Abstract
This chapter covers the known effects of endocrine disrupting chemicals (EDCs) on reproductive disorders. The EDCs represented are highly studied, including plasticizers (bisphenols and phthalates), chemicals in personal care products (parabens), persistent environmental contaminants (polychlorinated biphenyls), and chemicals in pesticides or herbicides. Both female and male reproductive disorders are reviewed in the chapter. Female disorders include infertility/subfertility, irregular reproductive cycles, early menopause, premature ovarian insufficiency, polycystic ovarian syndrome, endometriosis, and uterine fibroids. Male disorders include infertility/subfertility, cryptorchidism, and hypospadias. Findings from both human and animal studies are represented.
1. Introduction
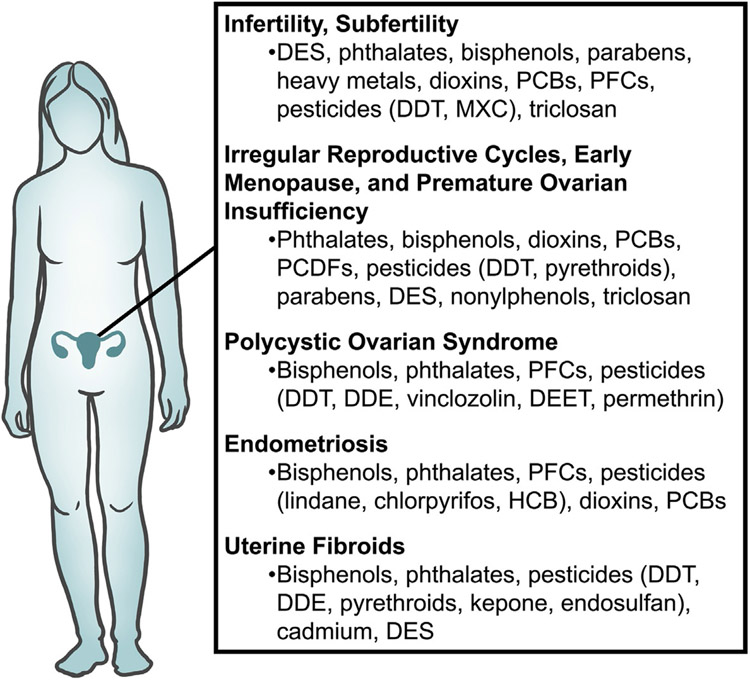
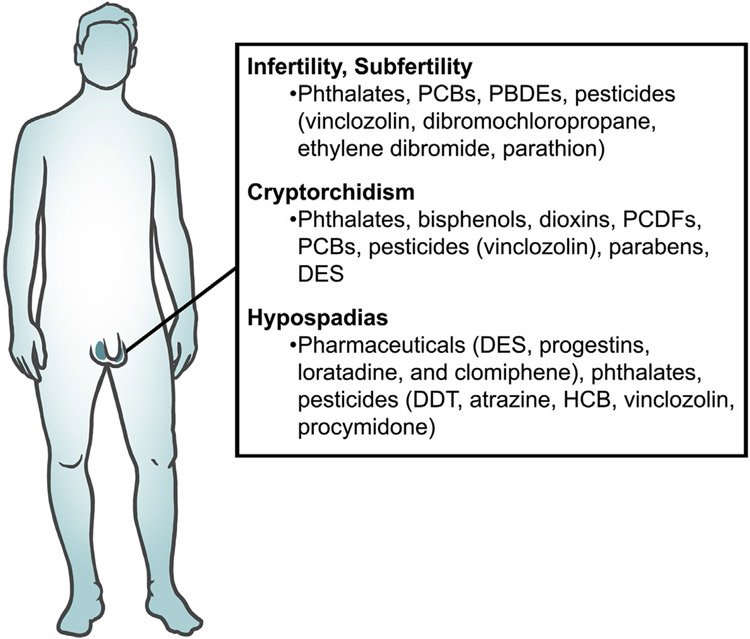
Endocrine disrupting chemicals (EDCs) are exogenous agents that can interfere with the synthesis, storage, release, transport, metabolism, binding, action, and/or elimination of endogenous hormones. EDCs are encountered in everyday life as they are present in plastic food containers, water bottles, personal care products, air pollution, and pesticides. Substantial evidence shows that EDCs impact human and animal reproductive health as well as contribute to reproductive disorders (Sifakis, Androutsopoulos, Tsatsakis, & Spandidos, 2017). In the female, EDCs are associated with subfertility, aberrations in the reproductive cycle, polycystic ovarian syndrome, endometriosis, and uterine fibroids (Fig. 1). In the male, EDCs have been implicated in male subfertility, cryptorchidism, and hypospadias (Fig. 2). The EDCs reviewed in this chapter are those that are the most studied including, but not limited to, bisphenols, phthalates, polychlorinated biphenyls (PCBs), perfluorochemicals (PFCs), polybrominated diphenyl ethers, dioxins, and pesticides. Furthermore, the EDCs reviewed in this chapter are those that have been linked to reproductive disorders such as subfertility/infertility, acyclicity, polycystic ovarian syndrome, endometriosis, and uterine fibroids in women and subfertility, cryptorchidism, and hypospadias in men. Finally, some of the EDCs reviewed in this chapter have been shown to cause reproductive disorders in mammalian animal models.
Fig. 1.
A summary of EDCs associated with female reproductive disorders.
Fig. 2.
A summary of EDCs associated with male reproductive disorders.
2. Female reproductive disorders
2.1. Female infertility, subfertility
The female reproductive system is a primary target for EDCs. Exposures to pharmaceuticals, chemicals used in consumer goods and industrial practices, as well as pesticides and herbicides can have an immediate impact on female fertility and in some cases, affect the fertility of future generations (Gore et al., 2015). Because the mammalian female is born with a finite number of oocytes, exposure to EDCs at any point in life can affect female fertility as an adult (Hannon & Flaws, 2015). Furthermore, the development of the reproductive tract is profoundly influenced by steroid hormones during development, making the developmental period a vulnerable time for EDC effects (Yao, Lopez-Tello, & Sferruzzi-Perri, 2020).
Perhaps one of the most tragic histories of an EDC and its effect on reproduction is that of diethylstilbestrol (DES), a synthetic form of the female hormone estrogen, prescribed beginning in 1940 to prevent complications of pregnancy. Although a study published in 1953 reported that DES did not improve pregnancy complications (Dieckmann, Davis, Rynkiewicz, & Pottinger, 1953), it was prescribed for two more decades. Several studies clearly indicate that prenatal exposure to DES reduces fertility in the female offspring in humans and animal models (Goldberg & Falcone, 1999).
Plasticizers, such as phthalates and bisphenol A (BPA), found in plastics are synthetic chemicals that have adverse effects on female fertility. Phthalates were introduced in commercial products in the 1920s and are still used in many consumer goods, including plastic food containers, blood bags and medical tubing, as well as personal care products. Prospective clinical studies indicate that higher phthalate concentrations in urine were associated with longer time to pregnancy (TTP) (Jukic et al., 2016; Thomsen et al., 2017). Recent studies of medically assisted pregnancy outcomes reported that higher urinary phthalates were associated with lower probabilities of implantation, clinical pregnancy, and live birth (Al-Saleh et al., 2019; Messerlian et al., 2016; Mínguez-Alarcón et al., 2019). Basic science studies support the conclusion that exposure to phthalates decreases fertility for females exposed to the chemical as well as females of subsequent generations (Chiang & Flaws, 2019; Patiño-García, Cruz-Fernandes, Buñay, Palomino, & Moreno, 2018; Rattan, Brehm, Gao, & Flaws, 2018; Yang, Arcanjo, & Nowak, 2020; Zhou et al., 2017a, 2017b).
BPA is found in a number of consumer products such as plastic bags, bottles, and packaging, including food and drink cans. BPA concentrations were found to be significantly higher in infertile patients compared with fertile subjects (Caserta et al., 2013). Additionally, BPA was associated with a reduction in fertility and fecundability, as well as with increased embryo implantation failure and decreased estrogen, fertilized oocytes, and oocyte counts in patients undergoing in vitro fertilization (IVF) (Ehrlich et al., 2012; Radwan et al., 2020; Wang et al., 2018). However, some human studies report a lack of association of BPA exposure with reproductive endpoints (Jukic et al., 2016; Mínguez-Alarcón et al., 2019; Vélez et al., 2015a; Yeum et al., 2019). Studies in laboratory animals clearly indicate an impairment of female reproductive capacity with BPA as well as with BPA alternatives, bisphenol S and bisphenol E (Acevedo, Rubin, Schaeberle, & Soto, 2018; Shi, Whorton, Sekulovski, MacLean, & Hayashi, 2019; Srivastava & Dhagga, 2019).
EDCs found in personal care products and cosmetics, such as parabens and glycol ethers, may be associated with poor fertility outcomes. Parabens are a class of preservatives widely used in the majority of personal care products, cosmetics, medicines, and food products. The Longitudinal Investigation of Fertility and the Environment (LIFE) Study found that female urinary methyl paraben and ethyl paraben levels were associated with an increase in TTP, whereas a variety of parabens were associated with decreases in ovarian antral follicle counts and estradiol levels (Jurewicz et al., 2020; Smarr, Sundaram, Honda, Kannan, & Louis, 2017). However, parabens were not associated with adverse IVF outcomes (Mínguez-Alarcón et al., 2019). In animal studies, developmental exposure to n-butylparaben reduced fertility compared to controls in rats (Maske, Dighe, & Vanage, 2018).
Several heavy metals termed metalloestrogens have been shown to modulate estrogen signaling and affect female fertility (Darbre, 2006). Lead exposure was associated with an increase in spontaneous abortions and infertility in women (Borja-Aburto et al., 1999; Rahman, Fatima, Chowdhury, & Rahman, 2013). The Study of Metals in Assisted Reproductive Technologies found that both mercury and cadmium were associated with decreases in clinical and biochemical pregnancies, and the LIFE study showed that cadmium was associated with reduced odds of fertility (Bloom et al., 2012; Buck Louis et al., 2012). Additionally, mercury exposure was associated with reduced fecundability (Cole et al., 2006). However, another study showed no association between mercury and fertility (Wright et al., 2015). A meta-analysis revealed that arsenic was associated with stillbirth and spontaneous abortion (Quansah et al., 2015). Copper was associated with negative IVF outcomes (Kumar et al., 2018).
Several EDCs that are products or byproducts of commercial and industrial practices have long half-lives in our environment. Many of these chemicals, such as dioxins, PCBsand PFCs negatively impact female fertility. The most well studied dioxin, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), was associated with longer TTP (Eskenazi et al., 2010). Rat studies have shown that in utero exposure to TCDD reduced fertility in the exposed rats as well as in three subsequent generations, suggesting that TCDD exerts effects in an epigenetic manner (Bruner-Tran & Osteen, 2011). PCBs have been associated with longer TTP (Björvang et al., 2020; Chevrier et al., 2013). Exposure to a PCB mixture reduced fertility with a significant increase in atretic ovarian follicles in mice (Pocar et al., 2012).
Perfluorononanoic acid (PFNA) has been associated with longer TTP (Jørgensen et al., 2014). Perfluorooctanoic acid (PFOA) and perfluorohexane sulfonate were associated with reduced fertility and fecundity (Vélez et al., 2015b). Other studies, however, report no association between PFCs and fecundity (Bach et al., 2015; Crawford et al., 2017).
A number of pesticides and herbicides have been shown to act as EDCs and negatively impact female fertility. Discontinued in the US in 1972, dichloro-diphenyl-trichloroethane (DDT) was withdrawn as a pesticide due to increased evidence of declining benefits and in public response to the 1962 publication Rachel Carson’s Silent Spring, which drew concern to the dangers of pesticide use. DDT is still used in many countries and persists in the environment. Based on laboratory studies, DDT has long been suspected of negatively impacting female fertility (Tiemann, 2008). Subsequent studies found that DDT was associated with implantation failure in IVF patients, and females exposed in utero to DDT experienced reduced fertility (Al-Hussaini et al., 2018; Cohn et al., 2003). Methoxychlor (MXC), an insecticide developed to replace DDT, has an unexpected affinity for the estrogen receptor (ER), and it is toxic to ovarian follicles and causes implantation failure in laboratory rodents (Cummings & Gray, 1989; Paulose, Tannenbaum, Borgeest, & Flaws, 2012).
A phenolic compound with antibacterial properties, triclosan, has been used for over 40years in consumer products. Little is known about the effects of this endocrine disrupter on fertility. The LIFE study showed no association between triclosan and TTP (Smarr et al., 2017). However, the Maternal-Infant Research on Environmental Chemicals study showed an association between triclosan in first trimester urine and longer TTP (Vélez et al., 2015a) and the Shanghai Birth Cohort Study reported longer TTP with higher levels of urine triclosan in preconception urine (Zhu et al., 2019).
2.2. Irregular reproductive cycles, early menopause, and premature ovarian insufficiency
The reproductive capacity of females is determined by many different factors, including regular reproductive cycles, fluctuation in hormones, and number of viable and healthy oocytes. The reproductive cycle of women is the menstrual cycle that consists of the proliferative phase, the secretory phase, and menses, with the average cycle lasting for 28 days (Nowak, 2018). The reproductive cycle of rodents is the estrous cycle. It consists of proestrus, estrus, metestrus, and diestrus, and the estrous cycle typically lasts 4–5 days (Nowak, 2018). Changes in reproductive cycles depend on the cyclic changes in the hypothalamic-pituitary-ovarian (HPO) axis, specifically fluctuations in estrogen, progesterone, luteinizing hormone, and follicle-stimulating hormone (FSH).
In addition to reproductive cycles and hormonal changes, the health and quantity of the ovarian follicle pool is vital for successful reproduction. Females are born with a finite primordial follicle pool and a natural decline of follicles occurs mainly by atresia, but some primordial follicles develop into more mature follicle types for possible ovulation (Hannon & Flaws, 2015). Primordial follicles grow into primary, preantral, and eventually antral follicles, and antral follicles are important for successful reproduction and the production of sex steroid hormones, specifically estradiol (Hannon & Flaws, 2015).
The natural decline in cycle lengths, dysregulation of the HPO axis, and decline in the ovarian follicle pool occurs in all females, leading to reproductive aging, or menopause in women. However, the occurrence of irregular cyclicity, early menopause, and premature or primary ovarian insufficiency (POI) can negatively affect the health of females. Irregular cycles can be due to hormonal abnormalities in the hypothalamus, pituitary, or ovary. Irregular cyclicity is considered a biomarker of reproductive aging, or menopause, and early menopause can cause many detrimental effects on overall female health (Barbieri, 2014; Burger, Hale, Robertson, & Dennerstein, 2007; Cauley, 2015; dos Santos, da Silva, Ribeiro, & Stefanon, 2014; Toffoletto, Lanzenberger, Gingnell, Sundstrom-Poromaa, & Comasco, 2014). Menopause consists of dysregulation of the hormones in the HPO axis, irregular cyclicity, and a decreased follicle pool, which together lead to decreased fertility. Early menopause can have negative effects on reproductive health by decreasing time for the possibility of pregnancy, and it can cause negative effects on non-reproductive health such as heart health, bone health, and cognitive function (Barbieri, 2014; Burger et al., 2007; Cauley, 2015; dos Santos et al., 2014; Toffoletto et al., 2014). In addition to early menopause, many young women under the age of 40 experience POI. POI is defined as a loss of ovarian function, leading to decreased estradiol levels, increased FSH levels, premature loss of follicles, amenorrhea (absence of menses), and subfertility or infertility (De Vos, Devroey, & Fauser, 2010; Rudnicka et al., 2018; Vabre et al., 2017; Wesevich, Kellen, & Pal, 2020).
