Abstract
Acrylamide is one of the undesirable compounds created in food, which leads to oxidative stress. Under normal conditions of the body, there is a balance between the production and elimination of free radicals. Imbalance in this process causes oxidative stress. Ascorbic acid has antioxidant properties and can be used to prevent oxidative stress damage. In this study, we considered the effect of acrylamide as a substance that causes oxidative stress and ascorbic acid as an antioxidant. In this experiment, 28 rats were separated into 4 groups (n = 7). Mice were fed orally with acrylamide (10 mg/kg), ascorbic acid (200 mg/kg), both acrylamide and ascorbic acid, and water as AR, AA, AR&AA, and control groups, respectively. Blood and ovarian tissue samples were collected after the final feeding and anesthesia for hematological tests. Blood cells, anti-oxidation status and relative expression of BAX (Bcl-2 Associated X-protein), BCL-2 (B-cell lymphoma-2), Folliculogenesis Specific BHLH Transcription Factor (FIGLA), Follicle Stimulating Hormone Receptor (FSHR), and Klotho (KL) genes were assessed and compared between the study groups. Despite no change in the levels of other factors, white blood cells were significantly different between all groups. The total oxidant and antioxidant index had significant changes in the AR group compared to controls. Glutathione Peroxidase showed the least concentration in the AR group than others although this change was not significant. Gene expression of BAX, BCL-2, FIGLA, FSHR, and KL genes was not significant between the study groups (P > 0.05). The most ratio of BAX to BCL-2 belonged to the AR group compared to other groups. In conclusion, AR probably induces ovarian dysfunction by increasing the proportions of apoptosis-related genes, and the usage of antioxidants like AA can minimize its hazardous effects. However, more research is needed to uncover effective ways to limit AR exposure.
Keywords: Ovarian follicle development, Acrylamide, Ascorbic acid, Gene expression, Oxidative stress markers, Rat
Graphical Abstract
Highlights
-
•
Acrylamide may cause ovarian dysfunction by inducing oxidative stress.
-
•
The use of antioxidants like ascorbic acid can minimize the hazardous effects of acrylamide.
1. Introduction
Under normal conditions of the body, there is a balance between the production and elimination of free radicals, otherwise, it would cause oxidative stress [1]. Oxidative stress is caused by an increase in reactive oxygen species (ROS) because the cells' antioxidant capacity to remove these free radicals decreases [2]. Increased oxidative stress depletes tissue glutathione and leads to an increase in free radicals which lead to cytotoxic effects, because of oxidative degradation of biological macromolecules such as nucleic acids, etc. by free radicals [1]. Evidence suggests that high levels of oxidative stress cause pathological processes such as endometriosis, infertility of unknown cause, preeclampsia, recurrent miscarriage, and polycystic ovary syndrome. Thus, the regulation of oxidative stress can facilitate physiological reproductive functions in oocyte maturation, folliculogenesis, ovarian steroid production, luteolysis, ovulation, endometrial period changes, and menstruation. Prescribing antioxidants can reduce the destructive effects of ROS and thus increase the number, and quality of oocytes and follicles, as well as inhibit the apoptosis of ovarian follicles [3], [4]. Antioxidants, both enzyme-based and non-enzymatic, such as vitamin A, vitamin E, zinc, and selenium, are necessary for maintaining proper ROS levels in the cell by eliminating excess free radicals. It can be a sensitive environment for oxidative stress due to the generation of ROS in numerous areas of the ovulatory follicle. Follicular fluid (FF), endometrial fluid (EF), and peritoneal fluid (PF) can all be used to evaluate ROS levels [2]. Any disturbance in the antioxidant / ROS balance leads to oxidative stress in the cell, which has destructive consequences, so the physiological level of ROS is essential for the proper functioning of various biological pathways and the maintenance of homeostasis in the human body. Disruption at this level can lead to sexual issues in women, such as unexplained infertility, ovarian epithelial damage, and cellular apoptosis, and can also be linked to aging and infertility [2], [5], [6].
Acrylamide (CH2 = CH – CONH2) (AR) is a chemical compound that can use in various industries. Smoking and drinking coffee are other sources of its production [7]. AR is one of the undesirable compounds created in food that is formed when heated to temperatures above 120 °C, which can cause one-third of nutrition-related cancers [8]. Several studies have confirmed its tumorigenesis of it in the uterus, thyroid gland, pancreas, kidney, colon, and breast in mice and humans [9], [10]. Preliminary toxicological studies have shown that AR can act as a reproductive genetic toxin and lead to disorders such as impaired sperm production, decreased sperm motility, testicular atrophy, seminal vesicle atrophy, and testicular mesothelioma, mammary gland tumors, shrinkage Ability to mate in laboratory animals [7], [11], [12].
