Abstract
Background:
Grade III-IV acute graft-versus-host disease (aGVHD) is associated with high short-term morbidity and mortality following allogeneic hematopoietic cell transplantation (HCT). The long-term effects after recovery from grade III-IV aGVHD are unknown.
Objectives:
The objectives of this study were to analyze late medical comorbidities, quality of life, non-relapse mortality and survival in patients treated for grade III-IV aGVHD.
Study design:
Chart review identified late effects and patients were asked to complete annual surveys to collect patient reported outcomes (PRO). Outcomes were compared between patients with grade 0-I aGVHD and grade III-IV aGVHD who were transplanted between 2001–2019 and survived at least one year after HCT.
Results:
Patients with a history of grade III-IV aGVHD (n=192) had significantly higher rates of late medical comorbidities (p <0.001) and worse physical (p=0.01) and mental (p=0.04) functioning compared to patients who only had grade 0-I aGVHD (n=615). Patients who survived >1 year post-transplant and had prior grade III-IV aGVHD also had worse 5-year overall survival (OS) (77.5% vs. 83.6%, p=0.006) and higher non-relapse mortality (NRM) (19.2% vs. 10.6%, p <0.001) than those with a history of grade 0-I aGVHD. No difference was found in cumulative incidence of chronic GVHD.
Conclusions:
Patients who recover from severe aGVHD remain vulnerable to developing late comorbidities. These patients would likely benefit from continued monitoring and supportive care to try to prevent late effects and improve survival.
Keywords: acute graft-versus-host disease, allogeneic transplant, quality of life
Introduction
Graft versus host disease (GVHD) continues to be a significant cause of morbidity and mortality after allogeneic hematopoietic cell transplant (HCT). Acute GVHD (aGVHD) affects up to 70% of patients following transplantation [1, 2], and around 14% of patients develop grade III-IV (severe) aGVHD with standard GVHD prophylaxis [3, 4]. Patients who develop grade III-IV aGVHD have historically had a dismal prognosis with a one-year overall survival (OS) around 30% [5]. A more recent review showed moderate improvement with OS around 40% at one year [6]. Patients with aGVHD receive potent immunosuppressive treatment, often develop severe and life-threatening organ damage and infections, and are at higher risk to develop chronic GVHD (cGVHD). Counseling patients with grade III-IV aGVHD about their prognosis should include information on both short and long-term outcomes, but the long-term effects on patients who survived after developing severe aGVHD have not yet been reported.
A few studies have reported short-term effects of grade III-IV aGVHD. One study evaluated 76 patients for changes in physical function following HCT using knee extensor strength and a 6-minute walk test. Both patients with grade 0-I and grade III-IV aGVHD had worse physical function after transplantation, but patients with grade 0-I aGVHD had significant recovery by discharge whereas patients with grade III-IV aGVHD did not [7]. Patients with severe aGVHD reported lower quality of life scores (QOL), increased fatigue and sleep disturbances [8, 9] in the peri-transplant period. Another study of 164 patients showed increased permanent organ damage more than 6 months after transplant in patients who had severe aGVHD, using established criteria by the American Medical Association, but this study did not directly capture QOL or functional status [10].
We studied patients who developed grade III-IV aGVHD and survived at least one year after HCT to compare QOL, functional status, organ function and subsequent survival compared with patients who had grade 0-I aGVHD.
Methods
Patients
We retrospectively identified 3426 patients ≥18 years of age who underwent a first and only allogeneic stem cell transplantation between 2001–2019. Of these patients, 1877 with grade II aGVHD were excluded as our focus was evaluating outcomes in patients with severe (grade III-IV) aGVHD. A previous study evaluating outcomes after 6 months in patients with grade II vs grade III-IV showed a significant difference in OS and non-relapse mortality (NRM) between the two groups [11]. Grade II aGVHD was also excluded because of the more heterogenous clinical manifestations [12] making results more difficult to interpret. As the grade II aGVHD group included the largest number of patients, a separate analysis evaluating late medical comorbidities including grade II was performed. Supplemental Figure 1. An additional 33 patients were excluded due to lack of consent, 610 patients who did not survive at least one year after HCT and 99 who relapsed during the first year, resulting in a study cohort of 807 patients. This study was approved by the Fred Hutchinson Cancer Center Institutional Review Board. All subjects signed informed consent.
End points
Late comorbidities were defined as the development or worsening of medical conditions following transplantation. These were classified by the authors prior to the analysis as minor, intermediate, and major comorbidities based on typical severity and treatment burden. Both long-term effects, defined as events starting during the peri-transplant period and persisting, and late effects, defined as events starting after transplant is completed, operationally defined here as 1 year post-transplant, were included. Examples of minor complications include development of cataracts, hypertension, and hyperlipidemia. Examples of intermediate complications include adrenal insufficiency, deep venous thrombosis (DVT), and diabetes. Major complications include acute respiratory distress syndrome, heart failure, and renal failure requiring dialysis. Supplementary table 1 lists the conditions assigned to each group.
