Abstract
Adjacent regions of parietal cortex are thought to affiliate with distinct large-scale networks and thereby make different contributions to memory formation. We directly tested this putative functional segregation within parietal cortex by perturbing activity of anterior versus posterior parietal areas. We applied noninvasive theta-burst transcranial magnetic stimulation to these locations immediately before a semantic encoding task, and subsequently tested recollection memory. Consistent with previous findings, fMRI activity in left inferior frontal gyrus during semantic encoding correlated with subsequent high memory accuracy and strong subjective recollection. Stimulation of the posterior parietal cortex decoupled its network – the hippocampal-cortical network – from left inferior frontal gyrus. Furthermore, posterior parietal stimulation reduced highly accurate subjective recollection. Critically, both of these changes occurred relative to stimulation of the anterior parietal cortex. Stimulating anterior versus posterior parietal cortex therefore differentiated hippocampal network involvement in episodic memory. This provides direct evidence that distinct territories within close proximity of each other in parietal cortex make functionally distinct contributions to memory formation. Further, noninvasive stimulation has the spatial resolution required to differentially modulate the interaction of these adjacent parietal locations with distributed large-scale brain networks.
Keywords: Episodic memory, Semantic memory, Theta-burst stimulation, Parietal cortex, Subjective recollection
Graphical abstract
Highlights
-
•
Stimulation was used to segregate parietal-network contributions to memory.
-
•
Posterior parietal stimulation affected highly accurate recollection memory.
-
•
This stimulation decoupled the hippocampal network from task-related frontal cortex.
-
•
These effects occurred relative to stimulation of anterior parietal cortex.
-
•
Distinct anterior versus posterior parietal cortex roles during memory formation.
1. Introduction
Forming an episodic memory involves generating and storing an internal representation of event content (James, 1890; Mesulam, 1998). The success of memory formation can be measured by both the accuracy of later memory performance as well as in the subjective confidence of retrieval and awareness of event contextual details (Paller and Wagner, 2002; Rugg and Yonelinas, 2003; Yonelinas, 2001a, 2001b). Accumulating evidence suggests that the subjective experience of rich episodic recall may depend on activity in posterior regions of the parietal cortex (Cabeza et al., 2012; Cabeza et al., 2008; Humphreys et al., 2021; Qin et al., 2011; Yazar et al., 2012). Patients with focal posterior parietal lesions (Ciaramelli et al., 2017; Drowos et al., 2010; Simons et al., 2010) and healthy individuals with transient disruptions in posterior parietal activity (e.g., via transcranial magnetic stimulation, TMS) (Koen et al., 2018; Wynn et al., 2018; Yazar et al., 2014) typically show normal memory performance on objective measures, but impairments in subjective recall, especially when retrieving source or contextual details. The posterior parietal cortex has strong neuroanatomical connections to the hippocampus and its distributed cortical network (Eichenbaum et al., 2007; Kahn et al., 2008; Mesulam, 1990; Ranganath and Ritchey, 2012), and activity of this network has been associated with ratings of subjective confidence during recollection (Ren et al., 2018; Shrager et al., 2008; Smith et al., 2011).
Memory retrieval is influenced by the cognitive processes engaged during memory formation. For example, semantic strategies, including rating object or word animacy during encoding, can strongly influence the degree to which events are later remembered (i.e., the levels of processing effect (Craik and Lockhart, 1972)). Neuroimaging and TMS studies have shown that changes in brain regions associated with semantic categorization, especially in the left inferior frontal gyrus (IFG), typically predict subsequent memory outcomes – especially those reflecting subjective confidence (Demb et al., 1995; Kirchhoff et al., 2005; Kohler et al., 2004). IFG and associated semantic memory regions are thought to form a frontal memory network that is functionally distinct from the hippocampal memory network (Hurley et al., 2015; Mesulam, 1990; Mesulam et al., 2014).
Accumulating evidence from neuroimaging suggests that these two parallel memory networks together resemble the canonical default mode network (Andrews-Hanna et al., 2010; Braga and Buckner, 2017; Braga et al., 2019; Buckner and DiNicola, 2019). These two networks are differentiated by their fMRI connectivity patterns. Brain regions comprising the hippocampal network (i.e., default network-A in (Braga et al., 2019)) couple with the hippocampal formation, unlike the frontal network (default network-B). Critically, the hippocampal network includes relatively posterior areas of the parietal cortex, while the frontal network includes relatively anterior parietal cortex (Braga et al., 2019; Buckner and DiNicola, 2019; Kahn et al., 2008; Nelson et al., 2010; Wagner et al., 2005). However, there is some ambiguity over the degree to which these functional networks are segregated within parietal cortex, given the spatial overlap of fMRI activity across tasks and cognitive domains (Cabeza et al., 2012; Uncapher and Wagner, 2009). Further, small distinctions in fMRI activations or resting-state fMRI connectivity patterns within the parietal cortex may be artificially exaggerated by arbitrary statistical thresholding or by “winner-takes-all” parcellation approaches (Bijsterbosch et al., 2020; Woo et al., 2014).
The current study thus aimed to address the interrelated issues of how these networks may differentially contribute to the formation of strong memories, and whether these networks are spatially segregated within parietal cortex. We performed an experiment using noninvasive brain stimulation intended to influence fMRI connectivity of the hippocampal versus frontal networks via their putatively distinct territories within parietal cortex. We applied network-targeted transcranial magnetic stimulation to two parietal locations, which were defined based on their resting-state fMRI connectivity with left IFG versus hippocampus (Fig. 1). This design was motivated by previous findings demonstrating that this network-targeted stimulation approach can influence fMRI connectivity of the network, producing corresponding effects on cognitive functions that depend on the modulated network (Fox et al., 2012; Hebscher and Voss, 2020). The logic of this experiment is that posterior parietal targeted stimulation should modulate hippocampal network fMRI connectivity with brain regions that are important for task performance relative to anterior parietal stimulation, due to their putatively dissociable involvement in different memory networks.
Fig. 1.
Experiment design. (A) Before the experimental stimulation sessions, subjects completed resting-state fMRI and structural MRI scans used to guide TMS delivery. fMRI connectivity analyses were used to define subject-specific left posterior and anterior parietal targets. Posterior parietal sites (purple) were defined based on subject-specific connectivity to left hippocampal seeds (indicated by arrow). Anterior parietal sites (orange) were defined based on subject-specific connectivity to left IFG seeds (indicated by arrow). We further used vertex stimulation as a control condition. (B) Each subject participated in three experimental sessions. Each session began with one of the three stimulation conditions, followed by a resting-state MRI scan and a word encoding phase. This portion of the task occurred during the typical expected duration of stimulation-related effects on neural function. Recognition memory for words and word locations studied during encoding were tested after a delay, such that retrieval likely occurred after stimulation effects had decayed. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
In line with many previous fMRI studies of semantic encoding (e.g. Kirchhoff et al., 2005), we predicted that the brain regions likely recruited during successful memory formation would include left IFG and associated regions in the temporal cortex. We further hypothesized that stimulation conditions that modulated hippocampal network connectivity with these task-relevant regions immediately before encoding would produce corresponding changes in encoding success, as indicated by subsequent recognition memory performance.
Based on previous TMS studies employing a network-targeted approach (e.g. Fox et al., 2012; Hebscher and Voss, 2020; Hermiller et al., 2019; Wang et al., 2014), we predicted stimulation of posterior parietal cortex would influence hippocampal network connectivity with task-relevant brain regions. Critically, we predicted changes in brain activity following posterior parietal stimulation would occur alongside reductions in subjective recollection (cf. Koen et al., 2018; Wynn et al., 2018; Yazar et al., 2014). Correspondingly, we predicted that stimulation of anterior parietal cortex would affect frontal network connectivity with task-relevant brain areas. We expected that these connectivity-related changes would occur alongside changes in performance on a semantic categorization task, which is known to recruit frontal network regions (Demb et al., 1995; Kirchhoff et al., 2005). Such evidence of behavioral and functional dissociations would not only support the conclusion that relatively anterior versus posterior parietal cortex belong to distinct frontal versus hippocampal memory networks, but would further illustrate how these memory networks may uniquely contribute to both memory formation and subsequent confident memory retrieval.
2. Methods
2.1. Participants
21 healthy adults were recruited for the study from the greater Chicago area. All subjects passed safety screenings for MRI and for TMS (Rossi et al. 2020), reported normal or corrected-to-normal vision, and had no known history of substance abuse or psychiatric or neurological disorder. All subjects provided written informed consent to a protocol approved by the Northwestern University Institutional Review Board and received payment for their participation. Two participants were discontinued from the study due to poor behavioral performance (i.e., below chance accuracy of 50% when identifying either old or new test stimuli). One additional participant was discontinued from the study after failure to follow memory test instructions.
Reported MRI analyses include 18 adults (9 women; average age: 23.8 years; range: 18–31 years). Two participants were further excluded from behavioral analyses due to poor performance on this semantic categorization task (i.e., scoring lower than the group mean by over 3 SD). Reported behavior and correlation analyses therefore include 16 adults (9 women; average age: 24.1 years; range: 19–31 years).
