Abstract
The functional roles of the precuneus are unclear. Focal precuneus lesions are rare, making it difficult to identify robust brain–behavior relationships. Distinct functional subdivisions of the precuneus have been proposed based on unique connectivity profiles. This includes an association of the anterior division with bodily awareness, the central region with complex cognition, and the posterior division with visual processing. Our goal was to test the hypothesis that the central precuneus is preferentially involved (compared to the other sectors of the precuneus) in executive function, as estimated from performance on the trail-making test (TMT). 35 patients with focal brain lesions involving the precuneus were included from the University of Iowa and Montpellier University. Multivariate lesion symptom mapping of TMT performance was performed to evaluate whether lesion location was associated with impaired task performance. Lesion symptom mapping revealed a statistically significant association of central precuneus lesions with impaired TMT performance (r = 0.43, p < 0.01). Further, a functional network derived from this precuneus region showed connectivity to other cortical areas implicated in executive function, including the dorsolateral prefrontal cortex and inferior parietal lobe. This analysis provides support for the role of the central precuneus in executive function, consistent with the unique connectivity pattern of the central precuneus with a broader network implicated in cognitive control and executive function.
Keywords: Executive dysfunction, Trail-making test, Lesion symptom mapping, Brain lesions, Lesion network mapping
Introduction
The functional contribution of the precuneus to human behavior and consciousness has attracted considerable interest and speculation yet remains largely enigmatic. It is situated on the posteromedial parietal lobe, a deep cortical site that has made accurate brain mapping difficult (Cavanna and Trimble 2006). The precuneus is a highly connected hub region (Van den Heuvel and Sporns 2013) and a central node of the default mode network (Raichle et al. 2001). Robust brain–behavior relationships have been difficult to elucidate, due in large part to the rarity of focal precuneus lesions. The precuneus has redundant blood supply, which makes ischemic infarcts uncommon (Mavridis et al. 2016; Kalamatianos et al. 2019; Parvizi et al. 2021).
Despite these challenges, new insights have emerged over the last decade from several resting-state functional connectivity MRI (rs-fcMRI) studies revealing distinct functional subdivisions of the precuneus (Margulies et al. 2009; Zhang and Chiang-shan. 2012). The pattern of unique connectivity of each subdivision has informed the hypothesized functional correlates: an anterior division that represents the body and bodily awareness, a central division linked to complex cognition, and a posterior division involved in higher-order visual processing (Margulies et al. 2009). Support for this tripartite functional parcellation of the precuneus can be seen in a previous study in which patients underwent surgical resection of gliomas involving the precuneus who had subsequent bodily awareness disorders; the antero-dorsal precuneus, but not the postero-dorsal precuneus, was linked to bodily awareness disorders (Herbet et al. 2019). The link between the anterior precuneus and body awareness was further supported with electroencephalography in patients with seizures originating from the precuneus who reported disturbances in body awareness (Harroud et al. 2017; Yang et al. 2018; Parvizi et al. 2021). Additionally, the proposed role for the central precuneus in cognition was supported by task-based fMRI, in which significant activation of the precuneus was shown during N-back and relational processing tasks (Lyu et al. 2021), both of which require top-down cognitive control. Finally, a subset of 12 individuals with ischemic stroke of the precuneus had chronic executive dysfunction, measured as impairments in mental flexibility and response inhibition (Kumral et al. 2021), though the subregion within the precuneus was not determined.
With this previous evidence supporting the involvement of the central precuneus in higher-order complex cognition, we set out to evaluate the specific hypothesis that lesions of the central precuneus would be associated with impaired executive function. Executive function refers to a collection of core processes needed for effortful top-down control of cognition and for the support of goal-directed, adaptive behavior (Badre 2008; Diamond 2012). Examples include inhibitory control and task switching/cognitive flexibility (Miyake et al. 2000; Lehto et al. 2003). Together these processes contribute to several higher-order functions, such as decision making (Collins and Koechlin 2012), conflict monitoring and resolution (Fan et al. 2003; Wu et al. 2020), planning (García-Madruga et al. 2016), and reasoning (Lunt et al. 2012).
