Abstract
Conflicting evidence suggest that perturbations of GABAergic neurotransmission play crucial roles in disrupting cortical neuronal network oscillations, memory, and cognitive deficits in Alzheimer's disease (AD). However, the role and impact of sex differences on GABAergic transmission in AD are not well understood. Using an APP knock-in mouse model of AD, APPNLGF mice, we studied the effects of acute diazepam administration on memory and anxiety-like behavior to unveil sex-dependent dysregulation of GABAergic neurotransmission. We also examined sex differences in GABAA receptor subunit mRNA and protein expression and the role of epigenetic regulation in hippocampus of APPNLGF mice. We found that diazepam elicited dose-dependent suppression of locomotion in wildtype and APPNLGF mice. However, a low dose, which had no significant effect in both male and female wildtype as well as female APPNLGF mice, significantly suppressed locomotion in male APPNLGF mice. Furthermore, this low dose of diazepam was more efficacious at eliciting anxiolytic-like effects in male than female APPNLGF mice. The same low dose of diazepam disrupted recognition memory exclusively in male APPNLGF mice. Biochemical analyses revealed that hippocampal α1 and α5 GABAA receptor subunits mRNA and protein expression were significantly higher in male than female APPNLGF mice and were regulated by histone H3 tri-methylation (H3K4me3) but not histone H3 acetylation. The higher sensitivity of APPNLGF males to diazepam-induced behavioral effects may potentially be due to epigenetic-dependent upregulation of hippocampal α1 and α5 GABAA receptor subunits expression compared to female APPNLGF mice. These findings suggest that dysregulation of GABAergic neurotransmission plays a significant role in memory and affective behavior, particularly in male APPNLGF mice.
Keywords: GABA, Diazepam, Alzheimer's, Epigenetics, Behavior, Hippocampus
Highlights
-
•
A low dose of diazepam (0.2 mg/kg), which has no effect in both male and female wildtype as well as female APPNLGF mice, significantly reduces locomotor activity in male APPNLGF mice.
-
•
The same low dose of diazepam induces stronger anxiolytic-like effects in male than female APPNLGF mice.
-
•
Diazepam impairs recognition memory in male but not in female APPNLGF mice.
-
•
Hippocampal α1 and α5 GABAA receptor subunits expression is significantly higher in male than female APPNLGF mice and are regulated by histone H3 tri-methylation.
1. Introduction
Alzheimer's disease (AD) is the most common neurodegenerative disorder characterized by progressive memory declined combined with certain neuropsychiatric symptoms, pathological changes such as neurofibrillary tangles and senile plagues, and preferential neuronal loss in hippocampus and prefrontal cortex (Kwakowsky et al., 2018a; Braak and Braak, 1991; Alzheimer's Association, 2012). Years of extensive research into the mechanisms underlying AD have yielded several possible mechanisms including β-amyloid (Aβ) oligomer-induced neuronal death and synaptic dysfunction, glutamate-induced excitotoxicity (Muke and Selkoe, 2012), loss of cholinergic neurons, and reduced cortical choline acetyltransferase activity (Mufson et al., 2008). There is growing evidence in support of important contributions of the primary inhibitor neurotransmitter, gamma-aminobutyric acid (GABA), to the pathogenesis of AD (Fritschy, 2008; Rudolph and Mohler, 2014a; Solas et al., 2015; Francis, 2005). In particular, postmortem studies have reported that AD patients have increased GABA levels in the cerebrospinal fluid (Li et al., 2016a) as well as decreased GABA levels in the most brain areas (Govindpani et al., 2017). However, inconsistent findings of GABA levels in the frontal, parietal and temporal cortices, and the hippocampal regions of AD postmortem brains have also been reported (Govindpani et al., 2017; Imbimbo and Giardina, 2011). In a preclinical study, Bell and colleagues found significant increase in GABAergic presynaptic bouton density in a TgCRND8 mouse model of AD (Bell et al., 2003). Most significantly, administration of the GABAA receptor antagonists, bicuculline and picrotoxin rescued memory deficits in adult APP/PS1 mice (Yoshiike et al., 2008). However, previous studies painted a complex, convoluted, and often inconsistent pictures of the relationship between GABAergic system and AD. Given the importance of GABAergic neurotransmission in neuronal function and homeostasis, maintenance of excitatory/inhibitory balance, and learning and memory processes, alterations of GABAergic function could be a critical factor in AD pathogenesis. Therefore, a better understanding of the role of GABAergic remodeling in AD could help discovering new targets for the development of innovative therapeutic alternatives. In fact, there is increasing interest in developing novel therapeutic agents targeting the alterations of GABAergic neurotransmission that have been reported in AD (Kwakowsky et al., 2018b; Li et al., 2016b; Guzman et al., 2018).
Although mounting evidence linking dysregulated GABAergic neurotransmission and AD (Kwakowsky et al., 2018a, 2018b; Govindpani et al., 2017; Guzman et al., 2018), the molecular mechanisms and sex-related impact underlying this linkage remain elusive. In this study, we used a newly developed AD mouse model, namely APPNLGF mice, which expresses a humanized Aβ sequence harboring three familial AD mutations [Swedish (NL), Beyreuther/Iberian (F), and Artic (G)] to model Aβ amyloidosis without non-physiological overexpression of APP (Sasaguri et al., 2017a). We used this AD mouse model to examine the sex-dependent role of GABAergic neurotransmission in AD by focusing on the pharmacological effects of a positive allosteric modulator of GABA action at GABAA receptors. Specifically, we evaluated the behavioral alterations elicited by acute administration of diazepam, a typical and full positive allosteric modulator of GABA action at GABAA receptors (Guidotti et al., 2005), on locomotor activity, anxiety-like behavior, and memory function in male and female APPNLGF mice. Of note, we used acute diazepam administration as a pharmacological tool to better understand the functional status of the GABAergic neurotransmission, rather than exploring a possible “therapeutic effect” of this drug in AD mice. Moreover, we evaluated the expression of specific GABAA receptor subunits in the hippocampus of male and female APPNLGF mice. We chose the hippocampus because it is one of the brain areas linked to memory deficits in AD and one of the most vulnerable areas exhibiting neuronal loss in both postmortem brains of AD subjects and preclinical models of AD (Braak and Braak, 1991; Serrano-Pozo et al., 2011). In addition, we examined the possible epigenetic mechanisms underlying differential expression of GABAA receptor subunits in the hippocampus between male and female APPNLGF mice, and most likely, the differential sensitivity to diazepam-induced behavioral effects in these mice.
2. Materials and methods
A total of 116 (12-month-old) male (n = 58) and female (n = 58) APPNLGF mice [APP KM670/671NL (Swedish), APP 1716F (Iberian), APP E693G (Artic); modification: APP: knock-in; homozygous; C57BL/6J genetic background)] and their respective wildtype littermates were used for this study. APPNLGF breeders were kindly provided by RIKEN (Japan). Genotyping was done at 21–24 days after birth by PCR with APPNLGF primers (forward: 5′-CTCCTTGTGGCTGGCGGTCACAC-3’; reverse: 5′-CTATCGTGGACCGAGAATGGTCATG-3′). The offspring were then separated with their original littermates into cages based on sex. Heterozygous APPNLGF mice were not used in the current study. Animals were housed in groups of 5 on a 12-hr light/dark cycle (lights on at 8:00 a.m.) with access to chow and water made available ad libitum. All experiments and procedures were carried out in accordance with the National Institute of Health Guidelines for the treatment of animals and the Current Guide for the Care and Use of Laboratory Animals under protocol approved by the Northwestern University Animal Care and Use Committee.