Exposure to EDCs can lead to irregular cycles, early menopause, and POI. In human studies, a high sum of phthalate metabolites was associated with an increased risk of ever experiencing hot flashes, which is a biomarker of menopause (Ziv-Gal et al., 2016). Additionally, studies have shown that phthalates and/or phthalate metabolites have been associated with increased odds of POI in women (Cao et al., 2020; Özel et al., 2019). Further, animal studies have shown that prenatal exposure and adult exposure to both single phthalates and phthalate mixtures altered estrous cyclicity (Brehm & Flaws, 2019; Brehm, Zhou, Gao, & Flaws, 2020; Chiang & Flaws, 2019; Chiang, Lewis, Borkowski, & Flaws, 2020; Hannon, Niermann, & Flaws, 2016; Zhou, Chiang, Brehm, & Flaws, 2017; Zhou et al., 2017a, 2017b). Further, phthalate exposure has been shown to accelerate biomarkers of reproductive aging in rodents (Brehm & Flaws, 2019, Brehm et al., 2020; Chiang & Flaws, 2019; Chiang et al., 2020; Hannon et al., 2016). Finally, phthalates have been shown to affect estrous cyclicity and reproductive aging in both multigenerational and transgenerational manners, suggesting possible epigenetic mechanisms underlie the effects of phthalates on these endpoints ((Brehm & Flaws, 2019; Rattan et al., 2019).
Bisphenols and other plasticizers have been shown to affect cyclicity, reproductive aging, and POI. BPA has been associated with POI in women (Özel et al., 2019), possibly by downregulating microRNAs associated with decreased sex steroid hormones and POI (Ziv-Gal & Flaws, 2016). In rodent studies, BPA has been shown to alter estrous cyclicity, most likely via alterations in the regulation of estrogens (Brehm & Flaws, 2019; Rattan et al., 2017; Viguie et al., 2018; Ziv-Gal & Flaws, 2016).
TCDD is an environmental contaminant that has been shown to increase the risk of early menopause in women (Eskenazi et al., 2005; Rattan et al., 2017). Additionally PCBs and polychlorinated dibenzofurans (PCDFs) have been associated with abnormal menstruation in adolescent girls (Yang et al., 2005), and dioxin-like PCBs have been significantly associated with POI in women (Pan et al., 2019). Animal studies have shown that exposure to TCDD and PCB altered estrous cyclicity (Rattan et al., 2017; Zhang, Ji, et al., 2018) and TCDD alone caused early reproductive aging in female rodents (Franczak, Nynca, Valdez, Mizinga, & Petroff, 2006).
Increased body burdens of pesticides have been associated with an early age at menopause (Akkina, Reif, Keefe, & Bachand, 2004; Cooper, Savitz, Millikan, & Chiu Kit, 2002; Rattan et al., 2017). Additionally, pesticide exposure in girls hired as farmworkers has been associated with irregular menstrual cycles (Varnell et al., 2020). Further, insecticides such as DDT and pyrethroids have been associated with POI in women (Li et al., 2018; Pan et al., 2019). Pesticide exposure in rodents has been shown to cause irregular estrous cyclicity and advance the onset of reproductive aging (Armenti, Zama, Passantino, & Uzumcu, 2008; Gore, Walker, Zama, Armenti, & Uzumcu, 2011; Pascotto et al., 2015; Rattan et al., 2017). Moreover, studies suggest that pesticide exposure may alter neuroendocrine gene expression, DNA methylation, ovarian gene expression, and folliculogenesis, contributing to reproductive aging in female rodents (Armenti et al., 2008; Gore et al., 2011).
A few studies have examined other EDCs such as parabens, DES, nonylphenols, and triclosan and their associations with abnormal cyclicity or early menopause. A study examining triclosan effects found that high levels of triclosan were associated with an increased risk of abnormal menstruation in women planning to conceive (Zhu et al., 2019). In rodent studies, DES, nonylphenols, parabens, and triclosan have been shown to cause irregular estrous cyclicity (Bitencourt, Fortunato, Panis, Amorim, & de Arruda Amorim, 2019; Rattan et al., 2017; Zhou, Chiang, et al., 2017).
2.3. Polycystic ovarian syndrome
Polycystic ovarian syndrome (PCOS) is an endocrine disorder affecting nearly 10% of women of childbearing age (Neven, Laven, Teede, & Boyle, 2018). Although the exact etiology of PCOS is unknown, it is characterized by hyperandrogenism, polycystic ovaries, and anovulation (Neven et al., 2018). Furthermore, a subset of women diagnosed with PCOS present with peripheral insulin resistance, impaired glucose tolerance, and increased risk for the development of type 2 diabetes and metabolic syndrome (Diamanti-Kandarakis & Dunaif, 2012; Moran, Misso, Wild, & Norman, 2010). In addition to metabolic disorders, PCOS is linked with infertility. PCOS patients account for 80% of women diagnosed with anovulatory infertility (Balen et al., 2016). Women with PCOS undergoing IVF often produce poor quality oocytes, leading to lower fertilization, cleavage, and implantation rates and a higher miscarriage rate compared to women without PCOS (35.8% vs 23.6%, respectively) (Balen et al., 2016; Qiao & Feng, 2011). Moreover, PCOS patients that do conceive have a higher risk of pregnancy complications, including gestational diabetes, hypertension, pre-eclampsia, preterm birth, and perinatal mortality (Boomsma et al., 2006). Considering the associated health and fertility complications, it is critical to understand what factors contribute to the manifestation of PCOS. Recent studies have suggested a link between exposure to EDCs and PCOS.
Epidemiological studies have shown that BPA is elevated in serum, urine, and follicular fluid samples from women diagnosed with PCOS compared to women without PCOS (Kandaraki et al., 2011; Konieczna et al., 2018; Tarantino et al., 2013; Wang et al., 2017). Although one study reported no difference in serum BPA concentrations, higher serum levels of the BPA replacement chemical BPS in women with PCOS was found compared to women without PCOS (Jurewicz et al., 2020). Moreover, higher concentrations of BPA have been detected in serum and urine samples from adolescents with PCOS compared to those without PCOS, suggesting that BPA exposure plays an early role in the pathogenesis of PCOS (Akgül et al., 2019; Akın et al., 2015). In rodent-based studies, animals exposed neonatally to BPA had higher testosterone levels and exhibited polycystic ovaries compared to vehicle treated animals (Fernández, Bourguignon, Lux-Lantos, & Libertun, 2010; Yang et al., 2019).
In epidemiological studies, PCOS patients exhibited levels of phthalates and phthalate metabolites in blood and urine that are less than or equivalent to those seen in control samples (Akın et al., 2020; Vagi et al., 2014). One study identified a negative association between monoethyl phthalate levels in maternal antenatal blood and the occurrence of polycystic ovaries in the daughters (Hart et al., 2014). Conversely, di(2-ethylhexyl) phthalate (DEHP) levels were higher in follicular fluid collected from women with PCOS compared to non-PCOS women (Jin, Zhang, Pan, Wang, & Qu, 2019). Moreover, primary human granulosa cells exposed to DEHP in vitro exhibited increased androgen synthesis, increased cytochrome P450 17A1 (CYP17A1) expression, and decreased aromatase expression (Jin et al., 2019). In rodent studies, exposures to phthalates and phthalate mixtures have been shown to increase the occurrence of ovarian cysts compared to vehicle treated animals (Brehm, Rattan, Gao, & Flaws, 2018; Zhang,Mu, et al., 2018; Zhou et al., 2017a, 2017b). Interestingly, exposing pregnant mice to phthalates or mixtures containing phthalates and BPA increased cyst formation in F1-F3 generations, indicating these plasticizers have transgenerational effects (Brehm et al., 2018; Manikkam, Tracey, Guerrero-Bosagna, & Skinner, 2013; Zhou et al., 2017a, 2017b).
PFCs such as PFOA and perfluorooctanesulfonic acid (PFOS) are chemicals used in the manufacturing of textiles and non-stick cookware. Epidemiological studies show elevated levels of PFOS and PFOA in sera from women with PCOS compared to non-PCOS women and an increased risk of the development of PCOS in women with high serum concentrations of PFAS and PFOS (Heffernan et al., 2018; Vagi et al., 2014). Furthermore, PFOS levels were positively associated with irregular menstrual cycles in both the PCOS and control populations (Heffernan et al., 2018).
Pesticides are another group of environmental chemicals that have been linked to PCOS. Organochlorine pesticides such as DDT and dichlorodiphenyldichloroethylene (DDE) were present in higher concentrations in sera from PCOS patients (Guo et al., 2017; Yang et al., 2015). Moreover, serum DDT levels were positively correlated with testosterone and negatively correlated with FSH and sex hormone-binding globulin levels (Guo et al., 2017). In rodents, gestational exposure to the fungicide vinclozolin or pesticides such as permethrin and N, N-diethyl-meta-toluamide (DEET) promoted the development of polycystic ovaries in a transgenerational manner (Guerrero-Bosagna et al., 2012; Nilsson et al., 2012).
2.4. Endometriosis
Endometriosis is a reproductive disorder characterized by the formation of endometrial-like lesions on pelvic tissues, likely caused by retrograde menstruation of blood and endometrial cells into the abdomen (Bulun et al., 2019). Women with endometriosis often experience chronic pelvic pain, painful periods, and infertility (Bulun et al., 2019). Approximately 10% of women suffer from endometriosis, and 30–50% of these women are infertile (Bulletti, Coccia, Battistoni, & Borini, 2010). Endometriosis is associated with reduced ovarian reserve, poor oocyte quality, impaired embryo implantation, and reduced live birth rate (Bulletti et al., 2010; Macer & Taylor, 2012). Although the exact molecular mechanism driving endometriosis is unknown, endometriotic lesions exhibit reduced apoptosis, impaired stromal cell decidualization, and increased expression of factors involved in inflammation, angiogenesis, and tissue remodeling (Bulun et al., 2019; Kim, Kurita, & Bulun, 2013). Moreover, excessive estrogen production and progesterone resistance are common features in endometriosis (Kim et al., 2013). Given the hormone sensitivity of endometriosis, it is important to investigate potential links between EDCs and its pathogenesis.
Epidemiological studies show a positive correlation between urinary BPA levels and endometriosis (Peinado et al., 2020; Simonelli et al., 2017; Upson et al., 2014; Wen et al., 2020). Similarly, in an experimentally induced mouse model of endometriosis, BPA exposure induced endometrial ERβ expression and increased the size and number of endometriotic lesions compared to vehicle treated mice (Xue et al., 2020). Interestingly, co-administration of an ERβ antagonist blunted the effects of BPA on lesion number and volume, suggesting that BPA promotes endometriotic lesion formation and growth in an ERβ-dependent manner (Xue et al., 2020). Additionally, the BPA substitute, bisphenol AF, induced lesion growth in a mouse endometriosis model and exhibited a greater effect than BPA (Jones, Lang, Kendziorski, Greene, & Burns, 2018).
Several epidemiological studies show a positive association between blood and urinary phthalate/phthalate metabolite levels, including DEHP and mono-n-butyl phthalate, and incidence of endometriosis (Buck Louis et al., 2013; Chou et al., 2020; Cobellis et al., 2003; Huang et al., 2010; Kim et al., 2011, 2015; Reddy, Rozati, Reddy, & Raman, 2006). Interestingly, others report an inverse association between urinary mono-(2-ethylhexyl) phthalate, a metabolite of DEHP, and endometriosis (Upson, Sathyanarayana, et al., 2013; Weuve, Hauser, Calafat, Missmer, & Wise, 2010). In vitro studies using isolated human endometrial cells show that exposure to DEHP increased expression of factors involved in tissue remodeling and progesterone metabolism (Kim et al., 2015; Kim, Kim, Kim, & Cho, 2017). Moreover, human endometrial tissue implanted into immunocompromised mice exposed to DEHP showed greater growth, proliferation, and expression of matrix metalloproteinases 2 and 9 compared to vehicle treated mice (Kim et al., 2015).
Higher serum levels of PFOS, PFOA, and PFNA were reported in women with endometriosis compared to those without endometriosis (Campbell, Raza, & Pollack, 2016). Moreover, PFOA exposure was associated with both increased risk of endometriosis development and increased severity of the condition (Louis et al., 2012).
Many pesticides exhibit endocrine disrupting actions that could contribute to the development of endometriosis. In humans, serum, urine, and fat levels of organochlorine pesticides and pesticide byproducts, such as lindane, chlorpyrifos, and hexachlorobenzene (HCB), were positively associated with endometriosis (Buck Louis et al., 2012; Cooney, Buck Louis, Hediger, Vexler, & Kostyniak, 2010; Li, Chen, Lin, Buck Louis, & Kannan, 2020; Ploteau et al., 2017; Upson, De Roos, et al., 2013). In rats, HCB exposure increased lesion size and vascularization compared to vehicle treatment in an experimentally induced endometriosis model (Chiappini et al., 2019).
Elevated blood and adipose tissue levels of several PCB congeners, including anti-estrogenic, dioxin-like, and non-dioxin-like PCBs, as well as dioxins such as 1,2,3,7,8-pentachlorodibenzo-p-dioxin and TCDD were associated with endometriosis in women (Louis et al., 2005; Martínez-Zamora et al., 2015; Ploteau et al., 2017; Porpora et al., 2006, 2009). Rhesus monkeys chronically exposed to TCDD for 4 years showed an increased incidence of endometriosis 10 years after termination of exposure compared to control monkeys (Rier, Martin, Bowman, Dmowski, & Becker, 1993). In mice, exposure to dioxins and dioxin-like PCBs enhanced growth of endometriotic lesions in an experimentally induced model, whereas non-dioxin-like PCBs did not affect lesion growth (Huang et al., 2017; Johnson, Cummings, & Birnbaum, 1997).
2.5. Uterine fibroids
Uterine fibroids or leiomyomas are steroid hormone sensitive benign tumors that form in the myometrial layer of the uterus and are classified as subserosal, intramural, submucosal, or pedunculated, based on the location of the fibroid (Ikhena & Bulun, 2018; McWilliams & Chennathukuzhi, 2017). Uterine fibroids are the most common gynecologic tumors and are present in up to 80% of women by the age of 50 (Ikhena & Bulun, 2018). Approximately 15–30% of women with fibroids present with serious symptoms, including heavy and prolonged bleeding, chronic pelvic pain, and urinary incontinence (Zimmermann, Bernuit, Gerlinger, Schaefers, & Geppert, 2012). Further, symptomatic women experience pregnancy (reduced implantation rate, spontaneous abortion, infertility) and obstetric (preterm labor, placental abruption, postpartum hemorrhage) complications (Cook, Ezzati, Segars, & McCarthy, 2010). Uterine fibroids contribute to 30–50% of hysterectomies and the annual economic burden is estimated to be $34.4 billion, exceeding that of breast and ovarian cancer (Al-Hendy, Myers, & Stewart, 2017). Given their prevalence and financial burden, it is critical to understand the role of EDCs in the pathogenesis of uterine fibroids.
