Highlights
-
•
ExtTs decreases the ability of T. gondii to proliferate in J774 macrophages.
-
•
ExtTs-treatment decreases parasitism and inflammation in intestine of infected mice.
-
•
ExtTs-treatment preserves the Paneth and goblet cells in intestine of infected mice.
-
•
ExtTs-treatment decreases parasitism and inflammation in the liver of infected mice.
-
•
ExtTs-treatment preserves triglycerides and VLDL systemically as uninfected mice.
Keywords: J774 macrophages, Inflammation, Toxoplasma parasitism, Toxoplasmosis treatment, Trichoderma stromaticum extract (ExtTs)
Abstract
Trichoderma are fungi that are well-known to inhibit the growth of a variety of plant pathogens. Currently, there is an increasing search for new drugs to treat toxoplasmosis. The aims of this study were to investigate the effect of ExtTs in the control of Toxoplasma gondii proliferation in vitro and the course of toxoplasmosis in a mouse model. Firstly, the cytotoxicity of the ExtTs was evaluated by cultivating macrophages with different concentrations of the extract and cell viability was assessed by the MTT assay. Next, the infectivity of the T. gondii treated with extract was analyzed by infecting J774 macrophages. To evaluate the effect of the ExtTs in vivo, C57BL/6 mice were infected orally with T. gondii, ME-49, treated daily with ExtTs, and clinical, biochemical and histological changes were monitored. It was demonstrated that the extract did not affect the host cellular viability and, the treatment of parasites with ExtTs altered their morphology and decreased their ability to proliferate inside macrophages. Additionally, the treatment of mice with ExtTs decreased the parasitism and inflammation in the small intestine and liver of infected mice in parallel with increased IL-10/TNF ratio systemically and prevented alterations to serum VLDL and triglyceride levels. Thus, ExtTs could be considered an alternative/complementary therapy to control toxoplasmosis.
Graphical abstract
1. Introduction
T. gondii infection can be unnoticed in immunocompetent hosts as the signs and symptoms vary depending on the immune status of the patient (Montoya and Liesenfeld, 2004). The combination of pyrimethamine, sulfadiazine and folinic acid is the most typical drug combination against toxoplasmosis (Montoya and Liesenfeld, 2004). However, toxicity and adverse events attributed to current therapies against toxoplasmosis are common, as are clinical relapses (Haverkos, 1987; McAuley et al., 1994; Ben-Harari et al., 2017). Thus, the development of therapeutic alternatives with good efficacy and lower toxicity to treat toxoplasmosis has received considerable attention. Previous studies have shown the effects of other medications, such as enrofloxacin (Barbosa et al., 2012), azithromycin (Castro-Filice et al., 2014) and medicinal plants (Sharif et al., 2016) against T. gondii infection in vivo and in vitro. Trichoderma is a phytopathogenic fungus that has been used to control plant diseases which can exert control either directly by mycoparasitism or indirectly, by competing for space and nutrients, producing antibiotics, changing environmental conditions and inactivating the enzymes of the pathogens (Elad et al., 1980; Benítez et al., 2004). T. stromaticum is a filamentous fungus that was isolated from dried cacao brooms in Belém (Pará, Brazil) and has been used as a biopesticide called Tricovab® (Bastos, 1996; Samuels, 1996). T. stromaticum presents polypeptide antibiotics (Peptaibiotics) and trichostromaticins A-E, which may contribute to the potent bioactivity of the fungus (Degenkolb et al., 2006).
Previous studies have shown that metabolites derived from some specific genera of fungi have been able to generate antiparasitic effects in vitro against protozoa pathogens, such as Leishmania amazonensis amastigote, L. (Viannia) braziliensis, Plasmodium falciparum, L. donovani and Trypanosoma cruzi (Campos et al., 2008; Moreno et al., 2011; Cota et al., 2018). We have shown that the crude ethanolic extract of the fungus T. stromaticum (ExtTs) decreased the growth of P. falciparum in infected human red blood cells and was also able to increase survival and prevent the development of experimental cerebral malaria (Cariaco et al., 2018). Additionally, we also demonstrated that spores from T. stromaticum downregulated the response of murine phagocytes by decreasing the production of nitric oxide (NO) and reactive oxygen species (ROS) (Alves-Filho et al., 2011). However, no current studies have evaluated the effect of the ExtTs in T. gondii infection.
The current study aimed to evaluate the effect of ExtTs on the infectivity of T. gondii in vitro and in vivo. As oral T. gondii-infection of C57BL/6 mice provokes an intense inflammatory immune response leading to the death of animals (Liesenfeld et al., 1999) and T. stromaticum ExtTs reduces the proinflammatory cytokines in Plasmodium infection (Cariaco et al., 2018), the study investigated whether the ExtTs-treatment could affect the proinflammatory cytokine profile and inflammation provoked by oral infection. Additionally, the lipid profile was investigated in serum samples from animals infected and treated with ExtTs, as the host lipid contributes to parasite growth (Coppens et al., 2000; Nolan et al., 2017).
2. Material and methods
2.1. Maintenance of T. stromaticum cultures and crude ethanolic extract preparation (ExtTs)
The fungus Trichoderma stromaticum ALF 64 strain that is used in the preparation of Tricovab® (Cepec-Ceplac; Centro de Pesquisas do Cacau - Comissão Executiva do Plano da Lavoura Cacaueira) was utilized in this study. T. stromaticum was cultivated on potato dextrose agar (PDA) in Petri dishes at 25 °C in the dark until conidia production (7–15 days) was observed. After sporulation, the cultures were washed with 95% ethanol. The ethanolic solution was homogenized in a shaker for 24 h and centrifuged at 2200 × g for 20 min. The supernatant was collected and dried under a vacuum; the crude extract obtained was weighed, resuspended in sterile phosphate buffered saline (PBS) and stored at −20 °C until use (Fukuzawa et al., 2008).
2.2. Parasite strains
Tachyzoites of the T. gondii RH strain (2F1 clone), which constitutively express cytoplasmic β-galactosidase (Seeber and Boothroyd, 1996), were propagated in human cervix adenocarcinoma (HeLa) cell lines obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Cultilab, Campinas, SP, Brazil) supplemented with l-glutamine, penicillin, streptomycin (all from Sigma-Aldrich, St. Louis, USA) and 2% fetal bovine serum (FBS) (Cultilab) at 37 °C and 5% CO2 (Almeida et al., 2019). The RH strain was also used for in vivo experiments by infecting animals intraperitoneally.
T. gondii ME49 strain was used to infect animals orally and was maintained in Swiss Webster mice infected at least 1 month before.
2.3. Animals
C57BL/6 mice were bred and maintained with a 12/12 h light/dark cycle under specific pathogen-free conditions (SPF) at the Federal University of Uberlandia animal facilities (Rede de Biotérios de Roedores da Universidade Federal de Uberlândia, REBIR-UFU), with water and food ad libitum. In this experimental work it was used C57BL/6 female mice aged eight week old. The experimental procedures were approved by the Animal Experimental Ethics Committee (CEUA) of the Federal University of Uberlândia, under protocol number 110/11.
2.4. Cell culture
The murine macrophage J774 cell line was obtained from ATCC and cultured in 25 cm2 culture flasks in Dulbecco's Modified Eagle's (DMEM) (Cultilab) medium, supplemented with 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin and 10% heat-inactivated FBS. The cells were maintained in a humidified incubator at 37 °C and 5% CO2 atmosphere.