2.1. Drug treatment
Diazepam was obtained from Hoffman-La Roche (Nutley, NJ). The drug was dissolved in 5–10% of DMSO (depending on the desired final concentration) and subsequently diluted with vehicle containing 11% polyethylene glycol-400, 50% propylene glycol and 30% sterile water. Injection for vehicle and diazepam was 0.1 ml/10g body weight. Mice received intraperitoneal (i.p.) injection of vehicle or diazepam 30 min prior to behavioral testing or euthanasia.
2.2. Behavioral studies
Mice were handled once daily for five consecutive days before initiating behavioral experiments to minimize the potential stress that might be caused by interaction with the experimenter. A series behavioral test separated by 5-days inter-test intervals to allow complete drug washout were conducted beginning at 12 months of age; all behavioral tasks began at 9 a.m. Prior to testing, mice were transferred from their normal housing to the testing room 1 h before experimental session to allow for pretest-acclimation and to minimize potential stress due to exposure to new environment. Importantly, between each session, the chambers, tools, and instruments were carefully cleaned with 70% alcohol to eliminate olfactory odors. As previously described (Locci et al., 2021), the following testing sequence was used: a) locomotor activity/open field; b) novel object recognition; c) Y-maze; d) light-dark box.
2.2.1. Open field (OF)
The open field test was used to evaluate general locomotor activity and to assess anxiety behavior and the functional status of GABAergic neurotransmission in adult APPNLGF mice. A computerized automated system (Any-Maze) with an open field box chamber (50 cm × 50 cm x 40 cm) was used to evaluate locomotor activity as previously described (Locci and Pinna, 2019). Thirty minutes post vehicle or diazepam treatment, mice were placed in the center of the chambers. Horizontal sensor beam interruptions recorded as distance travelled in the open field box during 10 min trial sessions.
2.2.2. Light dark box (LD)
Mice were tested for anxiety-like behavior using the LD exploration/transition test (Kulesskaya and Voikar, 2014). The apparatus consists of a two-chamber metal grid floor shuttle box separated by a guillotine door. One compartment is dark, and the other is illuminated with a bright stimulus light (500 lux). Photobeams installed in each chamber detect the location and locomotor activity of the mice and report these activities in a Graphic State computer program (Colbourne), which controls the experimental processes. The testing equipment was housed within a sound-attenuating chamber, which contained a fan that provides 68 dB of background noise. On testing day, the animal was placed in the light chamber allowed to explore both the dark and light chambers for 10 min. Rodents prefer dark over light areas; however, when exposed to a novel environment, rodents tend to explore. These two conflicting “emotions” lead to observable anxiety-like behavior manifested as greater time spent in the safer, dark areas. The percent of time spent in the light and dark compartments represents anxiolytic or anxiogenic-like behavior.
2.2.3. Novel object recognition (NOR)
The Novel objective recognition is a well-established behavioral benchmark for assessing recognition memory, the human correlate of declarative memory (Broadbent et al., 2009a). The NOR test was performed in an apparatus consisting of an evenly illuminated plexiglass box (25 cm × 25 cm × 25 cm). The procedure consisted of 3 phases over a 24-day period: habituation, training, and retention. During the habituation phase (Day 1), each mouse was placed in the empty box and allowed to freely explore the box for 10 min. For the training phase (day 2–24) phase, two identical objects were placed in the box and positioned 6 cm away from the walls on opposite corners and each mouse allowed to explore the identical objects for 10 min and then returned to its home cage. The retention or testing (day 24) phase began 1 h after completion of the training phase; one of the two familiar objects was replaced with a novel object and the mouse allowed to explore the familiar and novel objects for 10 min. Object exploration was defined as sniffing, licking, or touching the objects with forepaw, but not leaning against, turning around, standing, or sitting on the objects. Object exploration was later scored from video recordings by an experimenter who was blinded to the treatment groups. The proportion of time spent exploring the novel object served as the measure of recognition memory for the familiar object. The data were expressed as discrimination index (DI) which represents the percentage of the time a mouse spent exploring the novel object (compared to the time spent exploring the familiar object) as a proportion of the time spent exploring both objects during retention testing.
2.2.4. Y-maze
The Y-maze is a spontaneous alternation behavioral paradigm used to assess short term and/or spatial working memory by evaluating the “willingness” of rodents to explore novel environments (Broadbent et al., 2009b). This spontaneous alternation Y-shaped apparatus consists of three opaque arms (5 cm wide × 21 cm long × 15.5 cm high) containing special cues positioned in the top inner part of each arm and oriented at 120° from each other. Mice are driven by an innate curiosity to explore previously unvisited areas; therefore, mice were allowed to explore the three arms of a maze placed on a stable table with an overhead video recorder. Mice were placed in the arm “A” (starting point, excluded by the analysis) facing the end of the arm and allowed to freely explore the apparatus for 5 min while a camera records their movements. A correct alternation is defined as discrete and successive entries into each open arm, including events where the animal directly progresses from one arm to the next in consecutive fashion (that is ABC, ACB, BAC, BCA, CAB, and CBA) without reentering two previously visited arms. The percentage of spontaneous alternation is expressed as the ratio of total successful alternations by the total number of possible alternations (i.e., the number of total entries minus two) multiplied by 100. The activity of each mouse was also recorded using an automated tracking system (Any-Maze).
2.3. Tissue collection, RNA extraction, and real-time polymerase chain reaction (PCR)
Five days after behavioral testing, mice were sacrificed by i.p. injection of Euthasol® solution followed by cervical dislocation and hippocampal tissue quickly dissected, frozen and stored at −80 °C. Only tissues belonging to vehicle-treated groups were used for relative quantitative real-time polymerase chain reaction (RT-qPCR), ChIP assay or Western blot. Total RNA was isolated from hippocampi tissue using TRIzol reagent and further purified using Qiagen RNeasy Kit and converted to cDNA using the Applied Biosystems (USA) High-Capacity Archive Kit (4368813). Relative qPCR was performed with the Applied Biosynthesis Real-Time PCR system using Maxima SYBR Green master mix (ThermoFisher Scientific, Waltham, MA). To ensure tissue-specific quantification of mRNA of interest and to avoid amplification of genomic DNA contamination, primers (see Supplementary Table 1) corresponding to mRNAs for genes of interest were designed to at least cross over one intron. Dissociation curves were conducted to establish the presence of a single amplicon at the predicted melting temperature and lack of primer-dimer formation. A comparative threshold cycle (CT) validation experiment was done to determine target and reference primer efficiency. For normalization of mRNAs expression, hypoxanthine-guanine phosphoribosyltransferase (Hprt1) was used as reference gene. CT values were used for relative quantification of target gene expression and normalized to Hprt1, and the relative expression levels were calculated as CT (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008).
2.4. Western blot (WB)
We performed WB analysis to investigate whether the changes in α1 and α5 GABAA receptor subunit mRNA expression observed were accompanied with changes in protein expression. As we previously described (Locci and Pinna, 2019; Locci et al., 2020), hippocampal tissues were homogenized in a mixture of ice-cold RIPA buffer (Sigma-Aldrich) and protease inhibitor cocktail solution (Fisher Scientific), centrifuged at 20,000 g for 10 min at 4 °C and supernatant collected for total protein concentration determination using Pierce® bicinchoninic acid (BCA) assay kit (Fisher Scientific). Protein lysates were re-suspended in Laemmli reducing buffer and 20 μg of each sample loaded and resolved by electrophoresis in 10% Criterion TGX Stain-Free Precast Gels at 100 V for 1.5 h (BioRad). Membranes were blocked using 5% milk and probed by overnight incubation at 4 °C with specific primary antibodies against α1 (1:200; 12410-1-AP, Proteintech) and α5 (1:500; NB300-195, Novus) GABAA receptor subunits, respectively. Blots were detected by Gel Doc EQ System Universal Hood II (Biorad) and quantified using ImageJ software (https://imageJ.nih.gov/ij/download.html). The expression levels for the respective proteins were normalized with β-actin (1:1000; sc-47778, Santa Cruz Biotechnology). In addition, we confirmed that β-actin expression was not impacted by sex by conducting a parallel experiment using Gapdh as primary antibody (1:1000; sc-32233, Santa Cruz Biotechnology).
