Highlights
-
•
Host soluble factors determine the fitness and persistence of bacterial biofilms.
-
•
Glucose promotes biofilm formation of Staphylococcus aureus and Enterococcus faecalis in a dose-dependent manner.
-
•
A medium enriched with blood plasma inhibits biofilm formation, whereas a surface coating with blood plasma can promote biofilm formation.
Keywords: Experimental parameter, Static biofilm model, Plasma glucose level, Human plasma, Blood-contacting implants
Abstract
The prevention of implant infections is a major challenge for implant developers and clinicians. Understanding biofilm dynamics and favorable implant or environmental characteristics will help to prevent biofilm formation. Blood-contact implants, such as cardiovascular implants, are particularly susceptible to infections as the blood provides a favorable growth environment for bacteria due to its rich supply of micro- and macro substances, such as glucose and plasma proteins. In this context, Staphylococcus aureus, Staphylococcus epidermidis and Enterococcus faecalis are the most reported causes accompanying foreign body-associated infections, mainly due to their ability to form an adherent, multilayered bacterial biofilm on a wide variety of surfaces. The present study demonstrates that the provision of glucose and human plasma to the growth medium or coating of the flask with human plasma differentially affects the biofilm formation of these three bacterial species, with human plasma being the most effective regulator. However, glucose supplementation promoted and stabilized biofilm formation of S. aureus and E. faecalis, while an opposite effect was observed for additional plasma. These findings highlight the urgent need to intensify studies on the impact of host soluble factors as risk factors promoting fitness and persistence of bacterial biofilms.
Graphical Abstract
Introduction
Medical devices or implants, including orthoses and prostheses, are on the one hand the basis for a longer and high-quality life, but on the other hand they carry the risk of life-threatening bacterial colonization, accounting for up to 70% of healthcare-associated infections (Scialla et al., 2021). Recent studies have shown that implant-associated infections are the most common cause of revision interventions in the first 5 years following implantation (Bai et al., 2020). A key feature of implant-associated infections is that the presence of a foreign body can increase susceptibility to infection by at least 10,000-fold. This response is highly dependent on the implant site and the conditions that prevail there (type of implant, tissue). In vivo inocula used for catheter-associated experiments are reported as 104 – 108 CFU/ml (Nowakowska et al., 2014). Often the causative bacteria are biofilm builders (Scialla et al., 2021; Bai et al., 2020), as two appropriate conditions come together here: First, the surgery itself provides an opportunity for the microorganisms to contaminate the implant and invade the body and second, the implant as foreign body offers a favorable attachment site. It is well known that the ability of bacterial cells to adhere to biotic or abiotic surfaces and to form microbial self-assembled communities, so-called biofilms, in the host environment is crucial for their pathogenicity. Hallmarks of this pathogenicity then include reduced sensitivity to antibiotics and limited host resistance to bacteria in a biofilm, which increases their responsibility for serious infections (Parrino et al., 2019). With attachment, bacteria begin to accumulate in a self-formed glycocalyx matrix. This matrix is composed of many different biomolecules, such as exopolysaccharides, nucleic acids, proteins and lipids (Karygianni et al., 2020). Attachment as well as maturation of the biofilm are influenced by the conditions prevailing in the host and the available resources (Seidl et al., 2008), both sugars and plasma proteins seem to play an important role in this process (Knobloch et al., 2002; Vaudaux et al., 1995; Waldrop et al., 2014). Biofilms on indwelling medical devices can consist of gram-positive or gram-negative bacteria or yeasts. Bacteria most frequently isolated from infected devices include gram-positive Enterococcus faecalis, Staphylococcus aureus, Staphylococcus epidermidis (Donlan, 2001; Baddour et al., 2010). Staphylococci and Enterococci are often associated with cardiovascular device infections (Agarwal et al., 2010), and are frequently listed as a cause of serious infections such as infectious endocarditis (Chirouze et al., 2013). The most common strategies to prevent microbial colonization of implants are anti-microbial properties of materials, release of anti-microbial drugs or immunostimulatory approaches (Grainger et al., 2013). However, only a few of these have been used in clinical practice so far. In addition to the material properties, which can be controlled by technical measures, it is also important to identify the key factors for bacterial growth on the implant recipient site in order to be able to combat them as effectively as possible. One of these key factors is glucose, which is often used in in vitro experiments to promote bacterial growth. In the clinical context, glucose is associated with diabetes as it is accompanied by hyperglycemia (Brownlee, 2001). Constantly high blood sugar levels combined with ageing and the increasing number of different implants in the body, such as heart valves, catheters, dental implants, or orthopedic implants, pose a major problem in the field of implant medicine. Persistent hyperglycemia is associated with tissue destruction and poor wound healing, e.g. at the implant site, caused by cellular dysfunction in the soft tissue and a decreased immune response (Giri et al., 2018). In vitro it has been shown that elevated glucose levels increase pathogenicity of bacteria as they form biofilms to a greater extent (Waldrop et al., 2014). Besides glucose, proteins also have an impact on the formation of biofilms. Mere seconds after implantation, medical implants are coated with host plasma (matrix) proteins (Francois et al., 2000; François et al., 1998) due to their relative abundance and their biochemical or electrical affinity to the surface (Vroman effect) (Brewster et al., 2014; Vroman and Adams, 1969). These plasma proteins serve as adhesive anchors for bacteria to bind to the implant surface (Foster, 1998). In this study, we analyzed the biofilm forming capacity of three clinically relevant bacteria species, Staphylococcus aureus, Staphylococcus epidermidis and Enterococcus faecalis under different experimental conditions regarding the concentrations of glucose levels and plasma proteins. Our experimental setting included various, physiological concentrations of glucose (0%, 0.1% (5 mM), 0.2% (10 mM) and 1% (50 mM)) as well as human plasma (supplemented or coated).