2.2. Experiment design
Prior to experimental sessions, subjects completed baseline structural and resting-state fMRI scans. These scans allowed for anatomical localization of subsequent noninvasive brain stimulation, as described below.
The experiment used a within-subjects design across three experimental sessions. At the beginning of each session, subjects received one of three transcranial magnetic stimulation (TMS) conditions. After stimulation, fMRI was acquired while subjects completed a resting-state scan, followed by a word and word location encoding task. Following a delay, subjects completed a memory test, during which fMRI was not recorded. The three experimental sessions occurred at least 48 h apart (mean inter-session interval = 4 days; range = 2–22 days).
2.3. Network-targeted transcranial magnetic stimulation
2.3.1. MRI acquisition and processing
Subjects completed 5 min structural MRI and 10 min resting-state fMRI scans at baseline to allow anatomical localization during TMS. During both scans, subjects were instructed to lie as still as possible. During the resting-state scan, subjects were further asked to keep their eyes open and fixed on a centrally presented fixation cross, letting their minds wander while avoiding specific thoughts or meditation.
MRI was collected via a Siemens 3T Prisma whole-body scanner with a 64-channel head-neck coil located in the Northwestern University Center for Translational Imaging. Structural images were acquired using a T1-weight MPRAGE sequence (176 frames; TE: 1.69 ms; TR: 2170 ms; TI: 1160 ms; flip angle: 7°; voxel resolution: 1.0 × 1.0 × 1.0 mm; 1 mm thick sagittal slices; 256 × 256 mm FOV; scan duration: 5.12 min). Functional images were acquired using a BOLD contrast sensitive gradient-echo echoplanar sequence (290 frames; TE: 20 ms; TR: 2000 ms; flip angle: 80°; voxel resolution: 1.6 × 1.6 × 1.6 mm; 70 ascending 1.6 mm thick axial slices; 210 × 203 mm FOV; multi-band factor of 2; scan duration: 10 min).
Structural and resting-state MRI data were preprocessed using AFNI software (AFNI_20.04) (Cox, 1996). Resting-state scan pre-processing included: co-registration to the skull-stripped (3dSkullStrip) anatomic scan (align_epi_anat), warping scans into Talairach-Tournoux (TT) stereotactic space using the TT_N27 atlas (auto_tlrc), slice-timing correction (to3d), removal of the first five EPI volumes to attain MR steady state (3dTcat), outlier suppression (3dDespike), and motion correction (3dvolreg). Volumes with excessive motion (>0.3 mm) were flagged for censoring. Following data preprocessing, spatial smoothing was applied using a 4 mm FWHM Gaussian kernel (3dmerge). Subsequently, bandpass filtering (0.01–0.10 Hz), motion censoring, and the nuisance motion time series (3dTproject) were linearly de-trended from each voxel (3dDeconvolve). We report all coordinates in MNI space (WhereamI).
2.3.2. Determination of subject-specific stimulation targets
Each session began with subjects receiving noninvasive brain stimulation delivered at one of three locations (Fig. 1). Stimulation was either administered at a posterior parietal cortical site determined by its resting-state connectivity to the left hippocampus, at an anterior parietal cortical site determined by its resting-state connectivity to the left inferior frontal gyrus, or at the vertex (i.e., posterior parietal, anterior parietal, control conditions). Stimulation order was counterbalanced across sessions. Each subject's stimulation locations were individually determined based on resting-state fMRI connectivity measured during the baseline session.
Subject-specific hippocampal sites were identified as the voxel central to the left hippocampal body and proximal to a default hippocampal coordinate (hippocampus default MNI: 29, −25, −13; mean distance from default: 2.0 mm, range = 0–5.8 mm). Hippocampal sites were then used as seeds in whole-brain resting-state functional connectivity analyses. For each subject, we identified the peak of a cluster of left posterior parietal cortex voxels with high functional connectivity to the hippocampal seed (mean seed-target connectivity: 0.35; range: 0.21–0.54), based on a default posterior parietal MNI coordinate (posterior parietal default MNI (−45, −70, 39) identified by group-average fMRI connectivity in the 1000 connectomes dataset available via Neurosynth (Yarkoni et al., 2011, cf. Wang et al., 2014); mean distance from default: 7.1 mm, range = 1–15.7 mm).
Similarly, subject-specific IFG sites were identified as the nearest adjacent brain surface to a default IFG coordinate (IFG default MNI: 54, 24, 3; mean distance from default: 1.5 mm, range = 0–3.6 mm). For each subject, we then identified the peak of a cluster of left anterior parietal cortex voxels with high functional connectivity to the IFG seed (mean seed-target connectivity: 0.44; range: 0.28–0.71), based on a default anterior parietal MNI coordinate (anterior parietal default MNI (−62, −50, 28) identified via Neurosynth, as above (Yarkoni et al., 2011, cf. Hurley et al., 2015); mean distance from default: 5.5 mm, range = 0–14.5 mm). Lastly, we determined subject-specific vertex sites as the nearest adjacent brain surface to a default interhemispheric fissure coordinate (control default MNI: 0, −15, 74; mean distance from default: 4.2 mm, range = 2–6.4 mm).
Stimulation targets were transformed from MNI space into subject-specific original MRI space for anatomically guided TMS. The mean distance between anterior and posterior stimulation targets was 32.2 mm (SD = 3.3 mm, range = 27.3–38.9 mm).
2.3.3. Stimulation protocol
At the beginning of each session, subjects received continuous theta-burst stimulation (cTBS) at one of the three stimulation targets. The cTBS protocol consisted of 50 Hz biphasic pulse triplets delivered every 200 ms (5 Hz) for 40 s continuously. This protocol has been employed in several previous studies involving modulation of the hippocampal network via stimulation of posterior parietal cortex (e.g. Hermiller et al., 2020; Hermiller et al., 2019; Tambini, Nee, & D'Esposito, 2018). Stimulation sensation was relatively similar across conditions and subjects were unaware of location-specific study hypotheses. The experiment had a within-subject design, in which all subjects received all three stimulation conditions, and the order of the stimulation conditions was counterbalanced across subjects.
TMS was delivered via a MagPro X100 system with a Cool-B65 coil (MagVenture A/S, Denmark) using frameless stereotactic guidance (Localite GmBH, Germany). Stimulation intensity was calibrated to 80% of each subject's resting motor threshold identified for the right abductor pollicis brevis before the experimental sessions (mean stimulation intensity: 42.8% of maximum system output, range = 38–59%). TMS was delivered to optimize induced electric field orientation, directed medial-laterally for parietal stimulation conditions and medial-caudally for the vertex (Janssen et al., 2015). Immediately following TMS, subjects performed a 2 min category fluency task and did not show any signs of impairment on this task (mean items named per category: 25.0, SD: 4.0, range: 19.3–33.3, within the range typical for cognitively healthy young adults aged 20–39 (Tombaugh et al., 1999)), suggesting suitable cognitive ability for performing the semantic encoding task.
2.4. Resting-state fMRI
2.4.1. Defining networks of interest from baseline resting-state fMRI
Resting-state fMRI acquired during the baseline session was analyzed to determine hippocampal and frontal networks of interest, such that networks were defined independently from data collected during the experimental sessions, which were subsequently analyzed to identify the effects of stimulation.
Each network was defined as a set of brain regions with robust group-level functional connectivity to a network-specific seed. The hippocampal network was identified via seed-based functional connectivity with a group-averaged hippocampal site based on targets determined for stimulation (hippocampus mean MNI: 29, −24, −13). We identified the peaks of clusters comprising of >100 contiguous voxels that were robustly connected with the hippocampal seed (p < 2 × 10−6 voxel-wise threshold). We generated 4 mm spheres centered at each of these nine cluster peaks to create a group-level hippocampal network (Table 1). As expected, this network corresponded to the canonical hippocampal-defined default mode (i.e., episodic memory, hippocampal, default mode-A) network (e.g. Buckner and DiNicola, 2019; Kahn et al., 2008).
Table 1.
Hippocampal and frontal networks of interest. The centroid MNI coordinates of each supra-threshold cluster listed for each network site. R and L indicate right and left brain.
| X | Y | Z | |
|---|---|---|---|
| Hippocampal network | |||
| R posterior cingulate cortex | 3 | −54 | 13 |
| R medial frontal gyrus | 0 | 52 | −7 |
| bL hippocampus | −21 | −21 | −13 |
| R hippocampus | 23 | −17 | −14 |
| L medial frontal gyrus | 0 | −12 | 58 |
| L superior frontal gyrus | −20 | 42 | 49 |
| L middle temporal gyrus | −68 | −28 | −7 |
| aL posterior parietal cortex | −47 | −71 | 34 |
| R anterior cingulate cortex | 3 | 52 | 20 |
| Frontal network | |||
| L inferior frontal gyrus | −58 | 20 | 12 |
| L superior frontal gyrus | −2 | 26 | 60 |
| R inferior frontal gyrus | 52 | 33 | −7 |
| aL anterior parietal cortex | −57 | −57 | 25 |
| L superior temporal gyrus | −65 | −26 | −2 |
| L middle temporal gyrus | −65 | −35 | 1 |
| R middle occipital gyrus | 38 | −86 | 9 |
Indicates the location of parietal cortex that was stimulated for the posterior and anterior stimulation conditions; these sites were removed from the network definition in control analyses of fMRI network connectivity that excluded the stimulated location in each network.
fMRI connectivity between this left hippocampal location and both networks of interest was examined in exploratory analyses.