The prefrontal cortex (PFC) and parietal lobe provide core neural substrates that support many aspects of executive function (Jung and Haier 2007; Badre 2008; Baddeley 1992, 2003). Together they include major nodes of large-scale functional networks, including the frontoparietal control (FPN) and default mode networks (DMN) (Menon and D’Esposito 2022). The role of the precuneus in executive function is less well established, but it may interact with these other networks. The precuneus is a central node of the DMN (Raichle et al. 2001), and the central precuneus region, specifically, shows unique connectivity with frontoparietal sites commonly invoked in executive function. This includes the dorsolateral prefrontal, dorsomedial prefrontal, and lateral inferior parietal cortex, both at rest (Margulies et al. 2009) and during tasks requiring executive control (Utevsky et al. 2014). As such, it is possible the central precuneus may interface with the functionally interconnected FPN and DMN to facilitate goal-directed cognition (Spreng et al. 2010).
In the current study, we set out to evaluate whether lesions of the central precuneus are associated with impairments in executive function, as measured by the trail-making test (TMT), a widely used test of executive function that requires cognitive flexibility, divided attention, response monitoring, and task switching. We performed multivariate lesion symptom mapping of TMT performance in 35 participants with focal brain lesions involving the precuneus. We hypothesized that lesions of the central precuneus would be associated with impaired TMT performance relative to lesions involving other precuneus regions. In addition, we used normative rs-fcMRI to evaluate the functional networks associated with precuneus regions implicated in impaired executive function.
Methods
Participants
Participants were identified retrospectively from two institutions. All participants provided informed consent and the study was approved by the ethical boards of the participating institutions. Participants included 16 individuals from the University of Iowa Neurological Patient Registry of the Division of Neuropsychology and Cognitive Neuroscience, Department of Neurology, and 19 individuals from the Montpellier University Medical Center’s Department of Neurosurgery.
Inclusion criteria for Iowa subjects included: (a) a single stable focal brain lesion involving the precuneus evident from structural neuroimaging data acquired in the chronic epoch, > 3 months from lesion onset, (b) the lesion was acquired at age 18 or older, and (c) availability of task performance on the TMT. Exclusion criteria included a history of significant alcohol or substance abuse, psychiatric disorder, or other neurologic disorder unrelated to the lesion. All participants had parenchymal damage of the precuneus with the etiology of lesion including ischemic stroke (n = 10), benign tumor and vascular malformation resections (n = 3), focal contusion from trauma (n = 2), and hemorrhagic stroke (n = 1). Fourteen of the nineteen Montpellier participants were studied previously with regard to bodily awareness, and the same inclusion and exclusion criteria were used for the five new participants (Herbet et al. 2019). All participants had a medial parietal resection for a low-grade glioma involving the precuneus. A summary of the participants’ demographics and clinical data from both sites is provided in Table 1. Lesion maps for each participant are shown in Supplementary Fig. 1.
Table 1.
Demographic and clinical data of study populations
| Iowa (N = 16) | Montpellier (N = 19) | All subjects (N = 35) | |
|---|---|---|---|
| Age (years) | |||
| Mean (± SD) | 55.44 (± 11.18) | 40.16 (± 10.24) | 47.14 (± 13.11) |
| Range | 29–73 | 22–56 | 22–73 |
| Education (years) | |||
| < 12 | 2 | 2 | 4 |
| 12–16 | 11 | 8 | 19 |
| 16 + | 3 | 9 | 12 |
| Gender (women/men) | 5/11 | 8/11 | 13/22 |
| Handedness (R/L/A)a | 12/3/1 | 16/2/1 | 28/5/2 |
| Lesion laterality (R/L/B)b | 5/6/5 | 12/7/0 | 17/13/5 |
| Etiology | |||
| Stroke | 11 | 0 | 11 |
| Resections | 3 | 19 | 22 |
| Contusion (trauma) | 2 | 0 | 2 |
R right-handed, L left-handed, A ambidextrous
R right hemisphere lesion, L left hemisphere lesion, B bilateral lesion
Behavioral assessment
All 35 participants completed TMT assessment in the chronic epoch, > 3 months after lesion onset at Iowa and at 3-months post operation at Montpellier. The TMT is comprised of parts A and B. Part A requires the patient to draw a line connecting 25 circled numbers in numeric order as fast as possible. The numbers are randomly scattered on a page. Part A is thought to test psychomotor functions and processing speed. Part B consists of both numbers and letters, and patients are required to connect the circles in an alternating sequence (i.e., 1-A-2-B-3-C, etc.), as fast as possible. Part B is more difficult and is considered to be a test of executive functions that include cognitive flexibility, divided attention, response monitoring, and task switching. Performance on this task is measured as time in seconds to complete part A and part B. Part A accounts for many task requirements other than executive function, including visual properties of the stimuli, the number of stimuli, and the motor responses required (i.e., connecting circles), which are similar for TMT part A and part B. Thus, subtraction of performance time for TMT part A from B provides a more specific assessment of executive function, and this score (part B time minus part A time) was used here for the main dependent variable of interest, as in previous work (Hwang et al. 2020). All patients’ data were normalized to account for age and years of education from population-derived data (Tombaugh 2004), and converted to Z-scores by subtracting the normative mean and dividing by the normative standard deviation. Impairment was defined as a Z-score ≤ −1.65, which corresponds with left-tailed alpha of 0.05, or 95% confidence the score is impaired.
