Highlights
-
•
Heavy metals are recalcitrant to degradation and cause concern.
-
•
Heavy metals exert a toxic effect on living beings.
-
•
Natural and anthropogenic sources contribute to HM pollution.
-
•
Heavy metal remediation future relies on composite or hybrid technologies.
Keywords: Ecotoxicity, Heavy metals, Pollution, Remediation, Sustainable
Abstract
Heavy metal (HM) pollution is extremely deleterious because of the toxicity they exert on human beings, animals, and plants. HMs are recalcitrant to degradation, and hence persistent in the environment for a longer duration adding to the concern. HMs at high concentrations have adverse effects on the production of food as they affect the metabolic activity of plants. HMs have serious implications for human health, reaching the tissue via direct ingestion, dermal contact, inhalation, and adsorption. Several methods have been explored for the eradication of HMs from the environment. Conventional methods of metal removal are constrained by the processing problems, expenses, and the generation of toxic sludge, therefore more research is now focused on the use of bacteria, fungi, plants, and diatoms for the removal of metal ions from the environment. In this context, this review article sheds light on the distribution of HMs in the environment, their sources, and the ecotoxicity they exert on the environment and living beings. The sustainable remedies to decontaminate the environment and the current knowledge and strategies to minimize HM toxicity are also discussed along with the recent developments in the use of nanoparticles and diatoms for HM removal.
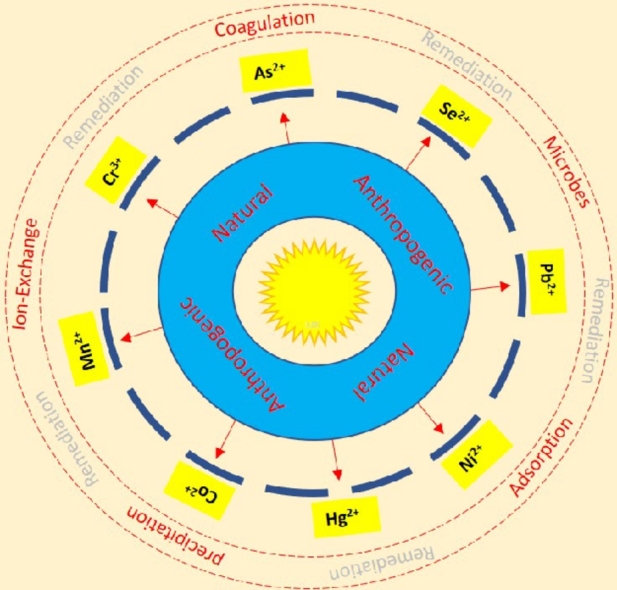
Graphical abstract
1. Introduction
Irrational use of mineral resources globally has resulted in the production of copious amounts of metal mining waste, affecting and damaging the environment (Gautam et al., 2016). The widespread use of HMs in the past few years, owing to accelerated industrialization and modernization has led to the exploitation of natural resources (Cao et al., 2021). Several pollutants including organic, inorganic, nanoparticles, organometallic compounds, and radioactive isotopes have polluted the environment at an alarming rate, thereby exacerbating the problem of pollution (Sharma et al., 2021). One of the emerging contaminants is HMs. They are designated as HMs because of their high density and atomic weight. The term HMs can be interchangeably used with toxic metals (Sadhu et al., 2015).HM pollution remains a global environmental challenge that poses a significant threat to human life. HMs cannot be degraded but can be converted to a lesser toxic form. Excessive concentration of HMs harms plant metabolism, hence affecting the production of food qualitatively and quantitatively. HMs affect the health of human beings and are regarded as a potent carcinogen and mutagen (Saravanan et al., 2021). According to the United States Environmental Protection Agency (USEPA), HMs are considered priority pollutants. According to US Agency for Toxic Substances and Diseases Registry (ATSDR), lead (Pb) is the most toxic substance followed by mercury (Hg), and arsenic (As) whereas cadmium (Cd) is the sixth most toxic metal on the list. The high concentration of HMs in terrestrial and aquatic ecosystems is regarded as a major cause of concern and acts as an ecological toxin (Budianta et al., 2021).
Anthropogenic sources are the major source of environmental pollution. Industrial discharge, automobiles, and roadways are major sources of HM pollution since the particulate matter in the emissions contains the HMs such as Cd, Pb, and As (Nogueira et al., 2013). Sewage sludge finds its way to fields which can result in HM accumulation in soil and plants. Adamu et al., 2015, reported HM contamination in the sludge and sewage water in River Kubanni (Adamu et al., 2015). According to Onat et al., 2013, every year, a tonne of HMs is released into soil, such as one million tonnes of Nickel (Ni) and 5 million tonnes of Pb are released into the soil each year (Onat et al., 2013). Groundwater contamination is direct via the leachate from solid waste disposal, mining, industrial waste, etc. (Tejaswini et al., 2022).
The toxic metals transfer in the food chain is a major challenge. It has been studied that HMs with no cellular role such as Cd and As are toxic even at lower (nM) concentrations whereas the ones which act as cofactors may be essential at lower (nM) concentrations but at higher concentrations (μM or mM) are toxic (Singh et al., 2011). Some HMs such as Zn, iron (Fe), Cu, Co, and molybdenum (Mo) are required by human beings in trace amounts but can induce toxicity at higher concentrations (Sodhi et al., 2019). Toxic HMs including As, Pb, Cd, and Hg are not required by human beings but are reported to induce carcinogenicity if accumulated for a longer time in the bodies (Balali-Mood et al., 2021). Toxic metals can accumulate in the body and disrupt the functioning of the kidney, bones, liver, heart, brain, etc. They replace the minerals in the body which disrupts biological functioning (Rai et al., 2019; Hamza et al., 2021).
In Southeast Asian countries like Thailand, Pakistan, Indonesia, India, and Bangladesh monitoring HM contamination is given huge importance (Shaji et al., 2021). Kapungwe, 2013, showed that toxic metals such as Cr, Cu, Ni, Pb, and Co are contaminated in the wastewater, crops, and soil in Zambia (Kapungwe, 2013). In Egypt, wastewater is used to irrigate crops which has led to HM accumulation in plants and soil above the permissible limits (Nguyen et al., 2018). In India, the central pollution control board (CPCB 2011), reported that Maharashtra, Andhra Pradesh, and Gujarat are responsible for 80% of the hazardous waste generation which includes toxic HMs also. Cd is readily available to crops as it is the most mobile metal. Industrial and waste discharge can lead to the contamination of soil. Toxic HMs such as Zn, Pb, Cd, As, and Cr were reported in parsley (Petroselinum crispum), beet leaf (Beta vulgaris), coriander (Coriandrum sativum), radish leaf (Raphanus sativus), and basil (Ocimum basilicum) in North East of Iran. These vegetables are hazardous for human consumption (Kumar et al., 2021).
Rapid modernization and industrialization have deteriorated the air, soil, and water quality as the industrial effluents harm the quality of water and leaching can ultimately affect the soil quality (Ruba et al., 2021). As Ni and Cobalt (Co) are dissolved toxic metals that are directly discharged into the water bodies. Mineral processing, electroplating, and paint formulation increase the concentration of toxic metals in the water (Samanta et al., 2017). The toxic metals intake via drinking water can result in pulmonary, digestive, and renal failures along with skin diseases (Mahurpawar, 2014). There is an urgent need for the treatment of industrial effluents before they get discharged to different water sources. Treatment methods include the likes of adsorption, membrane filtration, ion exchange, and electrochemical processes (Nayak et al., 2017). Adsorption is a cost-effective way to remove pollutants (Zhang et al., 2014). For the sanitization of water, various cost-effective adsorbents are developed (Hanfi et al., 2020). The adsorption having a high capacity of adsorption, low cost, and compatibility are indispensable. Traditional methods are prone to generate toxic sludge and maintenance is expensive so research has focussed majorly on the use of microorganisms and plants for the removal of pollutants (Sathya et al., 2022).
The spread and distribution of HMs in the environment are dependent on their chemical characteristic. The HM gets accumulated over a while, and when there is a change in the chemical and environmental conditions, the HMs may get activated and deteriorate the environment. Mainly, the HMs in the water, air, and soil are contributed via anthropogenic sources (Kubier et al., 2019). Table 1 shows the sources, adverse effects, and significance of toxic HMs.
Table 1.