The frontal network was identified via seed-based functional connectivity with a group-level left IFG site identified for targeted stimulation (IFG mean MNI: 54, 23, 3). Using the same cluster size and voxel-wise threshold as above, we generated 4 mm spheres centered at the seven peaks of clusters robustly connected with the IFG seed, creating a group-level frontal network. Predictably, this network overlapped with canonical regions of the IFG-defined default mode (i.e., semantic memory, frontal, default mode-B) network (e.g. Buckner and DiNicola, 2019; Hurley et al., 2015). Using 3dmask_tool, we found that there were no overlapping voxels between the two networks of interest. This confirms a premise of the experiment that left IFG and hippocampus are members of two distinct, distributed functional networks (Fig. 2).
Fig. 2.
Hippocampal and frontal networks of interest. Spheres centered at the peaks of clusters robustly connected with the hippocampal seed are plotted left, and spheres centered at the peaks of clusters robustly connected with the IFG seed are plotted right, creating group-level hippocampal and frontal networks, respectively.
2.4.2. Resting-state fMRI as a stimulation outcome
During each experimental session, stimulation was followed by a 10-min resting-state fMRI scan (mean time from last TMS pulse to scan start: 11 min, SD = 2 min, range: 9–20 min). The resting-state scan during the experimental sessions was acquired using the same scanner parameters and instruction protocol as the baseline scan, and the data were subject to the same fMRI preprocessing procedures described above.
Based on the networks of interest identified from the baseline data, which corresponded highly with canonical hippocampal and frontal networks reported for independent samples (Hurley et al., 2015; Kahn et al., 2008), we analyzed the effect of stimulation on resting-state fMRI data collected during experimental sessions. We examined how stimulation influenced hippocampal and frontal network connectivity to task-relevant brain regions (described below). For each subject, we computed the correlation coefficient between each task-relevant region and each of the network cluster peaks (3dTcorr1D), and subsequently applied Fisher Z-transformations to each correlation. We then calculated averaged correlations between each task-relevant region and each network of interest. These analyses were separated by stimulation condition, such that we could examine how stimulation affected connectivity between task-relevant regions and each network of interest. To do this, we performed 1-by-3 repeated-measures analysis of variance (RM-ANOVAs) with stimulation condition as the within-subject factor (posterior parietal, anterior parietal, and control stimulation). RM-ANOVAs were conducted in R (version R-3.6.2) and were followed by pairwise comparisons with Bonferroni corrections (corrected p values are indicated as pbf). For significant contrasts, we report Cohen's d.
2.5. fMRI memory task
2.5.1. Encoding phase
The resting-state scan was immediately followed by a memory encoding task during experimental sessions. fMRI was acquired while subjects completed the encoding task, which consisted of two blocks and lasted a total of 20 min (mean time from last TMS pulse to scan end: 42 min, SD = 2.4 min, range: 39–50 min). The TMS protocol used in this study has been shown to impact neural activity and cognitive function during this time period (Hoogendam et al.2010; Thut and Pascual-Leone, 2010).
Subjects were informed in the experiment instructions that they would be tested on the word and word location presented during the encoding task (i.e., item and source memory). Subjects performed a 5-min practice version of encoding and memory tests before each session.
During each session, 160 words were presented sequentially at one of two locations—either the left or right side of the screen (at 500 pixels left or right of screen center). Subjects were instructed to respond to each word via button-press (yes/no) to indicate whether or not the word was an animal (15% of the words were animals). Otherwise, subjects performed very well on semantic categorization, indicating they were attentive and alert during encoding (mean accuracy: 99.7%, range: 92–100%; mean response time: 1009 ms). We conducted a one-way RM-ANOVA on categorization task performance as a function of stimulation condition, and found semantic categorization accuracy did not vary across stimulation condition (F(2, 30) = 0.12, p = 0.89).
Words were drawn from a list of 480 total words. The mean SUBTLEX-US frequency count was 1098, mean word length was 6.0 letters, and mean concreteness rating was 4.8 (Brysbaert et al.2014). The 160 words presented during each session were matched across sessions on frequency, length, and concreteness; word presentation location (left or right of screen center) was randomized across trials. The experimental materials (i.e., the list of words and their locations) were counterbalanced across session and stimulation order. Each word was presented for 2000 ms. Between trials, a central fixation cross was displayed for 2000–6000 ms (mean = 4000 ms, SD = 1466.9 ms), with inter-stimulus interval randomized across trials.
The same fMRI preprocessing procedures were applied to task data as described above for baseline resting-state scans. After preprocessing, we performed generalized least squared regression on task-fMRI data, followed by restricted maximum likelihood estimation of temporal correlation structure (3dDeconvolve (BLOCK (4,1)); 3dREMLfit). Analyses were guided by behavioral findings, such that task trials were binned by memory performance (strong, moderate, and weak explicit memory trials; see Results). These analyses were performed separately for each subject, combining data across the three experimental sessions to yield an appropriate number of trials in each condition per subject. The results of the regression analyses were used to conduct mixed-effects meta analyses (3dMEMA) at the group level in order to model both within- and across-subject variability. Noise smoothness values were estimated (3dFWHMx) for permutation testing to identify the probability of false positive/noise clusters (3dClustsim). We then conducted stringent two-sided t-tests with α < 0.05 in order to reduce family-wise error contributions to the false positive rate (Chen et al., 2019). At a voxel-wise threshold of p < 0.001 (two-sided/two-tailed), we found the minimum significant cluster-size threshold for the task data was 50 voxels. We additionally identified clusters surviving 100% mask overlap (3dmask_tool) between strong vs. moderate and strong vs. weak memory masks. We applied a 200-voxel threshold overlap cutoff to identify task-relevant brain regions. The overlap mask was dilated, eroded, and filled exclusively for visualization purposes.
2.5.2. Recognition test phase
A recognition memory test was conducted following a 15-min delay after the encoding phase (mean time from last TMS pulse to test start: 57 min, SD = 2.4 min, range: 54–65 min). cTBS TMS protocols have been reported to effect cognitive function up to 60 min after stimulation (Hoogendam et al., 2010; Thut and Pascual-Leone, 2010), suggesting that recognition memory testing was primarily conducted outside of this window and stimulation-related effects on memory performance likely reflect differences induced during encoding.
We tested memory for studied words and their locations. Studied words were randomly intermixed with an equal number of novel lure words matched on length, frequency, and concreteness. For all studied words, subjects performed an item memory test followed by a source memory test. During the item memory test, subjects discriminated studied from lure words using a four-point confidence scale (confident old, guess old, guess new, and confident new); words were presented individually at the center of the screen above the confidence scale. During the source memory test, the word remained at the screen center above a four-point confidence scale that subjects used to indicate whether the word was presented on the left or right of the screen during the study phase (confident left, guess left, guess right, and confident right). Subjects were given up to 5 s to respond for item and source memory trials, and their response cued presentation of the next item (in each experimental session: 320 word recognition trials, 160 word location recall trials; mean test duration: 18 min).
Behavioral analyses were performed in R. Word location recall accuracy and confidence ratings were analyzed independently and as a function of word recognition success, in line with our a priori predictions. As described below, three memory trial conditions were defined based on the resultant pattern of confident memories: strong, moderate, and weak memory trials.
We examined the effects of brain stimulation on memory performance at the group level. For each stimulation condition, we calculated the proportion of strong, moderate, and weak memory trials out of the total number of studied-word trials (i.e., 160 trials) and submitted these values to 1-by-3 RM-ANOVAs with stimulation condition as the within-subject factor (posterior parietal, anterior parietal, and control stimulation), followed by pairwise comparisons with Bonferroni corrections (corrected p values are indicated as pbf). For significant contrasts, we report Cohen's d.
3. Results
3.1. Recognition memory performance
During the recognition memory test (Fig. 3A), subjects discriminated studied words from novel foils while simultaneously rating word memory confidence (item memory; Fig. 3B). On average, subjects were more successful discriminating old from new words when they reported confidence in the judgement (d′: M = 2.13, SD = 0.64) than when they reported that they had guessed (d′: M = 0.31, SD = 0.37) (mean d′ difference = 1.82; t(15) = 9.29, p < 0.001, d = 3.51). Location (left or right of the screen) memory was tested for all studied words following the item memory response (location memory; Fig. 3C). There was a relationship between source memory accuracy and subjective confidence, such that source memory varied as a function of subjective confidence in both item and source memory judgements (interaction of item and source memory confidence ratings on source memory accuracy: F(1, 15) = 11.36, p < 0.001, ηp 2 = 0.43). Confidence in source memories did not significantly vary when subjects reported that they had guessed the item (t(15) = 0.22, pbf = 0.83). However, when subjects confidently recalled items, confident source recollections (M = 0.87, SD = 0.11) were associated with higher source accuracy compared to guessed locations (M = 0.57, SD = 0.26; t(15) = 11.40, pbf < 0.001). Therefore, source memory strongly correlated with confidence across both item and source judgments.