Quality of life scores were obtained from annual patient-reported outcome (PRO) surveys that included the short form 12 questions (SF-12) to derive physical and mental functioning scores [13]. Scores on the SF-12 range from 0–100 where 50 indicates the general population norm and higher scores reflect better functioning. A clinically meaningful difference is greater than 5 points (half a standard deviation). Patients reported their overall health as excellent, very good, good, fair or poor and selected a Karnofsky performance score (KPS) that best reflected their functional status. Patients were sent surveys by postal mail or email, according to their preference, and non-responders received one reminder.
Chronic GVHD (cGVHD) diagnoses were determined by established NIH guidelines [14]. Non-relapse mortality, with relapse as a competing risk, and overall survival were measured from time of transplant until death, although all subjects had survived one year without relapse to be included in the analysis.
Statistical analysis
Characteristics of the study population were tabulated and compared by aGVHD grade groups using the Chi-square test and the Wilcoxon rank sum test, as appropriate. Cumulative incidence of late effects by severity and organ groups were calculated from time of transplant with relapse and death as competing risks and were compared by aGVHD group using Gray’s Test. Univariate and multivariable regression was used to compare the patient report of general health and KPS, and the mean SF12 physical and mental functioning scores per patient between the aGVHD grade groups. Non-relapse mortality, with relapse as a competing risk, cGVHD and overall survival were calculated from time of transplant and compared between aGVHD grade groups using Gray’s Test and the log-rank test, respectively. Multivariate modeling adjusted for significant clinical covariates with p≤0.10 in univariate analyses from the variables in table 1.
Table 1.
Population characteristics
| Characteristic | Total (N=807) | AGVHD grade 0-I (N=615) | AGVHD grade III-IV (N=192) | P-value1 |
|---|---|---|---|---|
| Age at transplant (years) | ||||
| Median (IQR) | 51.9 (39.5–61.6) | 53.1 (41.6–62.2) | 47.1 (34.8–58.7) | <.001 |
| Sex | 0.57 | |||
| Female | 322 (39.9%) | 242 (39.3%) | 80 (41.7%) | |
| Male | 485 (60.1%) | 373 (60.7%) | 112 (58.3%) | |
| Disease | 0.03 | |||
| Acute lymphoblastic leukemia | 74 (9.2%) | 44 (7.2%) | 30 (15.6%) | |
| Acute myeloid leukemia | 321 (39.8%) | 249 (40.5%) | 72 (37.5%) | |
| Chronic lymphocytic leukemia | 35 (4.3%) | 29 (4.7%) | 6 (3.1%) | |
| Chronic myeloid leukemia | 60 (7.4%) | 49 (8.0%) | 11 (5.7%) | |
| Myelodysplastic syndrome | 218 (27.0%) | 164 (26.7%) | 54 (28.1%) | |
| Non-Hodgkin lymphoma | 38 (4.7%) | 32 (5.2%) | 6 (3.1%) | |
| Non-malignant disorders | 41 (5.1%) | 31 (5.0%) | 10 (5.2%) | |
| Other | 20 (2.5%) | 17 (2.8%) | 3 (1.6%) | |
| Myeloablative | 0.001 | |||
| No | 245 (30.4%) | 205 (33.3%) | 40 (20.8%) | |
| Yes | 562 (69.6%) | 410 (66.7%) | 152 (79.2%) | |
