Highlights
-
•
Disruption of the Mla system in B. pertussis results in growth defects.
-
•
Mutant in the Mla system is rescued by inactivation of pldA.
-
•
Phospholipids accumulate in the outer leaflet of the outer membrane of pldA mlaF double mutant.
-
•
Double mutants shows increased vesiculation.
-
•
Double mutant may be useful in the development of OMV-based vaccines.
Keywords: Bordetella pertussis, Phospholipid transport, Outer-membrane vesicles, Mla system, Outer-membrane phospholipase A, Biofilms
Abstract
Bordetella pertussis is the causative agent of a respiratory infection known as whooping cough. With the goal of improving the production of outer-membrane vesicles (OMVs), we studied here the mechanisms that are involved in maintaining lipid asymmetry in the outer membrane of this organism. We identified homologues of the phospholipid (PL)-transport systems Mla and Pqi and of outer-membrane phospholipase A (OMPLA). Inactivation of mlaF, encoding the ATPase of the Mla system, together with pldA, which encodes OMPLA, resulted in an accumulation of PLs at the cell surface as demonstrated by the binding of a phosphatidylethanolamine-specific fluorescent probe to intact cells of this strain. The corresponding single mutations did hardly or not affect binding of the probe. These results are consistent with a retrograde transport directionality of the Mla system in B. pertussis and indicate that PLs accumulating at the cell surface in the mlaF mutant are degraded by OMPLA. Consequently, the mlaF mutant showed a conditional growth defect due to the production of free fatty acids by OMPLA, which could be compensated by inactivation of OMPLA or by sequestration of the produced fatty acids with starch. The mlaF pldA double mutant showed markedly increased OMV production, and representative antigens were detected in these OMVs as in wild-type OMVs. Further phenotypic characterization showed that the barrier function of the outer membrane of the mlaF pldA mutant was compromised as manifested by increased susceptibility to SDS and to several antibiotics. Moreover, inactivation of mlaF alone or together with pldA resulted in increased biofilm formation, which was, however, not directly related to increased vesiculation as the addition of purified OMVs to the wild-type strain decreased biofilm formation. We conclude that the absence of MlaF together with OMPLA results in PL accumulation in the outer leaflet of the outer membrane, and the increased vesiculation of the mutant could be useful in the development of novel, OMV-based pertussis vaccines.
Graphical abstract
1. Introduction
The Gram-negative bacterium Bordetella pertussis is the causative agent of an acute respiratory disease known as whooping cough or pertussis. The first vaccine against pertussis, based on inactivated whole cells, was introduced in the 1940s (Mattoo and Cherry, 2005). Although effective, reactogenicity, mostly due to endotoxin (Geurtsen et al., 2006), resulted in decreased acceptance of this vaccine. Therefore, the whole-cell vaccines were replaced in the 1990s by acellular pertussis vaccines (Mattoo and Cherry, 2005). These acellular vaccines contain one to five purified antigens and are less reactogenic. Although vaccination rates in high-income countries are high, the number of pertussis cases is resurgent, due to, amongst others, rapid waning of vaccine-induced immunity and lack of protection against colonization by acellular pertussis vaccines (Esposito et al., 2019). Therefore, the development of third-generation pertussis vaccines is needed.
Outer-membrane vesicles (OMVs) are considered as promising novel tools in the development of vaccines against Gram-negative bacteria (Micoli and MacLennan, 2020). OMVs are small (10–300 nm) blebs of the outer membrane (OM) and have strong immunogenic properties (Ellis and Kuehn, 2010). OMVs isolated from B. pertussis have demonstrated great promise as safe and effective pertussis vaccines. Subcutaneously administered OMVs provided protection in mice characterized by a mixed T helper (Th) 1/Th2/Th17 response and broad antibody responses against multiple antigens (Raeven et al., 2016, 2015). Moreover, intranasal immunization evoked, besides systemic immune responses, also mucosal immunity that prevented colonization of the lungs and the nasal cavity after challenge (Raeven et al., 2020). Unfortunately, spontaneous OMV production by B. pertussis is relatively low, frustrating development of cost-effective OMV-based vaccines (Hozbor et al., 1999). Therefore, we are exploring ways to improve vesiculation in B. pertussis.
In Escherichia coli and other Gram-negative bacteria, the accumulation of phospholipids (PLs) in the outer leaflet of the OM was shown to increase OMV production (Jan, 2017; Roier et al., 2016). The OM is an asymmetric lipid bilayer. The outer leaflet is made up of lipopolysaccharides (LPS, a.k.a. endotoxin), while the inner leaflet consists of PLs. This asymmetry is important for the OM barrier function, which protects the bacteria against noxious compounds from the environment, such as antibiotics and bile salts (Silhavy et al., 2010). In E. coli, OM lipid asymmetry is maintained by the maintenance of lipid asymmetry (Mla) system (Malinverni and Silhavy, 2009). It is composed of six proteins, i.e. lipoprotein MlaA in the OM, periplasmic protein MlaC, and the inner membrane (IM) complex MlaFEDB. The Mla system transfers PLs that end up in the outer leaflet of the OM back to the IM (Malinverni and Silhavy, 2009; Powers and Trent, 2018), although this directionality of the transport system has recently been questioned (Ercan et al., 2019; Hughes et al., 2019; Kamischke et al., 2019). Inactivation of the Mla system results in the accumulation of PLs in the outer leaflet of the OM and was shown to lead to a hypervesiculating phenotype in Haemophilus influenzae, Vibrio cholerae, Neisseria gonorrhoeae, and Campylobacter jejuni (Baarda et al., 2019; Davies et al., 2019; Roier et al., 2016). In the proposed model, OMV formation is induced by increased curvature of the OM caused by the presence of PLs in the outer leaflet of the OM (Jan, 2017; Roier et al., 2016).
Besides the Mla system, other machinery plays a role in maintaining OM lipid asymmetry. The substrate-binding protein MlaD of the Mla system has a mammalian cell entry (MCE) domain, presumably involved in PL binding (Isom et al., 2017). In E. coli, two other proteins with MCE domains have been identified, i.e. PqiB and YebT, which are encoded by genes that are part of the pqiABC and the yebST operons, respectively (Ekiert et al., 2017; Nakayama and Zhang-Akiyama, 2017). Like MlaD, PqiB and YebT are able to bind PLs (Ekiert et al., 2017), and the Pqi and Yeb systems are important for OM integrity, particularly in an mla mutant background (Nakayama and Zhang-Akiyama, 2017), suggesting that the Pqi and Yeb systems are also PL transport systems. PLs in the outer leaflet of the OM can also be degraded by OM phospholipase A (OMPLA) (Dekker, 2000; Snijder et al., 1999) or by PagP (Bishop et al., 2000). OMPLA resides as inactive monomers in the OM. When PLs are present in the outer leaflet of the OM, OMPLA dimerizes and becomes active (Dekker et al., 1997; Rangl et al., 2017). Hydrolysis of the PLs results in the release of the fatty acids from both positions sn-1 and sn-2 (Rangl et al., 2017), thereby dismantling the PL. On the other hand, PagP transfers a palmitate from position sn-1 of a PL to the lipid A moiety of LPS, leaving a lyso-PL behind (Bishop et al., 2000). Together, these systems prevent accumulation of PLs in the outer leaflet of the OM in E. coli.
The machinery involved in maintaining OM lipid asymmetry has, so far, not been studied in B. pertussis. A pagP gene is present in this organism, but it is not expressed due to an insertion in the putative promoter region (Preston et al., 2003). In this study, we identified the genes for homologues of other proteins involved in maintaining OM lipid asymmetry in E. coli and inactivated these genes. The mutants were phenotypically characterized, and OMV production was evaluated.
2. Materials and methods
2.1. Bacterial strains and growth conditions
All bacterial strains used in this study are listed in Supplementary Table S1. B. pertussis and Bordetella bronchiseptica strains were grown on Bordet-Gengou (BG) agar (Difco) plates supplemented with 15% (v/v) defibrinated sheep blood (bioTRADING) at 35 °C. For liquid cultures, bacteria were scraped from plates and grown in Verwey medium (Verwey et al., 1949) or Stainer-Scholte (SS) medium (Stainer and Scholte, 1971) at 35 °C with shaking at 175 rpm. Where indicated, SS medium was supplemented with 1 g/L of starch. E. coli strains were grown at 37 °C on lysogeny broth (LB) agar plates or in liquid LB medium while shaking at 200 rpm. When appropriate, media were supplemented with antibiotics: 100 µg/mL ampicillin, 10 µg/mL gentamicin, 300 µg/mL streptomycin, 50 µg/mL nalidixic acid, or 5 µg/mL cefotaxime.