Fig. 3.
Memory performance linked to item and source memory confidence. (A) Subjects performed a semantic categorization task (i.e., judging whether words were animals) while fMRI was recorded. Following a delay, a 2-stage memory test was administered whereby item recognition then location memory was tested. For the item recognition prompt, subjects attempted to discriminate studied words from novel foils while simultaneously rating confidence using a 4-point scale (confident/guess). After registering the recognition response, location memory was tested for each studied word (studied-left/studied-right) with simultaneous reporting of confidence (confident/guess). (B) Subjects were significantly more accurate in discriminating studied from novel words when they self-reported confident item memory. (C) Subjects were significantly more accurate when they reported they were confident making both item and source memory judgments. ***p < 0.001.
Reflecting the very strong relationship between item and source memory confidence (Table 2), we binned trials into strong, moderate, and weak memory conditions, as follows. The strong memory condition consisted of trials in which subjects confidently recognized both the studied word and its studied location. The moderate memory condition consisted of trials in which subjects accurately identified both the item and its location, but reported guessing on one or both of these judgments. The weak memory condition consisted of trials in which subjects responded inaccurately for either the item or location judgment, irrespective of their confidence levels.
Table 2.
Item recognition and source recall performance. Item recognition accuracy is reported as accurate item recognition (i.e., as old or new) in each response condition per total trials in the response condition. Source recall accuracy is reported as accurate source recall per total trials in the response condition (“All trials”) or per total accurate item recognition trials in the response condition (“Item hits only”). All reported vales are mean proportions followed by standard deviations.
|
Response condition |
Item recognition accuracy |
Source recall accuracy |
|
|---|---|---|---|
| All trials | Item hits only | ||
| Old/studied items | Hits | ||
| Item conf., source conf. | 0.95 (0.05) | 0.87 (0.12) | 0.87 (0.11) |
| Item guess, source conf. | 0.81 (0.25) | 0.61 (0.24) | 0.57 (0.26) |
| Item conf., source guess | 0.67 (0.17) | 0.59 (0.06) | 0.61 (0.07) |
| Item guess, source guess | 0.62 (0.14) | 0.54 (0.24) | 0.58 (0.11) |
| New/lure items | Correct rejections | ||
| Item conf. | 0.93 (0.06) | – | – |
| Item guess | 0.56 (0.23) | – | – |
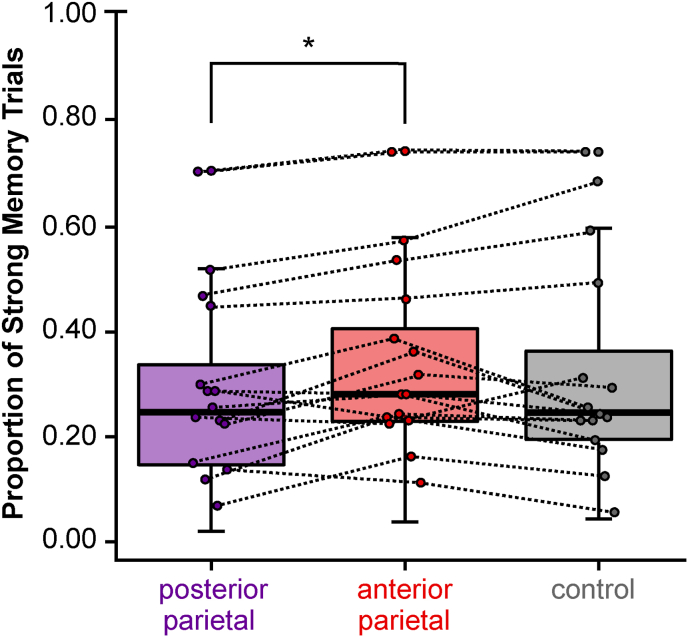
3.2. Stimulation condition affects proportion of strong memory trials
We examined the effect of stimulation condition on proportion of strong, moderate, and weak memory trials. Stimulation condition significantly influenced the proportion of strong memory trials (Fig. 4; main effect: F(2, 30) = 3.50, p = 0.04, ηp 2 = 0.19). Posterior parietal stimulation significantly reduced strong memory relative to anterior parietal stimulation (t(15) = 3.24, pbf = 0.02, d = 0.35). The proportion of strong memory trials were likewise numerically reduced following posterior parietal stimulation relative to control stimulation (t(15) = 1.41, pbf = 0.18, d = 0.08). The proportion of strong memory trials did not vary between anterior parietal and control stimulation conditions (t(15) = 0.98, pbf = 0.34, d = 0.14). Thus, the proportion of strong memory trials was lower following posterior parietal compared to anterior parietal stimulation. Stimulation condition did not significantly influence the proportion of weak memory trials (F(2, 30) = 0.10, p = 0.90, all pairwise pbf values > 0.99) or moderate memory trials (F(2, 30) = 1.30, p = 0.29, all pairwise pbf values > 0.25). The effects of posterior parietal stimulation were therefore specific to memory formation characterized by later item and location accuracy and confidence (cf. similar results of posterior parietal TMS in (Koen et al., 2018; Yazar et al., 2014)).
Fig. 4.
Posterior parietal stimulation reduces the proportion of strong memory trials. Subjects had fewer strong explicit memory trials following posterior relative to anterior parietal stimulation. Colored dots indicate individual subjects; box-and-whisker plots include whiskers marking first and third quartiles. *p < 0.05.
The order of stimulation conditions across the three experimental sessions did not impact the proportion of strong memory trials (all pairwise pbf values > 0.36), indicating that the effects of stimulation on memory were not driven by practice or order effects.
The above analyses were conducted on memory strength classification derived when item and source memory confidence and accuracy were considered together. We further analyzed whether stimulation condition influenced the proportion of confident item or source memories, considered separately. Stimulation condition did not significantly influence the proportion of confident, accurate item responses (F(2, 30) = 2.17, p = 0.13, all pairwise pbf values > 0.14). Likewise, stimulation condition only had marginal impact on the proportion of confident, accurate location responses (F(2, 30) = 2.94, p = 0.07), driven by a weak reduction of confident location trials following posterior compared to anterior parietal stimulation (t(15) = 2.41, pbf = 0.09, all other pairwise pbf values > 0.14). We interpret these findings to suggest that jointly considering item and source confidence yielded a more diagnostic indicator of strong explicit memory than either performance type considered separately, as it was more unlikely that subjects guessed correctly with high confidence on both item and item location judgments relative to either one independently. Thus, posterior parietal stimulation resulted in an overall reduction in the proportion of strong memory trials relative to anterior parietal stimulation.
3.3. Left inferior frontal and posterior temporal activity predicted memory formation
Strong, moderate, and weak memory conditions allowed us to identify the brain regions that were important for highly-effective versus less-effective encoding (Buckner et al., 2000). We compared task-related fMRI activity during all encoding trials for which subjects later had strong, moderate, or weak memory (i.e., pooled across data from all stimulation conditions). Strong memory trials were associated with greater activity of left IFG, left posterior temporal gyrus, bilateral medial frontal gyrus, and left inferior occipital gyrus relative to moderate explicit memory trials (Table 3). As expected, the contrast of strong versus weak memory trials identified increased activity of a broader set of regions compared to the strong versus moderate comparison (Table 3). There were no significant differences in activity for moderate versus weak memory trials.
Table 3.
Comparisons between task-related fMRI activity during strong versus moderate and strong versus weak explicit memory trials. Cluster size (mm3), centroid stereotaxic coordinates (MNI), peak standardized t-statistic (z[t]), and cluster-corrected p thresholds (p) are provided for each supra-threshold cluster. All t-tests were two-sided. Negative z values indicate greater activity during weak relative to strong memory trials. For primary clusters surviving overlap between strong vs. moderate and strong vs. weak explicit memory masks, cluster size (mm3) and center of mass coordinates (MNI) are displayed.