Sources of HMs in the environment.
| HM | Sources | Adverse effects of the HMs | Significance of Heavy metal | References | |
|---|---|---|---|---|---|
| Cu2+ | Fertilizers, Agricultural fungicides, electroplating, algicides | Wilson's disease, headaches, nausea, dizziness due to long-term exposure | An essential nutrient, Helps in the formation of RBC with Iron. Helps in bone formation, blood vessels, and the functioning of the immune system. | (Khalid et al., 2021) | |
| Cd2+ | Agricultural fungicides, electroplating, algicides, Cd & Ni batteries, welding | The cumulative toxin, carcinogenic, neural problems, and kidney failure. In plants, leads to tissue death | No significant function in the human body. Transported in the blood bound to metallothionein. |
(Genchi et al., 2020) | |
| Cr6+ | Chrome plating, paints, dyes, ceramics | Lung cancer, pulmonary illness | Helps in the breakdown of carbohydrates and fats. Stimulate the synthesis of cholesterol and fatty acids. Helps in the action of insulin and also in the breakdown of glucose. | (Tabelin et al., 2018) | |
| Ni2+ | Chrome plating, Cd & Ni batteries | Allergic contact dermatitis. oxidative stress, nutritional imbalance in plants | A micronutrient important for the proper functioning of the human body increases hormonal activity and helps in lipid metabolism. A micronutrient in plants too and helps in nitrogen fixation and urea metabolism. |
(Kuhn et al., 2022) | |
| Zn2+ | Refineries, metal plating, plumbing, brass manufacture | Headaches, nausea, dizziness due to long-term exposure, Night blindness | Zinc is important for the immune system to function properly and plays an important role in cell growth, cell division, also in wound healing. Helps in the breakdown of carbohydrates which is an important dietary nutrient. In zinc-deficient plants, chlorophyll synthesis is reduced significantly. | (Kuhn et al., 2022) | |
| As3+ | Mining, smelting, fossil fuels, dietary intake (cereals, poultry, fish, and dairy products) | Carcinogen leading to lung cancer and skin cancer, hyperpigmentation, keratosis | In industries as an alloying agent for the smelting, used in textiles and paper industries, pesticides, feed additives but no significant function in the human body | Sodhi et al., 2019 | |
2. Entry, effects, and transport of HMs into the environment
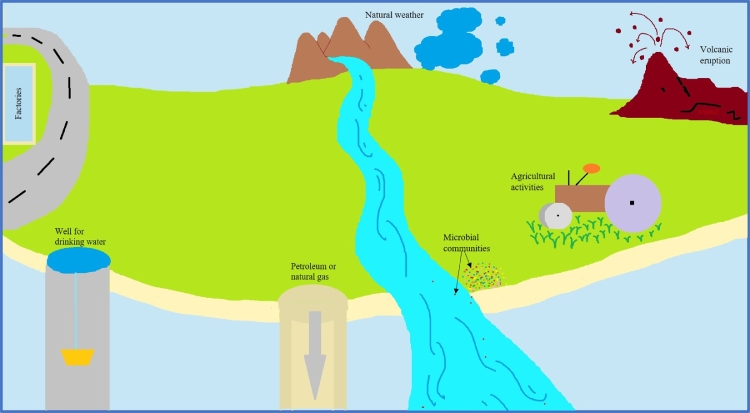
HMs find their way into the environment via natural sources such as weathering of rocks and volcanic eruptions, and anthropogenically due to industrial activity, mining, and sewage disposal (Fig. 1). Physical factors such as temperature, air circulation, wind speed, and water direction influence the stability of HMs in the environment (Rezapour et al., 2022).
Fig. 1.
Sources of HMs in the environment.
2.1. HMs-induced pollution of water and soil
Rapid industrialization coupled with urbanization is a major player in HMs pollution in the water bodies. Runoffs from villages, cities, and industries can accumulate in the sediments associated with water bodies. The toxic metals in trace amounts can be very toxic to the ecosystem (Paul, 2017). The toxicity to the living beings depends on the HMs speciation. For ex., hexavalent Cr [Cr (VI)] is more toxic than trivalent Cr [Cr (III)], and the duration of exposure is also important (Zaynab et al., 2022). The effect of bioaccumulation is most prominent in humans as they are last in the food chain. HMs are not removed effectively via the sewage treatment plant, they get degraded in the final sludge produced. Therefore, in raw sewage, they are found at very high concentrations (Masocha et al., 2022).
Irrigation with wastewater contaminated with HMs, pesticides containing HMs, leaded paint, and coal combustion residues can lead to pollution of the soil. The crops absorb the HMs from the agricultural lands irrigated by the sewage and wastewater containing HMs. The crops are in turn consumed by humans leading to dreadful diseases such as cancer. The major problem with HMs is that they are refractory to degradation thereby persisting in the environment for a longer time (Khan et al., 2015). HM enters the food chain thereby ruining the ecosystem's integrity. HMs can alter the biodegradation capacity of organic pollutants, making them persist in the environment for a longer time and accentuating their effect. They directly affect soil health by altering the pH, porosity, and increasing acidity of the soil, and indirectly via the absorption by plants which can be lethal for both the plants and also affect the food chain (Rascio et al., 2011).
3. Ecotoxicity of HMs to living beings
HM pollution is getting intensified due to the phytotoxicity they exert on the plants. Research is now focused mainly on the effect of HMs on plants and the mechanisms used by them to counter their detrimental effects (Singh et al., 2011). Along with exerting toxicity in plants, their bioaccumulation in the successive trophic levels in the food chain is a major cause of concern. The plants must adapt to different soils composition as they are sessile (Xun et al. 2018). The HMs can disturb photosynthesis, germination, development, and essential digestion. HMs can cause senescence, and putrefaction (Shi et al. 2018). Certain HMs are required by living beings in trace amounts, but at a higher concentration than normally required they can be a potential toxin (Sharma et al., 2019). Essential HMs such as Fe, Cu, Zn, Mo, and Mn play important physiological roles inside animals and plants. HMs help in the redox reactions and also are a part of enzymatic reactions, intricate for the elimination of superoxide radicals (e.g., ascorbate, oxidase, and superoxide dismutase) (Mahmood et al., 2007; Hasan et al., 2019). Ni is an important trace element for living beings as it is a part of the enzyme urease, which is crucial for the working and wellness of organisms. Cu helps as an electron donor in photosystem I and plays an important role as a cofactor of mono-oxygenase, di-oxygenase, and oxidase (Sarkar et al., 2021). Non-essential HMs Cd, Pb, Cr, Cu, Co, As, and Ni are toxic at even low concentrations. They follow the bioremediation process followed by organic contaminants which means they are recalcitrant to physicochemical and biological treatment methods (Balali Mood et al., 2021). The higher concentration of Cu can lead to the production of reactive oxygen species in plants and can result in oxidative stress (Filetti et al. 2018). Pb at high concentrations can instigate uneven morphology in plants (Kushwaha et al. 2018). High Ni concentration leads to nutrient imbalance (Mendez et al. 2014). At high Cr concentration. the photosynthesis process is affected especially the fixation of carbon dioxide, photophosphorylation, and electron transport are also disturbed. Phytotoxicity of As can lead to leaf shrinking and putrefaction and hinders the development of shoots (Kumari et al. 2018).
In human beings, they can disrupt the normal functioning of major organs such as the brain, heart, and liver, and also decreases the activity of the central nervous system. The metals enter the body via the consumption of beverages, food, and inhaled air and accumulated in the tissues of living beings. Among different heavy metals, chronic exposure to low doses of cancer-causing heavy metals may induce many types of cancer (Junaid et al., 2016). Occupational exposure to hexavalent Cr because of toxic metals is observed in cases of lung cancer (Mishra et al., 2019). According to Jiang et al., 2013, Cd-contaminated food can cause a risk of postmenopausal breast cancers. Both acute and chronic exposure to toxic metal arsenic can lead to liver cancer along with various neurological problems, teratogenesis, mutagenic, and genotoxic effects (Jiang et al., 2013). HMs can result in a delay in human growth, and disturbance of bioregulatory systems accountable for functional or psychological disorders, such as neurodegenerative pathologies including Alzheimer's, Parkinson's diseases, and chronic fatigue syndrome. Intoxication by Pb and Hg can result in autoimmune disorders such as rheumatoid arthritis (Lauwerys et al. 2007).
The levels of different HMs such as Cr, Zn, Cd, and Pb were detected in edible vegetables such as Coriander sativum, Ocimum basilicum, Beta vulgaris, and Raphanus sativus in Iran. In some of the vegetables, the HM concentration exceeded the maximum limit prescribed by World Health Organization (WHO). The concentration of HMs in vegetables is measured in mg/kg. These vegetables are harmful and unsafe for consumption by human beings and can increase the risk of chronic diseases such as cancer in humans (Manzoor et al., 2018). As a part of the risk assessment, the effects of HMs on the invertebrate species were studied. Invertebrates are more sensitive to toxic metals than vertebrates. Invertebrates form a vital part of the food chain and any change in their abundance can affect human beings also (Xin et al., 2015). A study by Mrozinska et al., 2020, conducted in the lake Lebsko of the Baltic sea and sediments, determined the effect of HMs Cr, Pb, Ni, and Cu on the invertebrates inhabiting the Baltic sea sediments (Mrozinska et al., 2020). The toxic metals concentration was determined using inductively coupled plasma-optical emission spectroscopy (ICP-OES) and it was found that HMs concentration is significantly higher in the sediments affecting the structure of the benthic community in the sediments. Another study conducted by Monchanin et al., 2021, showed that invertebrates are sensitive to toxic metal concentrations such as Cd and Pb and their exposure can lead to a decline in the biodiversity and abundance of terrestrial invertebrates (Monchanin et al., 2021).
4. Conventional methods of remediation
There are multiple physicochemical treatment methods for the remediation of HMs contaminated sites such as adsorption, membrane filtration, electro-dialysis, chemical precipitation, and photocatalysis. These methods have advantages which include ease of operation, and flexibility, and can also afford the discharge of complex pollutants along with lower space requirements and installation. The drawbacks include the consumption of higher energy, and high operational, and handling costs (Dhingra et al., 2020). The most commonly used method for HM removal from inorganic effluents is chemical precipitation. The pH can be adjusted in the range of 9–11 to provide basic conditions which significantly improve the removal efficiency of the HMs. Limestone and lime can be used as precipitating agents due to their low cost and easy availability (Aziz et l., 2008). Ion exchange can be used to remove contaminated wastewater from industries. The cations and anions can be exchanged from contaminated materials. The most commonly used matrix is synthetic organic ion exchange resins. The ion exchange has many disadvantages such as that it is a nonselective technique and sensitive to pH and the concentrated HM solution (Al-Enezi et al., 2004). Adsorption, on the other hand, involves the transfer of metal ions from the solution phase to a solid phase. Recently modified biopolymers are employed for HM removal from contaminated water. Conventional methods of HMs removal generate toxic sludge and the cost of operating these methods is very high so most of the research has focused on the use of living organisms as an eco-friendly and sustainable solution to removing HMs (Barakat, 2011).