Lesion mapping
Each participant included in the analysis had a focal brain lesion with visible boundaries evident from structural imaging from T1 to T2 sequences on MRI. All Iowa lesions were manually segmented and transformed to a common template brain (MNI152) using ANTs (Avants et al. 2009). Lesion segmentation methods for the Montpellier participants were similar and reported previously (Herbet et al. 2019). Overlap of the lesion mask with the Harvard–Oxford precuneus region was present in all 35 participants. To evaluate whether lesion location was associated with impaired task performance, we performed multivariate lesion symptom mapping using sparse canonical correlation analysis (SCCAN). This analysis was conducted with the LESYMAP package in R (Pustina et al. 2018). The SCCAN method involves an optimization procedure that finds voxel weights that maximize the multivariate correlation between voxel values and behavioral scores, which were coded as impaired or unimpaired based on Z-scores. The statistical significance and sparseness of the model is derived empirically using a fourfold, within-sample correlation between model-predicted and actual behavioral scores. The significance of the brain–behavior association is assessed on the entire map at once and thus avoids concerns related to multiple comparisons performed on a voxel-wise basis.
Lesion network mapping
Next, we evaluated the functional connectivity network associated with the precuneus region identified in the lesion symptom map. The motivation for this step was to evaluate the broader network of brain areas functionally connected to the precuneus region maximally associated with impaired TMT performance. To perform this analysis, the peak regional lesion symptom mapping result was used to ‘seed’ a functional connectivity analysis to evaluate the connectivity pattern of this region using rs-fcMRI data from 98 healthy adults (48 male, ages 22 ± 3.2 years), similar to prior work (Bowren et al. 2022). Participants completed two 6.2 min rs-fcMRI scans during which they were asked to rest in the scanner (3 T, Siemens) with their eyes open (TR = 3000 ms, TE = 30 ms, FA = 85°, 3 mm voxel size, FOV = 216, 47 axial slices with interleaved acquisition and no gap). The rs-fcMRI data were preprocessed in accordance with previously described methods (Van Dijk et al. 2010; Boes et al. 2018). Briefly, functional data were realigned using AFNI’s “3dvolreg” and this was followed by spatial smoothing using a Gaussian kernel of 6 mm full-width at half-maximum, temporal filtering (0.009 Hz < f < 0.08 Hz), and nuisance signal regression. Nuisance variables included the following: (a) six movement parameters computed by rigid body translation and rotation during preprocessing, (b) mean whole brain signal, (c) mean brain signal within the lateral ventricles, (d) mean signal within a deep white matter region of interest (ROI), and (e) mean signal from the posteromedial cortical territory defined using the Harvard–Oxford atlas’ precuneus and posterior cingulate ROIs. For this latter variable, regressing the signal from the surrounding region has the effect of amplifying the local connectivity patterns that are unique to the seed region relative to the surrounding cortex, as performed previously (Boes et al. 2018; Di Martino et al. 2008). The time course of the average BOLD signal within the ‘seed’ ROI derived from the lesion-symptom map was compared with the BOLD signal time course of other brain voxels to identify regions with positive and negative correlations. Pearson correlation coefficients were converted to normally distributed Z-scores using the Fisher transformation. For reference, a network map was also generated from each of the ROIs representing the main functional divisions of the precuneus derived from Margulies et al. (2009) (displayed in Fig. 1C). As in the prior analysis, we regressed the signal from the local surrounding cortical territory to emphasize the unique connectivity profile of each individual subdivision. In this analysis a 4 mm spherical ROI was placed at the coordinates of each subdivision from the Margulies paper to ‘seed’ a rs-fcMRI network analysis while regressing the signal from the other subdivisions. Again, this has the effect of amplifying differences in connectivity patterns between adjacent regions and producing four relatively distinct networks. The resulting network maps were compared to each other using spatial correlation to evaluate how similar the network derived from our LESYMAP seed was to the networks derived from the Margulies subdivisions of the precuneus.
Fig. 1.