2.5. Cytotoxicity activity of ExtTs
The effects of different concentrations of ExtTs on the viability of J774 macrophages were evaluated using the MTT [3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide] assay, according to Mosmann (1983). The cells were cultured into 96-well plates at a density of 3 × 104 cells/200 μL/well in DMEM medium with 10% FBS for 24 h at 37 °C and 5% CO2. Next, the macrophages were treated with increasing concentrations of ExtTs (15.6 ng/mL to 10 μg/mL) for an additional 24 h, and the MTT assay was conducted to verify whether ExtTs were toxic to the cells. The data were expressed as percent viable cells in relation to controls (untreated cells – 100% viability). Three independent experiments with six replicates were performed.
2.6. T. gondii inhibition proliferation in J774 macrophages
The T. gondii infectivity treated with ExtTs was evaluated in murine macrophages. For this purpose, tachyzoites of 2F1 clone were pre-incubated with different concentrations (62.5 ng/mL, 125 ng/mL, 250 ng/mL, 500 ng/mL or 1 µg/mL) of ExtTs for 3 h at 37 °C. After this time, the J774 cells that had been cultured into 96-well plates for 24 h (3 × 104 cells/200 μL/well), were infected with the pre-incubated T. gondii tachyzoites at a proportion of 5 parasites per cell for 3 h, washed to remove extracellular parasites and T. gondii proliferation was measured 24 h later. The supernatants were discarded and cells were analyzed for T. gondii intracellular proliferation by a colorimetric β-galactosidase assay using the color substrate, chlorophenol red-13-D-galactopyranoside (CPRG), as previously described (Teo et al., 2007). Untreated and infected cells were used as a positive control. T. gondii intracellular proliferation data were expressed as the number of tachyzoites corresponding to their enzyme activity obtained from a reference curve of 2F1 strain tachyzoites, ranging from 1 × 106 to 15.625 × 103 total parasites. Data were obtained from three independent experiments performed in six replicates.
In order to measure the DNA of T. gondii, J774 cells were cultured in 24-well plates (4 × 105 cells/300 µL/well) in DMEM medium with 10% FBS at 37 °C and 5% CO2 for 24 h. Subsequently, the cells were infected with tachyzoites of T. gondii (2F1 clone) (5:1), untreated or previously treated with ExtTs (250 ng/mL or 1 µg/mL) during 3 h, and incubated for additional 24 h at 37 °C and 5% CO2. Total DNA isolation of J774 cells infected with T. gondii was obtained by the phenol-choroform method with minor modifications (Miller et al., 1988). Briefly, the supernatant was discarded and cells were washed with sterile PBS, treated with 500µL of lysis buffer (50 mM Tris–HCl pH 8,0, 200 mM NaCl, 20 mM EDTA pH 8,0 and 1% SDS) and the DNA was extracted with Phenol-chloroform (1:1) solution. Real-time PCR quantitative DNA analyses were performed on the ABI Prism 7500 Sequence Detection System using SYBR green fluorescence (Applied Biosystems, Warrington, UK). The primers to amplify the 529 bp fragment (forward: 5′-GCTCCTCCAGCCGTCTTG-3′, reverse: 5′-TCCTCACCCTCGCCTTCAT-3′) was used to determine and quantify the presence of T. gondii, and β-actin (forward: 5′-CACTATTGGCAACGAGCGG-3′, reverse: 5′-GCCACAGGATTCCATACCCA-3′) was used to normalize the amount of total genomic DNA. The relative changes in target gene/ β-actin DNA ratio were determined by the formula: 2-ΔΔct. The linear regression was used to establish the absolute amount of parasite in each condition by the formula: P(log2) = 36,91–0,9158*CT.
2.7. Transmission electron microscopy
In order to verify the direct effect of ExtTs treatment on tachyzoites, we performed transmission electron microscopy (TEM). For this purpose, 5 × 106 free tachyzoites were added to microtubes and treated with 1 μg/mL ExtTs for 3 h in RPMI 1640 medium at 37 °C and 5% CO2. Untreated parasites were considered as control. Next, the parasites were fixed for 24 h in Karnovsky solution containing 2% paraformaldehyde and glutaraldehyde in a 0.1 M sodium cacodylate buffer (pH 7.4). After, the tachyzoites were treated with 1% osmium tetroxide in cacodylate solution for 1 h, then samples were dehydrated in increasing concentrations of ethanol, and embedded in propylene oxide and Epon resin. Ultrathin sections were stained with lead citrate and uranyl, and then analyzed in a transmission electron microscope (Zeiss EM 900, Germany).
2.8. Nitrite measurement
J774 cells were cultured for 24 h, as described above, were treated with 62.5 ng/mL, 125 ng/mL, 250 ng/mL, 500 ng/mL or 1 µg/mL of ExtTs in association or not with 1.5 ng/mL IFN-γ, or only stimulated with 1.5 ng/mL IFN-γ [obtained previously from the culture supernatant of L1210-lymphocytic leukemia cell line (ATCC)], and incubated for additional 24 h. The nitrite concentration in the supernatants of cells exposed to the conditions cited above was measured by Griess method (Green et al., 1982). Untreated cells were used as control. A culture medium was used as a blank and the nitrite level in each sample was calculated from a standard curve of sodium nitrite (NaNO2). Three independent experiments in six replicates were performed.
2.9. The effect of treatment of mice with ExtTs in the susceptibility to oral infection with T. gondii: experimental procedure and tissue processing
C57BL/6 female mice were infected by gavage with 10 cysts of the ME-49 T. gondii strain that were harvested from the brains of previously infected Swiss mice. One day after infection, animals were treated with 100 mg/Kg/day of ExtTs diluted in 100 µL PBS via the i.p. route and treatment persisted for 6 days. A group of animals were treated with sulfadiazine in drinking water (500 mg/L) one day after infection, and treatment persisted for six days. Control mice were orally infected and treated i.p. with PBS daily, similarly to ExtTs-treated mice. On day 8 post-infection (8 dp.i.), animals were injected with the anesthetics Ketamine (Syntec Brasil Ltda, SP, Brazil)/Xylazine (Schering-Plough Coopers, SP, Brazil) via the i.p. route; the blood samples were collected by puncture of the retro-orbital plexus and the animals were euthanized by cervical dislocation. Serum samples were stored at −80 °C for cytokine, alanine aminotransferase (ALT) and lipid analysis. Tissue samples of the liver, lung and small intestine were collected and fixed in 10% buffered formalin and processed routinely for paraffin embedding and sectioning. The small intestine was cut into four pieces (duodenum, proximal jejunum, distal jejunum and ileum) and each piece was rolled on itself to make a "Swiss roll", being the small intestine examined in all of the extensions. Tissue sections with a thickness of 5 μm were mounted on slides and stained with hematoxylin and eosin (H&E) for inflammatory score and Paneth cell quantification or Alcian blue for goblet cell quantification in the small intestine. The goblet and Paneth cells were analyzed using a 40 × objective in a light microscope in a blind manner. For Paneth cells, 400 crypts of the small intestine were analyzed, while the number of stained goblet cells was counted in 400 villi.
