Abstract
Damnacanthal is an anthraquinone, extracted, and purified from the root of Morinda citrifolia in Thailand. This study aimed to measure acute oral toxicity and to investigate the anticancer activity of damnacanthal in colorectal tumorigenesis. We found that the growth of human colorectal cancer cells was inhibited by damnacanthal in a dose- and a time-dependent manner. The growth inhibitory effect of damnacanthal was better than that of 5-FU used as a positive control in colorectal cancer cells, along with the downregulation of cell cycle protein cyclin D1. Similarly, an oral treatment of damnacanthal effectively inhibited the growth of colorectal tumor xenografts in nude mice, which was approximately 2–3-fold higher as compared to 5-FU by tumor size as well as expression of bioluminescence. Furthermore, the study of acute oral toxicity in mice exhibited a relatively low toxicity of damnacanthal with a LD50 cut-off value of 2500 mg/kg according to OECD Guideline 423. These results reveal the potential therapeutic activity of a natural damnacanthal compound as an anti-colorectal cancer drug.
Abbreviations: DMSO, Dimethyl sulfoxide; MTT, 3-(4,5-Dimethythiazol-2-yl)− 2,5-diphenyltetrazolium bromide; FBS, Fetal bovine serum; DPBS, Dulbecco’s phosphate buffered saline; 5-FU, 5-Fluorouracil; PMSF, Phenylmethanesulfonyl fluoride; BSA, Bovine serum albumin; VLC, Vacuum liquid chromatographic method; TLC, Thin layer chromatography; FTIR, Fourier transform infrared spectroscopy; MS, Mass spectrometry; NMR, Nuclear magnetic resonance spectroscopy; IC50, Half-maximal inhibitory concentration; TBST, Tris-buffered saline containing 0.05 % Tween 20; LD50, Median lethal dose; NC, Negative control; F20, 5-Fluorouracil at 20 mg/kg; D20, Damnacanthal at 20 mg/kg; D40, Damnacanthal at 40 mg/kg; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; BUN, Blood urea nitrogen
Keywords: Damnacanthal, Acute oral toxicity, Anticancer activity, Colorectal tumorigenesis
Graphical Abstract
Highlights
-
•
Acute oral toxicity of damnacanthal in mice showed a LD50 value of 2500 mg/kg.
-
•
Damnacanthal exhibited a better tumor growth inhibition compared with NC and 5-FU.
-
•
Bioluminescence imaging confirmed damnacanthal’s efficacy in tumor xenograft model.
1. Introduction
Cancer is an important cause of death worldwide. In 2020, colorectal cancer is the third most common cancer in terms of new cases and the second leading cause of cancer death [1]. The percentage of cumulative risk to develop colorectal cancer in males (2.71 %) is higher than in females (1.83 %), and this sexual dimorphism is a key factor for the initiation and progression of colorectal cancer [2]. The lower incident rate in women is probably caused by estrogen-regulated genes and cell signaling, which are protective pathways in colorectal cancer development. Colorectal cancer treatment includes surgery, radiation therapy, chemotherapy, targeted therapy, and immunotherapy [3]. Although chemotherapy is one of the most common cancer treatments that use medicines or drugs to kill cancerous cells, side effects and drug resistance associated with chemotherapeutic drugs prompt us to discover effective agents for colorectal cancer treatment derived from natural products including alkaloids, polysaccharides, polyphenols, diterpenoids, and unsaturated fatty acids [4].
Morinda citrifolia L., which is generally known as noni, is a tropical and subtropical tree that grows in Southeast Asia, Australasia, and the pacific islands. All parts of this tree including fruits, leaves, barks, and roots have been well-known to contain various phytochemical constituents to treat common diseases and to maintain good health [5]. Damnacanthal is an anthraquinone compound isolated from the root of Morinda citrifolia L. It is a promising natural compound with various pharmacological properties such as antioxidant [6], antinociceptive, anti-inflammatory [7], [8], cytotoxic, immunomodulatory [9], antiviral, antimicrobial [10], antibacterial [11], and antifungal activities [12]. In addition, several studies have demonstrated the anticancer activity of damnacanthal in many cancer cell lines such as colorectal [13], breast [14], hepatocellular [15], oral squamous [16], T-lymphoblastic leukemia [17], and ovarian cancer cells [18]. Damnacanthal exerts anticancer effects through (1) inhibiting cell migration, the Ras oncogene, and the p56lck tyrosine kinase, (2) inducing apoptosis, and (3) interfering cell cycle as well as (4) possessing photodynamic action by generating reactive oxygen species (ROS) [19].
In this study, we evaluated, for the first time, acute oral toxicity of damnacanthal in mice. Further, the anticancer activity of damnacanthal against colorectal cancer was investigated both in cell culture and in tumor xenograft. Our data clearly demonstrated to provide a promising therapeutic drug of damnacanthal with a low toxicity in colon cancer.
2. Materials and methods
2.1. Chemicals and reagents
Dichloromethane and dimethyl sulfoxide (DMSO) were purchased from ACI Labscan (Bangkok, Thailand). 3-(4,5-Dimethythiazol-2-yl)− 2,5-diphenyltetrazolium bromide (MTT) was bought from Invitrogen (Eugene, OR, USA). McCoy's 5A medium was purchased from Corning Inc. (Corning, NY, USA). Fetal bovine serum (FBS), Dulbecco’s phosphate-buffered saline (DPBS), and trypsin–EDTA were purchased from Gibco (Carlsbad, CA, USA). Dulbecco's Modified Eagle Medium (DMEM) was purchased from Thermo Fisher Scientific (Waltham, MA USA). 5-Fluorouracil (5-FU) and phenylmethanesulfonyl fluoride (PMSF) were obtained from Sigma–Aldrich (St. Louis, MO, USA) and bovine serum albumin (BSA) was obtained from Merck (Darmstadt, Germany). D-luciferin potassium salt was purchased from PerkinElmer (Boston, MA, USA). RIPA buffer and a protease inhibitor cocktail were bought from Cell Signaling Technology (Beverly, MA, USA). A phosphatase inhibitor cocktail was obtained from Boster Biological Technology (Pleasanton, CA, USA). Mouse monoclonal cyclin D1 (A-12) antibody and mouse monoclonal β-actin (C4) antibody conjugated to horseradish peroxidase (HRP) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and goat anti-mouse IgG H&L conjugated to HRP was purchased from Abcam (Cambridge, UK).
2.2. Damnacanthal
Damnacanthal was extracted from the roots of Morinda citrifolia L. in Thailand by maceration with dichloromethane for 6 days at room temperature and then isolated by vacuum liquid chromatographic method (VLC). The purification of damnacanthal was carried out by column chromatography and crystallization techniques. The purified damnacanthal was identified by various methods such as thin layer chromatography (TLC), Fourier transform infrared spectroscopy (FTIR), mass spectrometry (MS), and nuclear magnetic resonance spectroscopy (NMR) as previously described [8]. The purified damnacanthal was stored at − 20 °C until use in different studies.