Precuneus lesion mapping. A The lesion overlap of subjects with impaired executive function and B the lesion overlap of subjects with intact executive function. The color scale is shown as a proportion of the peak overlap, which was 7 of 13 subjects in A and 9 of 22 subjects in B. C The significant LESYMAP results highlighting a region of the central precuneus associated with impaired TMT B minus A performance. The strength of association is represented on a unitless scale of 0–1, with 1 representing the strongest brain-behavior association. The LESYMAP findings are displayed on an underlay depicting the three functional subdivisions of the precuneus, as well as a limbic network, derived from coordinates in Margulies et al. (2009). The blue ROI corresponds to the sensorimotor division, green to the cognitive division, yellow to the visual division, and the red denotes a limbic posterior cingulate region
Results
Demographics and behavioral results
Demographic data from the Iowa and Montpellier cohort are shown in Table 1. We observed some differences between cohorts, with the Montpellier cohort being younger than the Iowa cohort (40.16 ± 10.51 vs. 55.31 ± 11.70 years of age, respectively), t (33) = −3.98, p < 0.0001). On average, the Montpellier cohort was also more educated than the Iowa cohort (15.26 ± 2.51 vs. 13.31 ± 2.96 years of education, respectively), t (33) = 2.08, p < 0.05. For TMT performance our results showed 13 of 35 patients with TMT B minus A impairment (according to the cutoff of −1.65). Of the 13 impaired individuals, five had right hemisphere lesions, six had left hemisphere lesions, and two had bilateral lesions, with 7 of the 13 from Iowa (stroke in 3, resections in 3, focal contusion in 1). The proportion of patients who had left, right, and bilateral lesions did not differ between locations, X2 (2, N = 35) = 5.25, p = 0.07).
Lesion results
The lesions were distributed throughout the precuneus, and the lesion overlap map is shown for descriptive purposes for individuals with and without TMT B minus A impairment (Fig. 1A, B, respectively). The multivariate lesion symptom mapping analysis showed a statistically significant association of central precuneus lesions with impaired TMT B minus A performance (Fig. 1C; r = 0.43, p < 0.01, optimal sparseness = 0.01, peak MNI (mm) coordinates left: −9, −60, 38; right: 6, −64, 35). To evaluate this result relative to the central precuneus defined by Margulies et al. (2009), we used the MNI coordinates reported by those authors to create spherical ROIs for each precuneus subdivision to underlay with our LESYMAP. Notably, the significant results localized most closely with the region previously identified as the central ‘cognitive’ precuneus plotted in green in Fig. 1C. This result appeared most focal in the left hemisphere, while the right hemisphere findings extended into the visual subdivision of the precuneus.
To address the overlap into the visual subdivision of the precuneus, we calculated Euclidean distance between our LESYMAP result and coordinates provided from each of the Margulies precuneus subdivisions. Despite the overlap of the LESYMAP result with the visual subdivision, the peak voxels of the LESYMAP result are closest in distance to the cognitive subdivision (see Fig. 2 and Supplemental Fig. 2).
Fig. 2.
Our LESYMAP results projected onto the MNI152 brain. Peak voxels of the right (z = 38) and left (z = 35) are shown relative to the cognitive (green) and visual (yellow) coordinates from Margulies et al. (2009). The color scale of the LESYMAP finding is on a unitless scale from 0 to 1 reflecting the regional strength of association with impaired TMT performance
Lesion network results
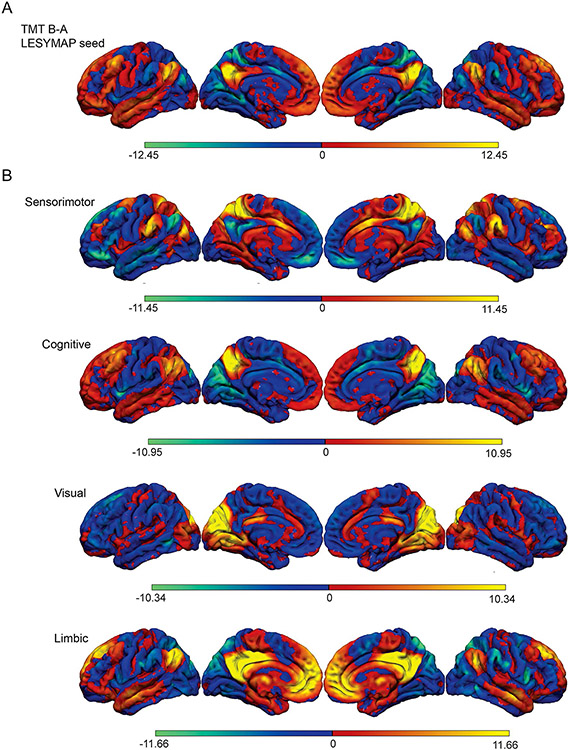
The fcMRI network derived from this lesion symptom map showed robust connectivity to the angular gyrus of the inferior parietal lobe and the caudal middle frontal gyrus of the dorsolateral prefrontal cortex (Fig. 3A). Next, we compared this LESYMAP-derived network map (Fig. 3A) to those derived from the Margulies’ subdivisions of the precuneus (Fig. 3B). The spatial correlation of the LESYMAP-derived map was highest with that of the central ‘cognitive’ precuneus network (r = 0.66, p < 0.001; the second highest r value was the limbic network at 0.56).