Materials and methods
Bacterial species and culture conditions
Snap frozen Staphylococcus (S.) aureus (ATCC35556), Staphylococcus (S.) epidermidis (ATCC35984) and Enterococcus (E.) faecalis (ATCC29212) were purchased from the American Type Culture Collection (ATCC). Clinical blood culture isolates of S. aureus, S. epidermidis and E. faecalis were provided by the Institute for Medical Microbiology, Virology and Hygiene from Rostock, Germany. A positive statement from the ethics committee of the University Medical Center Rostock is available under the number A 2022-0088. Table 1
Table 1.
Antibiogram of the clinical isolates.
| Staphylococcus aureus | Staphylococcus epidermidis | Enterococcus faecalis | |
|---|---|---|---|
| Oxacillin | S | R | n.d. |
| Ampicillin | n.d. | n.d. | S |
| Ampicillin/Sulbactam | S | R | S |
| Cefuroxime | S | R | n.d. |
| Meropenem | S | R | n.d. |
| Gentamicin | S | S | n.d. |
| Gentamicin high-grade aminoglycoside resistance | n.d. | n.d. | N |
| Tetracycline | S | R | n.d. |
| Cotrimoxazole | S | S | n.d. |
| Erythromycin | S | R | n.d. |
| Clindamycin | S | S | n.d. |
| Vancomycin | S | S | S |
| Teicoplanin | n.d. | n.d. | S |
| Fosfomycin | S | S | n.d. |
| Fusidic acid | S | R | n.d. |
| Rifampicin | S | S | n.d. |
| Linezolid | S | S | S |
| Daptomycin | S | S | n.d. |
| Tigecycline | S | S | S |
S = sensitive, R = resistant, N = negative, n.d. = not determined.
S. aureus was cultivated in Luria broth (LB, Sigma-Aldrich) according to the supplier's instructions, S. epidermidis was cultivated in tryptic soy broth (TSB, Sigma-Aldrich) and for the cultivation of E. faecalis Brain heart infusion broth (BHI, Sigma-Aldrich) was used. S. aureus ATCC 35556 and E. faecalis ATCC 29212 as well as the clinical isolates used are susceptible to methicillin and aminoglycosides. S. epidermidis ATCC 35984 is resistant to methicillin. For all experiments, the inoculum was prepared by adjusting the concentration of an overnight bacterial broth culture to an OD600 of 0.01 for growth curves and 0.1 for biofilm (optical density measured with wavelength 600 nm) in LB, TSB or BHI medium. All experiments were performed under aerobic conditions at 37 °C in 96-well polystyrene plates (Nunc, Thermofisher scientific, Germany) with a culture volume of 0.2 ml and performed in three independent replicates. Glucose was added in the following concentrations: 0%, 0.1% (5 mM), 0.2% (10 mM) and 1% (50 mM). In addition, different conditions (concentration, coating) of human plasma were analyzed: culture conditions without human plasma, with 50% of human plasma to mimic physiological condition, or overnight coating of the culture plate wells with human plasma. The human plasma was applied as pooled plasma from 11 individual, healthy donors.
Bacterial growth curves and plasma sensitivity assay
The bacterial response to the different growth conditions was determined generating growth curves by OD measurement. In brief, bacterial suspensions (OD600 0.01) were added to culture-well plates containing different glucose and plasma conditions and incubated for up to 24 h. Concentration of bacteria was monitored by measuring the OD at 600 nm hourly using a microplate reader (FLUOstar Omega, BMG Labtech).
Biofilm quantification
Crystal violet staining was performed to quantify the biofilm mass on the materials as described before (Kwasny and Opperman, 2010). In brief, 0.2 ml of the bacterial suspensions (OD600 0.1) were incubated under the different experimental settings for 6 h and 24 h. Supernatants were removed, and the wells were washed three times with double-distilled water to remove non- or loosely adherent bacteria. For heat fixation, the plates were incubated at 60 °C for 1 h. Fifty µl of 0.06% crystal violet was added into each well and incubated for 5 min. Afterwards, crystal violet was removed, and the wells were washed three times with double-distilled water. Stained biofilms in the wells were eluted with 0.2 ml of 30% acetic acid. Measurement of supernatants was performed at 600 nm in a multilabel microtiter plate (FLUOstar Omega, BMG Labtech).
Carbohydrate quantification in the biofilm
The carbohydrate fraction of the biofilm mass was determined using the phenol sulfuric acid method (DuBois et al., 1956). In brief, following 6 h or 24 h incubation the supernatants were thoroughly removed by three times washing with PBS and biofilms were scraped and resuspended in 50 µl PBS. After mixing, 150 µl of concentrated sulfuric acid and 30 µl of phenol (5%) were added and incubated for 20 min at 85 °C. The absorption was measured at 490 nm in a multilabel microtiter plate reader (FLUOstar Omega, BMG Labtech). The concentration was determined using a carbohydrate standard curve.