2.2. Construction of mutants
All primers and plasmids used in this study are listed in Supplementary Tables S2 and S3, respectively. To construct mutant strains, regions upstream of the targeted open reading frames were amplified by polymerase chain reaction (PCR) using primer pairs mlaF_up_Fw/mlaF_up_Rev, pldA_up_Fw/pldA_up_Rev, and pqiAB_up_Fw/pqiAB_up_Rev with the Expand High Fidelity PCR system (Roche) with genomic DNA of B. pertussis strain B213 as template. Similarly, downstream regions were amplified using primer pairs mlaF_down_Fw/mlaF_down_Rev, pldA_down_Fw/pldA_down_Rev, and pqiAB_down_Fw/pqiAB_down_Rev. All amplicons were ligated into pCRII, and the ligation mixtures were used to transform E. coli strain DH5α. The resulting plasmids were digested with XbaI and either SalI for plasmids containing mlaF- or pldA-flanking fragments or Eco81I for plasmids containing pqiAB-flanking fragments, and up- and downstream fragments corresponding to each target were joined together in one plasmid. A gentamicin-resistance cassette was amplified from plasmid pYRC by PCR using primer pairs Gm_Fw_S/Gm_Rev_S or Gm_Fw_E/Gm_Rev_E. The obtained gentamicin-resistance cassette was inserted in between up- and downstream regions of target genes by either SalI or Eco81I restriction digestion followed by ligation. The resulting mlaF and pqiAB knockout constructs were cleaved from their pCRII backbone with XbaI and SacI and ligated into suicide plasmid pKAS32, digested with the same restriction enzymes. The pldA knockout construct was cleaved from the pCRII vector with EcoRI and ligated into EcoRI-digested pKAS32. E. coli strain SM10λpir was transformed with pKAS32-mlaFKO, pKAS32-pldAKO or pKAS32-pqiABKO, and the strains obtained were used to transfer the knockout constructs via conjugation to B. pertussis strain B213 or B. bronchiseptica strain BB-D09-SR to inactivate the target genes by allelic exchange (Skorupski and Taylor, 1996). To construct the double mutant B213 ΔmlaF ΔpldA, pKAS32-pldAKO was conjugated to B213 ΔmlaF. Single crossovers were selected by using ampicillin or gentamicin for selection and nalidixic acid (B. pertussis) or cefotaxime (B. bronchiseptica) for counterselection against E. coli. After correct integration of the plasmid, double crossovers were selected by using gentamicin and streptomycin. Transconjugants were screened by PCR.
2.3. Detection of cell-surface-exposed phosphatidylethanolamine (PE)
Surface-exposed PE was detected using a large, membrane-impermeable, PE-specific fluorescent probe. The probe was prepared as described previously (Lin et al., 2018). Briefly, cinnamycin (BioAustralis) was coupled to FITC-PEG5000-NHS (PG2-FCNS-5k, Nanocs) by mixing them in a molar ratio of 1:2 in 50 mM NaHCO3 (pH 8.5) for 3–4 h at room temperature, after which the reaction was continued overnight at 4 °C. The conjugation process was stopped by the addition of 1 M lysine. Bacterial cells were grown in Verwey medium, washed with phosphate-buffered saline (PBS), and resuspended to an optical density at 600 nm (OD600) of 1 in PBS. Then, 500 µL of cells were incubated with 2 µM PE probe for 30 min at 35 °C. After three washes with PBS, binding of the probe to the cells was measured as fluorescence intensity at 485 nm (excitation) and 528 nm (emission).
2.4. Sensitivity to sodium dodecyl sulfate (SDS)
SDS sensitivity was determined as previously described (Lo Sciuto et al., 2014). Briefly, bacterial cells were grown in Verwey medium, washed with physiological salt solution, and resuspended to an OD600 of 1. Then, 0.5 mL of the bacterial suspension was mixed with 0.5 mL of physiological salt solution containing 0, 0.01 or 0.025% SDS. After 5 min incubation at room temperature, the OD600 was measured to determine cell lysis induced by SDS.
2.5. Sensitivity to antibiotics
Antibiotic susceptibility was determined by Etest. Bacteria were scraped from plates and suspended in physiological salt solution, washed, and resuspended to an OD600 of 0.8. Samples of 100 or 200 µL bacterial suspension were spread on 100 mm round or 120 mm square BG agar plates, respectively. The plates were dried for 30 min, and Etest strips (bioMérieux) were placed on the plates. MICs were determined after incubation for 3 days at 35 °C.
2.6. OMV isolation
OMVs were isolated as previously described (de Jonge et al., 2021). Briefly, precultures grown in Verwey medium without antibiotics were diluted to an OD600 of 0.05 in fresh medium. For the isolation of OMVs used in biofilm experiments, Verwey medium without starch but supplemented with 1 g/L of heptakis(2,6-di-O-methyl)-β-cyclodextrin (Sigma-Aldrich) was used. The cultures were grown for one day with an air:liquid ratio of 3.33:1 in conical tubes or for two days in Erlenmeyer flasks with an air:liquid ratio of 5:1. Bacterial cells were pelleted by centrifugation at 5000 x g for 10 min, and supernatants were passed through a 0.45-µm pore-size filter (Sarstedt). OMVs were pelleted by ultracentrifugation for 2 h at 40,000 rpm and 4 °C (Beckman Coulter Optima LE-80 K, Type 70 Ti rotor) and resuspended in PBS. OMVs were quantified based on protein content using a Lowry DC protein assay (Bio-Rad) according to the manufacturer's instructions.
2.7. SDS-polyacrylamide gel electrophoresis (PAGE) and Western blot analysis
Isolated OMVs or whole cells were mixed with sample buffer (Laemmli, 1970), and samples were boiled for 5 min. For analysis of LPS, whole-cell lysates were treated with 50 µg/mL of proteinase K (ThermoFisher) for 1 h at 65 °C before electrophoresis. Protein patterns and LPS were analyzed on SDS-polyacrylamide gels containing 14% acrylamide. After electrophoresis, proteins and LPS were stained with Bradford reagent (Bos et al., 2015) and silver (Tsai and Frasch, 1982), respectively. Alternatively, proteins were transferred to a 0.45-μm pore-size nitrocellulose membrane (GE Healthcare). Membranes were then incubated with primary antisera directed against BrkA, FauA, OmpP, or FtsH (de Jonge et al., 2021), and subsequently with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG antisera (ThermoFisher). Antibody binding was visualized by using Clarity Western ECL Blotting Substrate (Bio-Rad).
2.8. Biofilm formation
Biofilm formation was studied as described previously (Pérez-Ortega et al., 2021) with slight modifications. Briefly, bacteria were collected from plates and suspended in Verwey medium. Cells were washed and resuspended to an OD600 of 0.1. Samples of 0.5 mL were added to 24-wells plates, either supplemented with isolated OMVs or not, and incubated at 35 °C in static conditions. After 72 h, supernatants were discarded, and wells were washed with 1 mL of physiological salt solution. Biofilms were stained for 2 min with 0.5 mL of 0.5% crystal violet in 20% methanol, washed twice and solubilized with 0.75 mL of 33% acetic acid. Biofilms were quantified by measuring the OD595.
3. Results
3.1. Homologues of proteins involved in maintaining OM lipid asymmetry in B. pertussis
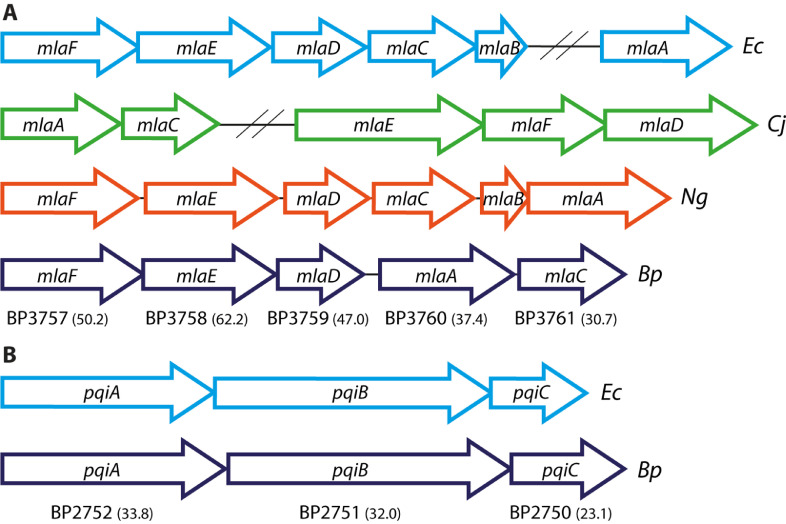
Using the sequences of E. coli proteins known to be involved in maintaining OM lipid asymmetry as probes, we first searched for homologues in B. pertussis with BLASTp. Homologues of components of the Mla and Pqi systems, but not of the Yeb system, could be identified (Fig. 1). All mla genes found are adjacent to each other and probably form an operon, but an MlaB homologue was not found (Fig. 1A). The most conserved proteins of the Mla system are MlaF, MlaE, and MlaD with 50.2, 62.2, and 47.0% amino-acid sequence identity to the E. coli proteins (Fig. 1A), respectively; they are all components of the IM complex. A PqiC homologue was not identified in the BLASTp search; however, the protein encoded by the gene downstream of pqiA and pqiB showed 23.1% identity to E. coli PqiC (Fig. 1B). Furthermore, a homologue of the PL-degrading enzyme OMPLA (locus tag BP0878) was found, with 28.6% identity to OMPLA of E. coli.
