartment (HPLC Column: TC-C18 (2), 170, 5 µm, 4,6 × 250 mm; thus, pore size of 170, particle size of 5.0 µ, inner diameter of 4.6 mm, length of 250 mm and carbon load of 12%) temperature regulated at 35 °C. The mobile phase was a mixture of water and acetonitrile at ratios of 25:75 (v/v), respectively, and an isocratic delivery mode was employed at a flow rate of 0.8 mL/min with an injection volume of 10 µl.
5. Validation
HPLC-FLD method was validated according to the guidelines of European Commission Decision 657/2002/EC for confirmatory analysis methods and the tested parameters were linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, precision and selectivity. The linearity was assessed by constructing five-point solvent matched calibrations in triplicate for AFM1 standard solutions in the concentration range of 0.05–0.8 mg/L. Calibration curves were drawn by plotting the peak area against AFM1 concentration, and linearity was evaluated by linear regression analysis expressed as coefficient of determination (r2).
Precision of the method was estimated in terms of % RSD of three identical extractions of milk samples spiked with AFM1 at the same as well as at three different spiking levels. Method selectivity was evaluated by analyzing AFM1 known negative milk matrix and reagent blank to determine any interference from endogenous substances around the retention time of the target analyte.
6. Risk assessment of exposure to AFM1 via consumption of milk
6.1. Estimation of exposure
Estimated Daily Intake (EDI) was considered by using the mean quantities of aflatoxins derived from the milk samples, the quantity of sample consumed daily, and the average body weight. The EDI for mean aflatoxin was premeditated according to the following formula (1) and expressed in μg/kg of body weight/day (μg/kg b.w/day) [26], [92].
| EDI = daily intake (food) X mean level of AFM1 average bodyweight | (1) |
Daily intake of milk in Ghana according to Omore et al. [76] is approximately 0.0137 kg/day (5.0 kg/year).
The different age categories according to EFSA (Panel on Dietetic Products and Allergies) (2009) and their corresponding estimated average weights in Ghana used in this study were done as follows; Infants- 2.9 (2.5–3.2) kg [4], [62], Toddler − 9.8 (7–12.6) kg [1], [39], Children − 26 (24–28) kg [19], [79], Adolescents- 46.25 (38.5–54) kg [10], Adults- 60.7 kg [108].
6.2. Margin of exposure characterization for aflatoxins
Genotoxic and carcinogenic compounds such as aflatoxins have their risk assessment fittingly computed based on the Margin of Exposure (MOEs) approach, which was estimated by dividing the Benchmark dose lower limit (BMDL) for aflatoxins 400 ng/kg bw/day by toxin exposure [9], [25], [29], [37] as expressed in Eq. (2).
| MOE= Benchmark dose lower limit EDI (Exposure) | (2) |
The EFSA [24] identified the liver carcinogenicity of aflatoxins as the critical consequence of the risk assessment; therefore, the benchmark dose lower confidence limit for a benchmark response of 10% (BMDL10) regarding the frequency of hepatocellular carcinomas (HCCs) in male rats was considered. In the absence of a specific BMDL10 for AFM1, the BMDL10 for HCCs related to the ingestion of AFB1 (0.4 μg/kg or 400 ng/kg body weight per day) [25], [90] was used in the present study for the definition of MOE applying a potency factor. A public health alarm is raised in instances where MOEs are less than 100,000 [9], [37], [57].
6.3. Estimated liver cancer risk due to consumption of raw cow milk
The ingestion of aflatoxins can be linked to the onset of liver cancer [95]. Therefore, liver cancer risk estimation for Ghanaian adult consumers was calculated for aflatoxins [95]. This involved estimating the population cancer risk per 100,000, which is a product of the EDI value and the average hepatocellular carcinoma (HCC) potency figure from individual potencies of Hepatitis B surface antigen (HBsAg) (HBsAg-positive and HBsAg-negative groups).
The JECFA [38] estimated potency values for AFM1 which corresponded to 0.3 cancers /year/100,000 population ng/kg bw/day (uncertainty range: 0.05–0.5) in HBsAg-positive individuals and 0.01 cancer/year/100, 000 population/ ng/kg bw/day (uncertainty range: 0.002–0.03) in HBsAg-negative individuals [95] were adopted for this calculation. Furthermore, the average HBsAg+ prevalence rate of 7.74% (adult-8.36%, 14.3%-adolescents, 0.55%-children) in Ghana [2] was adopted and 92.26% (100–7.74%) was extrapolated for HBsAg-negative groups. Hence, the average potency for cancer in Ghana was estimated as follows according to Eq. (7) as prescribed by Shephard [95] and Adetunji et al. [9]:
| Average potency = [0.03 x HBsAg –negative individuals in Ghana] + [0.01 x HBsAg- positive individuals/prevalence rate in Ghana] | (3) |
(0.3 × 0.077) + (0.01 × 0.9226).
= 0.0323.
Thus, the Cancer risk (cancers per year per 100,000 population per ng aflatoxin/kg bw/day) was estimated using the following formula in Eq. (8) [57], [9]:
| Cancer Risk = Exposure (EDI) × Average potency | (4) |
7. Statistical analysis
The aflatoxin concentrations were calculated using regression analysis from the curves generated from the standards of AFM1 with Excel for Microsoft Windows (version 10). One sample t-test was used to compare the means obtained at a 95% confidence interval and 5% level of significance. The statistical results were summarized as median, standard deviation, variance, skewness, standard error of skewness, kurtosis and standard error of kurtosis and mean values (range from the 25th percentile to the 75th percentile). SPSS 22 (Chicago, USA) was used in the analysis of data. Deterministic risk assessment models were used; dietary exposure (Estimated Dietary Intake), MOE values, Average potency, and cancer risk.
8. Results
8.1. Occurrence of aflatoxins
Most of the food samples tested produced good linearity or coefficients of correlations (R2 > 0.990) within the tested range. The mean recovery percentage of AFM1 in spiked milk samples were found between 80.5% and 84.07% with % RSD from 3.19 to 5.42. Since the recoveries and % RSD were within the EC regulation.
The number of raw cow milk samples contaminated with AFM1 is presented in Table 2, Table 4. The level of occurrence of the AFM1 ranged between 0 and 2.61 µg/kg, 0–3.04 µg/kg, 0–3.52 µg/kg, and 0–2.11 µg/kg respectively for Greater Accra, Volta, Western and Eastern Regions. Greater Accra Region (GTA) recorded 0.26, 0.010, and 0.371 µg/kg for mean, median, and variance, respectively, while the skewness and kurtosis were 2.81 and 7.77 respectively and showed that the data set of AFM1 obtained in this town was asymmetrical and heavy-tailed (Table 2). The lower and upper limits were 0.031 and 0.486, respectively, and showed statistical differences (P < 0.05) (Table 3). For Volta Region (VR), values of 0.54, 0.00, and 0.65 µg/kg were recorded from the summary statistics as mean, median, and variance, respectively, while 1.59 and 2.13 were recorded as skewness and kurtosis and implied moderate skewness and light-tailed. The upper and lower limits were 0.234 and 0.837. Values significantly differed (P < 0.05) (Table 2, Table 3). The mean, median, and variance recorded for Western Region (WR) were 0.86, 0.63, and 0.89 µg/kg, respectively. While the data set showed symmetrical and light-tailed as, the skewness and kurtosis were 0.92 and 0.33, respectively (Table 2). Values of 0.503 and 1.206 were recorded as upper and lower limits. There were significant differences (P < 0.05) observed (Table 3). For Eastern Region (ER), the recorded mean, median, and variance were 0.67, 0.67, and 0.40 µg/kg, respectively. The data set for ER was fairly symmetrical and light-tailed (0.49 and −0.67 for skewness and kurtosis, respectively. Upper and lower limits of 0.431 and 0.902 were, respectively, recorded. There were statistically significant (P < 0.05) differences (Table 2, Table 3).
