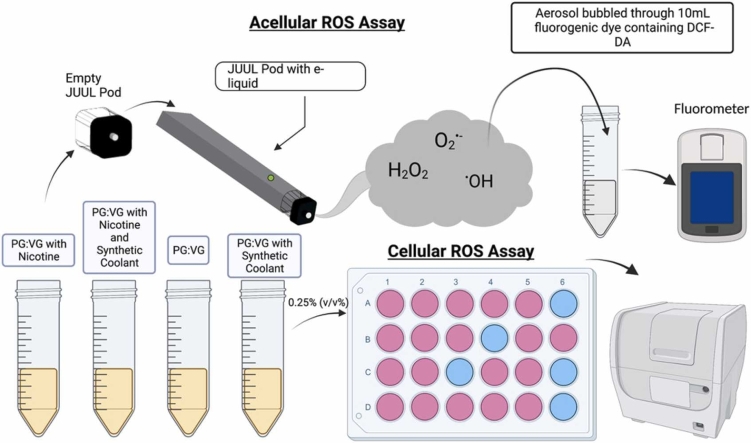
Abstract
There has been a substantial rise in e-cigarette (e-cig) use or vaping in the past decade, prompting growing concerns about their adverse health effects. Recently, e-cig manufacturers have been using synthetic cooling agents, like WS-23 and WS-3, to provide a cooling sensation without the “menthol taste”. Studies have shown that aerosols/vapes generated by e-cigs can contain significant levels of reactive oxygen species (ROS). However, studies investigating the role of synthetic coolants in modulating ROS levels generated by e-cigs are lacking. This study seeks to understand how synthetic coolants, e-cig additives that have become increasingly prevalent in e-liquids sold in the United States (US), impact acellular ROS production from e-liquid aerosols as well as cellular ROS levels from pulmonary epithelial cells exposed to these e-liquids. To further explain, our study aims to understand whether the addition of WS-3 and WS-23 to e-liquid base and e-liquid base with nicotine significantly modifies generated acellular ROS levels within aerosolized e-liquids, as well as cellular ROS within BEAS-2B cells treated with these same e-liquids. Aerosols were generated from e-liquids with and without synthetic coolants through a single-puff aerosol generator; subsequently, acellular ROS was semi-quantified in H2O2 equivalents via fluorescence spectroscopy. Our acellular ROS data suggest that adding WS-3 to e-liquid base (PG:VG), regardless of nicotine content, has a minimal impact on modifying e-cig generated acellular ROS levels. Additionally, we also measured cellular ROS in lung epithelial cells using both e-liquids containing and not containing synthetic coolants via the CellROX Green fluorescent sensor. Similar comparable results were found in BEAS2B cells though ROS was increased by WS-3 and WS-23 treated in e-cig nicotine groups. Altogether, our data suggest that neither the addition of WS-23 nor WS-3 to e-liquid base solution, with and without nicotine, significantly modifies e-cig generated acellular ROS levels within aerosolized e-liquids and cellular ROS levels within treated BEAS-2B cells. Together, our data provide insight into whether synthetic coolants added to e-liquids could impact vaping-induced oxidative stress in the lungs.
Keywords: E-cigarette vaping, Electronic nicotine delivery systems (ENDS), Synthetic coolants, Reactive Oxygen Species (ROS), Acellular ROS, Cellular ROS, W-3, WS-23, Cooling-agent, Oxidative stress
Graphical Abstract
Highlights
-
•
Synthetic cooling agents (WS-23 and WS-3) provide a cooling sensation.
-
•
Coolants in e-cigarettes modify acellular and ROS levels in lung epithelial cells.
-
•
Synthetic coolants in e-cigarette affect oxidative stress in lung cells.
1. Introduction
During the past few years, adolescent use of e-cigs or various electronic nicotine delivery systems (ENDS) has significantly increased, thus leading to an increase in the prevalence of E-cigarette (E-cig) or Vaping Associated Lung injury (EVALI) across the United States [1], [2]. As of February 18, 2020, a total of 2807 EVALI-related hospitalizations or deaths were reported to the Centers for Disease Control (CDC) from all 50 states [1], [2]. Consequently, the Food & Drug administration (FDA) implemented an enforcement policy to remove all flavored cartridge or/pod-based e-cigs except tobacco and menthol-flavored pods from the market [3].
Following the FDA’s 2020 flavor-enforcement policy, menthol-flavored e-cig sales had significantly increased in the US; specifically, there was a 54.5 % increase in the market share of menthol-flavored e-cigs over four weeks and an 82.8 % increase over eight weeks following the FDA’s ruling [4]. Menthol induces a perception of a cool sensation by activating the cold receptor found in the oral cavity [5], [6]. Menthol reduces the bitterness associated with inhaled nicotine and increases its smoothness upon inhalation, thus increasing e-cigarette appeal [7]. Other than menthol, several synthetic cooling agents have been added to e-cigarette/e-liquid formulations as a replacement for menthol due to these synthetic agents giving similar cooling sensations upon aerosol inhalation as menthol [8], [9], [10], [11]. Methyl diisopropyl propionamide (WS-23) and N-Ethyl-2-isopropyl-5-methylcyclohexanecarboxamide (WS-3) are examples of these added cooling agents.[5], [9].
A recent report has showed that e-cigarette flavors with "ice", "chilled", "Cooled", and "Polar" in their name, and other flavors consisting of flavor combinations with fruity and drink flavors like "melon-ice", "blueberry-ice", and "iced-pink punch" contained WS-23 and WS-3 in their formulations [12]. The significant increase in the marketing of “iced/cooled” flavored e-cigs in the U.S had occurred right around the time when sales of disposable e-cigs surged following the FDA’s implementation of its March 2020 e-cig flavor enforcement policy [12]. One study had found, via GC-MS and LC-HRMS/MS, that both WS-3 and WS-23 were major components found in nicotine-containing vaping fluids provide by patients apart of the 197 reported-cases of EVALI in New York State to the Wadsworth Center at the New York State Department of Heath from August 2019 through June 2021 [13]. Additionally, one study [9] found that WS-23 was present in e-cigs marketed in the US at levels that may potentially result in exceeding the Margin of Exposure (MOE), a risk assessment parameter for toxic compounds used by World Health Organization (WHO) [9]. Jabba et al. [14] results suggest that those who use e-liquids comprised of W-3 or WS-23 are potentially at risk for long-term pulmonary health issues [9].
Aerosols generated by e-cigs or other ENDS modalities have been found to contain dangerous chemicals, including formaldehyde and acetaldehyde, which are known to cause lung cancer and cardiovascular disease [15]. Also, consistently, it has been found that dysregulated inflammatory cytokine output is an effect of chronic e-cig exposure in both in vivo and in vitro models [16], [17], [18]. Moreover, previous studies have shown that aerosols generated by flavored e-cigs produce significant levels of acellular reactive oxygen species (ROS) and induce cellular ROS in small airway epithelial cells (SAEC) [19], [20], [21]. ROS, either exogenous or when produced in excess endogenously, can lead to a redox imbalance in the lungs [22]. One study found tobacco smoke to contain a significant amount of free radicals, ∼1 × 1015 radicals per puff [14], [23], [24]. ROS in smoke generated from conventional cigarettes, when inhaled, will react with antioxidants in the epithelial lining fluid (ELF) covering airway epithelial cells [23]. Moreover, ROS in tobacco smoke, after reaching the ELF of airways, can lead to the destruction of endogenous antioxidants, thus significantly reducing cellular antioxidant capacity [24]. Oxidative stress induced by this redox imbalance has been implicated in the pathology of many types of lung diseases, such as acute respiratory distress syndrome (ARDS), asthma, and chronic obstructive pulmonary disease (COPD) [22]. Similarly, studies have shown that exposure of e-liquids, e-liquid solvents, and e-cig aerosols have led to significant increases in the levels of cellular ROS produced by cultured pulmonary airway cells [25], [26].