Protein quantification in the biofilm
Protein content of the biofilm mass was measured using Pierce™ Modified Lowry Protein Assay Kit according to the manufacturer's instruction. In brief, following 6 h or 24 h incubation, supernatants were discarded, and the biofilms were resolved in 40 µl PBS by scratching and mixing. The Lowry Regent was added and incubated for 10 min. Following addition of 1-fold Folin-Ciocalteu Reagent, the absorption was measured at 750 nm using a multilabel microtiter plate reader (FLUOstar Omega, BMG Labtech). For determination of the protein concentration, a standard curve of bovine serum albumin (BSA) was used.
Statistics
Statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). Values are expressed as mean + SEM. Normal distribution was tested using the D'Agostino and Pearson Omnibus Normality Test. Non-normally distributed samples were compared using the Kruskal–Wallis test followed by a Dunn's post hoc test. For all statistical analyses, p values < 0.05 were considered significant. Significant results are indicated as: # p < 0.05; ## p<0.01; ### p<0.001 vs. Staphylococcus aureus without glucose or plasma supplementation; * p<0.05; ** p<0.01; *** p<0.001 vs. Staphylococcus epidermidis without glucose or plasma supplementation; + p<0.05; ++ p<0.01; +++ p<0.001 vs. Enterococcus faecalis without glucose or plasma supplementation.
Results
Glucose increases the biofilm formation of the studied strains of Staphylococcus aureus and Enterococcus faecalis, but not of Staphylococcus epidermidis
The growth of S. aureus ATCC 35556 increased steadily up to 24 h without the addition of glucose. Regardless of the glucose concentration, S. aureus ATCC 35556 growth stagnated after 10 h (Fig. 1A), whereas stagnation in clinical isolate of S. aureus started after 7 h (Fig. 1B). In contrast to S. aureus, the addition of different concentration of glucose had no negative effect on both S. epidermidis, the laboratory and the clinical isolate, as shown by the comparable growth curves (Fig. 1C+D). With regard to E. faecalis, glucose addition had a beneficial growth effect, irrespective of the concentration and origin of the strains (Fig. 1E+F).
Fig. 1.
Glucose increases biofilm formation of the studied strains of Staphylococcus (S.) aureus and Enterococcus (E.) faecalis, but not Staphylococcus (S.) epidermidis. The growth behavior of (A) S. aureus (ATCC 35556), (B) a clinical isolate of S. aureus, (C) S. epidermidis (ATCC 35984), (D) a clinical isolate of S. epidermidis, (E) E. faecalis (ATCC 29212) and (F) a clinical isolate of E. faecalis was assessed at 37 °C while shaking at 150 rpm in medium with different glucose concentrations. Growth was monitored hourly by measuring the optical density (OD600). Glu: glucose.
The biofilm masses of the ATCC and clinical isolates of S. aureus and E. faecalis were shown to be promoted by increasing concentrations of glucose. For S. aureus ATCC 35556 and E. faecalis ATCC 29212, but not for the clinical S. aureus and E. faecalis (Fig. 2D) the biofilm formation became detectable after 6 h (Fig. 2A+B) and increased steadily over the study period (Fig. 2C). The addition of glucose did not affect biofilm formation of both investigated S. epidermidis (Fig. 2A-D).
Fig. 2.
The addition of glucose increases the biofilm formation of Staphylococcus (S.) aureus and Enterococcus (E.) faecalis, but not Staphylococcus (S.) epidermidis. The biofilm formation of S. aureus (ATCC 35556), a clinical isolate of S. aureus, S. epidermidis (ATCC 35984), a clinical isolate of S. epidermidis, E. faecalis (ATCC 29212) and a clinical isolate of E. faecalis was quantified by crystal violet staining after (A+B) 6 h and (C+D) 24 h. Data are presented as mean + SEM of three independent experiments carried out in duplicates. Significant results are indicated as: ##p<0.01; ###p<0.001 vs. S. aureus without glucose or plasma supplementation; +p<0.05; +p<0.01; vs. E. faecalis without glucose or plasma supplementation.
Human plasma reduces the growth of Staphylococcus aureus, Enterococcus faecalis, and Staphylococcus epidermidis
The addition of human plasma (50%) to the medium significantly inhibited the growth of all bacteria investigated in this study: S. aureus (ATCC and clinical isolate), S. epidermidis (ATCC and clinical isolate) and E. faecalis (ATCC and clinical isolate). This growth inhibition could not be reversed by the formally seen growth-promoting effect of glucose (Fig. 3A-F). In line with these results, the biofilm formation of S. aureus and S. epidermidis was significantly reduced by human plasma after 6 and 24 h (Fig. 4A-D). The supplementation of glucose did not increase the biofilm formation in these two bacteria species. However, the reduction in E. faecalis biofilm mass due to plasma addition could be neutralized by all tested glucose concentrations (Fig. 4A+C). With regard to the clinical isolate of E. faecalis, biofilm formation was significantly reduced by the plasma without an effect of glucose addition (Fig. 4B+D).