Fig. 1.
Genetic organization of the Mla and Pqi systems in B. pertussis and other gram-negative bacteria. Each open reading frame encoding a component of the Mla (A) or Pqi (B) systems is depicted as an arrow with its length being proportional to the gene size. The locus tags of B. pertussis genes are depicted below the arrows and the percentage of amino-acid identity to the corresponding E. coli proteins is indicated in brackets. Non-adjacent genes are separated by two slashes. Ec, Escherichia coli; Cj, Campylobacter jejuni; Ng, Neisseria gonorrhoeae; Bp, Bordetella pertussis.
3.2. Disruption of OM lipid asymmetry
To determine if OMPLA, the Mla system, and/or the Pqi system are involved in maintaining lipid asymmetry in the OM of B. pertussis, we inactivated the pldA gene, which encodes OMPLA, the mlaF gene, which encodes the ATPase that energizes the Mla system, and the pqiAB genes in strain B213. The pldA gene is present in a monocistronic operon. The downstream-located gene with locus tag BP0877, encoding a homolog of the flagellar brake protein YcgR, is oppositely oriented relative to pldA. Downstream of the mla genes and separated from them by an intergenic region potentially containing a promoter are two genes for an ABC-2 transporter. The first of these genes, encoding the ATPase of the transporter, however, is a pseudogene (locus tag BP3762/3763), disrupted by a frameshift mutation; hence, no functional transporter can be produced from these genes. Downstream of the pqi genes and separated from them by an intergenic region, which could contain a promoter sequence, is a gene (locus tag BP2749) encoding a PutA homolog involved in proline utilization. Considering the genetic context of the target genes, polar effects of the constructed mutations on downstream genes are not to be expected.
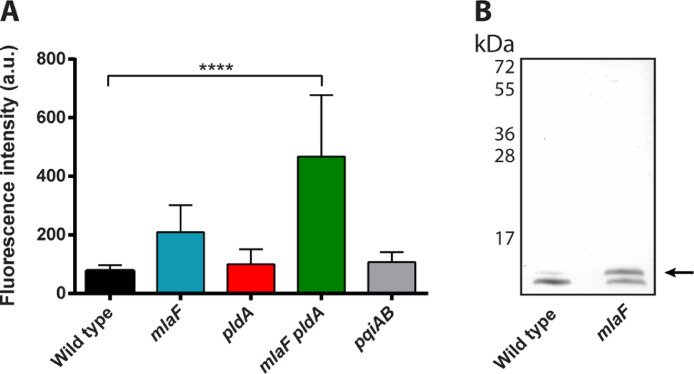
Although mlaF and other mla genes were identified as essential genes in B. pertussis (Belcher et al., 2020; Gonyar et al., 2019), we were able to construct a knockout mutant in strain B213. We also constructed an mlaF pldA double mutant, since inactivation of either one of them could potentially be compensated by activation of the other protein. To investigate if the lipid asymmetry in the OM is disrupted in our mutants, we assessed the presence of PLs at the cell surface by studying the binding of a large, membrane-impermeable, PE-specific fluorescent probe to intact cells. Binding of the PE probe was significantly increased in the mlaF pldA double mutant (Fig. 2A). Increased binding was also consistently observed in the mlaF single mutant, but the difference relative to the wild type was not statistically significant (p = 0.19). Binding of the probe was not affected by the pldA mutation alone or by the pqiAB mutation (Fig. 2A).
Fig. 2.
Disruption of OM lipid asymmetry. (A) The presence of PE at the cell surface was measured as fluorescence intensity after binding of a PE-specific fluorescent probe to intact B. pertussis cells. Mean values with standard deviations of five independent experiments are shown. Significant differences were determined by one-way ANOVA followed by Dunnett's multiple comparisons test using GraphPad Prism 6 and are indicated by asterisks (****, p < 0.0001). (B) Proteinase K-treated whole-cell lysates of B. bronchiseptica strain BB-D09-SR and its mlaF mutant derivative were analyzed by SDS-PAGE, and the LPS was stained with silver. Palmitoylated LPS (lipid A plus core moiety) is indicated with an arrow. Molecular weight markers are indicated on the left.
An alternative, yet indirect approach to demonstrate the accumulation of PLs in the outer leaflet of the OM is to determine the PagP-mediated palmitoylation of LPS (Malinverni and Silhavy, 2009). As the active site of PagP is in the outer leaflet of the OM, PagP can only transfer a palmitoyl chain from PL to LPS if the substrate is available there. Since pagP is not expressed in B. pertussis (Preston et al., 2003), we switched to B. bronchiseptica to apply this approach. Previous mass-spectrometry analysis showed that about 10% of the LPS is palmitoylated in wild-type B. bronchiseptica, and we have demonstrated that palmitoylation can be monitored by SDS-PAGE (Pérez-Ortega et al., 2021). After introducing the mlaF mutation in B. bronchiseptica strain BB-D09-SR, the LPS content in whole cells was analyzed by SDS-PAGE, and the results indicated a drastically increased amount of palmitoylated LPS in the mlaF mutant (Fig. 2B). Together, these results demonstrate the accumulation of PLs in the outer leaflet of the OM of the mlaF pldA double mutant of B. pertussis and in the mlaF mutant of B. bronchiseptica.
3.3. Growth characteristics of mutants with disturbed lipid asymmetry
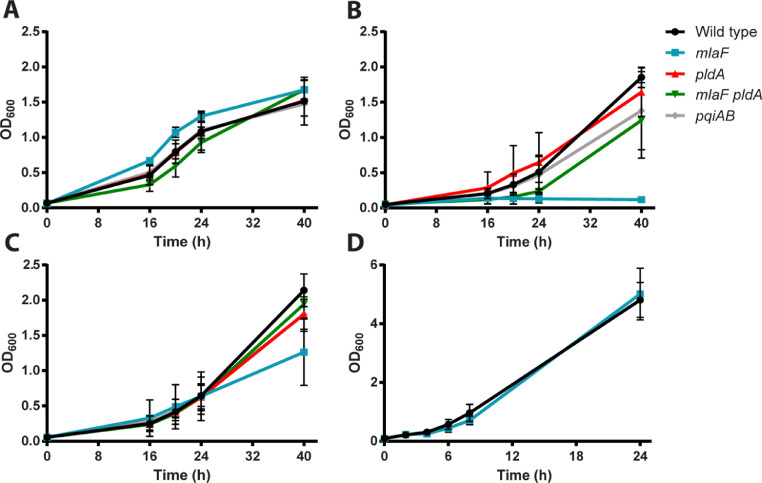
Disruption of lipid asymmetry may impact growth kinetics. To assess growth, strain B213 and its mutant derivatives were incubated in liquid medium, and the OD600 was measured in time. All strains grew similarly in Verwey medium (Fig. 3A). In contrast, growth of the mlaF single mutant, but not that of the mlaF pldA double mutant, was greatly impaired in SS medium (Fig. 3B). During growth, B. pertussis produces fatty acids, which are released into the medium and inhibit the growth of the bacteria (Frohlich et al., 1996). As growth of the mlaF pldA double mutant was not inhibited (Fig. 3B), the growth of the mlaF single mutant may be inhibited by fatty acids produced due to activation of OMPLA. One of the differences between Verwey medium and SS medium is the presence in the former of starch, which is known to adsorb free fatty acids (Schoch and Williams, 1944). To test whether starch rescues the growth of the mlaF mutant, growth was also assessed in SS medium supplemented with starch. The presence of starch indeed restored growth of the mlaF mutant in SS medium (Fig. 3C). To test the idea that the growth defect of the mlaF mutant in SS medium is due to the production of fatty acids further, we also determined growth of wild-type B. bronchiseptica strain BB-D09-SR and its mlaF mutant derivative in SS medium. In contrast to B. pertussis, growth of B. bronchiseptica is not inhibited by the presence of free fatty acids in the medium because of the presence of a functional efflux pump in this bacterium (MacArthur et al., 2019). Indeed, growth of the B. bronchiseptica mlaF mutant was not inhibited in SS medium (Fig. 3D). Thus, inactivation of the Mla system results in a growth defect due to activation of OMPLA, which can be compensated by (i) inactivation of OMPLA, (ii) sequestration of the free fatty acids produced by OMPLA activity, or (iii) efflux of the fatty acids by a functional efflux pump.
Fig. 3.
Growth curves of B. pertussis strain B213 and B. bronchiseptica strain BB-D09-SR and their mutant derivatives. B. pertussis strains (A–C) and B. bronchiseptica strains (D) were grown in Verwey medium (A) or SS medium (B–D). In panel (C), the SS medium was supplemented with starch. Growth was monitored by measuring the OD600. Graphs shown represent mean values with standard deviations of three independent experiments.