Epidemiological studies have shown higher concentrations of the plasticizer BPA in uterine fibroid tissue and urine from women with fibroids compared to women without fibroids (Othman, Al-Adly, Elgamal, Ghandour, & El-Sharkawy, 2016; Pollack et al., 2015). Interestingly, no differences in serum BPA levels were detected with the presence or absence of uterine fibroids (Han et al., 2011; Jeong et al., 2013). In vitro, BPA promoted cell cycle progression and proliferation in human leiomyoma cells (Li, Lu, Ding, Xu, & Shen, 2019; Wang, Kao, Chang, Lin, & Kuo, 2013; Yu et al., 2019). In rodents, the presence of leiomyomas was observed in rats neonatally exposed to BPA while no leiomyomas were detected in vehicle treated rats (Newbold, Jefferson, & Padilla-Banks, 2007). In adult rats, BPA exposure increased myometrial proliferation and thickness, although no leiomyomas were observed (Othman et al., 2016). This suggests timing of exposure is a critical component in fibroid development.
Several epidemiological studies have reported greater urine concentrations of phthalate metabolites in women with fibroids and positive associations between phthalate metabolites and fibroids (Huang et al., 2014, 2010; Kim et al., 2016; Lee et al., 2020; Sun et al., 2016). In human leiomyoma cells, in vitro exposure to DEHP decreased apoptosis and increased cell viability, proliferation, and collagen production (Kim, 2018; Kim, Kim, Oh, et al., 2017). Similarly, human fibroid tissue xenografted into immunodeficient mice that were fed DEHP showed greater proliferation, tumor growth, and collagen production than fibroid tissue implanted in vehicle fed mice (Kim et al., 2020).
Organochlorine pesticides DDT and DDE were both positively associated with fibroid development (Saxena et al., 1987; Trabert et al., 2015). Additionally, uterine fibroids were observed in rhesus monkeys chronically exposed to DDT (Takayama, Sieber, Dalgard, Thorgeirsson, & Adamson, 1999). In vitro, other organochlorine pesticides, such as kepone and endosulfan, stimulated proliferation of rat leiomyoma cells in an ER dependent manner (Hodges, Bergerson, Hunter, & Walker, 2000). Another study demonstrated that the pyrethroid insecticide fenvalerate promoted cell proliferation, cell cycle progression, collagen production, and suppressed apoptosis in both human leiomyoma and uterine smooth muscle cells (Gao et al., 2010).
Other EDCs have been associated with uterine fibroids in epidemiological studies. Cadmium is a heavy metal present in cigarette smoke that has been shown to have detrimental effects on female reproduction (Henson & Chedrese, 2004). Elevated blood cadmium levels were positively associated with uterine fibroid development (Johnstone et al., 2014; Ye et al., 2017). Additionally, DES, a synthetic estrogen once used to support pregnancy, was associated with higher incidence of uterine fibroids in women prenatally exposed to DES compared to women whose mothers did not take DES (Mahalingaiah et al., 2014).
3. Male reproductive disorders
3.1. Male infertility, subfertility
Few human studies have assessed the relationship between EDCs and male fertility rates. However, studies have examined the relationship between exposure to EDCs and markers of male reproductive health that collectively make up testicular dysgenesis syndrome (TDS), including semen quality, testicular cancer, cryptorchidism, hypospadias, low testosterone levels, and decreased anogenital distance (Skakkebaek et al., 2016). Of particular concern is sperm count, which has been declining in Western men over the past century (Swan, Elkin, & Fenster, 2000). From 1973 to 2011, sperm count and sperm concentration have declined over 50%, with no sign of leveling off (Levine et al., 2017). Evidence from humans, animals, and wildlife suggests that the mechanisms through which EDCs impact male fertility are mediated by the development of symptoms of TDS (Edwards et al., 2006).
Human studies indicate that prenatal phthalate exposure is associated with decreased anogenital distance, one known marker of decreased testosterone during development (Radke, Braun, Meeker, & Cooper, 2018). Extensive animal literature demonstrates that multiple phthalates exert anti-androgenic action, leading to TDS (Latini, Del Vecchio, Massaro, Verrotti, & De Felice, 2006).
Associations between male reproductive health and PCBs have been widely studied, identifying significant effects on sperm motility (Meeker & Hauser, 2010). Polybrominated diphenyl ethers (PBDEs) in sera were negatively associated with sperm quality in young men in Japan (Akutsu et al., 2008) and in men recruited from a fertility clinic in Canada (Abdelouahab, Ainmelk, & Takser, 2011). However, studies on adult exposure to dioxins and HCB have not identified associations with sperm parameters or hormone levels (Vested, Giwercman, Bonde, & Toft, 2014). In rodents, developmental exposures to PCBs have been shown to alter anogenital distance and testosterone levels in males (Ulbrich & Stahlmann, 2004).
Epidemiological studies show associations between occupational pesticide exposure and male infertility, especially semen quality and TTP (Bretveld, Brouwers, Ebisch, & Roeleveld, 2007). In the 1970s, pesticide workers exposed to dibromochloropropane were reported to have oligospermia and azoospermia due to suppression of spermatogenesis (Whorton, Krauss, Marshall, & Milby, 1977). Later studies on the same population following cessation of exposure found that some of the cases of azoospermia were permanent (Bretveld et al., 2007; Sheiner, Sheiner, Hammel, Potashnik, & Carel, 2003). Other pesticides associated with adverse male reproductive outcomes in men include ethylene dibromide, chlordecone, carbaryl, and methyl parathion, as well as mixtures of pesticides (Bretveld et al., 2007). In animal studies, vinclozolin and MXC have been shown to disrupt male reproductive function transgenerationally via epigenetic mechanisms (Anway & Skinner, 2006).
3.2. Cryptorchidism
Cryptorchidism is defined as undescended testes, in which one or both testes fail to descend into the scrotal sac. The normal descent of the testis has been described in a two stage model consisting of the transabdominal phase and the inguinoscrotal phase (Foresta, Zuccarello, Garolla, & Ferlin, 2008; Hughes & Acerini, 2008; Hutson et al., 2013; Kurpisz, Havryluk, Nakonechnyj, Chopyak, & Kamieniczna, 2010; Virtanen & Adamsson, 2012). Testicular descent is controlled mainly by testosterone, anti-Müllerian hormone (AMH), and insulin-like factor 3 (INSL3) (Hughes & Acerini, 2008; Klonisch, Fowler, & Hombach-Klonisch, 2004). INSL3 has been shown to control the transabdominal phase, and AMH and testosterone have been shown to control the inguinoscrotal phase (Bay, Main, Toppari, & Skakkebaek, 2011; Foresta et al., 2008; Hughes & Acerini, 2008; Hutson et al., 2013; Mamoulakis, Antypas, Sofras, Takenaka, & Sofikitis, 2015).
Cryptorchidism causes many negative effects on male reproduction. Without normal descent into the scrotal sac, the testes do not function at a lower ambient temperature, often leading to compromised spermatogenesis and/or subfertility/infertility. In addition to sperm alterations and infertility, cryptorchidism has been strongly associated with testicular cancer (Bay, Asklund, Skakkebaek, & Andersson, 2006; Bay et al., 2011; Foresta et al., 2008; Hughes & Acerini, 2008; Hutson et al., 2009, 2013; Klonisch et al., 2004; Kurpisz et al., 2010; Vigneswaran & Abern, 2018).
Although few studies have evaluated associations between phthalate exposure and cryptorchidism in humans, a French study revealed a positive correlation between self-reported exposure to phthalates and cryptorchidism (Wagner-Mahler et al., 2011). In contrast, studies from Copenhagen and Australia found no association between phthalate exposure and cryptorchidism (Hart et al., 2018; Jensen et al., 2015). Additionally, phthalates have been shown to increase the incidence of cryptorchidism in rodents (Chen et al., 2015; Ema & Miyawaki, 2001; Ema, Miyawaki, Hirose, & Kamata, 2003; Ema, Miyawaki, & Kawashima, 2000; Fisher, Macpherson, Marchetti, & Sharpe, 2003; Imajima, Shono, Zakaria, & Suita, 1997; Jiang, Ma, Yuan, Wang, & Zhang, 2007; Kim et al., 2010; Shen et al., 2017; Shono, Shima, Kondo, & Suita, 2005).
Very few studies have examined exposure to bisphenols and cryptorchidism in human and animal models. Human studies indicate that BPA concentrations in placenta, maternal sera, and sera from cryptorchid boys are higher than in boys without cryptorchidism (Fernandez et al., 2016; Fisher et al., 2020; Komarowska et al., 2015). Additionally, prenatal exposure to BPA increased the incidence of undescended testes in mice in the F1 and F2 generations, without affecting adult reproductive structures or functions later in life (Tyl et al., 2008).
Human studies have not been consistent in relating persistent environmental contaminants with cryptorchidism. For example, some studies have found associations between maternal exposure to polychlorinated dibenzo-p-dioxins, PCDFs, and PCBs and cryptorchidism (Brucker-Davis et al., 2008; Koskenniemi et al., 2015). In contrast, other epidemiological studies have found no associations between in utero exposure to PCBs, dioxins, or HCB and cryptorchidism (Axelsson et al., 2020; McGlynn et al., 2009; Virtanen et al., 2012).
Human studies on the associations of pesticide exposure and cryptorchidism in boys have been inconsistent. For example, many studies have examined pesticide exposure in mothers that mostly work in greenhouses or gardens and found positive associations with the occurrence of cryptorchidism in boys (Agopian, Lupo, Canfield, & Langlois, 2013; Andersen et al., 2008; Brucker-Davis et al., 2008; Carbone et al., 2006; Damgaard et al., 2006; Gabel et al., 2011; Weidner, Møller, Jensen, & Skakkebaek, 1998). However, other human studies have found no associations between pesticide exposure and cryptorchidism (Gaspari et al., 2012; Pierik, Klebanoff, Brock, & Longnecker, 2007; Rouget et al., 2020; Saiyed et al., 2003; Trabert, Longnecker, Brock, Klebanoff, & McGlynn, 2012; Waliszewski et al., 2005). Some animal studies have found that exposure to vinclozolin increased the incidence of undescended testes compared to control treated rodents (Shono et al., 2004a, 2004b; Wolf, LeBlanc, Ostby, & Gray, 2000).
Finally, other EDCs have been associated with cryptorchidism in both human and animal studies. Parabens, used as preservatives in personal care products, have been associated with cryptorchidism in boys in Spain (Fernandez et al., 2016). Additionally, prenatal exposure to DES has been associated with the occurrence of cryptorchidism in both the United States and Spain (Palmer et al., 2009; Tournaire et al., 2018). Animal studies have shown that DES exposure negatively affects the proper development of the gubernaculum (a structure involved in testis descent) and can lead to undescended testes (Emmen et al., 2000; Nomura & Kanzaki, 1977; Zhang, Li, Ma, Huang, & Jiang, 2012; Zhang et al., 2009). Additionally, DES exposure in rodents decreased protein and mRNA expression of INSL3 and down regulated steroidogenic factor 1, suggesting a possible mechanism for cryptorchidism (Emmen et al., 2000; Zhang et al., 2009).
3.3. Hypospadias
Hypospadias is a condition in which the urethral opening develops on the ventral side of the penis. It is one of the most common congenital malformations of the male reproductive tract, occurring in ~0.5% of live births in North America, and requires surgery to correct (Springer, van den Heijkant, & Baumann, 2016). Hypospadias is thought to be caused by both genetic and environmental factors; however, for the majority of cases, the etiology is unknown (Shih & Graham, 2014). In humans, the male reproductive tract develops between 8 and 16 weeks gestation in a process tightly controlled by genetics and sex steroid hormone signaling. Thus, the gestational peroid is an important exposure window in the development of male sex organs. Although hypospadias can occur in conjunction with cryptorchidism or other congenital conditions, it most commonly occurs alone and in mild form (Carmichael, Shaw, & Lammer, 2012). Pharmaceuticals and environmental chemicals have been implicated in the development of hypospadias in human and animal studies and generally follow either an estrogenic or anti-androgenic mode of action (Cunha, Sinclair, Risbridger, Hutson, & Baskin, 2015).
An early indication that environmental chemicals could be a factor in development of hypospadias came from studies of DES, an estrogen mimic that was given to pregnant women in the mid-20th century without proper testing for teratogenicity. Multiple studies of the sons of women exposed to DES in utero indicated statistically significant increases in hypospadias development of up to 20-fold higher than in the general population (Brouwers et al., 2006; Klip et al., 2002). Additional studies have found increased hypospadias development in grandsons of DES-exposed women, suggesting an epigenetic mode of action (Kalfa, Paris, Soyer-Gobillard, Daures, & Sultan, 2011). In rodents, prenatal and neonatal DES treatment induced development of hypospadias in male pups (Cunha et al., 2015; Sinclair, Cao, Baskin, & Cunha, 2015). Other pharmaceuticals, including progestins, loratadine, and clomiphene, have been associated with hypospadias in human and animal studies (Carmichael et al., 2005; Given et al., 2016; Meijer, De Jong-Van Den Berg, Van Den Berg, Verheij, & De Walle, 2006; Willingham, Agras, Vilela, & Baskin, 2006).
Although human evidence for associations between phthalates and hypospadias in male infants is mixed, rodent studies provide significant causal evidence. Occupational maternal exposure to phthalates is significantly associated with increased risk of hypospadias when exposure is approximated using a job matrix in multiple studies worldwide (Haraux et al., 2017; Morales-Surez-Varela et al., 2011; Nassar, Abeywardana, Barker, & Bower, 2010; Ormond et al., 2009). However, few studies find associations between phthalate metabolites in maternal urine or amniotic fluid and hypospadias (Anand-Ivell et al., 2018; Jensen et al., 2015; Raghavan, Romano, Karagas, & Penna, 2018). In rats, hypospadias has been induced following gestational exposure to a wide range of chemicals, including di-n-butyl phthalate, benzylbutyl phthalate, and DEHP (Gray Jr et al., 2000, 2004). Phthalates induced hypospadias through anti-androgenic modes of action by interfering with fetal Leydig cell differentiation and function, resulting in decreased androgen production during critical windows of development (Foster, 2006).
Non-pesticide persistent organic pollutants have been investigated in human studies for associations between maternal biomarkers of exposure and hypospadias incidence, with no associations found for PCBs or PBDEs (Raghavan et al., 2018). Although persistent pollutants such as PCBs, PBDEs, and dioxins are reproductive toxicants, their putative mechanism of action is through the aryl hydrocarbon receptor, and they did not induce hypospadias in animal models (Gray et al., 2004).
Numerous pesticides that bind to the androgen receptor and act through anti-androgen mechanisms have been implicated in the development of hypospadias in human and animal studies. A meta-analysis of parental occupational exposure to pesticides indicated a modest elevated risk (Rocheleau, Romitti, & Dennis, 2009). A large study that analyzed maternal levels of DDT and DDT metabolites found associations with hypospadias in offspring, although smaller studies found no statistically significant effects (Raghavan et al., 2018). Furthermore, epidemiological studies have identified associations with atrazine and HCB (Agopian et al., 2013; Giordano et al., 2010). In rodents, prenatal treatment with anti-androgenic pesticides, including vinclozolin, procymidone, DDT and its metabolites, linuron, and flutamide, caused hypospadias and other male reproductive tract abnormalities (Gray et al., 2004). Although hypospadias is not directly translatable to many non-mammalian species, wildlife exposed to pesticides exhibit TDS (Edwards et al., 2006). For example, juvenile alligators exposed to DDT via a chemical spill in Lake Apopka in Florida exhibited reduced penis length compared to unexposed populations (Guillette, Pickford, Crain, Rooney, & Percival, 1996).