4.1. Bioremediation is a potential alternative for HMs removal and environmental restoration
Fungi, bacteria, plants, and algae are instrumental in the removal of pollutants. Bacteria are ubiquitous in the environment and almost all biological processes of life require bacteria. Metals play an instrumental role in the metabolic processes of bacteria which allows the bacteria to grow in the presence of toxic metals (Igiri et al., 2018). But, at higher concentrations the toxic metals exert toxicity to microbes by disturbing the osmotic balance and oxidative phosphorylation, along with the alterations in the polypeptides and nucleic acids (Fashola et al., 2016). Bacteria tolerant to HMs stress can be utilized for HMs bioremediation. Bacteria that are isolated from an environment that has high metal stress can survive the pressure and can be exploited for HMs removal (Urra et al., 2019). HMs cannot be degraded and can only be transformed from one form to the other. The bacteria should transform the HMs into a lesser toxic and less bioavailable form (Ahemad, 2012). HMs stress has resulted in the selection of HMs-resistant bacteria, and to circumvent the HMs stress, the bacteria have evolved processes that aid the bacteria to tolerate the toxic metals i.e., the cell wall accumulation of HMs, redox reactions, entrapment (extracellular or intracellular), transportation across the cell membrane (Jasmin et al., 2020). The HMs resistance is mainly attributed to plasmid but some may be chromosomal also (Sun et al., 2021). Bioaccumulation is the accumulation of bacterial cells on living cells whereas biosorption is the passive removal of HMs via adsorption on non-living biomass (Diep et al., 2018). Various living organisms such as bacteria e.g., Bacillus, Alcaligenes, fungi e.g., Penicillium, Aspergillus, and plants e.g., Eichhornia, Pistia are involved in the bioremediation of HMs (Table 2) and Table 3 depicts the advantages and disadvantages of different treatment methods such as physical, chemical, and biological in the removal of HMs from the environment.
Table 2.
Various living organisms involved in HMs bioremediation.
| The living organism involved in Bioremediation | Living organism | HMs removed | Operating conditions for HMs removal | References |
|---|---|---|---|---|
| Bacillus sp. ATS-1 | Bacteria | Cu2+ | HM was removed at pH 5, temperature 25℃, and total metal adsorption 16.3 mg/g. | (Kanmani et al., 2012) |
| Zoogloea ramigera | Bacteria | Cu2+ | HM was removed at pH 4, temperature 45℃, and total metal adsorption 52.3 mg/g. | (Dourado et al., 2013) |
| Exiguobacterium sp. ZM-2 | Bacteria | Cr6+ | Anaerobic conditions required for Cr6+removal | (Rajesh et al., 2014) |
| Nesterenkonia sp. MF2 | Bacteria | Cr6+ | Aerobic conditions required for Cr6+removal | (Oyewole et al., 2019) |
| Burkholderia sp. strain | Bacteria | Cd2+ | HM was removed at temperatures 30℃ and 200 rpm and total metal adsorption was 53.70 mg/kg | (Prajapati et al., 2012) |
| Alcaligenes sp. MMA | Bacteria | Cu2+, Cd2+, Cr6+, Ni2+, and Zn2+ | At temperature 28℃ and pH 7, 88.45% Cu2+, 53.04% Cd2+, 48,93% Cu2+, Cd2+, Cr6+, Ni2+, and Zn2+at 20 mg/L of concentration. | (Sodhi et al., 2020) |
| Bacillus sp. strain | Bacteria | Cd2+ | HM was removed at temperatures 30℃ and 200 rpm and with total metal removal of 90.14% | (Miretzky et al., 2004) |
| Penicillium notatum | Fungi | Cd2+ | 77.67% biosorption in 28 days at temperature 28℃ and pH 7 | (Mishra et al., 2008) |
| Aspergillus niger | Fungi | Ni2+ | 81.07% biosorption in 28 days at temperature 28℃ and pH 7 | (Oyewole et al., 2019) |
| Pistia stratiotes | Plant root and leaves | Cr6+ | 100% Cr6+ removal in 72 h at an initial concentration of 5 mg/L | (Koul and Taak, 2018) |
| Pistia stratiotes | Plant root and leaves | Cr6+ | 97.3%, 72.2%, and 73.5% Cr6+removed at 1,2, and 4 mg/L of initial metal solution in 190 h. | (Shrestha et al., 2021) |
| Eichhornia crassipes | Plant root and leaves | Cr6+ | 84% removal of Cr6+ achieved in 11 days | (Sodhi et al., 2021) |
Table 3.
Advantages and disadvantages of HMs treatment methods.
| Methods to remove HMs | Way of treatments | Advantages and Disadvantages | References |
|---|---|---|---|
| Physical | Electro-kinetic remediation Mechanical separation |
Can be applied to different metals But the homogeneous distribution is the prerequisite for mechanical separation |
(Jacob et al., 2018) |
| Chemicals | Chemical precipitation Coagulation and flocculation Flotation |
Relatively less invasive method High cost and the generation of toxic sludge |
(Dakal et al., 2016) |
| Biological methods | Bioremediation Phytoremediation |
No side effects and eco-friendly method Large amounts of waste can be generated |
(Chandrangsu et al., 2017) |
4.2. HMs uptake by bacterial cell
HM uptake in the bacteria takes place majorly by two mechanisms mainly the ATP independent and dependent mechanism. ATP-independent uptake by bacterial cells is a non-specific mechanism that works on secondary active transport and uses the chemiosmotic gradient across the cell membrane. The HMs can be transported at a faster rate via this mechanism, whereas, the second process requires ATP utilization and is highly specific to the substrate and slower compared to the ATP-independent mechanism (Dakal et al., 2016). Bacteria including the likes of Escherichia coli can alter the porin production against toxicity caused by Cu. So, it can be noted that bacteria can alter their cell membrane or cell wall composition and decrease cell permeability to protect themselves from the stress of HMs (Rani et al., 2009). Metal ions encounter the bacterial cell wall and get accumulated in functional groups on the bacterial cell wall. Metal ions mainly bind to the carboxyl group which is the most abundant on the bacterial cell wall. The electrostatic interactions are facilitated by the negatively charged carboxyl ions which can bind the positively charged metal ions. HMs in Gram-positive bacteria get accumulated much more than the Gram-negative bacteria (Kang et al., 2007). Cu can bind to the carboxyl groups on Streptomyces pilosus. The amine group can chelate the negatively charged metals. Biosorption can be determined by scanning and transmission electron microscopy, FTIR, etc. Cu at low concentration is required for the enzyme biosynthesis and cofactor of cytochrome c oxidase, and hydroxylase. In the soil and water, bacteria are exposed to high concentrations of Cu as it is used in mining, agricultural, and industrial processes. At high concentrations, the Cu is highly toxic to bacteria thus different mechanisms are developed by bacteria to overcome the Cu toxicity (Issazadeh et al., 2013). Genetic determinants for the regulation of Cu are present in the plasmid in bacteria, few studies report the chromosomal and plasmid genes' co-ordination for Cu regulation in bacteria. P-type ATPases which are required to pump Cu out of cells regulate Cu resistance in bacteria (Mazhar et al., 2021). For e.g., in E.coli, multi-protein complex CusCBA controls the efflux of Cu. CusA is an RND protein, that drives Cu out of the cell with the aid of cusB and cusC gene products (Verma et al., 2019). In Pseudomonas sp. cop operon is inducible by Cu and located on plasmids and imparts resistance to Cu consisting of CopA, CopB, CopC, and CopD proteins. Zn is a trace element required by cellular enzymes and complexes. Zn enters the bacterial cell via an unspecific mechanism that is fast and also involved in magnesium uptake. But at high concentrations, zinc can inhibit enzymes. P-type ATPases encoded by chromosomal genes help in zin efflux across the plasma membrane by an active transport mechanism. RND transporter uses a proton gradient for the transport of materials across the plasma membrane. It is common in Gram-negative bacteria (Haferburg and Kothe, 2007). Ni tolerance is determined by a yohM (rcnA) gene encoding a transmembrane protein. The cadA system is responsible for Cd resistance in Gram-positive bacteria in response to the accumulation of Cd inside the cells. CadA is a plasmid-encoded system and helps in pumping Cd out of the cell actively (Ghazisaeedi et al., 2020).