Antioxidants are considered chemical protective agents against diseases related to oxidative stress, which counteract the oxidative stress process by reducing the production of free radicals [13], [14]. Vitamin C, also known as ascorbic acid (AA), is a water-soluble antioxidant vitamin that is necessary for a variety of biological processes in the body, including growth, collagen formation, and optimal organ function. AA, the most potent antioxidant to prevent oxidative damage, is the first line of antioxidant defense in many types of oxidation conditions that due to its solubility can trap free radicals before they reach the lipids in the aqueous phase [15], [16], [17], [18]. AA inhibits the production of reactive oxygen species. Water-soluble vitamins, particularly AA, protect the body from the damaging effects of acrylamide due to their strong antioxidant capacity [19].
One of the essential proteins that are essential in the control of apoptosis is a member of the Bcl-2 family of proteins. Anti-apoptotic proteins (Bcl-2) and pro-apoptotic proteins from the Bax family make up the Bcl-2 family. The mitochondrial apoptotic pathway is tightly controlled by these proteins. An anti-apoptotic protein called Bcl-2 participates in the internal apoptotic process and inhibits the action of caspases. Bax is another protein that triggers apoptosis by blocking Bcl-2's effects. It results in various histological alterations, including diminished or absent apoptotic cell adherence to neighboring cells, fragmentation of genomic DNA, the release of ribosomes, and other things. Apoptosis is induced when Bax levels are raised, whereas cell survival and repair are induced when Bax levels are lowered. Also, the increase in Bcl-2 values is in the direction of cell survival and repair and inhibits apoptosis [20]; Therefore, the balance between Bax/Bcl-2 is an important factor in determining the amount of apoptosis.
The human FIGLA gene encodes the protein germinal factor alpha (FIGalpha), also known as transcription factor FIGa, which is specifically involved in folliculogenesis. This gene is a major helix-loop-helix transcription factor that regulates a variety of oocyte-specific genes, including those involved in folliculogenesis and oocyte differentiation. The protein that this gene encodes is known as a helix-loop-helix transcription factor. Early ovarian failure is characterized by infertility and hypogonadotropic ovarian failure. Current thinking holds that the primary factor causing early ovarian failure in humans is FIGLA haploid deficiency, which prevents the growth of primordial follicles [21], [22]. The granulosa cell of the ovary and testicular Sertoli as the Follicle Stimulating Hormone Receptor (FSHR) gene, which its production triggers the creation of aromatase, the maturation of follicles and oocytes, and the start of ovulation. According to studies, FSHR gene mutations significantly reduce fertility. Small ovaries, infertility, and the stoppage of folliculogenesis are all effects of FSHR mutations[23], [24], [25], [26].
An anti-aging protein is klotho. By acting in a variety of ways, this protein protects the organ. The idea that Klotho functions as an anti-aging molecule in humans is supported by evidence. In humans, Klotho polymorphism is linked to long life, coronary artery disease, atherosclerosis, and osteoporosis. However, it has only recently been clear that the Klotho phenotype may have reproductive implications. 2017 research by Zenghui et al. suggested that KL may play a role in the death of GCs and subsequent development of PCOS [27].
As a result, since the indicated genes might indicate ovarian function, measuring them can also indicate ovarian function and, thus, can reveal the impact of acrylamide on the ovaries.
Therefore, because of the importance of this topic, we settle to study the effects of AR and AA on the expression of genes related to the development of ovarian follicles and serum levels of oxidative stress markers in rats.
2. Material and method
2.1. Study design
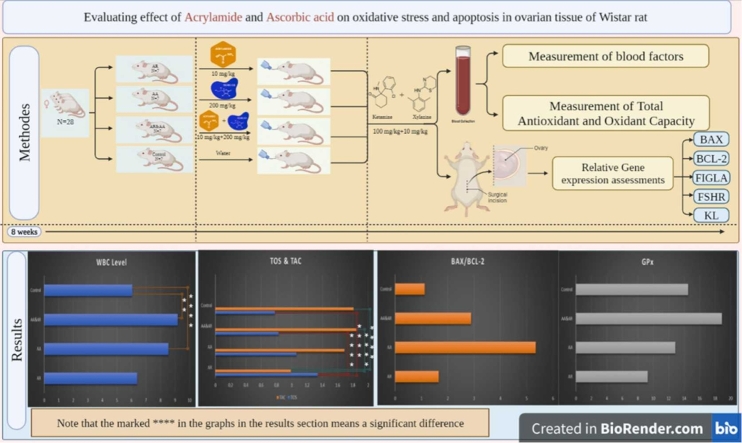
This experimental study with the code of ethics from the Iran National Committee of Ethics in Biomedical Research under the number IR.SSU.SPH.REC.1400.142 was conducted on 28 healthy female Wistar rats, weighing approximately 200–250 g, and a mean age of 8 weeks which were obtained from the animal house of Yazd Institute of Reproductive Sciences. They were split into 4 groups (n = 7) including 1) AR group includes rats that were gavage-fed with 10 mg/kg acrylamide (Sigma_Aldrich Chemical Co.) daily for eight weeks [28], 2) AA group includes rats that were gavage-fed with 200 mg/kg ascorbic acid (Sigma_Aldrich Chemical Co.) daily for eight weeks [29], 3) AA & AR group includes rats that were gavage-fed orally with 10 mg/kg acrylamide and 200 mg/kg ascorbic acid daily for eight weeks, 4) Control group includes rats that were kept in normal conditions with received sterile water as a placebo.