Studies so far have shown that exposure to e-cig aerosols induces oxidative and carbonyl stress in the lungs [21], [17], [27]. Regarding ROS-related e-cig studies, studies have shown that total acellular ROS levels in e-cig aerosols are dependent on brand, flavor, operational voltage, and puffing protocol, but no studies so far have sought to investigate the role synthetic coolants have in modifying total acellular ROS in aerosols generated from e-liquids and cellular ROS levels from BEAS-2B cells exposed to those e-liquids. In this study, we seek to understand the role of WS-23 and WS-3 in modifying acellular and cellular ROS levels due to exposure to e-liquids.
2. Materials and methods
2.1. Procurement of e-liquid constituents and composition of e-liquid solutions
Propylene Glycol (PG), Vegetable Glycerin (VG), WS-23 solution (30 % suspended in PG), and Koolada (10 % WS-3 in PG) were purchased online from Flavor Jungle. 100 mg/mL nicotine salt solution (50:50 PG-to-VG ratio) was purchased online from PERFECTVAPE. E-liquid solutions comprising of PG, VG, salt nicotine, Koolada, and WS-23 were made. For our acellular ROS assays, the following e-liquids were made (Table 1). Six different e-liquid formulations were used in this study; all of them containing a mixture of PG and VG at a 50:50 vol percentage ratio, and some of them differing in their volume concentrations of nicotine (0% or 5%) and Flavor Jungle synthetic coolant-containing solution (0% or 3%).
Table 1.
Composition of E-liquids Analyzed.
| Composition of E-liquid solution | PG:VG Ratio (by volume) | Nicotine concentration (% by volume) | Cooling solution added | Cooling solution concentration (% by volume) |
|---|---|---|---|---|
| PG:VG | 50:50 | 0.0 | None | 0.0 |
| PG:VG (Nicotine) | 50:50 | 5.0 | None | 0.0 |
| PG:VG +Koolada | 50:50 | 0.0 | FlavorJungle Koolada (10 % WS-3 in PG) | 3.0 |
| PG:VG + WS-23 |
50:50 | 0.0 | FlavorJungle WS-23 (30 % in PG) | 3.0 |
| PG:VG (Nicotine) + Koolada |
50:50 | 5.0 | FlavorJungle Koolada (10 % WS-3 in PG) | 3.0 |
| PG:VG (Nicotine) + WS-23 |
50:50 | 5.0 | FlavorJungle WS-23 (30 % in PG) | 3.0 |
2.2. Generation of aerosols, fluorescence spectroscopy, and acellular ROS quantification
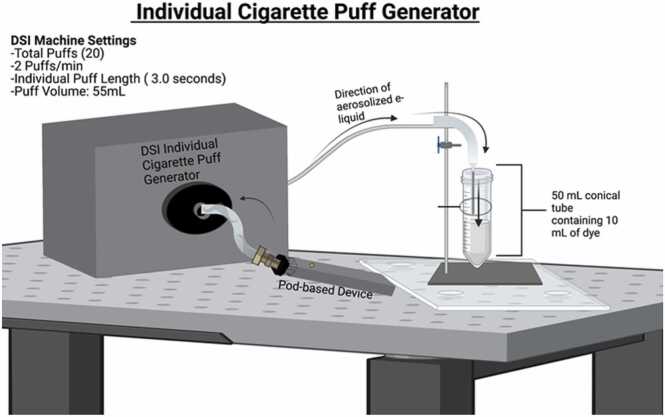
Each e-liquid solution was added to a new, empty refillable pod (OVNStech, Shenzen, GD, China) (Mo: WO1 Pods) and aerosolized using a pod-based device. Specifically, each pod-based device was attached to a Buxco Individual Cigarette Puff Generator (Data Sciences International (DSI), St. Paul, MN, USA) (Cat#601–2055–001), and subsequently, its component e-liquid was aerosolized and “bubbled” through 10 mL of freshly made fluorogenic dye within a 50 mL conical tube (Fig. 1).
Fig. 1.
This pictogram shows the e-cigarette exposure generation system used in the study. E-cigarette aerosol was generated from the e-cigarette device using the artificial lung present in the Individual Cigarette Puff Generator. The e-cigarette aerosol then traveled to and was exposed to 10 mL of fluorogenic dye for one puff regimen at 1.5 L/min. One puff regimen consists of 20 total puffs (2 puffs/min) for 10 min, with the volume of each puff being 55.0 mL and each individual puff length lasting 3.0 s. Each conical tube was wrapped in aluminum foil to protect the fluorogenic dye from light. The entirety of the aerosolization and exposure process using the DSI machine was performed inside a chemical fume hood.
Cell permeant 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (EMD Biosciences, San Diego, CA, USA) (Cat # 287810) dissolved in 0.01 N NaOH, phosphate buffer, PO4, and horseradish peroxidase (Thermo Fisher Scientific, Waltham, MA, USA (Cat# 31491) were used to make the fluorogenic dye. The aerosols generated from each e-liquid solution were individually bubbled through 10 mL of H2DCFDA solution at 1.5 L/min. A schematic of the e-cig aerosolization procedure is shown in Fig. 1. Each pod containing a respective e-liquid solution had undergone three separate puffing regimens to create three separate samples of bubbled dye solution. The same puffing regimen was used for “bubbling” filtered air through fluorogenic dye for a negative control. For our positive control, the smoke generated from a research cigarette (Kentucky Tobacco Research & Development Center in the University of Kentucky, Lexington, KY, USA) (Mo: 3R4F) was bubbled through the fluorogenic dye. After “bubbling,” each resulting fluorogenic dye sample was placed in a 37 °C degree water bath (VWR 1228 Digital Water Bath) for fifteen minutes; subsequently, the solution was analyzed via fluorescence spectroscopy using a spectrofluorometer (Turner Quantech fluorometer, Mo. FM109535) in fluorescence intensity units (FIU). Readings on the spectrofluorometer were measured as H2O2 equivalents using a standard curve generated using the 0–50 µM H2O2 standards made.
2.3. Cells culture conditions and treatments
BEAS-2B cell lines (ATCC, Manassas, VA) were used in this study for subsequent oxidative stress detection assays. Cells were maintained in DMEM/F12 media supplemented with 10 % FBS and 1 % antibiotic-antimycotic solution at 37 °C and 5 % of CO2. Cells for treatments were seeded at a concentration of 2.5 × 105 cells/well in 24 well plate (Corning).
Prior to treatment Cells were incubated overnight in low serum-containing media (FBS 0 %). Cells were exposed to six different e-liquids of various nicotine and synthetic coolant concentrations for 4 h to assess for differences in cellular ROS production. The treatments were as follow; An untreated group (only low serum-containing media (FBS 0 %) was added to culture media), PG:VG (50:50), PG:VG (50:50) containing nicotine (5%), PG:VG (50:50) +WS-23 (3 %), PG:VG (nicotine) + WS-23, PG:VG (50:50)+WS-3 (3 %) PG:VG (nicotine) + WS-3 (3 %). To clarify, 0.25 % of each of the previously listed e-liquids were used to treat the cells for 4 h. Afterward, cellular ROS production in BEAS-2B cells was assessed through a fluorescent probe, CellROX Green Reagent (ThermoFisher).