4.3. Phytoremediation: a sustainable solution for decontamination of HMs
The availability of the microbes and speciation along with physicochemical characteristics are key factors that govern the remediation of HMs (Abatenh et al., 2017). The metal degradation rate can get affected because of less interaction frequency between the HM and the bacteria. The metals and microbes are not uniform in the environment which may retard the biodegradation efficiency of microbes. Temperature, pH, temperature, and type of matrix also pose a constraint on the removal of metals (Nedjimi, 2021). As discussed earlier the HMs cannot be biodegraded but can be converted to a lesser toxic form, but the transformed products are more persistent and toxic than the parent compound. Owing to the specificity of the biological processes, optimum conditions and an appropriate amount of contaminants and nutrients are requisite for the successful remediation of HMs. Phytoremediation makes use of plants and rhizospheric microorganisms to transform or degrade pollutants in the environment, water, and soil (Nedjimi, 2020). Phytoremediation has come across as an efficient, sustainable, and cheap technology (Maestri et al., 2010). Mainly phytoremediation methods such as phytovolatilization, phytoextraction, phytodegradation, phytostabilization, and phytofiltration. The uptake of HMs is followed by translocation to the different structures of plants, and detoxification of HMs. It is dependent on plant species, solubility, and speciation of different HMs. Hyperaccumulator plants have high biomass and they can accumulate high concentrations of HMs in shoots. Hyperaccumulator plants tend to grow more in contaminated soils, which is one of the ways of toxin adaptation and an important trait that helps in the ecological and physiological adaptation of plants in terms of HMs resistance (Chaturvedi et al., 2014). Up to 1000 ppm of the HMs can be hyperaccumulation by the plants belonging to the Fabaceae, Poaceae, Brassicaceae, and Amaranthaceae families (Prasad and Freitas, 2005).
As the traditional methods of phytoremediation cannot be used on a pilot scale, so the research in phytoremediation has moved towards the role of plant growth-promoting bacteria in HMs removal, along with that the use of transgenic plants, and phytohormones assisted phytoremediation also can be used for decontamination of HMs. Transgenic plant species are modified by genome manipulation to integrate new genes which can accentuate the HMs removal. It is mainly playing with the plant genome to introduce foreign genes which were originally not present in the plant, a part of genetic engineering. Gene manipulation with the genes involved in the degradation and sequestration of HMs isolated from other plants, fungi, and bacteria are introduced into plants. For ex., the SbMT-2 gene from Salicornia brachiata confers tolerance to toxic metals such as Cd, Zn, and Cu along with modulating reactive oxygen species (ROS) in transgenic herbaceous plant Nicotiana tabacum (Nahar et al., 2017).). Research based on transgenic plants is majorly on the overexpression of genes involved in the biosynthesis of pathways of metal-binding peptides. For ex., in transgenic tobacco, the AtACR2 gene also known as arsenic reductase 2 which is isolated from Arabidopsis thaliana can help to detoxify arsenic (Ullah et al., 2015).
4.3.1. Plant growth-promoting rhizobacteria (PGPR) for the detoxification of HMs
PGPR colonizes the rhizosphere and is used in the bioremediation of pollutants. These PGPR can decontaminate the toxic HMs by transforming them from one form to another. By allowing the uptake of HMs from the root, they aid in enhancing the phytoremediation capability of plants (Yan et al., 2020). Many previous studies have shown the use of PGPR in the decontamination of HMs. The bacteria can help to enhance the HMs bioavailability in the soil by secreting iron chelators such as siderophores and some organic acids such as indole acetic acid, which can decrease the pH of soil. A study by Verma et al., 2019, showed that PGPR can help in the phytoremediation of Cd (Verma et al., 2019). Many plants such as Mung bean, Canola, Indian mustard, and Trifolium in association with Pseudomonas, Enterobacter, and Brevibacillus can help in the removal of Ni, Cu, Cr, and Pb (Khonsue et al., 2013). Ocimum gratissimum inoculated with Arthrobacter can induce the Cd phytoextraction by roots (Fan et al., 2018). Robinia pseudoacacia can associate with Mesorhizobium loti HZ76 to enhance its capacity for phytoremediation (Wang, 2015).
4.4. Diatoms mediated removal of HMs
Diatoms are the diverse group of phytoplanktons accounting for almost 45% primary productivity of oceans. Diatoms play an important role in the speciation, degradation, and detoxification of hazardous metals from contaminated sites. They are used as an indicator for HM pollution in wastewater. Diatoms aid in the phytoremediation of HMs by active assimilation and passive adsorption from the aqueous environment via the intracellular and extracellular mechanisms involved in the uptake of contaminants for circumventing the toxicity of HMs (Marella et al., 2020). Diatoms can adapt well to environmental conditions, including biotic and abiotic stress (Azimi et al., 2017). The use of diatoms is a novel approach that is cost-effective and eco-friendly (Hernandez-Avila et al., 2019). As compared to other algae species, diatoms exhibit evolutionary variances in their cellular organization and metabolic pathways. Diatoms are grown for aquaculture as they can reach high productivity. Diatoms can aid in bioremediation primarily by HM biosorption on the surface of the cell followed by bio-accumulation which is an active intracellular mechanism (Biswas and Choudhary, 2021). As compared to bioaccumulation, biosorption is a faster process, where the majority of the toxic metal gets bound to the cell surface and some metals may get transported into the cell. Benthic diatoms have a symbiotic relationship with bacteria, helping in the formation of diatom bacterial biofilms and secreting extracellular polysaccharides (EPS), thereby facilitating the adhesion of metal ions to the bacterial cell wall (Ayangbenro, and Babalola, 2017). Functional groups present in diatoms such as silanol (SI-O-H), amino (-NH2), ketone, aldehyde, carboxyl (-COOH), and esters help in the detoxification of metals. Diatoms play a potential role in phycoremediation and may prove to be the best eco-friendly material for HM adsorption. But their functional role is still underutilized due to tricky isolation and culturing. More research should be focused on the potential of the diatoms for pollutant removal as they can be a sustainable and eco-friendly approach towards the removal of emerging contaminants (Rabiee et al., 2021).
4.5. Limitations of bioremediation
The availability of the microbes and speciation along with physicochemical characteristics is key factors that govern the remediation of HMs (Chandra et al., 2020). The metal degradation rate can get affected because of less interaction frequency between the HM and the bacteria. The metals and microbes are not uniform in the environment which may retard the biodegradation efficiency of microbes. Temperature, pH, temperature, and type of matrix also pose a constraint on the removal of metals. As discussed earlier the HMs cannot be biodegraded but can be converted to a lesser toxic form, but the transformed products are more persistent and toxic than the parent compound. Owing to the specificity of the biological processes, optimum conditions and an appropriate amount of contaminants and nutrients are requisite for the successful remediation of HMs (Sodhi et al., 2019).
4.6. Nanoadsorbents in the removal of HMs
Nanoparticles are used in the removal of HMs. The nanomaterials mainly function by the adsorption of HMs on their surface (Wang et al., 2019). Ex., zeolite-based nanoparticles, and metal-oxide-based nanoparticles, among others, are used in HM removal. Metal-oxide nanoparticles are extensively used in the past few years. The metallic nanoparticles are used rarely as an adsorbent because of their instability. It is difficult to separate the nanoparticles from wastewater, so the functionalization of the nanoparticles is required to make the separation easy (Sun et al., 2016). Nano zero-valent iron nanoparticles Fe0 i.e., nano zero-valent iron (NZVI) are significant as they have a greater surface area, high stability, high adsorption capacity, and non-toxicity (Sun et al., 2016). Xiao et al., [2017] studied the synthesis of iron-based nanoparticles from Fecl3 using Syzygium jambos (SJA) and could eliminate the chromate (Cr6+), and another study by Kanet et al., [2006], studied the Arsenate (As5+) removal using iron-based nanoparticles. The stability of the iron-based nanoparticles can be improved further by adding a stabilizing agent such as chitosan carboxymethyl β-cyclodextrin complex, a nontoxic and biodegradable stabilizer, and used in the removal of Cu and Cr completely. Bimetallic nanoparticles are also used in the removal of metal ions (Kanet et al., 2006). Bimetallic Iron/Ni nanoparticles embedded in kaolinite (K-Fe/Ni) were synthesized by Cai et al., [2018], and were effective in the simultaneous removal of nitric oxide and Cu ions. Along with metallic nanoparticles, metal-oxide-based nanoparticles are used in the decontamination of wastewater. Because of their inherent magnetic character, metal oxide nanoparticles can be classified as magnetic and non-magnetic metal oxide nanoparticles (Cai et al., 2018). Oxides of Al, Cu, Mg, Zn, Fe, and Ce are used for metal ions removal. Studies by Gupta et at., [2010], used magnetron sputtering to synthesize CuO nanoparticles for the removal of Pb2+ and Cr6+. The removal of the metal ions by the nanoparticles depends on various factors such as ionic radii, atomic mass, and electronegativity (Gupta et al., 2010). The adsorption capacity of the nanoparticles can be enhanced by modifying the metal oxide surface with surfactants. Pham et al., [2017], observed the adsorption performance of sodium dodecyl sulfate (SDS) modified alumina (Al2O3@SDS) towards ammonium ions. The anionic surfactant coating helps to increase the removal efficiency significantly which is due to the change in surface charge. Both SDS and sodium tetradecyl sulfate (STS) modified ɤ-alumina nanoparticles were used in Cd2+ removal. Carbon-based nanomaterials are used as new-generation nano adsorbents. Carbonaceous-based nanoparticles have emerged as favorable nano adsorbents (Pham et al., 2017). Carbon nanotubes (CNTs) and graphene-based nano adsorbents are used for the decontamination of wastewater. CNTs have a small size, electrical conductivity, cylindrical and hollow structure, and larger surface area. CNTs are of 2 types single-cell walled and multi-cell walled. CNTs were able to remove Pb2+ and other HMs with a good adsorption rate (Pyrzynska and Stafiej, 2012 & Gupta et al., 2021). Multi-walled carbon nanotubes oxidized with MnO2 were used for the removal of Cd2+ ions. Graphene is also used as a carbon-based nanomaterial. Graphene oxide is hydrophilic due to the presence of oxygen as a functional moiety on graphene. The hydrophilic nature helps it to disperse in water. The high surface area of graphene oxide helps in the decontamination of wastewater (Gupta et al., 2021). The HMs removal is adsorption based and takes place via the complexation of metal ions with the functional group i.e., the oxide binding site in graphene (da Silva Alves et al., 2021). Table 4 gives an insight into the different adsorbents involved in HMs removal with their advantages and disadvantages.