2.2. Quantitative measurement of blood factors
After the feeding time, blood samples were collected from all study groups. After anesthesia with 100 mg/kg ketamine and 10 mg/kg xylazine, the chest cavity was opened and blood was collected from the heart with a sterile syringe and poured into tubes containing the anticoagulant ethylene diamine tetraacetic acid (EDTA). White blood cell count (WBC), red blood cell count (RBC), hemoglobin (HGB), hematocrit (HCT), and markers such as mean hemoglobin (MCV), mean body hemoglobin (MCH), mean concentrations of hemoglobin erythrocytes (MCHC), platelets (PLT), and lymphocytes (LYM) were examined by NIHON KOHDEN cell counter on the same day.
2.3. Measurement of total antioxidant and oxidant capacity
To measure and compare the capacity of total antioxidant and oxidant, serum was isolated from the obtained blood of all groups. Blood was poured into microtubes without anticoagulant to separate serum and kept at room temperature for about 30 min to coagulate blood. Finally, the sample serum was separated by centrifugation at 3500 rpm for 5 min. Blood serum was isolated and poured into a 2 mL microtube. Total antioxidant capacity (TAC), glutathione peroxidase (GPx), and total oxidant status (TOS) were assessed by using the ELISA-specific kit protocol of Navand Lab for all experimental and control groups.
2.4. Relative gene expression assessments
After the last feeding, all groups were anesthetized and ovarian tissue was removed. The left ovaries were used for genetic work. After preparing the ovarian tissue, by using RNX- Plus kit (Sinaclon Bioscience, Cat. No.: RN7713C), the total RNA was extracted. The RNA quantification and purity were determined using a NanoDrop spectrophotometer (2000 C; Thermo Scientific, Waltham, MA, USA) at 260 nm and 260/280, respectively. After RNA normalization for each sample, 500 ng/µL of the extracted RNA was used to synthesize the complementary DNA by a cDNA synthesis kit (Yektatajhiz Co. Cat No: YT4500) according to the manufacturer’s protocol. Finally, a quantitative real-time polymerase chain reaction (qRT-PCR) was performed to quantify the mRNA expression levels of the genes by applying the specific primers for all genes (Table 1). High ROX™ (Amplicon) and a Step One ABI thermocycler were used in the qRT-PCR process. To assess gene expression, the reaction efficiency was first determined, and even though the efficiency was greater than 90%, the comparative CT technique (2-△△ ct) was applied. In this research, B-Actin as housekeeping gene was considered as an internal control gene.
Table 1.
The sequence of primers used.
| Gene | Primer sequence (5′−3′) | Sequence amplified | Product size (bp) |
|---|---|---|---|
| KL | F_GAATACGCAAAGTAGCCACAAAG R_GAGCAAGACTCACTGAGGATG |
XM_039089809.1 | 104 |
| FSHR | F_ATCATTTGCTGGATTTGGAGAC R_TTGGGTAGGTTGGAGAACACAT |
NM_199237.2 | 96 |
| GDF-9 | F_CAATACCGTCCGGCTCTTCAG R_AGGGGTCCTGTCATCTGGTTG |
XM_017597502.2 | 72 |
| FIGLA | F_CAGGAAGCCCAGTAAAGTTGA R_CTTCTTTCTTCAAGCCACTTGC |
XM_575589.7 | 218 |
| BCL-2 | F_CGGGAGAACAGGGTATGA R_CAGGCTGGAAGGAGAAGA |
NM_016993.2 | 149 |
| BAX | F_CAGGACGCATCCACCAAGAAG R_TGCCACACGGAAGAAGACCTC |
NM_017059.2 | 135 |
| B-Actin | F_ CCCATCTATGAGGGTTACGC R_TTTAATGTCACGCACGATTTC |
NM_031144.3 | 150 |
bp: base pair, Kl: Rattus norvegicus Klotho, FSHR: follicle-stimulating hormone receptor, GDF-9: growth differentiation factor-9, FIGLA: folliculogenesis specific BHLH transcription factor, BCL-2: B-cell lymphoma 2, BAX: Bcl-2 Associated X-protein 4, B-Actin
2.5. Statistical analysis
All data obtained in this study were represented as the mean and the standard error of the mean by using SPSS software version 21. Mann-Whitney and Kruskal-Walis tests were used to compare data between two groups and all groups, respectively. P < 0.05 was considered a significant value.
3. Results
3.1. Quantification Effects on blood factors and cells
In this study, a significant difference was observed between all groups regarding the WBC factor (P = 0.01). Also, there were significantly higher levels of WBC in the AA and AA & AR groups compared to the controls (8.56 ± 1.14 & 9.18 ± 0.13 VS. 6.07 ± 0.21, P = 0.04). However, RBC, HGB, HCT, and markers such as MCV, MCH, MCHC, PLT, and LYM were not significantly different between/among the groups (P > 0.05) (Table 2).