2.5. Chromatin immunoprecipitation (ChIP) assay
To examine the role of epigenetic mechanisms in the differential expression of α1 and α5 GABAA receptor subunits in hippocampi of male and female APPNLGF and wildtype mice we measured the levels of histone acetylation and methylation at α1 (Gabra1) and α5 (Gabra5) GABAA receptor subunit gene promoters. We used specific antibodies against anti-acetylated histone H3 (Millipore Corp USA, catalog # 06–599) and tri-methyl histone H3 lysine4 (H3K4me3) (Cell Signaling, catalog # 9751S) for ChIP assay as previously described (Pandey et al., 2015; Gatta et al., 2021). Briefly, hippocampal tissues were weighed and fixed in methanol-free 1% formaldehyde by incubating on a rocking platform at room temperature for 15–20 min followed by homogenization in lysis buffer and DNA shearing by sonication. The resulting cross-linked DNA-chromatin complex was immunoprecipitated (IP) with anti-acetylated histone H3 or H3K4me3 antibody and antibody-chromatin complexes precipitated using protein A/G Plus-Agarose (Santa Cruz Biotech, Inc., Dallas, Texas). The final precipitated and purified DNA was concentrated using Chelex 100 Resin (BioRad, DesPlaines, IL) and quantified using qPCR with Maxima SYBR Green master mix and primers targeting two locations within Gabra1 (Forward: 24 to −3, Reverse: +83 to +63 [site 1] and Forward: 451 to −430, Reverse: 343 to −324 [site 2]) and Gabra5 (Forward: 74 to −56, Reverse: 3 to +17 [site 1] and Forward: 183 to −165, Reverse: 86 to −65 [site 2]) GABAA receptor promoter, respectively. Input DNA was used as internal standard for normalization. After subtraction of input DNA Ct value from the Ct values of the respective samples, the ΔΔCt method (Livak and Schmittgen, 2001) was used to determine the fold change in levels of H3K4me3 H3-acetylated at the Gabra1 and Gabra5 promoters. The percentages of IP DNA were calculated using the following formula: % (IP/total input) = 2(Ct (10% input−Ct (IP) x 100%. The sequences for the two sets of primer pair for Gabra1 and Gabra5 promoters are provided in Supplementary Table 2. Detailed information on the antibodies used for our experiments are reported in Supplementary Table 3.
2.6. Statistical analysis
All data are represented as mean ± SEM and were analyzed using Prism 9 statistical analysis software (GraphPad, San Diego, CA). For behavioral data, differences between mean values were determined using 3-way ANOVA followed by post-hoc comparison using Tukey's test when statistical significance for ANOVA was reached. Whereas differences between mean values for biochemical measurements were determined using 2-way ANOVA followed by post-hoc comparison with Tukey's test when significance for ANOVA was reached. Level of statistical significance for all experiments was set at p < 0.05.
3. Results
3.1. Male APPNLGF mice exhibit greater sensitivity to diazepam-induced impairment of locomotor activity
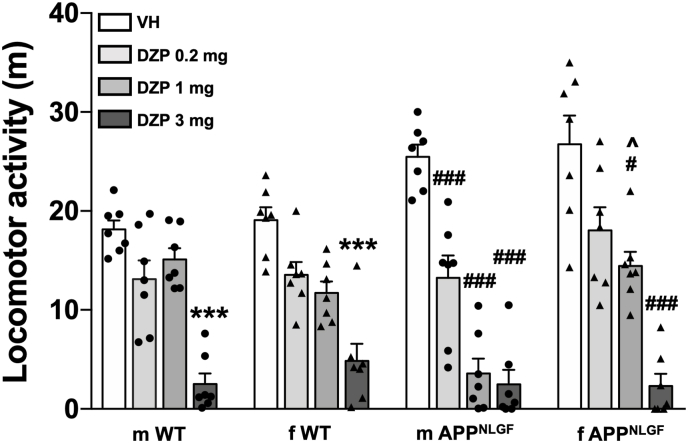
To evaluate the functional status of GABAergic neurotransmission in APPNLGF mice, we compared the effects of three doses (0.2, 1, and 3 mg/kg) of diazepam, a positive allosteric modulator of GABA action at a variety of GABAA receptor subtypes (Guidotti et al., 2005), on locomotor activity in male and female APPNLGF mice and their wildtype counterparts. It is noteworthy that acute or chronic administration of this dose-range of diazepam does not increase GABAA receptor subunit expression in the hippocampus (Impagnatiello et al., 1996). Three-way ANOVA revealed that 3 mg/kg of diazepam markedly suppressed locomotor activity in both wildtype and APPNLGF mice (Fig. 1, F [1,48] = 206, p < 0.0001). There was no significant interaction between drug - sex - genotype (F [1,48] = 0.0823, p = 0.3686). We also found that at 1 mg/kg, diazepam significantly suppressed locomotor activity (Fig. 1, F [1, 48] = 37.42, p < 0.0001); specifically, post hoc test showed significant effect between diazepam-treated wildtype male mice vs. diazepam-treated APPNLGF male mice (p < 0.0278) and diazepam-treated APPNLGF male mice vs diazepam-treated AppNLGF female mice (p < 0.0279). In addition, there was significant interaction between drug -sex -genotype (F [1,48] = 6.507, p = 0.0140) and between drug and genotype (F [1,48] = 16.60, p = 0.0002). Although three-way ANOVA analyses revealed statistically significant drug effect at 0.2 mg/kg of diazepam (Fig. 1, F[1,48] = 23.67, p < 0.0001), there was no significant interaction between drug-sex-genotype (F [1,48] = 1.035, p = 0.3141). However, there was significant effect of genotype (F [1,48] = 15.93, p = 0.0002) and a significant interaction between drug-genotype (F [1,48] = 7.374, p = 0.0092). All together, these data suggest that APPNLGF male and female mice have significantly higher basal locomotor activity than their wildtype counterparts. Most importantly, male APPNLGF mice showed greater sensitivity to the effect of diazepam than the female APPNLGF and their wildtype counterparts. Based on these observations, we chose 0.2 mg/kg of diazepam, which produced minimal suppression of locomotor activity and similar effects in male and female APPNLGF and wildtype mice, to further investigate the effects of diazepam on anxiolytic-like behavior, recognition, and spatial working memory paradigms.
Fig. 1.
Male APPNLGF mice exhibit greater sensitivity to diazepam-induced impairment of locomotor activity than female APPNLGF and wildtype (WT) mice in the open field test. Both male and female APPNLGF mice show significantly higher basal locomotor activity than their WT counterparts. While only 3 mg/kg of diazepam elicited significant suppression of locomotor activity in WT mice, 1 mg/kg of diazepam significantly suppressed locomotor activity in male and female APPNLGF mice, whereas only male APPNLGF mice were showed significant suppression of locomotor activity at 0.2 mg/kg of diazepam, suggesting that the male APPNLGF mice are more sensitivity to diazepam-induced impairment of locomotor activity than female APPNLGF and WT mice. Data represent mean ± SEM of 7 mice per group. ***p < 0.001 when compared to respective vehicle-treated male and female WT mice, #p < 0.05 and ###p < 0.001 when compared to respective vehicle-treated male and female APPNLGF mice, ∧p < 0.05 when compared to diazepam-treated male APPNLGF mice. Three-way ANOVA followed by Tukey's post hoc analysis.