3.4. Inactivation of mlaF together with pldA results in hypervesiculation
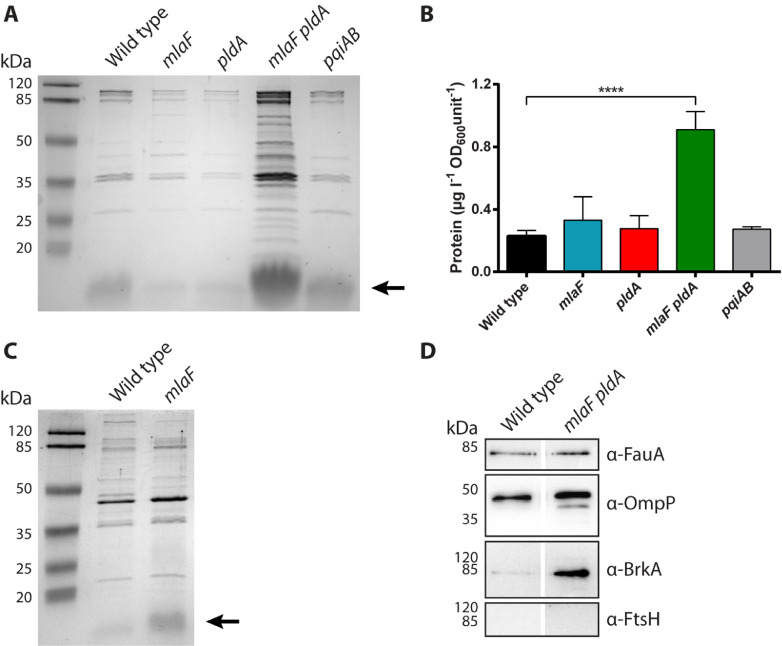
To determine if disruption of lipid asymmetry in the OM improves OMV production, OMVs were isolated from the culture medium of wild-type strain B213 and the constructed mutant derivatives and analyzed by SDS-PAGE. Initial results from bacteria grown in conical tubes indicated increased OMV release by the mlaF single and the mlaF pldA double mutant (Supplementary Fig. S1). However, OMV release under these conditions is still rather low and unsuitable for large-scale production. Previous results showed enhanced release of OMVs by B. pertussis and B. bronchiseptica after growth for two days in Erlenmeyer flasks with an air:liquid ratio of 5:1 (de Jonge et al., 2021; Gasperini et al., 2017). OMVs isolated from bacteria grown under these conditions were analyzed by SDS-PAGE. OMV production was considerably increased in the mlaF pldA double mutant as can be deduced from the increased intensity of protein bands on the gels (Fig. 4A). Similarly, the amount of LPS, which can also be detected on the gels, was increased (Fig. 4A). Quantification of the protein content indicated a ∼4-fold increase in OMV production in the double mutant compared to the wild type (Fig. 4B). Under these conditions, inactivation of mlaF, pldA, or pqiAB alone did not significantly influence vesiculation (Fig. 4A,B). OMV production by B. bronchiseptica BB-D09-SR and its mlaF mutant derivative was also analyzed by SDS-PAGE and appeared to be comparable in both strains (Fig. 4C).
Fig. 4.
Influence of disrupted lipid asymmetry on OMV production. (A) B. pertussis strain B213 and its mutant derivatives were grown for two days in Verwey medium in Erlenmeyer flasks after which OMVs were isolated. OMVs released from equal amounts of cells, based on OD600, were analyzed by SDS-PAGE. Proteins were stained with the Bradford reagent. The reagent also stains LPS, which is indicated with an arrow on the right. (B) Isolated OMVs were quantified based on protein content using a Lowry assay, and quantities are expressed as amount of protein per liter of bacterial culture per OD600 unit. Graph shows mean values with standard deviations from three independent experiments. Statistical significance was determined by one-way ANOVA followed by Dunnett's multiple comparisons test using GraphPad Prism 6 and is indicated by asterisks (****, p < 0.0001). (C) OMV production by B. bronchiseptica strain BB-D09-SR and its mlaF mutant derivative was assessed by SDS-PAGE as described for panel A. LPS (lipid A plus core moiety) is indicated with an arrow. (D) OMVs from B. pertussis strain B213 and its mlaF pldA mutant derivative were equally loaded, based on protein content, and analyzed by Western blotting using antisera directed against OMPs FauA (78 kDa), OmpP (39 kDa), BrkA (73 kDa), and inner-membrane protein FtsH (69 kDa) as indicated at the right. In panels A, C, and D, the positions of molecular weight standard proteins are indicated at the left.
Important for potential application of OMVs from the hypervesiculating mlaF pldA double mutant as a vaccine is the presence of immunogenic OM proteins (OMPs) in these preparations. To determine if the mutations affected the presence of OMPs, OMV preparations of the wild-type strain and the mlaF pldA mutant were loaded equally (based on protein concentration) on gel and analyzed for the presence of several representative OMPs by Western blotting. Autotransporter BrkA, siderophore receptor FauA, and major porin OmpP, which are all considered relevant vaccine antigens (Afonina et al., 2006; Alteri et al., 2009; Brickman and Armstrong, 1999; Hu et al., 2012; Marr et al., 2008; Zhang et al., 2019), were detectable in whole-cell lysates of the wild type (Supplementary Fig. S2) and in OMVs from both strains (Fig. 4D). We also analyzed the presence of inner-membrane protein FtsH, which should be absent in OMVs. Indeed, whilst FtsH could readily be detected in whole-cell lysates (Supplementary Fig. S2), we could not detect FtsH in the OMV preparations (Fig. 4D), indicating that the isolated OMVs are not contaminated with membrane material resulting from of cell lysis.
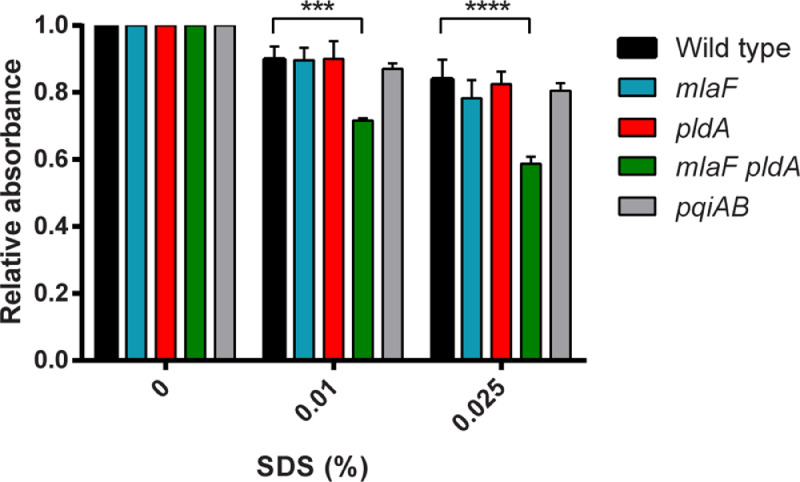
3.5. Disrupted lipid asymmetry results in loss of the OM barrier function
Next, we set out to characterize the phenotypes of the constructed mutants further. First, we wished to determine if the barrier function of the OM is affected by the mutations. The accumulation of PLs in the outer leaflet of the OM results in the presence of patches of PL bilayer, which is expected to increase the susceptibility of the cells to detergents and hydrophobic antibiotics. To assess the sensitivity of the mutants to detergents, whole-cell suspensions were incubated with various concentrations of SDS, and cell lysis was determined by measuring the OD600. Some lysis was already observed after exposure of the wild-type strain to concentrations of SDS as low as 0.01% (Fig. 5). Whereas the susceptibility of the single mutants to SDS was unaffected, the mlaF pldA double mutant showed increased lysis after incubation with SDS (Fig. 5). The susceptibility of the strains to antibiotics was assessed using Etest. The susceptibility of the mlaF pldA double mutant to rifampicin, which diffuses through the OM via PL bilayer regions (Nikaido, 1976), was drastically increased (Table 1) consistent with the disrupted lipid asymmetry in this strain. Its susceptibility to daptomycin and vancomycin was also increased compared to the wild-type strain with a more than 20-fold reduction in minimal inhibitory concentration (MIC) (Table 1). This demonstrates that the barrier function of the OM is defective, since daptomycin and vancomycin are normally not active against Gram-negative bacteria because they cannot pass the OM (Kohanski et al., 2010; Miller et al., 2016). The MIC of the mlaF pldA double mutant to ciprofloxacin, which enters the cells via porins (Hooper et al., 1987), was only marginally increased. Inactivation of mlaF alone also resulted in a slight increase in susceptibility to rifampicin and vancomycin, indicating that this mutation alone already affected the barrier function of the OM. In contrast, the pldA and pqiAB mutants were equally sensitive as the wild-type strain to all antibiotics tested (Table 1).
Fig. 5.
Cell lysis induced by SDS. Suspensions of B. pertussis strain B213 and its mutant derivatives were incubated for 5 min with different concentrations of SDS after which the OD600 was measured. Values were normalized to OD600 measurements after incubation with physiological salt solution. Mean values with standard deviations of three independent experiments are shown. Significant differences were determined by one-way ANOVA followed by Dunnett's multiple comparisons test using GraphPad Prism 6 and are indicated by asterisks (***, p < 0.001; ****, p < 0.0001).