Fig. 3.
Human plasma reduces the growth of Staphylococcus (S.) aureus, Enterococcus (E.) faecalis, and Staphylococcus (S.) epidermidis. The growth behavior of (A) S. aureus (ATCC 35556), (B) a clinical isolate of S. aureus, (C) S. epidermidis (ATCC 35984), (D) a clinical isolate of S. epidermidis, (E) E. faecalis (ATCC 29212) and (F) a clinical isolate of E. faecalis was assessed at 37 °C while shaking at 150 rpm in medium with addition of human plasma (50%) and different glucose concentrations. Growth was monitored hourly by measuring OD600. Glu: glucose.
Fig. 4.
Human plasma inhibits the biofilm formation of Staphylococcus (S.) aureus, Staphylococcus (S.) epidermidis and clinical isolate of Enterococcus (E.) faecalis. The biofilm formation of S. aureus (ATCC 35556), a clinical isolate of S. aureus, S. epidermidis (ATCC 35984), a clinical isolate of S. epidermidis, E. faecalis (ATCC 29212) and a clinical isolate of E. faecalis was quantified by crystal violet staining after (A+B) 6 h and (C+D) 24 h. Data are presented as mean + SEM of three independent experiments carried out in duplicates. Significant results are indicated as: #p< 0.05; ###p< 0.001 vs. S. aureus without glucose or plasma supplementation; * p< 0.05; ** p< 0.01; *** p< 0.001 vs. S. epidermidis without glucose or plasma supplementation; +p< 0.05; +p< 0.01; ++p< 0.001 vs. E. faecalis without glucose or plasma supplementation.
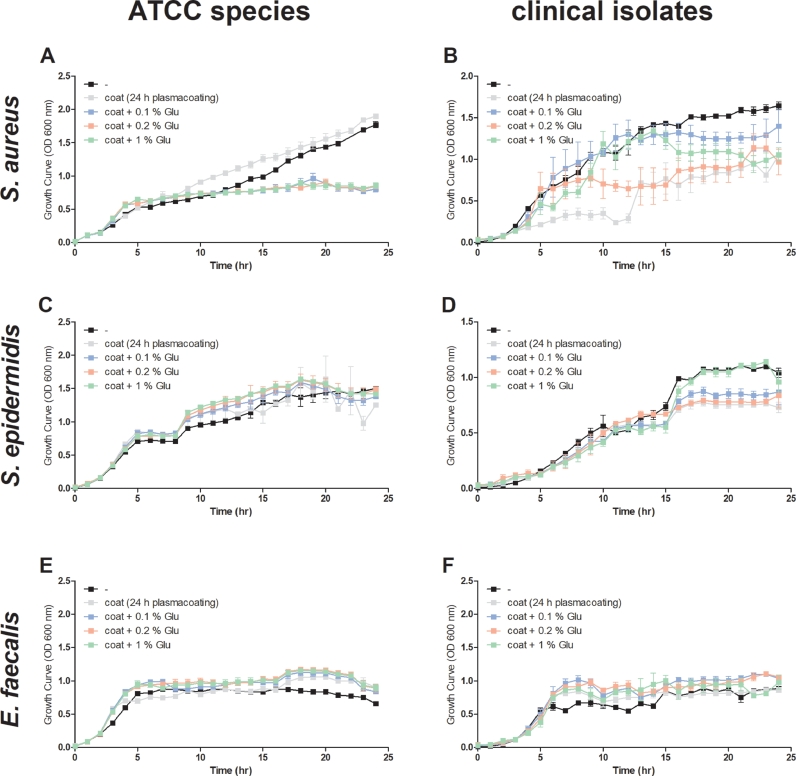
To give the observed effect of plasma proteins on bacterial growth more physiological appearance, the bacterial culture plates were coated with human plasma overnight. Interestingly, plasma coating had no effect on the growth of S. aureus ATCC 35556. Moreover, glucose supplementation reduced the growth of S. aureus ATCC 35556 on plasma-coated culture plates (Fig. 5A). In contrast, the growth of the clinical isolate of S. aureus was negatively affected by plasma coating and was improved by glucose (Fig. 5B). The investigated strains of S. epidermidis and E. faecalis appeared to be unaffected by human plasma coating with or without supplementation of glucose (Fig. 5C-F).
Fig. 5.
Human plasma coating increases the growth behavior of Staphylococcus (S.) aureus ATCC 35556 and Enterococcus (E.) faecalis, but not that of Staphylococcus (S.)epidermidis. The growth behavior of (A) S. aureus (ATCC 35556), (B) a clinical isolate of S. aureus, (C) S. epidermidis (ATCC 35984), (D) a clinical isolate of S. epidermidis, (E) E. faecalis (ATCC 29212) and (F) a clinical isolate of E. faecalis was assessed at 37 °C while shaking at 150 rpm in medium with different glucose concentrations. Before incubation, wells were coated with human plasma overnight. Growth was monitored hourly by measuring OD600. Glu: glucose.