3.2. A low dose of diazepam induced anxiolytic-like effect and worsen recognition memory impairment in male APPNLGF mice
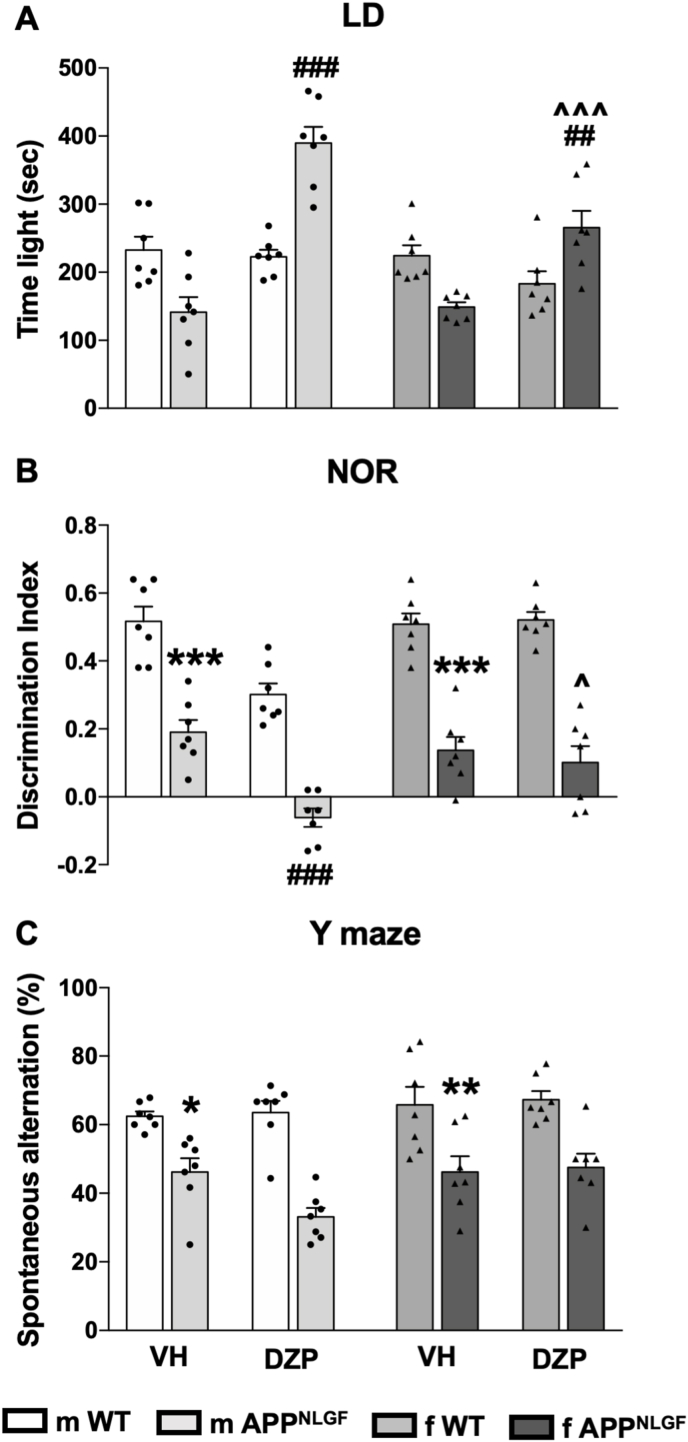
In the LD test for anxiety-like behavior (Fig. 2A), 0.2 mg/kg of diazepam produced significant increase of time spent in the light compartment in both wild type and APPNLGF male and female mice (F [1,48] = 34.45, p < 0.0001). In addition, post hoc comparison indicated that at baseline, male (p = 0.0305) but not the female (p = 0.1228) APPNLGF mice spent significantly (p = 0.0305) less time in the light compartment when compared to the wild type counterparts. Although this dose of diazepam did not produce statistically significant increase of time spent in the light compartment in male and female wild type mice, it produced significant anxiolytic-like effects in male (p < 0.0001) and female (p = 0.002) APPNLGF mice. However, diazepam was more efficacious in male than in female APPNLGF mice (diazepam-treated male APPNLGF mice vs. diazepam-treated female APPNLGF mice, p = 0.0005). Furthermore, three-way ANOVA analysis revealed significant effect of sex (F [1,48] = 9.770, p = 0.003), interactions of drug-sex (F [1,48] = 9.606, p < 0.0032), and drug - sex - genotype (F [1,48] = 3.4746, p < 0.0001) but no significant effect of genotype (F [1,48] = 2.606, p = 0.1130) or interaction of drug - sex-genotype (F [1,48] = 3.746, p = 0.0588). However, the number of crossings between light and dark compartment did not differ between all the experimental groups (Supplementary Fig. 1).
Fig. 2.
A low dose of diazepam produced greater alteration of affective behavior (A) and impaired recognition memory (B) in male than in female APPNLGF and wildtype (WT) mice. (A) Light/dark box (LD) exploration test for anxiety-like behaviors in male and female APPNLGF and wildtype mice. Following challenge with 0.2 mg/kg of diazepam, both male and female APPNLGF mice spent more time in the light compartment than male and female WT mice. Although diazepam elicited anxiolytic-like effects in male and female APPNLGF mice, it was more efficacious in male APPNLGF mice. (B) Novel object recognition (NOR) memory test shows baseline memory deficits in both male and female APPNLGF mice when compared to WT mice. This low dose of diazepam did not impair recognition memory in WT mice but produced significant impairment of recognition memory in male APPNLGF mice. (C) Although the Y maze test for spatial memory revealed baseline memory deficits in APPNLGF mice when compared to WT, acute challenge with the same low dose of diazepam tested in the LD and NOR behavioral paradigms failed to alter spatial memory performance. Data represent mean ± SEM of 7 mice per group. *p < 0.05, **p < 0.01, and ***p < 0.001 when compared to respective vehicle-treated male and female WT mice, ##p < 0.01 and ###p < 0.001 when compared to respective vehicle-treated male and female APPNLGF mice, ∧p < 0.05 and ∧∧∧p < 0.001 when compared to diazepam-treated male APPNLGF mice. Three-way ANOVA followed by Tukey's post hoc.
The result of the NOR test indicated that 0.2 mg/kg of diazepam resulted in a significant decrease of the discrimination index for the novel object in both wild type and APPNLGF mice (F [1,48] = 22.85, p < 0.0001) (Fig. 2B). Interestingly, we also found a highly significant interaction for genotype (F [1,48] = 208.2, p < 0.0001), suggesting memory deficits in both male and female APPNLGF mice at this age. Most importantly, post hoc comparison revealed that diazepam administration significantly decreased discrimination index in male (p = 0.0027) but not female (p > 0.999) wild type mice. Furthermore, post hoc comparison also indicated that while diazepam significantly decreased discrimination index in male (p = 0.0003) APPNLGF mice, it had no effect (p = 0.96) in female APPNLGF mice. Three-way ANOVA analysis revealed significant effect for sex (F [1,48] = 9.7580, p < 0.003), interactions between drug - sex (F [1,48] = 9.606, p < 0.0001), and of drug - sex - genotype (F [1,48] = 3.4746, p < 0.0001) but no there were significant interactions between drug - genotype, sex-genotype, or drug - sex - genotype.
In contrast to the LD and the NOR tests, three-way ANOVA analysis indicated no significant drug effect (F [1,48] = 0.7894, p = 0.3787) on the Y maze test for spatial memory (Fig. 2C) but a significant effect of sex (F [1,48] = 4.321, p < 0.0430) and genotype (F [1,48] = 9.7580, p < 0.0001). Post hoc comparison revealed no significant difference (p = 0.1222) between percent spontaneous alternation for diazepam-treated male APPNLGF mice and diazepam-treated female APPNLGF mice. Although this dose of diazepam failed to elicit spatial alternation memory deficits, the data revealed spatial alternation memory deficits in the vehicle-treated male and female APPNLGF mice when compared to their respective vehicle-treated wild type counterparts.