| mm3 | X | Y | Z | z[t] | p < | |
|---|---|---|---|---|---|---|
| Strong versus moderate explicit memory trials | ||||||
| L inferior frontal gyrus | 7348 | −53 | 25 | −9 | 5.48 | 0.0001 |
| L posterior temporal gyrus | 1380 | −57 | −52 | −19 | 4.41 | 0.0001 |
| R medial frontal gyrus | 991 | −2 | 18 | 53 | 5.56 | 0.0001 |
| L medial frontal gyrus | 471 | −8 | 42 | −13 | 6.09 | 0.001 |
| L inferior occipital gyrus | 258 | −21 | −97 | −8 | 5.14 | 0.02 |
| Strong versus weak explicit memory trials | ||||||
| L inferior frontal gyrus | 11796 | −53 | 25 | −9 | 4.68 | 0.0001 |
| L posterior temporal gyrus | 4309 | −57 | −58 | −24 | 4.69 | 0.0001 |
| L medial frontal gyrus | 2806 | −3 | 20 | 45 | 4.85 | 0.0001 |
| L inferior occipital gyrus | 1450 | −26 | −97 | −8 | 6.51 | 0.0001 |
| R inferior occipital gyrus | 1167 | 33 | −95 | −8 | 5.37 | 0.0001 |
| L inferior parietal lobule | 840 | −55 | −24 | 56 | 5.27 | 0.0001 |
| L precuneus | 774 | −34 | −63 | 42 | 4.80 | 0.0001 |
| R inferior frontal gyrus | 684 | 49 | 11 | 29 | 4.81 | 0.0001 |
| R cerebellum (Crus I) | 639 | 46 | −55 | −24 | 4.25 | 0.0001 |
| R medial frontal gyrus | 582 | 54 | 37 | 18 | 4.65 | 0.0002 |
| R cerebellum (VI) | 430 | 31 | −70 | −21 | 4.00 | 0.0005 |
| R inferior frontal gyrus | 410 | 23 | 27 | −17 | 5.32 | 0.001 |
| R middle frontal gyrus | 344 | 43 | 56 | 3 | −5.26 | 0.002 |
| R cuneus | 328 | 13 | −70 | 12 | 4.57 | 0.002 |
| R middle temporal gyrus | 295 | 67 | −20 | −13 | −4.05 | 0.005 |
| R cingulate gyrus | 270 | 2 | −2 | 30 | 5.42 | 0.01 |
| L parahippocampal gyrus | 238 | −31 | −6 | −33 | 4.10 | 0.02 |
| R inferior frontal gyrus | 217 | 38 | 25 | −5 | 4.51 | 0.03 |
| Overlapping clusters | ||||||
| L inferior frontal gyrus | 5751 | −44 | 27 | 6 | – | – |
| L inferior temporal gyrus | 991 | −48 | −51 | −17 | – | – |
The strong versus moderate and strong versus weak contrasts were similar in their peak differences, and formal analysis of overlap indicated that greater activity of left IFG and temporal areas were common to both comparisons. These regions are semantic memory hubs commonly identified as critical for memory formation in semantic encoding studies (Kirchhoff et al., 2005; Kohler et al., 2004; Yazar et al., 2014). The peaks of these regions were identified for subsequent analyses on the effects of stimulation on functional connectivity (IFG MNI: 53, 25, −9, temporal MNI: 57, −52, −19, displayed in Fig. 4, Fig. 6). These peaks were determined based on the more stringent contrast (strong vs. moderate memory) in order to best characterize regions associated with subjective confidence during recollection (but note that peak IFG MNI coordinates were identical for strong-moderate and strong-weak contrasts, and distance between strong-moderate and strong-weak peak temporal coordinates was only 7.80 mm).
Fig. 6.
Posterior parietal stimulation reduces connectivity between the hippocampal network and task-relevant brain regions. Hippocampal network fMRI connectivity with IFG and temporal-task relevant regions was consistently reduced following posterior parietal stimulation versus anterior parietal (left) and control (right) stimulation conditions. Dots indicate individual subjects, and correlations are plotted with regression lines including confidence intervals. *p < 0.05.
3.4. Posterior parietal stimulation reduced fMRI connectivity of task-relevant regions with the hippocampal network
We next assessed how stimulation parameters influenced resting-state fMRI connectivity of IFG and temporal task-relevant areas with either the frontal or hippocampal networks.
Stimulation condition significantly influenced fMRI connectivity between the hippocampal network and IFG (F(2, 34) = 4.52, p = 0.02, ηp 2 = 0.21) (Fig. 5). Posterior parietal stimulation decreased fMRI connectivity of the hippocampal network with the task-relevant IFG area relative to anterior temporal stimulation (t(17) = 3.79, pbf = 0.004, d = 0.67) and relative to control stimulation (t(17) = 2.68, pbf = 0.05, d = 0.58). This finding only emerged in comparisons to connectivity following posterior parietal stimulation, as anterior parietal stimulation did not influence fMRI connectivity of the hippocampal network with IFG relative to control stimulation (t(34) = 0.17, pbf = 0.87).
Fig. 5.
Posterior parietal stimulation reduces connectivity between the hippocampal network and task-relevant brain regions. Resting-state fMRI connectivity between the hippocampal network and task-relevant brain regions – (A) IFG and (B) posterior temporal cortex – was reduced following posterior parietal relative to anterior parietal or control stimulation. Lines are drawn between hippocampal network regions and the peaks of task-relevant IFG and temporal brain activity. Dots on bar plots indicate individual subjects; means are plotted alongside standard errors. **p < 0.01, *p < 0.05.
Stimulation condition resulted in marginal variation in fMRI connectivity of the hippocampal network with task-relevant temporal regions (F(2, 34) = 2.99, p = 0.06, ηp 2 = 0.15). fMRI connectivity of the task-relevant temporal area with the hippocampal network was significantly lower for posterior stimulation relative to anterior parietal stimulation (t(17) = 2.88, pbf = 0.03, d = 0.52). Neither network-targeted stimulation resulted in significantly different fMRI connectivity between temporal regions and the hippocampal network when compared to control stimulation (posterior parietal vs. control: t(17) = 1.83, pbf = 0.26; anterior parietal vs. control: t(17) = 0.42, pbf = 0.68).
Stimulation resulted in similar effects on fMRI connectivity of the hippocampal network to both task-relevant regions (Fig. 6). Within-subjects correlation found that subjects with greater reductions in hippocampal network connectivity with IFG due to posterior parietal stimulation relative to control stimulation also had greater reductions in hippocampal network connectivity to task-relevant temporal regions (r(18) = 0.58, p = 0.01). The same relationship was identified when comparing the effects of posterior to anterior parietal stimulation (r(18) = 0.56, p = 0.02). This suggests that posterior parietal stimulation elicited similar changes in fMRI connectivity response profiles for the hippocampal memory network and both task-relevant brain regions.
In contrast, stimulation condition did not significantly affect fMRI connectivity of the frontal network with either IFG (F(2, 34) = 0.66, p = 0.52, all pairwise pbf values > 0.70) or temporal regions (F(2, 34) = 0.29, p = 0.76, all pairwise pbf values > 0.99) (Fig. 7). Interestingly then, anterior parietal stimulation did not affect connectivity between task-relevant brain regions and the frontal memory network (of which the anterior parietal cortex is a network hub).
Fig. 7.
Stimulation condition does not affect connectivity between the frontal network and task-relevant brain regions. Resting-state fMRI connectivity between the frontal network and task-relevant brain regions – (A) IFG and (B) posterior temporal cortex – did not differ between stimulation conditions. Lines are drawn between frontal network regions and the peaks of task-relevant IFG and temporal brain activity. Dots on bar plots indicate individual subjects; means are plotted alongside standard errors. **p < 0.01, *p < 0.05.
3.5. Control analyses to test the network vs. local nature of stimulation effects
To evaluate the possibility that what we interpret as network-level effects instead merely reflect the local impact of stimulation on parietal activity, and therefore how the stimulated portions of parietal cortex interact with task-relevant regions, we repeated the analyses described above after removing parietal cortex stimulation locations used for the anterior and posterior parietal stimulation conditions from the analysis (i.e., the hippocampal network excluding its posterior parietal location and the frontal network excluding its anterior parietal location, see Table 1). As in the main analysis, stimulation condition did not significantly influence frontal network connectivity with IFG (F(2, 34) = 0.59, p = 0.56, all pairwise pbf values > 0.83) or temporal task-relevant regions (F(2, 34) = 0.34, p = 0.71, all pairwise pbf values > 0.99). Also as in the main analysis, stimulation condition significantly influenced fMRI connectivity of the hippocampal network with IFG (F(2, 34) = 4.11, p = 0.03, ηp 2 = 0.19). Hippocampal network fMRI connectivity with IFG was reduced for posterior relative to anterior parietal stimulation (t(17) = 3.78, pbf = 0.004, d = 0.68) and weakly reduced relative to control stimulation (t(17) = 2.31, pbf = 0.10, d = 0.52) stimulation, with little difference between control and anterior parietal stimulation conditions (t(17) = 0.35, pbf = 0.99). Also as in the main analysis, marginal effects of stimulation on hippocampal network connectivity with task-relevant temporal regions were identified in this analysis (F(2, 34) = 2.44, p = 0.10, ηp 2 = 0.13), driven as in the main analysis by reduced hippocampal network connectivity for posterior versus anterior parietal stimulation (t(17) = 2.93, pbf = 0.03, d = 0.49). As before, there were no meaningful differences in hippocampal network connectivity with task-relevant temporal regions when comparing either network-targeted stimulation condition to control stimulation (posterior parietal vs. control: t(17) = 1.20, pbf = 0.74; anterior parietal vs. control: t(17) = 0.81, pbf = 0.99). It is therefore unlikely that stimulation-related changes in connectivity of task-relevant regions with the hippocampal network were driven by the local effects of stimulation on parietal cortex.