Fig. 3.
Functional connectivity associated with the precuneus. A Our TMT B minus A LESYMAP result used as a seed for functional connectivity analysis, showing robust connectivity to the angular gyrus of the inferior parietal lobe and the caudal middle frontal gyrus of the dorsolateral prefrontal cortex. B Regions of interest within each functional subdivision defined by Margulies et al. (2009) were used to seed functional connectivity analyses. The spatial correlation of the map in 3a was highest with the central “cognitive” precuneus network (r = 0.66, p < 0.001)
A secondary, post hoc analysis was performed to compare the LESYMAP-derived network to superior parietal cortex regions parcellated using histology (Scheperjans et al. 2008a, b). The eight parcels of the superior parietal cortex were used as seeds to create eight separate network maps. The spatial correlation of the LESYMAP-derived network most closely matched that of region 7 M (r = 0.16, p < 0.0001); 7 M from the Scheperjans parcellation aligns most with the Margulies ‘cognitive’ subdivision. Additional details of this analysis are included in Supplemental Figs. 3 and 4.
Discussion
In this study, we evaluate executive function measured using the TMT in relation to lesion location in 35 individuals with precuneus lesions. Our results identify a region of the central precuneus where lesions are significantly associated with impaired TMT performance. Moreover, we show that this region is functionally connected to the inferior parietal lobe and dorsolateral prefrontal cortex regions also implicated in executive function. These findings provide additional support implicating the central precuneus in having a critical role in executive function, possibly through interactions with frontoparietal regions more commonly implicated in executive functioning. This adds to a small but growing literature showing cognitive deficits in association with precuneus lesions (Herbet et al. 2019; Kumral et al. 2021).
There is a rich literature implicating frontoparietal regions in executive function, contributing to core functions like attention, working memory (Baddeley 1992, 2003), and general intelligence (Jung and Haier 2007; Bowren et al. 2020). Our results build upon these models of fronto-parietal integration in executive function by supporting the central precuneus as another node in the distributed networks that support executive functions. The exact mechanistic contribution of the central precuneus to TMT performance and executive function more broadly is still unclear, but several lines of research provide emerging insights.
The precuneus is commonly thought of as a core node of the DMN, a network that is active in rest states and has decreased activity during tasks that require goal-directed attention, such as the TMT. However, this may be an over-simplified view of the precuneus. Relative to other nodes of the DMN, the precuneus is unique in two respects: (1) its activity has been found to increase in cognitively demanding tasks (Cavanna and Trimble 2006; Fletcher et al. 1995; Lundstrom et al. 2005; Maddock et al. 2001; Hayden et al. 2008), and (2) its connectivity pattern is state-dependent, showing increased connectivity with the DMN in a resting state and increased connectivity with the FPN during attention-demanding tasks (Utevsky et al. 2014; Li et al. 2019). The FPN spans prefrontal and parietal nodes and is thought to play a role in TMT performance and other executive functions (Seeley et al. 2007; Harding et al. 2015; Sadaghiani et al. 2019). Coordinated DMN and FPN state-dependent dynamic functional connectivity correlates with performance on tasks requiring cognitive flexibility (Douw et al. 2016). Based on the central precuneus connectivity profile with DMN and these frontoparietal regions implicated in executive function, it is possible this region is critical for coordinating processing between the DMN and FPN in a way that supports optimal performance in executive function tasks (Cocchi et al. 2013; Li et al. 2019). This function may be particularly well-developed in humans as the precuneus has undergone a significant expansion relative to other primates (Bruner et al. 2017; Bruner and Colom 2022).