2.4. Quantification of cellular ROS production measured by CellROX green fluorescence
BEAS-2B cells were treated with the respective e-liquids. ROS generation was calculated using the CellROX™ Green Oxidative Stress kit (ThermoFisher). Following the exposure of e-liquids to BEAS-2 cells, cell media was removed and the cells were incubated with 5 μM CellRox Green in DMEM at 37 °C for 30 min. The solution was then aspirated and fixed with 4% paraformaldehyde (PFA). Nuclei were counterstained with Hoechst stain in 1× PBS; subsequently cells were analyzed via fluorescence imaging through the Cytation 5 Cell Imaging Multi-Mode Reader (BioTek Instruments, Inc., VT, USA. CellROX fluorescent signals were then were analyzed using Image J.
2.5. Statistical analyses
One-way ANOVA, unpaired t-test, and Tukey’s post-hoc tests were used for pairwise comparisons via GraphPad Prism Software version 8.1.1. Samples were analyzed by triplicate. The results are shown as mean ± SEM. Data were considered to be statistically significant for p values < 0.05.
3. Results
3.1. WS-3 and WS-23 differentially modify acellular ROS levels with aerosolized e-liquid base solution, based of nicotine content
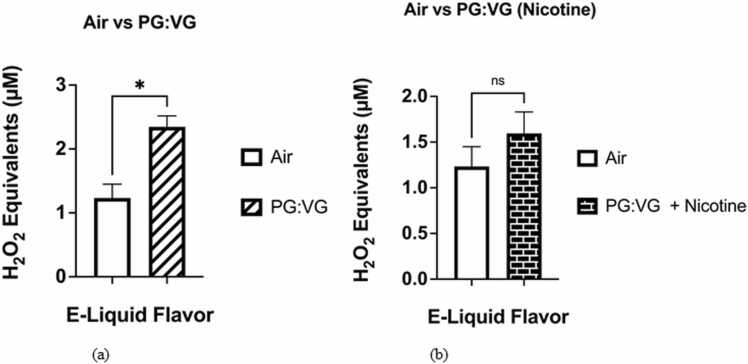
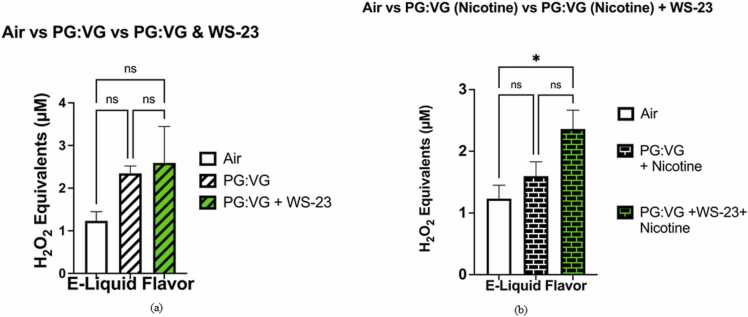
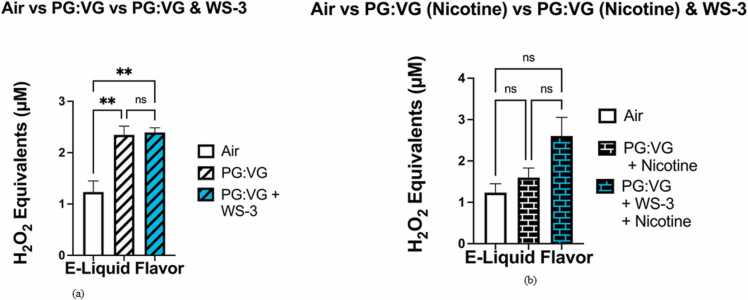
The levels of acellular ROS generated by the PG:VG solution (2.02–2.60 μM H2O2) were significantly higher (p < 0.05), than those generated by the filtered air control (0.96–1.66 μM H2O2) (Fig. 2a). When the levels of acellular ROS generated by the PG:VG solution containing nicotine (5 %) (1.13–1.84 μM H2O2) and the filtered air control (0.96–1.66 μM H2O2) were compared, the generated ROS levels did not significantly differ (p > 0.05) (Fig. 2b). The levels of ROS generated by the PG:VG with WS-23 solution (1.21–4.16 μM H2O2) did not significantly differ from those generated by the aerosolized PG:VG solution nor from the levels of acellular ROS generated by the filtered air control (p > 0.05) (Fig. 3a). However, the levels of acellular ROS generated by the aerosolized e-liquid solution containing PG:VG with nicotine (5 %) and WS-23 (3 %) (1.94–2.95 μM H2O2) were significantly higher than those generated by the filtered air control (p < 0.05) (0.96–1.66 μM H2O2) (Fig. 3b). In contrast, the levels of acellular ROS generated by the PG:VG solution containing nicotine and WS-23 (1.94–2.95 μM H2O2) did not differ significantly from those generated by the PG:VG solution containing nicotine (p > 0.05) (Fig. 3b). When the levels of acellular ROS generated by the PG:VG solution containing nicotine and WS-3 (2.27–2.57 μM H2O2) and the filtered air control were compared, the generated ROS levels were significantly different (p < 0.01) (Fig. 4a). However, the difference in acellular ROS levels between aerosolized PG:VG with WS-3 solution and aerosolized PG:VG solution was not significant (p > 0.05) (Fig. 4a). Additionally, the levels of ROS generated by the PG:VG solution with nicotine and WS-3 (1.79–3.35 μM H2O2) did not significantly differ from those generated by the aerosolized PG:VG with nicotine solution nor the filtered air control (p > 0.05) (Fig. 4b).
Fig. 2.
Comparative analysis of acellular ROS levels generated by aerosolized PG:VG, PG:VG with nicotine, and a filtered air control. Acellular ROS was measured through hydrogen peroxide standards within aerosols generated from the previously mentioned e-liquids. Specifically, the e-liquid solutions were aerosolized using a pod-based device inserted into the Buxco Individual Cigarette Puff Generator. Data are represented as mean ± SEM, and significance was determined using an unpaired t-test. * p < 0.05 and ‘NS’ is abbreviated for “Non-Significant” versus air control (p > 0.05). N = 3. It should be noted that the acellular ROS concentrations (H2O2 equivalents) within aerosols generated by the filtered air control used to draw Fig. 2A were also used for Fig. 2B.
Fig. 3.
Comparative analysis of acellular ROS levels generated by aerosolized PG:VG, PG:VG with nicotine, PG:VG + WS-23, PG:VG with nicotine + WS-23, and a filtered air control. Acellular ROS was measured through hydrogen peroxide standards within aerosols generated from the previously mentioned e-liquids. Specifically, the e-liquid solutions were aerosolized using a pod device inserted into the Buxco Individual Cigarette Puff Generator. Data are represented as mean ± SEM, and significance was determined One-way ANOVA. * p < 0.05 and ‘NS’ is abbreviated for “Non-Significant” versus air control (p > 0.05). N = 3. It should be noted that the acellular ROS concentrations (H2O2 equivalents) from aerosols generated by the filtered air control used to draw Fig. 3A were also used to prepare Fig. 3B. Additionally, it must be noted that the levels of H2O2 equivalents shown within the air, PG:VG, and PG:VG (Nic) aerosols shown in Fig. 3A-3B are the same as those used for Fig. 2A-2B. Fig. 3A-3B include these air, PG:VG, and PG:VG (Nic) aerosol groups in order compare the acellular ROS levels between specific e-liquid formulation (PG:VG, PG:VG (Nic)) and corresponding e-liquid formulations including WS-23.
Fig. 4.