Table 1.
Susceptibility of B. pertussis strain B213 and its mutant derivatives to antibioticsa.
| Wild type | ΔmlaF | ΔpldA | ΔmlaF ΔpldA | ΔpqiAB | |
|---|---|---|---|---|---|
| Ciprofloxacin | 0.25 | 0.125 – 0.19 | 0.25 | 0.094 – 0.125 | 0.19 – 0.25 |
| Rifampicin | 0.19 – 0.25 | 0.094 – 0.125 | 0.19 | 0.004 – 0.012 | 0.19 – 0.25 |
| Daptomycin | >256 | >256 | >256 | 1.5 – 12 | >256 |
| Vancomycin | >256 | 96 - 192 | >256 | 4 – 12 | >256 |
Values indicate the range of MIC values (µg/mL) as determined by Etest. MIC values were determined in three independent experiments.
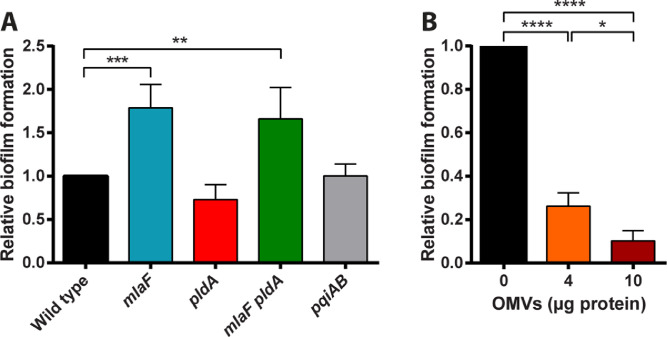
3.6. Inactivation of mlaF enhances biofilm formation
One of the many functions ascribed to OMVs is to facilitate biofilm formation (Schwechheimer and Kuehn, 2015), as has been demonstrated in Pseudomonas aeruginosa, Pseudomonas putida, and Helicobacter pylori (Schooling and Beveridge, 2006; Yonezawa et al., 2009). Therefore, it was of interest to investigate if the increased OMV production observed in the mlaF pldA double mutant increases biofilm formation. To test this possibility, wild-type strain B213 and its mutant derivatives were incubated for 72 h in static conditions after which the biofilms formed were quantified. Biofilm formation was significantly increased in the mlaF pldA mutant strain (Fig. 6A), suggesting that, indeed, the increased OMV production by this strain stimulates biofilm formation. Also the mlaF single mutant produced more biofilm (Fig. 6A), although OMV production in this strain appeared unaffected (Fig. 4A,B). It should be noticed, however, that OMV production in this strain was variable and depended on the culture conditions (compare results in Fig. 4A,B and Fig. S1). Hence, it is possible that this strain produced more OMVs than the wild type under the conditions of the biofilm assay. To directly investigate the influence of OMVs on biofilm formation, OMVs were isolated from the wild-type strain and added to the cells in the biofilm assay. The OMVs were isolated after growth of the strain in Verwey medium without starch to exclude any effect of starch, which pellets together with OMVs during ultracentrifugation. Isolated OMVs were added to wild-type cells and biofilms were allowed to form. Unexpectedly, the addition of OMVs reduced, rather than stimulated biofilm formation in a dose-dependent fashion (Fig. 6B), which indicates an inhibitory effect of OMVs on biofilm formation in B. pertussis. Thus, although biofilm formation is increased in the mlaF and mlaF pldA mutants (Fig. 6A), this phenotype appears unrelated to the increased OMV production by these strains. Interestingly, biofilm formation of the pldA mutant was lower than that of the wild type in each individual experiment, although the difference was statistically not significant in the combined results (p = 0.3) (Fig. 6A).
Fig. 6.
Biofilm formation. (A) B. pertussis strain B213 and its mutant derivatives were grown for 72 h in static conditions to allow for biofilm formation. Biofilms were stained with crystal violet and quantified by measuring the OD595. (B) Cultures of the wild-type B. pertussis strain B213 were supplemented with different amounts of isolated OMVs and biofilm formation was determined as in panel (A). Graphs show mean values and standard deviations of four (A) or three (B) independent experiments relative to biofilm formation by the wild-type strain (without OMV supplementation), which was set at 1. Significant differences were determined by one-way ANOVA followed by Dunnett's (A) or Tukey's (B) multiple comparisons test using GraphPad Prism 6 and are indicated by asterisks (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001).
4. Discussion
In this study, we investigated the molecular systems that are potentially involved in maintaining lipid asymmetry in the OM of B. pertussis. We identified homologues of the PL-transport systems Mla and Pqi and of the phospholipase OMPLA. The genetic organization of the mla operon differs between bacterial species (Fig. 1A). In B. pertussis, mlaA is present as a part of the mla operon, where it is located between mlaD and mlaC. Likewise, mlaA is present in the mla operon of N. gonorrhoeae, where it is the last gene of the operon. In contrast, mlaA is not part of the operon in E. coli, and in C. jejuni mlaA and mlaC are encoded in a chromosomal region separate from mlaEFD (Fig. 1A). Most noteworthy, we were not able to identify an MlaB homologue in B. pertussis (a β-proteobacterium). Similarly, MlaB is not present in the Mla systems of C. jejuni (Fig. 1A), H. pylori (both ε-proteobacteria), and Caulobacter crescentus (an α-proteobacterium) (Roier et al., 2016; Davies et al., 2019), but it is present in Neisseria meningitidis and N. gonorrhoeae (Fig. 1A) (both β-proteobacteria). MlaB is a cytoplasmic protein, and it is part of the IM complex MlaFEDB. In E. coli, MlaB was shown to be important for the stability of MlaF. Lack of MlaB could be compensated by the overproduction of MlaF, indicating that MlaB does not play an essential direct role in the PL transport process (Kolich et al., 2020). Perhaps, MlaF of B. pertussis and other bacteria without an MlaB homologue is intrinsically more stable than that of E. coli and can be assembled into the MlaFED complex without assistance of an MlaB homologue.
The genes for the Mla system, BP3757-BP3761, were previously identified as essential for the in vitro and in vivo growth of B. pertussis (Belcher et al., 2020; Gonyar et al., 2019). Nevertheless, we succeeded to create a viable mlaF mutant. This discrepancy can be explained by the observed dependence of growth of the mlaF mutant on the medium used, as growth in the commonly used SS medium was very poor (Fig. 3B). This growth defect is exceptional as no growth defect of any mla mutant in any bacterium has been reported so far (Powers et al., 2020 and references therein). The growth defect of the mlaF mutant could be suppressed by introducing a pldA mutation or by supplementing the medium with starch. OMPLA, which is encoded by the pldA gene, produces fatty acids when activated, whilst starch adsorbs them (Schoch and Williams, 1944). B. pertussis is known to be very sensitive to fatty acids (Imaizumi et al., 1983), and it was recently demonstrated that this is due to a defect in the AcrAB efflux system for hydrophobic compounds (MacArthur et al., 2019). Thus, we postulate that the poor growth of the mlaF mutant in SS medium is due to the toxicity of fatty acids produced by activated OMPLA. Consistently, an mlaF mutant of B. bronchiseptica, which does produce an active AcrAB efflux pump (MacArthur et al., 2019), was not inhibited in SS medium. It has also been reported previously that the growth of wild-type B. pertussis in commonly used media is limited because these bacteria release fatty acids into the culture medium during growth (Frohlich et al., 1996), which is somewhat enigmatic considering their defective efflux pump for fatty acids (MacArthur et al., 2019). Thus, we hypothesize that also those results could be explained by OMPLA producing fatty acids in the OM.
The directionality of the Mla system has been under discussion. In E. coli, the system was originally described as having a retrograde directionality (Malinverni and Silhavy, 2009). The evidence for this directionality was somewhat indirect, i.e. the demonstration of PagP-mediated palmitoylation of LPS in an mla mutant, which is dependent on the presence of PL in the outer leaflet of the OM. Later in vitro studies, however, showed the transfer of PLs from the IM component MlaD to the periplasmic component MlaC, suggesting anterograde PL trafficking (Ercan et al., 2019; Hughes et al., 2019). Anterograde directionality was further supported by the accumulation of newly synthesized PLs in the IM of an mlaC mutant of Acinetobacter baumannii (Kamischke et al., 2019), but these results have later been disputed (Powers et al., 2020). As a pagP gene is present but not expressed in B. pertussis (Preston et al., 2003), we could use LPS palmitoylation as a criterion to assess the presence of PLs in the outer leaflet of the OM only in B. bronchiseptica, where an increased abundance of palmitoylated LPS was indeed detected in the mlaF mutant (Fig. 2B). For B. pertussis, we used a direct approach to assess the presence of PLs at the cell surface of intact cells by using a large, membrane-impermeable, PE-specific probe. Whilst a slight increase in the binding of the probe was already observed in the mlaF single mutant, a drastic and statistically significant increase was observed in the mlaF pldA double mutant (Fig. 2A). As the pldA mutation alone did not affect binding of the probe, these results demonstrate that the Mla system in B. pertussis transports PLs in a retrograde direction. This result is consistent with the results of a recently developed in vitro assay, which demonstrated the transfer of PLs from proteoliposomes containing MlaA and OmpF of E. coli to proteoliposomes containing the MlaFEDB complex in the presence of soluble MlaC provided that ATP was available (Tang et al., 2021).