The inflammatory score of the small intestine was represented as arbitrary units: 0–2, mild; 2–4, moderate; 4–6, severe; and above 6, very severe and was evaluated in the entire section, as previously described (Oliveira et al., 2020). In the lung, the alveolar septa were measured in 10 microscopic fields using the ImageJ software version 1.50i and the inflammatory foci in the liver were quantified in 20 microscopic fields. The inflammatory score analyses were performed using a 10 × objective in a light microscope and in a blind manner.
2.10. Immunohistochemistry for tissue parasitism detection
As previously described, the tissue parasitism was evaluated in tissue sections by immunohistochemistry (Oliveira et al., 2020). The stained parasites were counted under a light microscope using a 40 × objective in fifty microscopic fields in the lung, liver and each section of the small intestine (200 fields in total).
2.11. Cytokines measurement
The cytokines tumor necrosis factor (TNF), interferon (IFN)-γ, interleukin (IL)−6, and IL-10, were measured in serum samples using a cytometric bead array assay (CBA) (BD, San Jose, CA, USA) following the manufacturer's instructions. Samples were processed using a FACSCanto II Flow Cytometer (BD, Biosciences, Mexico) and analyzed with FACSDiva software (BD). According to the instruction manual of the kit, the cytokine theoretical limit of detection (pg/mL) is: TNF, 0.9; IFN-γ, 0.5; IL-6, 1.4; IL-10, 16.8.
2.12. Measurement of lipids in serum samples
The serum lipid levels were determined using a commercial kit (Lab-test Diagnóstica S.A., Lagoa Santa, MG, Brazil). Blood samples were centrifuged at 800 × g for 10 min and serum aliquots were transferred to a clean tube. Total cholesterol, HDL and triglycerides concentrations in the sera were measured following the kit instructions for each parameter. LDL and VLDL serum levels were estimated using the Friedewald equation (Friedwald, 1972).
2.13. ALT measurement
The ALT was determined using a commercial kit (Lab-test Diagnóstica S.A., Lagoa Santa, MG, Brazil), according to the manufacturer's instructions. The absorbance was obtained at 505 nm.
2.14. Statistical analysis
Statistical analyzes were performed using GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA). Differences between groups were analyzed using the Unpaired Student's t or Mann-Whitney test when assuming or not Gaussian distribution, respectively. In addition, the one-way ANOVA was used for multiple comparison followed by Sidak's multiple comparison test or Kruskal Wallis followed by Dunn's post-test, when appropriate. Values of P < 0.05 were considered statistically significant.
3. Results
3.1. The treatment of J774 macrophages with ExtTs was not toxic to cells, nor did it stimulate the production of nitric oxide (NO)
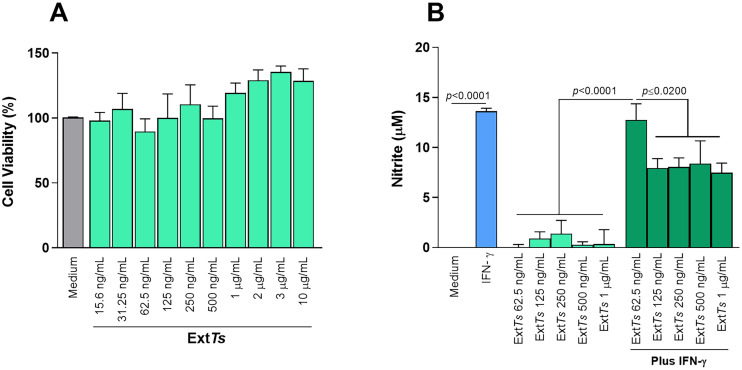
Initially, whether the ExtTs could be cytotoxic to macrophages was investigated. For this purpose, J774 cells were treated with ExtTs and analyzed by the MTT assay. It was observed that the treatment of cells with ExtTs in concentrations ranging from 15.6 ng/mL to 10 μg/mL did not alter the cellular viability when compared with untreated cells (Fig. 1A), demonstrating no cytotoxicity to the cells.
Fig. 1.
Effect of ExtTs-treatment on J774 macrophages cellular viability and nitrite production. J774 cells were cultured into 96-well plates (3 × 104 cells/200 μL/well) in DMEM medium supplemented with 10% FBS for 24 h, treated with or without ExtTs in different concentrations and cellular viability analyzed by MTT assay (A). Cells were cultured and treated with ExtTs as described or with IFN-γ, incubated for additional 24 h, and evaluated for nitrite production (B). Data were expressed as mean ± SEM from three independent experiments and were analyzed by one-way ANOVA followed by Sidak's multiple comparison test.
The IFN-γ, is known to stimulate T. gondii control by macrophages through NO production (Adams et al., 1990). It was shown that, J774 macrophages treated with different concentrations of ExtTs presented similar NO production as non-treated cells (Fig. 1B). When cells were stimulated with IFN-γ, a high production of NO was observed; however, the treatment of IFN-γ-stimulated cells with ExtTs at doses of 125 ng/mL – 1 μg/mL was able to significantly decrease the NO production (Fig. 1B).
3.2. The ExtTs-treatment induced structural changes in morphology of parasites and decreased their ability to proliferate in J774 macrophages
It was shown that the treatment of T. gondii with ExtTs at concentrations of 62.5, 125, 250, 500 ng/mL or 1 μg/mL significantly reduced the T. gondii proliferation (number of tachyzoites) compared with untreated parasites (medium condition) (Fig. 2A). It was also showed by T. gondii DNA detection when J774 cells were infected with parasites pretreated with ExtTs (Supplementary Fig. 1).
Fig. 2.
Effect of treatment of T. gondii tachyzoites with ExtTs on their ultra-structural morphology and capability to proliferate in J774 macrophages. J774 cells were cultured into 96-well plates (3 × 104 cells/200 μL/well) for 24 h. After, T. gondii tachyzoites were incubated with different concentrations of ExtTs or with medium (control) for 3 h and, the cells were infected with the pre-incubated parasites for additional 24 h. Then, T. gondii intracellular proliferation was measured by β-galactosidase assay (A). Data were expressed as the number of tachyzoites (mean ± SEM) in relation to standard curve and are representative from one of three independent experiments performed in six replicates. Data were analyzed by one-way ANOVA followed by Sidak's multiple comparison test. Tachyzoites were treated with 1 μg/mL ExtTs for 3 h in RPMI 1640 medium at 37 °C and 5% CO2, and submitted to transmission electron microscopy. Representative eletromicrography of untreated (B, C) and treated (D, E) tachyzoites. Rhoptries (Rp); dense granule (G); arrows indicate duple membrane and arrowheads indicate single membrane. Bar scale: 500 nm or 200 nm.
After verifying reduced T. gondii intracellular proliferation in J774 cells when tachyzoites were pretreated with ExtTs, the parasite structural morphology was investigated by TEM. It was shown a complete duple membrane (black arrows) and well-delimited rhoptries (Rp) and dense granules (G), representing the morphology of a typical viable parasite (Fig. 2B, C). On the other hand, when tachyzoites were treated with ExtTs, a single membrane was detected in parasites, suggesting damage of the duple membrane (black arrowhead) (Fig. D, E). In addition, definition of organelles was not observed in treated tachyzoites, including absence of well-defined rhoptries, confirming significant ultra-structural changes in parasites (Fig. D, E).