2.3. Cell culture
Human colorectal cancer HCT116-Red-FLuc cell line, which is stably transfected with firefly luciferase gene from Luciola Italica (Red-FLuc), and Caco-2 cell line were purchased from PerkinElmer (Waltham, MA, USA) and American Type Culture Collection (ATCC, Manassas, VA, USA), respectively. HCT116-Red-FLuc cells were cultured in McCoy's 5A medium supplemented with 10 % FBS. Caco-2 cells were maintained in DMEM medium supplemented with 20% FBS and 1% penicillin-streptomycin solution. Both cells were incubated in a humidified 5 % CO2 atmosphere at 37 °C in 75-cm2 culture flasks.
2.4. Cytotoxicity assay
HCT116-Red-FLuc cells were seeded into 96-well culture plates at a cell density of 15,000 cells/well and incubated for 24 h, while Caco-2 cells were plated at a density of 10,000 cells/well and incubated for 48 h. After incubation, the cells were treated either with DMSO alone (solvent) used as a control or with different concentrations (1.95–500 μM) of damnacanthal or 5-FU in DMSO for 24, 48, and 72 h. The final DMSO concentration in each well was ≤ 1 %. At the indicated time points, the cytotoxicity was analyzed by 3-(4,5-dimethythiazol-2-yl)− 2,5-diphenyltetrazolium bromide (MTT) assay. Briefly, 20 µL of MTT solution (5 mg/mL) was added to each well and incubated for 3 h in a humidified 5 % CO2 atmosphere at 37 °C. The whole medium was carefully removed and then 100 µL of DMSO was added to each well to solubilize formazan crystals. The absorbance of each well was measured at 590 nm with a microplate reader spectrophotometer (Thermo Fisher Scientific, Vantaa, Finland). The results were expressed as the percentage of cell viability relative to the control. All determinations were done as three independent experiments in triplicate. The half-maximal inhibitory concentration (IC50) of damnacanthal and 5-FU on HCT116-Red-FLuc cancer cells was analyzed by using OriginPro 8 software (OriginLab Corporation, Northampton, MA, USA).
2.5. Western blot analysis for in vitro protein expression
HCT116-Red-FLuc and Caco-2 cells were cultured in 25-cm2 culture flasks or 6-well culture plates until the confluence of 60–70 %. Both cells were treated with 1 % DMSO (negative control) or different concentrations of damnacanthal (1, 10, 25, and 50 µM) or 5-FU in DMSO (50 µM) for 24 h. At the end of each treatment, the cells were washed with DPBS and then harvested in RIPA buffer supplemented with 1 mM PMSF, protease inhibitor cocktail, and phosphatase inhibitor cocktail. The cell lysates were briefly sonicated and then centrifuged at 14,000× g for 15 min at 4 °C. Supernatant was collected and protein concentration was measured by BCA protein assay (Thermo Scientific, Rockford, IL, USA) using BSA as a standard. The proteins (∼30 µg) were separated by 12 % SDS-PAGE followed by transferring them onto a nitrocellulose membrane (GE Healthcare Life Sciences, Marlborough, MA, USA). Precision Plus Protein™ Kaleidoscope™ Prestained protein standards were used as markers. The membranes were blocked with 5 % skim milk in Tris-buffered saline containing 0.05 % Tween 20 (TBST) for 1 h at room temperature and then incubated with the primary antibody cyclin D1 (1:1000) overnight at 4 °C. After three washes with TBST, the membranes were probed with the HRP-conjugated secondary antibody (1:5000) for 1 h at room temperature followed by washing three times with TBST. For loading control, the membranes were incubated with β-actin conjugated to HRP (1:5000) for 1 h at room temperature. The bands were detected by chemiluminescence using Western ECL Substrate (Bio-Rad, Hercules, CA, USA), visualized by UVP ChemStudio imaging systems (Analytik Jena, Upland, CA, USA), and quantified using VisionWorks analysis software (Analytik Jena, Upland, CA, USA).
2.6. Animals, housing, diet, and water
All animal experiments were conducted under Ethical Principles and Guidelines for the Use of Animals, National Research Council of Thailand and approved by the Animal Care and Use Committee of Thammasat University (Ethic number: 006/2560). Animals were purchased from Nomura Siam International Company Limited (Bangkok, Thailand) and acclimatized for 7 days before starting experiment.
In an acute oral toxicity study, 9–10 weeks old male Jcl:ICR mice were housed in plastic cages. The conditions of laboratory room were controlled at a temperature of 22 ± 2 °C and relative humidity of 30–70 % with a 12-h light/darkness cycle. Standard pellet diet and water were provided ad libitum.
In the anticancer study, 6–8 weeks old male athymic BALB/cAJcl-nu/nu nude mice have housed in individually ventilated cages (IVC) under specific pathogen-free conditions. They were fed sterile diet and sterile water ad libitum.
For in vivo tumor xenograft, male nude mice were used because sex hormone may affect development of colorectal cancer and the incident rate of colorectal cancer was high in males. For this reason, acute oral toxicity was also studied in male mice.
2.7. Acute oral toxicity
In vivo toxicity of damnacanthal was investigated by the acute toxic class method according to OECD Guideline 423, which is a stepwise procedure with the use of three animals of the same-sex per step [20]. Approximately 2–4 steps may be necessary to define the acute toxicity of the test substance. Because there is no information about in vivo toxicity test of damnacanthal, the starting dose of 300 mg/kg was used in this study. Mice were divided into 3 groups of 3 animals each, except for a control group having one animal. Group I (control group) received 10% DMSO/Tween80 in DPBS (vehicle). Groups II and III received damnacanthal at doses of 300 and 2000 mg/kg (next higher dose level), respectively. All test substances were prepared in 10 % DMSO/Tween80 in DPBS and administered as a single dose by oral gavage after the animals were fasted for 2–3 h. After dosing, the animals were fasted further for 1–2 h. Sign and behavior including changes in skin and fur, eyes, mucous membranes, respiratory, tremors, convulsions, salivation, diarrhea, lethargy, sleep, coma, and death were observed according to OECD Guideline 423 [20]. Toxic signs, time of onset, length of recovery period, and mortality were observed after treatment for the first 4 h, and then daily thereafter for a total period of 14 days. The body weights of the animals were monitored every week. At the end of experiment, all survival animals were euthanized by carbon dioxide inhalation followed by cervical dislocation method and then subjected to gloss necropsy. Liver and kidneys were collected for histopathological examination. In brief, the tissues were preserved in 10 % neutral buffered formalin, trimmed, and embedded in paraffin followed by cutting the sections into 5 µm thickness. The sections were stained with haematoxylin and eosin (H&E) and evaluated histopathological lesions under a light microscope. The severity of the lesion was classified into 4 levels as follows: minimal (1–2 foci), mild (3–6 foci), moderate (many foci), and severe (coalescing to diffuse lesion) based on an increasing extent and/or complexity of abnormal change in the tissue.