Table 2.
Comparison of complete blood count between the study groups.
| Analytic variables | Study groups (N = 28) |
P-value | |||
|---|---|---|---|---|---|
| ARa (N = 7) | AAb (N = 7) | AA&ARc (N = 7) | Controld (N = 7) | ||
| White blood cell (103/µL) | 6.40 ± 0.62 | 8.56 ± 1.14 | 9.18 ± 0.13 | 6.07 ± 0.21 |
0.01 0.20a,b 0.1a,c 0.62a,d 0.7b,c 0.04b,d 0.04c,d |
|
Red blood cell (106/ µL) |
6.78 ± 0.15 | 7.01 ± 0.06 | 6.73 ± 0.12 | 7.08 ± 0.02 | 0.08 |
|
Hemoglobin (g/dL) |
13.53 ± 0.12 | 14.10 ± 0.15 | 13.77 ± 0.14 | 14.20 ± 0.23 | 0.08 |
|
platelet count (103/µL) |
921 ± 49.08 | 825 ± 79.42 | 720 ± 12.03 | 809 ± 56.16 | 0.28 |
Abbreviations: Ascorbic acid (AA) Acrylamide (AR) Ascorbic acid &Acrylamide (AA&AR) Data were presented as Mean ± SEM by using the Mann-Whitney and the Kruskal-Walis tests to compare two groups and all groups, respectively. P < 0.05 were considered a significant value.
3.2. Oxidation potential status
According to Table 3, TOS showed significantly higher rates in the AR group than that in the AA & AR group (1.34 ± 0.15 VS. 0.83 ± 0.15, P = 0.04). In other words, the highest levels of TOS in serum samples belonged to the AR group (1.34 ± 0.15) than that factor in others. In contrast, the lowest serum levels of TAC belonged to the AR group (0.99 ± 0.49) compared to samples in other groups. A significant difference was observed in the AR group (0.99 ± 0.49) compared to both controls as well as the AA& AR groups (P = 0.02 and P = 0.04, respectively). The lowest rate of GPx index was seen in serum samples of the AR group; however, this level was not significant compared to other study groups (P > 0.05).
Table 3.
Comparison of oxidant and antioxidant status between study groups.
| Variables | Study groups (N = 28) |
P-Value | |||
|---|---|---|---|---|---|
| ARa (N = 7) | AAb (N = 7) | AA&ARc (N = 7) | Controld (N = 7) | ||
|
TOS (µmol Equiv./L) |
1.34 ± 0.15 | 1.06 ± 0.17 | 0.83 ± 0.15 | 0.78 ± 0.13 | 0.09 0.26a,b 0.04a,c 0.01a,d 0.37b,c 0.32b,d 0.65c,d |
|
TAC (mmol Fe2+/L) |
0.99 ± 0.49 | 1.69 ± 0.56 | 1.86 ± 0.11 | 1.81 ± 0.23 | 0.09 0.15a,b 0.02a,c 0.04a,d 0.33b,c 0.38b,d 0.59c,d |
|
GPx+ (mU/mL) |
9.28 ± 1.82 | 12.85 ± 2.84 | 18.87 ± 4.77 | 14.52 ± 3.04 | 0.30 |
Abbreviations: Ascorbic acid (AA), Acrylamide (AR), Ascorbic acid &Acrylamide (AA&AR), Total antioxidant capacity (TAC), glutathione peroxidase (GPx), and total oxidant status (TOS). Data were presented as Mean ± SEM by using the Mann-Whitney and the Kruskal-Walis tests to compare two groups and all groups, respectively. P < 0.05 were considered a significant value.
3.3. Gene expression profile
The relative expression of BAX, BCL-2, BAX / BCL-2, FIGLA, FSHR, and KL genes in the study groups was shown in Table 4. There was no significant change in the relative expression of BAX, BCL-2, FIGLA, FSHR, and KL genes between all groups (P > 0.05). However, the median ratio of BAX to BCL2 was found to be AA = 1.66, AR = 5.35, AA & AR = 2.89, and control = 1.13.
Table 4.
Comparison of gene expression profiles between the study groups.