4. Conclusion
EDCs are an integral part of our lives. These chemicals are used in plastics, personal care products, industrial practices, herbicides, and pesticides. EDCs can contribute to male and female reproductive disorders. As EDCs are identified and removed from everyday products, other chemicals will fill the functional void. Thus, more studies on the effects of current and emerging/replacement EDCs on reproductive health are essential, ideally before a chemical is mass produced and used in everyday items and applications.
Acknowledgments
The authors thank Catheryne (Katie) Chiang for Figs. 1 and 2. This work was supported by the National Institutes of Health [R01 ES028661 to JAF, K99 ES031150 to GRW, and T32 ES007326 to GRW and AMN].
Abbreviations
- AMH
anti-Müllerian hormone
- BPA
bisphenol A
- DDE
dichlorodiphenyldichloroethylene
- DDT
dichloro-diphenyl-trichloroethane
- DEET
N, N-diethyl-meta-toluamide
- DEHP
di(2-ethylhexyl) phthalate
- DES
diethylstilbestrol
- EDC
endocrine disrupting chemical
- ER
estrogen receptor
- FSH
follicle-stimulating hormone
- HCB
hexachlorobenzene
- HPO
hypothalamic-pituitary-ovarian
- INSL3
insulin-like factor 3
- IVF
in vitro fertilization
- LIFE
Longitudinal Investigation of Fertility and the Environment Study
- MXC
methoxychlor
- PBDEs
polybrominated diphenyl ethers
- PCB
polychlorinated biphenyl
- PCDF
polychlorinated dibenzofuran
- PCOS
polycystic ovarian syndrome
- PFC
perfluorochemical
- PFNA
perfluorononanoic acid
- PFOA
perfluorooctanoic acid
- PFOS
perfluorooctanesulfonic acid
- POI
primary ovarian insufficiency
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- TDS
testicular dysgenesis syndrome
- TTP
time to pregnancy
Footnotes
Conflict of interest statement
The authors have no conflicts of interest.
References
- Abdelouahab N, Ainmelk Y, & Takser L (2011). Polybrominated diphenyl ethers and sperm quality. Reproductive Toxicology, 31(4), 546–550. 10.1016/j.reprotox.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Acevedo N, Rubin BS, Schaeberle CM, & Soto AM (2018). Perinatal BPA exposure and reproductive axis function in CD-1 mice. Reproductive Toxicology, 79, 39–46. 10.1016/j.reprotox.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agopian AJ, Lupo PJ, Canfield MA, & Langlois PH (2013). Case-control study of maternal residential atrazine exposure and male genital malformations. American Journal of Medical Genetics. Part A, 161A(5), 977–982. 10.1002/ajmg.a.35815. [DOI] [PubMed] [Google Scholar]
- AkgÜl S, Sur U, Düzçeker Y, Balcı A, Kızılkan MP, Kanbur N, et al. (2019). Bisphenol A and phthalate levels in adolescents with polycystic ovary syndrome. Gynecological Endocrinology, 35(12), 1084–1087. 10.1080/09513590.2019.1630608. [DOI] [PubMed] [Google Scholar]
- Akın L, Kendirci M, Narin F, Kurtoğlu S, Hatipoğlu N, & Elmalı F (2020). Endocrine disruptors and polycystic ovary syndrome: Phthalates. Journal of Clinical Research in Pediatric Endocrinology, 12(4), 393–400. 10.4274/jcrpe.galenos.2020.2020.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akın L, Kendirci M, Narin F, Kurtoglu S, Saraymen R, Kondolot M, et al. (2015). The endocrine disruptor bisphenol A may play a role in the aetiopathogenesis of polycystic ovary syndrome in adolescent girls. Acta Paediatrica, 104(4), e171–e177. 10.1111/apa.12885. [DOI] [PubMed] [Google Scholar]
- Akkina J, Reif J, Keefe T, & Bachand A (2004). Age at natural menopause and exposure to organochlorine pesticides in Hispanic women. Journal of Toxicology and Environmental Health. Part A, 67(18), 1407–1422. 10.1080/15287390490483845. [DOI] [PubMed] [Google Scholar]
- Akutsu K, Takatori S, Nozawa S, Yoshiike M, Nakazawa H, Hayakawa K, et al. (2008). Polybrominated diphenyl ethers in human serum and sperm quality. Bulletin of Environmental Contamination and Toxicology, 80(4), 345–350. 10.1007/s00128-008-9370-4. [DOI] [PubMed] [Google Scholar]
- Al-Hendy A, Myers ER, & Stewart E (2017). Uterine fibroids: Burden and unmet medical need. Seminars in Reproductive Medicine, 35(6), 473–480. 10.1055/s-0037-1607264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hussaini TK, Abdelaleem AA, Elnashar I, Shabaan OM, Mostafa R, El-Baz MAH, et al. (2018). The effect of follicullar fluid pesticides and polychlorinated biphenyls concentrations on intracytoplasmic sperm injection (ICSI) embryological and clinical outcome. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 220, 39–43. 10.1016/j.ejogrb.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Al-Saleh I, Coskun S, Al-Doush I, Abduljabbar M, Al-Rouqi R, Al-Rajudi T, et al. (2019). Couples exposure to phthalates and its influence on in vitro fertilization outcomes. Chemosphere, 226, 597–606. 10.1016/j.chemosphere.2019.03.146. [DOI] [PubMed] [Google Scholar]
- Anand-Ivell R, Cohen A, Nørgaard-Pedersen B, Jönsson BAG, Bonde JP, Hougaard DM, et al. (2018). Amniotic fluid INSL3 measured during the critical time window in human pregnancy relates to cryptorchidism, hypospadias, and phthalate load: A large case-control study. Frontiers in Physiology, 9, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen HR, Schmidt IM, Grandjean P, Jensen TK, Budtz-Jorgensen E, Kjaerstad MB, et al. (2008). Impaired reproductive development in sons of women occupationally exposed to pesticides during pregnancy. Environmental Health Perspectives, 116(4), 566–572. 10.1289/ehp.10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, & Skinner MK (2006). Epigenetic transgenerational actions of endocrine disruptors. Endocrinology, 147(6 Suppl), S43–S49. 10.1210/en.2005-1058. [DOI] [PubMed] [Google Scholar]
- Armenti AE, Zama AM, Passantino L, & Uzumcu M (2008). Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicology and Applied Pharmacology, 233(2), 286–296. 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson J, Scott K, Dillner J, Lindh CH, Zhang H, Rylander L, et al. (2020). Exposure to polychlorinated compounds and cryptorchidism; a nested case-control study. PLoS One, 15(7), e0236394. 10.1371/journal.pone.0236394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach CC, Bech BH, Nohr EA, Olsen J, Matthiesen NB, Bossi R, et al. (2015). Serum perfluoroalkyl acids and time to pregnancy in nulliparous women. Environmental Research, 142, 535–541. 10.1016/j.envres.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, et al. (2016). The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Human Reproduction Update, 22(6), 687–708. 10.1093/humupd/dmw025. [DOI] [PubMed] [Google Scholar]
- Barbieri RL (2014). The endocrinology of the menstrual cycle. Methods in Molecular Biology, 1154, 145–169. 10.1007/978-1-4939-0659-8_7. [DOI] [PubMed] [Google Scholar]
- Bay K, Asklund C, Skakkebaek NE, & Andersson AM (2006). Testicular dysgenesis syndrome: Possible role of endocrine disrupters. Best Practice & Research. Clinical Endocrinology & Metabolism, 20(1), 77–90. 10.1016/j.beem.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Bay K, Main KM, Toppari J, & Skakkebaek NE (2011). Testicular descent: INSL3, testosterone, genes and the intrauterine milieu. Nature Reviews. Urology, 8(4), 187–196. 10.1038/nrurol.2011.23. [DOI] [PubMed] [Google Scholar]
- Bitencourt G, Fortunato ED, Panis C, Amorim EMP, & de Arruda Amorim JP (2019). Maternal exposure to triclosan causes fetal development restriction, deregulation of the oestrous cycle, and alters uterine tissue in rat offspring. Environmental Toxicology, 34(10), 1105–1113. 10.1002/tox.22812. [DOI] [PubMed] [Google Scholar]
- Björvang RD, Gennings C, Lin PI, Hussein G, Kiviranta H, Rantakokko P, et al. (2020). Persistent organic pollutants, pre-pregnancy use of combined oral contraceptives, age, and time-to-pregnancy in the SELMA cohort. Environmental Health, 19(1), 67. 10.1186/s12940-020-00608-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MS, Fujimoto VY, Steuerwald AJ, Cheng G, Browne RW, & Parsons PJ (2012). Background exposure to toxic metals in women adversely influences pregnancy during in vitro fertilization (IVF). Reproductive Toxicology, 34(3), 471–481. 10.1016/j.reprotox.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, & Macklon NS (2006). A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Human Reproduction Update, 12(6), 673–683. 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- Borja-Aburto VH, Hertz-Picciotto I, Rojas Lopez M, Farias P, Rios C, & Blanco J (1999). Blood lead levels measured prospectively and risk of spontaneous abortion. American Journal of Epidemiology, 150(6), 590–597. 10.1093/oxfordjournals.aje.a010057. [DOI] [PubMed] [Google Scholar]
- Brehm E, & Flaws JA (2019). Transgenerational effects of endocrine-disrupting chemicals on male and female reproduction. Endocrinology, 160(6), 1421–1435. 10.1210/en.2019-00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm E, Rattan S, Gao L, & Flaws JA (2018). Prenatal exposure to di(2-ethylhexyl) phthalate causes long-term transgenerational effects on female reproduction in mice. Endocrinology, 159(2), 795–809. 10.1210/en.2017-03004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm E, Zhou C, Gao L, & Flaws JA (2020). Prenatal exposure to an environmentally relevant phthalate mixture accelerates biomarkers of reproductive aging in a multiple and transgenerational manner in female mice. Reproductive Toxicology, 98, 260–268. 10.1016/j.reprotox.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretveld R, Brouwers M, Ebisch I, & Roeleveld N (2007). Influence of pesticides on male fertility. Scandinavian Journal of Work, Environment & Health, 33(1), 13–28. 10.5271/sjweh.1060. [DOI] [PubMed] [Google Scholar]
- Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, de Gier RP, & Roeleveld N (2006). Hypospadias: A transgenerational effect of diethylstilbestrol? Human Reproduction, 21(3), 666–669. 10.1093/humrep/dei398. [DOI] [PubMed] [Google Scholar]
- Brucker-Davis F, Wagner-Mahler K, Delattre I, Ducot B, Ferrari P, Bongain A, et al. (2008). Cryptorchidism at birth in Nice area (France) is associated with higher prenatal exposure to PCBs and DDE, as assessed by colostrum concentrations. Human Reproduction, 23(8), 1708–1718. 10.1093/humrep/den186. [DOI] [PubMed] [Google Scholar]
- Bruner-Tran KL, & Osteen KG (2011). Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reproductive Toxicology, 31(3), 344–350. 10.1016/j.reprotox.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Chen Z, Peterson CM, Hediger ML, Croughan MS, Sundaram R, et al. (2012). Persistent lipophilic environmental chemicals and endometriosis: The ENDO study. Environmental Health Perspectives, 120(6), 811–816. 10.1289/ehp.1104432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Peterson CM, Chen Z, Croughan M, Sundaram R, Stanford J, et al. (2013). Bisphenol A and phthalates and endometriosis: The endometriosis: Natural history, diagnosis and outcomes study. Fertility and Sterility, 100(1), 162–169.e161-162. 10.1016/j.fertnstert.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, et al. (2012). Heavy metals and couple fecundity, the LIFE Study. Chemosphere, 87(11), 1201–1207. 10.1016/j.chemosphere.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulletti C, Coccia ME, Battistoni S, & Borini A (2010). Endometriosis and infertility. Journal of Assisted Reproduction and Genetics, 27(8), 441–447. 10.1007/s10815-010-9436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, et al. (2019). Endometriosis. Endocrine Reviews, 40(4), 1048–1079. 10.1210/er.2018-00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger HG, Hale GE, Robertson DM, & Dennerstein L (2007). A review of hormonal changes during the menopausal transition: Focus on findings from the Melbourne Women’s Midlife Health Project. Human Reproduction Update, 13(6), 559–565. 10.1093/humupd/dmm020. [DOI] [PubMed] [Google Scholar]
- Campbell S, Raza M, & Pollack AZ (2016). Perfluoroalkyl substances and endometriosis in US women in NHANES 2003-2006. Reproductive Toxicology, 65, 230–235. 10.1016/j.reprotox.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Cao M, Pan W, Shen X, Li C, Zhou J, & Liu J (2020). Urinary levels of phthalate metabolites in women associated with risk of premature ovarian failure and reproductive hormones. Chemosphere, 242, 125206. 10.1016/j.chemosphere.2019.125206. [DOI] [PubMed] [Google Scholar]
- Carbone P, Giordano F, Nori F, Mantovani A, Taruscio D, Lauria L, et al. (2006). Cryptorchidism and hypospadias in the Sicilian district of Ragusa and the use of pesticides. Reproductive Toxicology, 22(1), 8–12. 10.1016/j.reprotox.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, & Lammer EJ (2012). Environmental and genetic contributors to hypospadias: A review of the epidemiologic evidence. Birth Defects Research. Part A, Clinical and Molecular Teratology, 94, 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, & Lammer EJ (2005). Maternal progestin intake and risk of hypospadias. Archives of Pediatrics and Adolescent Medicine, 159, 957–962. [DOI] [PubMed] [Google Scholar]
- Caserta D, Bordi G, Ciardo F, Marci R, La Rocca C, Tait S, et al. (2013). The influence of endocrine disruptors in a selected population of infertile women. Gynecological Endocrinology, 29(5), 444–447. 10.3109/09513590.2012.758702. [DOI] [PubMed] [Google Scholar]
- Cauley JA (2015). Estrogen and bone health in men and women. Steroids, 99(Pt. A), 11–15. 10.1016/j.steroids.2014.12.010. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu S, Wen S, Shen L, Peng J, Yan C, et al. (2015). The mechanism of environmental endocrine disruptors (DEHP) induces epigenetic transgenerational inheritance of cryptorchidism. PLoS One, 10(6), e0126403. 10.1371/journal.pone.0126403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier C, Warembourg C, Gaudreau E, Monfort C, Le Blanc A, Guldner L, et al. (2013). Organochlorine pesticides, polychlorinated biphenyls, seafood consumption, and time-to-pregnancy. Epidemiology, 24(2), 251–260. 10.1097/EDE.0b013e31827f53ec. [DOI] [PubMed] [Google Scholar]
- Chiang C, & Flaws JA (2019). Subchronic exposure to di(2-ethylhexyl) phthalate and diisononyl phthalate during adulthood has immediate and long-term reproductive consequences in female mice. Toxicological Sciences, 168(2), 620–631. 10.1093/toxsci/kfz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Lewis LR, Borkowski G, & Flaws JA (2020). Late-life consequences of short-term exposure to di(2-ethylhexyl) phthalate and diisononyl phthalate during adulthood in female mice. Reproductive Toxicology, 93, 28–42. 10.1016/j.reprotox.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappini F, Sánchez M, Miret N, Cocca C, Zotta E, Ceballos L, et al. (2019). Exposure to environmental concentrations of hexachlorobenzene induces alterations associated with endometriosis progression in a rat model. Food and Chemical Toxicology, 123, 151–161. 10.1016/j.fct.2018.10.056. [DOI] [PubMed] [Google Scholar]