Comparative analysis of acellular ROS levels generated by aerosolized PG:VG, PG:VG with nicotine, PG:VG + WS-3, PG:VG with nicotine + WS-3, and a filtered air control. Acellular ROS was measured through hydrogen peroxide standards within aerosols generated from the previously mentioned e-liquids. Specifically, the e-liquid solutions were aerosolized using a pod device inserted into the Buxco Individual Cigarette Puff Generator. Data are represented as mean ± SEM, and significance was determined using One-Way ANOVA. p < 0.01 and ‘NS’ is abbreviated for “Non-Significant” versus air control (p > 0.05). N = 3. It should be noted that the acellular ROS concentrations (H2O2 equivalents) from aerosols generated by the filtered air control used to prepar Fig. 4A were also used to draw Fig. 4B. Additionally, it must be noted that the levels of H2O2 equivalents shown within the air, PG:VG, and PG:VG (Nic) aerosols shown in Fig. 4A-4B are the same as those used for Fig. 2A-2B. Fig. 4A-4B include these air, PG:VG, and PG:VG (Nic) aerosol groups in order to compare the acellular ROS levels between specific e-liquid formulation (PG:VG, PG:VG (Nic)) and corresponding e-liquid formulations including WS-3.
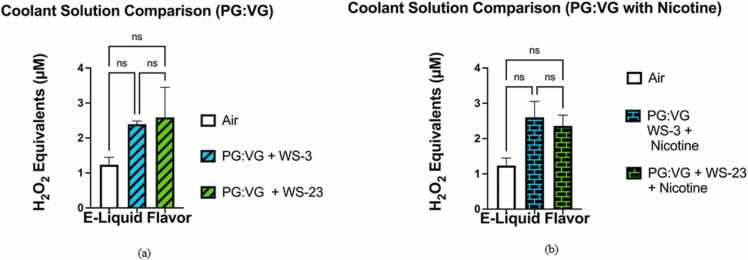
The levels of acellular ROS generated by the PG:VG (50:50) with WS-3 (3 %) solution did not significantly differ from those generated by the PG:VG (50:50) with WS-23 (3%) solution (p > 0.05) (Fig. 5a). Additionally, neither the difference in acellular ROS levels between the aerosolized PG:VG + WS-3 solution and the filtered air control nor that between the aerosolized PG:VG + WS-23 solution and the filtered air control were significant (p > 0.05) (Fig. 5a). When comparing the levels of ROS generated by the PG:VG with WS-3 and nicotine solution to those generated by the PG:VG with WS-23 and nicotine solution, it did not significantly differ (p > 0.05) (Fig. 5b). Moreover, neither the difference in acellular ROS levels between aerosolized PG:VG + WS-3 solution and the filtered air control nor that between the aerosolized PG:VG +WS-23 solution and filtered air control were significant (p > 0.05) (Fig. 5b). Our data show that regardless of nicotine content (0% or 5%), minimal differences in acellular ROS levels exist when comparing the addition of WS-3 and WS-23 to e-liquid base (PG:VG) (Fig. 5a-b). For the acellular ROS assays conducted, smoke generated from a 3R4F research cigarette was used as a positive control with 45.87–49.42 μM H2O2 equivalents (n = 2).
Fig. 5.
Comparative analysis of acellular ROS levels generated by aerosolized PG:VG + WS-3, PG:VG with nicotine + WS-3, PG:VG + WS-23, PG:VG with nicotine + WS-23, and a filtered air control. Acellular ROS was measured through hydrogen peroxide standards within aerosols generated from the previously mentioned e-liquids. Specifically, the e-liquid solutions were aerosolized using a pod device inserted into the Buxco Individual Cigarette Puff Generator. Data are represented as mean ± SEM, and significance was determined using One-Way ANOVA. ‘NS’ is abbreviated for “Non-Significant” versus air control (p > 0.05). N = 3. It should be noted that the acellular ROS concentrations (H2O2 equivalents) from aerosols generated by the filtered air control used to prepare Fig. 5A were also used to prepare Fig. 5B. Additionally, it must be noted that the levels of H2O2 equivalents shown within the air, PG:VG, PG:VG (Nic), PG:VG with WS-3, PG:VG with WS-23, PG:VG (Nic) with WS-3 groups, and PG:VG (Nic) with WS-23 groups are the same as those in Fig. 3, Fig. 4. Fig. 5A-5B include the air, PG:VG, PG:VG (Nic), PG:VG + WS-3, PG:VG + WS-23, PG:VG (Nic) + WS-3 groups, and PG:VG (Nic) + WS-23 groups in order to compare the acellular ROS levels between specific e-liquid formulations.
3.2. WS-3 and WS-23 modify ROS levels in BEAS-2B cells
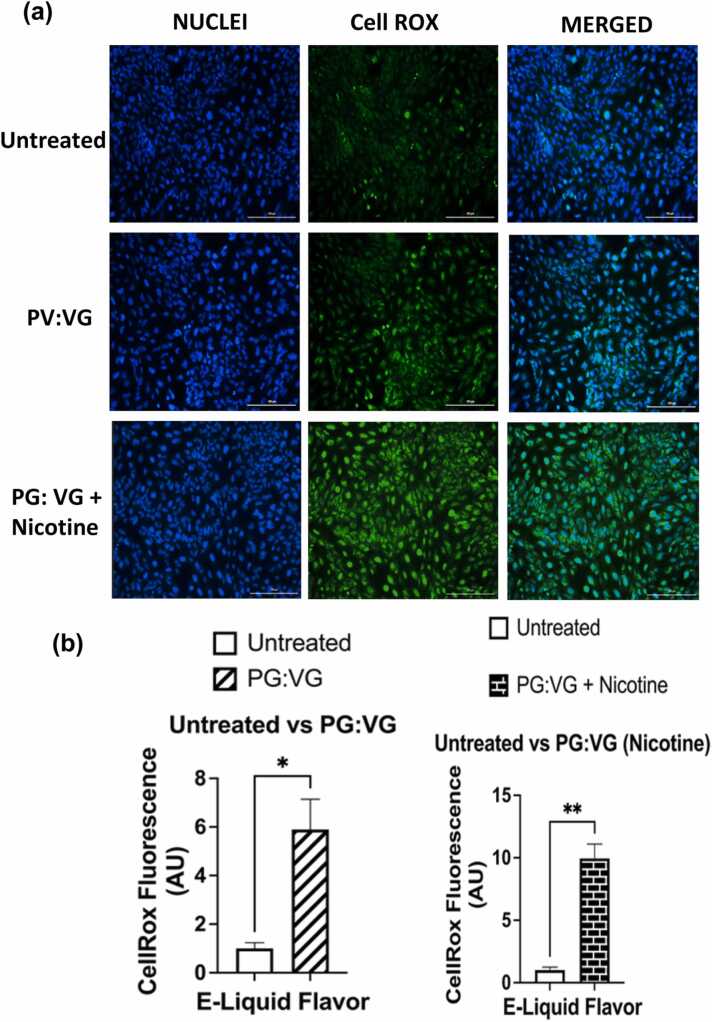
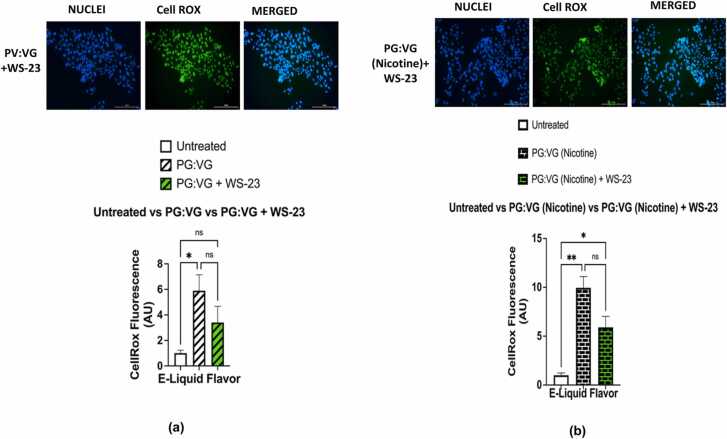
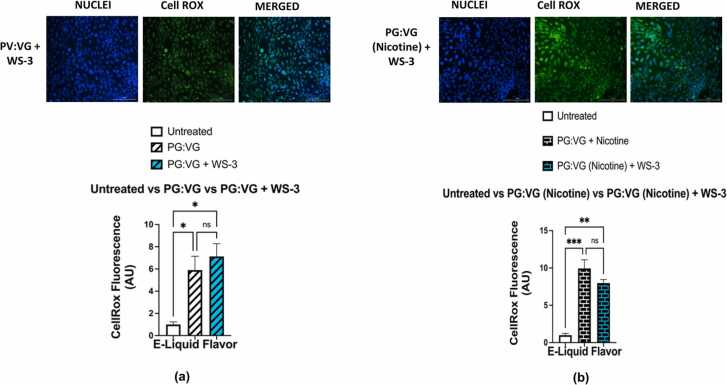
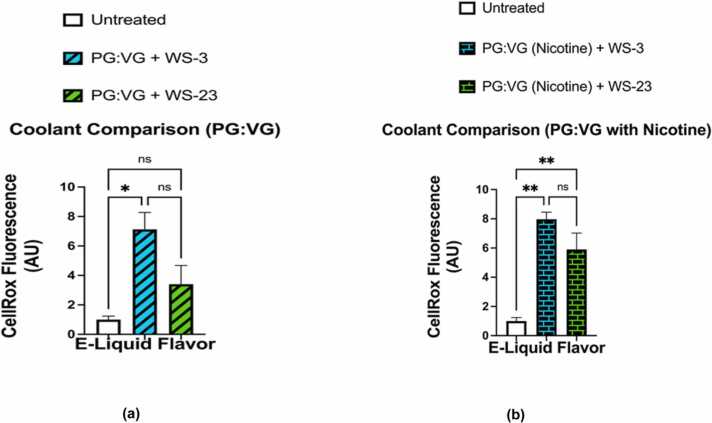
Cellular oxidative stress in BEAS-2B cells was assessed through using analyses of fluorescent intensities within cells exposed to CellROX Green reagent; these fluorescent intensities being used as a measure of ROS levels. Fluorescent imagining showed that cells exposed to e-liquid containing PG:VG and cells exposed to PG:VG (nicotine) contained significantly higher levels of cellular ROS than untreated cells; (p < 0.05) and (p < 0.01), respectively (Fig. 6a-b). However, the levels of cellular ROS generated by BEAS-2B exposed to PG:VG with WS-23 solution did not significantly differ from those generated by cells exposed to the PG:VG (p > 0.05) nor those generated by untreated cells (p > 0.05) (Fig. 7a). In contrast, the levels of the cellular ROS generated by BEAS-2B cells exposed to PG:VG (nicotine) with WS-23 were significantly higher than those generated by untreated cells (p < 0.05), but they did