Disruption of the lipid asymmetry in the OM is expected to affect the barrier function of this membrane. As PLs and LPS do not mix, PLs in the outer leaflet of the OM will segregate into patches leading to the formation of PL bilayer domains (Nikaido, 2005). This allows lipophilic toxic molecules, such as SDS and rifampicin, to enter the cells by diffusion through these patches. Remarkably, wild-type B. pertussis was already very sensitive to SDS. Even when exposed to concentrations of SDS as low as 0.01%, some lysis of the cells was observed (Fig. 5). For comparison, incubation of wild-type E. coli and P. aeruginosa strains with up to 0.5% SDS did not result in any significant lysis (Fernández-Piñar et al., 2015; Lo Sciuto et al., 2014; Malinverni and Silhavy, 2009). The high sensitivity of B. pertussis to SDS is probably related to the disrupted AcrAB efflux pump (MacArthur et al., 2019) as discussed above. Cells lacking both mlaF and pldA were significantly more sensitive to SDS than the wild type. As expected, they also showed a drastic increase in susceptibility to the lipophilic antibiotic rifampicin (Table 1). In addition, high susceptibility to vancomycin and daptomycin, which are hydrophilic antibiotics that are too large to pass the OM by diffusion through the porins, was observed. This can be explained by the hydrophobic mismatch between LPS and PLs resulting in transient “cracks” in the OM at the boundaries between PL domains and LPS allowing for the passage of large hydrophilic molecules (Nikaido, 2005; Ruiz et al., 2006). In accordance with our results in B. pertussis, inactivation of the Mla system in E. coli led to disruption of the OM barrier function as evidenced by sensitivity of the mutants to SDS in combination with EDTA, whereas pldA inactivation did not have such an effect (Malinverni and Silhavy, 2009). Furthermore, the phenotype of the mlaC mutant used in that study was aggravated in combination with a pldA mutation and could be suppressed by OMPLA overproduction, suggesting that the Mla system is the key player in removing PLs from the outer leaflet of the OM. Inactivation of the Mla system then leads to PL accumulation and activation of OMPLA. Also in A. baumannii, disruption of the Mla system resulted in increased OM permeability (Kamischke et al., 2019), whereas in C. jejuni, it did not (Davies et al., 2019).
The Pqi system has not been studied in great detail in any organism. However, studies in E. coli suggest its involvement in PL transport since PLs were co-purified with PqiB (Ekiert et al., 2017; Isom et al., 2017). In our assays, we could not detect any phenotype of the B. pertussis pqiAB mutant. The absence of a growth defect indicates that no PLs are degraded by OMPLA after reaching the outer leaflet of the OM of the pqiAB mutant. Thus, the Mla system appears to be the main system involved in maintaining OM lipid asymmetry in B. pertussis.
In contrast to the constructed pqiAB and pldA mutants, the mlaF mutant showed increased vesiculation (Fig. S1), consistent with results described for mla mutants in several other bacterial species (Baarda et al., 2019; Davies et al., 2019; Roier et al., 2016). The hypervesiculating phenotype of the mlaF mutant, however, could not be reproduced under large-scale OMV production conditions needed for vaccine development (Fig. 4A,B). This discrepancy could be related to the differences in aeration between the small-scale and large-scale conditions. However, a robust increase in OMV production in large-scale conditions was observed in the mlaF pldA double mutant (Fig. 4A,B). The strong synergistic effect of the mlaF and pldA mutations is consistent with the notion that the two systems inactivated are independently involved in the same process, i.e. the removal of PLs from the outer leaflet of the OM. Previously, it has been proposed that OMV formation is initiated by budding of the OM due to accumulation of PLs in the outer leaflet of the OM (Roier et al., 2016). The direct demonstration of PLs present in the outer leaflet of the OM of the B. pertussis mlaF pldA mutant, together with its hypervesiculating phenotype, is consistent with this model.
Several studies suggested an involvement of OMVs in biofilm formation (Schooling and Beveridge, 2006; Yonezawa et al., 2009). Therefore, we were interested to determine whether the hypervesiculating B. pertussis mutants showed increased biofilm formation. Indeed, the mlaF single and mlaF pldA double mutants showed increased biofilm formation (Fig. 6A). However, this phenotype could not be correlated to the increased OMV production of these strains since the addition of purified OMVs to wild-type B. pertussis cells inhibited, rather than increased biofilm formation (Fig. 6B). It remains to be investigated what property stimulates biofilm formation in these mutant strains.
This study was undertaken to explore the possibility of increasing OMV production in B. pertussis by disrupting lipid asymmetry in the OM. In previous studies, we have described alternative methods to increase OMV production in bordetellae, i.e. by applying a heat shock to wild-type cells (de Jonge et al., 2021) or by depleting the cells of the peptidoglycan-associated lipoprotein Pal (de Jonge et al., 2022). We have shown that OMVs obtained after heat shock contain similar protein patterns as spontaneously released OMVs. Nevertheless, we cannot exclude the possibility that the heat shock altered the tertiary structure of these OMPs. Moreover, the heat shock could change the OM lipid composition as illustrated by the drastic increase in lysophospholipid content in OMVs of B. bronchiseptica exposed to heat shock, which was shown to be caused by the activation of OMPLA (Balhuizen et al., 2021) and PagP (Pérez-Ortega et al., 2021). These changes could affect the immunogenicity of the OMVs. Pal is an essential lipoprotein in bordetellae (de Jonge et al., 2022). By placing the pal gene under control of an IPTG-inducible promoter, it was possible to deplete the cells of Pal, which resulted in increased OMV production. The protein profile of these OMVs was deviant from that of spontaneoulsly released OMVs and was more similar to that of isolated OMs of the bacteria (de Jonge et al., 2022). However, while feasible on a laboratory scale, it might be difficult to establish reproducible Pal-depletion conditions on an industrial large scale needed for vaccine production. In this study, we showed that inactivation of mlaF together with pldA resulted in hypervesiculation. The protein patterns of the OMVs isolated from the mutant were similar to those of the wild type (Fig. 4A). Although it is still possible that some minor OMPs are lacking in the mutant OMVs due to misassembly in the altered OM, this is not likely to affect the strength of immunity potentially induced by these OMVs as (i) minor antigens individually usually evoke poor protective immune responses because bound antibodies are too much dispersed over the bacterial cell surface to trigger complement activation, although synergistic activity by antibodies against multiple minor antigens could still be expected (Weynants et al., 2007), and (ii) only few relevant antigens are required to evoke effective immune responses as illustrated by the current acellular vaccines. In any case, several relevant antigens were detected in the isolated OMVs by Western blotting (Fig. 4D). Moreover, the OMVs were not notably contaminated with inner membranes. Although it cannot totally be excluded at this stage that the disturbed lipid asymmetry influences the immunogenicity of the OMVs, the mlaF pldA double mutant strain is a good candidate for the cost-effective production of an OMV-based third-generation pertussis vaccine. In future experiments, the immunogenicity of OMVs produced by the mlaF pldA double mutant will be determined in animal models and compared with that of OMVs produced by the Pal-depletion strain and of those released from wild-type cells after heat shock. Moreover, since OMVs contain LPS, the endotoxicity of the OMV preparations needs to be determined. If deemed necessary, it can be reduced by lipid A engineering as described previously (Arenas et al., 2020).
Funding
This work was supported by the domain Applied and Engineering Sciences (TTW) of The Netherlands Organization for Scientific Research (NWO) (TTW Perspectief grant number 14,921) which received financial contributions for this grant from GlaxoSmithKline Biologicals SA and PULIKE Biological Engineering Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. All authors declare no other competing interests.
Appendix A. Supplementary data
CRediT authorship contribution statement
Eline F. de Jonge: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. Lana Vogrinec: Investigation. Ria van Boxtel: Investigation. Jan Tommassen: Conceptualization, Supervision, Funding acquisition, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Federica Galimberti for initial experiments and Jesús Pérez Ortega and Eefjan Breukink for advices.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.crmicr.2022.100172.
Appendix. Supplementary materials
Data Availability
Data will be made available on request.