3.6. Control analyses to test hippocampal network disconnection via stimulation
We additionally examined whether effects of posterior parietal stimulation on hippocampal memory network connectivity were limited to task-relevant regions in the parallel frontal memory network (e.g., default network-A connections to default network-B regions), or if posterior parietal stimulation resulted in global disconnection of the hippocampal network. Occipital cortex and M1 regions were identified based on prior evidence indicating that these regions are not typically activated during memory formation (right lateral occipital cortex mean MNI: 29, −24, −13, (Dave et al., 2020); right M1 mean MNI: 33, −23, 67 (Sarfeld et al., 2012)). Importantly, these brain regions did not show differences in task-related fMRI activity during strong memory trials vs. weak or moderate memory trials (Table 3), indicating that these brain regions are unlikely to be recruited in the formation of strong memories in the current paradigm. Stimulation condition did not significantly affect fMRI connectivity of the hippocampal network with either right lateral occipital cortex (F(2, 34) = 0.07, p = 0.93, all pairwise pbf values > 0.99) or right M1 regions (F(2, 34) = 0.36, p = 0.70, all pairwise pbf values > 0.99). It is thus unlikely that posterior parietal stimulation globally disconnected the hippocampal network from other brain regions, i.e., these effects were not global, as all brain regions, including these control locations, would be expected to change in connectivity given stimulation-related global disconnection of the hippocampal network. Instead, it is more likely that posterior parietal stimulation specifically impacted hippocampal network connections to its parallel frontal memory network.
3.7. Evaluation of hippocampus as a potential “task-relevant” region
Effective encoding of item and source memories has frequently been tied to the hippocampus (reviewed in Paller and Wagner, 2002), though notably, semantic encoding strategies often do not directly engage hippocampal activity (Kirchhoff et al., 2005; Murray and Ranganath, 2007; Reber et al., 2002), as seen in the current study. It is possible that we could have identified hippocampal involvement in the current task, e.g., if trial counts would have been suitable for comparing source memory hits versus misses with subjective confidence held constant (e.g. Rugg and Yonelinas, 2003). We therefore scrutinized change in hippocampal connectivity to both memory networks. The left hippocampus was identified by its membership in the hippocampal network (see Methods). We performed control analyses to determine if stimulation condition affected connectivity between the hippocampus and either the frontal or hippocampal memory network (i.e., the hippocampal network excluding the left hippocampal location). Stimulation condition did not modulate hippocampal connectivity with either the frontal network (F(2, 34) = 1.13, p = 0.33, all pairwise pbf values > 0.67) or non-left hippocampal regions of the hippocampal network (F(2, 34) = 0.94, p = 0.40, all pairwise pbf values > 0.66). Therefore, while posterior parietal stimulation decoupled hippocampal network activity from task-relevant brain regions, it did not do so by directly modulating left hippocampal connectivity with frontal or hippocampal memory networks.
4. Discussion
Noninvasive theta-burst stimulation targeting a parietal component of the hippocampal memory network decreased this network's fMRI connectivity with IFG and temporal regions implicated via task-based fMRI in memory formation. This decreased connectivity occurred with corresponding reduction in subjective recollection. These findings indicate that task-relevant IFG and temporal brain regions support memory formation via their interaction with the distributed hippocampal network. This is notable because semantic encoding that improves memory (Craik and Lockhart, 1972) is associated with greater activity in IFG and temporal brain areas (Blumenfeld and Ranganath, 2007; Kirchhoff et al., 2005), i.e., regions which are distinct from the hippocampal network. Across numerous similar paradigms, semantic encoding elicits consistent activity in semantic memory regions but not the hippocampus (Kirchhoff et al., 2005; Murray and Ranganath, 2007; Reber et al., 2002), though hippocampal network activity supports successful memory formation. We likewise found that posterior parietal cTBS effectively “decoupled” the hippocampal network from IFG and posterior brain regions which were crucial for encoding in the current task.
Effects of posterior parietal stimulation emerged relative to stimulation of the anterior parietal cortex. Differential stimulation effects provide direct evidence for distinct hippocampal versus frontal network territories comprising relatively posterior versus relatively anterior parietal cortex, respectively. Such distinction has been hypothesized based on previous fMRI evidence (Braga and Buckner, 2017; Braga et al., 2019; Cabeza et al., 2012; Vilberg and Rugg, 2008), but interpretation has been limited due to variability in the loci of fMRI activity peaks across studies (Cabeza et al., 2012; Uncapher and Wagner, 2009) and due to interpretive limitations in assigning regions to specific networks based on fMRI alone (Bijsterbosch et al., 2020; Woo et al., 2014). Thus, these findings are important for directly confirming this hypothesized functional network subdivision within parietal cortex.
These results further support the relatively high effective resolution of the stimulation protocol used (single-session cTBS), given the proximity of parietal stimulation locations (∼32 mm apart). Although some evidence suggests that TMS effects on neural activity might be highly focal, on the order of 1–2 mm (Romero et al., 2019), and highly focal stimulation effects can be observed within primary motor cortex, the current findings provide novel evidence of functional distinctions given stimulation of two nearby locations within association cortex in the same experimental design. Although our individualized network-targeted stimulation protocol is based on the premise of such selectivity, future studies are needed to determine whether individualized targeting is more effective than what would have been achieved using relatively anterior versus posterior parietal cortex targets based on group average connectivity.
cTBS has been proposed (Hebscher and Voss, 2020) to mimic and thereby influence the endogenous preferred theta-nested-gamma activity rhythm of the hippocampal network (Lega et al., 2012; Zhang and Jacobs, 2015). The directionality of stimulation effects on the hippocampal network may be linked to the cognitive processes engaged during encoding. Several previous studies using perceptual encoding paradigms have found posterior parietal cTBS increased performance on strongly hippocampal-dependent memory tasks, relative to other (non-theta) stimulation rhythms and relative to stimulation locations outside of the hippocampal network (Hebscher et al., 2021; Hermiller et al., 2020; Hermiller et al., 2019; Tambini, Nee, & D'Esposito, 2018). These studies primarily employed non-word visual paradigms to investigate effects of parietal cTBS on recall of object locations in visual scenes (Tambini et al., 2018) and recall or recognition of videos and scenes (Hebscher et al., 2021; Hermiller et al., 2020). Although Hermiller et al., 2019a, Hermiller et al., 2019b found memory enhancement using a word memory task, subjects in that experiment associated words with the color they were presented in during study, which is not believed to involve deep semantic processing (Craik and Lockhart, 1972). Studies reporting enhancement thus have used tasks that involve primarily perceptual associative memory demands.
In contrast, subjects in the current study completed a word animacy categorization task known to engage frontal semantic brain regions during study. Our findings are more similar to previous semantic encoding studies, which found posterior parietal cTBS selectively reduced subjective recollection (Koen et al., 2018; Kohler et al., 2004; Yazar et al., 2014) and resulted in subjects generating fewer details during tasks that require semantic simulation or divergent thinking (Thakral et al., 2017, 2020). Critically, the current findings demonstrate that posterior parietal cTBS also “decoupled” the hippocampal network from brain regions supporting semantic encoding, suggesting a parallel neural pathway by which the acute effects of cTBS may differ for memory tasks with perceptual associative encoding versus semantic encoding demands.
It is notable that anterior parietal cTBS did not modulate fMRI connectivity between task-relevant brain regions and the frontal network relative to control stimulation, inconsistent with our a priori prediction. Our prediction was based on well-established associations been anterior parietal cortex and semantic processing (reviewed in Humphreys et al., 2021). Thus, the evidence we provide is conceptually similar to a neuropsychological “single dissociation” (Teuber, 1955), as we were able to differentially modulate hippocampal network via posterior versus anterior stimulation, but were not able to correspondingly modulate frontal network via anterior versus posterior stimulation. While frontal network involvement in episodic memory is thought to rely on synchronous theta band activity, frontal network function may depend more on activity in higher frequency bands, such as beta (Backus et al., 2016). It is thus plausible that cTBS was less effective for modulating this network because it was an inappropriate stimulation rhythm. Further research is needed to evaluate this possibility. We additionally did not find anterior parietal cTBS modulated performance on semantic categorization tasks; however, semantic categorization accuracy was at or near ceiling across all stimulation conditions in most subjects, suggesting this task may not have the sensitivity required to show stimulation-related modulation. Nonetheless, anterior parietal cTBS provided an ideal comparison for the effects of posterior parietal stimulation, as these areas were nearby within parietal cortex and felt subjectively similar for participants. Our findings of different outcomes across stimulation conditions provide novel and direct evidence that hippocampal and frontal networks are functionally segregated within parietal cortex, validating previous evidence for dissociation based on neuroimaging (Cabeza et al., 2012; Vilberg and Rugg, 2008), as discussed above.
Although item confidence is often considered distinct from relational memory thought to support retrieval of studied locations and similar memory expressions (i.e., source or context (Davachi and Wagner, 2002; Eichenbaum, 2004)), item and location confidence were highly correlated in this study. The strong relationship between item and source confidence supported our use of memory strength categories, which is consistent with previous work suggesting that performance in some item and source memory tasks can be accounted for by memory strength (Smith et al., 2011; Squire, Genzel, bWixted, & Morris, 2015; Yazar et al., 2014). We suggest that subjects may have employed a semantic encoding strategy benefiting memory for both words and their left/right locations, producing highly verbalizable memories that were thereby strongly coupled with event and event context confidence. This is consistent with the relationship identified between strong memory formation, involving confidence item and source recognition, and increased activity in IFG and temporal regions of the semantic memory network during semantic encoding (Kirchhoff et al., 2005; Kohler et al., 2004; Yazar et al., 2014). It is possible that careful analyses could have isolated hippocampal correlates of memory formation (see above) had trial counts been sufficient for such comparisons. Yet, stimulation did not affect interaction of either network with the hippocampus, so such analyses are unlikely to affect the current conclusions. Instead, effects of stimulation on task performance seem to be related to altering interactions between the hippocampal network and frontal regions critical for task performance. Notably, while stimulation condition affected strong memory responses, stimulation did not impact item memory or item location memory performances separately. It is possible that item memory or item location memory scores may be less sensitive to the effects of stimulation and therefore observing effects on these variables may require a larger sample than collected in the current study.