- Chou YC, Chen YC, Chen MJ, Chang CW, Lai GL, & Tzeng CR (2020). Exposure to mono-n-butyl phthalate in women with endometriosis and its association with the biological effects on human granulosa cells. International Journal of Molecular Sciences, 21(5), 1794–1809. 10.3390/ijms21051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobellis L, Latini G, De Felice C, Razzi S, Paris I, Ruggieri F, et al. (2003). High plasma concentrations of di-(2-ethylhexyl)-phthalate in women with endometriosis. Human Reproduction, 18(7), 1512–1515. 10.1093/humrep/deg254. [DOI] [PubMed] [Google Scholar]
- Cohn BA, Cirillo PM, Wolff MS, Schwingl PJ, Cohen RD, Sholtz RI, et al. (2003). DDT and DDE exposure in mothers and time to pregnancy in daughters. Lancet, 361(9376), 2205–2206. 10.1016/s0140-6736(03)13776-2. [DOI] [PubMed] [Google Scholar]
- Cole DC, Wainman B, Sanin LH, Weber JP, Muggah H, & Ibrahim S (2006). Environmental contaminant levels and fecundability among non-smoking couples. Reproductive Toxicology, 22(1), 13–19. 10.1016/j.reprotox.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Cook H, Ezzati M, Segars JH, & McCarthy K (2010). The impact of uterine leiomyomas on reproductive outcomes. Minerva Ginecologica, 62(3), 225–236. [PMC free article] [PubMed] [Google Scholar]
- Cooney MA, Buck Louis GM, Hediger ML, Vexler A, & Kostyniak PJ (2010). Organochlorine pesticides and endometriosis. Reproductive Toxicology, 30(3), 365–369. 10.1016/j.reprotox.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GS, Savitz DA, Millikan R, & Chiu Kit T (2002). Organochlorine exposure and age at natural menopause. Epidemiology, 13(6), 729–733. Retrieved from https://journals.lww.com/epidem/Fulltext/2002/11000/Organochlorine_Exposure_and_Age_at_Natural.21.aspx. [DOI] [PubMed] [Google Scholar]
- Crawford NM, Fenton SE, Strynar M, Hines EP, Pritchard DA, & Steiner AZ (2017). Effects of perfluorinated chemicals on thyroid function, markers of ovarian reserve, and natural fertility. Reproductive Toxicology, 69, 53–59. 10.1016/j.reprotox.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings AM, & Gray LE Jr. (1989). Antifertility effect of methoxychlor in female rats: Dose- and time-dependent blockade of pregnancy. Toxicology and Applied Pharmacology, 97(3), 454–462. 10.1016/0041-008x(89)90250-0. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Sinclair A, Risbridger G, Hutson J, & Baskin LS (2015). Current understanding of hypospadias: Relevance of animal models. Nature Reviews Urology, 12, 271–280. [DOI] [PubMed] [Google Scholar]
- Damgaard IN, Skakkebaek NE, Toppari J, Virtanen HE, Shen H, Schramm KW, et al. (2006). Persistent pesticides in human breast milk and cryptorchidism. Environmental Health Perspectives, 114(7), 1133–1138. 10.1289/ehp.8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbre PD (2006). Metalloestrogens: An emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. Journal of Applied Toxicology, 26(3), 191–197. 10.1002/jat.1135. [DOI] [PubMed] [Google Scholar]
- De Vos M, Devroey P, & Fauser BCJM (2010). Primary ovarian insufficiency. The Lancet, 376(9744), 911–921. 10.1016/s0140-6736(10)60355-8. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, & Dunaif A (2012). Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocrine Reviews, 33(6), 981–1030. 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann WJ, Davis ME, Rynkiewicz LM, & Pottinger RE (1953). Does the administration of diethylstilbestrol during pregnancy have therapeutic value? American Journal of Obstetrics and Gynecology, 66(5), 1062–1081. 10.1016/s0002-9378(16)38617-3. [DOI] [PubMed] [Google Scholar]
- dos Santos RL, da Silva FB, Ribeiro RF Jr., & Stefanon I (2014). Sex hormones in the cardiovascular system. Hormone Molecular Biology and Clinical Investigation, 18(2), 89–103. 10.1515/hmbci-2013-0048. [DOI] [PubMed] [Google Scholar]
- Edwards TM, Moore BC, Guillette LJ, Olea N, McLachlan J, & Page D (2006). Reproductive dysgenesis in wildlife: A comparative view. International Journal of Andrology, 29, 109–121. [DOI] [PubMed] [Google Scholar]
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, et al. (2012). Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Human Reproduction, 27(12), 3583–3592. 10.1093/humrep/des328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, & Miyawaki E (2001). Adverse effects on development of the reproductive system in male offspring of rats given monobutyl phthalate, a metabolite of dibutyl phthalate, during late pregnancy. Reproductive Toxicology, 15(2), 189–194. 10.1016/S0890-6238(01)00111-3. [DOI] [PubMed] [Google Scholar]
- Ema M, Miyawaki E, Hirose A, & Kamata E (2003). Decreased anogenital distance and increased incidence of undescended testes in fetuses of rats given monobenzyl phthalate, a major metabolite of butyl benzyl phthalate. Reproductive Toxicology, 17(4), 407–412. 10.1016/s0890-6238(03)00037-6. [DOI] [PubMed] [Google Scholar]
- Ema M, Miyawaki E, & Kawashima K (2000). Critical period for adverse effects on development of reproductive system in male offspring of rats given di-n-butyl phthalate during late pregnancy. Toxicology Letters, 111(3), 271–278. 10.1016/S0378-4274(99)00192-7. [DOI] [PubMed] [Google Scholar]
- Emmen JMA, McLuskey A, Adham IM, Engel W, Verhoef-Post M, Themmen APN, et al. (2000). Involvement of insulin-like factor 3 (Insl 3) in diethylstilbestrol-induced cryptorchidism. Endocrinology, 141(2), 846–849. 10.1210/endo.141.2.7379. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner M, Marks AR, Samuels S, Gerthoux PM, Vercellini P, et al. (2005). Serum dioxin concentrations and age at menopause. Environmental Health Perspectives, 113(7), 858–862. 10.1289/ehp.7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Warner M, Marks AR, Samuels S, Needham L, Brambilla P, et al. (2010). Serum dioxin concentrations and time to pregnancy. Epidemiology, 21(2), 224–231. 10.1097/EDE.0b013e3181cb8b95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MF, Arrebola JP, Jimenez-Diaz I, Saenz JM, Molina-Molina JM, Ballesteros O, et al. (2016). Bisphenol A and other phenols in human placenta from children with cryptorchidism or hypospadias. Reproductive Toxicology, 59, 89–95. 10.1016/j.reprotox.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Fernández M, Bourguignon N, Lux-Lantos V, & Libertun C (2010). Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environmental Health Perspectives, 118(9), 1217–1222. 10.1289/ehp.0901257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JS, Macpherson S, Marchetti N, & Sharpe RM (2003). Human ‘testicular dysgenesis syndrome’: A possible model using in-utero exposure of the rat to dibutyl phthalate. Human Reproduction, 18(7), 1383–1394. 10.1093/humrep/deg273. [DOI] [PubMed] [Google Scholar]
- Fisher BG, Thankamony A, Mendiola J, Petry CJ, Frederiksen H, Andersson AM, et al. (2020). Maternal serum concentrations of bisphenol A and propyl paraben in early pregnancy are associated with male infant genital development. Human Reproduction, 35(4), 913–928. 10.1093/humrep/deaa045. [DOI] [PubMed] [Google Scholar]
- Foresta C, Zuccarello D, Garolla A, & Ferlin A (2008). Role of hormones, genes, and environment in human cryptorchidism. Endocrine Reviews, 29(5), 560–580. 10.1210/er.2007-0042. [DOI] [PubMed] [Google Scholar]
- Foster PMD (2006). Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. International Journal of Andrology, 29, 140–147. Discussion 181–185. [DOI] [PubMed] [Google Scholar]
- Franczak A, Nynca A, Valdez KE, Mizinga KM, & Petroff BK (2006). Effects of acute and chronic exposure to the aryl hydrocarbon receptor agonist 2,3,7, 8-tetrachlorodibenzo-p-dioxin on the transition to reproductive senescence in female Sprague-Dawley rats. Biology of Reproduction, 74(1), 125–130. 10.1095/biolreprod.105.044396. [DOI] [PubMed] [Google Scholar]
- Gabel P, Jensen MS, Andersen HR, Baelum J, Thulstrup AM, Bonde JP, et al. (2011). The risk of cryptorchidism among sons of women working in horticulture in Denmark: A cohort study. Environmental Health, 10, 100. 10.1186/1476-069X-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Yu L, Castro L, Moore AB, Hermon T, Bortner C, et al. (2010). An endocrine-disrupting chemical, fenvalerate, induces cell cycle progression and collagen type I expression in human uterine leiomyoma and myometrial cells. Toxicology Letters, 196(3), 133–141. 10.1016/j.toxlet.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari L, Sampaio DR, Paris F, Audran F, Orsini M, Neto JB, et al. (2012). High prevalence of micropenis in 2710 male newborns from an intensive-use pesticide area of Northeastern Brazil. International Journal of Andrology, 35(3), 253–264. 10.1111/j.1365-2605.2011.01241.x. [DOI] [PubMed] [Google Scholar]
- Giordano F, Abballe A, De Felip E, Di Domenico A, Ferro F, Grammatico P, et al. (2010). Maternal exposures to endocrine disrupting chemicals and hypospadias in offspring. Birth Defects Research. Part A, Clinical and Molecular Teratology, 88, 241–250. [DOI] [PubMed] [Google Scholar]
- Given JE, Loane M, Luteijn JM, Morris JK, de Jong van den Berg LTW, Garne E, et al. (2016). EUROmediCAT signal detection: An evaluation of selected congenital anomaly-medication associations. British Journal of Clinical Pharmacology, 82(4), 1094–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, & Falcone T (1999). Effect of diethylstilbestrol on reproductive function. Fertility and Sterility, 72(1), 1–7. 10.1016/s0015-0282(99)00153-3. [DOI] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. (2015). EDC-2: The endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocrine Reviews, 36(6), E1–E150. 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Walker DM, Zama AM, Armenti AE, & Uzumcu M (2011). Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Molecular Endocrinology, 25(12), 2157–2168. 10.1210/me.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE Jr., Ostby J, Furr J, Price M, Veeramachaneni DNR, & Parks L (2000). Perinatal exposure to the phthalates DEHP, BBP, and DINP, but Not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicologial Sciences, 58, 350–365. [DOI] [PubMed] [Google Scholar]
- Gray LE Jr., Ostby J, Furr J, Wolf C, Lambright C, Wilson V, et al. (2004). Toxicant-induced hypospadias in the male rat. Advances in Experimental Medicine and Biology, 545, 217–241. 10.1007/978-1-4419-8995-6_14. [DOI] [PubMed] [Google Scholar]
- Guerrero-Bosagna C, Covert TR, Haque MM, Settles M, Nilsson EE, Anway MD, et al. (2012). Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reproductive Toxicology, 34(4), 694–707. 10.1016/j.reprotox.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette LJ, Pickford DB, Crain DA, Rooney AA, & Percival HF (1996). Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. General and Comparative Endocrinology, 101, 32–42. [DOI] [PubMed] [Google Scholar]
- Guo Z, Qiu H, Wang L, Wang L, Wang C, Chen M, et al. (2017). Association of serum organochlorine pesticides concentrations with reproductive hormone levels and polycystic ovary syndrome in a Chinese population. Chemosphere, 171, 595–600. 10.1016/j.chemosphere.2016.12.127. [DOI] [PubMed] [Google Scholar]
- Han MS, Byun JC, Park JE, Kim JY, Chung JY, & Kim JM (2011). Bisphenol-A concentrations from leiomyoma patients by LC/MS. Toxicology Research, 27(1), 49–52. 10.5487/tr.2011.27.1.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon PR, & Flaws JA (2015). The effects of phthalates on the ovary. Frontiers in Endocrinology (Lausanne), 6, 8. 10.3389/fendo.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon PR, Niermann S, & Flaws JA (2016). Acute exposure to di(2-ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Toxicological Sciences, 150(1), 97–108. 10.1093/toxsci/kfv317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraux E, Braun K, Buisson P, Stéphan-Blanchard E, Devauchelle C, Ricard J, et al. (2017). Maternal exposure to domestic hair cosmetics and occupational endocrine disruptors is associated with a higher risk of hypospadias in the offspring. International Journal of Environmental Research and Public Health, 14, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart R, Doherty DA, Frederiksen H, Keelan JA, Hickey M, Sloboda D, et al. (2014). The influence of antenatal exposure to phthalates on subsequent female reproductive development in adolescence: A pilot study. Reproduction, 147(4), 379–390. 10.1530/rep-13-0331. [DOI] [PubMed] [Google Scholar]
- Hart RJ, Frederiksen H, Doherty DA, Keelan JA, Skakkebaek NE, Minaee NS, et al. (2018). The possible impact of antenatal exposure to ubiquitous phthalates upon male reproductive function at 20 years of age. Frontiers in Endocrinology (Lausanne), 9, 288. 10.3389/fendo.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan AL, Cunningham TK, Drage DS, Aylward LL, Thompson K, Vijayasarathy S, et al. (2018). Perfluorinated alkyl acids in the serum and follicular fluid of UK women with and without polycystic ovarian syndrome undergoing fertility treatment and associations with hormonal and metabolic parameters. International Journal of Hygiene and Environmental Health, 221(7), 1068–1075. 10.1016/j.ijheh.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Henson MC, & Chedrese PJ (2004). Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Experimental Biology and Medicine (Maywood, N.J.), 229(5), 383–392. 10.1177/153537020422900506. [DOI] [PubMed] [Google Scholar]
- Hodges LC, Bergerson JS, Hunter DS, & Walker CL (2000). Estrogenic effects of organochlorine pesticides on uterine leiomyoma cells in vitro. Toxicological Sciences, 54(2), 355–364. 10.1093/toxsci/54.2.355. [DOI] [PubMed] [Google Scholar]
- Huang Q, Chen Y, Chen Q, Zhang H, Lin Y, Zhu M, et al. (2017). Dioxin-like rather than non-dioxin-like PCBs promote the development of endometriosis through stimulation of endocrine-inflammation interactions. Archives of Toxicology, 91(4), 1915–1924. 10.1007/s00204-016-1854-0. [DOI] [PubMed] [Google Scholar]
- Huang PC, Li WF, Liao PC, Sun CW, Tsai EM, & Wang SL (2014). Risk for estrogen-dependent diseases in relation to phthalate exposure and polymorphisms of CYP17A1 and estrogen receptor genes. Environmental Science and Pollution Research International, 21(24), 13964–13973. 10.1007/s11356-014-3260-6. [DOI] [PubMed] [Google Scholar]
- Huang PC, Tsai EM, Li WF, Liao PC, Chung MC, Wang YH, et al. (2010). Association between phthalate exposure and glutathione S-transferase M1 polymorphism in adenomyosis, leiomyoma and endometriosis. Human Reproduction, 25(4), 986–994. 10.1093/humrep/deq015. [DOI] [PubMed] [Google Scholar]