not significantly differ from those generated by cells exposed to PG:VG (nicotine) (p > 0.05) (Fig. 7b). Regarding the levels of ROS generated from cells treated with PG:VG containing WS-3; while they were significantly higher than those generated by untreated cells (p < 0.05), they did not differ significantly from those generated from cells exposed to PG:VG solution (p > 0.05) (Fig. 8a). Similarly, while our data showed that the difference in generated cellular ROS levels between the PG:VG (nicotine) + WS-3 treatment and the PG:VG treatment was not significant (p > 0.05), cells exposed to PG:VG (nicotine) + WS-3 did produce significantly higher levels of ROS than untreated cells (p < 0.01) (Fig. 8b). Additionally, the levels of ROS generated by cells exposed to PG:VG with WS-3 solution did not significantly differ from those generated by the PG:VG with WS-23 solution nor from those generated by the untreated cells (p > 0.05) (Fig. 9a). Similar results were observed when comparing ROS levels generated by cells exposed to both treatments of e-liquid formulations containing nicotine and synthetic coolants. Overall, there was no significant difference between the levels of ROS generated by the PG:VG (nicotine) with WS-3 treatment and the PG:VG (nicotine) with WS-23 treatment (p > 0.05) (Fig. 9b). Cellular ROS levels from cells exposed to e-liquid formulations containing either WS-3 or WS-23 were significantly higher than the untreated cells. It should be noted that cell viability was slightly reduced in the PG:VG (Nicotine) + WS-23 treatment groups (personal observation).
Fig. 6.
Comparative analysis of cellular ROS levels generated from treatments with PG:VG, PG:VG with nicotine, and an untreated control. Comparisons between the cellular ROS levels was conducted using CellROX green reagent generated by BEAS-2B which were left untreated and, (a) cells treated with PG:VG (50:50) and PG:VG containing nicotine for four hours. After an additional four hours, cell medium was aspirated, and the live cells were stained with 5 μM CellROX Green Reagent (in DMEM 0% FBS). After PFA fixation, nuclei were counterstained with Hoechst stain. Data are represented as mean ± SEM, and significance was determined using an unpaired t-test.* p < 0.5 and **p <0.01 (n=3). (b) ROS labelled nuclei were assessed by Cytation 5 imaging (BioTek) reader and CellROX fluorescent signals were analyzed using Image J software (n = 3). Scale bar = 200 μm. The fluorescent images showing stained untreated cells were common to both the treatment groups, and were used for comparative analyses between the groups. Histograms as Arbitrary Unit (AU) representing CellRox Fluorescence in untreated cells (a) were used to prepare the comparative graph shown (b).
Fig. 7.
Comparative analysis of cellular ROS levels generated by PG:VG , PG:VG +WS-23, PG:VG with nicotine, PG:VG with nicotine + WS-23, and an untreated control. Comparisons between the cellular ROS levels was conducted using CellROX green reagent generated by BEAS-2B which were left untreated and, (a) Cells were treated with PG:VG and PG:VG+WS-23, (b) Cells were treated with both PG:VG containing nicotine and PG:VG containing nicotine and WS-23 for four hours. After an additional four hours, cell medium was aspirated and the live cells were stained with 5 μM CellROX Green Reagent (in DMEM 0% FBS). After PFA fixation, nuclei were counterstained with Hoechst stain. Data are represented as mean ± SEM, and significance was determined using One-way ANOVA.* p < 0.05 and **p < 0.01(n = 3). ‘NS’ is abbreviated for “Non-Significant” versus air control (p > 0.05). Data were compared with images on Fig 6. ROS labelled nuclei were analyzed by Cytation 5 imaging (BioTek) reader and CellROX fluorescent signals were measured using Image J software. Scale bar = 200μm.
Fig. 8.
Comparative analysis of cellular ROS levels generated by PG:VG,PG:VG +WS-3, PG:VG with nicotine, PG:VG with nicotine + WS-3, and an untreated control. Comparisons between the cellular ROS levels was conducted using CellRox green reagent generated by BEAS-2B which were left untreated and those which where treated; treatments are as follow: (a) Cells were treated with PG:VG and PG:VG+WS-3, (b) Cells were treated with PG:VG containing nicotine and PG:VG containing nicotine + WS-3 for four hours. After an additional four hours cell medium was aspirated and the live cells were stained with 5 μM CellRox Green Reagent (in DMEM O% FBS). After PFA fixation, nuclei were counterstained with Hoechst stain. Data are represented as mean ± SEM, and significance was determined using One-Way ANOVA.* p < 0.05 and **p < 0.01 (n = 3). ‘NS’ is abbreviated for “Non-Significant” versus air control (p > 0.05). Data were compared with images on Fig 6. ROS labeled nuclei were assessed by Cytation 5 imaging (BioTek) reader and CellROX fluorescent signals were analyzed using Image J software. Scale bar = 200 µm.