The biofilm formation of S. aureus ATCC 35556 and E. faecalis ATCC 29212 remained unchanged under the different experimental conditions after 6 h but rose significantly after 24 h (Fig. 6A+C). With regard to the clinical S. aureus, biofilm formation was significantly increased by plasma coating (Fig. 6B) and by 1% glucose supplementation after 24 h (Fig. 6D). In contrast, S. epidermidis ATCC 35984 biofilm formation was halted following human plasma coating with or without glucose supplementation (Fig. 6A+C) and for the clinical isolate of S. epidermidis it was significantly decreased after 6 and 24 h (Fig. 6B+D).
Fig. 6.
Human plasma coating increases the biofilm formation of Staphylococcus (S.) aureus and Enterococcus (E.) faecalis and decreases that of Staphylococcus (S.) epidermidis. The biofilm formation of S. aureus (ATCC 35556), a clinical isolate of S. aureus, S. epidermidis (ATCC 35984), a clinical isolate of S. epidermidis, E. faecalis (ATCC 29212) and a clinical isolate of E. faecalis was quantified by crystal violet staining after (A+B) 6 h and (C+D) 24 h. Before incubation, wells were coated with human plasma overnight. Data are presented as mean + SEM of three independent experiments carried out in duplicates. Significant results are indicated as: #p< 0.05; ###p< 0.001 vs. S. aureus without glucose or plasma supplementation; * p< 0.05; ** p< 0.01; *** p< 0.001 vs. S. epidermidis without glucose or plasma supplementation; +p< 0.05; ++p< 0.001 vs. E. faecalis without glucose or plasma supplementation.
The investigated strains of Enterococcus faecalis and to a lesser extent the Staphylococcus aureus and Staphylococcus epidermidis accumulate carbohydrate sources in their biofilm mass
Extra glucose or human plasma in the growth environment display a suitable carbohydrate source for bacteria. The increase in saccharide concentration measured in the biofilms of the investigated S. aureus and E. faecalis strains correlated with the increasing glucose concentration in the medium (Fig. 7A+C). This effect appeared even more pronounced following human plasma coating, particularly for E. faecalis ATCC 35984 after 24 h (Fig. 7C). A similar picture emerged for the storage of proteins. The protein concentration in E. faecalis ATCC 35984 biofilms correlated with increasing glucose concentration and was even more pronounced on plasma coated culture wells (Fig. 8A+C). The protein concentration of the clinical isolate of S. aureus is highest on plasma coated wells without glucose and decreases with higher glucose concentration (Fig. 8B+D), whereas S. aureus ATCC 35556 remains unaffected.
Fig. 7.
Enterococcus (E.) faecalis and to a lesser extent the Staphylococci accumulate carbohydrate sources in their biofilm mass. Glucose uptake and recovery in the biofilm were quantified using the phenol sulfuric acid method. Staphylococcus (S.) aureus (ATCC 35556 and clinical isolate), Staphylococcus (S.) epidermidis (ATCC 35984 and clinical isolate) and E. faecalis (ATCC 29212 and clinical isolate) were grown with different glucose concentrations, with overnight plasma coating. Saccharide content was quantified by measurement at OD490 after (A+C) 6 h and (B+D) 24 h. Data are presented as mean + SEM of three independent experiments carried out in duplicates. Significant results are indicated as: #p< 0.05; ##p< 0.01; ###p< 0.001 vs. S. aureus without glucose; * p< 0.05; ** p< 0.01; *** p< 0.001 vs. S. epidermidis with 1% glucose in B, vs. S. epidermidis without glucose in D; +p< 0.05; +p< 0.01; ++p< 0.001 vs. E. faecalis without glucose.
Fig. 8.
Enterococcus (E.) faecalis accumulate protein sources in their biofilm mass. Protein uptake and recovery in the biofilm were quantified using the lowry method. Staphylococcus (S.) aureus (ATCC 35556 and clinical isolate), Staphylococcus (S.) epidermidis (ATCC 35984 and clinical isolate) and E. faecalis (ATCC 29212 and clinical isolate) were grown with different glucose concentrations, with overnight plasma coating. Protein content was quantified by measurement at OD750 after (A+C) 6 h and (B+D) 24 h. Data are presented as mean + SEM of three independent experiments carried out in duplicates. Significant results are indicated as: #p< 0.05; ##p< 0.01; vs. S. aureus without glucose; * p< 0.05 vs. S. epidermidis without glucose; +p< 0.05; +p< 0.01 vs. E. faecalis without glucose.
Discussion
The present study demonstrates that glucose supplementation to the culture medium promotes the biofilm formation of both investigated Staphylococcus (S.) aureus, the ATCC and the clinical isolate as well as the ATCC Enterococcus (E.) faecalis in a dose-dependent manner (Fig. 2A+C), although the effect was less pronounced for the respective clinical isolates of these species. The both investigated strains of Staphylococcus (S.) epidermidis appeared to be unaffected. Moreover, our results point to a different susceptibility of the tested bacteria species to human plasma. This is reflected by the fact that the ATCC and the clinical isolates of S. aureus and S. epidermidis, and the clinical isolate of E. faecalis are far more sensitive to human plasma than E. faecalis ATCC 29212, shown by growth inhibition (Fig. 3A-F) and significantly reduced biofilm formation (Fig. 4A-D).