- Hughes IA, & Acerini CL (2008). Factors controlling testis descent. European Journal of Endocrinology, 159(Suppl. 1), S75–S82. 10.1530/EJE-08-0458. [DOI] [PubMed] [Google Scholar]
- Hutson JM, Baker ML, Griffiths AL, Momose Y, Goh DW, Middlesworth W, et al. (2009). Endocrine and morphological perspectives in testicular descent. Reproductive Medicine Review, 1(2), 165–177. 10.1017/s096227990000051x. [DOI] [Google Scholar]
- Hutson JM, Southwell BR, Li R, Lie G, Ismail K, Harisis G, et al. (2013). The regulation of testicular descent and the effects of cryptorchidism. Endocrine Reviews, 34(5), 725–752. 10.1210/er.2012-1089. [DOI] [PubMed] [Google Scholar]
- Ikhena DE, & Bulun SE (2018). Literature review on the role of uterine fibroids in endometrial function. Reproductive Sciences, 25(5), 635–643. 10.1177/1933719117725827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajima T, Shono T, Zakaria O, & Suita S (1997). Prenatal phthalate causes cryptorchidism postnatally by inducing transabdominal ascent of the testis in fetal rats. Journal of Pediatric Surgery, 32(1), 18–21. 10.1016/S0022-3468(97)90083-X. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Anand-Ivell R, Nørgaard-Pedersen B, Jönsson BAG, Bonde JP, Hougaard DM, et al. (2015). Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology, 26, 91–99. [DOI] [PubMed] [Google Scholar]
- Jeong EH, Hong GY, Kim BR, Park SN, Lee HH, Na YJ, et al. (2013). The relationship between uterine myoma growth and the endocrine disruptor in postmenopausal women. Journal of Menopausal Medicine, 19(3), 130–134. 10.6118/jmm.2013.19.3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Ma L, Yuan L, Wang X, & Zhang W (2007). Study on developmental abnormalities in hypospadiac male rats induced by maternal exposure to di-n-butyl phthalate (DBP). Toxicology, 232(3), 286–293. 10.1016/j.tox.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Jin Y, Zhang Q, Pan JX, Wang FF, & Qu F (2019). The effects of di(2-ethylhexyl) phthalate exposure in women with polycystic ovary syndrome undergoing in vitro fertilization. The Journal of International Medical Research, 47(12), 6278–6293. 10.1177/0300060519876467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KL, Cummings AM, & Birnbaum LS (1997). Promotion of endometriosis in mice by polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls. Environmental Health Perspectives, 105(7), 750–755. 10.1289/ehp.97105750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone EB, Louis GM, Parsons PJ, Steuerwald AJ, Palmer CD, Chen Z, et al. (2014). Increased urinary cobalt and whole blood concentrations of cadmium and lead in women with uterine leiomyomata: Findings from the ENDO study. Reproductive Toxicology, 49, 27–32. 10.1016/j.reprotox.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Lang SA, Kendziorski JA, Greene AD, & Burns KA (2018). Use of a mouse model of experimentally induced endometriosis to evaluate and compare the effects of bisphenol A and bisphenol af exposure. Environmental Health Perspectives, 126(12), 127004. 10.1289/ehp3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen KT, Specht IO, Lenters V, Bach CC, Rylander L, Jönsson BA, et al. (2014). Perfluoroalkyl substances and time to pregnancy in couples from Greenland, Poland and Ukraine. Environmental Health, 13, 116. 10.1186/1476-069x-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukic AM, Calafat AM, McConnaughey DR, Longnecker MP, Hoppin JA, Weinberg CR, et al. (2016). Urinary concentrations of phthalate metabolites and bisphenol a and associations with follicular-phase length, luteal-phase length, fecundability, and early pregnancy loss. Environmental Health Perspectives, 124(3), 321–328. 10.1289/ehp.1408164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurewicz J, Majewska J, Berg A, Owczarek K, Zajdel R, Kaleta D, et al. (2020). Serum bisphenol A analogues in women diagnosed with the polycystic ovary syndrome—Is there an association? Environmental pollution (Barking, Essex: 1987), 272, 115962. 10.1016/j.envpol.2020.115962. [DOI] [PubMed] [Google Scholar]
- Jurewicz J, Radwan M, Wielgomas B, Karwacka A, Klimowska A, Kałużny P, et al. (2020). Parameters of ovarian reserve in relation to urinary concentrations of parabens. Environmental Health, 19(1), 26. 10.1186/s12940-020-00580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalfa N, Paris F, Soyer-Gobillard MO, Daures JP, & Sultan C (2011). Prevalence of hypospadias in grandsons of women exposed to diethylstilbestrol during pregnancy: A multigenerational national cohort study. Fertility and Sterility, 95, 2574–2577. [DOI] [PubMed] [Google Scholar]
- Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, et al. (2011). Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. The Journal of Clinical Endocrinology and Metabolism, 96(3), E480–E484. 10.1210/jc.2010-1658. [DOI] [PubMed] [Google Scholar]
- Kim JH (2018). Analysis of the in vitro effects of di-(2-ethylhexyl) phthalate exposure on human uterine leiomyoma cells. Experimental and Therapeutic Medicine, 15(6), 4972–4978. 10.3892/etm.2018.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Cho S, Ihm HJ, Oh YS, Heo SH, Chun S, et al. (2015). Possible role of phthalate in the pathogenesis of endometriosis: In vitro, animal, and human data. The Journal of Clinical Endocrinology and Metabolism, 100(12), E1502–E1511. 10.1210/jc.2015-2478. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chun S, Jang JY, Chae HD, Kim CH, & Kang BM (2011). Increased plasma levels of phthalate esters in women with advanced-stage endometriosis: A prospective case-control study. Fertility and Sterility, 95(1), 357–359. 10.1016/j.fertnstert.2010.07.1059. [DOI] [PubMed] [Google Scholar]
- Kim TS, Jung KK, Kim SS, Kang IH, Baek JH, Nam HS, et al. (2010). Effects of in utero exposure to di (n-butyl) phthalate on development of male reproductive tracts in Sprague-Dawley rats. Journal of Toxicology and Environmental Health. Part A, 73(21–22), 1544–1559. 10.1080/15287394.2010.511579. [DOI] [PubMed] [Google Scholar]
- Kim YA, Kho Y, Chun KC, Koh JW, Park JW, Bunderson-Schelvan M, et al. (2016). Increased urinary phthalate levels in women with uterine leiomyoma: A case-control study. International Journal of Environmental Research and Public Health, 13(12), 1247–1259. 10.3390/ijerph13121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Kim MR, Kim JH, & Cho HH (2017). Aldo-keto reductase activity after diethylhexyl phthalate exposure in eutopic and ectopic endometrial cells. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 215, 215–219. 10.1016/j.ejogrb.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Kim SH, Oh YS, Heo SH, Kim KH, Kim DY, et al. (2020). Effects of phthalate esters on human myometrial and fibroid cells: Cell culture and NOD-SCID mouse data. Reproductive Sciences, 28, 479–487. 10.1007/s43032-020-00341-0. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim SH, Oh YS, Ihm HJ, Chae HD, Kim CH, et al. (2017). In vitro effects of phthalate esters in human myometrial and leiomyoma cells and increased urinary level of phthalate metabolite in women with uterine leiomyoma. Fertility and Sterility, 107(4), 1061–1069.e1061. 10.1016/j.fertnstert.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Kurita T, & Bulun SE (2013). Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocrine Reviews, 34(1), 130–162. 10.1210/er.2012-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klip H, Verloop J, van Gool JD, Koster ME, Burger CW, & van Leeuwen FE (2002). Hypospadias in sons of women exposed to diethylstilbestrol in utero: A cohort study. Lancet, 359(9312), 1102–1107. 10.1016/s0140-6736(02)08152-7. [DOI] [PubMed] [Google Scholar]
- Klonisch T, Fowler PA, & Hombach-Klonisch S (2004). Molecular and genetic regulation of testis descent and external genitalia development. Developmental Biology, 270(1), 1–18. 10.1016/j.ydbio.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Komarowska MD, Hermanowicz A, Czyzewska U, Milewski R, Matuszczak E, Miltyk W, et al. (2015). Serum Bisphenol A level in boys with cryptorchidism: A step to male infertility? International Journal of Endocrinology, 2015, 973154. 10.1155/2015/973154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczna A, Rachoń D, Owczarek K, Kubica P, Kowalewska A, Kudłak B, et al. (2018). Serum bisphenol A concentrations correlate with serum testosterone levels in women with polycystic ovary syndrome. Reproductive Toxicology, 82, 32–37. 10.1016/j.reprotox.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Koskenniemi JJ, Virtanen HE, Kiviranta H, Damgaard IN, Matomaki J, Thorup JM, et al. (2015). Association between levels of persistent organic pollutants in adipose tissue and cryptorchidism in early childhood: A case-control study. Environmental Health, 14, 78. 10.1186/s12940-015-0065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Mishra V, Thaker R, Gor M, Perumal S, Joshi P, et al. (2018). Role of environmental factors & oxidative stress with respect to in vitro fertilization outcome. The Indian Journal of Medical Research, 148(Suppl), S125–s133. 10.4103/ijmr.IJMR_1864_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpisz M, Havryluk A, Nakonechnyj A, Chopyak V, & Kamieniczna M (2010). Cryptorchidism and long-term consequences. Reproductive Biology, 10(1), 19–35. 10.1016/s1642-431x(12)60035-7. [DOI] [PubMed] [Google Scholar]
- Latini G, Del Vecchio A, Massaro M, Verrotti A, & De Felice C (2006). Phthalate exposure and male infertility. Toxicology, 226(2–3), 90–98. 10.1016/j.tox.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Lee J, Jeong Y, Mok S, Choi K, Park J, Moon HB, et al. (2020). Associations of exposure to phthalates and environmental phenols with gynecological disorders. Reproductive Toxicology, 95, 19–28. 10.1016/j.reprotox.2020.04.076. [DOI] [PubMed] [Google Scholar]
- Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, et al. (2017). Temporal trends in sperm count: A systematic review and meta-regression analysis. Human Reproduction Update, 23(6), 646–659. 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Cao M, Ma L, Ye X, Song Y, Pan W, et al. (2018). Pyrethroid pesticide exposure and risk of primary ovarian insufficiency in Chinese women. Environmental Science & Technology, 52(5), 3240–3248. 10.1021/acs.est.7b06689. [DOI] [PubMed] [Google Scholar]
- Li AJ, Chen Z, Lin TC, Buck Louis GM, & Kannan K (2020). Association of urinary metabolites of organophosphate and pyrethroid insecticides, and phenoxy herbicides with endometriosis. Environment International, 136, 105456. 10.1016/j.envint.2019.105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lu Q, Ding B, Xu J, & Shen Y (2019). Bisphenol A promotes the proliferation of leiomyoma cells by GPR30-EGFR signaling pathway. The Journal of Obstetrics and Gynaecology Research, 45(7), 1277–1285. 10.1111/jog.13972. [DOI] [PubMed] [Google Scholar]
- Louis GM, Peterson CM, Chen Z, Hediger ML, Croughan MS, Sundaram R, et al. (2012). Perfluorochemicals and endometriosis: The ENDO study. Epidemiology, 23(6), 799–805. 10.1097/EDE.0b013e31826cc0cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis GM, Weiner JM, Whitcomb BW, Sperrazza R, Schisterman EF, Lobdell DT, et al. (2005). Environmental PCB exposure and risk of endometriosis. Human Reproduction, 20(1), 279–285. 10.1093/humrep/deh575. [DOI] [PubMed] [Google Scholar]
- Macer ML, & Taylor HS (2012). Endometriosis and infertility: A review of the pathogenesis and treatment of endometriosis-associated infertility. Obstetrics and Gynecology Clinics of North America, 39(4), 535–549. 10.1016/j.ogc.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingaiah S, Hart JE, Wise LA, Terry KL, Boynton-Jarrett R, & Missmer SA (2014). Prenatal diethylstilbestrol exposure and risk of uterine leiomyomata in the nurses’ health study II. American Journal of Epidemiology, 179(2), 186–191. 10.1093/aje/kwt250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamoulakis C, Antypas S, Sofras F, Takenaka A, & Sofikitis N (2015). Testicular descent. Hormones (Athens, Greece), 14(4), 515–530. 10.14310/horm.2002.1634. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, & Skinner MK (2013). Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One, 8(1), e55387. 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Zamora MA, Mattioli L, Parera J, Abad E, Coloma JL, van Babel B, et al. (2015). Increased levels of dioxin-like substances in adipose tissue in patients with deep infiltrating endometriosis. Human Reproduction, 30(5), 1059–1068. 10.1093/humrep/dev026. [DOI] [PubMed] [Google Scholar]
- Maske P, Dighe V, & Vanage G (2018). n-Butylparaben exposure during perinatal period impairs fertility of the F1 generation female rats. Chemosphere, 213, 114–123. 10.1016/j.chemosphere.2018.08.130. [DOI] [PubMed] [Google Scholar]
- McGlynn KA, Guo X, Graubard BI, Brock JW, Klebanoff MA, & Longnecker MP (2009). Maternal pregnancy levels of polychlorinated biphenyls and risk of hypospadias and cryptorchidism in male offspring. Environmental Health Perspectives, 117(9), 1472–1476. 10.1289/ehp.0800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliams MM, & Chennathukuzhi VM (2017). Recent advances in uterine fibroid etiology. Seminars in Reproductive Medicine, 35(2), 181–189. 10.1055/s-0037-1599090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, & Hauser R (2010). Exposure to polychlorinated biphenyls (PCBs) and male reproduction. Systems Biology in Reproductive Medicine, 56(2), 122–131. 10.3109/19396360903443658. [DOI] [PubMed] [Google Scholar]
- Meijer WM, De Jong-Van Den Berg LTW, Van Den Berg MD, Verheij JBGM, & De Walle HEK (2006). Clomiphene and hypospadias on a detailed level: Signal or chance? Birth Defects Research. Part A, Clinical and Molecular Teratology, 76, 249–252. [DOI] [PubMed] [Google Scholar]
- Messerlian C, Wylie BJ, Mínguez-Alarcón L, Williams PL, Ford JB, Souter IC, et al. (2016). Urinary concentrations of phthalate metabolites and pregnancy loss among women conceiving with medically assisted reproduction. Epidemiology, 27(6), 879–888. 10.1097/ede.0000000000000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mínguez-Alarcón L, Messerlian C, Bellavia A, Gaskins AJ, Chiu YH, Ford JB, et al. (2019). Urinary concentrations of bisphenol A, parabens and phthalate metabolite mixtures in relation to reproductive success among women undergoing in vitro fertilization. Environment International, 126, 355–362. 10.1016/j.envint.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Surez-Varela MM, Toft GV, Jensen MS, Ramlau-Hansen C, Kaerlev L, Thulstrup AM, et al. (2011). Parental occupational exposure to endocrine disrupting chemicals and male genital malformations: A study in the Danish national birth cohort study. Environmental Health: A Global Access Science Source, 10, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LJ, Misso ML, Wild RA, & Norman RJ (2010). Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Human Reproduction Update, 16(4), 347–363. 10.1093/humupd/dmq001. [DOI] [PubMed] [Google Scholar]