Table 2.
Summary of statistics of AFM1 concentration in raw cow milk obtained from four different regions of Ghana.
| Greater Accra Region | Volta Region | Western Region | Eastern Region | |
|---|---|---|---|---|
| No. of samples | 30 | 30 | 30 | 30 |
| Mean | 0.26 | 0.54 | 0.86 | 0.67 |
| Std. Error of Mean | 0.111 | 0.147 | 0.172 | 0.115 |
| Median | 0.010 | 0.000 | 0.625 | 0.665 |
| Std. Deviation | 0.608 | 0.807 | 0.942 | 0.631 |
| Variance | 0.371 | 0.652 | 0.887 | 0.398 |
| Skewness | 2.814 | 1.590 | 0.916 | 0.494 |
| Std. Error of Skewness | 0.427 | 0.427 | 0.427 | 0.427 |
| Kurtosis | 7.769 | 2.133 | 0.334 | -0.670 |
| Std. Error of Kurtosis | 0.833 | 0.833 | 0.833 | 0.833 |
| Range | 2.61 | 3.04 | 3.52 | 2.11 |
| Percentiles 25 | 0.000 | 0.000 | 0.000 | 0.000 |
| 50 | 0.010 | 0.000 | 0.625 | 0.665 |
| 75 | 0.130 | 0.968 | 1.705 | 1.100 |
Table 4.
Proportions of AFM1 concentrations of raw cow milk samples that exceeded limits of the European Food Safety Authority (EFSA) and Ghana Standard Authority (GSA).
| Region | No. of samples | Exceeding EFSA regulation | Exceeding GSA regulation | Overall positive with AFM1 | ||
|---|---|---|---|---|---|---|
| Yes (%) | Range | Yes (%) | Range | |||
| Greater Accra | 30 | 13 (43.3%) | 0.06 ± 0.001–2.61 ± 0.7 | 4 (13.3%) | 1.21 ± 0.15–2.61 ± 0.6 | 15 (50.0%) |
| Volta | 30 | 12 (40%) | 0.55 ± 0.02–3.04 ± 0.7 | 12 (40%) | 0.55 ± 0.02–3.04 ± 0.7 | 12 (40.0%) |
| Western | 30 | 18 (60%) | 0.08 ± 0.001–3.52 ± 0.5 | 17 (56.7%) | 0.50 ± 0.03–3.52 ± 0.5 | 20 (66.7%) |
| Eastern | 30 | 20 (66.7%) | 0.09 ± 0.001–2.11 ± 0.4 | 17 (56.7%) | 0.61 ± 0.02–2.11 ± 0.4 | 20(66.7%) |
| Total | 120 | 63 (52.5%) | 0.06 ± 0.001–3.52 ± 0.5 | 50 (41.7%) | 0.50 ± 0.03–3.52 ± 0.5 | 67(55.8%) |
European Union Food Safety (EFSA) limit for AFM1 = 0.05 µg/kg
Ghana Standards Authority (GSA) limit for AFM1 = 0.5 µg/kg.
Table 3.
Statistics of the AFM1 concentrations using one sample t-test of raw cow milk samples from different regions.
| 95% confidence interval of the difference | ||||||
|---|---|---|---|---|---|---|
| t | df | Sig. (2-tailed) | Mean Difference | Lower | Upper | |
| GTA | 2.327 | 29 | 0.027 | 0.259 | 0.031 | 0.486 |
| ER | 5.787 | 29 | 0.000 | 0.667 | 0.431 | 0.902 |
| VR | 3.635 | 29 | 0.001 | 0.536 | 0.234 | 0.837 |
| WR | 4.969 | 29 | 0.000 | 0.855 | 0.503 | 1.2064 |
The European Food Safety Authority (EFSA) and Ghana Standards Authority (GSA) regulatory limits for AFM1 (Table 4) were used in this study. Toxin quantity thresholds prescribed by the Ghana Standards Authority are a subset of the European Food Safety Authority (EFSA). Regarding the frequency and (percentage %) of positive (Yes) AFM1 contaminated milk samples above the various permissible limits, The Greater Accra Region (GTA) recorded 13(43.3%) and ranged between 0.06 ± 0.001–2.61 ± 0.7 µg/kg and 4(13.3%) with 1.21 ± 0.15–2.61 ± 0.6 µg/kg tested positive for EFSA and GSA, respectively. The overall AFM1 positive samples were 15 (50.0%). For Volta Region (VR), AFM1 values of 12(40.0%) which ranged between 0.55 ± 0.02–3.04 ± 0.7 and 12(40.0%) of 0.55 ± 0.02–3.04 ± 0.7 µg/kg. An overall positive AFM1 of 12(40.0%) was recorded. Western Region (WR) recorded total aflatoxin values of 18 (60%) within the range of 0.08 ± 0.001–3.52 ± 0.5 µg/kg and 17 (56.7%) within the range of 0.50 ± 0.03–3.52 ± 0.5 µg/kg were recorded. Values of 20 (66.7%) were recorded as overall positive AFM1 for the samples tested. In the Eastern Region (ER), AFM1 values of 20(66.7%) within a range of 0.09 ± 0.001–2.11 ± 0.4 µg/kg and 17(56.7%) within a range of 0.61 ± 0.02–2.11 ± 0.4 µg/kg were recorded. Values of 20(66.7%) were recorded as positive AFM1 among the samples. Out of the 120 samples analyzed for AFM1, 63% exceeded the limits of EFSA and were within the range of 0.06 ± 0.001–3.52 ± 0.5 µg/kg. While for GSA, 50 (41.7%) of samples exceeded and ranged between 0.50 ± 0.03–3.52 ± 0.5 µg/kg. Values of 67(55.8%) were recorded as the grand frequency and percentage of positive AFM1 among the samples.
8.2. Risk assessment
The Estimated Daily Intakes (EDI) of total aflatoxins in the raw cow milk samples from Greater Accra (GTA) Region were 0.61, 0.36, 0.14, 0.08, and 0.06 ng/kg bw/day for infants, toddlers, children, adolescents, and adults respectively. The Margin of Exposure (MOE) values recorded were 655.74, 1111.11, 2857.14, 5000.00, and 6666.67, respectively. The average potency of the aflatoxins was 0.0323 aflatoxins ng/kg bw/day and produced cancer risks of 0.02, 0.01, 4.5 × 10-3, 2.58 × 10-3 and 1.94 × 10-3 cases/100,000 person/yr respectively (Table 5). Samples from Volta Region (VR) recorded EDI values of 1.28, 0.75, 0.29, 0.16, and 0.12 ng/kg bw/day for infants, toddlers, children, adolescents, and adults respectively. MOE values of 312.50, 533.33, 1379.31, 2500.00, and 3333.33. Average potency was same. Cancer risks of 0.04, 0.02, 9.37 × 10-3, 5.1 × 10-3, and 3.88 × 10-3 cases/100,000 person/yr respectively for these age categories were recorded (Table 5). In Western Region (WR), the EDI values recorded for infants, toddlers, children, adolescents, and adults were 2.03, 1.20, 0.45, 0.25, and 0.19 ng/kg bw/day respectively. MOE values recorded were 197.04, 333.33, 888.89, 1600.00, and 2105.26, respectively. The average potency was the same as other regions, while the cancer risks were 0.07, 0.04, 0.014, 8.08 × 10-3 and 6.14 × 10-3 cases/100,000 person/yr respectively (Table 5).
Table 5.