2.8. Nude mice experiment
To investigate in vivo anticancer activity of damnacanthal against human colorectal tumor xenograft, 5 × 105 HCT116-Red-FLuc cells suspended in 100 µL of McCoy's 5A medium without FBS were subcutaneously injected into the right flank of each nude mouse (a total of 24 nude mice). Tumor sizes were measured twice weekly by a digital caliper. Tumor volumes were calculated by the formula: 1/2 × length × width2 [21], [22]. When the tumor volume reached approximately 50 mm3, the nude mice were randomly divided into 4 groups of 6 animals each. Group I received vehicle only (10 % DMSO/Tween80 in DPBS) served as a negative control (NC). Group II receive 5-FU at 20 mg/kg (F20). Group III and IV received damnacanthal at 20 mg/kg (D20) and 40 mg/kg (D40), respectively. All test substances were prepared in 10 % DMSO/Tween80 in DPBS and orally administered via gavages to tumor-bearing nude mice every 2 days for 26 days. The tumor volumes and body weights of the nude mice were monitored twice a week. Additionally, the nude mice were imaged once a week by in vivo bioluminescence imaging as described below. At the end of experiment, blood samples were collected for biochemistry evaluation including aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine. All animals were sacrificed by isoflurane overdose followed by cervical dislocation method. Tumor, lungs, liver, kidneys, and intestine were isolated and weighed. Tumor samples were stored at − 80 °C for further western blot analysis. In addition, samples of liver and kidneys were collected for histopathological examination by H&E staining and evaluated histopathological lesions under a light microscope.
2.9. Bioluminescence imaging
To verify whether the luminescence intensity correlated with the number of HCT116-Red-FLuc cells stably expressing firefly luciferase, the cells were seeded in 96-well plates at a cell number of 49–100,000 cells/well in duplicate. An equal volume of D-luciferin solution in DPBS (300 µg/mL) was added to each well and then incubated at 37 °C for 10 min prior to imaging. Bioluminescence imaging was performed using an In Vivo Imaging System MS FX PRO (Carestream Health Inc., Rochester, NY, USA) according to the instructions of the manufacturer. The parameters were used for imaging as follows: exposure time of 2 min, f-stop of 2.50, field of view (FOV) of 150 mm, and X-Y binning of 4 × 4.
For in vivo imaging, the tumor-bearing nude mice were intraperitoneally injected with D-luciferin solution in DPBS at a dose of 150 mg/kg. After injection for 10–15 min, the nude mice were anesthetized with isoflurane and then placed onto a tray inside of the imaging cabinet with a continuous exposure to the isoflurane. Two to three nude mice were imaged with the same imaging parameters except for the exposure time of 1 min. For ex vivo imaging, the nude mice were intraperitoneally injected with 150 mg/kg of D-luciferin solution in DPBS prior to euthanasia. Tumors were immediately removed and then placed into 12-well or 24-well culture plates depending on the size of each organ. After adding D-luciferin solution in DPBS (300 µg/mL) to cover the organs, the imaging was performed with the same imaging parameters as above. The bioluminescence signals were visually displayed as color images that were overlayed on the white light images. The luminescence intensity was quantified as photon/sec by using Bruker Molecular Imaging software version 7.1.3.20550 (Bruker, Billerica, MA, USA).
2.10. Western blot analysis for in vivo protein expression
Tumor samples were ground in the presence of liquid nitrogen and immediately homogenized in RIPA buffer containing 1 mM PMSF, protease inhibitor cocktail, and phosphatase inhibitor cocktail. The tissue lysates were sonicated briefly and then centrifuged at 14,000g for 20 min at 4 °C. The supernatant was collected and centrifuged again. Total protein concentration was determined by BCA method. The Western immunoblotting was performed according to the Section 2.5 except for the dilutions of primary antibodies that were used as 1:500 for cyclin D1 and 1:8000 for β-actin.
2.11. Statistical analysis
Results are expressed as the mean ± standard deviation (SD). All comparisons were analyzed by the unpaired Student’s t test and one-way analysis of variance (ANOVA) using IBM SPSS Statistics 20. To compare the significance of differences between two groups, the post hoc Tukey’s and Dunnett’s T3 pairwise comparison tests were used when the variances were equal and unequal, respectively. The values of p < 0.05 were considered statistically significant.
3. Results and discussion
3.1. Effect of damnacanthal on cell growth
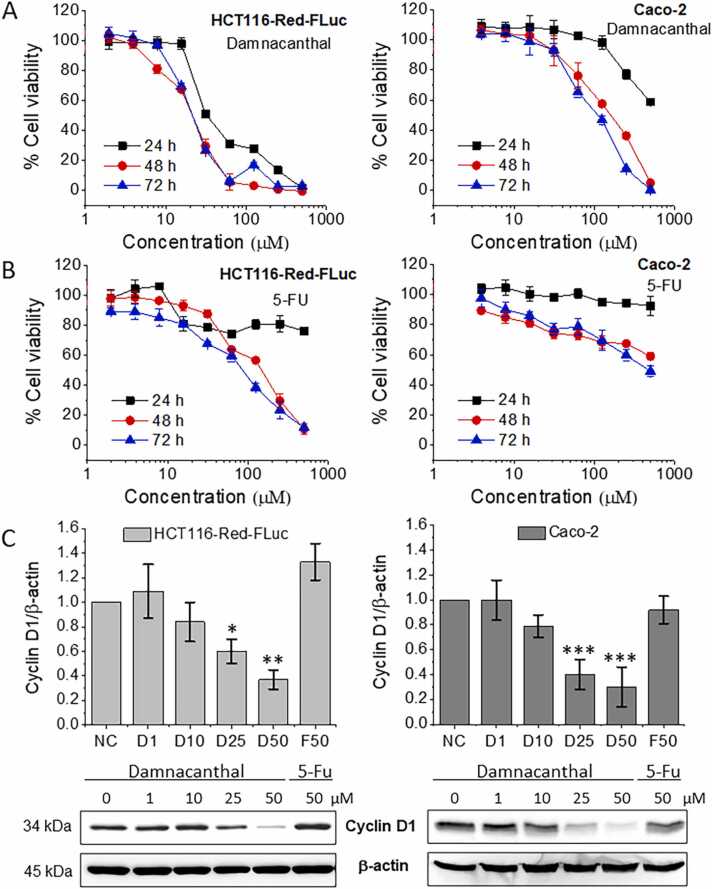
To investigate the cytotoxicity of damnacanthal, human colorectal cancer cells (HCT116-Red-FLuc and Caco-2) were treated with various concentrations of damnacanthal for 24, 48, and 72 h, and cell viability was measured by MTT assay. In this study, 5-FU was used as a positive control because it was one of the first-line treatments for patients with colorectal cancer [23]. As shown in Fig. 1A and B, the cell growth was inhibited by both damnacanthal and 5-FU in a dose- and a time-dependent manner. The cell growth inhibition of damnacanthal was apparently faster and better than that of 5-FU in both cancer cells as exhibited by ≤ 60 % of cell viability after 24 h of treatment with damnacanthal (31.25–500 µM for HCT116-Red-FLuc cells and 500 µM for Caco-2 cells), whereas this effect was not observed in 5-FU treatment. Damnacanthal had IC50 values of 29.38 ± 3.31, 21.02 ± 2.21, and 19.14 ± 0.71 µM after treatment with HCT116-Red-FLuc cells for 24, 48, and 72 h, respectively (Table 1). The IC50 values of damnacanthal were significantly lower than 5-FU during all treatment periods (p < 0.001), suggesting that the inhibitory effect of damnacanthal on cancer cell growth had stronger than that of 5-FU. This effect may be a result from a chemoresistance of both HCT116-Red-FLuc and Caco-2 cells. A degree of 5-FU resistance to HCT116 cells was approximately intermediate in comparison to other colon cancer cells such as LS174T, LoVo, TC7, SW480, LS513, and HCT-EB [24], while Caco-2 cells with a mutant p53 showed the most resistance to 5-FU when compared with colorectal cancer RKO, HT-29, and DLD1 cells [25]. It has been reported that damnacanthal had cytotoxic effect both in p53-wild type (HCT116) and p53-mutated (SW480) colorectal cancer cells [13]. Thus, it was considered that damnacanthal affects many cancer cells for the cell growth retardation regardless genetic background of the cells.