The glucose dependent promotion of the biofilm formation goes in line with an observation made by Croes et al. who investigated biofilm formation in distinct clonal lineages of S. aureus at different glucose concentrations. In this study a positive correlation between glucose concentration and biofilm formation was shown (Croes et al., 2009). In addition, a correlation between a methicillin-resistance and the biofilm formation has been shown (Zhang et al., 2021). In this study, S. epidermidis ATCC 35984 is the only methicillin-resistant strain used in our experimental setting. And moreover, it is the only strain in our study that displayed a glucose-unaffected biofilm formation. Further studies would be needed to examine the relationship between methicillin resistance and glucose utilization by S. epidermidis ATCC 35984. Further supporting this, for E. faecalis an amoxicillin and aminoglycoside resistance have likewise been described in connection with biofilm formation (Gowda et al., 2021; Pinheiro et al., 2004). However, the E. faecalis strains used in the present study is susceptible to both antibiotics (Chai et al., 2007).
In the context of plasma susceptibility, Pont et al. has compared the diverse behavior of different Pseudomonas aeruginosa (P. aeruginosa) strains to Escherichia (E.) coli after 3 h incubation in plasma and whole blood. While E. coli was eradicated in both conditions, the P. aeruginosa strains showed varying survival patterns from resistance to complete eradication (Pont et al., 2020). Taha et al. investigated the viability of different bacterial strains that were incubated in whole blood. They divided the bacteria in dependence on their reaction in three groups, bacteria with proliferation, bacteria which remained viable or showed low proliferation and bacteria with decreased or even lost viability (Taha et al., 2019). Furthermore, it is well accepted that implanted biomaterials are rapidly coated with plasma proteins (Brewster et al., 2014; Vroman and Adams, 1969; Ekdahl et al., 2011; Andersson et al., 2005) and that some proteins can enhance the adhesion of bacteria. Accordingly, fibronectin and fibrinogen have been shown to promote attachment of different S. aureus strains to biomaterials (Herrmann et al., 1988; Vaudaux et al., 1993). In view of our results, we can confirm that the biofilm formation of the investigated S. aureus strains used in the present study is significantly elevated on well plates coated with human plasma. The same effect was observed for E. faecalis ATCC 29212 after 24 h. However, for both investigated S. epidermidis (ATCC and clinical isolate) an opposite behavior was detected (Fig. 6). This finding contradicts a previous study by Loza-Correa et al. (Loza-Correa et al., 2017), where storage bags for platelet concentrates were evaluated for the ability of S. epidermidis to adhere to the inner walls of the bags preconditioned with platelet rich/poor plasma (PRP/PPP) compared to bags without PRP/PPP. S. epidermidis has been shown to adhere to preconditioned bags. However, two different S. epidermidis isolates, ST11003 (biofilm negative) and AZ39 (biofilm positive), were used and, in contrast to our study, bacterial concentrations in the bags were examined after 7 days.
Furthermore, an enrichment of saccharides was found in the biofilm mass of E. faecalis and to a lesser extent in that of the investigated Staphylococci (Fig. 7). These results might be of importance in regard to an increasingly aging population, in which the use of biomedical implants is becoming more diverse and patients' concomitant diseases, such as hyperglycemia in type II diabetes, are also increasing (Brownlee, 2001). Diabetes have been shown to be an increased risk factor for catheter-related infections (Bomberg et al., 2015a,b). The glucose concentrations used in this study span physiological hyperglycemic condition ranging from 90 to 900 mg/dl (Waldrop et al., 2014). The main finding of this study is that three of the six examined microorganisms exhibited increased biofilm formation in association with clinically relevant glucose levels and showed different susceptibility to human plasma. These results provide important information on the relevance of physiological parameters in biofilm models of different bacteria species. These findings will help to optimize the performance of in vitro investigations on medical devices and underscore the importance of questioning the culture conditions used in in vitro material studies.
Conclusion
The relationship between glucose or plasma concentration and the biofilm formation of different clinically relevant bacterial species is important to mimic the best physiological conditions for in vitro testing of implant materials. Among the species used, glucose supplementation enhanced biofilm formation of S. aureus and E. faecalis, but not S. epidermidis. All bacteria species responded to the plasma supplementation with a reduction of biofilm formation, except for ATCC of E. faecalis. Contrary, the coating of culture plates with human plasma increased biofilm formation of both S. aureus und E. faecalis (ATCC and clinical isolates) after 24 h. S. epidermidis, on the other hand, responded with a decrease in biofilm mass. This highlights that different bacterial backgrounds or origins must not be neglected in experimental settings. Targeting biofilm-specific conditions in experimental approaches as physiologically as possible is the only way to develop effective anti-biofilm approaches and an improved translation into clinical application.
Funding
This study was financially supported by the Federal Ministry of Education and Research (BMBF) with RESPONSE “Partnership for Innovation in Implant Technology.”
CRediT authorship contribution statement
Franziska Woitschach: Conceptualization, Investigation, Formal analysis, Visualization, Writing – original draft, Project administration. Marlen Kloss: Visualization, Writing – original draft. Niels Grabow: Funding acquisition, Resources. Emil C. Reisinger: Funding acquisition, Resources, Supervision. Martina Sombetzki: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We gratefully thank Liza Denski for her excellent technical support.