| Source | <.001 | |||
| Bone marrow | 108 (13.4%) | 85 (13.8%) | 23 (12.0%) | |
| Peripheral blood stem cell | 654 (81.0%) | 515 (83.7%) | 139 (72.4%) | |
| Cord blood | 45 (5.6%) | 15 (2.4%) | 30 (15.6%) | |
| Donor match | <.001 | |||
| Related donor | 318 (39.4%) | 279 (45.4%) | 39 (20.3%) | |
| Haploidentical | 14 (1.7%) | 11 (1.8%) | 3 (1.6%) | |
| Unrelated donor | 430 (53.3%) | 310 (50.4%) | 120 (62.5%) | |
| Cord | 45 (5.6%) | 15 (2.4%) | 30 (15.6%) | |
| Donor female/recipient male | 0.51 | |||
| No | 605 (76.0%) | 467 (76.6%) | 138 (74.2%) | |
| Yes | 191 (24.0%) | 143 (23.4%) | 48 (25.8%) | |
| Missing | [N=11] | [N=5] | [N=6] | |
| HCT-CI | ||||
| Median (IQR) | 2 (0–3) | 2 (0–3) | 2 (1–3) | 0.04 |
| N | 507 | 386 | 121 | |
| 0 | 132 (26.0%) | 111 (28.8%) | 21 (17.4%) | 0.04 |
| 1–2 | 170 (33.5%) | 127 (32.9%) | 43 (35.5%) | |
| 3+ | 205 (40.4%) | 148 (38.3%) | 57 (47.1%) | |
| Missing | [N=198] | [N=139] | [N=59] | |
| Donor/recipient CMV | 0.92 | |||
| −/− | 255 (33.5%) | 198 (33.2%) | 57 (34.5%) | |
| +/− | 189 (24.8%) | 146 (24.5%) | 43 (26.1%) | |
| −/+ | 100 (13.1%) | 79 (13.3%) | 21 (12.7%) | |
| +/+ | 217 (28.5%) | 173 (29.0%) | 44 (26.7%) | |
| Missing | [N=46] | [N=19] | [N=27] | |
| Year of transplant | 0.58 | |||
| 2001–2004 | 182 (22.6%) | 144 (23.4%) | 38 (19.8%) | |
| 2005–2009 | 172 (21.3%) | 131 (21.3%) | 41 (21.4%) | |
| 2010–2014 | 176 (21.8%) | 136 (22.1%) | 40 (20.8%) | |
| 2015–2019 | 277 (34.3%) | 204 (33.2%) | 73 (38.0%) |
Abbreviations: AGVHD, acute graft-versus-host disease; URD, unrelated donor; CMV, cytomegalovirus; HCT-CI, hematopoietic cell transplant-comorbidity index
based on the Chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables
Results
Participant characteristics
Table 1 shows the characteristics and Figure 1 shows the selection of the study cohort. There were 615 patients who had been diagnosed with grade 0-I aGVHD and 192 patients diagnosed grade III-IV aGVHD who were still alive without relapse one-year post-transplant. The median follow-up time for survivors was 7.2 years (interquartile range, 3.4–13.0). Most of the patients had received myeloablative conditioning and mobilized peripheral blood stem cells (PBSC). Significant differences were seen in disease, type of conditioning regimen, stem cell source, donor type, patient age at transplant, and baseline hematopoietic cell transplant-comorbidity index (HCT-CI) between the aGVHD groups. There were no differences in donor-recipient sex match, CMV match or year of transplant.
Figure 1.

Consort diagram of the population
Severity of late comorbidities
Patients who developed grade III-IV aGVHD had significantly higher cumulative incidences of minor (HR 1.3, 95% CI 1.08–1.58), intermediate (HR 1.94, 95% CI 1.59–2.37), and major late effects (HR 2.03, 95% CI 1.57–2.62) than patients who had grade 0-I aGVHD. The cumulative incidence of patients developing a major late effect at 10 years was 42% in the grade 0-I and 61% in the grade III-IV aGVHD cohorts. Figure 2
Figure 2.
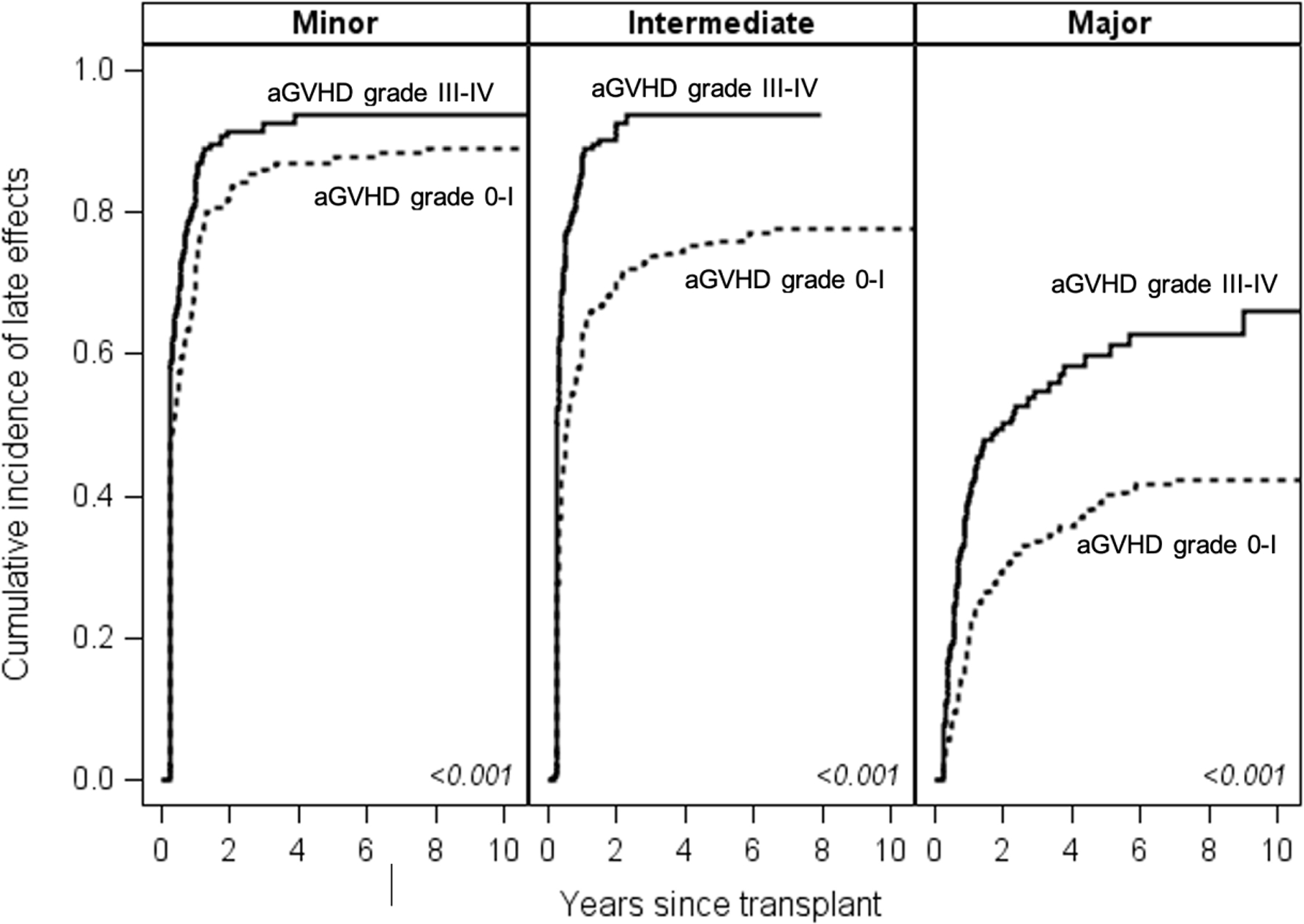