Risk evaluation for AFM1 via consumption of raw cow milk.
| Region | Age category | Av.body Wgt. (kg) | Estimated Daily Intake (EDI) (ng/ kgbw/day) | MOE | Cancer Risk (Cases/100,000 person/yr) |
|---|---|---|---|---|---|
| Greater Accra |
Infants (0–11mths) |
2.9 | 0.61 | 655.74 | 0.02 |
| Toddlers (12–35mths) | 9.8 | 0.36 | 1111.11 | 0.01 | |
| Children (36mths-10yrs) |
26 | 0.14 | 2857.14 | 4.5 × 10-3 | |
| Adolescents (11–17 yrs) |
46.25 | 0.08 | 5000.00 | 2.58 × 10-3 | |
| Adults (18–64 yrs) |
60.7 | 0.06 | 6666.67 | 1.94 × 10-3 | |
| Volta | Infants (0–11mths) |
2.9 | 1.28 | 312.50 | 0.04 |
| Toddler (12–35mths) | 9.8 | 0.75 | 533.33 | 0.02 | |
| Children (36mths-11yrs) |
26 | 0.29 | 1379.31 | 9.37 × 10-3 | |
| Adolescents (11–17 yrs) |
46.25 | 0.16 | 2500.00 | 5.17 × 10-3 | |
| Adults (18–64 yrs) |
60.7 | 0.12 | 3333.33 | 3.88 × 10-3 | |
| Western | Infants (0–11mths) |
2.9 | 2.03 | 197.04 | 0.07 |
| Toddlers (12–35mths) | 9.8 | 1.20 | 333.33 | 0.04 | |
| Children (36mths-10yrs) |
26 | 0.45 | 888.89 | 0.014 | |
| Adolescents (11–17 yrs) |
46.3 | 0.25 | 1600.00 | 8.08 × 10-3 | |
| Adults (18–64 yrs) |
60.7 | 0.19 | 2105.26 | 6.14 × 10-3 | |
| Eastern | Infants (0–11mths) |
2.9 | 1.58 | 253.16 | 0.05 |
| Toddlers (12–35mths) | 9.8 | 0.94 | 425.53 | 0.03 | |
| Children (36mths-10yrs) |
26 | 0.35 | 1142.85 | 0.01 | |
| Adolescents (11–17 yrs) |
46.3 | 0.20 | 2000.00 | 6.46 × 10-3 | |
| Adults (18–64 yrs) |
60.7 | 0.15 | 2666.67 | 4.85 × 10-3 |
Margin of Exposure-MOE
Mean of AFM1- GTA= 0.26 µg/kg, VR= 0.54 µg/kg
Daily intake of milk for infants was halved (0.5 ×0.0137 kg)
Average potency of aflatoxin= 0.0323
Average Body weights were obtained from the different ranges referenced by the authors 1 µg= 1000 ng
1 µg= 1000 ng
Means of AFM1- WR-0.86 µg/kg, ER- 0.67 µg/kg.
Lastly, for the Eastern Region (ER), the EDI values recorded for infants, toddlers, children, adolescents, and adults were 1.58, 0.94, 0.35, 0.20, and 0.15 ng/kg bw/day respectively. MOE values recorded were 253.16, 425.53, 1142.85, 2000.00, and 2666.67, respectively. The average potency was the same as other regions, while the cancer risks were 0.05, 0.03, 0.01, 6.46 × 10-3 and 4.85 × 10-3 cases/100,000 person/yr respectively (Table 5).
9. Discussion
9.1. Occurrence of AFM1
Milk is consumed by most Ghanaian across all age categories; infants, toddlers, children, adolescents, and adults. Milk consumed by children and adults which are typically added to boiled tea and porridge while infants and toddlers are fed with raw milk. Considering the present findings, we detected comparatively moderate levels of AFM1 contamination in raw cow milk samples obtained from the regions of Southern Ghana as investigated. Our results of 0–3.52 µg/kg (3520 ng/kg) compared favorably well with some published findings of some researchers around the globe. In Ghana, Addo-Boadu [6] recorded AFM1 levels of range of 0.35–3.76 µg/L (350–3760 ng/L) in raw milk and milk products samples from Greater Accra Region. In Tanzania, studies conducted on raw cow milk samples revealed 83.3% (31/37) of aflatoxin contamination in cows that fed on sunflower cake in the range of 0.026–2.007 µg/kg (26–2007 ng/kg) (exceeding both Tanzania‘s and EC allowable value which is 0.05 µg/kg [69].
Several studies have reported the occurrence of AFM1 in milk and dairy products. Milk samples from urban centers in Kenya contained AFM1 up to 6800 ng/L [52]. In Sudan, 95% of milk was contaminated with AFM1 ranging between 220 and 6800 ng/L [28], whereas 6–527 ng/L of AFM1 was detected in 15% of cow milk samples from Cameroon [102]. The concentration of AFM1 varied between 150 and 170 ng/L in commercial and rural milk in South Africa [70], while 100% of milk samples in Nigeria contained AFM1 and the levels were within the range of 0.004–0.845 µg/L (4–8450 ng/l). Goncalves et al., [40] reported AFM1 levels in fresh bovine milk to be in the range of 0.09–3.385 µg/L (90–3385 ng/L) per their work. The least amount of data is available from African countries, nonetheless the available data suggest the highest prevalence and frequent detection levels [34]. Lower values were recorded by [110] in milk from Sudan and found 33% of the milk samples with the highest occurrence (82.4%) in cow milk (35.3% ranged between 0.05 and 0.1 μ/kg and 47.1% ranged between 0.1 and 0.15 μ/kg) and milk samples from camel in semi-intensive systems (15.6% ranged between 0.05 and 0.1 μg/kg). Again, all samples of milk from traditional nomadic systems indicated an absence of AFM1.
Makun et al. [67] also reported contamination of raw cow milk with AFM1 at levels higher than the EU permitted levels in Nigeria. In their study, contamination of raw cow milk (from nomadic cows) with AFM1 ranged from 0.0109 to 1.3543 µg/L (10.9–1354.3 ng/L) with and an average concentration of 0.5308 ± 0.0938 µg/L (530.8 ng/L). For commercial cow’s milk, AFM1 contamination ranged between 0.0464 and 0.0992 (46.4–99.2 ng/L) and 0.0584 ± 0.0052 µg/L (58 ng/L) as the mean. A similar trend regarding levels of AFM1 contamination of 0.05 µg/kg has also been reported in a study conducted in Brazil by Jager et al. [51]. In Egypt, Amer and Ibrahim [13] reported a prevalence of 38 positive samples with a range of 0.023–0.073 µg/L (23–73 ng/L) in raw milk. Moreover, Rahimi et al. [87] reported mean values of 0.0601, 0.0319, 0.0190, 0.0281, and 0.0301 µg/L (60.1, 31.9, 19.0, 28.1 and 30.1 ng/L) respectively for raw cow, water buffalo, camel, sheep and goat milk from Iran.
Worthy of note, milk in Europe is time and again analyzed for AFM1 and is also averred to be of low AFM1 content and perceived to be the safest [12]. In Portugal, Duarte et al. [23] reported values of n.d-0.069 µg/L (nd-69.0 ng/L) in 99.4% positive raw milk samples. In Spain, Rodríguez-Blanco et al., [89] and Cano-Sancho et al. [20] reported ranges of n.d-0.2 µg/L (n.d-200 ng/L) and 0.009–1.36 µg/L (9–1360 ng/L) respectively for raw milk samples. In Serbia, Kos et al. [58] and Tomašević et al. [103] reported ranges of 0.01–1.2 µg/L (10–1200 ng/L) and 0.09–0.145 µg/L (90–1450 ng/L) respectively for milk. Furthermore, in Croatian milk, values of 0.006—0.027 µg/L (6–27 ng/L) were reported by Bilandžić et al. [18]. AFM1 levels recorded in Italy by Bellio et al. [17] and De Roma et al. [21], all pointed at results below 0.05 µg/kg.