Table 4.
Different adsorbents involved in HMs removal with their advantages and disadvantages.
| Adsorbents based methods | Overview | Disadvantages | References |
|---|---|---|---|
| Chitosan-based adsorbents | Chitosan has an affinity toward pollutants because of the hydroxyl and amino groups | Low porosity, surface area, and high crystallinity | (Jain et al., 2021) |
| Carbon-based adsorbents | Graphene-activated carbon and carbon nanotubes have a high surface area and are used to remove HMs. Phenyl, carboxy, and lactone groups can enhance the HMs uptake | Surface modification demands high pressure, heat, intensive oxidation/reduction reactions which makes the process expensive | (Gu et al., 2019) |
| Mineral adsorbents | Clay, zeolite, and silica are considered good adsorbents because of their low operating costs. Extraordinary cation exchange capacity and selectivity along with surface hydrophilicity and surface electronegativity are seen in clay. | After a few cycles, the removal efficiency is decreased which is the only disadvantage. | (Qasem et al., 2021) |
| Magnetic adsorbents | They have a specific material matrix that hosts iron particles such as Fe3O4. Easily synthesized, low cost, extraordinary surface charge, and reusability are the main advantages of magnetic adsorbents. | pH, irradiation time, the concentration of the adsorbent, temperature, and the initial dosage of pollutants affect the HMs removal | (Dang et al., 2017) |
| Adsorbents based on metal-organic framework (MOFs) | MOFs are synthesized using reticular synthesis. The metal ions are bonded strongly to organic linkers. | Organic ligands which form MOFs are toxic and expensive | (Chen et al., 2020) |
4.7. Capacitive deionization and electrosorption for the removal of HMs
Capacitive deionization (CDI) is an upcoming technology that gained attention for the desalination and purification of water. It is a cost-effective technology and enables the use of widely available porous carbon materials used in electrosorption research (Kim et al., 2018). The porous carbon can be modified and function as a redox material for selective metal ion binding. The adsorption of a dissolved species on the electrode and the binding to the surface of the cell is promoted because of an electric field. The electrodes are made up of porous carbon. During the electrodes' charging, the oppositely charged ions are attracted to each other by coulombic forces. The metal ions form an electric double layer on the surface of the polarized electrode (Bui et al., 2016). CDI refers to the phenomenon of the removal of metal ions from the solution. Ions remain in a double layer till the charge is reversed or stopped and then discharged. Once discharged, the electrodes are regenerated and ions are liberated. Electrosorption is the energy-saving removal technique as the CDI technique stores charge, hence the energy used in electrosorption can be recovered partially. CDI and electrosorption processes can be combined with any removal technology such as desalination techniques, and ion exchange resins, and enhance the efficiency of HM removal (McNair et al., 2022). Activated carbon has been used frequently in CDI studies as they are not expensive and have a high specific surface area (Xing et al., 2020). CDI is efficient when it has high specific surface areas as the electrode material can participate in double-layer formation. The carbon layer is porous and can capture the ionic species from water without discrimination. But more research needs to be carried out on designing materials that can remove selective ions of interest from competing ions (Wang et al., 2021)
5. Metagenomics approach in the bioremediation of heavy metals
The metagenomics approach reveals the knowledge of microbial communities in the uncultivable samples using research based on the conserved sequence and functions. Metagenomics has proved to be a vital tool that does not involve the culturing of the microorganisms, directly the DNA extraction can be done from environmental samples (Ronholm, 2018). Microbes have already proved to be an eco-friendly option for the remediation of pollutants. The goal of metagenomics is to uncover the potential of uncultivable Proteobacteria, Actinobacteria, and Chloroflexi can thrive at high metal concentrations. Many bioactive enzymes and compounds have an important role in the clean-up of pollutants and are characterized using metagenomics (Mohapatra et al., 2022). Enzymes such as dioxygenases, laccases, and esterases are extracted using metagenomic techniques and can be used to remove toxins from the environment. Hydrocarbon-degrading enzymes are also obtained from metagenomic sequencing and can be used to remove microbes along with acknowledging the role of the microbial community in the removal of heavy metals from the environment (Dash et al., 2022). Metagenomics is an emerging and promising area of research. Both sequence and function-based metagenomic strategies can be used to get insight into the uncultivable microbes to unravel their potential for HMs remediation. Gene and studying the metabolic processes, genome assemblies, and a conserved sequence-based identification from communities are a part of metagenomic sequencing. Genes that can serve as biomarkers of pollution can be used for the clean-up of pollutants (Shweta et al., 2021). Many bacteria belong to the phylum Firmicutes, Bacteroidetes, a pollutant from the environment. Novel genes-producing bacteria aid to enhance the strategy of remediation. Next-generation genomic sequencing techniques, such as pyrosequencing, and ligation sequencing are proving to be very time efficient and vital for studying the metal-contaminated environment. Previous studies reported the presence of β-proteobacteria such as Burkholderia, and γ-proteobacteria such as Pseudomonas from metal-contaminated sites. The rapid annotation search tool used for metagenomics read analysis (MG-RAST) is used as an advanced tool for the functional analysis of metagenomes along with aiding in quantitative insights on the microbiome (Devarapalli and Kumawath, 2015). Bioinformatic analysis can range from nucleic acid sequencing to quality control, and protein prediction. The raw data obtained as a FASTA sequence can be used for downstream analysis. Metabolic reconstruction and annotations can give insight into the enzymes that can be used in the bioremediation of metal-contaminated ecosystems (Kumar et al., 2020).
6. Conclusion
The contamination of the environment by anthropogenic activities has increased the hazard of HMs. HMs have both acute and chronic effects on both the fauna and flora. The HMs are discharged into the environment in a partially treated or untreated form. HMs are extremely toxic at low concentrations thereby adding to the concern. Increasing awareness among the public of environmental pollution such as the menace caused by the HMs has influenced the development of eco-friendly and sustainable technologies for the clean-up of pollutants. The HM cannot be completely removed from the environment as their degradation is difficult but we can reduce the HM pollution using several remediation techniques and the HM can be converted into a nontoxic form. The use of living organisms such as bacteria, algae, fungi, and plants is a sustainable way. Bioremediation is a socially acceptable, low-cost, and environmental-friendly technology as compared to Physico-chemical technologies. With the recent advancements in molecular biology and nanotechnology, new techniques can be designed to detoxify HMs. Remediation strategies such as low-cost adsorbent and chelating agents can also be used. The major aim is to level down the problems linked with food security and human diseases caused by HMs. Particularly in countries having rapid urbanization and modernization eco-friendly technologies should be used to control the menace caused by HMs.
Research involving human participants and/or animals
The study does not relate to animals or humans.
Informed consent
N/A
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgements
The author KKS and CKS would like to acknowledge Department of Zooogy, MK would like to thank Hindu college (DU) for providing the infrastructural facility and funds for the ongoing innovation project (SC/2019-2020/05 and SC/2019-2020/06). The corresponding author acknowledges Prof. D.K. Singh for his support and guidance always.
Contributor Information
Kushneet Kaur Sodhi, Email: kushneetsodhi936@gmail.com.
Lokesh Chandra Mishra, Email: lokesh20sept@yahoo.co.in.
Chandra Kant Singh, Email: singh007ck@gmail.com.
Mohit Kumar, Email: mohitzoologydu@gmail.com.
Data availability
No data was used for the research described in the article.