Cumulative incidences of minor, intermediate and major late effects, according to prior 0-I or III-IV acute GVHD.
A separate analysis including survivors of grade II aGVHD showed that patients with grade III-IV aGVHD has significantly higher rates of major late comorbidities (HR 1.42 95% CI 1.13 – 1.78, p=0.003) compared to patients with grade II aGVHD. The two groups had similar rates of minor (HR 1.06, 95% CI 0.88–1.26, p=0.55) and intermediate (HR 1.18, 95% CI 0.99–1.41, p=0.06) comorbidities. Supplemental Figure 1.
Organ dysfunction
Late medical comorbidities were also characterized by organ involvement. Patients with grade III-IV aGVHD had significantly higher cumulative incidences of cardiac (p=0.006), endocrine (p<0.001), genitourinary/renal (p<0.001), hematologic (p<0.001) and infectious diseases complications (p<0.001) when compared to patients who had grade 0-I aGVHD. There was no significant difference in the cumulative incidence of gastrointestinal complications between the two groups (p=0.22).
Chronic graft-versus-host disease
No significant difference was found in the cumulative incidence of NIH-defined chronic GVHD between patients with grade III-IV aGVHD and patients with grade 0-I aGVHD in unadjusted analysis (p=0.18, Table 2 and Figure 3) or multivariable analysis (HR 1.22, 95% CI 0.98–1.51, p=0.08).
Table 2.
Unadjusted Posttransplant Outcomes
| Outcomes | Total (N=807) | AGVHD grade 0-I (N=615) | AGVHD grade III-IV (N=192) | P-value1 |
|---|---|---|---|---|
| Relapsed after 1 year post-HCT | 0.02 | |||
| No | 734 (91.0%) | 551 (89.6%) | 183 (95.3%) | |
| Yes | 73 (9.0%) | 64 (10.4%) | 9 (4.7%) | |
| Chronic GVHD | 0.17 | |||
| No | 329 (41.3%) | 259 (42.7%) | 70 (37.0%) | |
| Yes | 467 (58.7%) | 348 (57.3%) | 119 (63.0%) | |
| Missing | [N=11] | [N=8] | [N=3] | |
| SF-12 PCS, mean (STD) | 45.2 (10.8) | 45.9 (10.5) | 42.5 (11.8) | 0.01 |
| SF-12 MCS, mean (STD) | 51.6 (9.1) | 52.0 (8.7) | 49.7 (10.6) | 0.04 |
| Missing | [N=386] | [N=273] | [N=113] | |
| Overall health | ||||
| Poor, Fair | 64 (14.8%) | 44 (12.5%) | 20 (24.7%) | 0.005 |
| Good, Very Good, Excellent | 370 (85.2%) | 309 (87.5%) | 61 (75.3%) | |
| Missing | [N=373] | [N=262] | [N=111] | |
| KPS | ||||
| <80 | 61 (14.4%) | 41 (11.9%) | 20 (25.6%) | 0.002 |
| 80+ | 362 (85.6%) | 304 (88.1%) | 58 (74.4%) | |
| Missing | [N=384] | [N=270] | [N=114] | |
| Died after 1 year post-HCT | 0.02 | |||
| No | 609 (75.5%) | 476 (77.4%) | 133 (69.3%) | |
| Yes | 198 (24.5%) | 139 (22.6%) | 59 (30.7%) | |
| Follow up years among survivors | ||||
| Median (IQR) | 7.2 (3.4–13.0) | 7.3 (3.7–13.1) | 6.3 (2.5–12.0) | 0.06 |
HCT, hematopoietic stem cell transplant; GVHD, graft-versus-host disease; SF-12, short form survey 12; PCS, physical component summary; MCS, mental component summary; STD, standard deviation; KPS, Karnofsky performance scale; IQR, interquartile range
based on the Chi-square test for categorical variables, the T-test where means are reported, and the Wilcoxon rank sum test where medians are reported
Figure 3.