| Variables | Study groups (N = 28) |
P-Value | |||
|---|---|---|---|---|---|
| ARa (N = 7) | AAb (N = 7) | AA&ARc (N = 7) | Controld (N = 7) | ||
| FSHR | 1.72 ± 0.76 | 1.29 ± 0.34 | 1.28 ± 0.39 | 1.98 ± 1.20 | 0.99 |
| GDF-9 | 1.36 ± 0.34 | 2.26 ± 0.78 | 1.36 ± 0.33 | 2.64 ± 1.32 | 0.92 |
| KL | 2.32 ± 1.17 | 1.28 ± 0.37 | 3.05 ± 2.01 | 4.68 ± 2.22 | 0.9 |
| FIGLA | 2.24 ± 1.37 | 1.11 ± 0.21 | 2.16 ± 1.07 | 1.81 ± 0.62 | 0.97 |
| BAX | 4.69 ± 3.80 | 1.11 ± 0.19 | 1.17 ± 0.25 | 1.17 ± 0.31 | 0.94 |
| BCL-2 | 1.40 ± 0.36 | 1.37 ± 0.38 | 2.53 ± 1.54 | 1.47 ± 0.65 | 0.93 |
| BAX/BCL-2 | 1.66 | 5.35 | 2.89 | 1.13 | – |
Abbreviations: AA: Ascorbic acid, AR: Acrylamide, AA&AR: Ascorbic acid &Acrylamide Kl: Rattus norvegicus Klotho, FSHR: follicle-stimulating hormone receptor, GDF-9: growth differentiation factor-9, FIGLA: folliculogenesis specific BHLH transcription factor, BCL-2: B-cell lymphoma 2, BAX: Bcl-2 Associated X-protein 4, B-Actin. Data were presented as Mean ± SEM by using the Mann-Whitney and the Kruskal-Walis tests to compare two groups and all groups, respectively.
4. Discussion
AR is a toxic compound that has been described by previous studies [7], [8], [9], [10], [11], [12]. Pathological evaluations associated with women's infertility with unexplained origins, recurrent miscarriage, and polycystic ovarian syndromes have emerged from high levels of oxidative stress. Therefore, balancing the oxidative stress would improve physiological reproductive functions such as oocyte maturation and ovulation features. The use of useful and safe antioxidants is considered one of the treatment methods to reduce the destructive effects of oxidative stress. In this study, the effects of AR and AA on oxidative stress by applying three oxidation potential markers in rats were investigated. Regarding TOS levels, it was discovered that rats in the AR group had a significantly greater amount of total oxidant than rats who consumed AA and AR at the same time as well as those in the control group. The lowest rate of GPx index, as another antioxidant marker, was seen in serum samples of the AR group; however, this level was not significant compared to other study groups (P > 0.05). In contrast, the TAC value in AR was significantly lower compared to other groups. Erdemli et al. conducted a study in 2019 to assess the effects of AR and vitamin E on the ovarian tissue of adult mice (8 weeks) who were the offspring of pregnant mice given AR and vitamin E orally. The findings of this investigation revealed that AR has a deleterious impact on oxidant-antioxidant parameters and produces oxidative stress in ovarian tissue, which may be alleviated by supplementing with vitamin E [30]. Our findings were consistent with Erdemli et al. research. Also, in 2020, YENER et al., by researching the effect of AA on the level of TAC in plasma, found that AA increases oxidative stress and causes cell membrane damage [31]. Overall, the findings of our study stated that AR toxicity can cause oxidative stress and may cause dysfunction in various parts of the female reproductive tract such as the ovary. AA can neutralize the harmful effects of AR on the body and reduce TOS levels.
Evaluating the effects of AR and AA on apoptosis-related genes in the ovaries showed the most increased ratio of BAX / BCL-2 in the AR group compared to AA and controls, which may indicate the induction of apoptosis through this toxic compound. The lower ratio of BAX / BCL-2 in the AA & AR group in comparison with AR demonstrated that the antioxidant competence of AA may be able to reduce induction of apoptosis caused by extrinsic exposure to chemical and toxic agents like AR. However, due to the fact that we did not investigate other factors affecting apoptosis in the ovary, we cannot definitely confirm this result. In 2019, Shokoohi et al. reported that the use of olive leaf extract, as an antioxidant, could reduce ROS and apoptosis in the ovarian tissue of rats with ovarian torsion. They demonstrated a significant increase in GPx, estrogen, superoxide dismutase, and BCL-2 mRNA levels, as well. In contrast, malondialdehyde levels and BAX expression were also lower in the olive leaf extract-treated group compared to the controls. As a result of the aforementioned study, antioxidant-rich extracts/supplements/food such as olive leaf extract and AA may prevent tissue damage induced by oxidative stress and apoptosis in the ovary after exposure to either chemical and toxic materials, drugs, or physical and physiological damages [32]. As a result of the findings of the previous studies, antioxidant-rich supplements like AA may assist the immune system's function, after exposure to hazardous substances like AR. Also, Chen et al. investigated the impact of AR on mitochondrial breakdown and apoptosis in human astrocytoma cells in 2013. The goal of this research was to discover the harmful processes generated by AR in human astrocytoma U-1240 MG cells. As result, Cell viability reduced as time passed after exposure to 1 and 2 mM AR. After 2 mM AR treatment, the sub-G1 phase increased by 87.5-fold, and pro-caspase 3 and PARP protein expression reduced by 35% and 54.5%, respectively, compared to the control. Under 2 mM AR exposure, the BAX / BCL-2 ratio and cytochrome c expression rose 8.86-fold and 6.81-fold, respectively, whereas procaspase 9 reduced 67.8% [33]. Also, in the 1999 research conducted by Perlman et al. to investigate the relationship between BAX / BCL-2 and the initiation of apoptosis of prostate epithelial cells, it was understood that the increase in the level of BAX / BCL-2 causes the initiation of apoptosis, which is consistent with our results [34]. Additionally, no difference in gene expression was observed in any of the groups, which could be explained by the AR and AA doses and exposure times, which might vary in a larger dose and longer exposure time. Because, based on our research, little research has been done on the effect of acrylamide and the genes under study, we investigated the relationship between their expression in the ovary and other environmental factors. This observation was in line with research published in 2018 by Ramezani et al. on the effects of freezing and in vitro culture on follicular development and gene expression in stimulated human ovarian tissue. According to this study, freezing the stimulated ovarian tissue did not affect the expression of FIGLA and KL genes in primary follicles, as well as GDF9 and FSHR genes in primary and secondary follicles. As a result of the data, it is possible to infer that external stimuli do not affect the development of ovarian tissue [35]. As a result of the foregoing investigations, it can be inferred that compounds like AR might raise the risk of triggering apoptosis in the human body, and antioxidants may be a useful way to counteract these substances' detrimental effects.