Taken together, the behavioral data demonstrate that both male and female APPNLGF mice showed memory impairment but not anxiety-like behavior at 12 months of age. However, a low dose of diazepam (0.2 mg/kg) which did not suppress locomotor activity or impaired spatial working memory, induced stronger anxiolytic-like effects in male than female APPNLGF mice. Importantly, diazepam was able to significantly worsen recognition memory in male but not in female APPNLGF mice, confirming a sex-biased pharmacological effect.
3.3. The expression of α1 and α5 GABAA receptor subunits is significantly higher in male APPNLGF mice
To investigate the molecular mechanisms underlying enhanced sensitivity of male APPNLGF mice to diazepam-induced anxiolytic-like effects and impairment of recognition memory, we studied the expression of α1 and α5 GABAA receptor subunits in hippocampus of the same cohort of male and female APPNLGF and wild type mice used in the behavioral experiments. We chose the hippocampus because it is the most studied brain area that has been shown to regulate the cellular aspects of synaptic plasticity, long-term potentiation, and long-term depression which underlie the cognitive, learning, and memory deficits that have been reported in AD subjects and preclinical models of AD (LaFerla and Green, 2012; Puzzo et al., 2015). Additionally, previous studies showed that α1-containing GABAA receptors are responsible for the sedative, ataxic, and cognitive impairment associated with routinely prescribed benzodiazepine recognition site ligands which act as full positive allosteric modulators of GABA action (Rudolph et al., 1999). In contrast, the α5-containing GABAA receptors which are abundantly expressed in hippocampus, regulate tonic GABA-currents, learning, and memory functions (Caraiscos et al., 2004).
Two-way ANOVA analysis for α1 GABAA receptor subunit mRNA expression in hippocampus indicated statistically significant (F [1,26] = 14.23, p = 0.0008) differences between groups, significant effect of sex (F [1,26] = 6.896, p = 0.014) but not genotype (Fig. 3A) on α1 GABAA mRNA expression. In addition, post hoc comparison revealed that α1 GABAA mRNA expression was significantly lower in female APPNLGF than male APPNLGF (p = 0.001) and female wild type (p = 0.048) mice (Fig. 3A). The results for α5 GABAA receptor subunit mRNA expression revealed no significant interaction between groups (Fig. 3B).
Fig. 3.
mRNA and protein levels of α1 and α5 GABAA receptor subunits in hippocampus of APPNLGF mice. Relative mRNA quantification of α1 (A) and α5 (B) GABAA receptor subunit mRNA in adult mice hippocampus shows that the expression of the α1 GABAA receptor subunit was significantly lower in female than in male APPNLGF mice. Protein levels of α1 (C) and α5 (D) GABAA receptor subunits in adult mice hippocampus show significantly higher expression of the α1 and α5 subunit in male than in female APPNLGF mice. Upper panels above the respective graphical representation are representative Western blots for the respective GABAA receptor subunit proteins and β-actin. GABAA receptor subunit mRNA levels are expressed as 2ΔCt while protein levels are expressed in percentage; the average of the respective WT mice considered as 100%. Data represent mean ± SEM of 8 mice per group. *p < 0.05 when compared to respective male and female WT mice, #p < 0.001 when compared to male APPNLGF mice. Two-way ANOVA followed by Tukey's post hoc.
For levels of α1 GABAA receptor subunit protein expression (Fig. 3C), two-way ANOVA analysis revealed statistically significant effect of genotype (F [1,26] = 6.44, p = 0.0198) but not sex for α1 GABAA protein expression. Post hoc comparison revealed significantly higher expression (p = 0.04) of α1 GABAA protein in APPNLGF male when compared to male wild type, near statistically significant difference (p = 0.0512) when compared to female wild type mice, but no significant difference when compared to APPNLGF female mice (Fig. 3C). We also found significant effects of sex (F [1,26] = 4.674, p = 0.04) and genotype (F [1,26] = 6.44, p = 0.002) for α5 GABAA receptor subunit protein expression (Fig. 3D). Furthermore, post hoc comparison revealed significantly higher α5 GABAA protein expression in APPNLGF male mice when compared to male (p = 0.01) and female (p = 0.002) wild type, and near statistically significant difference (p = 0.07) when compared to their APPNLGF female counterparts (Fig. 3D). All together these data indicate that α1 and α5 GABAA protein expression was higher in male APPNLGF than in female APPNLGF mice.
3.4. H3K4me3 epigenetic mark occupancy at Gabra1 and Gabra5 promoters correspond with the transcript levels for these receptors in hippocampus of male APPNLGF mice
We investigated the role of epigenetic mechanisms on the increased α1 and α5 GABAA receptor subunits transcript levels observed in the hippocampus of male APPNLGF mice. To this end, we studied acetylated histone H3 and tri-methyl histone H3 lysine4 (H3K4me3) occupancy at Gabra1 and Gabra5 promoters using specific antibodies against acetylated histone H3 and tri-methyl histone H3 lysine4 (H3K4me3) for ChIP assay (Fig. 4). Two-way ANOVA analysis revealed that there were no statistically significant differences between groups for acetylated-H3 occupancy at site 1 (F [1,24] = 0.4439, p = 0.5116) and site 2 (F [1,24] = 0.3041, p = 0.5864) of Gabra1 promoter, and at site 1 (F [1,24] = 0.1059, p = 0.7477) or at site 2 (F [1,24] = 0.32369, p = 0.6309) of Gabra5 promoter (Fig. 4A–D). This data suggests that histone acetylation mechanisms do not play a major role in the epigenetic regulation of these GABAA receptor transcripts in hippocampus of male APPNLGF mice., However, two-way ANOVA analysis indicated statistically significant differences between groups for H3K4me3 occupancy at site 1 (F [1,22] = 9.617, p = 0.00052) and site 2 (F [1,24] = 10.17, p = 0.0042) of Gabra1 promoter (Fig. 4 E and F, respectively). Post hoc comparison revealed that H3K4me3 occupancy at site 1 of the Gabra1 promoter was significantly higher in male (p = 0.0007) APPNLGF mice when compared to female APPNLGF mice, female wild type (p = 0.0015) and male wild type (p = 0.006) mice (Fig. 4E). For the occupancy of H3K4me3 at site 2 of the Gabra1 promoter, post hoc analysis revealed statistically significant higher occupancy in male (p = 0.0002) APPNLGF mice when compared to female APPNLGF mice, female wild type (p = 0.0009) and male wild type (p = 0.003) mice (Fig. 4F).
Fig. 4.
The occupancy of H3K4me3 but not acetylated-H3 epigenetic histone mark at Gabra1 and Gabra5 promoters regulate α1 and α5 GABAA receptor subunit transcript levels in hippocampus of male APPNLGF mice. (A, B, C, & D) Chromatin immunoprecipitation (ChIP) analysis for the occupancy of acetylated-H3 at two sites of Gabra1 (A & B) and Gabra5 (C & D) promoters was not significantly different in wildtype (WT) and APPNLGF mice. (E, F, G, & H) ChIP analysis of H3K4me3 at two sites of Gabra1 (E & F) and Gabra5 (G & H) show that H3K4me3 occupancy at both Gabra1 and Gabra5 promoters was significantly higher in male than in female APPNLGF mice. Data represent mean ± SEM of 8 mice per group. *p < 0.05 and ***p < 0.001 when compared to respective male and female WT mice, #p < 0.05 and ###p < 0.001 when compared to male APPNLGF mice. Two-way ANOVA followed by Tukey's post hoc.