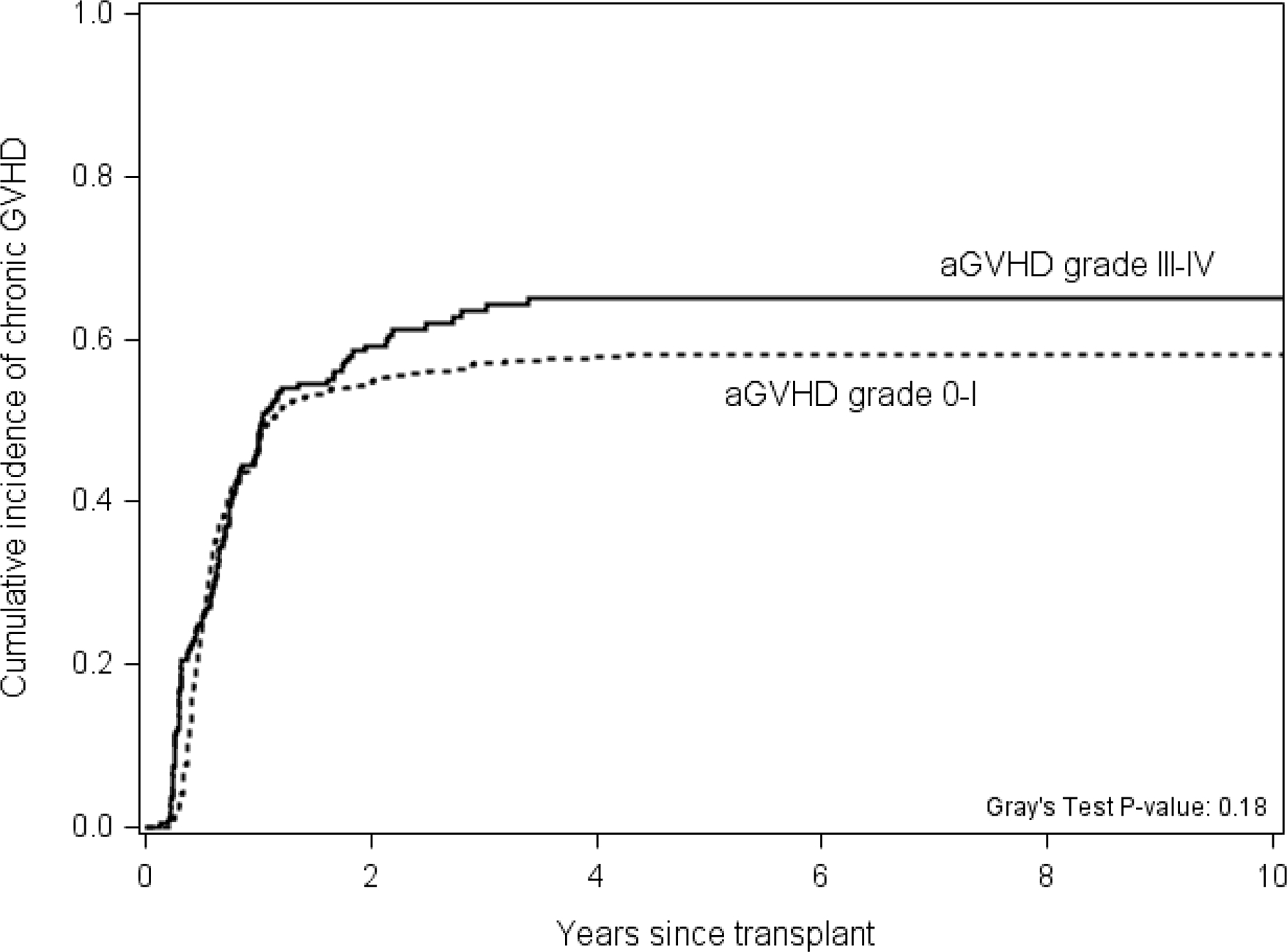
The cumulative incidence of chronic GVHD by prior grade 0-I or grade III-IV acute graft-versus-host disease group.