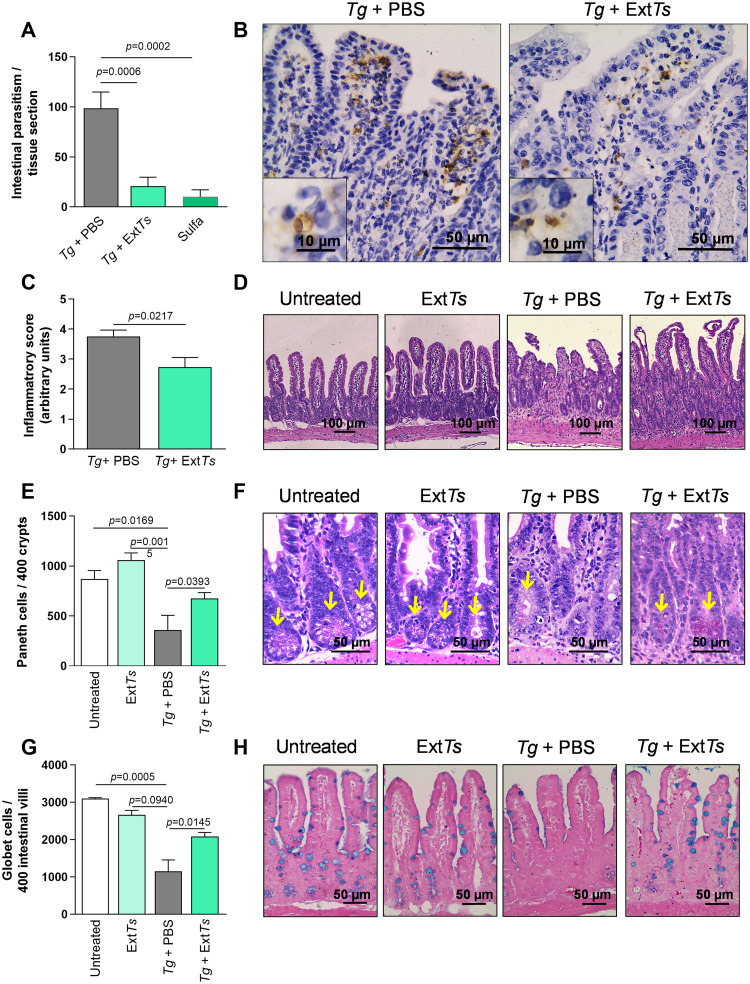
3.3. ExtTs treatment decreased the parasitism and ameliorated the intestinal pathology of C57BL/6 mice orally infected with ME-49 T. gondii strain
It was previously shown that the treatment of C57BL/6 mice with 100 mg/Kg/day of ExtTs improved the survival rate and prevented neurological signs of experimental cerebral malaria (Cariaco et al., 2018). In the present study, mice were inoculated with 10 ME-49 T. gondii cysts via an oral route, treated with 100 mg/kg/day ExtTs and the parasite load was examined in the small intestine. Analysis of the intestinal tissue showed that the treatment with 100 mg/kg/day ExtTs significantly decreased the parasite load in the organ compared to PBS-treated mice (Fig. 3A and B). The treatment with sulfadiazine also decreased the parasite load in the organ, similarly to ExtTs-treated mice (Fig. 3A). Furthermore, the small intestine was examined histologically and showed inflammatory cell infiltrations in the lamina propria (LP) and submucosa of C57BL/6 mice PBS-treated and infected with T. gondii. In some areas, shortening and necrosis of the villi were verified (Fig. 3D). When animals were ExtTs-treated, they presented less severe inflammatory changes in the organ when compared with PBS-treated infected mice; in the majority of the organ, the epithelial architecture was preserved (Fig. 3C and D). Uninfected animals which were PBS- or ExtTs-treated did not present any histological alterations in the organ. Next, the number of Paneth and goblet cells in the small intestine was analyzed. It was shown that the number of Paneth cells decreased in T. gondii-infected mice compared to with non-infected animals or in animals which were non-infected and treated with ExtTs (Fig. 3E and F). Interestingly, the infection with T. gondii did not substantially decrease the Paneth cell numbers when animals were treated with ExtTs, preserving this cell phenotype in the organ (Fig. 3E and F). Regarding goblet cells, the infection with the parasite significantly decreased their numbers in the small intestine (Fig. 3G and H), whereas the ExTs-treatment preserved the number of these cells.
Fig. 3.
Tissue parasitism, histological changes, quantification of Paneth and Goblet cells in the small intestine of C57BL/6 mice infected and treated with ExtTs. C57BL/6 female mice were infected by oral route with 10 ME-49 T. gondii cysts and treated intraperitoneally with ExtTs 100 mg/kg/day or PBS from day 2 to 7 post-infection (n = 5 mice per group). Groups of animals were treated with PBS or ExtTs 100 mg/kg/day during 6 days, as uninfected untreated or uninfected treated controls (n = 5 mice per group). An additional group of animals was treated with sulfadiazine in drinking water (500 mg/L) one day after infection, and for 5 additional days (n = 5 mice per group). The parasitism was detected by immunohistochemistry and quantified in a light microscope using a 40 × objective by counting the number of stained parasites in 50 microscopic fields of each small intestine segment (200 fields total) (A); representative photomicrographs of the specific immunostaining (B). The inflammatory score in the small intestine was determined in H&E stained tissue sections (C). Representative photomicrographs of histological alterations in the small intestine of PBS- or ExtTs-treated uninfected mice or PBS- or ExtTs-treated infected mice (D). Paneth cells were quantified in 400 crypts of H&E (E) and Goblet cell in 400 intestinal villi of toluidin blue (G) stained tissue sections of the small intestine. Representative photomicrographs of tissue sections of the small intestine from PBS- or ExtTs-treated uninfected mice or PBS- or ExtTs-treated infected mice stained by H&E to show Paneth (F) or toluidin blue to show Goblet cell (H). Data were analyzed by Unpaired t-test (C) or one-way ANOVA followed by Sidak's multiple comparison test (A, E and G).
3.4. ExtTs treatment reduced the parasitism and inflammatory alterations in the liver of T. gondii-infected mice
Tissue parasitism was also analyzed in the lung and liver of mice that were orally infected with T. gondii and treated with or without ExtTs (Fig. 4). It was shown that ExtTs or sulfadiazine treatment did not alter the tissue parasitism in the lung (Fig. 4A). The pulmonary tissue of T. gondii-infected C57BL/6 mice presented inflammatory infiltrates of mononucleated cells within the alveolar walls, enlarging the pulmonary septa (Fig. 4C). The intensity of pulmonary lesions was analyzed by measuring the pulmonary septa area. It was observed that these areas were enlarged in infected compared to non-infected mice, irrespective of whether the infected animals were treated with ExtTs or not (Fig. 4B). In the liver, the ExtTs treatment decreased the parasite load (Fig. 4D). Sulfadiazine markedly decreased the parasitism in the liver, however, it was not statistically different from animals ExtTs-treated (Fig. 4D). When analyzing the histological changes, it was observed that the liver of T. gondii-infected mice presented inflammatory foci of mononucleated cells scattered by parenchyma (Fig. 4F) in association with higher ALT levels in serum samples (Fig. 4G). The numbers of inflammatory foci were lower in ExtTs-treated and infected animals than in PBS-treated and infected mice (Fig. 4E). The ExtTs-treated infected mice presented similar ALT levels to non-infected mice (Fig. 4G).
Fig. 4.