The central precuneus may provide a neural substrate that interfaces between the DMN and FPN to mediate broader coordinated processing states between internally mediated versus attentionally demanding tasks (Li et al. 2019). Within this framework, impairments in TMT performance following lesions of the central precuneus may relate to disruptions in the coordinated transition from passive states to those requiring focused attention, or difficulty sustaining attention-demanding focus, both processes that would normally correspond with enhanced FPN activity and suppressed DMN activity. While our results are consistent with this possibility, this interpretation remains speculative. The exact contribution the central precuneus makes to facilitate normal TMT performance is not evident from the current study design and will require further study. In particular, combined approaches such as intracranial electrophysiology and fMRI could be used to elucidate high spatiotemporal information on activity patterns while relating them to more global network dynamics (Daitch and Parvizi 2018).
Strengths of our study include the use of one of the largest sample sizes to date of patients with lesions to the precuneus, which allows a unique opportunity to infer causal links between cognition and the precuneus. This study also has limitations. First, while we use the TMT to measure executive function, we acknowledge that this single task does not capture the complexity of the broad construct of executive function. We felt TMT was an optimal task for the current analysis in that it combines several core features of executive function (cognitive flexibility, divided attention, response monitoring, and task switching (Lehto et al. 2003; Arbuthnott and Frank 2000; Salthouse 2011)) along with controlling for performance-related features that do not invoke these processes, but may be influenced by a brain lesion (e.g., hemiparesis or visual deficits that would impair TMT part A and part B performance, and thus were controlled for through subtraction of A from B). It will be important to conduct further work with a wider array of executive function tests (Toba et al. 2020). Whether the impairment observed in TMT performance can be generalized to other domains of executive function will also require further study.
In an effort to maximize the sample size and generalizability of our analysis, we included lesions that involve the precuneus but in many cases were not exclusively located within the precuneus. In addition, we included lesions of multiple different etiologies in the same analysis (e.g., tumor resection, stroke, contusion). It should be acknowledged that patients with lesions in the same locations can show differences in cognitive and behavioral outcomes depending on if their lesion resulted from stroke or from a tumor (Anderson et al. 1990). We recognize meaningful differences could exist between different etiologies, but we did not have the sample size to explore the effects of each lesion etiology independently. However, there is recent work showing that lesions resulting from stroke versus traumatic brain injury have very similar cognitive and behavioral outcomes, obviating to some extent concerns about different lesion etiologies (Harris et al. 2022). Moreover, each lesion was mapped to a common template brain based on the anatomical location of the lesion. This approach assumes a similar anatomical-functional organization exists within the precuneus across different subjects, which is another point of caution. Prior work shows that pronounced inter-individual differences in precuneus anatomy can exist (Bruner et al. 2015) possibly related to developmental differences in somato-visual integration (Huntenburg et al. 2018).
Some of these limitations can be addressed in future studies. A larger sample size with varied etiologies would allow evaluation of the role of lesion etiology with precuneus lesions. It will also be important to utilize a wider array of executive function tests to identify the extent to which impairments in other aspects of executive function are associated with precuneus lesions.
In conclusion, this study provides supporting evidence for a critical role of the central precuneus in executive function. This functional role is consistent with the unique connectivity pattern of this central precuneus region with a broader frontoparietal network implicated in cognitive control and executive function.
Supplementary Material
Funding
This study was supported by the National Institutes of Health Predoctoral Training Grant T32-NS007421, the National Institute of Neurological Disorders and Stroke RO1-NS114405, and the National Institute of Mental Health-R21MH120441. This work was conducted, in part, on an MRI instrument funded by 1S10OD025025-01.
Abbreviations
- TMT
Trail-making test
- ANTs
Advanced normalization tools
- ROI
Region of interest
- SCCAN
Sparse canonical correlation analysis
Footnotes
Conflict of interest The authors report no conflict of interest.