Patient-reported outcomes
Of the 807 patients in the analysis, 434 (54%) returned at least one posttransplant survey and were including in the PRO subanalysis. Patients with a history of severe aGVHD were more likely to report poor or fair health (24.7% vs. 12.5%, p=0.005) and a KPS of 70 or lower (25.6% vs. 11.9%, p=0.002) than patients with grade 0-I aGVHD. Table 2. These results were confirmed in multivariable modeling adjusting for significant clinical covariates, both including and excluding HCT-CI scores which were associated with PROs but where almost 40% of patients were missing data. The adjusted odd ratios (95% confidence intervals) for both measures in the models including HCT-CI were 2.5 (1.25–5.0) when the overall health model was adjusted for patient age at transplant, patient sex, patient-donor CMV serostatus, HCT-CI and presence of cGVHD and the KPS model was adjusted for patient age at transplant, patient-donor CMV serostatus, year of transplant and HCT-CI. Mean PCS and MCS scores were worse in survivors of grade III-IV aGVHD compared to patients who had 0-I aGVHD (PCS difference −3.34 points, p=0.01; MCS difference −2.3 points, p=0.04). These relationships persisted after adjustment for age at transplant, conditioning intensity, donor match, gender mismatch with female donor to male recipient, patient-donor CMV serostatus, year of HCT, cGVHD and HCT-CI for PCS, and for gender, age at transplant and HCT-CI for MCS. Table 3. The mean difference between the two aGVHD groups was less than 5 points for both the physical and mental functioning scores, so although they were statistically different, the differences did not meet the threshold for being clinically meaningful.
Table 3.
Multivariable analysis of SF-12 scores associated with 0-I or III-IV acute graft-vs.-host disease
| SF-12 score | Parameter | Estimate | p-value |
|---|---|---|---|
| Physical subscale | Acute GVHD III-IV vs. 0-I | −4.37 | 0.02 |
| Myeloablative | 0.66 | 0.69 | |
| Donor match | 0.20 | ||
| Related | reference | ||
| Haploidentical | −19.29 | 0.07 | |
| Unrelated | −0.29 | 0.84 | |
| Donor female/patient male | −3.31 | 0.04 | |
| Donor/recipient CMV status | 0.74 | ||
| +/+ | reference | ||
| +/− | −1.22 | 0.49 | |
| −/+ | −0.86 | 0.72 | |
| −/− | −1.96 | 0.27 | |
| Year HCT | 0.53 | ||
| 2005–2009 | reference | ||
| 2010–2014 | −1.76 | 0.40 | |
| 2015–2019 | −0.24 | 0.91 | |
| Chronic GVHD | −2.23 | 0.12 | |
| Age at transplant, per year | −0.09 | 0.19 | |
| HCT-CI | 0.006 | ||
| 0 | reference | ||
| 1–2 | −1.65 | 0.36 | |
| 3+ | −5.47 | 0.002 | |
| Mental subscale | Acute GVHD III-IV vs. 0-I | −3.58 | 0.01 |
| Sex (male) | 0.80 | 0.47 | |
| Age at transplant, per year | 0.14 | 0.002 | |
| HCT-CI | 0.001 | ||
| 0 | reference | ||
| 1–2 | −3.50 | 0.01 | |
| 3+ | −4.91 | <0.001 |
SF-12, short form survey 12; GVHD, graft-versus-host disease; CMV, cytomegalovirus; HCT hematopoietic stem cell transplant; HCT-CI hematopoietic cell transplant-comorbidity index
Relapse and Non-relapse mortality
The relapse rate was lower in patients with grade III-IV aGVHD compared to patients with grade 0-I aGVHD, 4.7% vs 10.4%, p=0.02. No multivariable analysis was performed because of the small number of events. Non-relapse mortality (NRM) beyond one year was significantly higher in patients with grade III-IV aGVHD compared to those with grade 0-I aGVHD (p<0.001). The 5-year NRM, after already surviving for one-year post-transplant, was 19.2% for patients with a history of grade III-IV aGVHD compared to 10.6% in the grade 0-I aGVHD group. Table 2 and Figure 4. In multivariable analysis, the severe aGVHD group had higher NRM (HR 2.61, 95% CI 1.81–3.76, p<0.001) adjusting for factors that differed between the groups. Supplementary Table 2. As HCT-CI was only available for around 60% of the patients, it was excluded from the multivariable analysis. In an exploratory multivariable analysis, baseline HCT-CI was not significantly associated with NRM (p=0.46).
Figure 4.

The cumulative incidence of non-relapse mortality by prior grade 0-I or grade III-IV acute graft-versus-host disease (aGVHD) group.
Overall survival
Despite only including those who have survived to one-year post-transplantation, overall survival beyond one year was significantly worse in patients with grade III-IV aGVHD compared to patients with grade 0-I aGVHD. The 5-year OS for patients who had survived at least one-year post-transplant was 77.5% for patients with grade III-IV aGVHD compared with 83.6% for patients with grade 0-I aGVHD (p=0.006). Figure 5. In multivariable analysis, the severe aGVHD group had worse OS (HR 1.87, 95% CI 1.35–2.60, p<0.001) adjusting for factors that differed between the groups. In an exploratory multivariable analysis, baseline HCT-CI was not significantly associated with OS (p=0.5).