References
- Agarwal A., Singh K.P., Jain A. Medical significance and management of staphylococcal biofilm. FEMS Immunol. Med. Microbiol. 2010;58:147–160. doi: 10.1111/j.1574-695X.2009.00601.x. [DOI] [PubMed] [Google Scholar]
- Andersson J., Ekdahl K.N., Lambris J.D., Nilsson B. Binding of C3 fragments on top of adsorbed plasma proteins during complement activation on a model biomaterial surface. Biomaterials. 2005;26:1477–1485. doi: 10.1016/j.biomaterials.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Baddour L.M., Epstein A.E., Erickson C.C., Knight B.P., Levison M.E., Lockhart P.B., Masoudi F.A., Okum E.J., Wilson W.R., Beerman L.B., et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- Bai D., Chen J., Li P., Huang W. In: Racing For the Surface: Pathogenesis of Implant Infection and Advanced Antimicrobial Strategies. Li B., Moriarty T.F., Webster T., Xing M., editors. Springer International Publishing; Cham: 2020. Perspectives on biomaterial-associated infection: pathogenesis and current clinical demands; pp. 75–93. ISBN 978-3-030-34475-7. [Google Scholar]
- Bomberg H., Albert N., Schmitt K., Gräber S., Kessler P., Steinfeldt T., Hering W., Gottschalk A., Standl T., Stork J., et al. Obesity in regional anesthesia–a risk factor for peripheral catheter-related infections. Acta Anaesthesiol. Scand. 2015;59:1038–1048. doi: 10.1111/aas.12548. [DOI] [PubMed] [Google Scholar]
- Bomberg H., Kubulus C., List F., Albert N., Schmitt K., Gräber S., Kessler P., Steinfeldt T., Standl T., Gottschalk A., et al. Diabetes: a risk factor for catheter-associated infections. Reg. Anesth. Pain Med. 2015;40:16–21. doi: 10.1097/AAP.0000000000000196. [DOI] [PubMed] [Google Scholar]
- Brewster L., Brey E.M., Greisler H.P. Elsevier; 2014. Blood Vessels. Principles of Tissue Engineering; pp. 793–812. ISBN 9780123983589. [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Chai W.L., Hamimah H., Cheng S.C., Sallam A.A., Abdullah M. Susceptibility of Enterococcus faecalis biofilm to antibiotics and calcium hydroxide. J. Oral Sci. 2007;49:161–166. doi: 10.2334/josnusd.49.161. [DOI] [PubMed] [Google Scholar]
- Chirouze C., Athan E., Alla F., Chu V.H., Ralph Corey G., Selton-Suty C., Erpelding M.-.L., Miro J.M., Olaison L., Hoen B. Enterococcal endocarditis in the beginning of the 21st century: analysis from the International Collaboration on Endocarditis-Prospective Cohort Study. Clin. Microbiol. Infect. 2013;19:1140–1147. doi: 10.1111/1469-0691.12166. [DOI] [PubMed] [Google Scholar]
- Croes S., Deurenberg R.H., Boumans M.-L.L., Beisser P.S., Neef C., Stobberingh E.E. Staphylococcus aureus biofilm formation at the physiologic glucose concentration depends on the S. aureus lineage. BMC Microbiol. 2009;9:229. doi: 10.1186/1471-2180-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlan R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001;7:277–281. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Ekdahl K.N., Lambris J.D., Elwing H., Ricklin D., Nilsson P.H., Teramura Y., Nicholls I.A., Nilsson B. Innate immunity activation on biomaterial surfaces: a mechanistic model and coping strategies. Adv. Drug Deliv. Rev. 2011;63:1042–1050. doi: 10.1016/j.addr.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–488. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- Francois P., Schrenzel J., Stoerman-Chopard C., Favre† H., Herrmann M., Foster T.J., Lew D.P., Vaudaux P. Identification of plasma proteins adsorbed on hemodialysis tubing that promote Staphylococcus aureus adhesion. J. Lab. Clin. Med. 2000;135:32–42. doi: 10.1016/s0022-2143(00)70018-7. [DOI] [PubMed] [Google Scholar]
- François P., Vaudaux P., Lew P.D. Role of plasma and extracellular matrix proteins in the physiopathology of foreign body infections. Ann. Vasc. Surg. 1998;12:34–40. doi: 10.1007/s100169900112. [DOI] [PubMed] [Google Scholar]
- Giri B., Dey S., Das T., Sarkar M., Banerjee J., Dash S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: an update on glucose toxicity. Biomed. Pharmacother. 2018;107:306–328. doi: 10.1016/j.biopha.2018.07.157. [DOI] [PubMed] [Google Scholar]
- Gowda J., Tavarageri A., Kulkarni R., Anegundi R.T., Janardhan A., Bhat M.A. Comparative Assessment of the Antimicrobial Efficacy of Triclosan, Amoxicillin and Eugenol against Enterococcus faecalis. Int. J. Clin. Pediatr. Dent. 2021;14:59–62. doi: 10.5005/jp-journals-10005-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger D.W., van der Mei H.C., Jutte P.C., van den Dungen J.J.A.M., Schultz M.J., van der Laan B.F.A.M., Zaat S.A.J., Busscher H.J. Critical factors in the translation of improved antimicrobial strategies for medical implants and devices. Biomaterials. 2013;34:9237–9243. doi: 10.1016/j.biomaterials.2013.08.043. [DOI] [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P.E., Pittet D., Auckenthaler R., Lew P.D., Schumacher-Perdreau F., Peters G., Waldvogel F.A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J. Infect. Dis. 1988;158:693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- Karygianni L., Ren Z., Koo H., Thurnheer T. Biofilm matrixome: extracellular components in structured microbial communities. Trends Microbiol. 2020;28:668–681. doi: 10.1016/j.tim.2020.03.016. [DOI] [PubMed] [Google Scholar]