References
- Abatenh E., Gizaw B., Tsegaye Z., Wassie M. The role of microorganisms in bioremediation-A review. Open J. Environ. Biol. 2017;2(1):038–046. [Google Scholar]
- Adamu C.I., Nganje T.N., Edet A. Heavy metal contamination and health risk assessment associated with abandoned barite mines in Cross River State, southeastern Nigeria. Environ. Nanotechnol. Monitoring & Manag. 2015;3:10–21. [Google Scholar]
- Ahemad M. Implications of bacterial resistance against heavy metals in bioremediation: a review. J. Institute of Integrative Omics and Appl. Biotechnol. (IIOAB) 2012;3(3) [Google Scholar]
- Al-Enezi G., Hamoda M.F., Fawzi N. Ion exchange extraction of heavy metals from wastewater sludges. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2004;39(2):455–464. doi: 10.1081/ese-120027536. [DOI] [PubMed] [Google Scholar]
- Ayangbenro A.S., Babalola O.O. A New Strategy for Heavy Metal Polluted Environments: a Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health. 2017;14(1):94. doi: 10.3390/ijerph14010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimi A., Azari A., Rezakazemi M., Ansarpour M. Removal of heavy metals from industrial wastewaters: a review. ChemBioEng. Rev. 2017;4(1):37–59. [Google Scholar]
- Aziz H.A., Adlan M.N., Ariffin K.S. Heavy metals (Cd, Pb, Zn, Ni, Cu and Cr (III)) removal from water in Malaysia: post treatment by high quality limestone. Bioresour. Technol. 2008;99(6):1578–1583. doi: 10.1016/j.biortech.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Balali-Mood M., Naseri K., Tahergorabi Z., Khazdair M.R., Sadeghi M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021:12. doi: 10.3389/fphar.2021.643972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas R.K., Choudhury A.K. Diatoms: miniscule biological entities with immense importance in synthesis of targeted novel bioparticles and biomonitoring. J. Biosci. 2021;46(4):1–14. [PubMed] [Google Scholar]
- Budianta W. THe influence of mineralogical composition on the adsorption capacity of heavy metals solution by java natural clay, Indonesia. ASEAN Eng. J. 2021;11(2):64–76. [Google Scholar]
- Bui M.P.N., Brockgreitens J., Ahmed S., Abbas A. Dual detection of nitrate and mercury in water using disposable electrochemical sensors. Biosensors and Bioelectron. 2016;85:280–286. doi: 10.1016/j.bios.2016.05.017. [DOI] [PubMed] [Google Scholar]
- Cai T., Wang L., Liu Y., Zhang S., Dong W., Chen H.…Luo S. Ag3PO4/Ti3C2 MXene interface materials as a Schottky catalyst with enhanced photocatalytic activities and anti-photocorrosion performance. Appl. Catalysis B: Environ. 2018;239:545–554. [Google Scholar]
- Cao Y., Zhao M., Ma X., Song Y., Zuo S., Li H., Deng W. A critical review on the interactions of microplastics with heavy metals: mechanism and their combined effect on organisms and humans. Sci. Total Environ. 2021;788 doi: 10.1016/j.scitotenv.2021.147620. [DOI] [PubMed] [Google Scholar]
- Chandra P., Singh R., Arora P.K. Microbial lipases and their industrial applications: a comprehensive review. Microb. Cell Fact. 2020;19(1):1–42. doi: 10.1186/s12934-020-01428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrangsu P., Rensing C., Helmann J.D. Metal homeostasis and resistance in bacteria. Nat. Rev. Microbiol. 2017;15(6):338–350. doi: 10.1038/nrmicro.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi A.K., Patel M.K., Mishra A., Tiwari V., Jha B. The SbMT-2 gene from a halophyte confers abiotic stress tolerance and modulates ROS scavenging in transgenic tobacco. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0111379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhao Y., Zhao X., Wu J., Zhu L., Zhang X.…He P. Selective pressures of heavy metals on microbial community determine microbial functional roles during composting: sensitive, resistant and actor. J. Hazard. Mater. 2020;398 doi: 10.1016/j.jhazmat.2020.122858. [DOI] [PubMed] [Google Scholar]
- da Silva Alves D.C., Healy B., Pinto L.A., Cadaval T.R., Breslin C.B. Recent developments in chitosan-based adsorbents for the removal of pollutants from aqueous environments. Molecules. 2021;26(3):594. doi: 10.3390/molecules26030594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakal T.C., Kumar A., Majumdar R.S., Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016;7:1831. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang S., Zhu Q.L., Xu Q. Nanomaterials derived from metal–organic frameworks. Nat. Rev. Mater. 2017;3(1):1–14. [Google Scholar]
- Dash S.P., Mohapatra M., Rastogi G. Bioprospecting of Microbial Diversity. Elsevier; 2022. Microbial diversity and bioprospecting potential of Phragmites rhizosphere microbiome through genomic approaches; pp. 503–528. [Google Scholar]
- Devarapalli P., Kumavath R.N. Advances in Bioremediation of Wastewater and Polluted Soil. IntechOpen; 2015. Metagenomics-a technological drift in bioremediation; pp. 73–91. [Google Scholar]
- Dhingra N., Singh N.S., Parween T., Sharma R. Modern Age Waste Water Problems. Springer; Cham: 2020. Heavy metal remediation by natural adsorbents; pp. 233–250. [Google Scholar]
- Diep P., Mahadevan R., Yakunin A.F. Heavy metal removal by bioaccumulation using genetically engineered microorganisms. Front. Bioeng. Biotechnol. 2018;6:157. doi: 10.3389/fbioe.2018.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourado M.N., Martins P.F., Quecine M.C., Piotto F.A., Souza L.A., Franco M.R.…Azevedo R.A. Burkholderia sp. SCMS54 reduces cadmium toxicity and promotes growth in tomato. Ann. of Appl. Biol. 2013;163(3):494–507. [Google Scholar]
- Fan M., Xiao X., Guo Y., Zhang J., Wang E., Chen W.…Wei G. Enhanced phytoremdiation of Robinia pseudoacacia in heavy metal-contaminated soils with rhizobia and the associated bacterial community structure and function. Chemosphere. 2018;197:729–740. doi: 10.1016/j.chemosphere.2018.01.102. [DOI] [PubMed] [Google Scholar]
- Fashola M.O., Ngole-Jeme V.M., Babalola O.O. Heavy metal pollution from gold mines: environmental effects and bacterial strategies for resistance. Int. J. Environ. Res. Public Health. 2016;13(11):1047. doi: 10.3390/ijerph13111047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam P.K., Gautam R.K., Banerjee S., Chattopadhyaya M.C., Pandey J.D. Vol. 60. Nova Sci Publishers; 2016. pp. 101–130. (Heavy Metals in the Environment: Fate, Transport, Toxicity, and Remediation Technologies). [Google Scholar]
- Genchi Giuseppe, et al. Nickel: human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health. 21 Jan. 2020;17(3):679. doi: 10.3390/ijerph17030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazisaeedi F., Ciesinski L., Bednorz C., Johanns V., Pieper L., Tedin K.…Günther S. Phenotypic zinc resistance does not correlate with antimicrobial multi-resistance in fecal E. coli isolates of piglets. Gut Pathog. 2020;12(1):1–10. doi: 10.1186/s13099-019-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S., Kang X., Wang L., Lichtfouse E., Wang C. Clay mineral adsorbents for heavy metal removal from wastewater: a review. Environ. Chem. Lett. 2019;17(2):629–654. [Google Scholar]
- Gupta A.K., Deva D., Sharma A., Verma N. Fe-grown carbon nanofibers for removal of arsenic (V) in wastewater. Ind. Eng. Chem. Res. 2010;49(15):7074–7084. [Google Scholar]
- Gupta A., Sharma V., Sharma K., Kumar V., Choudhary S., Mankotia P.…Mishra P.K. A review of adsorbents for heavy metal decontamination: growing approach to wastewater treatment. Materials (Basel) 2021;14(16):4702. doi: 10.3390/ma14164702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haferburg G., Kothe E. Microbes and metals: interactions in the environment. J. Basic Microbiol. 2007;47(6):453–467. doi: 10.1002/jobm.200700275. [DOI] [PubMed] [Google Scholar]
- Hamza M.F., Hamad N.A., Hamad D.M., Khalafalla M.S., Abdel-Rahman A.A.H., Zeid I.F.…Salem W.M. Synthesis of eco-friendly biopolymer, alginate-chitosan composite to adsorb the heavy metals, Cd (II) and Pb (II) from contaminated effluents. Materials (Basel) 2021;14(9):2189. doi: 10.3390/ma14092189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanfi M.Y., Yarmoshenko I.V. Health risk assessment quantification from heavy metals contamination in the urban soil and urban surface deposited sediment. J. Taibah University for Sci. 2020;14(1):285–293. [Google Scholar]
- Hasan M., Uddin M., Ara-Sharmeen I., Alharby F., H, Alzahrani Y., Hakeem K.R., Zhang L. Assisting phytoremediation of heavy metals using chemical amendments. Plants. 2019;8(9):295. doi: 10.3390/plants8090295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Avila J., Serrano-Mejía E.O., Salinas-Rodríguez E., Cerecedo-Sáenz E., Reyes-Valderrama M.I., del Pilar, Gutiérrez-Amador M., Rodríguez-Lugo V. Trace Metals in the Environment-New Approaches and Recent Advances. IntechOpen; 2019. Use of porous no metallic minerals to remove heavy metals, precious metals and rare earths, by cationic exchange. [Google Scholar]