Figure 5.

The probability of overall survival by prior grade 0-I or grade III-IV acute graft-versus-host-disease (aGVHD) group
Discussion
Severe (grade III-IV aGVHD) is associated with significant morbidity and mortality during the peri-transplant period. Lasting side effects for patients who survive grade III-IV aGVHD have not been extensively studied but this information would be helpful for patient counseling and to guide long-term follow-up care. We analyzed patients with a history of grade III-IV aGVHD who survived without evidence of disease relapse for at least one year after transplant. These patients continued to have higher non-relapse mortality and lower overall survival compared to patients with a history of grade 0-I aGVHD. Although patients with grade III-IV aGVHD had statistically higher baseline HCT-CI scores, a multivariable analysis showed that baseline HCT-CI did not have a significant association with either OS or NRM in our population. Patients with prior grade III-IV aGVHD also developed significantly higher rates of minor, intermediate and major late comorbidities compared to patients with grade 0-I and significantly higher rates of major late comorbidities when compared to patients with grade II aGVHD. When these late effects were categorized by organ system, the survivors of grade III-IV aGVHD had persistently higher rates of organ dysfunction than patients with prior grade 0-I aGVHD. Patients with grade III-IV aGVHD can develop multiorgan damage due to complications of aGVHD and/or the side effects of the treatments for aGVHD. They usually require higher doses of steroids and longer duration of steroids, which can lead to many complications such as hyperglycemia, insulin dependence, adrenal insufficiency, and immune suppression. These patients also require ongoing treatment with other immunosuppressive agents, which can further exacerbate organ dysfunction contributing to the worse OS and higher NRM seen in this group.
Additionally, patients with grade III-IV aGVHD reported worse physical and mental functioning compared to patients with grade 0-I aGVHD although this difference did not quite meet the threshold for being clinically meaningful on the SF-12 PCS and MCS. It is possible that a more sensitive multi-dimensional instrument would be better able to detect a meaningful difference because patients with severe aGVHD were more than twice as likely to report worse health using other measures. One limitation is that we are missing PRO data on half the population who did not return their surveys. Nevertheless, the available data indicate that grade III-IV aGVHD is not only associated with worse QOL measures but can also continue to impact functional status after recovery from aGVHD.
Given the lasting higher morbidity and mortality seen in the population with grade III-IV aGVHD, one issue to consider is how to improve supportive care for this population of vulnerable patients, as integrative palliative care or supportive care programs are rarely provided for patients with severe aGVHD [15]. Comprehensive supportive care during the time of severe aGVHD treatment is likely helpful given the life-threatening nature and high symptom burden of their situations. For those who survive, our study highlights the importance of continued monitoring for late complications and organ dysfunction. Developing a specialized post-transplant program for detecting and providing early treatment for anticipated complications for these patients may be of significant benefit.
These data also highlight the necessity for improving therapeutic options for the prevention and treatment of severe aGVHD. Patients who have had grade III-IV aGVHD and survived one-year post-transplant continue to have significantly worse outcomes compared to patients who only had grade 0-I aGVHD. Ultimately, preventing the development of grade III-IV aGVHD will be the most effective way of reducing these complications.
Interestingly, the cumulative incidence of cGVHD was not statistically different among the two groups although aGVHD is a known risk factor for cGVHD [16]. One explanation for this finding could be that in our population, cord blood grafts were overrepresented among survivors of grade III-IV aGVHD compared to grade 0-I aGVHD (15.6% vs 2.4%). Cord blood transplantation has been associated with a decreased incidence of cGVHD [17]. Another notable difference was that the survivors in the grade 0-I group were older than the patients in the grade III-IV aGVHD group (median age 53 vs 47, p<0.001), and older age is associated with increased rates of cGVHD [18]. The grade 0-I aGVHD group also had higher rates of peripheral blood stem cell (PBSC) transplants compared to the grade III-IV aGVHD group (83.7% vs 72.4%), and PBSC is associated with higher rates of cGVHD [19]. Given the low number of patients in the grade III-IV aGVHD group, this study may not be powered to see a statistically significant difference in rates of cGVHD between the two groups. Patients who died prior to one year were not included in this analysis, and many may have developed cGVHD as the median time to diagnosis of chronic GVHD is 159 (overlap) to 244 (classic chronic) days post-transplant [20]. Finally, patients with grade II aGVHD were excluded from our analysis and most studies that found an association between aGVHD and cGVHD included all patients.
This study also showed that patients with grade III-IV aGVHD had lower relapse rates compared to patients with grade 0-I aGVHD. This association has been previously described and is thought to be due to an increased graft-versus-leukemia effect in patients with either aGVHD or cGVHD [21, 22].