Tissue parasitism, histological changes in the lung and liver, and ALT measurement in serum samples of C57BL/6 mice infected with T. gondii and treated with ExtTs. C57BL/6 mice were infected by oral route with 10 ME-49 T. gondii cysts and treated intraperitoneally with ExtTs 100 mg/kg or PBS from day 2 to 7 post-infection and analyzed on day 8 p.i. when animals were euthanized. Where appropriate data of groups of animals non-infected and treated with PBS or ExtTs 100 mg/kg/day during 6 days; or infected and treated with sulfadiazine in drinking water (500 mg/L) for 6 days (n = 5 mice per group) were shown. The tissue parasitism was detected by immunohistochemistry and quantified in a light microscope using a 40 × objective by counting the number of stained parasites in the lung (A) and liver (D). The inflammatory score was evaluated by measuring the pulmonary parenchyma area in histological sections stained by H&E in 10 microscopic fields and analyzed with ImageJ software (B). In the liver the inflammatory foci were quantified in 20 microscopic fields of histological sections stained by H&E (E). Representative photomicrography of the lung (C) and liver (F) tissue sections stained by H&E. The alanine aminotransferase (ALT) was measured in serum samples from PBS or ExtTs-treated infected and PBS or ExtTs-treated uninfected animals using a commercial kit (G). Data are representative of two independent experiments with 5 mice per group, and were analyzed by Unpaired t-test (E) and one-way ANOVA followed by Sidak's multiple comparison test (A, B, D and G). Arrows point inflammatory foci. Bar scale: 100 µm.
3.5. The ExtTs treatment improves the IL-10/TNF ratio systemically in T. gondii infection
It has been shown that the exacerbated inflammatory immune response is detrimental to the host in T. gondii infection (Liesenfeld et al., 1999; Suzuki et al., 2000). Our group previously verified that the ExtTs-treatment decreased the expression of proinflammatory mediators in the brain, improving experimental cerebral malaria (Cariaco et al., 2018). Herein, it was shown that the treatment of infected mice with ExtTs did not interfere significantly with the cytokine profile in serum samples in relation to PBS-treated infected mice (Fig. 5). The infection with T. gondii increased the IFN-γ levels in serum samples, irrespective of whether the animals were treated with or without ExtTs (Fig. 5A). The TNF levels were higher in PBS- or ExtTs-treated T. gondii-infected mice than uninfected untreated mice and in PBS-treated T. gondii-infected mice compared with uninfected ExtTs-treated mice (Fig. 5B). The IL-6 was higher in serum samples from PBS-treated T. gondii-infected mice than in uninfected untreated and uninfected ExtTs-treated mice, and in T. gondii-infected ExtTs-treated mice in relation to uninfected untreated mice (Fig. 5C). Despite not being statistically significant, the ExtTs treatment decreased the TNF and IL-6 levels in relation to PBS-treated mice when animals were infected with T. gondii (Fig. 5B and C). In relation to IL-10, PBS-treated T. gondii-infected mice presented higher levels compared with uninfected mice. Despite not being statistically significant, PBS-treated T. gondii-infected mice showed higher IL-10 levels than uninfected ExtTs-treated mice. ExtTs-treated T. gondii-infected mice also presented higher levels than PBS or ExtTs-treated uninfected mice (Fig. 5D). Interestingly, the IL-10/TNF ratio was higher in ExtTs-treated T. gondii infected mice than PBS-treated infected mice (Fig. 5F). However, the IL-10/IFN-γ and IL-10/IL-6 ratio was not significantly altered in ExtTs-treated infected mice compared to infected untreated mice (Fig. 5E and G).
Fig. 5.
Cytokine measurement in serum samples of mice infected with T. gondii treated with or without ExtTs. C57BL/6 mice were infected by oral route with 10 ME-49 T. gondii cysts and treated intraperitoneally with ExtTs 100 mg/kg/day or PBS from day 2 to 7 post-infection and the cytokine levels were measured in serum samples on day 8 of infection (n = 5 mice per group). The levels of IFN-γ (A), TNF (B), IL-6 (C) and IL-10 (D) were measured by the CBA (Cytometric bead array) assay. The ratio between IL-10 and IFN-γ (E), IL-10 and TNF (F) or IL-10 and IL-6 (G) levels were calculated. Data were analyzed by Kruskal Wallis followed by Dunn's post-test (A, B, C, D) and Unpaired t-test (F).
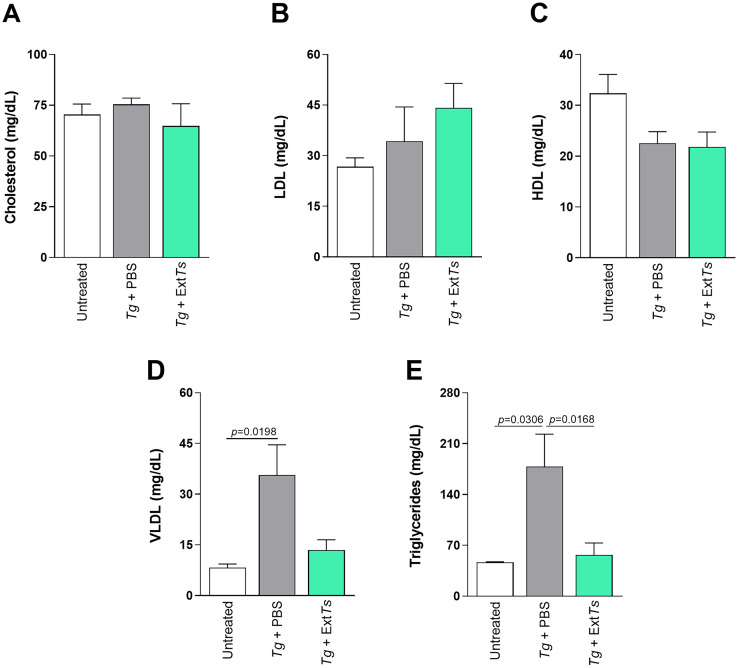
3.6. ExtTs treatment influences the serum lipid profile of C57BL/6 mice infected with T. gondii
Serum samples from animals infected or not with T. gondii and treated with ExtTs or PBS were collected on day 8 of the infection to measure lipid levels. T. gondii infection did not alter the cholesterol, LDL or HDL levels, irrespective of whether the animals were treated or not with ExtTs (Fig. 6A–C). The PBS-treated infected mice presented increased levels of VLDL and triglycerides compared with non-infected mice (Fig. 6D and E). The ExtTs-treatment decreased the triglyceride levels in infected animals compared to infected PBS-treated mice (Fig. 6E), maintaining the triglyceride and VLDL levels at the same level as those of non-infected mice (Fig. 6D and E).
Fig. 6.
Lipid serum profile of mice infected with T. gondii and treated with or without ExtTs. C57BL/6 mice were orally infected with 10 T. gondii cysts, treated intraperitoneally with ExtTs 100 mg/kg/day or PBS from day 2 to 7 post-infection and examined on day 8 of infection (n = 5 mice per group). Serum concentrations of total cholesterol (A), LDL-c (B), HDL-c (C), VLDL-c (D), and triglycerides (E) were measured using a commercial kit. Results were expressed as means ± SEM. Data were analyzed by Kruskal Wallis followed by Dunn's post-test and are representative of at least two independent experiments.