- Knobloch J.K.-M., Horstkotte M.A., Rohde H., Mack D. Evaluation of different detection methods of biofilm formation in Staphylococcus aureus. Med. Microbiol. Immunol. 2002;191:101–106. doi: 10.1007/s00430-002-0124-3. [DOI] [PubMed] [Google Scholar]
- Kwasny S.M., Opperman T.J. Static biofilm cultures of Gram-positive pathogens grown in a microtiter format used for anti-biofilm drug discovery. Curr. Protoc. Pharmacol. 2010 doi: 10.1002/0471141755.ph13a08s50. Chapter 13, Unit 13A.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loza-Correa M., Kalab M., Yi Q.-.L., Eltringham-Smith L.J., Sheffield W.P., Ramirez-Arcos S. Comparison of bacterial attachment to platelet bags with and without preconditioning with plasma. Vox Sang. 2017;112:401–407. doi: 10.1111/vox.12513. [DOI] [PubMed] [Google Scholar]
- Nowakowska J., Landmann R., Khanna N. Foreign body infection models to study host-pathogen response and antimicrobial tolerance of bacterial biofilm. Antibiotics. 2014;3:378–397. doi: 10.3390/antibiotics3030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrino B., Schillaci D., Carnevale I., Giovannetti E., Diana P., Cirrincione G., Cascioferro S. Synthetic small molecules as anti-biofilm agents in the struggle against antibiotic resistance. Eur. J. Med. Chem. 2019;161:154–178. doi: 10.1016/j.ejmech.2018.10.036. [DOI] [PubMed] [Google Scholar]
- Pinheiro E.T., Gomes B.P.F.A., Drucker D.B., Zaia A.A., Ferraz C.C.R., Souza-Filho F.J. Antimicrobial susceptibility of Enterococcus faecalis isolated from canals of root filled teeth with periapical lesions. Int. Endod. J. 2004;37:756–763. doi: 10.1111/j.1365-2591.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- Pont S., Fraikin N., Caspar Y., van Melderen L., Attrée I., Cretin F. Bacterial behavior in human blood reveals complement evaders with some persister-like features. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialla S., Martuscelli G., Nappi F., Singh S.S.A., Iervolino A., Larobina D., Ambrosio L., Raucci M.G. Trends in managing cardiac and orthopaedic device-associated infections by using therapeutic biomaterials. Polymers. 2021;13 doi: 10.3390/polym13101556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidl K., Goerke C., Wolz C., Mack D., Berger-Bächi B., Bischoff M. Staphylococcus aureus CcpA affects biofilm formation. Infect. Immun. 2008;76:2044–2050. doi: 10.1128/IAI.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha M., Kyluik-Price D., Kumaran D., Scott M.D., Toyofuku W., Ramirez-Arcos S. Bacterial survival in whole blood depends on plasma sensitivity and resistance to neutrophil killing. Transfusion. 2019;59:3674–3682. doi: 10.1111/trf.15550. [DOI] [PubMed] [Google Scholar]
- Vaudaux P., Pittet D., Haeberli A., Lerch P.G., Morgenthaler J.J., Proctor R.A., Waldvogel F.A., Lew D.P. Fibronectin is more active than fibrin or fibrinogen in promoting Staphylococcus aureus adherence to inserted intravascular catheters. J. Infect. Dis. 1993;167:633–641. doi: 10.1093/infdis/167.3.633. [DOI] [PubMed] [Google Scholar]
- Vaudaux P.E., François P., Proctor R.A., McDevitt D., Foster T.J., Albrecht R.M., Lew D.P., Wabers H., Cooper S.L. Use of adhesion-defective mutants of Staphylococcus aureus to define the role of specific plasma proteins in promoting bacterial adhesion to canine arteriovenous shunts. Infect. Immun. 1995;63:585–590. doi: 10.1128/iai.63.2.585-590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroman L., Adams A.L. Identification of rapid changes at plasma-solid interfaces. J. Biomed. Mater. Res. 1969;3:43–67. doi: 10.1002/jbm.820030106. [DOI] [PubMed] [Google Scholar]
- Waldrop R., McLaren A., Calara F., McLemore R. Biofilm growth has a threshold response to glucose in vitro. Clin. Orthop. Relat. Res. 2014;472:3305–3310. doi: 10.1007/s11999-014-3538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Wang P., Shi X., Tan H. Inhibitory properties of Chinese Herbal Formula SanHuang decoction on biofilm formation by antibiotic-resistant Staphylococcal strains. Sci. Rep. 2021;11:7134. doi: 10.1038/s41598-021-86647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]