- Igiri B.E., Okoduwa S.I., Idoko G.O., Akabuogu E.P., Adeyi A.O., Ejiogu I.K. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: a review. J. Toxicol. 2018 doi: 10.1155/2018/2568038. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issazadeh K., Jahanpour N., Pourghorbanali F., Raeisi G., Faekhondeh J. Heavy metals resistance by bacterial strains. Ann. Biol. Res. 2013;4(2):60–63. [Google Scholar]
- Jacob J.M., Karthik C., Saratale R.G., Kumar S.S., Prabakar D., Kadirvelu K., Pugazhendhi A. Biological approaches to tackle heavy metal pollution: a survey of literature. J. Environ. Manag. 2018;217:56–70. doi: 10.1016/j.jenvman.2018.03.077. [DOI] [PubMed] [Google Scholar]
- Jain A., Kumari S., Agarwal S., Khan S. Water purification via novel nano-adsorbents and their regeneration strategies. Process Safety and Environ. Protection. 2021;152:441–454. [Google Scholar]
- Jasmin C., Anas A., Singh D., Purohit H.J., Gireeshkumar T.R., Nair S. Aberrations in the microbiome of cyanobacteria from a tropical estuary polluted by heavy metals. Mar. Pollut. Bull. 2020;160 doi: 10.1016/j.marpolbul.2020.111575. [DOI] [PubMed] [Google Scholar]
- Jiang M., Zeng G., Zhang C., Ma X., Chen M., Zhang J.…Liu L. Assessment of heavy metal contamination in the surrounding soils and surface sediments in Xiawangang River, Qingshuitang District. PLoS ONE. 2013;8(8):e71176. doi: 10.1371/journal.pone.0071176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junaid M., Hashmi M.Z., Malik R.N., Pei D.S. Toxicity and oxidative stress induced by chromium in workers exposed from different occupational settings around the globe: a review. Environ. Sci. Pollut. Res. 2016;23(20):20151–20167. doi: 10.1007/s11356-016-7463-x. [DOI] [PubMed] [Google Scholar]
- Kanel S.R., Greneche J.M., Choi H. Arsenic (V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environ. Sci. Technol. 2006;40(6):2045–2050. doi: 10.1021/es0520924. [DOI] [PubMed] [Google Scholar]
- Kang S.J., Kocabas C., Ozel T., Shim M., Pimparkar N., Alam M.A.…Rogers J.A. High-performance electronics using dense, perfectly aligned arrays of single-walled carbon nanotubes. Nat. Nanotechnol. 2007;2(4):230–236. doi: 10.1038/nnano.2007.77. [DOI] [PubMed] [Google Scholar]
- Kanmani P., Aravind J., Preston D. Remediation of chromium contaminants using bacteria. Int.l J. Environ. Sci. Technol. 2012;9(1):183–193. [Google Scholar]
- Kapungwe E.M. Heavy metal contaminated water, soils and crops in peri urban wastewater irrigation farming in Mufulira and Kafue towns in Zambia. J. Geography and Geol. 2013;5(2):55. [Google Scholar]
- Khalid N., Aqeel M., Noman A., Khan S.M., Akhter N. Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environments. Environ. Pollut. 2021;290 doi: 10.1016/j.envpol.2021.118104. [DOI] [PubMed] [Google Scholar]
- Khan A., Khan S., Khan M.A., Qamar Z., Waqas M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ. Sci. Pollut. Res. 2015;22(18):13772–13799. doi: 10.1007/s11356-015-4881-0. [DOI] [PubMed] [Google Scholar]
- Khonsue N., Kittisuwan K., Kumsopa A., Tawinteung N., Prapagdee B. Inoculation of soil with cadmium-resistant bacteria enhances cadmium phytoextraction by Vetiveria nemoralis and Ocimum gratissimum. Water, Air, & Soil Pollut. 2013;224(10):1–9. [Google Scholar]
- Kim S., Park C.M., Jang M., Son A., Her N., Yu M.…Yoon Y. Aqueous removal of inorganic and organic contaminants by graphene-based nanoadsorbents: a review. Chemosphere. 2018;212:1104–1124. doi: 10.1016/j.chemosphere.2018.09.033. [DOI] [PubMed] [Google Scholar]
- Koul B., Taak P. Biotechnological Strategies for Effective Remediation of Polluted Soils. Springer; Singapore: 2018. In Situ Soil Remediation Strategies; pp. 59–75. [Google Scholar]
- Kubier A., Wilkin R.T., Pichler T. Cadmium in soils and groundwater: a review. Appl. Geochem. 2019;108 doi: 10.1016/j.apgeochem.2019.104388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R., Bryant I.M., Jensch R., Böllmann J. Applications of environmental nanotechnologies in remediation, wastewater treatment, drinking water treatment, and agriculture. Appl. Nano. 2022;3(1):54–90. [Google Scholar]
- Kumar V., Thakur I.S., Singh A.K., Shah M.P. Emerging Technologies in Environmental Bioremediation. Elsevier; 2020. Application of metagenomics in remediation of contaminated sites and environmental restoration; pp. 197–232. [Google Scholar]
- Maestri E., Marmiroli M., Visioli G., Marmiroli N. Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ. Exp. Bot. 2010;68(1):1–13. [Google Scholar]
- Mahmood T., Islam K.R., Muhammad S. Toxic effects of heavy metals on early growth and tolerance of cereal crops. Pak. J. Bot. 2007;39(2):451. [Google Scholar]
- Manzoor J., Sharma M., Wani K.A. Heavy metals in vegetables and their impact on the nutrient quality of vegetables: a review. J. Plant Nutr. 2018;41(13):1744–1763. [Google Scholar]
- Marella T.K., Saxena A., Tiwari A. Diatom mediated heavy metal remediation: a review. Bioresour. Technol. 2020;305 doi: 10.1016/j.biortech.2020.123068. [DOI] [PubMed] [Google Scholar]
- Masocha B.L., Dikinya O., Moseki B. Bioavailability and contamination levels of Zn, Pb, and Cd in sandy-loam soils, Botswana. Environ. Earth Sci. 2022;81(6):1–11. [Google Scholar]
- Mazhar S.H., Li X., Rashid A., Su J., Xu J., Brejnrod A.D.…Rensing C. Co-selection of antibiotic resistance genes, and mobile genetic elements in the presence of heavy metals in poultry farm environments. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142702. [DOI] [PubMed] [Google Scholar]
- McNair R., Szekely G., Dryfe R.A. Sustainable processing of electrodes for membrane capacitive deionization (MCDI) J. Clean. Prod. 2022;342 [Google Scholar]
- Miretzky P., Saralegui A., Fernandez C.A. Aquatic macrophytes potential for the simultaneous removal of heavy metals (Buenos Aires, Argentina) Chemosphere. 2004;57:997–1005. doi: 10.1016/j.chemosphere.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Mohapatra B., Malhotra H., Saha B.K., Dhamale T., Phale P.S. Current Developments in Biotechnology and Bioengineering. Elsevier; 2022. Microbial metabolism of aromatic pollutants: high-throughput OMICS and metabolic engineering for efficient bioremediation; pp. 151–199. [Google Scholar]
- Monchanin C., Devaud J.M., Barron A.B., Lihoreau M. Current permissible levels of metal pollutants harm terrestrial invertebrates. Sci. Total Environ. 2021;779 doi: 10.1016/j.scitotenv.2021.146398. [DOI] [PubMed] [Google Scholar]
- Mrozińska N., Bąkowska M. Effects of heavy metals in lake water and sediments on bottom invertebrates inhabiting the brackish coastal lake Łebsko on the southern Baltic coast. Int. J. Environ. Res. Public Health. 2020;17(18):6848. doi: 10.3390/ijerph17186848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar N., Rahman A., Nawani N.N., Ghosh S., Mandal A. Phytoremediation of arsenic from the contaminated soil using transgenic tobacco plants expressing ACR2 gene of Arabidopsis thaliana. J. Plant Physiol. 2017;218:121–126. doi: 10.1016/j.jplph.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Nayak V., Jyothi M.S., Balakrishna R.G., Padaki M., Deon S. Novel modified poly vinyl chloride blend membranes for removal of heavy metals from mixed ion feed sample. J. Hazard. Mater. 2017;331:289–299. doi: 10.1016/j.jhazmat.2017.02.046. [DOI] [PubMed] [Google Scholar]
- Nedjimi B. Germination characteristics of Peganum harmala L (Nitrariaceae) subjected to heavy metals: implications for the use in polluted dryland restoration. Int. J. Environ. Sci. Technol. 2020;17(4):2113–2122. [Google Scholar]
- Nedjimi B. Phytoremediation: a sustainable environmental technology for heavy metals decontamination. SN Appl. Sci. 2021;3(3):1–19. [Google Scholar]
- Nguyen T.C., Loganathan P., Nguyen T.V., Kandasamy J., Naidu R., Vigneswaran S. Adsorptive removal of five heavy metals from water using blast furnace slag and fly ash. Environ. Sci. Pollut. Res. 2018;25(21):20430–20438. doi: 10.1007/s11356-017-9610-4. [DOI] [PubMed] [Google Scholar]
- Nogueira T.A.R., Franco A., He Z., Braga V.S., Firme L.P., Abreu-Junior C.H. Short-term usage of sewage sludge as organic fertilizer to sugarcane in a tropical soil bears little threat of heavy metal contamination. J. Environ. Manag. 2013;114:168–177. doi: 10.1016/j.jenvman.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Onat B., Sahin U.A., Akyuz T. Elemental characterization of PM2. 5 and PM1 in dense traffic area in Istanbul, Turkey. Atmos Pollut. Res. 2013;4(1):101–105. [Google Scholar]
- Oyewole O.A., Zobeashia S.S.L.T., Oladoja E.O., Raji R.O., Odiniya E.E., Musa A.M. Biosorption of heavy metal polluted soil using bacteria and fungi isolated from soil. SN Appl. Sci. 2019;1(8):1–8. [Google Scholar]
- Pham T.D., Do T.T., Doan T.H.Y., Nguyen T.A.H., Mai T.D., Kobayashi M., Adachi Y. Adsorptive removal of ammonium ion from aqueous solution using surfactant-modified alumina. Environ. Chem. 2017;14(5):327–337. [Google Scholar]