Fig. 9.
Comparative analysis of cellular ROS levels generated by E-Liquids of PG:VG , PG:VG +WS-3, PG:VG +WS-23, PG:VG with nicotine, PG:VG with nicotine+WS-3 , PG:VG with nicotine+WS-23 and an untreated control. Comparisons between the cellular ROS levels was conducted using CellROX green reagent generated by BEAS-2B which were left untreated and treatment as follow: (a) Cells were treated PG:VG + WS-3 and PG:VG+WS-23, (b) Cells were treated with both PG:VG containing nicotine and WS-3 and PG:VG containing nicotine and WS-23 for four hours. After an additional four hours cell medium was aspirated and the live cells were stained with 5 μM CellROX Green Reagent (in DMEM O% FBS). After PFA fixation, nuclei were counterstained with Hoechst stain. Data are represented as mean ± SEM, and significance was determined using One-way ANOVA. *p < 0.05 and **p < . 0.01 (n = 3). ‘NS’ is abbreviated for “Non-Significant” versus air control (p > 0.05). Data were compared with images on Figs 6 and 8. ROS labeled nuclei were assessed by Cytation 5 imaging (BioTek) reader and CellROX fluorescent signals were analysed using Image J software. Scale bar = 200 µm.
4. Discussion
With the surge in e-cig use amongst youth in the United States in 2021 and the recent influx of "iced" e-cig flavors in US marketplaces, there is a greater need to fill the knowledge gap on the safety of inhaling synthetic-coolant additives [28]. Our study sought to determine whether adding a widely used synthetic coolants, WS-3 and WS-23, in e-liquids modifies the level of acellular ROS generated in e-cig aerosols and cellular ROS levels generated by the BEAS-2B airway epithelial cell lines. When comparing acellular ROS levels generated by the PG:VG with nicotine and WS-23 (3.0%) solution and the PG:VG with nicotine solution, the levels of acellular ROS did not differ significantly. Similarly, there was no significant difference between acellular ROS levels from PG:VG + WS-23 and PG:VG. Regarding WS-3 containing e-liquids, there was no significant difference between acellular ROS levels from PG:VG (Nic) + WS-3 and PG:VG (Nic). Similarly, there was no significant difference between acellular ROS levels from PG:VG + WS-23 and PG:VG (p > 0.05). Likewise, our data suggests that neither the addition of WS-23 nor WS-3 to nicotine and non-nicotine-containing e-liquids significantly modifies the levels of acellular ROS generated by e-liquids nor the levels of cellular ROS generated by treated BEAS-2B cells.
When comparing the levels of cellular ROS generated by BEAS-2B cells exposed to the PG:VG with nicotine and WS-23 (3 %) solution and the PG:VG with nicotine solution, the levels of generated cellular ROS did not significantly differ. However, we did note that the difference in BEAS-2B cell generated ROS levels between the PG:VG (50:50) treatment and the untreated cells) (p < 0.05) was lower than the difference in BEAS-2B generated ROS levels between the PG:VG (nicotine) treatment and the untreated cells (p < 0.01). Also, we observed significantly higher levels of ROS production by BEAS-2B cell treated with PG:VG (nicotine) with WS-23 (3 %) when compared to untreated cells (p < 0.01). Also, we observed a significant difference in BEAS-2B ROS production between the PG:VG with WS-3 (3 %) treatment and untreated cells (p < 0.05). Our data suggests that the addition of WS-23 and WS-3 by themselves to e-liquid base solution, PG:VG, and e-liquid based solution containing nicotine, PG:VG (nicotine), does not lead to significantly modifying cellular ROS levels generated by BEAS-2B cells. However, these differences in generated cellular ROS levels between e-liquid base solution treatments and synthetic coolant-containing e-liquid base solution treatments are noteworthy and were observed regardless of nicotine content (5 %). Cellular ROS levels were increased by WS-3 and WS-23 treated in e-cig nicotine groups.
Regarding our acellular ROS data, we see that the difference in acellular ROS levels between the aerosolized WS-23 (3 %) solution and the filtered air control (p < 0.05) is higher than that between the aerosolized PG:VG with nicotine solution and the filtered air control (p > 0.05). Likewise, our acellular ROS data suggest that adding WS-23 to nicotine-containing e-liquid base leads to noteworthy changes in generated acellular ROS levels. Our findings are similar to that of previous studies showing that treatments with e-liquids induce significant levels of ROS production in BEAS-2B cells when compared to untreated cells [29]. Regarding our understanding of the potentially harmful effects of WS-23, our data seems to suggest that WS-23 itself has a limited impact in altering e-cig-generated acellular ROS levels as well as a limited effect on modifying the levels of ROS generated by BEAS-2B cells. Similarly, our data also seems to suggest that the addition of WS-3 to e-liquids does not significantly modify e-cig generated acellular ROS levels nor cellular ROS levels generated by treated BEAS-2B cells. Concerning the findings of other studies investigating the physiological effects of using coolant containing e-liquids, using human bronchial epithelial cell cultures, previously, another study found that treatment with menthol significantly increased mitochondrial ROS via the TRPM8 receptor [30]. BEAS-2B cell exposures reported by these investigators consisted of aerosol treatments, in which cells were exposed for two separate 1.5-minute durations, separated by an incubation period; however, our cell treatment protocol conducted cell culture exposures through direct stimulation (e-liquid being directly pipetted into cell-culture media) as reported recently [31], [32].
Recent studies investigating the potential of exposures to synthetic coolant-containing e-liquids may have an effect on pulmonary pathophysiology including cytotoxicity evaluations on BEAS-2B cells exposed to aerosols generated by various flavored e-cigs containing either WS-3, WS-23, or both [33]. Omaiye et al. [33] used Lactase Dehydrogenase (LDH), Neutral Red Uptake (NRU), and MTT (3-(4,5-dimethylthiazol-2-yl)− 2,5-diphenyltetrazolium bromide) tetrazolium reduction assays to assess the role of exposure to aerosols generated by e-liquids containing WS-3 and WS-23 have an effect in inducing cytotoxicity in human bronchial epithelial cells [33]. Similar to this study [29], our study involved analyzing the cellular responses of BEAS-2B cells to e-liquids containing either WS-3 or WS-23, and showed some cytotoxic responses when WS-3 and WS-23 combined with e-cig nicotine. However, n contrast to Omaiye et al. [33], which assessed the cytotoxicity induced by different treatments, our study assessed differences in ROS production. Additionally Omaiye et al. [33] used aerosol exposures, whereas our study conducted cell-culture exposures via direct stimulation [32]. We further determined the cytotoxicity of the cooling agents, when WS-23 was treated to BEAS2B cells for hazard characterization. Various toxicological parameters were calculated in a dose-response using a linear response phase (0.05–3 mg/mL), and found the dose > 2.0 mg/mL was more cytotoxic (based on significant LDH release). Further work is in progress to determine the LC50/IC50 of these synthetic coolants.