Ethical approval Approval was obtained from the ethics committees of the University of Iowa and Montpellier University. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to participate Informed consent was obtained from all individual participants included in the study.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00429-022-02556-0.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- Anderson SW, Damasio H, Tranel D (1990) Neuropsychological impairments associated with lesions caused by tumor or stroke. Arch Neurol 47(4):397–405 [DOI] [PubMed] [Google Scholar]
- Arbuthnott K, Frank J (2000) Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol 22(4):518–528 [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison N, Song G (2009) Advanced normalization tools (ANTS). Insight J 2(365):1–35 [Google Scholar]
- Baddeley A (1992) Working memory. Science 255(5044):556–559 [DOI] [PubMed] [Google Scholar]
- Baddeley A (2003) Working memory: looking back and looking forward. Nat Rev Neurosci 4(10):829–839 [DOI] [PubMed] [Google Scholar]
- Badre D (2008) Cognitive control, hierarchy, and the rostro–caudal organization of the frontal lobes. Trends Cogn Sci 12(5):193–200 [DOI] [PubMed] [Google Scholar]
- Boes AD, Fischer D, Geerling JC, Bruss J, Saper CB, Fox MD (2018) Connectivity of sleep-and wake-promoting regions of the human hypothalamus observed during resting wakefulness. Sleep 41(9):zsy108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowren M, Adolphs R, Bruss J, Manzel K, Corbetta M, Tranel D, Boes AD (2020) Multivariate lesion-behavior mapping of general cognitive ability and its psychometric constituents. J Neurosci 40(46):8924–8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowren M, Bruss J, Manzel K, Edwards D, Liu C, Corbetta M, Boes AD (2022) Post-stroke outcomes predicted from multivariate lesion-behaviour and lesion network mapping. Brain 145(4):1338–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner E, Colom R (2022) Can a Neandertal meditate? An evolutionary view of attention as a core component of general intelligence. Intelligence 93:101668 [Google Scholar]
- Bruner E, Román FJ, de la Cuétara JM, Martin-Loeches M, Colom R (2015) Cortical surface area and cortical thickness in the precuneus of adult humans. Neuroscience 286:345–352 [DOI] [PubMed] [Google Scholar]
- Bruner E, Preuss TM, Chen X, Rilling JK (2017) Evidence for expansion of the precuneus in human evolution. Brain Struct and Funct 222(2) 1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129(3):564–583 [DOI] [PubMed] [Google Scholar]
- Cocchi L, Zalesky A, Fornito A, Mattingley JB (2013) Dynamic cooperation and competition between brain systems during cognitive control. Trends Cogn Sci 17(10):493–501 [DOI] [PubMed] [Google Scholar]
- Collins A, Koechlin E (2012) Reasoning, learning, and creativity: frontal lobe function and human decision-making. PLoS Biol 10(3):e1001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daitch AL, Parvizi J (2018) Spatial and temporal heterogeneity of neural responses in human posteromedial cortex. Proc Natl Acad Sci 115(18):4785–4790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP (2008) Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex 18:2735–2747 [DOI] [PubMed] [Google Scholar]
- Diamond A (2012) Activities and programs that improve children’s executive functions. Curr Dir Psychol Sci 21(5):335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douw L, Wakeman DG, Tanaka N, Liu H, Stufflebeam SM (2016) State-dependent variability of dynamic functional connectivity between frontoparietal and default networks relates to cognitive flexibility. Neuroscience 339:12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI (2003) Cognitive and brain consequences of conflict. Neuroimage 18(1):42–57 [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak RS, Dolan RJ (1995) The mind’s eye—precuneus activation in memory-related imagery. Neuroimage 2(3):195–200 [DOI] [PubMed] [Google Scholar]
- García-Madruga JA, Gómez-Veiga I, Vila JÓ (2016) Executive functions and the improvement of thinking abilities: the intervention in reading comprehension. Front Psychol 7:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding IH, Yücel M, Harrison BJ, Pantelis C, Breakspear M (2015) Effective connectivity within the frontoparietal control network differentiates cognitive control and working memory. Neuroimage 106:144–153 [DOI] [PubMed] [Google Scholar]
- Harris S, Bowren M, Anderson SW, Tranel D (2022) Does brain damage caused by stroke versus trauma have different neuropsychological outcomes? A lesion-matched multiple case study. Appl Neuropsychol. 10.1080/23279095.2022.2033242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harroud A, Boucher O, Tran TPY, Harris L, Hall J, Dubeau F, Nguyen DK (2017) Precuneal epilepsy: clinical features and surgical outcome. Epilepsy Behav 73:77–82 [DOI] [PubMed] [Google Scholar]
- Hayden BY, Nair AC, McCoy AN, Platt ML (2008) Posterior cingulate cortex mediates outcome-contingent allocation of behavior. Neuron 60(1):19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbet G, Lemaitre AL, Moritz-Gasser S, Cochereau J, Duffau H (2019) The antero-dorsal precuneal cortex supports specific aspects of bodily awareness. Brain 142(8):2207–2214 [DOI] [PubMed] [Google Scholar]
- Huntenburg JM, Bazin PL, Margulies DS (2018) Large-scale gradients in human cortical organization. Trends Cogn Sci 22(1):21–31 [DOI] [PubMed] [Google Scholar]