Fig. 1.
Effect of damnacanthal on cell viability and protein expression in colorectal cancer cells. (A) HCT116-Red-FLuc (left panel) and Caco-2 cells (right panel) were treated with ≤ 1 % DMSO or different concentrations of damnacanthal or (B) 5-FU in DMSO for 24, 48, and 72 h. The cell viability was measured by MTT assay as described in the method section. Data represent the mean ± SD of triplicate determinations from three independent experiments. (C) For cyclin D1 protein expression, HCT116-Red-FLuc (left panel) and Caco-2 cells (right panel) were incubated with 1 % DMSO (negative control, NC) or various concentrations of damnacanthal (1, 10, 25, and 50 µM) or 5-FU (50 µM) for 24 h. The treated cells were harvested and total cell lysates were subjected to Western blot analysis. β-actin was used as the loading control. Graphs display the relative intensities of cyclin D1 normalized against β-actin (top panel) and the data are presented as the mean ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 indicate significant difference from NC. The representative Western blots show cyclin D1 and β-actin expression (bottom panel).
Table 1.
The IC50 values of damnacanthal and 5-FU in HCT116-Red-FLuc cells after 24, 48, and 72 h of treatment.
| Time after treatment | IC50 values (µM) |
|
|---|---|---|
| Damnacanthal | 5-FU | |
| 24 h | 29.38 ± 3.31*** | > 500 |
| 48 h | 21.02 ± 2.21*** | 186.59 ± 9.02 |
| 72 h | 19.14 ± 0.71*** | 113.49 ± 5.39 |
Values represent the mean ± SD of triplicate determinations from three independent experiments.
Significant different from 5-FU (p < 0.001).
3.2. Effect of damnacanthal on cyclin D1 protein expression
Cyclin D1 plays an important role in the regulation of cell cycle progression and cell proliferation in many types of cancer cells [26]. In this study, HCT116-Red-FLuc and Caco-2 cells were treated with 1, 10, 25, and 50 µM of damnacanthal as well as 50 µM of 5-FU for 24 h to investigate cyclin D1 protein expression by Western blot analysis. As shown in Fig. 1C, the expression of cyclin D1 in both cell lines was significantly reduced by damnacanthal at concentrations of 25 and 50 µM (p < 0.05) as compared to DMSO-treated cells (negative control, NC). The effect of damnacanthal on cyclin D1 expression was in a dose-dependent manner, which was correlated with the cell growth inhibition of damnacanthal in Fig. 1A. It is suggested that damnacanthal induced a down-regulation of cyclin D1 protein expression, leading to the suppression of cell proliferation in both cell lines. While cyclin D1 was increased in HCT116-Red-FLuc cells or slightly decreased in Caco-2 cells treated with 50 µM of 5-FU when compared with the NC (p > 0.05), possibly due to the resistance of both HCT116-Red-FLuc and Caco-2 cells to 5-FU. This result was in accordance with a study of Liu et al. that reported an increase of cyclin D1 level in 5-FU-resistant HCT116 cells when compared with the parent HCT116 cells [27]. In a previous study, cyclin D1 was degraded by damnacanthal at the post-translational level via proteasomal-dependent pathway, with the ubiquitination at the lysine sites of cyclin D1 [28]. Since anticancer agents can induce down-regulation and/or degradation of cyclin D1 expression, our data suggested that damnacanthal could be an effective compound for chemoprevention and treatment of cancer. However, there are several reports indicating that anticancer activity of damnacanthal is mediated by many molecular target proteins such as the induction of pro-apoptotic protein nonsteroidal anti-inflammatory activated gene-1 (NAG-1) and CCAAT enhancer binding protein beta (C/EBPβ) expression [13] and the suppression of chromosome maintenance protein 1 (CRM1) expression [29]. Taken together, such molecular mechanisms of damnacanthal may contribute to the anticancer activity of damnacanthal in human colorectal cancer.
3.3. Acute oral toxicity of damnacanthal
Because in vivo toxicology data for damnacanthal has never been reported, the acute oral toxicity of damnacanthal in mice was investigated. This study was performed using a stepwise procedure with the starting dose of 300 mg/kg according to the animal welfare reasons of OECD Guideline 423 [20]. We found that there was no mortality of the animals received damnacanthal at 300 mg/kg, the next step was also tested with a higher dose level (2000 mg/kg). Body weights and clinical observations in mice that orally received damnacanthal at doses of 300 and 2000 mg/kg are summarized in Table 2. A control group received only vehicle (10 % DMSO/Tween80 in DPBS) showed no signs of toxic effects and behavioral changes including changes in the skin, fur, eyes, mucous membrane, tremors, salivation, diarrhea, lethargy, and coma, whereas some treated mice with damnacanthal exhibited diarrhea or dehydration. After dosing with 300 mg/kg of damnacanthal, mild watery diarrhea was observed in 2 mice (out of 6 mice) at approximately 19–26 h and disappeared within 29–46 h after the onset time. The remaining mice showed no toxic symptoms similar to the control group. While 5 mice (out of 6 mice) had moderate watery diarrhea and one mouse had severe dehydration following 19–24.5 h of treatment with 2000 mg/kg of damnacanthal. Most of the mice recovered from moderate watery diarrhea within 18–48 h after appearing the symptom except that one mouse took 5 days until diarrhea disappeared. Additionally, one mouse (out of 6 mice) was dead after 42.5 h of dosing with the symptom of severe dehydration and subsequent body weight loss (13.38 %), eyes slightly open, slow breathing, pale pink mucus membrane, tremor, and lethargy. However, the mean body weights of all survival mice were not statistically different throughout the 14 days of the acute oral toxicity study (p > 0.05). These results exhibited that damnacanthal had a relatively low acute oral toxicity, which was classified as GHS category 5 with the median lethal dose (LD50) cut-off value of 2500 mg/kg according to OECD Guideline 423 [20].
Table 2.
Body weights and clinical observations in mice treated with damnacanthal during acute oral toxicity study.
| Group | Dose (mg/kg) | No. dosed | Body weight (g)a |
Symptom observation (no. animal) | No. death | ||
|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 14 | |||||
| I | Control | 1 | 39.50 | 39.12 | 40.30 | Not found | 0 |
| II | 300 | 3 + 3 | 38.04 ± 2.12 | 37.72 ± 2.38 | 38.63 ± 3.30 | Mild watery diarrhea (n = 2) | 0 |
| III | 2000 | 3 + 3 | 38.04 ± 1.95 | 38.92 ± 4.03 | 40.35 ± 3.69 | Moderate watery diarrhea (n = 5) | 1 |
| Severe dehydration (n = 1) | |||||||
Values are the mean ± SD (n = 5–6) except for a control group.