In rodent studies, rats exposed to aerosolized e-liquid containing WS-23 at tested doses (via acute and subacute exposures) found no substantial changes in histopathologic analyses of vital organs nor relative organ weights [34]. This same study, via a bronchioalveolar lavage fluid (BALF) analysis, found no significant difference in neutrophil concentration between rats which had undergone repeated 28-day WS-23 exposure and those apart of the respective control group [34]. Neutrophils are a major sources of endogenous ROS production. Likewise, future studies aimed at understanding the role of WS-23 in modulating e-cig induced oxidative stress should involve measurements of intracellular and extracellular ROS using isolated Polymorphonuclear Neutrophils (PMNs) [35]. More specifically, PMNs isolated from blood collected from mice exposed to aerosolized e-liquids of varying WS-23 concentrations can be analyzed via luminol enhanced chemiluminescence exposure [35]. The proposed experiment can provide insight into the differences between intra-and extra-cellular ROS of PMNs isolated from mice exposed to various concentration of WS-23 as reported recently [35]. Regarding our understanding of the effects of other e-liquid coolant additives, using human bronchial epithelial cell cultures, one study found that treatment with menthol significantly increased mitochondrial ROS via the TRPM8 receptor [30]. However, in contrast to our study, the studies of Nair et al. [30] BEAS-2B cell exposures consisted of aerosol treatments, in which cells were exposed for two separate 1.5-minute durations, separated by an incubation period; however, our cell treatment protocol consisted of a direct e-liquid treatment for 4 h. Hence, understanding the role of WS-3 and WS-23 in modulating e-cig-induced oxidative stress should involve measurements of intracellular and extracellular ROS using isolated Polymorphonuclear Neutrophils (PMNs) [35] and macrophages, airway immune cells and epithelial cells.
Our findings concur with previous studies showing that aerosolized e-liquids contain significant levels of acellular ROS and induce significant levels of cellular ROS in pulmonary epithelial cells [19], [21]. Regarding previous studies that analyzed acellular ROS levels within “cool/iced” flavored e-cigs, one study found differences in generated-acellular ROS levels between Tobacco-Derived Nicotine (TDN) and Tobacco-Free Nicotine (TFN) among cool/iced flavored e-cigs were minimal compared to tobacco and fruit flavors [20].
Regarding limitations in our study, our study did not include the treatment of airway epithelial cells with aerosolized e-liquids. Previous studies have shown that treatments with aerosolized e-liquids induce significant levels of ROS production in Human Bronchial Epithelial cells (BEAS-2B) [29]. Epithelial cells lining the airways are the first structural cell targets of any inhaled substances [36] and, the inhalation of e-cigs results in pulmonary epithelial cells being exposed to aerosols generated from e-liquids. In comparison to aerosol exposures, e-cig cell exposures using direct stimulation allows for a more precise control of dosage and a more expeditious analysis of different types of e-liquids consisting of various flavors, nicotine concentrations, and coolant concentrations [32]. However, cell cultures conducted with e-liquids via direct stimulation do not emulate the process associated with the actual usage of e-cigs which is the inhalation of aerosolized e-liquids into the lungs (“vaping”).
Future studies analyzing the role of WS-3 and WS-23 in potentially modifying ROS generated from BEAS-2B cells should utilize cell culture exposures via e-cig aerosols for analyzing the cellular oxidative-stress levels [37], [29]. Through this proposed assay, an understanding of how exposure to aerosolized synthetic coolants affects mitochondrial ROS production can be obtained. The reasoning for our reason to conduct e-cig cell exposures via direct stimulation rather than using aerosols lied in our understanding of that more studies investigating how the direct addition of e-liquids to pulmonary cells impacts cellular ROS responses are needed [31]. Consequently, after having observed significant levels of ROS generated by e-liquids containing WS-3 and WS-23 in cell-free conditions, we also determined how the addition of these synthetic coolants to e-liquids directly treated to pulmonary cells impact the cellular ROS levels. However, our study has shown that the addition of WS-3 and WS-23 to e-liquids, either 0 % or 5 % nicotine, has a minimal effect on modifying the acellular ROS levels from aerosolized e-liquid base solution or the cellular ROS levels generated by BEAS-2B cells exposed to e-liquid base solution. However, ROS levels were increased by WS-3 and WS-23 in e-cig/e-liquid nicotine groups. Thus, these preliminary findings do strongly suggest the need for further evaluation on the potential health risks associated with inhaling the newly marketed e-cigs containing synthetic coolants. Specifically, our findings do underscore the need for further investigation into the role of WS-3 and WS-23 in e-cigarettes in disrupting the endogenous oxidant and antioxidant balance in airways upon inhalation.
Funding
This research was supported by our TCORS Grant: CRoFT 1 U54 CA228110.
CRediT authorship contribution statement
Conceptualization, I.R.; methodology, I.R.; assay performance: S.Y, SBS, software, S.Y., SBS; validation, S.Y, SBS, I.R.; formal analysis, S.Y., SBS; investigation, S.Y. SBS; re-sources, I.R.; data curation, S.Y., SBS; writing—original draft preparation, S.Y, SBS and I.R.; writing—review and editing, S.Y., SBS, M. M., H.S.C., and I.R.; visualization, S.Y.; supervision, I.R.; project administration, I.R.; funding acquisition, I.R. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Fig. 1 and the Graphical Abstract were made using BioRender and AdobeIllustrator. Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9 were made using GraphPadPrism. Joseph Lucas (JL) and Dr. Thivanka Muthumalage (TM) for technical help and discussions.
Informed consent statement
Not applicable; no human subjects were involved.
Conflicts of interest
The authors declare no conflicts of interest.
Handling Editor: Lawrence Lash
Data availability
We declare that we have provided all the data in figures.
References
- 1.King B.A., Jones C.M., Baldwin G.T., Briss P.A. E-cigarette, or vaping, product use-associated lung injury: looking back, moving forward. Nicotine Tob. Res. 2020;22(Suppl 1):S96–S99. doi: 10.1093/ntr/ntaa186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Lancet Respiratory M. The EVALI outbreak and vaping in the COVID-19 era. Lancet Respir. Med. 2020;8(9):831. doi: 10.1016/S2213-2600(20)30360-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu X., Sun L., Xie Z., Li D. Perception of the food and drug administration electronic cigarette flavor enforcement policy on Twitter: observational study. JMIR Public Health Surveill. 2022;8(3) doi: 10.2196/25697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz M.C., Donovan E.M., Schillo B.A., Vallone D. Menthol e-cigarette sales rise following 2020 FDA guidance. Tob. Control. 2021;30(6):700–703. doi: 10.1136/tobaccocontrol-2020-056053. [DOI] [PubMed] [Google Scholar]
- 5.Davis D.R., Morean M.E., Bold K.W., Camenga D., Kong G., Jackson A., Simon P., Krishnan-Sarin S. Cooling e-cigarette flavors and the association with e-cigarette use among a sample of high school students. PLOS One. 2021;16(9) doi: 10.1371/journal.pone.0256844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diomede L., Salmona M. The soothing effect of menthol, eucalyptol and high-intensity cooling agents. Nutrafood. 2017;16(79) doi: 10.17470/NF-017-1006-3. [DOI] [Google Scholar]