- Prajapati S.K., Meravi N.&., Singh S. Phytoremediation of Chromium and Cobalt using Pistia stratiotes: a sustainable approach. Proceed. Int. Acad. Ecol. Environ. Sci. 2012;2(2):136. [Google Scholar]
- Prasad M.N.V., Freitas H. Trace Elements in the Environment. CRC Press; 2005. Metal-tolerant plants: biodiversity prospecting for phytoremediation technology; pp. 501–524. [Google Scholar]
- Pyrzynska K., Stafiej A. Sorption behavior of Cu (II), Pb (II), and Zn (II) onto carbon nanotubes. Solvent Extraction and Ion Exchange. 2012;30(1):41–53. [Google Scholar]
- Qasem N.A., Mohammed R.H., Lawal D.U. Removal of heavy metal ions from wastewater: a comprehensive and critical review. Npj Clean. Water. 2021;4(1):1–15. [Google Scholar]
- Rabiee N., Khatami M., Jamalipour Soufi G., Fatahi Y., Iravani S., Varma R.S. Diatoms with invaluable applications in nanotechnology, biotechnology, and biomedicine: recent advances. ACS Biomater. Sci. Eng. 2021;7(7):3053–3068. doi: 10.1021/acsbiomaterials.1c00475. [DOI] [PubMed] [Google Scholar]
- Rai P.K., Lee S.S., Zhang M., Tsang Y.F., Kim K.H. Heavy metals in food crops: health risks, fate, mechanisms, and management. Environ. Int. 2019;125:365–385. doi: 10.1016/j.envint.2019.01.067. [DOI] [PubMed] [Google Scholar]
- Rajesh P., Athiappan M., Paul R., Raj K.D. Bioremediation of cadmium by Bacillus safensis (JX126862), a marine bacterium isolated from mangrove sediments. Int. J. Curr. Microbiol. Appl. Sci. 2014;3(12):326–335. [Google Scholar]
- Rani A., Souche Y.S., Goel R. Comparative assessment of in situ bioremediation potential of cadmium resistant acidophilic Pseudomonas putida 62BN and alkalophilic Pseudomonas monteilli 97AN strains on soybean. Int. Biodeterior. Biodegradation. 2009;63(1):62–66. [Google Scholar]
- Rascio N., Navari-Izzo F. Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci. 2011;180(2):169–181. doi: 10.1016/j.plantsci.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Rezapour S., Siavash Moghaddam S., Nouri A., Khosravi Aqdam K. Urbanization influences the distribution, enrichment, and ecological health risk of heavy metals in croplands. Sci. Rep. 2022;12(1):1–16. doi: 10.1038/s41598-022-07789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronholm J. Game changer-next generation sequencing and its impact on food microbiology. Front. Microbiol. 2018;9:363. doi: 10.3389/fmicb.2018.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruba U.B., Chakma K., Senthi J.Y., Rahman S. Impact of industrial waste on natural resources: a review in the context of Bangladesh. Current World Environ. 2021;16(2):348. [Google Scholar]
- Sadhu A., Upadhyay P., Singh P.K., Agrawal A., Ilango K., Karmakar D.…Dubey G.P. Quantitative analysis of heavy metals in medicinal plants collected from environmentally diverse locations in India for use in a novel phytopharmaceutical product. Environ. Monit. Assess. 2015;187(8):1–11. doi: 10.1007/s10661-015-4764-3. [DOI] [PubMed] [Google Scholar]
- Samanta S., Amrutha K., Dalai T.K., Kumar S. Heavy metals in the Ganga (Hooghly) River estuary sediment column: evaluation of association, geochemical cycling and anthropogenic enrichment. Environ. Earth Sci. 2017;76(4):140. [Google Scholar]
- Saravanan A., Kumar P.S., Jeevanantham S., Karishma S., Tajsabreen B., Yaashikaa P.R., Reshma B. Effective water/wastewater treatment methodologies for toxic pollutants removal: processes and applications towards sustainable development. Chemosphere. 2021 doi: 10.1016/j.chemosphere.2021.130595. [DOI] [PubMed] [Google Scholar]
- Sarkar D.J., Sarkar S.D., Das B.K., Sahoo B.K., Das A., Nag S.K.…Samanta S. Occurrence, fate and removal of microplastics as heavy metal vector in natural wastewater treatment wetland system. Water Res. 2021;192 doi: 10.1016/j.watres.2021.116853. [DOI] [PubMed] [Google Scholar]
- Sathya K., Nagarajan K., Carlin Geor Malar G., Rajalakshmi S., Raja Lakshmi P. A comprehensive review on comparison among effluent treatment methods and modern methods of treatment of industrial wastewater effluent from different sources. Appl Water Sci. 2022;12(4):1–27. doi: 10.1007/s13201-022-01594-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaji E., Santosh M., Sarath K.V., Prakash P., Deepchand V., Divya B.V. Arsenic contamination of groundwater: a global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021;12(3) [Google Scholar]
- Sharma N., Sodhi K.K., Kumar M., Singh D.K. Heavy metal pollution: insights into chromium eco-toxicity and recent advancement in its remediation. Environ. Nanotechnol. Monitoring & Manag. 2021;15 [Google Scholar]
- Sharma S., Nagpal A.K., Kaur I. Appraisal of heavy metal contents in groundwater and associated health hazards posed to human population of Ropar wetland, Punjab, India and its environs. Chemosphere. 2019;227:179–190. doi: 10.1016/j.chemosphere.2019.04.009. [DOI] [PubMed] [Google Scholar]
- Shrestha R., Ban S., Devkota S., Sharma S., Joshi R., Tiwari A.P.…Joshi M.K. Technological trends in heavy metals removal from industrial wastewater: a review. J. Environ. Chem. Eng. 2021 [Google Scholar]
- Shweta N., Samatha S., Keshavkant S. Microbial Ecology of Wastewater Treatment Plants. Elsevier; 2021. Mechanisms, types, effectors, and methods of bioremediation: the universal solution; pp. 41–72. [Google Scholar]
- Singh R., Gautam N., Mishra A., Gupta R. Heavy metals and living systems: an overview. Indian J. Pharmacol. 2011;43(3):246. doi: 10.4103/0253-7613.81505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi K.K., Kumar M., Agrawal P.K., Singh D.K. Perspectives on arsenic toxicity, carcinogenicity and its systemic remediation strategies. Environ. Technol. Innovation. 2019;16 [Google Scholar]
- Sodhi K.K., Kumar M., Singh D.K. Multi-metal resistance and potential of Alcaligenes sp. MMA for the removal of heavy metals. SN Appl. Sci. 2020;2(11):1–13. [Google Scholar]
- Sun M.H., Huang S.Z., Chen L.H., Li Y., Yang X.Y., Yuan Z.Y., Su B.L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016;45(12):3479–3563. doi: 10.1039/c6cs00135a. [DOI] [PubMed] [Google Scholar]
- Tabelin C.B., Igarashi T., Villacorte-Tabelin M., Park I., Opiso E.M., Ito M., Hiroyoshi N. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: a review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ. 2018;645:1522–1553. doi: 10.1016/j.scitotenv.2018.07.103. [DOI] [PubMed] [Google Scholar]
- Ullah A., Heng S., Munis M.F.H., Fahad S., Yang X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ. Exp. Bot. 2015;117:28–40. [Google Scholar]
- Urra J., Alkorta I., Mijangos I., Epelde L., Garbisu C. Application of sewage sludge to agricultural soil increases the abundance of antibiotic resistance genes without altering the composition of prokaryotic communities. Sci. Total Environ. 2019;647:1410–1420. doi: 10.1016/j.scitotenv.2018.08.092. [DOI] [PubMed] [Google Scholar]
- Verma M., Mishra J., Arora N.K. Environmental biotechnology: For Sustainable Future. Springer; Singapore: 2019. Plant growth-promoting rhizobacteria: diversity and applications; pp. 129–173. [Google Scholar]
- Wang, J.K. (2015). An Absorbent and method of application for the treatment of heavy metal waste water. Chinene Patent: 201410072325.0.
- Wang L., Wang Y., Ma F., Tankpa V., Bai S., Guo X., Wang X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: a review. Sci. Total Environ. 2019;668:1298–1309. doi: 10.1016/j.scitotenv.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li Q., Tang G., Zhang N. Recent progress on carbon based desalination membranes and carbon nanomaterial incorporated non-polyamide desalination membranes. J. Environ. Chem. Eng. 2021;9(4) [Google Scholar]
- Xiao Z., Zhang H., Xu Y., Yuan M., Jing X., Huang J.…Sun D. Ultra-efficient removal of chromium from aqueous medium by biogenic iron based nanoparticles. Sep. Purif. Technol. 2017;174:466–473. [Google Scholar]
- Xin Z., Wenchao Z., Zhenguang Y., Yiguo H., Zhengtao L., Xianliang Y., Xiaonan W., Tingting L., Liming Z. Species sensitivity analysis of heavy metals to freshwater organisms. Ecotoxicology. 2015;24(7–8):1621–1631. doi: 10.1007/s10646-015-1500-2. [DOI] [PubMed] [Google Scholar]
- Xing W., Liang J., Tang W., He D., Yan M., Wang X.…Huang M. Versatile applications of capacitive deionization (CDI)-based technologies. Desalination. 2020;482 [Google Scholar]
- Yan A., Wang Y., Tan S.N., Mohd Yusof M.L., Ghosh S., Chen Z. Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020;11:359. doi: 10.3389/fpls.2020.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaynab M., Al-Yahyai R., Ameen A., Sharif Y., Ali L., Fatima M.…Li S. Health and environmental effects of heavy metals. J. King Saud University-Sci. 2022;34(1) [Google Scholar]
- Zhang Q., Ye J., Chen J., Xu H., Wang C., Zhao M. Risk assessment of polychlorinated biphenyls and heavy metals in soils of an abandoned e-waste site in China. Environ. Pollut. 2014;185:258–265. doi: 10.1016/j.envpol.2013.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.