Histopathological changes were observed in the liver and kidney of some survival mice treated with 2000 mg/kg of damnacanthal. As shown in Fig. 2, the moderate midzonal hepatocellular necrosis with the retention of cellular architecture, mild hemorrhage, and mild inflammatory cell infiltration was found in the liver of one survival mouse, whereas the minimal to mild periportal inflammation was observed in the liver of 2 survival mice (data not shown). Furthermore, there was minimally and focally single-cell necrosis in renal tubules of one survival mouse (Fig. 2). This is the first study that showed the toxic effects of damnacanthal by a single-dose oral administration in mice. The diarrhea was predominantly an acute toxic sign of damnacanthal, which was a dose-dependent manner. Since anthraquinone compounds have been widely used as stimulative laxatives such as senna anthraquinones and rhubarb anthraquinones [30], the toxic effect may be caused by the anthraquinone properties of damnacanthal. It also has been reported that the purgative effect may be due to the presence of hydroxyl groups at the position of 1 and 8 (or 9) of the anthracene ring (a tricyclic aromatic ring) [31]. After digestion of anthraquinone compounds, their unabsorbed parts are moved to the large intestine and then metabolized into the active aglycones by intestinal bacterial flora [32]. The metabolites affect the increment of transportation of electrolytes into the colonic lumen and intestinal motility through stimulation of myenteric plexuses [33]. The inhibition of chloride ion transportation through colon cells is a major target of the laxative action. Additionally, the metabolites can damage the intestinal epithelial cells resulting in alterations of absorption, secretion, and motility of the intestine [34]. Taking all results into account, damnacanthal would be rather safe and useful for therapeutic applications.
Fig. 2.
Histopathological examination of liver and kidney sections of mice. Mice were orally administered with vehicle (control) or damnacanthal (300 and 2000 mg/kg). Liver and kidney were excised and stained with H&E. Multifocal areas of coagulative hepatocellular necrosis with the retention of cellular architecture and loss of cellular detail, scattered inflammatory cells, and occasionally mild hemorrhage (asterisks) were present in the midzonal regions of the liver from a mouse treated with 2000 mg/kg of damnacanthal (magnification ×10). There were a few single-cell necrosis in the renal tubules (arrows) of a mouse treated with 2000 mg/kg of damnacanthal (magnification ×40). Others did not find the histopathological changes (magnification ×10).
3.4. Anticancer activity of damnacanthal in tumor xenograft model
In this experiment, human colorectal cancer HCT116-Red-FLuc cells, stably expressed firefly luciferase gene were subcutaneously injected into the right flank of nude mice. The nude mice were orally administered with vehicle (negative control, NC), 5-FU at 20 mg/kg (F20), and damnacanthal at 20 mg/kg (D20) or 40 mg/kg (D40) every 2 days for 26 days. Tumor growth was assessed by tumor volume from a caliper measurement as well as firefly luciferase expression using in vivo bioluminescence imaging system. According to the measurement of tumor volume, the tumor growth rate of damnacanthal (D20 and D40) and 5-FU (F20) treatments was slower than that of the NC group as shown in Fig. 3A. However, the similar tumor growth rates of both D20 and D40 groups were highly slower as compared to F20 group. After 26 days of treatment, the final tumor volume of the D20, D40, and F20 groups was 314 ± 119 mm3, 266 ± 104 mm3, and 699 ± 84 mm3, respectively, which was significantly smaller than that of the NC group (1277 ± 209 mm3, p < 0.001). Both doses of damnacanthal treatments demonstrated similar tumor volumes (p > 0.05), which were considerably 4–5-fold and 2–3-fold smaller when compared with the NC (p < 0.001) and 5-FU treatment (p < 0.01), respectively.
Fig. 3.
Growth of tumor xenografts in nude mice after oral administration of damnacanthal. Nude mice bearing tumor xenografts established by subcutaneous injection of HCT116-Red-FLuc cells stably expressing firefly luciferase were randomly divided into four groups. Animals received vehicle only served as a negative control (NC); treated animals received 5-FU at 20 mg/kg (F20) or damnacanthal at 20 mg/kg (D20) or 40 mg/kg (D40) by orally gavages every 2 days for 26 days. (A) Tumor volume measured by a digital caliper, (B) luminescence intensity and (C) the representative luminescence images of tumor xenografts in nude mice using in vivo bioluminescence imaging, and (D) body weight of tumor-bearing nude mice during 26 days of treatment. The data presented are the mean ± SD (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001 indicate significant difference from NC group. #p < 0.05, ##p < 0.01 indicate significant difference from F20 group.
In addition to the classical caliper measurement, the growth of tumor xenografts in nude mice was also monitored by bioluminescence imaging technique. Bioluminescence intensity of the serially diluted HCT116-Red-FLuc cancer cells were varied in color from blue to red, which was highly correlated with the increase in the cell number (R2 = 0.9965) as shown in Supplementary material: Fig. S1. Interestingly, the initially significant reduction of the tumor volume was observed after the mice received all treatments for 6 days as compared to the NC group (Fig. 3A), whereas the bioluminescence intensity of both NC and treatment groups at the same period was not different (Fig. 3B). This result suggested that all groups had similar numbers of cancer cells despite the different tumor sizes. It is important to note that the smaller tumor size/volume does not always mean the treatment response decreases or inhibits the number of cancer cells. Furthermore, the luminescence intensity of tumor xenograft also represents the functional cancer cells through the expression of firefly luciferase and reaction with a luciferin substrate to emit visible light from living cells [35]. For this reason, in vivo bioluminescence imaging is an effective technique for evaluating or confirming tumor burden and treatment response of anticancer agents in tumor xenograft models [36]. As illustrated in Fig. 3B, bioluminescence data revealed that tumor growth of all treatment groups was much slower than that of the NC group after treatment for 12–26 days. The final luminescence intensity of tumor-bearing nude mice in the NC group (8.84 × 109 ± 1.90 × 109 photon/sec) was greatly higher (p < 0.001) when compared with that in F20 (4.89 × 109 ± 1.25 × 109 photon/sec), D20 (2.10 × 109 ± 9.32 × 108 photon/sec), and D40 groups (1.90 × 109 ± 9.71 × 108 photon/sec), following treatment for 26 days (Fig. 3B and C). The luminescence intensities of both D20 and D40 groups were not significantly different (p > 0.05), indicating that they had a similar tumor growth retardation. Their bioluminescence signals were significantly 4–5-fold and 2–3-fold lower than the NC (p < 0.001) and F20 groups (p < 0.01), respectively, which was in accordance with the result of tumor volume measurement. It indicated that anticancer activities of both D20 and D40 treatments were highly effective than F20 treatment. However, on day 18 of treatment, the D20 group exhibited a faster and stronger anticancer effect than the D40 group because it had significantly decreased by 2.7-fold the luminescence intensity of tumor xenografts when compared with F20 (p < 0.05), while the luminescence intensities of D40 and F20 groups were not significantly different.