- 7.Leventhal A., Cho J., Barrington-Trimis J., Pang R., Schiff S., Kirkpatrick M. Sensory attributes of e-cigarette flavours and nicotine as mediators of interproduct differences in appeal among young adults. Tob. Control. 2020;29(6):679–686. doi: 10.1136/tobaccocontrol-2019-055172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erythropel H.C., Anastas P.T., Krishnan-Sarin S., O'Malley S.S., Jordt S.E., Zimmerman J.B. Differences in flavourant levels and synthetic coolant use between USA, EU and Canadian Juul products. Tob. Control. 2020;30(4):453–455. doi: 10.1136/tobaccocontrol-2019-055500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jabba, S.V., H.C. Erythropel, D.G. Torres, L.A. Delgado, J.G. Woodrow, P.T. Anastas, J.B. Zimmerman and S.E. Jordt , 2022. Synthetic Cooling Agents in US-marketed E-cigarette Refill Liquids and Popular Disposable Ecigarettes: Chemical Analysis and Risk Assessment." Nicotine Tob Res. [DOI] [PMC free article] [PubMed]
- 10.Omaiye E.E., McWhirter K.J., Luo W., Pankow J.F., Talbot P. High-nicotine electronic cigarette products: toxicity of JUUL fluids and aerosols correlates strongly with nicotine and some flavor chemical concentrations. Chem. Res. Toxicol. 2019;32(6):1058–1069. doi: 10.1021/acs.chemrestox.8b00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pankow J.F., Luo W., McWhirter K.J., Gillette S., Cohen J.E. "Menthol-Plus': a major category of cigarette found among 'concept' descriptor cigarettes from Mexico. Tob. Control. 2022;31(e1):e18–e24. doi: 10.1136/tobaccocontrol-2020-056173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leventhal A., Dai H., Barrington-Trimis J., Sussman S. 'Ice' flavoured e-cigarette use among young adults. Tob. Control. 2021 doi: 10.1136/tobaccocontrol-2020-056416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu S.J., Li L., Duffy B.C., Dittmar M.A., Durocher L.A., Panawennage D., Delaney-Baldwin E.R., Spink D.C. Investigation of vaping fluids recovered from New York state e-cigarette or vaping product use-associated lung injury patients. Front Chem. 2021;9 doi: 10.3389/fchem.2021.748935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pryor W.A., Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann. N. Y Acad. Sci. 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. discussion 27-18. [DOI] [PubMed] [Google Scholar]
- 15.Ogunwale M.A., Li M., Ramakrishnam Raju M.V., Chen Y., Nantz M.H., Conklin D.J., Fu X.A. Aldehyde detection in electronic cigarette aerosols. ACS Omega. 2017;2(3):1207–1214. doi: 10.1021/acsomega.6b00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis L.C., Sapey E., Thickett D.R., Scott A. Predicting the pulmonary effects of long-term e-cigarette use: are the clouds clearing? Eur. Respir. Rev. 2022;31(163) doi: 10.1183/16000617.0121-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noël A., Hossain E., Perveen Z., Zaman H., Penn A.L. Sub-ohm vaping increases the levels of carbonyls, is cytotoxic, and alters gene expression in human bronchial epithelial cells exposed at the air-liquid interface. Respir. Res. 2020;21(1):305. doi: 10.1186/s12931-020-01571-1. PMID: 33213456; PMCID: PMC7678293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinkston R., Zaman H., Hossain E., Penn A.L., Noël A. Cell-specific toxicity of short-term JUUL aerosol exposure to human bronchial epithelial cells and murine macrophages exposed at the air-liquid interface. Respir. Res. 2020;21(1):269. doi: 10.1186/s12931-020-01539-1. PMID: 33069224; PMCID: PMC7568376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yogeswaran S., Muthumalage T., Rahman I. Comparative reactive oxygen species (ROS) content among various flavored disposable vape bars, including cool (Iced) flavored bars. Toxics. 2021;9(10) doi: 10.3390/toxics9100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yogeswaran S., Rahman I. Differences in acellular reactive oxygen species (ROS) generation by E-cigarettes containing synthetic nicotine and tobacco-derived nicotine. Toxics. 2022;10(3) doi: 10.3390/toxics10030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J., Zhang Y., Sisler J.D., Shaffer J., Leonard S.S., Morris A.M., Qian Y., Bello D., Demokritou P. Assessment of reactive oxygen species generated by electronic cigarettes using acellular and cellular approaches. J. Hazard. Mater. 2018;344:549–557. doi: 10.1016/j.jhazmat.2017.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuo L., Wijegunawardana D. Redox role of ROS and inflammation in pulmonary diseases. Adv. Exp. Med. Biol. 2021;1304:187–204. doi: 10.1007/978-3-030-68748-9_11. [DOI] [PubMed] [Google Scholar]
- 23.Valavanidis A., Vlachogianni T., Fiotakis K. Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health. 2009;6(2):445–462. doi: 10.3390/ijerph6020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Toorn M., Rezayat D., Kauffman H.F., Bakker S.J., Gans R.O., Koeter G.H., Choi A.M., van Oosterhout A.J., Slebos D.J. Lipid-soluble components in cigarette smoke induce mitochondrial production of reactive oxygen species in lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297(1):L109–L114. doi: 10.1152/ajplung.90461.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chatterjee S., Tao J.Q., Johncola A., Guo W., Caporale A., Langham M.C., Wehrli F.W. Acute exposure to e-cigarettes causes inflammation and pulmonary endothelial oxidative stress in nonsmoking, healthy young subjects. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;317(2):L155–L166. doi: 10.1152/ajplung.00110.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams M., Ventura J., Loza A., Wang Y., Talbot P. Chemical elements in electronic cigarette solvents and aerosols inhibit mitochondrial reductases and induce oxidative stress. Nicotine Tob. Res. 2020;22(Supplement_1):S14–S24. doi: 10.1093/ntr/ntaa193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J., Zhang T., Johnston C.J., Kim S.Y., Gaffrey M.J., Chalupa D., Feng G., Qian W.J., McGraw M.D., Ansong C. Protein thiol oxidation in the rat lung following e-cigarette exposure. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen-Sankey J., Bover Manderski M.T., Young W.J., Delnevo C.D. Examining the survey setting effect on current e-cigarette use estimates among high school students in the 2021 national youth tobacco survey. Int. J. Environ. Res. Public Health. 2022;19(11) doi: 10.3390/ijerph19116468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L., Wang Y., Chen J., Yang X.M., Jiang X.T., Liu P., Li M. Comparison of biological and transcriptomic effects of conventional cigarette and electronic cigarette smoke exposure at toxicological dose in BEAS-2B cells. Ecotoxicol. Environ. Saf. 2021;222 doi: 10.1016/j.ecoenv.2021.112472. [DOI] [PubMed] [Google Scholar]
- 30.Nair V., Tran M., Behar R.Z., Zhai S., Cui X., Phandthong R., Wang Y., Pan S., Luo W., Pankow J.F., Volz D.C., Talbot P. Menthol in electronic cigarettes: a contributor to respiratory disease? Toxicol. Appl. Pharmacol. 2020;407 doi: 10.1016/j.taap.2020.115238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lerner C.A., Sundar I.K., Yao H., Gerloff J., Ossip D.J., McIntosh S., Robinson R., Rahman I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLOS One. 2015;10(2) doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snoderly H.T., Nurkiewicz T.R., Bowdridge E.C., Bennewitz M.F. E-cigarette use: device market, study design, and emerging evidence of biological consequences. Int. J. Mol. Sci. 2021;22(22) doi: 10.3390/ijms222212452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omaiye E.E., Luo W., McWhirter K.J., Pankow J.F., Talbot P. Disposable puff bar electronic cigarettes: chemical composition and toxicity of E-liquids and a synthetic coolant. Chem. Res. Toxicol. 2022 doi: 10.1021/acs.chemrestox.1c00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Z.H., Liu Y.S., Li X.D., Xu T., Xu J., Yang X.M., Ma R.Q., Jiang X.T. Acute and subacute inhalation toxicity assessment of WS-23 in Sprague-Dawley rats. J. Appl. Toxicol. 2021;41(11):1826–1838. doi: 10.1002/jat.4166. [DOI] [PubMed] [Google Scholar]
- 35.Kuhns D.B., Priel D.A.L., Chu J., Zarember K.A. Isolation and functional analysis of human neutrophils. Curr. Protoc. Immunol. 2015;111 doi: 10.1002/0471142735.im0723s111. 7 23 21-27 23 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hiemstra P.S., Tetley T.D., Janes S.M. Airway and alveolar epithelial cells in culture. Eur. Respir. J. 2019;54(5) doi: 10.1183/13993003.00742-2019. [DOI] [PubMed] [Google Scholar]
- 37.Muthumalage T., Lamb T., Friedman M.R., Rahman I. E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-51643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We declare that we have provided all the data in figures.