In summary, this study showed that patients who recover from grade III-IV aGVHD have significantly worse long-term outcomes when compared to patients with grade 0-I aGVHD, with one-year survivors having increased long-term morbidity, higher mortality, and worse quality of life. As these patients continue to have late comorbidities, especially with higher rates of organ impairment, this study highlights the need for specialized supportive care and close follow up for this population of patients to further enhance the recovery process with the goal of preventing late effects and improving survival and QOL measures. These data can provide patients with a more in depth understanding of post-transplant complications and the recovery process after severe aGVHD to allow them to better participate in their health care.
Supplementary Material
Highlights.
Survivors of grade III-IV aGVHD have high rates of late comorbidities
Patients with severe aGVHD surviving for at least a year had 5-year survival of 78%
Funding
CA018029(SJL); T32HL007093 (NR)
Footnotes
Conflict of Interest
The authors do not have any conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin PJ, et al. , First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant, 2012. 18(8): p. 1150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flowers ME, et al. , Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood, 2011. 117(11): p. 3214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeiser R and Blazar BR, Acute Graft-versus-Host Disease - Biologic Process, Prevention, and Therapy. N Engl J Med, 2017. 377(22): p. 2167–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Souza A, et al. , Current Use and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant, 2017. 23(9): p. 1417–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamani K, et al. , Prognosis of grade 3–4 acute GVHD continues to be dismal. Bone Marrow Transplant, 2013. 48(10): p. 1359–61. [DOI] [PubMed] [Google Scholar]
- 6.El-Jawahri A, et al. , Improved Treatment-Related Mortality and Overall Survival of Patients with Grade IV Acute GVHD in the Modern Years. Biol Blood Marrow Transplant, 2016. 22(5): p. 910–8. [DOI] [PubMed] [Google Scholar]
- 7.Hamada R, et al. , Effect of the severity of acute graft-versus-host disease on physical function after allogeneic hematopoietic stem cell transplantation. Support Care Cancer, 2020. 28(7): p. 3189–3196. [DOI] [PubMed] [Google Scholar]
- 8.Pidala J, Anasetti C, and Jim H, Quality of life after allogeneic hematopoietic cell transplantation. Blood, 2009. 114(1): p. 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bevans MF, Mitchell SA, and Marden S, The symptom experience in the first 100 days following allogeneic hematopoietic stem cell transplantation (HSCT). Support Care Cancer, 2008. 16(11): p. 1243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adachi Y, et al. , Permanent Impairment-Free, Relapse-Free Survival: A Novel Composite Endpoint to Evaluate Long-Term Success in Allogeneic Transplantation. Biol Blood Marrow Transplant, 2020. 26(5): p. 1005–1012. [DOI] [PubMed] [Google Scholar]
- 11.Ringden O, et al. , What is the outcome in patients with acute leukaemia who survive severe acute graft-versus-host disease? J Intern Med, 2018. 283(2): p. 166–177. [DOI] [PubMed] [Google Scholar]
- 12.Mielcarek M, et al. , Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica, 2015. 100(6): p. 842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ware J Jr., Kosinski M, and Keller SD, A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care, 1996. 34(3): p. 220–33. [DOI] [PubMed] [Google Scholar]
- 14.Jagasia MH, et al. , National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant, 2015. 21(3): p. 389–401 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wenzel F, et al. , Burden and Needs of Patients with Severe GvHD from the Supportive and Palliative Care Perspective-A Literature Review. Cancers (Basel), 2021. 13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storb R, et al. , Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med, 1983. 308(6): p. 302–7. [DOI] [PubMed] [Google Scholar]
- 17.Lazaryan A, et al. , Risk Factors for Acute and Chronic Graft-versus-Host Disease after Allogeneic Hematopoietic Cell Transplantation with Umbilical Cord Blood and Matched Sibling Donors. Biol Blood Marrow Transplant, 2016. 22(1): p. 134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remberger M, et al. , Risk factors for moderate-to-severe chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant, 2002. 8(12): p. 674–82. [DOI] [PubMed] [Google Scholar]
- 19.Cutler C, et al. , Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol, 2001. 19(16): p. 3685–91. [DOI] [PubMed] [Google Scholar]
- 20.Jagasia M, et al. , Incidence and outcome of chronic graft-versus-host disease using National Institutes of Health consensus criteria. Biol Blood Marrow Transplant, 2007. 13(10): p. 1207–15. [DOI] [PubMed] [Google Scholar]
- 21.Weisdorf D, et al. , Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biol Blood Marrow Transplant, 2012. 18(11): p. 1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeshurun M, et al. , The impact of the graft-versus-leukemia effect on survival in acute lymphoblastic leukemia. Blood Adv, 2019. 3(4): p. 670–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