In other parts of the world, greater quantities of AFM1 have been reported across the globe. Lee et al. [63] from South Korea reported values of 0.22–6.9 µg/l (220–6900 ng/L) in raw milk. In Pakistan, Iqbal and Asi [50] reported values of 0.02–3.09 µg/l (20–3090 ng/L). Iha et al. [49] from Brazil, reported 83% of the milk samples tested positive for AFM1, in a range of 0.008–0.760 ng/g and in India, almost half of the analyzed milk was contaminated, with 44% being above EU limit [72]. Recently, Kaur et al. [53] reported AFM1 values of 0.314 ± 0.35 ppb in milk samples in India.
The contamination prevalence and levels of AFM1 in raw milk obtained in this study may be due to the reason that dairy animals kept in local dairy farms were fed with compound rations stored under poor conditions, which can be contaminated with aflatoxins. Hot and humid climatic conditions prevailing in Ghana (Table 1) are very conducive for fungal invasion, growth, and production of mycotoxins including aflatoxins in food and feed commodities [93]. Unseasonal rains and related flash floods are widespread, and this increases the moisture content of the grains and other feedstuff, and therefore its vulnerability to fungal attack. Indeed, a number of previous reports indicated the presence of high levels of aflatoxins in dairy animals, feed and ingredients from Ghana.
Moreover, most of the dairy farmers prefer to feed cereals (maize, wheat etc) or agricultural or oilseed byproducts (peanuts, soybean, etc.) to their dairy animals and such aflatoxin susceptible feed materials constitute more than 70% of cattle feed [93]. Therefore, if such high aflatoxin contaminated the feedstuff included in the diet of dairy animals, there is always a great possibility of AFM1 appearance in milk at high levels. Other probable factors which may play an important role in the high levels of AFM1 in milk in this study include poor farm management practices specially feed storage practices, no legal limits of aflatoxins exist for livestock feed and lack of knowledge among dairy farmers in relation to aflatoxins.
Aflatoxin exposure early in life has been associated with impaired growth, particularly stunting [43]. Furthermore, early exposure to aflatoxins is a potential risk for synergistic interactions with other toxins as subjects grow [14], [55]. A recent scoping review by Soriano et al., [99] has shown the presence of these aflatoxins appeared in greater proportion in kwashiorkor in children and in different organs and in biological samples including brain [81], heart [81], kidney [8], liver [80], lung [82], serum [77], stool [22], and urine [102], [46], [77], whereas in marasmic kwashiorkor they were detected in liver [47], serum [77], and urine [22], [77]. Weaning, a transition period of a child from breast milk to other sources of food, often results in a marked decrease in nutrient intake in developing countries [61]. One possible variable contributing to poor child health in developing countries is the increased exposure to aflatoxin contaminated foods following weaning [41].
Gizachew et al. [36] and Škrbić et al. [98] emphasized several factors such as geographical region, season, type and quality of feed, feed storage conditions, and processing methods and conditions are responsible for the variability of AFM1 in milk and dairy products. Lack of fresh forage as feed might have led to longer storage of hay or feed leading to contamination of Aspergillus sp. leading to AFB1 contamination and ultimately biotransformed into AFM1 [68].
9.2. Risk assessment
Aflatoxins are unaffected by many food processing techniques such as boiling or pasteurization, etc. as they are heat stable [59]. There is always a risk involved with their association with food or feed. Risk estimations as explained by Liu and Wu [65] as well as Kuiper-Goodman [60] are modeled to predict the magnitude of adverse health implications of mycotoxin exposure and guide food regulators to set thresholds for these toxins in foodstuffs. MOE results obtained in this study implied a high risk for infants, children, and adolescents (total aflatoxins). Our results showed a high risk for cancer due to AFM1 exposure from milk consumption for infants, toddlers, children, adolescents, and adults. Considering the EDI values obtained in a study by Addo-Boadu [6] in Ghana for infants, i.e., 3.679 ± 2.213 and 2.445 ± 2.001 ng/kg bw/day, it exceeded 1 ng/kg bw/day by far and indicated a serious risk to AFM1 through raw cow milk consumption for this age category.
Our results again agreed with the published findings of Kaur et al. [53] as they reported EDI and HCC values of 2.30 and 0.0020–0.0106, respectively. Their health risk assessment indicated that consumers, especially children, in the study area are at comparatively higher health risk to AFM1 owing to their low body weight and higher milk intake.
The incidence of liver cancer in Iran was 3.53 cancers per year per 105 persons or 3530 cancers/yr/108 persons [33] and AFM1 intake through yoghurt contributed 0.023–0.048 cancers/yr/108 person for mean consumers and 0.028–0.069 cancers/yr/108 person for high consumers. Therefore, their findings indicated AFM1 in yoghurt contributed a slight part of the overall incidence of liver cancer in the Iranian population. The intake of AFM1 and liver cancer incidence due to the consumption of this mycotoxin through yoghurt and milk have been reported in other countries including China, Spain, Greece, and Serbia [20], [45], [105]. The range of liver cancer incidence or hepatocellular carcinoma (HCC) due to AFM1 intake through milk and yoghurt was 0.025–0.033 case or cancers/yr/108 person in China, was similar to the results of this study in Serbia and Greece was 3.6–0.4.7 and 0.7–0.9 case or cancers/yr/108 person, respectively that it was higher than the current study. These distributes were related to the AFM1 level and consumption value of yoghurt.
Findings of studies by Serraino et al. [94] from Italy showed the EDI of AFM1 in different population groups was in the range of 0.025– 0.328 ng kg-1 body weight (bw) per day, based on the average consumption levels and weighted mean contamination of milk in the study period. The estimated fractions of HCC incidences attributable to AFM1 intake were 0.005 and 0.004 cases per 100,000 individuals in the 0–0.9 and 1–2.9-year age groups, respectively, and below 0.004 cases in the other age categories which posed adverse health consequences. Trevisani et al. [104] in a related study, we reported 0.011–0.057 cases/100,000 people in different age categories in an Italian population. The estimated fraction of the incidence of HCC in the Italian population projected a slight increase in cases due to milk consumption.
Contrariwise, a recent study by Njombwa et al. [73] from Malawi, reported a probable mean daily exposure to AFM1 for adults as 4.98 ± 7.25 ng/kg bw/day and almost double for children (8.28 ± 11.82 ng/kg bw/day). Estimated risks of AFM1-induced HCC associated with consumption of milk among children and adults were 0.038 and 0.023 cases per 100,000 individuals per year, respectively. Their results suggested a low risk of hepatocellular carcinoma (HCC).
In this study, EDI were moderate compared with other EDI values reported globally. This implies a reasonably significant impact of aflatoxins on the nutritional status of humans. Gong et al. [42] observed that aflatoxin exposures were to some extent linked to nutrient deficiency in humans following the suggestion that aflatoxin exposure expedites intestinal damage resulting in a decline in nutrient absorption. A noteworthy positive link between aflatoxin prevalence and zinc and vitamin A deficiency was also established by Watson et al. [109]. Furthermore, a study in Ghana reported that subjects with high exposure to the toxin were more likely to suffer deficiencies of vitamins A and E [75]. A strong association between anemia and aflatoxin has been reported in Ghana [96] showed that aflatoxin exposure may contribute partly to the high iron deficiency prevalent in children in developing countries including Ghana.
The consumption of aflatoxins at high levels in a single dose or repeatedly for a brief period induces acute intoxication, henceforward labeled aflatoxicosis in humans and animals with typical symptoms, including jaundice, lethargy, nausea, edema, hemorrhagic necrosis of liver tissues, bile duct hyperplasia, and eventually death (10–60%) subsequent to severe liver damage [84]. While there is no accord on the specific dose of aflatoxins that triggers acute toxicity in humans, it is well recognized that such a dose is highly adjustable depending on many factors, including age, gender, health and nutritional status, presence or absence of underlying factors such as chronic viral hepatitis, alcoholism, smoking, cirrhosis, exposure to hepatotoxic microcystins); and it is lowest in youngsters, as validated by the highest death rates of this age-group in aflatoxicosis outbreaks [78].