Several recent proposals have suggested that subregions of lateral parietal cortex may make similar functional contributions during semantic and episodic tasks when episodic content has semantic properties (Binder and Desai, 2011). While some have suggested that parietal activation generally reflects the reinstatement of earlier semantic processing in order support successful event retrieval (Renoult et al.2019), others have posited that parietal activation supports the online convergence of multimodal representations (e.g., “event-semantic” information binding) (Humphreys et al., 2021; Rugg and King, 2018). Further work should thus directly examine whether activation across the parietal cortex reflect similar processes across multiple domains, or unique contributions to distinct functions.
One limitation for the present study is the use of vertex as a control stimulation location. While posterior parietal stimulation reduced fMRI connectivity between task-relevant regions and the hippocampal network relative to both anterior parietal and vertex stimulation conditions, only directional (non-significant) reductions in behavioral performance followed posterior parietal versus vertex stimulation. Vertex stimulation has had variable application as a control condition for TMS, with recent evidence suggesting low-frequency vertex stimulation may deactivate default network regions, including the posterior parietal cortex (Jung et al., 2016). There is unlikely to ever be a perfect control condition in brain stimulation studies and it is important to therefore take into account the pattern of findings across multiple stimulation locations. Critically, our primary comparison between parietal stimulation locations was sufficient to test their differential functions and resulted in reductions in both fMRI connectivity and subjective recollection following posterior versus anterior parietal stimulation, and the same pattern was evident relative to vertex.
In sum, by employing a network-targeted stimulation approach, we found evidence for distinct parietal contributions to hippocampal versus frontal memory networks and supported the theory that frontal regions involved in semantic categorization contribute to subjective recollection by interacting with the distributed hippocampal network. These findings are consistent with the pervasive, yet rarely tested, assumption of shared information processing by distributed brain networks in support of cognition (Eichenbaum, 2017; McIntosh et al., 1997; Mesulam, 1990). This comports with a recent emphasis on the use of network-targeted brain stimulation to evaluate theories of the network organization of cognition (Fox et al., 2012; Hebscher and Voss, 2020), such as by directly testing relevant network functional organization suggested by methods such as fMRI. Further studies in this vein could lead to the ability to control network activity via stimulation, thereby yielding potential treatments for the many neurologic and psychiatric disorders that involve brain network dysfunction.
Funding
This research was supported by the National Institutes of Health [grant numbers R01-MH106512, F32-MH118718]. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
CRediT authorship contribution statement
Shruti Dave: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Stephen VanHaerents: Supervision, Writing – review & editing. Borna Bonakdarpour: Supervision, Writing – review & editing. M.- Marsel Mesulam: Supervision, Writing – review & editing. Joel L. Voss: Conceptualization, Methodology, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank B.E. Durr, M.M. Gunlogson, M.S. Hermiller, E. Karp, A.S. Nilakantan, and S. Wert for assistance with the experiment. Neuroimaging was performed at the Northwestern University Center for Translational Imaging, supported by the Northwestern University Department of Radiology. This research was supported in part through the computational resources and staff contributions provided for Quest, the high-performance computing facility at Northwestern University, which is jointly supported by the Office of the Provost, the Office for Research, and Northwestern University Information Technology.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crneur.2022.100030.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus A.R., Schoffelen J.M., Szebenyi S., Hanslmayr S., Doeller C.F. Hippocampal-Prefrontal theta oscillations support memory integration. Curr. Biol. 2016;26:450–457. doi: 10.1016/j.cub.2015.12.048. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch J., Harrison S.J., Jbabdi S., Woolrich M., Beckmann C., Smith S., Duff E.P. Challenges and future directions for representations of functional brain organization. Nat. Neurosci. 2020;23(12):1484–1495. doi: 10.1038/s41593-020-00726-z. [DOI] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H. The neurobiology of semantic memory. Trends Cognit. Sci. 2011;15:527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld R.S., Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Braga R.M., Buckner R.L. Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron. 2017;95:457–471 e455. doi: 10.1016/j.neuron.2017.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga R.M., Van Dijk K.R.A., Polimeni J.R., Eldaief M.C., Buckner R.L. Parallel distributed networks resolved at high resolution reveal close juxtaposition of distinct regions. J. Neurophysiol. 2019;121:1513–1534. doi: 10.1152/jn.00808.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brysbaert M., Warriner A.B., Kuperman V. Concreteness ratings for 40 thousand generally known English word lemmas. Behav. Res. Methods. 2014;46:904–911. doi: 10.3758/s13428-013-0403-5. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., DiNicola L.M. The brain's default network: updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 2019;20:593–608. doi: 10.1038/s41583-019-0212-7. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Logan J., Donaldson D.I., Wheeler M.E. Cognitive neuroscience of episodic memory encoding. Acta Psychol. 2000;105:127–139. doi: 10.1016/s0001-6918(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Ciaramelli E., Moscovitch M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cognit. Sci. 2012;16:338–352. doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Ciaramelli E., Olson I.R., Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat. Rev. Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A.H., Ge W., Metcalf W., Jakobsson E., Mainzer L.S., Lipka A.E. An assessment of true and false positive detection rates of stepwise epistatic model selection as a function of sample size and number of markers. Heredity. 2019;122:660–671. doi: 10.1038/s41437-018-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E., Faggi G., Scarpazza C., Mattioli F., Spaniol J., Ghetti S., Moscovitch M. Subjective recollection independent from multifeatural context retrieval following damage to the posterior parietal cortex. Cortex. 2017;91:114–125. doi: 10.1016/j.cortex.2017.03.015. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craik F.I., Lockhart R. Levels of processing: a framework for memory research. J. Verb. Learn. Verb. Behav. 1972;11:13. [Google Scholar]
- Davachi L., Wagner A.D. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J. Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Dave S., VanHaerents S., Voss J.L. Cerebellar theta and beta noninvasive stimulation rhythms differentially influence episodic memory versus semantic prediction. J. Neurosci. 2020;40:7300–7310. doi: 10.1523/JNEUROSCI.0595-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb J.B., Desmond J.E., Wagner A.D., Vaidya C.J., Glover G.H., Gabrieli J.D. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J. Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drowos D.B., Berryhill M., Andre J.M., Olson I.R. True memory, false memory, and subjective recollection deficits after focal parietal lobe lesions. Neuropsychology. 2010;24:465–475. doi: 10.1037/a0018902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Prefrontal-hippocampal interactions in episodic memory. Nat. Rev. Neurosci. 2017;18:547–558. doi: 10.1038/nrn.2017.74. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H., Yonelinas A.P., Ranganath C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Halko M.A., Eldaief M.C., Pascual-Leone A. Measuring and manipulating brain connectivity with resting state functional connectivity magnetic resonance imaging (fcMRI) and transcranial magnetic stimulation (TMS) Neuroimage. 2012;62:2232–2243. doi: 10.1016/j.neuroimage.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebscher M., Kragel J.E., Kahnt T., Voss J.L. Enhanced reinstatement of naturalistic event memories due to hippocampal-network-targeted stimulation. Curr. Biol. 2021;31:1428–1437. doi: 10.1016/j.cub.2021.01.027. e1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebscher M., Voss J.L. Testing network properties of episodic memory using non-invasive brain stimulation. Curr Opin Behav Sci. 2020;32:35–42. doi: 10.1016/j.cobeha.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiller M.S., Chen Y.F., Parrish T.B., Voss J.L. Evidence for immediate enhancement of hippocampal memory encoding by network-targeted theta-burst stimulation during concurrent fMRI. J. Neurosci. 2020;40:7155–7168. doi: 10.1523/JNEUROSCI.0486-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiller M.S., Karp E., Nilakantan A.S., Voss J.L. Episodic memory improvements due to noninvasive stimulation targeting the cortical-hippocampal network: a replication and extension experiment. Brain Behav. 2019;9 doi: 10.1002/brb3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiller M.S., VanHaerents S., Raij T., Voss J.L. Frequency-specific noninvasive modulation of memory retrieval and its relationship with hippocampal network connectivity. Hippocampus. 2019;29:595–609. doi: 10.1002/hipo.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendam J.M., Ramakers G.M., Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Humphreys G.F., Ralph M.A.L., Simons J.S. T.i.N. 2021. A Unifying Account of Angular Gyrus Contributions to Episodic and Semantic Cognition. [DOI] [PubMed] [Google Scholar]