Previous research has indicated that consuming antioxidants, particularly AA, boosts and improves the immune system's performance [36]. Nsonga et al. studied the effects of various doses of dietary AA on the growth, survival, and hematology of Oreochromi skarongae in 2009. They discovered that the group that consumed AA had a considerable increase in the number of their WBCs [37]. Also, Ching et al. studied the effect of combining vitamins C and E on the production of cytokine by peripheral blood mononuclear cells in adults in 1996 and discovered that while consuming either of these compounds alone can boost the body’s immune system, combining them has a better effect than consuming them separately [38]. So in the current study, by evaluating the effects of AR and AA on blood factors, we observed a meaningful difference in the numbers of WBC levels in all groups. The number of WBCs was probably greater in the AA and AA & AR groups than in the control group, indicating that AA consumption activates the immune system and increases the number of WBCs due to its antioxidant capabilities. These findings are consistent with the findings of the aforementioned investigations. Due to limited financial support, we did not work out complimentary assessments on the histopathological and morphometric aspects of affected ovaries as well as other oxidative stress markers; however, these tests have already been initiated in our lab to achieve a more comprehensive view of the different effects of antioxidants in protecting the ovary against the destructive effect of AR.
5. Conclusion
The results of this study suggest that AR may cause ovarian dysfunction by causing oxidative stress (disturbing the balance between oxidant and antioxidant agents) and may also cause ovarian dysfunction by inducing apoptosis (the ratio of pro-apoptotic BAX to anti-apoptotic BCL-2 gene expression). The use of antioxidants like AA can minimize the hazardous effects of this chemical substance. However, more research is needed to uncover effective ways to limit AR exposure, whether in the workplace or when cooking starchy meals, as well as to evaluate the histopathological effects of AR on ovarian tissue and follicular developments.
Disclosure
The funders have developed the formulation, conceived the idea, developed the protocol, and prepared the manuscript, but had no role in the execution of the study, sample collection, analysis, and interpretation of data.
Authors’ Contributions
All authors made substantial contributions to the (a) conception or design, acquisition, analysis, and interpretation of data for the work; (b) drafting the article or revising it critically for important intellectual content and (c) final approval of the version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CRediT authorship contribution statement
FF and FZ; Contributed to conception and design. AF, MI, FZZ, MY, MP and NG; Contributed to all experimental work, data and statistical analysis, and interpretation of data. FF and FZ were responsible for overall supervision. AF and FF; Drafted the manuscript, which was revised by AF. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Lawrence Lash
Data Availability
The final analyzed data used to support the findings of this study are included within the article. The raw data would be available from the corresponding author upon request.