Two-way ANOVA analysis of H3K4me3 occupancy at Gabra5 promoter revealed statistically significant differences at site 1(F [1,22] = 4.420, p = 0.0467) but not at site 2 (F [1,23] = 0.1211, p = 0.7310) of Gabra5 promoter (Fig. 4G and H, respectively). Post hoc comparison indicated that H3K4me3 occupancy at site 1 of Gabra5 promoter was significantly higher in male (p = 0.028) APPNLGF mice when compared to female APPNLGF mice, female wild type (p = 0.037) and male wild type (p = 0.0286) mice (Fig. 4G). These data indicate that the expression of α1 and α5 GABAA receptor subunit transcripts observed in male APPNLGF mice is regulated by H3K4me3 epigenetic mark, however the occupancy of this epigenetic mark is more remarkable at Gabra1 than at Gabra5 promoter. All together our ChIP data demonstrate that the expression of α1 and α5 GABAA receptor subunit transcripts in hippocampus of male APPNLGF is regulated by activating H3K4me3 epigenetic mark.
3.5. Downregulation of H3K4me3 demethylases in hippocampus of APPNLGF mice
Histone methyltransferases (Kmts) and demethylases (Kdms) are epigenetic regulators of H3K4me3 which is associated with gene activation (Santos-Rosa et al., 2002). Thus, H3K4 methylation could be enhanced and repressed by increased catalytic activity of Kmts and Kdms, respectively. To determine the controlling influence of Kdms and Kmts on H3K4 methylation and consequently the transcription of α1 and α5 GABAA receptor subunits, we measured hippocampal expression of Kdm1a, Kdm1b, and Kmt2b in the hippocampus of mice because changes in expression levels for these enzymes have been shown to contribute to cognitive and synaptic deficits in AD mouse models (Collins et al., 2019). We found that Kdm1a expression was significantly lower in males than female wildtype mice (p = 0.0012) in the hippocampus. Moreover, both male (p = 0.05) and female (p = 0.0002) APPNLGF mice showed statistically significant (F [1,26] = 20.26, p = 0.0001) decrease Kdm1a expression as compared to their respective wildtype control mice (Fig. 5A). Additionally, Kdm1b expression was significantly lower in female (p = 0.02) APPNLGF mice as compared to wildtype controls (Fig. 5B). However, we did not find sex differences in male and female for both Kdm1a and Kdm1b expression in APPNLGF mice. Also, no significant differences for the expression of Kmt2b in hippocampus of wild type and APPNLGF mice (Fig. 5C). These data suggest that decreased hippocampal expression of kdm1a and Kdm1b but not Kmt2b may lead to increased H3K4 methylation and consequently increased occupancy of this epigenetic mark at Gabra1 and Gabra5 promoters thereby activating the expression of α1 and α5 GABAA receptor subunit transcripts in hippocampus of male APNLGF mice.
Fig. 5.
The expression levels of H3K4me3 demethylases (Kdm1a and Kdm1b) are downregulated in hippocampus of APPNLGF mice. Relative mRNA analysis of Kdm1a (A), Kdm1b (B), and Kdm2b (C) expression in hippocampus of male and female wildtype (WT) and APPNLGF mice. Data represent mean ± SEM of 8 mice per group. *p < 0.05 and ***p < 0.001 when compared to respective male and female WT mice, ##p < 0.01 when compared to male APPNLGF mice. Two-way ANOVA followed by Tukey's post hoc.
4. Discussion
The main focus of this study was to examine the sex-dependent role of GABA-mediated neurotransmission and the impact of these changes on memory and behavior in an APP knock-in (APPNLGF) mouse model of AD. Accordingly, for the first-time we showed enhanced sensitivity of male APPNLGF mice to diazepam-induced anxiolytic-like effects as well as impairment of recognition memory when compared to female APPNLGF and their wild type counterparts. Indeed, we showed that a low dose of diazepam, which does not induce anxiolytic-like effects in wild type mice, induces stronger anxiolytic-like effects in male than female APPNLGF mice. Of note, the same low dose of diazepam significantly impairs recognition memory only in male APPNLGF mice. It is unlikely that these sex-specific behavioral effects of acute diazepam challenge are due to pharmacokinetic differences since it has been reported that diazepam is more rapidly metabolized in males than female rats because of higher hepatic enzymatic activity in male rats (Reilly et al., 1990). Notably, the effects of diazepam are long-lasting because it is metabolized into desmethyldiazepam and oxazepam, which are both pharmacologically active and are used as drug entities (Bertilsson et al., 1990). Moreover, it has been reported that female C57BL/6NCrlBR mice are more sensitive to the sedative effects of acute diazepam challenge than their male counterparts (Podhorna et al., 2002). Additionally, we demonstrated that the sex differences in response to diazepam-mediated behavioral effects is due to increased H3Kme3 occupancy at Gabra1 and Gabra5 promoters, which in turn leads to the increased expression of α1 and α5 GABAA receptor subunit transcripts in hippocampus of male APPNLGF mice. Lastly, the epigenetic reprogramming of hippocampal H3K4me3 found in our study may, at least in part, be due to dysfunction of Kdm1a and Kdm1b demethylases catalytic activity which resulted in the enhancement of H3K4me3-mediated gene activation. Interestingly, we found that Kdm1a expression was higher in wildtype female mice, suggesting that a sex-dependent function of Kdm1a might exist. Indeed, previous studies suggest that Kdm1 is essential for zygotic genome activation and ovarian cancer cells migration (Ancelin et al., 2016; Shao et al., 2015). However, the possible existence of sex-dependent function of Kdm1a in brain needs further investigation. Although we found sex difference in baseline expression of Kdma1, we did not observe sex differences in Kdm1a and Kdm1b deficits APPNLGF mice, suggesting that these demethylases may regulate different epigenetic processes and/or targets in male and female APPNLGF mice. In summary, our data suggests enhanced GABA-mediated inhibitory neurotransmission particularly in APPNLGF mice given a low dose of diazepam which fail to produce significant behavioral alteration in wild type mice, elicits anxiolytic-like effects in both male and female APPNLGF mice. Most significantly, we demonstrated that the sex differences in the sensitivity to diazepam-mediated effects may in part, be attributed to enhanced hippocampal α1-and α5-containing GABAA receptors-mediated inhibitory neurotransmission in male APPNLGF mice.
It is well known that alteration of GABAergic neurotransmission, the primary inhibitory neurotransmission in brain, plays a major role in a number of neuropsychiatric disorders, including AD (Li et al., 2016b; Rudolph and Mohler, 2014b; Schouseshoe et al., 2014). In addition, it has been suggested that facilitation of memory decline associated with Aβ accumulation, may be due to upregulation of GABA-mediated inhibitory neurotransmission in the brain (Sasaguri et al., 2017b). Furthermore, it has been reported that the upregulation of GABAergic neurotransmission observed in both clinical pathology and preclinical models of AD is due to a compensatory response to the aberrant excitatory neurotransmission found in hippocampus of hAPP mouse model of AD (Palop et al., 2007). In addition, Kwakowsky and colleagues reported prominent increases in the expression of α1 and α5 GABAA receptor subunits in all layers of the CA3 region and in CA1 region, respectively, of AD patients (Kwakowsky et al., 2018a). Moreover, Yoshiike et al. (2008) reported that hippocampal LTP and memory deficits observed in both adult (9–15 months) transgenic APP/PS1 and old (19–25 months) non-transgenic mice can be rescued by bicuculline, a competitive inhibitor of the GABA recognition site on GABAA receptor complex and picrotoxin, a non-competitive antagonist of GABA action on the GABAA receptor respectively. Taken together, these data suggests that the upregulation of α1 and α5 GABAA receptor subunits in hippocampus of APPNLGF mice may underlie the increased sensitivity to diazepam-mediated behavioral effects.