Both assessments of tumor volumes and bioluminescence signals in tumor-bearing nude mice on day 26 of treatment showed that approximately 75 % tumor growth inhibition was observed in both D20 and D40 groups, whereas about 45 % inhibition was found in the F20 group in comparison to the NC group. These findings indicated that in vivo anticancer activity of damnacanthal was dramatically higher than that of 5-FU, which was in line with in vitro observations on the inhibition of colorectal cancer cell growth. During the treatment period of 26 days, the body weights of all treatment groups were not significantly different from those of the NC group (Fig. 3D). Additionally, there were no toxic symptoms and behavioral changes among different groups. To confirm the anticancer activity of damnacanthal, the isolated tumors from the nude mice at the end of the experiment were weighed and measured the size using the caliper as well as the firefly luciferase expression using ex vivo bioluminescence imaging. Fig. 4A–C exhibit that the volumes and bioluminescence intensities of the isolated tumors were not significantly different (p > 0.05) between the D20 group (248 ± 112 mm3 and 2.36 × 109 ± 5.47 × 108 photon/sec) and D40 group (216 ± 53 mm3 and 2.54 × 109 ± 5.22 × 108 photon/sec). Similarly, both damnacanthal treatments greatly inhibited the tumor growth by approximately 4–5-fold and 2–3-fold compared with the NC (1204 ± 213 mm3 and 8.88 × 109 ± 1.91 × 109 photon/sec, p < 0.001) and 5-FU treatment (580 ± 128 mm3 and 4.88 × 109 ± 1.43 × 109 photon/sec, p < 0.05), respectively, as clearly indicated by a potent decrease of tumor volumes as well as luciferase activities in the isolated tumors. As presented in Fig. 4D, although 5-FU treatment significantly reduced the tumor weight by 1.6-fold (0.67 ± 0.14 g, p < 0.01), the stronger reduction in tumor weight (about 3.5-fold) was observed in damnacanthal treatments (0.30 ± 0.14 g for D20 and 0.32 ± 0.08 g for D40, p < 0.001) when compared with the NC group (1.08 ± 0.22 g). The weights of other organs such as lungs, liver, kidneys, and intestine were not significantly different between the NC group and all treatment groups (Fig. 4D, p > 0.05). Moreover, the damnacanthal treatments induced a slight decrease in cyclin D1 protein expression in the tumor tissues as compared to the NC group by using Western blot assays (p > 0.05, Fig. 4E and F). It could be possible that the doses of damnacanthal at 20 and 40 mg/kg were not sufficient for a significant decrease in the protein level of cyclin D1 in tumor tissues. In another way, the oral administration of damnacanthal could be metabolized by nude mice, which could result in a generation of bioactive compounds. These metabolites could enhance the anticancer activity in tumor-bearing nude mice without affecting the cyclin D1 down-regulation. The protein level of cyclin D1 in the F20 group was not decreased when compared with the NC group (p > 0.05), which may be caused by the resistance of HCT116-Red-FLuc tumor xenograft to 5-FU. The serum biochemistry of the NC group including AST, ALT, BUN, and creatinine was not significantly different from that of all treatment groups (Table 3). The result of serum biochemistry was in accordance with the histopathological examination, in which no histopathological lesions or abnormal changes were observed in the liver and kidney tissues between the NC group and all treatment groups (Supplementary material: Fig. S2). It indicated that both damnacanthal and 5-FU treatments were not toxic to the health of nude mice during 26 days of oral treatment. These findings were consistent with the order of anticancer activity of D20 ≅ D40 > F20, which showed in the in vitro experiment and the in vivo inhibition of tumor growth rate.
Fig. 4.
Effect of damnacanthal on tumor xenograft at day 26 of treatment. (A) Tumor volume was measured by a digital caliper, (B) luminescence intensity derived from the ex vivo bioluminescence imaging, and (C) the representative photographs and luminescence images of the tumor xenografts excised from nude mice on day 26 after treatment with vehicle only (negative control, NC), 5-FU at 20 mg/kg (F20), or damnacanthal at 20 mg/kg (D20) or 40 mg/kg (D40). (D) Organ weight of the tumors and interested tissues on day 26 after treatment. Data in graphs A, B, and D represent the mean ± SD (n = 6). The protein level of cyclin D1 in tumor xenograft was determined by Western blot assay. β-actin was used as the loading control. (E) The representative Western blots and (F) the relative intensities of cyclin D1 normalized against β-actin. The data are presented as the mean ± SD (n = 3). **p < 0.01, ***p < 0.001 indicate significant difference from NC group. #p < 0.05, ##p < 0.01 indicate significant difference from F20 group.
Table 3.
Serum biochemistry parameters of tumor-bearing nude mice on day 26 after treatment with vehicle only (negative control, NC), 5-FU at 20 mg/kg (F20), or damnacanthal at 20 mg/kg (D20) or 40 mg/kg (D40).
| Parameters | Value of serum biochemistrya |
|||
|---|---|---|---|---|
| NC | F20 | D20 | D40 | |
| ALT (U/L) | 34 ± 5 | 33 ± 5 | 30 ± 3 | 31 ± 6 |
| AST (U/L) | 114 ± 38 | 87 ± 21 | 91 ± 36 | 76 ± 17 |
| Creatinine (mg/dL) | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| BUN (mg/dL) | 25 ± 2 | 28 ± 9 | 23 ± 3 | 23 ± 4 |
ALT: alanine aminotransferase, AST: aspartate aminotransferase, BUN: blood urea nitrogen.
Values are the mean ± SD (n = 6).
Damnacanthal exerted the substantial anticancer effects on nude mice bearing HCT116-Red-FLuc tumor xenografts and this result may be due to its stronger inhibitory effect on cell proliferation and its down-regulation of cyclin D1 at protein level in the colorectal cancer cells as shown by in vitro cytotoxicity and western blot analysis. The cyclin D1 is a cellular proto-oncogene that is a critical regulator of the G1 to S-phase transition of the cell cycle progression in many cell types [37]. Overexpression of cyclin D1 is associated with tumor development and progression. Damnacanthal decreased the expression of cyclin D1 not only in HCT116 or HCT116-Red-FLuc and Caco-2 human colorectal cancer cells, but also in PC-3 prostate and MCF-7 breast cancer cells, which resulted in the growth suppression in various cancer cells [28]. In addition, damnacanthal was found to induce apoptosis and cell cycle arrest by increasing p27Kip1 protein with reducing cyclin D1 protein levels in SKVO3 ovarian cancer cells [18] as well as accumulating cell population in the G1 phase of MCF-7 breast [14] and in the S phase of H400 oral cancer cells [38]. These mechanisms may lead to a potent anticancer activity of damnacanthal. In a previous study, damnacanthal had more selective anticancer activity against several types of cancer cells including liver adenocarcinoma, prostate adenocarcinoma [39], leukemia [40], and ovarian cancer cells [18] with minimal toxicity on normal cells. This may be a reason that no toxicity of damnacanthal was observed in the tumor-bearing nude mice. Many studies have reported on in vitro anticancer activities and molecular mechanisms of damnacanthal, but little is known about in vivo anticancer activity of damnacanthal. Recently, the anticancer effect of damnacanthal has been exhibited in a xenograft nude mouse model of SKVO3 ovarian cancer cells by intraperitoneal injection [18]. Our study firstly showed anti-colorectal cancer effect of damnacanthal on HCT116-Red-FLuc tumor xenografts in nude mice by oral administration. Damnacanthal at 20 and 40 mg/kg of was safe for tumor-bearing nude mice, and it was about 2-fold more effective in anti-colorectal cancer activity when compared with the same dose of 5-FU. Nevertheless, an oral administration of damnacanthal at 20 mg/kg was enough to suppress the growth of human colorectal tumors rapidly and effectively. A low dose of damnacanthal would be better reduction of adverse side effects for a long-term treatment of human colorectal cancer.