- Hwang K, Bruss J, Tranel D, Boes AD (2020) Network localization of executive function deficits in patients with focal thalamic lesions. J Cogn Neurosci 32(12):2303–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ (2007) The Parieto-Frontal integration theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci 30(2):135–154 [DOI] [PubMed] [Google Scholar]
- Kalamatianos T, Mavridis IN, Karakosta E, Drosos E, Skandalakis GP, Kalyvas A, Stranjalis G (2019) The parieto-occipital artery revisited: a microsurgical anatomic study. World Neurosurg 126:e1130–e1139 [DOI] [PubMed] [Google Scholar]
- Kumral E, Bayam FE, Özdemir HN (2021) Cognitive and behavioral disorders in patients with precuneal infarcts. Eur Neurol 84(2):1–11 [DOI] [PubMed] [Google Scholar]
- Lehto JE, Juujärvi P, Kooistra L, Pulkkinen L (2003) Dimensions of executive functioning: evidence from children. Br J Dev Psychol 21(1):59–80 [Google Scholar]
- Li R, Utevsky AV, Huettel SA, Braams BR, Peters S, Crone EA, van Duijvenvoorde AC (2019) Developmental maturation of the precuneus as a functional core of the default mode network. J Cogn Neurosci 31(10):1506–1519 [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM (2005) The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage 27(4):824–834 [DOI] [PubMed] [Google Scholar]
- Lunt L, Bramham J, Morris RG, Bullock PR, Selway RP, Xenitidis K, David AS (2012) Prefrontal cortex dysfunction and ‘jumping to conclusions’: bias or deficit? J Neuropsychol 6(1):65–78 [DOI] [PubMed] [Google Scholar]
- Lyu D, Pappas I, Menon DK, Stamatakis EA (2021) A precuneal causal loop mediates external and internal information integration in the human brain. J Neurosci 41(48):9944–9956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH (2001) Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104(3):667–676 [DOI] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C et al. (2009) Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci 106(47):20069–20074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavridis IN, Kalamatianos T, Koutsarnakis C, Stranjalis G (2016) Microsurgical anatomy of the precuneal artery: does it really exist? Clarifying an ambiguous vessel under the microscope. Oper Neurosurg 12(1):68–76 [DOI] [PubMed] [Google Scholar]
- Menon V, D’Esposito M (2022) The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology 47(1):90–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000) The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol 41(1):49–100 [DOI] [PubMed] [Google Scholar]
- Parvizi J, Braga RM, Kucyi A, Veit MJ, Pinheiro-Chagas P, Perry C, Markert M (2021) Altered sense of self during seizures in the posteromedial cortex. Proc Natl Acad Sci 118(29):e2100522118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustina D, Avants B, Faseyitan OK, Medaglia JD, Coslett HB (2018) Improved accuracy of lesion to symptom mapping with multivariate sparse canonical correlations. Neuropsychologia 115:154–166 [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci 98(2):676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Dombert PL, Løvstad M, Funderud I, Meling TR, Endestad T, D’Esposito M (2019) Lesions to the fronto-parietal network impact alpha-band phase synchrony and cognitive control. Cereb Cortex 29(10):4143–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2011) What cognitive abilities are involved in trail-making performance? Intelligence 39(4):222–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Hömke L, Mohlberg H, Hermann K, Amunts K, Zilles K (2008a) Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex 18(9):2141–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K (2008b) Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex 18(4):846–867 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27(9):2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL (2010) Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 53(1):303–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toba MN, Malkinson TS, Howells H, Mackie MA, Spagna A (2020) Same or different? A multi-method review on the relationships between processes underlying executive control. PsyArXiv [Pre-Print]. 10.31234/osf.io/6zcvn [DOI] [PubMed] [Google Scholar]
- Tombaugh TN (2004) Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol 19(2):203–214 [DOI] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV, Huettel SA (2014) Precuneus is a functional core of the default-mode network. J Neurosci 34(3):932–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel MP, Sporns O (2013) Network hubs in the human brain. Trends Cognit Sci 17(12):683–696 [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL (2010) Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103(1):297–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Chen C, Spagna A, Wu X, Mackie MA, Russell-Giller S, Fan J (2020) The functional anatomy of cognitive control: a domain-general brain network for uncertainty processing. J Comp Neurol 528(8):1265–1292 [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang H, Zhou W, Qian T, Sun W, Zhao G (2018) Electroclinical characteristics of seizures arising from the precuneus based on stereoelectroencephalography (SEEG). BMC Neurol 18(1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Chiang-shan RL (2012) Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage 59(4):3548–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.