In contrast to histone acetylation, it has been reported that perturbations of histone methylation repress or activate gene transcription depending on the methylation status of specific histone residues (Wood, 2018). For example, methylation of histone H3 lysine 4 leads to activation of gene transcription while methylation of histone H3 lysine 9 is associated with repression of gene transcription (Rea et al., 2000; Kuzmichev et al., 2002). More specifically, it has been reported that increased histone methylation negatively impacts cognitive function in AD (Collins et al., 2019). The important role of H3K4me in learning, memory (Gupta et al., 2010) and synaptic transmission (Cheung et al., 2010), has raised interest on the role of H3K4me3 in neurodegenerative disease, including AD. In a recent study, Cao, and colleagues (Cao et al., 2020) reported significant increases of H3K4me3 epigenetic mark in postmortem prefrontal cortex of AD subjects and AD mouse model. Interestingly, these authors also found that administration of WDR5-0103, a specific H3K4me3 inhibitor, restored synaptic function and resulted in improvement of memory-related behavioral deficits in an AD mouse model. Our findings of significant increases in H3K4me3 occupancy at Gabra1 and Gabra5 promoter in male APPNLGF mice which associated with memory and behavioral changes were consistent with these observations. Given evidence suggesting that H3K4me3 is enriched at the transcriptional active sites of genes (Guenther et al., 2005), we hypothesize that the increase of hippocampal H3K4me3 occupancy at Gabra1 and Gabra5 promoters in male APPNLGF mice leads to increased expression of α1 and α5 GABAA receptor subunit transcripts and may consequently be responsible for the increase sensitivity to diazepam-induced behavioral effects in these mice. However, further studies using lentiviral approach to overexpress Kdm1a and/or Kdm1b in hippocampus or behavioral studies with negative allosteric modulators of α1- (β-carboline-3-carboxylate-t-butyl ester, β-CCt) and α5- (MRK-016) containing GABAA receptors will be needed in order to validate our hypothesis. H3K4me3 could be regulated by many factors including Kdms; we found that increased occupancy of H3K4me3 at Gabra1 and Gabra5 promoters is associated with decreased Kdm1a and Kdm1b expression in male but not in female APPNLGF mice, suggesting differential regulation of these histone demethylases on the different genes in male and female in male APPNLGF mice. Based on our results, we propose that the decrease Kdm1a and Kdm1b expression contributes to H3K4me3-mediated enhancement of α1 and α5 GABAA receptor subunit transcription in male APPNLGF mice, whereas the changes in these demethylases observed in female APPNLGF mice may regulate epigenetic marks that occupy promoters of different target genes. This speculation is based on our preliminary findings indicating preferential upregulation of inflammatory markers (Gfap, Nlrp3, Cd11b, and Tlr2) in female APPNLGF mice (Supplementary Fig. 2) and the observation that increased H3K4me3 epigenetic mark has also been linked with the regulation of immune functions (Gjoneska et al., 2015). However, further studies will be needed to determine if the promoters of these inflammatory markers are also preferential regulated by H3K4me3 epigenetic marks in female than in male APPNLGF mice.
5. Conclusions
Our study provided first evidence showing sex-dependent sensitivity to the action of a positive allosteric modulator of GABA action at GABAA receptors in a knock-in (APPNLGF) mouse model of Alzheimer's disease. Additionally, our data showed sex-dependent epigenetic regulation in the expression of GABAA receptor subunit transcripts in APPNLGF mice. Most importantly, our results suggest that perturbations of GABA neurotransmitter systems or epigenetic mechanisms may underlie the sex difference in memory deficits of AD. Future studies will continue this line of work by examining sex differences in GABA-mediated postsynaptic inhibitory currents and the modulatory effects of diazepam using electrophysiological recordings, and the epigenetic regulation on the of GABAA receptor subunit expression and functioning in other brain areas such as prefrontal cortex of APPNLGF mice. Additionally, the specific genes that are regulated by Kdm-H3K4m3 pathways differently between male and females in the mouse model of AD need to be determined.
Funding and disclosure
This work was supported by National Institute of Health grants RF1AG057884 and R01AG062249 to Dr. Hongxin Dong. The authors have no competing interest to disclose.
Authors statement
All the authors of this manuscript declare:
-
•
no conflicts of interest;
-
•
the data is not published and submitted elsewhere;
-
•
all the procedures reported in this manuscript have been approved;
-
•
the final version of the manuscript has been approved by all authors;
-
•
This study was supported by RF1AG057884 and R56AG053491 to Hongxin Dong.
CRediT authorcontribution statement
James Auta: Conceptualization, Investigation, Data curation, Validation, Writing – original draft, preparation, Visualization. Andrea Locci: Conceptualization, Investigation, Data curation, Validation, Writing – original draft, preparation, Visualization, Writing – review & editing, Visualization. Alessandro Guidotti: Conceptualization, Writing – review & editing. John M. Davis: Writing – review & editing. Hongxin Dong: Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crneur.2021.100025.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alzheimer’s Association Alzheimer's disease facts and figures. Alzheimer's Dementia. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Ancelin K., Syx L., Borenszstein M., Ranisavljevic R., Vassilev I. Maternal LSD1/KDM1A is an essential regulator of chromatin and transcription landscapes during zygotic genome activation. Elife. 2016;5 doi: 10.7554/eLife.08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell K.F.S., de Kort G.J.L., Steggerda S., Shigemoto R., Ribeiro-da-Silva A., Cuello A.C. Structural involvement of glutamatergic presynaptic boutons in a transgenic mouse model expressing early onset amyloid pathology. Neurosci. Lett. 2003;353:143–147. doi: 10.1016/j.neulet.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Bertilsson L., Baillie T.A., Reviriego J. Factors influencing the metabolism of diazepam. Pharmacol. Ther. 1990;45:85–91. doi: 10.1016/0163-7258(90)90009-q. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Broadbent N.J., Gaskin S., Squire L.R., Clark Object recognition memory and the rodent hippocampus. Learn. Mem. 2009;17(1):5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent N.J., Gaskin S., Squire L.R., Clark Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2009;101(40):14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q., Wang W., Williams J.B., Yang F., Wang Z., Yan Z. Targeting histone K4 trimethylation for treatment of cognitive and synaptic deficits in mouse models of Alzheimer's disease. Sci. Adv. 2020;6(50) doi: 10.1126/sciadv.abc8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos V.B., Elliot E.M., You-Ten K.E., Cheng V.Y., et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by a5 subunit-containing g-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. U.S.A. 2004;101(10):3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung I., Shulha H.P., Jiang Y., Matevossian A., Wang J., et al. Developmental regulation and individual differences of neuronal H3K4me3 epigenomes in the prefrontal cortex. Proc. Natl.Acad. Sic USA. 2010;107:8824–8829. doi: 10.1073/pnas.1001702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B.E., Greer C.B., Coleman B.C., Sweatt J.D. Histone H3 lysine K4 methylation and its role in learning and memory. Epigenet. Chromatin. 2019;12(1):7. doi: 10.1186/s13072-018-0251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis P.T. The interplay of neurotransmitters in Alzheimer's disease. CNS Spectr. 2005;10:6–9. doi: 10.1017/s1092852900014164. [DOI] [PubMed] [Google Scholar]