- Hurley R.S., Bonakdarpour B., Wang X., Mesulam M.M. Asymmetric connectivity between the anterior temporal lobe and the language network. J. Cognit. Neurosci. 2015;27:464–473. doi: 10.1162/jocn_a_00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. H. Holt and company; New York: 1890. The Principles of Psychology. [Google Scholar]
- Janssen A.M., Oostendorp T.F., Stegeman D.F. The coil orientation dependency of the electric field induced by TMS for M1 and other brain areas. J. NeuroEng. Rehabil. 2015;12:47. doi: 10.1186/s12984-015-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J., Bungert A., Bowtell R., Jackson S.R. Vertex stimulation as a control site for transcranial magnetic stimulation: a concurrent TMS/fMRI study. Brain Stimul. 2016;9:58–64. doi: 10.1016/j.brs.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I., Andrews-Hanna J.R., Vincent J.L., Snyder A.Z., Buckner R.L. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100:129–139. doi: 10.1152/jn.00077.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff B.A., Schapiro M.L., Buckner R.L. Orthographic distinctiveness and semantic elaboration provide separate contributions to memory. J. Cognit. Neurosci. 2005;17:1841–1854. doi: 10.1162/089892905775008670. [DOI] [PubMed] [Google Scholar]
- Koen J.D., Thakral P.P., Rugg M.D. Transcranial magnetic stimulation of the left angular gyrus during encoding does not impair associative memory performance. Cognit. Neurosci. 2018;9:127–138. doi: 10.1080/17588928.2018.1484723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S., Paus T., Buckner R.L., Milner B. Effects of left inferior prefrontal stimulation on episodic memory formation: a two-stage fMRI-rTMS study. J. Cognit. Neurosci. 2004;16:178–188. doi: 10.1162/089892904322984490. [DOI] [PubMed] [Google Scholar]
- Lega B.C., Jacobs J., Kahana M. Human hippocampal theta oscillations and the formation of episodic memories. Hippocampus. 2012;22:748–761. doi: 10.1002/hipo.20937. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R., Nyberg L., Bookstein F.L., Tulving E. Differential functional connectivity of prefrontal and medial temporal cortices during episodic memory retrieval. Hum. Brain Mapp. 1997;5:323–327. doi: 10.1002/(SICI)1097-0193(1997)5:4<323::AID-HBM20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Mesulam M.M. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann. Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Mesulam M.M. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mesulam M.M., Rogalski E.J., Wieneke C., Hurley R.S., Geula C., Bigio E.H., Thompson C.K., Weintraub S. Primary progressive aphasia and the evolving neurology of the language network. Nat. Rev. Neurol. 2014;10:554–569. doi: 10.1038/nrneurol.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L.J., Ranganath C. The dorsolateral prefrontal cortex contributes to successful relational memory encoding. J. Neurosci. 2007;27:5515–5522. doi: 10.1523/JNEUROSCI.0406-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S.M., Cohen A.L., Power J.D., Wig G.S., Miezin F.M., Wheeler M.E., Velanova K., Donaldson D.I., Phillips J.S., Schlaggar B.L., Petersen S.E. A parcellation scheme for human left lateral parietal cortex. Neuron. 2010;67:156–170. doi: 10.1016/j.neuron.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller K.A., Wagner A.D. Observing the transformation of experience into memory. Trends Cognit. Sci. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Qin S., van Marle H.J., Hermans E.J., Fernandez G. Subjective sense of memory strength and the objective amount of information accurately remembered are related to distinct neural correlates at encoding. J. Neurosci. 2011;31:8920–8927. doi: 10.1523/JNEUROSCI.2587-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C., Ritchey M. Two cortical systems for memory-guided behaviour. Nat. Rev. Neurosci. 2012;13:713–726. doi: 10.1038/nrn3338. [DOI] [PubMed] [Google Scholar]
- Reber P.J., Siwiec R.M., Gitelman D.R., Parrish T.B., Mesulam M.M., Paller K.A. Neural correlates of successful encoding identified using functional magnetic resonance imaging. J. Neurosci. 2002;22:9541–9548. doi: 10.1523/JNEUROSCI.22-21-09541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Nguyen V.T., Sonkusare S., Lv J., Pang T., Guo L., Eickhoff S.B., Breakspear M., Guo C.C. Effective connectivity of the anterior hippocampus predicts recollection confidence during natural memory retrieval. Nat. Commun. 2018;9:4875. doi: 10.1038/s41467-018-07325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoult L., Irish M., Moscovitch M., Rugg M.D. From knowing to remembering: the semantic-episodic distinction. Trends Cognit. Sci. 2019;23:1041–1057. doi: 10.1016/j.tics.2019.09.008. [DOI] [PubMed] [Google Scholar]
- Romero M.C., Davare M., Armendariz M., Janssen P. Neural effects of transcranial magnetic stimulation at the single-cell level. Nat. Commun. 2019;10:2642. doi: 10.1038/s41467-019-10638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg M.D., King D.R. Ventral lateral parietal cortex and episodic memory retrieval. Cortex. 2018;107:238–250. doi: 10.1016/j.cortex.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg M.D., Yonelinas A.P. Human recognition memory: a cognitive neuroscience perspective. Trends Cognit. Sci. 2003;7:313–319. doi: 10.1016/s1364-6613(03)00131-1. [DOI] [PubMed] [Google Scholar]
- Sarfeld A.S., Diekhoff S., Wang L.E., Liuzzi G., Uludag K., Eickhoff S.B., Fink G.R., Grefkes C. Convergence of human brain mapping tools: neuronavigated TMS parameters and fMRI activity in the hand motor area. Hum. Brain Mapp. 2012;33:1107–1123. doi: 10.1002/hbm.21272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y., Kirwan C.B., Squire L.R. Activity in both hippocampus and perirhinal cortex predicts the memory strength of subsequently remembered information. Neuron. 2008;59:547–553. doi: 10.1016/j.neuron.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons J.S., Peers P.V., Mazuz Y.S., Berryhill M.E., Olson I.R. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cerebr. Cortex. 2010;20:479–485. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.N., Wixted J.T., Squire L.R. The hippocampus supports both recollection and familiarity when memories are strong. J. Neurosci. 2011;31:15693–15702. doi: 10.1523/JNEUROSCI.3438-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L.R., Genzel L., Wixted J.T., Morris R.G. Memory consolidation. Cold Spring Harbor Perspect. Biol. 2015;7:a021766. doi: 10.1101/cshperspect.a021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A., Nee D.E., D'Esposito M. Hippocampal-targeted theta-burst stimulation enhances associative memory formation. J. Cognit. Neurosci. 2018;30:1452–1472. doi: 10.1162/jocn_a_01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber H.L. Physiological psychology. Annu. Rev. Psychol. 1955;6:267–296. doi: 10.1146/annurev.ps.06.020155.001411. [DOI] [PubMed] [Google Scholar]
- Thakral P.P., Madore K.P., Schacter D.L. A role for the left angular gyrus in episodic simulation and memory. J. Neurosci. 2017;37:8142–8149. doi: 10.1523/JNEUROSCI.1319-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakral P.P., Madore K.P., Schacter D.L. The core episodic simulation network dissociates as a function of subjective experience and objective content. Neuropsychologia. 2020;136:107263. doi: 10.1016/j.neuropsychologia.2019.107263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G., Pascual-Leone A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 2010;22:219–232. doi: 10.1007/s10548-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh T.N., Kozak J., Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch. Clin. Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- Uncapher M.R., Wagner A.D. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol. Learn. Mem. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg K.L., Rugg M.D. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A.D., Shannon B.J., Kahn I., Buckner R.L. Parietal lobe contributions to episodic memory retrieval. Trends Cognit. Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wang J.X., Rogers L.M., Gross E.Z., Ryals A.J., Dokucu M.E., Brandstatt K.L., Hermiller M.S., Voss J.L. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345:1054–1057. doi: 10.1126/science.1252900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C.W., Krishnan A., Wager T.D. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn S.C., Hendriks M.P.H., Daselaar S.M., Kessels R.P.C., Schutter D. The posterior parietal cortex and subjectively perceived confidence during memory retrieval. Learn. Mem. 2018;25:382–389. doi: 10.1101/lm.048033.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazar Y., Bergstrom Z.M., Simons J.S. What is the parietal lobe contribution to long-term memory? Cortex. 2012;48:1381–1382. doi: 10.1016/j.cortex.2012.05.011. discussion 1383-1387. [DOI] [PubMed] [Google Scholar]
- Yazar Y., Bergstrom Z.M., Simons J.S. Continuous theta burst stimulation of angular gyrus reduces subjective recollection. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas A.P. Components of episodic memory: the contribution of recollection and familiarity. Phil. Trans. Biol. Sci. 2001;356:1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas A.P. Consciousness, control, and confidence: the 3 Cs of recognition memory. J. Exp. Psychol. Gen. 2001;130:361–379. doi: 10.1037//0096-3445.130.3.361. [DOI] [PubMed] [Google Scholar]
- Zhang H., Jacobs J. Traveling theta waves in the human Hippocampus. J. Neurosci. 2015;35:12477–12487. doi: 10.1523/JNEUROSCI.5102-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.