In the face of the anticipated risk of cancer incidence that can be gotten from AFM1 in this study, the effects of AFM1 on health, and especially the combined effects of mixtures of mycotoxins, the additive effects of aflatoxins, other dietary contaminants, alcohol consumption, and poor diet on cancer risk still remains largely unknown.
10. Conclusion
The findings of this study suggested that a moderate percentage 55% of raw cow milk samples collected in different locations of southern Ghana; Greater Accra, Volta, Western and Eastern regions of Ghana proved to have AFM1 contents, it further showed a public health concern considering the adverse health especially hepatocellular carcinoma (HCC) outcome of the health risk assessments in all age categories since the calculated MOEs were less than 100,000. Rigorous regulation and monitoring of livestock feeds and regular inspection of milk and milk products is vital in the lessening of AFM1 contamination of dairy products.
Funding
This work received no funding.
Consent to publish
Not applicable.
CRediT authorship contribution statement
NKK, TA, AAB, VK-B, NOB and COT performed the experiments and wrote the manuscript. NKK, VK-B, EKE were responsible for statistical analysis. NKK, VK-B. NOB and AAB helped conceive the experiments and prepared the manuscript. NKK, TA, EKE, and COT conceived the original study and VK-B, NKK, and EKE led the sampling and study in Ghana. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge and sincerely extend our heartfelt appreciation to Mr. Francis Nyasem and Mr. Precious Agbemeseli, both Department of Nutrition and Dietetics, University of Health and Allied Sciences for their enormous efforts in data collection. A big thank you also goes to the staff of Food Chemistry and Nutrition Research Division, CSIR- Food Research Institute, Ghana for the AFM1 analysis.
Handling Editor: Lawrence Lash
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.Abeshu M.A., Lelisa A., Geleta B. Complementary feeding: review of recommendations, feeding practices, and adequacy of homemade complementary food preparations in developing countries–lessons from Ethiopia. Front. Nutr. 2016;3:41. doi: 10.3389/fnut.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abesig J., Chen Y., Wang H., Sompo F.M., Wu I.X. Prevalence of viral hepatitis B in Ghana between 2015 and 2019: a systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrar M., Anjum F.M., Butt M.S., Pasha I., Randhawa M.A., Saeed F., Waqas K. Aflatoxins: biosynthesis, occurrence, toxicity and remedies. Crit. Rev. Food Sci. Nutr. 2013;53:862–874. doi: 10.1080/10408398.2011.563154. [DOI] [PubMed] [Google Scholar]
- 4.Abubakari A., Kynast-Wolf G., Jahn A. Prevalence of abnormal birth weight and related factors in Northern region, Ghana. BMC Pregnancy Childbirth. 2015;15:1–8. doi: 10.1186/s12884-015-0790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Achaglinkame M.A., Opoku N., Amagloh F.K. Aflatoxin contamination in cereals and legumes to reconsider usage as complementary food ingredients for Ghanaian infants: a review. J. Nutr. Intermed. Metab. 2017;10:1–7. [Google Scholar]
- 6.Addo-Boadu C. Aflatoxin M1 Contamination of Raw Cow Milk, Milk Products and Dietary Exposure. 2019. [Google Scholar]
- 7.Adegbeye M.J., Reddy P.R.K., Chilaka C.A., Balogun O.B., Elghandour M.M., Rivas-Caceres R.R., Salem A.Z. Mycotoxin toxicity and residue in animal products: prevalence, consumer exposure and reduction strategies–A review. Toxicon. 2020;177:96–108. doi: 10.1016/j.toxicon.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Adelusola O.O.S.M.K., Oyelese T.A.A. Aflatoxins in autopsy kidney specimens from children in Nigeria. J. Toxicol. Environ. Health Part A. 1998;55:317–323. doi: 10.1080/009841098158368. [DOI] [PubMed] [Google Scholar]
- 9.Adetunji M.C., Alika O.P., Awa N.P., Atanda O.O., Mwanza M. Microbiological quality and risk assessment for aflatoxins in groundnuts and roasted cashew nuts meant for human consumption. J. Toxicol. 2018;2018 doi: 10.1155/2018/1308748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afrifa–Anane E., Agyemang C., Codjoe S.N.A., Ogedegbe G., Aikins A. d-G. The association of physical activity, body mass index and the blood pressure levels among urban poor youth in Accra, Ghana. BMC Public Health. 2015:1–9. doi: 10.1186/s12889-015-1546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agriopoulou S., Stamatelopoulou E., Varzakas T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods. 2020;9:137. doi: 10.3390/foods9020137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahlberg S., Grace D., Kiarie G., Kirino Y., Lindahl J. A risk assessment of aflatoxin M1 exposure in low and mid-income dairy consumers in Kenya. Toxins. 2018;10:348. doi: 10.3390/toxins10090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amer A.A., Ibrahim M.E. Determination of aflatoxin M1 in raw milk and traditional cheeses retailed in Egyptian markets. J. Toxicol. Environ. Health Sci. 2010;2:50–52. [Google Scholar]
- 14.Asare G.A., Bronz M., Naidoo V., Kew M.C. Interactions between aflatoxin B1 and dietary iron overload in hepatic mutagenesis. Toxicology. 2007;234:157–166. doi: 10.1016/j.tox.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Asi M.R., Iqbal S.Z., Ariño A., Hussain A. Effect of seasonal variations and lactation times on aflatoxin M1 contamination in milk of different species from Punjab, Pakistan. Food Control. 2012;25:34–38. [Google Scholar]
- 16.Authority E.F.S. Opinion of the scientific committee on a request from EFSA related to exposure assessments. EFSA J. 2005;3:249. [Google Scholar]
- 17.Bellio A., Bianchi D.M., Gramaglia M., Loria A., Nucera D., Gallina S., Gili M., Decastelli L. Aflatoxin M1 in cow’s milk: method validation for milk sampled in northern Italy. Toxins. 2016;8:57. doi: 10.3390/toxins8030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilandžić N., Varenina I., Solomun B. Aflatoxin M1 in raw milk in Croatia. Food Control. 2010;21:1279–1281. [Google Scholar]
- 19.Biritwum R., Gyapong J., Mensah G. The epidemiology of obesity in Ghana. Ghana Med. J. 2005;39:82. [PMC free article] [PubMed] [Google Scholar]
- 20.Cano-Sancho G., Marin S., Ramos A.J., Peris-Vicente J., Sanchis V. Occurrence of aflatoxin M1 and exposure assessment in Catalonia (Spain) Rev. Iberoam. Micol. 2010;27:130–135. doi: 10.1016/j.riam.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 21.De Roma A., Rossini C., Ritieni A., Gallo P., Esposito M. A survey on the Aflatoxin M1 occurrence and seasonal variation in buffalo and cow milk from Southern Italy. Food Control. 2017;81:30–33. [Google Scholar]
- 22.De Vries H., Maxwell S., Hendrickse R. Aflatoxin excretion in children with kwashiorkor or marasmic kwashiorkor—a clinical investigation. Mycopathologia. 1990;110:1–9. doi: 10.1007/BF00442763. [DOI] [PubMed] [Google Scholar]
- 23.Duarte S., Almeida A., Teixeira A., Pereira A., Falcão A., Pena A., Lino C. Aflatoxin M1 in marketed milk in Portugal: assessment of human and animal exposure. Food Control. 2013;30:411–417. [Google Scholar]
- 24.EFSA Panel on Contaminants in the Food Chain (CONTAM), Schrenk D., Bignami M., Bodin L., Chipman J.K., del Mazo J., Grasl-Kraupp B., Hogstrand C., Hoogenboom L., Leblanc J.C., et al. Risk assessment of aflatoxins in food. EFSA J. 2020;18 doi: 10.2903/j.efsa.2020.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.EFSA Panel on Contaminants in the Food Chain (CONTAM), Schrenk D., Bignami M., Bodin L., Chipman J.K., del Mazo J., Grasl-Kraupp B., Hogstrand C., Hoogenboom L., Leblanc J.C., et al. Risk assessment of aflatoxins in food. EFSA J. 2020;18 doi: 10.2903/j.efsa.2020.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.EFSA Panel on Contaminants in the Food Chain (CONTAM), Schrenk D., Bignami M., Bodin L., Chipman J.K., del Mazo J., Grasl‐Kraupp B., Hogstrand C., Hoogenboom L., Leblanc J.C., et al. Risk assessment of aflatoxins in food. EFSA J. 2020;18 doi: 10.2903/j.efsa.2020.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elsanhoty R.M., Salam S.A., Ramadan M.F., Badr F.H. Detoxification of aflatoxin M1 in yoghurt using probiotics and lactic acid bacteria. Food Control. 2014;43:129–134. [Google Scholar]
- 28.Elzupir A.O., Elhussein A.M. Determination of aflatoxin M1 in dairy cattle milk in Khartoum State, Sudan. Food Control. 2010;21:945–946. [Google Scholar]
- 29.F. Joint, Matters of interest arising from FAO and WHO and from the 68th meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 40th Session, Joint FAO/WHO Food Standards Programme Codex Committee on Food Additives, Beijing, China, 2008.