4. Discussion
The treatment of toxoplasmosis is often based on a combination of sulfadiazine and pyrimethamine. The limited efficiency of these drugs and the microbial tolerance to them urged further research to find novel therapeutic agents (Shubar et al., 2011). Therefore, new products that are active against T. gondii and less toxic to the host were intensively investigated. Various studies in vitro and in vivo have been performed to verify the efficacy of herbal medicines against T. gondii (Sharif et al., 2016); however, studies using fungus products against the parasite are scarce (Paugam et al., 2002). In search for new approaches to treat toxoplasmosis that effectively control the parasite and do not present toxicity to the host, and based on our recent study which showed that ExtTs-treatment was able to improve experimental cerebral malaria (Cariaco et al., 2018), the effect of the fungus extract on the control of T. gondii in vitro and in vivo was investigated.
T. gondii can parasitize macrophages, surviving and replicating within the potentially hostile environment of the host cell; however, when activated by IFN-γ, macrophages show a potent microbiostatic effect through NO production (Adams et al., 1990). Thus, the effect of ExtTs in the mechanisms that could be involved in the control of T. gondii by J774 murine macrophages was investigated. Initially, it was verified that the ExtTs was not toxic to the host cells. Considering macrophages activation, the ExtTs treatment did not induce significant NO production and, on the other hand, decreased the NO production by IFN-γ stimulated macrophages. Previous studies using Agaricus blazei Murill mushroom water extract showed that NO production or inducible nitric oxide synthase expression was not observed in murine peritoneal macrophages after stimulation with the extract (Valadares et al., 2011). Thus, besides not presenting cytotoxicity to the host cells in the concentrations utilized here, ExtTs does not stimulate the microbicidal mechanism of J774 macrophages, which is important to T. gondii control.
As the T. stromaticum extract did not activate the NO production by J774 macrophages, the parasites were incubated with ExtTs before the infection of host cells. In this way, ExtTs was able to significantly decrease the parasite proliferation inside J774 cells. Similarly, when L. amazonensis, L. chagasi and L. major were pre-incubated with the A. blazei Murill mushroom water extract and later used to infect murine peritoneal macrophages, they were able to infect only 12.7%, 24.5% and 19.7% of the phagocytic cells, respectively (Valadares et al., 2011). This direct effect on the parasite was also observed in Plasmodium, as we previously showed that ExtTs presented a dose-dependent ability to control P. falciparum in infected human red blood cells (Cariaco et al., 2018). In order to verify possible mechanisms involved in the parasite control induced by ExtTs-treatments, we explored possible ultra-structural changes in pretreated parasites. ExtTs caused damage to tachyzoites, since single membrane and absence of well-defined organelles, including rhoptries, were detected.
It has previously been shown that the ExtTs at doses of 100 mg/kg/day do not cause toxicity in mice via i.p. route (Cariaco et al., 2018). In the present study, ExtTs at doses of 100 mg/kg/day was used to treat C57BL/6 mice, to investigate whether there is a protective effect against toxoplasmosis in vivo, as demonstrated in vitro. C57BL/6 mice infected with T. gondii present parasites in the lung, small intestine and liver on day 8 of infection in association with inflammatory changes (Oliveira et al., 2020). The present investigation demonstrated parasites in the lung, small intestine and liver on day 8 of infection and the ExtTs-treatment was shown to decrease parasite load in the small intestine and liver, at a similar level as sulfadiazine treatment. In agreement with this, it was previously shown that ExtTs-treatment decreased the parasitemia in the initial phase of experimental infection of C57BL/6 mice with P. berghei ANKA (Cariaco et al., 2018). Trichoderma species have been evaluated in Peptaibiomics level and they produce a group of polypeptide antibiotics named peptaibiotics. T. stromaticum presents 18-residue peptaibol compounds, named trichostromaticins, that contribute to the potent bioactivity of the fungus against plant pathogens and, they also produces eight-residue, peptaibiotics (Degenkolb et al., 2006). Despite it is not known the mechanism of action of ExtTs in T. gondii and P. berghei ANKA control, the peptaibols and other components of the extracts may contribute.
Oral infection with T. gondii can cause immunopathology in a large number of animal species in addition to mice (Schreiner and Liesenfeld, 2009); specifically, C57BL/6 mice develop intestinal, lung and liver pathology when infected orally with the parasite (Oliveira et al., 2020; Miranda et al., 2021). In the present work, it was verified that ExtTs-treatment decreased the liver and intestinal pathology. Paneth cells are specialized in producing α-defensins, CRS, cryptdin-related sequence peptides (in mice), as well as Lysozyme C, sPLA2, secretory group IIA phospholipase A2, REG3γ, Regenerating islet-derived protein III-gamma in mice and ANG4, angiogenin 4, which are antimicrobial proteins that are important for the maintenance of intestinal homeostasis (reviewed by Bevins and Salzman, 2011).
The infection of C57BL/6 mice with the T. gondii ME-49 strain via oral route decreases the number of Paneth cells in the small intestine in the acute phase of infection (Raetz et al., 2013; Villeret et al., 2013; Oliveira et al., 2020; Miranda et al., 2021) which was demonstrated be mediated by IL-1 signaling (Villeret et al., 2013) and IFN-γ production (Raetz et al., 2013). Interestingly, herein, it was detected that ExtTs-treated infected mice preserved the majority of Paneth cells in the small intestine.
Goblet cells are another intestinal cell phenotype responsible for the production and release of mucins, which form a film that lubricates and protects the intestinal epithelium (Miller, 1987). Previous studies demonstrated that C57BL/6 mice infected with the ME-49 strain via the oral route presented a low density of goblet cells in the small intestine on day 8 following oral infection (Miranda et al., 2021). The present study demonstrated that the infection decreased the goblet cell numbers in the small intestine and ExtTs treatment was able to preserve this cell phenotype, at least partially. Thus, these results show the effect of ExtTs in maintaining the intestinal homeostasis of animals infected with T. gondii which could improve the intestinal pathology provoked by the infection.
In our previous studies, mouse phagocytes treated with T. stromaticum spores were demonstrated to show decreased respiratory burst and NO production, while BALB/c mice sensitized via the intranasal route showed decreased IFN-γ and IL-10 production by spleen cells in vitro (Alves-Filho et al., 2011). Furthermore, Cariaco et al. showed that the ExtTs-treatment decreased the inflammatory alterations in the liver, lungs and brain in experimental cerebral malaria, preserving the blood-brain barrier and decreasing the cerebral expression levels of IFN-γ, ICAM-1, VCAM-1 and CCR5 (Cariaco et al., 2018). In the present study, infected mice treated with ExtTs had reduced levels of TNF and IL-6 in serum samples compared to untreated infected mice, but there was no statistical difference. Interestingly, ExtTs-treated animals showed a higher IL-10/TNF ratio systemically when infected compared to PBS-treated infected mice. This indicates that the ExtTs induces a response that is prone to being anti-inflammatory, which could contribute to the decreased inflammatory changes observed in ExtTs-treated mice.
T. gondii is unable to synthesize sterols and cholesterol, thus depending on the uptake of host cholesterol for its own development (Coppens et al., 2000). The fat storage organelle lipid droplet (LD) contains an accumulation of neutral lipids (mostly triacylglycerols and cholesteryl esters) (Bartz et al., 2007). When mammalian cells are infected with T. gondii, the host LD numbers increase until the onset of parasite multiplication and the parasite growth is reduced in cells depleted of LD (Nolan et al., 2017). It is known that certain fungi are able to decrease the lipid fractions systemically (Jo et al., 2019; Lai et al., 2020). In the present study, it was observed that in T. gondii-infected animals’ levels of triglycerides and VLDL were enhanced systemically. Interestingly, the ExtTs-treatment decreased the triglyceride and VLDL levels in infected animals. These data suggest that the enhancement of triglycerides and VLDL could contribute to parasite growth in the first days of infection. The ExtTs-treatment maintained the levels of these lipid fractions similar to those of non-infected animals, which could be indirectly involved in the lower parasite growth observed in treated animals.