References
- Afonina G., Leduc I., Nepluev I., Jeter C., Routh P., Almond G., Orndorff P.E., Hobbs M., Elkins C. Immunization with the Haemophilus ducreyi hemoglobin receptor HgbA protects against infection in the swine model of chancroid. Infect. Immun. 2006;74:2224–2232. doi: 10.1128/IAI.74.4.2224-2232.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alteri C.J., Hagan E.C., Sivick K.E., Smith S.N., Mobley H.L.T. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas J., Pupo E., Phielix C., David D., Zariri A., Zamyatina A., Tommassen J., van der Ley P. Shortening the lipid A acyl chains of Bordetella pertussis enables depletion of lipopolysaccharide endotoxic activity. Vaccines (Basel) 2020;8:594. doi: 10.3390/vaccines8040594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarda B.I., Zielke R.A., Le Van A., Jerse A.E., Sikora A.E. Neisseria gonorrhoeae MlaA influences gonococcal virulence and membrane vesicle production. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balhuizen M.D., Versluis C.M., van Harten R.M., de Jonge E.F., Brouwers J.F., van de Lest C.H.A., Veldhuizen E.J.A., Tommassen J., Haagsman H.P. PMAP-36 reduces the innate immune response induced by Bordetella bronchiseptica-derived outer membrane vesicles. Curr. Res. Microb. Sci. 2021;2 doi: 10.1016/j.crmicr.2020.100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher T., MacArthur I., King J.D., Langridge G.C., Mayho M., Parkhill J., Preston A. Fundamental differences in physiology of Bordetella pertussis dependent on the two-component system Bvg revealed by gene essentiality studies. Microb. Genomics. 2020;6 doi: 10.1099/mgen.0.000496. mgen000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop R.E., Gibbons H.S., Guina T., Trent M.S., Miller S.I., Raetz C.R.H. Transfer of palmitate from phospholipids to lipid A in outer membranes of Gram-negative bacteria. EMBO J. 2000;19:5071–5080. doi: 10.1093/emboj/19.19.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos M.P., Tommassen-van Boxtel R., Tommassen J. Experimental methods for studying the BAM complex in Neisseria meningitidis. Methods Mol. Biol. 2015;1329:33–49. doi: 10.1007/978-1-4939-2871-2_3. [DOI] [PubMed] [Google Scholar]
- Brickman T.J., Armstrong S.K. Essential role of the iron-regulated outer membrane receptor FauA in alcaligin siderophore-mediated iron uptake in Bordetella species. J. Bacteriol. 1999;181:5958–5966. doi: 10.1128/jb.181.19.5958-5966.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C., Taylor A.J., Elmi A., Winter J., Liaw J., Grabowska A.D., Gundogdu O., Wren B.W., Kelly D.J., Dorrell N. Sodium taurocholate stimulates Campylobacter jejuni outer membrane vesicle production via down-regulation of the maintenance of lipid asymmetry pathway. Front. Cell. Infect. Microbiol. 2019;9:177. doi: 10.3389/fcimb.2019.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge E.F., Balhuizen M.D., van Boxtel R., Wu J., Haagsman H.P., Tommassen J. Heat shock enhances outer-membrane vesicle release in Bordetella spp. Curr. Res. Microb. Sci. 2021;2 doi: 10.1016/j.crmicr.2020.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge E.F., van Boxtel R., Balhuizen M.D., Haagsman H.P., Tommassen J. Pal depletion results in hypervesiculation and affects cell morphology and outer-membrane lipid asymmetry in bordetellae. Res. Microbiol. 2022;173 doi: 10.1016/j.resmic.2022.103937. [DOI] [PubMed] [Google Scholar]
- Dekker N. Outer-membrane phospholipase A: known structure, unknown biological function. Mol. Microbiol. 2000;35:711–717. doi: 10.1046/j.1365-2958.2000.01775.x. [DOI] [PubMed] [Google Scholar]
- Dekker N., Tommassen J., Lustig A., Rosenbusch J.P., Verheij H.M. Dimerization regulates the enzymatic activity of Escherichia coli outer membrane phospholipase A. J. Biol. Chem. 1997;272:3179–3184. doi: 10.1074/jbc.272.6.3179. [DOI] [PubMed] [Google Scholar]
- Ekiert D.C., Bhabha G., Isom G.L., Greenan G., Ovchinnikov S., Henderson I.R., Cox J.S., Vale R.D. Architectures of lipid transport systems for the bacterial outer membrane. Cell. 2017;169:273–285. doi: 10.1016/j.cell.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis T.N., Kuehn M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010;74:81–94. doi: 10.1128/mmbr.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan B., Low W.Y., Liu X., Chng S.S. Characterization of interactions and phospholipid transfer between substrate binding proteins of the OmpC-Mla system. Biochemistry. 2019;58:114–119. doi: 10.1021/acs.biochem.8b00897. [DOI] [PubMed] [Google Scholar]
- Esposito S., Stefanelli P., Fry N.K., Fedele G., He Q., Paterson P., Tan T., Knuf M., Rodrigo C., Olivier C.W., Flanagan K.L., Hung I., Lutsar I., Edwards K., O'Ryan M., Principi N. Pertussis prevention: reasons for resurgence, and differences in the current acellular pertussis vaccines. Front. Immunol. 2019;10:1344. doi: 10.3389/fimmu.2019.01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Piñar R., Lo Sciuto A., Rossi A., Ranucci S., Bragonzi A., Imperi F. In vitro and in vivo screening for novel essential cell-envelope proteins in Pseudomonas aeruginosa. Sci. Rep. 2015;5:17593. doi: 10.1038/srep17593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich B.T., d'Alarcao M., Feldberg R.S., Nicholson M.L., Siber G.R., Swartz R.W. Formation and cell-medium partitioning of autoinhibitory free fatty acids and cyclodextrin's effect in the cultivation of Bordetella pertussis. J. Biotechnol. 1996;45:137–148. doi: 10.1016/0168-1656(95)00155-7. [DOI] [PubMed] [Google Scholar]
- Gasperini G., Arato V., Pizza M., Aricò B., Leuzzi R. Physiopathological roles of spontaneously released outer membrane vesicles of Bordetella pertussis. Future Microbiol. 2017;12:1247–1259. doi: 10.2217/fmb-2017-0064. [DOI] [PubMed] [Google Scholar]
- Geurtsen J., Steeghs L., Hamstra H.J., ten Hove J., de Haan A., Kuipers B., Tommassen J., van der Ley P. Expression of the lipopolysaccharide-modifying enzymes PagP and PagL modulates the endotoxic activity of Bordetella pertussis. Infect. Immun. 2006;74:5574–5585. doi: 10.1128/IAI.00834-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonyar L.A., Gelbach P.E., McDuffie D.G., Koeppel A.F., Chen Q., Lee G., Temple L.M., Stibitz S., Hewlett E.L., Papin J.A., Damron F.H., Eby J.C. In vivo gene essentiality and metabolism in Bordetella pertussis. mSphere. 2019;4 doi: 10.1128/msphere.00694-18. e00694-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D.C., Wolfson J.S., Ng E.Y., Swartz M.N. Mechanisms of action of and resistance to ciprofloxacin. Am. J. Med. 1987;82:12–20. [PubMed] [Google Scholar]
- Hozbor D., Rodriguez M.E., Fernández J., Lagares A., Guiso N., Yantorno O. Release of outer membrane vesicles from Bordetella pertussis. Curr. Microbiol. 1999;38:273–278. doi: 10.1007/PL00006801. [DOI] [PubMed] [Google Scholar]
- Hu Y.H., Dang W., Sun L. A TonB-dependent outer membrane receptor of Pseudomonas fluorescens: virulence and vaccine potential. Arch. Microbiol. 2012;194:795–802. doi: 10.1007/s00203-012-0812-3. [DOI] [PubMed] [Google Scholar]
- Hughes G.W., Hall S.C.L., Laxton C.S., Sridhar P., Mahadi A.H., Hatton C., Piggot T.J., Wotherspoon P.J., Leney A.C., Ward D.G., Jamshad M., Spana V., Cadby I.T., Harding C., Isom G.L., Bryant J.A., Parr R.J., Yakub Y., Jeeves M., Huber D., Henderson I.R., Clifton L.A., Lovering A.L., Knowles T.J. Evidence for phospholipid export from the bacterial inner membrane by the Mla ABC transport system. Nat. Microbiol. 2019;4:1692–1705. doi: 10.1038/s41564-019-0481-y. [DOI] [PubMed] [Google Scholar]