- 30.Fakhri Y., Rahmani J., Oliveira C.A.F., Franco L.T., Corassin C.H., Saba S., Khaneghah A.M. Aflatoxin M1 in human breast milk: a global systematic review, meta-analysis, and risk assessment study (Monte Carlo simulation) Trends Food Sci. Technol. 2019;88:333–342. [Google Scholar]
- 31.Fallah A.A. Assessment of aflatoxin M1 contamination in pasteurized and UHT milk marketed in central part of Iran. Food Chem. Toxicol. 2010;48:988–991. doi: 10.1016/j.fct.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Fallah A.A., Jafari T., Fallah A., Rahnama M. Determination of aflatoxin M1 levels in Iranian white and cream cheese. Food Chem. Toxicol. 2009;47:1872–1875. doi: 10.1016/j.fct.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 33.Fazli Z., Fatemeh S., Abdi A., Pourhosseingholi M., Taghinejad H. Studying of liver cancer mortality and morbidity burden in Iran. Sci. J. Ilam Univ. Med. Sci. 2012;4:117–122. [Google Scholar]
- 34.Flores-Flores M.E., Lizarraga E., de Cerain A.L., González-Peñas E. Presence of mycotoxins in animal milk: a review. Food Control. 2015;53:163–176. [Google Scholar]
- 35.G.S. Bbosa, D. Kitya, J. Ogwal-Okeng, Aflatoxins Metabolism, Effects on Epigenetic Mechanisms and Their Role in Carcinogenesis, 2013.
- 36.Gizachew D., Szonyi B., Tegegne A., Hanson J., Grace D. Aflatoxin contamination of milk and dairy feeds in the Greater Addis Ababa milk shed, Ethiopia. Food Control. 2016;59:773–779. [Google Scholar]
- 37.Joint FAO/WHO Expert Committee on Food Additives . Evaluation of certain food additives and contaminants: Forty nineth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO; Geneva: 2001. [Google Scholar]
- 38.Joint FAO/WHO Expert Committee on Food Additives . World Health Organization; 1999. Safety evaluation of certain mycotoxins in foods. [Google Scholar]
- 39.Glover-Amengor M., Agbemafle I., Hagan L.L., Mboom F.P., Gamor G., Larbi A., Hoeschle-Zeledon I. Nutritional status of children 0–59 months in selected intervention communities in northern Ghana from the africa RISING project in 2012. Arch. Public Health. 2016;74:1–12. doi: 10.1186/s13690-016-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goncalves L., Dalla Rosa A., Gonzales S.L., Feltes M.M.C., Badiale-Furlong E., Dors G.C. Incidence of aflatoxin M1 in fresh milk from small farms. Food Sci. Technol. 2017;37:11–15. [Google Scholar]
- 41.Gong Y., Egal S., Hounsa A., Turner P., Hall A., Cardwell K., Wild C. Determinants of aflatoxin exposure in young children from Benin and Togo, West Africa: the critical role of weaning. Int. J. Epidemiol. 2003;32:556–562. doi: 10.1093/ije/dyg109. [DOI] [PubMed] [Google Scholar]
- 42.Gong Y.Y., Turner P.C., Hall A.J., Wild C.P. Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade. 2008. Aflatoxin exposure and impaired child growth in West Africa: an unexplored international public health burden; pp. 53–65. [Google Scholar]
- 43.Gong Y.Y., Cardwell K., Hounsa A., Egal S., Turner P.C., Hall A.J., Wild C.P. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. BMJ. 2002;325:20–21. doi: 10.1136/bmj.325.7354.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Granito A., Muratori L., Lalanne C., Quarneti C., Ferri S., Guidi M., Lenzi M., Muratori P. Hepatocellular carcinoma in viral and autoimmune liver diseases: Role of CD4+ CD25+ Foxp3+ regulatory T cells in the immune microenvironment. World J. Gastroenterol. 2021;14(27(22)):2994–3009. doi: 10.3748/wjg.v27.i22.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo Y., Yuan Y., Yue T. Aflatoxin M1 in milk products in China and dietary risk assessment. J. Food Prot. 2013;76:849–853. doi: 10.4315/0362-028X.JFP-12-419. [DOI] [PubMed] [Google Scholar]
- 46.Hatem N.L., Hassab H.M., Al-Rahman E.M.A., El-Deeb S.A., Ahmed R.L.E.-S. Prevalence of aflatoxins in blood and urine of Egyptian infants with protein–energy malnutrition. Food Nutr. Bull. 2005;26:49–56. doi: 10.1177/156482650502600106. [DOI] [PubMed] [Google Scholar]
- 47.Hendrickse R. Kwashiorkor: 50 years of myth and mystery. Do aflatoxins provide a clue. Acta Leiden. 1985;53:11–30. [PubMed] [Google Scholar]
- 48.Huong B.T.M., Brimer L., Dalsgaard A. Dietary exposure to aflatoxin B1, ochratoxin A and fuminisins of adults in Lao Cai province, Viet Nam: a total dietary study approach. Food Chem. Toxicol. 2016;98:127–133. doi: 10.1016/j.fct.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Iha M.H., Barbosa C.B., Okada I.A., Trucksess M.W. Aflatoxin M1 in milk and distribution and stability of aflatoxin M1 during production and storage of yoghurt and cheese. Food Control. 2013;29:1–6. [Google Scholar]
- 50.Iqbal S.Z., Asi M.R. Assessment of aflatoxin M1 in milk and milk products from Punjab, Pakistan. Food Control. 2013;30:235–239. [Google Scholar]
- 51.Jager A., Tedesco M., Souto P., Oliveira C. Assessment of aflatoxin intake in São Paulo, Brazil. Food Control. 2013;33:87–92. [Google Scholar]
- 52.Kang’ethe E.K., Lang’a K. Aflatoxin B1 and M1 contamination of animal feeds and milk from urban centers in Kenya. Afr. Health Sci. 2009:9. [PMC free article] [PubMed] [Google Scholar]
- 53.Kaur S., Bedi J.S., Dhaka P., Vijay D., Aulakh R.S. Exposure assessment and risk characterization of aflatoxin M1 through consumption of market milk and milk products in Ludhiana,Punjab. Food Control. 2021;126 doi: 10.1016/j.foodcont.2021.107991. [DOI] [Google Scholar]
- 54.Ketney O., Santini A., Oancea S. Recent aflatoxin survey data in milk and milk products: a review. Int. J. Dairy Technol. 2017;70:320–331. [Google Scholar]
- 55.Kew M.C. Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int. 2003;23:405–409. doi: 10.1111/j.1478-3231.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 56.Kortei N.K., Agyekum A.A., Akuamoa F., Baffour V.K., Alidu H.W. Risk assessment and exposure to levels of naturally occurring aflatoxins in some packaged cereals and cereal based foods consumed in Accra, Ghana. Toxicol. Rep. 2019;6:34–41. doi: 10.1016/j.toxrep.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kortei N.K., Annan T., Akonor P.T., Richard S.A., Annan H.A., Kyei-Baffour V., Akuamoa F., Akpaloo P.G., Esua-Amoafo P. The occurrence of aflatoxins and human health risk estimations in randomly obtained maize from some markets in Ghana. Sci. Rep. 2021;11:1–13. doi: 10.1038/s41598-021-83751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kos J., Lević J., Đuragić O., Kokić B., Miladinović I. Occurrence and estimation of aflatoxin M1 exposure in milk in Serbia. Food Control. 2014;38:41–46. [Google Scholar]