In conclusion, the extract of T. stromaticum was able to interfere with the ability of T. gondii to proliferate in mammalian host cells. In parallel, this phenomenon was also verified in vivo, since the treatment of infected mice with ExtTs reduced tissue parasitism and the inflammatory alterations in the liver and small intestine, preserving the Paneth and goblet cell numbers. Associated with the diminished tissue parasitism and inflammation, it was observed that ExtTs-treatment was able to maintain VLDL and triglycerides at the levels of those detected in non-infected mice. Based on these results, the extract of T. stromaticum could be a promising alternative/complementary treatment to control T. gondii and the inflammatory alterations induced by the infection.
CRediT authorship contribution statement
Layane Alencar Costa Nascimento: Investigation, Writing – original draft. Romulo Oliveira Sousa: Investigation. Marcos Paulo Oliveira Almeida: Investigation, Writing – original draft. Yusmaris Cariaco: Investigation, Writing – original draft. Angelica Oliveira Gomes: . Natália Carnevalli Miranda: Investigation. Flávia Batista Ferreira França: Investigation. Mariele de Fátima Alves Venâncio: Investigation. Carlos Antonio Trindade Silva: Investigation. Wânia Rezende Lima: Supervision, Writing – review & editing. Bellisa Freitas Barbosa: Supervision, Writing – review & editing. Jane Lima Santos: Supervision, Writing – review & editing. Neide Maria Silva: Conceptualization, Supervision, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Conselho Nacional de Pesquisa Científica e Tecnológica (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). In addition, this study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Financial Code 001. N. M. Silva is research fellow from CNPq. We would like to thank the REBIR-UFU for the maintenance and animal welfare.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.crmicr.2022.100173.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- Adams L.B., Hibbs J.B., Jr, Taintor R.R., Krahenbuhl J.L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J. Immunol. 1990;144:2725–2729. [PubMed] [Google Scholar]
- Almeida M.P.O., Ferro E.A.V., Briceño M.P.P., Oliveira M.C., Barbosa B.F., Silva N.M. Susceptibility of human villous (BeWo) and extravillous (HTR-8/SVneo) trophoblast cells to Toxoplasma gondii infection is modulated by intracellular iron availability. Parasitol. Res. 2019;118:1559–1572. doi: 10.1007/s00436-019-06257-2. [DOI] [PubMed] [Google Scholar]
- Alves-Filho E.R., Maioli T.U., Faria A.M.C., Noronha F.S.M., Silva N.M., Costa M.G.C., Santos J.L. The biocontrol fungus Trichoderma stromaticum downregulates respiratory burst and nitric oxide in phagocytes and IFN-gamma and IL-10. J. Toxicol. Environ. Health, Part A. 2011;74:943–958. doi: 10.1080/15287394.2011.573747. [DOI] [PubMed] [Google Scholar]
- Barbosa B.F., Gomes A.O., Ferro E.A., Napolitano D.R., Mineo J.R., Silva N.M. Enrofloxacin is able to control Toxoplasma gondii infection in both in vitro and in vivo experimental models. Vet. Parasitol. 2012;187:44–52. doi: 10.1016/j.vetpar.2011.12.039. [DOI] [PubMed] [Google Scholar]
- Bartz R., Li W.H., Venables B., Zehmer J.K., Roth M.R., Welti R., Anderson R.G.W., Liu P., Chapman K.D. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid. Res. 2007;48:837–847. doi: 10.1194/jlr.M600413-JLR200. [DOI] [PubMed] [Google Scholar]
- Bastos C.N. Mycoparasitic nature of the antagonism between Trichoderma viride and Crinipellis perniciosa. Fitopatol. Bras. 1996;21:50–54. [Google Scholar]
- Ben-Harari R.R., Goodwin E., Casoy J. Adverse event profile of pyrimethamine-based therapy in toxoplasmosis: a systematic review. Drugs R D. 2017;17:523–544. doi: 10.1007/s40268-017-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benítez T., Rincón A.M., Limón M.C., Codón A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004;7:249–260. [PubMed] [Google Scholar]
- Bevins C.L., Salzman N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- Campos F.F., Rosa L.H., Cota B.B., Caligiorne R.B., Rabello A.L.T., Alves T.M.A., Rosa C.A., Zani C.L. Leishmanicidal metabolites from Cochliobolus sp., an endophytic fungus isolated from Piptadenia adiantoides (Fabaceae) PLoS Negl. Trop. Dis. 2008;2:1–11. doi: 10.1371/journal.pntd.0000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariaco Y., Lima W.R., Sousa R., Nascimento L.A.C., Briceño M.P., Fotoran W.L., Wunderlich G., Santos J.L., Silva N.M. Ethanolic extract of the fungus Trichoderma stromaticum decreases inflammation and ameliorates experimental cerebral malaria in C57BL/6 mice. Sci. Rep. 2018;8:1–15. doi: 10.1038/s41598-018-19840-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Filice L.S., Barbosa B.F., Angeloni M.B., Silva N.M., Gomes A.O., Alves C.M.O.S., Silva D.A.O., Martins-Filho O.A., Santos M.C., Mineo J.R., Ferro E.A.V. Azithromycin is able to control Toxoplasma gondii infection in human villous explants. J. Transl. Med. 2014;12:1–12. doi: 10.1186/1479-5876-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens I., Sinai A.P., Joiner K.A. Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J. Cell Biol. 2000;149:167–180. doi: 10.1083/jcb.149.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota B.B., Tunes L.G., Maia D.N.B., Ramos J.P., Oliveira D.M., Kohlhoff M., Alves T.M.A., Souza-Fagundes E.M., Campos F.F., Zani C.L. Leishmanicidal compounds of Nectria pseudotrichia, an endophytic fungus isolated from the plant Caesalpinia echinata (Brazilwood) Mem. Inst. Oswaldo Cruz. 2018;113:102–110. doi: 10.1590/0074-02760170217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenkolb T., Gräfenhan T., Berg A., Nirenberg H.I., Gams W., Brückner H. Peptaibiomics: screening for polypeptide antibiotics (peptaibiotics) from plant-protective Trichoderma species. Chem. Biodivers. 2006;3:593–610. doi: 10.1002/cbdv.200690063. [DOI] [PubMed] [Google Scholar]
- Elad Y., Chet I., Katan J. Trichoderma harzianum: a biocontrol agent effective against Sclerotium rolfsii and Rhizoctonia solani. Phytopathol. 1980;70:119–121. doi: 10.1094/Phyto-70-119. [DOI] [Google Scholar]
- Friedwald W.T. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Fukuzawa M., Yamaguchi R., Hide I., Chen Z., Hirai Y., Sugimoto A., Yasuhara T., Nakata Y. Possible involvement of long chain fatty acids in the spores of Ganoderma lucidum (Reishi Houshi) to its anti-tumor activity. Biol. Pharm. Bull. 2008;31:1933–1937. doi: 10.1248/bpb.31.1933. [DOI] [PubMed] [Google Scholar]
- Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Haverkos H.W. Assessment of therapy for Toxoplasma encephalitis: the TE study group. Am. J. Med. 1987;82:907–914. doi: 10.1016/0002-9343(87)90151-3. [DOI] [PubMed] [Google Scholar]
- Jo K.J., Ghim J., Kim J., Lee H., Lee T.G., Kim J.I., Kim Y., Byun J.W., Min B.S., Son J.S., Shim S.G., Cheon W.J., Ryu S.H. Water extract of Pleurotus eryngii var. ferulae prevents high-fat diet-induced obesity by inhibiting pancreatic Lipase. J.Med. Food. 2019;22:178–185. doi: 10.1089/jmf.2018.4255. [DOI] [PubMed] [Google Scholar]
- Lai P., Cao X., Xu Q., Liu Y., Li R., Zhang J., Zhang M. Ganoderma lucidum spore ethanol extract attenuates atherosclerosis by regulating lipid metabolism via upregulation of liver X receptor alpha. Pharm. Biol. 2020;58:760–770. doi: 10.1080/13880209.2020.1798471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesenfeld O., Kang H., Park D., Nguyen T.A., Parkhe C.V., Watanabe H., Abo T., Sher A., Remington J.S., Suzuki Y. TNF-α, nitric oxide and IFN-γ are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 1999;21:365–376. doi: 10.1046/j.1365-3024.1999.00237.x. [DOI] [PubMed] [Google Scholar]
- McAuley J., Boyer K.M., Patel D., Mets M., Swisher C., Roizen N., Wolters C., Stein L., Stein M., Schey W., Remington J., Meier P., Johnson D., Heydeman P., Holfels E., Withers S., Mack D., Brown C., Patton D., McLeod R. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago collaborative treatment trial. Clin. Infect. Dis. 1994;18:38–72. doi: 10.1093/clinids/18.1.38. [DOI] [PubMed] [Google Scholar]
- Miller H. Gastrointestinal mucus, a medium for survival and for elimination of parasitic nematodes and protozoa. Parasitol. 1987;94:S77–S100. doi: 10.1017/s0031182000085838. [DOI] [PubMed] [Google Scholar]
- Miller S.A., Dykes D.D., Polesky H.F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda N.C., Araujo E.C.B., Justino A.B., Cariaco Y., Mota C.M., Costa-Nascimento L.A., Espindola F.S., Silva N.M. Anti-parasitic activity of Annona muricata L. leaf ethanolic extract and its fractions against Toxoplasma gondii in vitro and in vivo. J. Ethnopharmacol. 2021;273 doi: 10.1016/j.jep.2021.114019. [DOI] [PubMed] [Google Scholar]
- Montoya J.G., Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- Moreno E., Varughese T., Spadafora C., Arnold A.E., Coley P.D., Kursar T.A., Gerwick W.H., Cubilla-Rios L. Chemical constituents of the new endophytic fungus Mycosphaerella sp. nov. and their anti-parasitic activity. Nat. Prod. Commun. 2011;6:835–840. doi: 10.1177/1934578X1100600620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nolan S.J., Romano J.D., Coppens I. Host lipid droplets: an important source of lipids salvaged by the intracellular parasite Toxoplasma gondii. PLoS Pathog. 2017;13:1–38. doi: 10.1371/journal.ppat.1006362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira M.C., Coutinho L.B., Almeida M.P.O., Briceño M.P., Araujo E.C.B., Silva N.M. The availability of iron is involved in the murine experimental Toxoplasma gondii infection outcome. Microorganisms. 2020;8:1–18. doi: 10.3390/microorganisms8040560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugam A., Creuzet C., Dupouy-Camet J., Roisin P. In vitro effects of gliotoxin, a natural proteasome inhibitor, on the infectivity and proteolytic activity of Toxoplasma gondii. Parasitol. Res. 2002;88:785–787. doi: 10.1007/s00436-002-0644-1. [DOI] [PubMed] [Google Scholar]
- Raetz M., Hwang S.H., Wilhelm C.L., Kirkland D., Benson A., Sturge C.R., Mirpuri J., Vaishnava S., Hou B., Defranco A.L., Gilpin C.J., Hooper L.V., Yarovinsky F. Parasite-induced T H 1 cells and intestinal dysbiosis cooperate in IFN-γ-dependent elimination of Paneth cells. Nat. Immunol. 2013;14:136–142. doi: 10.1038/ni.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels G.J. Trichoderma: a review of biology and systematics of the genus. Mycol. Res. 1996;100:923–935. doi: 10.1016/S0953-7562(96)80043-8. [DOI] [Google Scholar]
- Schreiner M., Liesenfeld O. Small intestinal inflammation following oral infection with Toxoplasma gondii does not occur exclusively in C57BL/6 mice: review of 70 reports from the literature. Mem. Inst. Oswaldo Cruz. 2009;104:221–233. doi: 10.1590/S0074-02762009000200015. [DOI] [PubMed] [Google Scholar]
- Seeber F., Boothroyd J.C. Escherichia coli β-galactosidase as an in vitro and in vivo reporter enzyme and stable transfection marker in the intracellular protozoan parasite Toxoplasma gondii. Gene. 1996;169:39–45. doi: 10.1016/0378-1119(95)00786-5. [DOI] [PubMed] [Google Scholar]
- Sharif M., Sarvi S., Pagheh A.S., Asfaram S., Rahimi M.T., Mehrzadi S., Ahmadpour E., Gholami S., Daryani A. The efficacy of herbal medicines against Toxoplasma gondii during the last 3 decades: a systematic review. Can. J. Physiol. Pharmacol. 2016;94:1237–1248. doi: 10.1139/cjpp-2016-0039. [DOI] [PubMed] [Google Scholar]
- Shubar H.M., Lachenmaier S., Heimesaat M.M., Lohman U., Mauludin R., Mueller R.H., Fitzner R., Borner K., Liesenfeld O. SDS-coated atovaquone nanosuspensions show improved therapeutic efficacy against experimental acquired and reactivated toxoplasmosis by improving passage of gastrointestinal and blood–brain barriers. J. Drug Target. 2011;19:114–124. doi: 10.3109/10611861003733995. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Sher A., Yap G., Park D., Neyer L.E., Liesenfeld O., Fort M., Kang H., Gufwoli E. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J. Immunol. 2000;164:5375–5382. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- Teo C.F., Zhou X.W., Bogyo M., Carruthers V.B. Cysteine protease inhibitors block Toxoplasma gondii microneme secretion and cell invasion. Antimicrob. Agents Chemother. 2007;51:679–688. doi: 10.1128/AAC.01059-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadares D.G., Duarte M.C., Oliveira J.S., Chávez-Fumagalli M.A., Martins V.T., Costa L.E., Leite J.P.V., Santoro M.M., Régis W.C.B., Tavares C.A.P., Coelho E.A.F. Leishmanicidal activity of the Agaricus blazei Murill in different Leishmania species. Parasitol. Int. 2011;60:357–363. doi: 10.1016/j.parint.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Villeret B., Brault L., Couturier-Maillard A., Robinet P., Vasseur V., Secher T., Dimier-Poisson I., Jacobs M., S Zheng G., Quesniaux V.F., Ryffel B. Blockade of IL-1R signaling diminishes Paneth cell depletion and Toxoplasma gondii induced ileitis in mice. Am. J. Clin. Exp. Immunol. 2013;2:107. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.