- Imaizumi A., Suzuki Y., Ono S., Sato H., Sato Y. Heptakis(2,6-O-dimethyl)β-cyclodextrin: a novel growth stimulant for Bordetella pertussis phase I. J. Clin. Microbiol. 1983;17:781–786. doi: 10.1128/jcm.17.5.781-786.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom G.L., Davies N.J., Chong Z.-.S., Bryant J.A., Jamshad M., Sharif M., Cunningham A.F., Knowles T.J., Chng S.-.S., Cole J.A., Henderson I.R. MCE domain proteins: conserved inner membrane lipid-binding proteins required for outer membrane homeostasis. Sci. Rep. 2017;7:8608. doi: 10.1038/s41598-017-09111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan A.T. Outer membrane vesicles (OMVs) of Gram-negative bacteria: a perspective update. Front. Microbiol. 2017;8:1053. doi: 10.3389/fmicb.2017.01053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamischke C., Fan J., Bergeron J., Kulasekara H.D., Dalebroux Z.D., Burrell A., Kollman J.M., Miller S.I. The Acinetobacter baumannii Mla system and glycerophospholipid transport to the outer membrane. Elife. 2019;8:e40171. doi: 10.7554/elife.40171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanski M.A., Dwyer D.J., Collins J.J. How antibiotics kill bacteria: from targets to networks. Nat. Rev. Microbiol. 2010;8:423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolich L.R., Chang Y.T., Coudray N., Giacometti S.I., MacRae M.R., Isom G.L., Teran E.M., Bhabha G., Ekiert D.C. Structure of MlaFB uncovers novel mechanisms of ABC transporter regulation. Elife. 2020;9:e60030. doi: 10.7554/eLife.60030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin Y., Bogdanov M., Lu S., Guan Z., Margolin W., Weiss J., Zheng L. The phospholipid-repair system LplT/Aas in Gram-negative bacteria protects the bacterial membrane envelope from host phospholipase A2 attack. J. Biol. Chem. 2018;293:3386–3398. doi: 10.1074/jbc.RA117.001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Sciuto A., Fernández-Piñar R., Bertuccini L., Iosi F., Superti F., Imperi F. The periplasmic protein TolB as a potential drug target in Pseudomonas aeruginosa. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur I., Belcher T., King J.D., Ramasamy V., Alhammadi M., Preston A. The evolution of Bordetella pertussis has selected for mutations of acr that lead to sensitivity to hydrophobic molecules and fatty acids. Emerg. Microbes Infect. 2019;8:603–612. doi: 10.1080/22221751.2019.1601502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinverni J.C., Silhavy T.J. An ABC transport system that maintains lipid asymmetry in the gram-negative outer membrane. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8009–8014. doi: 10.1073/pnas.0903229106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr N., Oliver D.C., Laurent V., Poolman J., Denoël P., Fernandez R.C. Protective activity of the Bordetella pertussis BrkA autotransporter in the murine lung colonization model. Vaccine. 2008;26:4306–4311. doi: 10.1016/j.vaccine.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Mattoo S., Cherry J.D. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micoli F., MacLennan C.A. Outer membrane vesicle vaccines. Semin. Immunol. 2020;50 doi: 10.1016/j.smim.2020.101433. [DOI] [PubMed] [Google Scholar]
- Miller W.R., Bayer A.S., Arias C.A. Mechanism of action and resistance to daptomycin in Staphylococcus aureus and enterococci. Cold Spring Harb. Perspect. Med. 2016;6 doi: 10.1101/cshperspect.a026997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Zhang-Akiyama Q.M. pqiABC and yebST, putative mce operons of Escherichia coli, encode transport pathways and contribute to membrane integrity. J. Bacteriol. 2017;199:e00606–e00616. doi: 10.1128/JB.00606-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Restoring permeability barrier function to outer membrane. Chem. Biol. 2005;12:507–509. doi: 10.1016/j.chembiol.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium transmembrane diffusion of some hydrophobic substances. Biochim. Biophys. Acta. 1976;433:118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Pérez-Ortega J., van Harten R.M., van Boxtel R., Plisnier M., Louckx M., Ingels D., Haagsman H.P., Tommassen J. Reduction of endotoxicity in Bordetella bronchiseptica by lipid A engineering: characterization of lpxL1 and pagP mutants. Virulence. 2021;12:1452–1468. doi: 10.1080/21505594.2021.1929037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers M.J., Simpson B.W., Trent M.S. The Mla pathway in Acinetobacter baumannii has no demonstrable role in anterograde lipid transport. Elife. 2020;9:e56571. doi: 10.7554/eLife.56571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers M.J., Trent M.S. Phospholipid retention in the absence of asymmetry strengthens the outer membrane permeability barrier to last-resort antibiotics. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E8518–E8527. doi: 10.1073/pnas.1806714115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston A., Maxim E., Toland E., Pishko E.J., Harvill E.T., Caroff M., Maskell D.J. Bordetella bronchiseptica PagP is a Bvg-regulated lipid A palmitoyl transferase that is required for persistent colonization of the mouse respiratory tract. Mol. Microbiol. 2003;48:725–736. doi: 10.1046/j.1365-2958.2003.03484.x. [DOI] [PubMed] [Google Scholar]
- Raeven R.H.M., Brummelman J., Pennings J.L.A., van der Maas L., Tilstra W., Helm K., van Riet E., Jiskoot W., van Els C.A.C.M., Han W.G.H., Kersten G.F.A., Metz B. Bordetella pertussis outer membrane vesicle vaccine confers equal efficacy in mice with milder inflammatory responses compared to a whole-cell vaccine. Sci. Rep. 2016;6:38240. doi: 10.1038/srep38240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeven R.H.M., Rockx-Brouwer D., Kanojia G., van der Maas L., Bindels T.H.E., ten Have R., van Riet E., Metz B., Kersten G.F.A. Intranasal immunization with outer membrane vesicle pertussis vaccine confers broad protection through mucosal IgA and Th17 responses. Sci. Rep. 2020;10:7396. doi: 10.1038/s41598-020-63998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeven R.H.M., van der Maas L., Tilstra W., Uittenbogaard J.P., Bindels T.H.E., Kuipers B., van der Ark A., Pennings J.L.A., van Riet E., Jiskoot W., Kersten G.F.A., Metz B. Immunoproteomic profiling of Bordetella pertussis outer membrane vesicle vaccine reveals broad and balanced humoral immunogenicity. J. Proteome Res. 2015;14:2929–2942. doi: 10.1021/acs.jproteome.5b00258. [DOI] [PubMed] [Google Scholar]
- Rangl M., Rima L., Klement J., Miyagi A., Keller S., Scheuring S. Real-time visualization of phospholipid degradation by outer membrane phospholipase A using high-speed atomic force microscopy. J. Mol. Biol. 2017;429:977–986. doi: 10.1016/j.jmb.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Roier S., Zingl F.G., Cakar F., Durakovic S., Kohl P., Eichmann T.O., Klug L., Gadermaier B., Weinzerl K., Prassl R., Lass A., Daum G., Reidl J., Feldman M.F., Schild S. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat. Commun. 2016;7:10515. doi: 10.1038/ncomms10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz N., Wu T., Kahne D., Silhavy T.J. Probing the barrier function of the outer membrane with chemical conditionality. ACS Chem. Biol. 2006;1:385–395. doi: 10.1021/cb600128v. [DOI] [PubMed] [Google Scholar]
- Schoch T.J., Williams C.B. Adsorption of fatty acid by the linear component of corn starch. J. Am. Chem. Soc. 1944;66:1232–1233. doi: 10.1021/ja01235a508. [DOI] [Google Scholar]
- Schooling S.R., Beveridge T.J. Membrane vesicles: an overlooked component of the matrices of biofilms. J. Bacteriol. 2006;188:5945–5957. doi: 10.1128/JB.00257-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C., Kuehn M.J. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 2015;13:605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy T.J., Kahne D., Walker S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010;2 doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorupski K., Taylor R.K. Positive selection vectors for allelic exchange. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- Snijder H.J., Ubarretxena-Belandia I., Blaauw M., Kalk K.H., Verheij H.M., Egmond M.R., Dekker N., Dijkstra B.W. Structural evidence for dimerization-regulated activation of an integral membrane phospholipase. Nature. 1999;401:717–721. doi: 10.1038/401717a0. [DOI] [PubMed] [Google Scholar]
- Stainer D.W., Scholte M.J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 1971;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Tang X., Chang S., Qiao W., Luo Q., Chen Y., Jia Z., Coleman J., Zhang K., Wang T., Zhang Z., Zhang C., Zhu X., Wei X., Dong C., Zhang X., Dong H. Structural insights into outer membrane asymmetry maintenance in gram-negative bacteria by MlaFEDB. Nat. Struct. Mol. Biol. 2021;28:81–91. doi: 10.1038/s41594-020-00532-y. [DOI] [PubMed] [Google Scholar]
- Tsai C.M., Frasch C.E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-X. [DOI] [PubMed] [Google Scholar]
- Verwey W.F., Thiele E.H., Sage D.N., Schuchardt L.F. A simplified liquid culture medium for the growth of Hemophilus pertussis. J. Bacteriol. 1949;58:127–134. [PMC free article] [PubMed] [Google Scholar]
- Weynants V.E., Feron C.M., Goraj K.K., Bos M.P., Denoël P.A., Verlant V.G., Tommassen J., Peak I.R., Judd R.C., Jennings M.P., Poolman J.T. Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect. Immun. 2007;75:5434–5442. doi: 10.1128/IAI.00411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa H., Osaki T., Kurata S., Fukuda M., Kawakami H., Ochiai K., Hanawa T., Kamiya S. Outer membrane vesicles of Helicobacter pylori TK1402 are involved in biofilm formation. BMC Microbiol. 2009;9:197. doi: 10.1186/1471-2180-9-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhang H., Xiong B., Fan G., Cao Z. Immunogenicity of recombinant outer membrane porin protein and protective efficacy against lethal challenge with Bordetella bronchiseptica in rabbits. J. Appl. Microbiol. 2019;127:1646–1655. doi: 10.1111/jam.14451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.