4. Conclusion
Damnacanthal exhibited a decrease in the cell viability of human colorectal cancer cells, which was mediated by down-regulation of cyclin D1 protein expression. Damnacanthal was safe enough for use as a therapeutic agent with a high LD50 cut-off value of 2500 mg/kg. Furthermore, damnacanthal productively inhibited colorectal tumor xenografts in nude mice with a greater inhibitory effect than 5-FU. Therefore, damnacanthal can be a safe and potential anticancer drug candidate for chemoprevention and/or treatment of human colorectal cancer. However, further in vivo pharmacokinetic study of damnacanthal is necessary for understanding how the drug behaves in the body including absorption, distribution, metabolism, and elimination, which would be useful for various therapeutic applications.
Funding
The study was supported by Thailand Integrated Research and Innovation Grant (Contract No. 49/2561 and 45/2562) and Thammasat University Research Unit in Mechanisms of Drug Action and Molecular Imaging.
CRediT authorship contribution statement
Warunya Woradulayapinij: Methodology, Validation, Formal analysis, Investigation, Visualization, Project administration, Writing - review & editing. Apipu Pothiluk: Methodology, Investigation. Thararat Nualsanit: Conceptualization, Methodology, Writing - review & editing. Thunyatorn Yimsoo: Methodology, Investigation. Werayut Yingmema: Methodology, Investigation. Pleumchitt Rojanapanthu: Conceptualization. Yukyung Hong: Methodology, Investigation. Seung Joon Baek: Writing - review & editing, Supervision. Worapapar Treesuppharat: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Sarinya Wongsanit (Thailand Institute of Nuclear Technology) for her suggestion in tumor xenograft of nude mice and Dr. Aurapa Sakulpanich (Faculty of Pharmacy, Thammasat University) for her support in the purification and characterization of damnacanthal.
Handling Editor: Prof. L.H. Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2022.10.015.
Appendix A. Supplementary material
Supplementary material
.
Data availability
No data was used for the research described in the article.
References
- 1.Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M. International Agency for Research on Cancer; Lyon: 2020. Global Cancer Observatory: Cancer Today. (https://gco.iarc.fr/today, accessed April 2022) [Google Scholar]
- 2.Abancens M., Bustos V., Harvey H., McBryan J., Harvey B.J. Sexual dimorphism in colon cancer. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.607909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dekker E., Tanis P.J., Vleugels J.L.A., Kasi P.M., Wallace M.B. Colorectal cancer. Lancet. 2019;394(10207):1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 4.Huang X.M., Yang Z.J., Xie Q., Zhang Z.K., Zhang H., Ma J.Y. Natural products for treating colorectal cancer: a mechanistic review. Biomed. Pharm. 2019;117 doi: 10.1016/j.biopha.2019.109142. [DOI] [PubMed] [Google Scholar]
- 5.Krishnaiah D., Nithyanandam R., Sarbatly R. Phytochemical constituents and activities of Morinda citrifolia L. Phytochem. – A Glob. Perspect. Their Role Nutr. Health. 2012:127–150. [Google Scholar]
- 6.Saha K., Lam K.W., Abas F., Hamzah A.S., Stanslas J., Hui L.S., et al. Synthesis of damnacanthal, a naturally occurring 9,10-anthraquinone and its analogues, and its biological evaluation against five cancer cell lines. Med. Chem. Res. 2013;22:2093–2104. [Google Scholar]
- 7.Okusada K., Nakamoto K., Nishida M., Fujita-Hamabe W., Kamiya K., Mizushina Y., et al. The antinociceptive and anti-inflammatory action of the CHCl3-soluble phase and its main active component, damnacanthal, isolated from the root of Morinda citrifolia. Biol. Pharm. Bull. 2011;34(1):103–107. doi: 10.1248/bpb.34.103. [DOI] [PubMed] [Google Scholar]
- 8.Nualsanit T., Rojanapanthu P., Gritsanapan W., Kwankitpraniti T., Min K.W., Baek S.J. Damnacanthal-induced anti-inflammation is associated with inhibition of NF-kappaB activity. Inflamm. Allergy Drug Targets. 2011;10(6):455–463. doi: 10.2174/187152811798104908. [DOI] [PubMed] [Google Scholar]
- 9.Noorjahan B.M.A., Ali A.M., Yeap S.K., Suhaimi M., Lajis N.H., Mashitoh A.R., et al. Cytotoxicity and Immunomodulatory Effects of Damnacanthal and Nordamnacanthal Isolated from Roots of Morinda elliptica. J. Agrobiotechnol. 2010;1:29–42. [Google Scholar]
- 10.Ali A.M., Ismail N.H., Mackeen M.M., Yazan L.S., Mohamed S.M., Ho A.S., et al. Antiviral, cyototoxic and antimicrobial activities of anthraquinones isolated from the roots of morinda elliptica. Pharm. Biol. 2000;38(4):298–301. doi: 10.1076/1388-0209(200009)3841-AFT298. [DOI] [PubMed] [Google Scholar]
- 11.Comini L.R., Montoya S.C., Paez P.L., Arguello G.A., Albesa I., Cabrera J.L. Antibacterial activity of anthraquinone derivatives from Heterophyllaea pustulata (Rubiaceae) J. Photochem. Photobiol. B. 2011;102(2):108–114. doi: 10.1016/j.jphotobiol.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Singh D.N., Verma N., Raghuwanshi S., Shukla P.K., Kulshreshtha D.K. Antifungal anthraquinones from Saprosma fragrans. Bioorg. Med. Chem. Lett. 2006;16(17):4512–4514. doi: 10.1016/j.bmcl.2006.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nualsanit T., Rojanapanthu P., Gritsanapan W., Lee S.H., Lawson D., Baek S.J. Damnacanthal, a noni component, exhibits antitumorigenic activity in human colorectal cancer cells. J. Nutr. Biochem. 2012;23(8):915–923. doi: 10.1016/j.jnutbio.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aziz M.Y., Omar A.R., Subramani T., Yeap S.K., Ho W.Y., Ismail N.H., et al. Damnacanthal is a potent inducer of apoptosis with anticancer activity by stimulating p53 and p21 genes in MCF-7 breast cancer cells. Oncol. Lett. 2014;7(5):1479–1484. doi: 10.3892/ol.2014.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Vilas J.A., Quesada A.R., Medina M.A. Damnacanthal, a noni anthraquinone, inhibits c-Met and is a potent antitumor compound against Hep G2 human hepatocellular carcinoma cells. Sci. Rep. 2015;5:8021. doi: 10.1038/srep08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaghayegh G., Alabsi A.M., Ali-Saeed R., Ali A.M., Vincent-Chong V.K., Ismail N.H., et al. Effects of damnacanthal and nordamnacanthal on proliferation, apoptosis, and migration of oral squamous cell carcinoma cells. Asian Pac. J. Cancer Prev. 2017;18(12):3333–3341. doi: 10.22034/APJCP.2017.18.12.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latifah S.Y., Gopalsamy B., Abdul Rahim R., Manaf Ali A., Haji Lajis N. Anticancer potential of damnacanthal and nordamnacanthal from morinda elliptica roots on T-lymphoblastic leukemia cells. Molecules. 2021;26(6) doi: 10.3390/molecules26061554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li R., Li H., Lan J., Yang D., Lin X., Xu H., et al. Damnacanthal isolated from morinda species inhibited ovarian cancer cell proliferation and migration through activating autophagy. Phytomedicine. 2022;100 doi: 10.1016/j.phymed.2022.154084. [DOI] [PubMed] [Google Scholar]
- 19.Abu N., Ali N.M., Ho W.Y., Yeap S.K., Aziz M.Y., Alitheen N.B. Damnacanthal: a promising compound as a medicinal anthraquinone. Anticancer Agents Med Chem. 2014;14(5):750–755. doi: 10.2174/18715206113136660366. [DOI] [PubMed] [Google Scholar]
- 20.OECD. Test No. 423: Acute Oral toxicity - Acute Toxic Class Method 2002.