- Nassar N, Abeywardana P, Barker A, & Bower C (2010). Parental occupational exposure to potential endocrine disrupting chemicals and risk of hypospadias in infants. Occupational and Environmental Medicine, 67, 585 LP–589. [DOI] [PubMed] [Google Scholar]
- Neven ACH, Laven J, Teede HJ, & Boyle JA (2018). A summary on polycystic ovary syndrome: Diagnostic criteria, prevalence, clinical manifestations, and management according to the latest international guidelines. Seminars in Reproductive Medicine, 36(1), 5–12. 10.1055/s-0038-1668085. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, & Padilla-Banks E (2007). Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reproductive Toxicology, 24(2), 253–258. 10.1016/j.reprotox.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova MI, & Skinner MK (2012). Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS One, 7(5), e36129. 10.1371/journal.pone.0036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, & Kanzaki T (1977). Induction of urogenital anomalies and some tumors in the progeny of mice receiving diethylstilbestrol during pregnancy. Cancer Research, 37(4), 1099–1104. Retrieved from https://cancerres.aacrjournals.org/content/canres/37/4/1099.full.pdf. [PubMed] [Google Scholar]
- Nowak RA (2018). Estrous and menstrual cycles. In Skinner M (Ed.), Encyclopedia of reproduction (2nd ed., pp. 114–120). Elsevier. [Google Scholar]
- Ormond G, Nieuwenhuijsen MJ, Nelson P, Toledano MB, Iszatt N, Geneletti S, et al. (2009). Endocrine disruptors in the workplace, hair spray, folate supplementation, and risk of hypospadias: Case-control study. Environmental Health Perspectives, 117, 303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman ER, Al-Adly DM, Elgamal DA, Ghandour N, & El-Sharkawy S (2016). Bisphenol A concentrates preferentially in human uterine leiomyoma and induces proliferation in rat myometrium. Reproductive Sciences, 23(4), 508–514. 10.1177/1933719115608001. [DOI] [PubMed] [Google Scholar]
- Özel Ş, Tokmak A, Aykut O, Aktulay A, Hançerlioğulları N, & Engin Ustun Y (2019). Serum levels of phthalates and bisphenol-A in patients with primary ovarian insufficiency. Gynecological Endocrinology, 35(4), 364–367. 10.1080/09513590.2018.1534951. [DOI] [PubMed] [Google Scholar]
- Palmer JR, Herbst AL, Noller KL, Boggs DA, Troisi R, Titus-Ernstoff L, et al. (2009). Urogenital abnormalities in men exposed to diethylstilbestrol in utero: A cohort study. Environmental Health, 8, 37. 10.1186/1476-069X-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Ye X, Yin S, Ma X, Li C, Zhou J, et al. (2019). Selected persistent organic pollutants associated with the risk of primary ovarian insufficiency in women. Environment International, 129, 51–58. 10.1016/j.envint.2019.05.023. [DOI] [PubMed] [Google Scholar]
- Pascotto VM, Guerra MT, Franci JA, de Camargo JL, Kempinas WG, & Franchi CA (2015). Effects of a mixture of pesticides on the adult female reproductive system of Sprague-Dawley, Wistar, and Lewis rats. Journal of Toxicology and Environmental Health. Part A, 78(9), 602–616. 10.1080/15287394.2015.1010467. [DOI] [PubMed] [Google Scholar]
- Patiño-García D, Cruz-Fernandes L, Buñay J, Palomino J, & Moreno RD (2018). Reproductive alterations in chronically exposed female mice to environmentally relevant doses of a mixture of phthalates and alkylphenols. Endocrinology, 159(2), 1050–1061. 10.1210/en.2017-00614. [DOI] [PubMed] [Google Scholar]
- Paulose T, Tannenbaum LV, Borgeest C, & Flaws JA (2012). Methoxychlor-induced ovarian follicle toxicity in mice: Dose and exposure duration-dependent effects. Birth Defects Research. Part B, Developmental and Reproductive Toxicology, 95(3), 219–224. 10.1002/bdrb.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado FM, Lendínez I, Sotelo R, Iribarne-Durán LM, Fernández-Parra J, Vela-Soria F, et al. (2020). Association of urinary levels of bisphenols A, F, and S with endometriosis risk: Preliminary results of the EndEA study. International Journal of Environmental Research and Public Health, 17(4), 1194–1206. 10.3390/ijerph17041194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik FH, Klebanoff MA, Brock JW, & Longnecker MP (2007). Maternal pregnancy serum level of heptachlor epoxide, hexachlorobenzene, and beta-hexachlorocyclohexane and risk of cryptorchidism in offspring. Environmental Research, 105(3), 364–369. 10.1016/j.envres.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploteau S, Cano-Sancho G, Volteau C, Legrand A, Vénisseau A, Vacher V, et al. (2017). Associations between internal exposure levels of persistent organic pollutants in adipose tissue and deep infiltrating endometriosis with or without concurrent ovarian endometrioma. Environment International, 108, 195–203. 10.1016/j.envint.2017.08.019. [DOI] [PubMed] [Google Scholar]
- Pocar P, Fiandanese N, Secchi C, Berrini A, Fischer B, Schmidt JS, et al. (2012). Effects of polychlorinated biphenyls in CD-1 mice: Reproductive toxicity and inter-generational transmission. Toxicological Sciences, 126(1), 213–226. 10.1093/toxsci/kfr327. [DOI] [PubMed] [Google Scholar]
- Pollack AZ, Buck Louis GM, Chen Z, Sun L, Trabert B, Guo Y, et al. (2015). Bisphenol A, benzophenone-type ultraviolet filters, and phthalates in relation to uterine leiomyoma. Environmental Research, 137, 101–107. 10.1016/j.envres.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porpora MG, Ingelido AM, di Domenico A, Ferro A, Crobu M, Pallante D, et al. (2006). Increased levels of polychlorobiphenyls in Italian women with endometriosis. Chemosphere, 63(8), 1361–1367. 10.1016/j.chemosphere.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Porpora MG, Medda E, Abballe A, Bolli S, De Angelis I, di Domenico A, et al. (2009). Endometriosis and organochlorinated environmental pollutants: A case-control study on Italian women of reproductive age. Environmental Health Perspectives, 117(7), 1070–1075. 10.1289/ehp.0800273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, & Feng HL (2011). Extra- and intra-ovarian factors in polycystic ovary syndrome: Impact on oocyte maturation and embryo developmental competence. Human Reproduction Update, 17(1), 17–33. 10.1093/humupd/dmq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quansah R, Armah FA, Essumang DK, Luginaah I, Clarke E, Marfoh K, et al. (2015). Association of arsenic with adverse pregnancy outcomes/infant mortality: A systematic review and meta-analysis. Environmental Health Perspectives, 123(5), 412–421. 10.1289/ehp.1307894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke EG, Braun JM, Meeker JD, & Cooper GS (2018). Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environment International, 121(Pt. 1), 764–793. 10.1016/j.envint.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan P, Wielgomas B, Radwan M, Krasiński R, Klimowska A, Kaleta D, et al. (2020). Urinary bisphenol A concentrations and in vitro fertilization outcomes among women from a fertility clinic. Reproductive Toxicology, 96, 216–220. 10.1016/j.reprotox.2020.07.009. [DOI] [PubMed] [Google Scholar]
- Raghavan R, Romano ME, Karagas MR, & Penna FJ (2018). Pharmacologic and environmental endocrine disruptors in the pathogenesis of hypospadias: A review. Current Environmental Health Reports, 5, 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman SN, Fatima P, Chowdhury AQ, & Rahman MW (2013). Blood level of lead in women with unexplained infertility. Mymensingh Medical Journal, 22(3), 508–512. [PubMed] [Google Scholar]
- Rattan S, Beers HK, Kannan A, Ramakrishnan A, Brehm E, Bagchi I, et al. (2019). Prenatal and ancestral exposure to di(2-ethylhexyl) phthalate alters gene expression and DNA methylation in mouse ovaries. Toxicology and Applied Pharmacology, 379, 114629. 10.1016/j.taap.2019.114629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S, Brehm E, Gao L, & Flaws JA (2018). Di(2-ethylhexyl) phthalate exposure during prenatal development causes adverse transgenerational effects on female fertility in mice. Toxicological Sciences, 163(2), 420–429. 10.1093/toxsci/kfy042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S, Zhou C, Chiang C, Mahalingam S, Brehm E, & Flaws JA (2017). Exposure to endocrine disruptors during adulthood: Consequences for female fertility. The Journal of Endocrinology, 233(3), R109–R129. 10.1530/JOE-17-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BS, Rozati R, Reddy BV, & Raman NV (2006). Association of phthalate esters with endometriosis in Indian women. BJOG, 113(5), 515–520. 10.1111/j.1471-0528.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- Rier SE, Martin DC, Bowman RE, Dmowski WP, & Becker JL (1993). Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundamental and Applied Toxicology, 21(4), 433–441. 10.1006/faat.1993.1119. [DOI] [PubMed] [Google Scholar]
- Rocheleau CM, Romitti PA, & Dennis LK (2009). Pesticides and hypospadias: A meta-analysis. Journal of Pediatric Urology, 5, 17–24. Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouget F, Kadhel P, Monfort C, Viel JF, Thome JP, Cordier S, et al. (2020). Chlordecone exposure and risk of congenital anomalies: The timoun mother-child cohort study in Guadeloupe (French West Indies). Environmental Science and Pollution Research International, 27(33), 40992–40998. 10.1007/s11356-019-06031-y. [DOI] [PubMed] [Google Scholar]
- Rudnicka E, Kruszewska J, Klicka K, Kowalczyk J, Grymowicz M, Skorska J, et al. (2018). Premature ovarian insufficiency—Aetiopathology, epidemiology, and diagnostic evaluation. Przeglad Menopauzalny, 17(3), 105–108. 10.5114/pm.2018.78550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiyed H, Dewan A, Bhatnagar V, Shenoy U, Shenoy R, Rajmohan H, et al. (2003). Effect of endosulfan on male reproductive development. Environmental Health Perspectives, 111(16), 1958–1962. 10.1289/ehp.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena SP, Khare C, Farooq A, Murugesan K, Buckshee K, & Chandra J (1987). DDT and its metabolites in leiomyomatous and normal human uterine tissue. Archives of Toxicology, 59(6), 453–455. 10.1007/bf00316214. [DOI] [PubMed] [Google Scholar]
- Sheiner EK, Sheiner E, Hammel RD, Potashnik G, & Carel R (2003). Effect of occupational exposures on male fertility: Literature review. Industrial Health, 41(2), 55–62. 10.2486/indhealth.41.55. [DOI] [PubMed] [Google Scholar]
- Shen H, Liao K, Wu HF, Lu HC, Li Y, Li Z, et al. (2017). In utero exposure of high-dose di- n-butyl phthalate resulted in opposite effects on testicular cell apoptosis in late embryonic and pubertal male rat offspring. Human & Experimental Toxicology, 36(12), 1236–1247. 10.1177/0960327116685886. [DOI] [PubMed] [Google Scholar]
- Shi M, Whorton AE, Sekulovski N, MacLean JA, & Hayashi K (2019). Prenatal exposure to Bisphenol A, E, and S induces transgenerational effects on female reproductive functions in mice. Toxicological Sciences, 170(2), 320–329. 10.1093/toxsci/kfz124. [DOI] [PubMed] [Google Scholar]
- Shih EM, & Graham JM (2014). Review of genetic and environmental factors leading to hypospadias. European Journal of Medical Genetics, 57, 453–463. Elsevier Masson SAS. [DOI] [PubMed] [Google Scholar]
- Shono T, Shima Y, Kondo T, & Suita S (2005). In utero exposure to mono-n-butyl phthalate impairs insulin-like factor 3 gene expression and the transabdominal phase of testicular descent in fetal rats. Journal of Pediatric Surgery, 40(12), 1861–1864. 10.1016/j.jpedsurg.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Shono T, Suita S, Kai H, & Yamaguchi Y (2004a). The effect of a prenatal androgen disruptor, vinclozolin, on gubernacular migration and testicular descent in rats. Journal of Pediatric Surgery, 39(2), 213–216. 10.1016/j.jpedsurg.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Shono T, Suita S, Kai H, & Yamaguchi Y (2004b). Short-time exposure to vinclozolin in utero induces testicular maldescent associated with a spinal nucleus alteration of the genitofemoral nerve in rats. Journal of Pediatric Surgery, 39(2), 217–219. Discussion 217–219 10.1016/j.jpedsurg.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Sifakis S, Androutsopoulos VP, Tsatsakis AM, & Spandidos DA (2017). Human exposure to endocrine disrupting chemicals: Effects on the male and female reproductive systems. Environmental Toxicology and Pharmacology, 51, 56–70. 10.1016/j.etap.2017.02.024. [DOI] [PubMed] [Google Scholar]
- Simonelli A, Guadagni R, De Franciscis P, Colacurci N, Pieri M, Basilicata P, et al. (2017). Environmental and occupational exposure to bisphenol A and endometriosis: Urinary and peritoneal fluid concentration levels. International Archives of Occupational and Environmental Health, 90(1), 49–61. 10.1007/s00420-016-1171-1. [DOI] [PubMed] [Google Scholar]
- Sinclair AW, Cao M, Baskin L, & Cunha GR (2015). Diethylstilbestrol-induced mouse hypospadias: "Window of susceptibility". Differentiation, 91, 1–18. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, et al. (2016). Male reproductive disorders and fertility trends: Influences of environment and genetic susceptibility. Physiological Reviews, 96(1), 55–97. 10.1152/physrev.00017.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarr MM, Sundaram R, Honda M, Kannan K, & Louis GM (2017). Urinary concentrations of parabens and other antimicrobial chemicals and their association with couples’ fecundity. Environmental Health Perspectives, 125(4), 730–736. 10.1289/ehp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer A, van den Heijkant M, & Baumann S (2016). Worldwide prevalence of hypospadias. Journal of Pediatric Urology, 12, 152.e151–152.e157. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- Srivastava S, & Dhagga N (2019). Dose exposure of Bisphenol-A on female Wistar rats fertility. Hormone Molecular Biology and Clinical Investigation, 38(2). 10.1515/hmbci-2018-0061, 20180061. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhang MR, Zhang LQ, Zhao D, Li SG, & Chen B (2016). Phthalate monoesters in association with uterine leiomyomata in Shanghai. International Journal of Environmental Health Research, 26(3), 306–316. 10.1080/09603123.2015.1111310. [DOI] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, & Fenster L (2000). The question of declining sperm density revisited: An analysis of 101 studies published 1934–1996. Environmental Health Perspectives, 108(10), 961–966. 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Sieber SM, Dalgard DW, Thorgeirsson UP, & Adamson RH (1999). Effects of long-term oral administration of DDT on nonhuman primates. Journal of Cancer Research and Clinical Oncology, 125(3–4), 219–225. 10.1007/s004320050266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantino G, Valentino R, Di Somma C, D’Esposito V, Passaretti F, Pizza G, et al. (2013). Bisphenol A in polycystic ovary syndrome and its association with liver-spleen axis. Clinical Endocrinology, 78(3), 447–453. 10.1111/j.1365-2265.2012.04500.x. [DOI] [PubMed] [Google Scholar]