- Fritschy J.M. Epilepsy, E/I balance and GABAA receptor plasticity. Front. Mol. Neurosci. 2008;1:5. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatta E., Grayson D.R., Auta J., Saudagar V., Dong E., Chen Y. Genome-wide methylation in alcohol used disorder subjects: implications for an epigenetic regulation of the cortico-limbic glucocorticoid receptor (NR3C1) Mol. Psychiatr. 2021;26:1029–1041. doi: 10.1038/s41380-019-0449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjoneska E., Pfenning A.R., Mathys H., Quon G., Kundaje A., et al. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer's disease. Nature. 2015;518:365–369. doi: 10.1038/nature14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindpani K., Guzman B.C.G., Vinnakota C., Waldvogel H.J., Faull R.L., et al. Towards a better understanding of GABAergic remodeling in Alzheimer's disease. Int. Mol. Sci. 2017;18(8):1813. doi: 10.3390/ijms18081813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther M.G., Jenner R.G., Chevalier B., Nakamura T., Croce C.M., et al. Global and Hox-specific roles for MLL1 methyltransferase. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8603–8608. doi: 10.1073/pnas.0503072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A., Auta J., Davis J.M., Dong E., Grayson D.R., et al. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology. 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Gupta S., Kim S.Y., Artis S., Molfese D.L., Schumacher A., et al. Histone methylation regulates memory formation. J. Neurosci. 2010;30:3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman B.C.F., Vinnakota C., Govindpani K., Waldvogel H.J., Richard L.M., Faull R.L., et al. The GABAergic system as a therapeutic target for Alzheimer's disease. J. Neurochem. 2018;146:649–669. doi: 10.1111/jnc.14345. [DOI] [PubMed] [Google Scholar]
- Imbimbo B.P., Giardina G.A. Gamma-secretase inhibitors and modulators for the treatment of Alzheimer's disease: disappointment and hopes. Curr. Top. Med. Chem. 2011;11:1555–1570. doi: 10.2174/156802611795860942. [DOI] [PubMed] [Google Scholar]
- Impagnatiello F., Pesold C., Longone P., Caruncho H., Fritschy J.M., Costa E., Guidotti A. Modifications of gamma-aminobutyric acidA receptor subunit expression in rat neocortex during tolerance to diazepam. Mol. Pharm. 1996;49(5):822–831. [PubMed] [Google Scholar]
- Kulesskaya N., Voikar V. 2014. Assessment of Mouse Anxiety-like Behavior in the Light-Dark Box and Open-Field Arena: Role of Equipment and Procedure. [DOI] [PubMed] [Google Scholar]
- Kuzmichev A., Nishioka K., Erdjument-Bromage H., Tempst P., Reiberg D. Histone methyltransferase activity associated with human multiprotein complex containing the enhancer Zeste protein. Genes Dev. 2002;16:2893–2905. doi: 10.1101/gad.1035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakowsky A., Guzman B.C.F., Pandya M., Turner C., Waldvogel H.J., Faull R.L. GABAA receptor subunit expression changes in the human Alzheimer's disease hippocampus, subiculum, entorhinal cortex, and superior temporal gyrus. J. Neurochem. 2018;145:374–392. doi: 10.1111/jnc.14325. [DOI] [PubMed] [Google Scholar]
- Kwakowsky A., Calvo-Flores G.B., Govindpani K., Waldvogel H.J., Faull R.L. Gamma-aminobutyric acid A receptors in Alzheimer's disease: highly localized remodeling of a complex diverse signaling pathway. Neural Regen. Res. 2018;13(8):1362–1363. doi: 10.4103/1673-5374.235240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla F.M., Green K.N. Animal models of Alzheimer’ disease. Cold Spring Harb. Perspect Med. 2012;2 doi: 10.1101/cshperspect.a006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Sun H., Chen Z., Xu H., Bu G., Zheng H. Implications of GABAergic neurotransmission in Alzheimer's disease. Front. Aging Neurosci. 2016;8 doi: 10.3369/fnagi.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Sun H., Chen Z., Xu H., Bu G., Zheng H. Implications of GABAergic neurotransmission in Alzheimer's disease. Front. Aging Neurosci. 2016;8:31. doi: 10.3389/fnagi.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Locci A., Pinna G. Stimulation of peroxisome proliferator-activated receptor-a by N-palmitoylethanolamine engages allopregnanolone biosynthesis to modulate emotional behavior. Biol. Psychiatr. 2019;85(12):1036–1045. doi: 10.1016/j.biopsych.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Locci A., Yan Y., Rodriguez G., Dong H. Sex differences in CRF1, CRF, and CRFBP expression in C57Bl/6J mouse brain across lifespan and in response to acute stress. J. Neurochem. 2020 doi: 10.1111/jnc.15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci A., Oreland H., Rodriguez G., Gottliebson M., McClarty B., et al. Comparison of memory, affective behavior, and neuropathology in APPNLGF knock-in mice to 5xFAD and APP/PS1 mice. Behav. Brain Res. 2021;404:113192. doi: 10.1016/j.bbr.2021.113192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson E.J., Counts S.E., Perez S.E., Ginsberg S.D. Cholinergic system during the progression of Alzheimer's disease: therapeutic implication. Expert Rev. Neurother. 2008;8(11):1703–1718. doi: 10.1586/14737175.8.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muke L., Selkoe D.J. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb. Perspect. Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop J.J., Chin J., Roberson E.D., Wang J., Thwin M.T., et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S.C., Sakharkar A.J., Tang L., Zhang H. Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol. Dis. 2015;82:607–619. doi: 10.1016/j.nbd.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podhorna J., McCabe S., Brown E.R. Male and female C57BL/6 mice respond differently to diazepam challenge in avoidance learning tasks. Pharmacol. Biochem. Behav. 2002;72:13–21. doi: 10.1016/s0091-3057(01)00783-3. [DOI] [PubMed] [Google Scholar]
- Puzzo D., Gulisano W., Palmeri A., Arancio O. Rodent models of Alzheimer's disease drug discovery. Expet Opin. Drug Discov. 2015;10:703–711. doi: 10.1517/17460441.2015.1041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea S., Eisenhaber F., O’carroll D., Strahl B.D., Sun Z., et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- Reilly P.E.B., Thompson D.A., Mason S.R., Hooper W.D. Cytochrome p450IIIA enzymes in rat live microsomes: involvement in C3-Hydroxylation of diazepam and nordiazepam but not N-dealkylation of diazepam and temazepam. Mol. Pharmacol. 1990;37:767–774. [PubMed] [Google Scholar]
- Rudolph U., Mohler H. GABAA receptor subtypes: therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu. Rev. Pharmacol. Toxicol. 2014;54:483–507. doi: 10.1146/annurev-pharmtox-011613-135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U., Mohler H. GABAA receptor subtypes: therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu. Rev. Pharmacol. Toxicol. 2014;54:483–507. doi: 10.1146/annurev-pharmtox-011613-135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U., Crestani F., Benke D., Brunig I., Benson J.A., et al. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H., Schneider R., Bennister A.J., Sherriff J., Bernstein B.E., et al. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419:407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- Sasaguri H., Nilsson P., Hashimoto S., Nagata K., Saito T., Strooper B.D. APP mouse models for Alzheimer's disease preclinical studies. EMBO J. 2017;36:2473–2487. doi: 10.15252/embj.201797397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaguri H., Nilsson P., Hashimoto S., Nagata K., Saito T., Strooper B.D. APP mouse models for Alzheimer's disease preclinical studies. EMBO J. 2017;36:2473–2487. doi: 10.15252/embj.201797397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schouseshoe A., Madsen K.K., Barker-Haliski M.L., White H.S. The GABA synapse as a target for antiepileptic drugs: a historical overview focused on GABA transporters. Neurochem. Res. 2014;39:1980–1987. doi: 10.1007/s11064-014-1263-9. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer's disease. Cold Spring Harb. Perspect Med. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao G., Wang J., Li Y., Liu X., Xie X., et al. Lysine-specific demethylase 1 mediates epidermal growth factor signaling to promote cell migration via ovarian cancer cells. Sci. Rep. 2015;5:15344. doi: 10.1038/srep15344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solas M., Puerta E., Ramirez M.J. Treatment options in Alzheimer’s disease: the GABA story. Curr. Pharmaceut. Des. 2015;21:4960–4971. doi: 10.2174/1381612821666150914121149. [DOI] [PubMed] [Google Scholar]
- Wood I.C. The contribution and therapeutic potential of epigenetic modifications in Alzheimer's disease. Front. Neurosci. 2018;12:649. doi: 10.3389/fnins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiike Y., Kimura T., Yamashita S., Furudate H., Mizoroki Y., et al. GABAA receptor-mediated acceleration of aging-associated memory decline in APP/PS1 mice and its pharmacological treatment by picrotoxin. PLoS One. 2008;3(8) doi: 10.1371/journal.pone.0003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.