References
- 1.Chumnantana R., Yokochi N., Yagi T. Vitamin B6 compounds prevent the death of yeast cells due to menadione, a reactive oxygen generator. Biochim. Biophys. Acta - Gen. Subj. 2005;1722(1):84–91. doi: 10.1016/j.bbagen.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Gupta S., Ghulmiyyah J., Sharma R., Halabi J., Agarwal A. Power of proteomics in linking oxidative stress and female infertility. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/916212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duleba A.J. Medical management of metabolic dysfunction in PCOS. Steroids. 2012;77(4):306–311. doi: 10.1016/j.steroids.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruder E.H., Hartman T.J., Blumberg J., Goldman M.B. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum. Reprod. Update. 2008;14(4):345–357. doi: 10.1093/humupd/dmn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuo T., Zhu M., Xu W. Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxid. Med. Cell. Longev. 2016;2016:8589318. doi: 10.1155/2016/8589318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammadi M.. Oxidative Stress and Polycystic Ovary Syndrome: A Brief Review. Int J Prev Med [Internet]. 2019 May 17;10:86. Available from: https://pubmed.ncbi.nlm.nih.gov/31198521. [DOI] [PMC free article] [PubMed]
- 7.Kermani-Alghoraishi M., Anvari M., Talebi A.R., Amini-Rad O., Ghahramani R., Miresmaili S.M. The effects of acrylamide on sperm parameters and membrane integrity of epididymal spermatozoa in mice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010;153(1):52–55. doi: 10.1016/j.ejogrb.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Mohammed A., Sawicka B., Umachandran K. Food safety of potato processed in the aspect of acrylamide risk. MOJ Food Process. Technol. 2018;6(1):96–102. [Google Scholar]
- 9.Timilsena Y.P., Khanal J.S., Anal A.K. Acrylamide: thermally induced toxicant in foods and its control measures. J. Food Sci. Technol. 2013;6:19–30. [Google Scholar]
- 10.Knight M., McWilliam S., Peck S., Koutsidis G., Chope G., Puddephat I., et al. Kinetic modelling of acrylamide formation during the frying of potato chips. Food Chem. 2021;352 doi: 10.1016/j.foodchem.2021.129305. [DOI] [PubMed] [Google Scholar]
- 11.Rivadeneyra-Domínguez E., Becerra-Contreras Y., Vázquez-Luna A., Díaz-Sobac R., Rodríguez-Landa J.F. Alterations of blood chemistry, hepatic and renal function, and blood cytometry in acrylamide-treated rats. Toxicol. Rep. 2018;5:1124–1128. doi: 10.1016/j.toxrep.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng X., Cheng K.-W., Jiang Y., Lin Z.-X., Shi J.-J., Ou S.-Y., et al. Inhibition of acrylamide formation by vitamins in model reactions and fried potato strips. Food Chem.] 2009;116(1):34–39. 〈https://www.sciencedirect.com/science/article/pii/S0308814609001538〉 (Available from) [Google Scholar]
- 13.Chen J., Guo Q., Pei Y.-H., Ren Q.-L., Chi L., Hu R.-K., et al. Effect of a short-term vitamin E supplementation on oxidative stress in infertile PCOS women under ovulation induction: a retrospective cohort study. BMC Women’s Health. 2020;20(1):69. doi: 10.1186/s12905-020-00930-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sargazi Z., Reza Nikravesh M., Jalali M., Reza Sadeghnia H., Rahimi, Anbarkeh F. The protective effect of vitamin E on rats’ ovarian follicles following an administration of diazinon: an experimental study. Int. J. Reprod. Biomed. 2019;17(2):79–88. doi: 10.18502/ijrm.v17i2.3985. 〈https://pubmed.ncbi.nlm.nih.gov/31435588〉 (Available from) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim E.J., Won R., Sohn J.H., Chung M.A., Nam T.S., Lee H.J., et al. Anti-oxidant effect of ascorbic and dehydroascorbic acids in hippocampal slice culture. Biochem. Biophys. Res. Commun. 2008;366(1):8–14. doi: 10.1016/j.bbrc.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 16.Talley V.H.C., Wicks M.N., Carter M., Roper B. Ascorbic acid does not influence consciousness recovery after anesthesia. Biol. Res. Nurs. 2009;10(3):292–298. doi: 10.1177/1099800408323222. [DOI] [PubMed] [Google Scholar]
- 17.Abramsson-Zetterberg L., Vikström A.C., Törnqvist M., Hellenäs K.-E. Differences in the frequency of micronucleated erythrocytes in humans in relation to consumption of fried carbohydrate-rich food. Mutat. Res. 2008;653(1–2):50–56. doi: 10.1016/j.mrgentox.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Knol J.J., Viklund G.Å.I., Linssen J.P.H., Sjöholm I.M., Skog K.I., van Boekel M.A.J.S. Kinetic modelling: a tool to predict the formation of acrylamide in potato crisps. Food Chem. 2009;113(1):103–109. doi: 10.1016/j.foodchem.2008.07.032. (Available from) [DOI] [PubMed] [Google Scholar]
- 19.Rezvani M.E., Mirgalili A., Dashti-Rahmatabadi M.H., Talebi A.R. Effect of glycyrrhiza glabra and vitamin C on acrylamide-induced motor dysfunction TT - اثر عصاره آبی شیرین بیان و ویتامین C بر اختلالات حرکتی ناشی از آکریل آمید در موش صحرایی. SSU_J. 2014;22(5):1567–1576. 〈http://jssu.ssu.ac.ir/article-1-2890-en.html〉 (Available from) [Google Scholar]