- 59.Kuboka M.M., Imungi J.K., Njue L., Mutua F., Grace D., Lindahl J.F. Occurrence of aflatoxin M1 in raw milk traded in peri-urban Nairobi, and the effect of boiling and fermentation. Infect. Ecol. Epidemiol. 2019;9 doi: 10.1080/20008686.2019.1625703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuiper-Goodman T. Uncertainties in the risk assessment of three mycotoxins: aflatoxin, ochratoxin, and zearalenone. Can. J. Physiol. Pharmacol. 1990;68:1017–1024. doi: 10.1139/y90-155. [DOI] [PubMed] [Google Scholar]
- 61.Kumi J., Dotse E., Asare G.A., Ankrah N.-A. Urinary aflatoxin M1 exposure in Ghanaian children weaned on locally prepared nutritional food. Afr. J. Sci. Res. 2015;4:28–32. [Google Scholar]
- 62.Lartey A., Manu A., Brown K., Peerson J., Dewey K. Predictors of growth from 1 to 18 months among breast-fed Ghanaian infants. Eur. J. Clin. Nutr. 2000;54:41–49. doi: 10.1038/sj.ejcn.1600891. [DOI] [PubMed] [Google Scholar]
- 63.Lee J.E., Kwak B.-M., Ahn J.-H., Jeon T.-H. Occurrence of aflatoxin M1 in raw milk in South Korea using an immunoaffinity column and liquid chromatography. Food Control. 2009;20:136–138. [Google Scholar]
- 64.Li L., Ye T., Zhang Q., Li X., Ma L., Yan J. The expression and clinical significance of TPM4 in hepatocellular carcinoma. Int. J. Med. Sci. 2021;18(1):169–175. doi: 10.7150/ijms.49906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y., Wu F. Global burden of aflatoxin-induced hepatocellular carcinoma: a risk assessment. Environ. Health Perspect. 2010;118:818–824. doi: 10.1289/ehp.0901388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Magoha H., Kimanya M., De Meulenaer B., Roberfroid D., Lachat C., Kolsteren P. Risk of dietary exposure to aflatoxins and fumonisins in infants less than 6 months of age in R ombo, N orthern T anzania. Matern. Child Nutr. 2016;12(3):516–527. doi: 10.1111/mcn.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Makun H., Mwanza M., Iheanacho H., Apeh D., Abdulrahim S., Jamiu S. Comparative study of aflatoxin M1 in livestock livers from Minna, Nigeria. J. Liver. 2017;6:2167. 0889.1000205. [Google Scholar]
- 68.Min L., Fink-Gremmels J., Li D., Tong X., Tang J., Nan X., Yu Z., Chen W., Wang G. An overview of aflatoxin B1 biotransformation and aflatoxin M1 secretion in lactating dairy cows. Anim. Nutr. 2021;7:42–48. doi: 10.1016/j.aninu.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohammed S., Munissi J.J., Nyandoro S.S. Aflatoxin M1 in raw milk and aflatoxin B1 in feed from household cows in Singida, Tanzania. Food Addit. Contam. Part B. 2016;9:85–90. doi: 10.1080/19393210.2015.1137361. [DOI] [PubMed] [Google Scholar]
- 70.Mulunda M., Mike D. Occurrence of aflatoxin M1 from rural subsistence and commercial farms from selected areas of South Africa. Food Control. 2014;39:92–96. [Google Scholar]
- 71.Nahle S., El Khoury A., Savvaidis I., Chokr A., Louka N., Atoui A. Detoxification approaches of mycotoxins: by microorganisms, biofilms and enzymes. Int. J. Food Contam. 2022;9(3):1–14. doi: 10.1186/s40550-022-00089-2. [DOI] [Google Scholar]
- 72.Nile S.H., Park S.W., Khobragade C. Occurrence and analysis of aflatoxin M1 in milk produced by Indian dairy species. Food Agric. Immunol. 2016;27:358–366. [Google Scholar]
- 73.Njombwa C.A., Moreira V., Williams C., Aryana K., Matumba L. Aflatoxin M 1 in raw cow milk and associated hepatocellular carcinoma risk among dairy farming households in Malawi. Mycotoxin Res. 2021;37:89–96. doi: 10.1007/s12550-020-00417-5. [DOI] [PubMed] [Google Scholar]
- 74.O’Connor D. Folate in goat milk products with reference to other vitamins and minerals: a review. Small Rumin. Res. 1994;14:143–149. [Google Scholar]
- 75.Obuseh F.A., Jolly P.E., Jiang Y., Shuaib F.M., Waterbor J., Ellis W.O., Piyathilake C.J., Desmond R.A., Afriyie-Gyawu E., Phillips T.D. Aflatoxin B. Int. J. Vitam. Nutr. Res. 2010;80:355–368. doi: 10.1024/0300-9831/a000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Omore A.O., Mulindo J.C.o., Khan M., Islam S.F., Staal S., Nurah G., Dugdill B. Employment Generation through Small-scale Dairy Marketing and Processing: Experiences from Kenya, Bangladesh and Ghana: A Joint Study by the ILRI Market-oriented Smallholder Dairy Project and the FAO Animal Production and Health Division. Food & Agriculture Organisation; 2004. [Google Scholar]
- 77.Onyemelukwe G.C., Ogoina D., Ibiam G., Ogbadu G. Aflatoxins in body fluids and food of Nigerian children with protein-energy malnutrition. Afr. J. Food Agric. Nutr. Dev. 2012;12:6553–6566. [Google Scholar]
- 78.Opoku N., Achaglinkame M.A., Amagloh F.K. Aflatoxin content in cereal-legume blends on the Ghanaian market far exceeds the permissible limit. Food Secur. 2018;10:1539–1545. [Google Scholar]
- 79.Organization W.H. World Health Organization; 2006. WHO Child Growth Standards: Length/height-for-age, Weight-for-age, Weight-for-length, Weight-for-height and Body Mass Index-for-age: Methods and Development. [Google Scholar]
- 80.Oyelami O., Maxwell S., Aladekomo T., Adelusola K. Two unusual cases of kwashiorkor: can protein deficiency explain the mystery? Ann. Trop. Paediatr. 1995;15:217–219. doi: 10.1080/02724936.1995.11747775. [DOI] [PubMed] [Google Scholar]
- 81.Oyelami O., Maxwell S., Adelusola K., Aladekoma T., Oyelese A. Aflatoxins in the autopsy brain tissue of children in Nigeria. Mycopathologia. 1995;132:35–38. [Google Scholar]
- 82.Oyelami O., Maxwell S., Adelusola K., Aladekoma T., Oyelese A. Aflatoxins in the lungs of children with kwashiorkor and children with miscellaneous diseases in Nigeria. J. Toxicol. Environ. Health. 1997;51:623–628. doi: 10.1080/00984109708984048. [DOI] [PubMed] [Google Scholar]
- 83.Pardakhti A., Maleki S. Risk assessment of aflatoxin M1 contamination of milk in Iran. Int. J. Environ. Res. 2019;13(2):265–271. [Google Scholar]
- 84.Peraica M., Radić B., Lucić A., Pavlović M. Toxic effects of mycotoxins in humans. Bull. World Health Organ. 1999;77:754. [PMC free article] [PubMed] [Google Scholar]