- Thomsen AM, Riis AH, Olsen J, Jönsson BA, Lindh CH, Hjollund NH, et al. (2017). Female exposure to phthalates and time to pregnancy: A first pregnancy planner study. Human Reproduction, 32(1), 232–238. 10.1093/humrep/dew291. [DOI] [PubMed] [Google Scholar]
- Tiemann U (2008). In vivo and in vitro effects of the organochlorine pesticides DDT, TCPM, methoxychlor, and lindane on the female reproductive tract of mammals: A review. Reproductive Toxicology, 25(3), 316–326. 10.1016/j.reprotox.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Toffoletto S, Lanzenberger R, Gingnell M, Sundstrom-Poromaa I, & Comasco E (2014). Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain: A systematic review. Psychoneuroendocrinology, 50, 28–52. 10.1016/j.psyneuen.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Tournaire M, Devouche E, Epelboin S, Cabau A, Dunbavand A, & Levadou A (2018). Birth defects in children of men exposed in utero to diethylstilbestrol (DES). Thérapie, 73(5), 399–407. 10.1016/j.therap.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Trabert B, Chen Z, Kannan K, Peterson CM, Pollack AZ, Sun L, et al. (2015). Persistent organic pollutants (POPs) and fibroids: Results from the ENDO study. Journal of Exposure Science & Environmental Epidemiology, 25(3), 278–285. 10.1038/jes.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabert B, Longnecker MP, Brock JW, Klebanoff MA, & McGlynn KA (2012). Maternal pregnancy levels of trans-nonachlor and oxychlordane and prevalence of cryptorchidism and hypospadias in boys. Environmental Health Perspectives, 120(3), 478–482. 10.1289/ehp.1103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyl RW, Myers CB, Marr MC, Sloan CS, Castillo NP, Veselica MM, et al. (2008). Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicological Sciences, 104(2), 362–384. 10.1093/toxsci/kfn084. [DOI] [PubMed] [Google Scholar]
- Ulbrich B, & Stahlmann R (2004). Developmental toxicity of polychlorinated biphenyls (PCBs): A systematic review of experimental data. Archives of Toxicology, 78(5), 252–268. 10.1007/s00204-003-0519-y. [DOI] [PubMed] [Google Scholar]
- Upson K, De Roos AJ, Thompson ML, Sathyanarayana S, Scholes D, Barr DB, et al. (2013). Organochlorine pesticides and risk of endometriosis: Findings from a population-based case-control study. Environmental Health Perspectives, 121(11–12), 1319–1324. 10.1289/ehp.1306648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upson K, Sathyanarayana S, De Roos AJ, Koch HM, Scholes D, & Holt VL (2014). A population-based case-control study of urinary bisphenol A concentrations and risk of endometriosis. Human Reproduction, 29(11), 2457–2464. 10.1093/humrep/deu227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upson K, Sathyanarayana S, De Roos AJ, Thompson ML, Scholes D, Dills R, et al. (2013). Phthalates and risk of endometriosis. Environmental Research, 126, 91–97. 10.1016/j.envres.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabre P, Gatimel N, Moreau J, Gayrard V, Picard-Hagen N, Parinaud J, et al. (2017). Environmental pollutants, a possible etiology for premature ovarian insufficiency: A narrative review of animal and human data. Environmental Health, 16(1), 37. 10.1186/s12940-017-0242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagi SJ, Azziz-Baumgartner E, Sjödin A, Calafat AM, Dumesic D, Gonzalez L, et al. (2014). Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol A in polycystic ovary syndrome: A case-control study. BMC Endocrine Disorders, 14, 86. 10.1186/1472-6823-14-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnell RR, Arnold TJ, Quandt SA, Talton JW, Chen H, Miles CM, et al. (2020). Menstrual cycle patterns and irregularities in hired latinx child farmworkers. Journal of Occupational and Environmental Medicine, 63(1), 38–43. 10.1097/JOM.0000000000002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vélez MP, Arbuckle TE, & Fraser WD (2015a). Female exposure to phenols and phthalates and time to pregnancy: The maternal-infant research on environmental chemicals (MIREC) study. Fertility and Sterility, 103(4), 1011–1020.e1012. 10.1016/j.fertnstert.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Vélez MP, Arbuckle TE, & Fraser WD (2015b). Maternal exposure to perfluorinated chemicals and reduced fecundity: The MIREC study. Human Reproduction, 30(3), 701–709. 10.1093/humrep/deu350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vested A, Giwercman A, Bonde JP, & Toft G (2014). Persistent organic pollutants and male reproductive health. Asian Journal of Andrology, 16(1), 71–80. 10.4103/1008-682X.122345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneswaran HT, & Abern M (2018). Testicular cancer. In Skinner M (Ed.), Encyclopedia of reproduction (2nd ed., pp. 479–483). Elsevier. [Google Scholar]
- Viguie C, Mhaouty-Kodja S, Habert R, Chevrier C, Michel C, & Pasquier E (2018). Evidence-based adverse outcome pathway approach for the identification of BPA as en endocrine disruptor in relation to its effect on the estrous cycle. Molecular and Cellular Endocrinology, 475, 10–28. 10.1016/j.mce.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Virtanen HE, & Adamsson A (2012). Cryptorchidism and endocrine disrupting chemicals. Molecular and Cellular Endocrinology, 355(2), 208–220. 10.1016/j.mce.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Virtanen HE, Koskenniemi JJ, Sundqvist E, Main KM, Kiviranta H, Tuomisto JT, et al. (2012). Associations between congenital cryptorchidism in newborn boys and levels of dioxins and PCBs in placenta. International Journal of Andrology, 35(3), 283–293. 10.1111/j.1365-2605.2011.01233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner-Mahler K, Kurzenne JY, Delattre I, Berard E, Mas JC, Bornebush L, et al. (2011). Prospective study on the prevalence and associated risk factors of cryptorchidism in 6246 newborn boys from Nice area, France. International Journal of Andrology, 34 (5 Pt. 2), e499–e510. 10.1111/j.1365-2605.2011.01211.x. [DOI] [PubMed] [Google Scholar]
- Waliszewski SM, Infanzon RM, Arroyo SG, Pietrini RV, Carvajal O, Trujillo P, et al. (2005). Persistent organochlorine pesticides levels in blood serum lipids in women bearing babies with undescended testis. Bulletin of Environmental Contamination and Toxicology, 75(5), 952–959. 10.1007/s00128-005-0842-5. [DOI] [PubMed] [Google Scholar]
- Wang KH, Kao AP, Chang CC, Lin TC, & Kuo TC (2013). Bisphenol A at environmentally relevant doses induces cyclooxygenase-2 expression and promotes invasion of human mesenchymal stem cells derived from uterine myoma tissue. Taiwanese Journal of Obstetrics & Gynecology, 52(2), 246–252. 10.1016/j.tjog.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhou W, Zhu W, Chen L, Wang W, Tian Y, et al. (2018). Associations of female exposure to bisphenol A with fecundability: Evidence from a preconception cohort study. Environment International, 117, 139–145. 10.1016/j.envint.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhu Q, Dang X, He Y, Li X, & Sun Y (2017). Local effect of bisphenol A on the estradiol synthesis of ovarian granulosa cells from PCOS. Gynecological Endocrinology, 33(1), 21–25. 10.1080/09513590.2016.1184641. [DOI] [PubMed] [Google Scholar]
- Weidner IS, Møller H, Jensen TK, & Skakkebaek NE (1998). Cryptorchidism and hypospadias in sons of gardeners and farmers. Environmental Health Perspectives, 106(12), 793–796. 10.1289/ehp.98106793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X, Xiong Y, Jin L, Zhang M, Huang L, Mao Y, et al. (2020). Bisphenol A exposure enhances endometrial stromal cell invasion and has a positive association with peritoneal endometriosis. Reproductive Sciences, 27(2), 704–712. 10.1007/s43032-019-00076-7. [DOI] [PubMed] [Google Scholar]
- Wesevich V, Kellen AN, & Pal L (2020). Recent advances in understanding primary ovarian insufficiency. F1000Res, 9, 1–13. 10.12688/f1000research.26423.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Hauser R, Calafat AM, Missmer SA, & Wise LA (2010). Association of exposure to phthalates with endometriosis and uterine leiomyomata: Findings from NHANES, 1999-2004. Environmental Health Perspectives, 118(6), 825–832. 10.1289/ehp.0901543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorton D, Krauss RM, Marshall S, & Milby TH (1977). Infertility in male pesticide workers. Lancet, 2(8051), 1259–1261. 10.1016/s0140-6736(77)92665-4. [DOI] [PubMed] [Google Scholar]
- Willingham E, Agras K, Vilela M, & Baskin LS (2006). Loratadine exerts estrogen-like effects and disrupts penile development in the mouse. Journal of Urology, 175, 723–726. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, LeBlanc GA, Ostby JS, & Gray LE Jr. (2000). Characterization of the period of sensitivity of fetal male sexual development to vinclozolin. Toxicological Sciences, 55(1), 152–161. 10.1093/toxsci/55.1.152. [DOI] [PubMed] [Google Scholar]
- Wright DL, Afeiche MC, Ehrlich S, Smith K, Williams PL, Chavarro JE, et al. (2015). Hair mercury concentrations and in vitro fertilization (IVF) outcomes among women from a fertility clinic. Reproductive Toxicology, 51, 125–132. 10.1016/j.reprotox.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Yao X, Ting G, Ling J, Huimin L, Yuan Q, et al. (2020). BPA modulates the WDR5/TET2 complex to regulate ERβ expression in eutopic endometrium and drives the development of endometriosis. Environmental Pollution (Barking, Essex: 1987), 268(Pt. B), 115748. 10.1016/j.envpol.2020.115748. [DOI] [PubMed] [Google Scholar]
- Yang S, Arcanjo RB, & Nowak RA (2020). The effects of the phthalate DiNP on reproduction. Biology of Reproduction, 104(2), 305–316. 10.1093/biolre/ioaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Shi J, Guo Z, Chen M, Wang C, He C, et al. (2019). A pilot study on polycystic ovarian syndrome caused by neonatal exposure to tributyltin and bisphenol A in rats. Chemosphere, 231, 151–160. 10.1016/j.chemosphere.2019.05.129. [DOI] [PubMed] [Google Scholar]
- Yang CY, Yu ML, Guo HR, Lai TJ, Hsu CC, Lambert G, et al. (2005). The endocrine and reproductive function of the female Yucheng adolescents prenatally exposed to PCBs/PCDFs. Chemosphere, 61(3), 355–360. 10.1016/j.chemosphere.2005.02.089. [DOI] [PubMed] [Google Scholar]
- Yang Q, Zhao Y, Qiu X, Zhang C, Li R, & Qiao J (2015). Association of serum levels of typical organic pollutants with polycystic ovary syndrome (PCOS): A case-control study. Human Reproduction, 30(8), 1964–1973. 10.1093/humrep/dev123. [DOI] [PubMed] [Google Scholar]
- Yao S, Lopez-Tello J, & Sferruzzi-Perri AN (2020). Developmental programming of the female reproductive system-a review. Biology of Reproduction, 104(4), 745–770. 10.1093/biolre/ioaa232. [DOI] [PubMed] [Google Scholar]
- Ye S, Chung HW, Jeong K, Sung YA, Lee H, Park SY, et al. (2017). Blood cadmium and volume of uterine fibroids in premenopausal women. Annals of Occupational and Environmental Medicine, 29, 22. 10.1186/s40557-017-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeum D, Ju S, Cox KJ, Zhang Y, Stanford JB, & Porucznik CA (2019). Association between peri-conceptional bisphenol A exposure in women and men and time to pregnancy—The HOPE study. Paediatric and Perinatal Epidemiology, 33(6), 397–404. 10.1111/ppe.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Das P, Vall AJ, Yan Y, Gao X, Sifre MI, et al. (2019). Bisphenol A induces human uterine leiomyoma cell proliferation through membrane-associated ERα36 via nongenomic signaling pathways. Molecular and Cellular Endocrinology, 484, 59–68. 10.1016/j.mce.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ji M, Tan X, Yu K, Liu X, Li N, et al. (2018). Impairment of ovaries by 2,3,7,8-tetrachlorobenzo-p-dioxin (TCDD) exposure in utero associated with BMP15 and GDF9 in the female offspring rat. Toxicology, 410, 16–25. 10.1016/j.tox.2018.08.015. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li JH, Ma L, Huang TH, & Jiang XW (2012). Diethylstilbestrol impairs the morphology and function of mouse gubernaculum testis in culture. Cell Biology and Toxicology, 28(6), 397–407. 10.1007/s10565-012-9231-0. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mu X, Gao R, Geng Y, Liu X, Chen X, et al. (2018). Foetal-neonatal exposure of di (2-ethylhexyl) phthalate disrupts ovarian development in mice by inducing autophagy. Journal of Hazardous Materials, 358, 101–112. 10.1016/j.jhazmat.2018.06.042. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zheng XM, Hubert J, Zheng H, Yang ZW, & Li SW (2009). Prenatal exposure to diaethylstilbestrol in the rat inhibits transabdominal testicular descent with involvement of the INSL3/LGR8 system and HOXA10. Chinese Medical Journal, 122(8), 967–971. [PubMed] [Google Scholar]
- Zhou C, Chiang C, Brehm E, & Flaws JA (2017). Personal care products and cosmetics. In Reproductive and developmental toxicology (2nd ed., pp. 857–899). Academic Press. [Google Scholar]
- Zhou C, Gao L, & Flaws JA (2017a). Exposure to an environmentally relevant phthalate mixture causes transgenerational effects on female reproduction in mice. Endocrinology, 158(6), 1739–1754. 10.1210/en.2017-00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Gao L, & Flaws JA (2017b). Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicology and Applied Pharmacology, 318, 49–57. 10.1016/j.taap.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Zhou W, Huo X, Zhao S, Gan Y, Wang B, et al. (2019). Triclosan and female reproductive health: A preconceptional cohort study. Epidemiology, 30(Suppl. 1), S24–s31. 10.1097/ede.0000000000001011. [DOI] [PubMed] [Google Scholar]
- Zimmermann A, Bernuit D, Gerlinger C, Schaefers M, & Geppert K (2012). Prevalence, symptoms and management of uterine fibroids: An international internet-based survey of 21,746 women. BMC Womens Health, 12, 6. 10.1186/1472-6874-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv-Gal A, & Flaws JA (2016). Evidence for bisphenol A-induced female infertility: A review (2007–2016). Fertility and Sterility, 106(4), 827–856. 10.1016/j.fertnstert.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv-Gal A, Gallicchio L, Chiang C, Ther SN, Miller SR, Zacur HA, et al. (2016). Phthalate metabolite levels and menopausal hot flashes in midlife women. Reproductive Toxicology, 60, 76–81. 10.1016/j.reprotox.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]