- 21.Tsukihara H., Nakagawa F., Sakamoto K., Ishida K., Tanaka N., Okabe H., et al. Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, together with bevacizumab, cetuximab, or panitumumab on human colorectal cancer xenografts. Oncol. Rep. 2015;33(5):2135–2142. doi: 10.3892/or.2015.3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y.Y., Li T.M., Zang L.Q., Liu B., Wang G.X. Effects of berberine on tumor growth and intestinal permeability in HCT116 tumor-bearing mice using polyamines as targets. Biomed. Pharmacother. 2018;107:1447–1453. doi: 10.1016/j.biopha.2018.08.130. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham D., Sirohi B., Pluzanska A., Utracka-Hutka B., Zaluski J., Glynne-Jones R., et al. Two different first-line 5-fluorouracil regimens with or without oxaliplatin in patients with metastatic colorectal cancer. Ann. Oncol. 2009;20(2):244–250. doi: 10.1093/annonc/mdn638. [DOI] [PubMed] [Google Scholar]
- 24.Violette S., Poulain L., Dussaulx E., Pepin D., Faussat A.M., Chambaz J., et al. Resistance of colon cancer cells to long-term 5-fluorouracil exposure is correlated to the relative level of Bcl-2 and Bcl-X(L) in addition to Bax and p53 status. Int. J. Cancer. 2002;98(4):498–504. doi: 10.1002/ijc.10146. [DOI] [PubMed] [Google Scholar]
- 25.Thant A.A., Wu Y., Lee J., Mishra D.K., Garcia H., Koeffler H.P., et al. Role of caspases in 5-FU and selenium-induced growth inhibition of colorectal cancer cells. Anticancer Res. 2008;28(6A):3579–3592. [PMC free article] [PubMed] [Google Scholar]
- 26.Yang K., Hitomi M., Stacey D.W. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006;1:32. doi: 10.1186/1747-1028-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu T., Wang X., Hu W., Fang Z., Jin Y., Fang X., et al. Epigenetically down-regulated acetyltransferase PCAF increases the resistance of colorectal cancer to 5-fluorouracil. Neoplasia. 2019;21(6):557–570. doi: 10.1016/j.neo.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sukamporn P., Rojanapanthu P., Silva G., Zhang X., Gritsanapan W., Baek S.J. Damnacanthal and its nanoformulation exhibit anti-cancer activity via cyclin D1 down-regulation. Life Sci. 2016;152:60–66. doi: 10.1016/j.lfs.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 29.Chaichanasak N., Rojanapanthu P., Yoon Y., Gritsanapan W., Chirachanchai S., Sathirakul K., et al. Chitosan-based nanoparticles with damnacanthal suppress CRM1 expression. Oncol. Lett. 2018;16(6):7029–7034. doi: 10.3892/ol.2018.9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z., Cheng Y., Su W., Zhang H., Li C., Routledge M.N., et al. Organ specific differences in alteration of aquaporin expression in rats treated with sennoside A, senna anthraquinones and rhubarb anthraquinones. Int. J. Mol. Sci. 2021;22(15) doi: 10.3390/ijms22158026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paneitz A., Westendorf J. Anthranoid contents of rhubarb (Rheum undulatum L.) and other Rheum species and their toxicological relevance. Eur. Food Res Technol. 1999;210(2):97–101. [Google Scholar]
- 32.Fouillaud M., Caro Y., Venkatachalam M., Grondin I L.D. In: Phenolic Compounds in Food Characterization and Analysis. Nollet L.M.L., Gutiérrez-Uribe J.A., editors. CRC Press; 2018. Anthraquinones; pp. 130–170. [Google Scholar]
- 33.Portalatin M., Winstead N. Medical management of constipation. Clin. Colon Rectal Surg. 2012;25(1):12–19. doi: 10.1055/s-0032-1301754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Gorkom B.A., de Vries E.G., Karrenbeld A., Kleibeuker J.H. Review article: anthranoid laxatives and their potential carcinogenic effects. Aliment Pharmacol. Ther. 1999;13(4):443–452. doi: 10.1046/j.1365-2036.1999.00468.x. [DOI] [PubMed] [Google Scholar]
- 35.Close D.M., Xu T., Sayler G.S., Ripp S. In vivo bioluminescent imaging (BLI): noninvasive visualization and interrogation of biological processes in living animals. Sensors. 2011;11(1):180–206. doi: 10.3390/s110100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sim H., Bibee K., Wickline S., Sept D. Pharmacokinetic modeling of tumor bioluminescence implicates efflux, and not influx, as the bigger hurdle in cancer drug therapy. Cancer Res. 2011;71(3):686–692. doi: 10.1158/0008-5472.CAN-10-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alao J.P., Gamble S.C., Stavropoulou A.V., Pomeranz K.M., Lam E.W., Coombes R.C., et al. The cyclin D1 proto-oncogene is sequestered in the cytoplasm of mammalian cancer cell lines. Mol. Cancer. 2006;5:7. doi: 10.1186/1476-4598-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaghayegh G., Alabsi A.M., Ali-Saeed R., Ali A.M., Vincent-Chong V.K., Zain R.B. Cell cycle arrest and mechanism of apoptosis induction in H400 oral cancer cells in response to Damnacanthal and Nordamnacanthal isolated from Morinda citrifolia. Cytotechnology. 2016;68(5):1999–2013. doi: 10.1007/s10616-016-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin F.L., Hsu J.L., Chou C.H., Wu W.J., Chang C.I., Liu H.J. Activation of p38 MAPK by damnacanthal mediates apoptosis in SKHep 1 cells through the DR5/TRAIL and TNFR1/TNF-alpha and p53 pathways. Eur. J. Pharmacol. 2011;650(1):120–129. doi: 10.1016/j.ejphar.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Alitheen N.B., Mashitoh A.R., Yeap S.K., Shuhaimi M., Abdul Manaf A., Nordin L. Cytotoxic effect of damnacanthal, nordamnacanthal, zerumbone and betulinic acid isolated from Malaysian plant sources. Int. Food Res. J. 2010;17:711–719. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
No data was used for the research described in the article.