- 85.Pereira P.C. Milk nutritional composition and its role in human health. Nutrition. 2014;30:619–627. doi: 10.1016/j.nut.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 86.Prandini A., Tansini G., Sigolo S., Filippi L., Laporta M., Piva G. On the occurrence of aflatoxin M1 in milk and dairy products. Food Chem. Toxicol. 2009;47:984–991. doi: 10.1016/j.fct.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 87.Rahimi E., Bonyadian M., Rafei M., Kazemeini H. Occurrence of aflatoxin M1 in raw milk of five dairy species in Ahvaz, Iran. Food Chem. Toxicol. 2010;48:129–131. doi: 10.1016/j.fct.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 88.Richard S.A., Manaphraim N.Y., Kortei N.K. The novel neurotoxic and neuroimmunotoxic capabilities of aflatoxin B1 on the nervous system: a review. Adv. Biosci. Clin. Med. 2020;8:1–8. [Google Scholar]
- 89.Rodríguez-Blanco M., Marín Sillué S., Sanchís Almenar V., Ramos Girona A.J. Mycotoxin contamination and fungal populations in silages for dairy cows in Spain. Toxins. 2019;11(7):21–22. [Google Scholar]
- 90.Roila R., et al. A study of the occurrence of aflatoxin M1 in milk supply chain over a seven-year period (2014–2020): human exposure assessment and risk characterization in the population of central Italy. Foods. 2021;10:1529. doi: 10.3390/foods10071529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sangoyomi T., Owoseni A., Okerokun O. Prevalence of enteropathogenic and lactic acid bacteria species in wara: a local cheese from Nigeria. Afr. J. Microbiol. Res. 2010;4:1624–1630. [Google Scholar]
- 92.dos Santos J.S., Souza T.M., Ono E.Y.S., Hashimoto E.H., Bassoi M.C., de Miranda M.Z., Itano E.N., Kawamura O., Hirooka E.Y. Natural occurrence of deoxynivalenol in wheat from Paraná State, Brazil and estimated daily intake by wheat products. Food Chem. 2013;138:90–95. doi: 10.1016/j.foodchem.2012.09.100. [DOI] [PubMed] [Google Scholar]
- 93.Schöneberg T., Kibler K., Wettstein F., Bucheli T., Forrer H., Musa T., Mascher F., Bertossa M., Keller B., Vogelgsang S. Influence of temperature, humidity duration and growth stage on the infection and mycotoxin production by Fusarium langsethiae and Fusarium poae in oats. Plant Pathol. 2019;68:173–184. [Google Scholar]
- 94.Serraino A., Bonilauri P., Kerekes K., Farkas Z., Giacometti F., Canever A., Zambrini A.V., Ambrus Á. Occurrence of aflatoxin M1 in raw milk marketed in Italy: exposure assessment and risk characterization. Front. Microbiol. 2019;10:2516. doi: 10.3389/fmicb.2019.02516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shephard G.S. Risk assessment of aflatoxins in food in Africa. Food Addit. Contam. 2008;25:1246–1256. doi: 10.1080/02652030802036222. [DOI] [PubMed] [Google Scholar]
- 96.Shuaib F.M., Jolly P.E., Ehiri J.E., Yatich N., Jiang Y., Funkhouser E., Person S.D., Wilson C., Ellis W.O., Wang J.S. Association between birth outcomes and aflatoxin B1 biomarker blood levels in pregnant women in Kumasi, Ghana. Trop. Med. Int. Health. 2010;15:160–167. doi: 10.1111/j.1365-3156.2009.02435.x. [DOI] [PubMed] [Google Scholar]
- 97.Singh P.K., Singh R.P., Singh P., Singh R.L. Food Safety and Human Health. Elsevier; 2019. Food hazards: physical, chemical, and biological; pp. 15–65. [Google Scholar]
- 98.Škrbić B., Antić I., Živančev J. Presence of aflatoxin M1 in white and hard cheese samples from Serbia. Food Control. 2015;50:111–117. [Google Scholar]
- 99.Soriano J.M., Rubini A., Morales-Suarez M., Merino-Torres J.F., Silvestre D. Aflatoxins in organs and biological samples from children affected by kwashiorkor, marasmus and marasmic-kwashiorkor: a scoping review. Toxicon. 2020 doi: 10.1016/j.toxicon.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 100.Sun G., Wang S., Hu X., Su J., Zhang Y., Xie Y., Zhang H., Tang L., Wang J.-S. Co-contamination of aflatoxin B1 and fumonisin B1 in food and human dietary exposure in three areas of China. Food Addit. Contam. 2011;28:461–470. doi: 10.1080/19440049.2010.544678. [DOI] [PubMed] [Google Scholar]
- 101.Tabari M., Karim G., Ghavami M., Chamani M. Method validation for aflatoxin M1 determination in yoghurt using immunoaffinity column clean-up prior to high-performance liquid chromatography. Toxicol. Ind. Health. 2011;27:629–635. doi: 10.1177/0748233710394236. [DOI] [PubMed] [Google Scholar]
- 102.Tchana A.N., Moundipa P.F., Tchouanguep F.M. Aflatoxin contamination in food and body fluids in relation to malnutrition and cancer status in Cameroon. Int. J. Environ. Res. Public Health. 2010;7:178–188. doi: 10.3390/ijerph7010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tomašević I., Petrović J., Jovetić M., Raičević S., Milojević M., Miočinović J. Two year survey on the occurrence and seasonal variation of aflatoxin M1 in milk and milk products in Serbia. Food Control. 2015;56:64–70. [Google Scholar]
- 104.Trevisani M., Serraino A., Canever A., Varisco G., Boni P. Quantitative Risk Assessment of Aflatoxicosis Associated with Milk Consumption in Italy (2000–2004). Towards A Risk-based Chain Control. 2006. pp. 91–114. [Google Scholar]
- 105.Udovicki B., Djekic I., Kalogianni E.P., Rajkovic A. Exposure assessment and risk characterization of aflatoxin M1 intake through consumption of milk and yoghurt by student population in Serbia and Greece. Toxins. 2019;11:205. doi: 10.3390/toxins11040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Unusan N. Occurrence of aflatoxin M1 in UHT milk in Turkey. Food Chem. Toxicol. 2006;44:1897–1900. doi: 10.1016/j.fct.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 107.V.K. Rao, V. Navale, S. Ajmera, V. Dhuri, Aspergillus derived mycotoxins in food and the environment: prevalence, detection, and toxicity, Toxicol. Rep., 2021. [DOI] [PMC free article] [PubMed]
- 108.Walpole S.C., Prieto-Merino D., Edwards P., Cleland J., Stevens G., Roberts I. The weight of nations: an estimation of adult human biomass. BMC Public Health. 2012;12:1–6. doi: 10.1186/1471-2458-12-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Watson S., Diedhiou P., Atehnkeng J., Dem A., Bandyopadhyay R., Srey C., Routledge M., Gong Y. Seasonal and geographical differences in aflatoxin exposures in Senegal. World Mycotoxin J. 2015;8:525–531. [Google Scholar]
- 110.Yousof S., El Zubeir I. Chemical composition and detection of Aflatoxin M1 in camels and cows milk in Sudan. Food Addit. Contam. Part B. 2020;13:298–304. doi: 10.1080/19393210.2020.1796826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