- 20.Phaneuf S., Leeuwenburgh C. Apoptosis and exercise. Med. Sci. Sports Exerc. 2001;33(3):393–396. doi: 10.1097/00005768-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Okunomiya A., Horie A., Tani H., Sato Y., Takamatsu S., Brown J.B., et al. Figla promotes secondary follicle growth in mature mice. Sci. Rep. 2021;11(1):9842. doi: 10.1038/s41598-021-89052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y., Sun J., Wang J., Wang L., Bai Y., Yu M., et al. Characterization of female germ-like cells derived from mouse embryonic stem cells through expression of GFP under the control of Figla promoter. J. Cell. Biochem. 2012;113(4):1111–1121. doi: 10.1002/jcb.24044. [DOI] [PubMed] [Google Scholar]
- 23.Heckert L.L., Daley I.J., Griswold M.D. Structural organization of the follicle-stimulating hormone receptor gene. Mol. Endocrinol. 1992;6(1):70–80. doi: 10.1210/mend.6.1.1738373. [DOI] [PubMed] [Google Scholar]
- 24.Simoni M., Gromoll J., Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr. Rev. 1997;18(6):739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- 25.Edson M.A., Nagaraja A.K., Matzuk M.M. The mammalian ovary from genesis to revelation. Endocr. Rev. 2009;30(6):624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruwanpura S.M., McLachlan R.I., Meachem S.J. Hormonal regulation of male germ cell development. J. Endocrinol. 2010;205(2):117–131. doi: 10.1677/JOE-10-0025. [DOI] [PubMed] [Google Scholar]
- 27.Mao Z., Fan L., Yu Q., Luo S., Wu X., Tang J., et al. Abnormality of klotho signaling is involved in polycystic ovary syndrome. Reprod. Sci. 2018;25(3):372–383. doi: 10.1177/1933719117715129. [DOI] [PubMed] [Google Scholar]
- 28.Anvari M., Talebi A.R., Mangoli E., Shahedi A., Ghasemi M.R., Pourentezari M. Effects of acrylamide in the presence of vitamin E on sperm parameters, chromatin quality, and testosterone levels in mice. Clin. Exp. Reprod. Med. 2020;47(2):101–107. doi: 10.5653/cerm.2019.03230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yousef J.M., Chen G., Hill P.A., Nation R.L., Li J. Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics. J. Antimicrob. Chemother. 2012;67(2):452–459. doi: 10.1093/jac/dkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erdemli Z., Erdemli M.E., Turkoz Y., Gul M., Yigitcan B., Gozukara, Bag H. The effects of acrylamide and Vitamin E administration during pregnancy on adult rats testis. Andrologia. 2019;51(7) doi: 10.1111/and.13292. [DOI] [PubMed] [Google Scholar]
- 31.Yener Y., YerlIkaya F.H. Acrylamide reduces plasma antioxidant vitamin levels in rats due to increased oxidative damage. Rev. Nutr. 2019;32:1–8. [Google Scholar]
- 32.Shokoohi M., Soltani M., Abtahi-Eivary S.H., Niazi V., Poor M., Ravaei H., et al. Effect of hydro-alcoholic extract of Olea europaea on apoptosis-related genes and oxidative stress in a rat model of torsion/detorsion-induced ovarian damage. Asian Pac. J. Reprod. 2019;8(4):148–156. [Google Scholar]
- 33.Chen J.-H., Yang C.-H., Wang Y.-S., Lee J.-G., Cheng C.-H., Chou C.-C. Acrylamide-induced mitochondria collapse and apoptosis in human astrocytoma cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013;51:446–452. doi: 10.1016/j.fct.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 34.Perlman H., Zhang X., Chen M.W., Walsh K., Buttyan R. An elevated bax/bcl-2 ratio corresponds with the onset of prostate epithelial cell apoptosis. Cell Death Differ. 1999;6(1):48–54. doi: 10.1038/sj.cdd.4400453. [DOI] [PubMed] [Google Scholar]
- 35.Ramezani M., Salehnia M., Jafarabadi M. Vitrification and in vitro culture had no adverse effect on the follicular development and gene expression of stimulated human ovarian tissue. J. Obstet. Gynaecol. Res. 2018;44(3):474–487. doi: 10.1111/jog.13530. [DOI] [PubMed] [Google Scholar]
- 36.Eicher-Pruiett S.D., Morrill J.L., Blecha F., Higgins J.J., Anderson N.V., Reddy P.G. Neutrophil and lymphocyte response to supplementation with vitamins C and E in young calves1. J. Dairy Sci. 1992;75(6):1635–1642. doi: 10.3168/jds.S0022-0302(92)77920-X. 〈https://www.sciencedirect.com/science/article/pii/S002203029277920X〉 (Available from) [DOI] [PubMed] [Google Scholar]
- 37.Nsonga A., Kang’ombe J., Mfitilodze W.M., Soko C.K., Mtethiwa A. Effect of varying levels of dietary vitamin C (ascorbic acid) on growth, survival and hematology of juvenile tilapia, Oreochromis karongae (Trewavas 1941) reared in aquaria. Braz. J. Aquat. Sci. Technol. 2009;13:17–23. [Google Scholar]
- 38.Jeng K.C., Yang C.S., Siu W.Y., Tsai Y.S., Liao W.J., Kuo J.S. Supplementation with vitamins C and E enhances cytokine production by peripheral blood mononuclear cells in healthy adults. Am. J. Clin. Nutr. 1996;64(6):960–965. doi: 10.1093/ajcn/64.6.960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The final analyzed data used to support the findings of this study are included within the article. The raw data would be available from the corresponding author upon request.